Graphical abstract
Keywords: E-cigarettes, Developmental toxicity, Cigarettes, Nicotine, Human induced pluripotent stem cells, In vitro reproduction assay
Abbreviations: DNPH, 2,4-dinitrophenylhydrazine; bPBS, bubbled phosphate buffered saline; ATRA, All-trans-retinoic acid; TP, cell viability toxicity potential concentration; CDC, Centers for Disease Control and Prevention; CV, coefficient of variation; DART, developmental and reproductive toxicity; dTP, developmental toxicity potential concentration; dTT, developmental toxicity threshold; devTOXqP, devTOX quickPredict; e-cigarettes, electronic cigarettes; EVP, electronic vapour product; ECVAM, European Center for the Validation of Alternative Methods; FDR, false discovery rate; HPHCs, Harmful and Potentially Harmful Constituents; HTP, heated tobacco product; HPLC-DAD, high-performance liquid chromatography with a diode-array detector; HYB, hybrid product; iPS cells, induced pluripotent stem cells; ISTD, internal standard; ISO, International Organization for Standardisation; LC-MS/MS, liquid chromatography with tandem mass spectrometry; LOQ, limit of quantification; NICE, National Institute for Health and Care Excellence; nAChRs, nicotinic acetylcholine receptors; ND, No effect was detected within the exposure range tested; OECD, Organisation for Economic Co-operation and Development; ODC, ornithine decarboxylase; o/c, ornithine/cystine ratio; PBS, phosphate buffered saline; POD, point of difference; PG/VG, propylene glycol/vegetable glycerine; Q-TOF, Quadrupole Time-of-Flight; ROS, reactive oxygen species; TT21C, toxicity testing in the 21st century; UPLC-HRMS, ultra-high performance liquid chromatography coupled high resolution mass spectrometry; COT, United Kingdom Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment; NHS, United Kingdom National Health Service; EPA, United States Environmental Protection Agency
Highlights
-
•
Effective in vitro strategies are required to predict early developmental toxicity.
-
•
devTOXqP is a metabolomics biomarker assay using iPSCs.
-
•
Sample smoke/aerosol captured in bPBS, was tested up to 10% concentration.
-
•
Cigarettes & HTP bPBS extracts were predicted as potentially developmentally toxic.
-
•
HYB & EVP aerosols were not predicted as having developmentally toxic potential in devTOXqP.
Abstract
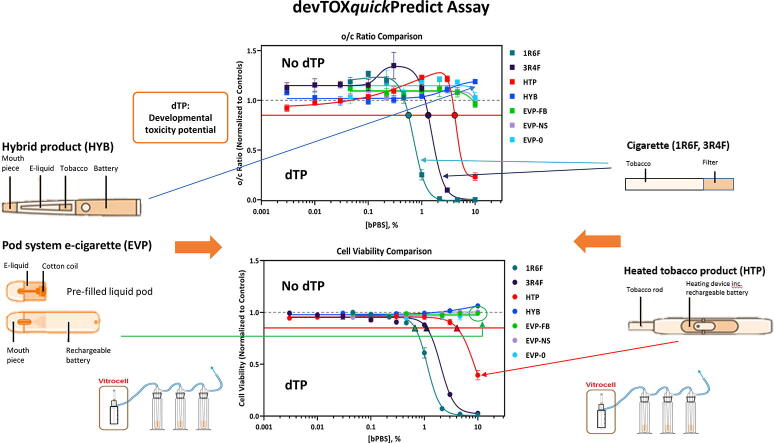
devTOX quickPredict (devTOXqP) is a metabolomics biomarker-based assay that utilises human induced pluripotent stem (iPS) cells to screen for potential early stage embryonic developmental toxicity in vitro. Developmental toxicity potential is assessed based on the assay endpoint of the alteration in the ratio of key unrelated biomarkers, ornithine and cystine (o/c).
This work aimed to compare the developmental toxicity potential of tobacco-containing and tobacco-free non-combustible nicotine products to cigarette smoke. Smoke and aerosol from test articles were produced using a Vitrocell VC10 smoke/aerosol exposure system and bubbled into phosphate buffered saline (bPBS). iPS cells were exposed to concentrations of up to 10% bPBS. Assay sensitivity was assessed through a spiking study with a known developmental toxicant, all-trans-retinoic acid (ATRA), in combination with cigarette smoke extract.
The bPBS extracts of reference cigarettes (1R6F and 3R4F) and a heated tobacco product (HTP) were predicted to have the potential to induce developmental toxicity, in this screening assay. The bPBS concentration at which these extracts exceeded the developmental toxicity threshold was 0.6% (1R6F), 1.3% (3R4F), and 4.3% (HTP) added to the cell media. Effects from cigarette smoke and HTP aerosol were driven largely by cytotoxicity, with the cell viability and o/c ratio dose–response curves crossing the developmental toxicity thresholds at very similar concentrations of added bPBS. The hybrid product and all the electronic cigarette (e-cigarette) aerosols were not predicted to be potential early developmental toxicants, under the conditions of this screening assay.
1. Introduction
The Centers for Disease Control and Prevention (CDC) and US Surgeon General state that cigarette smoking harms every phase of reproduction (CDC, 2004, US Department of Health Human Services, 2014). The impact of cigarette smoking on pregnancy has been reported in published scientific literature and by public health bodies (CDC, 2004, RCP, 2010), with maternal smoking during pregnancy being described in one review as ‘perhaps the single most avoidable cause of adverse pregnancy outcomes’ (Greene and Pisano, 2019). The reported adverse effects of cigarette smoking during pregnancy include an increased risk of: low birth weight, underdeveloped organs, congenital abnormalities, preterm birth, and foetal mortality (Greene and Pisano, 2019). A recent meta-analysis, including studies from 1985 to 2016, has estimated that more than 10% of women smoke during pregnancy globally with the highest prevalence observed in Ireland (38%), Uruguay (29.7%), and Bulgaria (29.4%) (Lange et al., 2018). Within the UK, around 10.6% of women smoke during pregnancy and it has been estimated that tobacco smoking during pregnancy causes approximately 2200 premature births, between 3000 and 5000 miscarriages, and 300 perinatal deaths per year (RCP, 2010, National Health Service, 2019).
Non-combustible nicotine products are growing in popularity. These products deliver nicotine to the adult smoker without tobacco combustion and therefore with substantially fewer and significantly reduced levels of harmful or potentially harmful constituents (HPHCs) than found in tobacco smoke (Rudd et al., 2020, Tayyarah and Long, 2014). E-cigarettes are battery-powered devices that deliver an aerosol (popularly referred to as “vapor”) to users from an e-liquid. The form of nicotine typically used in e-liquids is termed as ‘freebase’ nicotine, which is volatile on heating. Further e-liquid developments include nicotine protonation (commonly referred to as nicotine salts), which is less volatile and gives a ‘cigarette-like’ pulmonary delivery of nicotine and greater adult smoker satisfaction (O’Connell et al., 2019). By contrast, heated tobacco products (HTPs) electronically heat a tobacco stick to release the tobacco flavoured and nicotine containing aerosol. HTPs typically heat up to 350 °C (WHO, 2018), with research showing no tobacco combustion occurs at this lower operating temperature compared to cigarettes (which heat tobacco to approximately 600–850 °C) (Cozzani et al., 2020, Baker, 1974). Hybrid products (HYB) combine the technologies of e-cigarettes and HTPs, where aerosol generated via e-liquid heating is passed through a tobacco ‘plug’ to infuse tobacco aroma before being inhaled by the adult smoker (Poynton et al., 2017). The temperature of the aerosol in the HYB product has an average maximum reported temperature of 35 °C before reaching the tobacco plug, which decreases to an average maximum of 32 °C after passing through the tobacco plug (Poynton et al., 2017). There is a growing body of evidence that non-combustible nicotine products can be an effective tool in helping adult smokers to quit smoking and are a less harmful alternative to cigarettes (McRobbie et al., 2014, Hartmann-Boyce et al., 2016, Hajek et al., 2019, McNeill et al., 2018). It is important that their potential biological effects, including those focused on developmental toxicity, can be assessed quickly and accurately.
Reproduction is a highly complex process, involving multiple cell types, tissues and cell signalling feedback loops which directly or indirectly depend on other organ systems; developmental and reproductive toxicity (DART) testing encompasses a range of toxicological endpoints with multiple potential targets in both males and female animals (Bal-Price and Jennings, 2014, Mattison and Thomford, 1989). Currently, the gold-standard for assessing DART is through the use of laboratory animal studies (Iyer, 2017). DART studies are time and labour intensive, require a large number of animals, involving the analysis of both parental and offspring generations, and may also require testing in multiple species (Beekhuijzen, 2017). A review by Bailey et al. (2005), emphasised the poor predictivity and concordance of the current animal DART studies with developmental and reproductive effects reported in humans, highlighting most human developmental toxicants have been discovered during case reports and clinical studies, despite the huge amounts of animal data already available (Bailey et al., 2005). The current Organisation for Economic Co-operation and Development (OECD) animal DART studies are not helping to accurately to predict human developmental toxicants due to species differences in genetics, absorption, distribution, metabolism and excretion of xenobiotics (Bailey et al., 2005).
The poor predictivity of the rat model in OECD DART assays for cigarette smoke has been highlighted in in vivo inhalation studies. Rats were exposed to either the reference cigarettes 1R4F or 2R4F (designed as an American blend product, supplied by the University of Kentucky). Cigarette smoke was administered via the inhalation route (up to 600 mg/m3 for 2 h a day, 7 days a week). No adverse effects were observed on the full range of DART endpoints including resorption rates, litter size, sex ratio, developmental landmarks, and learning or memory in the next generation of pups raised to day 65. The only treatment related effects reported were decreased body weight gain in both dams and pups, which in the pups was associated with delayed ossification of occipital bones and sternebrae. In further work by Carmines et al. (2008), groups of 27 pregnant SD rats were exposed via the nasal route for 2 h/day 7 days/week. The test target concentrations were designed to produce the same plasma levels of biomarkers as exposure to 2R4F reference cigarette smoke at a concentration of 600 mg/m3 total particulate matter. The smoke constituents evaluated included carbon monoxide (CO), nicotine, and a mixture of aldehydes (acrolein, acetaldehyde, and formaldehyde). Exposure to nicotine only led to reduced maternal body weight gain but had no effect on fetal weight. Exposure to CO only had no effect on maternal body weight gain but did lead to reduced fetal weight to a degree comparable to that seen with the cigarette smoke exposed group (Carmines and Rajendran, 2008).
