Abstract
Background
COVID-19 is a complex disease targeting many organs. Previous studies highlight COVID-19 as a probable risk factor for acute cardiovascular complications. We aimed to quantify the risk of acute myocardial infarction and ischaemic stroke associated with COVID-19 by analysing all COVID-19 cases in Sweden.
Methods
This self-controlled case series (SCCS) and matched cohort study was done in Sweden. The personal identification numbers of all patients with COVID-19 in Sweden from Feb 1 to Sept 14, 2020, were identified and cross-linked with national inpatient, outpatient, cancer, and cause of death registers. The controls were matched on age, sex, and county of residence in Sweden. International Classification of Diseases codes for acute myocardial infarction or ischaemic stroke were identified in causes of hospital admission for all patients with COVID-19 in the SCCS and all patients with COVID-19 and the matched control individuals in the matched cohort study. The SCCS method was used to calculate the incidence rate ratio (IRR) for first acute myocardial infarction or ischaemic stroke following COVID-19 compared with a control period. The matched cohort study was used to determine the increased risk that COVID-19 confers compared with the background population of increased acute myocardial infarction or ischaemic stroke in the first 2 weeks following COVID-19.
Findings
86 742 patients with COVID-19 were included in the SCCS study, and 348 481 matched control individuals were also included in the matched cohort study. When day of exposure was excluded from the risk period in the SCCS, the IRR for acute myocardial infarction was 2·89 (95% CI 1·51–5·55) for the first week, 2·53 (1·29–4·94) for the second week, and 1·60 (0·84–3·04) in weeks 3 and 4 following COVID-19. When day of exposure was included in the risk period, IRR was 8·44 (5·45–13·08) for the first week, 2·56 (1·31–5·01) for the second week, and 1·62 (0·85–3·09) for weeks 3 and 4 following COVID-19. The corresponding IRRs for ischaemic stroke when day of exposure was excluded from the risk period were 2·97 (1·71–5·15) in the first week, 2·80 (1·60–4·88) in the second week, and 2·10 (1·33–3·32) in weeks 3 and 4 following COVID-19; when day of exposure was included in the risk period, the IRRs were 6·18 (4·06–9·42) for the first week, 2·85 (1·64–4·97) for the second week, and 2·14 (1·36–3·38) for weeks 3 and 4 following COVID-19. In the matched cohort analysis excluding day 0, the odds ratio (OR) for acute myocardial infarction was 3·41 (1·58–7·36) and for stroke was 3·63 (1·69–7·80) in the 2 weeks following COVID-19. When day 0 was included in the matched cohort study, the OR for acute myocardial infarction was 6·61 (3·56–12·20) and for ischaemic stroke was 6·74 (3·71–12·20) in the 2 weeks following COVID-19.
Interpretation
Our findings suggest that COVID-19 is a risk factor for acute myocardial infarction and ischaemic stroke. This indicates that acute myocardial infarction and ischaemic stroke represent a part of the clinical picture of COVID-19, and highlights the need for vaccination against COVID-19.
Funding
Central ALF-funding and Base Unit ALF-Funding, Region Västerbotten, Sweden; Strategic funding during 2020 from the Department of Clinical Microbiology, Umeå University, Sweden; Stroke Research in Northern Sweden; The Laboratory for Molecular Infection Medicine Sweden.
Introduction
COVID-19, caused by SARS-CoV-2, has led to a global health crisis. More than 190 million people have tested positive for SARS-CoV-2 worldwide, with more than 4 million deaths due to COVID-19 (WHO epidemiological update: July 20, 2021).1 Although initially the main concern focused on the risk of pneumonia progressing to acute respiratory distress syndrome with high mortality,2 there are increasing reports of cardiovascular manifestations and thrombotic complications following COVID-19.3 The prognosis is worse in patients with COVID-19 who have these complications, highlighting an acute need to determine the magnitude of cardiovascular complications and identify populations at risk.4
Evidence focusing on the association between COVID-19 and cardiovascular complications is based on relatively small studies, limited to the pandemic's early phase, and includes mainly hospitalised patients—ie, those with severe disease. Consequently, there is a need for studies at the population level to identify the burden of acute cardiovascular events following COVID-19. The aim of this study was to quantify the relative risk of acute myocardial infarction and ischaemic stroke following COVID-19 using two different methods: (1) the self-controlled case series (SCCS) method in a large, nationwide register-based cohort of all patients with COVID-19 in Sweden; and (2) a matched cohort study to identify the increased risk of acute cardiovascular events that COVID-19 confers compared with the background population.
Research in context.
Evidence before this study
Infection and inflammation are known to transiently increase the risk of stroke and acute myocardial infarction; therefore SARS-CoV-2 that causes COVID-19 might increase the risk of acute myocardial infarction and ischaemic stroke. We searched PubMed from database inception to March 10, 2021, for peer-reviewed studies and preprints published in English using the search terms “COVID-19” AND “acute myocardial infarction” AND “stroke”; and the combination of “COVID-19” AND “acute myocardial infarction”; or “COVID-19” AND “stroke”. Additionally, the bibliographies of identified studies were searched and Google Scholar was used to search for manuscripts and unpublished reports.
