Graphical abstract
Keywords: LLNA, Non-animal methods, Overload effect, Danger signal, Chemistry-based read-across
Highlights
-
•
Irregular dose–response patterns occur quite frequently in LLNA datasets.
-
•
These involve an overload effect, giving a bell-shaped or inverse dose–response.
-
•
A mechanistic rationale is presented to account for the overload effect.
-
•
The overload effect can lead to both over- and under-estimation of potency.
-
•
Chemistry input is useful in such cases for risk assessment and assessing Non-animal methods.
Abstract
There is a large body of information on testing of chemicals for skin sensitization in the murine local lymph node assay (LLNA), in which potency is quantified by the EC3 value, derived from dose-response data. This information finds use in risk assessment and regulatory classification, and also in assessing the performance of non-animal methods. However, some LLNA results are not straightforward to interpret, and in some cases published EC3 values are questionable. These cases usually arise where the dose–response does not show a monotonic increasing pattern but is bell-shaped, or shows a decrease in response with increasing dose over the whole dose range tested. By analogy with a long-recognised phenomenon in guinea pig sensitization, this is referred to as the overload effect. Here a mechanistic rationale is presented to explain the overload effect, and at the same time to explain the production of danger signals even when the sensitizer is non-irritant. Some illustrative examples are presented where the overload effect can lead to misinterpretation of LLNA results, and chemistry-based read-across is applied to reinterpret the data.
1. Introduction
The ability of chemicals to cause skin sensitization has traditionally been assessed, for risk assessment purposes, by animal testing; in particular, since the mid-1990s, by the murine local lymph node assay (LLNA) (Basketter et al., 2007). Historic LLNA data are still widely used for regulatory and risk assessment purposes. Because of increasing regulatory and ethical demands, a risk assessment approach without animal testing is desirable, and development and assessment of non-animal methods for skin sensitization testing has become a topic of high priority (Nishijo et al., 2020, Schultz et al., 2016).
For the assessment of non-animal methods, be they in vitro, in chemico, in silico or in cerebro, historic animal data are necessary. There is a large body of information on testing of chemicals in the LLNA, in which potency is quantified by the EC3 value. The EC3 value is derived from dose-response data, and is defined as the dose, in weight percent, that would give a stimulation index (SI) value of 3.0, which is regarded as the threshold for positive sensitization (Basketter et al., 2007). The LLNA is not the only source of skin sensitization data – many compounds were tested in guinea pig assays before the LLNA became the preferred method. The guinea pig data are usually less easy to interpret quantitatively and do not give a simple potency value. Nevertheless, guinea pig data led to quantitative models and provided a basis for structure–activity relationships (Roberts and Williams, 1982, Roberts et al., 1983, Roberts, 1987, Roberts and Basketter, 1990, Franot et al., 1994).
In the course of work on structure–activity and quantitative modelling with LLNA data, it has become apparent that some LLNA results are not straightforward to interpret, and in some cases published EC3 values are questionable. These cases often arise where the dose–response pattern does not show a monotonic increase of the SI value with increasing dose, but is bell-shaped or shows a decrease in SI with increasing dose over the whole dose range tested. This is analogous to a long-recognised feature in guinea pig sensitization, the “overload effect” (Roberts and Williams, 1982, Claman et al., 1980), whereby above some threshold, increasing the induction dose of sensitizer, or modifying the sensitizer structure so as to make it more reactive or more hydrophobic, leads to a lower degree of sensitization. It is most clearly apparent in situations where with increasing induction dose of sensitizer, the degree of sensitization decreases (Claman et al., 1980). It is also seen in plots of sensitization potential against the Relative Alkylation Index, RAI (the RAI being in effect a measure of the “dose” of protein binding), where the slope becomes negative at higher RAI values (Roberts and Williams, 1982). The large dataset on a series of alk-2-ene-1,3-sultones tested in a guinea pig single injection adjuvant test at a range of induction concentrations and a range of challenge concentrations (Ritz et al., 1975) and mathematically modelled by Roberts and Williams (1982) demonstrates the overload effect very clearly, as illustrated in Fig. 1, taken from Roberts and Williams (1982).
Fig. 1.
Sensitization score vs RAIi for unsaturated sultones, from Roberts and Williams (Roberts and Williams, 1982). Sensitization score is the number of guinea pigs, expressed as a percentage of the animals tested (in most cases 15), responding positively to challenge with the test compound at 20 nanomolar concentration. RAIi is the RAI value calculated for the induction dose (each sultone was tested at 6 induction doses).
The overload effect was originally assumed to result from induction of suppressor cells at high levels of protein binding (Claman et al., 1980). The suppressor cells, referred to as Ts-cells, were assumed to suppress the action of effector cells, referred to as Te-cells (Claman et al., 1980). Subsequently this interpretation has become less convincing, the proposed suppressor cells having proved elusive to detect.
In an analysis (Roberts et al., 2007) of a published LLNA dataset (Ashby et al., 1995), several examples of the overload effect were found. Table 1 lists all the 12 compounds (11% of the total) from that dataset of 106 chemicals for which a decrease in SI is seen on going to a higher test concentration. These are not the only examples of LLNA overload effects – later datasets, such as those of Gerberick et al., 2005, Kern et al., 2010, and the NICEATM LLNA database (ntp) contain further examples – but they serve to illustrate the point. In some cases the decrease is small and could possibly be attributed to statistical noise, but in others the difference is large. In other cases there is a negative dose–response trend over all three test concentrations, while in others it is only apparent for the two highest concentrations. For comparison and for use in an RAI plot, several cases where an overload effect is not observed are also shown in Table 1.
Table 1.
