Abstract
Bifunctional quinolinonyl DKA derivatives were first described as nonselective inhibitors of 3′-processing (3′-P) and strand transfer (ST) functions of HIV-1 integrase (IN), while 7-aminosubstituted quinolinonyl derivatives were proven IN strand transfer inhibitors (INSTIs) that also displayed activity against ribonuclease H (RNase H). In this study, we describe the design, synthesis, and biological evaluation of new quinolinonyl diketo acid (DKA) derivatives characterized by variously substituted alkylating groups on the nitrogen atom of the quinolinone ring. Removal of the second DKA branch of bifunctional DKAs, and the amino group in position 7 of quinolinone ring combined with a fine-tuning of the substituents on the benzyl group in position 1 of the quinolinone, increased selectivity for IN ST activity. In vitro, the most potent compound was 11j (IC50 = 10 nM), while the most active compounds against HIV infected cells were ester derivatives 10j and 10l. In general, the activity against RNase H was negligible, with only a few compounds active at concentrations higher than 10 μM. The binding mode of the most potent IN inhibitor 11j within the IN catalytic core domain (CCD) is described as well as its binding mode within the RNase H catalytic site to rationalize its selectivity.
Graphical Abstract
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) integrase (IN) and reverse trascriptase (RT) are two enzymes encoded by the pol gene and are essential for virus replication. IN mediates insertion of the double-stranded DNA provirus into the host genome, while RT synthesizes this DNA using viral RNA as a template and degrading RNA of the RNA–DNA replication intermediate in the process. As such, both events have been desirable therapeutical targets.1
The role of IN during HIV-1 replication begins in the cytoplasm after completion of reverse trascription. The resulting DNA double-stranded DNA is subjected to two reactions. The first of these involves removing of the 3′-terminal GT dinucleotides adjacent to the highly conserved CA at 3′-end creating reactive 3′-OH ends in both strands of the viral DNA and is termed 3′-processing (3′-P). During this step, IN, viral DNA, and both host (barrier to autointegration factor (BAF), heat-shock protein 60 (HSP60), high mobility group protein (HMG), lens epithelium-derived growth factor (LEDGF))2 and viral (matrix, viral protein R (Vpr), p7/nucleocapsid and RT)3 proteins form a preintegration complex (PIC), which is transported into the nucleus. This is followed by a strand transfer (ST) step, involving a concerted nucleophilic attack by the two reactive 3′-OH recessed ends of the viral DNA (DNA donor), formed during 3′-P, on the host DNA (DNA acceptor). The gap formed after the nucleophilic attack between the 5′-end of DNA donor and 3′-end of DNA acceptor is filled probably by the cellular DNA repair enzymes, finishing the integration process.
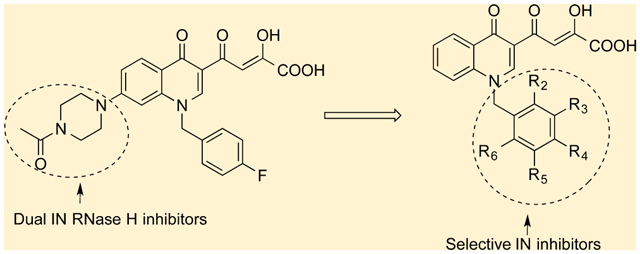
Integration is the final step before irreversibile and productive HIV-1 infection of the target cell.4 Although identified as an attractive target more then 12 years ago,5 the first drug targeting IN (raltegravir, 1) was approved by the Food and Drug Administration (FDA) (Figure 1) only in late 20076 as part of combination antiretroviral therapy.
Figure 1.
Structures of dual IN and RNase H inhibitors.
RT is the most actively and longest studied among the enzymes encoded by the pol gene, displaying both DNA polymerase and ribonuclease H (RNase H) activities, the latter catalyzing degradation of RNA of the RNA–DNA hybrid intermediate to permit the synthesis of (+) strand DNA. Although RT is a multifunctional enzyme, all approved nucleoside and non-nucleoside RT inhibitors target only its DNA polymerase function. IN and the RNase H domain of RT belong to a broader class of polynucleotidyl trasferases7 and share structural similarities.8 Both enzymes require Mg2+ for catalysis, and the metal ion is chelated by a highly conserved motif consisting of three acidic amino acid residues, also referred to as DDE motif. Within the IN catalytic core, this motif is represented by the three conserved acidic residues D64, D116, and E152 involved in the coordination of the two catalytic Mg2+ ions.9 In the RNase H domain, this motif comprises D443, E478, D498, and D549 amino acids which also chelate two magnesium ions.10
Given the structural similarities between the catalytic domains of these HIV-1 enzymes, several compounds initially developed as IN inhibitors, including diketo acids (DKAs) 2 and 3 (Figure 1)11 and DNA aptamers12 have also been screened against RNase H. Recently, three structurally different compounds have been described as dual inhibitors: tropolone 4,13 2-hydroxyisoquinolin-1,3(2H,4H)-dione 5,14 and madurahydroxylactone 615 (Figure 1).
Each one of these classes of inhibitors characteristically presents three aligned heteroatoms capable of binding divalent metal ions.
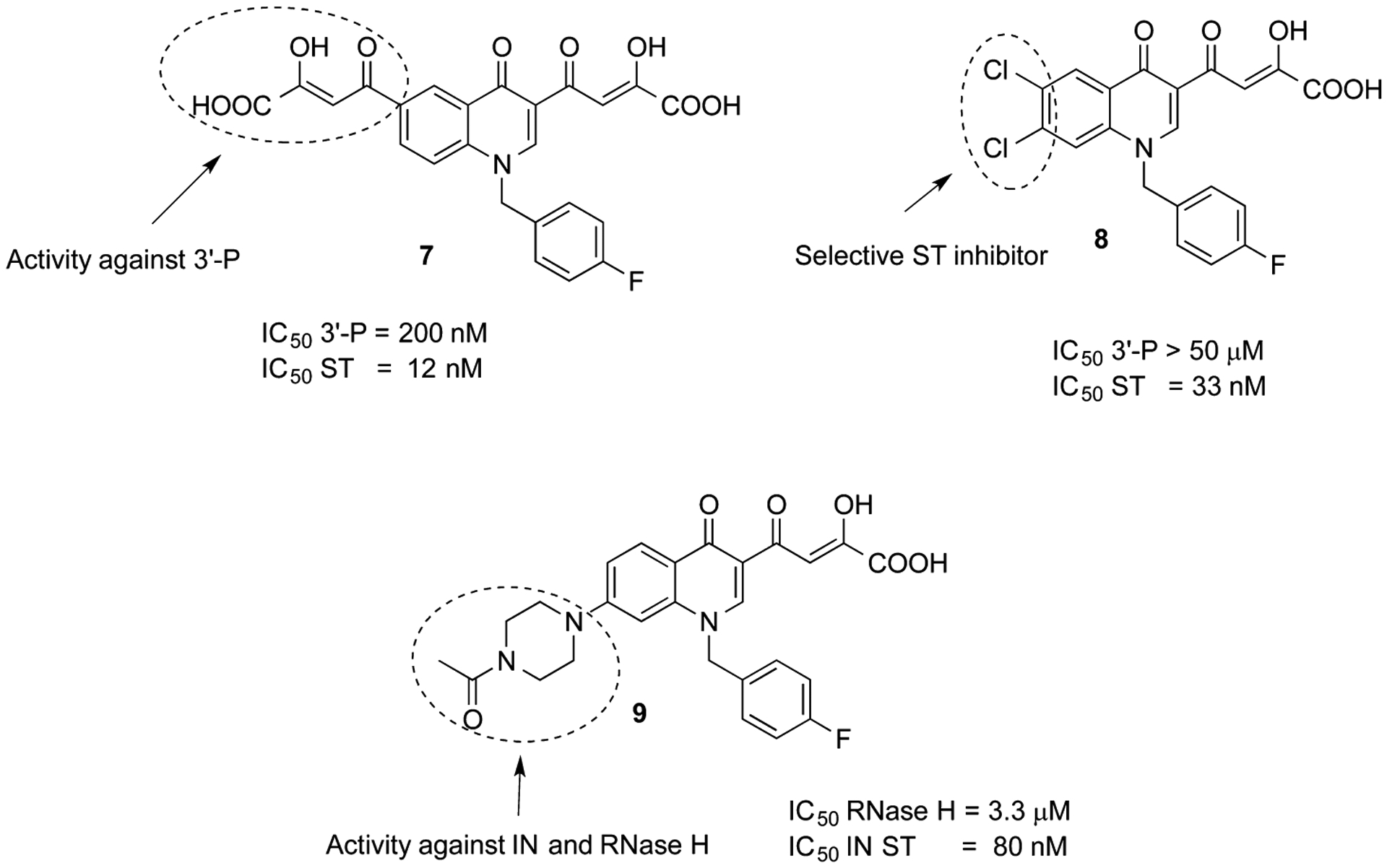
Recently, quinolinonyl diketo acid-derived HIV-1 IN inhibitors were described.16 First bifunctional DKA 7 (Figure 2) was reported as nonselective 3′-P and ST inhibitor, interacting with both the acceptor and donor DNA bindig sites of the enzyme. Indeed, 3′-P was inhibited with an IC50 of 0.20 μM, in comparison with ST reaction that was inhibited with IC50 of 0.012 μM. This compound was modified to obtain a series of quinolinonyl diketo acid derivatives in which the 6-diketo acid chain responsable of 3′-P inhibition was removed, providing selective ST inhibitors. In particular, compound 8 showed good inhibitory activity with an IC50 value of 33 nM with no activity against 3′-P step up to a 50 μM concentration (Figure 2). The proven capability of the DKA chain to chelate the catalytically critical metal ions made these potential inhibitors of both enzymes.17
Figure 2.
HIV-1 IN inhibitors of the mono- and bifunctional quinolinonyl DKA class.
In recent years, a number of IN inhibitors have been tested against RNase H function, and recently we reported derivatives of 9 as IN inhibitors endowed with a moderate activity against RNase H.18 However, the amino function that was introduced in that series does not increase either the potency against the IN enzyme or the activity against RNase H. Thus, we decided to design new quinolinone derivatives to better understand the structure–activity relationships within this series of inhibitors. In particular, we describe herein the design, synthesis, and the biological evaluation of a series of quinolinonyl DKA derivatives 10a–aa and 11a–aa that are characterized by a variously substituted benzyl group or by an aryl or heteroaryl alkyl moiety on nitrogen atom of the quinolinone ring (Figure 3). In fact, the benzyl portion of IN strand transfer inhibitors (INSTIs) is known to be relevant for the binding of these inhibitors to the IN/DNA complex. Thus, a study of its influence on the activity of this series of quinolinonyl DKAs against IN and its role on the inhibition of RNase H fuction is vital. Moreover, the role of the arylmethyl group linked on quinolonyl nitrogen was not previously studied within this series of inhibitors.
Figure 3.
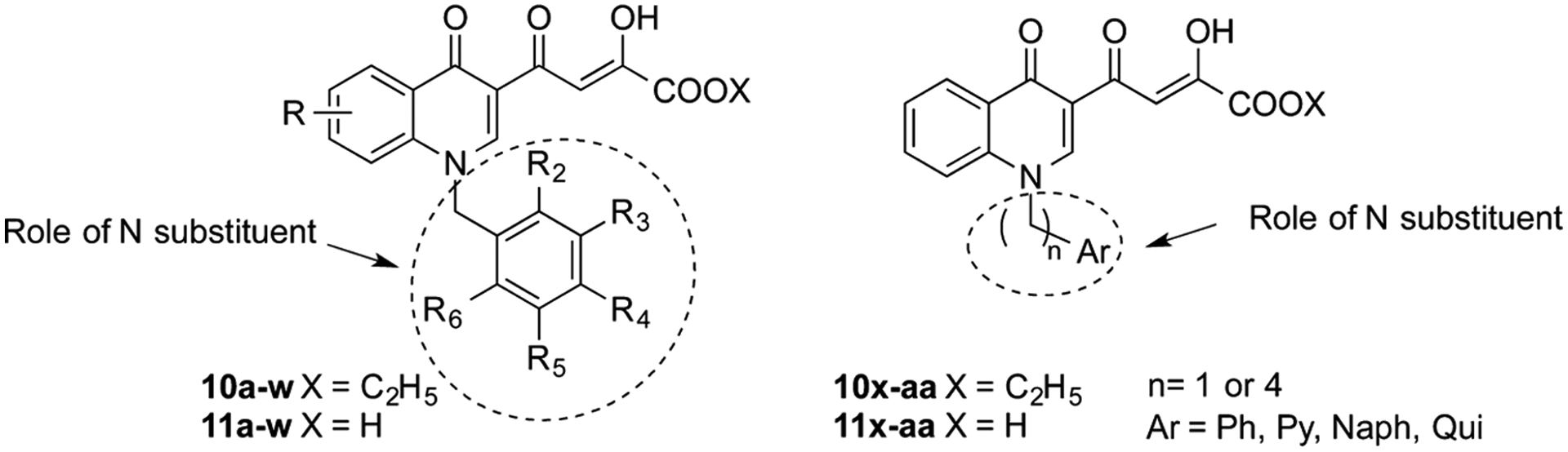
Stuctures of the newly designed quinolinonyl diketo acid derivatives 10a–aa and 11a–aa (Ph = phenyl, Py = 2-pyridinyl, Naph = 2-naphthyl, Qui = 2-quinolinyl). For specific structures and molecules, see Tables 1 and 2.
RESULT AND DISCUSSION
Chemistry.
Compounds 10a–aa and 11a–aa were synthesized according to the pathway reported in Scheme 1. 3-Acetyl-4(1H)-quinolinones 12a–c were prepared by reaction of aniline with ethyl orthoformate and ethyl acetoacetate, which were thermally condensed in the presence of an inert heating medium (Dowtherm A) under argon atmosphere, according to the Yoshizawa procedure.19 N-1 substituted quinolones 13a–aa were obtained via alkylation of compounds 12a–aa with the proper aryl alkyl bromide in alkaline medium (K2CO3). Compounds 12a–aa were then subjected to a Claisen condensation reaction with diethyl oxalate and sodium ethoxide as the catalyst to provide ethyl esters 10a–aa, which were in turn hydrolyzed with 1 N NaOH to afford the corresponding acids 11a–aa (Scheme 1). Chemical, physical, and spectroscopic data of final products 10a–aa and 11a–aa are described in the Experimental Section, while data of intermediates 12a–c, 13a–aa and analyses of 10a–aa and 11a–aa are reported in the Supporting Information.
