Abstract
Myopia is the most common eye disorder in the world, which is caused by a mismatch between the optical power of the eye and its excessively long axial length. Recent studies revealed that the regulation of the axial length of the eye occurs via a complex signaling cascade, which originates in the retina and propagates across all ocular tissues to the sclera. The complexity of this regulatory cascade has made it particularly difficult to develop effective anti-myopia drugs. The current pharmacological treatment options for myopia are limited to atropine and 7-methylxanthine, which have either significant side effects or low efficacy. This review focuses on the recent advances in genome-wide studies of the signaling pathways underlying myopia development and discusses the potential of systems genetics and pharmacogenomic approaches for the development of anti-myopia drugs.
Keywords: refractive eye development, emmetropization, signaling pathways, pharmacogenomics, drug discovery, myopia
Role of optical defocus and molecular signaling in etiology of myopia
Myopia (see Glossary) is the most common eye disorder in the world. Its worldwide prevalence is predicted to increase from the current 23% to 50% by 2050 [1]. Because of its increasing prevalence, myopia is rapidly becoming one of the leading causes of vision loss due to serious blinding complications such as retinal tears, retinal detachment, and myopic macular degeneration [2]. The etiology of myopia and the mechanisms of refractive eye development have been the subjects of intense investigation for at least a century (Box 1) [3]. These studies revealed that refractive eye development is controlled by both environmental and genetic factors, which shape optical geometry of the eye in a process called emmetropization [4, 5]. Several environmental factors appear to influence refractive eye development and susceptibility to myopia, including intensity of ambient lighting and light cycle [4, 6–9]; however, human population studies suggest that the leading environmental factors causing human myopia are nearwork and urban indoor environments [5, 10, 11]. Nearwork is believed to be associated with negative optical defocus (Box 2) produced by lag of accommodation [12–16]. The optical blur produced by the negative defocus appears to be an important signal that drives excessive eye growth which causes myopia. The role of optical defocus in emmetropization is supported by animal studies where it was revealed that postnatal eye growth is regulated by the sign of optical defocus [17–22]. Negative optical defocus stimulates eye growth and causes myopia, while positive defocus suppresses it and leads to the development of hyperopia (Box 2, Figure I, lower panel).
Box 1. Major milestones in the history of myopia research.
| 350 BC | Aristotle | Introduction of the term myopia [3] |
| 1280–1311 | Bacon, R. | Introduction of concave beryl lenses for optical correction of myopia [3] |
| 1855 | Von Graefe, F.W. | Proposed that myopia is caused by excessive axial elongation of the eyeball [3] |
| 1965 | Young, F.A. | Experimental confirmation that atropine can suppress myopia in animal models [36] |
| 1977 | Wiesel, T.N. and Raviola, E. | Discovery that visual form deprivation causes myopia; development of the monkey model of myopia [22] |
| 1987 | Troilo, D. et al. | Discovery that refractive eye development is controlled locally by the retina [85] |
| 1988 | Schaeffel, F. et al. | Discovery that negative optical defocus stimulates eye growth and positive optical defocus inhibits it [21] |
| 1990 | Schwartz, M. et al. | Mapping of the first genetic locus linked to myopia [24] |
| 1995 | Norton, T.T. and Rada, J.A. | Discovery that development of myopia is accompanied by restructuring of the sclera [124] |
| 2000 | Fischer, A.J. and Reh, T.A. | Discovery that development of myopia is accompanied by increased neurogenesis at the retinal periphery [93] |
| 2006 | Tkatchenko, A.V. et al. | Discovery that development of myopia is accompanied by large-scale changes in gene expression in the eye [30] |
| 2013 | Smith et al. | Discovery that peripheral optical defocus plays an important role in refractive eye development [88] |
| 2013 | CREAM and 23andMe | Discovery that refractive error development is controlled by multiple chromosomal loci [25, 26] |
| 2015 | Tkatchenko, A.V. et al. | Experimental confirmation of gene-environment interaction in myopia; identification of APLP2 as one of the genes responsible for gene-environment interaction [106] |
| 2018 | Tkatchenko, T.V. et al. | Experimental confirmation that the retina can recognize the sign of optical defocus and discovery that information about the sign of optical defocus is processed by the retina via BESOD mechanism; identification of putative signaling pathways underlying retinal response to optical defocus [31] |
| 2018 | Wu, H. et al. | Discovery that restructuring of the sclera in myopia is caused by the transdifferentiation of fibroblasts into myofibroblasts; identification of putative signaling pathways underlying scleral restructuring in myopia [129] |
Box 2. Refractive eye development.
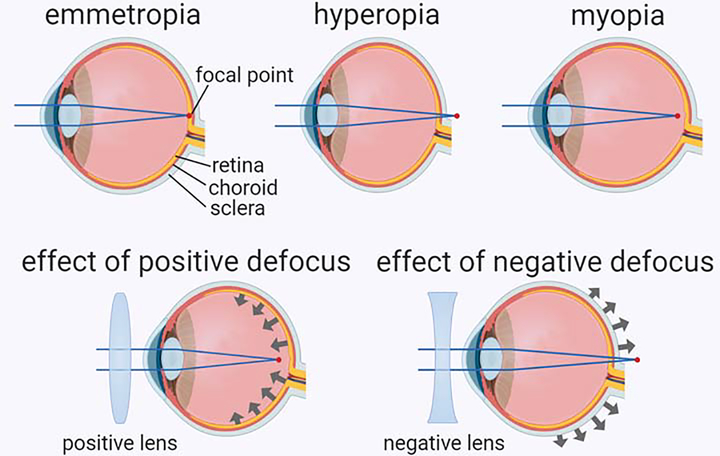
Depending on the relationship between the optical power of the eye and its axial length, the eye can acquire three refractive states (Figure I, upper panel), i.e., emmetropia, hyperopia, and myopia [4, 95]. In emmetropic eyes, the image focuses on the photoreceptors of the retina which ensures sharp vision (Box 2, Figure I, upper left panel). Hyperopic eyes are too short for the optical power of the eye. In hyperopic eyes, the image focuses behind the retina which results in a hyperopic (negative) optical defocus (Box 2, Figure I, upper middle panel). Myopic eyes are too long for the optical power of the eye. In myopic eyes, the image focuses in front of the retina which leads to a myopic (positive) defocus (Box 2, Figure I, upper right panel). Positive optical defocus, which can be imposed by placing a positive lens in front of the eye (Box 2, Figure I, lower left panel), inhibits growth of the eye and leads to the development of hyperopia [4, 17, 19–21]. Negative optical defocus, which can be imposed by placing a negative lens in front of the eye (Box 2, Figure I, lower right panel), stimulates eye growth and leads to the development of myopia [4, 17, 18, 20, 21]. Increasing evidence suggests that the information about optical defocus is integrated across the entire surface of the retina and the balance between positive and negative defocus ultimately determines the growth rate of the eye [4, 87, 88].
Box 2, Figure I. Refractive states of the eye and effect of optical defocus on eye growth.
Upper panel shows the three refractive states of the eye that include emmetropia (left, focal point located at the level of the retina), hyperopia (middle, focal point located behind the retina), and myopia (right, focal point located in front of the retina). Lower panels show the effects of positive (left) and negative (right) optical defocus. While positive optical defocus imposed by a positive lens inhibits eye growth, negative optical defocus imposed by a negative lens accelerates eye growth.
Although the increase in the prevalence of myopia in recent decades is primarily attributed to rapidly increasing exposure of young children to nearwork [11], the contribution of genetic factors to myopia development has been estimated to be between 60% and 80% [5]. Common myopia is a complex genetic disease, which is controlled by hundreds of genes; similar to height and weight [5, 23]. Genetic mapping studies have identified over 250 chromosomal loci linked to human myopia [5, 23–26]. These loci account for less than 10% of variance in refractive error in the human population because the majority of genetic variants causing myopia appear to be either low-frequency or low-effect variants, which are not readily identified by current genetic mapping methodologies [27]. Gene expression profiling studies have uncovered that development of myopia is accompanied by large-scale changes in gene expression in the retina, choroid, and sclera, suggesting that optical defocus triggers sign-of-defocus-specific signaling which regulates refractive eye development [23, 28–32]. Genetic variations in these signaling pathways appear to modulate the impact of visual environment on refractive eye development and determine whether a particular subject becomes emmetropic, hyperopic, or myopic [5, 23].
Optical correction using single-vision corrective lenses, which is the most widely used treatment option for myopia, does not stop the progression of myopia and does not prevent the pathological complications associated with the disease [33]. Several experimental optics-based and drug-based clinical interventions to slow myopia progression, such as multifocal lenses imposing positive defocus at the retinal periphery and atropine eye drops, have shown some promise; however, these treatment options are either associated with significant side effects or have low efficacy [34]. Recent identification of potential signaling pathways that control the eye’s response to optical defocus and susceptibility to myopia provide a framework for the development of new drugs for myopia control. Here we review recent developments in the genome-wide studies of signaling pathways underlying myopia and discuss the potential of emerging pharmacogenomics-based approaches for the development of anti-myopia drugs.
The current state of anti-myopia drug development
While experimental optics-based treatment options for myopia such as orthokeratology (OrthoK) and multi-focal contact lenses have recently gained much attention; however, the best estimates suggest that these methods can only slow the progression of myopia, but not stop it (reviewed in [34]). Therefore, the development of anti-myopia drugs remains a very high priority. The current pharmacological options for myopia control, however, are essentially limited to two drugs, atropine and 7-methylxanthine, which have significant side effects and/or relatively low efficacy.