We identified only one study that used the self-controlled case series method to calculate the incidence rate ratio of acute myocardial infarction and ischaemic stroke following COVID-19 and found increased risks of both acute myocardial infarction and ischaemic stroke in the first 2 weeks following COVID-19. Furthermore, two studies were identified that used the retrospective cohort study and retrospective case-control method to determine the risk of ischaemic stroke following COVID-19. A retrospective cohort study compared patients with COVID-19 with patients with influenza. The odds of stroke following COVID-19 was greater than the odds following influenza. A small (n=41) retrospective case-control study found patients with COVID-19 being associated with increased odds of acute ischaemic stroke. Current evidence focusing on the association between COVID-19 and cardiovascular complications is based on small studies, and includes mainly hospitalised patients (ie, those with severe disease), therefore presenting a high risk of bias.
Added value of this study
To our knowledge, this is the largest study using all patients diagnosed with COVID-19 to identify the risk of first acute myocardial infarction and first ischaemic stroke using two separate methods, the self-controlled case series method and the matched cohort study (control individuals adjusted for important cardiovascular risk factors). This study determines laboratory-diagnosed COVID-19 as an independent risk factor for acute myocardial infarction and ischaemic stroke, even after adjusting for the effect of important confounders.
Implications of all the available evidence
The evidence indicates that acute cardiovascular complications might represent an essential clinical manifestation of COVID-19 and the long-term effects might be a challenge for the future. These findings could change clinical practice and warrant a prioritisation of preventive and diagnostic strategies, which can affect treatment and, therefore, reduce the burden of morbidity and mortality in this patient group.
Methods
Participants and databases
This SCCS and matched cohort study was done in Sweden. Since Feb 1, 2020, COVID-19 has been a notifiable disease in Sweden, and diagnosed individuals are reported to SmiNet (Swedish Public Health Agency) daily. All personal identification numbers from SmiNet at the Swedish Public Health Agency were extracted (including all patients registered as having COVID-19 until Sept 14, 2020) and cross-linked with the following registers administered by the Swedish National Board of Health and Welfare: inpatient register (Jan 1, 1987, to Oct 11, 2020); outpatient register (Jan 1, 1997, to Oct 11, 2020); cancer register (Jan 1, 2000, to Dec 31, 2018), and cause of death register (up to Oct 5, 2020).5 Socioeconomic data were obtained by cross-linking the COVID-19 cohort with the Longitudinal Integrated Database for Health Insurance and Labor Market Studies registry using the personal identification number for each patient. All data were pseudonymised by Statistics Sweden and the National Board of Health and Welfare.
The weighted Charlson Comorbidity Index (wCCI)6 was calculated to consider the burden of comorbidities, based on historical registry data from the outpatient register, inpatient register, and the cancer register using a method specifically adapted to Swedish National Registers.7 To ensure complications due to COVID-19 were not included in the wCCI, the calculation was stopped 2 months before COVID-19 onset (see Procedures for details of COVID-19 onset date). If there were no wCCI diagnosis codes in the outpatient register, inpatient register, or the cancer register, the individual was presumed healthy until we stopped calculations 2 months before the COVID-19 date and given a wCCI of 0.
Control individuals were identified by Statistics Sweden and their data provided to us. Four control individuals without a positive test for SARS-CoV-2 infection were randomly matched by age, sex, and county of residence by Statistics Sweden for every patient with COVID-19 with the index date based on the date of report in SmiNet. The socioeconomic risk factors of income (annual disposable income divided into quintiles), education (primary, secondary, and tertiary), and birth country (divided into Sweden, high-income, middle-income, and low-income countries according to the World Bank gross national income Atlas method) were identified for each patient with COVID-19 and control individuals.
The study received ethical approval from the Swedish Ethical Review Authority (DNR 2020-02150).
Procedures
The study period encompassed the time from Feb 1 to Sept 14, 2020. The optimal COVID-19 date for both the SCCS and matched cohort studies is the date of infection with SARS-CoV-2. However, this date was not available in the dataset, instead the reporting physician entered the date of disease onset, sample date, diagnosis date, and the date of report to SmiNet. To identify the closest date possible to the date of SARS-CoV-2 infection from the registry database, each individual was assigned a COVID-19 date, reflecting the earliest available date from the SmiNet register or the outpatient or inpatient registers according to (1) date of disease onset; then (2) the earliest date of either hospital admission or visit to an outpatient clinic due to COVID-19 or sample date; then (3) diagnosis date; and finally (4) date of report to SmiNet.
The International Classification of Diseases (ICD) version 9 and 10 codes were identified in the main and contributing causes of hospital admission, thereby identifying the first acute myocardial infarction or ischaemic stroke since Jan 1, 1987, for patients with COVID-19 in the SCCS study and matched cohort study and since Jan 1, 1997, for control individuals in the matched cohort study. The diagnosis codes were ICD-9 codes 433–434 and ICD-10 codes I63–64 for ischaemic stroke and ICD-9 code 410 and ICD-10 codes I21–22 for acute myocardial infarction. The event date for acute myocardial infarction or ischaemic stroke was either the date of hospital admission due to acute myocardial infarction or stroke (data from the inpatient register), or the date of intervention (see appendix pp 1–2 for intervention codes) if the date of intervention and hospital admission differed by more than 3 days.
