Abstract
Klinefelter syndrome (KS, 47,XXY) is a common sex chromosome aneuploidy in males that is associated with a wide range of cognitive, social and emotional characteristics. The neural bases of these symptoms, however, are unclear. Brain structure in 19 pre- or early-pubertal boys with KS (11.5 ± 1.8 years) and 22 typically developing (control) boys (8.1 ± 2.3 years) was examined using surface-based analyses of cortical gray matter volume, thickness and surface area. Boys in the KS group were treatment-naïve with respect to testosterone replacement therapy. Reduced volume in the insula and dorsomedial prefrontal cortex was observed in the KS relative to the TD group, as well as increased volume in the parietal, occipital and motor regions. Further inspection of surface-based metrics indicated that whereas KS-associated increases in volume were driven by increases in thickness, KS-associated reductions in volume were associated with decreases in surface area. Exploratory analyses indicated correlations between brain structure and behavior, providing initial support for a neural basis of cognitive and emotional symptoms of this condition. Taken together, these data add support for a neuroanatomical phenotype of KS and extend previous studies through clarifying the precise neuroanatomical structural characteristics of that give rise to volumetric alterations.
Keywords: brain structure, Klinefelter syndrome, XXY, testosterone, MRI, genetics
1. Introduction
Klinefelter syndrome (KS) is the most common sex chromosome aneuploidy (47,XXY), affecting as many as 1 in 500 males (Smyth et al. 1998). Typical physical characteristics of the condition include androgen insufficiency, tall stature, gynecomastia and impaired spermatogenesis. A variety of cognitive, social and emotional features also accompany KS, including language and learning problems, executive dysfunction, poor concentration, low self-esteem, increased shyness, worry and depressed mood (Herlihy et al. 2011). Such features of KS often go underappreciated, yet are frequently cited as being the greatest clinical concern by parents (Bourke et al. 2014).
To better understand the nature of KS-associated alterations in behavior, investigators have turned to the use of neuroimaging to examine brain structural patterns in this population. Findings from our group (Bryant et al. 2011; Hong et al. 2014; A. J. Patwardhan et al. 2002) and others has documented a variety of KS-related reductions in gray matter, including in the temporal lobe (Bryant et al. 2011; DeLisi et al. 2005; Giedd et al. 2007; Goddard et al. 2016; Itti et al. 2006; Patwardhan et al. 2000; Skakkebæk et al. 2014), insula (Bryant et al. 2011; Goddard et al. 2016; Hong et al. 2014; Shen et al. 2004; Skakkebæk et al. 2014), and inferior frontal cortex (Bryant et al. 2012; DeLisi et al. 2005; Goddard et al. 2016; Skakkebæk et al. 2014). Anomalous increases in cortical gray matter have also been noted in the sensorimotor (Bryant et al. 2011; Hong et al. 2014; Savic et al. 2014), cuneus/precuneus (Hong et al. 2014; Skakkebæk et al. 2014) and parietal-occipital cortices (Bryant et al. 2012; Hong et al. 2014; Skakkebæk et al. 2014). Importantly however, there are inconsistencies in findings, with many research groups reporting no alterations in these regions (DeLisi et al. 2005; Giedd et al. 2007; Goddard et al. 2016; Itti et al. 2006; Shen et al. 2004).
Investigations of brain-behavior correlations have resulted in similarly mixed findings. Reduced left temporal lobe volume in KS was correlated in one report with poorer performance on language-related tasks (Itti et al. 2006). Others however have reported no observable correlations between brain and behavioral measures (Bryant et al. 2011; Skakkebæk et al. 2014) or did not include an examination of these associations (DeLisi et al. 2005; Giedd et al. 2007; Goddard et al. 2016; Hong et al. 2014). Clarification of the relation between structural alterations in the brain and cognitive and psychosocial difficulties nevertheless represent a critical research goal, given that the severity of behavioral difficulties can vary considerably between affected individuals, and can negatively influence long-term academic, adaptive (Boada et al. 2009) and psychological functioning (Bruining et al. 2009). Indeed, early identification and intervention for cognitive, social and emotional difficulties represents a pressing research priority for individuals with KS and for treating healthcare providers.
Factors contributing to inconsistencies in findings include age, androgen treatment status and neuroimaging analysis methods. Notably, most studies have included participant samples that have varied with respect to pubertal status and to history of testosterone supplementation, both of which have a significant effect on brain structure (Blakemore et al. 2010; Foland-Ross et al. 2019; Giedd et al. 2006; Patwardhan et al. 2000; Samango-Sprouse et al. 2013, but see Skakkebæk et al. 2014b). Moreover, existing work has largely relied on voxel based morphometry (VBM) and manual region-of-interest (ROI) based methods in the quantification of cortical gray matter volume. In contrast to these approaches, newer surface-based modelling packages can measure the components that make up gray matter volume: cortical thickness and surface area. These metrics follow different neurodevelopmental trajectories (Fjell et al. 2015; Hogstrom et al. 2013), have distinct genetic influences (Panizzon et al. 2009; Winkler et al. 2010) and exhibit unique associations with cognitive functions and psychiatric conditions (Noble et al. 2015; Schnack et al. 2015; Vuoksimaa et al. 2016). Moreover, the biological processes that drive surface area are separate from those that drive thickness (Geschwind et al. 2013; Rakic 1988). Thus, studies that incorporate measures of cortical thickness and surface area in addition to volume are likely to yield additional clarity into the underlying neural characteristics of different clinical conditions and their associated behaviors.
