Abstract
Brain-derived neurotrophic factor (BDNF) regulates a variety of physiological processes, and several studies have explored the role of BDNF in addiction-related brain regions like the nucleus accumbens core (NAcore). We sought to understand the rapid effects of endogenous BDNF on cocaine seeking. Rats were trained to self-administer cocaine and extinguished. We then microinjected two inhibitors of BDNF stimulation of tropomyosin receptor kinase B (TrkB), the non-competitive receptor antagonist ANA-12 and TrkB/Fc, a fusion protein that binds BDNF and prevents TrkB stimulation. Blocking TrkB or inactivating BDNF in NAcore potentiated active lever pressing, showing that endogenous BDNF tone was present and supplying inhibitory tone on cue-induced reinstatement. To determine if exogenous BDNF also negatively regulated reinstatement, BDNF was microinjected into NAcore 15 minutes before cue-induced reinstatement. BDNF decreased cocaine seeking through TrkB receptor binding, but had no effect on inactive lever pressing, spontaneous or cocaine-induced locomotion, or on reinstated sucrose seeking. BDNF-infusion potentiated within trial extinction when microinjected in the NAcore during cue- and context + cue induced reinstatement, and the inhibition of lever pressing lasted at least 3 days post injection. Although decreased reinstatement endured for 3 days when BDNF was administered prior to a reinstatement session, when microinjected before an extinction session or in the home cage, BDNF did not alter subsequent cued-reinstatement. Together, these data show that endogenous BDNF acts on TrKB to provide inhibitory tone on reinstated cocaine seeking, and this effect was recapitulated by exogenous BDNF.
Keywords: BDNF, cocaine seeking, nucleus accumbens, rats, self-administration, sucrose-seeking
INTRODUCTION
Cocaine addiction is a chronic disorder that is characterized by high vulnerability to relapse in response to cues or contexts associated with drugs, even after long periods of abstinence (Sinha 2011). One key region for initiating cue-induced cocaine seeking is the nucleus accumbens core (NAcore). The NAcore receives glutamatergic projections from the prelimbic cortex (PL) that are necessary for cocaine-induced (Capriles et al. 2003), context-induced (Fuchs et al. 2007), stress-induced (Capriles et al. 2003) and cue-induced (Stefanik, Kupchik, & Kalivas 2016) reinstatement of cocaine-seeking in animal models of relapse.
Synaptic plasticity within the glutamatergic PL-NAcore pathway plays a central role in reinstatement (Mulholland, Chandler, & Kalivas 2016), and brain-derived neurotrophic factor (BDNF) modulates activity-dependent plasticity involved in cocaine-seeking (Li & Wolf 2015; Koskela et al. 2016). Abstinent cocaine addicts show a correlation between serum BDNF and relapse risk (D’Sa et al. 2011; Corominas-Roso et al. 2015). Similarly, single and repeated non-contingent cocaine injections (Le Foll, Diaz, & Sokoloff 2005; Filip et al. 2006; Liu et al. 2006; Fumagalli et al. 2007) or self-administered cocaine (Graham et al. 2007; Sadri-Vakili et al. 2010; Schmidt et al. 2012; Li et al. 2013) increase BDNF mRNA and protein levels in the prefrontal cortex (PFC), nucleus accumbens (NAcc) and ventral tegmental area (VTA). Other studies also show time-dependent increases of BDNF levels in the VTA, NAcc and amygdala associated with the incubation of drug craving (Grimm et al. 2003). Cocaine-induced changes in BDNF-TrkB signaling are similarly observed when cocaine is administered at prenatal (McCarthy et al. 2016) or early developmental stages (Giannotti et al. 2014). Also, contingent cocaine increases the expression of the primary BDNF receptor, tropomyosin receptor kinase B (TrkB) in the shell subregion of the NAcc (NAshell) (Graham et al. 2009).
Consistent with a positive relationship between cocaine exposure and BDNF levels, repeated BDNF infusions into the VTA or NAcc increase cocaine-induced behavioral sensitization (Horger et al. 1999; Pierce, Pierce-Bancroft, & Prasad 1999) and self-administration (SA), and neutralizing BDNF in the NAcc reduces SA (Graham et al. 2007). Moreover, virus-mediated deletion of TrkB in NAcc reduces cocaine SA and cocaine conditioned place preference (CPP) (Graham et al. 2009), and virus-mediated over expression of TrkB in the NAshell regulates chronic cocaine-induced increases in spine density; although, this morphological regulation is unrelated to rates of cocaine SA (Anderson et al. 2017). Finally, systemic administration of a brain-penetrant TrkB antagonist reduces cocaine intake, motivation and reinstatement of cocaine seeking (Verheij et al. 2016).
BDNF also modulates context-induced and cue-induced cocaine seeking (Barker et al. 2015; Li et al, 2015). A single BDNF injection into the PFC activates the ERK signaling pathway, changes the phosphorylation of NMDA receptors in the PL by activating Src family kinases (Barry & McGinty 2017) and produces enduring inhibition through normalizing cocaine-mediated extracellular glutamate spillover in the NAcc (Berglind et al. 2009). In contrast, acute BDNF microinjection into the VTA promotes context-induced and cue-induced cocaine seeking through activation of the MAPK pathway (Lu et al. 2004). While BDNF is produced locally in the NAcc (Graham et al. 2007), BDNF is also anterogradely transported to the striatum from the PFC and VTA (Altar & DiStefano 1998), posing the possibility that BDNF release may differentially regulate reinstatement depending on the anatomical source to the NAcc. Other studies report differential effects of BDNF on relapse between the core and the shell subregions of the NAcc (Li et al. 2013). Differential regulation in NAcc is indicated by the fact that although the TrkB gene is expressed in both D1- and D2-receptor expressing medium spiny neurons (MSNs) (Freeman, Soghomonian, & Pierce 2003), BDNF differentially regulates cocaine CPP via selective D1- or D2-MSN TrkB activation (Lobo et al. 2010).
The studies outlined above examining enduring manipulations of BDNF or TrKB levels likely involved the well-established transcriptional regulation produced by BDNF. Here, we primarily focused on the acute, non-transcriptional effects of endogenous and exogenous BDNF in the NAcore on cue-induced reinstatement after cocaine SA, but also longer lasting effects in subsequent reinstatement testing. We found that both endogenous and exogenous BDNF in NAcore inhibit cue-induced cocaine seeking by stimulating TrKB, and this effect arises from accelerating within reinstatement trial extinction of lever pressing. The inhibitory effect of acute BDNF on cued reinstatement endured for at least 3 days, but only if BDNF was microinjected in conjunction with a reinstatement session, indicating that the enduring inhibition required BDNF in conjunction with activating cue-associated memories.
MATERIAL AND METHODS
Animals and surgery
Male Sprague Dawley rats (200–250 g, Charles River Laboratories) were housed individually in a temperature-controlled environment on a 12-hour reverse light cycle. After 1 week of acclimation, animals were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (7 mg/kg, i.p.) and implanted with indwelling jugular catheter and bilateral 26 gauge cannulae (PlasticsOne, VA) were inserted targeting the NAcore (+1.5 A/P, +1.7 M/L, −5.5 D/V), secured in place with dental cement and maintained open with 0.009″ × 30′″ internal stylets. Rats undergoing sucrose SA were only implanted with bilateral cannulae. All rats received ketorolac analgesic (0.28–0.32 mg/kg) and prophylactic antibiotic (Cefazolin, 200 mg/ml, subcutaneous; West-Ward Pharmaceuticals, NJ). Animals had 1 week to recover from surgery before starting behavioral training.
Reagents
All reagents were dissolved in 1 percent DMSO in saline. Vehicle (VEH) refers 1 percent DMSO in saline. Human BDNF (Peprotech, Rocky Hill, NJ, USA) was microinjected at 2 μg/μl (i.e. 1 μg/0.5 μl/side), a dose within the order of magnitude used in the literature (0.24–2.5 μg/0.5 μl/side) (Lu et al. 2004; Graham et al. 2007; Berglind et al. 2009). The TrkB selective and non-competitive antagonist ANA-12 (SML0209, Sigma-Aldrich, St Louis, MO, USA) (Cazorla et al. 2011) was microinjected in combination with BDNF or alone at 0.2 pg/μl (i.e. 0.1 pg/0.5 μl/side (Shirayama et al. 2015). We also tested a higher dose of [ANA-12] = 2 pg/μl (i.e.1 pg/0.5 μl/side), but this dose decreased spontaneous locomotion (data not shown). Human recombinant TrkB/Fc chimera (T8694; Sigma-Aldrich, St Louis, MO, USA), a soluble scavenger form of the TrkB receptor, was microinjected in combination with BDNF or alone at 1.3 μg/μl (i.e. 650 ng/0.5 μl/side) (Revest et al. 2014).
Cocaine self-administration (SA) procedure
Two days before starting cocaine SA, rats were food deprived overnight (22 g chow) and food trained under a FR1 reinforcement schedule to press for chow (45 mg, Bio-Serv, Flemington, NJ) for a single 2-hour session in standard rat modular test chambers (Med Associates, Fairfax, VT). Food training facilitated cocaine SA acquisition. Cocaine SA consisted of daily 2-h sessions on FR1 reinforcement schedule during which active lever presses resulted in cocaine delivery (0.25 mg/ infusion, NIDA) paired with a compound cue (light and tone, 78 dB, 4.5 kHz). Programs integrated a 20-second time out and a maximum of 80 infusions per session to prevent cocaine overdose. Following a minimum of 10 days of 10 infusions or more, rats entered extinction training, during which time lever presses had no consequences. Extinction criterion was reached when the average of active lever presses during the last 3 days of extinction was equal or less than 30 percent of the average of the last 3 days of SA, which usually happened after 10–12 days of extinction training. One group of animals underwent forced abstinence. Acquisition of SA was identical to extinguished animals, but instead of extinction training, these animals stayed in their home cage and were handled daily to prevent handling bias.
