Abstract
Lignans are secondary plant constituents with dibenzylbutane skeletons found in cereals, oilseeds, and nuts. Two members of this class, syringaresinol (Syr) and secoisolariciresinol (Seco), occur at relatively high levels in cereals and processed food products as well as in coniferous trees. In vitro studies have shown that Seco and its metabolites enterodiol (END) and enterolactone (ENL), which are formed by intestinal microbes, exhibit strong antioxidant activity because of their phenolic character. The biological activity and discussion of dietary supplementation with these substances led to questions about the potential adverse health effects of these compounds, which are explored here. Syr and the metabolites END and ENL were investigated by combining structural information generated in silico with practical testing in vitro. An in silico structure-activity analysis was performed using ToxTree and NexusPrediction to suggest plausible mechanisms of toxicity and estimate toxicological endpoints of these compounds. Structural alerts were generated based on the presence of phenolic units with coordinating substituents that could potentially form quinoid structures, promote reactive oxygen species (ROS) formation, bind to cellular structures, or damage chromosomes. To assess the in silico results, the cytotoxicity and genotoxic potential of the studied compounds were tested in vitro using the resazurin reduction and comet assays, respectively. Incubating HepG2 and HT29 cells for 1 h or 24 h with 0–100 μM Syr, END, or ENL induced no cytotoxic effects. Additionally, even the highest tested concentrations of END and ENL showed no modulation of background and total DNA damage. The initial in silico screen thus generated structural alerts linked to toxicological endpoints, but experimental assessments of the studied compounds revealed no detectable toxicity, demonstrating the need for individual mechanistic experimental verification of in silico predictions. This approach makes it possible to connect known biological activity, such as reported antioxidative effects, to underlying mechanisms such as proton abstraction or donation. This in turn can yield insights – for example, that a compound's tendency to act as a pro- or anti-oxidant (and hence to exert adverse or beneficial health effects) may depend on its concentration and the cellular state.
Keywords: Syringaresinol, Lignans, In silico, Cytotoxicity, DNA damage
Graphical abstract

Highlights
-
•
Potential of toxicologic mechanisms: cellular stress and chromosomal damage were identified in silico for syringaresinol, enterdiol and enterlactone.
-
•
However, in confirmatory in vitro assays (cytotoxicity, DNA damage and DNA strand breaks) in HepG2 and HT29 cells no such toxicities were induced by physiological and higher concentrations of syringaresinol and enterolignans.
-
•
This study serves as a cautionary tale of using in silico prediction of toxicity mechanisms. Experimental verification of in silico predictions is needed as these methodologies are still under development.
1. Introduction
Lignans are secondary plant constituents that occur, inter alia, in cereals, oilseeds, and nuts but also in high amounts in coniferous trees (Milder et al., 2005). The biosynthesis of their dibenzylbutane skeleton involves the dimerization of two coniferyl alcohols (Jenab et al., 1999). In structural terms, they are diphenolic derivatives exhibiting diverse patterns of oxidation, hydroxylation, methylation, and/or condensation. They thus exhibit striking structural similarity to the basic building blocks of the polyphenolic cell wall polymer, lignin (Touré and Xueming, 2010; Ford et al., 2001). The polyphenol content of foodstuffs correlates with their fibre content, and Begum and coworkers (Begum et al., 2004) identified lignin as a precursor of the enterolignans. The enterolignans enterodiol (END) and enterolactone (ENL), which do not occur in plants, are produced by metabolic degradation of lignans by bacteria in the intestinal tract as shown in Fig. 1. Ford et al. (2001) identified two genes encoding enzymes catalysing the biosynthesis of two lignans, pinoresinol (Pin) and lariciresinol (Lari), and showed that they were formed by the coupling of two monolignol radicals.
Fig. 1.
Syringaresinol (Syr), its diglucoside (Syr-DG), and its metabolites enterodiol (END) and enterolactone (ENL), which are formed via multi-step processes.
A study on the Dutch population found that the main dietary sources of lignans are beverages (37%), followed by vegetables (24%), nuts and seeds (14%), bread (9%), and fruit (7%) (Milder et al., 2005). Conversely, French women consumed lignans mainly through vegetables and fruits (Touillaud et al., 2007). Diet composition strongly affects the amount and identity of the lignans people consume. The daily intake of Seco and mataresinol (Mat) in western countries, was estimated to be 0.15–1.1 mg/person/day (Horn-Ross et al., 2000). Flaxseed was found to have the highest lignan content of the tested foodstuffs (about 30 mg/kg), with secoisolariciresinol (Seco) being the most abundant individual lignan among the analysed substances (Milder et al., 2005; Thompson et al., 2006). Grain-based intermediate products such as brewers' spent grains (BSG), a by-product of beer production with the potential for further valorisation, are also rich sources of lignans. During beer production, the more water-soluble lignans in the fermented grains are transferred to the beer, while the more lipophilic and otherwise bound grains remain in the grain. The most abundant lignans in dried BSG are Syringaresinol (Syr) and Seco, which account for 73% and 17%, respectively, of its total lignan content (Niemi et al., 2012). The content and composition of BSG will be significantly changed by further processing, and particularly by steps such as protease treatment and alkaline extraction. For example, the total lignan content of alkaline BSG extracts was 6.3 times higher than that in the original material, which was attributed to the degradation of lignin. This illustrates the influence of processing steps (especially those that increase solubility or involve heating) on lignan content and composition, which must be accounted for evaluating lignan-rich ingredients used in food.
After consumption, lignans undergo two major biotransformation processes. The first transformation occurs in the intestinal lumen caused by intestinal bacteria. Inter- and intraspecific differences in lignan absorption depend on diet composition, intestinal transit time, and intestinal redox status, but are primarily related to the composition and activity of the intestinal microbiota (Landete, 2012). Upon incubation with microbiota from human faeces for 24 h, Seco as well as the glycosidic compounds syringaresinol diglycoside (Syr-DG) and pinoresinol diglycoside (Pin-DG) underwent 72%, 4%, and 55% conversion, respectively, into END and ENL (Heinonen et al., 2001). The reactions by which lignans are degraded depend heavily on the bacteria prevalent in the intestinal tract. Consequently, lignan metabolism exhibits variable kinetics and can generate many different products. Intestinal bacteria can be classified as slow, moderate, or fast END or ENL producers (Setchell et al., 2014). In general, O-deglycosylation of glycosidic lignans occurs in the intestinal lumen. ENL can be formed by the oxidative lactonization of END, which can itself be formed from various lignans via O-hydroxylation, O-demethylation, reduction, and dehydrogenation reactions. The diversity of the intestinal microbiome suggests that these metabolites may be formed by many different metabolic pathways; Fig. 1 shows a highly simplified version of one such pathway (Touré and Xueming, 2010; Ford et al., 2001; Xie et al., 2003). Once the microbiotic products are absorbed into the intestinal epithelium, their distribution depends on their hydrophilic or lipophilic character. In humans, lignan metabolism occurs primarily in enterocytes, the liver, and the cells of the colon. Lignans and their metabolites undergo a series of hydrolyses and conjugations that form O-glucuronides, sulfate esters, and O-methyl ethers (Rechner, 2004; Jansen et al., 2005). Although seco-diglycoside (Seco-DG) is highly soluble in water, sesaminol-triglycoside (Ses-TG) becomes more lipophilic after deglucosylation. This influences its toxicokinetics, i.e. its absorption, distribution, metabolisation and excretion (ADME) in the human organism. Certain unconjugated lignans including lariciresinol and matairesinol as well as END and ENL have been detected at low nanomolar concentrations in human serum from healthy individuals not consuming any special diet (Grace et al., 2003; Morton et al., 2002; Smeds et al., 2006; Valentín-Blasini et al., 2003).