Atropine
Atropine, a nonselective muscarinic antagonist, is an alkaloid produced by Atropa belladonna, which has been traditionally used in ophthalmic practice as a mydriatic and cycloplegic drug [35]. Atropine has been receiving a lot of attention as a potential anti-myopia drug in recent years [36, 37]. Although the exact mechanism of the suppressive effect of atropine on myopia is unknown, Fischer et al. demonstrated that cholinergic signaling in the retina is not required for the atropine-mediated suppression of myopia [38] in spite of several earlier reports which suggested that cholinergic amacrines might be involved [18, 36, 37, 39]. Carr et al. found that myopia-inhibiting effect of atropine might be mediated by α2a-adrenergic receptors [40], whereas Barathi et al. suggested that gamma-aminobutyric-acid-mediated (GABAergic) signaling is involved [41]. Other studies demonstrated that administration of atropine caused a significant increase in the release of retinal dopamine [42] and implicated nitric oxide (NO) downstream of dopamine in myopia signaling [43, 44].
Several clinical trials have evaluated the effects of different concentrations of atropine on myopia development and its long-term effects on visual function in children. The first trial, Atropine for the treatment of Myopia 1 (ATOM1), revealed that the 1% atropine eye drops retard the progression of myopia by approximately 76% over the 2-year treatment period [45]. However, the follow up study found that the discontinuation of treatment led to a strong rebound effect resulting in the 300% increase in the myopia progression rate compared to placebo during the first 12 months after the cessation of atropine, which eliminated approximately 60% of the 2-year treatment effect [46]. Moreover, 1% atropine caused uncomfortable side effects such as photophobia, reduced accommodation amplitude, and blurred vision. The follow up trial, ATOM2, evaluated the effects of 0.5%, 0.1%, and 0.01% atropine on the progression of myopia in children and found that 0.5% atropine suppressed the progression of myopia by 75%, while 0.1% and 0.01% atropine retarded progression by 68% and 59% respectively [47]. The cessation of treatment caused a 218% rebound increase in the progression rate compared to placebo in the group treated with 0.5% atropine and 170% increase in the group treated with 0.1% atropine during the first 12 months after stopping the administration of the drug [48]. However, the progression rate dropped by approximately 30% in the group treated with 0.01% atropine, suggesting that 0.01% concentration represents a good option for myopia control [48]. These findings were reinforced by the recent 5-year follow up study, which revealed that a higher initial atropine dose predisposed children to greater myopia progression after the cessation of treatment and suggested that 0.01% atropine provides the best long-term outcome [49]. These findings were refined by a recent trial, Low-Concentration Atropine for Myopia Control (LAMP) study, which suggested that low-dose atropine has a dose-dependent suppressive effect on myopia progression [50]. This study found that 0.01% atropine retarded progression of myopia by 27% over 1-year period, compared to 43% and 67% achieved with 0.025% and 0.05% atropine respectively. Considering that all these concentrations were well tolerated by patients and caused minimal side effects, these results suggested that low-dose atropine could be used to control myopia progression in children. However, recent data on the long-term adverse effects of atropine on the development of ocular components and emmetropization in juvenile marmosets put in doubt the utility of atropine as anti-myopia drug [51].
7-methylxanthine
7-methylxanthine (7-MX), a nonselective adenosine receptor antagonist, is a natural metabolite of caffeine and theobromine, two alkaloids produced by several plant species and major constituents of cacao, coffee, and tea [52]. The first indication that 7-MX might be a potential medication for myopia control came from an observation that 7-MX causes thickening of the sclera and an increase in the diameter of the scleral collagen fibrils [53], i.e. it causes changes in the sclera opposite to those observed in myopic eyes. A small follow-up clinical trial analyzed the effect of a daily oral consumption of 400 mg (~15 mg/kg) of 7-MX on the progression of myopia in children and revealed that 7-MX can potentially suppress myopia by approximately 22% in subjects with slow progressing myopia, while had no effect on myopia progression in the subjects with high rates of progression [54]. In guinea pigs, a 300 mg/kg dose of 7-MX was shown to suppress myopia by 49% [55]. Similarly, a 30 mg/kg dose of 7-MX reduced the extent of induced myopia in rabbits by ~67% [56]. The latest data from a study in monkeys also suggested that 7-MX can suppress myopia in primates, but the effect strongly depended on the genetic background of a specific subject [57]. Thus, preliminary data suggest that 7-MX has therapeutic potential for myopia control in subjects with slow progressing myopia, but the questions of the effective dose and efficacy in humans remain to be clarified. It also should be noted that the safety profile and long-term effects of daily oral consumption of 7-MX in children is currently unknown.
Other pharmacological compounds with anti-myopic potential
Several other compounds have been demonstrated to suppress myopia. The muscarinic receptor antagonists pirenzepine and himbacine were shown to inhibit the development of experimental myopia in tree shrews, rhesus monkeys, and chickens [18, 58, 59]. While pirenzepine was found to suppress the progression of myopia in children by ~40%, clinical trials were eventually discontinued [60–62]. Several GABAB and GABAC receptor antagonists, such as (1,2,5,6-tetrahydropyridin-4yl) methylphosphinic acid (TPMPA), CGP46381, and (3-aminocyclopentanyl) butylphosphinic acid (3-ACPBPA) were shown to suppress myopia development in chickens and guinea pigs [63–66]. Further, α-adrenergic agonists, such as clonidine and guanfacine, were shown to inhibit experimentally induced myopia in chickens [67], while brimonidine suppressed myopia in chickens [67] and guinea pigs [68]. Moreover, apomorphine, a dopamine receptor agonist, was found to inhibit myopia development in several animal models, such as chicken, mouse and non-human primates [69–73], and an intraocular-pressure-lowering drug latanoprost was found to reduce progression of myopia in guinea pigs [74]. Finally, a recent drug screen in a mouse model of myopia identified crocetin, a natural carotenoid found in the crocus flowers and Gardenia jasminoides fruits, as a potential anti-myopia agent [75].
Pharmacogenomics and development of new drugs
Pharmacogenomic approaches are increasingly being used to identify new pharmacological compounds and identify existing drugs that can be repurposed for other diseases [76]. One of the strategies is to use information about disease-causing genes obtained from genome wide association studies (GWAS) and gene linkage studies to identify molecular targets that are then pursued for drug development [76, 77]. This approach is based on the observation that genes, which are associated with disease traits, are more likely to code for druggable proteins than the rest of the genome [78]. The increasingly used approach is to identify potential drugs by matching a disease-associated gene expression profile with a transcription signature of a particular drug [79]. This method seeks to identify drugs, which can reverse a disease-associated gene expression signature and “normalize” the phenotype [76]. Considering that disease-causing genes are often not amenable as drug targets or cannot be identified due to small effect or low frequency of disease-causing genetic variants (as is the case for myopia), a very promising emerging approach is identification of druggable targets within genetic networks and signaling pathways underlying a disease, reconstructed using genome-wide gene expression profiling data. This approach also allows the identification of drug combinations targeting several disease-associated pathways. Such multidrug therapy can increase efficacy by targeting multiple pathways at once and reduce side effects because the effective concentration of each individual drug can be significantly reduced. This strategy has been successfully used to identify a number of drugs and drug combinations for the treatment of several disorders such as Charcot-Marie-Tooth disease [80], CMT1A neuropathy [81, 82], Alzheimer’s disease [83], Parkinson’s disease [84], and many others [76]. Recent successes in identification of signaling pathways regulating refractive eye development provide a solid foundation for the application of pharmacogenomic approaches to the development of anti-myopia drugs.
