Abstract
Background
Aging increases the risk of tuberculosis (TB) and its adverse outcomes, but most studies are based on secondary analyses, and few are in Hispanics. Diabetes is a risk factor for TB in adults, but its contribution in the elderly is unknown. We aimed to identify the role of diabetes and other risk factors for TB in elderly Hispanics.
Methods
Cross-sectional study among newly-diagnosed TB patients, recent contacts (ReC), or community controls (CoC) totaling 646 participants, including 183 elderly (>60 years; 43 TB, 80 ReC, 60 CoC) and 463 adults (18 to 50 years; 80 TB, 301 ReC and 82 CoC). Host characteristics associated with TB and latent Mycobacterium tuberculosis infection (LTBI) were identified in the elderly by univariable and confirmed by multivariable logistic regression.
Results
LTBI was more prevalent among the elderly CoC (55% vs. 23.2% in adults; p<0.001), but not in ReC (elderly 71.3% vs. adult 63.8%); p = 0.213). Risk factors for TB in the elderly included male sex (adj-OR 4.33, 95% CI 1.76, 10.65), smoking (adj-OR 2.55, 95% CI 1.01, 6.45) and low BMI (adj-OR 12.34, 95% CI 4.44, 34.33). Unexpectedly, type 2 diabetes was not associated with TB despite its high prevalence (adj-OR 0.38, 95% CI 0.06, 2.38), and BCG vaccination at birth was protective (adj-OR 0.16, 95% CI 0.06, 0.45).
Conclusions
We report novel distinctions in TB risk factors in the elderly vs. adults, notably in diabetes and BCG vaccination at birth. Further studies are warranted to address disparities in this vulnerable, understudied population.
Introduction
Aging is associated with immune function decline consequent to cellular immunosenescence and inflammaging, and is identified as a risk factor for respiratory tract infections such as tuberculosis (TB) [1, 2]. TB caused an estimated 1.4 million deaths and 10 million new cases in 2019. Approximately one-fourth of the world’s population has latent Mycobacterium tuberculosis infection (LTBI) [3]. The relative risk of TB in the elderly is 1.5-fold higher than in adults (21 to 64 years old), and the risk of mortality is ≥ 5-fold higher, with rates ranging from 20 to 30% [4–6]. Elderly patients are difficult to diagnose due to the lack of classical symptoms and to impaired or reduced responses to diagnostics such as the tuberculin skin test. This leads to frequent delays in treatment initiation and in some cases to post-mortem diagnosis [7–9].
Most studies in older people with TB are based on secondary analysis of data [10–12]. These studies have identified risk factors for TB in the elderly, including aging itself, male sex, smoking, and malnutrition, undernutrition or low body mass index (BMI) [11]. Although diabetes increases the risk of TB by 3-fold among adults [13], the link between TB and diabetes in the elderly has not been formally studied. Instead, association studies among all adults suggest a stronger link to diabetes among middle-aged vs. older adults (e.g. ≥ 60 years) [14–17]. Our prior studies among Hispanics in the states of Tamaulipas (Mexico) and Texas (US) identified diabetes as a major contributor to TB, with a 25% population attributable risk fraction [17], but elderly individuals were mostly excluded [18].
Here we sought to identify risk factors for LTBI or TB among the elderly in a Hispanic community across the US-Mexico border [19]. We conducted a cross-sectional study with enrollment of newly-diagnosed TB patients, recent contacts (ReC), and community controls (CoC) among elderly individuals (>60 years), and used adults (18 to 50 years old) as reference. We found shared risk factors for TB between the elderly and adult populations, but most importantly, we identified unique aspects in the elderly, most notably the lack of association between TB and diabetes and a potential protective role of BCG vaccination at birth.
Methods
Ethical statement
This study was approved by the institutional review boards in Mexico (SST/SCAME/DCES/597/2017, Secretaría de Salud de Tamaulipas) and the United States (HSC-SPH-17-0990, University of Texas Health Houston), and all participants signed an informed consent.
Participant enrollment and characteristics
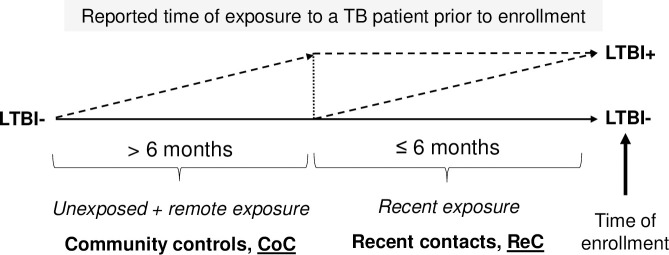
Adults (18 to 50 years) and elderly (>60 years) participants were enrolled at pulmonary and community clinics in South Texas and northern Tamaulipas, Mexico, between 2017 and 2020. Participants included newly-diagnosed active pulmonary TB patients enrolled prior to or within one week of TB treatment (TB), recent TB contacts (ReC), and community controls (CoC) (Fig 1). TB diagnosis was based on isolation of M. tuberculosis or positive sputum smear (laboratory diagnosis), or by clinical symptoms consistent with pulmonary TB and an abnormal chest X-ray (clinical TB) [20]. ReC were defined by exposure for ≥ 5 h to an active TB case ≤ 6 months prior to enrollment. CoC were enrolled in the community and reported no previous exposure or a remote exposure > 6 months before enrollment (Fig 2). LTBI was assessed by Interferon Gamma Release Assays (IGRA) [QuantiFERON-Gold-in-tube or QuantiFERON-plus (Qiagen, Germantown, MD) or T-SPOT.TB (Oxford Immunotec, Oxford)]. Socio-demographic information was recorded at enrollment as described previously [21]. Briefly, macrovascular and microvascular diseases, use of diabetes medications in the past month, or taking non-steroidal anti-inflammatory drugs (NSAIDs) in the past week was based on self-reporting. Excessive alcohol intake was based on a validated questionnaire [22]. Drug abuse was self-reported as daily or weekly use of recreational injectable or non-injectable drugs. Diabetes was based on hyperglycemia (fasting ≥126 mg/dL in most cases, or random ≥200 mg/dL), chronic hyperglycemia (HbA1c ≥6.5%), or self-reported diagnosis [23]. Prediabetes was based on HbA1c between 5.7% and 6.4% or fasting glucose between 110 and 125 mg/dL. HIV infection was based on self-reporting in non-TB cases, or blood test confirmed in TB patients. Total cholesterol, HDL cholesterol and triglycerides were determined using Lipid Panel Test Strips (PTS Diagnostics), and low-density cholesterol (cLDL) was calculated [24].