Scheme 1. Synthetic Route to Quinolinonyl DKAs 10a–aa and 11a–aaa.
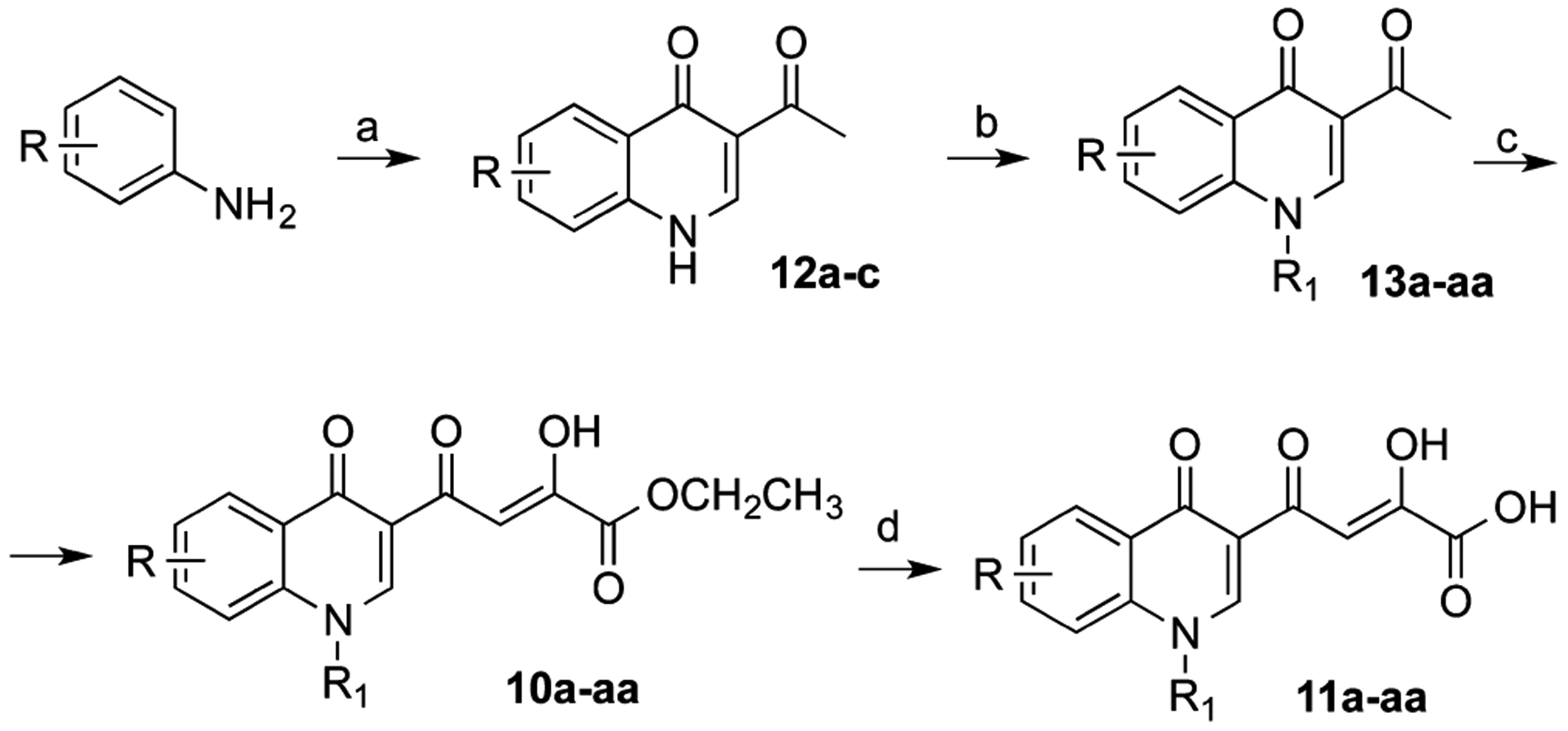
aReagents and conditions: (a) ethyl orthoformate, ethyl acetoacetate, Dowtherm A, 95–254 °C, 8 h, 52–58% yield; (b) arylalkyl bromide, K2CO3, DMF, 100 °C, 50–75% yield; (c) diethyl oxalate, C2H5ONa, THF, room temp, 10 min, 62–100% yield; (d) 1 N NaOH, THF/CH3OH, room temp, 40 min, 38–100% yield.
Evaluation of Biological Activities: In Vitro Assays.
Newly synthesized compounds 10a–aa and 11a–aa were tested in vitro with respect to RT-associated RNase H function as well as IN-associated 3′-P and ST activities. ST and 3′-P were evaluated using gel-based assay carried out in the presence of Mg2+, and the corresponding IC50 values were calculated using a dose–response curves. The results of these assays are summarized in Tables 1 and 2.
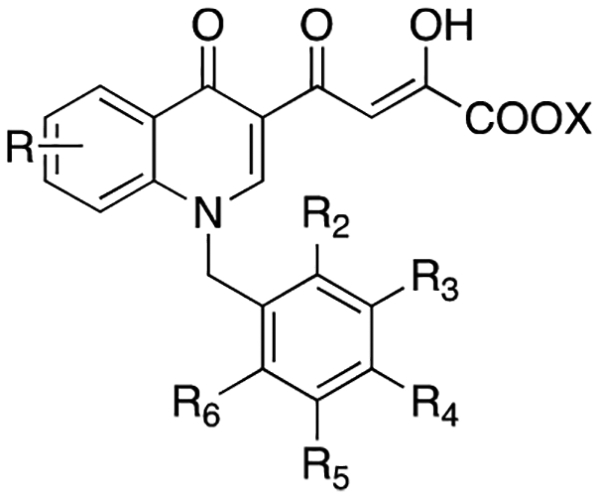
Table 1.
Cytotoxicity, Antiviral, Anti-IN, and Anti-RNase H Activities of Compounds 10a–w and 11a–w
 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| activity in enzyme assays | ||||||||||||||
| IN IC50 (μM)a | RNase H | antiviral activity and cytotoxicity | ||||||||||||
| compd | R | R2 | R3 | R4 | R5 | R6 | X | 3′-P | ST | % in. at 10 μMb | IC50 (μM) | EC50 (μM)c | CC50 (μM)d | SIe |
| 10a | H | H | H | H | H | Et | >1000 | 0.70 | −3.3 | ndf | 1 | >50 | >50 | |
| 10b | F | H | H | H | H | Et | 125 | 0.28 | 2.8 | nd | 1.5 | >50 | >33.4 | |
| 10c | och3 | H | H | H | H | Et | >333 | 1.4 | 11.4 | nd | 2.6 | >50 | >19.2 | |
| 10d | H | F | H | H | H | Et | >12.3 | 4.9 | −9.4 | nd | 1.7 | >50 | >29.4 | |
| 10e | H | OCH3 | H | H | H | Et | >333 | 16 | 4.9 | nd | 8.5 | 44 | 5.2 | |
| 10f | H | H | Cl | H | H | Et | >37 | >37 | 16.1 | nd | >50 | nd | ||
| 10g | H | H | OH | H | H | Et | >333 | 1.4 | 26.0 | nd | 30.9 | >50 | >1.6 | |
| 10h | H | H | och3 | H | H | Et | >37 | >37 | 10.0 | nd | >50 | nd | ||
| 10i | H | H | NO2 | H | H | Et | >37 | >37 | 31.9 | nd | >50 | nd | ||
| 10j | F | H | F | H | H | Et | 2.2 | 3 | nd | 0.58 | >50 | >86.2 | ||
| 10k | F | H | H | F | H | Et | 3.7 | 30 | nd | >50 | nd | |||
| 10l | F | H | H | H | F | Et | >12.3 | 0.25 | 4.1 | nd | <0.2 | >50 | >250 | |
| 10m | H | F | F | H | H | Et | 5.5 | 24 | nd | 9.8 | >50 | >5.1 | ||
| 10n | H | F | H | F | H | Et | 11 | 24 | nd | 32.4 | >50 | >1.5 | ||
| 10o | Cl | H | Cl | H | H | Et | >111 | 51 | >50 | >50 | nd | |||
| 10p | Cl | H | H | H | Cl | Et | 32 | 55.0 | >50 | 30.2 | >50 | >1.7 | ||
| 10q | ch3 | H | ch3 | H | H | Et | >111 | 33 | >50 | >50 | nd | |||
| 10r | H | CH3 | H | CH3 | H | Et | 1.3 | 21 | nd | >50 | nd | |||
| 10s | F | Cl | H | H | H | Et | 1.08 | 1.08 | 21 | nd | 10 | >50 | >5 | |
| 10t | H | Cl | F | H | H | Et | 266 | 3.1 | 18 | nd | 17.4 | >50 | >2.9 | |
| 10u | 8-Cl | H | Cl | F | H | H | Et | >333 | 25.08 | 24 | nd | >50 | nd | |
| 10w | 7-Cl | F | H | H | H | H | Et | 2.8 | nd | 11 | >50 | >4.5 | ||
| 11a | H | H | H | H | H | H | 4.0 | 0.034 | 9.6 | nd | 15.5 | >50 | ||
| 11b | F | H | H | H | H | H | 1.2 | 0.016 | 45.1 | >50 | 2.6 | >50 | >19.2 | |
| 11c | OCH3 | H | H | H | H | H | >4.1 | 0.038 | 45.2 | 16.3 ± 0.5 | 9.3 | >50 | >5.4 | |
| 11d | H | F | H | H | H | H | 4.6 | 0.015 | 2.4 | nd | 28.2 | >50 | >1.8 | |
| 11e | H | och3 | H | H | H | H | >4.1 | 0.14 | 1.9 | nd | >50 | nd | ||
| 11f | H | H | Cl | H | H | H | 22 | 0.54 | 56.2 | >50 | 0.87 | >50 | >57.5 | |
| 11g | H | H | OH | H | H | H | >37 | 0.45 | 44.4 | 9.5 ± 0.4 | >50 | nd | ||
| 11h | H | H | OCH3 | H | H | H | 28 | 0.51 | 45.8 | >50 | >50 | nd | ||
| 11i | H | H | no2 | H | H | H | 27 | 0.64 | 42.4 | 47.0 ± 1.5 | >50 | nd | ||
| 11j | F | H | F | H | H | H | 0.010 | 46 | 35.9 ± 0.8 | 13.8 | >50 | >3.6 | ||
| 11k | F | H | H | F | H | H | 3.2 | 37 | >50 | >50 | nd | |||
| 11l | F | H | H | H | F | H | 0.7 | 0.019 | 57.9 | 10.8 ± 0.4 | 8.1 | >50 | >6.2 | |
| 11m | H | F | F | H | H | H | 0.11 | 44 | >50 | 16.2 | >50 | >3.1 | ||
| 11n | H | F | H | F | H | H | 0.22 | 30 | >50 | >50 | nd | |||
| 11o | Cl | H | Cl | H | H | H | 2.3 | 68 | 44.7 ± 2.0 | >50 | nd | |||
| 11p | Cl | H | H | H | Cl | H | 0.27 | 86.3 | 10.0 ± 0.3 | >50 | nd | |||
| 11q | ch3 | H | ch3 | H | H | H | 7.0 | 52 | 19.6 ± 0.6 | >50 | nd | |||
| 11r | H | ch3 | H | ch3 | H | H | 0.05 | 47 | 37.8 ± 0.9 | >50 | nd | |||
| 11s | F | Cl | H | H | H | H | 1.75 | 0.018 | 12 | nd | 23.4 | >50 | >2.1 | |
| 11t | H | Cl | F | H | H | H | 21 | 0.19 | 25 | nd | >50 | nd | ||
| 11u | 8-Cl | H | Cl | F | H | H | H | 9.8 | 0.096 | 16 | nd | 50 | nd | |
| 11v | 8-Cl | F | H | H | H | H | H | 20.5 | 0.13 | 45 | >50 | >50 | nd | |
| 11w | 7-Cl | F | H | H | H | H | H | 36.5 | 22.6 ± 0.5 | 17 | >50 | >2.9 | ||
| 1 | 12.8 ± 6g | 0.087 ± 0.008h | nd | >100 | 0.0236 ± 0.0046 | >50 | >2118 | |||||||
| EVG | 8.1 ± 4.2g | 0.028 ± 0.006g | nd | 91 ± 8 | 0.0142 ± 0.0052 | >50 | >3521 | |||||||
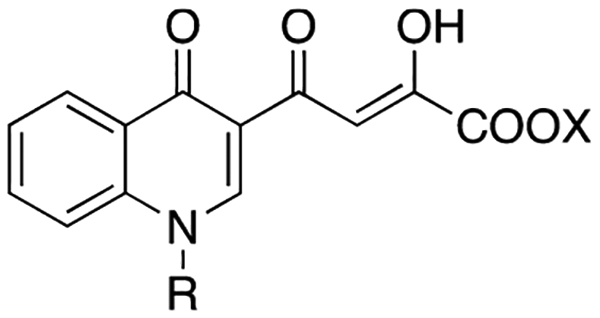
Table 2.
Cytotoxicity, Antiviral, Anti-IN, and Anti-RNase H Activities of Compounds 10x–aa and 11x–aa
 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Activity in enzyme assays | |||||||||
| IN IC50 (μM)a | RNaseH | Antiviral activity and cytotoxicity | |||||||
| R | X | 3’-P | ST | % in. at 10 μMb | IC50 (μM)x | EC50(μM)c | CC50 (μM)d | SIe | |
| 10x |  |
Et | >333 | 120 | 19.4 | nd | >50 | ndf | |
| 10y | 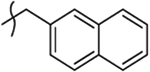 |
Et | >333 | 32 | 29.7 | nd | >50 | nd | |
| 10z | 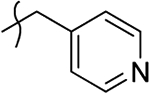 |
Et | >333 | 14 | −5.6 | nd | >50 | nd | |
| 10aa | 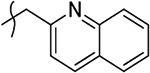 |
Et | >1000 | 3.5 | 4.4 | nd | >50 | nd | |
| 11x | 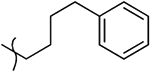 |
H | >333 | <0.45 | 33.0 | nd | >50 | nd | |
| 11y | 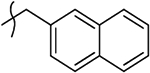 |
H | 18 | <0.45 | 40.7 | nd | 4.8 | >50 | >10.4 |
| 11z | 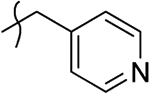 |
H | >1000 | 16 | −2.6 | nd | >50 | nd | |
| 11aa | 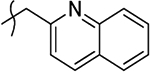 |
H | 70 | 0.40 | 16.4 | nd | >50 | nd | |
Inhibitory concentration 50% (μM) determined from dose response curves.