Signaling pathways regulating refractive eye development
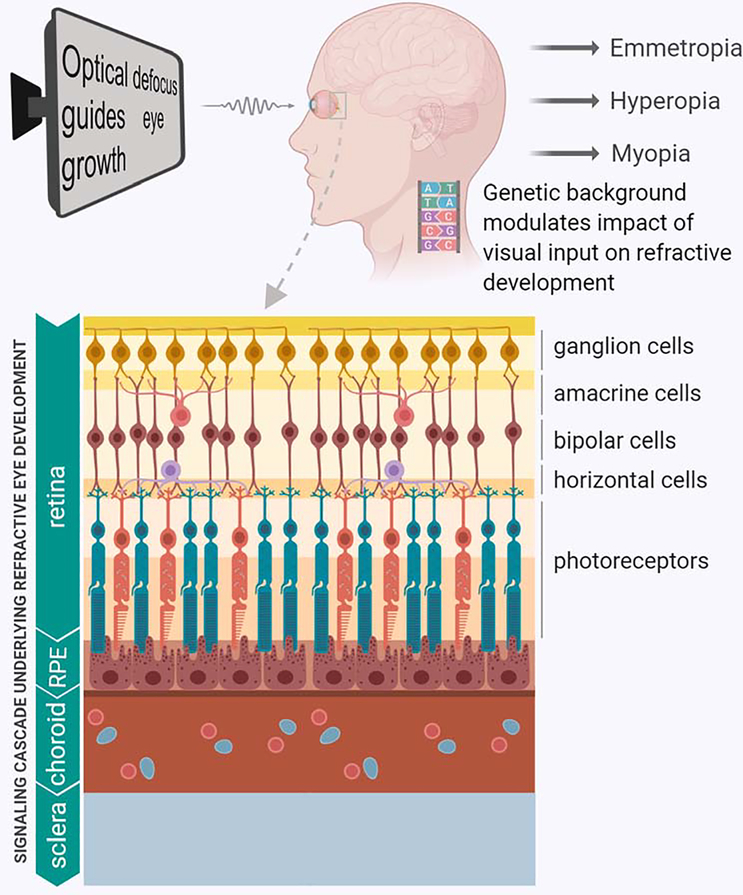
Evidence suggests that refractive eye development is regulated by optical defocus [4]. Animal studies in which experimental myopia was induced with all the feedback from the central nervous system (CNS) to the eye interrupted by sectioning the optic nerve, revealed that refractive eye development is largely controlled locally by the retina [85, 86]. These findings were further extended by work which found that simultaneous exposure of different parts of the same retina to the defocus of opposite signs triggers opposite changes in different parts of the same eye, thus suggesting that refractive eye development is regulated by the sign of optical defocus [4, 87, 88]. This information about the sign of optical defocus is processed by the retina and then converted into molecular signals that regulate the peripheral retinal growth and connective tissue turnover in the sclera, resulting in either increased or decreased rate of growth of the posterior segment of the eye [4, 30, 31, 89–93]. The balance between optical power of the eye (combined optical power of the cornea and crystalline lens) and its axial length determines the refractive state of the eye [94, 95]. The molecular signals produced by the retina in response to optical defocus have to propagate across several ocular tissues before they reach neuronal progenitors at the peripheral retina and scleral fibroblasts. This appears to be achieved via a multilayered signaling cascade which comprises signaling pathways in the retina, retinal pigment epithelium (RPE), choroid, and sclera (Figure 1) [4].
Figure 1. Gene-environment interaction in refractive eye development.
The refractive state of the eye is determined by a combination of environmental and genetic factors. Optical defocus is the chief environmental factor that drives refractive eye development. Genetic background modulates the impact of optical defocus on refractive eye development via a multilayered signaling cascade and determines whether an individual becomes emmetropic, hyperopic, or myopic (upper panel). The retina processes information about optical defocus and converts this information into molecular signals, which propagate across the retina, RPE, and choroid to the sclera, which undergoes structural changes depending on the sign of optical defocus (lower panel).
Signaling in the retina
The retina is a multicellular tissue with complex intercellular signaling [96]. The composition of the retinal neuronal network that processes the response to optical defocus is unknown. However, electrophysiological studies suggest that the inner retina plays an important role in refractive eye development [97]. Chicken studies found that wide-spread chemical ablation of amacrine cells blocked the development of form-deprivation myopia, suggesting that amacrine cells are necessary for emmetropization and myopia development [98]. Dopaminergic amacrine cells were among the first to be implicated in myopia development because retinal concentration of dopamine was found to be reduced in form-deprivation myopia and administration of dopamine receptor agonists apomorphine and quinpirole suppressed the development of myopia in chickens [44, 70–72, 99]. A discovery that the early growth response 1 (Egr-1) transcription factor exhibits optical defocus-specific expression in the chicken model of myopia directly implicated glucagonergic amacrine cells in the development of myopia [100]. Although glucagonergic amacrine cells have not been found in mammals [101], Egr-1 is also regulated by optical defocus in mammals and is expressed in a subset of GABAergic amacrines which are positive for vasoactive intestinal peptide (VIP) [101, 102]. Interestingly, VIPergic amacrines were the first to be directly implicated in myopia development [103]. The findings that opiate, serotonin, and gamma-aminobutyric acid (GABA) antagonists suppress the development of experimental myopia in chicks pointed to a possible involvement of other retinal interneurons in myopia development [63, 104, 105]. A recent finding that a human myopia gene amyloid beta precursor like protein 2 (APLP2) most likely exerts its effect on refractive eye development by modulating synaptic transmission at the level of glycinergic amacrines also supports the involvement of retinal interneurons in refractive development [106]. Several studies also implicated synaptic transmission at the level of bipolar cells and photoreceptors [107, 108]. Optical blur also triggers an increase in retinal neurogenesis at the retinal periphery, which further complicates the overall understanding of the retinal mechanisms of emmetropization [30, 93].
It is hypothesized that the visual environment modulates refractive eye development via the Bidirectional Emmetropization by the Sign of Optical Defocus (BESOD) mechanism, which propagates via two largely distinct sign-of-defocus-sensitive signaling cascades [31]. In support of this, several recent studies shed light on the retinal signaling pathways involved in the visual regulation of eye growth (Table 1). A genome-wide gene expression profiling study in marmosets found that the retinal responses to optical defocus of opposite signs propagate via two largely distinct sets of pathways and that the signaling underlying retinal response to defocus undergoes dynamic changes, which depend on the duration of the retinal exposure to defocus [31]. This study performed in marmosets exposed to either negative or positive optical defocus revealed that the early retinal response to negative defocus primarily involved pathways regulating glycogen degradation, ephrin receptor signaling, and choline biosynthesis, whereas the sustained response was mediated by several canonical pathways, including ß-adrenergic signaling, protein kinase A (PKA) signaling, calcium signaling, neuronal nitric oxide synthase (nNOS) signaling, ras-related nuclear protein (RAN) signaling, Hippo signaling, phosphatase and tensin homolog (PTEN) signaling, phototransduction pathway, synaptic long term potentiation, androgen signaling, Wnt/Ca+ pathway, and dopamine-DARPP32 feedback signaling [31]. In contrast, in the retinae exposed to positive defocus, there was transition from receptor activator of nuclear factor κB (RANK) signaling, stress-activated protein kinase/Jun amino-terminal kinase (SAPK/JNK) signaling, nerve growth factor (NGF) signaling, and gap junction signaling during the early response to α-adrenergic signaling, protein ubiquitination pathway, eukaryotic initiation factor 2 (EIF2) signaling, eukaryotic initiation factor 4 (eIF4) and ribosomal protein S6 kinase beta-1 (p70S6K) signaling, mammalian target of rapamycin (mTOR) signaling, glutamate receptor signaling, oncostatin M and somatostatin receptor 2 signaling, glucocorticoid receptor signaling, and cAMP response element-binding protein (CREB) signaling during the sustained response [31].
Table 1.
Signaling pathways involved in refractive eye development.
| Chicken | Mouse | Marmoset | Human | Canonical signaling pathways | Retina | RPE/choroid | Sclera | Baseline RD | Susceptibility | (+) Early | (+) Sustained | (−) Early | (−) Sustained | Reference |
| • | • | 4-hydroxyproline degradation I | • | • | [23] | |||||||||
| • | • | • | Actin cytoskeleton signaling | • | • | • | [23, 109, 129] | |||||||
| • | • | • | Aldosterone signaling in epithelial cells | • | • | • | [23, 31, 109] | |||||||
| • | • | • | Amyloid processing | • | • | • | • | [23, 106, 110] | ||||||
| • | • | • | Amyotrophic lateral sclerosis signaling | • | • | • | [23, 31, 109] | |||||||
| • | • | • | Androgen receptor signaling | • | • | • | • | • | [23, 31, 109, 129] | |||||
| • | • | Antiproliferative role of somatostatin receptor 2 signaling | • | • | • | [23, 31] | ||||||||
| • | • | Antiproliferative role of TOB in cell signaling | • | • | • | • | [23, 31, 129] | |||||||
| • | • | • | • | Calcium signaling | • | • | • | [23, 31, 109] | ||||||
| • | • | Choline biosynthesis III | • | • | • | [23, 31] | ||||||||
| • | • | Chondroitin sulfate biosynthesis | • | • | [23] | |||||||||
| • | • | • | • | Circadian rhythm signaling | • | • | • | • | [23, 31, 109, 113, 129] | |||||
| • | • | • | Clathrin-mediated endocytosis signaling | • | • | • | • | • | [23, 31] | |||||
| • | • | • | CREB signaling in neurons | • | • | • | [23, 31, 109] | |||||||
| • | • | • | • | CXCR4 signaling | • | • | • | • | [23, 31, 109] | |||||
| • | • | Diphthamide biosynthesis | • | • | • | [23] | ||||||||
| • | • | • | Dopamine receptor signaling | • | • | • | [23, 31, 109] | |||||||
| • | • | • | • | Dopamine-DARPP32 feedback signaling | • | • | • | [23, 31, 109] | ||||||
| • | • | EGF signaling | • | • | • | • | [23, 28, 31] | |||||||
| • | • | • | EIF2 signaling | • | • | • | • | • | [23, 31, 129] | |||||
| • | • | eNOS signaling | • | • | [23] | |||||||||
| • | • | • | Ephrin B signaling | • | • | • | [23, 31, 109] | |||||||
| • | • | • | • | Ephrin receptor signaling | • | • | • | [23, 31, 109] | ||||||
| • | • | • | • | Epithelial adherens junction signaling | • | • | • | • | • | [23, 31, 109, 113, 129] | ||||
| • | • | ERK/MAPK signaling | • | • | • | • | [23, 31] | |||||||
