Abstract
Developmental scientists have examined the independent effects of peer presence, social cues, and rewards on adolescent decision-making and cognitive control. Yet, these contextual factors often co-occur in real world social situations. The current study examined the combined effects of all three factors on cognitive control, and its underlying neural circuitry, using a task to better capture adolescents’ real world social interactions. A sample of 176 participants ages 13–25, was scanned while performing an adapted go/no-go task alone or in the presence of a virtual peer. The task included brief positive social cues and sustained periods of positive arousal. Adolescents showed diminished cognitive control to positive social cues when anticipating a reward in the presence of peers relative to when alone, a pattern not observed in older participants. This behavioral pattern was paralleled by enhanced orbitofrontal activation. The results demonstrate the synergistic impact of social and reward influences on cognitive control in adolescents.
Keywords: adolescents, cognitive control, fMRI, orbitofrontal cortex, peers, reward
1 |. INTRODUCTION
Adolescence is a transitional period of development characterized by heightened sensitivity to peer influence (Chein, Albert, O’Brien, Uckert, & Steinberg, 2011; Smith, Steinberg, Strang, & Chein, 2015; Weigard, Chein, Albert, Smith, & Steinberg, 2014), social cues (Cohen, Breiner, et al., 2016; Somerville, Hare, & Casey, 2011), and rewards (Cohen-Gillbert et al., 2014; Galvan et al., 2006; Geier, Terwilliger, Teslovich, Velanova, & Luna, 2009; Van Leijenhorst et al., 2010). Laboratory studies have typically examined the independent effects of peers and rewarding stimuli on behavior, and on its underlying neural circuitry. Yet, rarely do these factors occur in isolation. In the real world, social interactions that take place among teenagers involve multiple contextual factors. To the extent that these influences have synergistic effects, prior research may underestimate the impact of social influences and rewards on adolescent choices and actions. In the current study, we examine the combined effect of peer presence, social cues, and rewards on cognitive control in adolescents relative to adults.
There is a rich literature on how social influences and rewards differentially impact adolescent behavior relative to that of adults. Adolescents make riskier decisions when with peers (Chein et al., 2011), have more automobile accidents when driving with same-aged passengers (Williams, 2003), drink more alcohol in social contexts (Cooper, 1994), and commit more crimes in groups than do adults (Zimring, 1998). Laboratory studies have shown that social cues and the opportunity for immediate reward can increase risky choices and impulsive actions (Cauffman et al., 2010; Cohen, Breiner, et al., 2016; Dreyfuss et al., 2014; Figner, Mackinlay, Wilkening, & Weber, 2009; Jones et al., 2014; Somerville et al., 2011; Steinberg et al., 2009). Each of these contextual factors can independently overwhelm cognitive control.
Cognitive control and the execution of goal-directed behavior are supported in part by prefrontal circuitry. The prefrontal cortex is highly interconnected with other cortical and subcortical regions to enable complex regulation of attention, actions, emotions, and desires (Buhle et al., 2014; Casey, 2015; Chiew & Braver, 2011; de la Vega et al., 2016; Duijvenvoorde, Van Achterberg, Braams, Peters, & Crone, 2016; Ochsner & Gross, 2005). The orbitofrontal cortex (OFC), in particular, has been associated with goal valuation and decision-making in humans (Plassmann, Doherty, & Rangel, 2010), rodents (Balleine & O’Doherty, 2010), and non-human primates (Schultz & Tremblay, 2006), undergoes substantial development (Galvan et al., 2006) and tracks rewarding outcomes in adolescents (Chein et al., 2011; Galvan et al., 2006). Dense interconnections between orbitofrontal and subcortical regions (Haber & Knutson, 2009) have been associated with value updating, reward prediction, and motivated behavior (Fiorillo, Tobler, & Schultz, 2003; Hare, Doherty, Camerer, Schultz, & Rangel, 2008; Rangel & Hare, 2010; Schultz, Dayan, & Montague, 1997).
Cortico-subcortical and cortico-cortical connections within dorsolateral prefrontal (dlPFC) and orbitofrontal (OFC) circuits undergo extensive and dynamic neurobiological development from childhood into adulthood (Casey, Galván, & Somerville, 2016). These changes are evidenced in MRI-based structural (Achterberg, Peper, van Duijvenvoorde, & Mandl, 2016; Gogtay et al., 2004; Sowell, 2004; Sowell, Thompson, Colin, Jernigan, & Toga, 1999) and functional connectivity studies (Dosenbach et al., 2011; Fair et al., 2009) within and between prefrontal circuits. This protracted development parallels age-dependent changes in cognitive control in arousing situations (Cohen, Breiner, et al., 2016; Dreyfuss et al., 2014; Luna, Paulsen, Padmanabhan, & Geier, 2013; Silvers et al., 2016; Somerville et al., 2011) that continue into the early 20s (Cohen, Breiner, et al., 2016; Silvers et al., 2016). Recent evidence suggests that functional connectivity between prefrontal cognitive control and reward circuitry increases from adolescence to adulthood (Duijvenvoorde et al., 2016; van den Bos et al., 2015), demonstrating that adolescents may have weaker prefrontal-reward connectivity compared to older age groups and thus diminished control in the presence of potential rewards.
Dynamic changes throughout adolescence in frontostriatal circuitry involving the orbitofrontal cortex and ventral striatum (Ernst, Pine, & Hardin, 2006; Galvan et al., 2006; Geier & Luna, 2009; Richards, Plate, & Ernst, 2013; Silverman, Jedd, & Luciana, 2015; Van Leijenhorst et al., 2010 for review) have been associated with risky decision-making in the presence of a peer (Chein et al., 2011). Laboratory studies show that the mere presence of a peer (Chein et al., 2011; Smith et al., 2015; Weigard et al., 2014) or positive social cues (Somerville et al., 2011) yield greater OFC and ventral striatum activation in adolescents relative to adults. Positive social cues (Cohen, Breiner, et al., 2016; Silva, Shulman, Chein, & Steinberg, 2015; Somerville et al., 2011; Van Hoorn et al., 2016) and social feedback (Jones et al., 2014) are associated with increased activation of prefrontal and reward circuitry, diminished cognitive control (Cohen, Breiner, et al., 2016; Somerville et al., 2011), and enhanced motivated behavior (Jones et al., 2014) relative to adults.