Percentage of inhibition determined at compound concentration of 10 μM.
Effective concentration 50% (μM).
Cytotoxic concentration 50% (μM).
SI = CC50/EC50.
nd: not determined.
Several of the newly synthesized compounds were potent IN inhibitors showing high activity against the ST reaction and low activity against 3′-P and RNase H function. As expected from previous findings, acid derivatives were always more active then the corresponding esters when tested against IN. Moreover, the N-benzyl substituted derivatives 11a–w were more active than the N-arylalkyl substituted counterparts 11x–aa on both IN and RNase H (Tables 1 and 2).
Anti-IN Activities.
Acid derivatives 11a–w were very active ST inhibitors, showing IC50 values in the nanomolar range (640–10 nM), with the exception of 11k,o,q, which exibited IC50 values in the micromolar range (3.2–7.0 μM).
Mono- (11a–i) and disubstituted (11j–w) benzyl derivatives showed comparable activities against IN. In fact, in the first case the IC50 values were in the range of 0.64–0.015 μM, while in the second one, values were in the range of 0.22–0.010 μM.
The most active compound of the series was the 2,4-F2 acid derivative 11j, with an IC50 value of 10 nM. Four additional compounds, the 2-F, 3-F, 2,6-F2 and 2,3-F,Cl derivatives (11b,d,l,s), demonstrated comparable potencies (IC50 values of 16, 15, 19, and 18 nM, respectively). Interestingly, the fluorine substituent was best tolerated, particularly when bound in position 2 of the benzyl ring, both in mono and disubstituted-benzyl derivatives. This trend was highlighted in the series of the disubstituted benzyl derivatives, comparing the most active compound 11j with the other compounds 11l–u. In fact, when the fluorine atom was bound in position 2 of the benzyl ring, inhibitory activity increased even if there was a second substituent on the phenyl ring. For example, compounds 11l (2,6-F2) and 11s (2,3-F,Cl) showed inhibitory activities comparable to 11j. When the fluorine atom in position 2 was replaced by a chlorine atom (11o–p) or a methyl group (11q), activity decreased by 1–2 orders of magnitude (IC50 values of 2.3, 0.27, and 7.0 μM, respectively). An exception was the derivative 11v, 2-fluorobenzyl substituted, characterized by a chlorine atom in 8-position of the aromatic ring of the quinolinonic nucleus. This derivative was 10 times less active than its parent 2-fluoro benzyl substituted counterpart 11b (IC50 values of 0.13 and 0.016 μM, respectively).
Among monosubstituted benzyl derivatives 11a–i, replacing the fluorine atom in the 2-position with a methoxy group led to a decrease in the activity (11c, IC50 value of 38 nM), as well as its substitution with an hydrogen atom (11a, IC50 value of 34 nM). The shift of the fluorine atom from postition 2 to 3 gave a compound that had the same inhibitory activity (11d, IC50 value of 15 nM), while its shift to position 4 slightly decreased activity (8, IC50 value of 40 nM). When the fluorine atom in position 4 was replaced by other atoms (11f) or group (11g–i), activity decreased by an order of magnitude (IC50 values of 0.45–0.64 μM).
Anti-RH Activities.
The quinolinonyl diketo acid derivatives 10a–aa and 11a–aa were tested for RNase H inhibition, calculated as a percentage of inhibition at a concentration of 10 μM. IC50 values have been determined for derivatives showing % of inhibition >30%.
In general, the ester derivatives 10a–aa poorly inhibited the RNase H function at 10 μM, and in all cases the measured IC50s were always >50 μM. Although the acid counterparts were more effective, their IC50 were poor compared to their anti-IN potency. The most active compounds were the acid derivatives 11g, 11l, and 11p, showing IC50 values of ~10 μM. Among the monosubstituted benzyl derivatives (10a–i and 11a–i), substitution in position 4 was best tolerated. In particular, in the ester series, a nitro group (10i) increased the inhibitory activity of the molecule (31.9% inhibition); among this series, inhibitory activity decreased in the following order: NO2 > OH > Cl > OCH3 > F. In the acid series, increased activity was highlighted by the presence of a chlorine atom (11f, 56.2% inhibition); among this series, inhibitory activity decreased in the order: Cl > F > OCH3 ≈ OH ≈ NO2.
Interestingly, compound 8 was tested as a representative example of the nonbasic quinolonyl DKA series previously reported16b and was confirmed weak inhibitor, showing IC50 = 42 μM against recombinant RNase H.
The disubstituted benzyl derivatives 10j–w and 11j–w showed a good inhibitory activity when two chlorine atoms were the substituents of the benzyl moiety (10o–p, 51 and 55% inhibition, respectively; 11o–p, 68 and 86.3% inhibition, respectively). In general, the fluorine atom led to decreased activity, even then in the F2-benzyl and Cl,F-benzyl disubstituted derivatives (10j–n and 10s–u, 3–24% inhibition; 11j–n and 11s–u, 30–57.9% inhibition), with respect to the most active acid compound 2,6-Cl2-disubstituted derivative 11p (86.3% inhibition).
Cell-Based Assays.
Newly synthesized compounds were tested for antiviral activity and cytotoxicity using HeLa-CD4-LTR-β-gal cells infected by HIV-1(IIIB). Elvitegravir (EVG) and 1 were used as reference compounds. EC50 and CC50 values for compounds 10a–aa and 11a–aa are reported in Tables 1 and 2. Among these, derivatives 10a–e,g,j,l–n,p,s,t,w and 11a–d,f,j,l,m,s,w,y show EC50 < 50 μM. It is noteworthy that although less active in biochemical assays, esters 10a–e,g,j,l–n,p,s,t,w and acids 11a–d,f,j,l,m,s,w,y are almost equipotent in cell-based assays. In particular, the most active compounds are the ester derivatives 10j and 10l and acid 11f that are active in the submicromolar range (EC50 = 0.58, <0.2, and 0.87 μM, respectively), with selectivity indices (SI) higher than 50 (>86.2, >250, >57.5, respectively). Notably, compound 10l showed an increased antiviral activity if compared with quinolinonyl DKA reported in our previous papers.16,18 All active compounds were characterized by low cytotoxicity against the same HeLa-CD4-LTR-β-gal cells, showing CC50 values >50 μM, with the sole exception of 10e showing a CC50 of 44 μM.
Molecular Modeling.
To better rationalize structure–activity relationships (SARs) for the newly identified quinolinonyl DKAs, molecular docking studies were performed on compound 11j, which is the most potent INSTI (10 nM) within this series while displaying insignificant activity against RNase H. Computations were aimed at elucidating the structural requisites responsible for binding at the IN active sites but with residual activity against RNase H.
Our recently built homology model of the IN catalytic core domain (CCD)/viral DNA was used to carry out docking studies.18 This model was constructed starting from the coordinates of the full-length structure of prototype foamy virus (PFV) IN in complex with 1 (PDB code 3OYA) and two active site Mg2+ ions required for both catalytic activity and chelation by INSTIs.21 According to docking results, compound 11j lies at the IN/DNA interface with the O, O, O donor triad of the DKA branch chelating the Mg2+ ions, and the central hydroxyl anionic oxygen atom bridging between the two cations (Figure 4a). This results in formation of 5- and 6-membered chelate rings with the two metal centers. Interestingly, the 5-,6-membered chelate ring binding motif appears to be one of the most critical features of highly effective INSTIs, as underscored by recent theoretical studies on the role of the metal binding groups within this class of anti HIV-1 drugs.22 Additionally, chelation by the carboxylate group might explain the higher inhibitory potency of acidic compounds with respect to the corresponding esters (see 10a–aa vs 11a–aa). Besides coordination of the metal centers, additional key interactions are established by our reference compound within the active site. In particular, the quinolinonyl ring establishes hydrophobic contacts with the Cβ and Cγ carbons of Q148 and is involved in a parallel-displaced π–π interaction with the terminal adenosine at the 3′ end of the nucleic acid substrate, which is known to be displaced through an induced-fit mechanism.21 Furthermore, the p-fluorobenzyl group fits into a narrow pocket created by rearrangement of the aforementioned terminal adenosine and the enzyme surface. Herein, the p-F-benzyl branch establishes a π-stacking interaction with the penultimate cytidine of the DNA reactive strand and favorable hydrophobic contacts with Q146 and P145. Interestingly, these two amino acids are directly involved in separation of the viral DNA strands upon the ST reaction.23 Indeed, these interactions appear to influence the inhibitory potency of these INSTIs as demonstrated by the potencies of compounds bearing the fluorine atoms in different positions of the aromatic ring (compounds 11b,d,j–n,s,t,u,v). This is in line with SARs data achieved for different INSTIs by us and other groups.24 Nevertheless, it should be pointed out that the role of the fluorobenzyl group in the IN recognition is rather “enigmatic” as outlined by Hare and co-workers.23 On the other hand, the importance of the fluorine substitution on the benzyl group is also proved by compounds bearing different halogens (11f,o,p) and different groups (11e,g–i,o–q), which are generally less active than the fluorinated analogues. Additionally, limited extension of the pocket at the IN/DNA interface would also explain why replacement of the benzyl substituent with bulkier groups (compounds 11x–aa) results in a loss of the inhibitory activity against IN.
Figure 4.
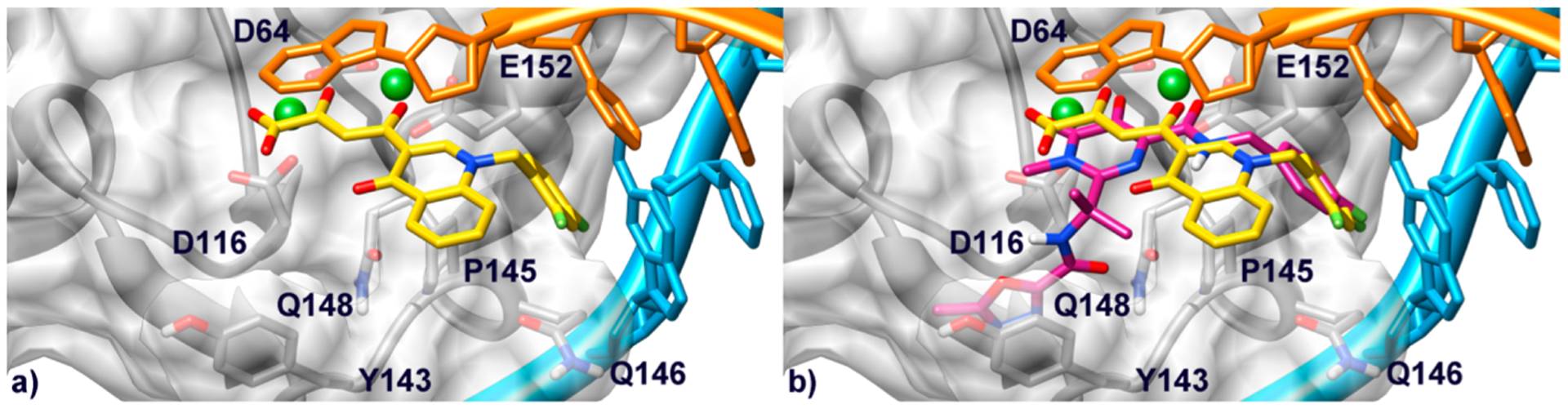
(a) Binding mode of compound 11j (yellow) within the HIV-1 IN/DNA model. Catalytic core domain is depicted as transparent light-gray surface and ribbons. Amino acid side chains important for the ligand binding are represented as sticks. The nontransferred (cyan) and reactive (orange) viral DNA strands are shown as ribbon and sticks. Mg2+ metal ions are represented as green spheres. (b) Overlay of compound 11j and 1 (magenta) in the IN binding pocket.
Overall, these results support the theory according to which INSTIs should inhibit the ST reaction by contacting the Mg2+ cofactors and displacing the terminal 3′-adenosine of the viral DNA, thus interfering with the recognition between IN/viral DNA binary complex and the host cell DNA (see Supporting Information Figure S1).23 Indeed, the binding mode provided by our docking calculations is shared by the majority of INSTIs including other quinolinonyl DKAs25–28 and also 1 (Figure 4b).21
A rationalization for residual activity against RNase H shown by some compounds of this series was also performed. The 2.09 Å resolution crystal structure of full-length HIV-1 RT in complex with a RNase H pyrimidinol carboxylic acid inhibitor and the nonnucleoside inhibitor nevirapine (PDB code 3QIP) was selected for docking calculations. This structure shows the RNase H domain in complex with an inhibitor which is more structurally related to our DKA compounds as compared to the other ligands cocrystallized with the full-length enzyme or the isolated RNase H domain.29–31 Before running docking calculations, Mn2+ ions in complex with this structure were replaced with physiologically relevant Mg2+ cations, which were also present in the biochemical and cell-based assays. In the predicted binding conformation, 11j chelates both catalytic Mg2+ cations with its DKA branch (Figure 5) in a 5-, 6-membered chelate ring binding arrangement, whereas the carboxylate terminal group H-bonds with the amide of N474. As already observed for IN, such a chelate geometry would explain the general higher inhibitory activity displayed by acidic ligands compared to the corresponding esters. In addition to chelating the metal cations, 11j establishes favorable lipophilic interactions with the Cβ and Cγ carbons of Q475 through its quinolinonyl nucleus, while the benzyl substituent, although being partially solvent exposed, is able to contact the lipophilic residues Q500, and W535. Indeed, the generally lower RNase H activity of this series with respect our previous study18 can be ascribed to the lack of additional interactions established with residues such as Y501, which is highly conserved and is a critical component of the RNase H primer grip. Moreover, recent experimental data demonstrate that mutating this residue is also negatively affecting the inhibithory potency of other chemo-types bearing the DKA moiety.32
Figure 5.
