Abstract
Objectives
Coronavirus disease 2019 (COVID-19) has emerged as a pandemic, affecting nearly 180 million people worldwide as of June 22, 2021. Previous studies have examined the association between the mean vitamin D (Vit D) concentration of each country and COVID-19 infection and mortality rate in European countries. The aim of the present study was to critically evaluate the relationship between prevalence of mild and severe Vit D deficiency in each country and COVID-19 infection, recovery, and mortality using updated data and a different methodological approach.
Methods
Information on Vit D concentration or deficiency for each country was retrieved through a literature search. COVID-19 infections and mortalities per million people and total recoveries, as of June 22, 2021, were obtained. The associations between Vit D deficiency and COVID-19 infection, recovery, and mortality were explored using correlation coefficients and scatterplots.
Results
Non-significant correlations were observed between both number of COVID-19 infections (r = 0.363, P = 0.116) and number of recoveries (r = 0.388, P = 0.091) and the prevalence of mild Vit D deficiency (<50 nmol/L). Similarly, non-significant correlations were observed between both infections (r = 0.215, P = 0.392) and recoveries (r = 0.242, P = 0.332) and the prevalence of severe Vit D deficiency (<30 nmol/L). Significant correlations were found between COVID-19 mortality and prevalence of both mild Vit D deficiency (r = 0.634, P = 0.003) and severe Vit D deficiency (r = 0.538, P = 0.021).
Conclusions
The prevalence of neither mild nor severe Vit D deficiency was associated with the number of COVID-19 infections in European countries. Thus, it is an important parameter to consider when implementing preventive measures to face COVID-19.
Keywords: Vitamin D, Deficiency, COVID-19, Infections, Mortalities, Europe
Introduction
COVID-19 has become a global public health emergency, affecting more than 180 million people from 222 countries and territories [1] in less than a year from the first outbreak in Wuhan, China [2]. As of June 22, 2021, the lowest and highest number of confirmed cases were reported in Oceania (∼70 000) and Europe (∼47 500 000) [1]. This substantial variation in the number of infections, as well as the severity and mortality of the disease, can be accredited to several factors, both at the state level and the individual level. State-level parameters include diverse factors, such as a country's preparedness, actions of the governments, health infrastructure, timing of lockdowns, rapid border closures, implementation of social distancing, and socioeconomic status [3], whereas the individual level includes sociodemographic factors and other determinants of health status, such as sex, age, chronic diseases, obesity, and malnutrition [4,5].
It is well known that malnutrition constitutes a risk factor for increased mortality and morbidity in several diseases [6]. Protein and energy malnutrition and other specific micronutrient deficiencies have been shown to manifest adverse effects in immunity and thereby cause poor prognosis in viral infections [7]. Regarding micronutrients, the association between vitamin D (Vit D) deficiency and the prevalence and severity of various diseases, such as autoimmune disorders, diabetes, skeletal diseases, and acute respiratory tract infections, have been adequately established in the past years [8,9]. However, evidence with regard to Vit D concentration and preventive or curative mechanisms of SARS-CoV-2 infection is limited [10] or presents some controversies [11], [12], [13]. Recent studies have demonstrated the mechanisms for possible interactions between serum vitamin D concentration and rate of COVID-19 infections [14]. Particularly, Vit D modulates the expression of angiotensin-converting enzyme 2, angiotensin (1-7), and the MAS receptor axis and plays a crucial role in protection against lung infection [15], [16], [17]. It thereby acts as a renin-angiotensin system inhibitor in treating people with COVID-19 and underlying comorbidities [18,19] and can lead to a weakening of the cytokine storm and the risk of acute respiratory syndrome in people with COVID-19. But all this evidence lacks clinical validation [20,21].
In three recently published studies of the relationship between mean concentration of Vit D and number of cases and deaths of COVID-19 per million people in 20 European countries, negative correlations are reported [10,22,23]. In this study we aimed to critically evaluate the relationships between Vit D status and COVID-19 infections, recoveries, and mortalities in European countries, using more recent data and a different methodological approach. We also examined the relationships between severe Vit D deficiency (<30 nmol/L) and COVID-19 infections, recoveries, and mortalities.
