Abstract
Background and Purpose:
Intracerebral hemorrhage (ICH) is a devastating subtype of stroke with high mortality and disability. Inflammatory response promotes secondary brain injury after ICH. Triggering receptor expressed on myeloid cells 1 (TREM-1) is a key regulator of inflammation. The aim of this study was to evaluate the role of TREM-1 in neuroinflammatory response after ICH in mice.
Methods:
CD1 mice (n=275) were used in this study. Mice were subjected to ICH by autologous blood injection. TREM-1 knockout CRISPR was administered intracerebroventricularly to evaluate the role of TREM-1 after ICH. A selective TREM-1 inhibitor, LP17 was administered intranasally 2h after ICH. To elucidate TREM-1 signaling pathway, CARD9 activation CRISPR was administered with LP17 and TREM-1 activating anti-mouse TREM-1 antibody (mAb) was administered with Rottlerin, a specific PKC δ inhibitor. Lastly, to evaluate the role of HMGB1 in TREM-1 mediated microglia activation, Glycyrrhizin, an inhibitor of HMBG1 was administered with TREM-1 activating mAb. Neurobehavioral test, brain water content, western blot, immunofluorescence staining and co-immunoprecipitation was performed.
Results:
TREM-1 knockout reduced ICH-induced neurobehavioral deficits and neuroinflammatory response. The temporal expression of HMGB1, TREM-1, PKC δ and CARD9 increased after ICH. TREM-1 was expressed on microglia. Intranasal administration of LP17 significantly decreased brain edema and improved neurobehavioral outcomes at 24h and 72h after ICH. LP17 promoted M2 microglia polarization and reduced proinflammatory cytokines after ICH, which was reversed with CARD9 activation CRISPR. TREM-1 mAb increased neurobehavior deficits, proinflammatory cytokines and reduced M2 microglia after ICH, which was reversed with Rottlerin. HMBG1 interaction with TREM-1 increased after ICH, and Glycyrrhizin reduced neuroinflammation and promoted M2 microglia which was reversed with TREM-1 mAb.
Conclusions:
This study demonstrated that TREM-1 enhanced neuroinflammation by modulating microglia polarization after ICH, and this regulation was partly mediated via PKC δ/CARD9 signaling pathway and increased HMGB1 activation of TREM-1.
Keywords: Intracerebral hemorrhage (ICH), Inflammation, Treatment, Triggering receptor expressed on myeloid cells 1 (TREM-1), Microglia
Introduction
Intracerebral hemorrhage (ICH) is a common subtype of stroke with high mortality and disability, which affects approximately 2 million individuals worldwide per year1. There are currently no specific treatments that can effectively improve outcomes after ICH. Surgical removal of hematoma is mainly supportive and can reduce mortality rate after ICH but clinical outcomes usually remain poor2. Thus, there is a critical need to develop new and effective therapies for ICH.
Neuroinflammation, including the activation of microglia and release of proinflammatory mediators, play an important role in progression of ICH-induced brain injury3, 4. Triggering receptor expressed on myeloid cells 1 (TREM-1) is an inflammation amplifier, expressed on the surface of myeloid cells5. TREM-1 played a crucial role in a number of inflammatory related diseases such as ischemia, subarachnoid hemorrhage, colitis, Alzheimer’s disease, encephalitis, and so on6-8. Activated TREM-1 provides a docking site for spleen tyrosine kinase (SYK) via phosphorylation of DAP12, and SYK can activate phospholipase-C-gamma (PLC-γ) and ERK pathways9, 10. Furthermore, PLC-γ has been reported to activate protein kinase C δ (PKC δ), which was recently shown to activate microglia via CARD9/NF-κB signaling11. Although TREM-1 ligands are not clearly known, in an animal model of liver cancer high mobility group box 1 (HMGB1) was found to be a potential TREM-1 ligand involved in the inflammatory response12. HMGB1 plays a critical role in ICH-induced secondary injury by amplifying inflammatory response13, 14. However, the relation between HMGB1 and TREM-1 after ICH has not been studied. The objective of this study was to determine whether TREM-1 promotes inflammatory response after ICH and whether TREM-1 inhibition can attenuate ICH-induced neuroinflammation. We hypothesized that TREM-1 modulates microglia polarization via PKC δ/CARD9 signaling pathway and thereby promotes early inflammatory response after ICH in mice, and furthermore, TREM-1 may be activated by endogenous HMGB1 after ICH.
Methods
Data Availability
All data are available within the article and additional data can be acquired from the corresponding author.
Animals
A total of 275 adult male CD1 mice were used (Table I). Mice were housed in a temperature and humidity-controlled room with a 12-hour light/dark cycle with adequate food and water. All experimental procedures were approved by Institutional Animal Care and Use Committee at Loma Linda University and complied with National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Study Design
Experimental design consisted of six parts (Figure I). Mice were randomly assigned to different experimental groups by generating random numbers. Information of experimental groups was blinded to researchers who performed surgeries, neurobehavioral assessments, Western blot, immunofluorescence staining, and data analysis.
Experiment 1
To explore the temporal expression and cellular localization of TREM-1 in the ipsilateral hemisphere after ICH. Total 36 mice were randomly divided into six groups (n=6 per group): sham and ICH groups at 6h, 12h, 24h, 72h and 7d after ICH. Western blot was performed to detect TREM-1 expression after ICH. Additionally, sham mice and ICH mice at 24h (n=2 per group) were used to assess cellular localization of TREM-1 via double immunohistochemistry staining.
Experiment 2
To evaluate the effect of TREM-1 knockout (KO) on neurobehavioral outcome and inflammatory response after ICH in mice. Total 16 mice were divided into four groups (n=4 per group): sham, ICH, ICH+control CRISPR and ICH+TREM-1 KO CRISPR. Neurobehavioral tests and western blot were conducted at 24h after ICH. Additionally, 16 mice were used to evaluate the knockdown efficiency of TREM-1 KO CRISPR in naive and ICH groups. Additional 4 mice were used to evaluate the effect of TREM-2 KO on TREM-1 expression after ICH.