Together, this body of work implicates ventral orbitofrontostriatal circuitry to social and arousing stimuli. However, the combined effects of different positively arousing factors on lateral prefrontal control circuitry at varying stages of development remain unexplored. The goal of the current study was to examine the combined influence of peer presence, social cues, and rewards on cognitive control, and the neural regions that underlie this ability in adolescents (13–17), young adults (18–21), and adults (over 21). We used a task that included brief and prolonged arousal states and social manipulations. We sought to determine whether the confluence of positively arousing conditions differentially impacted cognitive control and underlying neural processing in adolescents, young adults, and adults.
Based on previous studies, we hypothesized that compared to older age groups and compared to when completing the task alone, adolescents would exhibit worse cognitive control performance compared to young adults and adults (Chein et al., 2011; Silva et al., 2015). Specifically, we predicted poorer performance by adolescents in the presence of peers when under a positive state of arousal (i.e., anticipation of winning up to $100) and while viewing positive social (smiling) cues. We further predicted that this behavioral effect would be paralleled by enhanced activity in the orbitofrontal cortex and ventral striatum (Hare et al., 2008; Rangel & Hare, 2010) in teens than in older individuals, specifically in the presence of peers than when alone.
2 |. METHODS
2.1 |. Participants
One hundred and seventy-six healthy, right-handed individuals from a larger sample of 198 participants between the ages of 13–25 completed a variant of a go/no-go task (Cohen, Dellarco, et al., 2016) while undergoing an fMRI scan. Participants were recruited from Los Angeles and New York City as part of a larger study. Participants were21.6% African American, 14.8% Asian, 36.9% Caucasian, 22.7% Hispanic, and 4.0% “other.” Participants were randomly assigned to one of two conditions—“Alone” (97 completed the go/no-go task as reported in Cohen, Dellarco, et al., 2016) or “Peer” (79 completed the go/no-go task under the impression that a same-aged, same-gender peer was watching and evaluating their performance). Assignment into the peer versus alone group was counterbalanced across subjects and sites.
Twenty-two of the original 198 participants were excluded, due to poor behavioral performance on the task (defined as >2 SD from the group mean as measured by d′, n = 1); variation in scanning parameters (n = 1); or excessive motion in the scanner (defined as >10% of time points within a run exceeding >1.56 mm translational motion, half a voxel, or >1 degree rotational motion, n = 20). The final participant sample consisted of 71 adolescents (ages 13–17 years old, M = 15.48, SD = 1.24; 33 males, 38 females); 48 young adults (ages 18–21 years-old, M = 19.64, SD = 1.03; 25 males, 23 females); and 57 adults (ages 22–25 years-old, M = 23.34, SD = 1.01; 28 males, 29 females) (Supplementary Table S1). There were no differences between race (c2(4, N = 176) = 3.75, p = .441), study site (c2(1, N = 176) = .44, p = .505), age group (c2(2, N = 176) = .79, p = .674), or gender (c2(1, N = 176) = .53, p = .467) for the peer condition. Analyses of data from 97 participants in the alone condition of the current study were previously reported in a separate article (Cohen, Breiner, et al., 2016).
2.2 |. Experimental task
2.2.1 |. Procedure
Upon study enrollment, participants were randomly assigned to either complete the task in the “presence” of a peer (peer condition) or to complete the task alone (alone condition). Participants who were assigned to the peer condition arrived at the study location and were led to believe that another participant was running late but would be arriving soon—and that once s/he arrived, the two would be introduced to each other. In reality, there was no peer participant, but instead audio recordings would later be played to create the impression that a peer had arrived and was viewing the participant’s performance from a different room.
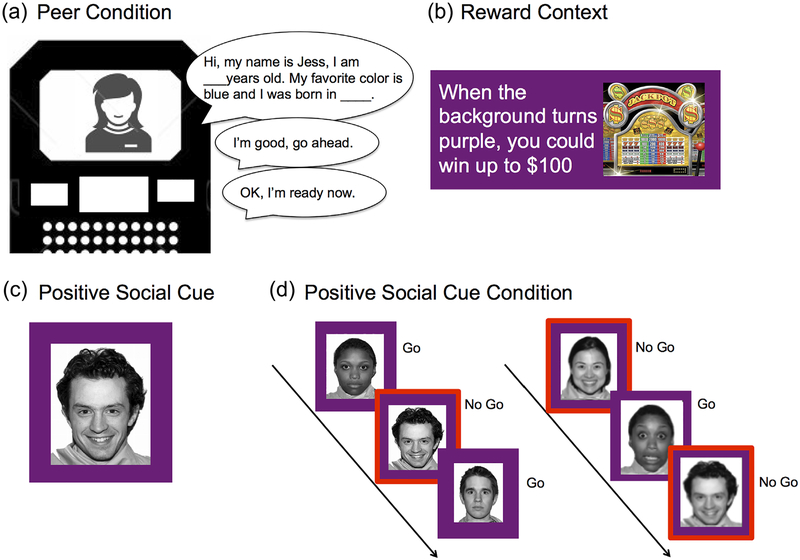
Once participants entered the scanner bore and prior to the start of the task, the experimenter alerted him/her (via the Resonance Technology (ResTech) communications system) that the other “participant” had now arrived, and that s/he was instructed to make judgments about the actual participant’s performance. The participant was told that they would have a chance to meet at the end of the study. The experimenter then instructed both participants to introduce themselves by reading aloud from a template script that included prompts regarding the participants’ name, favorite color, and place of birth. A recording from a same aged (adolescent/adult), same gender (male/female) peer reading from the same script was played into the ResTech microphone. Prior to the start of the task, the participant indicated that s/he was ready to begin, and a recording of the peer was played indicating that s/he was also ready to begin. A total of three recordings were played to the participant, including: “Hi! My name is John/Jess. My favorite color is blue and I was born in L.A./New York”; “OK, I’m ready now”; “I’m good, go ahead.” Abbreviated versions of some of the recordings were played prior to the start of each run of the task either asking the participant if s/he was doing well, and indicating that the session was about to begin (e.g., “I’m good”; “OK,” etc.) (Figure 1).
