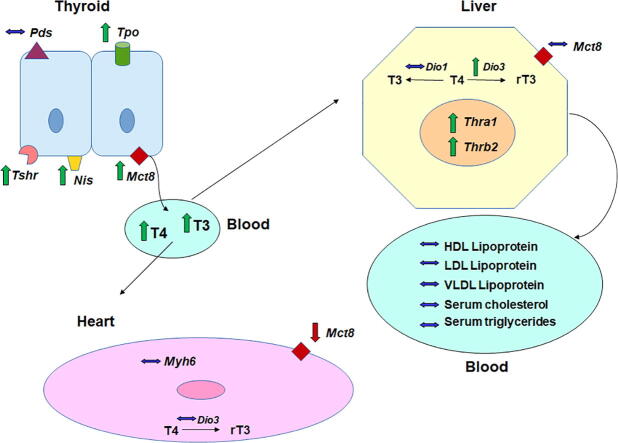
Graphical abstract
Abbreviations: AA, acrylamide; BW, body weight; DIO1, iodothyronine deiodinase 1; DIO2, iodothyronine deiodinase 2; DIO3, iodothyronine deiodinase 3; EDCs, endocrine-disrupting chemicals; HDL, high-density lipoproteins; HPT, hypothalamus-pituitary-thyroid axis; LDL, low lipoproteins; LOAEL, lowest Observed Adverse Effect Level; MCT-8, monocarboxylate transporter 8; MYH6, myosin heavy chain 6; NIS, sodium/iodide symporter; NOAEL, no Observed Adverse Effect Level; PDS, pendrin; PND, postnatal day; TDI, tolerable daily intake; RfD, reference dose; T3, triiodothyronine; T4, thyroxine; TH, thyroid hormones; THRA1, thyroid hormone receptor alpha 1; THRB2, thyroid hormone receptor beta 2; TPO, thyroid peroxidase; TRH, thyrotropin releasing hormone hormone; TRHR, thyrotropin releasing hormone receptor; TSH, thyrotropin; TSH, thyroid hormone receptor
Keywords: Acrylamide, Thyroid, Endocrine-disrupting chemicals, Thyroid hormone metabolism
Highlights
-
•
Acrylamide acts as endocrine disruptor for the thyroid gland function.
-
•
Acrylamide increases the transcript expression of proteins related to THs synthesis.
-
•
Exposure to acrylamide alters the hypothalamus-pituitary-thyroid axis homeostasis.
-
•
Acrylamide induces allostatic regulation of the hypothalamus-pituitary-thyroid axis.
Abstract
Some endocrine-disrupting chemicals (EDCs) can affect the endocrine system through covalent interactions with specific sites, leading to deregulation of physiological homeostasis. The acrylamide (AA) present in some fried or baked foods is an example of an electrophile molecule that is able to form adducts with nucleophilic regions of nervous system proteins leading to neurological defects. A positive correlation between increased urinary AA metabolite concentration and reduced levels of thyroid hormones (TH) was described in adolescents and young adults. Thus, this study aimed to evaluate whether AA affects the physiology of the hypothalamus-pituitary-thyroid (HPT) axis and the possible repercussions in peripheral TH-target systems. For this, male Wistar rats were exposed to doses of 2.5 or 5.0 mg AA/Kg/day, based on the LOAEL (Lowest Observed Adverse Effect Level) during prepubertal development. The expression of molecular markers of HPT functionality was investigated in the hypothalamus, pituitary, thyroid, heart and liver, as well as the hormonal and lipid profiles in blood samples. Herein, we showed that AA acts as EDCs for thyroid gland function, increasing the transcript expression of several proteins related to TH synthesis and altering hypothalamus-pituitary-thyroid axis homeostasis, an effect evidenced by the higher levels of THs in the serum. Compensatory mechanisms were observed in TH-target tissues, such as an increase in Dio3 mRNA expression in the liver and a reduction in Mct8 transcript content in the hearts of AA-treated rats. Together, these results pointed out an allostatic regulation of the HPT axis induced by AA and suggest that chronic exposure to it, mainly associated with food consumption, might be related to the higher prevalence of thyroid dysfunctions.
1. Introduction
Human exposure to several chemicals has increased due to the use by industries as well as the current consumption patterns of the population. Many of these products can interfere with different biological systems in humans, animals and plants, although it is difficult to establish their short- and long-term effects on human health. The consequences for human health vary not only to chemical per se but also according to the window of exposure, being particularly important when it occurs in early life stages of development, such as embryonic life and the pre-puberty phase. Thus, it is necessary to investigate the consequence of chemical exposure on the physiology of biological systems, especially neuroendocrine systems, considering its paramount importance for the regulation of several functions, such as growth, metabolism and reproduction (Gore, 2010, Karwacka et al., 2017, Rattan et al., 2017, Sifakis et al., 2017).
Polychlorinated biphenyls (PCBs), phthalates, bisphenol A, brominated and perfluorinated flame retardants (PBDEs), pesticides (chlorinated and phenols), polycyclic aromatic hydrocarbons (PAHs), phytoestrogens, some sunscreens used in cosmetic creams, perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) (found in Teflon pans, furniture coatings, carpets and paints) are examples of endocrine-disrupting chemicals (EDCs) identified for the thyroid axis (Boas et al., 2012, de Cock et al., 2014, Coperchini et al., 2017). EDCs affect the synthesis, regulation, transport, metabolism and/or action of thyroid hormones through interaction with different elements of the thyroid axis, such as enzymes, receptors, carrier proteins, among others (Diamanti-Kandarakis et al., 2009).
Most EDCs that affect thyroid function have electrophilic characteristics (electron deficiency), which form covalent bonds with electron-rich (nucleophilic) sites in target organs. The covalent binding of these substances at specific sites can trigger several cellular pathways leading to deregulation of physiological homeostasis and processes, interfering with enzyme and protein functions, e.g., (LoPachin et al., 2012, De Campos et al., 2015). The acrylamide present in some fried or baked foods is an example of an electrophile molecule that is able to form adducts with nucleophilic regions of nervous system proteins, altering their structure/function and impairing synaptic transmission, which could lead to several neurological defects (Barber and LoPachin, 2004).
In addition, some processed foods that undergo heating stages constitute a major source of acrylamide (AA) contamination in humans (Tareke et al., 2000, Hileman, 2002). Thus, fried, baked and roasted foods favor acrylamide formation from the chemical reactions between nutrients. In Sweden, the National Food Agency conducted the first determination of acrylamide on commercial food products, followed by other in European countries and the USA that confirmed the presence of acrylamide in several food products. High acrylamide levels were found in rich-carbohydrate foods processed at elevated temperatures, such as French fries, potato chips, toast, crackers, breakfast cereals and coffee. In contrast, the levels of acrylamide in protein-rich or water-cooked foods were not even detected [reviewed by Matoso, Bargi-Souza, Ivanski, Romano and Romano (Matoso et al., 2019)].
