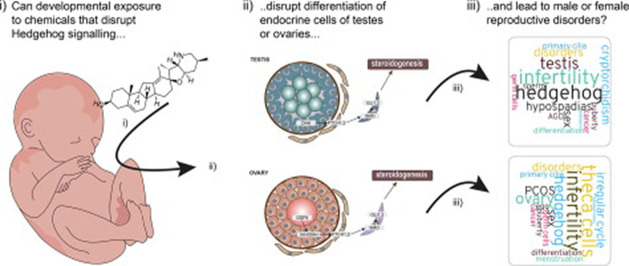
Graphical abstract
Keywords: Hedgehog signaling, Endocrine disruption, Reproductive disorders, Environmental chemicals, Adverse outcome pathway, AOP
Highlights
-
•
Hedgehog signaling is required for reproductive development.
-
•
Exposure to environmental chemicals can disrupt Hedgehog signaling and cause disease.
-
•
Perturbed Hedgehog signaling can disrupt Leydig cell differentiation and thereby sex hormone synthesis.
-
•
Perturbed Hedgehog signaling can disrupt theca cell differentiation and thereby sex hormone synthesis.
Abstract
Developmental exposure to chemicals that can disrupt sex hormone signaling may cause a broad spectrum of reproductive disorders. This is because reproductive development is tightly regulated by steroid sex hormones. Consequently, non-animal screening methods currently used to test chemicals for potential endocrine disrupting activities typically include steroidogenesis and nuclear receptor assays. In many cases there is a correlation between in vitro and in vivo data examining endocrine disruption, for example between blocked androgen receptor activity and feminized male genitals. However, there are many examples where there is poor, or no, correlation between in vitro data and in vivo effect outcomes in rodent studies, for various reasons. One possible, and less studied, reason for discordance between in vitro and in vivo data is that the mechanisms causing the in vivo effects are not covered by those typically tested for in vitro. This knowledge gap must be addressed if we are to elaborate robust testing strategies that do not rely on animal experimentation. In this review, we highlight the Hedgehog (HH) signaling pathway as a target for environmental chemicals and its potential implications for reproductive disorders originating from early life exposure. A central proposition is that, by disrupting HH signal transduction during critical stages of mammalian development, the endocrine cells of the testes or ovaries fail to develop normally, which ultimately will lead to disrupted sex hormone synthesis and sexual development in both sexes. If this is the case, then such mechanism must also be included in future test strategies aimed at eliminating chemicals that may cause reproductive disorders in humans.
1. Introduction
There is an increasing push towards relying more on alternative, non-animal, test methods for chemical hazard identification and risk assessment than what is currently the case. This is based on well-founded arguments and aligns with the 3R principles for animal experimentation (Replacement, Reduction and Refinement), but there are also several challenges associated with animal-free approaches. This is particularly relevant for chemicals with the potential to cause reproductive disorders through endocrine disrupting mechanisms, as the endocrine system involves regulatory signaling circuits between many, and distantly located, organs and tissues. This in itself makes it difficult to recapitulate the in vivo system in vitro, albeit not impossible. Another challenge when testing chemicals for potential endocrine disrupting effects using in vitro methods is that this often relies on well-established ‘endocrine modes of action’ such as disrupted steroidogenesis or interference with nuclear receptor activity.
In reproductive toxicology this often means androgen and estrogen receptors, albeit not limited to these. The potential failure of this approach is that it does not account for other effect modalities, for instance disruptions to cell differentiation or tissue integrity caused by interference with other regulatory pathways. Examples of relevant pathways include Wingless-like (WNT), retinoic acid (RA) and Hedgehog (HH) signaling, which are evolutionary conserved morphoregulatory pathways involved in a plethora of biological processes. Disruption to these signaling pathways can have severe consequences for development and function in all tissues and organs. With regard to the reproductive system, it can result in adverse reproductive outcomes reminiscent of those caused by disruption of more classical endocrine modalities such as steroidogenesis and receptor binding/activation. Whether or not disrupting these pathways would render a chemical an endocrine disrupting chemical (EDC), however, would depend on its mode of action.
In accordance with the World Health Organization (WHO), an EDC is “an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub) populations” (IPCS, 2002). By definition, this means that chemicals not directly perturbing classical EATS (estrogen, androgen, thyroid, and steroidogenesis) modalities, can also be considered EDCs provided it involves disruption to the endocrine system. The importance of considering alternative pathways for ED effects was highlighted by OECD in a Detailed Review Paper back in 2012, albeit not including the HH pathway (OECD, 2012). Especially when we move towards relying more on non-animal test methods will the concept of other effect modalities become highly relevant. Otherwise we run the risk of not detecting chemicals that will cause reproductive disorders without affecting classical in vitro methods such as nuclear receptor (ant)agonism.
In this review, we will focus on the HH signaling pathway. We will outline what we know about the central principles of how HH signaling is involved in testis, ovary (Fig. 1) and phallus development before discussing environmental chemicals that can perturb HH signaling; in particular in the context of gonadal development. Finally, we will discuss how exposure to HH signal disrupting chemicals potentially can give rise to reproductive disorders in both sexes. By so doing, we aim to highlight a need to think differently about how we test and assess chemicals for endocrine disrupting activities.
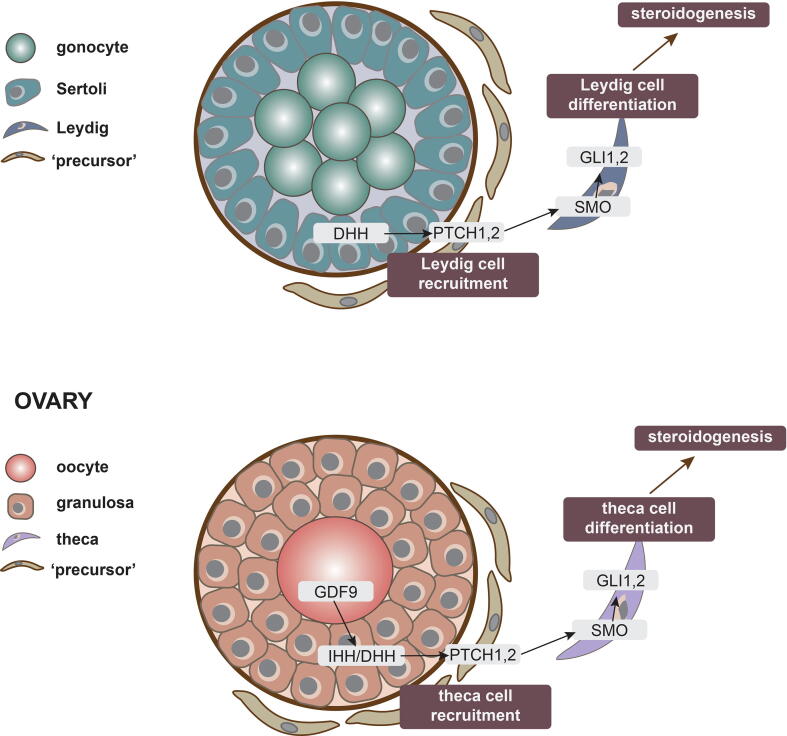
Fig. 1.