Statistical analysis
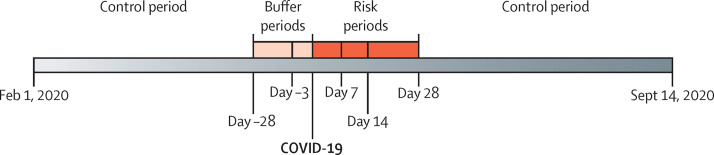
We used the SCCS method8 to calculate the incidence rate ratio (IRR) of acute myocardial infarction and ischaemic stroke in temporal risk periods following COVID-19 onset, using only individuals who were diagnosed with COVID-19 and had an acute myocardial infarction or ischaemic stroke (figure 1 ). The SCCS method is a conditional Poisson regression method that compares intraperson incidence rates of the outcome of interest (acute myocardial infarction or ischaemic stroke) in different study periods relative to an exposure (COVID-19).8 We fitted an SCCS deaths-adjusted model to allow for the fact that acute myocardial infarction and ischaemic stroke can increase short-term mortality.9 We adjusted for calendar month (February to September, 2020). A buffer period before COVID-19 was included (day −28 to −4) because hospital admission is a probable risk factor for contracting SARS-CoV-2. There was an increased number of first acute myocardial infarctions or ischaemic stroke events at day 0 (COVID-19) leading to concerns about potential test bias related to event and exposure occurring on the same day. Therefore, two analyses were done, one excluding day 0 (analysis 1) and one including day 0 (analysis 2). Analysis 1 had the buffer period, a pre-exposure period from day −3 to day 0, and risk periods from day 1 to 7, day 8 to 14, and day 15 to 28. Analysis 2 had the buffer period, a pre-exposure period from day −3 to −1, and risk periods from day 0 to 7, day 8 to 14, and day 15 to 28 (figure 1). Any time between Feb 1 and Sept 14, 2020, that was not included in the buffer, pre-exposure, or risk periods was the control period in both analyses. The sample size needed to identify a clinically relevant IRR of 2 in the SCCS study for acute myocardial infarction and ischaemic stroke using a risk period of 14 days was 154 acute myocardial infarction or ischaemic stroke events to achieve 70% power, and 199 acute myocardial infarction or ischaemic stroke events to achieve 80% power. Effect modification by sex and age (≤70 vs >70 years) were investigated, and these were prespecified analyses before study onset. The IRR and 95% CIs of acute myocardial infarction and ischaemic stroke following COVID-19 onset were estimated using the package SCCS version 1.29 in R version 4.0.2.
Figure 1.
Overview of the self-controlled case series study design
The matched cohort study compared the risk of a first-time acute myocardial infarction or ischaemic stroke in the 2 weeks following the COVID-19 date with the risk in the background population. Individuals with a previous myocardial infarction or ischaemic stroke were excluded from the matched cohort study. A matched cohort study compares the incidence of an outcome (acute myocardial infarction or ischaemic stroke) in two groups with or without an exposure (COVID-19) resulting in an odds ratio (OR) of the outcome. The matched cohort analyses were also done excluding and including day 0. For the matched cohort study, allowing for four control individuals per case, about 65 events were required in the 14-day follow-up period for 70% power and 83 events for 80% power. Unadjusted and adjusted conditional logistic regressions were done, including the wCCI and relevant socioeconomic variables such as income, education level, and country of birth. These variables were selected a priori. For the adjusted analyses, a complete case only analysis was done. OR and 95% CI of acute myocardial infarction and ischaemic stroke were estimated using the function clogit, package survival version 3.2-710 in R version 4.0.2.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
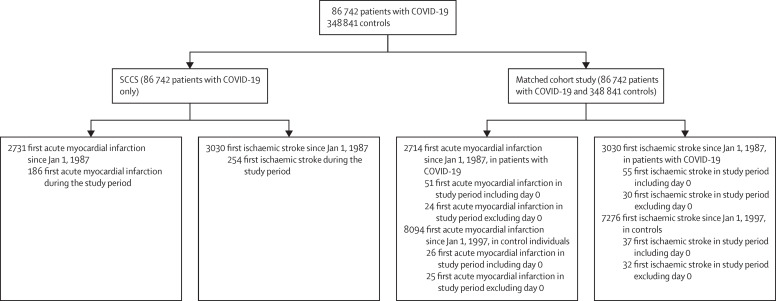
Between Feb 1 and Sept 14, 2020, 86 742 individuals were diagnosed with COVID-19 in Sweden (figure 2 ). The median age was 48 years (IQR 31–62), and 37 235 (43%) were male and 49 507 (57%) were female. For the SCCS and matched cohort studies, the COVID-19 date was mainly based on symptom onset, contact with outpatient clinics or hospitalisation, or sample date due to COVID-19. For acute myocardial infarction and ischaemic stroke events that occurred on day 0, the COVID-19 date was mainly based on the date of contact with the outpatient clinic or date of hospitalisation (appendix p 4). There were more events than required to achieve 70% power to detect an IRR of 2, with the exception of the matched cohort study for acute myocardial infarction excluding day 0 from the 14-day risk period.
Figure 2.
Flow diagram of study
The study period in the overall population and in the SCCS is from Feb 1 to Sept 14, 2020. The study period in the matched cohort study refers to the 2 weeks following COVID-19. SCCS=self-controlled case series.
In the SCCS study, there were 186 first acute myocardial infarction events during the study period (appendix p 9), and 36 patients died (for overview of events per day in the first 2-week period following COVID-19 see appendix p 5). The median age of people in the SCCS who had an acute myocardial infarction during the study period was 73 years (IQR 64–82), with 77 (41%) women and 109 (59%) men. When day 0 was excluded from the first risk period (analysis 1), compared with the control period, the risk of acute myocardial infarction following COVID-19 was significantly increased in the first and second weeks, but also during the pre-exposure period (day −3 to 0; table 1 ). In the analysis including day 0 (analysis 2), the risk for an acute myocardial infarction following COVID-19 was similarly increased in the first and second weeks following COVID-19. This effect was transient as the risk of acute myocardial infarction in the third and fourth weeks was not significantly increased compared with the control period. There was a significant association between acute myocardial infarction and COVID-19 in the month before COVID-19 (buffer period; table 1). The effect modification for sex and age was not significant for either analysis including or excluding day 0 (appendix p 6).