Lignans reportedly have diverse biological effects. In vitro studies indicated that Seco, END and ENL all reduce the levels of reactive oxygen species and thus act as antioxidants (Prasad, 2000). They were also found to protect against DNA damage and lipid peroxidation, which was attributed to the abstraction of benzylic hydrogens and the potential resonance stabilization of phenoxy radicals in aqueous environments (Hu et al., 2007). Because of their antioxidative activity, the therapeutic effects of lignans against inflammatory symptoms such as hypercholesterolemia, hyperglycaemia, and atherosclerosis have been investigated (Prasad, 2000). In addition to their ability to capture reactive oxygen species (ROS), lignans have been shown to affect various signalling pathways. For instance, in vitro treatment with 20–60 μM ENL inhibited proliferation and migration of prostate cancer cells by interfering insulin-like growth factor signalling, indicating possible anticarcinogenic activity (Chen et al., 2009). In vivo in mice with established human breast cancer ENL suppressed the extracellular vascular endothelial growth factor (VEGF), an important influencing factor while carcinogenesis (Dabrosin et al., 2002). Lignans may also exhibit anticarcinogenic effects resulting from their phytoestrogenic potential. This activity is probably related to their underlying diethylstilbestrol skeleton, whose conformation resembles that of 17β-estradiol, enabling it to bind to the estrogen receptor. By acting as endocrine modulator, these compounds could have positive estrogenic effects as seen in postmenopausal women. Accordingly, flaxseed supplementation was found to shift estrogen metabolism from the 16-α-hydroxylation pathway to the less cancerogenic 2-hydroxylation pathway (Brooks et al., 2004; Brooks and Skafar, 2004). However, some adverse effects were also seen, including an elevated risk of prostate cancer in men (Jackson et al., 2010). The antiviral and cytotoxic activity of podophyllotoxin-derived lignans has been discussed in relation to their potential as therapeutic agents against hormone-dependent cancers (Petersen and Alfermann, 2001).
These observations raise questions about the potential cytotoxic and other biological effects of lignans as pure substances. Lignans as secondary plant constituents in complex food matrices will inevitably be consumed as part of any plant-rich diet (Milder et al., 2005). However, because the enrichment of processed foods with these substances has been mooted, there is a need for thorough toxicological screening of these compounds.
In silico methods are powerful tools for screening compounds to prioritise for further testing in cases where toxicological data are lacking. They are particularly useful for implementing the 3R principles (reduce, refine, and replace) for animal testing, which have been integrated into European law via the REACH regulations. Some programs can also perform quantitative comparisons to better-characterized substances, making it possible to assign specific target endpoints and supporting the development of hypotheses regarding underlying reaction mechanisms. In this work, assessments of toxicological potential were performed using the program ToxTree, and the Nexus software package was used to identify mechanisms that might contribute to the predicted toxicity. Knowledge generated in this way can be used to guide and support targeted in vitro testing (Myatt et al., 2018; Hasselgren et al., 2019).
2. Materials and methods
2.1. In silico testing
A two-step computational testing procedure based on the chemical structures of the target compounds was adopted. The program ToxTree (version v2.6.13) was initially used to assign the tested compounds into Cramer classes by querying a decision tree based on relevant structural characteristics. Compounds belonging to Cramer class I are considered to be of no/low concern, while compounds assigned to class III have structural features associated with high toxicological potential, such as unsubstituted aromatic carbohydrates. The Cramer classification is embedded into the threshold of toxicological concern (TTC) concept, which ranks food ingredients with insufficient toxicological data based on their chemical structures and levels of human exposure. It should be noted that substance groups with a risk of health hazard above 1 in 106 (e.g. chlorinated dioxins or N-nitroso compounds) are excluded from the outset. The estimates are based on linearised low-dose extrapolations (Kroes et al., 2004). Organophosphates are assigned a threshold of 18 μg/person/day; all other compounds are assigned a conservative threshold of 1.5 μg/person/day as an initial evaluation level. The intake of compounds allocated to Cramer classes I, II or III should not exceed 1800, 540 or 90 μg/person/day, respectively. These values are based on NOEL data from chronic animal studies on rats multiplied by a safety factor of 100. There is a > 95% probability that intakes below these thresholds will be below the 5% confidence interval for the NOEL of the substance in question (Kroes et al., 2005).
For quantitative analysis Derek Nexus (version 5.0.1), Sarah Nexus (version 2.0.1; model version 1.1.19), and the ICH M7 tools of Nexus Prediction (version 2.1.0) was used. Derek Nexus Prediction does not provide a threshold for a daily intake but is considerably more specific in its ability to relate structural features to defined toxicological endpoints. It identifies compounds structurally similar to those studied in order to suggest potential mechanisms of action based on literature from the Lhasa database. The degree of structural conformity (expressed as a percentage) enables gradual assignment of specific toxicological endpoints. Other structure-based parameters are also calculated, such as the skin permeability coefficient (log Kp), which depends on the molecular weight and the estimated distribution coefficient (log P), as benchmark for its lipophilicity. Sarah Nexus Prediction focuses even more specifically on potential mammalian mutagenicity in vitro, and generates a quantitative estimate of the percentage probability of mutagenicity by comparing similar (relevant) structural components.
Summary reports of the outputs of Derek Nexus and Sarah Nexus were generated using ICH M7 Nexus Prediction.
2.2. In vitro testing
2.2.1. Substances and cell lines
All chemicals and solvents used in this work were of analytical grade or compliant with standards required for cell culture experiments. Formamidopyrimidine glycosylase (FPG) was kindly provided by Prof. Andrew Collins (University of Oslo, Norway).
Syringaresinol (4,4′-(1S,3aR,4S,6aR)-Tetrahydro-1H,3H-furo[3,4-c]furan-1,4-diylbis(2,6-dimethoxy-phenol); Syr) was purchased from BOC Science (NewYork, USA). Enterodiol ((2R,3R)-2,3-bis[(3-hydroxyphenyl)methyl]butane-1,4-diol; END), enterolactone ((3R,4R)-3,4-bis[(3-hydroxyphenyl) methyl]oxolan-2-one; ENL), dimethyl sulfoxide (DMSO) 99,6%, sodium dodecylsulfate (SDS), absolute ethanol (≥99.8%), trypan blue solution, resazurin, and ethidium bromide were obtained from Sigma-Aldrich (Munich, Germany). Low and normal melting agarose were obtained from Bio-Rad (Munich, Germany), and trypsin was obtained from Serva (Heidelberg, Germany). Cell culture media (RPMI 1640, DMEM) were purchased from Thermo Fisher Scientific (Dreieich, Germany) while the supplements fetal calf serum (FCS) and penicillin/streptomycin (P/S) were obtained from Invitrogen (Karlsruhe, Germany). Cell culture materials (petri dishes, flasks, etc.) were acquired from Greiner Bio-One (Essen, Germany). The cell lines HepG2 and HT29 were purchased from DSMZ (Braunschweig, Germany).