Involvement of HH signaling in the recruitment of endocrine cells of the ovary and testis. The regulatory role of HH signaling in specification of endocrine cells is very similar between testis and ovary, one major difference being when during development the cells are specified. A) In the testis, Sertoli cells express DHH, which act by paracrine signaling on PTCH-positive precursor cells. This triggers SMO release and activation, followed by activation of GLI transcription factors and differentiate into endocrine Leydig cells. B) In the ovary, GDF9 signaling from the oocyte triggers granulosa cells to express DHH and IHH, which then act by paracrine signaling on PTCH-positive precursor cells. This triggers SMO release and activation, followed by activation of GLI transcription factors and differentiation into endocrine theca cells.
2. Brief overview of the HH signaling pathway
In mammals, there are three principle HH ligands: Sonic hedgehog (SHH), Indian hedgehog (IHH) and Desert hedgehog (DHH). The HH ligands can interact with many surface receptors to promote intracellular signaling, as reviewed elsewhere (Pak and Segal, 2016), with Patched (PTCH) considered the canonical receptors (Pak and Segal, 2016). In vertebrate cells, the core components of the HH pathway localizes to the primary cilium; a singular microtubule-based, non-motile organelle extending from the basal body of the cell (Nozawa et al., 2013, Anvarian et al., 2019). At the primary cilium, and in the absence of HH ligand, the 12-transmembrane protein PTCH1 (or PTCH2) represses the accumulation of the G-protein coupled receptor Smoothened (SMO). HH ligand binding leads to endocytic clearance of PTCH1 from the primary cilium, allowing for the enrichment and activation of SMO in the primary cilium and, ultimately, activation of a downstream intracellular signaling cascade culminating in transcriptional regulation of HH target genes. Central to this signaling cascade are the GLI-Kruppel transcription factors GLI1, GLI2 and GLI3 (Anvarian et al., 2019, Ingham and McMahon, 2001).
HH signaling is involved in the regulation of organogenesis and body organization, a role that is evolutionary conserved across metazoans (Ingham and McMahon, 2001, Briscoe and Thérond, 2013). Because HH signaling is critical for a broad spectrum of developmental processes, disrupted HH signaling has also been linked to a large number of disorders and diseases, from severe birth morbidities to cancers (Pak and Segal, 2016, Varjosalo and Taipale, 2008). The HH pathway is also critical for gonad differentiation and reproductive development, as will be discussed in the following.
3. Gonad sex determination and reproductive development at a glance
Sexual development initiates during fetal life and completes with puberty in young adulthood. From conception, the male and female embryos are morphologically indistinct and develop similarly until gonadal sex determination. This marks the stage at which the two sexes diverge down two separate developmental trajectories. With the expression of the Y chromosome-specific gene SRY within a subset of precursor cells of the immature gonads of XY fetuses, testis differentiation is initiated (Svingen and Koopman, 2013). In contrast, the absence of SRY in XX fetuses allows for an opposing regulatory pathway to instruct the primitive gonads to differentiate into ovaries (Nicol and Yao, 2014). Up until this developmental stage, which corresponds to around week 7 in humans and mid-gestation in mice and rats, sexual development is largely genetically regulated. Afterwards, the bifurcation in developmental trajectories between the two sexes is heavily influenced by the steroid sex hormone milieu, with high androgen levels directing a male phenotype and low androgen levels directing a female phenotype.
Both the testes and ovaries are compartmentalized structures carrying out dual functions, namely sperm or egg production and sex steroid synthesis. In the testes, the Leydig cells are primarily responsible for steroid hormone synthesis, whereas in the ovaries both granulosa and theca cells are the primary source (Svingen and Koopman, 2013, Nicol and Yao, 2014). In consequence, the whole process of sex hormone-dependent development, from fetal life onwards, is intimately linked to the correct differentiation and maintenance of these endocrine cell lineages within the gonads. Perturbation to their differentiation or function could thus affect all aspects of sexual development and function throughout life.
3.1. HH and testis development
In the testis, the Leydig cells are responsible for testosterone synthesis that, during development, is critical for differentiation of secondary male sex organs and general masculinization of the body. Leydig cell specification itself is dependent on cues from the Sertoli cells, which are the first somatic cells to differentiate in the testis (Svingen and Koopman, 2013). One important Sertoli cell-derived factor is DHH, which is required for Leydig cell specification.
DHH is secreted from Sertoli cells and activates PTCH1 receptors expressed by precursor cells located in the testis interstitium. Upon binding and receptor activation, Ptch1-positive cells differentiate into fetal Leydig cells that organize themselves in small clusters between the testis cords. Once differentiated, the Leydig cells start expressing key enzymes of the steroidogenic pathway such as CYP11, CYP17 and HSD3β, allowing them to synthesize steroid sex hormones. The Leydig cells also synthesize INSL3, which is required for gubernacular differentiation and testis descent.
The role for DHH in specifying the fetal Leydig cell population was first shown in knockout mouse models. By inactivating Dhh, Leydig cell numbers were markedly reduced, resulting in suboptimal androgen synthesis and ultimately under-masculinization of the male fetuses (Yao et al., 2002, Pierucci-Alves et al., 2001). As in other organs and tissues, a hallmark of activated HH signaling and SMO recruitment in the (precursor) Leydig cells, is the upregulation of GLI1 and GLI2 transcription factors. The activation of both appears necessary in order to recruit cells to the Leydig cell lineage, but subsequent maintenance of expression is partly HH independent (Barsoum and Yao, 2011). That is, in the testis Gli2 expression does not require HH signaling for its expression, whereas Gli1 does, which is opposite to what is generally observed in other tissues as far as Gli1 and Gli2 regulation is concerned. Thus, the GLI factors operate somewhat redundantly, and semi-independently, of HH in the fetal testis that have already acquired a Leydig cell population. Although the aforementioned studies pertain to the mouse, studies involving various gene mutations have clearly shown the importance of DHH in testis development in rats (Kawai, et al., 2011), as well as in humans (Canto et al., 2005, Das et al., 2011, Umehara et al., 2000).
