Abstract
Neutralizing antibodies (NAbs) form the basis of immunotherapeutic strategies against many important human viral infections. Accordingly, we studied the prevalence, titer, genotype-specificity, and mechanism of action of anti-polyomavirus BK (BKV) NAbs in commercially available human immune globulin (IG) preparations designed for intravenous (IV) use. Pseudovirions (PsV) of genotypes Ia, Ib2, Ic, II, III, and IV were generated by co-transfecting a reporter plasmid encoding luciferase and expression plasmids containing synthetic codon-modified VP1, VP2, and VP3 capsid protein genes into 293TT cells. NAbs were measured using luminometry. All IG preparations neutralized all BKV genotypes, with mean EC50 titers as high as 254 899 for genotype Ia and 6,666 for genotype IV. Neutralizing titers against genotypes II and III were higher than expected, adding to growing evidence that infections with these genotypes are more common than currently appreciated. Batch to batch variation in different lots of IG was within the limits of experimental error. Antibody mediated virus neutralizing was dose dependent, modestly enhanced by complement, genotype-specific, and achieved without effect on viral aggregation, capsid morphology, elution, or host cell release. IG contains potent NAbs capable of neutralizing all major BKV genotypes. Clinical trials based on sound pharmacokinetic principles are needed to explore prophylactic and therapeutic applications of these anti-viral effects, until effective small molecule inhibitors of BKV replication can be developed.
Introduction
Polyomavirus BK (BKV) imposes a significant burden on the organ transplant recipient community. It is estimated that 4.6–28% of 60 000 kidney transplants performed worldwide are potentially compromised by BKV nephropathy (BKVN) or BK viremia (1–4). BKVN can be seen in up to 17% of all biopsies 6–12 months posttransplant (5). When nephropathy develops, approximately 20% of grafts are lost. At present, there are no highly effective antiviral drugs for treating polyomavirus infections and the only established intervention is reduction in immunosuppressive therapy. In addition to BKVN, persistent graft dysfunction is observed in 24% of patients (6). BKV also causes symptomatic hemorrhagic cystitis in approximately 5% of 50 000 hematopoietic stem cell transplants performed annually worldwide, and in 5% of cancer patients treated with high dose cyclophosphamide. Hemorrhagic cystitis causes prolonged hospitalization and severe cases can be fatal, due to blood loss or co-existing morbidities. Thus, there is an urgent need to develop anti-BKV therapies.
The ideal intervention for BKV infection would be an orally available small molecule that can block viral replication, but no such compound is currently available. In an in vitro screening of approximately 1500 compounds conducted over a period of 10 years, the most potent anti-BKV effect was found in human immunoglobulins (7), with some preparations showing greater than 95% inhibition at a concentration of 0.1 μg/mL with no cytotoxicity. This corresponds to a selectivity index of >1000, compared to selectivity indexes of 3.8 and 2.3 for cidofovir and leflunomide respectively (8). In the current study, we have tested the virus-neutralizing activity of human immunoglobulins against a panel of pseudovirions representing all the major known BKV genotypes circulating in humans. The mechanism of action has been investigated and potential clinical applications are discussed.
Methods
Genotype-specific BKV reporter vectors (pseudovirions)
VP1 expression constructs used in this work were reported previously (9,10). Viral isolates BKV-Ia (BK-D; accession number JF894228), BKV-Ib2 (PittVR2; DQ989796), BKV-Ic (RYU-2; AB211377), BKV-II (GBR-12; AB263920), BKV-III (KOM-3; AB211386), and BKV-IVb (THK-8; AB211390) were used to design codon-modified ORFs, which were chemically synthesized by Blue Heron Biotech. Gateway recombination technology (Invitrogen) was used to transfer the codon-modified open reading frames into expression plasmid pGwf (4). The codon-modified VP2/VP3 minor capsid protein genes based on BKV isolate A-66H were used for production of all BKV pseudoviruses.
Generation of pseudovirions
Briefly, capsid protein expression plasmids containing synthetic codon-modified VP1, VP2, and VP3 genes were co-transfected with a reporter plasmid encoding Gaussia luciferase (phGluc) into 293TT cells. Forty eight hours after transfection, the cells were suspended at >100 million cells/mL in Dulbecco’s phosphate buffered saline (DPBS) followed by a 15 min incubation at 37°C with neuraminidase V (Sigma). Cells were then lysed by addition of 0.5% Triton X-100, and RNase A/T1 cocktail (Ambion). The lysate was buffered by addition of 25 mM ammonium sulfate pH 9 and incubated at 37°C overnight to allow capsid maturation, then clarified by spinning twice for 10 min at 5,000 × g, with gentle mixing of the supernatant between spins. Reporter vector particles were purified out of the clarified supernatant by ultracentrifugation through a 27–33–39% iodixanol (Optiprep, Sigma) step gradient prepared in DPBS with a total of 0.8 M NaCl.