2.2.2. Cell culture and incubation procedure
HepG2 and HT29 cells were cultured in 175 cm2 flasks using RPMI 1640 and DMEM as base media, respectively. Both media were supplemented with 10% FCS and 1% P/S to stimulate proliferation after subcultivation. The cells were frequently subcultured by washing with PBS, trypsination, and dilution with fresh media. For use in assays, the subcultivated cells were seeded in fresh flasks, incubated for 24 h, washed with PBS, then transferred to the appropriate incubation media; (i.e. the base media was supplemented with 5% FCS and 1% P/S). The cells were maintained at 37 °C under a humidified atmosphere with 5% CO2 at all times. All assays were performed using both cell lines. The confluence was 80–90%, and trypan blue assays were performed before each comet assay to verify that the cell vitality was >80%.
2.2.3. Determination of cytotoxicity
Cytotoxicity was evaluated using the resazurin reduction assay according to Page et al. (1993) with slight modifications. Here the intracellular redox state is determined to assess cellular vitality. In vital cells, reducing equivalents such as NAD(P)H/H+ reduce the non-fluorescent resazurin to the photometrically detectable fluorescent compound resorufin. Any damage-induced reduction in the cells' metabolic activity thus also reduces the conversion to resorufin. Cytotoxicity was expressed in terms of vitality relative to a negative control.
Briefly, 180,000 cells per well were seeded on a 48 well plate and then after 24 h exposed separately to 0.1, 1, 10, 100 μM Syr, END, or ENL for 1 h or 24 h. For this purpose, stock solutions of the compounds to be tested in DMSO were diluted 1:100 with incubation media to obtain media with the desired compound concentration. On each plate, each combination of compound and cell type was tested on triplicate alongside negative controls (1% DMSO), positive controls (0.1% SDS), media controls, and blanks (media without cells). After incubation, the cells were washed with PBS, 500 μL resazurin working solution was added to each well, and the cells were left to stand for an hour. The resazurin working solution was prepared by adding 5 mL resazurin stock solution (11.05 mg resazurin, 100 μL dimethylformamide, 900 mg NaCl, 14.4 mg KH2PO4, 52.8 mg Na2HPO4, and 100 mL H2O) to 45 mL basic media containing 1% P/S.
Photometric measurements were performed using excitation and emission wavelengths of 544 nm and 590 nm, respectively, with a fluoroscan ascent FL (labsystems Thermos Fisher scientific, Dreieich, Germany). Relative vitality was calculated by relating the measured values to those for the negative control after subtracting the blank value and is expressed as a percentage.
2.2.4. Determination of DNA damage
The comet assay was performed according to Collins et al. (1996) and Bakuradze et al., 2010, Bakuradze et al., 2019, with slight modifications. Alkaline gel electrophoresis (pH 13) was conducted to measure DNA damage in the form of DNA strand breaks. Initially, one million cells per petri dish (10 mm) were exposed 24 h to 10, 100 μM END or ENL dissolved in DMSO and diluted 1:1000 in incubation media. A 0.1% DMSO solution was used as a negative control. For positive controls, cells were carried along untreated and then exposed to 10 J/m2 UVC radiation (254 nm) immediately after incubation with the test substances. Aliquots of 70,000 cells were centrifuged (10 min, 2000 rpm, 4 °C), then the pellets were resuspended in 65 μL low melting agarose (0.5%) and placed on microscope slides pre-coated with normal melting agarose (1.5%). After overnight lysis at 4 °C, the slides were covered with 50 mL of either enzyme buffer or a solution of the enzyme formamidopyrimidine-DNA-glycosylase (FPG) to differentiate between background DNA strand breaks and total damage (background strand breaks and oxidized bases). After DNA unwinding (pH 13.5, 20 min, 4 °C) and horizontal gel electrophoresis (20 min, 25 V, 300 mA), slides were washed and fixed with ethanol (95%, 3 min) and stored at room temperature in the dark. Thereafter, the slides were stained with ethidium bromide and analysed using a fluorescence microscope (Imager, A1, filter set 15, Zeiss, Germany) and computerized image analysis (Comet Assay IV, Perceptive Instruments, Suffolk, GB), scoring 2 × 50 cells per slide. DNA migration was calculated as mean tail intensity (TI%: DNA in the comet tail in percent of total DNA).
2.2.5. Statistics
Results are presented as means ± SD of three independent experiments. For reason of just screening the FPG treated samples were measured in two independent experiments. Data were analysed for significant differences between control and samples by unpaired two-sided student's t-test (OriginLab Corporation, Northampton, MA, USA, version 2019).
3. Results and discussion
3.1. In silico testing
To screen the toxicological potential of the three entero−/lignans, structural alerts were identified using ToxTree and interpreted in accordance with the TTC concept. Then, specific target endpoints and underlying mechanisms were characterized using Nexus Prediction.