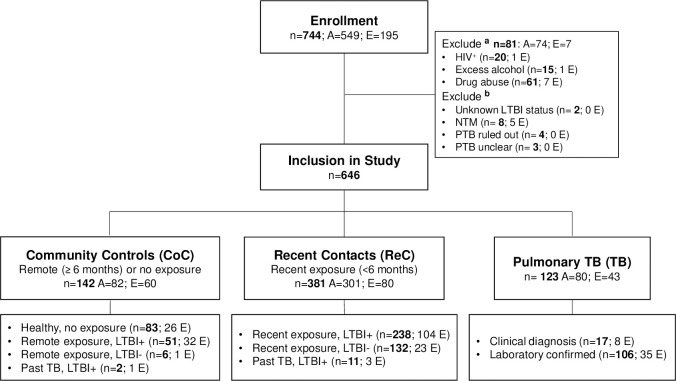
Fig 1. Enrollment algorithm.
Adults (A) and elderly (E) individuals identified in the community (Community controls, CoC), or in pulmonary clinics as newly-diagnosed pulmonary TB patients (TB), or contacts of TB cases with reported exposure ≤6 months before enrollment (Recent contacts, ReC), were invited to participate. TB diagnosis was based on laboratory findings (positive culture and/or acid-fast sputum smear) or clinical criteria based on physician diagnosis and abnormal chest X-ray. LTBI, Latent TB infection. aExclusion based on HIV infection, excessive alcohol use, and drug abuse, with some having more than one of these conditions. bExclusion due to unknown LTBI status, non-tuberculosis mycobacteria (NTM) or pulmonary TB diagnosis ruled out/unclear.
Fig 2. Definitions for community controls and recent contacts.
Community controls were identified in the community (not in pulmonary clinics) and reported no previous exposure or a remote exposure to a pulmonary TB patient >6 months before enrollment. Recent contacts reported exposure to an active TB case ≤6 months before the time of enrollment. Community controls and recent contacts with at least one positive IGRA assay were classified as LTBI+, and when both negative, then as LTBI-.
Data analysis
The study design followed the STROBE guidelines [25]. Data were entered into Microsoft Access and exported into SAS version 9.4 (Cary, NC) for quality control and analysis. Additional analyses were performed using GraphPad Prism version 9.0 (La Jolla, CA). Categorical variables were compared with the Chi-square or Fisher’s exact test. Continuous variables were analyzed for differences in medians using Wilcoxon test or Kruskal-Wallis with Dunn’s multiple comparisons test for > 2 groups. For multivariable logistic regression models, variables were selected based on a p value ≤ 0.099 or biological relevance. Given the low incidence of TB in our study population, we report odds ratios and refer to them as risk factors given their close approximation to risk ratios [17]. Significance was set as p ≤ 0.050 and marginal significance at p ≤ 0.099.
Results
Participant enrollment and characteristics
A total of 744 participants were enrolled (549 adults and 195 elderly). Adults that had a higher prevalence of HIV, excessive alcohol consumption or drug abuse, were excluded given our primary interest in identifying risk factors for TB in the elderly (Fig 1). We further excluded TB suspects with non-tuberculous mycobacteria, unclear or ruled out TB, or ReC with unknown LTBI status. The remaining 646 participants comprised 123 new-diagnosed pulmonary TB (43 elderly and 80 adults), 381 ReC (80 elderly and 301 adults), and 142 CoC (60 elderly and 82 adults; Fig 1). More than half of the participants were females (62.8%) and 72.9% married. Only 39% had an education beyond middle school, 74.8% had health insurance, 71.2% were non-smokers with only 12.6% current smokers. Two-thirds were obese or overweight (66.1%), 71.9% had central obesity, and 28.9% had diabetes (98% type 2 diabetes). Most were BCG vaccinated (88.5%) at birth, and only 2.5% had a history of TB (Table 1).
Table 1. Characteristics of the elderly vs. adults, by TB study groups.
| All | Non-TB | TB | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adults | Elderly | Adults | Elderly | |||||||||
| n | % or | n | % or | n | % or median (IQR) | p value | n | % or median (IQR) | n | % or median (IQR) | p value | |
| median (IQR) | median (IQR) | |||||||||||
| Sociodemographics | ||||||||||||
| Age, in years | 646 | 43.0 (28.0) | 383 | 38 (14) | 140 | 67 (10.5) | <0.001 | 80 | 37.0 (21.0) | 43 | 68.0 (11.0) | <0.001 |
| Male sex | 240 | 37.2% | 126 | 32.9% | 38 | 27.1% | 0.209 | 45 | 56.3% | 31 | 72.1% | 0.085 |
| Marital status | <0.001 | 0.003 | ||||||||||
| Never married | 104 | 16.1% | 68 | 17.8% | 7 | 5.0% | 21 | 26.3% | 8 | 18.6% | ||
| Ever married * | 471 | 72.9% | 306 | 79.9% | 84 | 60.0% | 56 | 70.0% | 25 | 58.1% | ||
| Widowed | 71 | 11.0% | 9 | 2.3% | 49 | 35.0% | 3 | 3.8% | 10 | 23.3% | ||
| Smoking | 0.716 | 0.099 | ||||||||||
| Never | 459 | 71.2% | 284 | 74.2% | 106 | 75.7% | 49 | 62.0% | 20 | 46.5% | ||
| Past or Current | 186 | 28.8% | 99 | 25.8% | 34 | 24.3% | 30 | 38.0% | 23 | 53.5% | ||
| Smoking—pack per year | 644 | 0.01 (0.40) | 383 | 0 (0.1) | 140 | 0 (0.0) | 0.697 | 79 | 0.01 (1.50) | 42 | 0.55 (5.10) | 0.017 |
| Socioeconomic indicators | ||||||||||||
| Highest education | <0.001 | <0.001 | ||||||||||
| Up to Middle School | 394 | 61.0% | 197 | 51.4% | 113 | 80.7% | 46 | 57.5% | 38 | 88.4% | ||
| High School or College | 252 | 39.0% | 186 | 48.6% | 27 | 19.3% | 34 | 42.5% | 5 | 11.6% | ||
| Health insurance | 482 | 74.8% | 269 | 70.2% | 116 | 82.9% | 0.004 | 66 | 82.5% | 31 | 73.8% | 0.259 |
| Household family size | 566 | 4.0 (2.0) | 331 | 4.0 (2.0) | 119 | 3.0 (3.0) | <0.001 | 81 | 4.0 (2.0) | 38 | 3.0 (4.0) | 0.228 |
| Health conditions | ||||||||||||
| Diabetes (2 categories) | 187 | 28.9% | 69 | 18.0% | 66 | 47.1% | <0.001 | 31 | 38.8% | 21 | 48.8% | 0.280 |
| Diabetes (3 categories) | <0.001 | <0.001 | ||||||||||
| No diabetes | 311 | 48.1% | 233 | 60.8% | 32 | 22.9% | 39 | 48.8% | 7 | 16.3% | ||
| Pre-diabetes | 148 | 22.9% | 81 | 21.1% | 42 | 30.0% | 10 | 12.5% | 15 | 34.9% | ||
| Diabetes | 187 | 28.9% | 69 | 18.0% | 66 | 47.1% | 31 | 38.8% | 21 | 48.8% | ||
| Obesity, BMI | 646 | 27.2 (7.5) | 383 | 28.6 (7.5) | 140 | 27.8 (6.9) | 0.266 | 80 | 22.2 (7.0) | 43 | 21.7 (5.5) | 0.443 |
| Obesity, BMI categories | 0.148 | 0.125 | ||||||||||
| Under/Normal (<24.9) | 219 | 33.9% | 86 | 22.5% | 40 | 28.6% | 57 | 71.3% | 36 | 83.7% | ||