Thus, considering the sensitivity of thyroid function to chemical compounds, the potential of acrylamide as EDC and the lack of information regarding acrylamide-induced thyroid toxicity, the aim of this study was to evaluate whether acrylamide affects the physiology of the hypothalamic-pituitary-thyroid (HPT) axis and the possible repercussions in peripheral TH-target systems. For this, male Wistar rats were exposed to low doses of acrylamide and subjected to the pubertal development protocol.
2. Material and methods
2.1. Experimental design
The experimental design was based on a prepubertal protocol from the Endocrine Disrupting Screening and Testing Advisory Committee (EDSTAC) and includes the evaluation of endocrine-disrupting chemical (EDC) effects in thyroid function (Stoker et al., 2000). Forty-five newly weaned male Wistar rats (Rattus norvergicus var. albinus) were obtained from twenty pregnant female rats that had been monitored from the 17th day of pregnancy to determine the exact day of their birth. On postnatal day 4 (PND4), 10 litters were culled to 8 pups (4 males/4 females) per female and kept at this size until weaning (PND21). At weaning, they were housed (5 animals/cage) in polypropylene cages (43x43x20 cm) with 5 cm of wood shavings, free access to commercial feed (Nuvilab CR-1, Nuvital, PR, Brasil) and water ad libitum at a controlled temperature of 23 ± 1 °C under a 12:12 h light/dark cycle.
On PND23, animals were randomly assigned into 3 groups (15 rats/group) according to acrylamide (AA) (CAS 79-06-1 Sigma–Aldrich Co., St. Louis, USA) dose administered: 0 (control), 2.5 mg/body weight (BW) kg or 5 mg/ BW kg of AA daily. The higher dose was chosen based on the LOAEL of 5 mg/kg due to a reduction observed in testosterone levels (Hamdy et al., 2012, Yang et al., 2005). Considering the human risk assessment, AA is a genotoxic substance, and for this reason, any level of tolerable daily intake (TDI) of AA in food cannot be set. As an alternative for the purpose of comparative risk assessment in this study, we calculated the reference dose (RfD) dividing LOAEL by 5 mg by uncertainty factors of 10,000 (10 for human variability, 10 for an animal study, 10 for less than chronic exposure, and 10 for use of an LOAEL instead of a NOAEL) (Dankovic et al., 2015, [43]). This RfD was estimated at 0.5 and 0.25 µg of AA/kg BW/day, which could be considered the acceptable safety level for chronic noncarcinogenic and developmental effects in human exposure. Animals were weighed every day and received oral AA diluted in water by gavage between 7 and 8 am using 0.25 mL/100 g BW from 23 to 60 days postnatal (PND). Afterwards, animals were sacrificed under deep anesthesia, and blood/tissues were collected for further analysis. All procedures were in accordance with the Brazilian College of Animal Experimentation and approved by the Ethics Committee for Animal Research from Universidade Estadual do Centro-Oeste (protocol CEUA #013/2017).
2.2. Blood and tissue collection
Blood was collected via cardiac puncture and centrifuged at 3500 RPM (Excelsa II 206 BL, Sao Paulo, SP, Brazil) for 15 min. Serum samples were aliquoted, frozen and stored at −80 °C for subsequent analysis of hormonal and lipid profiles. Hypothalamus, pituitary, thyroid and 50 mg of heart and liver tissues were immediately excised, frozen in liquid nitrogen, pulverized in liquid nitrogen and stored at −80 °C for later total RNA extraction.
2.3. Reverse transcription followed by real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from pulverized tissues (hypothalamus, pituitary, thyroid, heart and liver) using TRIzol® reagent according to the manufacturer’s instructions (Life Technologies, Carlsbad, USA) (Chomczynski and Sacchi, 1987). Total RNA concentration was estimated with a nanospectrophotometer (Kasvi model k23-002, Brazil), and RNA integrity was confirmed by electrophoresis on a 1.2% agarose gel in TBE buffer (Tris/Acid boric/EDTA) through visualization of 18S and 28S ribosome bands stained with ethidium bromide. Afterwards, 2.5 μg of total RNA was used for first-strand cDNA synthesis (reverse transcription) using the GoScript™ Reverse Transcription System (Promega, Madison, USA) according to the manufacturer’s instructions. Real-time PCR from the product of reverse transcription (RT-qPCR) was performed using Platinum® SYBR® Green qPCR SuperMix-UDG (Life Technologies, Carlsbad, USA). Amplification was performed using the Applied Biosystems StepOnePlus™ Real-Time PCR System (Applied Biosystems, Singapore), and the PCR conditions were as follows: 50 °C (2 min), 95 °C (2 min), and 40 cycles of 95 °C (15 s) and 60 °C (30 s). At the end of the reaction, a melting curve was generated and analyzed to confirm the amplification specificity for each sample. The average cycle threshold (Ct) was automatically determined using StepOne™ Software v2.3 (Applied Biosystems). The quantification was calculated by the 2−ΔΔCt method using ribosomal protein L19 (Rpl19) as an endogenous control, as described previously (Livak and Schmittgen, 2001). The primer sequences and GenBank access number of genes are shown in Table 1.
Table 1.
Primers used for RT-qPCR analyses.
| Gene | Primers sequences (5′-3′) | GenBank |
|---|---|---|
| Trh | F:AACTCTACCCAGCCAGTTTGC | NM_013046.3 |
| (thyrotropin releasing hormone) | R: GCATCCTGGAGTCTGCGAAGT | |
| Slc16a2 (Mct8) | F: TGCCCTTGGTTACTTCGTCC | NM_147216.1 |
| (solute carrier family 16 member 2) | R: CAGGAATGAGAGGACCTGCAA | |
| Thrα1 | F: ACCTCCGCATGATCGGGGC | NM_001017960.1 |
| (thyroid hormone receptor alpha isoform 1) | R: CCTGATCCTCAAAGACCTC | |
| Thrb2 | F: TGAAGGCTGCAAGGGTTTCT | NM_001270854.1 |
| (thyroid hormone receptor beta isoform 2) | R: GCACTGGTTACGGGTGACTT | |
| Trhr | F: TGCTAACTACAGTGTGGCCC | NM_013047.3 |
| (thyrotropin releasing hormone receptor) | R: AACTGGGTCCATTCTTCTCGG | |
| Tshb | F: GGCAAACTGTTTCTTCCCAA | NM_013116.2 |
| (thyroid stimulating hormone beta) | R: GTTGGTTTTGACAGCCTCGT | |
| Tshr | F: CGCATTCCAGGGACTATGCAA | NM_012888.1 |
| (thyroid stimulating hormone receptor) | R: GTGGAAGACACGTCTAGCAAA | |
| Slc5a5 (Nis) | F: TCTTGCCGATCTTCTACCGC | NM_052983.2 |
| (solute carrier family 5 member 5) | R: ATGTCCAACCCGGTCACTTG | |
| Slc26a4 (Pds) | F: CAAGTGGGTTCTTGCCTCCT | NM_019214.1 |
| (solute carrier family 26 member 4) | R: TTGGTGGCGTAGACTTTCCC | |
| Tpo | F: CACGGCTTACCAGGCTACAA | NM_019353.2 |
| (thyroid peroxidase) | R: GCCTCCCAACCAGACATCAA | |
| Myh6 | F: ACAAGGTTAAAAACCTGACAGAGG | NM_017239.2 |
| (myosin heavy chain 6) | R: TACTGTTCTGCTGACTGATGTCAA | |
| Dio1 | F: GCCATTCCCCTGCTGTAACT | NM_021653.4 |
| (deiodinase type 1) | R: CCGTCAGTCCAAAGCCATCT | |
| Dio2 | F: ACGCCTACAAACAGGTTAAATTGG | NM_031720.3 |
| (deiodinase type 2) | R: CCGTCTTCTCTGAGGCACAA | |
| Dio3 | F: GCCCGTTGGTGCTCAATTTT | NM_017210.4 |
| (deiodinase type 3) | R: CTGTGGGATGACGTAGGGTG | |
| Rpl19 | F: CAATGAAACCAACGAAATCG | NM_031103.1 |
| (ribossomal protein L19) | R: TCAGGCCATCTTTGATCAGCT |
F, forward; R, reverse
2.4. Serum hormone concentrations and lipid profile
Serum TSH concentrations were determined using Luminex xMAP technology (Milliplex MAP rat pituitary panel, Billerica, MA, USA), and serum triiodothyronine (T3) and thyroxine (T4) concentrations were measured using the Siemens ADVIA Centaur test kit (Siemens, Dublin, Ireland) according to the manufacturer’s instructions. The serum dosages of triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol and VLDL cholesterol were measured by the enzymatic colorimetric method using the commercial kits Gold Analisa® (Gold Analisa Diagnostica Ltda, Brasil).
