Abstract
Background
The progesterone receptor (PR) is variably expressed in most meningiomas and was found to have prognostic significance. However, the correlation with patient age, tumor location, time to recurrence, and pattern of regrowth has scarcely been discussed.
Methods
A surgical series of 300 patients with meningiomas is reviewed. The PR expression was classified as: 0. absent; 1. low (<15%); 2. moderately low (16–50%); 3. moderately high (51–79%); 4. high (≥80%). The PR values were correlated with the patient age and sex, meningioma location, WHO grade, Ki-67 MIB1, recurrence rate, pattern of recurrence (local-peripheral versus multicentric diffuse), and time to recurrence.
Results
The PR expression has shown lower rate of high expression in the elderly group (p = 0.032) and no sex difference (including premenopausal versus postmenopausal women), higher expression in medial skull base and spinal versus other locations (p = 0.0036), inverse correlation with WHO grade and Ki67-MIB1 (p < 0.0001). Meningiomas which recurred showed at initial surgery higher rates of low or moderately low PR expression than the non-recurrent ones (p = 0.0004), whereas the pattern of regrowth was not significant. Higher rates of PR values ≥80% were found in cases with time to recurrence >5 years (p = 0.036).
Conclusion
The higher PR expression in medial skull base meningiomas, the significant correlation with the time to recurrence, the lack of difference of PR expression between premenopausal and postmenopausal women and between local-peripheral versus multicentric-diffuse recurrences are the most relevant unreported findings of this study. The rate of PR expression must be included in the routine pathological diagnosis of meningiomas because of its prognostic significance.
Keywords: meningioma, progesterone receptor, WHO grade, proliferation index Ki 67 MIB1, meningioma recurrence
Introduction
The presence of sex steroid hormone receptors in meningiomas is known since about 40 years (1, 2).
Some clinical evidence suggests that sex steroids play a role in the growth of meningiomas; these include the clear female predominance (female/male ratio 2:1), the reported rapid growth during pregnancy (3, 4), and women who receive oral contraceptives or hormone replacement therapy (5, 6). The progesterone receptor (PR) expression is found in variable and often very high rate meningiomas (39 to 88%) in some studies (7–9), whereas the estrogen receptor (ER) expression is lower than 10% and often undetectable. The PR expression was found to be correlated with the WHO grade and recurrence in ours (10) and other studies (11–14), with low expression associated with WHO grade II and recurrence. On the other hand, other factors, including patient age, intracranial tumor location, spinal meningiomas, time to recurrence, and patterns of regrowth, have scarcely been discussed.
This study reviews a surgical series of meningiomas and discusses the pathological correlation and prognostic significance of the PR expression.
Materials and Methods
Patient Population
Three hundred fifty-two patients who underwent neurosurgery for intracranial and spinal tumors diagnosed as meningiomas at the neurosurgical clinic of the “Federico II” University of Naples between 2006 and 2016 were reviewed. Two children with neurofibromatosis, five patients with post-irradiation meningiomas, and forty-two patients with recurrences were excluded. Thus, 300 consecutive patients with primary intracranial or spinal meningiomas were included in the study. Besides, the 42 patients with recurrence observed in this period and 33 observed between 2000 and 2006 were included in a recurrence group for a total of 75 patients, all with recurrent intracranial meningiomas.
An ethics committee approval was not required according to local and national legislation.
Analyzed Factors
The factors analyzed in the study included patient age and sex, meningioma location, WHO grade, PR expression, Ki67 MIB-1, recurrence rate, regrowth or recurrence pattern, and time to recurrence.
According to the patient age, two main groups were identified: group I or elderly ≥70 years old and group II <70 years. For the sex evaluation, the female patients were divided in two groups: A, premenopausal and B, postmenopausal. The tumor location was defined from the review of the magnetic resonance (MR) images and the surgical descriptions. Four groups were identified: group 1 or medial skull base included olfactory groove, ethmoidal-sphenoidal planum, tuberculum sellae, parasellar, clival-petroclival, and foramen magnum meningiomas; group 2 or lateral skull base included middle and lateral sphenoid wing and temporal fossa meningiomas and those of the petrous bone and occipital fossa; group 3 or non-skull base included convexity, parasagittal or falx meningiomas, and those of the tentorium, cerebellar convexity, pineal region and lateral ventricle; group 4 included spinal meningiomas.
The surgical specimens were reviewed independently by two pathologists (MC and EG) who were unaware of the clinical data. The WHO grade was defined according to the 2007 WHO classification (15), which was used at the observation period. The immunohistochemical studies were performed to evaluate the Ki67 MIB-1 and the PR expression. The specimens were fixed in neutral buffered 10% formalin, embedded in paraffin, and cut into sections of 5 mm thickness.
The expression of PR was determined in all specimens with monoclonal antibody against the progesterone (DAKO, Italy 1:400, overnight incubation). The quantitative evaluation was expressed as percentage for positive nuclei among 100 cells, for a total of 500 cells. The percentage of PR positivity was determined by a semiquantitative scoring scale with respect to staining intensity, according to the recommendations for immunohistochemistry of hormonal receptors (16) and slightly modified.
The PR expression was graded as follows: 0. absence of positive nuclei; 1. low (<15%); 2. moderately low (16–50%); 3. moderately high (51–79%); 4. high (≥80%) ( Figure 1 ).
Figure 1.
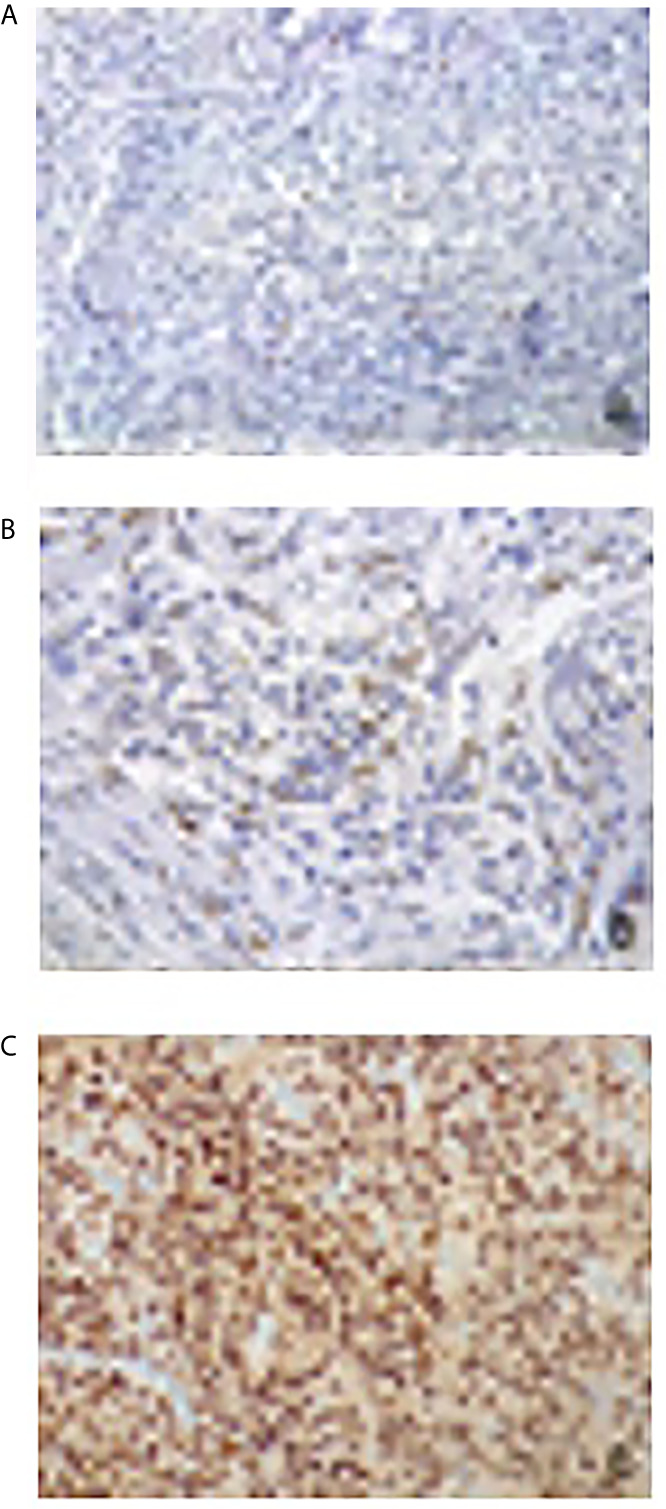
Immunohistochemical evaluation of progesterone receptor antibody expression: nuclear signal respectively in less than 1% (A), in 15% (B) and in 95% (C) of neoplastic cells (×200 magnification).