Sucrose self-administration (SA) procedure
Sucrose SA consisted of daily 2-hour sessions in standard rat modular test chambers (Med Associates, Fairfax, VT) on an FR1 reinforcement schedule during which active lever presses resulted in delivery of a sucrose pellet (45 mg, Bio-Serv, Flemington, NJ) paired with a compound cue (light and tone, 78 dB, 4.5 kHz). Following a minimum of 10 days of 10 pellets or more, rats entered extinction training, during which time lever presses had no consequences. Extinction criterion was reached, and animals were tested when the average of active lever presses during the last 3 days of extinction was equal or less than 30 percent of the average of the last 3 days of SA, which usually happened after 10–12 days of extinction training. Animals were maintained on food restriction (22 g chow per day) throughout behavioral testing. Behavioral sessions were carried out in the afternoon.
Reinstatement, microinjections and histology
Cocaine and sucrose 2-hour reinstatement tests were induced by contingent exposure to the cues previously paired with cocaine or sucrose, but without reward delivery. Active lever presses were interpreted as cocaine or sucrose seeking. For animals undergoing forced abstinence, cocaine seeking was induced by reintroducing rats in the chamber previously associate with cocaine delivery and presenting contingent cues, without any cocaine reward.
Rats were microinjected 2 mm below the cannula tip using 33-gauge microinjectors at 0.25 μl/min for a total volume of 0.5 μl per hemisphere. Microinjectors were left in place to allow diffusion for 2 minutes. Animals returned to home cage for 15 additional minutes before starting reinstatement sessions to allow recovery from the microinjection procedure. For all experiments, rats are tested as between-subjects and there are no counter-balanced crossovers due to the long-lasting effects of BDNF. At the end of each experiment, anesthetized rats were transcardially perfused with 1X PBS followed by a 10 percent phosphate buffered formalin solution. Brains were sliced at 100 μm on a vibratome and stained with cresyl violet to verify cannula placement (Fig. S1B).
Locomotor activity
The effect of reagents on spontaneous locomotion and cocaine-induced locomotion was performed on the same animals that underwent cocaine self-administration and reinstatement procedures included in Figure 2a–c, in a randomized fashion controlling for previous treatment. Rats were microinjected with BDNF, ANA-12 or TrkB/Fc followed by 15-minute recovery in house cage, followed by a 1-hour open field session in the Digiscan Animal Activity Monitor system (Omnitech Electronics Model RXYZCM, Columbus, OH). For cocaine-induced locomotion, BDNF was infused in the NAcore 15 minutes before administering 20 mg/kg cocaine i.p. and recording the locomotion for 1 hour.
Figure 2. Acute exogenous BDNF infusion into NAcore potentiated within trial extinction and inhibited cue-induced cocaine seeking through TrkB.
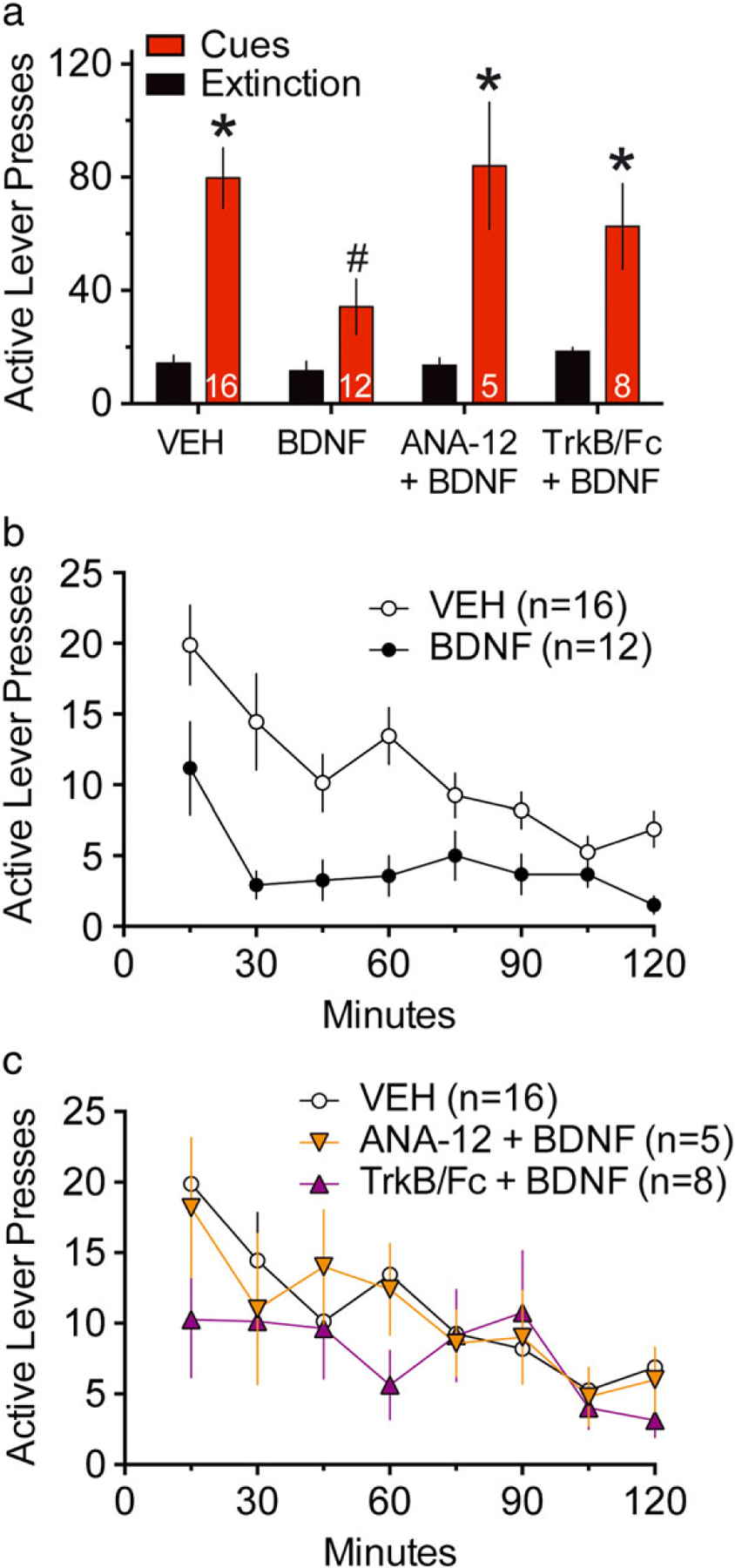
(a) Active lever pressing during 2 h cued-reinstatement test, vehicle VEH (0.5 μl/side), BDNF (1 μg/0.5 μl/side), BDNF (1 μg/0.5 μl/side) + ANA-12 (0.1 pg/0.5 μl/side) or BDNF (1 μg/0.5 μl/side) + TrkB/Fc (650 ng/0.5 μl/side) were administered into NAcore 15 minutes prior to initiating the session. A two-way ANOVA for active lever presses revealed effects of extinction/reinstatement F(3,37) = 55.54, P < 0.0001, treatment F(3,37) = 3.04, P = 0.041 and interaction F(3,37) = 3.21, P = 0.034. *P < 0.05 comparing extinction to reinstatement within groups. #P < 0.05 comparing BDNF to VEH or ANA-12 + BDNF during cued-reinstatement, Sidak’s multiple comparison test. (b) Time course of active lever pressing during 2-hour cued-reinstatement for VEH and BDNF microinjected groups. Two-way ANOVA for active lever revealed effects of time F(7,259) = 8.91, P < 0.0001, treatment F(3,37) = 4.34, P = 0.010 but no significant interaction F(21,259) = 1.29, P = 0.181. (c) Time course of active lever pressing during 2-hour cued-reinstatement for VEH, ANA-12 + BDNF and TrkB/Fc + BDNF microinjected groups. The vehicle group is the same as in b, and the four treatment groups were divided between panels b and c for illustrative clarity. For statistics, see ANOVA results in b
Experimental design and statistical analysis
For comparing multiple measurements in the same experiment, the data were analyzed using two-way ANOVA as indicated in the text describing each experiment. Sidak’s post hoc test was applied for multiple comparisons, and P < 0.05 was considered statistically significant. Locomotor activity comparison between vehicle or BDNF infused rats were analyzed using unpaired Student’s t-tests. All statistical tests were conducted using the Prism (Graphpad, La Jolla, CA) software package.
RESULTS
Cocaine self-administration (SA)
During the acquisition of SA, rats quickly learned to self-administer cocaine and discriminated between the active and the inactive lever by more than 80 percent at the end of acquisition (Fig. S1A). The number of cocaine infusions remained stable throughout SA (~35 infusions/2 hour session). After reaching SA criteria of 10 days with ≥10 infusions per day and more than 70 percent discrimination between active and inactive levers, rats underwent extinction training (Fig. S1A), during which active lever presses were not rewarded by cocaine or cues. After a burst of pressing on extinction day 1 rats rapidly reduced pressing both levers (Fig. S1A). Once active lever pressing in extinction reached <30 percent of the mean of the last 3 days of cocaine SA during acquisition, cue-induced cocaine seeking was tested. Figure S1B shows a representative micrograph, and Figure S1C shows cannula tip placements for all animals included in the experiments described below, divided by experiment for clarity.