Over the last decade, multiple in vitro assays have been developed or are under evaluation for developmental toxicity, including three tests validated by the European Center for the Validation of Alternative Methods (ECVAM): the ex vivo rodent whole embryo culture test, the micromass test based on day 14 limb buds from rat embryos, and mouse embryonic stem cell tests (Bal-Price and Jennings, 2014, Luijten et al., 2008). Due to the complexity of reproductive and developmental physiology there is no one in vitro system available to model the entire process, therefore individual elements of developmental and reproductive physiology should be studied separately, then integrated into an in vitro testing strategy (Luijten et al., 2008, Palmer et al., 2017). More recently, there has been an increased focus on the use of more human relevant in vitro cell systems (National Research Council, 2007), including the use of human-derived stem cells. Stemina’s devTOX quickPredict (devTOXqP) assay is a human induced pluripotent stem (iPS) cell-based assay, that predicts a test article’s developmental toxicity potential. This assay is a human-based cell system, which helps to eliminate the risk of false negative results due to inter-species differences in developmental pathways (Scott et al., 2013). The devTOXqP assay measures changes in the abundance of two metabolic biomarkers, ornithine and cystine, across a broad dose–response range. Ornithine and cystine are key biomarkers involved in normal cell proliferation and differentiation during embryo development, and disruptions in their signalling is a marker for developmental toxicity (Palmer et al., 2013). The devTOXqP platform has been successfully used by the United States Environmental Protection Agency (EPA) to support their ToxCast and Virtual Tissue Model programs. 1065 phase I and II compounds have been screened in the devTOXqP assay, as part of the ToxCast Initiative (Zurlinden et al., 2020). Within the screened compounds, 19% were predicted to have the potential to cause developmental toxicity, and when compared to in vivo models, the assay had a ‘79% to 82% accuracy with high specificity (greater than 84%) but modest sensitivity (<67%)’ (Zurlinden et al., 2020).
We previously assessed the impact of neat e-liquids or total particulate matter from cigarettes on a variety of cellular endpoints (Czekala et al., 2019); however, cells should ideally be exposed to both the particulate and volatile constituents of the whole smoke and aerosol. The emissions found within cigarette smoke are known to be biologically active and linked to a range of health endpoints in humans, including reproductive and developmental toxicity (FDA, 2012). The trapping of smoke/aerosol in bPBS is a commonly used method and allows for the direct addition of bPBS into in vitro cell system (Johnson et al., 2009). The bubbling procedure captures largely the water-soluble gaseous fraction of the smoke or aerosol, making this an alternative delivery mechanism to the historically used filter trapped total particulate matter or aerosol collected mass. Work by Buratto et al., has shown that a number of carbonyls can be successfully trapped and quantified within bPBS from both combustible cigarettes and HTPs (Buratto et al., 2018).
Known reproductive or developmentally toxic compounds found within tobacco or tobacco smoke include arsenic, 1,3-butadiene, lead, mercury and nicotine (FDA, 2012). In this study, human iPS cells were exposed to neat nicotine to determine whether nicotine on its own caused any developmental toxicity prediction in the devTOXqP assay. To address regulatory concerns, further research is required to assess whether non-combustible nicotine product aerosols present a risk to the developing foetus. In this study, the human iPS cell based devTOXqP assay was used to screen the developmental toxicity potential of three non-combustible nicotine product aerosols (HTP, e-cigarettes and HYB) and compared to reference cigarette smoke (3R4F and 1R6F), where both the aerosol or smoke were trapped directly in bPBS, as well as to the effects of nicotine. The assay sensitivity to complex mixtures was also assessed through a spiking study, using a known positive developmental toxicant (ATRA) and the highest non-responsive dose of 1R6F.
2. Material and Methods
2.1. Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
2.2. Test articles
The test samples used to generate smoke/aerosols are described in Table 1.
Table 1.
Test articles evaluated in the devTOXqP assay.
| Sample | Product category | Coding | Source |
|---|---|---|---|
| Reference cigarettes | Cigarette | 3R4F 1R6F |
University of Kentucky University of Kentucky |
| iQOS with Amber HEETs | Heated tobacco product | HTP | Philip Morris International |
| iFuse with Kent Neopods 109 | Hybrid product | HYB | British American Tobacco |
| myblu with tobacco flavour, 0% nicotine1 | E-cigarette | EVP-0 | Fontem Ventures |
| myblu with tobacco flavour, 1.6% nicotine1 | E-cigarette | EVP-FB | Fontem Ventures |
| myblu with tobacco flavour, 1.6% nicotine salt1 | E-cigarette | EVP-NS | Fontem Ventures |
| All-trans-retinoic acid | – | ATRA | Sigma-Aldrich |
| Nicotine | – | – | Sigma-Aldrich |
All myblu e-liquids were custom made by Nerudia, a subsidiary of Fontem Ventures, for research purposes only.
Reference cigarettes, 3R4F (batch code V351X61B5) and 1R6F (batch code V062X53D1) and HTP (iQOS) Amber HEETs tobacco sticks were stored in an airtight container at 4 °C until use. The cigarettes and HEETs sticks were allowed to come to room temperature for 15 min before opening and conditioned for at least 48 h in a standard humidified chamber following the International Organization for Standardisation (ISO) 3402 Guideline (International Organization for Standardization, 1999).
HYB (iFuse) samples were obtained with Kent Neopods 109 Tobacco Flavour capsules. The e-cigarette product was the myblu™, a closed pod system device, which was used to generate aerosols from samples specified in section 2.3. The myblu device is a rechargeable e-cigarette, consisting of two segments: a rechargeable battery section (battery capacity, 350mAh) and a replaceable e-liquid containing pod (volume, 1.5 mL; coil resistance, 1.3 Ω). Experimental e-liquids, for research purpose only, were manufactured using materials from the usual supply chain by Nerudia (Liverpool, UK). Three different tobacco flavoured e-liquids were tested: tobacco flavoured 1.6% unprotonated (freebase) nicotine, tobacco flavoured 1.6% protonated nicotine (nicotine lactate) and tobacco flavoured 0% nicotine. All e-liquids were formulated to have a 45/55 propylene glycol/vegetable glycerine (PG/VG) ratio. PG/VG ratios were fixed to remove the variable of PG/VG formulation within this study. The test e-liquids were filled into pods prior to aerosolization with the myblu system. The e-cigarettes, HTP and HYB devices were all stored at room temperature and batteries fully charged before use.
2.3. Smoke and aerosol extract generation
The VITROCELL VC10 exposure system (VITROCELL systems GMBH, Waldkirch, Germany) was used to generate all smoke and aerosol extracts. The smoke from reference cigarettes 3R4F and 1R6F was generated using the ISO 20779:2018 intense smoking regime (bell-shaped puff profile, 55 mL puff volume, 2 s duration, 30 s interval with 100% vent blocking) (International Organization for Standardization, 2018). The HTP aerosol was generated using the ISO 20779:2018 intense smoking regime (bell-shaped puff profile, 55 mL puff volume, 2 s duration, 30 s interval) but ventilation blocking was not applied. myblu e-cigarette and HYB aerosols were generated using the ISO 20768:2018 vaping regime which specifies a square-wave puff profile, 55 mL puff volume, 3 s duration and a 30 s interval (International Organization for Standardization, 2018). The collection time was 30 min for all samples.
Smoke or aerosol extracts were prepared by bubbling the sample generated smoke or aerosol into 3 in-line impingers, each containing 10 mL PBS. The three 10 mL samples were then combined to make a total stock solution of 30 mL and quantified (Section 2.4) prior to being aliquoted, rapidly frozen, and stored at −70 °C. For the 3R4F reference cigarette a total of 54 puffs (9 puffs, 6 cigarette sticks) per 30 mL PBS was used, equating to 1.8 puffs per mL, based on previous studies evaluating cytotoxicity (Trelles-Sticken et al., 2019). For the 1R6F reference cigarette a total of 56 puffs (8 puffs, 7 cigarette sticks) per 30 mL PBS was used (due to a lower number of puffs per stick for 1R6F compared to 3R4F). For all non-combustible products, a total of 120 puffs (for HTP: 10 puffs, 12 HEETS sticks; for iFUSE 60 puffs, 2 devices; for EVP 60 puffs, 2 devices) per 30 mL PBS, equating to 4 puffs per mL of PBS were used.
Samples were stored frozen at −70 °C, until being shipped to Stemina on dry ice, where they were stored at −80 °C and defrosted on the day of treatment.
2.4. Characterisation of bubbled PBS
Trapped nicotine and carbonyls were quantified within the aerosol and smoke bPBS samples. All samples were stored frozen at −70 °C prior to analysis. Nicotine was quantified using liquid chromatography with tandem mass spectrometry (LC-MS/MS) with an AB Sciex API 6500 QTRAP (SCIEX, Framingham, MA, USA) using nicotine-d4 as the internal standard. After 1:200 methanol dilution, data was collected over a 4-minute solvent gradient with 0.05% formic acid in water and methanol, using a Gemini NXC18 Column (Phenomenex, CA, USA).
Carbonyl detection methodology in bPBS was adapted from ISO 21160:2018 methodology for carbonyl detection in whole smoke (International Organization for Standardization, 2018). bPBS samples were diluted 1:2 with 2 mL of a 12 mM 2,4-dinitrophenylhydrazine (DNPH) in acetonitrile (greater than 99.9% purity), 12% orthophosporic acid (greater than 85% purity) solution to derivatize the trapped carbonyl compounds. After 30 min, 6 mL of Trisma® solution (16.5 mM) was added to quench the derivatization reaction and avoid the formation of poly-derivatized carbonyl adducts. A RP C18 column (Phenomenex, CA, USA) was used for chromatographic separation of the carbonyl-DNPH derivates, which were quantified using high-performance liquid chromatography with a diode-array detector (HPLC-DAD, Agilent Technologies 1100 Series, Agilent Technologies, Santa Clara, CA, USA). Eight working standard solutions of formaldehyde-DNPH (98% purity) covering 0.045–0.075 μg/mL were used.
2.5. Human iPS cell culture and devTOXqP assay
ATCC-HYR0103 human iPS cells (ATCC®ACS-1007™, Manassas, VA, USA) were maintained in mTeSR1 media (StemCell Technologies, Vancouver, BC, Canada) as previously described (Palmer et al., 2017). The test articles were tested in three separate batches between 2018 and 2020. All samples for the studies described in this paper were analysed within 3–4 months of production. For all experimental runs both positive and negative controls were used to ensure correct functioning of the assays. Assay endpoint (o/c ratio, cell viability, ornithine, and cystine) reproducibility has been evaluated using the positive control, negative control, and vehicle treatments from over 50 independently cultured 96-well plates analyzed over a 3-year period at Stemina to generate historical data. Historically, the average intraplate coefficient of variation (CV) across endpoints is 4.1% and the average interplate CV across endpoints is 9.9% for the assay (unpublished historical values from Stemina). The reproducibility of the predictive model has been evaluated using 34 independent replicates of two chemical treatments (carbamazepine and methotrexate) conducted by multiple technicians with various iPS cell lines, freeze lots and reagents over the course of 5 years. For both test chemicals, all interpolated dTP values were within 3-fold of the mean (data not shown).
Human iPS cells were exposed to 16 concentrations of nicotine ranging from 0.01 to 1000 µg/mL in the mTeSR1 culture medium. For smoke and aerosol bPBS testing, cells were exposed to eight concentrations of each test article, ranging from 0.003 to 10% with half-log dilutions (3R4F, HTP and HYB bPBS) or 0.046–10% with third-log dilutions (1R6F, EVP-FB, EVP-NS, and EVP-0 bPBS).
Additionally, 1R6F bPBS was tested in the presence of a positive compound, ATRA (0.03–30 nM). 0.22% 1R6F bPBS was defined as the highest non-responsive dose of 1R6F in the devTOXqP assay and therefore selected for the assay sensitivity study. ATRA was also tested alone at eight concentrations (0.01–30 nM).