Experiment 3
To evaluate the role of TREM-1 on outcomes 24h and 72h after ICH in mice. The selective TREM-1 inhibitor, LP17 and TREM-1 specific agonist, Anti-TREM-1 mAb were administered to ICH mice. In the first part, mice were randomly divided into six groups: sham, ICH, ICH+control peptide, ICH+LP17 (0.3 μg/g), ICH+LP17 (1.0 μg/g) and ICH+LP17 (3.0 μg/g). In the second part, mice were divided into six groups: sham, ICH, ICH+control peptide, ICH+LP17 (1.0 μg/g), ICH+control IgG and ICH+Anti-TREM-1 mAb (0.25 μg/g). Animal number per group are listed in Fig. I. Neurobehavioral tests, brain water content (BWC) and hematoxylin-eosin (HE) staining were analyzed.
Experiment 4
To explore the role of TREM-1 on microglia polarization after ICH in mice. Total 12 mice were divided into three groups (n=4 per group): sham, ICH, and ICH+LP17 (1.0 μg/g). Double immunohistochemistry staining was used to evaluate the number of M1 or M2 surrounding the hematoma at 24h after ICH.
Experiment 5
To verify TREM-1 downstream signaling pathway that modulates inflammatory response and microglia polarization after ICH. Brain samples from experiment 2 were used to evaluate temporal expression of downstream proteins using western blot and to determine cellular localization of CARD9 using immunohistochemistry staining. Meanwhile, 16 mice were used to evaluate the knockdown efficiency of CARD9 activation (ACT) CRISPR in naïve mice. Next, 36 mice were divided into six groups (n=6 per group): sham, ICH, ICH+control peptide, ICH+LP17 (1.0 μg/g), ICH+LP17 (1.0 μg/g)+control CRISPR and ICH+LP17 (1.0 μg/g)+CARD9 ACT CRISPR. Additionally, 36 mice were divided into six groups (n=6 per group): sham, ICH, ICH+control IgG, ICH+Anti-TREM-1 mAb (0.25 μg/g), ICH+Anti-TREM-1 mAb (0.25 μg/g)+DMSO and ICH+Anti-TREM-1 mAb (0.25 μg/g)+Rottlerin (8 μg/g). The expression of pathway proteins, microglia polarization related factors and inflammatory cytokines were assessed by western blot 24h after ICH.
Experiment 6
To identify the endogenous ligands of TREM-1 activation after ICH. Temporal expression of HMBG1 was analyzed using brain samples from experiment 2. Co-immunoprecipitation was performed to evaluate interaction between HMGB1 and TREM-1. Additionally, 16 mice were divided into four groups (n=4 per group): sham, ICH, ICH+Glycyrrhizin (75 μg/g) and ICH+Glycyrrhizin (75 μg/g)+Anti-TREM-1 mAb (0.25 μg/g). Western blot was used to assess microglia polarization related factors and inflammatory cytokines 24h after ICH.
ICH Model
ICH model was established by injecting autologous blood into right basal ganglia as previously described15. Mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) in a ratio of 2:1 given via intraperitoneal injection. A 1-mm cranial burr hole was drilled under a stereotaxic head frame (Kopf Instruments, USA) with the coordinates 0.2 mm posterior and 2.2 mm right lateral to bregma. Femoral arterial blood 40-50 μL was collected using a 1ml 27 G syringe (BD Biosciences, USA) under a surgical microscope (ZEISS, Germany). A total volume of 30 μL blood with no anticoagulants was injected into right basal ganglia through the burr hole. Blood volume 5 μL was infused at a depth 3.0 mm below the dura which was followed by 25 μL infusion at a depth 3.5 mm below the dura 5 min later. Blood was injected using a micro-injection pump (Stoelting, MA) at a rate of 2 μL/min. The needle was left in place for an additional 5 min and then withdrawn slowly 1 mm per min. The burr hole was covered with bone wax and scalp was sutured. Normal saline 400 μL was injected subcutaneously. Mice were allowed to recover fully under close observation. Sham surgery was performed following same protocol but without blood injection. For sham manipulation, the needle was inserted similar to blood injection method but nothing was injected into the brain, as previously published16.
Drug Administration
Selective TREM-1 inhibitor, LP17 (LQVTDSGLYRCVIYHPP) and a control peptide (TDSRCVIGLYHPPLQVY) were synthesized (GenScript, USA) as previously reported6. Three different doses of LP17 (0.3 μg/g, 1.0 μg/g, and 3.0 μg/g) or control peptide was administered intranasally at 2h after ICH. The specific TREM-1 agonist, anti-mouse TREM-1 rat IgG2a mAb (0.25 μg/g) or control rat IgG2a (both Thermo Fisher Scientific, USA) was administered intracerebroventricularly at 1h before ICH induction. Rottlerin (8 μg/g), a selective inhibitor of PKC δ and Glycyrrhizin (75 μg/g), an inhibitor of HMBG1 (both Sigma Aldrich, USA) were dissolved in dimethyl sulfoxide (DMSO) and administered intraperitoneally. TREM-1 KO CRISPR, TREM-2 KO CRISPR and CARD9 ACT CRISPR (all Santa Cruz Biotechnology, USA) were administered 48h before ICH. CRISPR KO or ACT (1 μg) was injected intracerebroventricularly at a rate of 1 μL/min using a micro-injection pump. Control CRISPRs (Santa Cruz Biotechnology, USA) was injected using the same protocol.
Intracerebroventricular Injection
Intracerebroventricular administration was performed as previously described17. The 26G needle of a 10 μL Hamilton syringe was stereotactically inserted into left lateral ventricle using the coordinates 0.3 mm posterior, 1.0 mm lateral, and 2.3 mm below the dura.