Chemicals with overload effects, mainly from reference Roberts et al. (Roberts et al., 2007).
| Name | LLNA test concentrations and responses | |||||
|---|---|---|---|---|---|---|
| Cinnamaldehyde | % | 5 | 10 | 25 | ||
| SI | 12.5 | 18.4 | 15.4 | |||
| Methyl dodecanesulfonatea,b | % | 1 | 2.5 | 5 | ||
| SI | 21.6 | 39.9 | 48.6 | |||
| % | 5 | 10 | 25 | |||
| SI | 46.3 | 43.6 | 34.1 | |||
| Methyl hexadec-3-ene sulfonate | % | 5 | 10 | 25 | ||
| SI | 26.7 | 35.4 | 32.9 | |||
| p-Nitrobenzyl chlorideb | % | 5 | 10 | 25 | ||
| SI | 40.0 | 31.7 | 23.8 | |||
| Octyl gallate | % | 5 | 10 | 25 | ||
| SI | 72.0 | 63.9 | 63.5 | |||
| 2-Methyl-4,5-trimethylene-4-isothiazolin-3-one | % | 3 | 10 | 30 | ||
| SI | 4.5 | 8.9 | 7.0 | |||
| 4-Nitroso-N,N-dimethylaniline | % | 2.5 | 5 | 10 | ||
| SI | 43.4 | 60.4 | 48.5 | |||
| Dimethyl sulfateb | % | 0.25 | 0.5 | 1.0 | ||
| SI | 3.8 | 6.0 | 5.7 | |||
| 7,12-Dimethylbenz[a]anthracene | % | 0.025 | 0.5 | 1.0 | ||
| SI | 7.6 | 17.7 | 15.6 | |||
| 1-Ethyl-3-nitro-1-nitrosoguanidine | % | 0.05 | 0.1 | 0.25 | ||
| SI | 5.7 | 9.6 | 8.4 | |||
| Octadecanoyl chloride | % | 5 | 10 | 25 | ||
| SI | 3.8 | 4.5 | 4 | |||
| 2-Hydroxyethyl acrylate | % | 10 | 25 | |||
| SI | 9.0 | 8.2 | ||||
| Diethyl maleate | % | 1 | 2.5 | 5 | 10 | 25 |
| SIc | 2.1 | 3.3 | 3.5 | 7.5 | 16 | |
| % | 25 | 50 | 100 | |||
| SId | 16.3 | 22.6 | 13.1 | |||
| Cases not showing an overload effect | ||||||
| Benzoquinone | % | 0.5 | 1 | 2.5 | ||
| SI | 36.4 | 42.3 | 52.3 | |||
| Hexylcinnamic aldehyde | % | 10 | 25 | 50 | ||
| SI | 3.2 | 6 | 10 | |||
| 1-Bromopentadecane | % | 5 | 10 | 25 | ||
| SI | 2.9 | 7.8 | 19.6 | |||
| 1-Bromo-dodecan-12-olb | % | 5 | 10 | 25 | ||
| SI | 1.3 | 2 | 3.9 | |||
| 12-Bromo-dodecanoic acid | % | 5 | 10 | 25 | ||
| SI | 2.2 | 4.3 | 9.8 | |||
| Dodecyl methanesulfonateb, | % | 5 | 10 | 25 | ||
| SI | 2.1 | 3.3 | 9 | |||
| Diethyl sulfateb, | % | 1 | 2.5 | 10 | ||
| SI | 0.8 | 1.9 | 12 | |||
| Methyl methanesulfonateb, | % | 0.25 | 1 | 10 | ||
| SI | 0.7 | 0.7 | 3.6 | |||
a Ref. Roberts and Basketter (2000)
b Data used in RAI plot, Fig. 3
c Ref. Basketter et al. (1999).
d Ref. Ryan et al. (2000).
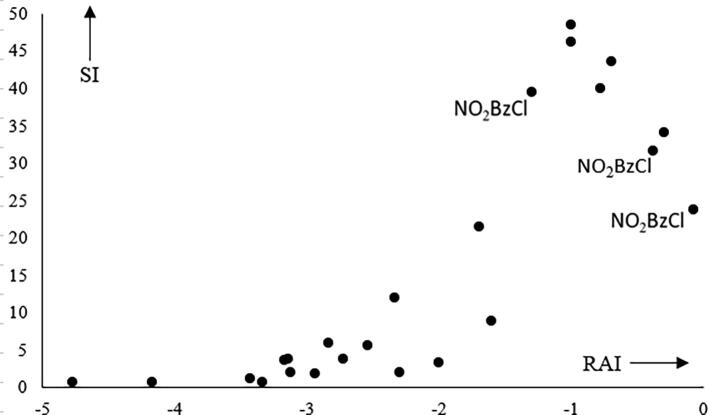
Fig. 2 shows dose response plots for the two compounds in Table 1 that have SI values at more than 4 different concentrations, In these the overload effect is very obvious and too large to be attributed to statistical noise. Fig. 3 shows an RAI plot in which the SI values for several structurally different chemicals, all having chemical reactivity alerts corresponding to the H-polar SN2 reaction mechanistic domain (Roberts et al., 2007, Roberts et al., 2016), are plotted against RAI values calculated as a function of the dose, relative reactivity and hydrophobicity as detailed in ref. Roberts et al. (2007). The overload effect is again very obvious and the plot has the same general shape as the guinea pig RAI plot in Fig. 1.
Fig. 2.
SI vs logC for LLNA data showing overload effect.
Fig. 3.
RAI plot for LLNA data on diverse H-polar SN2 electrophiles Based on dose–response data from Table 1. The nine points with SI greater than 20 correspond to methyl hexadecanesulfonate (six points) and p-nitrobenzyl chloride, NO2BzCl, (three points, indicated).
The principle of the LLNA is that if a compound is a skin sensitizer it will cause Langerhans cells to migrate to the draining lymph node, leading to increased lymph node activity which can be detected by increased lymph node uptake of thymidine in sensitizer-treated animals relative to controls. This ratio, referred to as the stimulation index, SI, is a measure of the extent to which the animals have been sensitized (Basketter et al., 2007).
Since the SI is simply a measure of the degree of cell proliferation in the lymph node and does not discriminate as to the type of cells that are proliferating, it follows that if the overload effect occurs by induction of suppressor cells, it would not be apparent in the LLNA – the induction of suppressor cells would contribute to the overall increase in lymph node cell proliferation.
That the overload effect is observed in the LLNA is therefore further evidence against the involvement of suppressor cells. It seems more likely that the overload effect arises not in the lymph node, but at the Langerhans cell.
It is nowadays considered that a further requirement for sensitization is production of a “danger signal” which stimulates the alkylated dendritic cells to migrate (McFadden and Basketter, 2000). Many sensitizers also have irritant properties, and it has been proposed that the irritation they produce can be the source of the danger signal. However, many sensitizers have very low irritancy, to the extent that they can be tested at 100%. One of several examples that can be found in the dataset of Gerberick et al. (2005) is diethyl maleate, shown in Table 1 with SI values of 16.3, 22.6 and 13.1 at 25%, 50% and 100% respectively.
Here a mechanistic rationale is presented to explain the overload effect, and at the same time to explain the production of danger signals even when the sensitizer is non-irritant.
2. Rationalising the bell-shaped dose–response
For skin sensitization to occur, dendritic cells in the epidermis must acquire proteins which have been modified by reaction with the sensitizer. This acquisition of modified proteins will be referred to as alkylation of the dendritic cells. For present purposes there is no need to consider whether the proteins which react with the sensitizer are originally associated with the dendritic cells or whether they only become associated with the dendritic cells after being modified.