3.2. HH and ovary development
In the ovary, steroidogenesis takes place in a two-cell system involving granulosa and theca cells. The theca cells are responsible for synthesis of androgens, which are subsequently converted into estrogens by the adjacent granulosa cells (Erickson et al., 1985). Expression of HH signaling components in the theca and granulosa cells has been known for the last 15 years (Wijgerde et al., 2005, Russell et al., 2007) and their potential role in inducing target gene expression in developing theca cells was suggested early on (Wijgerde et al., 2005). Morphologically, theca cells appear when follicles reach the secondary stage, having more than one layer of granulosa cells around the oocyte (Young and McNeilly, 2010) (see (Richards et al., 2017) for detailed review on theca cells). In mice, secondary follicles with a theca cell layer start to develop approximately a week after birth (Edson et al., 2009), whereas in humans they start to appear in the beginning of the third trimester (Cole et al., 2006). The specification of theca cell fate occurs before they are visible around the secondary follicles (Honda et al., 2007) and require HH signaling by a mechanism similar to Leydig cell specification in the testis; one apparent difference being that only DHH seems necessary in the testis, whereas both DHH and IHH seem necessary in the ovary. Females lacking both DHH and IHH experiences a marked loss of theca cells, followed by disrupted hormone synthesis and infertility, a phenotype not observed in single knockouts (Liu et al., 2018, Liu et al., 2015).
DHH and IHH are expressed by granulosa cells and act by paracrine signaling on interstitial precursor cells, marked by Gli1 expression, to differentiate into theca cells. The theca precursor cells appear to originate from two different sources, either the mesonephros or the ovarian mesenchyme, giving rise to two different populations of theca cells. The mesonephros-derived cells become the androgen producing cells located to the basal lamina, whereas the majority of theca cells, including smooth muscle cells, surrounding the follicle seem to be originating from the ovarian mesenchyme (Liu et al., 2015). In mouse models where both Dhh and Ihh are ablated, both types of theca cells are compromised as evident by the lack of the smooth muscle cell marker α-SMA for the one type, and lack of the steroidogenesis markers HSD3β and CYP17A1 for the other type (Liu et al., 2015, Liu et al., 2018). Interestingly, constitutive activation of HH signaling in early development also affects the theca layer, reducing the number of smooth muscle cells surrounding developing follicles, ultimately leading to ovulatory failure (Ren et al., 2009, Ren et al., 2012). Additionally, both inhibition and constitutive activation of HH signaling have been implicated in polyovular follicles (Ren et al., 2012, Terauchi et al., 2020).
As described above, HH pathway components are present in the somatic cells of the ovaries, but HH signaling seems to be controlled, at least in part, by the oocyte-derived factor GDF9. In ovaries depleted of oocytes, as well as in Gdf9 knockout mice, expression of Dhh, Ihh and Gli1 is reduced. When GDF9 is supplemented to oocyte-depleted ovaries, expression of Dhh, Ihh and Gli1 is increased, showing a role for GDF9 in stimulating synthesis of the two ligands in the granulosa cells (Liu et al., 2015).
As in the testis, the role for HH signaling in specifying the steroidogenic theca cell lineage in the ovary has been elegantly shown in mouse genetic models. HH signaling in ovary development and function is most likely evolutionary conserved across mammalian species, but there is no clear evidence from human patients similarly to the association seen in XY gonad dysgenesis. Notably though, disruption of the HH pathway has been associated with polycystic ovary syndrome (PCOS) in women (Makrinou et al., 2020).
3.3. HH and external genitalia development
HH signaling plays a direct role in the development of external genitalia. This is most clearly shown with Shh knockout mice, which present with complete genital tubercle agenesis (Haraguchi, 2001, Perriton et al., 2002). HH signaling is also involved in the actual growth and patterning of the genital tubercle, including urethral closure, as shown in various mouse studies where compromised signaling (rather than complete ablation of SHH early on) can lead to phallus disorders beyond its complete failure in Shh knockouts (Seifert et al., 2009, Seifert et al., 2010, Lin et al., 2009, Miyagawa, et al., 2009). Importantly, the development of the genital tubercle has two distinct phases with respect to endocrine influence. The early phase of development is androgen-independent whereas the late phase is androgen-dependent, but with HH signaling being involved during both phases (Hyuga et al., 2019, Chew et al., 2014). The direct involvements of androgens in the late phase of genital tubercle development, including the induction of hypospadias in response to compromised androgen signaling, is well established (MacLeod, et al., 2010, Welsh et al., 2010, Sinclair et al., 2017). The interplay between the HH and androgen signaling pathways during genital tubercle development is not fully understood, but it is reasonable to assume that chemicals disrupting HH signaling during the early phase would also affects HH signaling broadly across the body plan, whereas chemicals affecting HH signaling only during the late phase would be downstream of androgen signaling and potentially only affect the genital tubercle, or other tissues with an active androgen-HH signaling axis. In fact, there is strong evidence for, not least from prostate cancers, that HH signaling itself can support androgen signaling (Chen, et al., 2009, Chen et al., 2010, Hyuga, et al., 2019).
Studies on phallus development in the Tammar wallaby, a marsupial species, has shed more light on how HH function directly regulate phallus differentiation, further illuminating a close relationship with androgen signaling. As mentioned above, during the early phase of genital development SHH function appears androgen-independent, whereas during late genital development SHH function appears androgen-dependent (Hyuga et al., 2019, Chew et al., 2014). Recent studies in the same marsupial model have revealed a delicate spatiotemporal expression pattern of SHH and IHH during phallus development and it is clear that disrupted HH signaling can cause genital disorders such as hypospadias (Tarulli et al., 1237). As for extrapolations to humans, HH signaling components are expressed in the phallus during urethral closure (Shehata et al., 2011) and polymorphisms in HH-related genes are associated with a higher risk for boys being born with hypospadias (Carmichael et al., 2013). These are not concrete cause-effect relationships, but HH signaling appear to be an evolutionary conserved signaling pathway critical for external genitalia development across mammalian species.