Neurobehavior Tests
Neurobehavioral outcomes were evaluated at 24h and 72h after ICH by using modified Garcia test, forelimb placement test and corner turn test as previously described15.
Brain Water Content
Brain water content was measured by wet/dry method as described previously18. Whole brain was divided into ipsilateral cortex, ipsilateral basal ganglia, contralateral cortex, contralateral basal ganglia and cerebellum, and BWC was calculated as [(wet weight–dry weight)/wet weight]×100%.
Western Blot
Western blot was performed as previously described19. Equal amounts of samples from right hemispheres were loaded on an SDS-PAGE gel, electrophoresed, and transferred to a nitrocellulose membrane. Membranes were incubated overnight at 4°C with primary antibodies (Table II). Relative density of bands was analyzed using Image J software (NIH, Bethesda, USA).
Immunofluorescence Staining
Brain samples were collected and cut into 10 μm thick slices with a cryostat (Leica Microsystems, CM3050S, Germany). Double immunofluorescence staining was performed as described previously20. Brain sections were incubated overnight at 4°C with primary antibodies (Table III).
Co-immunoprecipitation
Co-immunoprecipitation (Co-IP) was performed as described previously12. Supernatant from brain samples 200 μL was mixed with 1 μg rabbit anti-HMGB1 antibody and 50 μL protein A/G sepharose (both Abcam, USA) and allowed to incubate at 4°C overnight. The beads were collected by centrifugation at 3,000g for 5 min at 4°C, and eventually western blot was performed using rabbit anti-TREM-1 antibody (Abcam, USA).
Statistical Analysis
Data were presented as mean and standard deviation (mean±SD). Statistical analysis was performed with GraphPad Prism 6.0. Multiple comparisons were statistically analyzed using one-way analysis of variance (ANOVA) followed by Tukey's test. P<0.05 was considered statistically significant.
Results
Mortality
The mortality rate in this study was 1.09% (3/275). A total of 3 mice died in ICH group and there was no mortality in sham or naïve groups.
Temporal expression of TREM-1 after ICH
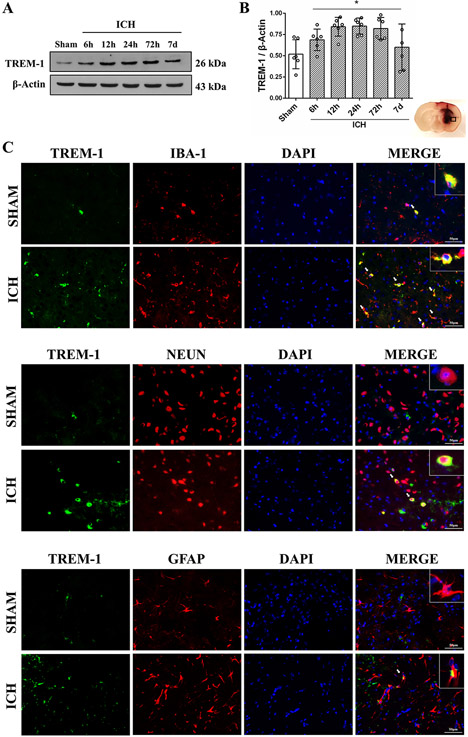
TREM-1 expression in the ipsilateral hemisphere increased as early as 6h after ICH and reached a peak around 24h (P<0.05, Fig. 1A and B). TREM-1 colocalized with microglia, neurons, and astrocytes. TREM-1 was mostly expressed on microglia 24h after ICH, and in sham mice only microglia expressed TREM-1 (Fig. 1C).
Fig. 1. Time course expression and cellular localization of TREM-1 after ICH.
(A-B) Representative immunoblots and quantitative analysis of TREM-1 at 6h, 12h, 24h, 72h and 7days after ICH. The error bars represent mean±SD. *P<0.05 vs sham. N=6 per group. (C) Double immunofluorescence staining for TREM-1 (green) with microglia (IBA-1, red), neuron (NeuN, red) and astrocytes (GFAP, red) in perihematomal region at 24h after ICH. N=2 per group. Scale bar, 50 μm. IBA-1, ionized calcium binding adaptor molecule-1; NeuN, neuronal nuclear; GFAP, glial fibrillary acidic protein; DAPI, 4′,6-diamidino2-phenylindole.
Effects of TREM-1 knockout in ICH mice
TREM-1 KO CRISPR improved neurobehavior compared to ICH+control CRISPR (P<0.05, Fig. IIA). TREM-1 and IL-1β expression was downregulated with TREM-1 KO CRISPR while Arg-1 expression was upregulated compared to control CRISPR (P<0.05, Fig. IIB). We validated the efficacy of TREM-1 KO CRISPR in naïve mice, which showed TREM-1 expression was significantly downregulated with TREM-1 KO CRISPR compared to control CRISPR (P<0.05, Fig. IIC and D). Double-immunofluorescence staining showed that TREM-1 KO CRISPR lowered the expression of TREM-1 on microglia after ICH (Fig. IID).