Table 1.
IRR of acute myocardial infarction following COVID-19 in the SCCS
| Number of events | IRR (95% CI) | p value | |
|---|---|---|---|
| Acute myocardial infarction analysis 1 (day 0 out of the risk period) | |||
| Control period | 90 | 1 (ref) | .. |
| Buffer period: days −28 to −4 | 30 | 2·04 (1·30–3·21) | 0·0021 |
| Pre-exposure period: days −3 to 0 | 30 | 16·20 (10·24–25·63) | <0·0001 |
| Risk period: days 1 to 7 | 12 | 2·89 (1·51–5·55) | 0·0014 |
| Risk period: days 8 to 14 | 12 | 2·53 (1·29–4·94) | 0·0067 |
| Risk period: days 15 to 28 | 12 | 1·60 (0·84–3·04) | 0·16 |
| Acute myocardial infarction analysis 2 (day 0 in the risk period) | |||
| Control period | 90 | 1 (ref) | .. |
| Buffer period: days −28 to −4 | 30 | 2·06 (1·31–3·24) | 0·0017 |
| Pre-exposure period: days −3 to −1 | 3 | 2·52 (0·78–8·09) | 0·12 |
| Risk period: days 0 to 7 | 39 | 8·44 (5·45–13·08) | <0·0001 |
| Risk period: days 8 to 14 | 12 | 2·56 (1·31–5·01) | 0·0059 |
| Risk period: days 15 to 28 | 12 | 1·62 (0·85–3·09) | 0·14 |
IRR=incidence rate ratio. SCCS=self-controlled case series.
For the matched cohort study, 83 937 patients with COVID-19 and 340 432 control individuals were included in the analysis. Of these, 24 patients with COVID-19 and 25 control individuals had their first acute myocardial infarction during the 2 weeks after the COVID-19 date, where day 0 was excluded. When day 0 was included in the analysis, 83 938 patients with COVID-19 and 340 432 control individuals were included in the study. Of these, 51 patients with COVID-19 and 26 control individuals had their first acute myocardial infarction during the first 2 weeks following the COVID-19 date. Individuals (regardless of COVID-19 status) who had had an acute myocardial infarction previously were excluded from this analysis. The results of the matched cohort study for acute myocardial infarction excluding day 0 are shown in table 2 , and including day 0 are shown in the appendix (p 7). The overall number of patients with missing information was 22 533 (5·3%). Three patients with an acute myocardial infarction (three [6·12%] excluding day 0; three [3·9%] including day 0) had missing information for level of education (table 2; appendix p 7). The odds for first acute myocardial infarction in the 2 weeks following COVID-19 (excluding day 0) were significantly increased compared with the control individuals (in both the univariable and multivariable analyses; table 2). The odds for first acute myocardial infarction in the 2 weeks following COVID-19 were also significantly increased compared with the control group when day 0 was included in the analysis (both adjusted and unadjusted analysis; appendix p 7). The adjusted odds for an acute myocardial infarction following COVID-19 were significantly increased for each wCCI point when excluding day 0 from the analysis (table 2) and when including day 0 in the analysis (appendix p 7). Following adjustment, the socioeconomic factors were not significantly associated with acute myocardial infarction (table 2; appendix p 7).
Table 2.
Unadjusted and adjusted conditional logistic regression models for acute myocardial infarction within 14 days following COVID-19 onset in the matched cohort study
|
Acute myocardial infarction |
Univariable models |
Multivariable model |
||
|---|---|---|---|---|
| No (n=424 320) | Yes (n=49) | OR (95% CI; p value) | OR (95% CI; p value) | |
| COVID-19 diagnosis | ||||
| No | 340 407 (99·99%) | 25 (0·01%) | 1 (ref) | 1 (ref) |
| Yes | 83 913 (99·97%) | 24 (0·03%) | 4·06 (2·27–7·25; p<0·0001) | 3·41 (1·58–7·36; p=0·0017) |
| wCCI | ||||
| Mean | 1 (2·02) | 3·53 (2·48) | 1·22 (1·08–1·38; p=0·0012) | 1·41 (1·18–1·68; p=0·0002) |
| Income quintile* | ||||
| 1 | 82 972 (99·99%) | 7 (0·01%) | 1 (ref) | 1 (ref) |
| 2 | 83 053 (99·99%) | 5 (0·01%) | 1·01 (0·28–3·60; p=0·98) | 1·14 (0·26–5·06; p=0·87) |
| 3 | 82 607 (99·99%) | 11 (0·01%) | 1·53 (0·48–4·88; p=0·47) | 1·76 (0·41–7·55; p=0·45) |
| 4 | 80 249 (99·98%) | 14 (0·02%) | 1·30 (0·44–3·85; p=0·64) | 0·96 (0·23–3·98; p=0·96) |
| 5 | 81 296 (99·99%) | 12 (0·01%) | 3·05 (0·94–9·88; p=0·064) | 4·17 (0·76–22·8; p=0·10) |
| Data missing | 14 143 (3·33%) | 0 | .. | .. |
| Education | ||||
| Tertiary | 154 667 (99·99%) | 8 (0·01%) | 1 (ref) | 1 (ref) |
| Secondary | 172 934 (99·99%) | 19 (0·01%) | 2·11 (0·83–5·34; p=0·12) | 1·51 (0·50–4·53; p=0·46) |
| Primary | 76 245 (99·98%) | 19 (0·02%) | 2·34 (0·91–6·04; p=0·079) | 1·25 (0·37–4·23; p=0·72) |
| Data missing | 20 474 (4·83%) | 3 (6·12%) | .. | .. |
| Country of birth | ||||
| Sweden | 324 733 (99·99%) | 37 (0·01%) | 1 (ref) | 1 (ref) |
| Other high-income country | 33 377 (99·99%) | 5 (0·01%) | 1·09 (0·37–3·20; p=0·88) | 1·30 (0·32–5·24; p=0·71) |
| Middle-income country | 45 128 (99·99%) | 6 (0·01%) | 5·76 (1·54–21·63; p=0·094) | 1·71 (0·23–12·97; p=0·60) |
| Low-income country | 20 870 (100%) | 1 (0%) | 1·30 (0·13–12·58; p=0·82) | 0·98 (0·06–17·32; p=0·99) |
| Data missing | 212 (0·04%) | 0 | .. | .. |
Data are n (%) or mean (SD) except where otherwise stated. Percentages are calculated as a proportion of the included study population; individuals with a previous acute myocardial infarction or stroke were excluded from the study. Day 0 is excluded from the study period due to risk of selection bias. OR=odds ratio. wCCI=weighted Charlson Comorbidity Index.