FIGURE 1.
Behavioral paradigm. (a) Participants in the peer condition were introduced to the peer via the scanner intercom. (b) In the reward context, participants were told that when the background of the screen changed colors (e.g., purple) that they could win up to $100.(c) Example of a positive social cue. (d) Participants were instructed to either withhold a response (no go) or respond (go) to a positive social cue (smiling face) during the go/no-go task
After completion of the task, participants were informed that the peer had to leave early and that they would not have a chance to meet. Participants completed self-report surveys pertaining to the peer condition to verify its effectiveness, and were not debriefed on the deception until after data had been collected from all participants in order to avoid potential contamination of the sample.
2.2.2 |. Task
All participants completed the Cognitive Control Under Emotion (CCUE) task (Cohen, Dellarco, et al., 2016, Supplementary Figure S1) programmed using E-Prime 2.0. In the task, participants were presented with smiling, fearful, and neutral face cues while under sustained anticipation of a negative event (an aversive sound), positive event (winning up to $100), or neutral event (no risk of a positive or negative event). Events were depicted by different colored backgrounds behind the cues (e.g., yellow, blue, purple) and (along with the cues) were pseudorandomized and counterbalanced between participants and within runs to control for item and order effects (see Cohen, Breiner, et al., 2016; Cohen, Dellarco, et al., 2016; and Supplementary Figure S1 for more task detail). Participants were informed that the occurrence of the positive or negative event was not based on their performance. Although participants were told that these events could occur at any time over the experiment, they only experienced the anticipated events once during the session. The aversive noise or bonus event always occurred near the end of the block, and subsequent trials were not included in analyses. To test the primary hypothesis, the current analyses focus on the combined effects of peer presence, exposure to brief positive social cues, and sustained positive state (anticipating winning up to $100) on performance (Figure 1) and treating other conditions and trial types as regressors in the analyses. In addition, gender and study site were treated as regressors.
Participants practiced the task prior to entering the scanner to encourage familiarization with the instructions and conditions. Participants were instructed to press their index finger upon viewing one type of cue (go trials; e.g., calm faces) and to inhibit responding to all other types of cues (no-go trials; e.g., fearful faces and smiling faces) (Supplementary Figure S1a). Prior to each run, participants were instructed to which face cue type they should press the button, and to which they should inhibit responding (Supplementary Figure S1b).
Participants completed six runs of the task, each lasting 8 min and 2 s, taking a total of 48 min and 12 s for task completion. Each run consisted of a combination of sustained arousal and state cues (e.g., smiling-go/calm-no go; smiling-go/fearful-no go; calm-go/ smiling-no go; calm-go/fearful-no go; fearful-go/smiling-no go; fearful-go/calm-no go) presented in a mixed-block event-related design. Each arousal state manipulation (reward, threat, or neutral) was presented in blocks of 75 s each that occurred twice during each run. Each stimulus (face) was presented for 500 ms prior to a jittered inter-trial interval (lasting 2–7 s). Each run consisted of 114 trials (84 go, 30 no-go).
After exiting the scanner, participants answered debriefing questions (e.g., “Did you expect to win money more during the purple blocks than the blue or yellow blocks?”) that probed the believability of the emotional state on a seven-point Likert scale (1 = not at all; 7 = very much). In addition, participants in the peer condition were asked how much they liked the peer and how often they thought about them during the task on a four-point Likert scale (1 = not at all; 4 = very much). We specifically did not ask about believability of the peer to avoid potential contamination of the sample.
2.2.3 |. Behavioral analyses
Behavioral data were analyzed using R 3.1.2. Stimulus timing and emotional-state timing were extracted and analyzed using MATLAB and Statistics Toolbox Release 2013b (The MathWorks, Natic, MA). Accuracy on the task was determined using the sensitivity index d′, which accounts for both hits and false alarms (Macmillan & Creelman, 2005). d′ was calculated by subtracting normalized false alarm rates from normalized accuracy on go trials. Because our a priori hypotheses focused on the effects of positive state and cue on response inhibition, a mixed linear model was used to compare differences in d′ for interactions between age group (adolescents, young adults, adults) and peer condition (alone, peer) under the reward state in response to positive social cues—controlling for study site and gender. To test for specificity of effects to these positive conditions, a difference score between d′ values in the positive condition of anticipation of reward and a happy face relative to the non arousing condition and calm face, was generated for each subject in the alone and peer conditions. A second mixed linear model was used to test for age group and peer effects in these difference scores. Student’s t-tests were performed to test whether responses to the debriefing questions were significantly different from 1 (the lowest value on the seven-point scale).
2.3 |. fMRI
2.3.1 |. fMRI image acquisition
Whole-brain fMRI images were collected using Siemens Magnetom Trio 3.0-T scanner at Weill Cornell Medical College Citigroup Biomedical Imaging Center and University of California, Los Angeles: Staglin IMHRO Center for Cognitive Neuroscience. Identical scanning parameters were used at both sites. Biomedical Informatic Research Network (Jovicich et al., 2016) optimized sequences were used to acquire T1-weighted magnetization-prepared rapid-acquisition gradient-echo (MPRAGE) sequence scan (repetition time (TR) = 2170 ms, echo time (TE) = 4.33 ms, slice thickness = 1.2 mm, sagittal slice number = 160, and 256-mm field of view (FOV)). T2*-sensitive echo planar pulse sequences were used to acquire functional images (TR = 2,500 ms, TE = 30 ms, slice thickness = 4-mm, axial slice number = 38, FOV = 200 mm, flip angle = 90°, and 3.1 × 3.1 × 4.0 mm voxels).