A 10% stock solution was prepared for each test article in mTeSR1 medium containing 0.1% DMSO. For the spiking experiment, a stock solution of ATRA was prepared in 100% DMSO at 30 µM. This stock was diluted (1:1000) in either mTeSR1 or mTeSR1 containing 0.22% 1R6F bPBS. Subsequent dilutions were performed with the same media used to prepare the media containing the top exposure level (mTeSR1 + 0.1% DMSO or mTeSR1 + 0.1% DMSO + 0.22% 1R6F bPBS). The final concentration of DMSO used was 0.1% in all treatments.
All experimental treatments were carried out in a 96-well plate format. Each test plate included cells exposed to 1 µM methotrexate (Selleck Chemicals, Houston, Texas) as the positive control (n = 3 wells), 0.005 µM methotrexate as the negative control (n = 3 wells), 0.1% DMSO as the reference (vehicle) control (n = 3 wells), eight concentrations of two test articles (n = 3 wells per concentration), and media controls for each treatment (lacking cells, ± test article). Cells were plated as a single-cell suspension and maintained in an undifferentiated state during test article exposure as described previously (Palmer et al., 2017, Palmer et al., 2013). Briefly, exposure began approximately 24 h after plating and iPS cells were exposed to the extracts or chemicals for 48 h. The media ± extract/chemicals was replaced every 24 h. The spent media from the last 24-hour treatment period was collected for analysis and added to acetonitrile (final acetonitrile concentration 40%, Fisher Chemical, Fair Lawn, NJ, USA). Cell viability was assessed after sample collection using the CellTiter-Fluor Cell Viability Assay (Promega, Madison, WI, USA). A single biological replicate (or repeat) was performed for each test article.
2.6. Sample preparation
High molecular weight constituents (greater than 3KDa) of the spent media samples were removed using a Pall AcroPrep™ Advance Omega 3 K MWCO filter plate (Pall Corporation, Port Washington, NY, USA). The filtrate was collected and concentrated overnight in a Savant High Capacity Speedvac Plus Concentrator (Savant Instruments, Inc., Holbrook, NY, USA). The concentrated sample was resolubilized in a 1:1 mixture of 0.1% formic acid in ultra-pure water (Fisher Chemical) to 0.1% formic acid in acetonitrile (Fisher Chemical) containing L-ornithine-13C5 hydrochloride and L-cystine-13C6, 15N2 (Cambridge Isotope Laboratories, Tewksbury, MA, USA) as internal standards (ISTDs).
2.7. Mass spectrometry
Ultra-performance liquid chromatography-high resolution mass spectrometry (UPLC-HRMS) data was acquired as described in Palmer et al. (Palmer et al., 2017, Palmer et al., 2013). Briefly, data was obtained using two separate UPLC-HRMS systems, consisting of an Agilent 1290 Infinity LC system (Agilent Technologies) interfaced with either a Quadrupole Time-of-Flight (Q-TOF) mass spectrometer or a triple-quadrupole (QqQ) system (Agilent Technologies) with a Waters Acquity UPLC BEH Amide column (Waters, Milford, MA, USA) for chromatographic separation of metabolites. Data was collected over a 6.5-minute solvent gradient with 0.1% formic acid in water and 0.1% formic acid in acetonitrile.
2.8. Quality control
Two quality control procedures were included to ensure correct plate readout. Firstly, the vehicle control (0.1% DMSO) sample CV for the viability relative fluorescent units (RFU) could not exceed 10%. Second, the positive and negative control treatments had to be correctly predicted to ensure that the iPS cell metabolism was within the assay specifications. Quality control data is provided in Appendix 1.
2.9. Data analytics
The extracted ion chromatogram areas for ornithine, cystine, and the ISTDs were determined using the Agilent MassHunter Quantitative Analysis software, version B.07.00 (Agilent Technologies). The areas of endogenous ornithine and cystine in each sample were normalized to the spiked-in ISTDs by dividing the endogenous metabolite area by the corresponding isotopically labelled ISTD area. Relative fold changes were then calculated for each ISTD-normalized metabolite in each sample by dividing by the median response of the vehicle treatment samples, producing a vehicle-normalized value for both metabolites for each sample. The data for ATRA + 0.22% 1R6F bPBS was further normalized to the median 0.22% 1R6F control sample. The o/c ratio was calculated for each sample by dividing the vehicle-normalized ornithine value by the vehicle-normalized cystine value.
Dose-response analyses for the o/c ratio, cell viability, ornithine response, and cystine response were performed with GraphPad Prism (version 7.3 or newer, GraphPad Software, San Diego, CA, USA). Each data set was fit with a four-parameter log-logistic or multiphasic non-linear dose–response model. The best fitting model was selected using Akaike’s information criterion. The developmental toxicity potential (dTP, o/c ratio) and toxicity potential (TP, cell viability) concentrations were predicted from the respective dose–response curves using the iPS cell developmental toxicity threshold (dTT, 0.85).
An extra sum-of-squares F test was used to determine if the dose–response curve of each endpoint (viability, ornithine, cystine, and o/c ratio) for the cigarette reference samples (3R4F and 1R6F bPBS) were significantly different from each other, under the null hypothesis that one curve adequately fits all data sets (i.e., the data sets have the same top, bottom, IC50, and hill slope best-fit values), and the alternative hypothesis that there is a different dose–response curve for each data set (i.e., the data sets have different top, bottom, IC50, and Hillslope best-fit values). If it was determined that the data sets have independent dose–response curves, an unpaired t test was conducted for each endpoint (viability, o/c ratio, ornithine, and cystine) at each closest or overlapping concentration to determine the concentration where the curves differed from one another. The resulting p values were adjusted for multiple comparisons (q value) using Benjamini and Hochberg's method to control the false discovery rate (FDR). These analyses were also used to determine if the dose–response curves for the reference cigarette samples (3R4F and 1R6F bPBS) were significantly different from the other extracts (HTP, HYB, EVP-0, EVP-FB, and EVP-NS bPBS) and if the response following HTP bPBS exposure was significantly different from HYB, EVP-0, EVP-FB, and EVP-NS bPBS. Additionally, the dose–response curves for ATRA alone and ATRA + 0.22% 1R6F bPBS were compared using these analyses. All values in figures are given as mean ± standard error. If the standard error is not shown, the error bars are smaller than the size of the symbol. The significance threshold was set at p < 0.05 for all statistical tests.
3. Results
3.1. Characterisation of bubbled PBS for reference cigarettes and non-combustible products
Nicotine and eight carbonyls were quantified in the PBS matrix following the capture of the 3R4F and 1R6F smoke and non-combustible product aerosols. Quantification results are displayed as µg/mL of bPBS in Table 2. 3R4F and 1R6F bPBS samples contained the highest level of carbonyls. Total quantified carbonyls were greatly reduced in non-combustible product samples. Low levels were detected for HTP for all eight quantified carbonyls, whereas the majority of these were below LOQ for HYB and e-cigarette samples. Formaldehyde levels in the EVP-NS bPBS samples were elevated compared to the seven other carbonyls but did not exceed those measured in the 1R6F bPBS.
Table 2.
Nicotine and eight selected carbonyl content (µg/mL) of bPBS test articles used in the devTOXqP assay. The number of puffs per mL of PBS for each test article was: 54 for 3R4F, 56 for 1R6F and 120 for HTP, HYB, EVP-FB, EVP-NS and EVP-0.
| Concentration (µg/mL) | 1R6F | 3R4F | HTP | HYB | EVP-FB | EVP-NS | EVP-0 | LOQ (µg/mL) |
|---|---|---|---|---|---|---|---|---|
| Nicotine | 127.1 | 82.5 | 123.0 | 53.0 | 165.1 | 187.0 | 0.5 | 0.01 |
| Formaldehyde | 12.6 | 5.9 | 0.9 | 1.0 | 5.0 | 9.0 | 4.4 | 0.25 |
| Acetaldehyde | 190.3 | 157.1 | 52.9 | <LOQ | <LOQ | 3.3 | <LOQ | 1.5 |
| Acetone | 55.8 | 24.0 | 5.4 | <LOQ | <LOQ | <LOQ | <LOQ | 1.0 |
| Acrolein | 4.0 | 9.4 | 1.3 | 0.5 | <LOQ | <LOQ | <LOQ | 0.5 |
| Propionaldehyde | 10.5 | 9.5 | 3.5 | <LOQ | <LOQ | <LOQ | <LOQ | 0.5 |
| Crotonaldehyde | 8.3 | 6.2 | 0.6 | <LOQ | <LOQ | <LOQ | <LOQ | 0.5 |
| 2-Butanone | 11.9 | 6.3 | 1.3 | <LOQ | <LOQ | <LOQ | <LOQ | 0.5 |
| n-Butyraldehyde | 4.0 | 3.6 | 2.8 | <LOQ | <LOQ | <LOQ | <LOQ | 0.5 |
HTP, EVP-FB and EVP-NS aerosols delivered higher levels of nicotine to PBS compared to the cigarette smoke, whereas slightly lower nicotine levels were delivered by HYB. Low levels of nicotine (0.5 µg/mL) were detected in the EVP-0 bPBS which was not recorded in the neat e-liquid sample, as per the Certificate of Analysis (determined using gas chromatography).
A preliminary, one-year stability study was performed (data not shown) for the 3R4F, HTP, HYB and EVP-FB bPBS samples. Slight reductions were observed in the nicotine content for the EVP-FB bPBS sample after one year, the reason for this was not known as none of the other samples were affected. There was a loss of highly reactive acrolein, in the 3R4F bPBS and in the HTP bPBS samples after a year of storage. The results indicated general stability of nicotine and most carbonyls during storage at −70 °C for up to a year.
3.2. Nicotine devTOXqP results
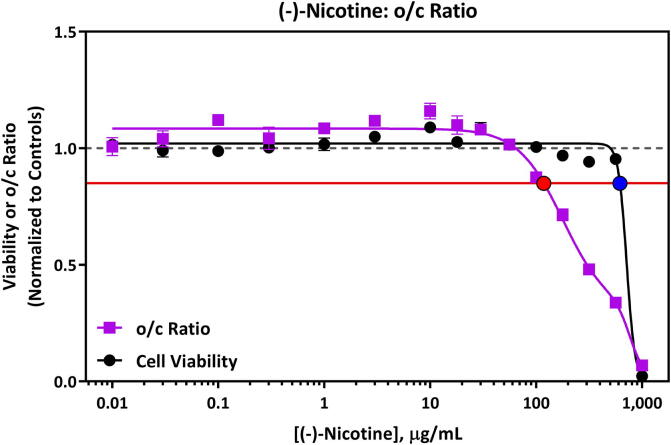
Nicotine caused a metabolic perturbation indicative of the potential for developmental toxicity at concentrations lower than those that affected cell viability (Fig. 1). The predicted dTP (o/c ratio) for nicotine was 118 µg/mL and the cell toxicity potential (TP, iPS cell viability) was 618 µg/mL. The o/c ratio and viability plot for nicotine is shown in Fig. 1. An increase in cystine response was observed at concentrations of approximately 100 µg/mL nicotine and higher (Fig. A3.4), whereas a decrease in ornithine release was observed at the two highest concentrations tested only (562 and 1000 µg/mL, Fig. A3.4). Nicotine was soluble in mTeSR1 media up to 1000 µg/mL. Information related to individual metabolite response curves for nicotine are detailed in Appendix 3 (Fig. A3.4) and replicate values for each endpoint are in Appendix 4 (Table A4.10 and A4.11).
