Abstract
Background
Altered amygdala activation in response to the emotional matching faces (EMF) task, a task thought to reflect implicit emotion detection and reactivity, has been found in some patients with internalizing disorders; mixed findings from the EMF suggest individual differences (within and/or across diagnoses) that may be important to consider. Attention Bias Modification (ABM), a mechanistic attention-targeting intervention, has demonstrated efficacy in treatment of internalizing disorders. Individual differences in neural activation to a relatively attention-independent task, such as the EMF, could reveal novel neural substrates relevant in ABM’s transdiagnostic effects, such as the brain’s generalized threat reactivity capacity.
Methods
In a sample of clinically anxious patients randomized to ABM (n = 43) or sham training (n = 18), we measured fMRI activation patterns during the EMF and related them to measures of transdiagnostic internalizing symptoms (i.e., anxious arousal, general distress, anhedonic depression, and general depressive symptoms).
Results
Lower baseline right amygdala activation to negative (fearful/angry) faces, relative to shapes, predicted greater pre-to-post reduction in general depression symptoms in ABM-randomized patients. Greater increases in bilateral amygdalae activation from pre-to-post ABM were associated with greater reductions in general distress, anhedonic depression, and general depression symptoms.
Conclusions
ABM may lead to greater improvement in depressive symptoms in individuals exhibiting blunted baseline amygdalar responses to the EMF task, potentially by enhancing neural-level discrimination between negative and unambiguously neutral stimuli. Convergently, longitudinal increases in amygdala reactivity from pre-to-post-ABM may be associated with greater improvement in depression, possibly secondary to improved neural discrimination of threat and/or decreased neurophysiological threat avoidance in these specific patients.
Keywords: Amygdala, Attention bias modification, Depression, Anxiety, Neuroimaging, Emotional context insensitivity
Introduction
Internalizing disorders (i.e., depressive and anxiety disorders) are associated with significant morbidity and mortality (Richards 2011; World Health Organization 2017). However, many treatments for internalizing disorders are costly, time-intensive, difficult to administer, not effective for all patients, or not easily accessible (Bystritsky 2006). One potential treatment to help fill this void is attention bias modification training (ABM), which has demonstrated potential efficacy for internalizing disorders in randomized controlled trials (Linetzky et al. 2015). ABM involves modulating attentional bias from threatening stimuli towards neutral stimuli, thereby allowing individuals to attend more to non-threatening stimuli, ultimately decreasing stress reactivity and anxiety (Mogg and Bradley 2016). However, ABM may only be effective in select patient populations with certain internalizing problems, given mixed results from studies of depressed patients, making identification of these patient populations a critical area for both treatment refinement and personalization (Price et al. 2016a, b). In studies of depressed patients, ABM typically includes depression-relevant stimuli (e.g. sad words) rather than threat-relevant stimuli; however, even when using these diagnostically tailored stimulus sets, the efficacy of ABM in depressed patients is unclear, often depending on study and patient-specific factors (Jones and Sharpe 2017). Meta-analyses generally support a link between successful attentional modulation following ABM and degree of anxiety reduction (Price et al. 2016a, b). Because anxiety and depression are highly comorbid conditions, anxious patients may also exhibit reductions in their comorbid depressive symptoms following ABM (Sartorius et al. 1996). Yet relatively few studies have examined effects of ABM targeting threat-related attentional biases on depressive symptoms. Thus, both the overall efficacy and the potential individual difference factors moderating outcomes, for patients with depressive symptoms who undertake these more widely-used, threat-focused variants of ABM, remain unclear.
Given ABM’s transdiagnostic benefits, understanding the mechanisms of ABM required for such benefits is important to deliver the most optimal treatment. A reduction in threat attentional bias (a main effect of ABM) in certain anxious individuals appears to be helpful. However, additional benefits of ABM may reach beyond attention per se, impacting other neurocognitive processes that subserve emotional regulation and promoting more generalized improvements, such as improvement in depressive symptoms.
To better understand the potential mechanisms of ABM’s transdiagnostic efficacy, researchers have increasingly started to examine the neural substrates of ABM’s effects to uncover the “active ingredients” of ABM treatment. To best capture transdiagnostic effects of ABM, fMRI tasks are required that capture neural patterns which are (1) well-validated in healthy samples, (2) relevant in both depressive and anxiety disorders, and (3) not necessarily dependent upon explicit attentional manipulation, but instead index constituent neural processes that may contribute to attentional patterns as well as other aspects of emotion regulation. The “emotional matching faces” (EMF) task fits these criteria. The EMF task involves presentation of sustained (e.g., 10–15 s) blocks of explicit angry and fearful faces, as well as blocks of neutral stimuli, to participants who are asked to determine which of the stimuli represent an identical visual match. This task is reliably and robustly associated with amgydalar activation even in healthy samples (Hariri et al. 2002). Amygdalar activation is a core neural substrate involved in normative threat detection, and also implicated in anxiety and depression. The degree of amygdala activation to negative, relative to neutral, stimuli during the EMF is conventionally interpreted as an index of automatic/non-volitional detection and reactivity to emotional stimuli, with higher levels of amygdala response indexing greater threat discrimination, reactivity, and/or less implicit regulation of stimulus-driven emotional response (Frank et al. 2014).
Neural patterns on the EMF have broadly been associated with internalizing disorder pathology (Gentili et al. 2016; Shin and Liberzon 2010; Stuhrmann et al. 2011), but findings are not uniform across diagnoses and studies. Several studies have shown that heightened amygdala activation to negative faces is associated with social phobia (Etkin and Wager 2007; Evans et al. 2008; Stein et al. 2002), generalized anxiety disorder (Bradley et al. 1999), and depression (Mingtian et al. 2012). However, other studies examining samples of patients with comorbid anxiety and depression find that anxiety and depressive symptoms are not significantly associated with amygdala activation (Hägele et al. 2016; Kiefer 2018; MacNamara et al. 2017; Sambuco et al. 2019). In fact, a recent meta-analysis of amygdalar response during emotional face processing tasks (including those using the EMF task) in patients with MDD found hypoactivation of the amygdala to negative visual stimuli compared to healthy controls (Schulze et al. 2019), with similar findings in studies in panic disorder (Sobanski and Wagner 2017). Thus, while higher levels of amygdala response may index less regulation of emotion reactivity, lower levels may reflect impaired bottom-up threat detection and/or blunted emotional reactivity that can prevent an individual from developing and engaging in appropriate emotion regulation strategies spurred by changes in emotional arousal. Individual differences across this hypo-to-hyper continuum, appearing within and/or across diagnostic boundaries, may thus be a critical factor to consider, particularly with the overarching goal of matching patients to appropriate, mechanistically targeted treatments.