If a dendritic cell is alkylated sufficiently, it gains the potential to migrate to the lymph node, where via interaction with T-cells the sensitized state can be produced. It is assumed that:
-
1.
The degree of sensitization is dependent on the number of dendritic cells which migrate to the lymph node as a result of being alkylated.
-
2.
When a sensitizer is applied to the skin, dendritic cells become alkylated. Some dendritic cells will be more alkylated than others (i.e. they will acquire more modified protein molecules). The numbers of cells n with each degree of alkylation will follow a Boltzman-type distribution (the underlying mathematics is exactly the same).
-
3.
For an individual dendritic cell, there is a threshold degree of alkylation below which the cell does not have the potential to migrate.
-
4.
For an individual dendritic cell, there is also a higher threshold degree of alkylation, above which the cell is immobilised and a danger signal is produced. The simplest interpretation of this effect, although not the only possibility, is that at high alkylation levels the cell is killed and its death produces the danger signal.
Fig. 4 shows the consequences of these assumptions, as the overall degree of alkylation is increased. In a), only a small proportion of dendritic cells are alkylated sufficiently to be able to migrate, and an even smaller proportion are able to produce a danger signal. If the sensitizer is non-irritant at the dose supplied, little if any sensitization will occur. However, if irritation is provided from another source, leading to danger signals being produced, then sensitization may still occur from the small proportion of dendritic cells which are able to migrate. LLNA studies with dinitrochlorobenzene (DNCB) at sub-optimal doses, with or without an irritant, give results in agreement with this reasoning (Cumberbatch et al., 1993).
Fig. 4.
Effects of alkylation on dendritic cell migration The number of dendritic cells migrating, and hence the degree of sensitization, is an increasing function of the area under the curve between the vertical lines indicating migration and immobilisation thresholds. In the curves below, based on the standard deviation curve, n (y-axis) is the number of cells having a given degree of alkylation (x-axis).
In b), a larger proportion of dendritic cells are able to migrate, and also a larger proportion are able to produce a danger signal. This degree of alkylation is in the range optimal for producing sensitization.
In c), the proportion of dendritic cells able to migrate is lower than in b), because many of them are alkylated above the second threshold. Thus, the degree of sensitization produced will be less than in b) even though the overall degree of alkylation is higher. This rationalises how the overload effect can manifest itself in the LLNA.
The argument so far is based on the concept that both sensitization and cell-deactivation result from alkylation of dendritic cells, depending on the degree of alkylation. If alkylation of dendritic cells were the only mechanism for sensitization and cell-deactivation, then the dose–response patterns for all sensitizers would be similar to the bell-shaped curves in Fig. 4, with the vertical axis now indicting the SI value and the horizontal axis the dose. Depending on the different alkylating abilities the curves for different chemicals would be shifted to the right or the left, and for less potent chemicals the downward side of the curve (overload effect) might be at a dose level too high to be realisable in practice. However, at this level of simplicity not all of the observed facts are explained. It is clear from Table 1 that in some cases the overload effect comes into play at low dose levels before large SI values are attained. For example, dimethyl sulfate and octadecanoyl chloride only attain single figure SI values before the overload effect becomes evident. In other cases high SI values are observed before the overload effect is observed, and in other cases it is not observed at all even at high doses or high SI values. The simplest explanation is that although alkylation is the key mechanism for sensitization, there is a diversity of mechanisms by which chemicals can produce cytotoxicity – for example physical disruption of membrane function, non-covalent receptor binding.
Interpretation of LLNA data without consideration of the overload effect can lead to both underestimation and overestimation of potency, as discussed below for a few illustrative examples listed in Table 2.
Table 2.
Chemicals analysed in detail.
| Name and CAS | 2-D structure | LLNA test concentrations and responses |
|
|---|---|---|---|
| % | SI | ||
| 2-Amino-3-hydroxypyridinea |  |
5 | 2.9 |
| CAS 16867-03-1 | 25 | 1.7 | |
| 50 | 1.9 | ||
| 2,5-diaminotolueneb |  |
0.5 | 4.9 |
| 1.5 | 4.2 | ||
| CAS 615-50-9 | 5 | 3.7 | |
| 4ʹ-Hydroxychalconec | 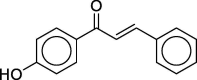 |
1 | 8.6 |
| CAS 2657-25-2 | 10 | 10.6 | |
| 20 | 10.8 | ||
| 4-Nitrophthalonitriled |  |
5 | 2.52 |
| CAS 31643-49-9 | 10 | 2.50 | |
| 25 | 1.75 | ||
| Pleuromutilin-22-mesylated |  |
10 | 2.46 |
| CAS 60924-38-1 | 25 | 2.40 | |
| 50 | 1.54 | ||
3. Alternative explanations for the bell-shaped dose–response
The physical chemistry of the vehicle and test substance might conceivably account for some cases of non-monotonic dose-responses. The simplest case would be where the test substance reaches its maximum solubility in the vehicle. At higher nominal concentrations the amount of test material in the vehicle would remain the same and the effective dose reaching the site of action would not increase. This effect would be more likely to give an S-shaped curve than a bell-shaped curve. A related possibility is that at high concentrations the rheology of the solution might change so as to reduce the mass-transfer to the site of action. In this situation an increased nominal dose could result in a reduced effective dose at the site of action, leading to a bell-shaped dose–response. This could be a plausible explanation for a bell-shaped dose–response when the test compound is a solid, but is less plausible for cases such as cinnamic aldehyde and diethyl maleate, where the test compound is a liquid and can act as its own vehicle. For a bell-shaped RAI plot with different compounds fitting the curve in the overload region, as in Fig. 3, the physical chemistry explanation is less plausible. For example, to explain Fig. 3 would require the ad hoc assumption that methyl dodecane sulfonate and 4-nitrobenzyl chloride, despite their different structures, different reactivities and different hydrophobicities, happen by chance to reach their mass-transfer maxima at very similar RAI values.