Fig. 1.
devTOXqP Assay Results for (-)-nicotine evaluated at 0.01–1,000 µg/mL. Nicotine was predicted to have the potential to cause developmental toxicity at 118 µg/mL or above via a non-cytotoxic mechanism. The horizontal red line represents the developmental toxicity threshold (0.85), the dashed horizontal grey line represents 1.0, and the red and blue filled circles indicate the dTP (118 µg/mL) and TP (618 µg/mL) concentrations, respectively. The x-axis is the concentration (µg/mL) of the test article. The y-axis is the vehicle-treatment normalized (fold change) values for the o/c ratio (purple line) and viability (black line). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Test article devTOXqP results
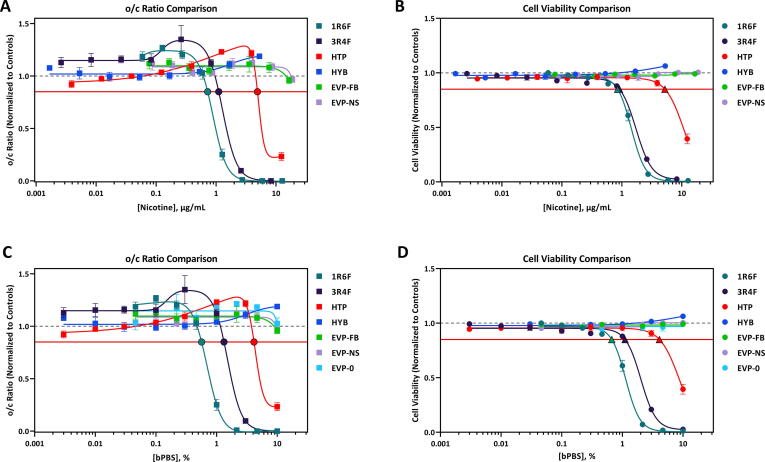
The calculated dTP (o/c ratio) and toxicity potential (TP, cell viability) concentrations for the seven test articles evaluated in this study are summarized in Table 3. Comparisons of test article response for the o/c ratio and cell viability are shown in Fig. 2. Changes in o/c ratio and cell viability for individual test articles, displayed as % bPBS and µg/mL nicotine in bPBS can be found in Appendix 2. A dose-dependent decrease in the o/c ratio and cell viability was observed following iPS cell exposure to 1R6F, 3R4F, and HTP bPBS (Table 3, Fig. 2). In contrast, exposure to HYB, EVP-FB, EVP-NS, and EVP-0 bPBS did not decrease the o/c ratio or cell viability (Table 3, Fig. 2). 1R6F bPBS was the most potent sample tested, causing a decrease in the o/c ratio and cell viability at lower concentrations than 3R4F and HTP bPBS (Fig. 2). For both 1R6F and 3R4F bPBS, similar trends in the o/c ratio were observed, with a large decrease in the release of ornithine into the cell media at approximately ≥ 1% (Appendix 3), with a steeper rate of decline seen for 1R6F bPBS when compared to 3R4F bPBS. Additionally, both cigarettes decreased the uptake of cystine from the media at ≥ 1% bPBS concentration (approximately 3-fold for 3R4F and 6-fold for the 1R6F bPBS, Appendix 3). For the HTP product, there was a decrease in ornithine at ≥ 3% bPBS and a large increase in cystine response at 10% bPBS concentration (approximately 1.5-fold increase) (Appendix 3).
Table 3.
Summary of developmental toxicity potential of smoke or aerosol trapped PBS from cigarettes, non-combustible tobacco products and e-liquid formulations tested between 0.003 and 10% bPBS added to the cell media.
| Sample ID | Concentration Range Tested (% bPBS) | o/c Ratio dTP1 (% bPBS) | Cell Viability TP (% bPBS) | Exposure Range (µg/mL Nicotine) | o/c Ratio dTP1 (µg/mL nicotine) | Cell Viability TP (µg/mL nicotine) |
|---|---|---|---|---|---|---|
| 1R6F | 0.046–10 | 0.6 | 0.7 | 0.06–12.71 | 0.8 | 0.9 |
| 3R4F | 0.003–10 | 1.3 | 1.1 | 0.003–8.25 | 1.1 | 0.9 |
| HTP | 0.003–10 | 4.3 | 4.1 | 0.004–12.3 | 5.3 | 5.0 |
| HYB | 0.003–10 | ND | ND | 0.0017–5.3 | ND | ND |
| EVP-FB | 0.046–10 | ND | ND | 0.08–16.51 | ND | ND |
| EVP-NS | 0.046–10 | ND | ND | 0.09–18.7 | ND | ND |
| EVP-0 | 0.046–10 | ND | ND | N/A | N/A | N/A |
dTP: Developmental Toxicity Potential. TP: Toxicity Potential. N/A: Not appropriate. ND: No effect was detected within the exposure range tested.
1Concentration in % bPBS and µg/mL nicotine where the developmental toxicity threshold of 0.85 is crossed.
Fig. 2.
Comparison of o/c Ratio Response Comparison (A, C) and Cell Viability Response Comparison (B, D) Following Exposure to 1R6F bPBS, 3R4F bPBS, HTP bPBS, HYB bPBS, and EVPs bPBS, displayed either as % bPBS or µg/mL nicotine. 1R6F bPBS was the most potent sample tested, causing a decrease in the o/c ratio and cell viability at lower concentrations than 3R4F and HTP bPBS. Similar response profiles were observed for HYB, EVP-FB, EVP-NS and EVP-0 bPBS The horizontal red line represents the developmental toxicity threshold (0.85) and the dashed horizonal grey line represents 1.0. The black outlined filled circles (A, C) indicate the predicted dTP and the black outlined filled triangles (B, D) indicate the predicted TP concentrations. The x-axis is the (A, B) concentration of nicotine in bPBS (µg/mL) or (C, D) concentration (%) of bPBS test article in culture media. The y-axis is the vehicle-treatment normalized (fold change) values for o/c ratio or cell viability. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
All product test articles were soluble in mTeSR1 media up to the maximum concentration (10%). Information related to individual metabolite response curves for each of the test articles and replicate values for each endpoint are detailed in Appendices 3–4.
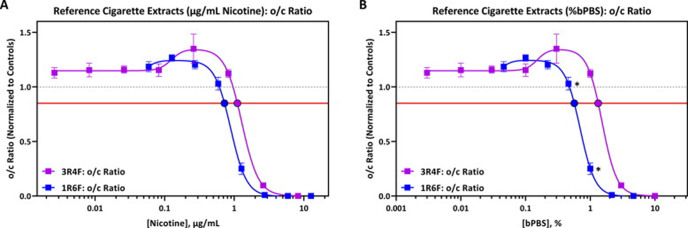
As two separate cigarette reference products were used in this study, the dTP and dose–response curves for 1R6F and 3R4F bPBS were compared to evaluate whether the dose-responses were deemed significantly different from one another. The dTP responses for 1R6F and 3R4F bPBS were 0.6% (0.8 µg/mL nicotine) and 1.3% (1.1 µg/mL nicotine) respectively, with approximately a 2-fold difference in dTP between the two products when compared on % bPBS concentration. A smaller difference was observed when results for 1R6F and 3R4F bPBS were normalised to nicotine concentration (Fig. 3). The difference in o/c ratio and associated dTP response, however, was not considered to be of biological significance due to these measurements falling within the historical range of inter-experimental variance.
Fig. 3.
o/c Ratio Response Comparison Following Exposure to 1R6F (blue line) and 3R4F (purple line), assessed by (A) µg/mL nicotine or (B) % bPBS. The difference in dose response curves for 1R6F and 3R4F bPBS were not considerd to be of biolgoical significance. The horizontal red line represents the developmental toxicity threshold (0.85), the dashed horizontal grey line represents 1.0 and the filled circles indicate the predicted dTP concentrations. The x-axis is the (A) concentration of nicotine in bPBS (µg/mL) or (B) concentration (%) of bPBS test article in culture media. The y-axis is the vehicle-treatment normalized (fold change) values for the o/c ratio. Asterisks (*) indicate a significant difference between indicated concentration for 1R6F and nearest 3R4F concentration for % bPBS only, (False Discovery Rate [FDR]-adjusted p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
When comparing dose–response curves of 1R6F and 3R4F bPBS using the extra sum of F test, the dose–response curves for o/c ratio were deemed different due to a significant difference in all measured curve parameters [top, bottom, EC50, and Hillslope best-fit values; (p < 0.001)] (Appendix 5). For the o/c ratio response, the response for 1R6F bPBS was significantly different from the 3R4F bPBS response at 0.46% (compared to 0.3% 3R4F bPBS) and 1% (FDR-adjusted p < 0.05, Fig. 3). Significant differences (p < 0.0001) in dose–response curves was also observed for cell viability, ornithine and cystine, detailed in Appendix 5.
Dose-response curves were compared for test products that elicited developmental toxicity potential within the devTOXqP assay (1R6F, 3R4F and HTP bPBS) and non-responsive test articles (HYB, EVP and EVP-0, EVP-FB, and EVP-NS bPBS). The dose–response curves for HYB, EVP-0, EVP-FB, and EVP-NS bPBS were all statistically significantly different from 1R6F, 3R4F and HTP bPBS (extra-sum-of-square F test, p < 0.0001). The point of difference (POD), the concentration where a consistent significant difference (FDR-adjusted p < 0.05) was observed between the non-combustible products and reference cigarette dose response curves, was assessed between reference cigarettes and other test articles. The POD evaluation for % bPBS was completed by comparing equivalent % bPBS concentrations in each sample. When expressed relative to nicotine concentration (Fig. 3A), since the nicotine concentrations at each dose differed for each test article, it was not possible to statistically compare the dose response curves. POD values for 1R6F and 3R4F bPBS for the o/c ratio and viability are shown in Table 4. PODs for ornithine and cystine are shown in Appendix 6. When compared to 1R6F bPBS, the o/c ratio POD for each of the test articles was 1% whereas this was higher (between 2.2% and 3%) for 3R4F bPBS (Table 4, Fig. 2). This pattern was not reflected in the viability POD, when compared to 1R6F bPBS, the POD values ranged between 0.5% and 1%, whereas values for 3R4F bPBS ranged between 0.1 and 3%. Both EVP-NS and EVP-FB bPBS had the same POD profiles (Table 4, Table 2). When compared to 1R6F bPBS, ornithine POD values ranged between 0.046% and 0.46%, whereas the ornithine POD for 3R4F bPBS was 1% for all test articles (Table A6.1, Fig. A6.1). In contrast, cystine POD for 1R6F was 1% for all test articles and between 0.3 and 3% for 3R4F (Table A6.2, Fig. A6.2).
Table 4.