The expression of Ki67 MIB-1 was evaluated in all specimens by using the monoclonal antibody MIB-1 Immunotech® (DAKO system, dilution 1:1,000, overnight incubation). The streptavidin–biotin system and the diaminobenzidine (DAB) were used for antigen detection and visualization. A specimen of breast carcinoma was used as a positive control. Ki67-LI count was performed by eye counting, taking the average on five adjacent representative fields of neoplastic cells in a hot spot area. The values of Ki67-LI were classified into two groups: group I ≤4%; group II >4%.
The histological types of WHO grade I meningiomas were classified as: meningothelial, transitional, fibroblastic, psammomatous, microcystic, secretory, and chordoid.
The 75 patients with tumor recurrence were classified into two groups: group I (50 cases) with local-peripheral recurrence, in which the recurrence occurred at the previous dural site or at the surrounding dura mater (within 2 cm); group II (25 cases) with multicentric-diffuse recurrence (at variable distance from the initial dural site). The data of these two groups have been compared with those of 100 consecutive patients operated between 2006 and 2010, who did not experience recurrence 9 years or more after the initial surgery.
The analyzed variables included patient age and sex, meningioma location, Simpson grade of surgical resection, PR expression, WHO grade, Ki67 Li.
The patient age was considered as median values; the data stratification between patients ≤70 years and those >70 years was avoided in the analysis of the recurrences because of very different life expectancies and length of the follow-up between the two groups.
In the overall group of 75 patients with recurrence, the PR expression was correlated to the recurrence time (≤5 years versus >5 years). Finally, the values of PR expression at the initial surgery were compared to those at the first recurrence.
Statistical Analysis
The values of PR expression were carefully analyzed and stratified in all cases according to the patient age and sex, tumor location, WHO grade, Ki67-LI, overall recurrence rate, and pattern of recurrence (local and peripheral versus multicentric and diffuse). The data were analyzed by one-way ANOVA test or Fisher’s exact test, and p-value was calculated. A p-value ≤0.05 was considered statistically significant. The group of 75 patients with recurrence was studied for PR expression, MIB-1 index, WHO grade, and tumor location, by a multivariate non-parametric statistical tests of hypotheses (Pearson linear correlation test, Spearman R test, Mann–Whitney U test). A Kaplan–Meier test was also performed for the time to recurrence.
Results
In the overall series of 300 patients with meningioma at first diagnosis, the PR expression was low (0–15%) in 54 (18%), moderately low (16–50%) in 68 (23%), moderately high in 60 (20%) and high (≥80%) in 118 (39%). No cases with complete absence of positive nuclei were found. The data of the PR expression according to the analyzed factors are as follows.
Patient Age and Sex
The patients were 223 women (74%) and 77 men (26%); their age was <70 years in 225 (75%) and ≥70 years in 75 (25%). The distribution of the PR expression in the age groups ( Table 1 ) has shown lower rate of cases with expression ≥80% in the elderly group (p = 0.032). According to the patient sex, no significant difference was evidenced between females and males ( Table 2 ). In the female group, 69 (30%) premenopausal and 154 (70%) postmenopausal women were considered separately; the distribution of the PR expression in these last two groups was not significantly different ( Table 2 ).
Table 1.
PR expression and patient age.
| PR expression | N. cases | Group 1 (≥70 years) 75 pts | Group 2 (<70 years) 225 pts | Statistical significance group 1 vs. group 2 |
|---|---|---|---|---|
| L (0–15%) | 54 | 20 (27%) | 34 (15%) | p = 0.99 |
| ML (16–50%) | 68 | 20 (27%) | 48 (22%) | p = 0.81 |
| MH (51–79%) | 60 | 12 (16%) | 48 (21%) | p = 0.17 |
| H (≥80%) | 118 | 23 (30%) | 95 (42%) | p = 0.032 |
Statistically significant values have been reported in bold.
Table 2.
PR expression and patient sex.
| PR expression | N. cases | Group 1 Females | Group 1A premenopausal women | Group 1B postmenopausal women | Group 2 Males | Statistical significance group 1 vs. group 2 | Statistical significance group 1A vs. group 1B |
|---|---|---|---|---|---|---|---|
| L (0–15%) | 54 | 41 (18%) | 10 (14%) | 31 (20%) | 13 (17%) | p = 0.55 | p = 0.23 |
| ML (16–50%) | 68 | 47 (21%) | 16 (23%) | 31 (20%) | 21 (27%) | p = 0.20 | p = 0.63 |
| MH (51–79%) | 60 | 43 (20%) | 17 (25%) | 26 (17%) | 17 (22%) | p = 0.39 | p = 0.81 |
| H (≥80%) | 118 | 92 (41%) | 26 (38%) | 66 (43%) | 26 (34%) | p = 0.86 | p = 0.24 |
| 300 | 223 | 69 | 154 | 77 |
PR Expression and Meningioma Location
The meningioma location was at the medial skull base in 72 patients (24%) and at the lateral skull base in 39 (13%); 161 (54%) were non-skull base and 28 (9%) were in the spinal canal. The distribution of the different locations within the four groups is summarized in Table 3 .
Table 3.
Meningioma location.
| Location | No. of cases |
|---|---|
| Medial skull base | |
| - Olfactory groove, planum ethmoidale-sphenoidale | 33 |
| - Tuberculum sellae | 18 |
| - Parasellar (anterior clinoid and optic canal) | 16 |
| - Clivus, petroclival, foramen magnum | 5 |
| - Total | 72 (24%) |
| Lateral skull base | |
| - Middle and lateral sphenoid wings, temporal fossa | 14 |
| - Spheno-orbital | 16 |
| - Petrous bone, occipital fossa | 9 |
| - Total | 39 (13%) |
| Non-skull base | |
| - Cerebral convexity, parasagittal, falx | 137 |
| - Tentorial, cerebellar convexity, pineal | 19 |
| - Lateral ventricles | 5 |
| - Total | 161 (54%) |
| Spinal | 28 (9%) |
| Total | 300 |
Medial skull base and spinal meningiomas showed significantly higher rate of cases with high PR expression and lower rate of cases with low expression than the lateral skull base and non-skull base meningiomas (p = 0.0036) ( Table 4 ).