One proposed explanation for amygdalar hypoactivation observed in depression is the emotion context insensitivity (ECI) model of depression, which posits that certain individuals with depression have lower emotional responsivity to both positive and negative stimuli and that this blunted reactivity partly stems from an impaired ability to differentiate emotional stimuli efficiently (Rottenberg and Hindash, 2015). If emotional stimulus detection is impaired in some patients (particularly depressed patients, according to the ECI model), a treatment such as ABM could theoretically help improve depressive symptoms by enhancing emotion detection and restoring normative emotional reactivity in patients with hypoactive amygdalar activity. Specifically, ABM involves repeated presentations of a pair of stimuli (one neutral and one threat-related) simultaneously, with subsequent replacement of the neutral item in the pair with a ‘probe’. In order to optimize performance on this task, patients first need to quickly identify and discriminate the threat vs. neutral cue, as this more fundamental skill underlies the ability to learn the task contingency between emotional stimulus placement and probe placement. Thus, the implicit training of attention over the course of ABM relies upon this more fundamental skill of efficient threat detection.
In summary, previous findings suggest ABM may improve anxiety symptoms (the primary clinical target) by reducing attention to threatening stimuli in patients with intact (and often hyper-reactive) threat detection at baseline (Price et al. 2018). Consistently, patients with greater baseline amygdalar activation to threat experience a greater reduction in anxiety symptoms after ABM (Britton et al. 2015; Price et al. 2018). However, for some individuals receiving ABM (e.g., those with comorbid anxiety and depressive symptoms), a distinct and more foundational mechanism—neural threat sensitivity and detection—may represent a complementary neurocognitive pathway through which additional clinical benefits (e.g., reduction in depression) might be achieved.
To better understand the neural and clinical correlates associated with ABM, we conducted a secondary analysis to examine baseline fMRI brain activation during the EMF task in a transdiagnostic cohort of clinically anxious patients with varying levels of depressive symptoms, who were then randomized to receive either ABM training or a sham version of ABM training. Following active ABM training, we then re-measured brain activation patterns in a subset of ABM-treated patients. We hypothesized that lower amygdala response to threat-relevant stimuli at baseline (a proxy for blunted threat reactivity) would predict greater clinical benefits from ABM, particularly in terms of depression reduction, given the potential of ABM to improve on emotional detection and reactivity deficits and thereby reduce depression via the ECI model. Similarly, we hypothesized that increases in amygdala reactivity from pre- to post-ABM would be associated with improvements in depressive symptoms.
Methods and Materials
Given the secondary nature of our analysis, our described sample has been used in analyses from prior published studies, which further detail study methods, inclusion, and exclusion criteria (Woody et al. 2019; Price et al. 2018).
Participant Sample
As described in previous papers, randomized participants included 70 unmedicated adults ages 18–55 with self-reported clinically significant anxiety and clinician-rated disability (Price et al. 2018). Of these, 61 participants completed the baseline EMF task in the scanner with usable data and were included in present analyses. Comparison of fMRI task completers to non-completers is available in the supplement, along with recruitment details and inclusion/exclusion (see Supplement; S1-Participant Details). Participants who completed the baseline visit were randomized in double-blind fashion to either an ABM treatment arm (n = 43) or a sham training arm (n = 18), although, due to budgeting constraints, our post-ABM fMRI analysis was restricted to 21 participants who were ABM-randomized and whom were able to complete the second fMRI scan, with selection of follow-up participants based on sequential order of completion of ABM and a usable baseline fMRI (clinicaltrials.gov: NCT02303691) (Price et al. 2018). The study was approved by the Institutional Review Board of the University of Pittsburgh and informed consent was obtained from all participants. This study was carried out in accordance with the Declaration of Helsinki.
ABM and Sham Training Conditions
All participants completed eight ~ 15-min sessions of a dot-probe task twice weekly over four weeks, with 300 dot-probe task trials per session. During trials, a word pair (either threat-neutral word pair or neutral–neutral word pair) was presented on a screen (one word in an upper position and another word in lower position) for 500 ms and was then followed by a probe (“E” or “F”) which replaced one of these words. Participants then had to press a button for the correct letter. In the ABM condition, in order to systematically train attention away from threat, probes replaced the neutral word in all the threat-neutral word pairings, while in the sham condition, probes were equally likely to replace either the neutral word or the threat word. Immediately after the baseline clinical interview, participants and clinical interviewers collaboratively selected ten idiographic threat words designed to capture the primary areas of concern/anxiety for the participant, which were then paired with ten neutral words (drawn from a normative corpus). These idiographic word pairs were supplemented by an additional 20 threat words and 20 neutral words derived from a normative corpus previously used in ABM research along with the idiographically selected words (Amir et al. 2009). All idiographic words were selected based on participant-rated Likert ratings of pleasantness on a scale of − 3 (very unpleasant) to 3 (very pleasant), with threat words required to be rated “− 2” or “− 3” and neutral words rated a “0”.
Clinical Measures
Outcome measures captured severity of transdiagnostic internalizing symptoms (see Supplement; S2-Outcome Measures). The primary outcome measure for the study was the Mood and Anxiety Symptoms Questionnaire Short-Form (MASQ) (Clark and Watson 1991), which has three sub-scales based on the tripartite model of depression and anxiety: Anxious Arousal (MASQ-AA), Anhedonic Depression (MASQ-AD), and General Distress (MASQ-GD). Because of our interest in understanding the impact and mechanisms of ABM with respect to depression, we also included a second depression measure, the Beck Depression Inventory II (BDI) (Beck et al. 1988). Diagnoses of anxiety/depressive disorders were established using the Mini-International Neuropsychiatric Interview, which was administered by experienced clinicians (with master’s degree or higher) trained to reliability. Clinical outcome measures were collected at a pre-training screening visit [~ 1.5 weeks prior to initial intervention (e.g., ABM or sham) training session and < 1 week prior to baseline fMRI imaging] and at a post-intervention visit (approximately 1 week after the final ABM/sham training session, which completed the 4-week ABM training). For reference for readers, means of depression and anxiety scores in our sample were comparable to patients in the general population diagnosed with MDD and GAD, as expected given the large preponderance of participants diagnosed with GAD in our sample. For example, mean baseline scores in our sample for the BDI (19.9) and PSWQ (64.8) were respectively comparable to average BDI scores in patients diagnosed with MDD (e.g. 20–25) (Honkalampi et al. 2001) and average PSWQ scores in patients diagnosed with GAD (e.g. 67.4) (Behar et al. 2003).
Functional MRI Task
The EMF task stimuli that were presented included an array of three similar stimuli presented simultaneously in a triangular arrangement, consisting of three negative faces (either angry or fearful), three neutral faces, or three shapes (circles or ovals) (see Supplement; S3-EMF). The stimulus set was comprised of faces from the Pictures of Facial Affect stimulus set developed by Ekman (1976). For the fMRI task, a total of nine 32-s task blocks were presented, starting with a shapes task block and alternating between shapes and faces (which were in a set sequence of fearful, neutral, angry, and then neutral faces). Only negative face blocks and shape blocks were included in the present analyses. On an a priori basis and consistent with methodology in prior studies (Fonzo et al. 2015), we chose to examine the negative faces minus shapes contrast (as opposed to the negative faces minus neutral faces contrast) given that shapes are an unambiguously neutral stimulus type (in parallel to the neutral words used in our variant of ABM, which were also unambiguously neutral according to idiographic ratings made by each participant). In addition, prior studies indicate that anxious individuals may perceive neutral faces negatively (Cooney et al. 2006; Hölzel et al. 2013). Such negative perception of ambiguous stimuli has been associated with elevated amygdala activity and could thus have reduced our ability to detect amygdalar reactivity to negative faces. However, such results are presented as a supplementary analysis for interested readers (with analysis conducting negative faces minus neutral faces contrast in identical methodology to main analysis of negative faces minus shapes contrast, see Supplement; S4-Negative Minus Neutral Faces).