4. Reinterpretation of LLNA data in light of the overload effect
4.1. 2-Amino-3-hydroxypyridine
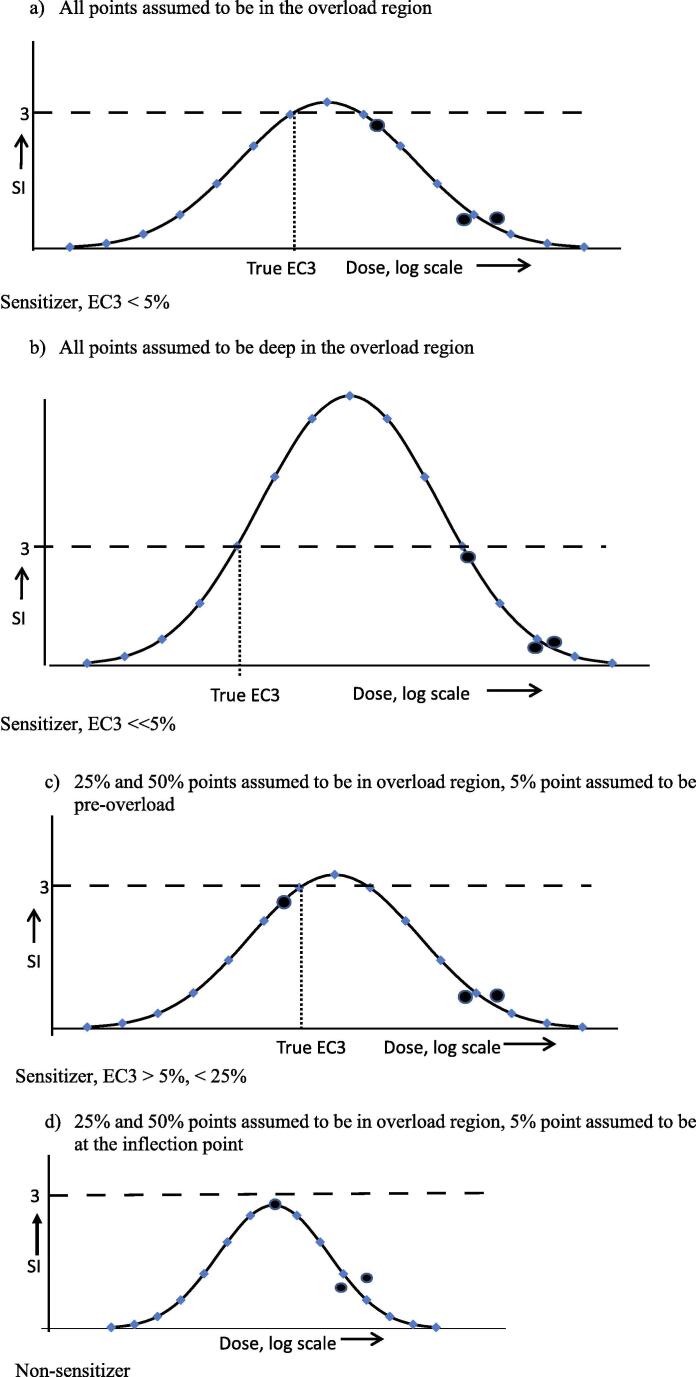
LLNA results for this compound are described in a 2008 SCCP (Scientific Committee on Consumer Products, now known as SCCS – S for Safety) report (SCCP, 2008): “The mean disintegrations per minute (DMP)/animal values for each test group were 113, 332, 192, and 220 for the 0%, 5%, 25%, and 50% dose groups, respectively. The SI were 2.9, 1.7, and 1.9 for the 5%, 25%, and 50% dose groups, respectively. There was no indication that 2-amino-3-hydroxypyridine could elicit an SI greater than 3. It was concluded that 2-amino-3-hydroxypyridine was not a skin sensitizer in this LLNA.” However, the non-sensitizer conclusion is highly questionable. The figures indicate an inverse dose–response, from which a reliable estimate of the EC3 cannot be obtained and it cannot be concluded that an SI value ≥3 could not have been obtained by testing at different concentrations. As the concentration increases from zero (at which SI = 1 by definition), the SI increases to a maximum, beyond which the SI decreases with increasing concentration. All that can be concluded confidently is that this maximum occurs somewhere between 0 and 25%, the maximum SI being not <2.9. There are 3 possible situations (Fig. 5):
-
1.
The maximum SI value (>2.9, i.e. ≥ 3) occurs at a concentration <5%, and the compound is a sensitizer with EC3 < 5% (a or b in Fig. 5)
-
2.
The maximum SI value (>2.9, i.e. ≥3) occurs at a concentration between 5% and 25%, and the compound is a sensitizer with EC3 between 5% and 25% (c in Fig. 5)
-
3.
The maximum SI value is 2.9, occurring at the 5% concentration and the compound is a non-sensitizer (d in Fig. 5)
Fig. 5.
Interpretations of dose–response for 2-amino-3-hydroxypyridine SI values of 2.9, 1.9 and 1.7 observed at 5%, 25% and 50% respectively.
There is a range of concentrations for which 1 could apply (between 0 and 5%) and a range of concentrations for which 2 could apply (between 5% and 25%), but only one concentration (5%) at which 3 could apply. Therefore, the probability that 1 or 2 applies is greater than the probability that 3 applies.
On a “chemistry-blind” basis, simply by critical examination of the LLNA data, it can be concluded that 2-amino-3-hydroxypyridine is, more likely than not, a sensitizer in the LLNA. However, on the basis of the LLNA data alone, it is not possible to assess its potency.
Using Structure Activity Relationship (SAR)/chemistry-based read-across, a rough estimate of the potency can be made, based on compounds with the same chemical mechanism of action, as follows (Scheme 1). EC3 values are taken from the dataset of Gerberick et al. (2005).
-
1.
It is well known in organic chemistry that nitrogen in a pyridine ring is quantitatively similar to a nitro-group in a benzene ring in its electronic effect, influencing chemical reactivity at other points in the aromatic ring. The EC3 of para-phenylenediamine is 0.16 and the EC3 of 2-nitro-para-phenylene diamine is 0.4; i.e. the effect of the nitro group is to increase the EC3 by a factor of 2.5.
-
2.
2-Amino-3-hydroxypyridine is predicted to be, in light of 1 above, similar in reactivity to 2-amino-3-nitrophenol, i.e. 2-aminophenol with a nitro-substituent.
-
3.
The EC3 value of 2-aminophenol is 0.4. Modifying the structure by introducing a ring nitrogen to make 2-amino-3-hydroxypyridine is predicted to increase the EC3 by a factor of 2.5, giving a predicted EC3 value of 1.0.
Scheme 1.
SAR/chemistry-based read across for 2-amino-3-hydroxypyridine.
Thus 2-amino-3-hydroxypyridine is predicted by SAR/chemistry-based read-across to be a class 1A sensitizer (GHS; United Nations, 2019), and the published LLNA data (SCCP, 2008) are not inconsistent with this classification.