Table 4.
PR expression and meningioma location.
| PR expression | ||||||||
|---|---|---|---|---|---|---|---|---|
| Meningioma location | N. cases | L (0–15%) | ML (16–50%) | MH (51–79%) | H (≥80%) | Statistical significance | ||
| Medial skull base | 72 | 5 (7%) | 13 | (18%) | 12 | (16.5%) | 42 (58.5%) | |
| Lateral skull base | 39 | 6 (15.5%) | 9 | (23%) | 10 | (25.5%) | 14 (36%) | Lateral skull base and non-skull base |
| Non-skull base | 161 | 39 (24%) | 39 | (24%) | 31 | (19.5%) | 52 (32.5%) | vs. |
| Spinal | 28 | 4 (14%) | 7 | (25%) | 7 | (25%) | 10 (36%) | medial skull base and spinal |
| Total | 300 | 54 (18%) | 68 | (23%) | 60 | (20%) | 118 (39%) | p=0.0036 |
Statistically significant values have been reported in bold.
PR Expression, WHO Grade, Ki67 MIB-1 and Histological Type
Atypical WHO grade II meningiomas have shown significantly lower rate (18%) of cases with high (≥80%) PR expression; on the other hand, benign WHO grade I tumors mainly showed high PR expression (82% of the examined cases). This correlation was statistically significant (p < 0.0001) ( Table 5 ).
Table 5.
PR expression, WHO grade and Ki67/MIB1.
| PR expression | N. cases | WHO grade | Statistical significance | Ki 67/MIB1 | Statistical significance | ||
|---|---|---|---|---|---|---|---|
| I | II | ≤4% | >4% | ||||
| L (0–15%) | 54 | 24 (47%) | 30 (53%) | p = 0.30 | 23 (42%) | 31 (58%) | p = 0.04 |
| ML (16–50%) | 68 | 32 (49%) | 36 (51%) | p = 0.40 | 28 (41%) | 40 (59%) | p = 0.017 |
| MH (51–79%) | 60 | 41 (69%) | 19 (31%) | p = 0.99 | 35 (58%) | 25 (42%) | p = 0.014 |
| H (≥80%) | 118 | 97 (82%) | 21 (18%) | p < 0.0001 | 100 (85%) | 18 (15%) | p < 0.0001 |
| 300 | 194 (65%) | 106 (35%) | 186 (62%) | 114 (38%) | |||
Statistically significant values have been reported in bold.
The correlation between PR expression and Ki67 MIB 1 has provided significant differences. Cases with Ki67 LI >4% showed significantly lower rate of high (≥80%) PR expression (p = 0.0001) and higher rates of low (p = 0.04) or moderately low (p = 0.017) expression ( Table 5 ). Thus, the study confirms an inverse correlation of the PR expression with both the WHO grade and Ki67 MIB-1.
The most frequent histological type of WHO I meningiomas was transitional (43%) followed by fibroblastic (22%) and meningothelial (15%). Tumors of meningothelial and psammomatous types showed slightly higher rates of high PR expression (76 and 77% respectively) than transitional (63%) and fibroblastic (52%), but with no statistical significance ( Table 6 ).
Table 6.
PR expression and histological type of 194 WHO grade I meningiomas.
| PR expression | Meningothelial | Transitional | Fibroblastic | Psammomatous | Microcystic | Secretory | Chordoid |
|---|---|---|---|---|---|---|---|
| L (0–15%) | 3 (10%) | 3 (3%) | 9 (22%) | 2 (9%) | 2 (22%) | – | - |
| ML (16–50%) | 1 (4%) | 14 (17%) | 8 (19%) | 2 (9%) | 2 (22%) | – | 2 (50%) |
| MH (51–79%) | 3 (10%) | 14 (17%) | 3 (7%) | 1 (5%) | 1 (11%) | – | - |
| H (≥80%) | 22 (76%) | 52 (63%) | 22 (52%) | 17 (77%) | 4 (45%) | 5 (100%) | 2 (50%) |
| Total (194) | 29 (15%) | 83 (43%) | 42 (22%) | 22 (11%) | 9 (5%) | 5 (2%) | 4 (2%) |
| Statistical significance | p = 0.08 | p = 0.5 | p = 0.09 | p = 0.096 | p = 0.15 |
PR Expression and Recurrence
The results of the clinical and pathological variables at the initial surgery were compared between the groups of patients with and without recurrence The data are summarized in Table 7 . The 75 patients with recurrence were 48 (64%) women and 27 (36%) men, with a median age of 55 years at initial diagnosis. The male rate was higher than in the group with no recurrence (25%) but with no significance. No differences of the median values of patient age and sex were evidenced. The analysis of the meningioma location has shown lower rate of medial skull base (13 versus 29%) and spinal meningiomas (0 versus 8%) and higher rate of lateral skull base meningiomas (31 versus 13%) in group I (recurrence). According to the extent of surgical resection, the recurrence groups, as expected, showed significantly lower number of Simpson grade I resection (33 versus 61%) and higher rate of grade III resections (27 versus 10%) than the no recurrence group. Meningiomas which recurred showed at initial examination higher rate of low and moderately low PR expression (69 versus 37%) and significantly lower rate of cases with high PR expression (12 versus 43%) (p = 0.0004) than the non-recurrent meningiomas. On the other hand, there were no significant differences between cases with local-peripheral versus multicentric-diffuse recurrences (p = 0.5). These data agree with the significantly higher rate of atypical forms (p > 0.00001) and of those with Ki67-LI >4% (p = 0.003) in meningiomas which recurred, as compared to the non-recurrent ones ( Table 7 ).
Table 7.
PR expression, WHO grade, Ki67/MIB1 at initial surgery and recurrence.
| Covariates | Group 1 overall recurrence (75 pts) | Group 1A Local-peripheral recurrences (50 pts) | Group 1B Multicentric diffuse recurrences (25 pts) | Group 2 No recurrence (100 pts) | Statistical significance group 1 vs. 2 | Statistical significance group 1A vs. 1B | |
|---|---|---|---|---|---|---|---|
| PR expression | |||||||
| L (0–15%) | 22 (29%) | 14 (28%) | 8 (32%) | 13 (13%) | p = 0.99 | p = 0.35 | |
| ML (16–50%) | 30 (40%) | 18 (36%) | 12 (48%) | 24 (24%) | p = 0.98 | p = 0.15 | |
| MH (51–79%) | 14 (19%) | 12 (24%) | 2 (8%) | 20 (20%) | p = 0.4 | p = 0.95 | |
| H (≥80%) | 9 (12%) | 6 (12%) | 3 (12%) | 43 (43%) | p = 0.0004 | p = 0.5 | |
| WHO grade | |||||||
| I | 23 (30%) | 18 (36%) | 5 (20%) | 72 (100%) | p = 0.000001 | p = 0.07 | |
| II | 52 (70%) | 32 (64%) | 20 (80%) | 28 (28%) | p = 0.00001 | p = 0.07 | |
| KI67/MIB1 | |||||||
| ≤4% | 27 (36%) | 22 (44%) | 5 (20%) | 62 (62%) | p = 0.003 | p = 0.07 | |
| >4% | 48 (64%) | 28 (56%) | 20 (80%) | 38 (38%) | p = 0.003 | p = 0.07 | |
Statistically significant values have been reported in bold.