Pseudovirion neutralization assays
293TT cells were seeded at a density of 3 × 104 per well in 96 well plates and allowed to attach for 3–5 h. Privigen (human immune globulin intravenous, IVIG) and Cytogam (cytomegalovirus immune globulin intravenous, CMVIG) were obtained from CSL Behring AG (Bern, Switzerland). Each IG preparation was tested at serial dilutions in triplicate: 24 μL of diluted antibodies are mixed with 96 μL of diluted and previously titrated pseudovirion reporter vector stocks corresponding to the appropriate BKV genotype, and placed at 4°C for 1 h. Dilutions were performed in cell culture medium (DMEM without phenol red supplemented with 25 mM HEPES, 10% heat-inactivated fetal bovine serum, 1% MEM non-essential amino acids, 1% Glutamax and 1% antibiotic-antimycotic, all from Invitrogen). One hundred micro liter of the antibody treated pseudovirion mixture warmed to room temperature was added to the cells which were then further incubated at 37°C for 72 h. Conditioned supernatants (20 μL) were harvested into white 96-well luminometry plates (Perkin Elmer) containing 50 μL of Gaussia Luciferase assay buffer prepared as per instructions contained in the BioLux Gaussia Luciferase Flex Assay Kit (New England BioLabs cat#E3308L; Kit containing BioLux Gluc Flex stabilizer). Following an incubation for 35–40 s at room temperature, luminescence was measured by a BioTek Synergy (Gen5 TM) HT Multidection Microplate Reader with a signal integration time of 8 s per well applied to groups of 6 wells at one time. Fifty percent neutralizing titers (EC50) were calculated based on dose-response curves with top and bottom values constrained to the average values of “no IVIG/CMVIG” and “no reporter vector” control wells, respectively (9).
Effect of complement on pseudovirion neutralization
Neutralization assays were performed as described above in 96 well plates, except that complement was present during the incubation of pseudovirions with IVIG. In initial experiments with pseudovirions 1a and 1b2, sterile filtered normal human serum without heat inactivation was used as the source of complement (Gemini Bio-Products, West Sacramento, CA, Cat #100–110, used at 3 dilutions 1:10, 1:50, 1:100). Since serum can contain virus neutralizing antibody and other undefined non-specific inhibitors, and its actual complement content was not known, subsequent experiments were performed on Pseudovirion Ia and IVc2 using defined concentrations of purified complement C3 from human serum (Sigma-Aldrich, St. Louis, MO, cat # C2910, molecular weight 185 kDa). Specifically, these experiments used two different dilutions of antibody (1: 6400 and 1:102 400) and three concentrations of C3 (10, 5, and 3 times the molar concentration of immunoglobulin in the culture wells).
Neutralization of infectious BKV particles
One million (1E + 06) 293 TT cells were plated in 6 well tissue culture plates containing DMEM medium with 10% fetal bovine serum, 1% glutamine, 1% HEPES, and 0, 100 or 1000 μg/mL IG. Cells were infected with the Gardner strain of BKV (genotype Ia) at a virus:cell ratio of 500:1 at 37°C for 2 h. Free virus was then removed with three washes of PBS and tissue culture medium containing different concentrations of IVIG was replenished. Aliquots of medium were collected on the 3rd, 5th, and 7th day of incubation. The cells were also harvested by treating with 0.05% Trypsin for 1–2 min at 37°C, washed thrice in cold PBS, pH 7.4, pelleted, and stored at −70°C until assayed for BKV genomic load using qPCR (11).
BKV cell binding and elution studies
Two million (2E + 06) 293 TT cells were plated in 6 well tissue culture plates containing cold (4°C) DMEM containing 0, 100 or 1000 μg/mL IVIG. Cells were incubated with BKV-Gardner at a virus: cell ratio of infection of 500:1 for 2 h at 4°C following which free virus was removed with three washes of PBS to determine the total amount of BKV bound to the target cells (0 h time point). In elution experiments parallel wells were maintained at 4°C to measure cell bound and supernatant released BKV 2, 4 and 24 h later using real time PCR. All cell washing steps during the harvesting procedure were also performed at 4°C.
Effect of IVIG on BKV release from host cells
Experimental set up was similar to that described above for neutralization of infectious BKV, except that infection was accomplished in the absence of IVIG. IVIG exposure was commenced 24 h later and continued till day 7. Aliquots of culture medium collected on day 4 and day 7 and cells harvested on day 7 were saved for BKV real time PCR.
Electron microscopy
BKV grown in WI-38 cells was purified over a cesium chloride gradient using standard methodology. Viral particle concentration in the purified preparation was estimated by real time PCR, and adjusted to 1E + 07 genomic equivalents per ml. Using an IVIG solution at a final concentration of 100 μg/mL IVIG. BKV neutralization was allowed to proceed for 2 h at 37°C. Antibody treated virus was deposited on 3 mm2 circular carbon coated copper grids previously activated by the glow discharge method. Grids were counterstained with a freshly prepared solution of 2% uranium acetate and examined using scanning electron microscopy.
Results
Demonstration of neutralizing antibodies
IVIG prepared from healthy subjects contained neutralizing antibodies to all pseudovirions tested (Table 1). EC50 titers varied from 2866.2 for genotype III to 254 899 for genotype Ia. The geometric standard deviations which are dimensionless multiplicative factors ranged from 1.1 to 3.2. The relative strengths of the titers for genotype Ia and IV reflect the prevalence of these genotypes in the US population. Thus, genotype I accounts for 46–82% of polyomavirus strains worldwide, while genotype IV accounts for most of the remaining subjects. On the other hand, urinary prevalence of genotypes II and III in the USA is typically quite low and in the range of 0–9% (12), and antibody titers to these two genotypes were higher than might be expected from their population prevalence.
Table 1:
Titers of neutralizing antibodies to six different BKV genotypes in four different commercially available human immunoglobin preparations
| Ia | Ib2 | Ic | II | III | IV | |
|---|---|---|---|---|---|---|
| IVIG #1 | 254,899.1 ± 1.1 | 14,918.1 ± 1.4 | 17,384.4 ± 1.6 | 18,368.5 ± 2.0 | 2,866.2 ± 1.3 | 3,359.0 ± 1.2 |
| IVIG #2 | 75,791.1 ± 1.7 | 17,321.4 ± 1.2 | 41,026.7 ± 3.2 | 18,169.4 ± 1.2 | 8,345.0 ± 2.0 | 6,666.8 ± 1.9 |
| CMVIG #1 | 157,370.0 ± 1.0 | 7,745.2 ± 1.5 | 15,827.3 ± 1.1 | 17,497.5 ± 1.4 | 1,893.4 ± 1.6 | 2,605.4 ± 1.1 |
| CMVIG #2 | 33,067.8 ± 2.3 | 9,544.4 ± 1.3 | 13,333.2 ± 1.7 | 8,124.2 ± 1.3 | 5,628.3 ± 2.7 | 3,412.2 ± 1.87 |
Data represents geometric means ± SE of six experiments for each condition evaluated.