Given these issues, we conducted a rigorous analysis of KS-associated alterations in cortical gray matter structure using a surface-based analysis of cortical volume, thickness and surface area. To control for the effects of puberty and testosterone replacement therapy, we focused on pre- or early-pubertal males who had not yet initiated testosterone replacement therapy. We hypothesized that males with KS would exhibit reduced cortical gray matter in the insula, temporal and frontal cortices relative to TD males, as well as increased cortical gray matter in the parietal and sensorimotor regions. Additionally, we explored whether KS-associated alterations in cortical gray matter were correlated with differences in cognitive and behavioral symptoms associated with this condition.
2. Methods
2.1. Participants
The study was approved by the Stanford University’s institutional review board. Boys provided written assent, and a parent provided written informed consent. Tanner staging was performed by a trained physician. A total of 19 boys with KS (11.5 ± 1.8 years) and 22 TD boys (8.1 ± 2.3 years) were included in the study. Details regarding recruitment and eligibility criteria can be found in the Supplement.
2.2. Cognitive and Behavioral Testing
Parents completed the Behavioral Assessment System for Children, Second Edition (BASC-II; Reynolds 2004) to provide information on their child’s emotions and behavior, and the Behavior Rating Inventory of Executive Function (BRIEF; Gioia et al. 2000), to provide information relating to their child’s executive functioning. Children were administered the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler 2003), as well as the Wide Range Assessment of Visual Motor Abilities to index visual-motor skills (WRAVMA; Adams et al. 1995), the Wide Range Achievement Test, Fourth Edition, to index academic skills (WRAT; Wilkinson et al. 2006), and the Developmental NEuroPSYchological Assessment (NEPSY, Second Edition), to index a variety of cognitive skills including executive functioning/attention, language, memory/learning, sensorimotor functioning, visuospatial processing, social perception (Korkman et al. 2007). Age-normed composite T scores were compared between KS and TD groups using independent sample t tests. Scores exhibiting a significant difference between groups were fed into secondary correlation analyses to test for brain-behavior associations.
2.3. MRI Acquisition and Analysis
Imaging data were acquired using a Signa 3.0 T whole-body MR system (GE Medical Systems, Milwaukee, WI) and processed using the FreeSurfer software package (version 5.3, http://surfer.nmr.mgh.harvard.edu). Details on MRI data acquisition can be found in the Supplement.
Statistical analyses were conducted to assess group differences in cortical gray matter volume, thickness and surface area. For each of these analyses, a general linear model (GLM) was fit at each vertex with the structural measure as the dependent variable and diagnostic group (KS, TD) as the independent variable. Age and total cortical tissue volume were included as covariates, centered to the sample mean. Correction for multiple comparisons was conducted using Monte-Carlo simulation.
2.4. Associations between cortical gray matter structure and cognition
Associations between brain and behavior were examined separately within the KS and TD groups using bivariate correlation analyses. After checking distributions with the Shapiro-Wilks test, associations between normally distributed variables were assessed by Pearson correlation. Non-normally distributed data was analyzed using Spearman correlation. Structural metrics were adjusted for age and total cortical tissue volume. To reduce the number of comparisons and because volume represents the direct product of thickness and surface area (Fischl et al. 2000), we constrained these exploratory analyses to volume measurements. Bonferonni correction for multiple comparisons was conducted across the number of brain regions tested. Due to the exploratory nature of these analyses however, we did not correct for the number of behavioral tests or sample groups.
3. Results
3.1. Participants
Participants in the KS and TD groups did not differ with respect to age, t(39) = −0.607, p = 0.547, total cortical tissue volume, t(39) = 1.686, p = 0.100, or Tanner stage, t(39) = 0.440, p = 0.663 (Table 1).
Table 1.
Participant characteristics
| KS | TD | p value | |
|---|---|---|---|
| N | 19 | 22 | - |
| Age (years) | 8.5 ± 2.1 | 8.1 ± 2.3 | 0.547 |
| Tanner stage | 1.1 ± 0.3 | 1.2 ± 0.4 | 0.663 |
| Total cortical tissue volume (cm3) | 992 ± 79 | 1041 ± 103 | 0.100 |
KS, Klinefelter syndrome. TD, typically developing. Values for the KS and TD groups are means ± standard deviation.
Comparisons of standardized scores from behavioral assessments indicated significant differences between the groups (Table 2), including measures of intelligence (Full Scale IQ, Perceptual Reasoning Index, Working Memory Index on the WISC-IV; ps < 0.01), visuospatial capabilities (Visual-Motor Integration Composite of the WRAVMA; p = 0.027), emotion and behavior (Behavioral Symptoms Index, Internalizing Problems composite and Adaptive Skills composite of the BASC-2; ps < 0.031), executive functioning (Behavioral Regulation Index, Metacognition Index and Global Executive Composite on the BRIEF, Response Set subtest on the NEPSY; ps < 0.0289), memory (Memory for Faces and Narrative Memory subtests on the NEPSY; ps < 0.049) and verbal skills (Verbal Comprehension Index on the WISC-IV, Word Reading and Spelling subtests of the WRAT; Comprehension of Instructions on the NEPSY; ps < 0.049).