LP17 improved short-term outcomes in ICH mice
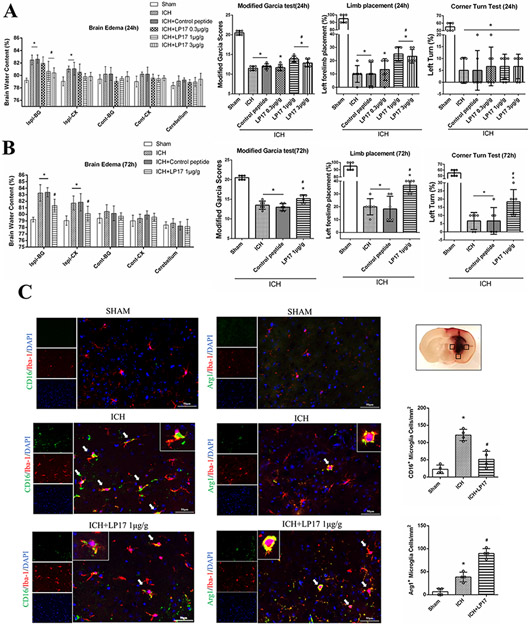
LP17 1.0 μg/g and 3.0 μg/g significantly decreased BWC 24h after ICH in ipsilateral basal ganglia (P<0.05, Fig. 2A), and there was no significant difference in BWC between the two doses. ICH mice that received 1.0 μg/g and 3.0 μg/g LP17 performed significantly better than ICH group in modified Garcia test and limb placement test without improvement in corner turn test (Fig. 2A). Based on 24h results, LP17 1.0 μg/g was chosen for further studies. We observed typical histopathological characteristics of brain edema such as widened lacunar spaces surrounding vessels and swollen cell bodies in the region surrounding the hemorrhage in ICH mice, and LP17 1.0 μg/g improved these characteristics (Fig. IIIC).
Fig. 2. The effect of TREM-1 on the short-term outcome and microglia polarization after ICH.
(A) Brain water content in different brain regions with the administration of three doses of LP17 at 24h after ICH. Modified Garcia test, limb placement test and corner turn test 24h after ICH. (B) Brain water content at 72h after ICH. Neurobehavioral tests with TREM-1 inhibitor and activator. (C) Double immunofluorescence staining for M1 microglia (CD16/Iba-1+) and M2 microglia (Arg1/Iba-1+) 24h after ICH. Three regions surrounding the hematoma were analyzed in each mice. The error bars represent mean±SD. *P<0.05 vs sham, #P<0.05 vs ICH. @P<0.05 vs LP17. N=6 per group (A and B). N=4 per group (C). Ispi, ipsilateral; Cont, contralateral; BG, basal ganglia; CX, cortex.
Outcomes evaluated at 72h after ICH showed that 1.0 μg/g LP17 significantly lowered BWC in ipsilateral basal ganglia and cortex as well as improved neurobehavior in ICH mice (Fig. 2B). Furthermore, Anti-TREM-1 mAb worsened neurobehavior in ICH mice (Fig. 2B).
TREM-1 modulated microglia polarization after ICH
M1 microglia (CD16 and Iba-1 positive cells) increased in perihematomal region 24h after ICH compared to sham, and it was downregulated with LP17 (Fig. 2C). Meanwhile, LP17 increased the number of M2 microglia (Arg1 and Iba-1 positive cells) surrounding the hematoma at 24h after ICH (Fig. 2C).
TREM-1 regulated microglia polarization via PKCδ/CARD9 signaling pathway
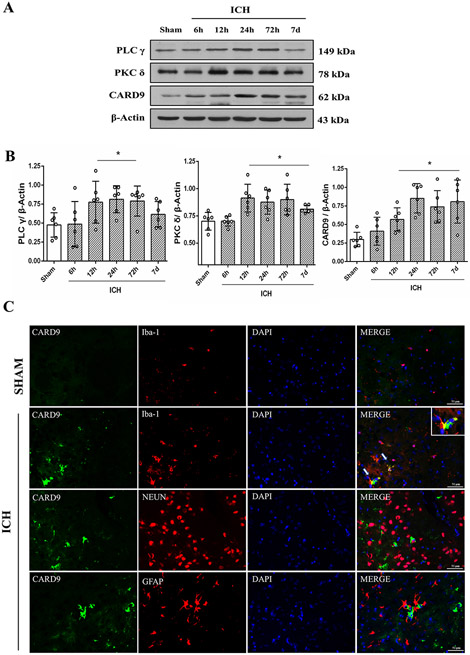
Temporal expressions of PLC-γ, PKC δ and CARD9 after ICH showed a similar upregulation as TREM 1 (Fig. 3A and B). CARD9 was mainly expressed in microglia and there was almost no expression in neurons and astrocytes at 24h after ICH (Fig. 3C).
Fig. 3. Time course expressions of TREM-1 related downstream signaling pathway proteins after ICH.
(A) Representative immunoblots of downstream signaling pathway proteins at 6h, 12h, 24h, 72h and 7days after ICH. (B) Quantitative analysis of PLC-γ, PKC δ and CARD9. The error bars represent mean±SD. *P<0.05 vs sham. N=6 per group. (C) Double immunofluorescence staining for CARD9 (green) with microglia (Iba-1, red), neuron (NeuN, red) and astrocytes (GFAP, red) in the perihematomal region. N=2 per group. Scale bar, 50 μm.
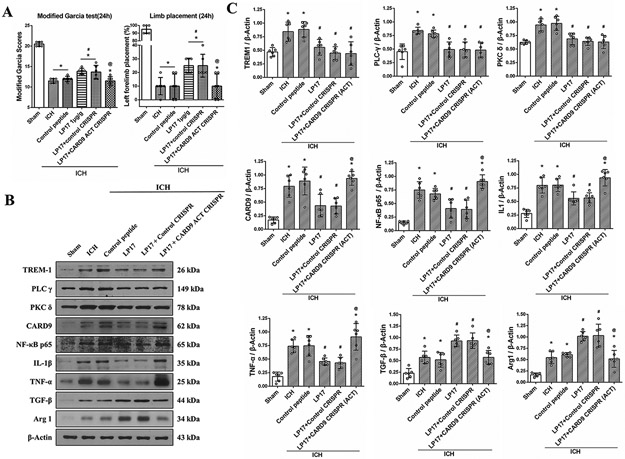
CARD9 ACT CRISPR significantly reversed the beneficial effects of LP17 on neurological function 24h after ICH (Fig. 4A). LP17 significantly reduced the expressions of TREM-1, PLC-γ, PKC δ, CARD9, NF-kB and M1 related factors while upregulating M2 related factors 24h after ICH (Fig. 4B and C). CARD9 ACT CRISPR did not change the expressions of TREM-1, PLC-γ and PKC δ, however, it significantly increased CARD9 and NF-kB expression compared to ICH+LP17 group. Likewise, CARD9 ACT CRISPR reversed the effect of LP17 by upregulating M1 related factors and downregulating M2 related factors (Fig. 4B and C). We performed western blot and immunofluorescence to validate CARD9 transcriptional activation, which showed that CARD9 ACT CRISPR significantly upregulated CARD9 expression and increased microglia CARD9 expression in brain samples of naïve mice compared to control CRISPR (Fig. IV)
Fig. 4. CARD9 activation (ACT) CRISPR abolished the beneficial effects of LP17 at 24h after ICH.