Endogenous BDNF applies inhibitory tone on cued-reinstatement
To determine if there is endogenous tone by BDNF on TrkB in the NAcore that is consequential in cued-reinstatement of cocaine seeking, we microinjected one of two different inhibitors of BDNF stimulation of TrkB into the NAcore 15 minutes before cued-reinstatement. A two-way ANOVA for active lever revealed effects of extinction/reinstatement F(1,21) = 77.12, P < 0.0001, treatment F(2,21) = 5.57, P = 0.012 and an interaction F(2,21) = 4.86, p = 0.019 (Fig. 1a). A Sidak’s post hoc comparison revealed a significant increase of active lever presses in the presence of cues compared to extinction for all groups. Moreover, microinjection of the TrkB non-competitive antagonist ANA-12 (0.1 pg/0.5 μl/side) significantly potentiated cued reinstatement compared to control. In contrast to ANA-12, there is no potentiation of cocaine seeking after microinjection of a TrkB receptor scavenger, the human recombinant TrkB/Fc chimera. However, when the time course of cue-induced active lever pressing was examined, both ANA-12 and TrkB/Fc augmented the first 15 minutes of cue-induced active lever pressing (Fig. 1b; two-way ANOVA with repeated measures over time for active lever revealed effects of treatment F(2,21) = 5.86, P = 0.001, time F(7,147) = 19.50, P < 0.0001 and interaction F(14,147) = 2.33, P = 0.006). A Sidak’s post hoc comparison revealed ANA-12 microinjection significantly increased active lever pressing compared to control at 15 minutes and 90 minutes, while TrkB/Fc potentiated lever pressing at 15 minutes. There was no effect on inactive lever pressing by either TrkB antagonist (Fig. S2, a two-way ANOVA for inactive lever revealed no significant effects of extinction/reinstatement F(1,21) = 0.01, P = 0.939, treatment F(2,21) = 0.49, P = 0.6 or interaction F(2,21) = 0.67, P = 0.524). These results indicate that endogenous BDNF provides inhibitory tone on cue-induced cocaine seeking.
Figure 1. Blockade of endogenous BDNF-induced TrkB signaling in the NAcore potentiated cue-induced cocaine seeking.
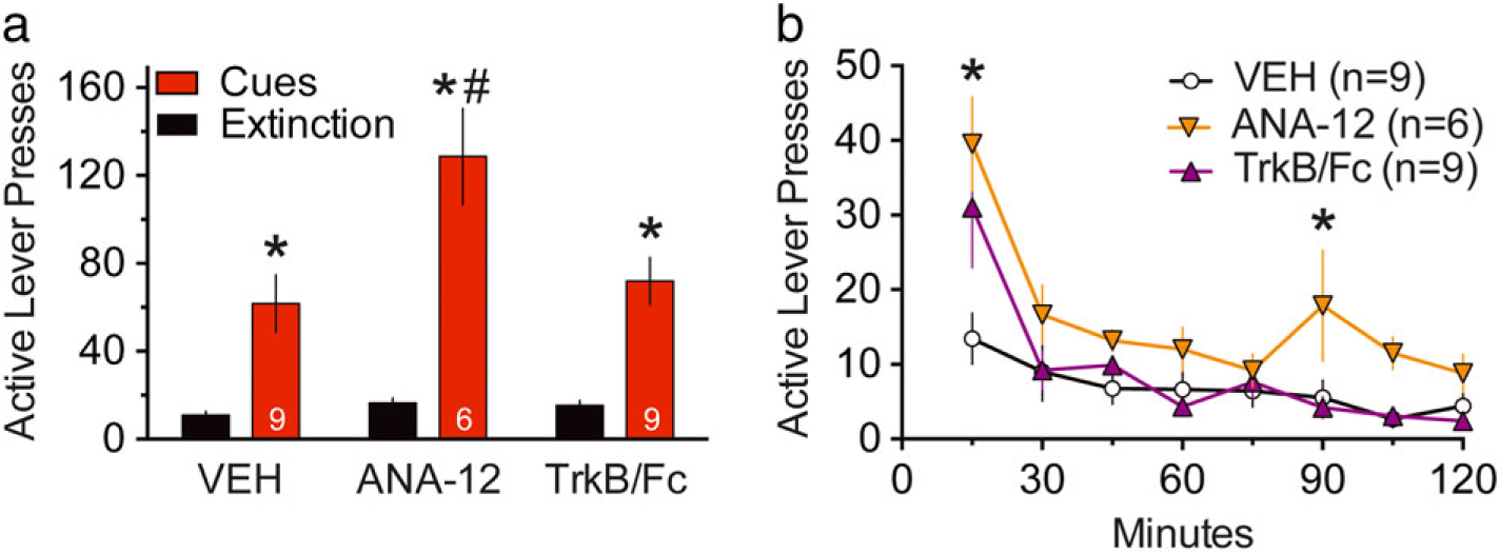
(a) Active lever pressing during 2-hour cued-reinstatement test, all drugs were administered into NAcore 15 minutes prior to initiating the session, including vehicle (VEH; 0.5 μl/side), ANA-12 (0.1 pg/0.5 μl/side) and TrkB/Fc (650 ng/0.5 μl/side). The number of animals is indicated in the columns. A two-way ANOVA for active lever revealed effects of extinction/reinstatement F(1,21) = 77.12, P < 0.0001, treatment F(2,21) = 5.57, P = 0.012 and an interaction F(2,21) = 4.86, P = 0.019. *P < 0.05 comparing extinction to reinstatement for all groups. #P < 0.05 comparing reinstatement of ANA-12 to VEH, Sidak’s multiple comparison test. (b) Time course of active lever pressing during 2-hour cued-reinstatement for VEH, ANA-12 and TrkB/Fc microinjected groups. A two-way ANOVA with repeated measures over time for active lever revealed effects of treatment F(2,21) = 5.86, P = 0.001, time F(7,147) = 19.50, P < 0.0001 and interaction F(14,147) = 2.33, P = 0.006. *P < 0.05 comparing ANA-12 or TrkB/Fc to VEH group at each time point, Sidak’s multiple comparison test
Acute exogenous BDNF in the NAcore recapitulates endogenous BDNF
An acute infusion of BDNF (1 μg/0.5 μl/side) into the NAcore 15 minutes before cue-induced reinstatement reduced cocaine seeking by ~50 percent (Fig. 2a). BDNF-induced reduction in active lever pressing was not different from extinction level pressing. A two-way ANOVA for active lever revealed effects of extinction/reinstatement F(3,37) = 55.54, P < 0.0001, treatment F(3,37) = 3.04, P = 0.041 and interaction F(3,37) = 3.21, P = 0.034. A Sidak’s post hoc comparison established a significant potentiation of active lever pressing in the presence of cues compared to extinction for animals microinjected with vehicle, ANA-12 + BDNF and TrkB/FC + BDNF, but not in the BDNF microinjected group. The post hoc test also revealed a significant inhibition of lever pressing in the presence of cues in the BDNF group compared to the vehicle control. A two-way ANOVA of inactive lever pressing revealed no significant effect of extinction/reinstatement F(1,37) = 0.46, P = 0.5, treatment F(3,37) = 0.76, P = 0.524 or interaction F(3,37) = 2.82, P = 0.052 (Fig. S3).
To determine if the inhibition of cued reinstatement by BDNF was mediated by TrkB, we microinjected a combination of BDNF and TrkB antagonist ANA-12 (0.1 pg/0.5 μl/side) (Fig. 2a). ANA-12 prevented the BDNF-induced decrease in cue-induced cocaine seeking, and restored levels of active lever pressing to vehicle treatment levels. To assure the acute role of TrkB in BDNF-induced decrease of cocaine seeking, we also blocked TrkB signaling by microinjecting TrkB/Fc in combination with the BDNF microinjection. Similar to ANA-12, TrkB/Fc + BDNF microinjected rats showed a significant increase in cocaine seeking during cued-reinstatement. There was no effect of the combination of BDNF+ANA-12 or BDNF + TrkB antagonist on inactive lever pressing (Fig. S3).
BDNF seemed to potentiate within session extinction of the cues, since a steep decrease of active lever pressing occurred between 15 and 30 minutes in the cued-reinstatement session, whereas VEH microinjected animals show a more gradual decrease over time (Fig. 2b). A two-way ANOVA for active lever revealed effects of time F(7,259) = 8.91, P < 0.0001, treatment F(3,37) = 4.34, P = 0.010 but no significant interaction F(21,259) = 1.29, P = 0.181. Figure 2c shows the time course of active lever pressing during cue-induced reinstatement in animals receiving microinjection of vehicle, a combination of ANA-12 + BDNF or TrkB + BDNF. The vehicle group is the same as in Figure 2b, the four treatment groups being divided between Figure 2b and c for clarity. The statistical analysis was performed jointly.
The inhibitory effect of acute exogenous BDNF is specific to cocaine seeking
Cocaine seeking measured during cued-reinstatement is a combination of complex behaviors including locomotion and motivation associated with non-drug rewards. In order to isolate the effect of BDNF on motivated seeking for cocaine reward, we tested the effect of BDNF on locomotion and cue-induced sucrose seeking. Acute BDNF microinjection in the NAcore 15 minutes before introducing rats to a new open field did not decrease exploratory behavior (Fig. 3a; an unpaired Student’s test revealed no differences between groups, t(17) = 1.53, P = 0.140), and did not reduce locomotion induced by cocaine (20 mg/kg, ip) (Fig. 3a; t(16) = 0.86, P = 0.40). We additionally tested spontaneous locomotion in a novel open field after ANA-12 and TrkB/Fc microinjections into the NAcore, and neither drug altered locomotion compared to vehicle (Fig. 3b). A one-way ANOVA revealed no differences between groups, F(2,26) = 0.43, P = 0.65.
Figure 3. Acute exogenous BDNF infusion in the NAcore was specific to cue-induced cocaine seeking behavior and had no effect on spontaneous or cocaine-induced locomotion or sucrose seeking.