| Over/Obese (≥25) | 427 | 66.1% | 297 | 77.5% | 100 | 71.4% | 23 | 28.8% | 7 | 16.3% | ||
| WHR (M ≥ 0.90; F ≥0.86) | 454 | 71.9% | 284 | 74.2% | 111 | 79.3% | 0.399 | 37 | 47.4% | 22 | 52.4% | 0.078 |
| Macrovascular diseases | 153 | 23.7% | 51 | 13.3% | 80 | 57.1% | <0.001 | 7 | 8.4% | 15 | 34.9% | <0.001 |
| Microvascular diseases | 169 | 26.2% | 68 | 17.8% | 65 | 46.4% | <0.001 | 20 | 24.1% | 18 | 41.9% | 0.040 |
| NSAIDs in past week | 128 | 23.3% | 48 | 12.5% | 40 | 28.6% | <0.001 | 24 | 31.2% | 16 | 42.1% | 0.247 |
| TB-related conditions | ||||||||||||
| Past TB | 13 | 2.5% | 9 | 2.3% | 4 | 2.9% | 0.742 | 10 | 12.1% | 4 | 9.3% | 0.642 |
| Latent TB infection ** | 211 | 55.1% | 212 | 55.4% | 91 | 65.0% | 0.048 | N/A | N/A | |||
| BCG vaccine | 570 | 88.5% | 341 | 89.0% | 131 | 93.6% | 0.158 | 70 | 87.5% | 28 | 65.1% | 0.003 |
Data expressed as column % for categorical variables or median (interquartile range, IQR) for continuous; Normal range values for each parameter shown in parenthesis
* Ever married includes married, cohabitation, divorced or separated; NSAIDs, non-steroidal anti-inflammatory drugs; WHR, waist-hip ratio or Central obesity; M, Male; F, Female; p values ≤ 0.099 shown in bold
** Latent TB infection based on a positive T-Spot.TB or QuantiFERON assay, LTBI only evaluated in non-TB study groups; BMI, Body-mass index
Age-associated characteristics amongst non-TB or TB participants
We first sought to identify host characteristics that distinguished elderly vs. adult groups, given their higher risk of active TB, or adverse TB outcomes [11, 12, 26]. When analyzing the non-TB groups (CoC and ReC groups), we found that most of the characteristics that distinguished the elderly from adults were similar (S1 Table). Therefore, for most analysis we merged both groups into one ‘non-TB category’. An exception was the analysis related to LTBI given its higher prevalence in the elderly vs. adults among CoCs (E 55.0% vs. A 23.2%; p <0.001), but similarity in ReCs (E 64.1% vs. A 71.3%; p 0.233; S1 Fig; S1 Table).
Among the non-TB participants, the following features distinguished the elderly vs. adults (Table 1; S1 and S2 Figs). Regarding socio-demographics, the elderly had differences in marital status (mostly widowed; p <0.001) and socioeconomic indicators [lower education (p <0.001) and smaller family size (p <0.001), but higher frequency of health insurance (p = 0.004)]. Regarding health conditions, the elderly had a higher prevalence of diabetes and pre-diabetes (p <0.001), LTBI (p 0.048), macrovascular and microvascular diseases (p <0.001), and use of anti-inflammatory medications (p <0.001). Glucose-related measurements (Table 2) were higher (hyperglycemia and HbA1c; p <0.001) which is consistent with their diabetes status. Lipid profiles (total, LDL and HDL cholesterols and triglycerides) were also higher in the elderly (Table 2). Regarding hematology indices, the elderly had higher eosinophils (p = 0.045) but lower platelets (p <0.001) and hemoglobin levels (p 0.047; S2 Table).
Table 2. Lipid and glucose profiles in elderly vs. adults, by TB status.
| Non-TB | TB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adults | Elderly | Adults | Elderly | |||||||
| n | % | n | % | p valuea | n | % | n | % | p value | |
| Lipid profiles, (mg/dl)b | ||||||||||
| High cholesterol (200) | 48 | 12.6% | 29 | 20.9% | 0.020 | 5 | 6.3% | 1 | 2.3% | 0.335 |
| Low HDL (40 M, 50 F) | 287 | 75.1% | 90 | 64.7% | 0.019 | 58 | 72.5% | 33 | 76.7% | 0.609 |
| High LDL (100) | 144 | 38.0% | 65 | 46.8% | 0.072 | 15 | 18.8% | 6 | 14.0% | 0.500 |
| High Triglycerides (150) | 97 | 25.3% | 46 | 32.9% | 0.087 | 6 | 7.5% | 3 | 7.0% | 0.915 |
| Glucose-related measurements | ||||||||||
| Glycemia | <0.001 | 0.153 | ||||||||
| Normoglycemia | 295 | 78.0% | 77 | 55.4% | 45 | 57.0% | 32 | 74.4% | ||
| Impaired Fasting Glucose | 28 | 7.4% | 22 | 15.8% | 8 | 10.1% | 2 | 4.7% | ||
| Hyperglycemia | 55 | 14.6% | 40 | 28.8% | 26 | 32.9% | 9 | 20.9% | ||
| Chronic hyperglycemia, HbA1c | <0.001 | 0.144 | ||||||||
| Normal (<5.7%) | 235 | 61.4% | 36 | 25.7% | 39 | 48.8% | 8 | 18.6% | ||
| Pre-diabetes (5.7–6.4%) | 89 | 23.2% | 56 | 40.0% | 11 | 13.8% | 18 | 41.9% | ||
| Chronic hyperglycemia (≥ 6.5%) | 59 | 15.4% | 48 | 34.3% | 30 | 37.5% | 17 | 39.5% | ||
a p values ≤ 0.099 shown in bold
b Normal range values for each parameter shown in parenthesis. Abbreviations: M, Male; F, Female.
Among TB patients, the following characteristics were significantly different between the elderly and adults (Table 1 and S1 Fig). Regarding sociodemographics, there was a higher prevalence of males (p = 0.085), widowed status (p = 0.003) and smoking-packs per year (p = 0.017) in the elderly. Regarding socioeconomics, the elderly had a lower education (p <0.001). Regarding health-related conditions, the elderly had more pre-diabetes or diabetes (p <0.001), macrovascular (p <0.001) and microvascular (p = 0.040) diseases, and lower prevalence of BCG vaccination (p = 0.003). Regarding lipid- and glucose-related measurements, there were no differences (Table 2). Regarding hematology indices, the ratios of neutrophils/lymphocytes and monocytes/lymphocytes were higher in the elderly (S2Table and S2 Fig).
Altogether, we found that known risk factors for TB in adults, such as lower education and dysglycemias, were significantly higher in the elderly vs. adults with or without TB [27, 28]. In the elderly, pre-diabetes and diabetes accounted for an overall 76.6% in non-TB and 83.7% in TB cases. Given the high prevalence of dysglycemias, we further examined if these were associated with TB status in our cohort.