Table 2.
Behavioral and cognitive functioning differences between groups
| Assessment | KS | TD | p value |
|---|---|---|---|
| WISC-IV | |||
| Full scale Intelligence Quotient (FSIQ)a | 94.7 ± 14.2 | 111.5 ± 8.5 | < 0.001 |
| Perceptual Reasoning Index (PRI)a | 102.3 ± 13.4 | 113.2 ± 11.5 | 0.008 |
| Verbal Comprehension Index (VCI)a | 95.3 ± 14.6 | 116.5 ± 14.5 | < 0.001 |
| Processing Speed Index (PSI)a | 92.4 ± 12.5 | 96.3 ± 12.1 | 0.321 |
| Working Memory Index (WMI)a | 88.0 ± 15.9 | 102.3 ± 9.3 | 0.003 |
| BASC-2 | |||
| Behavioral Symptoms Indexb | 55.6 ± 12.5 | 47.1 ± 5.7 | 0.012 |
| Adaptive Skills3 | 43.7 ± 10.6 | 50.3 ± 7.9 | 0.030 |
| Externalizing Problemsb | 54.1 ± 11.8 | 49.3 ± 8.3 | 0.145 |
| Internalizing Problemsb | 54.2 ± 15.8 | 45.9 ± 9.9 | 0.050 |
| BRIEF | |||
| Behavioral Regulation Indexb | 55.4 ± 13.4 | 45.7 ± 7.5 | 0.009 |
| Metacognition Indexb | 59.6 ± 14.5 | 49.9 ± 11.0 | 0.021 |
| Global Executive Compositeb | 58.5 ± 14.3 | 48.0 ± 9.5 | 0.011 |
| NEPSY-II* | |||
| Comprehension of Instructionsa | 10.1 ± 2.5 | 12.0 ± 2.0 | 0.020 |
| Response seta | 8.4 ± 3.6 | 11.3 ± 2.6 | 0.028 |
| Memory for faces (delayed)a | 10.1 ± 2.9 | 11.8 ± 2.0 | 0.048 |
| Narrative memory (free and cued recall)a | 8.3 ± 3.0 | 10.7 ± 3.4 | 0.035 |
| WRAVMA | |||
| Visual-Motor Integration Compositea | 94.6 ± 13.3 | 104.0 ± 11.4 | 0.027 |
| WRAT | |||
| Word readinga | 101.5 ± 11.1 | 111.5 ± 12.9 | 0.016 |
| Spellinga | 101.5 ± 16.4 | 111.8 ± 12.2 | 0.048 |
| Matha | 102.6 ± 13.2 | 107.9 ± 10.3 | 0.188 |
| Sentence comprehensiona | 90.3 ± 30.5 | 113.4 ± 35.4 | 0.061 |
| Reading compositea | 98.1 ± 13.7 | 117.8 ± 13.6 | 0.001 |
KS, Klinefelter syndrome. TD, typically developing. WISC-IV, Wechsler Intelligence Scale for Children, 4th Edition. BASC-2, Behavior Assessment System for Children 2nd Edition. BRIEF, Behavior Rating Inventory of Executive Functioning. NEPSY, A Developmental NEuroPSYchological Assessment. WRAVMA, Wide Range Assessment of Visual Motor Abilities. WRAT, Wide Range Achievement Test. A, higher scores indicate better performance. B, higher scores indicate worse performance.
See Supplement for more information.
3.2. Statistical analysis of a main effect of group on cortical gray matter structure
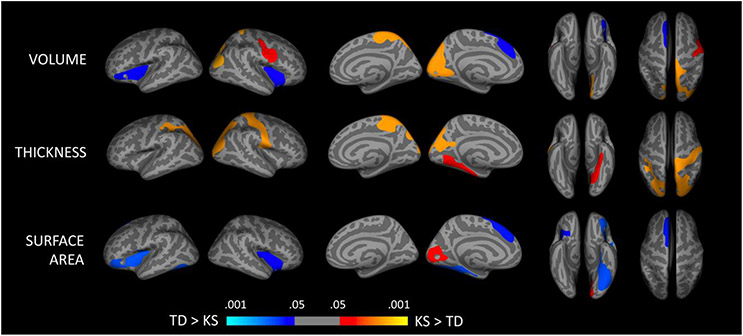
Group differences in cortical structure are presented in Table 3 and Figure 1. Vertex-based analyses of cortical gray matter volume indicated decreased volume in the KS relative to the TD group in the left and right insula and left dorsomedial prefrontal cortex. Increased volume in the KS group was observed in a cluster in the left occipital cortex that included the precuneus, cuneus, pericalcarine cortex and lingual gyrus, a cluster in the right parietal cortex that included the paracentral cortex and precuneus, and a cluster in the right precentral gyrus.
Table 3.