Summary of the point of difference (POD) values for o/c ratio and cell viability response, at concentrations where the test articles were statistically significantly different from reference cigarettes 1R6F bPBS and 3R4F bPBS (p < 0.05).
| o/c Ratio POD |
Viability POD |
|||
|---|---|---|---|---|
| Extract | 1R6F [% bPBS] | 3R4F [% bPBS] | 1R6F [% bPBS] | 3R4F [% bPBS] |
| HTP bPBS | 1% | 3% | 1% | 3% |
| HYB bPBS | 1% | 3% | 0.5% | 0.3% |
| EVP-FB bPBS | 1% | 2.2% | 0.5% | 0.1% |
| EVP-NS bPBS | 1% | 2.2% | 0.5% | 0.1% |
| EVP-0 bPBS | 1% | 2.2% | 1% | 1% |
3.4. ATRA spiking experiment results (a known developmental toxicant)
Changes in human iPS cell metabolism were measured following exposure to ATRA alone and in the presence of 0.22% 1R6F bPBS (equivalent to 0.03 µg/mL nicotine). ATRA was selected as a positive developmental toxicant control as it is a known human developmental toxicant that impacts ornithine and cystine metabolism at very low concentrations in the devTOXqP assay and via a non-cytotoxic mechanism (Palmer et al., 2017). The value of 0.22% 1R6F bPBS was selected as it was the highest non-responsive concentration of 1R6F bPBS (Appendix 2, Fig. A2.2). ATRA treatment decreased the o/c ratio at very low concentrations, and the dose–response curve crossed the developmental toxicity threshold at 0.16 nM (Fig. 4). The addition of 0.22% 1R6F bPBS did not cause a shift in the o/c ratio dose–response curve compared to ATRA alone (extra sum-of-squares F test, p = 0.1990) and yielded a dTP concentration of 0.13 nM (Fig. 4). Neither ATRA alone, nor in combination with 0.22% 1R6F, impacted cell viability at the concentrations tested (Fig. 4) and the dose–response curves for cell viability were not significantly different (extra sum-of-squares F test, p = 0.7271). A slight increase in ornithine was observed following exposure to ATRA (Fig. A3.3), which is consistent with previous results for this compound in the assay (Palmer et al., 2017). Addition of 0.22% 1R6F bPBS did not impact the ornithine response (extra-sum-of-squares F test p = 0.7659). Significant differences were observed in cystine dose–response curves between the two treatment groups (p = 0.0262), with significant differences at the ‘Top’ curve parameter only. Information related to individual metabolite response curves for each test article and replicate values for each endpoint are presented in Appendices 3–4.
Fig. 4.
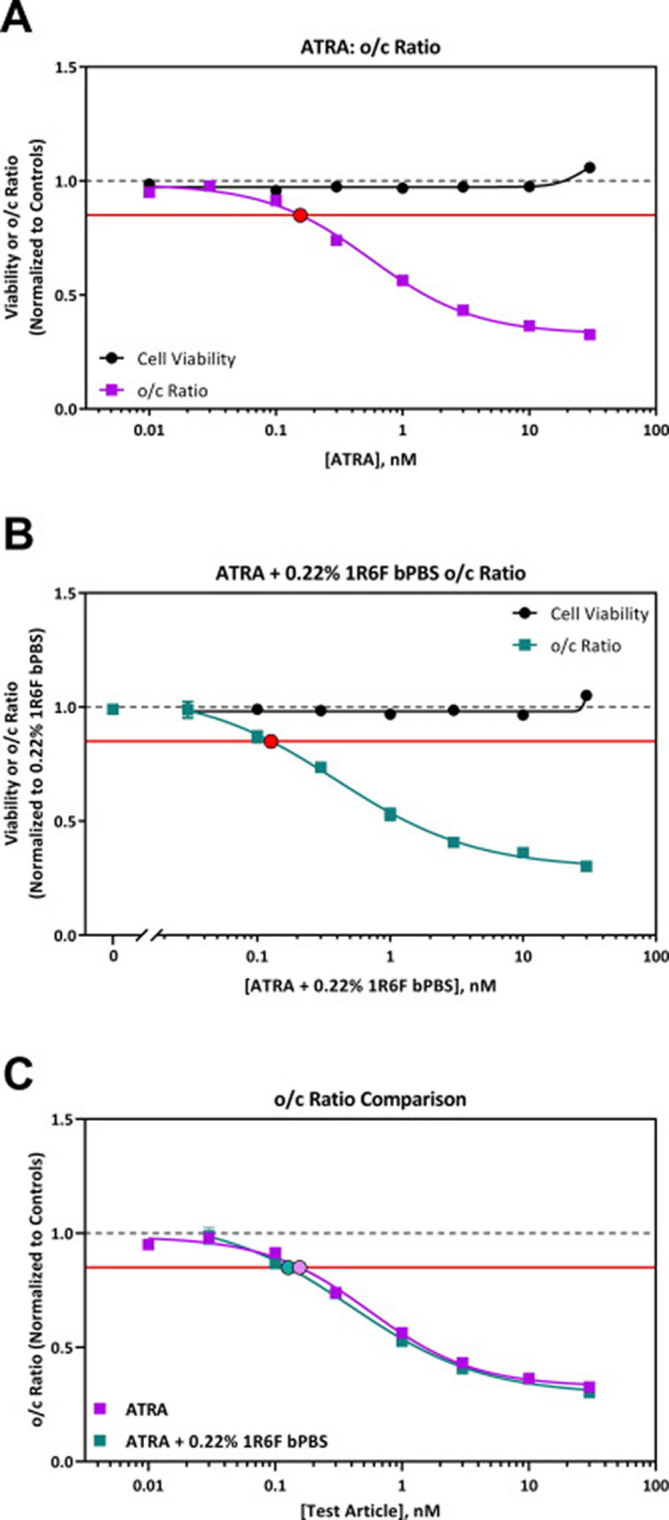
devTOXqP Assay Results for A) ATRA, B) ATRA + 0.22% 1R6F bPBS. C) Combined o/c ratio graph for ATRA and ATRA + 0.22% 1R6F bPBS. The addition of 0.22% 1R6F bPBS to the positive compound ATRA did not cause a shift in the o/c ratio dose–response curve compared to ATRA alone. In all panels, the horizontal red line represents the developmental toxicity threshold (0.85), the dashed horizontal grey line represents 1.0, and the filled circles indicate the predicted dTP concentrations. The x-axis is the concentration of test articles (nM). The y-axis is the vehicle-treatment normalized (fold change) values for the o/c ratio and viability. ATRA + 0.22% 1R6F bPBS values are normalized to 0.22% 1R6F bPBS control data. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
This is the first study reporting the use of the devTOXqP assay as part of the assessment of non-combustible nicotine products to screen for developmental toxicity potential. This study compared dose-responses over eight concentrations and allowed the relative toxicity of tobacco products to be compared. The use of in vitro assays using human derived cells is one of the aims of ‘Toxicity Testing in the 21st Century’ (TT21C) (National Research Council, 2007, Krewski et al., 2010), being more high throughput, cost effective, arguably more relevant to humans, by using human cells, and is in alignment with the 3Rs principles (Replacement, Reduction and Refinement) leading to the reduced use or ultimate replacement of animals (Hartung, 2017).
Currently, rat and rabbit models are the most widely used model to investigate a test article’s developmental and reproductive toxicity potential, although there is a shift to supplementing this data with in vitro models (Daston, 2011). Using animal models to evaluate developmental toxicity has significant limitations, including the lack of full mechanistic understanding of the toxicity endpoints measured and have only approximately 70–80% concordance to known human developmental toxicants (Daston, 2011, Daston and Knudsen, 2010). Given these issues and the fact that testing on animals for tobacco products is banned in several EU member states (Simms et al., 2019), our goal was to evaluate the devTOXqP assay as an alternative method for determining the developmental toxicity potential of non-combustible nicotine products and combusted cigarettes. This study evaluated the assay’s applicability/sensitivity for enabling the comparison of non-combustible product assessment when compared directly to combusted cigarettes, and to attempt to rank products based on potential harm following the TT21C and 3Rs principles. Whilst we acknowledge that this assay clearly does not cover all phases of embryonic and foetal development, and no single in vitro assay ever can, we believe it does cover a very important critical phase of early embryonic development.
4.1. Quantification of trapped carbonyls and nicotine in bubbled PBS
In this study, PBS-trapped smoke and aerosols were quantified for nicotine and eight carbonyls of public health interest. There were clear differences between the quantified constituents of the individual non-combustible product aerosols and cigarette smoke bPBS (Table 2). As expected, total carbonyl levels were the highest in both the cigarette bPBS, with lesser amounts captured for HTP bPBS. Limited carbonyls were detected in HYB and e-cigarette bPBS. This is reflective of the whole smoke and aerosol emissions profile typically reported for cigarettes and non-combustible products (Rudd et al., 2020, Poynton et al., 2017, Farsalinos et al., 2018, Jaccard et al., 2019, Schaller et al., 2016). For one e-cigarette sample (EVP-NS bPBS) the formaldehyde level was elevated in comparison to EVP-FB and EVP-0 bPBS, although this was still up to 3-fold less than combustible cigarettes on a per-puff basis. Specific carbonyls, including formaldehyde, have been shown to be produced from the thermal decomposition of glycerol and propylene glycol, which are common e-liquid components (Belushkin et al., 2020, Sleiman et al., 2016). Formaldehyde generation has been shown to increase with device coil temperature and is typically lower in closed systems (including pod-systems) e-cigarettes when compared to open systems (Belushkin et al., 2020). This study used the same myblu pod-system to produce all the e-cigarette test articles (based on the same PG/VG ratio), therefore it is anticipated the possible occurrence of insufficient wicking and resulting dry puffs may have possibly caused the increased formaldehyde levels measured (Farsalinos et al., 2015), however, it is worth highlighting that occurrence of dry puffs creates a distinctive, unpleasant taste for the consumer which adult smokers learn to avoid (Farsalinos et al., 2015).
Due to multiple studies being presented within this paper, both the 1R6F and 3R4F reference cigarette data have been included. The 3R4F Kentucky reference cigarettes have been widely used as monitor or comparator cigarettes for mainstream smoke analysis and used to generate in vitro and in vivo toxicological data of cigarettes to compare with novel tobacco products (Jaccard et al., 2019). The most recent version of the Kentucky Reference cigarette (Kentucky Tobacco Research and Development Centre, University of Kentucky), is the 1R6F reference cigarette having been manufactured to replace the depleted stocks of the 3R4F reference cigarette. Jaccard et al., compared the aerosol chemistry and in vitro toxicity of both reference cigarettes in the same laboratory during the same period of time to enable a direct comparison (Jaccard et al., 2019). That study concluded that the 1R6F reference cigarette was a suitable replacement for the 3R4F reference cigarette as a comparator/monitor cigarette due to only minor changes being detected in smoke analytes (including carbonyls) under multiple smoking regimes due to tobacco batch differences (Jaccard et al., 2019). In contrast, we found a significant difference in the trapping of nicotine and carbonyls between the 3R4F and 1R6F in PBS in our study, even when similar puff numbers were used (54 puffs for 1R6F and 56 for 3R4F). On a per-puff basis, 1R6F delivered 2.35 µg nicotine into the PBS whereas 3R4F delivered 1.47 µg showing differences between the two references cigarettes. This potentially indicates differences in manufacture and construction of the two products (Jaccard et al., 2019).