(A) Modified Garcia test and limb placement test at 24h after ICH. (B) Representative immunoblot bands and (C) Quantitative analysis of TREM-1, PLC-γ, PKC δ, CARD9, NF-κB p65, IL-1β, TNF-α, TGF-β and Arg-1 expressions in the ipsilateral hemisphere at 24h after ICH. The error bars represent mean±SD. *P<0.05 vs sham, #P<0.05 vs ICH, @P<0.05 vs LP17. N=6 per group.
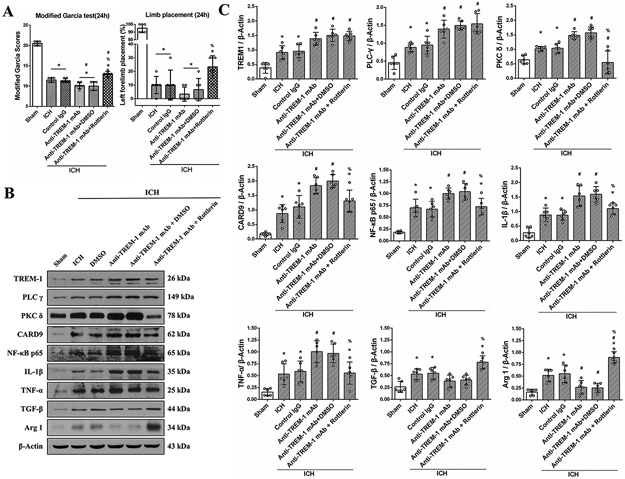
Furthermore, Rottlerin reversed deleterious effects of TREM-1 mAb on neurobehavior function in ICH mice (Fig. 5A). TREM-1 mAb promoted PKCδ/CARD9 signaling pathway and increased M1 related factors IL-1β and TNF-α. Rottlerin reversed the effect of Anti TREM-1 mAb on CARD9 expression and M1 polarization related factors (Fig. 5B and C).
Fig. 5. PKC δ inhibitor Rotterlin reversed the effect of TREM-1 activating mAb on M1 microglia polarization at 24h after ICH.
(A) Modified Garcia test and limb placement test at 24h after ICH. (B) Representative western blot bands and (C) Quantitative analysis of TREM-1, PLC-γ, PKC δ, CARD9, NF-κB p65, IL-1β, TNF-α, TGF-β and Arg-1 expressions in the ipsilateral hemisphere at 24h after ICH. The error bars represent mean±SD. *P<0.05 vs sham, #P<0.05 vs ICH, %P<0.05 vs Anti-TREM-1 mAb. N=6 per group.
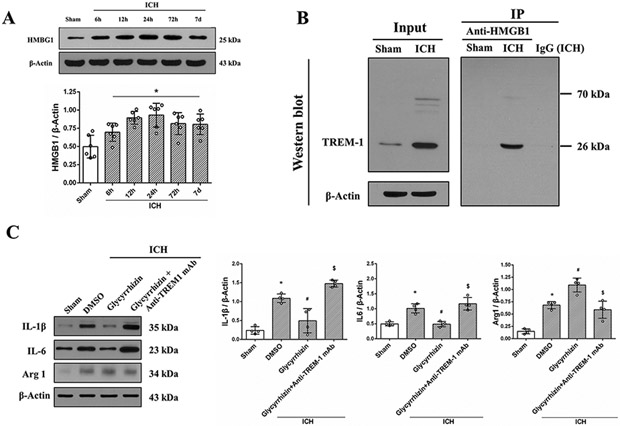
TREM-1 interaction with HMGB1 increased after ICH
HMGB1 expression increased within 6h after ICH and remained elevated until 7 days (Fig. 6A). TREM-1 interaction with HMGB1 increased at 24h after ICH, and there was no interaction between the two in sham group (Fig. 6B). Glycyrrhizin reduced IL-1β and IL-6 expression while promoting M2 polarization factor Arg1 after ICH and this effect was reversed by TREM-1 activating mAb (Fig. 6C).
Fig. 6. HMBG1 interaction with TREM-1 modulated microglia polarization after ICH.
(A) Representative immunoblots and quantitative analysis of HMGB1 expression after ICH. The error bars represent mean±SD. *P<0.05 vs sham. N=6 per group. (B) Co-IP showed TREM-1 binding to endogenous HMBG1 was detected after ICH. (C) HMGB1 selective inhibitor, Glycyrrhizin promoted M2 microglia polarization at 24h after ICH and TREM-1 activator, anti-TREM-1 mAb reversed this effect. The error bars represent mean±SD. *P<0.05 vs sham, #P<0.05 vs ICH, $P<0.05 vs Glycyrrhizin. N=4 per group.
Discussion
The study identified the following novel findings: 1) TREM-1 expression increased as early as 6h after ICH, and it was expressed on microglia. 2) TREM-1 knockout improved outcomes after ICH. 3) Intranasal administration of LP17 decreased brain edema and improved neurobehavioral outcomes at 24h and 72h after ICH. 4) TREM-1 promoted M1 microglia polarization after ICH via PKC δ/CARD9 signaling pathway. 5) HMGB1 interaction with TREM-1 increased after ICH which contributed to inflammatory response. Thus, these findings indicate that TREM-1 may play a detrimental role after ICH by promoting neuroinflammation.