| • | • | • | • | Estrogen receptor signaling | • | • | • | • | • | • | • | [23, 31, 109] | ||
| • | • | • | G2/M DNA damage checkpoint regulation | • | • | • | • | • | [23, 28, 31] | |||||
| • | • | • | • | GABA receptor signaling | • | • | • | • | • | [23, 28, 31, 109] | ||||
| • | • | • | Gap junction signaling | • | • | • | [23, 31, 109] | |||||||
| • | • | • | • | Glucocorticoid receptor signaling | • | • | • | • | • | [23, 31, 109, 129] | ||||
| • | • | • | • | Glutamate receptor signaling | • | • | • | • | [23, 31, 109] | |||||
| • | • | • | Glutathione biosynthesis | • | • | [23, 109] | ||||||||
| • | • | • | Glutathione redox reactions I | • | • | [23, 109] | ||||||||
| • | • | • | Glutathione-mediated detoxification | • | • | [23, 109] | ||||||||
| • | • | Glycoaminoglycan-protein linkage region biosynthesis | • | • | • | [23] | ||||||||
| • | • | • | Gαq signaling | • | • | • | • | • | • | [23, 31] | ||||
| • | • | Hypoxia signaling | • | • | [28, 129] | |||||||||
| • | • | HIPPO signaling | • | • | • | • | [23, 31] | |||||||
| • | • | • | HMGB1 signaling | • | • | • | [23, 31] | |||||||
| • | • | • | • | Huntington’s disease signaling | • | • | • | • | • | • | [23, 28, 31, 109, 129] | |||
| • | • | • | IGF-1 signaling | • | • | • | • | [23, 31] | ||||||
| • | • | • | IL-2 signaling | • | • | • | • | [23, 28, 31] | ||||||
| • | • | • | ILK signaling | • | • | • | [23, 109, 129] | |||||||
| • | • | • | Insulin receptor signaling | • | • | • | • | [23, 31] | ||||||
| • | • | • | Integrin signaling | • | • | • | [23, 31] | |||||||
| • | • | L-cysteine degradation I | • | • | • | [23] | ||||||||
| • | • | L-cysteine degradation III | • | • | [23, 31] | |||||||||
| • | • | Lysine degradation | • | • | • | • | • | [23, 28, 31] | ||||||
| • | • | Mismatch repair in eukaryotes | • | • | • | • | [23, 31] | |||||||
| • | • | • | mTOR signaling | • | • | • | • | • | [23, 31, 129] | |||||
| • | • | • | Mitochondrial dysfunction | • | • | • | • | [23, 31, 109, 129] | ||||||
| • | • | NF-κB signaling | • | • | • | [23] | ||||||||
| • | • | • | • | nNOS signaling | • | • | • | • | [23, 31, 109] | |||||
| • | • | • | NRF2-mediated oxidative stress response | • | • | • | • | • | • | [23, 31, 129] | ||||
| • | • | Oncostatin M signaling | • | • | • | [23, 31] | ||||||||
| • | • | p38 MAPK signaling | • | • | • | • | [23, 28] | |||||||
| • | • | Pentose phosphate pathway | • | • | • | [23, 28] | ||||||||
| • | • | • | • | Phenylalanine metabolism pathway | • | • | • | • | • | [23, 28, 31, 109] | ||||
| • | • | • | • | Phospholipase C signaling | • | • | • | • | [23, 31, 109] | |||||
| • | • | • | Phototransduction pathway | • | • | • | • | [23, 31, 108, 113] | ||||||
| • | • | • | • | PI3K/AKT signaling | • | • | • | • | [23, 31, 109] | |||||
| • | • | • | PPAR signaling | • | • | [23, 109] | ||||||||
| • | • | • | • | PPARα/RXRα activation | • | • | • | • | • | • | [23, 31, 109, 129] | |||
| • | • | • | Production of nitric oxide (NO) and reactive oxygen species | • | • | • | • | [23, 31] | ||||||
| • | • | • | • | Protein kinase A signaling | • | • | • | • | • | [23, 31, 109, 129] | ||||
| • | • | • | • | Protein ubiquitination pathway | • | • | • | • | [23, 31, 109, 129] | |||||
| • | • | • | PTEN signaling | • | • | • | • | [23, 31] | ||||||
| • | • | Purine nucleotides de novo biosynthesis II | • | • | [23, 109] | |||||||||
| • | • | • | Purine nucleotides degradation II (aerobic) | • | • | [23, 109] | ||||||||
| • | • | RAN signaling | • | • | • | [23, 31] | ||||||||
| • | • | • | RAR activation | • | • | • | • | [23, 31] | ||||||
| • | • | • | Regulation of eIF4 and p70S6K signaling | • | • | • | • | • | [23, 31, 129] | |||||
| • | • | • | • | Regulation of the epithelial-mesenchymal transition pathway | • | • | • | • | [23, 31, 109, 111] | |||||
| • | • | • | Relaxin signaling | • | • | • | • | [23, 31, 109] | ||||||
| • | • | • | RhoGDI signaling | • | • | • | • | [23, 109, 129] | ||||||
| • | • | • | SAPK/JNK signaling | • | • | • | • | [23, 28, 31, 109] | ||||||
| • | • | Semaphorin signaling in neurons | • | • | [23] | |||||||||
| • | • | • | • | Signaling by Rho family GTPases | • | • | • | • | • | • | [23, 31, 109] | |||
| • | • | • | Sumoylation pathway | • | • | • | [23, 109, 110] | |||||||
| • | • | Synaptic long-term depression | • | • | [23, 109] | |||||||||
| • | • | • | • | Synaptic long-term potentiation | • | • | • | [23, 31, 109] | ||||||
| • | • | • | TGF-β signaling | • | • | [23, 109] | ||||||||
| • | • | Tight junction signaling | • | • | • | [23, 109, 113] | ||||||||
| • | Toll-like receptor signaling | • | [28] | |||||||||||
| • | • | • | tRNA splicing | • | • | • | [23, 31, 109] | |||||||
| • | • | • | Wnt/Ca+ pathway | • | • | • | [23, 31] | |||||||
| • | • | • | Xenobiotic metabolism signaling | • | • | • | • | [23, 28, 31] | ||||||
| • | • | • | α-adrenergic signaling | • | • | • | [23, 31, 109] | |||||||
| • | • | • | β-adrenergic signaling | • | • | • | [23, 31, 109] |
Another recent study, which analyzed retinal genetic networks regulating baseline trajectory of refractive eye development versus genetic networks regulating susceptibility to myopia induced by visual form deprivation (usually induced by placing a frosted diffuser in front of the eye) in the mouse, found that baseline refractive development and susceptibility to myopia are regulated by overlapping but largely distinct genetic networks [23]. Multiple pathways were implicated in both baseline refractive development and susceptibility to myopia, including nuclear factor-erythroid 2-related factor 2 (NRF2) mediated oxidative stress response, PTEN signaling, opioid signaling pathway, EIF2 signaling, PKA signaling, regulation of eIF4 and p70S6K signaling, tight junction signaling, estrogen receptor signaling, GABA receptor signaling, mTOR pathway, HIPPO pathway, axonal guidance signaling, and peroxisome proliferator-activated receptor alpha/retinoid X receptor alpha (PPARα/RXRα) activation pathway [23]. Conversely, dopamine-DARPP32 feedback signaling, dopamine receptor signaling, α-adrenergic and β-adrenergic signaling, androgen signaling, glucocorticoid receptor signaling, aldosterone signaling, glutamate receptor signaling, protein ubiquitination pathway, ephrin receptor signaling, synaptic long-term potentiation, somatostatin receptor 2 signaling, and nNOS signaling were chiefly associated with baseline refractive development. The amyloid processing, retinoic acid receptor (RAR) activation, insulin-like growth factor 1 (IGF-1) signaling, RAN signaling, oncostatin M signaling, choline biosynthesis, epithelial-mesenchymal transition pathway, transforming growth factor beta (TGF-β) signaling, DNA methylation and transcriptional repression signaling were found to be involved in regulation of susceptibility to myopia [23].
Interestingly, many of the same pathways were implicated in refractive eye development by large-scale gene expression and proteomics studies in chickens too (Table 1) [109–113], highlighting the fact that retinal signaling pathways regulating refractive eye development are highly conserved across different vertebrate species. Taken together, these data suggest that the trajectory of refractive eye development and the sensitivity of the eye to visual input are regulated by the overlapping but largely unique signaling cascades.
Signaling in the RPE and choroid
The RPE and choroid were long implicated in refractive eye development by virtue of their location between the retina, which processes information about optical defocus, and the sclera, which undergoes restructuring in myopic eyes (Figure 1). The direct experimental evidence regarding the involvement of the RPE came from the RPE-sclera co-culture experiments, which demonstrated that the presence of the RPE significantly influenced scleral fibroblast proliferation believed to be underlying eye growth in myopia [114, 115]. The important role of the RPE in refractive development was also highlighted by the findings that mutations in the low density lipoprotein receptor-related protein 2 (LRP2) gene, encoding LRP2 protein expressed in the RPE, cause high myopia [116]. Studies in animal models also discovered that the choroid reacts to optical defocus with the sign-of-defocus-specific changes in thickness, thus, implicating the choroid in refractive eye development [117].
Although molecular signaling in the RPE and choroid during refractive eye development has not been investigated on a genome-wide scale, one gene expression profiling study performed in marmosets found that toll-like receptor (TLR) signaling, xenobiotic metabolism signaling, p38 mitogen-activated protein kinase (MAPK) signaling, SAPK/JNK signaling, GABA receptor signaling, epidermal growth factor (EGF) signaling, interleukin-2 (IL-2) signaling, hypoxia signaling, Huntington’s disease signaling, G2/M DNA damage checkpoint regulation, and pentose phosphate pathways were involved [28]. Other studies implicated a number of specific genes; however, no information about pathways could be ascertained due to small scale of the studies [118–120]. Several of these genes, such as BMP2, BMP4, BMP7, and TGF-β2, exhibited defocus-sensitive regulation in the RPE, suggesting that they might be involved in the processing of the defocus signal [121–123].