Quintile 1 is the highest income, quintile 5 is the lowest income.
In the SCCS study, there were 254 first ischaemic stroke events during the study period (see appendix p 10 for overview of all first ischaemic stroke events during the study, and appendix p 5 for overview of stroke events per day in the first 2-week period). The median age of people in the SCCS who had a stroke during the study period was 79 years (IQR 68–86), with 134 (43%) men and 120 (47%) women. With day 0 excluded from the risk period (analysis 1) the risk of ischaemic stroke was significantly increased in the first 2 weeks (by approximately three times) and in the last 2 weeks (by approximately two times) following COVID-19 compared with the control period (table 3 ). The risk of ischaemic stroke was also significantly increased in the short period before COVID-19 (day −3 to 0 in analysis 1 and day −3 to −1 in analysis 2; table 3). When day 0 was included in the risk period, the risk of ischaemic stroke was increased by approximately six times in the first week following COVID-19, by approximately three times in the second week following COVID-19, and by approximately two times in the third and fourth weeks following COVID-19, all compared with the control period (table 3). As for acute myocardial infarction, we observed an increased risk (to approximately twice the risk) of ischaemic stroke in the month before COVID-19 onset in analysis 1 and analysis 2) compared with the control period. The effect modification for sex and age for ischaemic stroke risk was not significant (appendix p 8).
Table 3.
IRR of ischaemic stroke following COVID-19 in the SCCS
| Number of events | IRR (95% CI) | p value | |
|---|---|---|---|
| Ischaemic stroke analysis 1 (day 0 out of the risk period) | |||
| Control period | 123 | 1 (ref) | .. |
| Buffer period: days −28 to −4 | 57 | 1·95 (1·25–3·04) | 0·0031 |
| Pre-exposure period: days −3 to 0 | 33 | 11·28 (7·15–17·80) | <0·0001 |
| Risk period: days 1 to 7 | 16 | 2·97 (1·71–5·15) | 0·0001 |
| Risk period: days 8 to 14 | 14 | 2·80 (1·60–4·88) | 0·0003 |
| Risk period: days 15 to 28 | 11 | 2·10 (1·33–3·32) | 0·0015 |
| Ischaemic stroke analysis 2 (day 0 in the risk period) | |||
| Control period | 123 | 1 (ref) | .. |
| Buffer period: days −28 to −4 | 57 | 1·89 (1·21–2·96) | 0·0048 |
| Pre-exposure period: days −3 to −1 | 8 | 3·96 (1·85–8·45) | 0·0004 |
| Risk period: days 0 to 7 | 41 | 6·18 (4·06–9·42) | <0·0001 |
| Risk period: days 8 to 14 | 14 | 2·85 (1·64–4·97) | 0·0002 |
| Risk period: days 15 to 28 | 11 | 2·14 (1·36–3·38) | 0·0011 |
IRR=incidence rate ratio. SCCS=self-controlled case series.
Results for ischaemic stroke from the matched cohort study analysis excluding day 0 are shown in table 4 , and for the analysis including day 0 are shown in the appendix (p 8). The overall number of patients with missing information was 22 460 (5·29%). Three patients with an ischaemic stroke event had missing information regarding level of education (one [1·61%] excluding day 0; two [2·17%] including day 0; table 4; appendix p 8). The odds for ischaemic stroke in the first 2 weeks following COVID-19 (excluding day 0) was significantly increased by approximately four times compared with the control group (in both univariable and multivariable analyses; table 4).
Table 4.