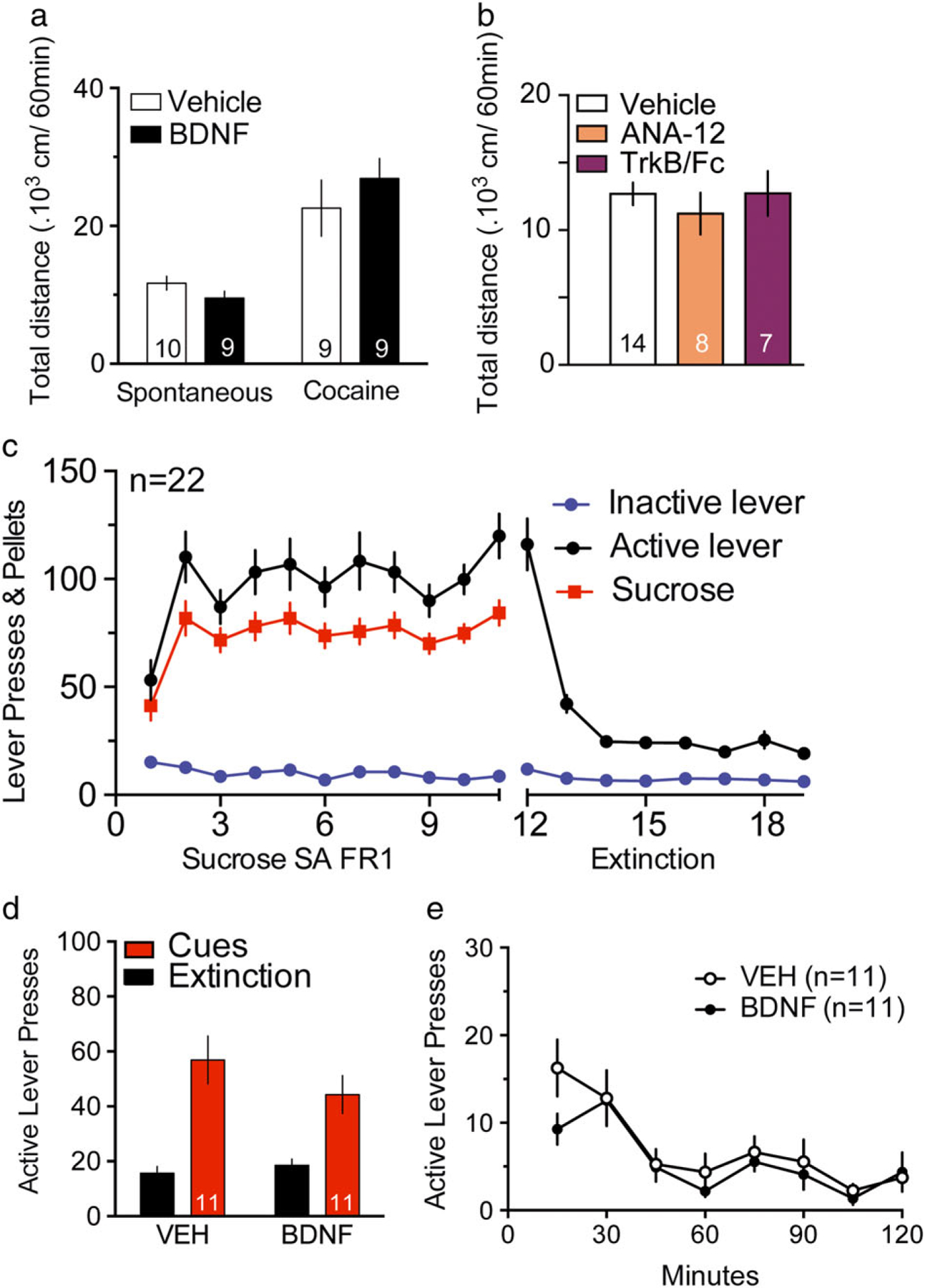
(a) 1-hour spontaneous locomotion in an unadapted open field was measured 15 minutes after VEH (0.5 μl/side) or BDNF (1 μg/0.5 μl/side) injections in the NAcore. An unpaired Student’s test revealed no differences between groups, t(17) = 1.53, P = 0.140. Cocaine-induced locomotion was measured immediately after cocaine injection (20 mg/kg, i.p.) in an adapted open field, 15 minutes after VEH (0.5 μl/side) or BDNF (1 μg/0.5 μl/side) infusions in the NAcore (unpaired Student’s test t(16) = 0.86, P = 0.40). (b) 1-hour spontaneous locomotion in an unadapted open field was measured 15 minutes after VEH (0.5 μl/side), ANA-12 (0.1 pg/0.5 μl/side) or TrkB/Fc (650 ng/0.5 μl/side) injections in the NAcore. A one-way ANOVA revealed no differences between groups F(2,26) = 0.43, P = 0.65. (c) Rats sucrose SA acquisition with fixed ratio 1 (FR1) and extinction training. (d) Vehicle VEH (0.5 μl/side) or BDNF (1 μg/0.5 μl/side) were administered into NAcore 15 minutes prior to initiating 2-hour cued reinstatement. The number of animals per group is located at the bottom of columns. A two-way repeated measures ANOVA for active lever presses revealed an effect of extinction/cue F(1,20) = 38.80, P < 0.0001, but no effect of treatment F(1,20) = 0.58, P = 0.456 or interaction F(1,20) = 2.01, P = 0.171. (e) Time course of active lever pressing during 2-hour cued-reinstatement for sucrose for VEH and BDNF microinjected groups. A two-way repeated measures ANOVA for active lever presses revealed an effect of time F(7,140) = 11.07, P < 0.001, but no significant effect of treatment F(1,20) = 1.3, P = 0.269 or interaction F(7,140) = 0.88, P = 0.521
To determine if BDNF inhibited seeking of a natural reward, a group of rats underwent sucrose SA, extinction training and cued-reinstatement (Fig. 3c–e). An acute microinjection of BDNF in the NAcore 15 minutes before cue-induced reinstatement did not modify sucrose seeking (Fig. 3d), suggesting that BDNF-mediated inhibition was related to adaptations produced by cocaine that were not also induced by sucrose. A two-way repeated measures ANOVA revealed an effect of extinction/cue F(1,20) = 38.80, P < 0.0001, but no effect of treatment F(1,20) = 0.58, P = 0.456 or interaction F(1,20) = 2.01, P = 0.171. A two-way ANOVA revealed no effect by BDNF on inactive lever pressing for extinction/cue F(1,20) = 2.67, P = 0.118, treatment F(1,20) = 0.15, P = 0.703 or interaction F(1,20) = 0.91, P = 0.352 (Fig. S4). The time course of cued-reinstatement to sucrose (Fig. 3e) shows similar within session extinction of sucrose-paired cues in vehicle- or BDNF-microinjected animals. Although BDNF-treated group shows a decreased active lever pressing at 15 minutes within the cue-induced sucrose reinstatement, a two-way repeated measures ANOVA for active lever presses revealed an effect of time F(7,140) = 11.07, P < 0.001, but no significant effect of treatment F(1,20) = 1.3, P = 0.269 or interaction F(7,140) = 0.88, P = 0.521.
Acute exogenous BDNF in the NAcore potentiates within session extinction during context + cue-induced reinstatement after forced abstinence
BDNF inhibited cocaine seeking in rats that underwent extinction training after cocaine SA. It was possible that extinction training was necessary for the actions of BDNF. Extinction training produces enduring changes in the NAcc compared to rats or mice placed in abstinence for an equivalent period without extinction training (Sutton et al. 2003; Knackstedt et al. 2010). Accordingly, we infused BDNF in the NAcore 15 minutes before context + cue-induced cocaine seeking after 12–14 days of abstinence without extinction training. We found no decrease in active (Fig. 4a) or inactive (Fig. S5) lever pressing over the 2-hour session in BDNF-infused rats; a two-way repeated measures ANOVA for active lever presses revealed an effect of last day of SA versus context + cue-induced reinstatement F(1,16) = 8.12, P = 0.012, but no effect of treatment F(1,16) = 2.62, P = 0.125 or interaction F(1,16) = 0.08, P = 0.783). A two-way repeated measures ANOVA for inactive lever presses revealed an effect of last day of SA versus context + cue-induced reinstatement F(1,16) = 32.62, P < 0001, but no effect of treatment F(1,16) = 0.60, P = 0.451 or interaction F(1,16) = 3, P = 0.103 (Fig. S5).
Figure 4. Acute exogenous BDNF infusion in the NAcore potentiated within trial extinction during context + cue-induced reinstatement after forced abstinence.