2.5. Statistical analysis
Parameters were first submitted to normality (Kolmogorov-Smirnov) and homoscedasticity (Bartlett) tests and then analyzed through ANOVA followed by Dunnett’s post hoc test. The Pearson correlation coefficient (r) was used to measure the linear correlation between the two variables. Linear correlation ranges from −1 to 1 and was classified as strong (|r > 0.7|), moderate (|0.5 < r < 0.7|) or weak (|0.3 < r < 0.5|). The results were considered significantly different when p < 0.05 and statistical tendency when 0.05 < P ≤ 0.1. All analyses were performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, California USA).
3. Results
3.1. Acrylamide increases the expression of Tshb mRNA content but not TSH serum concentration
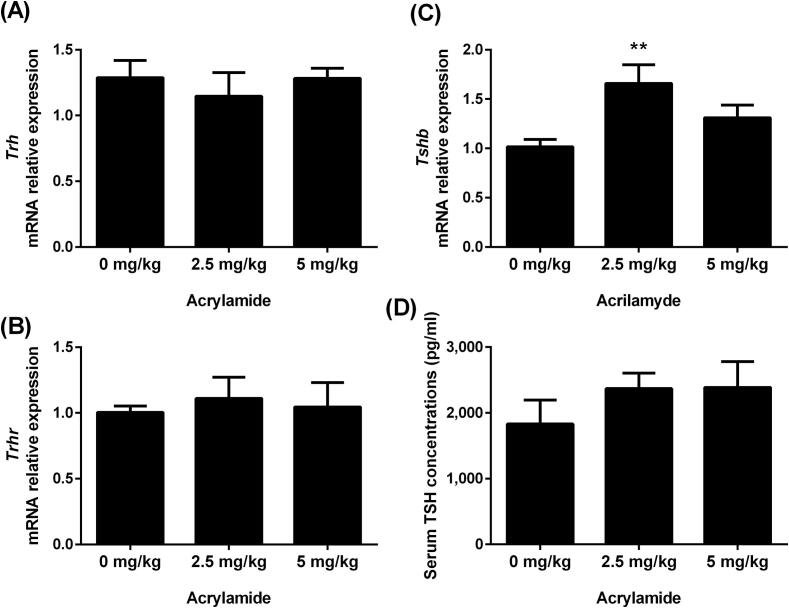
The components of the positive loop of the HPT axis were evaluated through the gene expression analysis of Trh, Trhr and Tshb, as well as the measurements of TSH serum concentration. In the hypothalamus, Trh mRNA content was not affected by acrylamide treatment (Fig. 1A). The expression of Trhr mRNA was not altered in the pituitary of rats treated with acrylamide compared to Control rats (Fig. 1B), while Tshb mRNA was increased in the group that received 2.5 mg AA/kg of BW (Fig. 1C). The TSH serum concentration was not altered by acrylamide administration (Fig. 1D).
Fig. 1.
Transcript expression of Trh mRNA in the hypothalamus (A), Trhr (B) and Tshb (C) in pituitary and serum TSH concentrations (D) of rats exposed to 0 (control), 2.5 or 5 mg/kg acrylamide of BW. The ribosomal protein L19 (Rpl19) mRNA content was used as an internal control. Values are expressed as the means ± SEM (ANOVA followed by the post-test of Dunnett), **P < 0.01 vs. control. Trh: thyrotropin releasing hormone; Trhr: thyrotropin releasing hormone receptor; Tshb: thyroid stimulating hormone beta; TSH: thyrotropin; BW: body weight.