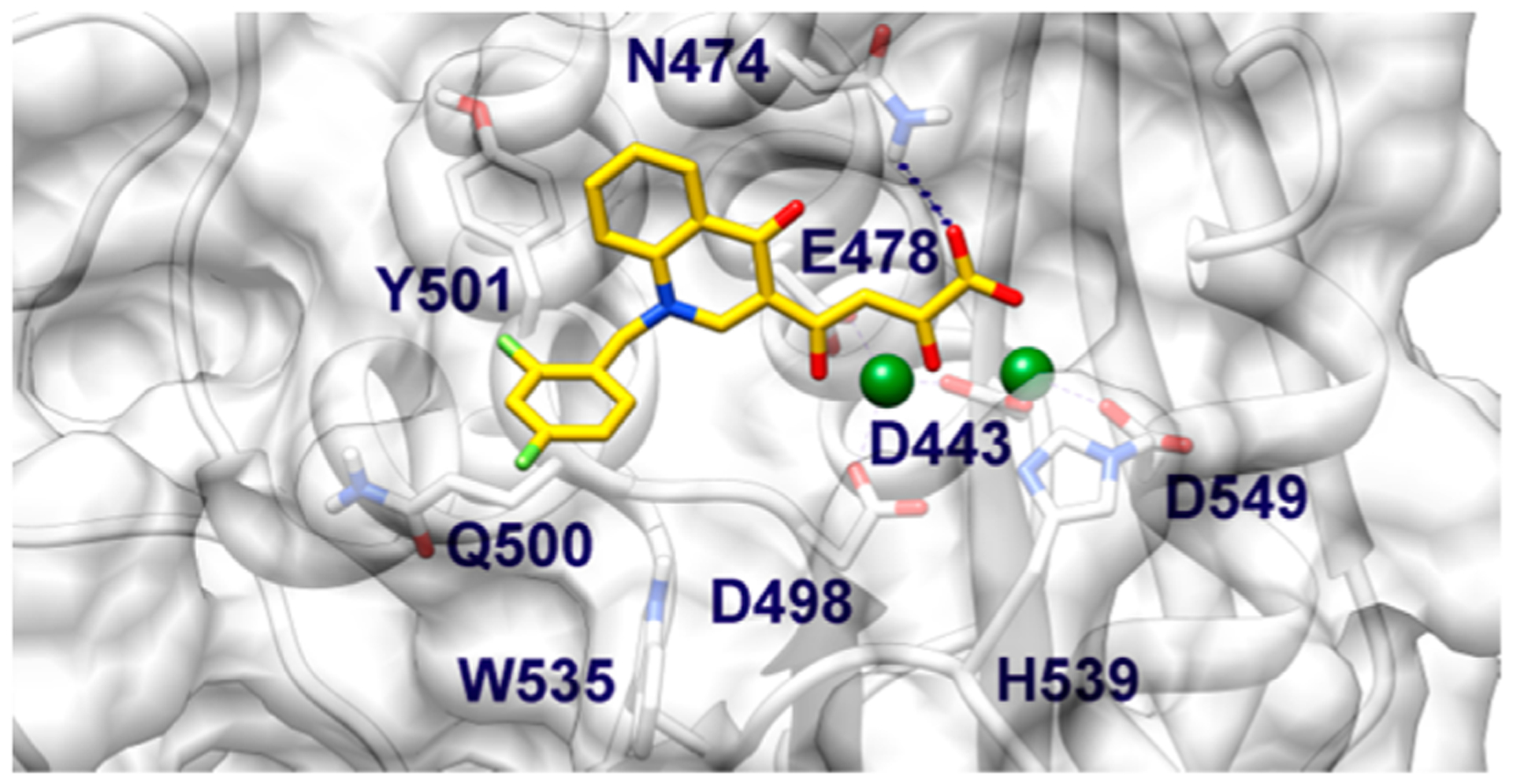
Binding mode of compound 11j (yellow) within the HIV-1 RNase H active site. CCD is shown as transparent white surface and ribbons. Amino acid side chains important for the ligand binding are represented as sticks. Mg2+ metal ions are depicted as green spheres.
In conclusion, and in support of previously reported series,18 we suggest that the quinolinonyl DKAs presented herein could exert their RNase H inhibitory activity by sterically hindering fitting of the viral DNA:RNA hybrid at the catalytitic site, thus hampering the degradation of the RNA strand (see Supporting Information Figure S2).18
A final consideration should be done on the higher activity of 10j in cell-based assays compared to that of 11j. Although ester 10j showed a similar binding pose of 11j at both the IN and RNase H active sites (see Supporting Information Figure S3 and compare with Figures 4a) and 5), its higher activity against HIV infected cells would be rationalized by hypothesizing that 10j is likely to better penetrate the cell membrane than its corresponding acid and, once in the cell, it would be hydrolyzed to 11j, which may represent the effective inhibiting form of both the IN and RNase H enzymes.
CONCLUSION
In this paper, we describe the design, synthesis, and biological assays of a series of new quinolinonyl diketo acid derivatives that are characterized by variously substituted alkylating groups on the nitrogen atom of the quinolinone ring. These derivatives were tested in both enzyme- and cell-based assays as anti-HIV-1 agents to selectively target the ST step of integration, testing in parallel the RNase H function of RT. We generated new compounds which are able to selectively inhibit IN and only marginally the RNase H. Five acid compounds resulted in being active against IN in the nanomolar range and, among them, compound 11j (IC50 = 10 nM against IN) inhibited RNase H activity with an IC50 = 35.9 μM. These compounds were also active against HIV-1 replication in acutely infected cells as well. However, the potency in cell-based assays was lower if compared to the activity against ST, suggesting that the acid derivatives are less prone to cell membrane penetration. Thus, the most active compounds against HIV infected cells turned out to be ester derivatives 10j and 10l.
In conclusion, the design of these new quinolinonyl DKAs was based on (i) removal of the second DKA branch of bifunctional DKAs that directed the activity against 3′-P, (ii) removal of the amino group in position 7 of quinolinone ring that addressed the activity on both IN and RNase H, and (iii) fine-tuning of the substituents on the benzyl group in position 1 of the quinolinone that preferentially addressed activity against IN ST. Further studies will be necessary to improve the activity of these compounds against HIV infected cells.
EXPERIMENTAL SECTION
Chemistry: General.
Melting points were determined on a Bobby Stuart Scientific SMP1 melting point apparatus and are uncorrected. Compounds purity were always >95% determined by high pressure liquid chromatography (HPLC). HPLC analysis were carried out with a Shimadzu LC-10AD VP CTO-10AC VP. Column used was generally Discovery Bio Wide Pore C18 (10 cm × 4.6 mm, 3 μm). IR spectra were recorded on a PerkinElmer Spectrum-One spectrophotometer. 1H NMR spectra were recorded at 400 MHz on a Bruker AC 400 Ultrashield 10 spectrophotometer (400 MHz). Dimethyl sulfoxide-d6 99.9% (code 44,139-2) (Aldrich) was used. Column chromatographies were performed on silica gel (Merck; 70–230 mesh). All compounds were routinely checked on TLC by using aluminum-baked silica gel plates (Fluka DC-Alufolien Kieselgel 60 F254). Developed plates were visualized by UV light. Solvents were reagent grade and, when necessary, were purified and dried by standard methods. Concentration of solutions after reactions and extractions involved the use of rotary evaporator (Büchi) operating at a reduced pressure (ca. 20 Torr). Organic solutions were dried over anhydrous sodium sulfate (Merck). All reactions were carried out under nitrogen; all solvents were freshly distilled under nitrogen and stored over molecular sieves for at least 3 h prior to use. Analytical results agreed to within ±0.40% of the theoretical values.
3-Acetyl-4(1H)-quinolinones 12a–c.
Ethyl orthoformate (4.0 g, 27 mmol), ethyl acetoacetate (3.5 g, 27 mmol), the proper aniline (27 mmol), and Dowtherm A (5.6 mL) were charged in a round-bottom three-necked flask equipped with a water separator. This mixture was stirred under argon atmosphere, while the temperature was increased to 95 °C in 1 h, then gradually up to 162 °C for a further hour. Then the mixture was stirred at this temperature for 6 h. After this time, the resulting solution was added in portions during 3 h into 42 mL of Dowtherm A stirred in a round-bottom three-necked flask equipped with thermometer and water separator and heated at 253–254 °C. After the addition, the mixture was heated at the same temperature for 2 h. Then the mixture was cooled at 90 °C, treated with 2-propanol (10 mL), cooled at 30 °C, filtered, and washed with 2-propanol and light petroleum ether in turn to give pure derivatives 12a–c. For chemical, physical, analytical, and spectroscopic data of compounds 12a–c, see Supporting Information.
General Procedure for the Synthesis of 3-Acetyl-1-(aryl)-methyl-4(1H)-quinolinones (13a–aa).
A mixture of 12a–c (1.1 mmol), the proper arylmethyl bromide (3.3 mmol), and anhydrous K2CO3 (210 mg, 1.5 mmol) in dry DMF (10 mL) was stirred at 100 °C for 1 h. After the mixture was cooled, water was added (40 mL), and the formed precipitate was filtered, washed with water and light petroleum ether in turn, and then dried under an IR lamp to provide pure derivatives 13a–aa. For chemical, physical, analytical, and spectroscopic data of compounds 13a–aa, see Supporting Information.
General Procedure for the Synthesis of Diketo Esters 10a–aa.
Sodium ethoxide (390 mg, 5.5 mmol) was added into a well stirred mixture of the appropriate acetyl derivative 13a–aa (2.7 mmol) and diethyl oxalate (790 mg, 5.4 mmol) in anhydrous THF (2.7 mL) under nitrogen atmosphere. The mixture was stirred at room temperature for 2 h, then was poured into n-hexane (50 mL). The collected precipitate was vigorously stirred for 30 min in 1 N HCl (50 mL). The formed yellow solid was filtered, washed with water, and dried under an IR lamp to afford the pure diketo esters 10a–aa. Yield (%), melting point (°C), recrystallization solvent, IR, 1H NMR, and analytical data for each of the following compounds are reported.
4-[1-Phenylmethyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10a).
Yield 89%; 141–142 °C; benzene. IR ν 3400 (OH), 1740 (C=O ester), 1648, 1629, and 1609 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.29 (t, 3H, CH2CH3), 4.29 (q, 2H, CH2CH3), 5.76 (s, 2H, CH2), 7.22–7.29 (m, 5H, benzene H), 7.48 (m, 1H, quinolinone C6–H), 7.70–7.71 (m, 2H, quinolinone C7-H and C8-H), 8.01 (s, 1H, butenoate C3-H), 8.31 (m, 1H, quinolinone C5-H), 9.12 (s, 1H, quinolinone C2-H), 15.50 (bs, 1H, OH). Anal. (C22H19NO5) C, H, N.
4-[1-(2-Fluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10b).
Yield 92%; 152–153 °C; toluene. IR ν 3200 (OH), 1744 (C=O ester), 1651, and 1612 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.28 (t, 3H, CH2CH3), 4.29 (q, 2H, CH2CH3), 5.81 (s, 2H, CH2), 7.12–7.28 (m, 4H, benzene H), 7.47 (m, 1H, quinolinone C6-H), 7.68–7.76 (m, 2H, quinolinone C7-H and C8-H), 7.99 (s, 1H, butenoate C3-H), 8.31 (m, 1H, quinolinone C5-H), 9.09 (s, 1H, quinolinone C2-H), 15.00 (bs, 1H, OH). Anal. (C22H18FNO5) C, H, F, N.
4-[1-(2-Methoxyphenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10c).
Yield 98%; 154–156 °C; toluene. IR ν 3200 (OH), 1726 (C=O ester), 1631, and 1601 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.29 (t, 3H, CH2CH3), 3.82 (s, 3H, CH3), 4.29 (q, 2H, CH2CH3), 5.63 (s, 2H, CH2), 6.89 (t, 1H, benzene H), 7.06 (d, 1H, benzene H), 7.13 (d, 1H, benzene H), 7.30 (t, 1H, benzene H), 7.49 (m, 1H, quinolinone C6-H), 7.71–7.76 (m, 2H, quinolinone C7-H and C8-H), 8.00 (s, 1H, butenoate C3-H), 8.31 (m, 1H, quinolinone C5-H), 9.05 (s, 1H, quinolinone C2-H), 14.20 (bs, 1H, OH). Anal. (C22H21NO6) C, H, N.
4-[1-(3-Fluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10d).
Yield 94%; 174–175 °C; methanol. IR ν 3138 (OH), 1740 (C=O ester), 1650 and 1613 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.27 (t, 3H, CH2CH3), 4.30 (q, 2H, CH2CH3), 5.78 (s, 2H, CH2), 7.01–7.20 (m, 3H, benzene H), 7.38 (m, 1H, benzene H), 7.49 (m, 1H, quinolinone C6-H), 7.65–7.74 (m, 2H, quinolinone C7-H and C8-H), 8.01 (s, 1H, butenoate C3-H), 8.31 (m, 1H, quinolinone C5-H), 9.12 (s, 1H, quinolinone C2-H), 15.00 (bs, 1H, OH). Anal. (C22H18FNO5) C, H, F, N.
4-[1-(3-Methoxyphenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10e).
Yield 93%; 177–179 °C; toluene. IR ν 3146 (OH), 1729 (C=O ester), 1628 and 1603 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.29 (t, 3H, CH2CH3), 3.69 (s, 3H, CH3), 4.27 (q, 2H, CH2CH3), 5.72 (s, 2H, CH2), 6.74 (d, 1H, benzene H), 6.84–6.89 (m, 2H, benzene H), 7.24 (t, 1H, benzene H), 7.49 (m, 1H, quinolinone C6-H), 7.69–7.75 (m, 2H, quinolinone C7-H and C8-H), 8.01 (s, 1H, butenoate C3-H), 8.31 (m, 1H, quinolinone C5-H), 9.10 (s, 1H, quinolinone C2-H), 15.40 (bs, 1H, OH). Anal. (C22H21NO6) C, H, N.