Pseudovirion neutralization was dose dependent and increasing antibody concentrations led to progressive reduction in the luminescence signal, thus generating a sigmoid inhibition curve (Figure 1). Batch to batch variation was evaluated by testing two different lots of each IG preparation (Figure 2). The geometric mean of the EC50 for antibodies to pseudovirion Ib2 and II were almost identical in lots 1 and 2. Antibodies to pseudovirions I, III, and IV were up to approximately 2–4 times higher in lot 2, whereas lot 1 had more reactivity with Pseudovirion Ia. Neutralizing antibody titers in CMVIG were broadly similar to those found in IVIG, and lot to lot variation was comparable (Table 1).
Figure 1: Titers of anti-BKV genotype Ia neutralizing antibodies in commercial preparations of human intravenous immunoglobulin (IVIG) and cytomegalovirus hyperimmune intravenous immunoglobulin (CMVIG).
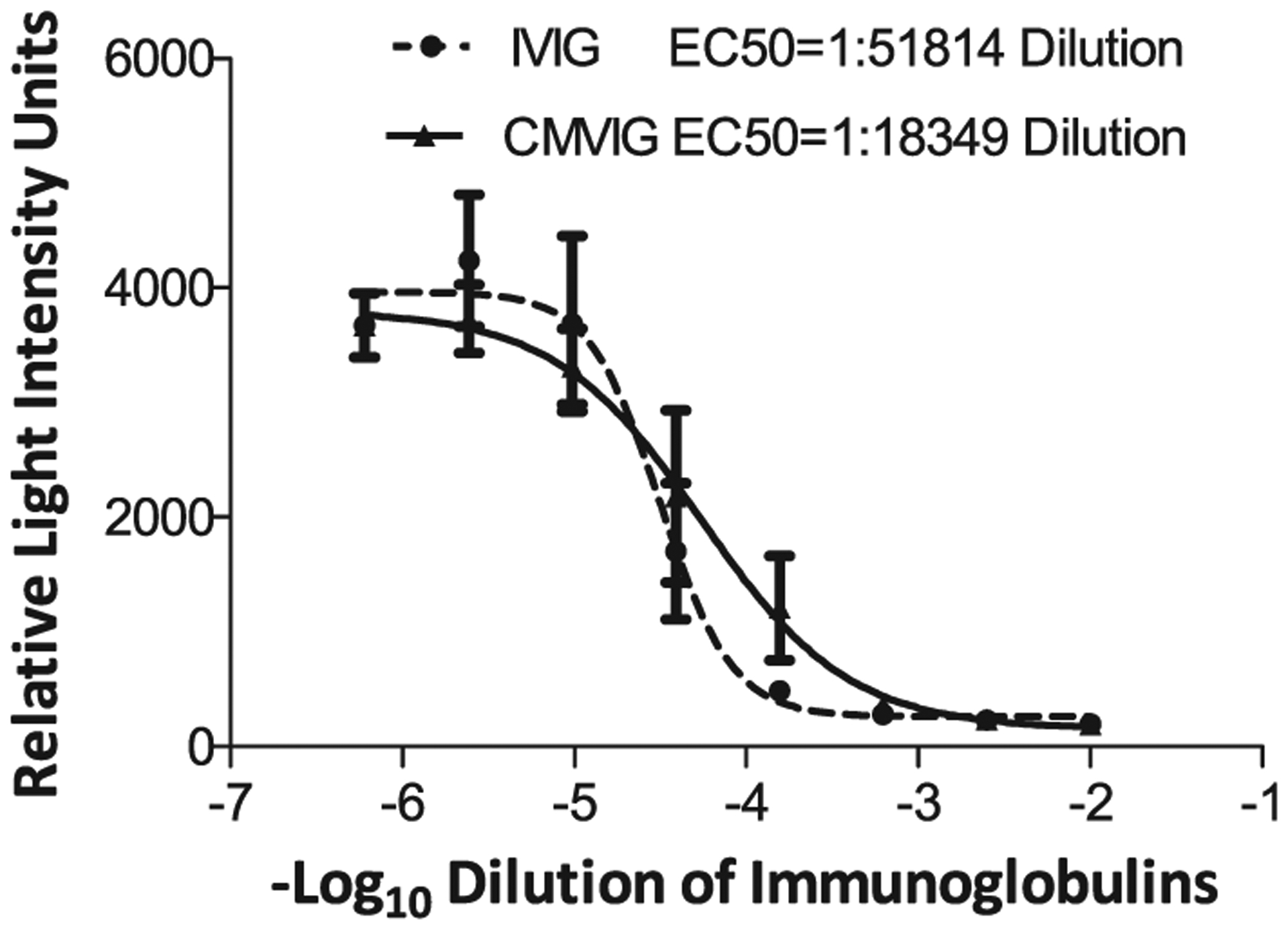
The x-axis depicts serial dilutions (log10) of IVIG and CMVIG added to the culture of 293TT cells exposed to BKV genotype Ia reporter vector. Luciferase release measured in relative light intensity units (RLUs) is plotted on the y-axis. Fifty percent inhibition (EC50) was obtained at a dilution of 1:51814 for IVIG and 1:18349 for CMVIG. Data is expressed as mean ± SE from triplicate wells of one representative experiment. The curves were plotted using Graph Pad Prism software.