The case of 2-amino-3-hydroxypyridine is analogous to that of 2,5-diaminotoluene tested as its sulfate in DMSO (Table 2), giving an inverse dose response, but in this assay all SI values were above 3.0. Tested at lower concentrations in AOO, this compound gave a normal dose–response, with an EC3 value, estimated by extrapolation, of 0.31% (SCCP, 2007).
4.2. Diethyl maleate
There are two entries in the NICEATM database (ntp) for LLNA studies on diethyl maleate, in both cases with acetone/olive oil as the vehicle. One entry, referring to a 1999 paper by Basketter et al. (1999) gives SI values corresponding to concentrations of 1, 2.5, 5, 10 and 25%, and the other, referring to the compilation of Gerberick et al. (2005), which in turn refers to a 2000 paper by Ryan et al. (2000) gives SI values corresponding to concentrations of 25, 50 and 100%. Although these appear to be two separate studies, the combined data plotted together as SI vs log(concentration) fit well to a bell-shaped curve with either one or two points in the overload region (Fig. 2b). The EC3 value of 5.8% given in Gerberick et al. (2005) was derived by logarithmic extrapolation from the SI values of 22.6 and 16.3 recorded at 50% and 25% respectively. This does not meet the applicability criteria of Ryan et al. (2007) for the log-linear extrapolation method (see under 4′-hydroxychalcone below) and an EC3 value of 2.1% derived by linear interpolation from the SI values reported by Basketter et al. (1999) of 2.1 and 3.3 at 1% and 2.5% respectively is a better representation of the potency.
4.3. 4ʹ-Hydroxychalcone
Kern et al. (2010) list an extremely low LLNA EC3 value, 0.002%, for this compound (structure shown in Table 2 together with the dose–response data). SI values above 3 were observed at all concentrations tested, and the EC3 value was derived by the log-linear extrapolation method of Ryan et al. (2007) from the two lowest concentrations. The SI values observed were 8.6, 10.6, and 10.8 at concentrations of 1%, 10%, and 20% respectively. Since the data show an increase of SI with increasing concentration, the first of the two recommended applicability criteria for estimating EC3 by extrapolation (Ryan et al., 2007) is met (specifically, there should be a positive dose–response trend covering the three points). However, this criterion is met only marginally, since the increase in SI between 10% and 20% is so small as to be almost insignificant. It is highly questionable whether the second applicability criterion, “ideally, the SI induced by the lowest dose should approach 3” (Ryan et al., 2007), is met, the lowest SI value being 8.6.
The simplest interpretation of the near-identical SI values at 10% and 20% is that these points are close to the peak in a bell-shaped dose–response curve. In this situation, extrapolation is liable to lead to an estimated EC3 value substantially lower than the true EC3, as shown in Fig. 6.
Fig. 6.
Schematic representation of dose–response for 4ʹ-hydroxychalcone.
A more realistic estimate of the EC3 can be made, based solely on the SI value of 8.6 at 1%. This point is clearly well below the peak in the bell-shaped curve and can therefore be used to derive an EC3 value by the single dose probit extrapolation method (SDPEM). This method was originally developed (Roberts, 2015) for the purpose of interpreting data from the single dose variant of the LLNA (rLLNA) and, when applied to the lowest observed SI value above 3, was found to give good agreement with EC3 values derived from the full LLNA by the interpolation method. The SDPEM is also applicable to deriving EC3 values when interpolation is not possible and log-linear extrapolation is inapplicable (Nishijo et al., 2020). For the SDPEM, the SI value, after subtraction of 1 (the nominal SI value at zero concentration) is expressed as a percentage of 78.5 and this percentage is converted to a probit value, Pr78.5(SI-1). The EC3 value is then calculated from the expression:
where D is the concentration tested, in units of weight percent. Using this expression with SI = 8.6 and D = 1 gives an estimated EC3 value of 0.18%. By this interpretation of the LLNA data 4ʹ-hydroxychalcone is still a strong sensitizer, but substantially less potent than estimated by the log-linear extrapolation.
4.4. 4-Nitrophthalonitrile and pleuromutilin-22-mesylate
These two compounds with their LLNA data are taken from the European Chemicals Agency (ECHA) database (echa). In both cases, all SI values are <3.0, but they show a decreasing dose–response trend consistent with the doses tested all being in the overload region. Both compounds have alerts for the high potency category (HPC) of chemicals that can be predicted from their structure as likely to have EC3 values below the reactive chemicals Dermal Sensitization Threshold (Reactive DST) of 0.26%, and for which the Reactive DST should not be used for risk assessment purposes (Roberts et al., 2015). For both chemicals there are suitable quantitative mechanistic models (QMMs) enabling their potency to be estimated by chemistry-based read across as shown below:
4-Nitrophthalonitrile has the structural features of an SNAr electrophile, with CN as the leaving group and ortho-CN plus para-NO2 as the two activating groups. For SNAr electrophiles potency (quantified as pEC3, the negative log of the EC3 in mole percent units) has been found to correlate well with a reactivity parameter (RP) calculated from the combined σ− values of the activating groups and the σ* value of the leaving group (RP = Σσ− + 0.24σ*) (Roberts and Aptula, 2014):
| pEC3 = 2.81(±0.12)RP − 5.44(±0.36) |
R2 = 0.987, adj R2 = 0.985, s = 0.13, F = 594
Using this equation, with the Hammett constant (σ−) values of 1.32 (o-CN) and 1.24 (p-NO2) and the Taft constant (σ*) value of 3.30 for the CN leaving group, pEC3 is predicted to be 3.98, corresponding to EC3 = 0.02% The lowest concentration, 5%, that was used in the LLNA for this compound was about 250 times larger than the predicted EC3.
Pleuromutilin-22-mesylate, despite its apparently complex structure, is simply a sulfonate ester that can be represented as R1SO2OCH2CO2R2, where R1 is an alkyl group (methyl in this case) and R2 is a tricyclic keto-alcohol entity. The reactive part of the molecule is indicated in red in Table 2. Sulfonate esters are SN2 electrophiles and enough is known about the relationship between their chemistry and their sensitization potency for chemistry-based read across to be applied as follows:
For SN2 electrophiles potency is dependent both on electrophilic reactivity and hydrophobicity, and the general relationship is:
| pEC3 = aRAI + C |
RAI, the Relative Alkylation index (Roberts and Williams, 1982), is a composite reactivity/hydrophobicity parameter that can be calculated as logk + 0.4 logP, where k is a rate constant with a model nucleophile and P is the octanol–water partition coefficient (Basketter and Roberts, 1990, Roberts et al., 2017).