The multivariate non-parametric statistical tests confirm strong correlation between PR expression ≥80%, low WHO grade, and low expression (≤4%) of Ki 67-Li. The WHO grade is the most efficient variable to predict recurrence. The high PR expression (≥80%) is a single efficient predictive factor (p = 0.017).
The PR expression at initial surgery was significantly correlated with the recurrence time, with higher rate of patients (23 versus 8%) with high PR values ≥80% in the group with recurrence time >5 years (p = 0.036) ( Table 8 and Figure 2 ).
Table 8.
PR expression and time to recurrence (75 pts).
| PR expression | Time to recurrence | Statistical significance | Statistical significance groups L+ML vs MH+H | |
|---|---|---|---|---|
| ≤5 years | >5 years | |||
| L (0–15%) | 17 (32%) | 5 (23%) | p = 0.78 | p = 0.009 |
| ML (16–50%) | 24 (45%) | 6 (27%) | p = 0.92 | |
| MH (51–79%) | 8 (15%) | 6 (27%) | p = 0.11 | p = 0.0096 |
| H (≥80%) | 4 (8%) | 5 (23%) | p = 0.036 | |
Statistically significant values have been reported in bold.
Figure 2.
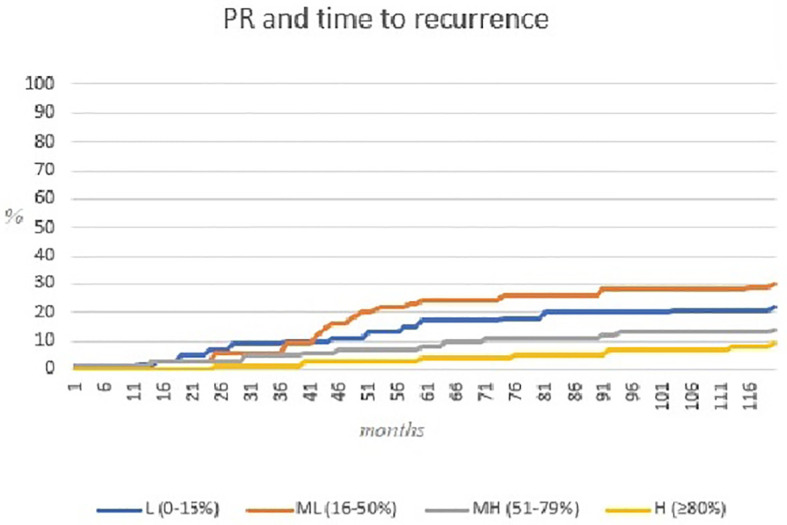
Kaplan–Meier curve representing relation between PR expression and time to recurrence.
Finally, the PR values of the surgical specimens at recurrence, as compared with those at initial surgery, had the same score (almost unchanged) in 42 cases (56%) and at a lower score in 33 (44%). This finding is associated with rather similar behavior of the Ki67 MIB-1, showing 44 cases (59%) with increased values, from ≤4 to >4%, and 31 (41%) with values in the same subgroup, both at initial surgery and recurrence.
Discussion
The possible pathological and prognostic implications of the PR expression in meningiomas have been discussed in several studies. However, the role of several factors is still controversial. Table 9 summarizes the results of 30 studies from the literature that focused on the epidemiological, pathological, and prognostic role of the PR expression in meningiomas (8, 10–14, 17–40).
Table 9.
Data of 30 reviewed studies on the progesterone receptor expression in meningiomas.
| Authors/year | N° of cases | Correlation of PR expression with epidemiological and pathological findings and recurrence | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Location | WHO grade | Ki67 MIB1 | Mitotic index | Histological type | Recurrence | ||
| Magdelenat et al., 1982 (17) | 42 | n.s. | p = 0.05 | n.s. | - | - | - | n.s. | - |
| Markwalder et al., 1983 (18) | 34 | n.s. | n.s | n.s. | – | – | n.s | ++meningothelial | – |
| Nagashima et al., 1995 (19) | 39 | - | p < 0.02 | - | p < 0.001 | p < 0.05 | - | - | - |
| Hsu et al., 1997 (8) | 70 | n.s. | – | – | p < 0.001 | – | p < 0.0001 | n.s | – |
| Fewings et al., 2000 (11) | 62 | - | - | - | b.m. | - | - | - | p = 0.013 |
| Perry et al., 2000 (20) | 175 | – | n.s. | – | p < 0.001 | – | – | n.s | – |
| Das et al., 2002 (21) | 90 | n.s. | n.s. | - | b.m. | - | - | - | n.s. |
| Gursan et al., 2002 (22) | 110 | n.s. | n.s. | n.s. | – | p < 0.05 | – | – | – |
| Strik et al., 2002 (23) | 30 | - | - | - | b.m. | n.s. | - | - | OR 3.533 |
| Konstantinidou et al., 2003 (24) | 51 | – | n.s | n.s | p = 0.036 | p = 0.041 | p = 0.009 | ++meningothelial p = 0.04 | – |
| Roser et al., 2004 (12) | 588 | n.s. | n.s | n.s. | p < 0.0001 | p < 0.001 | - | p < 0.0001 | p < 0.0005 |
| Wolfsberger et al., 2004 (25) | 82 | p=0.05 | n.s. | n.s. | n.s. | – | – | ++meningothelial p = 0.032 | – |
| Kohronen et al., 2006 (26) | 443 | n.s. | n.s. | - | n.s. | - | - | - | n.s. |
| Omulecka et al., 2006 (27) | 64 | – | – | – | s | – | – | p < 0.05 | – |
| Pravdenkova et al., 2006 (13) | 239 | - | - | - | p < 0.00009 | - | - | - | p = 0.002 |
| Maiuri et al., 2007 (10) | 100 | – | – | – | – | – | – | – | p < 0.0001 |
| Taghipour et al., 2007 (28) | 51 | n.s. | p < 0.021 | - | s | - | - | - | - |
| Metellus et al., 2008 (29) | 120 | – | – | – | – | – | – | – | p = 0.0025 |
| Takey et al., 2008 (30) | 57 | - | - | - | p = 0.0419 | n.s. | - | n.s. | - |
| Guevara et al., 2010 (31) | 42 | – | – | – | – | – | – | – | n.s |
| Kandemir et al., 2010 (32) | 53 | - | n.s. | - | n.s. | n.s. | - | n.s. | - |
| Karya et al., 2010 (33) | 59 | – | – | – | b.m. | – | – | – | n.s. |
| Shayanfar et al., 2010 (34) | 78 | - | p < 0.05 | - | p < 0.0001 | p < 0.0001 | - | - | - |
| Abdelzaher et al., 2011 (14) | 60 | – | – | – | b.m. | – | – | – | p = 0.028 |
| Tao et al., 2012 (35) | 102 | - | - | - | - | - | - | - | n.s. |
| Iplikcioglu et al., 2014 (36) | 48 | – | – | – | p = 0.01 | n.s. | p = 0.002 | – | n.s |
| Mukhopadhyay et al. 2017 (37) | 90 | - | - | - | p < 0.001 | - | - | - | - |
| Kuroi et al., 2018 (38) | 161 | – | – | +skull base p=0.00009 | – | – | – | – | – |
| Carvalho et al., 2020 (39) | 96 | - | - | - | b.m. | n.s. | - | - | n.s. |
| Portet et al., 2020 (40) | 90 | – | n.s. | n.s. | n.s. | – | – | n.s. | – |
| Present study | 300 | p = 0.032 | n.s. | lateral s.b and non-s.b. VS medial s.b. and spinal p=0.0036 | p < 0.0001 | p < 0.0001 | - | n.s. | p = 0.0004 |
n.s., not significant; not studied; s.b., skull base; b.m., only benign WHO grade I meningiomas included; s.g, referred as significant but with no statistical data.