Hypospadias is the most common birth defect observed in newborn boys after cryptorchidism, and has a prevalence rate of approximately 1 in 250 (Springer et al., 2016). It is thus possible, based on what has been discussed above, that disruption to HH signaling is involved in a fair proportion of cases involving phallus dysmorphologies. It is, however, important to distinguish between direct HH signal disruption and indirect disruption to androgen signaling via HH signal disruption. The former would most likely also manifest as body-wide effects downstream of perturbed HH signal transduction whereas the latter could be more limited to androgen sensitive tissues. And this would also suggest that, for disorders limited to the reproductive system caused by HH signal disrupting chemicals, the phallus would be the most sensitive organ since HH expression and action in the developing phallus is itself sensitive to androgens and estrogens in mice (Zheng et al., 2015) and wallabies (Tarulli et al., 1237, Chen, et al., 2018).
With respect to gonadal dysmorphologies caused by HH signal disruption, the most likely outcome would be disrupted development of the endocrine cell lineages that subsequently would result in compromised sex hormone synthesis and all the downstream effects that would entail. However, as these effects would likely result from sex hormone-independent mechanism, they would likely then also result in body wide effects in all tissues and organs that are dependent on HH signaling. Nevertheless, the jury is still out with regards to gonadal disruption in response to HH-disrupting chemicals and what downstream effects they may cause.
4. HH disrupting chemicals
One of the better known examples of how an environmental compound can cause severe birth defects by HH signal disruption dates back to the 1950s. Notably, Hedgehog wasn’t identified until 1980 by a genetic screen in fruit-flies (Nüsslein-Volhard and Wieschaus, 1980) and its mammalian orthologs even later, in the early 1990s as reviewed by (Briscoe and Thérond, 2013); but nevertheless, the case in question has become closely linked to what severe consequences exposure to HH-disrupting compounds can have on normal development. In 1957, sheep farmers in Idaho (USA) started reporting on strange cases of lambs being born with one eye in the middle of the forehead, known medically as cyclopia (DeSesso, 2020). After a decade of work by scientists, the root cause of this birth defect was traced back to ewes gracing on poisonous corn lily (Veratrum californicum) containing a steroidal alkaloid that later became known as cyclopamine. The definitive proof that the teratogenic defect was caused by cyclopamine interfering with SHH came decades later (Cooper et al., 1998).
The number of chemicals now known to interfere with HH signaling include potential cancer drugs (Galperin et al., 2019) or other pharmaceuticals such as acetazolamide (Schreiner et al., 2009) and itraconazole (Tiboni et al., 2006), and aspirin (Ming et al., 2017), but also environmental chemicals. Not surprisingly, fetal rat testes exposed ex vivo to cyclopamine show significant downregulation of HH pathway genes and other Leydig-cell specific genes, also indicating a general loss if differentiated Leydig cells (Brokken et al., 2009). Other examples include the insecticide synergist piperonyl butoxide (Wang, et al., 2012), the photolytic compounds of the insecticide methoprene (Smith et al., 2003) and the biocide tributyltin (Zhang et al., 2012), which all have been shown to inhibit HH signaling and cause severe developmental defects in fish. In rats, developmental exposure to DEHP was recently shown to downregulate Shh in male offspring, causing impaired neuromotor development (Fu et al., 2019), whereas intrauterine exposure to DBP can inhibit HH signaling and impair male reproductive development (Kim et al., 2010). In humans, maternal smoking is associated with suppressed DHH expression in the fetal testis and lead to impaired masculinization (Fowler, et al., 2008). Using the human endometrial cancer cell line RL95.2, bisphenol A exposure suppressed components of the HH pathway via upregulation of miR-107 (Chou et al., 2017).
5. HH disrupting chemicals and reproductive disease
The above-mentioned studies have examined effects of chemicals on HH signaling in various tissues and organs, even vastly different organisms. They clearly show that chemical exposure can disrupt HH signal transduction and cause adverse effects in intact organisms, but only some of them show effects on the reproductive system, and even fewer show clear evidence for an endocrine mode of action. There is good evidence that HH signal disruption can impact phallus development directly, as already discussed, but evidence that it can cause gonadal dysgenesis and subsequent reproductive disorders remain owing. This, broadly speaking, would point to one of two scenarios: i) disruption to HH signaling is not a mechanism underpinning reproductive disorders caused by chemical exposure or ii) chemicals can disrupt HH signaling and cause reproductive disorders, but there has not been enough studies aimed at characterizing this mode of action.
Although direct evidence remains scarce with respect to chemically induced HH signal disruption in the gonads, we suggest that the second scenario is likely. This opinion is based on the known role for HH in gonad development and function, as well as a rat study showing the disruption of HH signaling and Leydig cell function in explanted testes exposed to the AR antagonist flutamide (Brokken et al., 2009). The mechanism for the effect caused by flutamide in this instance is still unclear, but the authors propose that flutamide suppresses Dhh expression in Sertoli cells with subsequent consequences for Leydig cell differentiation. This is a reasonable assumption since HH ligand expression is sensitive to androgens and estrogens in various tissues and cells (Chen, et al., 2009, Chen, et al., 2018, Gowda et al., 2013, Koga, 2008), but it remains to be seen if an AR blocker can suppress Dhh expression directly, or if it is by an indirect or secondary mechanism. Another intriguing relationship between androgens and HH was recently shown in a Gli3 mutant mouse model (Kothandapani, et al., 2020). These mutant mice display both cryptorchidism and hypospadias, which can be attributed to impaired Leydig cell differentiation and subsequent INSL3 and testosterone synthesis. This is in line with what was described above for testis development. Surprisingly, though, by supplementing the mutants with androgens, cryptorchidism was partially rescued whereas hypospadias was not. This, again, shows that androgens and HH signaling work in parallel to ensure proper phallus development, and not simply up- or down-stream of each other (Kothandapani, et al., 2020).
Notably, it appears that there exists a significant degree of intrinsic compensatory, or redundant, mechanisms for HH signaling across tissues and organs. In the gonads, this is evident by, for instance, the Gdf9 knockout mouse (upstream regulator of HH ligands) causing more severe female reproductive outcomes than the Dhh/Ihh double knockout, which itself result in more severe phenotypes than the Dhh or Ihh single knockouts (Liu et al., 2015). Thus, many chemicals with HH signal-disrupting activity would perhaps not cause obvious reproductive effects in in vivo toxicity studies at doses below general maternal toxicity, or severe embryonic teratogenicity. But again, these are speculations that need further empirical validation beyond the fact that more subtle effects on the reproductive organs can give rise to compromised reproductive function in adult life.