Signaling in the sclera
The sclera plays a key role in refractive eye development and development of myopia [89]. The sclera forms a tunic for the eye and ultimately determines its shape and size in all species [89–91]. The main components of the mammalian sclera are an extracellular matrix (ECM) composed of collagen fibrils embedded in a matrix of proteoglycans and glycoproteins and fibroblasts [89]. The sclera undergoes substantial restructuring during emmetropization which affects its elasticity [89–92, 124]. In mammals, negative defocus leads to an increase in elasticity and a thinning of the sclera, whereas positive defocus causes a decrease in elasticity and a thickening of the sclera [90–92]. Molecular studies revealed that restructuring of the sclera during refractive eye development is primarily accomplished through changes in the production of type I collagen and several other extracellular matrix proteins [89, 125, 126]. Proteomics studies revealed that the most prominent changes occurred in expression of type I collagen, calcium-dependent activator protein for secretion 2 (CAPS2), crystallins, and several other proteins associated with cell adhesion, cytoskeleton and transcriptional regulation, as well as the restructuring of extracellular matrix [127, 128]. A genome-wide gene expression profiling study in a guinea pig model suggested that the signaling pathways regulating L-dopachrome, catecholamine, and eumelanin biosynthesis, as well as Gαi signaling were involved in the scleral restructuring during myopia development [32]. However, the biggest breakthrough in our understanding of the molecular signaling underlying the restructuring of the sclera in myopia came from a recent study, which analyzed genome-wide gene expression in the myopic sclera at the single cell level [129]. This study revealed that the changes in elastic properties of the sclera underlying myopia development result from a large-scale transdifferentiation of fibroblasts into myofibroblasts and subsequent activation or suppression of several signaling pathways, including EIF2 signaling, mTOR signaling, regulation of eIF4 and p70S6K signaling, protein ubiquitination pathway, Huntington’s disease signaling, PKA signaling, androgen and glucocorticoid receptor signaling, epithelial adherens junction signaling, and circadian rhythm signaling, among other pathways (Table 1). Importantly, it was found that hypoxia signaling plays a prominent role in the scleral restructuring underlying myopia [129]. In the work, the authors speculated that the hypoxia caused by the thinning of the choroid and reduced choroidal blood flow in myopia triggers activation of the hypoxia-inducible factor 1 alpha (HIF-1α) signaling pathway, which leads to the transdifferentiation of fibroblasts into myofibroblasts expressing low levels of type I collagen. This leads to an increased elasticity of the mammalian sclera and excessive growth of the posterior segment of the eye.
Common pathways underlie myopia signaling across different ocular tissues
Many of the studies reviewed above reveal a very intriguing aspect of the molecular signaling underlying myopia development [23, 31, 129]. It turns out that several of the same canonical pathways are involved in the signaling regulating refractive development in the retina, RPE/choroid, and sclera (Table 1). For example, xenobiotic metabolism signaling, p38 MAPK signaling, SAPK/JNK signaling, GABA receptor signaling, and EGF signaling are involved in myopia signaling in both the retina and the RPE/choroid. The hypoxia and Huntington’s disease signaling pathways are implicated in the signaling underlying refractive eye development in both RPE/choroid and sclera, while the Huntington’s disease signaling pathway is involved in the signaling in all three tissues.
Ultimately, taken together, these data suggest that refractive eye development is regulated by a multilayered and continuous signaling cascade which originates in the retina in response to optical defocus and propagates across the RPE and choroid to the sclera (Figure 1 and Table 1) where the RPE and choroid appear to serve as a signaling relay between the retina and sclera.
Concluding remarks and future directions
Myopia and refractive eye development have been a subject of intense investigation for the last four decades. These studies led to important insights into the mechanisms underlying visually guided eye growth and the key role of genetic factors and environment in this process. These in turn led to the development of several optics-based methods (such as OrthoK and multifocal lenses) for myopia control. Although the optics-based approaches provide a much needed hope to the myopic population at a time of rapidly increasing prevalence of the disease, the efficacy of these methods is low [34]. The development of drugs for myopia control has been based on sporadic, accidental findings and has been lacking a systematic approach; hence, identified drugs have low efficacy and/or significant side effects. However, recent developments in the field promise to change that trend. It is now becoming increasingly clear that refractive eye development is regulated by signaling pathways, which control refractive eye development by inhibiting eye growth in response to positive optical defocus and by stimulating eye growth in response to negative optical defocus. The discovery that the signals generated by the optical defocus of opposite signs propagate along different signaling cascades in the retina, as well as recent identification of signaling pathways underlying refractive error development in the RPE/choroid and sclera, open up a new and exciting venue for anti-myopia drug development. The pharmacogenomic approach (Figure 2), which combines whole-genome gene expression profiling, genetic mapping, reconstruction of signaling pathways using cutting edge bioinformatics, and identification of new drug targets and drug candidates using causal network analysis, has already produced very promising results. Well-established and recently developed animal models of myopia have allowed the application of genome-wide gene expression profiling methods such as RNA-seq and cutting-edge bioinformatics to reconstruct the signaling pathways involved in refractive eye development, while genetic mapping in the human and mouse populations have provided information about the impact of genetic variation on the signaling underlying refractive error development. The combination of gene expression profiling and genetic mapping has provided a systems genetics platform for the dissection of signaling pathways involved in refractive eye development (Figure 2). Once the signaling cascades are established, this information is processed using causal network analysis and other computational approaches, which allows identification of potential drug targets and drug candidates. Potential drug targets are passed through a medicinal chemistry pipeline, which allows for the identification of the existing pharmacological compounds and the development of novel drugs with anti-myopic activity (Figure 2). Both drug candidates predicted by the causal network analysis and those generated by the medicinal chemistry pipeline can be screened using a mouse model of myopia, which provides a convenient platform for preclinical drug testing.
Figure 2. Pharmacogenomic pipeline for anti-myopia drug development.
The pharmacogenomic approach is emerging as a promising avenue for the development of anti-myopia drugs. This approach takes advantage of the latest developments in systems genetics and pharmacogenomics. Several animal models of myopia allow the application of next-generation approaches to reconstruct the signaling pathways involved in refractive eye development, while genetic mapping in the human and mouse populations provide information about the impact of genetic variation on the signaling underlying refractive error development. Once the signaling cascades are established, this information is processed using cutting-edge bioinformatics to identify potential drug targets and drug candidates. Potential drug targets are further explored using a medicinal chemistry pipeline, which allows for the identification of the existing pharmacological compounds and the development of new drugs with anti-myopic activity. Both drug candidates predicted by the computational approaches and those generated by the medicinal chemistry pipeline can be screened using a mouse model of myopia, which provides a convenient platform for preclinical drug testing.
Several of the identified pathways were previously implicated in mediating the effect of drug compounds with anti-myopic activity. For example, dopamine, NO, α-adrenergic, and GABA receptor signaling were suggested to underlie the anti-myopic effect of atropine [40–44]. The efficacy of α-adrenergic and GABA receptor agonists in experimental myopia models also point to the involvement of α-adrenergic and GABA receptor signaling implicated by the systems genetics studies [63–68]. The suppressive effect of a dopamine receptor agonist apomorphine on myopia development in several animal models also in line with the findings that dopamine receptor signaling is involved in refractive eye development [69–73]. The pharmacogenomic approach combined with single-cell gene expression profiling in the sclera of mice with induced myopia led to the identification of the antihypoxic drugs salidroside and formononetin, as well as the eIF2α phosphorylation inhibitor GSK2606414, as potential anti-myopia drugs [129]. Preliminary work from our lab also show strong suppression of myopia in a mouse model by several drug compounds identified by the pharmacogenomic approach. These data point to the feasibility of the approach and a strong potential for anti-myopia drug development.
The future of myopia research and the prospects for the development of more effective anti-myopia drugs look bright. However, recent advances in the field also uncovered important challenges (see Outstanding Questions). The identified chromosomal loci linked to myopia explain less than 10% of phenotypic variance in refractive error in humans, leaving a large proportion of myopia heritability unexplained. The signaling cascades that control refractive eye development are complex and comprise hundreds, if not thousands, of genes/proteins. This diverse signaling network is compartmentalized within many different cell populations in the retina, RPE, choroid, and sclera. The identification of new drug targets and drug candidates for myopia control will depend to a large extent on our ability to identify and characterize the cell-specific signaling pathways underlying refractive eye development. Although recent advances in whole-genome approaches allow the dissection of the most complex signaling networks at the single-cell level, the identification of the key pathways underlying emmetropization and druggable targets within those pathways may not be a straightforward process. Nevertheless, these approaches have been increasingly used to dissect signaling cascades underlying various developmental and disease processes. In combination with the emerging pharmacogenomic approaches, these new technologies may provide a powerful platform for the development of new anti-myopia drugs in the near future.
Outstanding Questions Box.
What neurons are involved in the detection and processing of optical defocus in the retina?
How do retinal neuronal networks process information about optical defocus?
What cells are involved in the processing of the information about optical defocus in the choroid?