Within each task block, task instructions were initially given for 2 s. Participants were then asked to match one of two stimuli presented on the lower half of the screen with its identical matching target image on the upper half of the screen. Each stimulus was presented for five seconds, with six stimuli presentations per block. Matching of stimuli was done through pressing the left or right button to indicate that the left or right image, respectively, matched the target image. Participants’ overall accuracy on the EMF task was 86.3 ± 5.6%, with only one participant having accuracy below 75%.
fMRI Data Acquisition and Analysis
Functional MRI scans captured T2*-weighted images depicting blood-oxygen-level-dependent (BOLD) signal. Standard preprocessing steps were as described in prior manuscripts and the supplement (see Supplement; S5-fMRI Acquisition). A-priori interest existed for the bilateral amygdala brain regions, given their previously described role in internalizing disorders and normative activation in response to the EMF task (Fusar-Poli et al. 2009). Thus, after using whole-brain analysis to identify functional regions (see Supplement; S6-Whole Brain Analysis) that were robustly activated by negative faces during the task, we defined functional ROIs within the anatomical confines of both the left and right amygdala. After applying a small volume correction based on an anatomical mask (from the Montreal Neurological Institute atlas) of the bilateral amygdala, both left and right amygdalar functional ROIs were found that were significantly activated by negative face blocks during the task (p < 0.001). Data from both right and left amygdalar functional ROIs were extracted and used in all subsequent analyses.
Analysis of fMRI data was done in Analysis of Functional NeuroImages (AFNI) to obtain single-subject BOLD mean signal averages for each block type (see Supplement; S7-BOLD Signal). These block type means for brain regions were used to calculate contrast scores [negative (angry/fearful) faces minus shapes] for each participant at each timepoint, Winsorized to prevent any undue impact of outliers, and analyzed using the statistical software R version 3.5.2(R Core Team 2019). For interested readers, contrast scores are provided (both collapsed and separately for angry/fearful faces, and by ABM/sham randomization status) in the supplement (see Supplement; S6—Whole Brain Analysis). Given the preliminary nature of our analyses and modest sample sizes, and to provide a comprehensive picture balancing protection against both Type I and Type II error risk, we report both unadjusted p-values as well as those corrected for multiple comparisons among behavioral measures and left/right (e.g. 8 comparisons per outcome) using the False Discovery Rate (FDR) correction (“adjusted” p values below in the Results section) (Benjamini and Hochberg 1995).
All primary analyses focused on the ABM (not the sham) sample, as this group was the primary focus of the study and of a priori hypotheses regarding the neural mechanisms of ABM’s benefits, had a larger sample size (by design), and had (among a subset) both pre- and post-fMRI data available. Baseline fMRI data from the sham participants was used to provide an exploratory probe of the specificity of findings to ABM.
For descriptive purposes, baseline correlations between amygdala fMRI BOLD response and behavioral measures (along with clinical measure cross-correlations) are presented in the supplement (See Supplement; S8—BOLD Response and Behavioral Measure Correlation Matrix). In the supplement, we also present sensitivity analyses statistically adjusting for anxiety while examining amygdala BOLD response in relation to depression measures (see Supplement; S8).
Results
Sample Characteristics and Clinical Effects