3.2. Exposure to acrylamide did not affect the expression of genes involved in the negative feedback of the hypothalamus-pituitary-thyroid axis
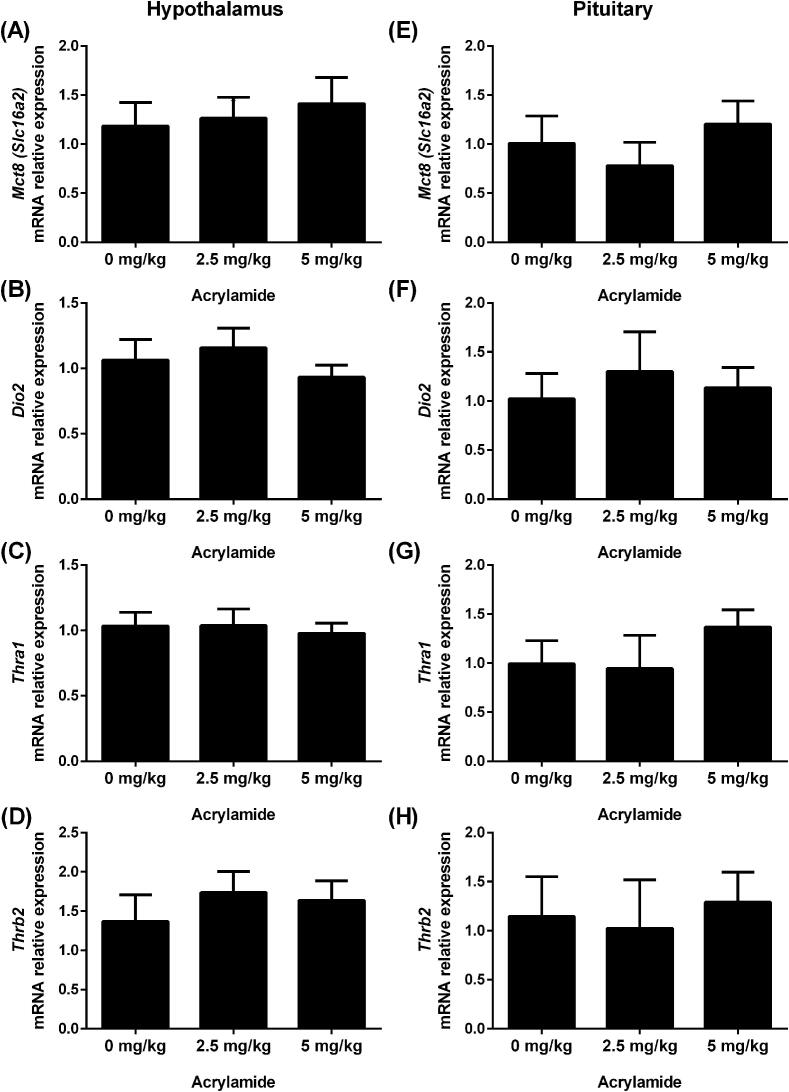
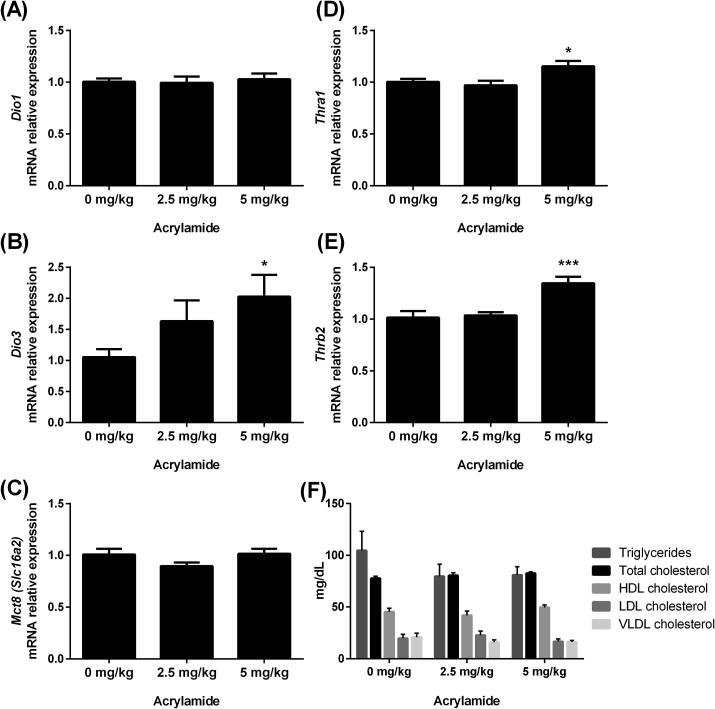
The expression of genes involved in the negative feedback of the HPT axis (Mct8/Slc16a2, Dio2, Thra1 and Thrb2) in the hypothalamus (Fig. 2A-D) and pituitary (Fig. 2E-H) was not significantly affected by AA pubertal exposure.
Fig. 2.
Transcript expression of Mct-8 (A and E), Dio2 (B and F), Thra1 (C and G) and Thrb2 (D and H) in the hypothalamus (left panel) and pituitary (right panel) of rats exposed to 0 (control), 2.5 or 5 mg/kg acrylamide of BW. The ribosomal protein L19 (Rpl19) mRNA content was used as an internal control. Values are expressed as the means ± SEM (ANOVA followed by the post-test of Dunnett), P > 0.05 vs. control. Mct-8: monocarboxylate transporter 8; Dio2: iodothyronine deiodinase 2; Thra1: thyroid hormone receptor alpha 1; Thrb2: thyroid hormone receptor beta 2; BW: body weight.
3.3. Acrylamide increases gene expression in the thyroid gland and the biosynthesis of thyroid hormones
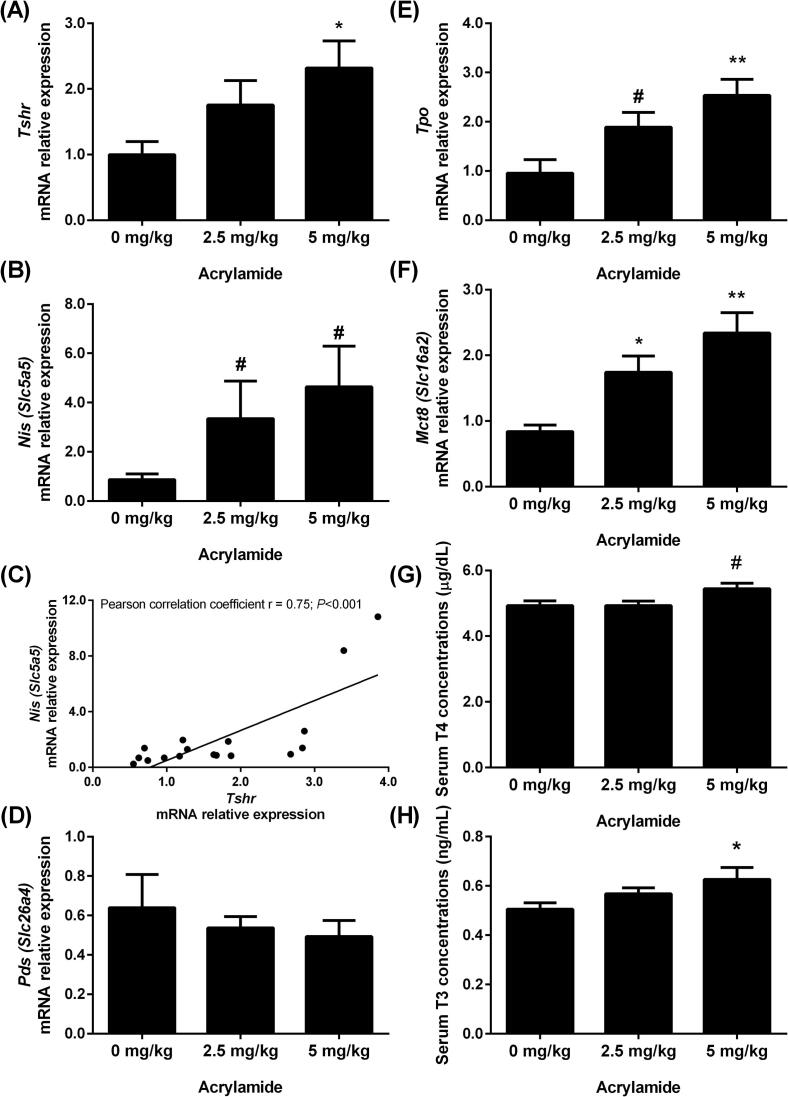
The expression of genes involved in thyroid hormone biosynthesis, as well as the T3 and T4 serum concentrations, was assessed in rats exposed to AA (Fig. 3). Exposure to the lower dose of AA (2.5 mg/kg BW) increased the Mct8 mRNA content (Fig. 3F), while the higher dose (5 mg/kg BW) upregulated the expression of Tshr (Fig. 3A), Tpo (Fig. 3E) and Mct8 (Fig. 3F) transcripts. Although not statistically significant, the content of Nis mRNA (Fig. 3B) tended to increase in animals treated with both doses of AA (indicated by #: 0.05 < P ≤ 0.1), as well as the Tpo mRNA content in rats treated with 2.5 mg AA/kg BW (Fig. 3E). A positive correlation between the contents of Tshr and Nis mRNAs was observed (Pearson correlation coefficient r = 0.75; P < 0.001; Fig. 3C). The content of Pds (Slc26a4) mRNA was not altered by acrylamide (Fig. 3D).
