The Oxford-AstraZeneca COVID-19 vaccine ChAdOx1 nCoV-19 is associated with a risk for vaccine-induced immune thrombosis with thrombocytopenia syndrome in the range of one to two cases per 100 000 vaccinations, with younger women showing the highest risk.1, 2 Additional cases have been reported for the Johnson & Johnson adenoviral vector-based Ad26.CoV2.S COVID-19 vaccine.3 Vaccine-induced antibodies against platelet factor 4 have been implicated in the pathogenesis.1, 2 These antibodies might be amplified by booster vaccination with an adenoviral vector, which prompted recommendations to boost with an mRNA-based vaccine instead, although data on safety and efficacy of heterologous prime–boost regimens are sparse.4
We quantified the vaccine-induced antibody response in vaccinees in Germany who received a heterologous COVID-19 vaccination scheme using ChAdOx1 nCoV-19 as prime and BNT162b2 mRNA (BioNTech-Pfizer) as boost vaccination. The results were compared with those of cohorts of health-care workers or volunteers who received homologous BNT162b2 or ChAdOx1 nCoV-19 vaccination regimens, respectively. Demographic data of the cohorts are presented in the appendix (pp 2–3).
To assess protective antibody responses, a surrogate neutralisation assay (NAb assay; Yhlo, Shenzen, China) based on the competition of serum antibodies with recombinant angiotensin-converting enzyme 2 for binding to the SARS-CoV-2 spike protein receptor-binding domain was used in two certified, university-based diagnostic laboratories in Munich and Erlangen, Germany. The surrogate neutralisation activity correlated closely with that in a cell culture-based SARS-CoV-2 infection-inhibition assay (appendix p 4).
A striking increase of vaccine-induced SARS-CoV-2 surrogate neutralisation activity was observed in 229 of 232 vaccinees who received a BNT162b2 boost vaccination 9–12 weeks after ChAdOx1 nCoV-19 prime vaccination. Sera were analysed on the day of BNT162b2 boost vaccination and 2 weeks after (appendix p 5). The single non-responder reported chronic lymphatic leukaemia. High antibody levels observed in two individuals after ChAdOx1 nCoV-19 prime vaccination most likely reflected previous, undetected SARS-CoV-2 infection.
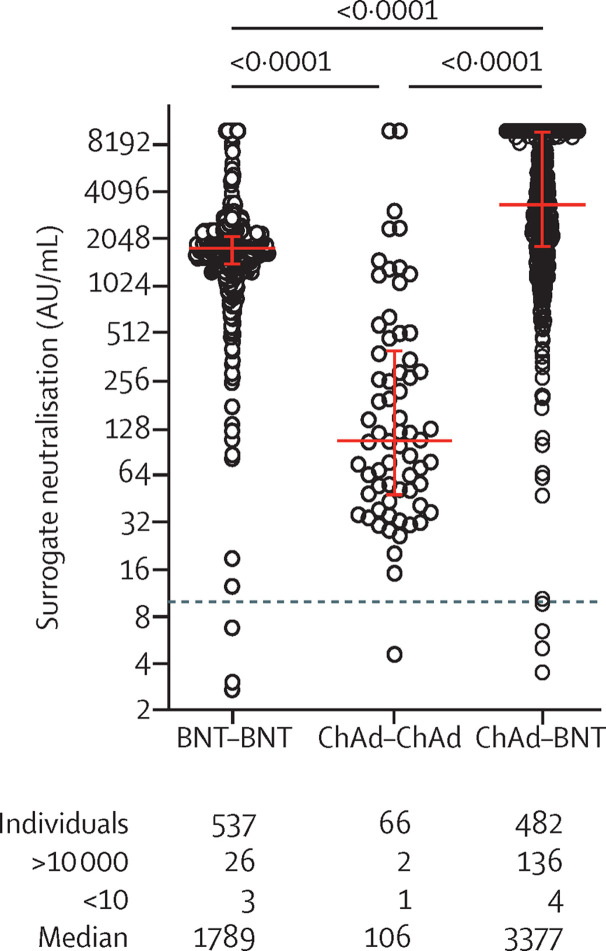
The figure shows the comparison of surrogate neutralisation activity observed 2 weeks after two doses of BNT162b2, two doses of ChAdOx1 nCoV-19, or one dose of ChAdOx1 nCoV-19 followed by one dose of BNT162b2. The heterologous vaccination regimen induced significantly higher surrogate neutralisation activity than homologous ChAdOx1 nCoV-19 or homologous BNT162b2 vaccination.
Figure.
Comparison of surrogate neutralisation activity induced by homologous and heterologous COVID-19 vaccine regimens
Dots represent the results from individual vaccinees analysed by the two study laboratories (appendix pp 2–3). p values from a Dunn's test for multiple comparisons are shown above the graph. Median and interquartile ranges are indicated by red horizontal lines. Below the graph, the total numbers of individual participants, the numbers below the lower (<10) and above the upper (>10 000) cutoff of the surrogate neutralisation assay, and median values of each group are shown.
Our results, in accordance with a recently published analysis4 and several studies available on public servers, indicate that a single dose of COVID-19 mRNA vaccine after a ChAdOx1 nCoV-19 prime vaccination is sufficient to achieve high serum neutralisation activity, predicting protection from SARS-CoV-2 infection.5 The heterologous vaccination regimen provided the highest surrogate neutralisation activity in our study. However, the shorter interval between the two mRNA vaccinations than between ChAdOx1 nCoV-19-prime and BNT162b2-boost vaccination might have contributed to the higher immunogenicity of the heterologous regimen.
Although we report a non-blinded and non-randomised study, the results obtained in more than 480 individuals who were primed with an adenoviral vector-based and boosted with an mRNA COVID-19 vaccine indicate increased efficacy of a heterologous prime–boost vaccination. This vaccination scheme is an interesting option if the thrombosis risk posed by adenoviral vector-based vaccines is a concern, and it increases flexibility in a setting of vaccine shortage. However, further studies need to address the safety and clinical efficacy of heterologous vaccination regimens.
JH reports grants and speaker honoraria from Pfizer, outside the study. UP reports grants from ALiOS and VirBio, and personal fees from AbbVie, Arbutus, Gilead, GSK, Johnson & Johnson, Roche, Sobi, and Vaccitech, outside the study. UP is co-founder and shareholder of SCG Cell Therapy. OAC reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, German Center for Infection Research (DZIF), EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer, and Scynexis; consulting fees from Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, PSI, Scynexis, and Seres; honoraria for lectures from Abbott, Al-Jazeera Pharmaceuticals, Astellas, Grupo Biotoscana/United Medical/Knight, Hikma, MedScape, MedUpdate, Merck, Mylan, and Pfizer; payment for expert testimony from Cidara; payment for participation on a data safety monitoring board or advisory board from Actelion, Allecra, Cidara, Entasis, IQVIA, Janssen, MedPace, Paratek, PSI, and Shionogi; and other from DGHO, DGI, ECMM, ISHAM, MSG-ERC, Wiley, outside the submitted work. All other authors declare no competing interests.
Contributor Information
DZIF-VACCELERATE-CoVaKo study team:
Hedwig Roggendorf, Otto Zelger, Catharina Christa, Samuel Jeske, Sarah Heringer, Rayya Alsalameh, Jan Esse, Jochen Mattner, and Monika Wytopil
Supplementary Material
References
- 1.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651–656. doi: 10.15585/mmwr.mm7017e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borobia AM, Carcas AJ, Pérez-Olmeda M, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021 doi: 10.1038/s41591-021-01377-8. published online May 17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.