Methods
Data sources and inclusion/exclusion criteria
Information on COVID-19 infections, recoveries, and mortalities was retrieved from the Worldometer website, which provides real-time statistics [1]. This source contains data derived directly from official government reports of individual countries and indirectly through reliable local media resources.
Data on the prevalence of Vit D deficiency in these countries were extracted by a comprehensive electronic search in the PubMed database (up to June 23, 2021). An advanced search was performed at the level of title/abstract by using keywords such as “Vitamin D” or “25-hydroxyvitamin D3,” combined with “deficiency,” “prevalence,” or “status” and the name of each European country. The final search string for each country and additional information about our search strategy are presented in Supplementary Table 1.
Inclusion criteria for our study were: population-based studies that reported data including the year 2010; studies reporting non-institutionalized adults (ages ≥ 18 y); studies defining mild Vit D deficiency as serum concentration < 20 ng/mL or < 50 nmol/L and/or severe deficiency as < 12 ng/mL or < 30 nmol/L; studies reporting the prevalence of Vit D deficiency in the sample population; European countries with population > 1 million; and European countries in which > 60 000 COVID-19 tests per million people were performed. Editorials, commentaries, book chapters, book reviews, and studies confined to a selective sample of community-dwelling people, such as pregnant women, menopausal women, and people with diagnosed illnesses, were excluded. As a last step, out of the articles screened for each country, the data on the prevalence of Vit D deficiency were retrieved from the most recently published study (with measurements completed not earlier than 2010), including the most representative sample for each country.
Data extraction
For each country, information on COVID-19 infections, recoveries, and mortalities per million people as of June 22, 2021, was extracted from the Worldometer website [1]. From the selected articles reporting Vit D deficiency in these countries, the name of the first author, year of publication, sample size, age range of the study population, mean Vit D concentration (nmol/L), and prevalence (%) of mild and severe Vit D deficiency were retrieved. All data were extracted by one reviewer (D. R. B.) using a standardized Excel form and were checked for accuracy by a second reviewer (M. C.).
Data analysis
The relationships between the prevalence of Vit D deficiency and variables such as the number of COVID-19 infections, recoveries, and mortalities per million people were explored with Pearson (r) correlation coefficients because the data were normally distributed. Scatterplots were used to visually represent the correlations. All countries were represented by a three-letter country code according to the ISO 3166 standard, as per the Terminology Bulletin for Country Names and the Country and Region Codes for Statistical Use maintained by the United Nations Statistics Divisions [24]. Pearson correlation (two-tailed) tests were performed using IBM SPSS version 25.0 software.
Results
A total of 20 European countries satisfying the inclusion and exclusion criteria were selected for the analysis [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44] (Table 1 ). Countries and territories excluded because of their limited population or lower number of COVID-19 tests were Andorra, the Channel Islands, the Faeroe Islands, Gibraltar, Iceland, the Isle of Man, Liechtenstein, Luxembourg, Malta, Monaco, Montenegro, San Marino, and Vatican City. Moreover, Albania, Belarus, Hungary, Latvia, Lithuania, Moldova, the Netherlands, North Macedonia, Serbia, Slovakia, and Sweden were not part of our analysis because of absent or non-updated evidence regarding Vit D concentration, or a non-representative sample. The prevalence of Vit D deficiency (<50 nmol/L) ranged from 6.9% to 75.8%, with the lowest and highest rates reported in Finland [30] and Bulgaria [28], respectively. The prevalence of severe Vit D deficiency (<30 nmol/L) ranged from 0.9% in Finland [30] to 30.2% in Germany [32]. In nine of the countries, the majority (>50%) of the adult population studied had Vit D deficiency (i.e., <20 ng/mL or <50 nmol/L) [27,28,32,33,37,38,40,42,44]. The size of study population used to retrieve data for the prevalence of Vit D deficiency varied from 280 (Slovenia) [40] to 74 235 (Italy) [35].
Table 1.