6. From biology to chemical risk assessment
The central modality proposed in this review is that chemicals can cause adverse reproductive outcomes by disrupting the HH pathway. The adverse outcomes caused by HH signal disruption may be similar to those typically associated with classical EDCs: disrupted steroidogenesis or androgen/estrogen receptor signaling can lead to short AGD, hypospadias, cryptorchidism, and reduced fertility in male offspring (Skakkebaek et al., 2016), and irregular cyclicity, reduced oocyte reserve and reduced fertility in female offspring (Johansson et al., 2017). But even though the adverse outcomes are the same, they are caused by very different effect modalities. HH signal disruption, as proposed herein, will for instance involve the failure of endocrine cells of the gonads to differentiate and function properly, so that reduced sex hormone synthesis or hormone signaling are secondary to perturbation pathway. Illuminating such cause-effect relationships between chemical exposure and adverse reproductive disorders is of paramount importance if we are to facilitate the current push towards animal-free toxicity testing of chemicals. This, because we need to know what to test for in vitro to accurately predict what will happen in vivo in the absence of animal testing.
To reiterate the argument; if we only test and assess chemicals using in silico or in vitro approaches using steroidogenesis or nuclear receptor assays only, some chemicals would appear safe based on negative results, whereas they could cause adverse outcomes in the intact organisms, including humans. This is not an issue if chemicals are tested in vivo, but a worrying scenario if only alternative test methods not covering HH signal disruption are used for testing. Therefore, we encourage future work looking at the relationship between HH signaling, environmental chemicals, and adverse reproductive outcomes to make use of the Adverse Outcome Pathway (AOP) framework (Ankley and Edwards, 2018). This would not only facilitate increased knowledge about how environmental chemicals can harm human reproductive health through HH signal disruption, but also facilitate the development of alternative test assays should the HH signaling pathway prove to be of relevance for chemical hazard identification and risk assessments.
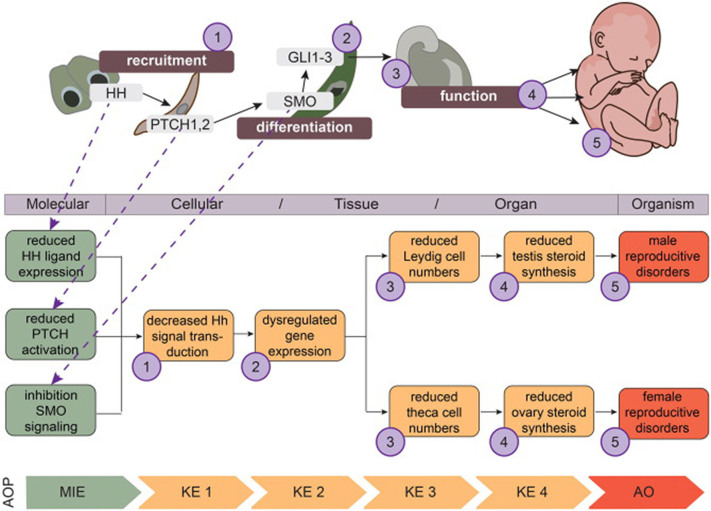
A description of the AOP concept is outside the scope of this review, but suffice to say it involves pragmatic descriptions of linear cause-effect relationships from initial perturbation to adverse outcomes in an intact organism (OECD, 2018). Of note, these descriptions are not meant as detailed descriptions of regulatory pathways covering all the molecular and cellular interactions actually taking place in the organism, but instead focusing on those key events that are essential for progression of the pathway toward the adverse outcome; and that are, at the same time, measurable; i.e. of use for regulatory toxicology. To exemplify this line of thought, we have constructed a smaller AOP network for HH pathway perturbation leading to reproductive disorders (Fig. 2). This small, putative AOP network present three individual pathways originating from three molecular initiating events. These would be considered separate AOPs since each individual event could in itself lead to the first downstream event: ‘decreased HH signal transduction’. The HH signaling pathway itself is much more complex than this representation, which is why it is of value from a risk assessment point of view. It is a question of ‘pragmatic essentiality’.
Fig. 2.
Proposed Adverse Outcome Pathway (AOP) network for disrupted Hedgehog (HH) signaling during development leading to reproductive disease. From what is currently known about the involvement of HH signaling in gonadal development, testes and ovaries, it is possible to extract putative AOPs for further elaboration. The numbers (purple circles) in the developmental pathway correspond to events that are believed to be essential to progress the cause-effect pathway towards the adverse outcome (AO), but the upper developmental pathway is far from a complete description of the HH signaling pathway as it takes place in cells and tissues. Being pragmatic descriptions of pathways between initial molecular perturbation to an AO in an intact organisms, the AOP serve as reference points for predicting toxic effects from effects on upstream events only; meaning, molecular initiating events (MIE) and key events (KE) should be measurable and applicable for chemical risk assessment. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
7. Perspectives
The idea that EDCs can cause adverse reproductive outcomes by mechanisms not classically considered EDC modes of action is supported by other studies. For example, when exposing pregnant rats to phthalates such as DBP or DEHP, fetal testosterone levels are significantly reduced which ultimately result in undervirilizaion of male fetuses (reviewed by (Schwartz et al., 2019). Because of the clear relationship between Leydig cell steroidogenesis and testosterone levels, it is natural to conjecture that phthalates disrupt steroidogenesis. This remains a prevailing notion, even though it remains unclear by what mechanisms phthalates reduces testosterone levels in rodents. An equally likely scenario is that phthalates disrupt Leydig cell differentiation or maintenance, which consequently lead to compromised androgen production. Recent studies suggests that phthalates, and indeed several other EDCs, can disrupt Leydig cell gap junctions and intercellular signaling (Yawer et al., 2020, Di Lorenzo et al., 2020). This ultimately means that it can be the Leydig cells themselves that are rendered dysfunctional by phthalate exposure and not steroidogenesis per se. With respect to HH pathway disruption, similar modalities could be what causes reproductive disorders. But despite studies suggesting this to be the case, as discussed in this review, it remains to be thoroughly examined and proven or disproven. To do so, we need more cross-disciplinary interactions between experts from basic biology, toxicology and chemical risk assessors. And, as recently advocated (Draskau et al., 2020), the AOP framework is a very good platform to facilitate such endeavors.