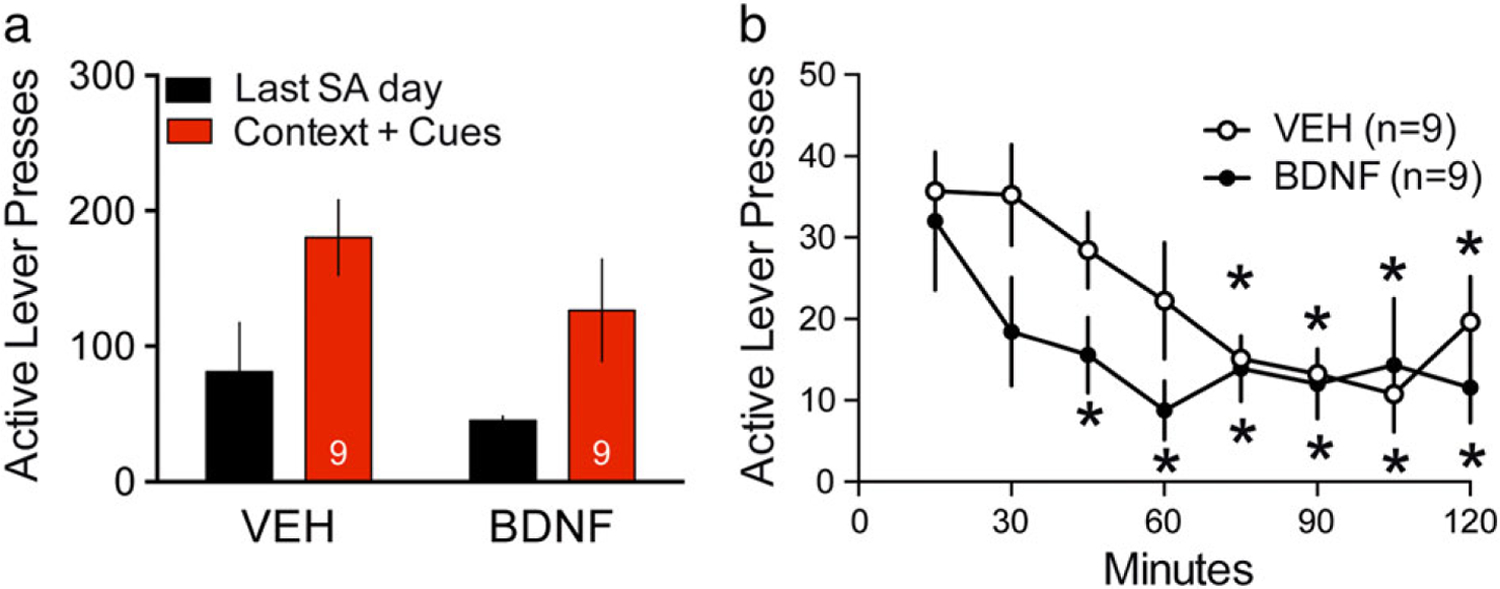
(a) Active lever pressing during 2-hour context + cue-reinstatement test after forced abstinence, all drugs were administered into NAcore 15 minutes prior to initiating the session, including vehicle VEH (0.5 μl/side) and BDNF (1 μg/0.5 μl/side). Two-way repeated measures ANOVA for active lever presses revealed an effect of last day of SA versus context + cue-induced reinstatement F(1,16) = 8.12, P = 0.012, but no effect of treatment F(1,16) = 2.62, P = 0.125 or interaction F(1,16) = 0.08, P = 0.783). (b) Time course of active lever pressing during 2-hour context + cue-reinstatement test after forced abstinence for VEH and BDNF microinjected groups. A two-way ANOVA revealed no effect of treatment F(1,16) = 1.31, P = 0.269, an effect of time F(7,112) = 9.36, P < 0.001 and a near significant interaction F(7,112) = 2.03, P = 0.057. *P < 0.05 comparing active lever pressing of each time point to t = 15 minutes within treatments, Sidak’s multiple comparison test
BDNF after forced abstinence produced highly variable results ranging from complete inhibition of cocaine seeking to no effect on cocaine seeking, and cannula placement could not account for these individual differences (Fig. S1C). We examined the time course of context + cue-induced reinstatement, and both groups showed robust within session extinction (Fig. 4b; a two-way ANOVA revealed no effect of treatment F(1,16) = 1.31, P = 0.269, an effect of time F(7,112) = 9.36, P < 0.001 and a near significant interaction F(7,112) = 2.03, P = 0.057. However, when each time point was compared to t = 15 minutes within treatment groups using a Sidak’s multiple comparison test, vehicle-infused rats showed a significant decrease from initial active lever pressing level at t = 75 minutes, while BDNF-infused rats decreased at t = 45 minutes. Each asterisk shows significant difference of each time point to t = 15 minutes within treatments. These results suggest BDNF-infusion potentiates within session extinction during context + cue-induced cocaine seeking in a less robust, but similar manner as during cue-induced reinstatement after extinction training (Fig. 2b).
BDNF acute effect is maintained up to 3 days after microinjection
Numerous studies show long lasting effects of BDNF through TrkB signaling via activation of transcriptional regulators (Ruiz, Shi, & Meffert 2014). Therefore, in a subgroup of animals shown in Figure 2, we examined possible long-lasting effects of BDNF by re-testing rats acutely microinjected with BDNF or vehicle 72 hours after the first reinstatement test. Rats were extinguished for 3 days after the first reinstatement session, and for the second reinstatement test all rats received a vehicle microinjection (Fig. 5a). Rats that previously received a BDNF microinjection showed significantly lower reinstated active lever pressing than rats microinjected with vehicle in the first session (Fig. 5b; a two-way repeated measures ANOVA for active lever presses revealed an effect of extinction/cue F(1,24) = 33.66, P < 0.001, treatment F(1,24) = 9.71, P = 0.005 and interaction F(1,24) = 9.33, P = 0.006). A Sidak’s post hoc comparison established a significant potentiation of active lever pressing in presence of cues compared to extinction in the group previously injected with vehicle, but not in the group previously injected with BDNF. The post hoc test also showed a significant decrease of active lever pressing in the presence of cues in the BDNF-microinjected group compared to vehicle control. A two-way ANOVA for inactive lever pressing (Fig. S6) revealed significant treatment factor F(1,24) = 4.83, P = 0.038, but no effect of extinction/cue factor F(1,24) = 0.17, P = 0.68 or interaction F(1,24) = 1.2, P = 0.28. These results suggest that in addition to the acute, non-transcriptional effect of BDNF through the TrkB signaling cascade, BDNF microinjections into the NAcore induce an enduring reduction in reinstated cocaine seeking. The time course of cue-induced active lever pressing on day 29 (Fig. 5c) was consistent with the total lever pressing data; a two-way ANOVA with repeated measures over time for active lever revealed effects of treatment F(1,24) = 8.06, P = 0.009 and time F(7,168) = 9.87, P < 0.0001 but no significant interaction F(7,168) = 1.36, P = 0.225.
Figure 5. BDNF inhibition on cocaine seeking was sustained for at least 3 days post infusion.
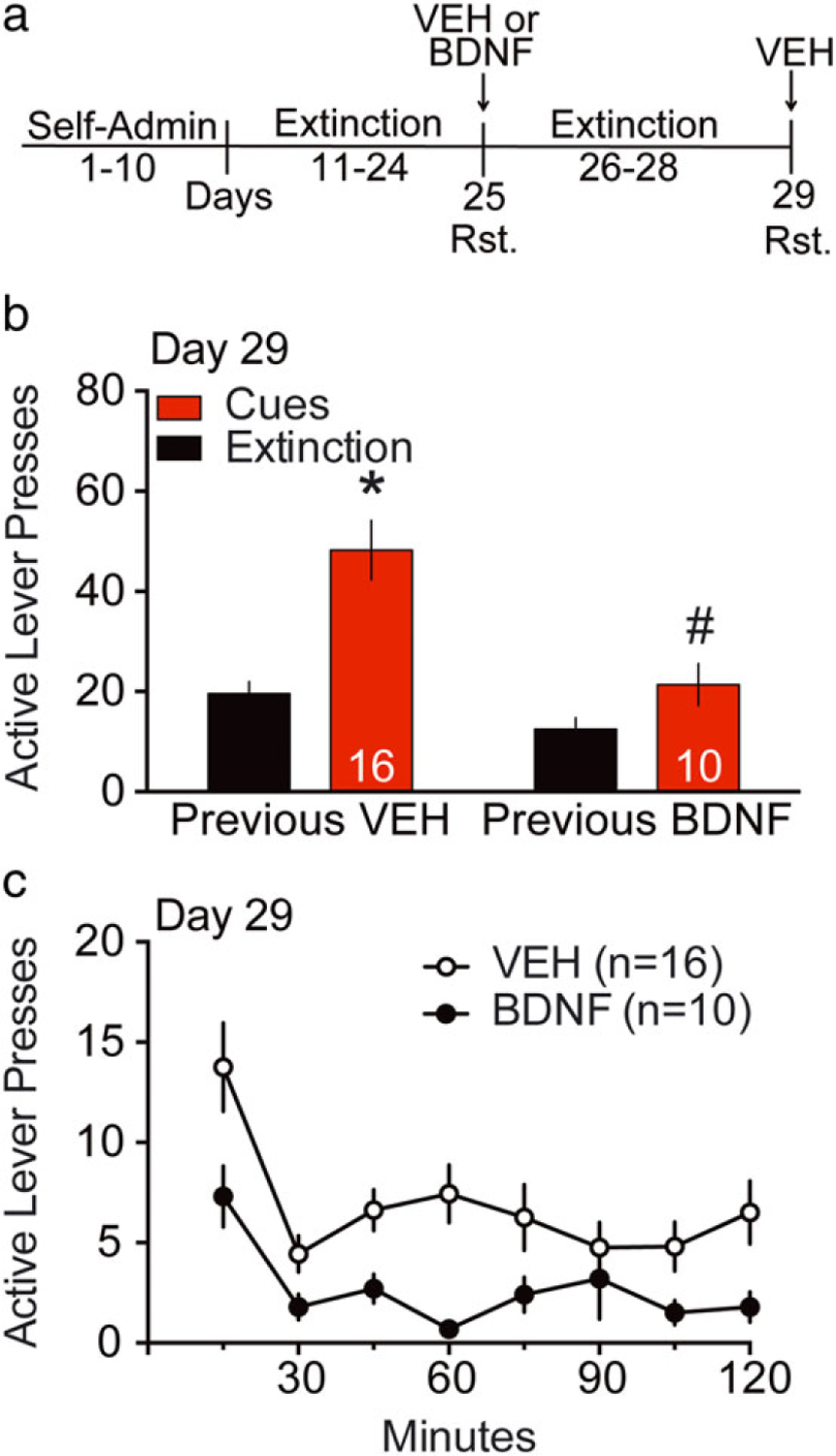
(a) Experiment timeline. Rats underwent at least 10 days of cocaine SA (Self-Admin), followed by at least 10 days of extinction training. Vehicle (VEH) or BDNF were microinjected into NAcore 15 minutes before the first cued-reinstatement (Rst, Day 25), while all animals received vehicle injections before the second cued-reinstatement (Rst, Day 29). (b) Active lever pressing during the second 2-hour cued-reinstatement test. Rats were microinjected VEH (0.5 μl/side) or BDNF (1 μg/0.5 μl/side) into the NAcore 15 minutes before the first cued-reinstatement. The second reinstatement presented here occurred 3 days after the first. Each animal received a maximum of two reinstatement trials. Number at the bottom of columns represents the N for each treatment group. A two-way repeated measures ANOVA for active lever presses revealed an effect of extinction/cue F(1,24) = 33.66, P < 0.001, treatment F(1,24) = 9.71, P = 0.005 and interaction F(1,24) = 9.33, P = 0.006). *P < 0.05 comparing extinction to cued-reinstatement within groups, #P < 0.05 comparing cued-reinstatement between VEH and BDNF, Sidak’s multiple comparison test. (c) Time course of active lever pressing during 2-hour cue-reinstatement test (Day 29) for VEH and BDNF microinjected groups. A two-way ANOVA with repeated measures over time for active lever revealed effects of treatment F(1,24) = 8.06, P = 0.009 and time F(7,168) = 9.87, P < 0.0001 but no significant interaction F(7,168) = 1.36, P = 0.225
Enduring inhibition by BDNF requires cue-memory reactivation
We next sought to determine if the enduring BDNF-induced inhibition of reinstated cocaine seeking required simultaneous stimulation of TrkB and activation of cue-induced plasticity in the NAcore. To test this, BDNF was microinjected into NAcore before an extinction session (in absence of cues) or in the home cage (in absence of cues and context) (Fig. 6a). An unpaired Student’s test revealed that rats infused with BDNF in the NAcore prior to an extinction session showed no difference in active or inactive lever pressing compared with vehicle treatment the day of the extinction session (Fig. 6b; unpaired Student’s test t(10) = 0.99, P = 0.347, Fig. S7A; t(10) = 1.23, P = 0.247). BDNF infusions before an extinction session 3 days prior to cued reinstatement session did not affect lever pressing (Fig. 6b; a two-way repeated measures ANOVA for active lever presses established an effect of extinction/cue F(1,10) = 70.06, P < 0.001, and no effect of treatment F(1,10) = 0.37, P = 0.556 or interaction F(1,10) = 0.01, P = 0.977. A two-way ANOVA revealed no effect by BDNF on inactive lever pressing (Fig. S7A, extinction/cue F(1,10) = 1.49, P = 0.251, treatment F(1,10) = 0.99, P = 0.344 and interaction F(1,10) = 2.36, P = 0.156). The time course of cue-induced active lever pressing on day 29 (Fig. 6c) showed no difference between vehicle and BDNF treatments; a two-way ANOVA with repeated measures over time for active lever revealed a significant effect of time F(7,70) = 5.41, P < 0.0001 and no significant effects of treatment F(1,10) = 0.13, P = 0.721 or interaction F(7,70) = 1.6, P = 0.149.