Definition of the Progesterone Receptor Expression
The score and cut-off values of PR expression have variably been considered in the reviewed reports. Many studies (11, 13, 17, 21, 26, 31, 39) only report negative or positive expression. Others consider as positive only those cases with strong staining in >10% or moderate staining in >50% (12, 34, 37). Two studies (16, 32) used stratification only for cases with positivity in <50% of the cells, whereas cases with >50% positive cells are considered as a unique group. Only three studies (14, 30, 35) have stratified all cases with different positivity, but the employed cut-off values are different. We have used the semiquantitative scoring scale recommended by the Group for Evaluation of Prognostic Factors using Immunohistochemistry, published in 1999 (16); we have only modified the cut-off of the lower expression (15% instead of 10%). We agree that the definition of negative and positive expression is not sufficient. The stratification of the data must be made for all cases with different positivity. In fact, our study shows significant correlation of PR expression with the WHO grade and recurrence only for cases with high PR expression (>80%).
Progesterone Receptor Expression and Patient Age and Sex
The PR expression of meningiomas in the different age groups is scarcely focused in the literature. Two recent reviews of reported studies on elderly patients do not include data on the PR expression (41, 42). We have found significantly higher rate of PR expression ≥80% in patients aged <70 years (p = 0.032), whereas lower PR values are not correlated. Our results agree with those of Wolfsberger et al. (25); on the other hand, Roser et al. (43) as well as others (8, 17, 18, 21, 22, 26, 28) did not find significant differences between younger and older patients. The discrepancy between our and these studies is likely due to the lesser stratification of the PR values.
The significant correlation between PR expression and patient sex was evidenced in four reviewed studies (17, 19, 28, 34). Others report slightly higher rate of expression in females (14, 22) or in males (25) but with no statistical significance or no relevant sex difference (12, 18, 20–22, 24–26, 32, 40), as in our series. All have considered the overall female group without no relation to the age and the sex female function. We did not find significant differences of PR expression between premenopausal and postmenopausal women. This confirms that the PR expression of meningiomas does not reflect the patient hormonal status.
Progesterone Receptor Expression and Meningioma Location
Seven reviewed studies (12, 17, 18, 22, 24, 25, 40) did not find significant correlation between PR status and tumor location. However, they consider the overall locations in a unique group. In several studies, as discussed in our recent report (35), the meningioma location was found to be correlated with the WHO grade and Ki67 MIB-1 in several studies which report significantly higher rates of WHO II grades and higher values of Ki67 LI in non-skull base meningiomas.
Only Kuroi et al. (38) reported significantly higher rate of positive PR expression in skull base meningiomas as compared to the non-skull base ones. In our series we have first studied the PR expression of medial skull base and lateral skull base meningiomas as distinct groups; our data show that medial skull base meningiomas have significantly higher rate of cases with higher PR expression and significantly lower rate of cases with low expression than lateral skull base ones.
The higher PR expression of medial skull base and spinal meningiomas, together with the lower values of Ki76-LI (44), may suggest an embryological explanation. Two studies (45, 46) have stated that the meninges around the brain stem develop from the cephalic mesoderm and those of the spinal canal from the somatic mesoderm, whereas the telencephalic meninges develop from the neural crest. This may explain the different PR expression levels and pathological features according to the meningioma location.
These different pathological features have some clinical significance. The skull base meningiomas may have different clinical behavior and recurrence rates. The medial skull base group includes locations, such as olfactory groove, tuberculum sellae, and foramen magnum, with more often slow course and lower recurrence rates (0 to 15%) (47, 48); on the other hand, the recurrence rates are higher for lateral skull base meningiomas (35–40%) (49, 50). This agrees with the different PR expression levels of such locations.
Progesterone Receptor Expression and Pathological Findings
The correlation between PR expression and pathological findings of meningiomas has largely been discussed, but the reported results are controversial. Among the 30 reviewed studies ( Table 9 ), six only included benign WHO grade I meningiomas (11, 14, 21, 23, 33, 39); thus the significance of the WHO grade was not possible. Among the 24 studies including all WHO grades, the correlation between PR expression and WHO grade was studied in 16; 12 found significantly higher rate of cases with high PR expression in benign WHO I tumors and low expression in atypical WHO II ones (8, 12, 13, 19, 20, 24, 27, 28, 30, 34, 36, 37) ( Table 9 ). The correlation between PR expression and Ki67 MIB-1 was studied in 10 reviewed series; 5 (12, 19, 22, 24, 34) found significantly lower PR expression in meningiomas with higher Ki67-Li; on the other hand, others (23, 30, 33, 36, 39) did not find significant differences.
The mitotic index was studied in four reports; three of them (8, 24, 36) found significant inverse correlation with the PR expression.
In a recent report (51), we studied the expression of p40, a shorter form of the p53 homolog gene p63, in a series of WHO I and II meningiomas; it was found to be significantly associated with Ki67 LI and recurrence and inversely correlated with the PR expression. All these data confirm that the decrease or loss of the PR expression is associated with histological and biological progression of meningiomas.
The histological subtypes of WHO I meningiomas were studied in 11 reviewed reports; 5 of them (12, 18, 24, 25, 27) have found significantly higher PR expression in the meningothelial ones, with no significant correlations with the other subtypes. In our study the difference of PR expression between the histological subtypes is not significant.
Presurgical information of the PR status of meningiomas, as for other pathological parameters, has recently been obtained with diffusion weighted imaging of magnetic resonance through histogram profiling of apparent diffusion coefficient (ACD) volumes. Skewness and entropy of the ACD are significantly associated with PR expression and Ki 67 LI values (52).
Progesterone Receptor Expression and Recurrence
Intracranial meningiomas are estimated to recur in 10 to 32% of the cases at 10 years (53–55). Fourteen reviewed studies focused on PR expression and recurrence of meningiomas; seven have found significant inverse correlation, with high recurrence rates in meningiomas with low PR expression at initial surgery (10–14, 23, 29). On the other hand, other studies (21, 26, 31, 33, 35, 36, 39) did not find significant results. Like our previous report (10), the present study confirms the inverse correlation between PR values and recurrence (p = 0.0004); the high PR expression (≥80%) is a single efficient predictive factor (p = 0.017).
The meningioma location may influence the recurrence rate. As discussed in our recent report (44), the medial skull base group includes locations, such as tuberculum sellae and olfactory groove meningiomas, at low recurrence rate; on the other hand, the lateral skull base group includes spheno-orbital meningiomas with dural and bone invasion and higher recurrence rate. This different distribution reflects the different possibilities of achieving resections of Simpson grades I and II.