Figure 2: Scatter plot of the geometric means of neutralizing antibodies to the pseudovirions panel in two different lots of IVIG.
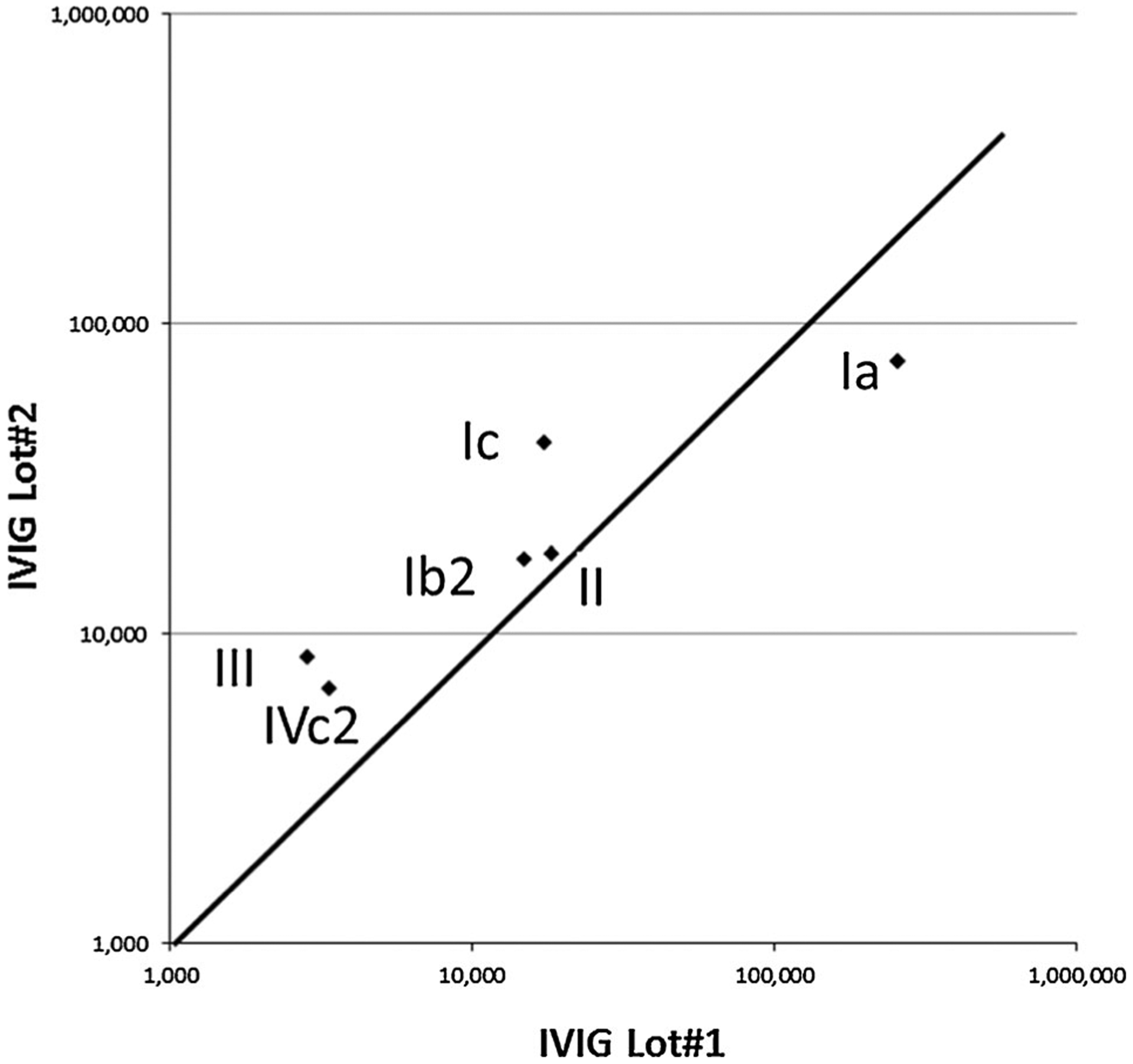
Lot 1 is represented on x-axis and lot 2 on y-axis.
Enhancement of pseudovirion neutralization by human complement
Pseudovirion neutralization experiments were performed in the presence or absence of C3 at two different dilutions of IVIG. At a 1:6400 dilution, the luminescence signal generated in the presence of 10× molar excess of C3 was approximately 60% of the signal obtained in the absence of C3, thus demonstrating neutralizing activity beyond that exerted by IVIG alone. This modest but consistent effect was dose dependent. At 1× molar excess of C3 the luminescence signal increased to 80% of the expected value (Figure 3, left panel). At a 1:102 400 dilution of IVIG the effect was weakened and measurements became more variable. Complement mediated enhancement of neutralization was also shown for Pseudovirion Ic using human complement C3 (Figure 3, right panel) and for Pseudovirions 1a and Ib2 using normal human serum as a source of complement (data not shown).