Figure 6. BDNF inhibition on cocaine seeking required cue-memory presentation.
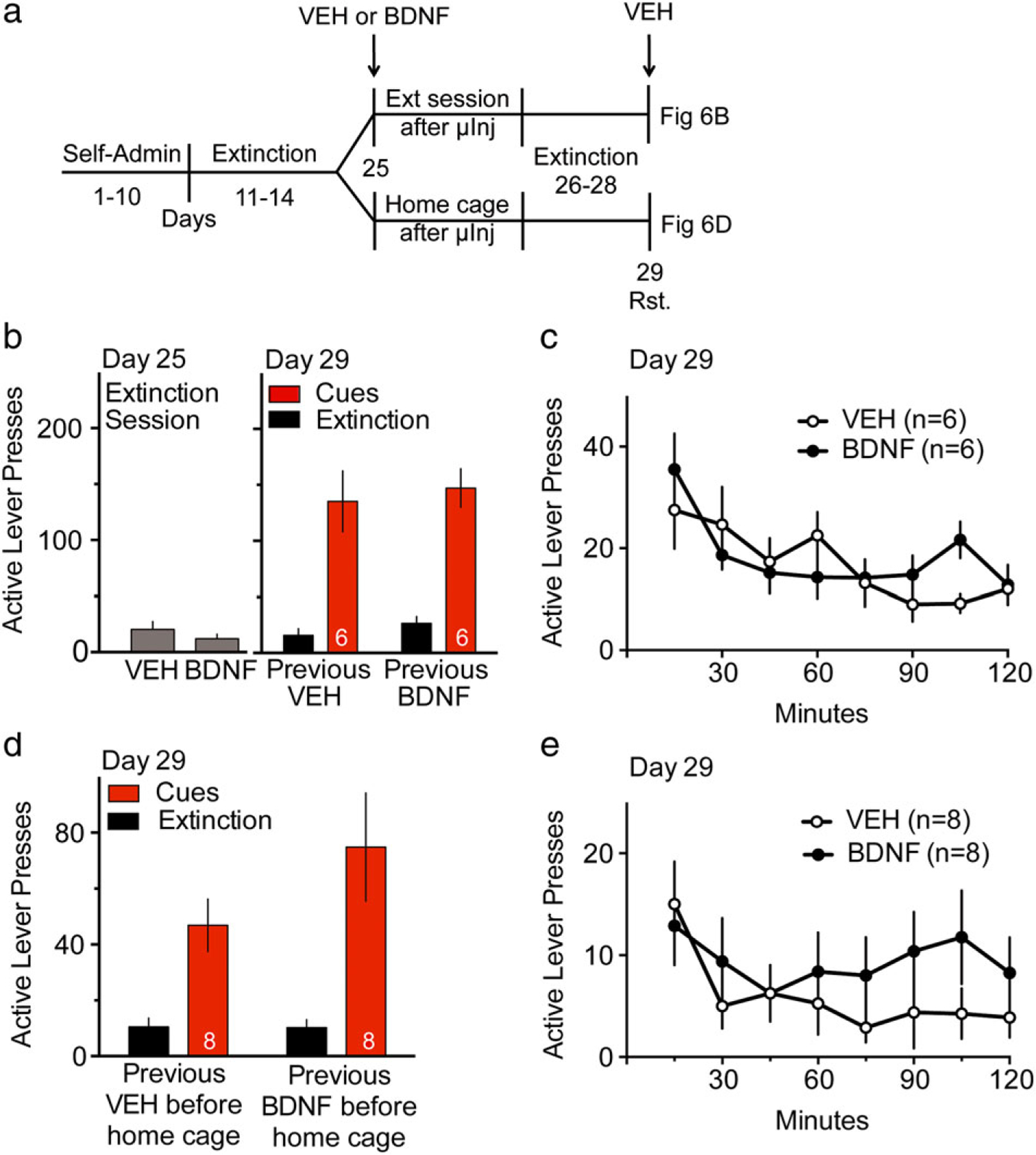
(a) Experiment timeline. Rats underwent at least 10 days of cocaine SA (Self-Admin), followed by at least 10 days of extinction training. Vehicle (VEH) or BDNF were microinjected into NAcore 15 minutes before an extinction (Ext) session or before returning to the home cage at Day 25, while all animals received vehicle injections before the second cued-reinstatement (Rst, Day 29). (b) Active lever pressing during the extinction session preceded by VEH (0.5 μl/side) or BDNF (1 μg/0.5 μl/side) infusions into the NAcore and during the first 2-hour cued-reinstatement test 3 days later. An unpaired Student’s t(10) = 0.99, P = 0.347 shows no difference between vehicle or BDNF microinjection on active lever pressing during extinction session Day 25. On day 29, a two-way repeated measures ANOVA for active lever presses revealed an effect of extinction/cue F(1,10) = 70.06, P < 0.001, and no effect of treatment F(1,10) = 0.37, P = 0.556 or interaction F(1,10) < 0.01, P = 0.977. (c) Time course of active lever pressing during 2-hour cue-reinstatement test (Day 29) for groups previously microinjected with VEH or BDNF in the NAcore before an extinction session. A two-way ANOVA with repeated measures over time for active lever revealed a significant effect of time F(7,70) = 5.41, P < 0.0001 and no significant effects of treatment F(1,10) = 0.13, P = 0.721 or interaction F(7,70) = 1.6, P = 0.149. (d) Active lever pressing during the first 2-hour cued-reinstatement test. Three days prior to cued-reinstatement, rats were microinjected VEH (0.5 μl/side) or BDNF (1 μg/0.5 μl/side) into the NAcore and placed back in their home cage, with no recording of lever pressing activity in the home cage. A two-way repeated measures ANOVA for active lever presses revealed an effect of extinction/cue F(1,14) = 22.35, P = 0.0003, and no effect of treatment F(1,14) = 1.50, P = 0.241 or interaction F(1,14) = 1.75, P = 0.207. The number of animals per group is located at the bottom of columns. (e) Time course of active lever pressing during 2-hour cue-reinstatement test (Day 29) for groups previously microinjected with VEH or BDNF in the NAcore prior to return to home cage. A two-way ANOVA with repeated measures over time for active lever revealed no significant effect of treatment F(1,14) = 1.71, P = 0.213, time F(7,98) = 1.65, P = 0.132 or interaction F(7,98) = 0.58, P = 0.771
Animals microinjected with BDNF and returned to the home cage had the same level of drug seeking as rats who received vehicle injections when tested for cue-induced reinstatement 3 days after the microinjection (Fig. 6d; a two-way ANOVA with repeated measures for active lever presses revealed an effect of extinction/cue F(1,14) = 22.35, P = 0.0003, and no effect of treatment F(1,14) = 1.50, P = 0.241 or interaction F(1,14) = 1.75, P = 0.207. A two-way ANOVA revealed no effect by BDNF on inactive lever pressing (Fig. S7B, extinction/cue F(1,14) = 0.636, P = 0.438, treatment F(1,14) = 0.01, P = 0.929 and interaction F(1,14) = 0.0, P > 1). Similarly, no difference between vehicle and BDNF treatments was observed in the time course of cue-induced active lever pressing on day 29 (Fig. 6e); a two-way ANOVA with repeated measures over time for active lever revealed no significant effect of treatment F(1,14) = 1.71, P = 0.213, time F(7,98) = 1.65, P = 0.132 or interaction F(7,98) = 0.58, P = 0.771.