Dodecyl methanesulfonate, CH3SO2OC12H25 has an EC3 value of 8.8% (Gerberick et al., 2005). Its logP value, calculated by the Hansch and Leo method (Hansch and Leo, 1979) is 5.49. The logP value of pleuromutilin-22-mesylate is 4.11, i.e. 1.38 lower than that of dodecyl methanesulfonate. The relative reactivity for SN2 substitution at a –CH2-CO2R reaction centre compared to that at a –CH2-Alkyl reaction centre is ca. 1400 (based on relative rate constants, for displacement of chloride by iodide ion, listed by Hine (1962). Thus, the difference in RAI values between pleuromutilin-22-mesylate and dodecyl methanesulfonate can be estimated as:
Assuming the coefficient relating RAI to pEC3 to be approximately 1, the molar EC3 of pleuromutilin-22-mesylate is estimated as 10−2.59 times that of dodecyl methanesulfonate. The molecular weight of pleuromutilin-22-mesylate (MW = 536) is about twice that of dodecyl methanesulfonate, so the EC3 of pleuromutilin-22-mesylate is estimated as 8.8 × 10−2.59 × 2 = 0.04%. This figure is of course a very approximate estimate and depends on the assumptions made regarding relative reactivity and the coefficient relating RAI to pEC3, but it indicates that the lowest concentration used in the LLNA for pleuromutilin-22-mesylate could have been more than 2 orders of magnitude greater than the EC3. Table 3 shows a sensitivity analysis in which EC3 values are predicted for different plausible combinations of assumptions regarding relative reactivity and the RAI coefficient. In all cases, the predicted EC3 value is significantly lower (more potent) than the lowest concentration actually tested in the LLNA.
Table 3.
Pleuromutilin-22-mesylate – predicted EC3 values for different assumptions regarding reactivity and RAI coefficient EC3 value in bold results from the calculation presented in the text.
| Estimated EC3 for k(CH2CO2R)/kCH2R = | |||
|---|---|---|---|
| RAI coefficient | 1000 | 1400 | 2000 |
| 1.5 | 0.004% | 0.002% | 0.001% |
| 1 | 0.06% | 0.04% | 0.03% |
| 0.5 | 1.1% | 0.9% | 0.7% |
In summary, the LLNA results for 4-nitrophtahlonitrile and pleuromutilin-22-mesylate cannot with any confidence be taken as evidence for these compounds being non-sensitizers, particularly bearing in mind that they have structural alerts for high reactivity corresponding to the SNAr and SN2 reaction mechanistic domains, respectively.
5. Conclusions
The overload effect, whereby the degree of sensitization induced decreases with increasing dose, has long been recognised in guinea pig sensitization. It can also occur in the LLNA, as the illustrative examples in Table 1, Table 2 clearly demonstrate.
The overload effect is unlikely to be due to induction of suppressor cell proliferation – this would be expected to be additive with induction of sensitization in contributing to SI, which is based on the overall degree of cell proliferation induced in the lymph node. It seems more likely that it is cytotoxicity, reducing the degree of dendritic cell migration to the lymph node, that causes the overload effect. The same cytotoxic effect could be associated with the danger signal as per the model in Fig. 4b.
That the overload effect is variable, and not observed in all cases even when high SI values are recorded and/or high doses are used (e.g. the last eighth entries in Table 1), is consistent with there being a wider diversity of cytotoxicity mechanisms than of sensitization mechanisms.
It might be possible to use in vitro methods, possibly involving reconstituted human epidermis, to evaluate the mechanism proposed here for the overload effect, and overload effects linkable to cell toxicity might also be observed in cell-based assays, but this is beyond the scope of the present paper. Irrespective of the exact mechanism, the overload effect in the LLNA is a real phenomenon with real consequences for interpretation of dose–response data.
As illustrated by the case of 4ʹ-hydroxychalcone, potency can be substantially overestimated if the EC3 values is estimated by log-linear extrapolation and the higher SI value is affected by the overload effect. However, with stricter adherence to the applicability criteria of Ryan et al. (2007) for the log-linear extrapolation method, such overestimates of potency should be largely avoidable.
As illustrated by the 3 cases of 2-amino-3-hydroxypyridine, 4-nitrophthalonitrile and pleuromutilin-22-mesylate, assignment of a non-sensitizer classification simply on the basis of failure to observe an SI ≥ 3 can be unsafe. In the first case, from a chemistry blind perspective, it seems more likely than not that the compound is a sensitizer. In the other two cases negative dose–response patterns with all SI values < 3 cannot give a clear indication as to whether or not the compounds are sensitizers. From their structures all three can be predicted by chemistry-based read-across to be strong sensitizers, and from a chemistry perspective it can be predicted with some confidence that all three compounds would test positive in non-animal methods such as GARD™skin (Johansson et al., 2019), DPRA (Gerberick et al., 2008), SENSIS (Cottrez et al., 2016), 2-out-of-3 (Urbisch et al., 2015) and would be indicated as sensitizers by in silico systems such as TIMES-SS (Dimitrov et al., 2005, Patlewicz et al., 2007) and DEREK Nexus (Marchant et al., 2008, Langton et al., 2006).
LLNA data are used not only for risk assessment and regulatory purposes, but also for evaluation of non-animal methods. There are ongoing efforts to develop non-animal methods for assessing the skin sensitisation potential and potency of chemicals (Ezendam et al., 2016, Casati et al., 2018). Several of the in vitro tests and combinations of them (e.g. 2-out-of-3) are under consideration as OECD guidelines and are recognised and accepted by ECHA as alternatives to animal testing for REACH hazard classification. Some other in vitro tests are ongoing formal validation and soon will be available to use as a method of choice (e.g. GARD™skin, Johansson et al., 2019). The performance of non-animal methods is often assessed against LLNA results, there being large compilations of historical LLNA data available. However, as illustrated by 2-amino-3-hydroxypyridine, 4-nitrophthalonitrile and pleuromutilin-22-mesylate discussed above, not all LLNA data are appropriate for this purpose. The three compounds above would almost certainly be predicted positive, but they are listed as negative and consequently would be treated as false positives in assessment of non-animal method performance. There is clearly a need for chemistry input and careful analysis of the in vivo datasets, with an awareness of the overload effect, before using them as a basis to assess the performance of non-animal methods. To a large extent the original “Gold Standard” LLNA datasets compiled by Gerberick et al., 2005, Kern et al., 2010 for the purpose of being used in modelling and non-animal method development, are suitable for this purpose, but the newer compilations of LLNA data, containing data submitted from various sources mainly for regulatory purposes are more liable to contain misleading cases.