What is the composition of the cell-specific signaling cascades underlying processing of optical defocus in different neuronal subpopulations of the retina?
Does sustained exposure to optical defocus cause stable or transient changes in the retinal signaling?
What are the key regulator proteins within each of the signaling pathways involved in refractive eye development and which proteins within these pathways can be targeted pharmacologically?
Highlights.
Current pharmacological options for myopia control are limited and can only slow the progression of myopia, but not stop it.
Eye growth is regulated by the Bidirectional Emmetropization by the Sign of Optical Defocus (BESOD) mechanism sensitive to the sign of optical defocus, where the information about positive and negative optical defocus is converted by the retina into molecular signals, which propagate via two different signaling cascades.
The signaling cascade underlying refractive eye development appears to be highly integrated and continuous.
Genetic background appears to modulate the impact of visual environment on refractive eye development and contribute to the final determination of the refractive state of the eye.
Emerging pharmacogenomic approaches based on whole-genome analyses of genetic networks provide a powerful platform for accelerated discovery and preclinical testing of new anti-myopia drugs.
Acknowledgments
This work was supported by the National Institutes of Health grant R01EY023839 to A.V.T., National Institutes of Health grant P30EY019007 (Core Support for Vision Research) to the Department of Ophthalmology, Columbia University, and unrestricted funds from Research to Prevent Blindness to the Department of Ophthalmology, Columbia University. Illustrations in this review were prepared using BioRender (https://biorender.com/).
Glossary
- Amacrine cells
a diverse group of interneurons localized in the inner nuclear layer of the retina. Amacrine cells lack typical axons and connect to the bipolar cells, ganglion cells and other amacrines through dendritic synapsis. The function of the amacrine cells is to integrate and modulate the output presented to the ganglion cells. More than 30 morphological subtypes of amacrines are distinguished in the retina. Amacrine cells can be classified by the neurotransmitters they release, such as the GABAergic, glycinergic, dopaminergic, cholinergic, VIPergic, or glucagonergic amacrines discussed in this review.
- Accommodation amplitude
the maximum increase in optical power that the eye can achieve during accommodation.
- Bidirectional Emmetropization by the Sign of Optical Defocus (BESOD)
a molecular mechanism underlying emmetropization, which utilizes sign of optical defocus to guide postnatal eye growth. BESOD converts information about positive and negative defocus into molecular signals, which propagate in the retina via two distinct signaling cascades.
- Bipolar cells
a group of secondary retinal neurons connecting the outer and inner retina. The function of the bipolar neurons is a transmission of electrical signals from the photoreceptors to the amacrine and ganglion cells.
- Cornea
an optically transparent, dome-shaped structure forming the front of the eye. It has a high refractive power. The main functions of the cornea are to refract the light and protect the inner structures of the eye.
- Crystalline lens
a transparent biconvex lens located between the anterior and posterior chambers of the eye. The main function of the crystalline lens is to refract light and focus images onto the retina. In the majority of mammals, the crystalline lens can change its shape in a process called accommodation; this allows the eye to focus the images of objects located at various distances on the retina.
- Cycloplegia
a paralysis of the ciliary muscle of the eye. The ciliary muscle regulates the thickness and the curvature of the crystalline lens underlying accommodation. Cycloplegia leads to loss of accommodation and is caused by so-called cycloplegic drugs.
- Emmetropization
a developmental process guided by the visual input, which coordinates the axial growth of the eye and ensures that the eye’s optical power matches its axial length.
- Form-deprivation myopia
myopia, which develops in response to elimination of high spatial frequency vision usually achieved by placing a frosted diffuser in front of the eye.
- Inner retina
the part of the retina which combines the inner nuclear layer, inner plexiform layer, and ganglion cell layer.
- Lag of accommodation
insufficiently strong accommodative response when a subject is engaged in nearwork, which places focal point slightly behind the retina, producing negative optical defocus.
- Mydriasis
a dilation of the pupil of the eye in response to reduced light intensity, certain diseases, or so-called mydriatic drugs.
- Myopia
also known as nearsightedness is a refractive disorder of the eye characterized by the difficulty seeing distant objects clearly. Myopia develops when the eyeball grows too long for the optical power of the eye, which leads to the images of distant objects focusing in front of the retina. Myopia can be subdivided into two large classes, i.e., syndromic myopia and common myopia. Syndromic forms of myopia are usually caused by mutations in a single gene and are transmitted in families as classical Mendelian diseases. Common myopia (discussed in this review) represents a complex genetic trait controlled by hundreds of genes with a large contribution of environmental factors.
- Optical defocus
blurring of the image projected onto the retina caused by refractive errors. Optical defocus is subdivided into hyperopic (negative) and myopic (positive) optical defocus. Hyperopic defocus occurs when the image is focused behind the retina; myopic defocus occurs when the image is focused in front of the retina.
- Photophobia
light sensitivity that leads to an intolerance of bright light. Photophobia can be caused mydriatic drugs, which dilate the pupil and prevent the eye from being able to regulate the amount light entering the eye.
- Photoreceptors
a group of primary retinal neurons comprising the outer nuclear layer of the retina. The function of the photoreceptor cells is to convert light into electrical signals, which are then processed by the secondary retinal neurons and transmitted to the visual cortex.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holden BA et al. (2016) Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 123 (5), 1036–42. [DOI] [PubMed] [Google Scholar]
- 2.Verhoeven VJ et al. (2015) Visual consequences of refractive errors in the general population. Ophthalmology 122 (1), 101–9. [DOI] [PubMed] [Google Scholar]
- 3.de Jong P (2018) Myopia: its historical contexts. Br J Ophthalmol 102 (8), 1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Troilo D et al. (2019) IMI - Report on Experimental Models of Emmetropization and Myopia. Invest Ophthalmol Vis Sci 60 (3), M31–M88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tedja MS et al. (2019) IMI - Myopia Genetics Report. Invest Ophthalmol Vis Sci 60 (3), M89–M105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French AN et al. (2013) Time outdoors and the prevention of myopia. Exp Eye Res 114, 58–68. [DOI] [PubMed] [Google Scholar]
- 7.Rose KA et al. (2008) Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 115 (8), 1279–85. [DOI] [PubMed] [Google Scholar]
- 8.Chung K et al. (2002) Undercorrection of myopia enhances rather than inhibits myopia progression. Vision Res 42 (22), 2555–9. [DOI] [PubMed] [Google Scholar]
- 9.Nickla DL (2013) Ocular diurnal rhythms and eye growth regulation: where we are 50 years after Lauber. Exp Eye Res 114, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saw SM et al. (2002) Nearwork in early-onset myopia. Invest Ophthalmol Vis Sci 43 (2), 332–9. [PubMed] [Google Scholar]
- 11.Huang HM et al. (2015) The Association between Near Work Activities and Myopia in Children-A Systematic Review and Meta-Analysis. PLoS One 10 (10), e0140419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwiazda J et al. (1995) A dynamic relationship between myopia and blur-driven accommodation in school-aged children. Vision Res 35 (9), 1299–304. [DOI] [PubMed] [Google Scholar]
- 13.Gwiazda JE et al. (2004) Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Invest Ophthalmol Vis Sci 45 (7), 2143–51. [DOI] [PubMed] [Google Scholar]
- 14.Gwiazda J et al. (2003) A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci 44 (4), 1492–500. [DOI] [PubMed] [Google Scholar]
- 15.Sun YY et al. (2017) Effect of uncorrection versus full correction on myopia progression in 12-year-old children. Graefes Arch Clin Exp Ophthalmol 255 (1), 189–195. [DOI] [PubMed] [Google Scholar]
- 16.Li SY et al. (2015) Effect of undercorrection on myopia progression in 12-year-old children. Graefes Arch Clin Exp Ophthalmol 253 (8), 1363–8. [DOI] [PubMed] [Google Scholar]
- 17.Hung LF et al. (1995) Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med 1 (8), 761–5. [DOI] [PubMed] [Google Scholar]
- 18.Cottriall CL and McBrien NA (1996) The M1 muscarinic antagonist pirenzepine reduces myopia and eye enlargement in the tree shrew. Invest Ophthalmol Vis Sci 37 (7), 1368–79. [PubMed] [Google Scholar]
- 19.Metlapally S and McBrien NA (2008) The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis 8 (3), 1 1–12. [DOI] [PubMed] [Google Scholar]
- 20.Howlett MH and McFadden SA (2009) Spectacle lens compensation in the pigmented guinea pig. Vision Res 49 (2), 219–27. [DOI] [PubMed] [Google Scholar]
- 21.Schaeffel F et al. (1988) Accommodation, refractive error and eye growth in chickens. Vision Res 28 (5), 639–57. [DOI] [PubMed] [Google Scholar]
- 22.Wiesel TN and Raviola E (1977) Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature 266 (5597), 66–8. [DOI] [PubMed] [Google Scholar]