Neuroinflammation driven by activated microglia contributes to high morbidity and mortality after ICH21. Microglia can adopt M1 or M2 phenotypes via polarization which initiate secondary brain injury and subsequent brain repair processes22. Thus, modulation of microglial phenotype could be a therapeutic target for ICH. TREM-1, expressed on majority of innate immune cells, played a role to amplify inflammatory response in many diseases but its role in ICH remained unclear. Consistent with the role of TREM-1 in inflammatory response23, we found proinflammatory cytokine was reduced and M2 anti-inflammatory factor was higher in TREM-1 KO mice. Additionally, TREM-1 KO mice performed better in neurobehavioral tests at 24h after ICH. Thus, we inferred that TREM-1 played a negative role in early stage after ICH. TREM-1 and TREM-2 are two important receptors of TREMs family expressed on myeloid cells such as microglia in the brain. The relation of the two receptors are poorly understood, and most studies suggest that TREM-1 promotes inflammation while TREM-2 inhibits inflammation via different signaling pathways9, 24 . Currently no study has clarified the relation between TREM-1 and TREM-2 after ICH. Previous study showed that TREM-2 activation attenuated neuroinflammation and neuronal apoptosis after ICH in mice25. Considering that TREM-2 participated in the inflammatory response after ICH, we evaluated whether TREM-1 KO CRISPR influenced the expression of TREM-2 in ICH, and a negative result was found (Fig. IIIA). Furthermore, TREM-2 KO CRISPR also did not change TREM-1 expression after ICH (Fig. IIIB). These results indicated the specificity of CRISPR knockdown in our study. Further studies are needed to clarify the interaction between TREM-1 and TREM-2 after ICH.
TREM-1 was found to increase early after cerebral ischemia and remained high until 28 days in middle cerebral artery occlusion (MACO) mice6. We measured TREM-1 in the acute stage after ICH and found it was upregulated at 6h post-ICH with expression on microglia, and the expression of TREM-1 at chronic stage needs further study. LP17 is a synthetic peptide considered as a decoy receptor for TREM-1 signaling26. As previously reported27, we found brain edema increased after ICH and LP17 reduced brain edema, inflammatory response and improved neurobehavior in ICH mice. We administered LP17 intranasally as previously described6. Intranasal administration is an easy and non-invasive method valuable for clinical translation to deliver various agents to brain bypassing the blood-brain barrier28. The dose and frequency of LP17 administration was based on previous study in MCAO mice6. In this study, three doses of LP17 were tested in the initial experiments. Based on the result of neurobehavioral outcome and brain edema at 24 hours after ICH, 1.0 μg/g was selected as the optimal dose which was used for further experiments. Additionally, we administered LP17 1.0 μg/g once daily for 3 days to evaluate outcomes at 3 days after ICH because no significant result was found with one time application. However, given the short half-life of peptides in vivo, more studies need to be designed to assess the frequency of LP17 administration.
LP17 is considered as a decoy receptor for TREM-1 signaling, and it comprises the complementary determining region-3 and “F” β-strand of the extracellular domain of TREM-129. In this study LP17 was administered by intranasal delivery to allow direct access to the CNS. However, the method to measure bioavailability of LP17 in brain has not been reported, and we measured TREM-1 as a surrogate to determine LP17 effect. We found that intranasal administration of LP17 reduced TREM-1 and its downstream intracellular signaling proteins as well as inflammatory response after ICH. Thus, we concluded that LP17 could affect TREM-1 receptor via intranasal administration.
Microglia respond to acute brain injury including ICH by polarizing into two main phenotypes: classic M1-like phenotype and alternative M2-like phenotype30. ICH outcomes were improved by inhibiting M1 activation or promoting transition of M1 to M2 phenotype4, 31. We evaluated the role of TREM-1 on microglia polarization and the result suggested that TREM-1 enhanced neuroinflammation by modulating microglia polarization after ICH. However, TREM-1 may also participate in neuroinflammation via infiltrated immune cells that release inflammation related factors32. This study focused on local inflammation mediated via microglia TREM-1 receptor. Further studies are necessary to assess the role of peripheral TREM-1 mediated inflammation.
Previous study reported that activated TREM-1 recruited PLC-γ via SYK 9. Furthermore, PLC-γ played a role in microglia polarization via PKC δ/CARD9 signaling33, 34. PKC δ, a member of the novel PKC isoform family is a critical regulator of inflammatory response11. Knockdown of PKC δ reduced microglial proinflammatory response, which was associated with NF-κB downregulation in Parkinson’s disease model35. Increased PKC α/δ was reported after ICH, and dihydrochloride (H7), a total PKC inhibitor, reduced ICH-induced injury36. CARD9 which is related with PKC δ, is a critical adaptor protein expressed in myeloid cells. CARD9 initiated inflammatory cytokine cascade and recruited neutrophil infiltration via NF-κB signaling37. The role of PKC δ and CARD9 in neuroinflammation after ICH has not been characterized. We observed that LP17 reduced TREM-1 downstream proteins and promoted M2 polarization after ICH, and these effects were reversed with CARD9 activation. Furthermore, the detrimental effects of TREM-1 activation were reversed with PKC δ inhibition. These findings suggested that PKC δ/CARD9 were key intracellular proteins mediating TREM-1 effects on microglia polarization after ICH.
There is currently limited data available about the ligand for TREM-1, and much remains unclear. We found that HMGB1 interaction with TREM-1 increased after ICH. Consistently, TREM-1 regulated inflammation via interacting with HMGB1 in a model of liver cancer12. Injured neurons released HMBG1 and induced inflammatory response after ICH, and an anti-HMGB1 neutralizing mAb improved outcomes13. Likewise, we found that HMGB1 inhibitor alleviated neuroinflammation after ICH which was abolished by TREM-1 activation. This suggested HMGB1 interaction with TREM-1 possibly activated PKC δ/CARD9 downstream signaling pathway to modulate inflammatory response after ICH.