4-[1-(4-Chlorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10f).
Yield 91%; 174–176 °C; toluene. IR ν 3130 (OH), 1745 (C=O ester), 1643 and 1611 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.30 (t, 3H, CH2CH3), 4.29 (q, 2H, CH2CH3), 5.77 (s, 2H, CH2), 7.30–7.32 (m, 2H, benzene H), 7.40–7.42 (m, 2H, benzene H), 7.50 (m, 1H, quinolinone C6-H), 7.67 (m, 1H, quinolinone C8-H), 7.73 (m, 1H, quinolinone C7-H), 8.01 (s, 1H, butenoate C3-H), 8.30 (m, 1H, quinolinone C5-H), 9.14 (s, 1H, quinolinone C2-H), 15.60 (bs, 1H, OH). Anal. (C22H18ClNO5) C, H, Cl, N.
4-[1-(4-Hydroxyphenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10g).
Yield 64%; 179–181 °C; methanol. IR ν 3141 (OH), 1733 (C=O ester), 1600 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.30 (t, 3H, CH2CH3), 4.31 (q, 2H, CH2CH3), 5.61 (s, 2H, CH2), 6.71–6.73 (m, 2H, benzene H), 7.13–7.15 (m, 2H, benzene H), 7.50 (m, 1H, quinolinone C6-H), 7.74 (m, 1H, quinolinone C7-H), 7.79 (m, 1H, quinolinone C8-H), 8.00 (s, 1H, butenoate C3-H), 8.31 (m, 1H, quinolinone C5-H), 9.06 (s, 1H, quinolinone C2-H), 9.51 (bs, 1H, OH phenole), 14.00 (bs, 1H, OH). Anal. (C22H19NO6) C, H, N.
4-[1-(4-Methoxyphenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10h).
Yield 90%; 154–156 °C; methanol. IR ν 1734 (C=O ester), 1638 and 1602 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.28 (t, 3H, CH2CH3), 3.69 (s, 3H, CH3), 4.29 (q, 2H, CH2CH3), 5.67 (s, 2H, CH2), 6.89–6.91 (m, 2H, benzene H), 7.24–7.26 (m, 2H, benzene H), 7.50 (m, 1H, quinolinone C6-H), 7.71–7.79 (m, 2H, quinolinone C7-H and C8-H), 8.00 (s, 1H, butenoate C3-H), 8.31 (m, 1H, quinolinone C5-H), 9.10 (s, 1H, quinolinone C2-H), 15.60 (bs, 1H, OH). Anal. (C22H21NO6) C, H, N.
4-[1-(4-Nitrophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10i).
Yield 88%; 175–176 °C; toluene. IR ν 1718 (C=O ester), 1638 and 1607 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.30 (t, 3H, CH2CH3), 4.32 (q, 2H, CH2CH3), 5.94 (s, 2H, CH2), 7.46–7.54 (m, 3H, benzene H and quinolinone C6-H), 7.60 (m, 1H, quinolinone C8-H), 7.68 (m, 1H, quinolinone C7-H), 8.02 (s, 1H, butenoate C3-H), 8.18–8.20 (m, 2H, benzene H), 8.33 (m, 1H, quinolinone C5-H), 9.18 (s, 1H, quinolinone C2-H), 15.60 (bs, 1H, OH). Anal. (C22H18N2O7) C, H, N.
4-[1-(2,4-Difluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10j).
Yield 96%; 180 °C; methanol. IR ν (C=O ester), and (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.35 (t, 3H, CH2CH3), 4.36 (q, 2H, CH2CH3), 5.84 (s, 2H, CH2), 7.10 (t, 1H, quinolinone C6-H), 7.41–7.57 (m, 3H, benzene H), 7.76–7.83 (m, 2H, quinolinone C8-H and quinolinone C7-H), 8.05 (s, 1H, butenoate C3-H), 8.38 (d, 1H, quinolinone C5-H), 9.14 (s, 1H, quinolinone C2-H), 14.60 (bs, 1H, OH). Anal. (C22H17F2NO5) C, H, F, N.
4-[1-(2,5-Difluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10k).
Yield 62%; 160–163 °C; toluene. IR ν 3432 (OH), 1736 (C=O ester), 1637 and 1602 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.35 (t, 3H, CH2CH3), 4.35 (q, 2H, CH2CH3), 5.86 (s, 2H, CH2), 7.18–7.41 (m, 3H, benzene H), 7.58 (t, 1H, quinolinone C6-H), 7.75 (m, 1H, quinolinone C8-H), 7.82 (m, 1H, quinolinone C7-H), 8.06 (s, 1H, butenoate C3-H), 8.38 (m, 1H, quinolinone C5-H), 9.16 (s, 1H, quinolinone C2-H), 14.60 (bs, 1H, OH). Anal. (C22H17F2NO5) C, H, F, N.
4-[1-(2,6-Difluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10l).
Yield 85%; 172–173 °C; benzene/toluene. IR ν 3432 (OH), 1736 (C=O ester), 1637 and 1602 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.28 (t, 3H, CH2CH3), 4.30 (q, 2H, CH2CH3), 5.84 (s, 2H, CH2), 7.14–7.19 (t, 2H, benzene H), 7.43–7.53 (m, 2H, benzene H and quinolinone C6-H), 7.68 (t, 1H, quinolinone C7-H), 7.80 (d, 1H, quinolinone C8-H), 8.00 (s, 1H, butenoate C3-H), 8.30 (m, 1H, quinolinone C5-H), 9.11 (s, 1H, quinolinone C2-H), 15.00 (bs, 1H, OH). Anal. (C22H17F2NO5) C, H, F, N.
4-[1-(3,4-Difluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10m).
Yield 100%; 175–176 °C; benzene. IR ν 3432 (OH), 1719 (C=O ester), 1648 and 1612 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.35 (t, 3H, CH2CH3), 4.38 (q, 2H, CH2CH3), 5.80 (s, 2H, CH2), 7.19 (t, 1H, quinolinone C6-H), 7.41–7.57 (m, 3H, benzene H), 7.72–7.80 (m, 2H, quinolinone C8-H and quinolinone C7-H), 8.06 (s, 1H, butenoate C3-H), 8.38 (d, 1H, quinolinone C5-H), 9.17 (s, 1H, quinolinone C2-H), 14.60 (bs, 1H, OH). Anal. (C22H17F2NO5) C, H, F, N.
4-[1-(3,5-Difluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10n).
Yield 90%; 174–175 °C; methanol. IR ν 3432 (OH), 1736 (C=O ester), 1642 and 1626 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.34 (t, 3H, CH2CH3), 4.36 (q, 2H, CH2CH3), 5.84 (s, 2H, CH2), 7.05–7.23 (m, 3H, benzene H), 7.58 (t, 1H, quinolinone C6-H), 7.69 (d, 1H, quinolinone C8-H), 7.79 (t, 1H, quinolinone C7-H), 8.04 (s, 1H, butenoate C3-H), 8.38 (m, 1H, quinolinone C5-H), 9.16 (s, 1H, quinolinone C2-H), 14.60 (bs, 1H, OH). Anal. (C22H17F2NO5) C, H, F, N.
4-[1-(2,4-Dichlorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10o).
Yield 94%; 200–201 °C; toluene. IR ν 1735 (C=O ester), 1641 and 1632 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.34 (t, 3H, CH2CH3), 4.35 (q, 2H, CH2CH3), 5.84 (s, 2H, CH2), 6.99 (d, 1H, benzene H), 7.37 (dd, 1H, benzene H), 7.50–7.61 (m, 2H, quinolinone C6-H and C8-H), 7.78–7.83 (m, 2H, benzene H and quinolinone C7-H), 8.06 (s, 1H, butenoate C3-H), 8.41 (dd, 1H, quinolinone C5-H), 9.13 (s, 1H, quinolinone C2-H), 15.00 (bs, 1H, OH). Anal. (C22H17Cl2NO5) C, H, Cl, N.
4-[1-(2,6-Dichlorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10p).
Yield 82%; 184–186 °C; toluene. IR ν (C=O ester), and (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.32 (t, 3H, CH2CH3), 4.35 (q, 2H, CH2CH3), 5.90 (s, 2H, CH2), 7.61–7.67 (m, 2H, benzene H), 7.73–7.75 (m, 2H, quinolinone C6-H and C8-H), 7.94 (t, 1H, benzene H), 8.03–8.04 (m, 2H, quinolinone C7-H and butenoate C3-H), 8.42 (d, 1H, quinolinone C5-H), 8.46 (s, 1H, quinolinone C2-H), 15.00 (bs, 1H, OH). Anal. (C22H17Cl2NO5) C, H, Cl, N.
4-[1-(2,4-Dimethylphenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10q).
Yield 95%; 181 °C; toluene. IR ν 1727 (C=O ester), 1638 and 1600 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.32 (t, 3H, CH2CH3), 2.24 (s, 3H, CH3), 2.36 (s, 3H, CH3), 4.32 (q, 2H, CH2CH3), 5.69 (s, 2H, CH2), 6.58 (d, 1H, benzene H), 6.90 (d, 1H, benzene H), 7.12 (s, 1H, benzene H), 7.53–7.61 (m, 2H, quinolinone C6-H and C8-H), 7.76 (m, 1H, quinolinone C7-H), 8.05 (s, 1H, butenoate C3-H), 8.37 (d, 1H, quinolinone C5-H), 8.94 (s, 1H, quinolinone C2-H), 15.00 (bs, 1H, OH). Anal. (C24H23NO5) C, H, N.
4-[1-(3,5-Dimethylphenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10r).
Yield 89%; 181 °C; methanol. IR ν 1716 (C=O ester), 1625 and 1601 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.35 (t, 3H, CH2CH3), 2.24 (s, 6H, CH3), 4.35 (q, 2H, CH2CH3), 5.72 (s, 2H, CH2), 6.89–6.96 (m, 3H, benzene H), 7.54 (t, 1H, quinolinone C6-H), 7.71–7.77 (m, 2H, quinolinone C7-H and C8-H), 8.05 (s, 1H, butenoate C3-H), 8.37 (d, 1H, quinolinone C5-H), 9.13 (s, 1H, quinolinone C2-H), 15.00 (bs, 1H, OH). Anal. (C24H23NO5) C, H, N.
4-[1-(3-Chloro-2-fluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10s).
Yield 79%; 167–169 °C; benzene. IR ν 1746 (C=O ester), 1648 and 1601 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.32 (t, 3H, CH2CH3), 4.32 (q, 2H, CH2CH3), 5.89 (s, 2H, CH2), 7.13–7.18 (m, 2H, benzene H and quinolinone C6-H), 7.55–7.77 (m, 4H, quinolinone C7-H, C8-H and benzene H), 8.03 (s, 1H, butenoate C3-H), 8.35 (d, 1H, quinolinone C5-H), 9.13 (s, 1H, quinolinone C2-H), 15.00 (bs, 1H, OH). Anal. (C22H17ClFNO5) C, H, Cl, F, N.
4-[1-(3-Chloro-4-fluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10t).
Yield 84%; 160–161 °C; methanol. IR ν 1746 (C=O ester), 1648 and 1601 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.32 (t, 3H, CH2CH3), 4.32 (q, 2H, CH2CH3), 5.77 (s, 2H, CH2), 7.30–7.72 (m, 6H, quinolinone C6-H, C7-H, C8-H and benzene H), 8.03 (s, 1H, butenoate C3-H), 8.33 (d, 1H, quinolinone C5-H), 9.15 (s, 1H, quinolinone C2-H), 15.00 (bs, 1H, OH). Anal. (C22H17ClFNO5) C, H, Cl, F, N.
8-Chloro-4-[1-(3-chloro-4-fluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10u).
Yield 70%; 166–167 °C; ethanol. IR ν 1746 (C=O ester), 1648 and 1601 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.32 (t, 3H, CH2CH3), 4.33 (q, 2H, CH2CH3), 6.10 (s, 2H, CH2), 7.11 (m, 1H, quinolinone C6-H), 7.34–7.50 (m, 3H, benzene H), 7.80–7.85 (m, 2H, quinolinone C7-H and C8-H) 7.95 (s, 1H, butenoate C3-H), 8.38 (d, 1H, quinolinone C5-H), 8.91 (s, 1H, quinolinone C2-H), 15.00 (bs, 1H, OH). Anal. (C22H16Cl2FNO5) C, H, Cl, F, N.
4-[7-Chloro-1-(2-fluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid Ethyl Ester (10w).
Yield 85%; 175 °C; methanol. IR ν 1721 (C=O ester), 1627 and 1585 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.35 (t, 3H, CH2CH3), 4.36 (q, 2H, CH2CH3), 5.88 (s, 2H, CH2), 7.22–7.48 (m, 4H, benzene H), 7.60 (t, 1H, quinolinone C6-H), 7.90 (m, 2H, quinolinone C7-H and C8-H), 8.00 (s, 1H, butenoate C3-H), 8.35 (d, 1H, quinolinone C5-H), 9.11 (s, 1H, quinolinone C2-H), 15.00 (bs, 1H, OH). Anal. (C22H17FClNO5) C, H, N, F, Cl.
2-Hydroxy-4-oxo-4-(4-oxo-1-(4-phenylbutyl)-1,4-dihydroquinolin-3-yl)but-2-enoic Acid Ethyl Ester (10x).
Yield 95%; 88–90 °C; cyclohxane. IR ν 1725 (C=O ester), 1630 and 1604 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.24 (t, 3H, CH2CH3), 1.60 (m, 2H, butyl H), 1.75 (m, 2H, butyl H), 2.58 (m, 2H, butyl H), 4.25 (m, 2H, butyl H), 4.45 (q, 2H, CH2CH3), 7.11–7.25 (m, 5H, benzene H and quinolinone C6-H), 7.49 (m, 1H, quinolinone C8-H), 7.74–7.78 (m, 2H, butenoate C3-H and quinolinone C7-H), 8.30 (d, 1H, quinolinone C5-H), 8.76 (s, 1H, quinolinone C2-H), 15.00 (bs, 1H, OH). Anal. (C25H25NO5) C, H, N.