Out of 70 patients qualifying for the study and potentially able to complete the faces task, 61 patients had usable fMRI data at baseline pre-ABM training, and 20 patients had usable fMRI data at both timepoints. At intake, participants were an average age of 30 ± 10.2 years old and 77% of participants were female. At baseline, all patients in this study analysis met criteria for an anxiety disorder, while 31.1% met criteria for any depressive disorder. Other clinical and demographic characteristics are further described in the supplemental material (see Supplement; S2-Outcome Measures and S9-Sample Characteristics).
Association of Baseline Amygdala fMRI Response with Change in Clinical Measures Over Time
To examine specific neural substrates of ABM’s efficacy, baseline amygdala BOLD response (faces > shapes contrast) was correlated with clinical symptoms reduction in the ABM group. Lower baseline right amygdala fMRI response was associated with greater decreases in BDI scores over time (greater score decreases indicate greater improvement of depression severity) for ABM participants (R2 = 0.11 (moderate effect size), r = 0.326, p = 0.043, adjusted p = n.s., df = 37, 95% CI [0.012, 0.582]). This pattern was not observed among sham participants (R2 = < 0.01 (negligible effect size), r = 0.033, p = 0.9, df = 16) (Fig. 1).
Fig. 1.
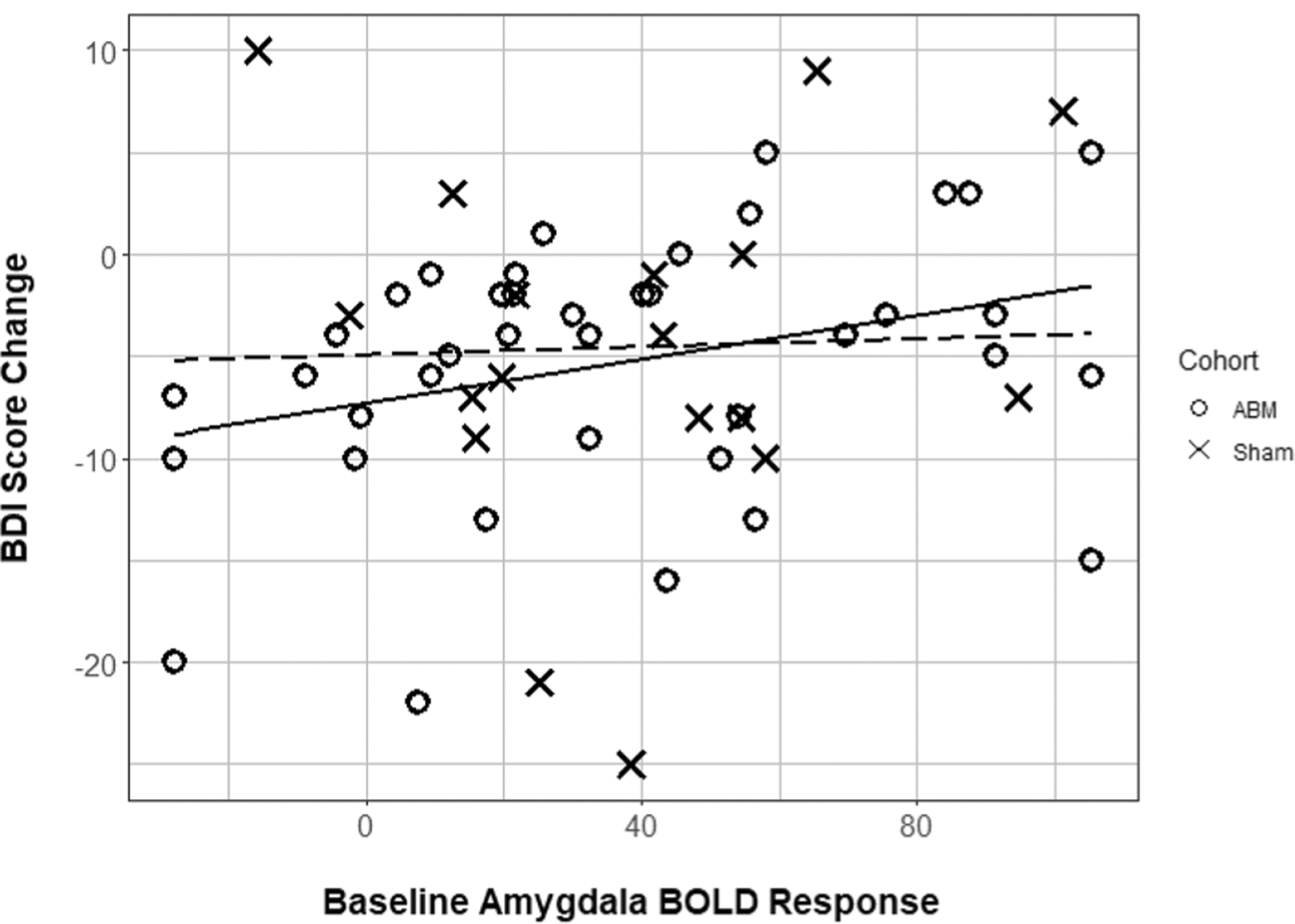
Baseline right amygdala BOLD response by BDI score change. Scatterplot of baseline right amygdala BOLD response (arbitrary units, reflecting negative faces vs. shapes contrast) with change in BDI scores over time in participants receiving ABM and participants receiving sham. Lines of best fit included for descriptive purposes (solid line = ABM cohort, dashed line = Sham cohort)
Baseline left amygdala response was not associated with decreases in BDI over time on either analysis of ABM randomized participants (R2 = 0.07, r = 0.264, p = 0.1, df = 37) or sham randomized participants (R2 = 0.09, r = 0.3, p = 0.23, df = 16). However, across all participants, lower baseline activation of the left amygdala (R2 = 0.07 (small effect size), r = 0.265, p = 0.046, adjusted p value = n.s., df = 55, 95% CI [0.005, 0.492]) was associated with greater decreases in BDI scores over time.
Association of Change in Amygdala fMRI Response with Change in Clinical Measures Over Time
Across the group of ABM treated participants, bilateral amygdala activation (faces > shapes contrast) did not change significantly between the baseline and post-ABM training timepoints (n = 20; lowest p = 0.89). However, across individual participants, greater increases in fMRI response over time (faces > shapes contrast) were correlated with greater improvement in BDI scores over time for the right amygdala [R2 = 0.31 (large effect size), r = − 0.553, p = 0.011, adjusted p value = 0.03, df = 18, 95% CI (− 0.8, − 0.146)] and left amygdala [R2 = 0.24 (large effect size), r = − 0.488, p = 0.029, adjusted p value = 0.046, df = 18, 95% CI (− 0.765, − 0.058)] (Fig. 2). Greater improvement in MASQ-AD scores over time were likewise associated with greater increases in fMRI activation over time for both the right amygdala [R2 = 0.25 (large effect size), r = − 0.504, p = 0.024, adjusted p value = 0.046, df = 18, 95% CI (− 0.774, − 0.079)] and left amygdala [R2 = 0.22 (large effect size), r = − 0.465, p = 0.039, adjusted p value = 0.051, df = 18, 95% CI (− 0.753, − 0.029)] (Fig. 3). Finally, greater improvements in MASQ-GD scores over time were likewise associated with increases in fMRI activation over time in both the right amygdala [R2 = 0.39 (large effect size), r = − 0.624, p = 0.003, adjusted p value = 0.013, df = 18, 95% CI (− 0.836, − 0.25)] and left amygdala [R2 = 0.53 (large effect size), r = − 0.726, p < 0.001, adjusted p value = 0.002, df = 18, 95% CI (− 0.884, − 0.418)] (Fig. 4).
Fig. 2.
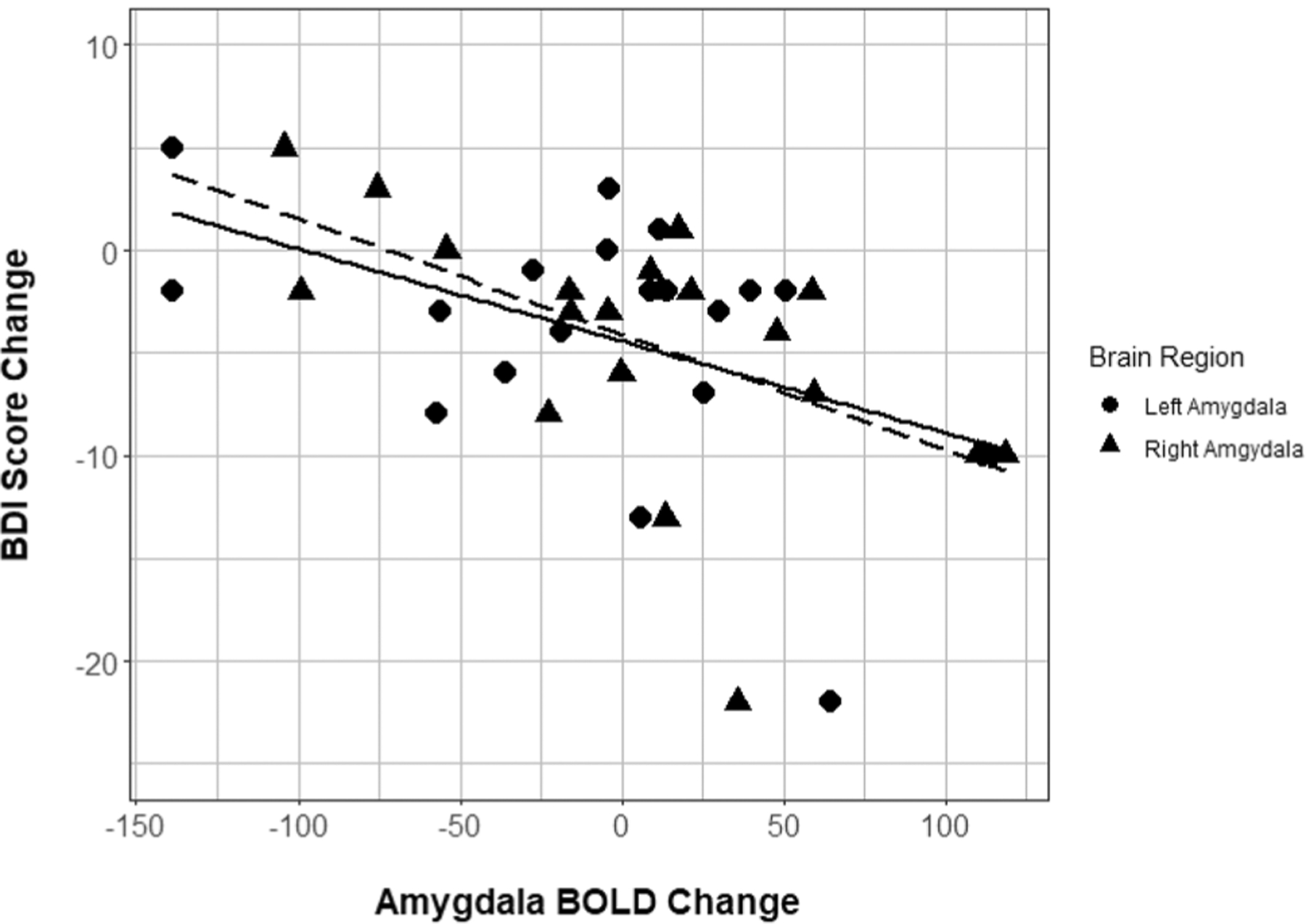
Amygdala BOLD response change by BDI score change. Scatterplot of bilateral amygdala BOLD response change over time (arbitrary units) with BDI score change over time. Lines of best fit included for descriptive purposes (right amygdala = dashed, left amygdala = solid). BDI Beck Depression Inventory II
Fig. 3.