Fig. 3.
Transcript expression of Tshr (A), Nis (B), Pds (D), Tpo (E) and Mct-8 (F) in thyroid and serum T4 (G) and T3 (H) concentrations of rats exposed to 0 (control), 2.5 or 5 mg/kg acrylamide of BW. The ribosomal protein L19 (Rpl19) mRNA content was used as an internal control. Correlation between Nis and Tshr mRNA contents (C) (Pearson correlation coefficient r = 0.75; P < 0.001). Values are expressed as the means ± SEM (ANOVA followed by the post-test of Dunnett), *P < 0.05 and **P < 0.01 vs. control, # 0.05 < P ≤ 0.1. Tshr: thyroid stimulating hormone receptor; Nis: sodium/iodide symporter; Pds: pendrin; Tpo: thyroid peroxidase; Mct-8: monocarboxylate transporter 8; T3: triiodothyronine; T4: thyroxine; BW: body weight.
Rats exposed to 5 mg AA/kg BW presented higher serum T3 concentrations (Fig. 3H) and a tendency of increasing total T4 serum concentrations (p = 0.056; Fig. 3G) compared to control rats.
3.4. Effects of acrylamide exposure to peripheral T3-target systems
The effects of acrylamide were investigated in the liver (Fig. 4) and heart (Fig. 5) as important peripheral T3-target organs. In the liver, the content of Dio3, Thra1 and Thrb2 mRNAs was increased in the 5 mg AA/kg BW group (Fig. 4B, D and E, respectively), while the transcript expression of Dio1 and Mct-8 and the lipid profile were not altered (Fig. 4A, C and F, respectively). In the heart, Mct-8 mRNA content was reduced in animals treated with 2.5 and 5 mg of AA/kg BW (Fig. 5A), whereas the content of Dio3 and Myh6 mRNAs was not altered (Fig. 5B and C, respectively).
Fig. 4.
Transcript expression of Dio1 (A), Dio3 (B), Mct-8 (C), Thra1 (D) and Thrb2 (E) in the liver and nonfasting lipid profile (F) of rats exposed to 0 (control), 2.5 or 5 mg/kg acrylamide of BW. The ribosomal protein L19 (Rpl19) mRNA content was used as an internal control. Values are expressed as the means ± SEM (ANOVA followed by the post-test of Dunnett), *P < 0.05 and ***P < 0.001 vs control. Dio1: iodothyronine deiodinase 1; Dio3: iodothyronine deiodinase 3; Mct-8: monocarboxylate transporter 8; Thra1: thyroid hormone receptor alpha 1; Thrb2: thyroid hormone receptor beta 2; BW: body weight; very low- (VLDL), low- (LDL) and high-density lipoproteins (HDL).
Fig. 5.
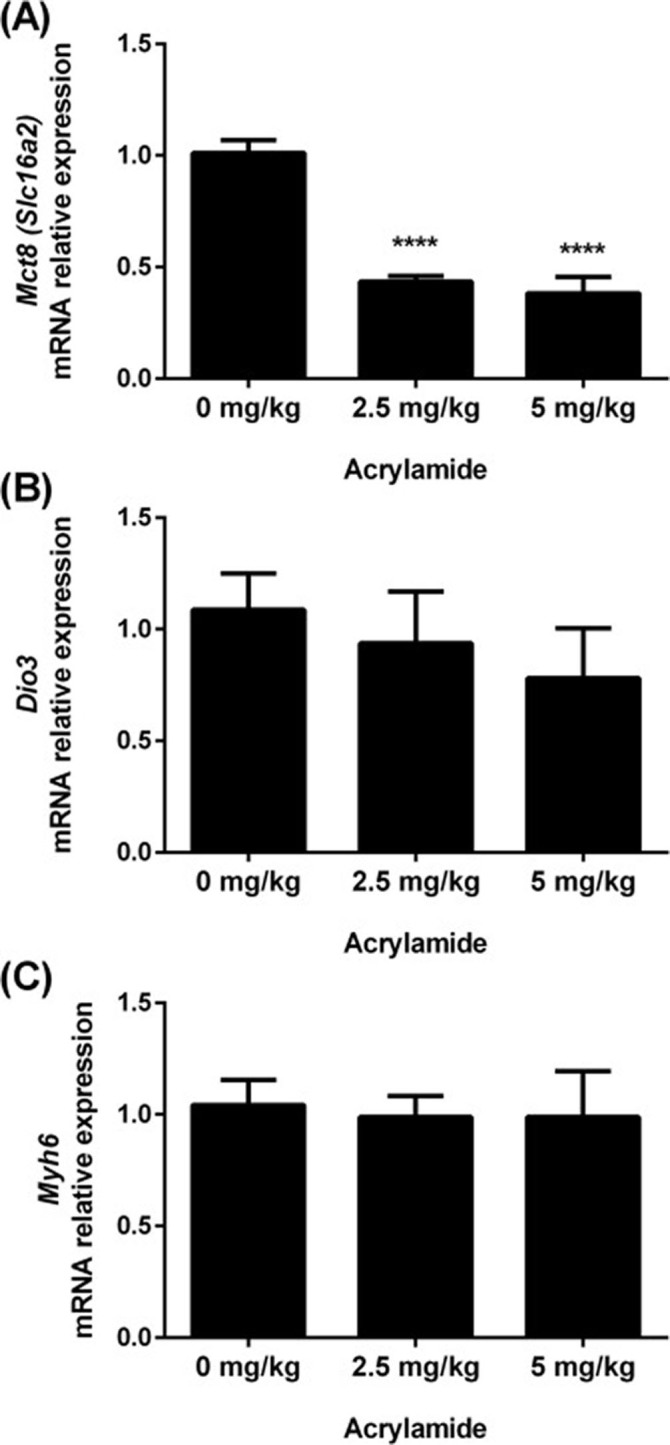
Transcript expression of Mct-8 (A), Dio3 (B) and Myh6 (C) in the hearts of rats exposed to 0 (control), 2.5 or 5 mg/kg acrylamide of BW. The ribosomal protein L19 (Rpl19) mRNA content was used as an internal control. Values are expressed as the means ± SEM (ANOVA followed by the post-test of Dunnett), ****P < 0.0001 vs control. Mct-8: monocarboxylate transporter 8; Dio3: iodothyronine deiodinase 3; Myh6: myosin heavy chain 6; BW: body weight.