- 23.Tkatchenko TV et al. (2019) Analysis of genetic networks regulating refractive eye development in collaborative cross progenitor strain mice reveals new genes and pathways underlying human myopia. BMC Med Genomics 12 (1), 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz M et al. (1990) X-linked myopia: Bornholm eye disease. Linkage to DNA markers on the distal part of Xq. Clin Genet 38 (4), 281–6. [PubMed] [Google Scholar]
- 25.Kiefer AK et al. (2013) Genome-wide analysis points to roles for extracellular matrix remodeling, the visual cycle, and neuronal development in myopia. PLoS Genet 9 (2), e1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verhoeven VJ et al. (2013) Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat Genet 45 (3), 314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manolio TA et al. (2009) Finding the missing heritability of complex diseases. Nature 461 (7265), 747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shelton L et al. (2008) Microarray analysis of choroid/RPE gene expression in marmoset eyes undergoing changes in ocular growth and refraction. Mol Vis 14, 1465–79. [PMC free article] [PubMed] [Google Scholar]
- 29.Schippert R et al. (2008) Microarray analysis of retinal gene expression in chicks during imposed myopic defocus. Mol Vis 14, 1589–99. [PMC free article] [PubMed] [Google Scholar]
- 30.Tkatchenko AV et al. (2006) Form deprivation modulates retinal neurogenesis in primate experimental myopia. Proc Natl Acad Sci U S A 103 (12), 4681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tkatchenko TV et al. (2018) Gene expression in response to optical defocus of opposite signs reveals bidirectional mechanism of visually guided eye growth. PLoS Biol 16 (10), e2006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivasalu N et al. (2018) Gene Expression and Pathways Underlying Form Deprivation Myopia in the Guinea Pig Sclera. Invest Ophthalmol Vis Sci 59 (3), 1425–1434. [DOI] [PubMed] [Google Scholar]
- 33.Ong E et al. (1999) Effects of spectacle intervention on the progression of myopia in children. Optom Vis Sci 76 (6), 363–9. [DOI] [PubMed] [Google Scholar]
- 34.Wildsoet CF et al. (2019) IMI - International Myopia Institute: Interventions for Controlling Myopia Onset and Progression Report. Invest Ophthalmol Vis Sci 60 (3), M106–M131. [DOI] [PubMed] [Google Scholar]
- 35.Rengstorff RH and Doughty CB (1982) Mydriatic and cycloplegic drugs: a review of ocular and systemic complications. Am J Optom Physiol Opt 59 (2), 162–77. [PubMed] [Google Scholar]
- 36.Young FA (1965) The Effect of Atropine on the Development of Myopia in Monkeys. Am J Optom Arch Am Acad Optom 42, 439–49. [DOI] [PubMed] [Google Scholar]
- 37.Bedrossian RH (1979) The effect of atropine on myopia. Ophthalmology 86 (5), 713–9. [DOI] [PubMed] [Google Scholar]
- 38.Fischer AJ et al. (1998) Cholinergic amacrine cells are not required for the progression and atropine-mediated suppression of form-deprivation myopia. Brain Res 794 (1), 48–60. [DOI] [PubMed] [Google Scholar]
- 39.Stone RA et al. (1991) Muscarinic antagonist effects on experimental chick myopia. Exp Eye Res 52 (6), 755–8. [DOI] [PubMed] [Google Scholar]
- 40.Carr BJ et al. (2018) Myopia-Inhibiting Concentrations of Muscarinic Receptor Antagonists Block Activation of Alpha2A-Adrenoceptors In Vitro. Invest Ophthalmol Vis Sci 59 (7), 2778–2791. [DOI] [PubMed] [Google Scholar]
- 41.Barathi VA et al. (2014) Involvement of GABA transporters in atropine-treated myopic retina as revealed by iTRAQ quantitative proteomics. J Proteome Res 13 (11), 4647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwahn HN et al. (2000) Effects of atropine on refractive development, dopamine release, and slow retinal potentials in the chick. Vis Neurosci 17 (2), 165–76. [DOI] [PubMed] [Google Scholar]
- 43.Carr BJ and Stell WK (2016) Nitric Oxide (NO) Mediates the Inhibition of Form-Deprivation Myopia by Atropine in Chicks. Sci Rep 6 (1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nickla DL et al. (2013) Nitric oxide synthase inhibitors prevent the growth-inhibiting effects of quinpirole. Optom Vis Sci 90 (11), 1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chua WH et al. (2006) Atropine for the treatment of childhood myopia. Ophthalmology 113 (12), 2285–91. [DOI] [PubMed] [Google Scholar]
- 46.Tong L et al. (2009) Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology 116 (3), 572–9. [DOI] [PubMed] [Google Scholar]
- 47.Chia A et al. (2012) Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 119 (2), 347–54. [DOI] [PubMed] [Google Scholar]
- 48.Chia A et al. (2014) Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol 157 (2), 451–457 e1. [DOI] [PubMed] [Google Scholar]
- 49.Chia A et al. (2016) Five-Year Clinical Trial on Atropine for the Treatment of Myopia 2: Myopia Control with Atropine 0.01% Eyedrops. Ophthalmology 123 (2), 391–9. [DOI] [PubMed] [Google Scholar]
- 50.Yam JC et al. (2019) Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology 126 (1), 113–124. [DOI] [PubMed] [Google Scholar]
- 51.Whatham AR et al. (2019) Effects of Monocular Atropinization on Refractive Error and Eye Growth in Infant New World Monkeys. Invest Ophthalmol Vis Sci 60 (7), 2623–2630. [DOI] [PubMed] [Google Scholar]
- 52.Beach CA et al. (1985) Metabolism, distribution, seminal excretion and pharmacokinetics of caffeine in the rabbit. J Pharmacol Exp Ther 233 (1), 18–23. [PubMed] [Google Scholar]
- 53.Trier K et al. (1999) Biochemical and ultrastructural changes in rabbit sclera after treatment with 7-methylxanthine, theobromine, acetazolamide, or L-ornithine. Br J Ophthalmol 83 (12), 1370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trier K et al. (2008) Systemic 7-methylxanthine in retarding axial eye growth and myopia progression: a 36-month pilot study. J Ocul Biol Dis Infor 1 (2–4), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cui D et al. (2011) Effects of 7-methylxanthine on the sclera in form deprivation myopia in guinea pigs. Acta Ophthalmol 89 (4), 328–34. [DOI] [PubMed] [Google Scholar]
- 56.Nie HH et al. (2012) Effects of 7-methylxanthine on form-deprivation myopia in pigmented rabbits. Int J Ophthalmol 5 (2), 133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hung LF et al. (2018) The Adenosine Receptor Antagonist, 7-Methylxanthine, Alters Emmetropizing Responses in Infant Macaques. Invest Ophthalmol Vis Sci 59 (1), 472–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tigges M et al. (1999) Effects of muscarinic cholinergic receptor antagonists on postnatal eye growth of rhesus monkeys. Optom Vis Sci 76 (6), 397–407. [DOI] [PubMed] [Google Scholar]
- 59.Cottriall CL et al. (2001) Inhibition of myopia development in chicks using himbacine: a role for M(4) receptors? Neuroreport 12 (11), 2453–6. [DOI] [PubMed] [Google Scholar]
- 60.Siatkowski RM et al. (2008) Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J AAPOS 12 (4), 332–9. [DOI] [PubMed] [Google Scholar]
- 61.Siatkowski RM et al. (2004) Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia: a 1-year, multicenter, double-masked, placebo-controlled parallel study. Arch Ophthalmol 122 (11), 1667–74. [DOI] [PubMed] [Google Scholar]
- 62.Tan DT et al. (2005) One-year multicenter, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology 112 (1), 84–91. [DOI] [PubMed] [Google Scholar]
- 63.Stone RA et al. (2003) GABA, experimental myopia, and ocular growth in chick. Invest Ophthalmol Vis Sci 44 (9), 3933–46. [DOI] [PubMed] [Google Scholar]
- 64.Chebib M et al. (2009) Novel, potent, and selective GABAC antagonists inhibit myopia development and facilitate learning and memory. J Pharmacol Exp Ther 328 (2), 448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng ZY et al. (2014) Inhibition of form-deprivation myopia by a GABAAOr receptor antagonist, (1,2,5,6-tetrahydropyridin-4-yl) methylphosphinic acid (TPMPA), in guinea pigs. Graefes Arch Clin Exp Ophthalmol 252 (12), 1939–46. [DOI] [PubMed] [Google Scholar]
- 66.Cheng ZY et al. (2015) GABAB receptor antagonist CGP46381 inhibits form-deprivation myopia development in guinea pigs. Biomed Res Int 2015, 207312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carr BJ et al. (2019) Alpha2 -adrenoceptor agonists inhibit form-deprivation myopia in the chick. Clin Exp Optom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y et al. (2017) alpha-adrenergic agonist brimonidine control of experimentally induced myopia in guinea pigs: A pilot study. Mol Vis 23, 785–798. [PMC free article] [PubMed] [Google Scholar]
- 69.Iuvone PM et al. (1991) Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci 32 (5), 1674–7. [PubMed] [Google Scholar]
- 70.Nickla DL et al. (2010) Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp Eye Res 91 (5), 715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmid KL and Wildsoet CF (2004) Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom Vis Sci 81 (2), 137–47. [DOI] [PubMed] [Google Scholar]
- 72.Rohrer B et al. (1993) Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci 10 (3), 447–53. [DOI] [PubMed] [Google Scholar]
- 73.Yan T et al. (2015) Daily Injection But Not Continuous Infusion of Apomorphine Inhibits Form-Deprivation Myopia in Mice. Invest Ophthalmol Vis Sci 56 (4), 2475–85. [DOI] [PubMed] [Google Scholar]
- 74.El-Nimri NW and Wildsoet CF (2018) Effects of Topical Latanoprost on Intraocular Pressure and Myopia Progression in Young Guinea Pigs. Invest Ophthalmol Vis Sci 59 (6), 2644–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mori K et al. (2019) Oral crocetin administration suppressed refractive shift and axial elongation in a murine model of lens-induced myopia. Sci Rep 9 (1), 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pushpakom S et al. (2019) Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 18 (1), 41–58. [DOI] [PubMed] [Google Scholar]