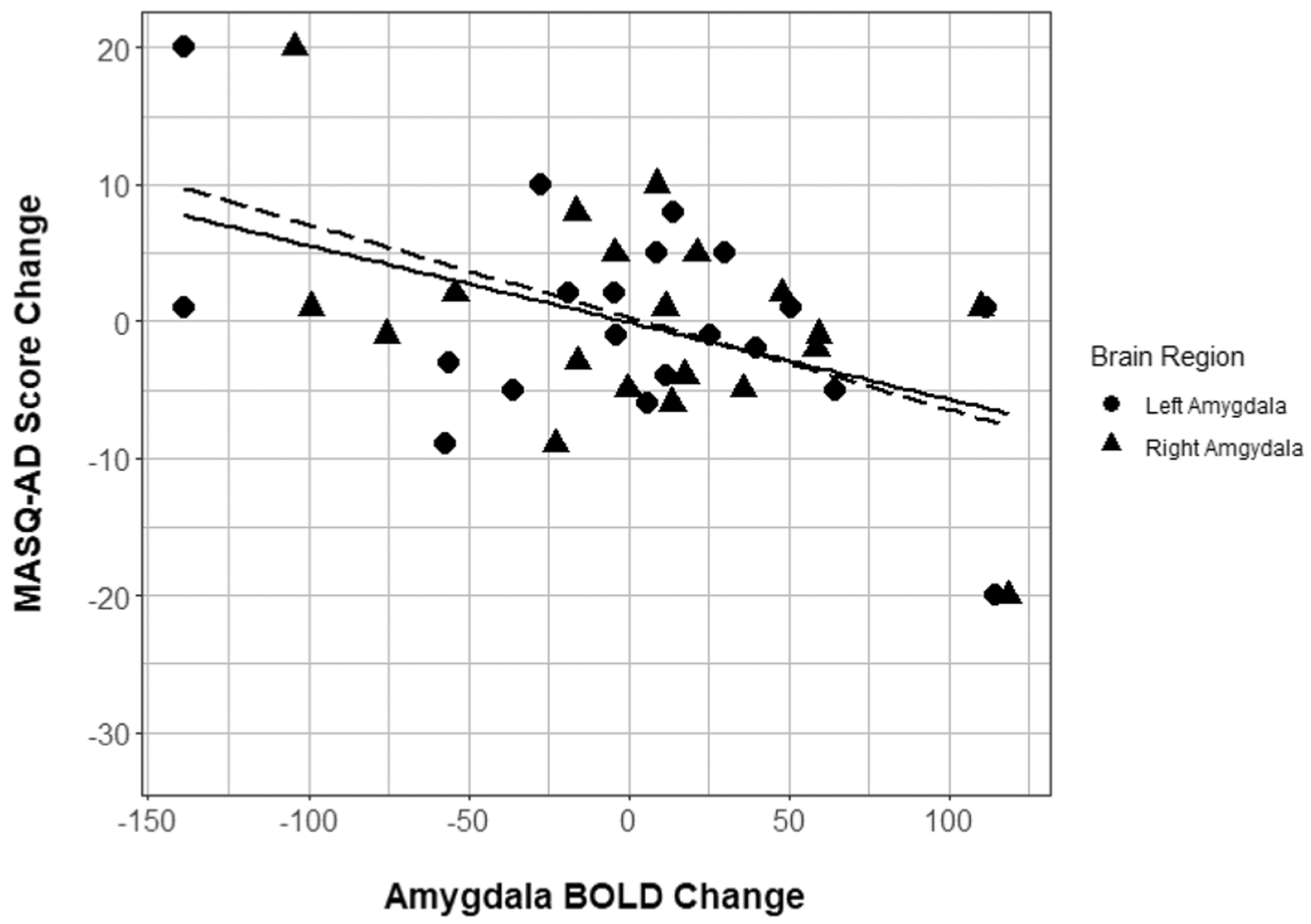
Amygdala BOLD response change by MASQ-AD score change. Scatterplot of bilateral amygdala BOLD response change over time (arbitrary units) with MASQ anhedonic depression score change over time. Lines of best fit included for descriptive purposes (right amygdala = dashed, left amygdala = solid). MASQ Mood and Anxiety Symptoms Questionnaire
Fig. 4.