DISCUSSION
We show that endogenous BDNF in the NAcore plays an inhibitory role during drug-free cue-induced cocaine seeking. Acute microinjection of exogenous BDNF into NAcore recapitulated the inhibition of cocaine seeking produced by endogenous BDNF. Both endogenous and exogenous BDNF effects were mediated by activation of the TrkB signaling. BDNF-induced decrease in cocaine seeking appeared within 15 minutes following intra-NAcore microinjection and was specific to cocaine seeking. Accordingly, no effect by BDNF was observed on inactive lever pressing, spontaneous or cocaine-induced locomotion, or on cue-induced sucrose seeking. BDNF infusions also potentiated within reinstatement session extinction of cocaine seeking after forced abstinence. In accordance with its well-established transcriptional effects, we found BDNF to have lasting effects and the inhibition of cue-induced reinstatement endured for at least 3 days post-infusion. Finally, enduring BDNF inhibition required administration during cue memory retrieval, since BDNF infusions before an extinction session or in the home cage had no effect on subsequent cue-induced reinstatement of cocaine seeking.
BDNF in the core (NAcore) and the shell (NAshell) subregions of the nucleus accumbens
Numerous studies (for review see (Li et al., 2015)) demonstrate that cocaine-induced BDNF alterations vary considerably depending on whether studies were conducted in the NAcore and NAshell subregions of the NAcc. For example, attenuation of BDNF–TrkB signaling in the NAcore increased cue-induced cocaine seeking only in early withdrawal (24 hours), while the same viral manipulation in the NAshell decreased cocaine seeking only in late withdrawal (90 days) (Li et al. 2013). Also, following cocaine self-administration, BDNF protein levels and TrkB signaling are increased in the shell, but not in the core (Graham et al. 2007). After 45 days of withdrawal, BDNF increase is only measured in the core while at 90 days of withdrawal, BDNF levels are increased in both the core and shell subregions (Li et al. 2013). Furthermore, increased BDNF protein expression in the NAcc (including both the core and the shell) induced by an acute intra-PFC exogenous BDNF injection decreases context-, cue- and cocaine-induced cocaine seeking (Berglind et al. 2007). Although these experiments are consistent with increases in BDNF levels after cocaine, a recent study showed that repeated non-contingent exposure to cocaine during adolescence decreased BDNF and its signaling in both the NAcore and NAshell (Caffino et al. 2018). Additionally, BDNF injections in the NAshell during cocaine self-administration increase cocaine-, cue- and stress-induced reinstatement for up to more than 4 weeks after BDNF treatment (Graham et al. 2007). BDNF transmission through TrkB is also necessary for cocaine-induced spine formation in the NAshell (Anderson et al. 2017). Similar BDNF injections have not been performed in the NAcore. In our study, we focused on the NAcore and found that BDNF injection minutes before cue-induced reinstatement to cocaine inhibited seeking.
Exogenous BDNF injection and bidirectional transport
BDNF is produced locally in the accumbens (Graham et al. 2007) and can undergo retro- and anterograde transport, although retrograde transport seems most prevalent (Zhao et al. 2014). Thus, it is likely that a portion of the exogenous BDNF we injected into NAcore traveled to regions both upstream and downstream from the NAcore and that the measured effect on cocaine seeking could be due to a BDNF role in these regions. However, BDNF travel velocity is estimated at 1.06 μm/sec in dendrites (Kwinter et al. 2009; Zhao et al. 2014), thus requiring nearly 50 minutes to reach a connected region such as the PFC that is ~3.1 mm from the injection site in the rat brain. Since the BDNF injection was microinjected 15 minutes before cue-induced reinstatement, it seems unlikely that biologically active amounts of the acutely microinjected BDNF reached distal sites creating off-target effects. Concerning the long-lasting effects of previous BDNF microinjections observed in Figure 5, we are unable to exclude a potential role of exogenous BDNF transport to brain regions other than the NAcore.
Transcriptional versus non-transcriptional effects of BDNF
Temporal and spatial specificity are key in the effects of BDNF on cocaine seeking. BDNF-induced activation of TrkB signaling results in receptor dimerization and initiation of a series of phosphorylation events, including phosphorylation of mitogen-activated protein kinase and extracellular signal-regulated kinases pathway (MAPK/ERK), phosphoinositide 3-kinase and protein kinase B (PI3K/Akt) and phospholipase C γ (PLCγ1) (Reichardt 2006). Some of these signaling events lead to activation of transcription factors such as cyclic AMP response element binding protein (CREB) (Lu et al. 2006), which regulates the expression of a wide array of target genes affected by BDNF. This effect of BDNF on gene expression most likely underlies the lasting effects of BDNF observed in the literature, including repeated BDNF infusions in the NAcc during cocaine SA potentiating cocaine intake, as well as context-, cue- and stress-induced cocaine seeking (Graham et al. 2007). In the same study, the authors neutralized BDNF by infusing BDNF antibodies in the NAcc during stable cocaine SA and measured a decrease of cocaine intake, as well as reduced footshock-induced reinstatement of cocaine seeking (Graham et al. 2007). When BDNF is infused in the PFC after the last cocaine exposure, it decreases context-, cue- and cocaine primed-induced reinstatement to cocaine seeking (Berglind et al. 2009). These studies focus on the long lasting, likely transcriptional effects of BDNF, while we focused here on faster mechanisms by administrating BDNF or TrkB antagonists only 15 minutes before cued-reinstatement and in the absence of cocaine. We hypothesize that transcriptional effects of BDNF underlie the inhibition of cued-reinstatement observed 3 days post infusion (Fig. 5b); although this could also be due to post-transcriptional changes induced by strong activation of the TrkB signaling and consequent phosphorylation cascade. For instance, BDNF-TrkB activation modulates alcohol-induced neuroadaptations by altering miRNA expression in the PFC (Heilig et al. 2017).
Role of context in the actions of BDNF
BDNF inhibits cue-induced cocaine seeking when it is infused minutes before cued-reinstatement. When infused before an extinction session (with presentation of the context but in the absence of cues, Fig. 6b) or in the home cage (Fig. 6d) BDNF had no effect on cocaine seeking. These results suggest that BDNF-induced inhibition of drug seeking requires a time window during which cue-induced reactivation of the neural circuit mediating seeking is paired with simultaneous TrkB signaling activation. Memory reactivation converts an inactive memory to a malleable state that can be reconsolidated (Gisquet-Verrier & Riccio 2012). Since BDNF decreases cue-induced cocaine seeking, we hypothesize that BDNF-induced activation of TrkB signaling weakens the memory of the cue associated to the drug during SA training. This would result in weaker activation of the circuit inducing seeking and thereby decrease cocaine seeking, however, the precise mechanism remains to be determined.
Role of D1- versus D2-MSNs
TrkB receptor gene is expressed by both D1- and D2-MSNs (Freeman et al. 2003); although TrkB mRNA expression is greater in D2-compared to D1-MSNs (Lobo et al. 2010). Genetically inhibiting the expression of TrkB specifically in D1-MSNs potentiates cocaine CPP and sensitization compared to control mice, while knocking out TrkB in D2-MSNs decreases CPP and sensitization (Lobo et al. 2010). Our study did not dissect the role of D1-versus D2-MSNs, it is however generally understood that activation of the D1 pathway promotes reward seeking, while D2 pathway activation can lead to aversion or reduced seeking (Kravitz, Tye, & Kreitzer 2012; Calipari et al. 2016). We hypothesize that BDNF could selectively activate D2-MSNs, potentiate within trial extinction and decrease cocaine seeking. Supporting this hypothesis, infusions of BDNF or systemic injections of a synthetic TrkB receptor agonist 7,8, dihydroxyflavone, enhance extinction of cocaine CPP (Otis, Fitzgerald, & Mueller 2014).
CONCLUSIONS
We investigated the effect of endogenous BDNF secretion on cocaine seeking in the NAcore and discovered that BDNF-induced TrkB signaling during cued-reinstatement promoted within session extinction and decreased cocaine seeking. We were able to pharmacologically recreate the inhibition of cocaine seeking by acutely microinfusing BDNF in the NAcore before cue-induced reinstatement. This effect was specific to cocaine seeking, and did not alter inactive lever pressing, spontaneous or cocaine-induced locomotion, or sucrose seeking. The inhibitory effect of BDNF transmission induced during cued cocaine seeking contrasts with some other studies examining effects of chronic or constitutive changes in BDNF levels and may indicate a distinction between non-translational effects of acute BDNF transmission and enduring effects mediated by changes in gene expression.
Supplementary Material
Figure S1: Rat cocaine self-administration (SA), extinction training and histology.
Figure S2: Blockade of endogenous BDNF-induced TrkB signaling in the NAcore potentiated cue-induced cocaine seeking.
Figure S5: Acute exogenous BDNF infusion in the NAcore potentiated within trial extinction during context + cue-induced reinstatement after forced abstinence.
Figure S4: Acute exogenous BDNF infusion in the NAcore had no effect on sucrose seeking.
Figure S3: Acute exogenous BDNF infusion in the NAcore potentiated within trial extinction and inhibited cue-induced cocaine seeking through TrkB.
Figure S6: BDNF inhibition on cocaine seeking was sustained for at least 3 days post infusion.
Figure S7: BDNF inhibition on cocaine seeking required cue-memory presentation.
Acknowledgements
The authors would like to thank Dr Jacqueline F. McGinty, Dr Sarah M. Barry, Dr Jamie Peters and Dr Ethan M. Anderson for helpful discussions and scientific insight, and Dr Heather A. Boger for allowing us to perform the rat locomotor measurement using her equipment.