The present study does not include recurrent spinal meningiomas (only one case in the observation period). Spinal meningiomas very rarely show diffuse growth (56) and are known to recur less frequently than intracranial ones, with reported rates ranging from 0 to 18% (57). Two reports (58, 59) have focused on the PR expression in spinal meningiomas and have found variable positivity in high rate of cases. In a recent study (57) we have first investigated the PR expression in recurrent versus non-recurrent tumors, and we did not find significant correlation, with high values in both groups. These data confirm that, differently from intracranial meningiomas, the PR expression is not a predictive factor for spinal meningiomas.
Intracranial meningiomas more often recur at the initial dural site or at the contiguous dural region; however, some patients show multicentric and diffuse recurrences, distant from the initial site, likely from undetected microscopic tumor nodules in distant regions. The reviewed studies which correlate PR expression and recurrence include the overall recurrent tumors, without considering the regrowth pattern. The present study first investigated the PR expression at initial surgery in patients who later experienced local-peripheral versus multicentric-diffuse recurrences; we did not find statistically significant differences of PR expression, although the values of Ki67 LI are significantly higher in meningiomas with multicentric and diffuse recurrences. This finding has not previously been reported.
Conclusion
The higher PR expression in medial skull base meningiomas, the significant correlation with the recurrence time, the lack of difference of PR expression between premenopausal and postmenopausal women and between local-peripheral versus multicentric-diffuse recurrences are the main findings of this study.
The immunohistochemical evaluation of the PR expression must be included in the routine histological study of meningiomas, together with the WHO grade and Ki67 LI. Percentages of the expression must be provided, whereas the definition of positive or negative expression is not sufficient.
The well-defined correlation of the PR status with the WHO grade, Ki67 LI, and recurrence is of prognostic significance. For atypical WHO grade II intracranial meningiomas, the low PR expression is a further risk factor of recurrence with the Ki67 LI. For WHO grade I meningiomas, even without high Ki67-LI, the low values of PR expression must suggest a closer follow-up. However, further biomolecular studies will contribute to stratify the group of patients with low PR expression and those at different recurrence risks.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Martuza RL, MacLaughlin DT, Ojemann RG. Specific Estradiol Binding in Schwannomas, Meningiomas, and Neurofibromas. Neurosurgery (1981) 9(6):665–71. 10.1227/00006123-198112000-00009 [DOI] [PubMed] [Google Scholar]
- 2. Maiuri F, Montagnani S, Gallicchio B. Estrogen and Progesteron Receptors in Meningiomas. Surg Neurol (1986) 26(5):435–40. 10.1016/0090-3019(86)90255-7. ISSN 0090- 3019. [DOI] [PubMed] [Google Scholar]
- 3. Lusis EA, Scheithauer BW, Yachnis AT, Fischer BR, Chicoine MR, Paulus W, et al. Meningiomas in Pregnancy: A Clinicopathologic Study of 17 Cases. Neurosurgery (2012) 71(5):951–61. 10.1227/NEU.0b013e31826adf65 [DOI] [PubMed] [Google Scholar]
- 4. Laviv Y, Bayoumi A, Mahadevan A, Young B, Boone M, Kasper EM. Meningiomas in Pregnancy: Timing of Surgery and Clinical Outcomes as Observed in 104 Cases and Establishment of a Best Management Strategy. Acta Neurochir (Wien) (2018) 160(8):1521–9. 10.1007/s00701-017-3146-8 [DOI] [PubMed] [Google Scholar]
- 5. Qi ZY, Shao C, Huang YL, Hui GZ, Zhou YX, Wang Z. Reproductive and Exogenous Hormone Factors in Relation to Risk of Meningioma in Women: A Meta-Analysis. PLoS One (2013) 8(12):e83261. 10.1371/journal.pone.0083261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harland TA, Freeman JL, Davern M, McCracken DJ, Celano EC, Lillehei K, et al. Progesterone-Only Contraception Is Associated With a Shorter Progression-Free Survival in Premenopausal Women With WHO Grade I Meningioma. J Neurooncol (2017) 136(2):327–33. 10.1007/s11060-017-2656-9 [DOI] [PubMed] [Google Scholar]
- 7. Brandis A, Mirzai S, Tatagiba M, Walter GF, Samii M, Ostertag H. Immunohistochemical Detection of Female Sex Hormone Receptors in Meningiomas: Correlation With Clinical and Histological Features. Neurosurgery (1993) 33(2):212–7. 10.1227/00006123-199308000-00005. discussion 217-8. [DOI] [PubMed] [Google Scholar]
- 8. Hsu DW, Efird JT, Hedley-Whyte ET. Progesterone and Estrogen Receptors in Meningiomas: Prognostic Considerations. J Neurosurg (1997) 86(1):113–20. 10.3171/jns.1997.86.1.0113 [DOI] [PubMed] [Google Scholar]
- 9. Claus EB, Park PJ, Carroll R, Chan J, Black PM. Specific Genes Expressed in Association With Progesterone Receptors in Meningioma. Cancer Res (2008) 1 68(1):314–22. 10.1158/0008-5472.CAN-07-1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maiuri F, De Caro MB, Esposito F, Cappabianca P, Strazzullo V, Pettinato G, et al. Recurrences of Meningiomas: Predictive Value of Pathological Features and Hormonal and Growth Factors. J Neurooncol (2007) 82:63–8. 10.1007/s11060-005-9078-9 [DOI] [PubMed] [Google Scholar]
- 11. Fewings PE, Battersby RD, Timperley WR. Long-Term Follow Up of Progesterone Receptor Status in Benign Meningioma: A Prognostic Indicator of Recurrence? J Neurosurg (2000) 92(3):401–5. 10.3171/jns.2000.92.3.0401 [DOI] [PubMed] [Google Scholar]
- 12. Roser F, Nakamura M, Bellinzona M, Rosahl SK, Ostertag H, Samii M. The Prognostic Value of Progesterone Receptor Status in Meningiomas. J Clin Pathol (2004) 57(10):1033–7. 10.1136/jcp.2004.018333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pravdenkova S, Al-Mefty O, Sawyer J, Husain M. Progesterone and Estrogen Receptors: Opposing Prognostic Indicators in Meningiomas. J Neurosurg (2006) 105(2):163–73. 10.3171/jns.2006.105.2.163 [DOI] [PubMed] [Google Scholar]