Unadjusted and adjusted conditional logistic regression models for ischaemic stroke within 14 days following COVID-19 onset in the matched cohort study
|
Stroke |
Univariable models |
Multivariable model |
||
|---|---|---|---|---|
| No (n=424 406) | Yes (n=62) | OR (95% CI; p value) | OR (95% CI; p value) | |
| COVID-19 diagnosis | ||||
| No | 340 920 (99·99%) | 32 (0·01%) | 1 (ref) | 1 (ref) |
| Yes | 83 486 (99·96%) | 30 (0·04%) | 4·52 (2·65–7·70; p<0·0001) | 3·63 (1·69–7·80; p=0·0009) |
| wCCI | ||||
| Mean | 1·05 (2·06) | 4·85 (3·69) | 1·41 (1·25–1·59; p<0·0001) | 1·46 (1·25–1·71; p<0·0001) |
| Income quintiles* | ||||
| 1 | 83 121 (99·99%) | 10 (0·01%) | 1 (ref) | 1 (ref) |
| 2 | 83 329 (99·99%) | 8 (0·01%) | 0·80 (0·28–2·29; p=0·68) | 0·40 (0·09–1·75; p=0·23) |
| 3 | 82 626 (99·99%) | 5 (0·01%) | 0·63 (0·19–2·07; p=0·44) | 0·44 (0·10–1·99; p=0·29) |
| 4 | 80 142 (99·97%) | 21 (0·03%) | 1·62 (0·65–4·04; p=0·30) | 1·24 (0·37–4·12; p=0·73) |
| 5 | 81 057 (99·98%) | 18 (0·02%) | 1·59 (0·63–4·00; p=0·32) | 0·48 (0·12–1·94; p=0·30) |
| Data missing | 14 131 (3·33%) | 0 | .. | .. |
| Education | ||||
| Tertiary | 154 519 (99·99%) | 17 (0·01%) | 1 (ref) | 1 (ref) |
| Secondary | 173 072 (99·99%) | 25 (0·01%) | 1·15 (0·58–2·29; p=0·68) | 1·95 (0·73–5·23; p=0·19) |
| Primary | 76 412 (99·98%) | 19 (0·02%) | 1·22 (0·58–2·56; p=0·59) | 1·63 (0·54–4·92; p=0·39) |
| Data missing | 20 406 (4·81%) | 1 (1·61%) | .. | .. |
| Country of birth | ||||
| Sweden | 324 554 (99·99%) | 45 (0·01%) | 1 (ref) | 1 (ref) |
| Other high-income country | 33 289 (99·98%) | 7 (0·02%) | 1·34 (0·52–3·44; p=0·55) | 1·44 (0·43–4·74; p=0·55) |
| Middle-income country | 45 440 (99·99%) | 5 (0·01%) | 2·00 (0·68–5·85; p=0·21) | 1·17 (0·25–5·45; p=0·84) |
| Low-income country | 20 911 (99·98%) | 5 (0·02%) | 10·13 (1·90–54·05; p=0·0067) | 11·92 (0·78–181·12; p=0·074) |
| Data missing | 212 (0·05%) | 0 | .. | .. |
Data are n (%) or mean (SD) except where otherwise stated. Percentages are calculated as a proportion of the included study population; individuals with a previous acute myocardial infarction or stroke were excluded from the study. Day 0 is excluded from the study period due to risk of selection bias. OR=odds ratio. wCCI=weighted Charlson Comorbidity Index.
Quintile 1 is the highest income, quintile 5 is the lowest income.
The odds for ischaemic stroke following COVID-19 were significantly increased for each wCCI point when excluding day 0 from the matched cohort analysis (table 4) and when including day 0 in both the univariable and multivariable analysis (appendix p 8).
Following adjustment, there was no significant increase in OR for ischaemic stroke for the socioeconomic risk factors either including or excluding day 0 (table 4; appendix p 8).
Discussion
In our study, we identified COVID-19 as an independent risk factor for ischaemic stroke and acute myocardial infarction. To the best of our knowledge, our study involving 86 742 patients with COVID-19 is the largest study done on the association between COVID-19 and acute cardiovascular events. The nationwide inclusion of all diagnosed COVID-19 patients in Sweden adds to the robustness of the data. We used two different methodological approaches to test our hypothesis. In the SCCS method, the cases act as their own controls, and confounders—eg, comorbidities or sociodemographic factors—are controlled for in the analyses.8 In addition, because we observed a high number of events at day 0, which could reflect test bias, we did two separate SCCS and match cohort analyses, one excluding and one including day 0. The mean incubation period for COVID-19 is 5·1 days, and fewer than 2·5% of patients develop symptoms within 2·2 days of infection; within 12·5 days, 97·5% of patients have developed symptoms.11 Therefore, it is highly likely that patients at day 0 were indeed infected with SARS-CoV-2 before their event, and that the systemic response to infection precipitated the event. How to handle the peak at day 0 reflects contrasting statistical perspectives (excluding day 0 due to risk of selection bias), and clinical perspectives (including day 0 in the risk period); however, the risk of acute myocardial infarction and ischaemic stroke was consistently and significantly increased in patients with COVID-19 compared with the control period, regardless of whether day 0 was included in the risk period. These effects were clinically significant, with the risk increasing by two times or more. The risk of acute myocardial infarction and ischaemic stroke was significantly increased during the buffer period (day −28 to −4), probably owing to reverse causality—ie, nosocomial COVID-19 during hospitalisation for acute myocardial infarction or ischaemic stroke. This hypothesis is further supported by Lauer and colleagues' study in which the majority of individuals developed symptoms within 12·5 days following SARS-CoV-2 infection.11 Our buffer period includes a time in which the majority of individuals develop symptoms of COVID-19 following SARS-CoV-2 infection. The increased number of acute myocardial infarctions and ischaemic strokes during the month before day 0 probably indicates nosocomial infection. This finding highlights the need for protecting patients from nosocomial COVID-19.
The matched cohort study enables a comparison with the background population and a quantification of how much COVID-19 increases the risk of acute myocardial infarction and ischaemic stroke. The historical data from several registers reaching back to 1987 (inpatient register) enabled us to quantify the wCCI with high specificity. However, even following adjustment for these comorbidities and sociodemographic risk factors, we consistently identified COVID-19 as an independent risk factor for acute myocardial infarction and ischaemic stroke.