4. Discussion
The thyroid endocrine system is highly susceptible to the effects of EDCs, which, in turn, are capable of altering the physiological homeostasis of the hypothalamic-pituitary-thyroid axis at different levels, including the synthesis, metabolism and biological effects of thyroid hormones. In this study, we showed that AA acts as EDCs for thyroid gland function, increasing the transcript expression of several proteins related to TH synthesis and altering hypothalamus-pituitary-thyroid axis homeostasis, an effect evidenced by the increased expression of pituitary Tshb mRNA, despite significant changes in TSH serum concentration and higher levels of THs in the serum. Compensatory mechanisms were observed in TH-target tissues, such as an increase in Dio3 mRNA expression in the liver and a reduction in Mct8 transcript content in the hearts of AA-treated rats. These mechanisms might contribute to reducing TH intake and increasing intracellular TH metabolization, suggesting peripheral adaptation to avoid thyrotoxicosis induced by AA.
In the thyroid gland, the activation of TSH receptor (TSHR) by TSH stimulates iodine uptake in thyrocytes through a sodium/iodide symporter (Nis) (Godlewska and Banga, 2019). Afterwards, iodine is carried out through the apical membrane to colloid by pendrin (Pds), an anionic channel (Gillam et al., 2004). In the colloid, the thyroperoxidase (Tpo) enzyme catalyzes iodide oxidation, iodination of the thyroglobulin tyrosine residue and iodothyronine coupling (Dohán and Carrasco, 2003); which are essential steps for T4 and T3 biosynthesis under TSH regulation. Then, THs are hydrolyzed from thyroglobulin protein and transported from the cytosol through the basolateral membrane to the bloodstream by monocarboxylate transporter 8 (Mct8) (Carvalho and Dupuy, 2017).
AA exposure increased Tshr expression, which was positively correlated with an increase in Nis transcript content, suggesting that even though TSH serum levels were not significantly altered, the thyroid gland was overstimulated in rats exposed to AA. In accordance with this, Tpo and Mct8 transcripts were augmented, as well as serum T4 and T3 concentrations. AA treatment did not alter Pds content; however, it has been shown that its expression is not regulated by TSH and that iodide efflux occurs even in the absence of PDS protein, suggesting that other channels may also be involved in this process (Pesce et al., 2012). Thus, AA exerts a stimulatory effect on the thyroid, increasing the expression of essential genes for TH synthesis and serum T3 and T4 concentrations.
The consequence of increasing serum THs induced by AA was investigated in the liver and heart, important TH peripheral targets. In the liver, THs play an essential role in the control of lipid metabolism (Araki et al., 2009). Within hepatocytes, the effects of THs are mediated by thyroid nuclear receptors encoded by the Thra1 and Thrb2 genes, whose expression was increased by the highest dose of AA. In the liver of rodents, THs stimulate the expression of enzymes involved in lipogenesis, reduce the synthesis of very low- and low-density lipoproteins (VLDL and LDL) and apolipoprotein B levels while increasing the expression of LDL receptors, the biosynthesis of apolipoprotein A1, a component of high-density lipoproteins (HDL), and HDL receptor expression (Davidson et al., 1988). THs also modulate the expression of cholesterol 7-a-hydroxylase (CYP7A1), an enzyme associated with cholesterol conversion to bile acids, increasing cholesterol excretion as bile acids (Mondal and Mugesh, 2017).
No significant alterations were observed in the lipid profile of AA-treated rats even though Thrb2 expression and serum T3 levels were augmented by AA. This discrepancy can be partially explained by the increased expression of deiodase type 3 (Dio3), which is involved in T4 and T3 metabolization by deiodination to reverse T3 (rT3) and 3,5-diiodothyronine (T2), thus reducing the T3 intracellular concentration and protecting the hepatocytes from possible AA-induced thyrotoxicosis. This hypothesis is corroborated by the reduced levels of Mct-8 mRNA content and unchanged expression of cardiac alpha myosin heavy chain (Myh6) transcript, an important target gene of TH modulation of contractility and cardiac structure (Jabbar et al., 2017), in the hearts of AA-treated animals. Thus, as observed for the liver, a compensatory (or adaptive) response is triggered in the heart, reducing TH uptake in cardiomyocytes and protecting cardiac tissue against excess TH levels.
Together, our data indicate that altered serum TH concentrations can reflect thyroid allostatic regulation induced by AA exposure. Allostasis is defined as a dynamic stress reaction that seeks to maintain homeostasis through transient changes but can potentially lead to the emergence of pathologies (Chatzitomaris et al., 2017). The type 2 allostatic response in the thyroid has increased circulating HT with normal or higher TSH levels and is observed in obesity, psychosocial stress, liver diseases, pregnancy, cold adaptation, acute schizophrenia, posttraumatic stress disorder and exposure to toxic chemicals (Chatzitomaris et al., 2017). Thus, the transient increase in TH levels induced by AA could chronically lead to hypothyroidism, which is consistent with the high acrylamide consumption and the hypothyroidism prevalence in the population (Taylor et al., 2018) and with the results obtained from a cohort study conducted between 1999 and 2000 that described a positive correlation between increased urinary AA metabolite concentration (N-acetyl-S-propionamide-cysteine) and decreased thyroxine (T4) levels in adolescents and young adults (Lin et al., 2015).
AA can also induce inflammatory processes in the gland, as described for amiodarone, denileucine, lithium, monoclonal antibodies, sodium valproate and tyrosine kinase inhibitors (Taylor et al., 2018, Sweeney et al., 2014); leading to thyroiditis, which could culminate in early hyperthyroidism or a short-term toxic phase, followed by transient or permanent hypothyroidism (Sweeney et al., 2014). In this sense, AA is metabolized by the cytochrome P450 enzyme (CYP2E1), which is found in the human thyroid gland, into glycidamide, an even more reactive metabolite (Bieche et al., 2007). Moreover, MCT8 exhibits T3 specificity due to the presence of histidine residues that interact with T3 in the internal part of the canal (Fischer et al., 2015, Braun et al., 2013). These histidine residues are targets of acrylamide nucleophilic reactions; which have two reactive sites in its molecule favoring its reaction with sulfhydryl (SH) groups present in cysteine, homocysteine and glutathione, amino terminal protein groups and NH group of histidine ring (Friedman, 2003). Therefore, AA could also interfere with T3 secretion, altering the specificity of the MCT8 transporter. More studies are required to test these hypotheses.
Controversially, a reduction in serum T4 and T3 levels were observed in Sprague Dawley male rats treated with 5, 10 or 15 mg AA/kg/day during 8 weeks (Hamdy et al., 2012), a longer period than our study. While any changes in analysis of gene expression of the HPT axis was observed in Fischer 344 rats treated with 2.5, 10 or 50 mg AA/kg/day during 14 days (Bowyer et al., 2008), a shorter period than our study. These divergent results may be related to the treatment duration, age at the start of the experiment, administration of AA (drinking water versus gavage). The exposure by drinking water, although it is the form that most closely matches the human exposure route, has important limitations, since the amount of water ingested by each animal can be variable and is usually measured by the consumption of water ingested per all animals in the cage. Thus, the results would be analyzed considering an estimate of the daily dose per kg of the animal, which can impair the dose-response analysis.