2-Hydroxy-4-(1-(naphthalen-2-ylmethyl)-4-oxo-1,4-dihydroquinolin-3-yl)-4-oxobut-2-enoic Acid Ethyl Ester (10y).
Yield 87%; 153–154 °C; DMF/H2O. IR ν 1734 (C=O ester), 1629 and 1603 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.28 (t, 3H, CH2CH3), 4.32 (q, 2H, CH2CH3), 5.94 (s, 2H, CH2), 7.49–7.56 (m, 4H, naphthyl H), 7.66–7.75 (m, 2H, naphthyl H), 7.79 (s, 1H, naphthyl H), 7.84–7.92 (m, 3H, quinolinone C6-H, C7-H and C8-H), 8.04 (s, 1H, butenoate C3-H), 8.32 (d, 1H, quinolinone C5-H), 9.20 (s, 1H, quinolinone C2-H), 15.00 (bs, 1H, OH). Anal. (C26H21NO5) C, H, N.
2-Hydroxy-4-oxo-4-(4-oxo-1-(pyridin-4-ylmethyl)-1,4-dihydroquinolin-3-yl)but-2-enoic Acid Ethyl Ester (10z).
Yield 66%; 168–169 °C; DMF/H2O. IR ν 1717 (C=O ester), 1645 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.28 (t, 3H, CH2CH3), 4.28 (q, 2H, CH2CH3), 5.82 (s, 2H, CH2), 7.08 (s, 2H, picoline H), 7.49 (t, 1H, quinolinone C6-H), 7.55 (d, 1H, quinolinone C8-H), 7.79 (t, 1H, quinolinone C7-H), 7.95 (s, 1H, butenoate C3-H), 8.32 (m, 1H, quinolinone C5-H), 8.50 (s, 2H, picoline H), 9.10 (s, 1H, quinolinone C2-H), 15.50 (bs, 1H, OH). Anal. (C21H18N2O5) C, H, N.
2-Hydroxy-4-oxo-4-(4-oxo-1-(quinolin-2-ylmethyl)-1,4-dihydroquinolin-3-yl)but-2-enoic Acid Ethyl Ester (10aa).
Yield 100%; 237–238 °C; DMF/H2O. IR ν 1712 (C=O ester), 1634 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.16 (t, 3H, CH2CH3), 4.30 (q, 2H, CH2CH3), 5.98 (s, 2H, CH2), 7.11–7.94 (m, 9H, quinolinone C6-H, C7-H, C8-H, quinoline H), 8.25–8.37 (m, 2H, quinolinone C5-H and quinoline H), 9.04 (s, 1H, quinolinone C2-H), 15.00 (bs, 1H, OH). Anal. (C25H20N2O5) C, H, N.
General Procedure for the Synthesis of Diketo Acids 11a–aa.
A mixture of 1 N NaOH (6.5 mL) and the appropriate ester 10a–aa (1.3 mmol) in 1:1 THF/methanol (12 mL) was stirred at room temperature for 40 min and then poured onto crushed ice. The aqueous layer was separated and treated with 1 N HCl until pH 3 was reached, and the formed yellow solid was collected by filtration, then washed with water, hot dry ethanol, and light petroleum ether to afford pure acids 11a–aa. Yield (%), melting point (°C), recrystallization solvent, IR, 1H NMR, and analytical data for each of the following compounds are reported.
4-[1-Phenylmethyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11a).
Yield 92%; 141–142 °C; DMF/H2O. IR ν 3424 (OH), 1726 (C=O acid), 1625 and 1603 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.75 (s, 2H, CH2), 7.22–7.35 (m, 5H, benzene H), 7.44 (m, 1H, quinolinone C6-H), 7.65–7.69 (m, 2H, quinolinone C7-H and C8-H), 7.90 (s, 1H, butenoate C3-H), 8.30 (m, 1H, quinolinone C5-H), 9.08 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C19H15NO5) C, H, N.
4-[1-(2-Fluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11b).
Yield 83%; 172–174 °C; toluene. IR ν 3200 (OH), 1748 (C=O acid), 1621 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.81 (s, 2H, CH2), 7.14–7.27 (m, 3H, benzene H), 7.35 (m, 1H, benzene H), 7.50 (m, 1H, quinolinone C6-H), 7.70–7.73 (m, 2H, quinolinone C7-H and C8-H), 7.95 (s, 1H, butenoate C3-H), 8.31 (d, 1H, quinolinone C5-H), 9.08 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C20H14FNO5) C, H, F, N.
4-[1-(2-Methoxyphenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11c).
Yield 94%; 172–175 °C; washed with dry ethanol. IR ν 3149 (OH), 1742 (C=O acid), 1620 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 3.82 (s, 3H, CH3), 5.61 (s, 2H, CH2), 6.89 (t, 1H, benzene H), 7.01–7.12 (m, 2H, benzene H), 7.0 (t, 1H, benzene H), 7.42–7.49 (m, 1H, quinolinone C6-H), 7.71–7.74 (m, 2H, quinolinone C7-H and C8-H), 7.94 (s, 1H, butenoate C3-H), 8.30 (d, 1H, quinolinone C5-H), 9.02 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C21H17NO6) C, H, N.
4-[1-(3-Fluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11d).
Yield 60%; 158–159 °C; washed with hot dry ethanol. IR ν 3392 (OH), 1719 (C=O acid), 1667 and 1620 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.76 (s, 2H, CH2), 7.05–7.18 (m, 3H, benzene H), 7.36 (m, 1H, benzene H), 7.47 (m, 1H, quinolinone C6-H), 7.66–7.68 (m, 2H, quinolinone C7-H and C8-H), 7.88 (s, 1H, butenoate C3-H), 8.30 (d, 1H, quinolinone C5-H), 9.07 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C20H14FNO5) C, H, F, N.
4-[1-(3-Methoxyphenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11e).
Yield 80%; 179–181 °C; washed with dry ethanol. IR ν 3002 (OH), 1748 (C=O acid), 1602 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 3.70 (s, 3H, CH3), 5.72 (s, 2H, CH2), 6.75 (d, 1H, benzene H), 6.84–6.89 (m, 2H, benzene H), 7.24 (t, 1H, benzene H), 7.49 (m, 1H, quinolinone C6-H), 7.69–7.74 (m, 2H, quinolinone C7-H and C8-H), 7.98 (s, 1H, butenoate C3-H), 8.30 (d, 1H, quinolinone C5-H), 9.08 (s, 1H, quinolinone C2-H), 13.90 (bs, 1H, OH), 15.60 (bs, 1H, OH). Anal. (C21H17NO6) C, H, N.
4-[1-(4-Chlorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11f).
Yield 96%; 177–179 °C; washed with hot dry ethanol. IR ν 3250 (OH), 1741 (C=O acid), 1643 and 1611 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.76 (s, 2H, CH2), 7.25–7.31 (m, 2H, benzene H), 7.39–7.41 (m, 2H, benzene H), 7.49 (m, 1H, quinolinone C6-H), 7.65–7.73 (m, 2H, quinolinone C8-H and C7-H), 7.96 (s, 1H, butenoate C3-H), 8.30 (d, 1H, quinolinone C5-H), 9.11 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C20H14ClNO5) C, H, Cl, N.
4-[1-(4-Hydroxyphenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11g).
Yield 77%; 207–208 °C; washed with dry ethanol. IR ν 3135 (OH), 1718 (C=O acid), 1600 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.59 (s, 2H, CH2), 6.71–6.73 (m, 2H, benzene H), 7.11–7.13 (m, 2H, benzene H), 7.47 (m, 1H, quinolinone C6-H), 7.67–7.78 (m, 2H, quinolinone C8-H and C7-H), 7.92 (s, 1H, butenoate C3-H), 8.29 (d, 1H, quinolinone C5-H), 9.00 (s, 1H, quinolinone C2-H), 9.50 (bs, 1H, OH phenole), 14.00–16.00 (bs, 2H, OH). Anal. (C20H15NO5) C, H, N.
4-[1-(4-Methoxyphenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11h).
Yield 100%; 152 °C; washed with dry ethanol. IR ν 3400 (OH), 1726 (C=O acid), 1620 and 1611 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 3.69 (s, 3H, CH3), 5.66 (s, 2H, CH2), 6.88–6.90 (m, 2H, benzene H), 7.24–7.26 (m, 2H, benzene H), 7.50 (m, 1H, quinolinone C6-H), 7.70–7.78 (m, 2H, quinolinone C7-H and C8-H), 7.93 (s, 1H, butenoate C3-H), 8.30 (d, 1H, quinolinone C5-H), 9.07 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C21H17NO6) C, H, N.
4-[1-(4-Nitrophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11i).
Yield 90%; 230–232 °C; washed with dry ethanol. IR ν 3100 (OH), 1731 (C=O acid), 1634 and 1602 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.87 (s, 2H, CH2), 7.45–7.52 (m, 3H, benzene H and quinolinone C6-H), 7.58 (m, 1H, quinolinone C8-H), 7.68 (m, 2H, quinolinone C7-H), 7.92 (s, 1H, butenoate C3-H), 8.18–8.20 (m, 2H, benzene H), 8.32 (d, 1H, quinolinone C5-H), 9.12 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C20H14N2O7) C, H, N.
4-[1-(2,4-Difluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11j).
Yield 84%; 182 °C; ethanol. IR ν 3400 (OH), 1729 (C=O acid), and 1620 and 1608 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.84 (s, 2H, CH2), 7.11 (t, 1H, quinolinone C6-H), 7.37–7.40 (m, 2H, benzene H), 7.57 (d, 1H, quinolinone C8-H), 7.77–7.81 (m, 2H, benzene H and quinolinone C7-H), 8.01 (s, 1H, butenoate C3-H), 8.37 (d, 1H, quinolinone C5-H), 9.13 (s, 1H, quinolinone C2-H), 14.60 (bs, 1H, OH), 15.90 (bs, 1H, OH). Anal. (C20H13F2NO5) C, H, F, N.
4-[1-(2,5-Difluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11k).
Yield 46%; 176–178 °C; washed with dry ethanol. IR ν 3402 (OH), 1746 (C=O acid), 1622 and 1602 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.85 (s, 2H, CH2), 7.21–7.38 (m, 3H, benzene H), 7.56–7.80 (m, 3H, quinolinone C6-H, C7-H and C8-H), 7.99 (s, 1H, butenoate C3-H), 8.38 (d, 1H, quinolinone C5-H), 9.11 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C20H13F2NO5) C, H, F, N.
4-[1-(2,6-Difluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11l).
Yield 89%; 171–172 °C; washed with dry ethanol. IR ν 3450 (OH), 1747 (C=O acid), 1621 and 1603 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.82 (s, 2H, CH2), 7.13–7.18 (t, 2H, benzene H), 7.45–7.49 (m, 2H, benzene H and quinolinone C6-H), 7.67 (d, 1H, quinolinone C8-H), 7.77 (t, 1H, quinolinone C7-H), 7.86 (s, 1H, butenoate C3-H), 8.29 (d, 1H, quinolinone C5-H), 9.06 (s, 1H, quinolinone C2-H), 13.00–16.00 (bs, 2H, OH). Anal. (C20H13F2NO5) C, H, F, N.
4-[1-(3,4-Difluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11m).
Yield 86%; 188–190 °C; washed with dry ethanol. IR ν 3000 (OH), 1730 (C=O acid), 1638 and 1610 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.79 (s, 2H, CH2), 7.17 (t, 1H, quinolinone C6-H), 7.45–7.54 (m, 3H, benzene H), 7.74–7.76 (m, 2H, quinolinone C8-H and quinolinone C7-H), 8.00 (s, 1H, butenoate C3-H), 8.37 (d, 1H, quinolinone C5-H), 9.15 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C20H13F2NO5) C, H, F, N.
4-[1-(3,5-Difluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11n).
Yield 85%; 188 °C; ethanol. IR ν 3200 (OH), 1721 (C=O acid), 1626 and 1602 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.83 (s, 2H, CH2), 7.05–7.22 (m, 3H, benzene H), 7.55 (t, 1H, quinolinone C6-H), 7.68 (d, 1H, quinolinone C8-H), 7.78 (t, 1H, quinolinone C7-H), 8.02 (s, 1H, butenoate C3-H), 8.36 (d, 1H, quinolinone C5-H), 9.14 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C20H13F2NO5) C, H, F, N.
4-[1-(2,4-Dichlorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11o).
Yield 83%; 220 °C (dec); washed with 2-propanole/isopropilic eter. IR ν 3200 (OH), 1722 (C=O acid), 1621 and 1608 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.83 (s, 2H, CH2), 6.97 (d, 1H, benzene H), 7.35 (d, 1H, benzene H), 7.49–7.59 (m, 2H, quinolinone C6-H and C8-H), 7.75–7.81 (m, 2H, benzene H quinolinone C7-H), 8.02 (s, 1H, butenoate C3-H), 8.39 (d, 1H, quinolinone C5-H), 9.10 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C20H13Cl2NO5) C, H, Cl, N.
4-[1-(2,6-Dichlorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11p).
Yield 98%; 193–195 °C; washed with dry ethanol. IR ν 3041 (OH), 1743 (C=O acid), 1630 and 1605 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.89 (s, 2H, CH2), 7.60–7.66 (m, 2H, benzene H), 7.72–7.74 (m, 2H, quinolinone C6-H and C8-H), 7.91–8.04 (m, 3H, benzene H, quinolinone C7-H and butenoate C3-H), 8.41 (d, 1H, quinolinone C5-H), 8.44 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C20H13Cl2NO5) C, H, Cl, N.
4-[1-(2,4-Dimethylphenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11q).
Yield 86%; 192 °C; washed with dry ethanol. IR ν 3500 (OH), 1731 (C=O acid), 1625 and 1602 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 2.27 (s, 3H, CH3), 2.38 (s, 3H, CH3), 5.72 (s, 2H, CH2), 6.61 (d, 1H, benzene H), 6.94 (d, 1H, benzene H), 7.15 (s, 1H, benzene H), 7.53–7.63 (m, 2H, quinolinone C6-H and C8-H), 7.78 (m, 1H, quinolinone C7-H), 8.05 (s, 1H, butenoate C3-H), 8.40 (d, 1H, quinolinone C5-H), 8.96 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C22H19NO5) C, H, N.