Funding
This work received funding from the Danish Environmental Protection Agency as a project under the Centre on Endocrine Disrupters (CEHOS).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ankley G.T., Edwards S.W. The adverse outcome pathway: A multifaceted framework supporting 21st century toxicology. Curr. Opin. Toxicol. 2018;9:1–7. doi: 10.1016/j.cotox.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anvarian Z., Mykytyn K., Mukhopadhyay S., Pedersen L.B., Christensen S.T. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 2019;15(4):199–219. doi: 10.1038/s41581-019-0116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum I., Yao H.H.C. Redundant and differential roles of transcription factors Gli1 and Gli2 in the development of mouse fetal Leydig cells. Biol. Reprod. 2011;84:894–899. doi: 10.1095/biolreprod.110.088997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J., Thérond P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013;14(7):416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- Brokken L.J., Adamsson A., Paranko J., Toppari J. Antiandrogen exposure in utero disrupts expression of desert hedgehog and insulin-like factor 3 in the developing fetal rat testis. Endocrinology. 2009;150:445–451. doi: 10.1210/en.2008-0230. [DOI] [PubMed] [Google Scholar]
- Canto P., Vilchis F., Söderlund D., Reyes E., Méndez J.P. A heterozygous mutation in the desert hedgehog gene in patients with mixed gonadal dysgenesis. Mol. Hum. Reprod. 2005;11:833–836. doi: 10.1093/molehr/gah216. [DOI] [PubMed] [Google Scholar]
- Carmichael S.L., Ma C., Choudhry S., Lammer E.J., Witte J.S., Shaw G.M. Hypospadias and genes related to genital tubercle and early urethral development. J. Urol. 2013;190(5):1884–1892. doi: 10.1016/j.juro.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. Androgenic regulation of hedgehog signaling pathway components in prostate cancer cells. Cell Cycle. 2009;8:149–157. doi: 10.4161/cc.8.1.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Hormone-responsive genes in the SHH and WNT/β-catenin signaling pathways influence urethral closure and phallus growth. Biol. Reprod. 2018;99:806–816. doi: 10.1093/biolre/ioy117. [DOI] [PubMed] [Google Scholar]
- Chen M., Feuerstein M.A., Levina E., Baghel P.S., Carkner R.D., Tanner M.J., Shtutman M., Vacherot F., Terry S., de la Taille A., Buttyan R. Hedgehog/Gli supports androgen signaling in androgen deprived and androgen independent prostate cancer cells. Mol Cancer. 2010;9(1):89. doi: 10.1186/1476-4598-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew K.Y., Pask A.J., Hickford D., Shaw G., Renfree M.B. A dual role for SHH during phallus development in a marsupial. Sex Dev. 2014;8(4):166–177. doi: 10.1159/000357927. [DOI] [PubMed] [Google Scholar]
- Chou W.-C., Lee P.-H., Tan Y.-Y., Lin H.-C., Yang C.-W., Chen K.-H., Chuang C.-Y. An integrative transcriptomic analysis reveals bisphenol A exposure-induced dysregulation of microRNA expression in human endometrial cells. Toxicol. In Vitro. 2017;41:133–142. doi: 10.1016/j.tiv.2017.02.012. [DOI] [PubMed] [Google Scholar]
- Cole B., Hensinger K., Maciel G.A.R., Chang R.J., Erickson G.F. Human fetal ovary development involves the spatiotemporal expression of p450c17 protein. J. Clin. Endocrinol. Metab. 2006;91:3654–3661. doi: 10.1210/jc.2006-0641. [DOI] [PubMed] [Google Scholar]
- Cooper M.K., Porter J.A., Young K.E., Beachy P.A. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- Das D.K., Sanghavi D., Gawde H., Idicula-Thomas S., Vasudevan L. Novel homozygous mutations in Desert Hedgehog gene in patients with 46,XY complete gonadal dysgenesis and prediction of its structural and functional implications by computational methods. Eur. J. Med. Genetics. 2011;54(6):e529–e534. doi: 10.1016/j.ejmg.2011.04.010. [DOI] [PubMed] [Google Scholar]
- DeSesso J.M. Of embryos and tumors: Cyclopia and the relevance of mechanistic teratology. Birth Defects Res. 2020;112(3):219–233. doi: 10.1002/bdr2.1636. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo M., Winge S.B., Svingen T., De Falco M., Boberg J. Intrauterine exposure to diethylhexyl phthalate disrupts gap junctions in the fetal rat testis. Curr. Res. Toxicol. 2020;1:5–11. doi: 10.1016/j.crtox.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draskau M.K., Spiller C.M., Boberg J., Bowles J., Svingen T. Developmental biology meets toxicology: contributing reproductive mechanisms to build Adverse Outcome Pathways. Mol. Hum. Reprod. 2020;26:111–116. doi: 10.1093/molehr/gaaa001. [DOI] [PubMed] [Google Scholar]
- Edson M.A., Nagaraja A.K., Matzuk M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson G.F., Magoffin D.A., Dyer C.A., Hofeditz C. The ovarian androgen producing cells: a review of structure/function relationships. Endocr. Rev. 1985;6:371–399. doi: 10.1210/edrv-6-3-371. [DOI] [PubMed] [Google Scholar]
- Fowler P.A. Maternal smoking during pregnancy specifically reduces human fetal desert hedgehog gene expression during testis development. J. Clin. Endocrinol. Metab. 2008;93:619–626. doi: 10.1210/jc.2007-1860. [DOI] [PubMed] [Google Scholar]
- Fu Y., Dong J., You M., Cong Z., Wei L., Fu H., Wang Y.i., Wang Y., Chen J. Maternal di-(2-ethylhexyl) phthalate exposure inhibits cerebellar granule precursor cell proliferation via down-regulating the Shh signaling pathway in male offspring. Chemosphere. 2019;215:313–322. doi: 10.1016/j.chemosphere.2018.10.040. [DOI] [PubMed] [Google Scholar]
- Galperin I., Dempwolff L., Diederich W.E., Lauth M. Inhibiting hedgehog: An update on pharmacological compounds and targeting strategies. J. Med. Chem. 2019;62(18):8392–8411. doi: 10.1021/acs.jmedchem.9b00188. [DOI] [PubMed] [Google Scholar]
- Gowda P.S., Deng J.D., Mishra S., Bandyopadhyay A., Liang S., Lin S., Mahalingam D., Sun L.-Z. Inhibition of hedgehog and androgen receptor signaling pathways produced synergistic suppression of castration-resistant prostate cancer progression. Mol. Cancer Res. 2013;11(11):1448–1461. doi: 10.1158/1541-7786.MCR-13-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi R. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128:4241–4250. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- Honda A., Hirose M., Hara K., Matoba S., Inoue K., Miki H., Hiura H., Kanatsu-Shinohara M., Kanai Y., Kono T., Shinohara T., Ogura A. Isolation, characterization, and in vitro and in vivo differentiation of putative thecal stem cells. Proc. Natl. Acad. Sci. 2007;104(30):12389–12394. doi: 10.1073/pnas.0703787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyuga T. Hedgehog signaling for urogenital organogenesis and prostate cancer: an implication for the epithelial-mesenchyme interaction (EMI) Int. J. Mol. Sci. 2019;21:58. doi: 10.3390/ijms21010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyuga T., Suzuki K., Acebedo A.R., Hashimoto D., Kajimoto M., Miyagawa S., Enmi J.-I., Yoshioka Y., Yamada G. Regulatory roles of epithelial-mesenchymal interaction (EMI) during early and androgen dependent external genitalia development. Differentiation. 2019;110:29–35. doi: 10.1016/j.diff.2019.08.004. [DOI] [PubMed] [Google Scholar]
- Ingham P.W., McMahon A.P. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- IPCS. in International Program on Chemical Safety, WHO/PCS/EDC/02.2 (eds T. Damstra et al.) (WHO, 2002).