- 14. Abdelzaher E, El-Gendi SM, Yehya A, Gowil AG. Recurrence of Benign Meningiomas: Predictive Value of Proliferative Index, BCL2, P53, Hormonal Receptors and HER2 Expression. Br J Neurosurg (2011) 25:707–13. 10.3109/02688697.2010.522743 [DOI] [PubMed] [Google Scholar]
- 15. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol (2007) 114(2):97–109. 10.1007/s00401-007-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Recommendations for the Immunohistochemistry of the Hormonal Receptors on Paraffin Sections in Breast Cancer. Update 1999. Group for Evaluation of Prognostic Factors Using Immunohistochemistry in Breast Cancer (GEFPICS-FNCLCC). Ann Pathol (1999) 19(4):336–43. [PubMed] [Google Scholar]
- 17. Magdelenat H, Pertuiset BF, Poisson M, Martin PM, Philippon J, Pertuiset B, et al. Progestin and Oestrogen Receptors in Meningiomas. Biochemical Characterization, Clinical and Pathological Correlations in 42 Cases. Acta Neurochir (Wien) (1982) 64(3-4):199–213. 10.1007/BF01406053 [DOI] [PubMed] [Google Scholar]
- 18. Markwalder TM, Zava DT, Goldhirsch A, Markwalder RV. Estrogen and Progesterone Receptors in Meningiomas in Relation to Clinical and Pathologic Features. Surg Neurol (1983) 20(1):42–7. 10.1016/0090-3019(83)90104-0 [DOI] [PubMed] [Google Scholar]
- 19. Nagashima G, Aoyagi M, Wakimoto H, Tamaki M, Ohno K, Hirakawa K. Immunohistochemical Detection of Progesterone Receptors and the Correlation With Ki-67 Labeling Indices in Paraffin-Embedded Sections of Meningiomas. Neurosurgery (1995) 37:478–82. 10.1227/00006123-199509000-00016. discussion 483. [DOI] [PubMed] [Google Scholar]
- 20. Perry A, Cai DX, Scheithauer BW, Swanson PE, Lohse CM, Newsham IF, et al. Merlin, DAL-1, and Progesterone Receptor Expression in Clinicopathologic Subsets of Meningioma: A Correlative Immunohistochemical Study of 175 Cases. J Neuropathol Exp Neurol (2000) 59(10):872–9. 10.1093/jnen/59.10.872 [DOI] [PubMed] [Google Scholar]
- 21. Das A, Tan WL, Teo J, Smith DR. Overexpression of Mdm2 and P53 and Association With Progesterone Receptor Expression in Benign Meningiomas. Neuropathology (2002) 22:194–9. 10.1046/j.1440-1789.2002.00443.x [DOI] [PubMed] [Google Scholar]
- 22. Gursan N, Gundogdu C, Albayrak A, Kabalar ME. Immunohistochemical Detection of Progesterone Receptors and the Correlation With Ki-67 Labeling Indices in Paraffin-Embedded Sections of Meningiomas. Int J Neurosci (2002) 112(4):463–70. 10.1080/00207450290025581 [DOI] [PubMed] [Google Scholar]
- 23. Strik HM, Strobelt I, Pietsch-Breitfeld B, Iglesias-Rozas JR, Will B, Meyermann R. The Impact of Progesterone Receptor Expression on Relapse in the Long-Term Clinical Course of 93 Benign Meningiomas. In Vivo (2002) 16(4):265–70. [PubMed] [Google Scholar]
- 24. Konstantinidou AE, Korkolopoulou P, Mahera H, Kotsiakis X, Hranioti S, Eftychiadis C, et al. Hormone Receptors in Non-Malignant Meningiomas Correlate With Apoptosis, Cell Proliferation and Recurrence-Free Survival. Histopathology (2003) 43(3):280–90. 10.1046/j.1365-2559.2003.01712.x [DOI] [PubMed] [Google Scholar]
- 25. Wolfsberger S, Doostkam S, Boecher-Schwarz HG, Roessler K, van Trotsenburg M, Hainfellner JA, et al. Progesterone-Receptor Index in Meningiomas: Correlation With Clinico-Pathological Parameters and Review of the Literature. Neurosurg Rev (2004) 27(4):238–45. 10.1007/s10143-004-0340-y [DOI] [PubMed] [Google Scholar]
- 26. Korhonen K, Salminen T, Raitanen J, Auvinen A, Isola J, Haapasalo H. Female Predominance in Meningiomas can Not be Explained by Differences in Progesterone, Estrogen, or Androgen Receptor Expression. J Neurooncol (2006) 80(1):1–7. 10.1007/s11060-006-9146-9 [DOI] [PubMed] [Google Scholar]
- 27. Omulecka A, Papierz W, Nawrocka-Kunecka A, Lewy-Trenda I. Immunohistochemical Expression of Progesterone and Estrogen Receptors in Meningiomas. Folia Neuropathol (2006) 44(2):111–5. [PubMed] [Google Scholar]
- 28. Taghipour M, Rakei SM, Monabati A, Nahavandi-Nejad M. The Role of Estrogen and Progesterone Receptors in Grading of the Malignancy of Meningioma. Iran Red Crescent Med J (2007) 9:17–21. [Google Scholar]
- 29. Metellus P, Nanni I, Dussert C, Trinkhaus M, Fuentes S, Chinot O, et al. Prognostic Implications of Biologic Markers in Intracranial Meningiomas: 120 Cases. Neurochirurgie (2008) 54:750–6. 10.1016/j.neuchi.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 30. Takei H, Buckleair LW, Powell SZ. Immunohistochemical Expression of Apoptosis Regulating Proteins and Sex Hormone Receptors in Meningiomas. Neuropathology (2008) 28(1):62–8. 10.1111/j.1440-1789.2007.00852.x [DOI] [PubMed] [Google Scholar]
- 31. Guevara P, Escobar-Arriaga E, Saavedra-Perez D, Martinez-Rumayor A, Flores-Estrada D, Rembao D, et al. Angiogenesis and Expression of Estrogen and Progesterone Receptors as Predictive Factors for Recurrence of Meningioma. J Neurooncol (2010) 98(3):379–84. 10.1007/s11060-009-0086-z [DOI] [PubMed] [Google Scholar]
- 32. Kandemir NO, Ege Gül A, Doğan Gün B, Karadayi N, Yurdakan G, Özdamar SO. Her- 2/Neu, Estrogen and Progesterone Receptor Expression In WHO Grade I Meningiomas. Trakya Univ Tip Fak Derg (2010) 27(3):292–6. 10.5174/tutfd.2009.01534.1 [DOI] [Google Scholar]
- 33. Kärjä V, Sandell PJ, Kauppinen T, Alafuzoff I. Does Protein Expression Predict Recurrence of Benign World Health Organization Grade I Meningioma? Hum Pathol (2010) 41(2):199–207. 10.1016/j.humpath.2009.06.020 [DOI] [PubMed] [Google Scholar]
- 34. Shayanfar N, Mashayekh M, Mohammadpour M. Expression of Progestrone Receptor and Proliferative Marker Ki 67 in Various Grades of Meningioma. Acta Med Iran (2010) 48(3):142–7. [PubMed] [Google Scholar]
- 35. Tao Y, Liang G, Li Z, Wang Y, Wu A, Wang H, et al. Clinical Features and Immunohistochemical Expression Levels of Androgen, Estrogen, Progesterone and Ki-67 Receptors in Relationship With Gross-Total Resected Meningiomas Relapse. Br J Neurosurg (2012) 26:700–4. 10.3109/02688697.2012.685780 [DOI] [PubMed] [Google Scholar]