There are several limitations in this study. First, TREM-1 was also expressed in neurons and endothelial cells as previously reported38, and TREM-1 knockout or LP17 treatment affected the global brain with no cell type-specific manipulation. Cell specific functions of TREM-1 need to be further explored. TREM-1 may also regulate inflammatory response though proteins other than PKC δ/CARD9. Second, HMGB1 can stimulate other receptors such as receptor for advanced glycation end products (RAGE) and toll-like receptors (TLRs). Third, Glycyrrhizin may also affect other targets and the specificity of Rottlerin remains controversial. Fourth, male mice were used in this study considering that estrogen would influence ICH outcomes. Use of only male animals may cause some bias. Another limitation that should be considered in this study is the age of mice, only young animals were used. Lastly, this study focused on early stage of ICH, and more study should be designed to evaluate LP17 effect on long-term ICH outcomes.
Summary
TREM-1 inhibition by LP17 improved neurobehavioral outcomes after ICH via modulating microglial polarization from M1 to M2 phenotype and reducing neuroinflammation, possibly mediated by PKC δ/CARD9 signaling. TREM-1 interaction with HMBG1 increased after ICH, which may be a potential endogenous ligand of TREM-1. Thus, microglial TREM-1 may be a therapeutic target for ICH patients.
Supplementary Material
Acknowledgments
QL and RL conducted experiments, analyzed data and drafted the manuscript. WH conducted experiments and analyzed data. PS and SY edited the manuscript. RR and YF helped in ICH model and neurobehavioral tests. YH, HS and LT participated in conducting the experiments. PS, JZ and JT worked on research design and manuscript preparation.
Sources of Funding
This work was supported by the National Institutes of Health (NS101284) to Jiping Tang.
Non-standard Abbreviations and Acronyms:
- ICH
intracerebral hemorrhage
- TREM-1
triggering receptor expressed on myeloid cells 1
- SYK
spleen tyrosine kinase
- PLC-γ
phospholipase-C-gamma
- PKC δ
protein kinase C δ
- CARD9
Caspase recruitment domain family member 9
- HMGB1
high mobility group box 1
- KO
knockout
- ACT
activation
- CO-IP
co-immunoprecipitation
- BWC
brain water content
Footnotes
Disclosures
None.
References
- 1.Cordonnier C, Demchuk A, Ziai W, Anderson CS. Intracerebral haemorrhage: Current approaches to acute management. Lancet. 2018;392:1257–1268 [DOI] [PubMed] [Google Scholar]
- 2.Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2015;46:2032–2060 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z, Zhang Z, Lu H, Yang Q, Wu H, Wang J. Microglial polarization and inflammatory mediators after intracerebral hemorrhage. Molecular neurobiology. 2017;54:1874–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Q, Gao L, Huang L, Ruan L, Yang J, Huang W, Li Z, Zhang Y, Jin K, Zhuge Q. Inhibition of mammalian target of rapamycin improves neurobehavioral deficit and modulates immune response after intracerebral hemorrhage in rat. Journal of neuroinflammation. 2014;11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arts RJ, Joosten LA, van der Meer JW, Netea MG. Trem-1: Intracellular signaling pathways and interaction with pattern recognition receptors. Journal of leukocyte biology. 2013;93:209–215 [DOI] [PubMed] [Google Scholar]
- 6.Xu P, Zhang X, Liu Q, Xie Y, Shi X, Chen J, Li Y, Guo H, Sun R, Hong Y, et al. Microglial trem-1 receptor mediates neuroinflammatory injury via interaction with syk in experimental ischemic stroke. Cell death & disease. 2019;10:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun XG, Duan H, Jing G, Wang G, Hou Y, Zhang M. Inhibition of trem-1 attenuates early brain injury after subarachnoid hemorrhage via downregulation of p38mapk/mmp-9 and preservation of zo-1. Neuroscience. 2019;406:369–375 [DOI] [PubMed] [Google Scholar]
- 8.Jiang T, Zhang YD, Gao Q, Zhou JS, Zhu XC, Lu H, Shi JQ, Tan L, Chen Q, Yu JT. Trem1 facilitates microglial phagocytosis of amyloid beta. Acta neuropathologica. 2016;132:667–683 [DOI] [PubMed] [Google Scholar]
- 9.Tammaro A, Derive M, Gibot S, Leemans JC, Florquin S, Dessing MC. Trem-1 and its potential ligands in non-infectious diseases: From biology to clinical perspectives. Pharmacology & therapeutics. 2017;177:81–95 [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Park SH, Baek JM, Erkhembaatar M, Kim MS, Yoon KH, Oh J, Lee MS. Harpagoside inhibits rankl-induced osteoclastogenesis via syk-btk-plcgamma2-ca(2+) signaling pathway and prevents inflammation-mediated bone loss. Journal of natural products. 2015;78:2167–2174 [DOI] [PubMed] [Google Scholar]
- 11.Yang Q, Langston JC, Tang Y, Kiani MF, Kilpatrick LE. The role of tyrosine phosphorylation of protein kinase c delta in infection and inflammation. International journal of molecular sciences. 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Li J, Salcedo R, Mivechi NF, Trinchieri G, Horuzsko A. The proinflammatory myeloid cell receptor trem-1 controls kupffer cell activation and development of hepatocellular carcinoma. Cancer research. 2012;72:3977–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D, Liu K, Wake H, Teshigawara K, Mori S, Nishibori M. Anti-high mobility group box-1 (hmgb1) antibody inhibits hemorrhage-induced brain injury and improved neurological deficits in rats. Scientific reports. 2017;7:46243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Y, Xiong KL, Lin S, Zhong Q, Lu FL, Liang H, Li JC, Wang JZ, Yang QW. Elevation of high-mobility group protein box-1 in serum correlates with severity of acute intracerebral hemorrhage. Mediators of inflammation. 2010;2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan J, Zuo G, Sherchan P, Huang L, Ocak U, Xu W, Travis ZD, Wang W, Zhang JH, Tang J. Ccr1 activation promotes neuroinflammation through ccr1/tpr1/erk1/2 signaling pathway after intracerebral hemorrhage in mice. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rynkowski MA, Kim GH, Komotar RJ, Otten ML, Ducruet AF, Zacharia BE, Kellner CP, Hahn DK, Merkow MB, Garrett MC, et al. A mouse model of intracerebral hemorrhage using autologous blood infusion. Nature protocols. 2008;3:122–128 [DOI] [PubMed] [Google Scholar]