- Johansson H.K.L., Svingen T., Fowler P.A., Vinggaard A.M., Boberg J. Environmental influences on ovarian dysgenesis — developmental windows sensitive to chemical exposures. Nat. Rev. Endocrinol. 2017;13(7):400–414. doi: 10.1038/nrendo.2017.36. [DOI] [PubMed] [Google Scholar]
- Kawai Y. A missense mutation of the Dhh gene is associated with male pseudohermaphroditic rats showing impaired Leydig cell development. Reproduction. 2011;141:217–225. doi: 10.1530/REP-10-0006. [DOI] [PubMed] [Google Scholar]
- Kim T.S., Jung K.K., Kim S.S., Kang I.H., Baek J.H., Nam H.-S., Hong S.-K., Lee B.M., Hong J.T., Oh K.W., Kim H.S., Han S.Y., Kang T.S. Effects of in utero exposure to DI(n -Butyl) phthalate on development of male reproductive tracts in sprague-dawley rats. J. Toxicol. Environ. Health Part A. 2010;73(21-22):1544–1559. doi: 10.1080/15287394.2010.511579. [DOI] [PubMed] [Google Scholar]
- Koga K. Novel link between estrogen receptor alpha and hedgehog pathway in breast cancer. Anticancer Res. 2008;28:731–740. [PubMed] [Google Scholar]
- Kothandapani A. GLI3 resides at the intersection of hedgehog and androgen action to promote male sex differentiation. PLoS Genet. 2020;16 doi: 10.1371/journal.pgen.1008810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Yin Y., Veith G.M., Fisher A.V., Long F., Ma L. Temporal and spatial dissection of Shh signaling in genital tubercle development. Development. 2009;136(23):3959–3967. doi: 10.1242/dev.039768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Peng J., Matzuk M.M., Yao H.-C. Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells. Nat. Commun. 2015;6(1) doi: 10.1038/ncomms7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Rodriguez K.F., Brown P.R., Yao H.H. Reproductive, physiological, and molecular outcomes in female mice deficient in Dhh and Ihh. Endocrinology. 2018;159:2563–2575. doi: 10.1210/en.2018-00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod D.J. Androgen action in the masculinization programming window and development of male reproductive organs. Int. J. Androl. 2010;33:279–287. doi: 10.1111/j.1365-2605.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- Makrinou E., Drong A.W., Christopoulos G., Lerner A., Chapa-Chorda I., Karaderi T., Lavery S., Hardy K., Lindgren C.M., Franks S. Genome-wide methylation profiling in granulosa lutein cells of women with polycystic ovary syndrome (PCOS) Mol. Cell. Endocrinol. 2020;500:110611. doi: 10.1016/j.mce:2019.110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming J., Sun B.o., Li Z., Lin L., Meng X., Han B.o., Wang R., Wu P., Li J., Cai J., Jiang C. Aspirin inhibits the SHH/GLI1 signaling pathway and sensitizes malignant glioma cells to temozolomide therapy. Aging. 2017;9(4):1233–1247. doi: 10.18632/aging.101224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa S. Dosage-dependent hedgehog signals integrated with Wnt/beta-catenin signaling regulate external genitalia formation as an appendicular program. Development. 2009;136:3969–3978. doi: 10.1242/dev.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol B., Yao H.-C. Building an ovary: insights into establishment of somatic cell lineages in the mouse. Sex Dev. 2014;8(5):243–251. doi: 10.1159/000358072. [DOI] [PubMed] [Google Scholar]
- Nozawa Y.I., Lin C., Chuang P.-T. Hedgehog signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction. Curr. Opin. Genet. Dev. 2013;23(4):429–437. doi: 10.1016/j.gde.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- OECD. Detailed Review Paper on the State of the Science on Novel In Vitro and In Vivo Screening and Testing Methods and Endpoints for Evaluating Endocrine Disruptors. (Paris, 2012).
- OECD. User’s handbook supplement to the guidance document for developing and assessing AOPs, 2018.