Risk factors for TB in the elderly
In order to determine if diabetes or other host characteristics are risk factors for TB in our elderly Hispanic cohort, we compared the elderly who had TB vs. those who did not (S3 Table and S3 Fig). For diabetes and related conditions, the elderly TB participants had lower prevalence of impaired fasting glucose (4.7 vs. 15.7%) or hyperglycemia (20.9 vs. 28.6%; p = 0.058). Regarding obesity and lipid profiles, the elderly with TB vs. no TB, had a higher prevalence of lower BMI and low central obesity (p <0.001), lower total cholesterol (p = 0.004), LDL cholesterol (p <0.001) and triglycerides (p <0.001). Regarding socio-demographics, the elderly with TB had a higher proportion of males or smoking index (p <0.001). The elderly TB participants had a lower prevalence of BCG vaccination (p <0.001) or macrovascular diseases (p = 0.011).
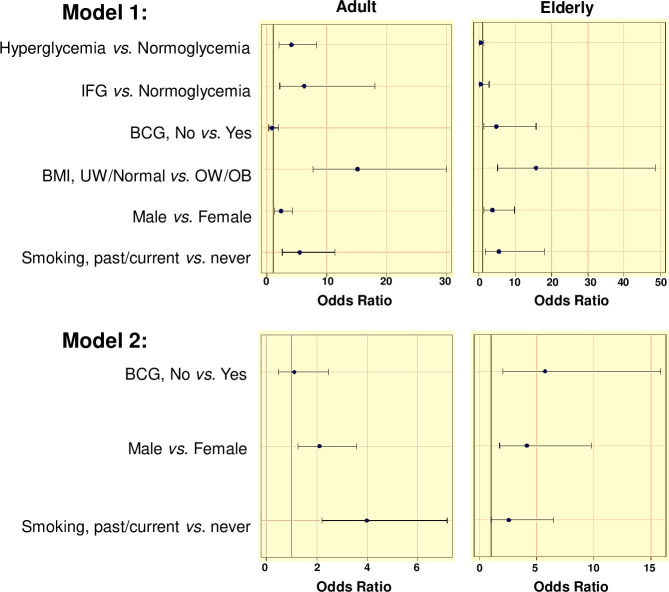
To determine if these host characteristics were independently associated with TB in the elderly, we conducted a multivariable logistic regression analysis. In our initial model 1, we evaluated glycemia as a diabetes-defining variable, together with sex, smoking history, BCG vaccination and BMI (selected from the variables defining obesity and lipid profiles). Our analysis showed that hyperglycemia and impaired fasting glucose were not associated, while male sex, BCG vaccination and BMI remained independently associated with TB, and smoking was associated when modeling ‘past’ vs. ‘never or current’ TB (Table 3; Fig 3). Given the unexpected association with BCG vaccination, in model 2 we removed BMI and dysglycemia since they could be a consequence of TB. However, absence of BCG vaccination remained associated with risk of TB in the elderly, along with male sex and smoking.
Table 3. Univariable and multivariable models for odds of TB vs. non-TB patients by host characteristics, in the elderly or adults.
| Elderly: TB vs non-TB | Adults: TB vs non-TB | |||||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Model 1 a: | Model 2 b: | OR (95% CI) | Model 1: | Model 2: | |||
| Adj-OR (95% CI) | Adj-OR (95% CI) | |||||||
| Adj-OR (95% CI) | Exclude BMI, glycemia | Adj-OR (95% CI) | Exclude BMI, glycemia | |||||
| Male vs female sex | 6.93 (3.23, 14.87) | 3.92 (1.41, 10.88) | 4.33 (1.76, 10.65) | 2.65 (1.61, 4.37) | 2.80 (1.49, 5.25) | 2.63 (1.56, 4.46) | ||
| Smoking, ’Past’ vs ’current or never’ | 5.37 (2.46, 11.70) | 5.43 (1.627, 18.122) | 2.55 (1.008, 6.449) | 4.71 (2.68, 8.29) | 5.41 (2.57, 11.38) | 3.99 (2.21, 7.19) | ||
| BCG vaccine, No vs Yes | 7.86 (3.13, 19.74) | 5.36 (1.68, 17.10) | 6.15 (2.24, 16.92) | 1.17 (0.56, 2.44) | 0.73 (0.30, 1.77) | 0.98 (0.45, 2.16) | ||
| Glycemia | ||||||||
| Normoglycemia | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Impaired Fasting Glucose | 0.22 (0.05, 0.99) | 0.38 (0.06, 2.38) | 2.11 (0.93, 4.76) | 5.84 (2.07, 16.49) | ||||
| Hyperglycemia | 0.54 (0.24, 1.25) | 0.52 (0.18, 1.46) | 3.30 (1.92, 5.80) | 4.37 (2.22, 8.58) | ||||
| Diabetes | 1.07 (0.54, 2.12) | 2.88 (1.71, 4.84) | ||||||
| Obesity | ||||||||
| Underweight/normal | 12.99 (5.34, 31.58) | 12.34 (4.44, 34.33) | 9.01 (5.26, 15.42) | 14.30 (7.48, 27.32) | ||||
| Overweight/Obese | 1.0 | 1.0 | 1.0 | 1.0 | ||||
a Model-1. Controlled for all variables in the table.
b Model-2. Model 1, except for BMI and glycemia; 95% CI with p values ≤ 0.099 shown in bold; In sensitivity analysis, additional models were evaluated: Model 1A. When glycemia was replaced by diabetes (2 categories) in Model 1, diabetes remained associated with TB in adults (adj-OR 4.08, 95% CI 2.13, 7.80), but not in the elderly [adj-OR 1.70 (0.65, 4.45)]. Model 1B. Addition of education or health insurance did not modify the associations already observed in Model 1.
Fig 3. Multivariable logistic regression models of odds of TB vs non-TB among adults or elderly, for different host characteristics.
Host characteristics with a p value ≤0.09 by univariable analysis were selected as indicated in the text for model 1. For model 2, BMI and glycemia were removed from model 2, given that they may be modified once TB develops. Graphs show odds ratio with Wald 95% CI. Abbreviations: IFG, impaired fasting glucose; UW, underweight (BMI <18.5); OW/OB, overweight/obese (BMI >25); BMI, body-mass index; BCG, Bacillus Calmette-Guérin vaccinated.
Previous studies in adults, including our own, indicated that diabetes is a risk factor for TB, and that BCG vaccination does not confer protection for TB development [17, 29]. To confirm if these findings would hold in our adult cohort, we ran the same multivariable models as described for the elderly. Our findings confirmed that in adults there are higher odds of hyperglycemia (adj-OR 4.37, 95% CI 2.22, 8.58) or impaired fasting glucose (adj-OR 5.84, 95% CI 2.07, 16.49) in TB patients (model 1), and a lack of association between TB and BCG vaccination (models 1 and 2; Table 3 and Fig 3). Past smoking, male sex and lower BMI were also associated with TB in both models (Fig 3).