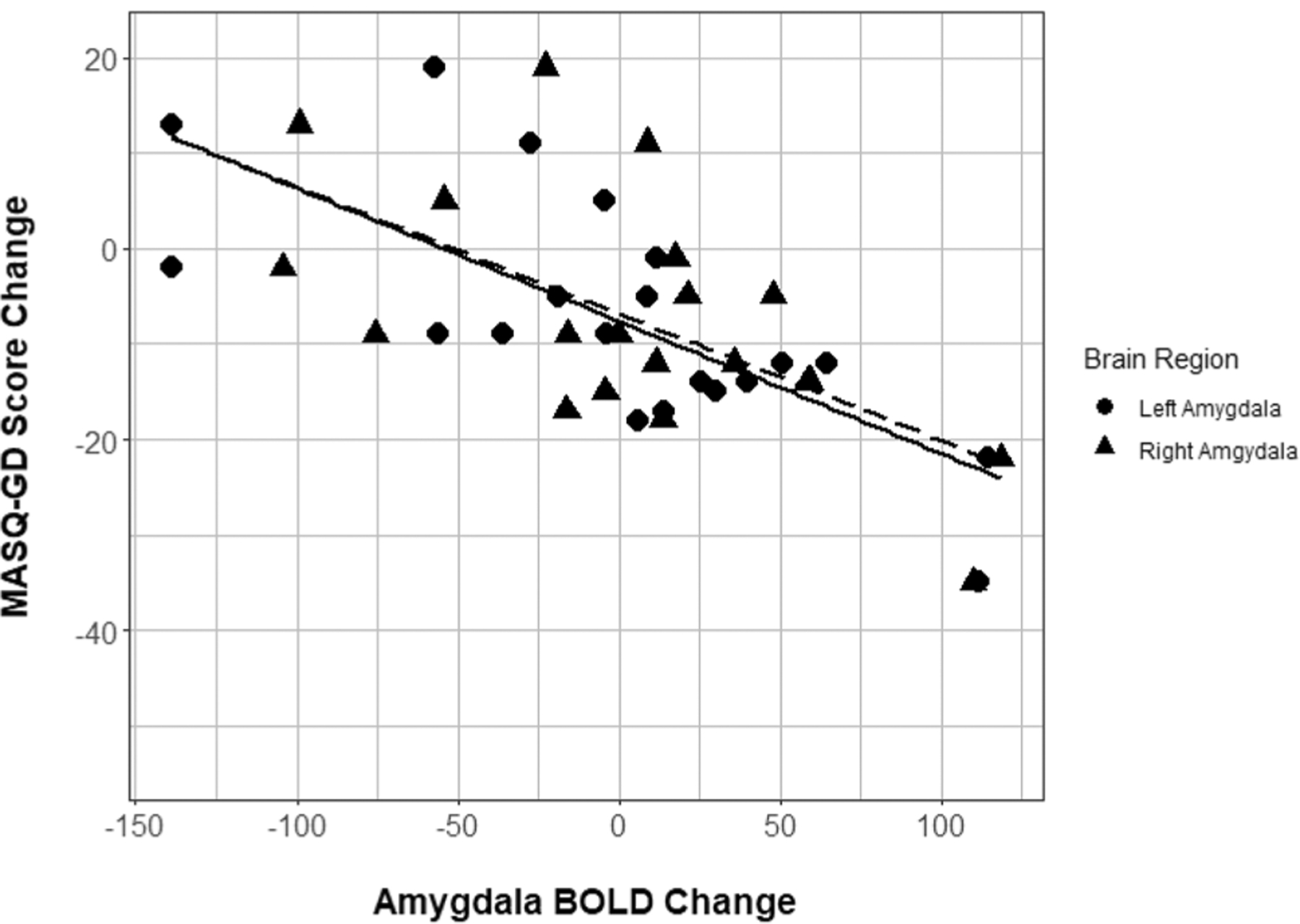
Amygdala BOLD response change by MASQ-GD score change. Scatterplot of bilateral amygdala BOLD response change over time (arbitrary units) with MASQ general distress score change over time. Lines of best fit included for descriptive purposes (right amygdala = dashed, left amygdala = solid). MASQ Mood and Anxiety Symptoms Questionnaire
Supplementary/Sensitivity Results
In supplementary analyses for interested readers (see Supplement; S4), in examining associations of clinical measures with amygdala BOLD response for the negative faces minus neutral faces contrast, greater baseline left amygdala BOLD response was associated with greater decreases in MASQ anhedonic depression levels on a trend level (p = 0.08), with no other significant associations between clinical measures and amygdala BOLD response (p’s > 0.13). In sensitivity analyses statistically adjusting for effects related to baseline or changes in anxiety (using the PSWQ), no appreciable change in findings were noted (see Supplement; S8).
Discussion
Few studies have examined relationships between neural indices and ABM’s effects on internalizing symptoms. Here, we specifically examined a neural biomarker with transdiagnostic relevance, amygdalar responses to the EMF task (contrasting negative faces vs shapes, an unambiguously neutral stimulus). The EMF robustly activates the amygdala in healthy samples (Hariri et al. 2002), and has shown neural alterations in both depression and anxiety, though varying patterns within and across specific diagnoses highlight critical individual differences that influence the nature of the aberration observed (e.g., hypo vs hyperactivation) (Bylsma et al. 2008). In our sample of anxious individuals with a range of transdiagnostic symptoms, we found that lower baseline right amygdala activation was associated with greater improvement in depression severity specifically following ABM training. A somewhat similar pattern was observed for the left amygdala, which was associated with greater improvement in depression severity following training (either ABM or sham) across the sample. We also found that, in a subset of 20 ABM recipients who completed both a pre-ABM and post-ABM fMRI scan, increases in fMRI activation (compared to pre-ABM baseline) in the bilateral amygdala tracked with greater improvements in symptoms of general depression, anhedonic depression, and general distress. The effect sizes of these findings were generally moderate to large, and mean reductions in depression scores were consistent with minimally clinical important differences previously reported in the literature. For example, for the BDI, minimally clinical important differences are reported at score reductions of 5 or greater, consistent with the average reduction of approximately 5 points in our sample (Masson 2013). These suggest for at least a portion of our sample, our findings potentially had clinical relevance. Of note, findings related to baseline amygdala activation did not persist after adjustment for multiple comparisons, while almost all findings related to change in amygdala activation (with exception of left amygdala—MASQ-AD associations) persisted after adjustment for multiple comparisons.
Our results suggest that ABM is associated with a greater reduction in depressive symptoms among patients who display blunted amygdalar responses to negative faces (relative to shapes) prior to treatment. Such findings may be reflective of changes consistent theoretically with the emotion context insensitivity (ECI) model in depression, which suggests that depression is characterized by indiscriminate, blunted responses to both positive and negative emotional stimuli, based on a large literature base (Bylsma et al. 2008; Rottenberg and Hindash 2015). In depression, such emotional insensitivity likely impairs potential for development of negative emotional regulation skills and limits emotional expression that might allow beneficial supportive behaviors by observers, hindering improvement of depression (Nyklíček et al. 2007). As would be suggested by the ECI model, patients with improvements in blunted amygdalar response from pre-to-post ABM were the most likely to experience ameliorated depressive and general distress symptoms. One possibility is that ABM might implicitly teach participants who exhibit greater emotional insensitivity at baseline to discriminate between negative and unambiguously neutral stimuli, as this fundamental skill underlies the ability to maximize task performance under the attention bias task contingencies (i.e., attend to neutral words and look away from negative threat words). Theoretically, such individuals could then more rapidly and automatically identify unambiguous neutral external stimuli as neutral (e.g., neutral words, rated idiographically as neutral by the participant—which were used as the comparator in ABM training; shapes—which are similarly unambiguously neutral and thus were used as the primary contrast in our EMF task analyses), allowing them to engage in more adaptive behavioral activities and cognitive processes that would predispose them to improvement in depression specifically, given that depression (but not anxiety) has been previously linked to emotional context insensitivity. Notably, our supplemental analyses of a negative vs. neutral face contrast suggested these findings were specifically relevant to unambiguously neutral stimuli but may not generalize to potentially more ambiguous stimuli (i.e., neutral faces).
Taken as a whole, these results suggest that improving the sensitivity of threat responses (at the level of the amygdala) may be one of the active ingredients in transdiagnostic ABM response, with particular relevance for depression symptoms and concomitant generalized distress. Although ABM training only explicitly targets attention bias, it may indirectly improve other constituent aspects of emotional processing that are likewise integral components of emotion regulation (Webb et al. 2012). Thus, heterogeneity across patients with internalizing disorders may result in heterogeneous impacts of ABM, particularly for individuals with low amygdalar responsivity at baseline (Schulze et al. 2019). Such findings may inform novel and/or refined mechanistic interventions designed to more explicitly enhance threat reactivity itself. Within a precision medicine framework, our findings suggest such interventions may be particularly beneficial for a subset of internalizing patients with comorbid anxiety and depression symptoms who exhibit impaired threat context sensitivity at the neurophysiological level.