ToxTree assigned the studied lignans to Cramer class III based on their structural features, indicating that they have a high toxicological potential. This assignment was based on the presence of phenolic units with complex substituents such as the lactone of ENL, which were identified as reactive structures. Heterocyclic aromatic structures contributed particularly strongly to these assignments. In the absence of additional toxicological information, Cramer class III compounds are assumed to have a TTC of 0.09 mg/person/day, corresponding to a NOEL of 0.15 mg/kg BW based on the fifth percentile of the cumulative NOAEL distribution. Nexus Prediction generated structural alerts based on the presence of 1,2-dihydroxy and alkylphenol motifs in the studied compounds; these motifs are associated with the formation of quinoid structures and ROS generation. As a result, Syr was assigned a skin-sensitizing potential, while END and ENL were attributed chromosome damaging potential. The compounds' n-octanol water distribution coefficients of log P < 3 represent lipophilic reluctance which enables drug-likeness. Plausible mechanisms for the formation of reactive intermediates from Syr can be drawn up, involving an initial O-dealkylation of the methoxy group on the aromatic group to form an 1,2-dihydroxybenzene that could then be oxidized to the corresponding quinone. This oxidation could occur as a result of a ROS-generating reaction with oxygen or it could be catalysed enzymatically by CYP2E1 (Seaton et al., 1994). There are thus two ways by which O-demethylated Syr could exert a skin-sensitizing effect. Oxidation of the prohaptenic catechol moiety would generate a haptenic quinone that can form covalent bonds with nucleophiles because it is an electrophilic Michael acceptor (Aptula and Roberts, 2006). The interaction of such antigens with endogenous carrier proteins can trigger an immune reaction in accordance with the hapten-carrier principle (Günther et al., 2007). The formation of a quinone methide structure is a less important activating mechanism: while Syr does have benzylic methine groups whose C—H bonds could be cleaved to generate a quinone methide, the formation of such a structure would be hindered by the steric demands of the substituted bis-tetrahydrofuran ring system. The opening of this ring system due to intestinal metabolism generates more reactive alkylphenol structures such as Seco and the dihydroxylated enterolactone precursors. Another way in which Syr and its metabolites could exert tissue-damaging effects is by promoting ROS generation, leading to increased cellular stress (Attia, 2010). All the studied compounds can undergo homolytic hydrogen abstraction to form phenolic radicals that are stabilized by the adjacent aromatic ring. Upon interacting with other cellular components, these phenolic radicals can initiate radical chain reactions involving ROS, with deleterious effects on cellular structure and metabolic pathways due to phenomena such as lipid peroxidation. Since END is an m-alkylphenol derivative, it cannot form reactive o- or p-quinone methide derivatives. However, as a phenol, it can undergo radical hydrogen abstraction from the hydroxyl group, generating ROS. Because it lacks an o- or p-hydroxy or ketone group, the resulting radical does not benefit from any mesomeric stabilization, unlike in the case of quinones. If ROS are not intercepted by the endogenous redox system, there is a risk of oxidative damage to the DNA via processes such as 8-hydroxyguanine formation (Gill and Tuteja, 2010). Despite the reported antioxidative activity of lignans, they have also been observed to induce ROS generation (Prasad, 2000). These contradictory results may indicate that their antioxidative and prooxidative effects are concentration-dependant. Both effects can be attributed to hydrogen abstraction from aromatic hydroxy groups. Since ENL is formed from END by oxidative condensation, the two structures differ only in that the former features a lactone formed from the two methylene hydroxy groups of the latter. The structural alerts generated for END are unaffected by this transformation, so the theoretical arguments for END can be applied directly to ENL. No structural alerts were found in silico for mutagenicity with a confidence of 39% for Syr, 35% for END and 26% for ENL. This shows that despite the presence of structural features associated with genotoxicity (determined using Derek Nexus Prediction) quantitative whole-structure comparisons (performed using Sarah Nexus Prediction) indicated that the studied compounds lack mutagenicity.
3.2. In vitro testing
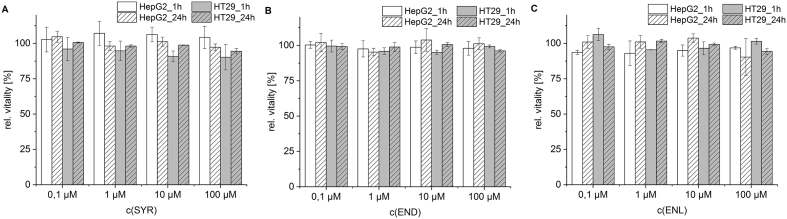
Because of their predicted toxicological potential, Syr, END, and ENL were tested in HepG2 and HT29 cells, which originate from human carcinoma of the liver respectively the colon. They were chosen because hepatocytes, enterocytes, and colonic cells are believed to be the main locations of lignan metabolism in the human body. Although permanent cell lines only reflect limited aspects of complex tissues, they are suitable for investigating mechanistic questions at the cellular level and have been used successfully to study the metabolism of (entero-)lignans (Jansen et al., 2005; Adlercreutz et al., 1992). The in silico prediction of skin-sensitizing effects was based on the formation of quinoid or quinone methide units that could potentially react with nucleophilic functional groups in proteins. Reactive units of these kinds can deactivate proteins, cause depletion of detoxifying compounds such as glutathione, or induce immunological reactions according to the hapten carrier principle. Since that mechanism is not limited to skin irritation, the basic effects of the three compounds on cell vitality were evaluated using the resazurin reduction assay. The results (Fig. 2) show no significant reductions of the relative vitality of HepG2 or HT29 cells after incubations of 1 h or 24 h with concentrations of 0–100 μM Syr, END or ENL. Because the in silico analysis indicated that END and ENL might cause chromosomal aberrations, the comet assay was used to assess their DNA damaging effects in HepG2 and HT29 cells (see Fig. 3). No significant modulation of background DNA-strand breaks was observed after 24 h incubation with END and ENL (10–100 μM). The screening for total DNA strand breaks with FPG treatment caused no significant modulation in %-TI, indicating that cells treated with 100 μM ENL for 24 h exhibited no more oxidative DNA damage than untreated controls.
Fig. 2.
Relative vitality of HepG2 and HT29 cells after 1 h and 24 h incubation with A Syringaresinol (Syr), B Enterodiol (END) and C Enterolactone (ENL) using resazurin reduction assay. Values are related to negative control 1% DMSO, as mean and SD of three independent experiments, each performed in triplicates.
Fig. 3.

Background and total DNA strand breaks of HepG2 and HT29 cells after 24 h incubation using comet assay. (−) no treatment with FPG, (+) treatment with FPG. As negative control 0,1% DMSO was used. For positive control cells were exposed to 10 J/m2 UVC radiation. The values are presented as mean and SD of three independent experiments, each performed in duplicates. For just screening the FPG treated samples were measured in two independent experiments, each as duplicates and the values presented as mean and range.
Syr, END, and ENL induced no detectable direct cytotoxicity or DNA strand breaking when applied to HepG2 and HT29 cells at concentrations of 0.1–100 μM for 24 h. In comparison to literature, the chosen test model and therefore the metabolic competence of it plays an important role. As discussed in silico, the biological activation of benzoid structures usually takes place via CYP2E1. However, the enzyme is expressed to a lesser extent in HepG2 and HT29 cells, the metabolic activation can be reduced accordingly (Hart et al., 2010; Altay et al., 2017). In human myelocytic HL-60 leukemia cancer cells, a significant concentration-dependent decrease in cell vitality after incubation with 0.5–40 μM Syr was observed by MTT assay (Park et al., 2008). However, a high CYP2E1 gene expression could also be measured in HL-60 (Nagai et al., 2002). This supports the assumption that metabolic activation is necessary for toxic effects in μM concentrations ranges of the investigated lignans. So as the skin-sensitizing and DNA damaging potentials cannot be strictly excluded by the negative effects regarding cytotoxicity and DNA damage in vitro, the observations are consistent with similar studies. In the human ovary carcinoma cell line ES-2 neither END nor ENL had shown a decrease in vitality after 48 h incubation in the concentrations used here. Only at 1 mM a significant decrease in vitality was observed (Liu et al., 2017). Testing the potential to induce DNA strand breaks using comet assay, Pool-Zobel and coworkers (Pool-Zobel et al., 2000) incubated HT29 clone 19A human colon tumor cells with phenolic phytoprotectants. Whereas after 15 min incubation with 100 μM END and ENL no differences to the negative control were detected, other phytoestrogens like genistein induced highly significant (p < 0.01) DNA strand breaks. But both metabolites of Syr even reduced the oxidased pyrimindine DNA bases while only ENL had such an impact on oxidized purine DNA bases as well.