Host factors associated with BCG vaccination
Given our observed protective effect of BCG vaccination for TB in the elderly (Fig 3), we evaluated if there was another host characteristic associated with BCG vaccination that we had not identified, that could influence TB risk (e.g. a confounding factor; S4 Table). Among all study participants, BCG vaccination was more prevalent in females vs. males (p 0.014), non-TB vs. TB participants (p <0.001), individuals with higher education (p = 0.027) and access to health insurance (p 0.027). After controlling for age, sex and TB, BCG vaccination remained associated with higher education level and access to health insurance. Therefore, we expanded the multivariable analysis shown in Fig 3, by adding education or health insurance to the models. However, neither of these variables affected the associations already noted, i.e. risk of TB and lack of BCG vaccination, and the lack of association with diabetes, among the elderly.
Clinical characteristics of the elderly with TB
Since the diagnosis of TB can be challenging in the elderly [30], we also evaluated whether the elderly differed in their microbiological or clinical presentation of active TB when compared to adults. Our results showed no significant differences in sputum acid-fast smear results or grade, nor the proportion with positive culture (Table 4). Signs and symptoms were similar for cough, productive cough, hemoptysis, chest pain or weight loss, but other important distinguishing features were observed in the elderly. Namely, elderly participants were less likely to report fever or chills, and had a longer history of cough or weight loss prior to TB diagnosis.
Table 4. TB diagnostic criteria and signs and symptoms, by age groups.
| Diagnostic criteria | ||||||
| Adults | Elderly | |||||
| n (%) | n (%) | p value | ||||
| Positive AFB Smear | 75 (91.5%) | 39 (90.7%) | 1.000 | |||
| AFB Smear grade | 0.581 | |||||
| Negative or 1+ | 40 (49.4) | 19 (44.2) | ||||
| 2+ or 3+ | 41 (50.6) | 24 (55.8) | ||||
| TB diagnosis | 0.319 | |||||
| Clinical | 10 (12.1%) | 8 (18.6%) | ||||
| Laboratory confirmed | 73 (87.9%) | 35 (81.4%) | ||||
| Signs and symptoms | Presence | Duration | ||||
| Adults | Elderly | Adults | Elderly | |||
| n (%) | n (%) | p value | Days | Days | p value | |
| Cough | 79 (95.2%) | 39 (92.9%) | 0.687 | 70 (70) | 90 (105) | 0.071 |
| Productive Cough | 68 (85%) | 35 (81.4%) | 0.616 | 30 (40) | 60 (70) | 0.610 |
| Hemoptysis | 28 (35%) | 13 (31%) | 0.691 | 0 (3) | 0 (1.5) | 0.904 |
| Fever/ Chills | 67 (80.7%) | 25 (58.1%) | 0.007 | 7 (18) | 7 (20) | 0.612 |
| Chest Pain | 43 (51.8%) | 21 (48.8%) | 0.706 | 14 (30) | 15 (30) | 0.419 |
| Weight Loss | 59 (73.8%) | 34 (79.1%) | 0.660 | 30 (40) | 60 (105) | 0.015 |
NAP, Not applicable; p values ≤ 0.099 shown in bold
Characteristics of the elderly with LTBI
Among the elderly CoC group, more than half had LTBI (Table 1), which puts them at higher risk for TB reactivation when compared to non-infected elderly [4]. However, we did not find any host factor associated with LTBI status among elderly CoC. Furthermore, among elderly ReC there were few differences when comparing positive vs. negative LTBI (S5 Table).
Discussion
We conducted a cross-sectional study to identify unique aspects of TB and LTBI in elderly Hispanics from the Texas-Mexico border region. TB risk factors such as HIV/AIDS, excess alcohol use and drug abuse were more prevalent in adults, and were excluded from analysis. We found that few host factors were associated with LTBI status. In contrast, the elderly had a higher prevalence of birth cohort characteristics that are known risk factors for active TB in adults, such as diabetes and lower education [31, 32]. Interestingly, despite the high prevalence of diabetes in the elderly population, we found that it was not associated with TB. Instead, being male, smoking and having a low BMI were risk factors for TB in the elderly. An additional unexpected finding was the protective effect of BCG vaccination at birth in the elderly.
Our finding that diabetes is not a risk factor for TB in the elderly contrasts with numerous studies in adults, including our own, where diabetes patients have a 1.5 to 3-fold higher risk of TB [15, 17, 33]. We confirmed that adult diabetes patients have a higher risk of TB, and we further observed a higher risk of TB in adults with impaired fasting glucose. However, hyperglycemia and impaired fasting glucose were not associated with TB in the elderly. In fact, impaired fasting glucose was protective by univariable analysis. A reduced strength in the association between TB and diabetes has been noted in past studies, but its implications for the elderly population have not been explicitly evaluated in multivariable models [14, 15]. In fact, diabetes continues to be regarded as a risk factor for TB among the elderly [34, 35]. We speculate that in the elderly, the lack of an association between type 2 diabetes and TB is explained by differences in the underlying pathophysiology of diabetes. Type 2 diabetes in the elderly is characterized by a milder hyperglycemia due to delayed responsiveness of pancreatic beta cells to release insulin versus high levels of insulin, insulin resistance and a more severe hyperglycemia in adults. We also cannot rule out a survivor effect of well-controlled diabetes in our elderly cohorts. We are currently evaluating these distinctions.
In experimental models, BCG vaccine can extend the survival of diabetic mice infected with M.tb through reduced immunopathology, potentially mediated by Th2/M2-mediated mechanisms [36]. Although these studies only evaluated mice for one year, equivalent to mid-life for mice [37], we can perhaps extrapolate that BCG would also afford older diabetic mice protection from TB. Specific studies to address BCG and diabetes in the context of TB have not been performed, but BCG vaccination of old mice [38] and guinea pigs [39] does provide protection against TB. Our findings raise the need to evaluate the occurrence of dysglycemia in old mice, and document its relationship to TB risk. Despite the lack of association between diabetes and TB in the elderly population, we recommend the screening for diabetes in all newly diagnosed elderly TB patients given that management of acute hyperglycemia is important for proper immune function against M. tuberculosis [40–42]. Furthermore, diabetes may be associated with adverse TB treatment outcomes in the elderly, as is the case for adults [5, 43].