Alternate explanations for our findings are possible. Regarding the correlations observed between pre-to-post-ABM neural patterns and symptoms, ABM’s rather rigid, repeated emphasis on attentional allocation away from threat could have led to an iatrogenic increase in avoidance of negative stimuli in some participants. Individuals who displayed decreased amygdalar activation on the EMF from pre-to-post-ABM may have learned such excessive avoidance, and as a result may have been left with greater residual depressive symptoms post-ABM—as excessive avoidance of threat has been found to prospectively confer risk for increased depressive symptoms among anxious patients (Price et al. 2016a, b). Additionally, task exposure itself may have affected depressive symptom change in certain individuals. Perhaps, merely being exposed repeatedly to the attentional bias task itself (irrespective of any consistent stimulus-probe contingency) may have beneficial effects in improving depressive symptoms among individuals with particular (left-lateralized) forms of amygdalar insensitivity at baseline.
The current analyses and findings are unique from prior publications in this sample (Woody et al. 2019; Price et al. 2018), as here we utilized a standardized, widely used, transdiagnostically relevant, relatively attention-independent fMRI task incorporating facial visual stimuli to provoke a normative (evolutionarily preserved) amygdalar response, and focused for the first time on the associations of neural response to depression and general distress outcomes (as opposed to solely anxiety) among this transdiagnostic sample. Additionally, this is the first analysis in this sample to incorporate post-ABM fMRI data in order to examine changes in neural activation longitudinally from pre-to-post-ABM—something that few prior studies of ABM’s neural substrates have done. Notably, unlike in our prior analyses using distinct neurocognitive and fMRI tasks also collected in this sample, here we found that anxiety (MASQ anxious arousal symptoms) was not associated with neural response. Placing this finding in the context of previous findings (in the current sample and others), changes in anxiety (the proximal symptom-level target of ABM) may track more closely with the proximal neurocognitive targets of ABM (e.g. attentional bias to threat and related fMRI patterns), while the current findings suggest a novel, distinct neural mechanism associated with other, more distal or generalized clinical outcomes (e.g., depression, general distress). Such novel, complex mechanistic relationships may be more likely to emerge when a given neurocognitive system is first perturbed through an experimental manipulation/intervention, rather than in cross-sectional designs.
Certain limitations exist for our findings. First, the secondary analysis nature of this data makes our analysis vulnerable to false positive discovery. Accordingly, to reduce multiple comparisons we selected a priori ROIs and used a conservative voxel-wise threshold p-value of 0.001. Also, the overall pattern of results did not appreciably change when adjusting for multiple comparisons. Also, our sample size was relatively small and due to uneven allocation across the two groups, was underpowered to assess the specificity of prediction patterns to the ABM versus sham groups with an explicit moderator analysis. Based on budgetary constraints, sham-randomized participants did not have a follow-up fMRI, limiting mechanistic inferences about longitudinal neural pattern changes linked to ABM. However, our sample was not receiving other treatment for anxiety/depression, and the brief 4-week intervention duration likely reduced the impact of uncontrolled factors. Our sample had a high number of GAD diagnoses, limiting generalizability to all clinical populations. Other limitations include the lack of depression-relevant stimuli and lack of measures examining emotional insensitivity per se (such as emotional awareness measures), restricting the interpretation of these findings in the context of the ECI hypothesis, and which require exploration in future studies. Future studies are needed to elucidate potential associations between amygdala activity in depressed patients and measures of emotional insensitivity (e.g., an emotional awareness measure). Prior studies suggest hypoactivation of the amygdala to negative visual stimuli in depressed patients (Schulze et al. 2019), but no prior work to our knowledge has directly linked such neural findings to emotional insensitivity metrics.
Finally, on an a priori basis and consistent with other studies (Fonzo et al. 2015), our analyses were performed based on a negative faces minus shapes contrast, rather than a perceptually similar stimulus contrast (neutral face), which limits the ability to isolate amygdalar responses to threat-related content from those that extend more broadly to facial stimuli. Consistent with prior studies suggesting negative perception of neutral faces in anxious individuals (Cooney et al. 2006), no significant associations were found between behavioral measures and amygdala BOLD response related to the threat minus neutral faces contrast in our supplementary analysis (see Supplement; S4), although these results and interpretations are preliminary in nature and require further studies to develop a complete understanding. Indeed, this presents an important limitation for interpretation of the present findings, as our neural index is less proximal to the process of threat discrimination per se. Future studies would benefit from use of an explicit threat discrimination task to quantify this capacity directly, at both the behavioral and the neural level.
In conclusion, in this sample of clinically anxious patients with varying levels of depressive symptoms, we examined amygdala response to the widely used EMF task and found that lower baseline right amygdala activation was associated with a more favorable depression response to ABM treatment. In addition, participants who exhibited increases in bilateral amygdala activation over time following ABM treatment reported greater reductions in general depression, anhedonic depression, and general distress severity. One possible interpretation of this findings is consistent with the emotional context insensitivity model of depression, suggesting that ABM potentially improved depressive symptoms by reversing emotional blunting and promoting more sensitive neural detection of evolutionarily salient negative stimuli. Future research is warranted to more comprehensively test the mechanisms of improvement of depressive and anxiety symptoms after ABM treatment. Such research will ideally yield insights for how to iteratively refine and personalize ABM and related automated intervention methods to successfully improve a wide range of transdiagnostic symptoms.
Supplementary Material
Acknowledgements
This project was supported by National Institute of Mental Health Grant (NIMH MH100259). This research was also supported by funding from the Ruth L. Kirschstein National Research Service Award Institutional Research Training Grants sponsored by the National Institutes of Health (NIH T32 MH018951: Brent) and by NIMH Grant K23 MH119225. The NIH had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10608-021-10205-9.
Conflict of Interest Manivel Rengasamy, Mary Woody, Tessa Kovats, Greg Siegle and Rebecca B. Price declare that they have no conflict of interest.
Informent Consent The study was approved by the Institutional Review Board of the University of Pittsburgh and informed consent was obtained from all participants. This study was carried out in accordance with the Declaration of Helsinki.
Animal Rights No animals were carried out by the authors for this article.