Declaration of Competing Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The author would like to acknowledge the contribution of Dr. A. Aptula by helpful discussions in the course of writing this paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Ashby J., Basketter D.A., Paton D., Kimber I. Structure activity relationships in skin sensitization using the murine local lymph node assay. Toxicology. 1995;103:177–194. doi: 10.1016/0300-483x(95)03132-y. [DOI] [PubMed] [Google Scholar]
- Basketter D.A., Lea L.J., Dickens A., Briggs D., Pate I., Dearman R.J., Kimber I. A comparison of statistical approaches to the derivation of EC3 values from local lymph node assay dose responses. J. Appl. Toxicol. 1999;19:261–266. doi: 10.1002/(sici)1099-1263(199907/08)19:4<261::aid-jat572>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Basketter D.A., Gerberick F., Kimber I. The local lymph node assay and the assessment of relative potency: status of validation. Contact Dermatitis. 2007;57:70–75. doi: 10.1111/j.1600-0536.2007.01141.x. [DOI] [PubMed] [Google Scholar]
- Basketter D.A., Roberts D.W. Structure/activity relationships in contact allergy. Int. J. Cosmet. Sci. 1990;12(2):81–90. doi: 10.1111/j.1467-2494.1990.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Casati S., Aschberger K., Barroso J., Casey W., Delgado I., Kim T.S., Kleinstreuer N., Kojima H., Lee J.K., Lowit A. Standardisation of defined approaches for skin sensitisation testing to support regulatory use and international adoption: position of the International Cooperation on Alternative Test Methods. Arch. Toxicol. 2018;92:611–617. doi: 10.1007/s00204-017-2097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H.N., Miller S.D., Sy M.S., Moorhead J.W. Suppressive mechanisms involving sensitization and tolerance in contact allergy. Immunol. Rev. 1980;50:105–132. doi: 10.1111/j.1600-065x.1980.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Cottrez F., Boitel E., Ourlin J.C., Peiffer J.L., Fabre I., Henaoui I.S., Mari B., Vallauri A., Paquet A., Barbry P., Auriault C., Aeby P., Groux H. SENS-IS, a 3D reconstituted epidermis based model for quantifying chemical sensitization potency: Reproducibility and predictivity results from an inter-laboratory study. Toxicol. In Vitro. 2016;32:248–260. doi: 10.1016/j.tiv.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M., Scott R.C., Basketter D.A., Scholes E.W., Hilton J., Dearman R.J., Kimber I. Influence of sodium lauryl sulphate on 2,4-dinitrochlorobenzene-induced lymph node activation. Toxicology. 1993;77:181–191. doi: 10.1016/0300-483x(93)90148-l. [DOI] [PubMed] [Google Scholar]
- Dimitrov S., Low L., Patlewicz G., Kern P., Dimitrova G., Comber M., Philips R., Niemela J., Bailey P., Mekenyan O. Skin sensitization: modeling based on skin metabolism simulation and formation of protein conjugates. Int. J. Toxicol. 2005;24(4):189–204. doi: 10.1080/10915810591000631. [DOI] [PubMed] [Google Scholar]
- <https://echa.europa.eu/information-on-chemicals/registered-substances>.
- Ezendam J., Braakhuis H.M., Vandebriel R.J. State of the art in non-animal approaches for skin sensitization testing: from individual test methods towards testing strategies. Arch. Toxicol. 2016;90:2861–2883. doi: 10.1007/s00204-016-1842-4. [DOI] [PubMed] [Google Scholar]
- Franot C., Roberts D.W., Basketter D., Benezra C., Lepoittevin J.-P. Structure-activity relationships for contact allergenic potential of γγ -dimethyl- γ -butyrolactone derivatives. 2. Quantititative structure-skin sensitisation relationships for α-substituted-α-methyl-γ, γ-dimethyl-γ-butyrolactones. Chem. Res. Toxicol. 1994;7:307–312. doi: 10.1021/tx00039a006. [DOI] [PubMed] [Google Scholar]
- Gerberick F., Aleksic M., Basketter D., Casati S., Karlberg A.T., Kern P., Kimber I., Lepoittevin J.-P., Natsch A., Ovigne J.M., Rovida C., Sakaguchi H., Schultz T.W. Chemical reactivity measurement and the predictive identification of skin sensitisers. ATLA Altern. Lab. Anim. 2008;36:215–242. doi: 10.1177/026119290803600210. [DOI] [PubMed] [Google Scholar]
- Gerberick G.F., Ryan C.A., Kern P.S., Schlatter H., Dearman R.J., Kimber I., Patlewicz G.Y., Basketter D.A. Compilation of historical local lymph node data for evaluation of skin sensitization alternative methods. Dermatitis. 2005;16:157–202. [PubMed] [Google Scholar]
- Hansch C., Leo A.J. Wiley and Sons; New York: 1979. Substituent Constants for Correlation Analysis in Chemistry and Biology. [DOI] [PubMed] [Google Scholar]
- Hine J. Physical Organic Chemistry, International Student Edition. second ed. McGraw-Hill; New York: 1962. p. 176. [Google Scholar]
- Johansson H., Gradin R., Johansson A., Adriaens E., Edwards A., Zuckerstätter V., Jerre A., Burleson F., Gehrke H., Roggen E.L. Validation of the GARD™skin assay for assessment of chemical skin sensitizers: ring trial results of predictive performance and reproducibility. Toxicol. Sci. 2019;170:374–381. doi: 10.1093/toxsci/kfz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P.S., Gerberick F.G., Ryan C.A., Kimber I., Aptula A., Basketter D.A. Local Lymph Node Data for the Evaluation of Skin Sensitization Alternatives: A Second Compilation. Dermatitis. 2010;21:8–32. [PubMed] [Google Scholar]
- Langton K., Patlewicz G.Y., Long A., Marchant C.A., Basketter D.A. Structure-activity relationships for skin sensitization: recent improvements to Derek for Windows. Contact Dermatitis. 2006;55:342–347. doi: 10.1111/j.1600-0536.2006.00969.x. [DOI] [PubMed] [Google Scholar]
- Marchant C.A., Briggs K.A., Long A. In silico tools for sharing data and knowledge on toxicity and metabolism: derek for windows, meteor, and vitic. Toxicol. Toxicol. Mech. Methods. 2008;18(2-3):177–187. doi: 10.1080/15376510701857320. [DOI] [PubMed] [Google Scholar]
- McFadden J.P., Basketter D.A. Contact allergy, irritancy and 'danger'. Contact Dermatitis. 2000;42:123–127. doi: 10.1034/j.1600-0536.2000.042003123.x. [DOI] [PubMed] [Google Scholar]
- Nishijo T., Api A.M., Gerberick G.F., Miyazawa M., Roberts D.W., Safford R.J., Sakaguchi H. Application of the dermal sensitization threshold concept to chemicals classified as high potency category for skin sensitization assessment of ingredients for consumer products. Regul. Toxicol. Pharm. 2020;117:104732. doi: 10.1016/j.yrtph.2020.104732. [DOI] [PubMed] [Google Scholar]
- <https://ntp.niehs.nih.gov/whatwestudy/niceatm/test-method-evaluations/immunotoxicity/llna/index.html#NICEATM-LLNA-Database>.