Regional differences in cortical gray matter structure between groups
| Region | KS | TD | x/y/z | Cluster Size (mm2) |
corrected p value |
||
|---|---|---|---|---|---|---|---|
| Volume (mm3) | |||||||
| Left insula | 5667 ± 319 | 6366 ± 393 | −26 | 24 | −6 | 2233 | 0.017 |
| Right insula | 4694 ± 435 | 5534 ± 493 | 33 | 4 | 7 | 1879 | 0.043 |
| Left dorsomedial prefrontal cortex | 4354 ± 589 | 5176 ± 491 | −7 | 34 | 50 | 1893 | 0.028 |
| Left occipital cortex | 10872 ± 1515 | 9541 ± 1387 | −12 | −67 | 35 | 4735 | <0.001 |
| Right precentral gyrus | 4533 ± 636 | 4081 ± 557 | 40 | −11 | 43 | 1854 | 0.039 |
| Right parietal/occipital cortex | 12723 ± 1303 | 10646 ± 1326 | 3 | −33 | 65 | 4656 | <0.001 |
| Thickness (mm) | |||||||
| Left parahippocampal gyrus | 2.819 ± 0.212 | 2.596 ± 0.169 | −35 | −23 | −23 | 2260 | 0.018 |
| Left parietal/occipital cortex | 2.248 ± 0.114 | 2.062 ± 0.123 | −47 | −28 | 53 | 4632 | <0.001 |
| Right parietal/occipital cortex | 2.319 ± 0.130 | 2.128 ± 0.148 | 62 | −10 | 29 | 11363 | <0.001 |
| Surface Area (mm2) | |||||||
| Left insula | 2297 ± 207 | 2567 ± 260 | −26 | 24 | −6 | 2917 | 0.002 |
| Right insula | 1468 ± 114 | 1710 ± 181 | 37 | −13 | 2 | 2140 | 0.015 |
| Left dorsomedial prefrontal cortex | 1674 ± 140 | 1870 ± 181 | −7 | 34 | 50 | 2312 | 0.011 |
| Left fusiform | 2060 ± 242 | 2500 ± 417 | −43 | −65 | −19 | 2809 | 0.002 |
| Left occipital cortex | 1770 ± 264 | 1635 ± 253 | −13 | −91 | 4 | 1932 | 0.017 |
KS, Klinefelter syndrome. TD, typically developing. Values for the KS and TD groups are means ± standard deviation. X/Y/Z, MNI coordinates of the peak significance within the cluster. Significance values indicate the cluster wise p value, corrected for multiple comparisons.
Figure 1.
Corrected statistical significance maps showing areas of reduced (blue) and increased (orange/red) cortical gray matter in boys with KS relative to typically developing (TD) boys. Colors are corrected significance (p) values, shown on the inflated surface of the average template.
Vertex-based analyses of cortical gray matter thickness indicated increased thickness in the KS relative to the TD group in 3 regions: a cluster that encompassed the left parahippocampal gyrus, a cluster that included the left occipital and parietal cortex and a third cluster that included the right precentral and parietal cortex. No areas of decreased thickness were observed in the KS relative to the TD group.
Finally, vertex-based analyses of pial surface area indicated decreased surface area in the KS relative to the TD group in the left and right insula, the left dorsomedial prefrontal cortex and the left fusiform gyrus. Increased surface area was observed in the KS relative to the TD group in the left occipital cortex. This cluster included the pericalcalarine cortex and lingual gyrus.
Posthoc analyses of regional measures of volume, thickness and surface area using a multivariate GLM indicated that the main effect of group across all regions remained significant when controlling for FSIQ in addition to age and total cortical tissue volume.
3.3. Secondary analyses of an association between structure and behavior
Exploratory correlations between assessment and brain measures were performed separately within the KS and TD groups. Within the KS group, increased volume in the left dorsomedial prefrontal cortex was correlated with higher FSIQ (WISC, r = 0.679, corrected p = 0.006), better adaptive skills (BASC-2 Adaptive Skills Composite, r = 0.586, corrected p = 0.048), fewer behavioral regulation problems (Behavioral Regulation Index of the BRIEF, r = −0.619, corrected p = 0.030), and improved visuomotor abilities (WRAVMA Visual-Motor Integration Composite, r = 0.634, corrected p = 0.048). Increased parietal volume in the right hemisphere was correlated in the KS group with fewer behavioral problems (Behavioral Symptoms Index of the BASC-2, r = −0.600, corrected p = 0.043). No associations between assessment scores and brain measures were observed within the TD group. Fisher’s r-to-z transformation indicated that correlation differences between groups were not significantly different, ps > 0.05.
4. Discussion
This study was conducted to examine KS-associated alterations in cortical gray matter volume, thickness and surface area in a sample that was recruited to avoid the confounds of puberty and testosterone supplementation. Using advanced surface-based procedures, we observed reduced volume in the insula and dorsomedial prefrontal cortex in the KS relative to the TD group, as well as increased volume in the parietal-occipital and sensorimotor regions. Further inspection of these differences indicated that whereas reductions in volume were associated with decreases in surface area, increases in volume were associated with greater regional cortical thickness. Finally, exploratory analyses indicated correlations between structure and behavior, suggesting a neural basis for KS-associated alterations in emotional and cognitive function (Temple et al. 2003; van Rijn et al. 2018). Taken together, these data add support for a neuroanatomical phenotype of KS and extend previous studies through clarifying the specific aspects of cortical morphometry that may underlie volume alterations in this genetic condition.