2.3.2 |. fMRI data processing
Analysis of Functional NeuroImages (AFNI) software version 16.0.00 was used to process functional imaging data (Cox, 1996). Preprocessing steps include slice-time correction using sinc interpolation, volume registration with 6-parameter rigid-body transformation accounting for head motions, and normalization to the Montreal Neurological Institute (MNI) 152 1-mm T1 template using a 12-parameter affine transformation as well as nonlinear transformation (AFNI 3dQWarp function). Transformed images were then resampled to 3-mm voxels and smoothed using a full-width/half-maximum Gaussian kernel of 6-mm. Signal intensity for each voxel time series was normalized to percent signal change.
2.3.3 |. Functional image analysis
A general linear model (GLM) was used to estimate the voxel-wise activation to different emotional cues and sustained-emotional-states. To examine the unique contribution of positive social cues in the reward state, we modeled each emotional cue in each sustained-emotional-states, to include 6 correct-response regressors (fearful, calm, or smiling faces on go trials, and fearful, calm, or smiling faces on no-go trials) under each of the 3 sustained-emotional-states (threat, neutral, or reward states) for a total of 18 regressors (e.g., fearful-go-reward, smiling-no-go-neutral). In addition, an incorrect-response regressor (for all trials) and 6 motion estimation parameters were included. Baseline trends were estimated to capture shifts in signal change. Responsive activations were modeled with a three-parameter gamma hemodynamic-response function (HRF). Time points with motion greater than half a voxel (1.56 mm), as well as the preceding and following time points, were censored.
Regression coefficients from individual GLMs were submitted to a group-level linear mixed-effect model analysis using the AFNI 3dMVM function with type III sums of squares. In the general model, random deviations from the group mean were used for participant intercepts with age group (adolescent, young adult, and adult), peer-condition, gender, and scanning site as between-subject variables; and emotional cues, sustained-emotional-states, and response types (go or no-go) as within-subject variables. Based on the behavioral results showing only a difference between the peer and alone conditions for teens younger than 18, a specific general linear test (GLT) was used to extract from the general model the interactive effect of collapsed age group (younger than 18 years and 18 or older) and peer-condition (peer and alone) towards positive social cues (go and no-go trials) under sustained reward state.
In addition to whole brain analysis, we performed a region of interest analysis using an anatomically defined orbitofrontal and a ventral striatum masks (AFNI MNI Atlas), based on our a priori hypothesis of developmental differences in reward circuitry engagement on this task (Chein et al., 2011; Galvan et al., 2006; Somerville et al., 2011). We used a conservative OFC anatomical mask contained within the parenchyma of the brain to ensure complete coverage. To quantitatively demonstrate this coverage, we extracted raw EPI signals from the OFC region (based on anatomical mask) and anatomical gray matter region. Analysis of signal intensity by region and age indicate no differences by region or signal dropout due to age (Supplementary Figure S4).
Individual voxels were thresholded at a p-value of 0.05. Clustersize was thresholded at an α of 0.05 after correction for multiple comparisons. Multiple comparison correction was performed using the 3dClustSim program in 2016 AFNI version 16.0.00 in which false positive rate is adjusted (Eklund, Nichols & Knutsson, 2016). In the 3dClustsim program, the new mixed model auto-correlation function option (-acf option) was used to further reduce false positive rate as recommended by Cox, Chen, Glen, Reynolds, & Taylor (2017). Post hoc analyses on extracted beta weights were conducted using R 3.1.2. ANOVA was conducted using type III sums of squares.
3 |. RESULTS
3.1 |. Validation of paradigm
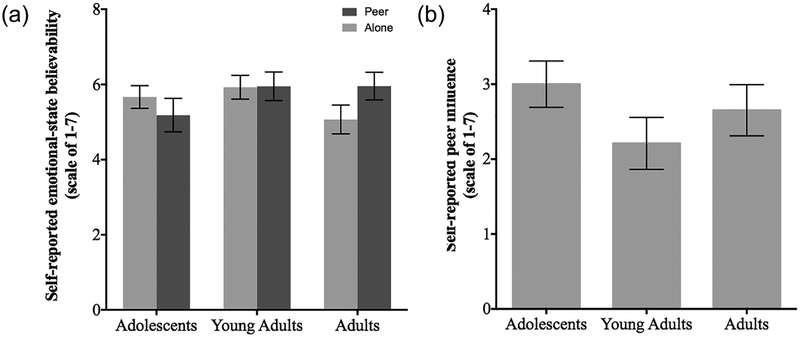
Participants provided self-report about the effectiveness of the experimental conditions to validate the manipulations. All ratings were significantly different from 1 across all age groups (Adolescents t = 17.5, df = 65, p < 0.0001; Young-Adults t = 20.48, df = 48, p < 0.0001; Adults t = 16.39, df = 52, p < 0.0001). There were no differences between age groups or peer groups in believability of the arousal states with each color background (p = 0.3387 and p = 0.6371, respectively), nor age group differences in response to the peer manipulation (p = 0.2613) (Figure 2). Six subjects reported being dubious of the peer manipulation after completion of the study, but these subjects were not excluded and did not differ by age group. Results did not change with removal of these participants from the analyses (p < 0.05).
FIGURE 2.
Self-report on believability of the emotional-state and virtual peer manipulations did not differ by age group. (a) All age groups reported significant believability (adolescents t = 17.5, df = 65, p < 0.0001; young-adults t = 20.48, df = 48, p < 0.0001; adults t = 16.39, df = 52, p < 0.0001). There was no effect of age group or peer condition on believability of the color background with the expected outcome of winning money or hearing noise (p = 0.3387 and p = 0.6371, respectively). (b) There was no age difference in the self-reported peer influence of the peer manipulation (p = 0.2613)
3.2 |. Behavior
A mixed linear model was performed to assess the interactive effects of age group (adolescents, young adults, adults) and peer condition (peer, alone), controlling for gender and study site. The dependent variable assessed was the overall d′ to positive social cues (both go and nogo) under the reward context.