Our findings are further strengthened by results from previous studies. The incidence of ischaemic stroke in patients with COVID-19 was reported to be between 0·9%12 and 4·6%;13 and incidence of acute myocardial infarction to be between 1·1%14 and 8·9%.4 In a study using a nationwide registry-based cohort of all patients with COVID-19 in Denmark, the risk of acute myocardial infarction following COVID-19 increased by five times, and the risk of ischaemic stroke following COVID-19 increased by ten times.15 Depending on whether day 0 was excluded or included in the risk period for the SCCS study, the risk in the first week following COVID-19 increased by either approximately three times or eight times for acute myocardial infarction or by approximately three times or six times for ischaemic stroke. In the matched cohort study adjusting for relevant risk factors, the risk of acute myocardial infarction and ischaemic stroke following COVID-19 increased by approximately three times in the analysis excluding day 0 and increased by approximately seven times in the analysis including day 0. These findings suggest that the true risk is increased by between three and eight times for acute myocardial infarction and by between three and seven times for ischaemic stroke following COVID-19. The results of the SCCS and matched cohort analyses are broadly consistent in our study. In addition, the absolute risks among both patients with COVID-19 and controls of acute myocardial infarction and ischaemic stroke obtained in the matched cohort study are less than 1 per 1000 people. Consequently, the ORs closely approximate relative risks, and thus are directly comparable to the IRRs obtained in the SCCS analysis. Previous COVID-19 studies4, 12, 13, 14, 15 might have had limitations regarding the small size of the study populations, a short study period, being limited to the pandemic's early phase, or including only hospitalised patients—ie, those with severe disease. Our study encompasses the whole first wave of the COVID-19 outbreak in Sweden and all diagnosed COVID-19 patients in Sweden are included regardless of whether they required hospitalisation or not.
Furthermore, our findings are in contrary to evidence showing a decrease in hospital admissions for acute myocardial infarction16 and stroke17 during the early phase of the pandemic. This paradox could be explained by either a real decrease in the overall incidence of cardiovascular events due to lifestyle changes during the lockdown, or by patient delay in seeking medical help due to physical distancing and fear of contagion.
In addition to COVID-19, other coronavirus infections (ie, MERS-CoV or SARS-CoV) were shown to increase the risk of cardiovascular disease.18 The nature of this observation is still unclear, but unique pathophysiological mechanisms such as viral pneumonia or downregulation of ACE2 are proposed as potential mechanisms.18 Moreover, atrial fibrillation, a well known risk factor for stroke,19 is common in patients with severe COVID-19.20
The identification of COVID-19 as an independent risk factor for acute myocardial infarction and ischaemic stroke in our and other studies is supported by previous studies in which infections with other viruses or bacteria increase transiently the risk of ischaemic stroke and acute myocardial infarction.21, 22, 23, 24 However, this risk seems to be higher following COVID-1925 (eg, the risk of stroke was 7·6 times higher with COVID-19 compared with influenza26), probably due to the disease's unique pathophysiological alterations.27 The exaggerated inflammatory response (cytokine storm)27 and the direct effect of the virus on endothelial cells28 are likely to precipitate cardiovascular events through ACE2 receptor downregulation, platelet activation, hypercoagulability,27 and effects on endothelial cells (activation, injury, dysfunction, and apoptosis).28 Long-term effects of COVID-19 on cardiovascular risk might also be a concern but need further analyses.
We also acknowledge limitations to this database study. The registry databases rely on Swedish nationwide reported data to the different registry stakeholders, with the implicit risk of incomplete data (as shown for the matched cohort study) and data inaccuracy. However, a previous study found a high validity for data, in particular with regards to acute cardiovascular events, from the Swedish inpatient register.29 Initially, acute myocardial infarction and ischaemic stroke diagnoses might be underestimated as critically ill patients with COVID-19 are too unstable to undergo diagnostic evaluation. Furthermore, patients with minor ischaemic stroke or acute myocardial infarction might not have sought health care due to fear of nosocomial infection.17 On the contrary, there is also a potential overestimation of the risk of acute myocardial infarction and ischaemic stroke because most asymptomatic or mildly symptomatic patients during the first wave of the pandemic were not tested for COVID-19. Moreover, an inaccurate diagnosis coding of a type II acute myocardial infarction as type I, or difficulties in distinguishing myocarditis from acute myocardial infarction, might also have contributed to an overestimation of the acute myocardial infarction incidence. Type II acute myocardial infarction due to demand ischaemia24 is probably not uncommon in patients with COVID-19,30 but its true incidence is still unknown. Unfortunately, the ICD-10 codes system does not distinguish between type I and type II acute myocardial infarction. However, previous analyses of the validity for acute myocardial infarction diagnosis codes is 100% and for stroke diagnosis codes is 98·6%.29
In conclusion, our findings suggest that COVID-19 is an independent risk factor for acute myocardial infarction and ischaemic stroke. Our results indicate that acute cardiovascular complications might represent an essential clinical manifestation of COVID-19 and the long-term effects might be a challenge for the future.