Our findings showed a lack of association between BCG vaccination and TB in adults, which is consistent with the literature [44]. However, a novel observation in our study was that the elderly with history of BCG vaccination were less likely to have TB. BCG may confer protection against LTBI in adults [45], but is mostly known for its protective effect against disseminated TB in children [46]. We are not aware of any other documentation that BCG vaccination at birth will confer protection from TB in elders. A recent meta-analysis concluded that BCG confers protection against TB shortly after vaccination, and hence, its effect is most notable in children but not in adults in high burden settings. Their results in adults do not support our findings, but there is limited data in individuals beyond 60 years of age [47]. Confirmation of our findings in other elderly cohorts would open the possibilities for considering a booster BCG in the elderly. Indeed, BCG induces a non-specific protective effect against other microbial infections in young children through the induction of long-lasting epigenetic changes in the bone marrow myeloid precursor for monocytes, a process known as trained immunity [48]. Evidence for BCG-induced trained immunity in elderly individuals was recently demonstrated by the ACTIVATE trial, in which BCG vaccination of individuals 65 years and older lowered the risk of viral respiratory infections [49], although preliminary analysis of the BCG-PRIME study shows no protection against COVID-19 [50]. We speculate that BCG-induced trained immunity at birth confers a “baseline” protection against active TB development that persists throughout life. This “baseline” protection is most notable when the immune system is still immature in newborns, and when it wanes in the elderly, but not in other age groups where other aspects of the immune system, including adaptive immunity, play a prominent role in M.tb infection control. Consistent with this possibility, BCG at birth can confer heterologous protection against other respiratory infections, potentially further shaping the pulmonary immune system through adult and elderly life [51].
TB delayed diagnosis or misdiagnosis and high mortality rates in the elderly are attributed to their increased likelihood of absent, altered, or delayed clinical symptoms, presence of age-associated conditions such as cognitive impairment, and clinical symptoms shared by active TB and old age such as fatigue, and weight loss [5, 30, 52, 53]. In our elderly cohort, we found clinical differences such as a lower prevalence of fever and chills, and longer duration of cough and weight loss. In fact, several elderly TB patients died prior to enrollment in our study or TB treatment initiation. There is a need for biomarkers to enhance TB diagnosis and prognosis in this population. Candidates include the higher neutrophil/lymphocyte and monocyte/lymphocyte ratios observed by others and us, associated with more severe TB in adults [54–59].
Study limitations are the small sample size of our elderly cohort, particularly for some sub-analysis. We also defined elderly participants as those 60 years and older, but TB risk increases with age among the elderly, with waning representation in the oldest age groups [4]. Our study could be affected by survival bias, with some TB patients dying prior to enrollment.
In summary, our findings in elderly Hispanics confirm the presence of shared risk factors for TB with adults, such as being male, smoking and low BMI. Importantly, our findings highlight differences between age groups, notably the lack of an association between TB and diabetes, and the potential protective effect of BCG vaccination in the elderly population. Despite its low prevalence in our study population, smoking was another independent risk factor for TB that deserves further evaluation in other elderly cohorts. Our results call for future studies in the elderly population, for tailored identification of risk factors for LTBI, active TB or adverse TB outcomes, in this unique, heterogeneous, vulnerable and understudied population.
Supporting information
Percentage of adults (A) and elderly (E) with TB and without TB (No TB) for select sociodemographic factors and health-related conditions. For smoking history, A and E with TB and No TB are shown as smoking pack per year. Student’s t test between age groups (A vs E) among participants with No TB or TB; *p ≤ 0.05. Dotted lines for BMI indicate cut-offs for underweight (<18.5), normal (18.5–24.9), overweight (25–30) and obese (<30); LTBI, latent TB infection; NSAIDs, nonsteroidal anti-inflammatory drugs.
(DOCX)
Percentage of adults (A) and elderly (E) with TB and without TB (No TB) with high cholesterol, high LDL, low HDL, and high triglycerides (top row). Bottom row shows complete blood counts (x1e3/μL) for immune cell populations and neutrophil: Lymphocyte and monocyte: Lymphocyte ratios by age and TB status (TB and No TB). Student’s t test between age groups (A vs E) among participants with No TB or TB; *p ≤ 0.05; # p between 0.051–0.099.
(DOCX)
Percentage of EL with TB and without TB (No TB) for select sociodemographic factors, health-related conditions, and lipids. Student’s t test between elderly (EL) with No TB and EL with TB; *p ≤ 0.05. IFG, Impaired fasting glucose.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to acknowledge Kristen Maynard, Danyelle Garza, Erica de Leon, Marielena Benavidez, Mateo Joya-Ayala and Fabiola Lopez for their technical support. We also thank the health professional at the clinics where participants were identified in South Texas (Hidalgo and Cameron County Department of Health and Human Services and Nuestra Clinica del Valle) and northeastern Mexico [TB clinics from the Secretaría de Salud (SSA) de Tamaulipas in Reynosa and Matamoros, and an adult care center from the Sistema para Desarrollo Integral de la Familia in Matamoros]. We thank the members of the Secretaría de Salud de Tamaulipas, including Q.F.B. Cristela Resendez-Cardoso, Drs. Francisco Garcia-Luna Martinez and Ariel Mercado-Cárdenas (administration) and Mr. Jorge Perez-Navarro (logistics). We dedicate this study to the memory of our team members who were passionate about TB research, and whom we lost to COVID-19 in 2020, Dr. Francisco Mora-Guzmán and R. Eminé Rodriguez-Reyna.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the National Institute of Aging (NIA) at the National Institutes of Health [P01-AG051428 to JT, BIR, LSS and JBT] and National Institute of Health (NIH)/NIA NRSA T32-AG021890 to JMS. URL: https://www.nia.nih.gov/research/grants-funding The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vesosky B, Turner J. The influence of age on immunity to infection with Mycobacterium tuberculosis. Immunol Rev. 2005;205:229–43. doi: 10.1111/j.0105-2896.2005.00257.x [DOI] [PubMed] [Google Scholar]
- 2.Piergallini TJ, Turner J. Tuberculosis in the elderly: Why inflammation matters. Exp Gerontol. 2018;105:32–9. doi: 10.1016/j.exger.2017.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Global Tuberculosis Report 2020. World Health Organization, 2020. [Google Scholar]