Very low levels of nicotine were detected in the EVP-0 bPBS sample (0.5 µg/mLl PBS). All neat experimental e-liquids were quantified for nicotine prior to the study according to internal quality control procedures at Nerudia and recorded on the Certificate of Analysis (data not shown). Nicotine was below LOQ in the EVP-0 e-liquid. Therefore the detection of nicotine in EVP-0 bPBS may be due to residual nicotine within laboratory equipment and the high detection sensitivity of the analytical equipment used to quantify the PBS extract (LC MS/MS), rather than the e-liquid itself containing nicotine.
4.2. Potential developmental toxicity screening for reference cigarettes and non-combustible nicotine products
In this study, three test articles (1R6F, 3R4F and HTP bPBS) tested in the devTOXqP assay elicited a metabolic response indicative of the potential for developmental toxicity at concentrations very similar to that impacting cell viability. Therefore, it is anticipated that these changes in o/c ratio were driven by cytotoxic mechanisms. Both ornithine and cystine have been associated with common mechanisms of developmental toxicity. Cystine is a cell media component that predominates over cysteine extracellularly due to the oxidative state of the medium. The transport of cystine into the cell, where it is rapidly converted to cysteine, is essential for numerous cellular processes, including glutathione production, providing cellular protection against oxidative stress and being used in protein synthesis (Shih et al., 2006). The cystine/cysteine redox cycle plays a critical role in the regulation of reactive oxygen species (ROS) generated in cells in the healthy state (Hansen, 2006, Conrad and Sato, 2012, Banjac et al., 2008).
Ornithine is important in nitrogen cycling, being produced as part of the urea cycle, and is a critical precursor to polyamine synthesis, which are required for cell proliferation, cell growth and differentiation (Hussain et al., 2017). the conversion of ornithine to putrescine by ornithine decarboxylase (ODC) is key for the normal development of murine embryos, with inhibition of ODC being linked to compromised cell growth, cellular transformation and arrest of cells in the G1 phase of the cell cycle (Pendeville et al., 2001). Ornithine is also linked to production of putrescine and reductions in the intracellular levels of the polyamines putrescine, spermidine, and spermine, appear to be essential for fundamental processes such as stabilisation of chromatin and cytoskeletal structure, translation, transcription, semiconservative DNA replication, and the protection of cells from DNA damage (Pendeville et al., 2001).
Chronic reductions in polyamine levels as reported in the scientific literature can lead to apoptosis, especially following exposure to oxidative stress (Seiler and Raul, 2005, Rhee et al., 2007). Paradoxically, ODC overexpression, which upregulates putrescine levels, can also trigger apoptosis (Seiler and Raul, 2005). Overall, these findings strongly support the concept that homeostasis of polyamine pools is a critical determinant of cell fate and an increased generation of glutathione (GSH) from cystine is often seen as a response to oxidative stress or other cellular stress caused by heat, acidification and/or ultraviolet light exposure (Dypbukt et al., 1994).
Dose-response curves from 1R6F and 3R4F reference cigarettes within the devTOXqP were shown to be statistically significantly different from each other when compared on percent bPBS. It is possible that the difference in the dose–response curves, and the resulting dTP and TP concentrations within the assay are driven by the possible differences in PBS trapping efficiency between the products. Although the dTP values differ slightly between reference cigarettes, this would not be deemed biologically significant due to the low (approximately 2-fold) difference in biological response. If the harmful trapped constituents of the 1R6F, 3R4F, and HTP bPBS achieve concentrations in vivo at or above those present in the dilution at the dTP in the devTOXqP assay they are predicted in the current assay to have the potential for developmental toxicity and/or embryo lethality.
It should be noted that this assay focuses specifically on early developmental toxicity (i.e., first trimester) and other stages of reproductive toxicity are not represented within this assay. The screening results from both 1R6F and 3R4F potentially support the published epidemiological data that cigarette smoking whilst pregnant can cause adverse developmental toxicity to the early stage embryo (Greene and Pisano, 2019). Due to the limited time on the market, there is insufficient evidence to define whether HTPs cause developmental toxicity using population and epidemiological data alone. Within our study, the HTP was classified as having developmental toxicity potential, but at bPBS concentrations approximately 4-fold (or more) higher than combustible cigarettes. This is aligned with the United Kingdom Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT), who have stated that ‘though the aim should be for pregnant women to stop smoking entirely, the risk to the unborn baby is likely to be reduced if using these [heated tobacco] products during pregnancy instead of smoking’ (UK COT, 2017).
Four test articles (HYB bPBS and the three e-cigarette extracts [EVP-0, EVP-FB, EVP-NS bPBS]) did not alter human iPS cell metabolism or cell viability at any of the concentrations evaluated under the conditions of the test. For these mixtures the o/c ratio and cytotoxicity were effectively the same, being unchanged at all concentrations and as expected did not alter when expressed as either percentage bPBS or nicotine concentration. Based on this data, both the HYB and all e-cigarette extracts tested were predicted in this screen to have little to no potential for developmental toxicity in vivo at the exposures tested up to 10% bPBS (the upper limit of PBS that can be added to the assay). Importantly, this should not be interpreted that these products are risk-free as these results are applicable only to the specific area covered by this assay (early blastocyst stage) and clearly cannot cover developmental toxicity as a whole.
When compared to non-combustible products both reference cigarettes elicited a positive response at a much lower concentration for both o/c ratio and cell viability. Typically, PODs were lower for 1R6F bPBS when compared to 3R4F bPBS, which is also reflective of the increase in quantified constituents captured in PBS for 1R6F over 3R4F. These changes also reflected differences in ROS measured in both IQOS and cigarettes (Salman et al., 2019) with the increased ROS measured linked with the decreased cystine seen for these products (Dypbukt et al., 1994). These results support the risk continuum positioning of non-combustible nicotine products relative to combustible cigarettes. The screening developmental toxicity potential of test articles, based on o/c ratio in the devTOXqP assay, can be ranked as: combustible cigarettes > HTP > HYB = e-cigarettes.
4.3. Nicotine effects seen at higher than physiologically relevant levels
Tobacco-containing and tobacco free non-combustible nicotine products typically aim to deliver nicotine and flavour to the adult smoker with reduced harmful emissions when compared to combusted cigarettes. However, non-combustible nicotine products as alternatives to smoking during pregnancy have been under scrutiny, as nicotine has been described in the literature as being a developmental neurotoxicant in vivo (Ross et al., 2015, Lantz-McPeak et al., 2015, England et al., 2017).
To support the understanding of test article responses, this study screened the developmental toxicity potential of neat nicotine in the devTOXqP assay. Nicotine was predicted to potentially elicit developmental toxicity and/or embryo lethality in vivo at concentrations of 118 µg/mL or above, significantly lower than the cytotoxic effects seen at 618 µg/mL. Nicotine is clearly affecting cell metabolism by non-cytotoxic mechanisms, unlike both cigarettes and HTP bPBS. Nicotine primarily acts as an agonist at nicotinic acetylcholine receptors (nAChRs) but has also been shown to bind to or change the expression of other enzymes and receptors, including the glucocorticoid (NR3C1), androgen, estrogen, estrogen-related, and nuclear subfamily 1 group I member 2 (NR1I2) receptors (Wishart et al., 2006, Williams et al., 2017). The NIH Stem Cell Data Management System Database indicates that human iPS cells express the nAChRs alpha-4, −5, and −7 (Mallon et al., 2013). Interestingly, prenatal exposure to nicotine has been shown to increase NR3C1 (glucocorticoid receptor) expression in rat offspring (Xu et al., 2012). NR3C1 is expressed in hPS cells and was the top gene associated with positive predictions in the recent devTOXqP study published by the EPA (Zurlinden et al., 2020).
Cystine is a component of the human iPS cell media used in the assay. Untreated human iPS cells utilize approximately 75% of the cystine in the media and increased cystine following chemical exposure indicates that less cystine is being utilized by the cell (Palmer et al., 2017, Palmer et al., 2013). Cystine is transported into the cell primarily through the cystine/glutamate antiporter system xc−, which transports a single molecule of cystine into the cell for each molecule of glutamic acid that is transported out of the cell (Conrad and Sato, 2012). This transport system consists of two protein components, the 4F2 heavy chain and the xCT protein, and xCT protein is responsible for the transport activity of system xc− (Conrad and Sato, 2012). xCT protein expression was decreased in rat brains following nicotine self-administration (Knackstedt et al., 2009). We hypothesize that the increased cystine observed following nicotine exposure is due to decreased uptake caused by decreased xCT protein expression.
The nicotine concentration that the cells were exposed to within the devTOXqP assay, from both the non-combustible test articles and nicotine-only studies, are in excess of typical human doses, achievable under either normal or excessive usage and represented exaggerated exposure. Plasma nicotine of adult cigarette smokers is reported to range from 10 to 37 ng/mL (equivalent to approximately 62–228 nMol/mL) (Schneider et al., 2001) and between 6 and 14 ng/mL for nicotine gum (Hansson et al., 2016). The dTP of 118 µg/mL for nicotine in more than 3000 times higher than typical human plasma levels (37 ng/mL). When the dTP for nicotine (118 µg/mL) was compared to the dTP for 1R6F bPBS (0.6%, containing 0.07 µg/mL nicotine), the dTP for nicotine alone was more than 1000-fold higher than the nicotine content in the 1R6F sample at the dTP. To note, it has been described that an in vitro response observed at ≤ 50 × the in vivo Cmax is considered to be relevant for prediction of in vivo toxicity (Talbert et al., 2015). How these observations apply directly to the human population cannot be determined due to the limited applicability of this screening assay to the overall reproductive process in humans (Ross et al., 2015).
Due to the potential for developmental toxicity elicited by cigarettes and HTP bPBS by cytotoxic mechanisms in this assay and lack of effects observed for HYB and e-cigarette extracts which have similar nicotine content to the cigarette and HTP bPBS, it is most probable that the trapped constituents other than nicotine in bPBS drive the developmental toxicity prediction in this study. This is further supported by the significant difference in the nicotine alone dTP concentration (118 µg/mL) and the nicotine content in the bPBS samples at their predicted dTP concentrations (≤5.3 µg/mL). In contrast to nicotine, there were significant differences between samples in the carbonyl concentrations. Furthermore, the concentrations of acetaldehyde, acetone, propionaldehyde, crotonaldehyde, and 2-butanone were higher in the 1R6F and 3R4F samples than the HTP sample. Based on this, we hypothesize that one or more of these carbonyls or other HPHCs are responsible for the changes in iPS cell metabolism and viability observed in this assay following exposure to 1R6F, 3R4F, and HTP bPBS.