A unique strength of the present study is the careful selection of the study sample. The influence of pubertal fluctuations testosterone and other sex hormones on brain structures been well documented in typical development (Bramen et al. 2011). Restricting our examination of brain structure to pre- or early-pubertal boys that have not yet begun treatment with testosterone replacement therapy therefore controls for the confounding influence of this hormone on gray matter structure. Indeed, because testosterone deficiency in KS typically begins at or after the onset of puberty (Salbenblatt et al. 1985, but see Gravholt et al. 2018), alterations in cortical gray matter observed here may likely be due to the genetic components of this condition. Support for this interpretation comes from Savic and Arver (2014), who found that sensorimotor cortical thickness was reduced in XY adult males compared with both XX adult females and XXY adult males, indicating an X-chromosome gene-dosage effect. Future studies that track whether testosterone supplementation in adolescents influences KS-associated differences in cortical gray matter structure is not yet known and is the focus of ongoing studies by our group.
Our finding of reduced insula volume in boys with KS are strikingly consistent with the small extant literature examining structural alterations in males with this condition (Bryant et al. 2011; Hong et al. 2014; Shen et al. 2004; Skakkebæk et al. 2014). This area of the brain is well recognized for its role in the identification, experience and regulation of emotions, as well as social functioning and empathy (Namkung et al. 2017; Singer 2006) – behaviors that are notably affected in KS (Boone et al. 2001; Ratcliffe 1999; van Rijn et al. 2006, 2008, 2014a, 2014b). Unlike previous studies however, the current investigation found that volume reductions in this region of XXY males were driven by alterations in surface area.
Indeed, use of surface-based analyses in the current study serves as a unique contribution to the literature. This method can identify spatially overlapping patterns of alterations in thickness and surface area – metrics that comprise cortical gray matter volume and that are genetically and phenotypically independent from one another (Panizzon et al. 2009; Winkler et al. 2010). While the precise neurobiological factors that drive each metric have yet to be understood, available evidence indicates that one determinant of surface area is the number of cortical columns (Rakic 1988). This number does not change following birth, despite significant increases in surface area seen in childhood (Hill et al. 2010). However, surface area is also influenced by the spacing between columns, and inter-columnar neuropil (Buxhoeveden et al. 2001). Additional research that tests whether these or other factors directly underlie the reductions in cortical gray matter volume and surface area observed here are needed.
Our observations of increased volume of the sensorimotor, cuneus and parietal-occipital areas of boys with KS are also remarkably consistent with those of previous studies (Bryant et al. 2011, 2012; Hong et al. 2014; Savic et al. 2014; Skakkebæk et al. 2014). In contrast to the insula, however, increased volume in these regions appear driven by differences in cortical thickness. Available research finds that across widespread areas of the cortex, there is an inverse relation between thickness and neuronal density (Cahalane et al. 2012; la Fougère et al. 2011). Thickness increases in the sensorimotor, cuneus and parietal-occipital cortices of boys with KS therefore may be driven by a reduction in neuron number. Alternatively, increased thickness in these regions may occur in the absence of an increase in number of neurons, leading to a decrease in density. It is also possible, however, that increased thickness in these regions may be the result of reduced myelination of cortical axons. Neurodevelopmental studies of typically developing youth, for example, find that regional cortical thinning during adolescence is tightly coupled with the expansion of white matter and an increased organization of cortical axons (Alemán-Gómez et al. 2013; Vandekar et al. 2015). Thus, cortical thinning at puberty is not entirely the result of reductions in the size or number of neuron cell bodies or their synaptic processes, but rather by an increase in the myelin coating of fibers in lower cortical layers (Sowell et al. 2004; Toga et al. 2006). Increased proliferation of myelin into the inner periphery of the cortical neuropil, in turn, leads to a change in the MR signal value from gray matter in young children to white matter in adolescents and young adults. Whether increased thickness in the sensorimotor, cuneus and parietal-occipital cortices of boys with KS is the result of reduced neuron number, or decreased myelination of cortical axons or another neurobiological process remains to be clarified. The functional significance of these alterations also remains to be understood. Increased gray matter in this region, for example, may reflect a relative sparing of these regions and their associated functions. In line with this formulation, visuospatial abilities are relatively unaffected in KS (Gravholt et al. 2018). Inconsistent with this interpretation, however, are observations of reduced sensorimotor function in KS (Verri et al. 2010).
The current study is the first, to our knowledge, to observe reductions in left dorsomedial prefrontal and fusiform gray matter in boys with KS. The latter area represents a key neural structure subserving the perception of social and emotional signals as well as structural features from human faces (Schultz et al. 2003). Thus, volumetric and surface area reductions in fusiform gray matter may underlie a reduced capacity of males with KS to recognize faces or identify emotional facial expressions (van Rijn et al. 2018). The dorsomedial prefrontal cortex, in turn, subserves a wider range of higher-order functions, including decision-making (Venkatraman et al. 2012), social processing (Lieberman et al. 2019) and emotion regulation (Downar et al. 2013). Volume reductions in this area may therefore contribute to KS-associated alterations in socio-emotional and executive functioning (Temple et al. 2003; van Rijn et al. 2018). In line with this possibility, exploratory correlations indicated increased volume of this region was associated with better adaptive skills (e.g., social skills, leadership skills, study skills, functional communication skills), improved behavioral regulation (e.g., ability to shift cognitive set and modulate emotions and behavior via appropriate inhibitory control), higher IQ, and increased visuomotor capabilities in XXY males.