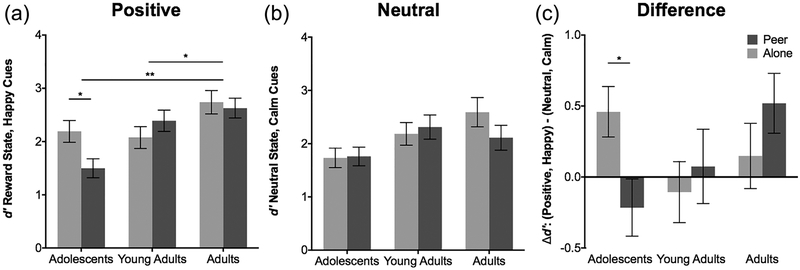
The analysis examining d′ to positive social cues during the reward state revealed a main effect of age group [F(2,168) = 8.19, p < 0.01] such that adults (M = 2.69, SD = 1.10) performed significantly better than young adults (M = 2.22, SD = 0.99), t = −2.2627, df = 102, p = 0.026 and adolescents (M = 1.91, SD = 1.23), t = −3.7967, df = 124, p <0.01. An interaction of age group by peer condition was revealed [F(2,168) = 4.49, p = 0.012] such that adolescents in the alone condition performed better (M = 2.19, SD = 1.08) than adolescents in the peer condition (M = 1.50, SD = 0.97), t = 2.5611, df = 67, p = 0.012, while this pattern was not observed in older age groups (Figure 3a and Supplementary Table S2).
FIGURE 3.
Behavioral performance for the neutral and positive conditions by age group, as a function of the peer manipulation. (a) Behavioral performance to positive social cues in the reward state by age group, shows that unlike the older age groups 18–25, the 13–17 year olds performed worse in the virtual peer condition (M = 1.50) than in the alone condition (M = 2.19) df = 67, p = 0.012. Adults (M = 2.68) performed significantly better than young adults (M = 2.22), df = 102, p = 0.026 and adolescents (M = 1.91), df = 124, p <0.001 in the reward state while detecting positive social cues. (b) Behavioral performance to calm cues in the neutral state showed a main effect of age group (F (2,165) = 5.161, p = 0.0067) with adults 22–25 performing better (M = 2.37) than adolescents (M = 1.74, df = 124, p = 0.011), but no effect of peer nor age × peer interaction. (c) Difference scores for the positive relative to the neutral condition showed that adolescents in the peer condition had diminished performance (M = −0.21) relative to the alone condition (M = 0.46), df = 67, p = 0.048, that was not observed for the older age groups. *p < 0.05 and **p < 0.001
To show specificity of the results to the positive condition, Figure 3b depicts the pattern of results for the neutral condition of a calm face in the no arousal condition as a function of the peer condition by age group. Behavioral performance to calm cues in the neutral state showed a main effect of age group (F(2,165) = 5.161, p = 0.0067) with adults 22–25 performing better (M = 2.37) than adolescents (M = 1.74, df = 124, p = 0.011), but no effect of peer nor age X peer interaction. Figure 3c further illustrates this effect for teens by depicting the data as a difference score between the positive condition of anticipation of reward and a happy face versus the non-arousing condition and calm face as a function of the peer condition by age group. Adolescents, unlike the older age groups, show worse performance when with a peer (M = −0.21), than when alone (M = 0.46) df = 67, p = 0.048 for the positive condition relative to the neutral condition. The analysis and results of the full experimental design are presented in Supplementary Figure S2 and Supplementary Table S3.
3.3 |. fMRI
3.3.1 |. Functional analysis
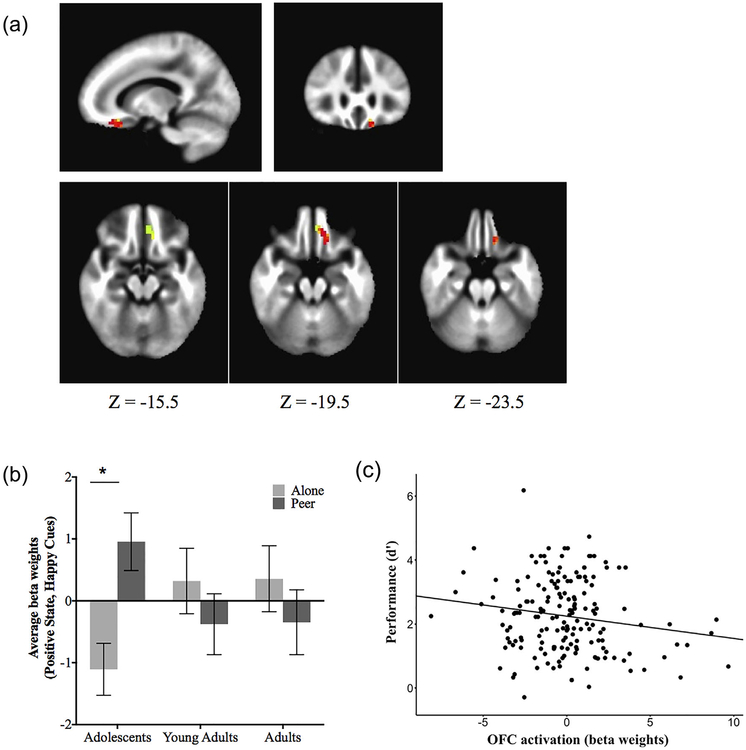
No clusters survived whole brain correction, but the region of interest analysis showed a significant cluster in the left orbitofrontal cortex for the interaction of age group (adolescent and non-adolescent) by peer condition (MNI coordinates: x = −6.5, y = 36.5, z = −20.5, 38 voxels; p < 0.05 corrected), to smiling faces when anticipating a reward (Figure 4a). This interaction is depicted in Figure 4b showing that adolescents 13–17 had greater left orbitofrontal activation in the peer condition compared to the alone condition (F(1, 66) = 5.549, p < 0.05), a pattern not observed for the young adults 18–21 or adults 22–25. This interaction held when excluding 3 outliers with extreme OFC beta-weights (>±3 standard deviations from the group mean) and when including them in the analysis (Supplementary Figure S3). No significant activations were found for the ventral striatum.