- Patlewicz G., Dimitrov S., Low L., Kern P., Dimitrova G., Comber M., Aptula A., Phillips R., Niemela J., Madsen C., Wedebye E., Roberts D., Bailey P., Mekenyan O. TIMES-SS - a promising tool for the assessment of skin sensitization hazard. A characterization with respect to the OECD validation principles for (Q)SARs and an external evaluation for predictivity. Regul. Toxicol. Pharm. 2007;48(2):225–239. doi: 10.1016/j.yrtph.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Ritz H.L., Connor D.S., Sauter E.D. Contact sensitization of guinea-pigs with unsaturated and halogenated sultones. Contact Dermatitis. 1975;1:349–368. doi: 10.1111/j.1600-0536.1975.tb05472.x. [DOI] [PubMed] [Google Scholar]
- Roberts D.W. Structure-activity relationships for skin sensitisation potential of diacrylates and dimethacrylates. Contact Dermatitis. 1987;17:281–289. doi: 10.1111/j.1600-0536.1987.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Roberts D.W. Estimating skin sensitization potency from a single dose LLNA. Regul. Toxicol. Pharm. 2015;71:437–443. doi: 10.1016/j.yrtph.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Roberts D.W., Api A.M., Safford R.J., Lalko J.F. Principles for identification of High Potency Category Chemicals for which the Dermal Sensitisation Threshold (DST) approach should not be applied. Regulatory Toxicology and Pharmacology. 2015;72:683–693. doi: 10.1016/j.yrtph.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Roberts D.W., Aptula A.O. Electrophilic reactivity and skin sensitization potency of SNAr electrophiles. Chem. Res. Toxicol.. 2014;27:240–246. doi: 10.1021/tx400355n. [DOI] [PubMed] [Google Scholar]
- Roberts D.W., Basketter D.A. A quantitative structure activity/dose response relationship for contact allergic potential of alkyl group transfer agents. Contact Dermatitis. 1990;23:331–335. doi: 10.1111/j.1600-0536.1990.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Roberts D.W., Basketter D.A. Quantitative structure-activity relationships: sulfonate esters in the local lymph node assay. Contact Dermatitis. 2000;42:154–161. doi: 10.1034/j.1600-0536.2000.042003154.x. [DOI] [PubMed] [Google Scholar]
- Roberts D.W., Goodwin B.F., Williams D.L., Jones K., Johnson A.W., Alderson C.J. Correlations between skin sensitisation potential and chemical reactivity for p–Nitrobenzyl compounds. Fd. Chem. Toxic. 1983;21:811–813. doi: 10.1016/0278-6915(83)90217-x. [DOI] [PubMed] [Google Scholar]
- Roberts D.W., Aptula A.O., Patlewicz G. Electrophilic chemistry related to skin sensitization. Reaction mechanistic applicability domain classification for a published data set of 106 chemicals tested in the mouse local lymph node assay. Chem. Res. Toxicol. 2007;20:44–60. doi: 10.1021/tx060121y. [DOI] [PubMed] [Google Scholar]
- Roberts D.W., Api A.M., Patlewicz G., Schultz T.W. Chemical applicability domain of the Local Lymph Node Assay (LLNA) for skin sensitization potency. Part 1. Underlying physical organic chemistry principles and the extent to which they are represented in the LLNA validation dataset. Regul. Toxicol. Pharm. 2016;80:247–254. doi: 10.1016/j.yrtph.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Roberts D.W., Williams D.L. The derivation of quantitative correlations between skin sensitization and physicochemical parameters for alkylating agents and their application to experimental data for sultones. J. Theor. Biol. 1982;99:807–825. doi: 10.1016/0022-5193(82)90199-0. [DOI] [PubMed] [Google Scholar]
- Roberts D.W., Aptula A., Api A.M. Structure−potency relationships for epoxides in allergic contact dermatitis. Chem. Res. Toxicol. 2017;30(2):524–531. doi: 10.1021/acs.chemrestox.6b00241. [DOI] [PubMed] [Google Scholar]
- Ryan C.A., Gerberick G.F., Cruse L.W., Basketter D.A., Lea L., Blaikie L., Dearman R.J., Warbrick E.V., Kimber I. Activity of human contact allergens in the murine local lymph node assay. Contact Dermatitis. 2000;43:95–102. doi: 10.1034/j.1600-0536.2000.043002095.x. [DOI] [PubMed] [Google Scholar]
- Ryan C.A., Chaney J.G., Gerberick G.F., Kern P.S., Dearman R.J., Kimber I., Basketter D.A. Extrapolating local lymph node assay EC3 values to estimate relative sensitizing potency. J. Cutan. Ocul. Toxicol. 2007;26:135–145. doi: 10.1080/15569520701212258. [DOI] [PubMed] [Google Scholar]
- SCCP, Scientific Committee on Consumer Products. Opinion on Toluene-2.5-diamine 2 October 2007. SCCP/1084/07.
- SCCP,Scientific Committee on Consumer Products. Opinion on 2-amino-3-hydroxy pyridine, 15 April 2008. SCCP/1126/07.
- Schultz T.W., Dimitrova G., Dimitrov S., Mekenyan O.G. The adverse outcome pathway for skin sensitisation: Moving closer to replacing animal testing. ATLA Altern. Lab. Anim. 2016;44:453–460. doi: 10.1177/026119291604400515. [DOI] [PubMed] [Google Scholar]
- United Nations. http://unece.org/ghs-rev8-2019.
- Urbisch D., Mehling A., Guth K., Ramirez T., Honarvar N., Kolle S., Landsiedel R., Jaworska J., Kern P.S., Gerberick F., Natsch A., Emter R., Ashikaga T., Miyazawa M., H., Sakaguchi, Assessing skin sensitization hazard in mice and men using non-animal test methods. Regul. Toxicol. Pharm. 2015;71:337–351. doi: 10.1016/j.yrtph.2014.12.008. [DOI] [PubMed] [Google Scholar]