- Pak E., Segal R. Hedgehog signal transduction: key players, oncogenic drivers, and cancer therapy. Dev. Cell. 2016;38(4):333–344. doi: 10.1016/j.devcel.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perriton C.L., Powles N., Chiang C., Maconochie M.K., Cohn M.J. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev. Biol. 2002;247(1):26–46. doi: 10.1006/dbio.2002.0668. [DOI] [PubMed] [Google Scholar]
- Pierucci-Alves F., Clark A.M., Russell L.D. A developmental study of the Desert hedgehog-null mouse testis. Biol. Reprod. 2001;65:1392–1402. doi: 10.1095/biolreprod65.5.1392. [DOI] [PubMed] [Google Scholar]
- Ren Y.i., Cowan R.G., Harman R.M., Quirk S.M. Dominant activation of the hedgehog signaling pathway in the ovary alters theca development and prevents ovulation. Mol. Endocrinol. 2009;23(5):711–723. doi: 10.1210/me.2008-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Cowan R.G., Migone F.F., Quirk S.M. Overactivation of hedgehog signaling alters development of the ovarian vasculature in mice. Biol. Reprod. 2012;86:174. doi: 10.1095/biolreprod.112.099176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J.S., Ren Y.A., Candelaria N., Adams J.E., Rajkovic A. Ovarian follicular theca cell recruitment, differentiation, and impact on fertility: 2017 update. Endocr. Rev. 2018;39:1–20. doi: 10.1210/er.2017-00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M.C., Cowan R.G., Harman R.M., Walker A.L., Quirk S.M. The hedgehog signaling pathway in the mouse ovary. Biol. Reprod. 2007;77:226–236. doi: 10.1095/biolreprod.106.053629. [DOI] [PubMed] [Google Scholar]
- Schreiner C.M., Bell S.M., Scott W.J., Jr. Microarray analysis of murine limb bud ectoderm and mesoderm after exposure to cadmium or acetazolamide. Birth Defect Res. A. 2009;85(7):588–598. doi: 10.1002/bdra.20577. [DOI] [PubMed] [Google Scholar]
- Schwartz C.L., Christiansen S., Vinggaard A.M., Axelstad M., Hass U., Svingen T. Anogenital distance as a toxicological or clinical marker for fetal androgen action and risk for reproductive disorders. Arch. Toxicol. 2019;93(2):253–272. doi: 10.1007/s00204-018-2350-5. [DOI] [PubMed] [Google Scholar]
- Seifert A.W., Bouldin C.M., Choi K.-S., Harfe B.D., Cohn M.J. Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development. 2009;136(23):3949–3957. doi: 10.1242/dev.042291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert A.W., Zheng Z., Ormerod B.K., Cohn M.J. Sonic hedgehog controls growth of external genitalia by regulating cell cycle kinetics. Nat. Commun. 2010;1(1) doi: 10.1038/ncomms1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata B.M., Elmore J.M., Bootwala Y., Steelman C.K., Bare J.B., Shoffeitt C.J., Wang R., Zhau H.E., He D., Zhu G., Chung L.W. Immunohistochemical characterization of sonic hedgehog and its downstream signaling molecules during human penile development. Fetal Pediatric. Pathol. 2011;30(4):244–251. doi: 10.3109/15513815.2011.555809. [DOI] [PubMed] [Google Scholar]
- Sinclair A.W., Cao M., Pask A., Baskin L., Cunha G.R. Flutamide-induced hypospadias in rats: A critical assessment. Differentiation. 2017;94:37–57. doi: 10.1016/j.diff.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Skakkebaek N.E., Rajpert-De Meyts E., Buck Louis G.M., Toppari J., Andersson A.-M., Eisenberg M.L., Jensen T.K., Jørgensen N., Swan S.H., Sapra K.J., Ziebe S., Priskorn L., Juul A. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol. Rev. 2016;96(1):55–97. doi: 10.1152/physrev.00017.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.G., Wilburn C., McCarthy R.A. Methoprene photolytic compounds disrupt zebrafish development, producing phenocopies of mutants in the sonic hedgehog signaling pathway. Mar. Biotechnol. 2003;5(2):201–212. doi: 10.1007/s10126-002-0062-5. [DOI] [PubMed] [Google Scholar]
- Springer A., van den Heijkant M., Baumann S. Worldwide prevalence of hypospadias. J. Pediatric Urol. 2016;12(3):152.e1–152.e7. doi: 10.1016/j.jpurol.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Svingen T., Koopman P. Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes Dev. 2013;27(22):2409–2426. doi: 10.1101/gad.228080.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarulli G.A., Pask A.J., Renfree M.B. Discrete hedgehog factor expression and action in the developing phallus. Int. J. Mol. Sci. 2020;21:1237. doi: 10.3390/ijms21041237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi K.J., Miyagawa S., Iguchi T., Sato T. Hedgehog signaling regulates the basement membrane remodeling during folliculogenesis in the neonatal mouse ovary. Cell Tissue Res. 2020;381(3):555–567. doi: 10.1007/s00441-020-03222-9. [DOI] [PubMed] [Google Scholar]
- Tiboni G.M., Marotta F., Del Corso A., Giampietro F. Defining critical periods for itraconazole-induced cleft palate, limb defects and axial skeletal malformations in the mouse. Toxicol. Lett. 2006;167(1):8–18. doi: 10.1016/j.toxlet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Umehara F., Tate G., Itoh K., Yamaguchi N., Douchi T., Mitsuya T., Osame M. A novel mutation of desert hedgehog in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am. J. Human Genetics. 2000;67(5):1302–1305. doi: 10.1016/S0002-9297(07)62958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varjosalo M., Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22(18):2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- Wang J. The insecticide synergist piperonyl butoxide inhibits hedgehog signaling: assessing chemical risks. Toxicol. Sci. 2012;128:517–523. doi: 10.1093/toxsci/kfs165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M., MacLeod D.J., Walker M., Smith L.B., Sharpe R.M. Critical androgen-sensitive periods of rat penis and clitoris development. Int. J. Androl. 2010;33:e144–152. doi: 10.1111/j.1365-2605.2009.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijgerde M., Ooms M., Hoogerbrugge J.W., Grootegoed J.A. Hedgehog signaling in mouse ovary: Indian hedgehog and desert hedgehog from granulosa cells induce target gene expression in developing theca cells. Endocrinology. 2005;146:3558–3566. doi: 10.1210/en.2005-0311. [DOI] [PubMed] [Google Scholar]
- Yao H.H., Whoriskey W., Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawer A., Sychrová E., Labohá P., Raška J., Jambor T., Babica P., Sovadinová I. Endocrine-disrupting chemicals rapidly affect intercellular signaling in Leydig cells. Toxicol. Appl. Pharmacol. 2020;404:115177. doi: 10.1016/j.taap.2020.115177. [DOI] [PubMed] [Google Scholar]
- Young J.M., McNeilly A.S. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140:489–504. doi: 10.1530/REP-10-0094. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zuo Z., Sun P., Wang H., Yu A., Wang C. Tributyltin exposure results in craniofacial cartilage defects in rockfish (Sebastiscus marmoratus) embryos. Marine Environ. Res. 2012;77:6–11. doi: 10.1016/j.marenvres.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Zheng Z., Armfield B.A., Cohn M.J. Timing of androgen receptor disruption and estrogen exposure underlies a spectrum of congenital penile anomalies. PNAS. 2015;112(52):E7194–E7203. doi: 10.1073/pnas.1515981112. [DOI] [PMC free article] [PubMed] [Google Scholar]