FIGURE 4.
Orbitofrontal activity by age group and behavioral performance. (a) Localization of orbitofrontal cluster showing an age group × peer condition interaction for positive social cues during the reward condition. (b) Mean activity in the orbitofrontal cortex in response to positive social (smiling) cues under the reward state, as a function of peer condition and age group. (c) Negative correlation between orbitofrontal activity and behavioral performance, as measured by d-prime (d′) (r(171) = −0.1631, p < 0.032). Error bars show ±1 SE. *p < 0.05
To constrain the interpretation of the imaging results in the context of the behavioral findings, we tested the association between beta-weights in the OFC on correct trials and overall task performance, as measured by d-prime. There was a negative correlation [r (171) = −0.1631, p = 0.0319, Figure 4c], with greater OFC activity being associated with poorer overall task performance (i.e., lower d-prime scores). This association was further examined using a linear model showing that OFC activity significantly contributed to the variance of task performance (t = −2.074, df = 164, p < 0.05) while controlling for age group, peer condition, gender, and sites. This association did not differ when including or excluding the three outliers (see Supplementary Figure S3).
4 |. DISCUSSION
The goal of this study was to test the combined effects of peer presence, exposure to positive social cues, and reward anticipation on adolescent cognitive control. Adolescents 13–17 showed diminished cognitive control when presented with positive social cues in a rewarding context and in the presence of peers relative to when alone. This pattern was not observed in adults 18 and older. This finding is consistent with previous studies that have separately examined the effects of peer (Chein et al., 2011; Gardner & Steinberg, 2005; Knoll, Magis-Weinberg, Speekenbrink, & Blakemore, 2015), rewards (Galvan et al., 2006), and positive social cues (Jones et al., 2014; Somerville et al., 2011) on cognitive control in adolescents. As there were no developmental differences in performance in the alone condition for the combined positive arousal conditions (positive social cue and anticipation of reward), these data suggest that diminished cognitive control under contextually exciting and rewarding conditions may be exacerbated or amplified by the presence of a peer in teens.
The deterioration of adolescent performance under the combined rewarding and positive social conditions was paralleled by increased orbitofrontal activity on correct trials. Given the role of the OFC in integrating regulatory, social, and affective computations (Roy, Shohamy, & Wager, 2012) through its direct projections to nuclei involved in affect and motivation (Price, 2007), one plausible explanation for this enhanced activation is that it provided additional cognitive resources to exert successful cognitive control when the teens were experiencing the most cognitive interference (i.e., when in the presence of peers, positive social cues, and potential reward). The confluence of socioemotional factors in the current experiment were more cognitively taxing for younger individuals. Less mature functional connectivity in cortico-cortical circuits (Hwang, Velanova, & Luna, 2010) may have limited cognitive regulation in the context of “triple arousal” (i.e., peers, rewards, and positive social cues).
Our findings suggest that previous empirical estimates of contextual influences on adolescent decision-making may under-represent the relevance of these influences when they co-occur, as is so frequently the case in naturalistic settings. For example, a recent study found no effects of peer presence on response inhibition in adolescents who were tested using a non-arousing or rewarding cognitive control task (Smith et al., in press). This finding suggests that focusing on a single social or emotional factor to assess adolescent behavior and neurobiology may not fully elucidate social influences on adolescent cognitive control and underlying functional circuitry. The utility of a paradigm that manipulates multiple social factors is that it may better reflect the real world situations in which teens often find themselves (e.g., having fun with good friends). This study highlights the importance of considering the combined effects of such motivational contexts on adolescent behavior and cognitive control.
Interpretation of the current findings should be considered in the context of potential limitations. The socioemotional manipulations and stimuli of our study may have impacted the results. We used a “virtual” rather than a real peer. The presence of an actual peer in lieu of our virtual peer could have led to an enhanced effect in adolescents and perhaps impacted performance more in the older age groups. However, the self report ratings suggest that the manipulation was effective for all age groups, as has been found in prior studies using similar methods (see Guyer, McClure-Tone, Shiffrin, Pine, & Nelson, 2009; Jones et al., 2011, 2014; Weigard et al., 2014). Notably, in the current study fewer than 15% of participants reported feeling dubious of the peer manipulation, and several indicated interest in meeting the peer following the study session. We also recognize that real social interactions may have yielded enhanced socioemotional responses from participants compared to the simple presentation of brief socioemotional cues (faces). Given that we did not include nonsocial stimuli (i.e., all the stimuli were faces), we cannot make strong claims on how these cues impact performance distinctly from nonsocial ones. In addition, we counterbalanced the order of emotional states and cues across subjects, but did not include order of these experimental conditions as regressors in our analysis. We recognize that presentation of a different affective cue or induction of a different affective state may impact the behavioral and neurobiological response to subsequent cues and states. However, by counterbalancing these factors, the effects of any given state or cue on subsequent responses was minimized. We acknowledge the difference in effects between sites, and suggest that while experimenters used the same language to introduce the task, different emphasis may have been placed on the potential for reward at one site compared to the other—yielding a difference in performance. To account for this difference, we controlled for site in our analyses, but suggest future studies consider the delivery of the instructions to participants. There were also fewer subjects in the peer condition than in the non-peer condition. Enrollment of participants in the peer condition was limited to individuals who had not been enrolled previously in studies with “peer” manipulations. However, future research may consider utilizing a confederate or real peer to determine whether the differences reported hold. Finally, the interpretation of findings would be strengthened with the addition of an objective measure of arousal. Although prior work using this paradigm (Cohen et al., 2015; Cohen, Dellarco, et al., 2016) has reported converging subjective (self report ratings) and objective (skin conductance) measures of arousal, there were insufficient skin conductance data across the two experimental groups for each age group in the current study.