Figure 3:
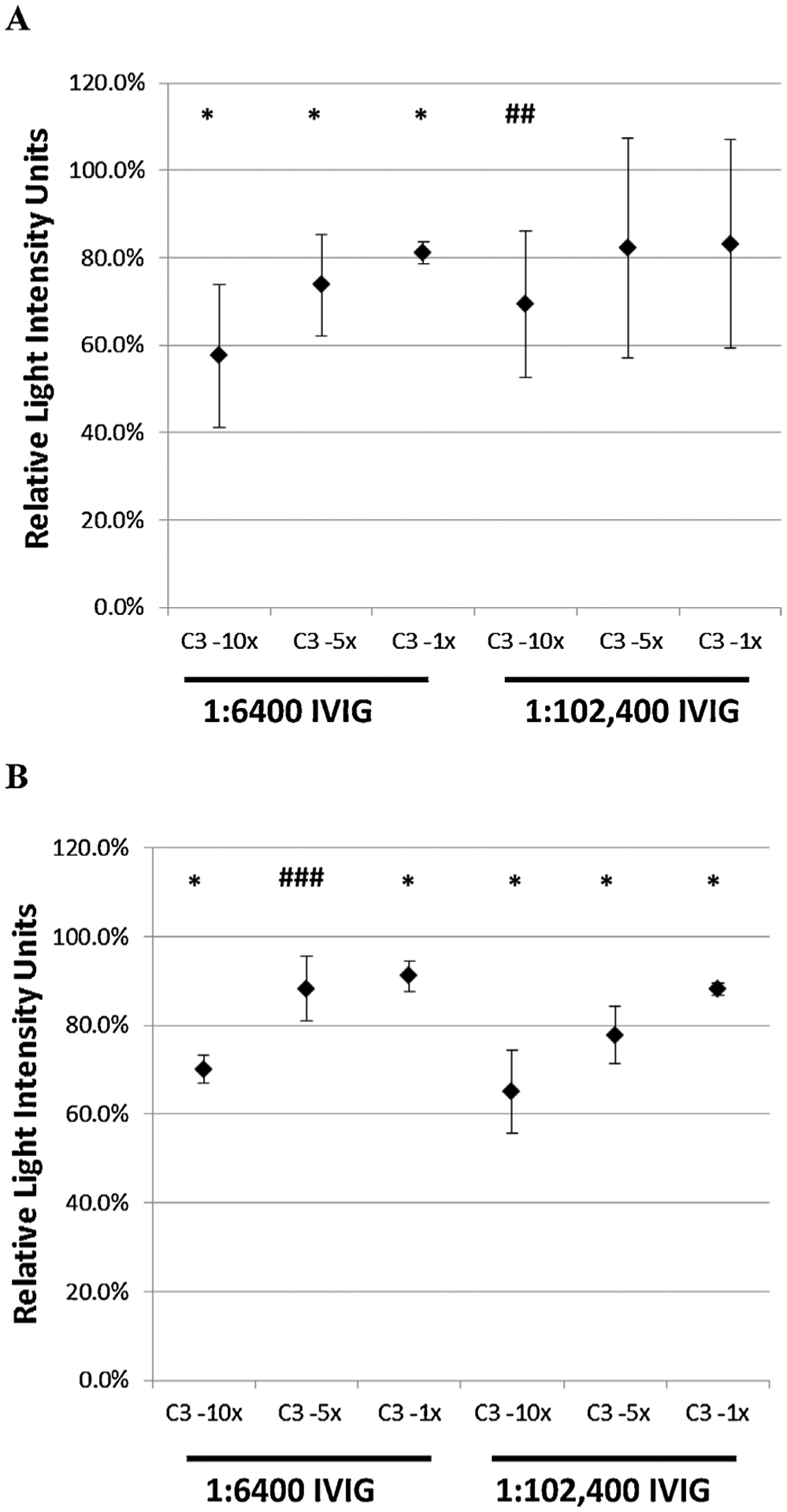
The effect of complement on neutralization of pseudovirions Ia (A) and 1Vc (B) by IVIG. Neutralizing experiments described in the Methods section were performed in the presence of two different dilutions of antibody (1: 6400 and 1:102,400) and thee concentrations of C3 (10×, 5× and 3× times the molar concentration of immunoglobulin in the culture wells). The luminescence signal obtained is expressed as a percentage of the maximal signal obtained in the presence of IVIG alone (without C3). Data represents mean +/− SD of 4 experiments for each condition evaluated. p-values refer to comparisons of the individual signals with the maximum signal. p < 0.05, ##p = 0.06, ###p = 0.07.
Neutralization of infectious BKV particles
The neutralizing effect of IVIG on pseudovirions was confirmed in a culture system that used the infectious BKV strain Gardner (genotype Ia) (Table 2). Cell associated BKV DNA on day 7 of culture was reduced by approximately 1 log at a concentration of 100 μg/mL and 3 logs at 1000 μg/mL. Assays of the supernatants demonstrated release of BKV from intact cells that had not reached the lytic phase of the viral life cycle. Viral release led to progressive accumulation of BKV DNA with time by a mechanism that was not affected by IVIG.
Table 2:
Neutralizing effect of IVIG on infectious BKV
| BKV released into medium1 | Cell associated BKV | |||
|---|---|---|---|---|
| 3 days | 5 days2 | 7 days2 | 7 days | |
| Control | 1.31E+07 ± 4.60E+06 | 8.31E+09 ± 2.14E+09 | 1.53E+10 ± 5.06E+09 | 5.32E+09 ± 1.04E+093 |
| IVIG 100 μg/mL | 1.17E+07 ± 3.86E+06 | 1.13E+08 ± 1.16E+08 | 1.47E+08 ± 1.46E+08 | 1.04E+08 ± 7.19E+07 |
| IVIG 1000 μg/mL | 1.13E+07 ± 2.22E+06 | 1.01E+07 ± 3.25E+06 | 2.26E+07 ± 3.06E+07 | 2.04E+06 ± 1.26E+06 |
BKV released into the tissue culture medium is expressed as total viral genomic equivalents (mean ± SD, n = 6) recovered from one well. Cell associated BKV was measured only once when cells were harvested on day 7.
p < 0.05 compared to 3 days (Mann–Whitney Rank Sum Test).
p < 0.05 compared to cells treated with IVIG at 100 or 1000 μg/mL.