- 4.Hochberg NS, Horsburgh CR Jr. Prevention of tuberculosis in older adults in the United States: obstacles and opportunities. Clin Infect Dis. 2013;56(9):1240–7. doi: 10.1093/cid/cit027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Goez JF, Velez JD, Mora BL, Parra-Lara LG, Pino-Escobar J, Cayla JA, et al. Tuberculosis in elderly patients in the city of Cali, Colombia: a hospital-based cohort study. J Bras Pneumol. 2020;46(5):e20200072. doi: 10.36416/1806-3756/e20200072 [DOI] [PubMed] [Google Scholar]
- 6.Abdelbary BE, Garcia-Viveros M, Ramirez-Oropesa H, Rahbar MH, Restrepo BI. Predicting treatment failure, death and drug resistance using a computed risk score among newly diagnosed TB patients in Tamaulipas, Mexico. Epidemiol Infect. 2017;145(14):3020–34. doi: 10.1017/S0950268817001911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajagopalan S. Tuberculosis and aging: a global health problem. Clin Infect Dis. 2001;33(7):1034–9. doi: 10.1086/322671 [DOI] [PubMed] [Google Scholar]
- 8.Byng-Maddick R, Noursadeghi M. Does tuberculosis threaten our ageing populations? BMC infectious diseases. 2016;16:119–. doi: 10.1186/s12879-016-1451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbara A, Collin SM, Kon OM, Buell K, Sullivan A, Barrett J, et al. Time to diagnosis of tuberculosis is greater in older patients: a retrospective cohort review. ERJ Open Res. 2019;5(4). doi: 10.1183/23120541.00228-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamini Y, Hochberg Y. A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57:289–300. [Google Scholar]
- 11.Cheng J, Sun YN, Zhang CY, Yu YL, Tang LH, Peng H, et al. Incidence and risk factors of tuberculosis among the elderly population in China: a prospective cohort study. Infect Dis Poverty. 2020;9(1):13. doi: 10.1186/s40249-019-0614-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajagopalan S. Tuberculosis in Older Adults. Clin Geriatr Med. 2016;32(3):479–91. doi: 10.1016/j.cger.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 13.Bigelow A, Freeland B. Type 2 Diabetes Care in the Elderly. The Journal for Nurse Practitioners. 2017;13(3):181–6. [Google Scholar]
- 14.Ponce-De-Leon A, Garcia-Garcia Md Mde L, Garcia-Sancho MC, Gomez-Perez FJ, Valdespino-Gomez JL, Olaiz-Fernandez G, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004;27(7):1584–90. doi: 10.2337/diacare.27.7.1584 [DOI] [PubMed] [Google Scholar]
- 15.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):1091–101. doi: 10.1371/journal.pmed.0050152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SJ, Hong YP, Lew WJ, Yang SC, Lee EG. Incidence of pulmonary tuberculosis among diabetics. Tuber Lung Dis. 1995;76(6):529–33. doi: 10.1016/0962-8479(95)90529-4 [DOI] [PubMed] [Google Scholar]
- 17.Restrepo BI, Camerlin AJ, Rahbar MH, Wang W, Restrepo MA, Zarate I, et al. Cross-sectional assessment reveals high diabetes prevalence among newly-diagnosed tuberculosis cases. Bull WHO. 2011;89(5):352–9. doi: 10.2471/BLT.10.085738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Restrepo BI, Schlesinger LS. Host-pathogen interactions in tuberculosis patients with type 2 diabetes mellitus. Tuberculosis (Edinb). 2013;93(S1):S10–S4. doi: 10.1016/S1472-9792(13)70004-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher-Hoch SP, Rentfro A, Salinas J, Perez A, Brown H, Reininger B, et al. Socioeconomic status and prevalence of obesity and diabetes in a Mexican American community, Cameron County, Texas, 2004–2007. Prev Chronic Dis. 2010;7(3):1–10. [PMC free article] [PubMed] [Google Scholar]
- 20.CDC. Diagnosis of Tuberculosis Disease 2016 [Available from: https://www.cdc.gov/tb/publications/factsheets/testing/diagnosis.htm.
- 21.Restrepo BI, Kleynhans L, Salinas AB, Abdelbary BE, Tshivhula H, Aguillon G, et al. Diabetes screen during tuberculosis contact investigations highlights opportunity for diabetes diagnosis and reveals metabolic differences between ethnic groups. Tuberculosis (Edinb). 2018;113:10–8. doi: 10.1016/j.tube.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rehm J, Greenfield TK, Walsh G, Xie X, Robson L, Single E. Assessment methods for alcohol consumption, prevalence of high risk drinking and harm: a sensitivity analysis. Int J Epidemiol. 1999;28(2):219–24. doi: 10.1093/ije/28.2.219 [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14–S31. doi: 10.2337/dc20-S002 [DOI] [PubMed] [Google Scholar]
- 24.Restrepo BI, Kleynhans L, Salinas AB, Abdelbary B, Tshivhula H, Aguillon-Duran GP, et al. Diabetes screen during tuberculosis contact investigations highlights opportunity for new diabetes diagnosis and reveals metabolic differences between ethnic groups. Tuberculosis (Edinb). 2018;113:10–8. doi: 10.1016/j.tube.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von EE, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 26.Behr MA, Edelstein PH, Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ. 2018;362:k2738. doi: 10.1136/bmj.k2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narasimhan P, Wood J, MacIntyre CR, Mathai D. Risk Factors for Tuberculosis. Pulmonary Medicine. 2013;2013:828939. doi: 10.1155/2013/828939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiamsakul A, Lee MP, Nguyen KV, Merati TP, Cuong DD, Ditangco R, et al. Socio-economic status and risk of tuberculosis: a case-control study of HIV-infected patients in Asia. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2018;22(2):179–86. doi: 10.5588/ijtld.17.0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moliva JI, Turner J, Torrelles JB. Prospects in Mycobacterium bovis Bacille Calmette et Guerin (BCG) vaccine diversity and delivery: Why does BCG fail to protect against tuberculosis? Vaccine. 2015;33(39):5035–41. doi: 10.1016/j.vaccine.2015.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson DA, Mailer K, Porter KA, Niemeier RT, Fearey DA, Pope L, et al. Challenges in assessing transmission of Mycobacterium tuberculosis in long-term-care facilities. Am J Infect Control. 2015;43(9):992–6. doi: 10.1016/j.ajic.2015.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Critchley JA, Restrepo BI, Ronacher K, Kapur A, Bremer AA, Schlesinger LS, et al. Defining a Research Agenda to Address the Converging Epidemics of Tuberculosis and Diabetes: Part 1: Epidemiology and Clinical Management. Chest. 2017;152(1):165–73. doi: 10.1016/j.chest.2017.04.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burusie A, Enquesilassie F, Addissie A, Dessalegn B, Lamaro T. Effect of smoking on tuberculosis treatment outcomes: A systematic review and meta-analysis. PLoS One. 2020;15(9):e0239333. doi: 10.1371/journal.pone.0239333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. Association between diabetes mellitus and active tuberculosis: A systematic review and meta-analysis. PLoS One. 2017;12(11):e0187967. doi: 10.1371/journal.pone.0187967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Chung PH, Leung CLK, Nishikiori N, Chan EYY, Yeoh EK. The strategic framework of tuberculosis control and prevention in the elderly: a scoping review towards End TB targets. Infect Dis Poverty. 2017;6(1):70. doi: 10.1186/s40249-017-0284-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y-H, Chen C-P, Chen P-Y, Huang J-C, Ho C, Weng H-H, et al. Screening for pulmonary tuberculosis in type 2 diabetes elderly: a cross-sectional study in a community hospital. BMC Public Health. 2015;15(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radhakrishnan RK, Thandi RS, Tripathi D, Paidipally P, McAllister MK, Mulik S, et al. BCG vaccination reduces the mortality of Mycobacterium tuberculosis-infected type 2 diabetes mellitus mice. JCI Insight. 