4.4. Detection of a known developmental toxicant in a spiked sample
The spiking of ATRA into the 1R6F bPBS was used to assess whether the devTOXqP assay could detect a known developmentally toxic compound within a complex mixture and if so at what concentration. ATRA was selected for this study due to its previously reported potency within the devTOXqP assay, and non-cytotoxic mechanism of action (Palmer et al., 2017). In this study changes in human iPS cell metabolism were measured following treatment with ATRA alone, or in combination with the highest non-responsive dose of 0.22% 1R6F bPBS. The o/c ratio dose–response curve for ARTA was assessed and the dTP was 0.16 nM, which is comparable to the previously reported dTP of 0.35 nM by Palmer et al., in 2017 (Palmer et al., 2017). As 1R6F, 3R4F and HTP bPBS appeared to be acting via a cytotoxicity-related mechanism, it was believed to be important to have an additional positive developmental compound that did not act via that mechanism. The dose–response curves for o/c ratio between ATRA and ATRA + 0.22% 1R6F bPBS were shown to be statistically similar and the dTP for ATRA and ATRA + 0.22% 1R6F bPBS were similar at 0.16 nM and 0.13 nM respectively. This data indicates that the devTOXqP assay is sufficiently sensitive to be able to detect known developmental toxicants in the bPBS media in the nM range without any interference from the trapped 1R6F constituents.
4.5. Study limitations
The major limitation with this study is that it is it covers a small but important part of the reproductive cycle focusing on the blastocyst and early implantation stage of development. However, no single in vitro assay can cover the entire cycle, and the currently regulatory approved reproductive assays are one in vitro and two ex vivo assay endpoints (Bal-Price and Jennings, 2014, Luijten et al., 2008). This assay uses human iPS cells as opposed to animal derived cells or tissues, being more human relevant and provides more mechanistic understanding, than that seen with the current ECVAM-approved methods. This method is in alignment with the 3Rs and is one of the battery of assays used in the US EPA ToxCast program (Zurlinden et al., 2020).
5. Conclusions
This study used a commercially available human iPS cell assay to screen for early developmental toxicity potential in vitro and compared the predicted developmental toxicity potential of reference cigarette smoke bPBS samples, to aerosols generated from a range of non-combustible nicotine products. Samples captured in PBS were applied directly in the devTOXqP assay, up to a maximum concentration of 10% bPBS added to the culture media. The trapped smoke samples from both reference cigarettes were classified as, or potentially as, developmentally toxic within the assay, as was HTP aerosol bPBS, albeit to a much lower extent (five times higher concentration required). Based on the screening response observed in the assay, trapped aerosols from HYB or e-cigarette with any of the three experimental e-liquid variants, were predicted to cause little to no developmental toxicity potential in this in vitro screening assay. The e-cigarette test articles at the maximum testing concentration, contained nicotine concentrations that were more than 3000 times above peak plasma nicotine levels found in adult smokers and adult e-cigarette users.
The spiking of ATRA in 0.22% 1R6F bPBS demonstrated that the devTOXqP assay was able to detect known developmentally toxic compounds within a complex mixture, within the nM range.
Although the devTOXqP assay shows promise at screening for potential developmental toxicity, as with other in vitro models, it cannot fully recapitulate all events contributing to the disruption of normal human development by exogenous chemicals. This is due to the many potential targets during both the maturation of gametes and embryonic development. However, the devTOXqP assay is sensitive to trapped cigarette smoke and non-combustible product aerosol constituents and has utility, when used as part of a weight-of-evidence approach, for the assessment of non-combustible nicotine products.
Overall, the devTOXqP assay was rapid, high through-put, and showed good screening predictivity potential with test products, suggesting its potential applicability for the pre-clinical assessment of non-combustible nicotine products. This is an important aspect of testing product designs and the devTOXqP can potentially enable the rapid screening of multiple product variants for potential developmental effects.
Funding
This work was supported by Imperial Brands PLC. Experimental work and statistical evaluation was carried out by Stemina Biomarker Discovery Inc and funded solely by Imperial Brands PLC.
Declaration of Competing Interest
The mybluTM devices used in this study were manufactured by Fontem Ventures B.V., a wholly owned subsidiary of Imperial Brands. All the authors were employed by Imperial Brands PLC or subsidiaries at time of writing, these were Simms, L., Rudd, K., Czekala, L., Yu, F., Chapman, F., Trelles-Sticken, E., Wieczorek, R., Bode, L., Stevenson, M., Walele, T.
Acknowledgements
The authors would like to thank Stemina Biomarker Discovery Inc for conducting the study and statistical evaluation and Imperial Brands PLC internal reading committee and peer reviewers for their critical review of the manuscript and useful comments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crtox.2020.11.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bailey, J., Knight, A., Balcombe, J., 2005. The future of teratology research is in vitro. Biogenic Amines 19(2) 97–145.
- Baker R.R. Temperature distribution inside a burning cigarette. Nature. 1974;247(5440):405–406. doi: 10.1038/247405a0. [DOI] [Google Scholar]
- Bal-Price, A., Jennings, P., 2014. In vitro Toxicology Systems. Springer.
- Banjac A. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27(11):1618–1628. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- Beekhuijzen M. The era of 3Rs implementation in developmental and reproductive toxicity (DART) testing: current overview and future perspectives. Reprod. Toxicol. 2017;72:86–96. doi: 10.1016/j.reprotox.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Belushkin Maxim, Tafin Djoko Donatien, Esposito Marco, Korneliou Alexandra, Jeannet Cyril, Lazzerini Massimo, Jaccard Guy. Selected harmful and potentially harmful constituents levels in commercial e-cigarettes. Chem. Res. Toxicol. 2020;33(2):657–668. doi: 10.1021/acs.chemrestox.9b00470.s001. [DOI] [PubMed] [Google Scholar]
- Buratto Roberto, Correia Daniela, Parel Monique, Crenna Maude, Bilger Mickaël, Debrick Audrey. Determination of eight carbonyl compounds in aerosols trapped in phosphate buffer saline solutions to support in vitro assessment studies. Talanta. 2018;184:42–49. doi: 10.1016/j.talanta.2018.02.048. [DOI] [PubMed] [Google Scholar]
- CDC, 2004. Smoking & Tobacco Use. 15/07/2015 [cited 2019 23/10/2019]. Available from: https://www.cdc.gov/tobacco/data_statistics/sgr/2004/highlights/reproductive/index.htm.
- UK Committee on Toxicity of Chemicals in Food Consumer Products and the Environment, 2017. Statement on the toxicological evaluation of novel heat not-burn tobacco products. 20/04/2020]. Available from: https://cot.food.gov.uk/cotstatements/cotstatementsyrs/cot-statements-2017/statement-on-heat-not-burn-tobacco-products.
- Carmines E, L, Rajendran N. Evidence for Carbon Monoxide as the Major Factor Contributing to Lower Fetal Weights in Rats Exposed to Cigarette Smoke. Toxicological sciences. 2008;102(2):383–391. doi: 10.1093/toxsci/kfn009. [DOI] [PubMed] [Google Scholar]
- Conrad Marcus, Sato Hideyo. The oxidative stress-inducible cystine/glutamate antiporter, system x c − : cystine supplier and beyond. Amino Acids. 2012;42(1):231–246. doi: 10.1007/s00726-011-0867-5. [DOI] [PubMed] [Google Scholar]
- Cozzani V. An experimental investigation into the operation of an electrically heated tobacco system. Thermochim. Acta. 2020;684:178475. [Google Scholar]
- Czekala L., Simms L., Stevenson M., Trelles-Sticken E., Walker P., Walele T. High Content Screening in NHBE cells shows significantly reduced biological activity of flavoured e-liquids, when compared to cigarette smoke condensate. Toxicol. In Vitro. 2019;58:86–96. doi: 10.1016/j.tiv.2019.03.018. [DOI] [PubMed] [Google Scholar]
- Daston G.P. Laboratory models and their role in assessing teratogenesis. Am. J. Med. Genet. C Semin. Med. Genet. 2011;157c(3):183–187. doi: 10.1002/ajmg.c.30312. [DOI] [PubMed] [Google Scholar]
- Daston G.P., Knudsen T.B. Comprehensive Toxicology. Elsevier; 2010. pp. 3–9. [DOI] [Google Scholar]
- Dypbukt J.M. Different prooxidant levels stimulate growth, trigger apoptosis, or produce necrosis of insulin-secreting RINm5F cells. The role of intracellular polyamines. J. Biol. Chem. 1994;269(48):30553–30560. [PubMed] [Google Scholar]
- England Lucinda J., Aagaard Kjersti, Bloch Michele, Conway Kevin, Cosgrove Kelly, Grana Rachel, Gould Thomas J., Hatsukami Dorothy, Jensen Frances, Kandel Denise, Lanphear Bruce, Leslie Frances, Pauly James R., Neiderhiser Jenae, Rubinstein Mark, Slotkin Theodore A., Spindel Eliot, Stroud Laura, Wakschlag Lauren. Developmental toxicity of nicotine: a transdisciplinary synthesis and implications for emerging tobacco products. Neurosci. Biobehav. Rev. 2017;72:176–189. doi: 10.1016/j.neubiorev.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K.E., Voudris V., Poulas K. E-cigarettes generate high levels of aldehydes only in 'dry puff' conditions. Addiction. 2015;110(8):1352–1356. doi: 10.1111/add.12942. [DOI] [PubMed] [Google Scholar]
- Farsalinos Konstantinos E., Yannovits Nikoletta, Sarri Theoni, Voudris Vassilis, Poulas Konstantinos, Leischow Scott J. Carbonyl emissions from a novel heated tobacco product (IQOS): comparison with an e-cigarette and a tobacco cigarette: carbonyl emissions in heated tobacco product. Addiction. 2018;113(11):2099–2106. doi: 10.1111/add.14365. [DOI] [PubMed] [Google Scholar]
- FDA, U.S., 2012. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke: Established List. 10/07/2020 [cited 2020 30/01/2020]; Available from: https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/harmful-and-potentially-harmful-constituents-tobacco-products-and-tobacco-smoke-established-list.
- Greene R.M., Pisano M.M. Developmental toxicity of e‐cigarette aerosols. Birth Defect. Res. 2019;111(17):1294–1301. doi: 10.1002/bdr2.1571. [DOI] [PubMed] [Google Scholar]
- Hajek, P., et al., 2019. E-cigarettes compared with nicotine replacement therapy within the UK Stop Smoking Services: the TEC RCT. Health Technol. Assess. 23(43) 1–82. [DOI] [PMC free article] [PubMed]
- Hansen Jason M. Oxidative stress as a mechanism of teratogenesis. Birth Defect. Res. C. 2006;78(4):293–307. doi: 10.1002/bdrc.20085. [DOI] [PubMed] [Google Scholar]
- Hansson A., Rasmussen T., Kraiczi H. Single-Dose and Multiple-Dose Pharmacokinetics of Nicotine 6 mg Gum. Nicotine Tob. Res. 2016;19(4):477–483. doi: 10.1093/ntr/ntw211. [DOI] [PubMed] [Google Scholar]
- Hartmann-Boyce, J., et al., 2016. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 9, CD010216. [DOI] [PMC free article] [PubMed]
- Hartung Thomas. Opinion versus evidence for the need to move away from animal testing. ALTEX. 2017:193–200. doi: 10.14573/altex.1703291. [DOI] [PubMed] [Google Scholar]
- Hussain Tarique, Tan Bi'e, Ren Wenkai, Rahu Najma, Kalhoro Dildar Hussain, Yin Yulong. Exploring polyamines: functions in embryo/fetal development. Animal Nutr. 2017;3(1):7–10. doi: 10.1016/j.aninu.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Organization for Standardization, 1999. ISO 3402:1999. In: Tobacco and tobacco products — Atmosphere for conditioning and testing. https://www.iso.org/standard/28324.html. p. 5.