Funding
This work was supported by a post-doctoral fellowship from the French Fyssen Foundation (A.C.B.) and the National Institute of Health NIH DA003906, DA12513 and DA015369 (P.W.K.).
Footnotes
Disclosure
The authors declare no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- Altar CA, DiStefano PS (1998) Neurotrophin trafficking by anterograde transport. Trends in Neurosciences 21:433–437. [DOI] [PubMed] [Google Scholar]
- Anderson EM, Wissman AM, Chemplanikal J, Buzin N, Guzman D, Larson EB, et al. (2017) BDNF-TrkB controls cocaine-induced dendritic spines in rodent nucleus accumbens dissociated from increases in addictive behaviors. Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Taylor JR, De Vries TJ, Peters J (2015). Brain-derived neurotrophic factor and addiction: pathological versus therapeutic effects on drug seeking. Brain Research 1628(Pt A): 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry SM, McGinty JF (2017). Role of Src family kinases in BDNF-mediated suppression of cocaine-seeking and prevention of cocaine-induced ERK, GluN2A, and GluN2B dephosphorylation in the prelimbic cortex. Neuropsychopharmacology. September; 42:1972–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW Jr, LaLumiere RT, Kalivas PW, McGinty JF (2009) A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. The Journal of neuroscience: the official journal of the Society for Neuroscience 29:3715–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW Jr, Miller SW, et al. (2007) A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. The European Journal of Neuroscience 26:757–766. [DOI] [PubMed] [Google Scholar]
- Caffino L, Giannotti G, Messa G, Mottarlini F, Fumagalli F (2018) Repeated cocaine exposure dysregulates BDNF expression and signaling in the mesocorticolimbic pathway of the adolescent rat. The World Journal of Biological Psychiatry 1–14. [DOI] [PubMed]
- Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Pena CJ, et al. (2016) vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proceedings of the National Academy of Sciences of the United States of America 113:2726–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J (2003) A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology 168:66–74. [DOI] [PubMed] [Google Scholar]
- Cazorla M, Premont J, Mann A, Girard N, Kellendonk C, Rognan D (2011) Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. The Journal of Clinical Investigation 121:1846–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corominas-Roso M, Roncero C, Daigre C, Grau-Lopez L, Ros-Cucurull E, Rodriguez-Cintas L, et al. (2015) Changes in brain-derived neurotrophic factor (BDNF) during abstinence could be associated with relapse in cocaine-dependent patients. Psychiatry Research 225:309–314. [DOI] [PubMed] [Google Scholar]
- D’Sa C, Fox HC, Hong AK, Dileone RJ, Sinha R (2011) Increased serum brain-derived neurotrophic factor is predictive of cocaine relapse outcomes: a prospective study. Biological Psychiatry 70:706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Faron-Gorecka A, Kusmider M, Golda A, Frankowska M, Dziedzicka-Wasylewska M (2006) Alterations in BDNF and trkB mRNAs following acute or sensitizing cocaine treatments and withdrawal. Brain Research 1071:218–225. [DOI] [PubMed] [Google Scholar]
- Freeman AY, Soghomonian JJ, Pierce RC (2003) Tyrosine kinase B and C receptors in the neostriatum and nucleus accumbens are co-localized in enkephalin-positive and enkephalin-negative neuronal profiles and their expression is influenced by cocaine. Neuroscience 117:147–156. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH (2007) Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. The European Journal of Neuroscience 26:487–498. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA (2007) Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. The European Journal of Neuroscience 26:2756–2763. [DOI] [PubMed] [Google Scholar]
- Giannotti G, Caffino L, Calabrese F, Racagni G, Riva MA, Fumagalli F (2014) Prolonged abstinence from developmental cocaine exposure dysregulates BDNF and its signaling network in the medial prefrontal cortex of adult rats. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 17:625–634. [DOI] [PubMed] [Google Scholar]
- Gisquet-Verrier P, Riccio DC (2012) Memory reactivation effects independent of reconsolidation. Learning & Memory 19:401–409. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW (2007) Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nature Neuroscience 10:1029–1037. [DOI] [PubMed] [Google Scholar]
- Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK, et al. (2009) Tropomyosin-related kinase B in the mesolimbic dopamine system: region-specific effects on cocaine reward. Biological Psychiatry 65:696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y (2003) Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. The Journal of neuroscience : the official journal of the Society for Neuroscience 23:742–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Barbier E, Johnstone AL, Tapocik J, Meinhardt MW, Pfarr S, et al. (2017) Reprogramming of mPFC transcriptome and function in alcohol dependence. Genes, Brain, and Behavior 16:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR (1999) Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. The Journal of Neuroscience 19:4110–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW (2010) Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. The Journal of neuroscience: the official journal of the Society for Neuroscience 30: 7984–7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskela M, Back S, Voikar V, Richie CT, Domanskyi A, Harvey BK, et al. (2016) Update of neurotrophic factors in neurobiology of addiction and future directions. Neurobiology of Disease. [DOI] [PMC free article] [PubMed]
- Kravitz AV, Tye LD, Kreitzer AC (2012) Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature Neuroscience 15:816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwinter DM, Lo K, Mafi P, Silverman MA (2009) Dynactin regulates bidirectional transport of dense-core vesicles in the axon and dendrites of cultured hippocampal neurons. Neuroscience 162:1001–1010. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P (2005) A single cocaine exposure increases BDNF and D3 receptor expression: implications for drug-conditioning. Neuroreport 16:175–178. [DOI] [PubMed] [Google Scholar]
- Li X, DeJoseph MR, Urban JH, Bahi A, Dreyer JL, Meredith GE, et al. (2013) Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance. The Journal of Neuroscience 33:1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wolf ME (2015) Multiple faces of BDNF in cocaine addiction. Behavioural Brain Research 279C:240–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Lu L, Zhu XG, Gong JP, Shaham Y, Uhl GR (2006) Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Research 1067:1–12. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Covington HE 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. (2010) Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y (2004) A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. The Journal of Neuroscience 24:1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y (2006) Role of ERK in cocaine addiction. Trends in Neurosciences 29:695–703. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Mueller KA, Cannon EN, Huizenga MN, Darnell SB, Bhide PG, et al. (2016) Prenatal cocaine exposure alters BDNF-TrkB signaling in the embryonic and adult brain. Developmental Neuroscience 38:365–374. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Chandler LJ, Kalivas PW (2016) Signals from the fourth dimension regulate drug relapse. Trends in Neurosciences 39:472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Fitzgerald MK, Mueller D (2014) Infralimbic BDNF/TrkB enhancement of GluN2B currents facilitates extinction of a cocaine-conditioned place preference. The Journal of neuroscience: the official journal of the Society for Neuroscience 34:6057–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Pierce-Bancroft AF, Prasad BM (1999) Neurotrophin-3 contributes to the initiation of behavioral sensitization to cocaine by activating the Ras/Mitogen-activated protein kinase signal transduction cascade. The Journal of neuroscience: the official journal of the Society for Neuroscience 19:8685–8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 361:1545–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revest JM, Le Roux A, Roullot-Lacarriere V, Kaouane N, Vallee M, Kasanetz F, et al. (2014) BDNF-TrkB signaling through Erk1/2 MAPK phosphorylation mediates the enhancement of fear memory induced by glucocorticoids. Molecular Psychiatry 19:1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz CR, Shi J, Meffert MK (2014). Transcript specificity in BDNF-regulated protein synthesis. Neuropharmacology 76 Pt C: 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, et al. (2010) Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. The Journal of neuroscience: the official journal of the Society for Neuroscience 30:11735–11744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Sangrey GR, Darnell SB, Schassburger RL, Cha JH, Pierce RC, et al. (2012) Increased brain-derived neurotrophic factor (BDNF) expression in the ventral tegmental area during cocaine abstinence is associated with increased histone acetylation at BDNF exon I-containing promoters. Journal of Neurochemistry 120:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Yang C, Zhang JC, Ren Q, Yao W, Hashimoto K (2015) Alterations in brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in the brain regions of a learned helplessness rat model and the antidepressant effects of a TrkB agonist and antagonist. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology 25:2449–2458. [DOI] [PubMed] [Google Scholar]
- Sinha R (2011) New findings on biological factors predicting addiction relapse vulnerability. Current Psychiatry Reports 13:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Kalivas PW (2016) Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Structure & Function 221:1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, et al. (2003) Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature 421:70–75. [DOI] [PubMed] [Google Scholar]
- Verheij MM, Vendruscolo LF, Caffino L, Giannotti G, Cazorla M, Fumagalli F, et al. (2016) Systemic delivery of a brain-penetrant TrkB antagonist reduces cocaine self-administration and normalizes TrkB signaling in the nucleus accumbens and prefrontal cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience 36:8149–8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhou Y, Weissmiller AM, Pearn ML, Mobley WC, Wu C (2014) Real-time imaging of axonal transport of quantum dot-labeled BDNF in primary neurons. Journal of visualized experiments: JoVE 91:51899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Rat cocaine self-administration (SA), extinction training and histology.
Figure S2: Blockade of endogenous BDNF-induced TrkB signaling in the NAcore potentiated cue-induced cocaine seeking.
Figure S5: Acute exogenous BDNF infusion in the NAcore potentiated within trial extinction during context + cue-induced reinstatement after forced abstinence.
Figure S4: Acute exogenous BDNF infusion in the NAcore had no effect on sucrose seeking.
Figure S3: Acute exogenous BDNF infusion in the NAcore potentiated within trial extinction and inhibited cue-induced cocaine seeking through TrkB.
Figure S6: BDNF inhibition on cocaine seeking was sustained for at least 3 days post infusion.
Figure S7: BDNF inhibition on cocaine seeking required cue-memory presentation.