Our findings suggest the importance of examining social influences on adolescent decision-making in combination rather than in isolation and have potential implications for informing social policies that protect and support youth. By providing evidence of a combined effect of social and reward influences on cognitive control, this research may help guide public health strategies and policies for modifying the environment to protect young people in these situations to establish lasting positive outcomes for teens.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge support from the John D. and Catherine T. MacArthur Foundation to Vanderbilt. Its contents reflect the views of the authors, and do not necessarily represent the official views of either the John D. and Catherine T. MacArthur Foundation or the MacArthur Foundation Research Network on Law and Neuroscience (www.lawneuro.org).
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- Achterberg M, Peper XJS, van Duijvenvoorde ACK, & Mandl CW (2016). Frontostriatal white matter integrity predicts development of delay of gratification: A longitudinal study. The Journal of Neuroscience, 36(6), 1954–1961. 10.1523/JNEUROSCI.3459-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, & O’Doherty JP (2010). Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology, 35, 48–69. 10.1038/npp.2009.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, … Ochsner KN (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology, 66, 295–319. 10.1146/annurev-psych-010814-015156 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Galván A, & Somerville LH (2016). Developmental Cognitive Neuroscience Beyond simple models of adolescence to an integrated circuit-based account: A commentary. Accident Analysis and Prevention, 17, 128–130. 10.1016/j.dcn.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauffman E, Shulman EP, Steinberg L, Claus E, Banich MT, Graham S, & Woolard J (2010). Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Developmental Psychology, 46(1), 193–207. 10.1037/a0016128 [DOI] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, & Steinberg L (2011). Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science, 14(2), F1–10. 10.1111/j.1467-7687.2010.01035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew KS, & Braver TS (2011). Positive affect versus reward: Emotional and motivational influences on cognitive control. Frontiers in Psychology, 2, 1–10. 10.3389/fpsyg.2011.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AO, Breiner K, Steinberg L, Bonnie RJ, Scott ES, Taylor-Thompson KA, … Casey BJ (2016). When is an adolescent an adult? assessing cognitive control in emotional and nonemotional contexts. Psychological Science, 27(4), 549–562. 10.1177/0956797615627625 [DOI] [PubMed] [Google Scholar]
- Cohen AO, Dellarco DV, Breiner K, Helion C, Heller AS, Rahdar A, …. Casey BJ (2016). The impact of emotional states on cognitive control circuitry and function. Journal of Cognitive Neuroscience, 28, 446–459. [DOI] [PubMed] [Google Scholar]
- Cohen-Gillbert J, Killgore WDS, White CN, Schwab ZJ, Crowley DJ, Covell MJ, … Silveri MM (2014). Differential influence of safe versus threatening facial expressions on decision-making during an inhibitory control task in adolescence and adulthood. Developmental Science, 17(2), 212–223. 10.1111/desc.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML (1994). Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychological Assessment, 6(2), 117–128. 10.1037/1040-3590.6.2.117 [DOI] [Google Scholar]
- Cox RW (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal, 29(3), 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017). FMRI clustering in AFNI: False-positive rates redux. Brain Connectivity 7, 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Vega A, De Chang XLJ, Banich MT, Wager XTD, & Yarkoni XT (2016). Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. The Journal of Neuroscience, 36(24), 6553–6562. 10.1523/JNEUROSCI.4402-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power D, Church JA, … Schlaggar BL (2011). Prediction of individual brain maturity using fMRI. Science, 329(5997), 1358–1361. 10.1126/science.1194144.Prediction [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss M, Caudle K, Drysdale AT, Johnston NE, Cohen AO, Somerville LH, … Casey BJ (2014). Teens impulsively react rather than retreat from threat. Developmental Neuroscience, 36(3–4), 220–227. 10.1159/000357755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijvenvoorde ACK, Van Achterberg M, Braams BR, Peters S, & Crone EA (2016). NeuroImage Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. Neuroimage, 124, 409–420. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Clusture failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Science of the United States of America, 113(28), 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Pine DS, & Hardin M (2006). Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine, 36, 299–312. 10.1017/S0033291705005891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, … Petersen SE (2009). Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology, 5(5), 1–14. 10.1371/journal.pcbi.1000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Mackinlay RJ, Wilkening F, & Weber EU (2009). Affective and deliberative processes in risky choice: Age differences in risk taking in the Columbia Card Task. Journal of Experimental Psychology. Learning, Memory, and Cognition, 35(3), 709–730. 10.1037/a0014983 [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, & Schultz W (2003). Discrete coding of reward probability and uncertainty by dopamine neurons. Science (New York, N.Y.), 299(5614), 1898–1902. 10.1126/science.1077349 [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, & Casey BJ (2006). Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(25), 6885–6892. 10.1523/JNEUROSCI.1062-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, & Steinberg L (2005). Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Developmental Psychology, 41(4), 625–635. 10.1037/0012-1649.41.4.625 [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, & Luna B (2009). Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex (New York, N.Y.: 1991), 20(7), 1613–1629. 10.1093/cercor/bhp225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C, & Luna B (2009). The maturation of incentive processing and cognitive control. Pharmacology Biochemistry and Behavior, 93(3), 212–221. 10.1016/j.pbb.2009.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, … Thompson PM (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101(21), 8174–8179. 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, & Nelson EE (2009). Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development, 80(4), 1000–1015. 10.1111/j.1467-8624.2009.01313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, & Knutson B (2009). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35, 4–26. 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Doherty JO, Camerer CF, Schultz W, & Rangel A (2008). Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. The Journal of Neuroscience, 28(22), 5623–5630. 10.1523/JNEUROSCI.1309-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Velanova K, & Luna B (2010). Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: A functional magnetic resonance imaging effective connectivity study. The Journal of Neuroscience, 30(46), 15535–15545. 10.1523/JNEUROSCI.2825-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Somerville LH, Li J, Ruberry EJ, Libby V, Glover G, … Casey BJ (2011). Behavioral and neural properties of social reinforcement learning. The Journal of Neuroscience, 31(37), 13039–13045. 10.1523/JNEUROSCI.2972-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Somerville LH, Li J, Ruberry EJ, Powers A, Mehta N, … Casey BJ (2014). Adolescent-specific patterns of behavior and neural activity during social reinforcement learning. Cognitive, Affective & Behavioral Neuroscience, 14(2), 683–697. 10.3758/s13415-014-0257-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Minati L, Marizzoni M, Marchitelli R, Sala-Llonch R, Bartrés-Faz D, … Frisoni GB (2016). Longitudinal reproducibility of default-mode network connectivity in healthy elderly participants: A multi-centric resting-state fMRI study. Neuroimage, 124, 442–454. 10.1016/j.neuroimage.2015.07.010 [DOI] [PubMed] [Google Scholar]
- Knoll LJ, Magis-Weinberg L, Speekenbrink M, & Blakemore S-J (2015). Social influence on risk perception during adolescence. Psychological Science, 26(5), 1–10. 10.1177/0956797615569578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Paulsen DJ, Padmanabhan A, & Geier C (2013). The teenage brain: Cognitivecontrol andmotivation. Current Directions in Psychological Science, 22(2), 94–100. 10.1177/0963721413478416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, & Creelman CD (2005). Detection theory: A user’s guide (2nd ed.). Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Ochsner KN, & Gross JJ (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–249. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Plassmann H, Doherty JPO, & Rangel A (2010). Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. The Journal of Neuroscience, 30(32), 10799–10808. 10.1523/JNEUROSCI.0788-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL (2007). Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Annals of the New York Academy of Sciences, 1121, 54–71. 10.1196/annals.1401.008 [DOI] [PubMed] [Google Scholar]
- Rangel A, & Hare T (2010). Neural computations associated with goal-directed choice. Current Opinion in Neurobiology, 20, 262–270. 10.1016/j.conb.2010.03.001 [DOI] [PubMed] [Google Scholar]
- Richards JM, Plate RC, & Ernst M (2013). A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: The impact of task design and implications for understanding neurodevelopment. Neuroscience and Biobehavioral Reviews, 37(5), 976–991. 10.1016/j.neubiorev.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, & Wager TD (2012). Ventromedial prefrontalsubcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–156. 10.1016/j.tics.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, & Montague PR (1997). A neural substrate of prediction and reward. Science, 275(5306), 1593–1599. 10.1126/science.275.5306.1593 [DOI] [PubMed] [Google Scholar]
- Schultz W, & Tremblay L (2006). Involvement of primate orbitofrontal neurons in reward, uncertainty, and learning. In Zald DH & Rauch S (Eds.), Oribitofrontal cortex (pp. 173–198). Oxford: Oxford University Press. [Google Scholar]
- Silva K, Shulman EP, Chein J, & Steinberg L (2015). Peers increase late adolescents’ exploratory behavior and sensitivity to positive and negative feedback. Journal of Research on Adolescence, 26(4), 696–705. 10.1111/jora.12219 [DOI] [PubMed] [Google Scholar]
- Silverman MH, Jedd K, & Luciana M (2015). Neural networks involved in adolescent reward processing: An activation likeli hood estimation meta-analysis of functional neuroimaging studies. Neuroimage, 122, 427–439. 10.1016/j.neuroimage,2015.07.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin R, … Ochsner KN (2016). The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-to-dorsal shift in medial prefrontal recruitment. Developmental Cognitive Neuroscience, 18(5), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, Steinberg L, Strang N, & Chein J (2015). Age differences in the impact of peers on adolescents’ and adults’ neural response to reward. Developmental Cognitive Neuroscience, 11, 75–82. 10.1016/j.dcn.2014.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare TA, & Casey BJ (2011). Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience, 23(9), 2123–2134. 10.1162/jocn.2010.21572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER (2004). Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience, 24(38), 8223–8231. 10.1523/JNEUROSCI.1798-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Colin J, Jernigan TL, & Toga AW (1999). In vivo evidence for maturation in frontal and striatal regions. Nature Neuroscience, 2(10), 859–861. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, & Banich M (2009). Age differences in future orientation and delay discounting. Child Development, 80(1), 28–44. 10.1111/j.1467-8624.2008.01244.x [DOI] [PubMed] [Google Scholar]
- van den Bos W, Rodriguez CA, Schweitzer JB, & Mcclure SM (2015). Adolescent impatience decreases with increased fronto striatal connectivity. Proceedings of the National Academy of Sciences USA, 112(29), E3765–E3774. 10.1073/pnas.1423095112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoorn J, Van Dijk E, Guroglu B, & Crone EA (2016). Neural correlates of prosocial peer influence on public goods game donations during adolescence. Social Cognitive Affective Neuroscience, 11(6), 923–933. https://doi.10.1093/scan/nsw013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Gunther Moor B, Op de Macks ZA, Rombouts SARB, Westenberg PM, & Crone EA (2010). Adolescent risky decision-making: Neurocognitive development of reward and control regions. Neuroimage, 51(1), 345–355. 10.1016/j.neuroimage.2010.02.038 [DOI] [PubMed] [Google Scholar]
- Weigard A, Chein J, Albert D, Smith A, & Steinberg L (2014). Effects of anonymous peer observation on adolescents’ preference for immediate rewards. Developmental Science, 17(1), 71–78. 10.1111/desc.12099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AF (2003). Teenage drivers: Patterns of risk. Journal of Safety Research, 34(1), 5–15. http://www.ncbi.nlm.nih.gov/pubmed/12535901 [DOI] [PubMed] [Google Scholar]
- Zimring FE (1998). American youth violence (1st ed.). New York, NY: Oxford University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.