It is noteworthy that our study was the first based on a validated protocol taking into account the sensitive window to endocrine disruption (Stoker et al., 2000). Thus, our study supports the Developmental Origin of Health and Disease (DOHaD) concept (Barouki et al., 2018) since fetus, children and teenagers (on prepubertal phase) are more susceptible to risks when exposed to substances and hormonal imbalances in these periods could lead to hormonal disorders in adults (Solomon and Schettler, 2000). Although the data regarding the AA general toxicity in humans is scarce, it is known to induces acute toxic effects when the oral doses are greater than 100 mg/kg of BW, and lethal doses are usually higher than 150 mg/kg of BW (FAO/WHO, 2005). Most of the information regarding toxicokinetics and toxicology, especially in the neurological, endocrine (reproductive and thyroid) systems, comes from animal studies (Hamdy et al., 2012, Bowyer et al., 2008, Miller et al., 1982, Kadry et al., 1999, Yilmaz et al., 2017). Considering the human risk assessment, AA is a genotoxic substance, and for this reason, any level of tolerable daily intake (TDI) of AA in food cannot be set. As an alternative for the purpose of comparative risk assessment in this study, we calculated the reference dose (RfD) dividing LOAEL by 5 mg by uncertainty factors of 10,000 (10 for human variability, 10 for an animal study, 10 for less than chronic exposure, and 10 for use of an LOAEL instead of a NOAEL - No Observed Adverse Effect Level) (Dankovic et al., 2015, [43]). This RfD was estimated at 0.5 and 0.25 µg of AA/kg BW/day, which could be considered the acceptable safety level for chronic noncarcinogenic and developmental effects in human exposure.
5. Conclusions
In summary, AA prepubertal exposure alters the expression of important thyroid gene transcripts, resulting in increased synthesis and secretion of THs and leading to allostatic regulation of the HPT axis. Thus, AA acts as an EDC for the thyroid system, and chronic exposure to AA might be associated with a higher prevalence of thyroid dysfunction.
Funding
This study was partially supported by the Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior – Brasil (CAPES) [Finance Code 001], Brazil.
CRediT authorship contribution statement
Viviane Matoso de Oliveira: Investigation, Writing - original draft. Fernanda Ivanski: Investigation. Isabela Medeiros de Oliveira: Investigation. Paula Bargi-Souza: . Dalton Luiz Schiessel: Writing - original draft. Marco Aurelio Romano: Conceptualization, Methodology, Formal analysis. Renata Marino Romano: Conceptualization, Methodology, Visualization, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Araki O., Ying H., Zhu X.G., Willingham M.C., Cheng S.Y. Distinct dysregulation of lipid metabolism by unliganded thyroid hormone receptor isoforms. Mol. Endocrinol. 2009;23(3):308–315. doi: 10.1210/me.2008-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber D.S., LoPachin R.M. Proteomic analysis of acrylamide-protein adduct formation in rat brain synaptosomes. Toxicol. Appl. Pharmacol. 2004;201(2):120–136. doi: 10.1016/j.taap.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Barouki R., Melen E., Herceg Z., Beckers J., Chen J., Karagas M., Puga A., Xia Y., Chadwick L., Yan W., Audouze K., Slama R., Heindel J., Grandjean P., Kawamoto T., Nohara K. Epigenetics as a mechanism linking developmental exposures to long-term toxicity. Environ. Int. 2018;114:77–86. doi: 10.1016/j.envint.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieche I., Narjoz C., Asselah T., Vacher S., Marcellin P., Lidereau R., Beaune P., de Waziers I. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet. Genomics. 2007;17(9):731–742. doi: 10.1097/FPC.0b013e32810f2e58. [DOI] [PubMed] [Google Scholar]
- Boas M., Feldt-Rasmussen U., Main K.M. Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2012;355(2):240–248. doi: 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Bowyer J.F., Latendresse J.R., Delongchamp R.R., Muskhelishvili L., Warbritton A.R., Thomas M., Tareke E., McDaniel L.P., Doerge D.R. The effects of subchronic acrylamide exposure on gene expression, neurochemistry, hormones, and histopathology in the hypothalamus–pituitary–thyroid axis of male Fischer 344 rats. Toxicol. Appl. Pharmacol. 2008;230(2):208–215. doi: 10.1016/j.taap.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Braun D., Lelios I., Krause G., Schweizer U. Histidines in potential substrate recognition sites affect thyroid hormone transport by monocarboxylate transporter 8 (MCT8) Endocrinology. 2013;154(7):2553–2561. doi: 10.1210/en.2012-2197. [DOI] [PubMed] [Google Scholar]
- Carvalho D.P., Dupuy C. Thyroid hormone biosynthesis and release. Mol. Cell. Endocrinol. 2017;458:6–15. doi: 10.1016/j.mce.2017.01.038. [DOI] [PubMed] [Google Scholar]
- Chatzitomaris A., Hoermann R., Midgley J.E., Hering S., Urban A., Dietrich B., Abood A., Klein H.H., Dietrich J.W. Thyroid allostasis-adaptive responses of thyrotropic feedback control to conditions of strain, stress, and developmental programming. Front. Endocrinol. (Lausanne) 2017;8:163. doi: 10.3389/fendo.2017.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coperchini F., Awwad O., Rotondi M., Santini F., Imbriani M., Chiovato L. Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) J. Endocrinol. Invest. 2017;40(2):105–121. doi: 10.1007/s40618-016-0572-z. [DOI] [PubMed] [Google Scholar]
- Dankovic D.A., Naumann B.D., Maier A., Dourson M.L., Levy L.S. The scientific basis of uncertainty factors used in setting occupational exposure limits. J. Occup. Environ. Hyg. 2015;12 Suppl 1(sup1):S55–S68. doi: 10.1080/15459624.2015.1060325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson N.O., Powell L.M., Wallis S.C., Scott J. Thyroid hormone modulates the introduction of a stop codon in rat liver apolipoprotein B messenger RNA. J. Biol. Chem. 1988;263(27):13482–13485. [PubMed] [Google Scholar]
- De Campos, P., Medeiros De Oliveira, I., Alvarenga, C., Romano, M.A., Romano, R., 2015. Thyroid Gland as a Target for Endocrine Chemical Disruptors.
- de Cock M., de Boer M.R., Lamoree M., Legler J., van de Bor M. Prenatal exposure to endocrine disrupting chemicals in relation to thyroid hormone levels in infants – a Dutch prospective cohort study. Environ. Health. 2014;13:106. doi: 10.1186/1476-069X-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E., Bourguignon J.P., Giudice L.C., Hauser R., Prins G.S., Soto A.M., Zoeller R.T., Gore A.C. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohán O., Carrasco N. Advances in Na+/I− symporter (NIS) research in the thyroid and beyond. Mol. Cell. Endocrinol. 2003;213(1):59–70. doi: 10.1016/j.mce.2003.10.059. [DOI] [PubMed] [Google Scholar]
- FAO/WHO, JOINT FAO/WHO EXPERT COMMITTEE ON FOOD ADDITIVES, WHO Press, 2005.