4-[1-(3,5-Dimethylphenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11r).
Yield 82%; 197–198 °C; washed with dry ethanol. IR ν 3200 (OH), 1736 (C=O acid), 1620 and 1602 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 2.23 (s, 6H, CH3), 5.72 (s, 2H, CH2), 6.85–7.16 (m, 3H, benzene H), 7.50–7.95 (m, 4H, butenoate C3-H, quinolinone C6-H, C7-H and C8-H), 8.49 (d, 1H, quinolinone C5-H), 9.26 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C22H19NO5) C, H, N.
4-[1-(3-Chloro-2-fluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11s).
Yield 38%; 164–165 °C; washed with dry ethanol. IR ν 3067 (OH), 1731 (C=O acid), 1622 and 1601 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.87 (s, 2H, CH2), 7.01–7.19 (m, 2H, benzene H and quinolinone C6-H), 7.51–7.75 (m, 4H, quinolinone C7-H, C8-H and benzene H), 7.85 (s, 1H, butenoate C3-H), 8.34 (d, 1H, quinolinone C5-H), 9.08 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C20H13ClFNO5) C, H, Cl, F, N.
4-[1-(3-Chloro-4-fluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11t).
Yield 85%; 162–163 °C; washed with dry ethanol. IR ν 3060 (OH), 1732 (C=O acid), 1620 and 1601 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.75 (s, 2H, CH2), 7.25 (m, 1H, quinolinone C6-H), 7.39 (t, 1H, quinolinone C7-H), 7.47–7.51 (m, 2H, quinolinone C8-H and benzene H), 7.60–7.7.72 (m, 3H, benzene H), 7.80 (s, 1H, butenoate C3-H), 8.33 (d, 1H, quinolinone C5-H), 9.08 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C20H13ClFNO5) C, H, Cl, F, N.
8-Chloro-4-[1-(3-chloro-4-fluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11u).
Yield 100%; 158–159 °C; washed with dry ethanol. IR ν 3040 (OH), 1730 (C=O acid), 1625 and 1601 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 6.08 (s, 2H, CH2), 7.08 (m, 1H, quinolinone C6-H), 7.37–7.48 (m, 4H, quinolinone C7-H and benzene H), 7.83 (s, 1H, butenoate C3-H), 8.40 (d, 1H, quinolinone C5-H), 8.86 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C20H12Cl2FNO5) C, H, Cl, F, N.
8-Chloro-4-[1-(2-fluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11v).
Yield 70%; 163–164 °C; washed with dry ethanol. IR ν 3400 (OH), 1728 (C=O acid), 1625 and 1601 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 6.14 (s, 2H, CH2), 6.90–6.96 (m, 2H, benzene H), 7.08–7.15 (m, 2H, benzene H), 7.22–7.37 (m, 2H, quinolinone C6-H and benzene H), 7.48 (m, 1H, quinolinone C7-H), 7.82 (s, 1H, butenoate C3-H), 8.38 (d, 1H, quinolinone C5-H), 8.88 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C20H13ClFNO5) C, H, Cl, F, N.
4-[7-Chloro-1-(2-fluorophenyl)methyl-4(1H)-quinolinon-3-yl]-2-hydroxy-4-oxo-2-butenoic Acid (11w).
Yield 83%; 186–187 °C; washed with dry ethanol; IR ν 3631 (OH), 1732 (C=O acid), 1600 and 1583 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.87 (s, 2H, CH2), 7.22–7.46 (m, 4H, benzene H), 7.60 (d, 1H, quinolinone C6-H), 7.90 (s, 1H, quinolinone C8-H), 7.93 (s, 1H, butenoate C3-H), 8.35 (d, 1H, quinolinone C5-H), 9.10 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C20H13FClNO5) C, H, N, F, Cl.
2-Hydroxy-4-oxo-4-(4-oxo-1-(4-phenylbutyl)-1,4-dihydroquinolin-3-yl)but-2-enoic Acid (11x).
Yield 90%; 136–138 °C; DMF/H2O. IR ν 3400 (OH), 1747 (C=O acid), 1623 and 1606 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 1.63 (m, 2H, butyl H), 1.77 (m, 2H, butyl H), 2.59 (m, 2H, butyl H), 4.46 (m, 2H, butyl H), 7.11–7.25 (m, 5H, benzene H and quinolinone C6-H), 7.49 (m, 1H, quinolinone C8-H), 7.77–7.83 (m, 2H, quinolinone C7-H and butenoate C3-H), 8.30 (d, 1H, quinolinone C5-H), 8.85 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C23H21NO5) C, H, N.
2-Hydroxy-4-(1-(naphthalen-2-ylmethyl)-4-oxo-1,4-dihydroquinolin-3-yl)-4-oxobut-2-enoic Acid (11y).
Yield 85%; 212–213 °C; washed with dry ethanol. IR ν (C=O acid), and (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.89 (s, 2H, CH2), 7.38–47 (m, 4H, naphthyl H), 7.64–7.71 (m, 2H, naphthyl H), 7.75 (s, 1H, naphthyl H), 7.85–7.91 (m, 3H, quinolinone C6-H, C7-H and C8-H), 8.12 (s, 1H, butenoate C3-H), 8.30 (d, 1H, quinolinone C5-H), 9.13 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C24H17NO5) C, H, N.
2-Hydroxy-4-oxo-4-(4-oxo-1-(pyridin-4-ylmethyl)-1,4-dihydroquinolin-3-yl)but-2-enoic Acid (11z).
Yield 65%; 225 °C; washed with dry ethanol. IR ν 3240 (OH), 1700 (C=O acid), 1620 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.79 (s, 2H, CH2), 7.18 (m, 2H, picoline H), 7.45–7.66 (m, 3H, quinolinone C6-H, C7-H and C8-H), 7.98 (s, 1H, butenoate C3-H), 8.33 (m, 1H, quinolinone C5-H), 8.51 (m, 2H, picoline H), 9.12 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C19H14N2O5) C, H, N.
2-Hydroxy-4-oxo-4-(4-oxo-1-(quinolin-2-ylmethyl)-1,4-dihydroquinolin-3-yl)but-2-enoic Acid (11aa).
Yield 62%; >300 °C; washed with dry ethanol. IR ν 3429 (OH), 1700 (C=O acid), 1633 (C=O ketone) cm−1. 1H NMR (DMSO-d6) δ 5.98 (s, 2H, CH2), 7.40–7.82 (m, 9H, quinolinone C6-H, C8-H, quinoline H), 7.93–7.95 (m, 2H, quinolinone C7-H and butenoate C3-H), 8.29–8.40 (m, 2H, quinolinone C5-H and quinoline H), 8.94 (s, 1H, quinolinone C2-H), 14.00–16.00 (bs, 2H, OH). Anal. (C23H16N2O5) C, H, N.
Biological Methods.
HIV-1 IN Inhibition.
HIV-1 IN gel-based assays were carried out as previously published.33 Briefly, drugs or an equivalent volume of 100% DMSO (dimethyl sulfoxide, used as the drug solvent) was added to a reaction mixture containing 20 nM 32P-labeled DNA substrate and 400 nM IN in 50 mM MOPS, pH 7.2, 7.5 mM MgCl2, and 14 mM 2-mercaptoethanol. Reactions were performed at 37 °C for 1 h and stopped by the addition of an equal volume of loading buffer [formamide containing 1% SDS (sodium dodecyl sulfate), 0.25% bromophenol blue, and xylene cyanol]. Products were separated in 16% polyacrylamide denaturing sequencing gels. Dried gels were visualized using a Typhoon 8600 (GE Healthcare). Densitometric analyses were performed using the ImageQuant 5.1 software from GE Healthcare. Data analyses (linear regression, IC50 determination, and standard deviation) were performed using Prism 5.0c software from GraphPad.
HIV-1 RT RNase H Inhibition.
IC50 values were determined as previously reported34 using an 18-nt 3′-fluorescein-labeled RNA annealed to a complementary 18-nt 5′-dabsyl-labeled DNA. To a 96-well plate was added 1 μL of each inhibitor (in DMSO), followed by 10 μL of the appropriate RT (15–80 ng/mL) in reaction buffer. Hydrolysis was initiated by adding 10 μL of RNA/DNA hybrid (2.5 μM). Final assay conditions were 50 mM Tris-HCl, pH 8.0, 60 mM KCl, 10 mM MgCl2, 1% DMSO, 150–800 ng of RT, 250 nM substrate, and increasing concentrations of inhibitor. Wells containing only DMSO were used as negative control. Plates were incubated at 37 °C in a Spectramax Gemini EM fluorescence spectrometer for 10 min, and fluorescence (λex = 485 nm; λem = 520 nm) was measured at 1 min intervals such that linear initial rates could be measured in the presence (vi) and absence (v0) of inhibitor. Percent inhibition was calculated as 100(v0 − vi)/v0, and plotted against log[I]. IC50 values were determined using Prism5 (GraphPad Software). All assays were performed in triplicate.
HIV-1 Replication Inhibition.
The compounds’ antiviral activity was determined in a cell-based assay according to the procedure described previously35 and modified as follows. HeLa-CD4-LTR-β-gal cells were maintained in DMEM with 10% serum and 0.5 mg/mL G418. The day prior experimentation, 96-well plates were prepared to contain 10000 cells per well in 100 μL of DMEM medium complemented with 10% serum. On day one, each drug was serial diluted directly on cells following a 3-fold dilution over six points, and each well was then filled to 200 μL with either fresh medium or concentrated viral supernatant (HIV-1(IIIB), Advanced Biotechnologies Inc.). The highest compound concentration tested was 50 μM. On day two, cells were washed three times with PBS before adding 200 μL of a solution containing 50 mM Tris-HCl pH 7.5, 100 mM β-mercaptoethanol, 0.05% Triton X100, and 5 mM 4-methyl-umbelliferyl-β-d-galactopyranoside (4-MUG, Sigma). On day three, sealed plates were read in a SpectraMax GEMINI-XS (Molecular Devices) with λex/em = 360/460 nm.
Cellular Toxicity.
Similar to the antiviral assays, plates were prepared with 10000 HeLa-CD4-LTR-β-gal cells per well and a serial dilution of compounds in 100 μL. After 24 h of culture, 100 μL of ATPlite reagent (PerkinElmer) was added to each well. After 5 min at room temperature, luminescence was quantified using an EnVision multilabel reader (PerkinElmer) according to manufacturer’s instructions.
Molecular Modeling.
Ligands Setup.
The 3D structures of all of the compounds were generated with the Maestro Build Panel36 and were then submitted to Polak–Ribiere conjugate gradient minimization (0.0005 kJ/(Å mol) convergence) using MacroModel (version 9.9).37 DKAs were modeled in their enolic tautomeric form because it has been clearly established that such compounds mainly exist in this form in solution and also given the influence of the two metal cations in the binding site.38
Proteins Setup.
The HIV-1 CCD/DNA homology model and the RNase H crystal structure (PDB code 3QIP) were prepared for docking calculations using the “Protein Preparation Wizard” panel of Schrödinger 2010 molecular modeling package.36 In particular, using the “preprocess and analyze structure” tool, the bond orders and disulfide bonds were assigned, all the hydrogen atoms were added, and all the water molecules in a distance greater than 5 Å from any heterogroup were deleted; in addition, both Mn2+ ions in the RNase H crystal structure were replaced with Mg2+ cations. Using Epik 2.0, a prediction of the side chains heterogroups ionization and tautomeric states was performed.39 An optimization of the hydrogen-bonding network was performed using the “H-bond assignment” tool. Finally, using the “impref utility”, the positions of the hydrogen atoms were optimized by keeping all the heavy atoms in place.
Docking Calculations.
Docking studies at both the HIV-1 IN and RNase H active sites were carried out with Glide v. 5.7 (Schrödinger).40 Glide is a grid-based ligand docking with energetics approach and searches for favorable interactions between ligands and receptors. The shape and properties of the receptor are represented on a grid by different sets of fields that provide progressively more accurate scoring of the ligand pose. These fields are generated as preprocessing steps in the calculation and hence need to be computed only once for each receptor. For the grid generation of both the receptors, a box centered on the Mg2+ cations was created. This box gives a more precise measure of the effective size of the search space. However, ligands can move outside this box during grid minimization. The Cartesian coordinates of the outer box, X, Y, and Z length, were set to 25 Å. The conformational space of the ligand is defined by Glide by several lowest-energy poses that are subjected to a Monte Carlo procedure that examines nearby torsional minima. This procedure is needed in some cases to properly orient peripheral groups and occasionally alters internal torsion angles. The default value (1.00) for the van der Waals radii scaling factor was chosen, which means no scaling for the nonpolar atoms was performed (no flexibility was simulated for the receptor). In the present study, the standard precision (SP) mode of GlideScore function was used to score the obtained binding poses. The force field used for the docking was the OPLS-2005.41 All of the pictures were rendered with the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco.42
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the Italian MIUR for financial support, PRIN 2010-2011 (2010W2KM5L_002) and “Sapienza” Ateneo C26A133H7B. R. Di Santo and R. Costi thank the FP7 CHAARM project for support. This work was also supported by the NIH Intramural Research Program, Center for Cancer Research, National Cancer Institute (Z01 BC 007333).
ABBREVIATIONS USED
- IN
integrase
- RT
reverse transcriptase
- 3′-P
3′-processing
- BAF
barrier to autointegration factor
- HSP60
heat-shock protein 60
- HMG
high mobility group protein
- LEDGF
lens epithelium-derived growth factor
- Vpr
viral protein R
- RNase H
ribonuclease H
- PIC
preintegration complex
- ST
strand transfer
- DKA
diketo acid
- rRT
recombinant reverse transcriptase
- rIN
recombinant integrase
- EVG
elvitegravir
- SI
selectivity index
- SAR
structure–activity relationship
- CCD
catalytic core domain
- PFV
prototype foamy virus
- INSTI
strand transfer inhibitor
Footnotes
Supporting Information
Chemical, physical and spectroscopic data of compounds 12a–c, 13a–aa, analyses of compounds 10a–aa 11a–aa, and molecular modeling. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.5b00159.
The authors declare no competing financial interest.