3.3. Further discussion
The apparently contradictory results obtained in silico and in vitro indicate that a more comprehensive assessment of the limitations and consistency of the applied methods is needed.
Regarding the in silico analysis, the decision tree of ToxTree identifies structural features that could in principle participate in certain reactions. However, some kinds of reactivity can have positive biological effects under certain conditions and negative effects under other conditions, as exemplified by the equilibrium of the anti- and prooxidative features of phenolic groups (Bellion et al., 2009). The structural alerts for Syr, END and ENL related mainly to their phenolic units (which bear substituents of varying complexity). While these structures were evaluated largely in terms of their potential contributions to ROS formation, it is important to note that compounds such as polyphenols can also have beneficial antioxidative effects (Leopoldini et al., 2011; Fraga et al., 2010). This illustrates the dependence of effects on concentrations and parameters such as the oxidative state of the cell. Because predictive toxicological tools are designed to search for adverse effects, they focus on worst-case scenarios.
Nexus Prediction compares larger structural units to known analogues. Since small differences can have decisive effects on toxicity, related compounds can have different effects even when they have very high similarity percentages based on comparisons of structural groups. Therefore, the transferability of the predicted mechanism of toxicity must be evaluated on a per-compound basis. In the compounds studied here, the relative positioning of the substituents of the aromatic ring plays a decisive role because of its effects on mesomeric stabilization and hence the rings' propensity to abstract or donate protons. While potentially reactive motifs were found and analysed using Derek Nexus Prediction, their relevance may be limited because Sarah Nexus Prediction indicated that the probabilities of mutagenicity for the compounds in vitro were low or even negative (albeit with low certainty). A limitation of in silico tools without specific modulation such as those used here is that they do not generally differentiate between enantiomers. However, chirality is indubitably important in many enzymatic reactions and when steric effects are considered. For the prooxidative reaction mechanism involving the formation of reactive quinoid or quinone methide structures, we do not anticipate chirality to be an important factor. It should also be noted that Nexus prediction was not explicitly designed to investigate natural compounds in complex food matrices, which can lead to false positive outcomes. For evaluation, it is crucial to screen for toxicological effects in an adequate in vitro model. But to support more targeted testing, the ability of the programme to generate hypotheses can be used (Raunio, 2011).
In vitro assays were performed to investigate specific toxicological endpoints. While genotoxicity was assessed on a molecular level in this work, the anticancerogenic effects mentioned in the introduction were shown to be due to impacts on signalling pathways (e.g. VEGF) or endocrine modulation (Brooks et al., 2004; Jackson et al., 2010). So, test systems based on single cell lines capture only specific endpoints and partially effects, especially those relating to absorption, distribution, metabolism and excretion (ADME). However, the simplification of studying single cell lines in mechanistic investigations has important advantages in that it reduces overlap and the influence of compensation mechanisms. Two human cancer cell lines were tested, one originating from the liver (HepG2) and one from the colon (HT29). Both lines were previously shown to be suitable for studying the metabolism of lignans and enterolignans (Jansen et al., 2005; Adlercreutz et al., 1992). Incubations were performed using the deglycosylated forms of the test compounds Syr, END, and ENL. The in silico predictions were based on the unconjugated versions because the active units of the substances are at least partly blocked by the glycosidic bonds, and the aim was to test the ‘worst-case scenario’. After intake, lignans undergo various de−/glycosylation steps. Deglycosylation by the intestinal microbiota enables absorption and further metabolization, which occurs mainly in the cells of the colon and liver. Assuming no accumulation of the deglycosylated lignan (Rechner, 2004), a 24 h exposure in vitro should significantly exceed the expected retention time. The negative results obtained under these overload conditions thus suggests that a safety factor of these compounds may be even higher. Given the incubation conditions and the good agreement of our results with literature data (see chapter 3.2), the negative effects observed in vitro are neither unreliable nor incompatible with the in silico results.
The physiological relevance of in vitro and in silico analysis must always be considered carefully. Here, pure compounds were investigated from a strictly structural point of view in silico and in an isolated test system on a cellular basis in vitro. This approach is a great simplification of the real-world situation in which the studied substances would most likely be ingested in complex food matrices that would then undergo ADME in the human body. Care should thus be taken when extrapolating the results presented here. In the absence of toxicological data, a TTC of 0.09 mg/person/day can be assigned by classifying Syr, END and ENL as Cramer class III compounds. To relate this value to the internal exposure, several factors must be considered. These include bioavailability, which is largely determined by the diverse intestinal microbiota that enable absorption through deglycosylation. The rate of this process can differ substantially between related compounds: experiments with human faecal microbiota yielded a turnover of 4% for Syr-DG but 55% for pinoresinol-diglycoside (Heinonen et al., 2001). After a correspondingly reduced actual uptake, rapid glycosylation occurs (Rechner, 2004). Conjugation tends to detoxify lignans by covering their reactive units, and plays a major role in distribution and excretion, so that it drastically reduces adverse internal impacts. In comparison to the estimated daily consumption of 0.15–1.1 mg/person/day lignans (Horn-Ross et al., 2000), up to 10 g flaxseed was applied to postmenopausal women in a human study that showed a positive impact on estrogen levels (Brooks et al., 2004). At an average lignan content of 301 mg/100 g flaxseed (Milder et al., 2005), this would correspond to a load of 83 μmol Seco (assuming for simplicity that Seco is the sole lignan in flaxseed). According to Heinonen and colleagues (Heinonen et al., 2001), intestinal microbiota is expected to convert 72% of Seco to END and ENL. Conversion at this rate would result in exposures of 60 μmol for END/ENL and 23 μmol for Seco. For comparative purposes, levels of lariciresinol, matairesinol, END, and ENL in the serum of non-supplemented participants were 190, 24, 6 and 110 nM, respectively (Grace et al., 2003; Morton et al., 2002; Smeds et al., 2006; Valentín-Blasini et al., 2003). While the lowest tested concentration of 100 nM is thus of a physiologically relevant magnitude (especially when considering potential supplementation and/or the effects of polymorphisms in a worst-case scenario), concentrations up of 100 μM in target cells are not considered realistic. These high dosages tested in this work clearly exceed a realistic daily intake, but even so no evidence of cytotoxicity or genotoxicity (indicated by single or double strand breaks in DNA) was found.
Thus, different sensitivities depending on the metabolic activation and an impact on the redox statue of cells are characteristics to be discussed further. The goal would be to assess the cyto- and genotoxicity of other common lignans, e.g. Seco. In addition, it will be necessary to evaluate the wider impacts of these compounds on the human body with respect to additional toxicological endpoints (e.g. endocrine signalling) if processed foodstuffs are deliberately supplemented with the studied compounds.