Cell binding and elution studies
Cell binding studies were conducted at 4°C to minimize cellular uptake. Baseline measurements indicated that approximately 10% of viral input (1E + 09 genomic equivalents) could be detected in the bound state under these conditions (Table 3). Cell binding was reduced by IG, particularly at the concentration of 1000 μg/mL. Maximal elution occurred from the cells in the first 2 h at which time cell bound BKV represented point only 30–40% of the baseline value. A fall in cell bound BKV was accompanied by an expected rise in eluted BKV at 4 h. The rate of viral elution was not altered by IG (data not shown).
Table 3:
Effect of IVIG on BKV binding to and subsequent elution from 293TT cells
| Cell bound BKV1 | ||||
|---|---|---|---|---|
| 0 h | 2 h2 | 4 h | 24 h | |
| Control | 1.62E+08 ± 1.41E+06 | 6.32E+07 ± 7.35E+06 | 9.44E+07 ± 3.39E+06 | 5.99E+07 ± 1.97E+07 |
| IVIG 100 μg/mL | 1.59E+08 ± 1.48E+ 07 | 5.70E+07 ± 1.33E+07 | 6.87E+07 ± 2.50E+07 | 3.84E+07 ± 6.12E+06 |
| IVIG 1000 μg/mL | 1.08E+08 ± 2.45E+07 | 3.90E+07 ± 1.20E+06 | 5.62E+07 ± 4.81E+06 | 1.16E+07 ± 5.66E+05 |
Cell bound BKV refers to total viral genomic equivalents (mean ± SD) recovered from adherent cells harvested from one well a 6-well tissue culture plate.
p < 0.05 compared to 0 hr cell bound BKV (Mann–Whitney Rank Sum Test).
Effect of IVIG on BKV release from host cells
The reduction in day 7 cell-associated BKV DNA noted above was likely the result of virus neutralization and hampered cell entry during the 2 h virus infection phase. To study the effect of IVIG on viral release independent of this initial effect on cell entry, experiments were performed in which IVIG exposure was initiated 24 h after the time of infection (Table 3). Viral release was again observed in the absence of cell lysis and not affected by IVIG. Cell associated BKV on day 7 was also similar in the presence or absence of IVIG. These data indicate that virus replication throughout the experiment was primarily intra-cellular. It appears that the cell culture system studied does not support amplification of viral replication by BKV release followed by infection of additional cells.
Electron microscopy
IVIG treatment did not alter viral morphology as examined at magnifications of up to 75 000. Virion measurements were in the range of 40–50 nm. No immune complexes were seen coating the BKV protein capsids. There was no evidence of viral aggregation at the potently neutralizing antibody concentration tested (data not shown).
Discussion
The experiments performed demonstrate that IVIG and CMVIG contain neutralizing antibodies directed against all major BKV genotypes. One would expect that the relative strength of titers to different pseudovirions would reflect the urinary prevalence of different genotypes and subtypes in the USA. This is true to the extent that antibody titers were highest against genotype Ia and IV. However, titers for antibodies to genotype II and III are higher than expected. The reasons for this are not clear. One potential explanation is that this reflects underestimation of the true prevalence of this genotype by PCR assays that are often biased in favor of genotype I reference sequences. A series of healthy subjects previously studied by us also had unexpectedly high titer antibodies to genotype II (10). Likewise, a BKV quasispecies analysis performed by us showed over-representation of genotype II sequences (13). Furthermore, in one recent investigation, 21% of urine specimens collected from transplant patients in Spain belonged to genotype II (14). Alternate explanations for the discrepancy between serology and PCR prevalence rates include genotype-specific differences in the rates of urinary BKV shedding and cross reactivity in the antibody assays. Competitive binding experiments would be needed to more rigorously evaluate the possibility that the same neutralizing antibody can bind to more than one pseudovirion.
Neutralizing titers against BKV-Ib2 were substantially lower than titers against Ia. This is surprising in light of the fact that the two strains differ by only a few amino acids. The result is consistent with our prior report showing that many individual subjects who robustly neutralized BKV-Ia showed little or no neutralization of BKV-Ib2 (9). Intriguingly, recent reports have shown that BKV-Ib2 is highly represented in kidney transplant recipients (15,16).
Titer differences of approximately 0.5 log were observed between different lots of IVIG and CMVIG. This is within the limits of experimental error for antibody titrations based on serial dilutions of the same sample. The extent to which different manufacturing techniques and donor characteristics affect antibody titers in various commercial immunoglobulin preparations remains to be systematically determined. However, given the fact that a batch of IG is typically derived from >40 000 units of plasma, this variation is likely to be small in magnitude.
EC90 values of the IGs studied were typically 10–100 fold lower than the EC50 value presented in Table 1. It has been suggested that vivo protection by some antibodies requires a serum concentration of the administered neutralizing antibody at least 100-fold higher than the concentration needed for viral neutralization in vitro (17). However, this is not always true as some antibodies can efficiently recruit effector functions in vivo and exert significant biologic effects at modest plasma concentrations (18,19).
The mechanism of pseudovirion neutralization presumably involves binding of antibodies to viral capsid protein VP1, and subsequent inhibition of transport across the host cell membrane. Interestingly the presence of complement seems to enhance the neutralizing efficacy of IVIG by a modest but consistent degree. The explanation of this phenomenon is not clear. As per the package insert provided with Privigen IVIG, this product does not directly activate the complement system. Fetal calf serum derived from the tissue culture system could potentially contain C3 convertases which would cleave C3 into C3a and C3b. However, the experimental system is free of leucocytes including macrophages and mechanisms such as opsonization and release of anaphylatoxins cannot be readily invoked. Maintenance of cell viability argues against the possibility that activation of the membrane attack complex led to complement mediated cell lysis. It is possible that viral antigens and C3 bind to form an immune complex which cannot readily infect the cells as efficiently as a free pseudovirion.