A unique strength of the present study is the careful selection of the study sample. Limiting our sample to pre- and early-pubertal males that have not been administered testosterone replacement therapy avoids potential confounds relating to androgen effects on the brain (Foland-Ross et al. 2019; Nguyen et al. 2013; Patwardhan et al. 2000). A primary limitation, however, regards sample size. The small number of participants in our study may have limited our power to detect group differences in brain structure and/or correlations with behavior. Second, while we speculate that regional alterations in thickness and surface area drove local volume differences between the two groups, additional testing is needed to confirm this conclusion. Third, the two groups differed with respect to IQ. Although our findings remained significant when IQ was added to our statistical model, additional research is needed to tease apart the influence of IQ from KS on cortical structure. Finally, although we limited our exploratory correlation analyses to specific domains affected in KS and corrected for multiple comparisons, we cannot exclude the possibility of Type II error.
In summary, in a carefully selected sample of boys designed to control for the potentially confounding effects of testosterone, we observed widespread alterations in cortical gray matter volume in pre- and early-pubertal boys with KS. Further inspection of surface-based metrics indicated that KS-associated increases in cortical gray matter volume were associated with increases in cortical thickness, and further, that reductions in volume were coupled with decreases in surface area. Exploratory analyses additionally indicated correlations between brain structure and behavior, providing initial support for a neural basis of cognitive and emotional symptoms of this condition. Taken together, these data add support for a neuroanatomical phenotype of KS and extend previous studies through clarifying the precise structural characteristics of cortical morphometry that may give rise to alterations in gray matter volume. Future studies that replicate and build upon these findings in larger samples are warranted, as are investigations that tease apart the influence of X-chromosome dosage and testosterone replacement therapy on the brain in boys with this genetic condition.
Supplementary Material
Highlights.
Alterations in cortical gray matter volume in males with Klinefelter syndrome arise early in development.
Increased cortical gray matter volume in boys with Klinefelter syndrome is driven by cortical thickness, whereas reductions in volume are driven by surface area.
Observed correlations between brain structure and behavior provide initial support for a neural basis of cognitive and emotional symptoms in this condition.
Acknowledgements
The authors thank the participants and their families. This work is supported by funding from the National Institute of Mental Health (NIMH; MH099630) and the National Institute of Child Health and Human Development (HD092847, HD049653). We also gratefully acknowledge AXYS for their assistance and support with subject recruitment, and Alexandra Ishak for help with data collection and management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors report no conflicts of interest.
References
- Adams W and Sheslow D (1995), 'Wide Range Assessment of Visual Motor Abilities (WRAVMA). Wilmington, DE: Wide Range', (Inc). [Google Scholar]
- Alemán-Gómez Y, Janssen J, Schnack H, Balaban E, Pina-Camacho L, Alfaro-Almagro F, Castro-Fornieles J, Otero S, Baeza I, Moreno D 2013. The human cerebral cortex flattens during adolescence. J. Neurosci 33: 15004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE 2010. The role of puberty in the developing adolescent brain. Hum. Brain Mapp 31: 926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada R, Janusz J, Hutaff-Lee C, Tartaglia N 2009. The cognitive phenotype in Klinefelter syndrome: a review of the literature including genetic and hormonal factors. Developmental disabilities research reviews. 15:284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone KB, Swerdloff RS, Miller BL, Geschwind DH, Razani J, Lee A, Gonzalo IG, Haddal A, Rankin K, Lu P 2001. Neuropsychological profiles of adults with Klinefelter syndrome. J. Int. Neuropsychol. Soc 7: 446–56. [DOI] [PubMed] [Google Scholar]
- Bourke E, Snow P, Herlihy A, Amor D, Metcalfe S 2014. A qualitative exploration of mothers’ and fathers’ experiences of having a child with Klinefelter syndrome and the process of reaching this diagnosis. Eur. J. Hum. Genet 22: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Dinov ID, Worthman CM, Sowell ER 2011. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb. Cortex 21: 636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruining H, Swaab H, Kas M, van Engeland H 2009. Psychiatric characteristics in a self-selected sample of boys with Klinefelter syndrome. Pediatrics. 123: e865–e70. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Hoeft F, Lai S, Lackey J, Roeltgen D, Ross J, Reiss AL 2011. Neuroanatomical phenotype of Klinefelter syndrome in childhood: a voxel-based morphometry study. The Journal of Neuroscience. 31: 6654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ---. 2012. Sex chromosomes and the brain: a study of neuroanatomy in XYY syndrome. Dev. Med. Child Neurol 54: 1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxhoeveden DP, Switala AE, Litaker M, Roy E, Casanova MF 2001. Lateralization of minicolumns in human planum temporale is absent in nonhuman primate cortex. Brain. Behav. Evol 57: 349–58. [DOI] [PubMed] [Google Scholar]