4. Conclusion
The in silico analysis emphasized structural features of Syr, END and ENL that are associated with ROS generation and reactivity towards cellular nucleophiles. The need to consider the impact of each mechanism identified in the analysis individually revealed limitations in the transferability of the hypotheses generated in the analysis and showed that potential negative impacts do not exclude additional potentially positive effects resulting from (for instance) antioxidative activity. These findings reinforce the need to consider the effects of concentration and cellular conditions when evaluating a compound's likely effects in vivo. The higher dosages tested in vitro corresponded to exposures higher than those that would be achieved through daily intake or by supplementation in human studies, even before taking ADME into account. Despite this, Syr, END, and ENL induced no detectable cytotoxicity in vitro at concentrations up to 100 μM in HepG2 and HT29 cells for exposures of up to 24 h. No modulation of background and total DNA strand breaks were observed in either cell line after exposure to 10 or 100 μM END or END after 24 h. These results are consistent with literature data, which suggests they have good reproducibility. Importantly, the findings presented here show that mechanistic hypotheses generated by in silico analysis based solely on chemical structure data can be used to interpret cellular effects observed in vitro to clarify the biological activity of lignans. Regarding the restrictions of in silico, for further evaluation a sufficient verification by representative in vitro models is necessary. After the initial screening in two cell lines, more complex 3D cell models are suggested for further investigations.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors gratefully acknowledge the gift of FPG enzyme from Prof. A.R. Collins (Institute for Nutrition Research, University of Oslo, Norway). The authors gratefully acknowledge financial support by the EU-INTERREG project BIOVAL (no. 018-4-09-021).
Funding
The EU-INTERREG project BIOVAL was supported by the “European Funds for Regional Development”, project no. 018-4-09-021.
References
- Adlercreutz H., Mousavi Y., Clark J., Höckerstedt K., Hämäläinen E., Wähälä K., Mäkelä T., Hase T. Dietary phytoestrogens and cancer: in vitro and in vivo studies. J. Steroid Biochem. Mol. Biol. 1992;3-8:331–337. doi: 10.1016/0960-0760(92)90359-q. https://10.1016/0960-0760(92)90359-Q [DOI] [PubMed] [Google Scholar]
- Altay A., İrtem K., Sadi G., Güray T., Yaprak A. Modulation of mRNA expression and activities of xenobiotic metabolizing enzymes, CYP1A1, CYP1A2, CYP2E1, GPx and GSTP1 by the Salicornia freitagii extract in HT-29 human colon cancer cells. Arch biol sci (Beogr) 2017;3:439–448. https://10.2298/ABS160825118A [Google Scholar]
- Aptula A.O., Roberts D.W. Mechanistic applicability domains for nonanimal-based prediction of toxicological end points. Chem. Res. Toxicol. 2006;8:1097–1105. doi: 10.1021/tx0601004. https://10.1021/tx0601004 [DOI] [PubMed] [Google Scholar]
- Attia S.M. Deleterious effects of reactive metabolites. Oxidative Med. Cell. Longev. 2010;4:238–253. doi: 10.4161/oxim.3.4.13246. https://10.4161/oxim.3.4.13246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakuradze T., Lang R., Hofmann T., Stiebitz H., Bytof G., Lantz I., Baum M., Eisenbrand G., Janzowski C. Antioxidant effectiveness of coffee extracts and selected constituents in cell-free systems and human colon cell lines. Mol. Nutr. Food Res. 2010;12:1734–1743. doi: 10.1002/mnfr.201000147. https://10.1002/mnfr.201000147 [DOI] [PubMed] [Google Scholar]
- Bakuradze T., Tausend A., Galan J., Maria Groh I.A., Berry D., Tur J.A., Marko D., Richling E. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free Radic. Res. 2019:1–11. doi: 10.1080/10715762.2019.1618851. https://10.1080/10715762.2019.1618851 [DOI] [PubMed] [Google Scholar]
- Begum A.N., Nicolle C., Mila I., Lapierre C., Nagano K., Fukushima K., Heinonen S.-M., Adlercreutz H., Rémésy C., Scalbert A. Dietary lignins are precursors of mammalian lignans in rats. J. Nutr. 2004;1:120–127. doi: 10.1093/jn/134.1.120. https://10.1093/jn/134.1.120 [DOI] [PubMed] [Google Scholar]
- Bellion P., Olk M., Will F., Dietrich H., Baum M., Eisenbrand G., Janzowski C. Formation of hydrogen peroxide in cell culture media by apple polyphenols and its effect on antioxidant biomarkers in the colon cell line HT-29. Mol. Nutr. Food Res. 2009;10:1226–1236. doi: 10.1002/mnfr.200800456. https://10.1002/mnfr.200800456 [DOI] [PubMed] [Google Scholar]
- Brooks S.C., Skafar D.F. From ligand structure to biological activity. Steroids. 2004;6:401–418. doi: 10.1016/j.steroids.2004.03.014. https://10.1016/j.steroids.2004.03.014 [DOI] [PubMed] [Google Scholar]
- Brooks J.D., Ward W.E., Lewis J.E., Hilditch J., Nickell L., Wong E., Thompson L.U. Supplementation with flaxseed alters estrogen metabolism in postmenopausal women to a greater extent than does supplementation with an equal amount of soy. Am. J. Clin. Nutr. 2004;2:318–325. doi: 10.1093/ajcn/79.2.318. [DOI] [PubMed] [Google Scholar]
- Chen L.-H., Fang J., Sun Z., Li H., Wu Y., Demark-Wahnefried W., Lin X. Enterolactone inhibits insulin-like growth factor-1 receptor signaling in human prostatic carcinoma PC-3 cells. J. Nutr. 2009;4:653–659. doi: 10.3945/jn.108.101832. https://10.3945/jn.108.101832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A.R., Dusinská M., Gedik C.M., Stĕtina R. Oxidative damage to DNA: do we have a reliable biomarker? Environ. Health Perspect. 1996:465–469. doi: 10.1289/ehp.96104s3465. https://10.1289/ehp.96104s3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrosin C., Chen J., Wang L., Thompson L.U. Flaxseed inhibits metastasis and decreases extracellular vascular endothelial growth factor in human breast cancer xenografts. Cancer Lett. 2002;1:31–37. doi: 10.1016/s0304-3835(02)00239-2. https://10.1016/S0304-3835(02)00239-2 [DOI] [PubMed] [Google Scholar]