- Fischer J., Kleinau G., Muller A., Kuhnen P., Zwanziger D., Kinne A., Rehders M., Moeller L.C., Fuhrer D., Gruters A., Krude H., Brix K., Biebermann H. Modulation of monocarboxylate transporter 8 oligomerization by specific pathogenic mutations. J. Mol. Endocrinol. 2015;54(1):39–50. doi: 10.1530/JME-14-0272. [DOI] [PubMed] [Google Scholar]
- Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review. J. Agric. Food Chem. 2003;51(16):4504–4526. doi: 10.1021/jf030204+. [DOI] [PubMed] [Google Scholar]
- Gillam M.P., Sidhaye A.R., Lee E.J., Rutishauser J., Stephan C.W., Kopp P. Functional characterization of pendrin in a polarized cell system evidence for pendrin-mediated apical iodide efflux. J. Biol. Chem. 2004;279(13):13004–13010. doi: 10.1074/jbc.M313648200. [DOI] [PubMed] [Google Scholar]
- Godlewska M., Banga P.J. Thyroid peroxidase as a dual active site enzyme: Focus on biosynthesis, hormonogenesis and thyroid disorders of autoimmunity and cancer. Biochimie. 2019 doi: 10.1016/j.biochi.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Gore A.C. Neuroendocrine targets of endocrine disruptors. Hormones. 2010;9(1):16–27. doi: 10.14310/horm.2002.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy S., Bakeer H., Eskander E., Sayed O. Effect of acrylamide on some hormones and endocrine tissues in male rats. Hum. Exp. Toxicol. 2012;31(5):483–491. doi: 10.1177/0960327111417267. [DOI] [PubMed] [Google Scholar]
- Hileman B. Acrylamide found in cooked foods. Chem. Eng. News. Arch. 2002;80(19):33. [Google Scholar]
- Jabbar A., Pingitore A., Pearce S.H., Zaman A., Iervasi G., Razvi S. Thyroid hormones and cardiovascular disease. Nat. Rev. Cardiol. 2017;14(1):39–55. doi: 10.1038/nrcardio.2016.174. [DOI] [PubMed] [Google Scholar]
- Kadry A.M., Friedman M.A., Abdel-Rahman M.S. Pharmacokinetics of acrylamide after oral administration in male rats. Environ. Toxicol. Pharmacol. 1999;7(2):127–133. doi: 10.1016/s1382-6689(99)00005-8. [DOI] [PubMed] [Google Scholar]
- Karwacka A., Zamkowska D., Radwan M., Jurewicz J. Exposure to modern, widespread environmental endocrine disrupting chemicals and their effect on the reproductive potential of women: an overview of current epidemiological evidence. Hum. Fertil. 2017;1–24 doi: 10.1080/14647273.2017.1358828. [DOI] [PubMed] [Google Scholar]
- Lin C.-Y., Lin L.-Y., Chen Y.-C., Wen L.-L., Chien K.-L., Sung F.-C., Chen P.-C., Su T.-C. Association between measurements of thyroid function and the acrylamide metabolite N-Acetyl-S-(propionamide)-cysteine in adolescents and young adults. Environ. Res. 2015;136:246–252. doi: 10.1016/j.envres.2014.08.043. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- LoPachin R.M., Gavin T., Decaprio A., Barber D.S. Application of the Hard and Soft, Acids and Bases (HSAB) theory to toxicant–target interactions. Chem. Res. Toxicol. 2012;25(2):239–251. doi: 10.1021/tx2003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoso V., Bargi-Souza P., Ivanski F., Romano M.A., Romano R.M. Acrylamide: a review about its toxic effects in the light of Developmental Origin of Health and Disease (DOHaD) concept. Food Chem. 2019;283:422–430. doi: 10.1016/j.foodchem.2019.01.054. [DOI] [PubMed] [Google Scholar]
- Miller M.J., Carter D.E., Sipes I.G. Pharmacokinetics of acrylamide in Fisher-344 rats. Toxicol. Appl. Pharmacol. 1982;63(1):36–44. doi: 10.1016/0041-008x(82)90024-2. [DOI] [PubMed] [Google Scholar]
- Mondal S., Mugesh G. Novel thyroid hormone analogues, enzyme inhibitors and mimetics, and their action. Mol. Cell. Endocrinol. 2017;458:91–104. doi: 10.1016/j.mce.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Pesce L., Bizhanova A., Caraballo J.C., Westphal W., Butti M.L., Comellas A., Kopp P. TSH regulates pendrin membrane abundance and enhances iodide efflux in thyroid cells. Endocrinology. 2012;153(1):512–521. doi: 10.1210/en.2011-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S., Zhou C., Chiang C., Mahalingam S., Brehm E., Flaws J.A. Exposure to endocrine disruptors during adulthood: consequences for female fertility. J. Endocrinol. 2017;233(3):R109–R129. doi: 10.1530/JOE-17-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifakis S., Androutsopoulos V.P., Tsatsakis A.M., Spandidos D.A. Human exposure to endocrine disrupting chemicals: effects on the male and female reproductive systems. Environ. Toxicol. Pharmacol. 2017;51:56–70. doi: 10.1016/j.etap.2017.02.024. [DOI] [PubMed] [Google Scholar]
- Solomon G.M., Schettler T. Environment and health: 6. Endocrine disruption and potential human health implications. CMAJ: Can. Med. Assoc. J. 2000;163(11):1471–1476. [PMC free article] [PubMed] [Google Scholar]
- Stoker T.E., Parks L.G., Gray L.E., Cooper R.L. Endocrine-disrupting chemicals: prepubertal exposures and effects on sexual maturation and thyroid function in the male rat. A focus on the EDSTAC recommendations. Endocrine Disrupter Screening and Testing Advisory Committee. Crit. Rev. Toxicol. 2000;30(2):197–252. doi: 10.1080/10408440091159194. [DOI] [PubMed] [Google Scholar]
- Sweeney L.B., Stewart C., Gaitonde D.Y. Thyroiditis: an integrated approach. Am. Fam. Physician. 2014;90(6) [PubMed] [Google Scholar]
- Tareke E., Rydberg P., Karlsson P., Eriksson S., Törnqvist M. Acrylamide: a cooking carcinogen? Chem. Res. Toxicol. 2000;13(6):517–522. doi: 10.1021/tx9901938. [DOI] [PubMed] [Google Scholar]
- Taylor P.N., Albrecht D., Scholz A., Gutierrez-Buey G., Lazarus J.H., Dayan C.M., Okosieme O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018;14(5):301–316. doi: 10.1038/nrendo.2018.18. [DOI] [PubMed] [Google Scholar]
- 43.US Environmental Protection Agency, E., Reference Dose (RfD): Description and Use in Health Risk Assessments Background Document 1A, 1993.
- Yang H.-J., Lee S.-H., Jin Y., Choi J.-H., Han D.-U., Chae C., Lee M.-H., Han C.-H. Toxicological effects of acrylamide on rat testicular gene expression profile. Reprod. Toxicol. 2005;19(4):527–534. doi: 10.1016/j.reprotox.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Yilmaz B., Yildizbayrak N., Aydin Y., Erkan M. Evidence of acrylamide- and glycidamide-induced oxidative stress and apoptosis in Leydig and Sertoli cells. Hum. Exp. Toxicol. 2017;36(12):1225–1235. doi: 10.1177/0960327116686818. [DOI] [PubMed] [Google Scholar]