- 77.Roden DM et al. (2019) Pharmacogenomics. Lancet 394 (10197), 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanseau P et al. (2012) Use of genome-wide association studies for drug repositioning. Nat Biotechnol 30 (4), 317–20. [DOI] [PubMed] [Google Scholar]
- 79.Dudley JT et al. (2011) Exploiting drug-disease relationships for computational drug repositioning. Brief Bioinform 12 (4), 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Attarian S et al. (2014) An exploratory randomised double-blind and placebo-controlled phase 2 study of a combination of baclofen, naltrexone and sorbitol (PXT3003) in patients with Charcot-Marie-Tooth disease type 1A. Orphanet J Rare Dis 9, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mandel J et al. (2015) A meta-analysis of randomized double-blind clinical trials in CMT1A to assess the change from baseline in CMTNS and ONLS scales after one year of treatment. Orphanet J Rare Dis 10, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chumakov I et al. (2014) Polytherapy with a combination of three repurposed drugs (PXT3003) down-regulates Pmp22 over-expression and improves myelination, axonal and functional parameters in models of CMT1A neuropathy. Orphanet J Rare Dis 9, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chumakov I et al. (2015) Combining two repurposed drugs as a promising approach for Alzheimer’s disease therapy. Sci Rep 5, 7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hajj R et al. (2015) Combination of acamprosate and baclofen as a promising therapeutic approach for Parkinson’s disease. Sci Rep 5, 16084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Troilo D et al. (1987) Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res 6 (8), 993–9. [DOI] [PubMed] [Google Scholar]
- 86.Raviola E and Wiesel TN (1990) Neural control of eye growth and experimental myopia in primates. Ciba Found Symp 155, 22–38; discussion 39–44. [DOI] [PubMed] [Google Scholar]
- 87.Diether S and Schaeffel F (1997) Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res 37 (6), 659–68. [DOI] [PubMed] [Google Scholar]
- 88.Smith EL 3rd et al. (2013) Effects of local myopic defocus on refractive development in monkeys. Optom Vis Sci 90 (11), 1176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harper AR and Summers JA (2015) The dynamic sclera: extracellular matrix remodeling in normal ocular growth and myopia development. Exp Eye Res 133, 100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McBrien NA et al. (2009) Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom Vis Sci 86 (1), E23–30. [DOI] [PubMed] [Google Scholar]
- 91.Phillips JR et al. (2000) Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Invest Ophthalmol Vis Sci 41 (8), 2028–34. [PubMed] [Google Scholar]
- 92.Siegwart JT Jr. and Norton TT (1999) Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res 39 (2), 387–407. [DOI] [PubMed] [Google Scholar]
- 93.Fischer AJ and Reh TA (2000) Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol 220 (2), 197–210. [DOI] [PubMed] [Google Scholar]
- 94.Goncharov AV et al. (2008) Reconstruction of the optical system of the human eye with reverse ray-tracing. Opt Express 16 (3), 1692–703. [DOI] [PubMed] [Google Scholar]
- 95.Atchison DA and Thibos LN (2016) Optical models of the human eye. Clin Exp Optom 99 (2), 99–106. [DOI] [PubMed] [Google Scholar]
- 96.Masland RH and Raviola E (2000) Confronting complexity: strategies for understanding the microcircuitry of the retina. Annu Rev Neurosci 23, 249–84. [DOI] [PubMed] [Google Scholar]
- 97.Chen JC et al. (2006) Evaluation of inner retinal function in myopia using oscillatory potentials of the multifocal electroretinogram. Vision Res 46 (24), 4096–103. [DOI] [PubMed] [Google Scholar]
- 98.Fischer AJ et al. (1998) Opiate and N-methyl-D-aspartate receptors in form-deprivation myopia. Vis Neurosci 15 (6), 1089–96. [DOI] [PubMed] [Google Scholar]
- 99.Stone RA et al. (1989) Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci U S A 86 (2), 704–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fischer AJ et al. (1999) Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat Neurosci 2 (8), 706–12. [DOI] [PubMed] [Google Scholar]
- 101.Mathis U and Schaeffel F (2007) Glucagon-related peptides in the mouse retina and the effects of deprivation of form vision. Graefes Arch Clin Exp Ophthalmol 245 (2), 267–75. [DOI] [PubMed] [Google Scholar]
- 102.Zhong X et al. (2004) Image defocus modulates activity of bipolar and amacrine cells in macaque retina. Invest Ophthalmol Vis Sci 45 (7), 2065–74. [DOI] [PubMed] [Google Scholar]
- 103.Stone RA et al. (1988) Increase in retinal vasoactive intestinal polypeptide after eyelid fusion in primates. Proc Natl Acad Sci U S A 85 (1), 257–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seltner RL et al. (1997) Endogenous opiates in the chick retina and their role in form-deprivation myopia. Vis Neurosci 14 (5), 801–9. [DOI] [PubMed] [Google Scholar]
- 105.George A et al. (2005) Retinal serotonin, eye growth and myopia development in chick. Exp Eye Res 81 (5), 616–25. [DOI] [PubMed] [Google Scholar]
- 106.Tkatchenko AV et al. (2015) APLP2 Regulates Refractive Error and Myopia Development in Mice and Humans. PLoS Genet 11 (8), e1005432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pardue MT et al. (2008) High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest Ophthalmol Vis Sci 49 (2), 706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tedja MS et al. (2018) Genome-wide association meta-analysis highlights light-induced signaling as a driver for refractive error. Nat Genet 50 (6), 834–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stone RA et al. (2011) Image defocus and altered retinal gene expression in chick: clues to the pathogenesis of ametropia. Invest Ophthalmol Vis Sci 52 (8), 576577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Riddell N et al. (2018) Short term optical defocus perturbs normal developmental shifts in retina/RPE protein abundance. BMC Dev Biol 18 (1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Riddell N and Crewther SG (2017) Integrated Comparison of GWAS, Transcriptome, and Proteomics Studies Highlights Similarities in the Biological Basis of Animal and Human Myopia. Invest Ophthalmol Vis Sci 58 (1), 660–669. [DOI] [PubMed] [Google Scholar]
- 112.Riddell N et al. (2017) The retina/RPE proteome in chick myopia and hyperopia models: Commonalities with inherited and age-related ocular pathologies. Mol Vis 23, 872–888. [PMC free article] [PubMed] [Google Scholar]
- 113.Riddell N et al. (2016) Bidirectional Expression of Metabolic, Structural, and Immune Pathways in Early Myopia and Hyperopia. Front Neurosci 10, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seko Y et al. (1997) Apomorphine inhibits the growth-stimulating effect of retinal pigment epithelium on scleral cells in vitro. Cell Biochem Funct 15 (3), 191–6. [DOI] [PubMed] [Google Scholar]
- 115.Seko Y et al. (1994) Scleral cell growth is influenced by retinal pigment epithelium in vitro. Graefes Arch Clin Exp Ophthalmol 232 (9), 545–52. [DOI] [PubMed] [Google Scholar]
- 116.Kantarci S et al. (2007) Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat Genet 39 (8), 957–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wallman J et al. (1995) Moving the retina: choroidal modulation of refractive state. Vision Res 35 (1), 37–50. [DOI] [PubMed] [Google Scholar]
- 118.He L et al. (2018) Altered gene expression in tree shrew retina and retinal pigment epithelium produced by short periods of minus-lens wear. Exp Eye Res 168, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.He L et al. (2014) Gene expression signatures in tree shrew choroid in response to three myopiagenic conditions. Vision Res 102, 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.He L et al. (2014) Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Exp Eye Res 123, 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y et al. (2016) Imposed Optical Defocus Induces Isoform-Specific Up-Regulation of TGFbeta Gene Expression in Chick Retinal Pigment Epithelium and Choroid but Not Neural Retina. PLoS One 11 (5), e0155356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y et al. (2012) Bidirectional, optical sign-dependent regulation of BMP2 gene expression in chick retinal pigment epithelium. Invest Ophthalmol Vis Sci 53 (10), 6072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Y et al. (2013) Effects of imposed defocus of opposite sign on temporal gene expression patterns of BMP4 and BMP7 in chick RPE. Exp Eye Res 109, 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Norton TT and Rada JA (1995) Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res 35 (9), 1271–81. [DOI] [PubMed] [Google Scholar]
- 125.Guo L et al. (2013) Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Invest Ophthalmol Vis Sci 54 (10), 6806–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schippert R et al. (2006) Changes in scleral MMP-2, TIMP-2 and TGFbeta-2 mRNA expression after imposed myopic and hyperopic defocus in chickens. Exp Eye Res 82 (4), 710–9. [DOI] [PubMed] [Google Scholar]
- 127.Zhou X et al. (2010) Changes in protein profiles of guinea pig sclera during development of form deprivation myopia and recovery. Mol Vis 16, 2163–74. [PMC free article] [PubMed] [Google Scholar]
- 128.Frost MR and Norton TT (2012) Alterations in protein expression in tree shrew sclera during development of lens-induced myopia and recovery. Invest Ophthalmol Vis Sci 53 (1), 322–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu H et al. (2018) Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]