- Ford J.D., Huang K.-S., Wang H.-B., Davin L.B., Lewis N.G. Biosynthetic pathway to the cancer chemopreventive secoisolariciresinol diglucoside−hydroxymethyl glutaryl ester-linked lignan oligomers in flax (Linumusitatissimum) seed †. J. Nat. Prod. 2001;11:1388–1397. doi: 10.1021/np010367x. https://10.1021/np010367x [DOI] [PubMed] [Google Scholar]
- Fraga C.G., Galleano M., Verstraeten S.V., Oteiza P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010;6:435–445. doi: 10.1016/j.mam.2010.09.006. https://10.1016/j.mam.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;12:909–930. doi: 10.1016/j.plaphy.2010.08.016. https://10.1016/j.plaphy.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Grace P.B., Taylor J.I., Botting N.P., Fryatt T., Oldfield M.F., Al-Maharik N., Bingham S.A. Quantification of isoflavones and lignans in serum using isotope dilution liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003;12:1350–1357. doi: 10.1002/rcm.1059. https://10.1002/rcm.1059 [DOI] [PubMed] [Google Scholar]
- Günther S., Weiser A.A., Ahmed J., Preissner R. Superhaptens and their potential role in contact dermatitis. Drug Discov. Today Ther. Strateg. 2007;1:11–17. https://10.1016/j.ddstr.2007.08.003 [Google Scholar]
- Hart S.N., Li Y., Nakamoto K., Subileau E.-a., Steen D., Zhong X.-b. A comparison of whole genome gene expression profiles of HepaRG cells and HepG2 cells to primary human hepatocytes and human liver tissues. Drug Metab. Dispos. 2010;6:988–994. doi: 10.1124/dmd.109.031831. https://10.1124/dmd.109.031831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselgren C., Ahlberg E., Akahori Y., Amberg A., Anger L.T., Atienzar F., Auerbach S., Beilke L., Bellion P., Benigni R., Bercu J., Booth E.D., Bower D., Brigo A., Cammerer Z., Cronin M.T.D., Crooks I., Cross K.P., Custer L., Dobo K., Doktorova T., Faulkner D., Ford K.A., Fortin M.C., Frericks M., Gad-McDonald S.E., Gellatly N., Gerets H., Gervais V., Glowienke S., van Gompel J., Harvey J.S., Hillegass J., Honma M., Hsieh J.-H., Hsu C.-W., Barton-Maclaren T.S., Johnson C., Jolly R., Jones D., Kemper R., Kenyon M.O., Kruhlak N.L., Kulkarni S.A., Kümmerer K., Leavitt P., Masten S., Miller S., Moudgal C., Muster W., Paulino A., Lo Piparo E., Powley M., Quigley D.P., Reddy M.V., Richarz A.-N., Schilter B., Snyder R.D., Stavitskaya L., Stidl R., Szabo D.T., Teasdale A., Tice R.R., Trejo-Martin A., Vuorinen A., Wall B.A., Watts P., White A.T., Wichard J., Witt K.L., Woolley A., Woolley D., Zwickl C., Myatt G.J. Genetic toxicology in silico protocol. Regul. Toxicol. Pharmacol. 2019;107:104403. doi: 10.1016/j.yrtph.2019.104403. https://10.1016/j.yrtph.2019.104403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen S., Nurmi T., Liukkonen K., Poutanen K., Wähälä K., Deyama T., Nishibe S., Adlercreutz H. In vitro metabolism of plant lignans. J. Agric. Food Chem. 2001;7:3178–3186. doi: 10.1021/jf010038a. https://10.1021/jf010038a [DOI] [PubMed] [Google Scholar]
- Horn-Ross P.L., Barnes S., Lee M., Coward L., Mandel J.E., Koo J., John E.M., Smith M. Assessing phytoestrogen exposure in epidemiologic studies. Cancer Causes Control. 2000;4:289–298. doi: 10.1023/a:1008995606699. https://10.1023/A:1008995606699 [DOI] [PubMed] [Google Scholar]
- Hu C., Yuan Y.V., Kitts D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007;11:2219–2227. doi: 10.1016/j.fct.2007.05.017. https://10.1016/j.fct.2007.05.017 [DOI] [PubMed] [Google Scholar]
- Jackson M.D., McFarlane-Anderson N.D., Simon G.A., Bennett F.I., Walker S.P. Urinary phytoestrogens and risk of prostate cancer in Jamaican men. Cancer Causes Control. 2010;12:2249–2257. doi: 10.1007/s10552-010-9648-9. https://10.1007/s10552-010-9648-9 [DOI] [PubMed] [Google Scholar]
- Jansen G.H.E., Arts I.C.W., Nielen M.W.F., Müller M., Hollman P.C.H., Keijer J. Uptake and metabolism of enterolactone and enterodiol by human colon epithelial cells. Arch. Biochem. Biophys. 2005;1:74–82. doi: 10.1016/j.abb.2004.12.015. https://10.1016/j.abb.2004.12.015 [DOI] [PubMed] [Google Scholar]
- Jenab M., Rickard S.E., Orcheson L.J., Thompson L.U. Flaxseed and lignans increase cecal beta-glucuronidase activity in rats. Nutr. Cancer. 1999;2:154–158. doi: 10.1207/S15327914NC330206. https://10.1207/S15327914NC330206 [DOI] [PubMed] [Google Scholar]
- Kroes R., Renwick A.G., Cheeseman M., Kleiner J., Mangelsdorf I., Piersma A., Schilter B., Schlatter J., van Schothorst F., Vos J.G., Würtzen G. Structure-based thresholds of toxicological concern (TTC) Food Chem. Toxicol. 2004;1:65–83. doi: 10.1016/j.fct.2003.08.006. https://10.1016/j.fct.2003.08.006 [DOI] [PubMed] [Google Scholar]
- Kroes R., Kleiner J., Renwick A. The threshold of toxicological concern concept in risk assessment. Toxicol. Sci. 2005;2:226–230. doi: 10.1093/toxsci/kfi169. https://10.1093/toxsci/kfi169 [DOI] [PubMed] [Google Scholar]
- Landete J.M. Plant and mammalian lignans. Food Res. Int. 2012;1:410–424. https://10.1016/j.foodres.2011.12.023 [Google Scholar]
- Leopoldini M., Russo N., Toscano M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011;2:288–306. https://10.1016/j.foodchem.2010.08.012 [Google Scholar]
- Liu H., Liu J., Wang S., Zeng Z., Li T., Liu Y., Mastriani E., Li Q.-H., Bao H.-X., Zhou Y.-J., Wang X., Hu S., Gao S., Qi Y., Shen Z., Wang H., Yu M., Gao T., Johnston R.N., Liu S.-L. Enterolactone has stronger effects than enterodiol on ovarian cancer. J Ovarian Res. 2017;1:49. doi: 10.1186/s13048-017-0346-z. https://10.1186/s13048-017-0346-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milder I.E.J., Arts I.C.W., van de Putte B., Venema D.P., Hollman P.C.H. Lignan contents of Dutch plant foods. BJN. 2005;03:393. doi: 10.1079/bjn20051371. https://10.1079/BJN20051371 [DOI] [PubMed] [Google Scholar]