References
- Amir N, Beard C, Burns M, & Bomyea J (2009). Attention modification program in individuals with generalized anxiety disorder. Journal of Abnormal Psychology, 118(1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Carbin MG (1988). Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review, 8(1), 77–100. [Google Scholar]
- Behar E, Alcaine O, Zuellig AR, & Borkovec T (2003). Screening for generalized anxiety disorder using the Penn State Worry Questionnaire: A receiver operating characteristic analysis. Journal of Behavior Therapy and Experimental Psychiatry, 34(1), 25–43. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological), 57, 289–300. [Google Scholar]
- Bradley BP, Mogg K, White J, Groom C, & De Bono J (1999). Attentional bias for emotional faces in generalized anxiety disorder. British Journal of Clinical Psychology, 38(3), 267–278. [DOI] [PubMed] [Google Scholar]
- Britton JC, Suway JG, Clementi MA, Fox NA, Pine DS, & Bar-Haim Y (2015). Neural changes with attention bias modification for anxiety: a randomized trial. Social Cognitive and Affective Neuroscience, 10(7), 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, & Rottenberg J (2008). A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review, 28(4), 676–691. [DOI] [PubMed] [Google Scholar]
- Bystritsky A (2006). Treatment-resistant anxiety disorders. Molecular Psychiatry, 11(9), 805. [DOI] [PubMed] [Google Scholar]
- Clark LA, & Watson D (1991). Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology, 100(3), 316. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Atlas LY, Joormann J, Eugène F, & Gotlib IH (2006). Amygdala activation in the processing of neutral faces in social anxiety disorder: Is neutral really neutral? Psychiatry Research: Neuroimaging, 148(1), 55–59. [DOI] [PubMed] [Google Scholar]
- Ekman P (1976). Pictures of facial affect. Consulting Psychologists Press. [Google Scholar]
- Etkin A, & Wager TD (2007). Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry, 164(10), 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, & Rauch SL (2008). A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depression and Anxiety, 25(6), 496–505. [DOI] [PubMed] [Google Scholar]
- Fonzo GA, Ramsawh HJ, Flagan TM, Sullivan SG, Letamendi A, Simmons AN, et al. (2015). Common and disorder-specific neural responses to emotional faces in generalised anxiety, social anxiety and panic disorders. The British Journal of Psychiatry, 206(3), 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D, Dewitt M, Hudgens-Haney M, Schaeffer D, Ball B, Schwarz N, et al. (2014). Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neuroscience and Biobehavioral Reviews, 45, 202–211. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surgu-ladze S, et al. (2009). Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience, 34, 418–432. [PMC free article] [PubMed] [Google Scholar]
- Gentili C, Cristea IA, Angstadt M, Klumpp H, Tozzi L, Phan KL, & Pietrini P (2016). Beyond emotions: A meta-analysis of neural response within face processing system in social anxiety. Experimental Biology and Medicine, 241(3), 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägele C, Friedel E, Schlagenhauf F, Sterzer P, Beck A, Berm-pohl F, et al. (2016). Affective responses across psychiatric disorders: A dimensional approach. Neuroscience Letters, 623, 71–78. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, & Weinberger DR (2002). The amygdala response to emotional stimuli: A comparison of faces and scenes. Neuroimage, 17(1), 317–323. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Hoge EA, Greve DN, Gard T, Creswell JD, Brown KW, et al. (2013). Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. NeuroImage: Clinical, 2, 448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkalampi K, Hintikka J, Laukkanen E, & Viinamäki JLH (2001). Alexithymia and depression: A prospective study of patients with major depressive disorder. Psychosomatics, 42(3), 229–234. [DOI] [PubMed] [Google Scholar]
- Jones EB, & Sharpe L (2017). Cognitive bias modification: A review of meta-analyses. Journal of Affective Disorders, 223, 175–183. [DOI] [PubMed] [Google Scholar]
- Kiefer C (2018). Neural correlates of emotional face processing in a community sample of adolescents with varying irritability and anxiety symptoms. San Diego: San Diego State University. [Google Scholar]
- Linetzky M, Pergamin-Hight L, Pine DS, & Bar-Haim Y (2015). Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depression and Anxiety, 32(6), 383–391. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Klumpp H, Kennedy AE, Langenecker SA, & Phan KL (2017). Transdiagnostic neural correlates of affective face processing in anxiety and depression. Depression and Anxiety, 34(7), 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson SC (2013). Minimum clinically important differences identified for commonly used depression rating scales. Journal of Clinical Epidemiology, 66(7), 805. [DOI] [PubMed] [Google Scholar]
- Mingtian Z, Shuqiao Y, Xiongzhao Z, Jinyao Y, Xueling Z, Xiang W, et al. (2012). Elevated amygdala activity to negative faces in young adults with early onset major depressive disorder. Psychiatry Research: Neuroimaging, 201(2), 107–112. [DOI] [PubMed] [Google Scholar]
- Mogg K, & Bradley BP (2016). Anxiety and attention to threat: Cognitive mechanisms and treatment with attention bias modification. Behaviour Research and Therapy, 87, 76–108. [DOI] [PubMed] [Google Scholar]
- Nyklíček I, Vingerhoets A, & Zeelenberg M (2007). Emotion regulation: Conceptual and clinical issues. New York: Springer. [Google Scholar]
- Price RB, Cummings L, Gilchrist D, Graur S, Banihashemi L, Kuo SS, & Siegle GJ (2018). Towards personalized, brain-based behavioral intervention for transdiagnostic anxiety: Transient neural responses to negative images predict outcomes following a targeted computer-based intervention. Journal of Consulting and Clinical Psychology, 86(12), 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Rosen D, Siegle GJ, Ladouceur CD, Tang K, Allen KB, et al. (2016a). From anxious youth to depressed adolescents: Prospective prediction of 2-year depression symptoms via attentional bias measures. Journal of Abnormal Psychology, 125(2), 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Wallace M, Kuckertz JM, Amir N, Graur S, Cummings L, et al. (2016b). Pooled patient-level meta-analysis of children and adults completing a computer-based anxiety intervention targeting attentional bias. Clinical Psychology Review, 50, 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2019). R: A language and environment for statistical computing. Austria: R Foundation for Statistical Computing. [Google Scholar]
- Richards D (2011). Prevalence and clinical course of depression: A review. Clinical Psychology Review, 31(7), 1117–1125. 10.1016/j.cpr.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, & Hindash AC (2015). Emerging evidence for emotion context insensitivity in depression. Current Opinion in Psychology, 4, 1–5. [Google Scholar]
- Sambuco N, Bradley M, Herring D, Hillbrandt K, & Lang PJ (2019). Transdiagnostic trauma severity in anxiety and mood disorders: Functional brain activity during emotional scene processing. Psychophysiology. 10.1111/psyp.13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius N, Üstün TB, Lecrubier Y, & Wittchen H-U (1996). Depression comorbid with anxiety: Results from the WHO study on psychological disorders in primary health care. The British Journal of Psychiatry, 168(S30), 38–43. [PubMed] [Google Scholar]
- Schulze L, Schulze A, Renneberg B, Schmahl C, & Niedtfeld I (2019). Neural correlates of affective disturbances: A comparative meta-analysis of negative affect processing in borderline personality disorder, major depressive disorder, and posttraumatic stress disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(3), 220–232. [DOI] [PubMed] [Google Scholar]
- Shin LM, & Liberzon I (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology, 35(1), 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobanski T, & Wagner G (2017). Functional neuroanatomy in panic disorder: Status quo of the research. World Journal of Psychiatry, 7(1), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LTE, & Brown GG (2002). Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry, 59(11), 1027–1034. [DOI] [PubMed] [Google Scholar]
- Stuhrmann A, Suslow T, & Dannlowski U (2011). Facial emotion processing in major depression: A systematic review of neuroimaging findings. Biology of Mood and Anxiety Disorders, 1(1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TL, Miles E, & Sheeran P (2012). Dealing with feeling: A meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychological Bulletin, 138(4), 775. [DOI] [PubMed] [Google Scholar]
- Woody ML, Yang JO, Cummings L, Gilchrist D, Graur S, Siegle GJ, & Price RB (2019). Protracted amygdalar response predicts efficacy of a computer-based intervention targeting attentional patterns in transdiagnostic clinical anxiety. Translational Psychiatry, 9, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2017). Depression and other common mental disorders: Global health estimates. Geneva: World Health Organization. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