Data sharing
The study protocol (R script) for the SCCS and the matched cohort study are available upon request from the corresponding author. The study used secondary registry data, which is regulated by the Public Access to Information and Secrecy Act (2009:400) and is protected by strict confidentiality. However, for the purpose of research, after formal application to access personal data, the responsible authority can grant access to data, although this is contingent on vetting by the Ethical Review Authority of Sweden, according to the Act (2003:460) concerning the Ethical Review of Research Involving Humans. Synthetic (ie, depersonalised and jittered) data can be provided on request from the corresponding author.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank Wolfgang Lohr for data management, without his data management this study would not be possible. This study was funded by: Central ALF-funding, Region Västerbotten, Sweden (RV-836351), Base Unit ALF-funding, Region Västerbotten, Sweden (RV-939769); Strategic Funding during 2020 from the Department of Clinical Microbiology, Umeå University, Sweden; and Stroke Research in Northern Sweden, Molecular Infection Medicine Sweden.
Contributors
IK did the study design, data interpretation, literature review, data presentation and visualisation, and writing of the original draft. OF-R did the study design, data curation, formal analysis, programming and software development, designing of computer programs, implementation of the computer code and supporting algorithms, testing of existing code components, data presentation and visualisation, data interpretation, and writing of the original draft. PF did the study design, formal analysis, programming and software development, designing of computer programs, implementation of the computer code and supporting algorithms, testing of existing code components, data presentation and visualisation, data validation, data interpretation, and writing, reviewing, and editing of the report. KL did the study design, supervision, data interpretation, and writing, reviewing, and editing of the report. A-MFC conceptualised the study; did the data collection, study design, investigation, funding acquisition, and methodology; supervised the study; did the project administration, literature review, data interpretation, and writing, reviewing, and editing of the report. OF-R, A-MFC, and PF have accessed and verified the underlying data. IK, OF-R, and A-MFC had full access to all the data; PF had accessed to jittered data (due to policies regarding data sharing from the national Swedish authorities). All authors had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.WHO Weekly epidemiological update—20 July 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-20-july-2021
- 2.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thakkar S, Arora S, Kumar A, et al. A systematic review of the cardiovascular manifestations and outcomes in the setting of coronavirus-19 disease. Clin Med Insights Cardiol. 2020;14 doi: 10.1177/1179546820977196. 1179546820977196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ludvigsson JF, Håberg SE, Knudsen GP, et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol. 2015;7:491–508. doi: 10.2147/CLEP.S90589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Ludvigsson JF, Appelros P, Askling J, et al. Adaptation of the Charlson Comorbidity Index for register-based research in Sweden. Clin Epidemiol. 2021;13:21–41. doi: 10.2147/CLEP.S282475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25:1768–1797. doi: 10.1002/sim.2302. [DOI] [PubMed] [Google Scholar]
- 9.Farrington P, Whitaker H, Weldeselassie YG. 2nd edn. Chapman and Hall/CRC Press; Boca Raton, FL: 2018. Self-controlled case series studies: a modelling guide with R. [Google Scholar]
- 10.Therneau T. A package for survival analysis in R. April 25, 2020. https://cran.r-project.org/web/packages/survival/vignettes/survival.pdf
- 11.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan YK, Goh C, Leow AST, et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis. 2020;50:587–595. doi: 10.1007/s11239-020-02228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Li M, Wang M, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5:279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modin D, Claggett B, Sindet-Pedersen C, et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142:2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mafham MM, Spata E, Goldacre R, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudilosso S, Laredo C, Vera V, et al. Acute stroke care is at risk in the era of COVID-19: experience at a comprehensive stroke center in Barcelona. Stroke. 2020;51:1991–1995. doi: 10.1161/STROKEAHA.120.030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Wu S, Qin M, Jiang W, Liu X. Prevalence of cardiovascular comorbidities in coronavirus disease 2019, severe acute respiratory syndrome, and Middle East respiratory syndrome: pooled analysis of published data. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 20.Gawałko M, Kapłon-Cieślicka A, Hohl M, Dobrev D, Linz D. COVID-19 associated atrial fibrillation: incidence, putative mechanisms and potential clinical implications. Int J Cardiol Heart Vasc. 2020;30 doi: 10.1016/j.ijcha.2020.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes M, Heywood AE, Mahimbo A, Rahman B, Newall AT, Macintyre CR. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart. 2015;101:1738–1747. doi: 10.1136/heartjnl-2015-307691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clayton TC, Thompson M, Meade TW. Recent respiratory infection and risk of cardiovascular disease: case-control study through a general practice database. Eur Heart J. 2008;29:96–103. doi: 10.1093/eurheartj/ehm516. [DOI] [PubMed] [Google Scholar]
- 23.Connolly-Andersen AM, Hammargren E, Whitaker H, et al. Increased risk of acute myocardial infarction and stroke during hemorrhagic fever with renal syndrome: a self-controlled case series study. Circulation. 2014;129:1295–1302. doi: 10.1161/CIRCULATIONAHA.113.001870. [DOI] [PubMed] [Google Scholar]
- 24.Musher DM, Abers MS, Corrales-Medina VF. Acute infection and myocardial infarction. N Engl J Med. 2019;380:171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 25.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77 doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magadum A, Kishore R. Cardiovascular manifestations of COVID-19 infection. Cells. 2020;9 doi: 10.3390/cells9112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefanini GG, Montorfano M, Trabattoni D, et al. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141:2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol (R script) for the SCCS and the matched cohort study are available upon request from the corresponding author. The study used secondary registry data, which is regulated by the Public Access to Information and Secrecy Act (2009:400) and is protected by strict confidentiality. However, for the purpose of research, after formal application to access personal data, the responsible authority can grant access to data, although this is contingent on vetting by the Ethical Review Authority of Sweden, according to the Act (2003:460) concerning the Ethical Review of Research Involving Humans. Synthetic (ie, depersonalised and jittered) data can be provided on request from the corresponding author.