- Morton M.S., Arisaka O., Miyake N., Morgan L.D., Evans B.A.J. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J. Nutr. 2002;10:3168–3171. doi: 10.1093/jn/131.10.3168. https://10.1093/jn/131.10.3168 [DOI] [PubMed] [Google Scholar]
- Myatt G.J., Ahlberg E., Akahori Y., Allen D., Amberg A., Anger L.T., Aptula A., Auerbach S., Beilke L., Bellion P., Benigni R., Bercu J., Booth E.D., Bower D., Brigo A., Burden N., Cammerer Z., Cronin M.T.D., Cross K.P., Custer L., Dettwiler M., Dobo K., Ford K.A., Fortin M.C., Gad-McDonald S.E., Gellatly N., Gervais V., Glover K.P., Glowienke S., van Gompel J., Gutsell S., Hardy B., Harvey J.S., Hillegass J., Honma M., Hsieh J.-H., Hsu C.-W., Hughes K., Johnson C., Jolly R., Jones D., Kemper R., Kenyon M.O., Kim M.T., Kruhlak N.L., Kulkarni S.A., Kümmerer K., Leavitt P., Majer B., Masten S., Miller S., Moser J., Mumtaz M., Muster W., Neilson L., Oprea T.I., Patlewicz G., Paulino A., Lo Piparo E., Powley M., Quigley D.P., Reddy M.V., Richarz A.-N., Ruiz P., Schilter B., Serafimova R., Simpson W., Stavitskaya L., Stidl R., Suarez-Rodriguez D., Szabo D.T., Teasdale A., Trejo-Martin A., Valentin J.-P., Vuorinen A., Wall B.A., Watts P., White A.T., Wichard J., Witt K.L., Woolley A., Woolley D., Zwickl C., Hasselgren C. In silico toxicology protocols. Regul Toxicol Pharmacol. 2018;96:1–17. doi: 10.1016/j.yrtph.2018.04.014. https://10.1016/j.yrtph.2018.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai F., Hiyoshi Y., Sugimachi K., Tamura H.-o. Cytochrome P450 (CYP) expression in human myeloblastic and lymphoid cell lines. Biol. Pharm. Bull. 2002;3:383–385. doi: 10.1248/bpb.25.383. https://10.1248/bpb.25.383 [DOI] [PubMed] [Google Scholar]
- Niemi P., Tamminen T., Smeds A., Viljanen K., Ohra-aho T., Holopainen-Mantila U., Faulds C.B., Poutanen K., Buchert J. Characterization of lipids and lignans in brewer’s spent grain and its enzymatically extracted fraction. J. Agric. Food Chem. 2012;39:9910–9917. doi: 10.1021/jf302684x. https://10.1021/jf302684x [DOI] [PubMed] [Google Scholar]
- Page B., Page M., Noel C. A new fluorometric assay for cytotoxicity measurements in vitro. Int. J. Oncol. 1993;3(3):473–476. https://10.3892/ijo.3.3.473 [PubMed] [Google Scholar]
- Park B.-Y., Oh S.-R., Ahn K.-S., Kwon O.-K., Lee H.-K. (−)-Syringaresinol inhibits proliferation of human promyelocytic HL-60 leukemia cells via G1 arrest and apoptosis. Int. Immunopharmacol. 2008;7:967–973. doi: 10.1016/j.intimp.2008.02.012. https://10.1016/j.intimp.2008.02.012 [DOI] [PubMed] [Google Scholar]
- Petersen M., Alfermann A.W. The production of cytotoxic lignans by plant cell cultures. Appl. Microbiol. Biotechnol. 2001;2:135–142. doi: 10.1007/s002530000510. https://10.1007/s002530000510 [DOI] [PubMed] [Google Scholar]
- Pool-Zobel B.L., Adlercreutz H., Glei M., Liegibel U.M., Sittlingon J., Rowland I., Wähälä K., Rechkemmer G. Isoflavonoids and lignans have different potentials to modulate oxidative genetic damage in human colon cells. Carcinogenesis. 2000;6:1247–1252. https://10.1093/carcin/21.5.247 [PubMed] [Google Scholar]
- Prasad K. Antioxidant activity of secoisolariciresinol diglucoside-derived metabolites, secoisolariciresinol, enterodiol, and enterolactone. Int. J. Angiol.2000;4:220–225. doi: 10.1007/BF01623898. https://10.1007/BF01623898 [DOI] [PubMed] [Google Scholar]
- Raunio H. In silico toxicology - non-testing methods. Front. Pharmacol. 2011;33 doi: 10.3389/fphar.2011.00033. https://10.3389/fphar.2011.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechner A. Colonic metabolism of dietary polyphenols. Free Radical Bio Med. 2004;2:212–225. doi: 10.1016/j.freeradbiomed.2003.09.022. https://10.1016/j.freeradbiomed.2003.09.022 [DOI] [PubMed] [Google Scholar]
- Seaton M.J., Schlosser P.M., Bond J.A., Medinsky M.A. Benzene metabolism by human liver microsomes in relation to cytochrome P450 2E1 activity. Carcinogenesis. 1994;9:1799–1806. doi: 10.1093/carcin/15.9.1799. https://10.1093/carcin/15.9.1799 [DOI] [PubMed] [Google Scholar]
- Setchell K.D.R., Brown N.M., Zimmer-Nechemias L., Wolfe B., Jha P., Heubi J.E. Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food Funct. 2014;3:491–501. doi: 10.1039/c3fo60402k. https://10.1039/C3FO60402K [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeds A.I., Hakala K., Hurmerinta T.T., Kortela L., Saarinen N.M., Mäkelä S.I. Determination of plant and enterolignans in human serum by high-performance liquid chromatography with tandem mass spectrometric detection. J. Pharm. Biomed. Anal. 2006;3:898–905. doi: 10.1016/j.jpba.2005.12.036. https://10.1016/j.jpba.2005.12.036 [DOI] [PubMed] [Google Scholar]
- Thompson L.U., Boucher B.A., Liu Z., Cotterchio M., Kreiger N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr. Cancer. 2006;2:184–201. doi: 10.1207/s15327914nc5402_5. https://10.1207/s15327914nc5402_5 [DOI] [PubMed] [Google Scholar]
- Touillaud M.S., Thiébaut A.C.M., Fournier A., Niravong M., Boutron-Ruault M.-C., Clavel-Chapelon F. Dietary lignan intake and postmenopausal breast cancer risk by estrogen and progesterone receptor status. J. Natl. Cancer Inst. 2007;6:475–486. doi: 10.1093/jnci/djk096. https://10.1093/jnci/djk096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touré A., Xueming X. Flaxseed lignans. CRFSFS. 2010;3:261–269. doi: 10.1111/j.1541-4337.2009.00105.x. https://10.1111/j.1541-4337.2009.00105.x [DOI] [PubMed] [Google Scholar]
- Valentín-Blasini L., Blount B.C., Caudill S.P., Needham L.L. Urinary and serum concentrations of seven phytoestrogens in a human reference population subset. J. Expo. Anal. Environ. Epidemiol. 2003;4:276–282. doi: 10.1038/sj.jea.7500278. https://10.1038/sj.jea.7500278 [DOI] [PubMed] [Google Scholar]
- Xie L.-H., Akao T., Hamasaki K., Deyama T., Hattori M. Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of enterococcus faecalis strain PDG-1 responsible for the transformation of (+)-Pinoresinol to (+)-Lariciresinol. Chem. Pharm. Bull. 2003;5:508–515. doi: 10.1248/cpb.51.508. https://10.1248/cpb.51.508 [DOI] [PubMed] [Google Scholar]