- Cahalane DJ, Charvet CJ, Finlay BL 2012. Systematic, balancing gradients in neuron density and number across the primate isocortex. Front. Neuroanat 6: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE, Maurizio AM, Svetina C, Ardekani B, Szulc K, Nierenberg J, Leonard J, Harvey PD 2005. Klinefelter's syndrome (XXY) as a genetic model for psychotic disorders. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 135: 15–23. [DOI] [PubMed] [Google Scholar]
- Downar J and Daskalakis ZJ 2013. New targets for rTMS in depression: a review of convergent evidence. Brain Stimul. 6: 231–40. [DOI] [PubMed] [Google Scholar]
- Fischl B and Dale AM 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 97: 11050–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Grydeland H, Krogsrud SK, Amlien I, Rohani DA, Ferschmann L, Storsve AB, Tamnes CK, Sala-Llonch R, Due-Tønnessen P 2015. Development and aging of cortical thickness correspond to genetic organization patterns. Proceedings of the National Academy of Sciences. 112: 15462–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross L, Ross J, Reiss A 2019. Androgen treatment effects on hippocampus structure in boys with Klinefelter Syndrome. Psychoneuroendocrinology. 100: 223–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH and Rakic P 2013. Cortical evolution: judge the brain by its cover. Neuron. 80: 633–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, Blumenthal JD, Tossell JW, Stayer C 2006. Puberty-related influences on brain development. Mol. Cell. Endocrinol 254: 154–62. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Wallace GL, Lenroot RK, Lerch JP, Wells EM, Blumenthal JD, Nelson JE, Tossell JW, Stayer C 2007. XXY (Klinefelter syndrome): a pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics. 119: e232–e40. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L (2000), Behavior Rating inventory of Executive Functions (Lutz, FL: Psychological Assessment Resources, Inc.). [Google Scholar]
- Goddard MN, Swaab H, Rombouts SA, van Rijn S 2016. Neural systems for social cognition: gray matter volume abnormalities in boys at high genetic risk of autism symptoms, and a comparison with idiopathic autism spectrum disorder. Eur. Arch. Psychiatry Clin. Neurosci 266: 523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravholt CH, Chang S, Wallentin M, Fedder J, Moore P, Skakkebæk A 2018. Klinefelter syndrome: integrating genetics, neuropsychology, and endocrinology. Endocr. Rev 39: 389–423. [DOI] [PubMed] [Google Scholar]
- Herlihy AS, McLachlan RI, Gillam L, Cock ML, Collins V, Halliday JL 2011. The psychosocial impact of Klinefelter syndrome and factors influencing quality of life. Genet. Med 13: 632–42. [DOI] [PubMed] [Google Scholar]
- Hill J, Dierker D, Neil J, Inder T, Knutsen A, Harwell J, Coalson T, Van Essen D 2010. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J. Neurosci 30: 2268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogstrom LJ, Westlye LT, Walhovd KB, Fjell AM 2013. The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cereb. Cortex 23: 2521–30. [DOI] [PubMed] [Google Scholar]
- Hong DS, Hoeft F, Marzelli MJ, Lepage J-F, Roeltgen D, Ross J, Reiss AL 2014. Influence of the X-chromosome on neuroanatomy: evidence from Turner and Klinefelter syndromes. The Journal of Neuroscience. 34: 3509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti E, Gaw Gonzalo I, Pawlikowska-Haddal A, Boone K, Mlikotic A, Itti L, Mishkin F, Swerdloff R 2006. The structural brain correlates of cognitive deficits in adults with Klinefelter’s syndrome. The Journal of Clinical Endocrinology & Metabolism. 91: 1423–27. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S (2007), Nepsy-II (San Antonio, TX: Pearson; ). [Google Scholar]
- la Fougère C, Grant S, Kostikov A, Schirrmacher R, Gravel P, Schipper HM, Reader A, Evans A, Thiel A 2011. Where in-vivo imaging meets cytoarchitectonics: the relationship between cortical thickness and neuronal density measured with high-resolution [18F] flumazenil-PET. Neuroimage. 56: 951–60. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Straccia MA, Meyer ML, Du M, Tan KM 2019. Social, self,(situational), and affective processes in medial prefrontal cortex (MPFC): causal, multivariate, and reverse inference evidence. Neurosci. Biobehav. Rev [DOI] [PubMed] [Google Scholar]
- Namkung H, Kim S-H, Sawa A 2017. The insula: an underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends Neurosci. 40: 200–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T-V, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, Israel M, Evans AC, Karama S, Group BDC 2013. Testosterone-related cortical maturation across childhood and adolescence. Cereb. Cortex 23: 1424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O 2015. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci 18: 773–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE 2009. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex 19: 2728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan A, Eliez S, Bender B, Linden M, Reiss A 2000. Brain morphology in Klinefelter syndrome Extra X chromosome and testosterone supplementation. Neurology. 54: 2218–23. [DOI] [PubMed] [Google Scholar]