With respect to the feasibility of antibody therapy for BKV infection it should be noted that neutralizing antibodies are believed to contribute significantly to the efficacy of many commonly used viral vaccines including those against hepatitis B, human papillomavirus, measles, mumps, rubella, polio (live and attenuated), and yellow fever. A commercially available monoclonal antibody, Palivizumab, is in use for both prevention and treatment of severe respiratory syncytial virus-induced pneumonia (20). In general antibody-based therapy is most effective as prophylactic agent in serologically naïve subjects, by serving to immediately neutralize the part of the infectious inoculum that enters the blood stream. Prophylactic use of IG could cover the period of maximum risk for BKV infection (1–9 months posttransplant), and be offered to patients with the highest risk, possibly elderly males with delayed graft function, or ureteric stents. Development of a risk scale developed by mathematical modeling may be useful in this regard.
A potential caveat is the short half-life of immunoglobulins (~ 3 weeks) which would limit the duration of protection. However, this drawback can be addressed by the use of higher doses, use of portable pumps for subcutaneous infusion (21), and genetic engineering of the antibody molecules, which can prolong antibody half life to 3 months (22). A vectored immunoprophylaxis approach can also circumvent the need for repeated injection of antibodies (23).
With respect to therapeutic (as opposed to preventive) treatment IGs, it is frequently argued that the high titers of anti-BKV antibodies in patients with BKV nephropathy imply that these antibodies are not protective (7,29–31). Certainly antibodies cannot enter the intra-cellular compartment or prevent cell to cell spread and are not expected to eradicate established viral infection. However, clinical observations made to date do not exclude the possibility that antibodies could aid T-cell mediated clearance of virus, particularly if infused to achieve high titers far exceeding those seen in natural infection, at a time when the virus load is relatively low. Neutralization of circulating virus load at such an early stage could conceivably have a favorable effect on the clinical course of disease. It is worth recalling that prior to the discovery of specific inhibitors of viral replication immunoglobulin preparations were successfully used to control (though not cure) CMV, hepatitis B and hepatitis C virus associated disease (24–27). As no effective small molecule therapy is currently available for BKV, it would be worthwhile to conduct appropriately designed trials of IG in patients with persistent BK viremia and early BKV nephropathy to determine if the viral neutralization observed by us in vitro translates into real clinical benefit.
The literature contains several reports that IVIG administration does not result in prompt resolution of responses in BKV nephropathy (3,28–32). Indeed, there are also anecdotal case reports where BKV load actually increased after initiating treatment (33). However, treatment in all published studies was (a) initiated at a late stage of disease, (b) not based on lot-specific stoichiometric measurements of actual antibody titer or half-life, and (c) typically monitored by PCR-based viral DNA assays that do not distinguish between infectious and neutralized virions. The doses used were empirical and varied widely from 2 g/Kg IV divided over 2–5 days to 150 mg/Kg every 2 weeks (7,32).
It is our contention that additional trials based on sound pharmacokinetic principles are needed. Such trials would specifically take into account the viral load, the antibody titer, its half-life, and the slope of its BKV-genotype appropriate neutralization curve. There is reason to believe that currently available IG preparations are sufficiently potent for clinical use: 5 g of a product tested by us was roughly estimated to be capable of inactivating a circulating virus load of 1.9E + 06 genomic equivalents per milliliter in a patient with a blood volume of 5000 mL (32). The cost of IG therapy for BKV would be approximately $10 000 if a dose of 2 g/Kg is used (as per pharmacy prices quoted by the University of Pittsburgh). Currently antibodies do cost more to manufacture than small molecules, but technological advances such as antibody production in bacteria, yeast and plant cells could bring costs down substantially in years to come.
Acknowledgments
This work was supported by CSL Behring, Inc.
Abbreviations:
- BKV
polyomavirus BK
- BKVN
BKV nephropathy
- CMVIG
cytomegalovirus hyperimmune intravenous immunoglobulin
- IG
immune globulin
- IVIG
intravenous immunoglobulin
- LT
liver transplantation
- MELD
model for end-stage liver disease
- NAbs
neutralizing antibodies
- OPTN
organ procurement and transplantation network
- PELD
pediatric end-stage liver disease
- US
United States
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Babel N, Fendt J, Karaivanov S, et al. Sustained BK viruria as an early marker for the development of BKV-associated nephropathy: Analysis of 4128 urine and serum samples. Transplantation 2009; 88: 89–95. [DOI] [PubMed] [Google Scholar]
- 2.Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation 2009; 87: 1019–1026. [DOI] [PubMed] [Google Scholar]
- 3.Smith JM, Dharnidharka VR, Talley L, Martz K, McDonald RA. BK virus nephropathy in pediatric renal transplant recipients: an analysis of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) registry. Clin J Am Soc Nephrol 2007; 2: 1037–1042. [DOI] [PubMed] [Google Scholar]
- 4.Koukoulaki M, Grispou E, Pistolas D, et al. Prospective monitoring of BK virus replication in renal transplant recipients. Transplant Infect Dis 2009; 11: 1–10. [DOI] [PubMed] [Google Scholar]
- 5.Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 2012; 12: 388–399. [DOI] [PubMed] [Google Scholar]