- 36. Iplikcioglu AC, Hatiboglu MA, Ozek E, Ozcan D. Is Progesteron Receptor Status Really a Prognostic Factor for Intracranial Meningiomas? Clin Neurol Neurosurg (2014) 124:119–22. 10.1016/j.clineuro.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 37. Mukhopadhyay M, Das C, Kumari M, Sen A, Mukhopadhyay B, Mukhopadhyay B. Spectrum of Meningioma With Special Reference to Prognostic Utility of ER, PR and Ki67 Expression. J Lab Physicians (2017) 9:308–13. 10.4103/JLP.JLP_158_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuroi Y, Matsumoto K, Shibuya M, Kasuya H. Progesterone Receptor Is Responsible for Benign Biology of Skull Base Meningioma. World Neurosurg (2018) 118:e918–24. 10.1016/j.wneu.2018.07.100 [DOI] [PubMed] [Google Scholar]
- 39. de Carvalho GTC, da Silva-Martins WC, de Magalhães KCSF, Nunes CB, Soares AN, Tafuri LSA, et al. Recurrence/Regrowth in Grade I Meningioma: How to Predict? Front Oncol (2020) 10:1144. 10.3389/fonc.2020.01144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Portet S, Banor T, Bousquet J, Simonneau A, Flores M, Ingrand P, et al. New Insights Into Expression of Hormonal Receptors by Meningiomas. World Neurosurg (2020) 140:e87–96. 10.1016/j.wneu.2020.04.168 [DOI] [PubMed] [Google Scholar]
- 41. Delgado-Fernández J, García-Pallero MA, Gil-Simoes R, Blasco G, Frade-Porto N, Pulido P, et al. Validation of Grading Scores and Outcome Prognostic Factors in Intracranial Meningiomas in Elderly Patients. World Neurosurg (2018) 114:e1057–65. 10.1016/j.wneu.2018.03.146 [DOI] [PubMed] [Google Scholar]
- 42. Ekşi MŞ, Canbolat Ç, Akbaş A, Özmen BB, Akpınar E, Usseli Mİ, et al. Elderly Patients With Intracranial Meningioma: Surgical Considerations in 228 Patients With a Comprehensive Analysis of the Literature. World Neurosurg (2019) 132:e350–65. 10.1016/j.wneu.2019.08.150 [DOI] [PubMed] [Google Scholar]
- 43. Roser F, Nakamura M, Ritz R, Bellinzona M, Dietz K, Samii M, et al. Proliferation and Progesterone Receptor Status in Benign Meningiomas Are Not Age Dependent. Cancer (2005) 104:598–601. 10.1002/cncr.21192 [DOI] [PubMed] [Google Scholar]
- 44. Maiuri F, Mariniello G, Guadagno E, Barbato M, Corvino S, Del Basso De Caro M. WHO Grade, Proliferation Index, and Progesterone Receptor Expression Are Different According to the Location of Meningioma. Acta Neurochir (Wien) (2019) 161:2553–61. 10.1007/s00701-019-04084-z [DOI] [PubMed] [Google Scholar]
- 45. O'Rahilly R, Müller F. The Meninges in Human Development. J Neuropathol Exp Neurol (1986) 45(5):588–608. 10.1097/00005072-198609000-00008 [DOI] [PubMed] [Google Scholar]
- 46. Catala M. Embryonic and Fetal Development of Structures Associated With the Cerebro-Spinal Fluid in Man and Other Species. Part I: The Ventricular System, Meninges and Choroid Plexuses. Arch Anat Cytol Pathol (1998) 46(3):153–69. [PubMed] [Google Scholar]
- 47. Nanda A, Vannemreddy P. Recurrence and Outcome in Skull Base Meningiomas: Do They Differ From Other Intracranial Meningiomas? Skull Base (2008) 18:243–52. 10.1055/s-2007-1016956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lam Shin Cheung V, Kim A, Sahgal A, Das S. Meningioma Recurrence Rates Following Treatment: A Systematic Analysis. J Neurooncol (2018) 136(2):351–61. 10.1007/s11060-017-2659-6 [DOI] [PubMed] [Google Scholar]
- 49. Maroon JC, Kennerdell JS, Vidovich DV, Abla A, Sternau L. Recurrent Spheno-Orbital Meningioma. J Neurosurg (1994) 80(2):202–8. 10.3171/jns.1994.80.2.0202 [DOI] [PubMed] [Google Scholar]
- 50. Mariniello G, Maiuri F, Strianese D, Donzelli R, Iuliano A, Tranfa F, et al. Spheno-Orbital Meningiomas: Surgical Approaches and Outcome According to the Intraorbital Tumor Extent. Zentralbl Neurochir (2008) 69:175–81. 10.1055/s-2008-1077077 [DOI] [PubMed] [Google Scholar]
- 51. Guadagno E, Del Basso De Caro M, Pignatiello S, Sciammarella C, Solari D, Cappabianca P, et al. Expression of P40 (δNp63) Protein in Meningiomas, an Unexpected Finding: Immunohistochemical Study and Evaluation of Its Possible Prognostic Role. J Neurooncol (2016) 129:405–13. 10.1007/s11060-016-2205-y [DOI] [PubMed] [Google Scholar]
- 52. Gihr GA, Horvath-Rizea D, Garnov N, Kohlhof-Meinecke P, Ganslandt O, Henkes H, et al. Diffusion Profiling via a Histogram Approach Distinguishes Low-Grade From High-Grade Meningiomas, Can Reflect the Respective Proliferative Potential and Progesterone Receptor Status. Mol Imaging Biol (2018) 20:632–40. 10.1007/s11307-018-1166-2 [DOI] [PubMed] [Google Scholar]
- 53. Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL. Meningioma: Analysis of Recurrence and Progression Following Neurosurgical Resection. J Neurosurg (1985) 62:18–24. 10.3171/jns.1985.62.1.0018 [DOI] [PubMed] [Google Scholar]
- 54. Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjani S, Martuza RL, et al. Barker Long-Term Recurrence Rates of Atypical Meningiomas After Gross Total Resection With or Without Postoperative Adjuvant Radiation. Neurosurgery (2009) 64(1):56–60. 10.1227/01.NEU.0000330399.55586.63 [DOI] [PubMed] [Google Scholar]
- 55. Nakasu S, Fukami T, Jito J, Nozaki K. Recurrence and Regrowth of Benign Meningiomas. Brain Tumor Pathol (2009) 26:69–72. 10.1007/s10014-009-0251-2 [DOI] [PubMed] [Google Scholar]
- 56. Mariniello G, Briganti F, De Caro ML, Maiuri F. Cervical Extradural "En-Plaque" Meningioma. J Neurol Surg A Cent Eur Neurosurg (2012) 73(5):330–3. 10.1055/s-0032-1304222 [DOI] [PubMed] [Google Scholar]
- 57. Maiuri F, Del Basso De Caro M, de Divitiis O, Guadagno E, Mariniello G. Recurrence of Spinal Meningiomas: Analysis of the Risk Factors. Br J Neurosurg (2019) 10:1–6. 10.1080/02688697.2019.1638886 [DOI] [PubMed] [Google Scholar]
- 58. Roser F, Nakamura M, Bellinzona M, Ritz R, Ostertag H, Tatagiba MS. Proliferation Potential of Spinal Meningiomas. Eur Spine J (2006) 15:211–5. 10.1007/s00586-005-0937-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barresi V, Alafaci C, Caffo M, Barresi G, Tuccari G. Clinicopathological Characteristich, Hormone Receptor Status and Matrix Metallo-Proteinase-9 (MMP-)) Immunoistochemical Expression in Spinal Meningiomas. Pathol Res Pract (2012) 208:350–5. 10.1016/j.prp.2012.02.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