- International Organization for Standardization, 2018. ISO 21160:2018. In: Cigarettes — Determination of selected carbonyls in the mainstream smoke of cigarettes — Method using high performance liquid chromatography. https://www.iso.org/standard/69993.html. p. 17.
- International Organization for Standardization, 2018. ISO 20779:2018. In: Cigarettes — Generation and collection of total particulate matter using a routine analytical smoking machine with an intense smoking regime. International Organization for Standardization.
- International Organization for Standardization, 2018. ISO 20768:2018. In: Vapour products — Routine analytical vaping machine — Definitions and standard conditions. International Organization for Standardization.
- Iyer P. Reproductive and Developmental Toxicology. Elsevier; 2017. pp. 179–192. [DOI] [Google Scholar]
- Jaccard Guy, Djoko Donatien Tafin, Korneliou Alexandra, Stabbert Regina, Belushkin Maxim, Esposito Marco. Mainstream smoke constituents and in vitro toxicity comparative analysis of 3R4F and 1R6F reference cigarettes. Toxicol. Rep. 2019;6:222–231. doi: 10.1016/j.toxrep.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, M.D., et al., 2009. Evaluation of in vitro assays for assessing the toxicity of cigarette smoke and smokeless tobacco. Cancer Epidemiol. Biomarkers Prev. 18(12) 3263–3304. [DOI] [PMC free article] [PubMed]
- Knackstedt Lori A., LaRowe Steven, Mardikian Pascale, Malcolm Robert, Upadhyaya Himanshu, Hedden Sarra, Markou Athina, Kalivas Peter W. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol. Psychiatry. 2009;65(10):841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krewski D. Toxicity testing in the 21st century: a vision and a strategy. J. Toxicol. Environ. Health B Crit. Rev. 2010;13(2–4):51–138. doi: 10.1080/10937404.2010.483176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S., Probst C., Rehm J., Popova S. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Global Health. 2018;6(7):e769–e776. doi: 10.1016/S2214-109X(18)30223-7. [DOI] [PubMed] [Google Scholar]
- Lantz-McPeak Susan, Guo Xiaoqing, Cuevas Elvis, Dumas Melanie, Newport Glenn D., Ali Syed F., Paule Merle G., Kanungo Jyotshna. Developmental toxicity assay using high content screening of zebrafish embryos: high content assay quantifies developmental toxicity in zebrafish embryos. J. Appl. Toxicol. 2015;35(3):261–272. doi: 10.1002/jat.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten, M., et al., 2008. Alternative methods in reproductive toxicity testing: State of the art. RIVM report 340720002.
- Mallon Barbara S., Chenoweth Josh G., Johnson Kory R., Hamilton Rebecca S., Tesar Paul J., Yavatkar Amarendra S., Tyson Leonard J., Park Kyeyoon, Chen Kevin G., Fann Yang C., McKay Ronald D.G. StemCellDB: the human pluripotent stem cell database at the national institutes of health. Stem Cell Res. 2013;10(1):57–66. doi: 10.1016/j.scr.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison D.R., Thomford P.J. The mechanisms of action of reproductive toxicants. Toxicol. Pathol. 1989;17(2):364–376. doi: 10.1177/019262338901700213. [DOI] [PubMed] [Google Scholar]
- McNeill, A., et al., 2018. Evidence review of e-cigarettes and heated tobacco products 2018. In: A report commissioned by Public Health England. London, Public Health England.
- McRobbie, H., et al., 2014. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst. Rev. 12, CD010216. [DOI] [PubMed]
- National Health Service, 2019. Statistics on Women's Smoking Status at Time of Delivery: England - Quarter 4, 2018-19. NHS Digital.
- National Research Council, 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy. National Academies Press.
- O’Connell G., Pritchard J.D., Prue C., Thompson J., Verron T., Graff D., Walele T. A randomised, open-label, cross-over clinical study to evaluate the pharmacokinetic profiles of cigarettes and e-cigarettes with nicotine salt formulations in US adult smokers. Intern Emerg. Med. 2019;14(6):853–861. doi: 10.1007/s11739-019-02025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J.A., Smith A.M., Egnash L.A., Conard K.R., West P.R., Burrier R.E., Donley E.L.R., Kirchner F.R. Establishment and assessment of a new human embryonic stem cell-based biomarker assay for developmental toxicity screening: biomarker-based HESC teratogenicity assay. Birth Defects Res. B. 2013;98(4):343–363. doi: 10.1002/bdrb.21078. [DOI] [PubMed] [Google Scholar]
- Palmer J.A., Smith A.M., Egnash L.A., Colwell M.R., Donley E.L.R., Kirchner F.R., Burrier R.E. A human induced pluripotent stem cell-based in vitro assay predicts developmental toxicity through a retinoic acid receptor-mediated pathway for a series of related retinoid analogues. Reprod. Toxicol. 2017;73:350–361. doi: 10.1016/j.reprotox.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Pendeville Hélène, Carpino Nick, Marine Jean-Christophe, Takahashi Yutaka, Muller Marc, Martial Joseph A., Cleveland John L. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol. Cell. Biol. 2001;21(19):6549–6558. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynton S., Sutton J., Goodall S., Margham J., Forster M., Scott K., Liu C., McAdam K., Murphy J., Proctor C. A novel hybrid tobacco product that delivers a tobacco flavour note with vapour aerosol (Part 1): product operation and preliminary aerosol chemistry assessment. Food Chem. Toxicol. 2017;106:522–532. doi: 10.1016/j.fct.2017.05.022. [DOI] [PubMed] [Google Scholar]
- RCP, 2010. Passive Smoking and Children: A Report. Royal College of Physicians (RCP). Tobacco Advisory Group.
- Rhee H.J., Kim E.J., Lee J.K. Physiological polyamines: simple primordial stress molecules. J. Cell Mol. Med. 2007;11(4):685–703. doi: 10.1111/j.1582-4934.2007.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E.J. Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacol. Official Publ. Am. College Neuropsychopharmacol. 2015;40(1):61–87. doi: 10.1038/npp.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd K., Stevenson M., Wieczorek R., Pani J., Trelles-Sticken E., Dethloff O., Czekala L., Simms L., Buchanan F., O'Connell G., Walele T. Chemical composition and in vitro toxicity profile of a pod-based e-cigarette aerosol compared to cigarette smoke. Appl. In Vitro Toxicol. 2020;6(1):11–41. doi: 10.1089/aivt.2019.0015. [DOI] [Google Scholar]
- Salman R. Free-base and total nicotine, reactive oxygen species, and carbonyl emissions from IQOS, a heated tobacco product. Nicotine Tob. Res. 2019;21(9):1285–1288. doi: 10.1093/ntr/nty235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller Jean-Pierre, Keller Daniela, Poget Laurent, Pratte Pascal, Kaelin Etienne, McHugh Damian, Cudazzo Gianluca, Smart Daniel, Tricker Anthony R., Gautier Lydia, Yerly Michel, Reis Pires Roger, Le Bouhellec Soazig, Ghosh David, Hofer Iris, Garcia Eva, Vanscheeuwijck Patrick, Maeder Serge. Evaluation of the Tobacco Heating System 2.2. Part 2: chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul. Toxicol. Pharm. 2016;81:S27–S47. doi: 10.1016/j.yrtph.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Schneider Nina G., Olmstead Richard E., Franzon Mikael A., Lunell Erik. The nicotine inhaler: clinical pharmacokinetics and comparison with other nicotine treatments. Clin. Pharmacokinet. 2001;40(9):661–684. doi: 10.2165/00003088-200140090-00003. [DOI] [PubMed] [Google Scholar]
- Scott C.W., Peters M.F., Dragan Y.P. Human induced pluripotent stem cells and their use in drug discovery for toxicity testing. Toxicol. Lett. 2013;219(1):49–58. doi: 10.1016/j.toxlet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Seiler Nikolaus, Raul Francis. Polyamines and apoptosis. J. Cellular Mol. Med. 2005;9(3):623–642. doi: 10.1111/j.1582-4934.2005.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih A.Y. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J. Neurosci. 2006;26(41):10514–10523. doi: 10.1523/JNEUROSCI.3178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms Liam, Clarke Anna, Paschke Thilo, Manson Andrew, Murphy James, Stabbert Regina, Esposito Marco, Ghosh David, Roemer Ewald, Martinez Javier, Freiesleben Jarl, Kim Hyo-Keun, Lindegaard Thomas, Scharfe Marc, Vincze Istvan, Vlachos Panagiotis, Wigotzki Diane, Pollner Gwen, Lutz Rolf. Assessment of priority tobacco additives per the requirements of the EU Tobacco Products Directive (2014/40/EU): Part 1: background, approach, and summary of findings. Regul. Toxicol. Pharm. 2019;104:84–97. doi: 10.1016/j.yrtph.2019.02.011. [DOI] [PubMed] [Google Scholar]
- Sleiman Mohamad, Logue Jennifer M., Montesinos V. Nahuel, Russell Marion L., Litter Marta I., Gundel Lara A., Destaillats Hugo. Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environ. Sci. Technol. 2016;50(17):9644–9651. doi: 10.1021/acs.est.6b01741.s001. [DOI] [PubMed] [Google Scholar]
- Talbert D.R. A multi-parameter in vitro screen in human stem cell-derived cardiomyocytes identifies ponatinib-induced structural and functional cardiac toxicity. Toxicol. Sci. 2015;143(1):147–155. doi: 10.1093/toxsci/kfu215. [DOI] [PubMed] [Google Scholar]
- Tayyarah R., Long G.A. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul. Toxicol. Pharm. 2014;70(3):704–710. doi: 10.1016/j.yrtph.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Trelles-Sticken E. Hamburg; Germany: 2019. Next generation product aerosol bubbled extracts show little to no effect in high content screening endpoints when compared to cigarette smoke bubbled extracts. In: Coresta Congress. [Google Scholar]
- US Department of Health Human Services, 2014. The health consequences of smoking—50 years of progress: a report of the Surgeon General.
- WHO, 2018. Heated tobacco products (HTPS): information sheet. [cited 2020 22/01/2020]; Available from: https://www.who.int/tobacco/publications/prod_regulation/heated-tobacco-products/en/.
- Williams Antony J., Grulke Christopher M., Edwards Jeff, McEachran Andrew D., Mansouri Kamel, Baker Nancy C., Patlewicz Grace, Shah Imran, Wambaugh John F., Judson Richard S., Richard Ann M. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J. Cheminform. 2017;9(1) doi: 10.1186/s13321-017-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D.S. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006;34(90001):D668–D672. doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Liang G., Yan Y.E., He W.W., Liu Y.S., Chen L.B., Magdalou J., Wang H. Nicotine-induced over-exposure to maternal glucocorticoid and activated glucocorticoid metabolism causes hypothalamic–pituitary–adrenal axis-associated neuroendocrine metabolic alterations in fetal rats. Toxicol. Lett. 2012;209(3):282–290. doi: 10.1016/j.toxlet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Zurlinden, T.J., et al., 2020. Profiling the ToxCast library with a pluripotent human (H9) stem cell line-based biomarker assay for developmental toxicity. Toxicol. Sci. official J. Soc. Toxicol. kfaa014. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.