- Patwardhan AJ, Brown WE, Bender BG, Linden MG, Eliez S, Reiss AL 2002. Reduced size of the amygdala in individuals with 47, XXY and 47, XXX karyotypes. Am. J. Med. Genet 114: 93–98. [DOI] [PubMed] [Google Scholar]
- Rakic P 1988. Specification of cerebral cortical areas. Science. 241: 170–76. [DOI] [PubMed] [Google Scholar]
- Ratcliffe S 1999. Long term outcome in children of sex chromosome abnormalities. Arch. Dis. Child 80: 192–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR (2004), Behavior assessment system for children (Wiley Online Library; ). [Google Scholar]
- Salbenblatt JA, Bender BG, Puck MH, Robinson A, Faiman C, Winter JS 1985. Pituitary-gonadal function in Klinefelter syndrome before and during puberty. Pediatr. Res 19: 82–86. [DOI] [PubMed] [Google Scholar]
- Samango-Sprouse CA, Sadeghin T, Mitchell FL, Dixon T, Stapleton E, Kingery M, Gropman AL 2013. Positive effects of short course androgen therapy on the neurodevelopmental outcome in boys with 47, XXY syndrome at 36 and 72 months of age. American Journal of Medical Genetics Part A. 161: 501–08. [DOI] [PubMed] [Google Scholar]
- Savic I and Arver S 2014. Sex differences in cortical thickness and their possible genetic and sex hormonal underpinnings. Cereb. Cortex 24: 3246–57. [DOI] [PubMed] [Google Scholar]
- Schnack HG, Van Haren NE, Brouwer RM, Evans A, Durston S, Boomsma DI, Kahn RS, Hulshoff Pol HE 2015. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb. Cortex 25: 1608–17. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, Skudlarski P 2003. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philosophical Transactions of the Royal Society B: Biological Sciences. 358: 415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Liu D, Liu H, Clasen L, Giedd J, Davatzikos C 2004. Automated morphometric study of brain variation in XXY males. Neuroimage. 23: 648–53. [DOI] [PubMed] [Google Scholar]
- Singer T 2006. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci. Biobehav. Rev 30: 855–63. [DOI] [PubMed] [Google Scholar]
- Skakkebæk A, Gravholt CH, Rasmussen PM, Bojesen A, Jensen JS, Fedder J, Laurberg P, Hertz JM, Østergaard JR, Pedersen AD 2014. Neuroanatomical correlates of Klinefelter syndrome studied in relation to the neuropsychological profile. NeuroImage: Clinical. 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth CM and Bremner WJ 1998. Klinefelter syndrome. Arch. Intern. Med 158: 1309–14. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW 2004. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci 24: 8223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple CM and Sanfilippo PM 2003. Executive skills in Klinefelter’s syndrome. Neuropsychologia. 41: 1547–59. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER 2006. Mapping brain maturation. Focus (Madison: ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S, de Sonneville L, Swaab H 2018. The nature of social cognitive deficits in children and adults with Klinefelter syndrome (47, XXY). Genes, Brain and Behavior. 17: e12465. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Swaab H, Aleman A, Kahn RS 2006. X Chromosomal effects on social cognitive processing and emotion regulation: A study with Klinefelter men (47, XXY). Schizophr. Res 84: 194–203. [DOI] [PubMed] [Google Scholar]
- ---. 2008. Social behavior and autism traits in a sex chromosomal disorder: Klinefelter (47XXY) syndrome. J. Autism Dev. Disord 38: 1634–41. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Barendse M, van Goozen S, Swaab H 2014a. Social attention, affective arousal and empathy in men with Klinefelter syndrome (47, XXY): Evidence from eyetracking and skin conductance. PLoS One. 9: e84721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rijn S, Stockmann L, Van Buggenhout G, van Ravenswaaij-Arts C, Swaab H 2014b. Social cognition and underlying cognitive mechanisms in children with an extra X chromosome: a comparison with autism spectrum disorder. Genes, Brain and Behavior. 13: 459–67. [DOI] [PubMed] [Google Scholar]
- Vandekar SN, Shinohara RT, Raznahan A, Roalf DR, Ross M, DeLeo N, Ruparel K, Verma R, Wolf DH, Gur RC 2015. Topologically dissociable patterns of development of the human cerebral cortex. J. Neurosci 35: 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V and Huettel SA 2012. Strategic control in decision-making under uncertainty. Eur. J. Neurosci 35: 1075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verri A, Cremante A, Clerici F, Destefani V, Radicioni A 2010. Klinefelter's syndrome and psychoneurologic function. MHR: Basic science of reproductive medicine. 16: 425–33. [DOI] [PubMed] [Google Scholar]
- Vuoksimaa E, Panizzon MS, Chen C-H, Fiecas M, Eyler LT, Fennema-Notestine C, Hagler DJ Jr, Franz CE, Jak AJ, Lyons MJ 2016. Is bigger always better? The importance of cortical configuration with respect to cognitive ability. Neuroimage. 129: 356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D 2003. Wechsler intelligence scale for children–Fourth Edition (WISC-IV). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wilkinson GS and Robertson GJ 2006. Wide range achievement test (WRAT4). Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC 2010. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 53: 1135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.