REFERENCES
- (1).Mehellou Y; De Clerck E Twenty-six years of anti-HIV drug discovery: where do we stand and where do we go? J. Med. Chem 2010, 53, 521–538. [DOI] [PubMed] [Google Scholar]
- (2).Gao K; Gorelick R; Johnson D; Bushman F Cofactors for HIV-1 cDNA integration in vitro. J. Virol 2003, 77, 1598–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Bukrinsky M; Sharova N; McDonald T; Pushkarskaya T; Tarpley W; Stevenson M Association of integrase, matrix, and reverse transcriptase antigens of HIV-1 with viral nucleic acids following acute infection. Proc. Natl. Acad. Sci. U. S. A 1993, 90, 6125–6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Pommier Y; Johnson AA; Marchand C Integrase inhibitors to treat HIV/AIDS. Nature Rev. Drug Discovery 2005, 4, 236–248. [DOI] [PubMed] [Google Scholar]
- (5).Farnet CM; Wang B; Russell Lipford J; Bushman FD Differential inhibition of HIV-1 preintegration complexs and purified integrase protein by small molecules. Proc. Natl. Acad. Sci. U. S. A 1996, 93, 9742–9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Grinsztejn B; Nguyen BY; Katlama C; Gallet JM; Lazzarin A; Vittecoq D; Gonzalez CJ; Chen J; Harvey CM; Isaacs RD Safety and efficacy of the HIV-1 integrase inhibitors raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: A phase II randomised controlled trials. Lancet 2007, 369, 1261–1269. [DOI] [PubMed] [Google Scholar]
- (7).(a) Yang W; Steitz TA Recombining the structures of HIV integrase, RuvC and RNase H. Structure 1995, 3, 131–134. [DOI] [PubMed] [Google Scholar]; (b) Haren L; Ton-Hoang B; Chandler M Integrating DNA: trasposase and retroviral integrases. Annu. Rev. Microbiol 1999, 53, 245–281. [DOI] [PubMed] [Google Scholar]
- (8).Andreola M Closely related antiretroviral agents as inhibitors of two HIV-1 enzymes, ribonuclease H and integrase: “Killing two birds with one stone”. Curr. Pharm. Des 2004, 10, 3713–3723. [DOI] [PubMed] [Google Scholar]
- (9).Métifiot M; Marchand C; Pommier Y HIV integrase inhibitors: 20-year landmark and challenges. Adv. Pharmacol 2013, 67, 75–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Davies JF II; Hostomska Z; Hostomsky Z; Jordan SR; Matthews DA Crystal structure of the ribonuclease H domain of HIV-1 reverse trascriptase. Science 1991, 252, 11536–11546. [DOI] [PubMed] [Google Scholar]
- (11).(a) Shaw-Reid CA; Munshi V; Graham P; Wolfe A; Witmer M; Danzeisen R; Olsen DB; Carroll SS; Embrey M; Wai JS; Miller MD; Cole JL; Hazuda DJ Inhibition of HIV-1 ribonuclease H by a novel diketo acid, 4-[5-(benzoylamino)thien-2-yl]-2,4-dioxobutanoic acid. J. Biol. Chem 2003, 278, 2777–2780. [DOI] [PubMed] [Google Scholar]; (b) Tramontano E; Esposito F; Badas R; Di Santo R; Costi R; La Colla P 6-[1-(4-Fluorophenyl)methyl-1H-pyrrol-2-yl)]-2,4-dioxo-5-hexenoic acid ethyl ester a novel diketo acid derivative which selectively inhibits the HIV-1 viral replication in cell culture and the ribonuclease H activity in vitro. Antiviral Res 2005, 65, 117–124. [DOI] [PubMed] [Google Scholar]
- (12).de Soultrait V; Lozach P; Altmeyer R; Tarrago-Litvak S; Andreola ML DNA aptamers derivated from HIV-1 RNase H inhibitors are strong anti-integrase agents. J. Mol. Biol 2002, 324, 195–203. [DOI] [PubMed] [Google Scholar]
- (13).(a) Didierjean J; Isel C; Querre F; Mouscadet JF; Aubertin AM; Valnot JY; Piettre SR; Marquet R Inhibition of human immunodeficiency virus type 1 reverse trascriptase, RNase H, and integrase activities by hydroxytropolones. Antimicrob. Agents Chemother 2005, 49, 4884–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Budihas S; Gorshkova I; Gaidamakov S; Wamiru A; Bona M; Parniak K; Crouch R; McMahon J; Beutler J; Le Grice S Selective inhibition of HIV-1 reverse trascriptase-associated ribonuclase H activity by hydroxylated tropolones. Nucleic Acid Res 2005, 33, 1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Semenova EA; Johnson AA; Marchand C; Davis DA; Yarchoan R; Pommier Y Preferential inhibition of the magnesium dependent strand transfer reaction of HIV-1 integrase by α-hydroxytropolones. Mol. Pharmacol 2006, 69, 1454–1460. [DOI] [PubMed] [Google Scholar]
- (14).(a) Billamboz M; Bailly F; Barreca ML; De Luca L; Mouscadet JF; Calmels C; Andreola ML; Witvrouw M; Christ F; Debyser Z; Cotelle P Design, synthesis, and biological evaluation of a series of 2-hydroxyquinoline-1,3(2H,4H)-diones as dual inhibitors of human immunodeficiency virus type 1 integrase and reverse trascriptase RNase H domain. J. Med. Chem 2008, 51, 7717–7730. [DOI] [PubMed] [Google Scholar]; (b) Billamboz M; Bailly F; Lion C; Calmels C; Andreola ML; Witvrouw M; Christ F; Debyser Z; De Luca L; Chimirri A; Cotelle P 2-Hydroxyisoquinoline-1,3(2H,4H)-diones as inhibitors of HIV-1 integrase and reverse transcriptase RNase H domain: influence of the alkylation of position 4. Eur. J. Med. Chem 2011, 46, 535–546. [DOI] [PubMed] [Google Scholar]
- (15).Marchand C; Beutler JA; Wamiru A; Budihas S; Möllmann U; Heinisch L; Mellors JW; Le Grice SF; Pommier Y Madurahydroxylactone derivatives as dual inhibitors of human immunodeficiency virus type 1 integrase and RNase H. Antimicrob. Agents Chemother 2008, 52, 361–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).(a) Di Santo R; Costi R; Roux A; Artico M; Lavecchia A; Marinelli L; Novellino E; Palmisano L; Andreotti M; Amici R; Galluzzo CM; Nencioni L; Palamara A; Pommier Y; Marchand C Novel bifunctional quinolonyl diketo acid derivatives as HIV-1 integrase inhibitors: design, synthesis, biological activities, and mechanism of action. J. Med. Chem 2006, 49, 1939–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Di Santo R; Costi R; Roux A; Miele G; Cuzzucoli Crucitti G; Iacovo A; Rosi F; Lavecchia A; Marinelli L; Di Giovanni C; Novellino E; Palmisano L; Andreotti M; Amici R; Galluzzo CM; Nencioni L; Palamara AT; Pommier Y; Marchand C Novel quinolinonyl diketo acid derivatives as HIV-1 integrase inhibitors: design, synthesis, and biological activities. J. Med. Chem 2008, 51, 4744–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Di Santo R Diketo acids derivatives as dual inhibitors of human immunodeficiency virus type 1 integrase and the reverse transcriptase RNase H domain. Curr. Med. Chem 2011, 18, 3335–3342. [DOI] [PubMed] [Google Scholar]
- (18).Costi R; Métifiot M; Chung S; Cuzzucoli Crucitti G; Maddali K; Pescatori L; Messore A; Madia VN; Pupo G; Scipione L; Tortorella S; Di Leva FS; Cosconati S; Marinelli L; Novellino E; Le Grice SFJ; Corona A; Pommier Y; Marchand C; Di Santo R Basic quinolinonyl diketo acid derivatives as inhibitors of HIV integrase and their activity against RNase H function of reverse transcriptase. J. Med. Chem 2014, 57, 3223–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Yoshizawa H Process for producing 5,7-dichloro-4-hydroxy quinoline. PCT Int. Appl WO 9523787, 1995; CAN 124: 29619, 1995.
- (20).Marinello J; Marchand C; Mott BT; Bain A; Thomas CJ; Pommier Y Comparison of raltegravir and elvitegravir on HIV-1 integrase catalytic reactions and on a series of drug-resistant integrase mutants. Biochemistry 2008, 47, 9345–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hare S; Vos AM; Clayton RF; Thuring JW; Cummings MD; Cherepanov P Molecular mechanisms of retroviral integrase inhibition and the evolution of viral resistance. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 20057–20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Agrawala A; DeSotoa J; Fullagara JL; Maddalib K; Rostamic S; Richmanc DD; Pommier Y; Cohena SM Probing chelation motifs in HIV integrase inhibitors. Proc. Natl. Acad. Sci. U. S. A 2012, 109, 2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Hare S; Gupta SS; Valkov E; Engelman A; Cherepanov P Retroviral intasome assembly and inhibition of DNA strand transfer. Nature 2010, 464, 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Tang J; Maddali K; Metifiot M; Sham YY; Vince R; Pommier Y; Wang Z 3-Hydroxypyrimidine-2,4-diones as an inhibitor scaffold of HIV integrase. J. Med. Chem 2011, 54, 2282–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Johnson AA; Marchand C; Patil SS; Costi R; Di Santo R; Burke TR Jr.; Pommier Y Probing HIV-1 integrase inhibitor binding sites with position-specific integrase-DNA cross-linking assays. Mol. Pharmacol 2007, 71, 893–901. [DOI] [PubMed] [Google Scholar]
- (26).Vandurm P; Guiguen A; Cauvin C; Georges B; Le Van K; Michaux C; Cardona C; Mbemba G; Mouscadet J-F; Hevesi L; Van Lint C; Wouters J Synthesis, biological evaluation and molecular modeling studies of quinolonyl diketo acid derivatives: new structural insight into the HIV-1 integrase inhibition. Eur. J. Med. Chem 2011, 46, 1749–1756. [DOI] [PubMed] [Google Scholar]
- (27).Tang J; Maddali K; Pommier Y; Sham YY; Wang Z Scaffold rearrangement of dihydroxypyrimidine inhibitors of HIV integrase: docking model revisited. Bioorg. Med. Chem. Lett 2010, 20, 3275–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).De Luca L; De Grazia S; Ferro S; Gitto R; Christ F; Debyser Z; Chimirri A HIV-1 integrase strand-transfer inhibitors: design, synthesis and molecular modeling investigation. Eur. J. Med. Chem 2011, 46, 756–764. [DOI] [PubMed] [Google Scholar]
- (29).Himmel DM; Maegley KA; Pauly TA; Bauman JD; Das K; Dharia C; Clark AD Jr.; Ryan K; Hickey MJ; Love RA; Hughes SH; Bergqvist S; Arnold E Structure of HIV-1 reverse transcriptase with the inhibitor β-thujaplicinol bound at the RNase H active site structure. Structure 2009, 17, 1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Su H-P; Yan Y; Prasad GS; Smith RF; Daniels CL; Abeywickrema PD; Reid JC; Loughran HM; Kornienko M; Sharma S; Grobler JA; Xu B; Sardana V; Allison TJ; Williams PD; Darke PL; Hazuda DJ; Munshi S Structural basis for the inhibition of RNase H activity of HIV-1 reverse transcriptase by RNase H active site-directed inhibitors. J. Virol 2010, 84, 7625–7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Lansdon EB; Liu Q; Leavitt SA; Balakrishnan M; Perry JK; Lancaster-Moyer C; Kutty N; Liu X; Squires NH; Watkins WJ; Kirschberg TA Structural and binding analysis of pyrimidinol carboxylic acid and N-hydroxy quinazolinedione HIV-1 RNase H inhibitors. Antimicrob. Agents Chemother 2011, 55, 2905–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Corona A; Di Leva FS; Thierry S; Pescatori L; Cuzzucoli Crucitti G; Subra F; Delelis O; Esposito F; Rigogliuso G; Costi R; Cosconati S; Novellino E; Di Santo R; Tramontano E Identification of highly conserved residues involved in inhibition of HIV-1 RNase H function by diketo acid derivatives. Antimicrob. Agents Chemother 2014, 58, 6101–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Métifiot M; Maddali K; Johnson BC; Hare S; Smith SJ; Zhao XZ; Marchand C; Burke TR Jr.; Hughes SH; Cherepanov P; Pommier Y Activities, crystal structures, and molecular dynamics of dihydro-1H-isoindole derivatives, inhibitors of HIV-1 integrase. ACS Chem. Biol 2013, 8, 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Chung S; Wendeler M; Rausch JW; Beilhartz G; Gotte M; O’Keefe BR; Bermingham A; Beutler JA; Liu S; Zhuang X; Le Grice SF Structure–activity analysis of vinylogous urea inhibitors of human immunodeficiency virus-encoded ribonuclease H. Antimicrob. Agents Chemother 2010, 54, 3913–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Métifiot M; Faure A; Guyonnet-Duperat V; Bellecave P; Litvak S; Ventura M; Andréola ML Cellular uptake of ODNs in HIV-1 human-infected cells: a role for viral particles in DNA delivery? Oligonucleotides 2007, 17, 151–165. [DOI] [PubMed] [Google Scholar]
- (36).Maestro, version 9.2; Schrodinger, LLC: New York, 2011. [Google Scholar]
- (37).MacroModel, version 9.9; Schrodinger, LLC, New York, 2011. [Google Scholar]
- (38).Maurin C; Bailly F; Buisine E; Vezin H; Mbemba G; Mouscadet JF; Cotelle P Spectroscopic studies of diketoacids-metal interactions. A probing tool for the pharmacophoric intermetallic distance in the HIV-1 integrase active site. J. Med. Chem 2004, 47, 5583–5586. [DOI] [PubMed] [Google Scholar]
- (39).Epik, version 2.2; Schrodinger, LLC: New York, 2011. [Google Scholar]
- (40).Glide, version 5.7; Schrödinger, LLC, New York, NY, 2009. [Google Scholar]
- (41).Jorgensen WL; Maxwell DS; Tirado-Rives J Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc 1996, 118, 11225–11236. [Google Scholar]
- (42).Pettersen EF; Goddard TD; Huang CC; Couch GS; Greenblatt DM; Meng EC; Ferrin TE UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem 2004, 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