- 17.Wang G, Manaenko A, Shao A, Ou Y, Yang P, Budbazar E, Nowrangi D, Zhang JH, Tang J. Low-density lipoprotein receptor-related protein-1 facilitates heme scavenging after intracerebral hemorrhage in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2017;37:1299–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luh C, Feiler S, Frauenknecht K, Meyer S, Lubomirov LT, Neulen A, Thal SC. The contractile apparatus is essential for the integrity of the blood-brain barrier after experimental subarachnoid hemorrhage. Translational stroke research. 2019;10:534–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navarro-Oviedo M, Roncal C, Salicio A, Belzunce M, Rabal O, Toledo E, Zandio B, Rodriguez JA, Paramo JA, Munoz R, et al. Mmp10 promotes efficient thrombolysis after ischemic stroke in mice with induced diabetes. Translational stroke research. 2019;10:389–401 [DOI] [PubMed] [Google Scholar]
- 20.Peng J, Pang J, Huang L, Enkhjargal B, Zhang T, Mo J, Wu P, Xu W, Zuo Y, Peng J, et al. Lrp1 activation attenuates white matter injury by modulating microglial polarization through shc1/pi3k/akt pathway after subarachnoid hemorrhage in rats. Redox biology. 2019;21:101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke. 2011;42:1781–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J Preclinical and clinical research on inflammation after intracerebral hemorrhage. Progress in neurobiology. 2010;92:463–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford JW, McVicar DW. Trem and trem-like receptors in inflammation and disease. Current opinion in immunology. 2009;21:38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li JT, Zhang Y. Trem2 regulates innate immunity in alzheimer's disease. Journal of neuroinflammation. 2018;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S, Peng J, Sherchan P, Ma Y, Xiang S, Yan F, Zhao H, Jiang Y, Wang N, Zhang JH, et al. Trem2 activation attenuates neuroinflammation and neuronal apoptosis via pi3k/akt pathway after intracerebral hemorrhage in mice. Journal of neuroinflammation. 2020;17:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelham CJ, Pandya AN, Agrawal DK. Triggering receptor expressed on myeloid cells receptor family modulators: A patent review. Expert opinion on therapeutic patents. 2014;24:1383–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X, Luo J, Liu H, Cui W, Guo K, Zhao L, Bai H, Guo W, Guo H, Feng D, et al. Recombinant adiponectin peptide ameliorates brain injury following intracerebral hemorrhage by suppressing astrocyte-derived inflammation via the inhibition of drp1-mediated mitochondrial fission. Translational stroke research. 2020 [DOI] [PubMed] [Google Scholar]
- 28.Topkoru BC, Altay O, Duris K, Krafft PR, Yan J, Zhang JH. Nasal administration of recombinant osteopontin attenuates early brain injury after subarachnoid hemorrhage. Stroke. 2013;44:3189–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibot S, Kolopp-Sarda MN, Bene MC, Bollaert PE, Lozniewski A, Mory F, Levy B, Faure GC. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. The Journal of experimental medicine. 2004;200:1419–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol. 2017;13:420–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei J, Wang M, Jing C, Keep RF, Hua Y, Xi G. Multinucleated giant cells in experimental intracerebral hemorrhage. Translational stroke research. 2020;10:1095–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Q, Johnson EM, Lam RK, Wang Q, Bo Ye H, Wilson EN, Minhas PS, Liu L, Swarovski MS, Tran S, et al. Peripheral trem1 responses to brain and intestinal immunogens amplify stroke severity. Nat Immunol. 2019;20:1023–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imai Y, Kohsaka S. Intracellular signaling in m-csf-induced microglia activation: Role of iba1. Glia. 2002;40:164–174 [DOI] [PubMed] [Google Scholar]
- 34.Lee SW, Hu YS, Hu LF, Lu Q, Dawe GS, Moore PK, Wong PT, Bian JS. Hydrogen sulphide regulates calcium homeostasis in microglial cells. Glia. 2006;54:116–124 [DOI] [PubMed] [Google Scholar]
- 35.Gordon R, Singh N, Lawana V, Ghosh A, Harischandra DS, Jin H, Hogan C, Sarkar S, Rokad D, Panicker N, et al. Protein kinase cdelta upregulation in microglia drives neuroinflammatory responses and dopaminergic neurodegeneration in experimental models of parkinson's disease. Neurobiology of disease. 2016;93:96–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui GY, Gao XM, Qi SH, Gillani A, Gao L, Shen X, Zhang YD. The action of thrombin in intracerebral hemorrhage induced brain damage is mediated via pkcalpha/pkcdelta signaling. Brain research. 2011;1398:86–93 [DOI] [PubMed] [Google Scholar]
- 37.Zhong X, Chen B, Yang L, Yang Z. Molecular and physiological roles of the adaptor protein card9 in immunity. Cell death & disease. 2018;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jolly L, Carrasco K, Derive M, Lemarie J, Boufenzer A, Gibot S. Targeted endothelial gene deletion of triggering receptor expressed on myeloid cells-1 protects mice during septic shock. Cardiovascular research. 2018;114:907–918 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available within the article and additional data can be acquired from the corresponding author.