2020;5(5). doi: 10.1172/jci.insight.133788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dutta S, Sengupta P. Men and mice: Relating their ages. Life Sci. 2016;152:244–8. doi: 10.1016/j.lfs.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 38.Ito T, Takii T, Maruyama M, Hayashi D, Wako T, Asai A, et al. Effectiveness of BCG vaccination to aged mice. Immun Ageing. 2010;7:12. doi: 10.1186/1742-4933-7-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komine-Aizawa S, Yamazaki T, Yamazaki T, Hattori S, Miyamoto Y, Yamamoto N, et al. Influence of advanced age on Mycobacterium bovis BCG vaccination in guinea pigs aerogenically infected with Mycobacterium tuberculosis. Clin Vaccine Immunol. 2010;17(10):1500–6. doi: 10.1128/CVI.00190-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Restrepo BI, Twahirwa M, Jagannath C. Hyperglycemia and dyslipidemia: Reduced HLA-DR expression in monocyte subpopulations from diabetes patients. Hum Immunol. 2020. doi: 10.1016/j.humimm.2020.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Restrepo BI, Khan A, Singh VK, Erica d-L, Aguillon-Duran GP, Ledezma-Campos E, et al. Human monocyte-derived macrophage responses to M. tuberculosis differ by the host’s tuberculosis, diabetes or obesity status, and are enhanced by rapamycin. Tuberculosis (Edinb). 2020;126:102047. doi: 10.1016/j.tube.2020.102047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ronacher K, van Crevel R, Critchley JA, Bremer AA, Schlesinger LS, Kapur A, et al. Defining a Research Agenda to Address the Converging Epidemics of Tuberculosis and Diabetes: Part 2: Underlying Biologic Mechanisms. Chest. 2017;152(1):174–80. doi: 10.1016/j.chest.2017.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Gennaro F, Vittozzi P, Gualano G, Musso M, Mosti S, Mencarini P, et al. Active Pulmonary Tuberculosis in Elderly Patients: A 2016–2019 Retrospective Analysis from an Italian Referral Hospital. Antibiotics (Basel). 2020;9(8). doi: 10.3390/antibiotics9080489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moliva JI, Turner J, Torrelles JB. Immune Responses to Bacillus Calmette-Guerin Vaccination: Why Do They Fail to Protect against Mycobacterium tuberculosis? Front Immunol. 2017;8:407. doi: 10.3389/fimmu.2017.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin CH, Kuo SC, Hsieh MC, Ho SY, Su IJ, Lin SH, et al. Effect of diabetes mellitus on risk of latent TB infection in a high TB incidence area: a community-based study in Taiwan. BMJ Open. 2019;9(10):e029948. doi: 10.1136/bmjopen-2019-029948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy A, Eisenhut M, Harris RJ, Rodrigues LC, Sridhar S, Habermann S, et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ. 2014;349:g4643. doi: 10.1136/bmj.g4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trauer JM, Kawai A, Coussens AK, Datta M, Williams BM, McBryde ES, et al. Timing of Mycobacterium tuberculosis exposure explains variation in BCG effectiveness: a systematic review and meta-analysis. Thorax. 2021. doi: 10.1136/thoraxjnl-2020-216794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Netea MG, Dominguez-Andres J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–88. doi: 10.1038/s41577-020-0285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, Antonakos N, Kotsaki A, Dominguez-Andres J, et al. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell. 2020;183(2):315–23 e9. doi: 10.1016/j.cell.2020.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.TB vaccine does not protect vulnerable elderly people against COVID-19. Netherland News Live. 2021 1/18/2021;Sect. Health.
- 51.de Castro MJ, Pardo-Seco J, Martinon-Torres F. Nonspecific (Heterologous) Protection of Neonatal BCG Vaccination Against Hospitalization Due to Respiratory Infection and Sepsis. Clin Infect Dis. 2015;60(11):1611–9. doi: 10.1093/cid/civ144 [DOI] [PubMed] [Google Scholar]
- 52.Teale C, Goldman JM, Pearson SB. The association of age with the presentation and outcome of tuberculosis: a five-year survey. Age Ageing. 1993;22(4):289–93. doi: 10.1093/ageing/22.4.289 [DOI] [PubMed] [Google Scholar]
- 53.Perez-Guzman C, Vargas MH, Torres-Cruz A, Villarreal-Velarde H. Does aging modify pulmonary tuberculosis?: A meta-analytical review. Chest. 1999;116(4):961–7. doi: 10.1378/chest.116.4.961 [DOI] [PubMed] [Google Scholar]
- 54.Russell CD, Parajuli A, Gale HJ, Bulteel NS, Schuetz P, de Jager CPC, et al. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: A systematic review and meta-analysis. The Journal of infection. 2019;78(5):339–48. doi: 10.1016/j.jinf.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stefaniuk P, Szymczyk A, Podhorecka M. The Neutrophil to Lymphocyte and Lymphocyte to Monocyte Ratios as New Prognostic Factors in Hematological Malignancies - A Narrative Review. Cancer Manag Res. 2020;12:2961–77. doi: 10.2147/CMAR.S245928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang W, Wang L-f, Liu Y-y, Yang F, Zhu L, Zhang X-h. Value of the Ratio of Monocytes to Lymphocytes for Monitoring Tuberculosis Therapy. Canadian Journal of Infectious Diseases and Medical Microbiology. 2019;2019:3270393. doi: 10.1155/2019/3270393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jun Wang YY, Wang Xuedong, Pei Hao, Kuai Shougang, Gu Lan, Xing Huiqin, Zhang Yu, Huang Qiusheng, Guan Bin. Ratio of monocytes to lymphocytes in peripheral blood in patients diagnosed with active tuberculosis. Brazilian Journal of Infectious Diseases. 2015;19(2). doi: 10.1016/j.bjid.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin Y, Kuai S, Liu J, Zhang Y, Shan Z, Gu L, et al. Pretreatment neutrophil-to-lymphocyte ratio in peripheral blood was associated with pulmonary tuberculosis retreatment. Arch Med Sci. 2017;13(2):404–11. doi: 10.5114/aoms.2016.60822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ault R, Dwivedi V, Koivisto E, Nagy J, Miller K, Nagendran K, et al. Altered monocyte phenotypes but not impaired peripheral T cell immunity may explain susceptibility of the elderly to develop tuberculosis. Exp Gerontol. 2018;111:35–44. doi: 10.1016/j.exger.2018.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percentage of adults (A) and elderly (E) with TB and without TB (No TB) for select sociodemographic factors and health-related conditions. For smoking history, A and E with TB and No TB are shown as smoking pack per year. Student’s t test between age groups (A vs E) among participants with No TB or TB; *p ≤ 0.05. Dotted lines for BMI indicate cut-offs for underweight (<18.5), normal (18.5–24.9), overweight (25–30) and obese (<30); LTBI, latent TB infection; NSAIDs, nonsteroidal anti-inflammatory drugs.
(DOCX)
Percentage of adults (A) and elderly (E) with TB and without TB (No TB) with high cholesterol, high LDL, low HDL, and high triglycerides (top row). Bottom row shows complete blood counts (x1e3/μL) for immune cell populations and neutrophil: Lymphocyte and monocyte: Lymphocyte ratios by age and TB status (TB and No TB). Student’s t test between age groups (A vs E) among participants with No TB or TB; *p ≤ 0.05; # p between 0.051–0.099.
(DOCX)
Percentage of EL with TB and without TB (No TB) for select sociodemographic factors, health-related conditions, and lipids. Student’s t test between elderly (EL) with No TB and EL with TB; *p ≤ 0.05. IFG, Impaired fasting glucose.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.