- 6.Wadei HM, Rule AD, Lewin M, et al. Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN). Am J Transplant 2006; 6: 1025–1032. [DOI] [PubMed] [Google Scholar]
- 7.Randhawa PS, Schonder K, Shapiro R, Farasati N, Huang Y. Polyomavirus BK neutralizing activity in human immunoglobulin preparations. Transplantation 2010; 89: 1462–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farasati NA, Shapiro R, Vats A, Randhawa P. Effect of leflunomide and cidofovir on replication of BK virus in an in vitro culture system. Transplantation 2005; 79: 116–118. [DOI] [PubMed] [Google Scholar]
- 9.Pastrana DV, Brennan DC, Cuburu N, et al. Neutralization serotyping of BK polyomavirus infection in kidney transplant recipients. PLoS Pathog 2012; 8: e1002650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pastrana DV, Ray U, Magaldi TG, Schowalter RM, Cuburu N, Buck CB. BK polyomavirus genotypes represent distinct serotypes with distinct entry tropism. J Virol 2013; 87: 10105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randhawa P, Ho A, Shapiro R, et al. Correlates of quantitative measurement of BK polyomavirus (BKV) DNA with clinical course of BKV infection in renal transplant patients. J Clin Microbiol 2004; 42: 1176–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo C, Bueno M, Kant J, Randhawa P. Biologic diversity of polyomavirus BK genomic sequences: Implications for molecular diagnostic laboratories. J Med Virol 2008; 80: 1850–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo C, Hirsch HH, Kant J, Randhawa P. VP-1 quasispecies in human infection with polyomavirus BK. J Med Virol 2012; 84: 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledesma J, Bouza E, Gonzalez-Nicolas MA, Garcia de Viedma D, Rodriguez-Sanchez B, Munoz P. BK polyomavirus genotyping at inter- and intra-patient level in Spain. J Med Virol 2013; 85: 1402–1408. [DOI] [PubMed] [Google Scholar]
- 15.Konietzny R, Fischer R, Ternette N, et al. Detection of BK virus in urine from renal transplant subjects by mass spectrometry. Clin Proteomics 2012; 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitt C, Raggub L, Linnenweber-Held S, Adams O, Schwarz A, Heim A. Donor origin of BKV replication after kidney transplantation. J Clin Virol 2014; 59: 120–5. [DOI] [PubMed] [Google Scholar]
- 17.Hessell AJ, Rakasz EG, Tehrani DM, et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus Type 1 gp41 membrane-proximal external region protect against mucosal challenge by Simian-Human Immunodeficiency Virus SHIVBa-L. J Virol 2010; 84: 1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hessell AJ, Rakasz EG, Poignard P, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. Plos Pathogens 2009; 5: e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watkins JD, Siddappa NB, Lakhashe SK, et al. An anti-HIV-1 V3 loop antibody fully protects cross-clade and elicits T-cell immunity in macaques mucosally challenged with an R5 clade C SHIV. Plos ONE 2011; 6: e18207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray J, Saxena S, Sharland M. Preventing severe respiratory syncytial virus disease: Passive, active immunisation and new antivirals. Arch Dis Child 2014; 99: 469–73. [DOI] [PubMed] [Google Scholar]
- 21.Berger M Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol 2004; 112: 1–7. [DOI] [PubMed] [Google Scholar]
- 22.Whaley KJ, Zeitlin L. Antibody-based concepts for multipurpose prevention technologies. Antiviral Research 2013; 100: S48–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson PR, Schnepp BC, Zhang JC, et al. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med 2009; 15: 901–U99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barin F, Jourdain G, Brunet S, et al. Revisiting the role of neutralizing antibodies in mother-to-child transmission of HIV-1. J Infect Dis 2006; 193: 1504–1511. [DOI] [PubMed] [Google Scholar]
- 25.Davis GL, Nelson DR, Terrault N, et al. A randomized, open-label study to evaluate the safety and pharmacokinetics of human hepatitis C immune globulin (Civacir) in liver transplant recipients. Liver Transplant 2005; 11: 941–949. [DOI] [PubMed] [Google Scholar]
- 26.Levitsky J, Doucette K, Practice AIDC. Viral hepatitis in solid organ transplant recipients. Am J Transplant 2009; 9: S116–S30. [DOI] [PubMed] [Google Scholar]
- 27.Bonaros N, Mayer B, Schachner T, Laufer G, Kocher A. CMV-hyperimmune globulin for preventing cytomegalovirus infection and disease in solid organ transplant recipients: A meta-analysis. Clin Transplant 2008; 22: 89–97. [DOI] [PubMed] [Google Scholar]
- 28.Sharma AP, Moussa M, Casier S, Rehman F, Filler G, Grimmer J. Intravenous immunoglobulin as rescue therapy for BK virus nephropathy. Pediatr Transplant 2009; 13: 123–129. [DOI] [PubMed] [Google Scholar]
- 29.Bodaghi S, Comoli P, Bosch R, et al. Antibody responses to recombinant polyomavirus BK large T and VP1 proteins in young kidney transplant patients. J Clin Microbiol 2009; 47: 2577–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hariharan S, Cohen EP, Vasudev B, et al. BK virus-specific antibodies and BKV DNA in renal transplant recipients with BKV nephritis. Am J Transplant 2005; 5: 2719–2724. [DOI] [PubMed] [Google Scholar]
- 31.Randhawa P, Bohl D, Brennan D, et al. longitudinal analysis of levels of immunoglobulins against BK virus capsid proteins in kidney transplant recipients. Clin Vaccine Immunol 2008; 15: 1564–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sener A, House AA, Jevnikar AM, et al. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: One-year follow-up of renal allograft recipients. Transplantation 2006; 81: 117–120. [DOI] [PubMed] [Google Scholar]
- 33.Maggiore U, Medici MC, Vaglio A, Buzio C. Increased viral load after intravenous immunoglobulin therapy for BK virus-associated nephropathy. Transplant Infect Dis 2010; 12: 470–472. [DOI] [PubMed] [Google Scholar]