Prevalence of mild and severe vitamin D deficiency and COVID-19 data in 20 European countries
| Country | Reference | Sample size (F/M) | Age range (y) | Mean ± SD vitamin D (nmol/L) | COVID-19 infections per million people* | COVID-19 deaths per million people* | COVID-19 recoveries per million people* | % Prevalence of vitamin D deficiency (<50 nmol/L) | % Prevalence of severe vitamin D deficiency (<30 nmol/L) |
|---|---|---|---|---|---|---|---|---|---|
| Austria | [25] | 541 (334/237) | 18–80 | No info | 71 742 | 1180 | 50 490.1 | 48.0 | 14.4† |
| Belgium | [26] | 905 (464/441) | 20–69 | 55.4 ± 22.7 | 92763 | 2160 | 87 344 | 44.8 | 6.4† |
| Bosnia and Herzegovina | [27] | 1830 (no info) | >18 | No info | 62 831 | 2959 | 55 518.7 | 66.4 | 28.5† |
| Bulgaria | [28] | 2016 (1068/948) | 20–80 | 38.75 ± 17.18 | 61 073 | 2611 | 57 135.6 | 75.8 | 21.3† |
| Croatia | [29] | 791 (660/131) | 45.5‡ | 54.4 | 88 042 | 2007 | 85 922.3 | 46.1 | 21 |
| Finland | [30] | 798 (no info) | 30–64 | 64.0 ± 28.8 | 17 028 | 176 | 8289.6 | 6.9 | 0.9 |
| France | [31] | 892 (429/463) | 18–89 | 60.0 ± 20 | 89 748 | 1694 | 85 361.3 | 34.6 | 9.9 |
| Germany | [32] | 6995 (3635/3360) | 18–79 | 45.6 | 44 396 | 1084 | 42 965.1 | 61.5 | 30.2 |
| Greece | [33] | 1084 (674/410) | ≥18 | 41.8 ± 25.5 | 40 386 | 1212 | 38 689.8 | 64.8 | 28.8 |
| Ireland | [34] | 1118 (no info) | 18–84 | No info | 53 992 | 998 | 50 912.7 | 43.7 | 11.4 |
| Italy | [35] | 74 235 (55 424/18 811) | 18–104 | 68.5 ± 39 | 70 464 | 2109 | 67 224.7 | 33.3 | 14.5 |
| Norway | [36] | 4465 (2424/2041) | 40–69 | 64 ± 19.2 | 23 756 | 145 | 16 284.2 | 24.7 | 1.9 |
| Portugal | [37] | 3092 (1995/1097) | ≥18 | 42.2 ± 17.1 | 85 253 | 1679 | 80 909.7 | 66.6 | 21.2† |
| Romania | [38] | 14 052 (12 347/1705) | >21 | 49.5 ± 25 | 56 525 | 1699 | 54 692.3 | 52.0 | 13.2† |
| Russia | [39] | 1011 (824/187) | 18–75 | 54.8 ± 22.3 | 36 772 | 897 | 33 577.1 | 47.9 | No info |
| Slovenia | [40] | 280 (152/128) | 18–74 | 49 ± 26.8 | 123 636 | 2124 | 120 835.4 | 61.0 | 21.4 |
| Spain | [44] | 12 912 (no info) | 30–105 | 30.25 ± 11.35 | 80 575 | 1726 | 76 319.8 | 64.6 | No info |
| Switzerland | [41] | 1291 (no info) | >60 | No info | 1248 | 1248 | 77 613.3 | 39.2 | 8† |
| United Kingdom | [42] | 6004 (3291/2713) | >50 | 48.7 | 68 178 | 1876 | 63 114.2 | 55.3 | 23.7 |
| Ukraine | [43] | 1639 (no info) | 18–82 | 56.7 ± 21.6 | 51 294 | 1197 | 49 538.4 | 41.9 | 4.8 |
Data up to June 23, 2021.
<25 nmol/L.
Mean age of participants.
As of June 22, 2021, with regard to the total number of COVID-19 infections per million people of total population, Finland reported the lowest, with 17 028/million people, and Slovenia had the highest, with 123 636/million people. Regarding COVID-19 mortalities per million people, the lowest number was documented in Norway (145), and the highest number was reported in Bosnia and Herzegovina (2959). Regarding recoveries per million people, the lowest number was reported in Finland (8289.6) and the highest number was observed in Slovenia (120 835.4).
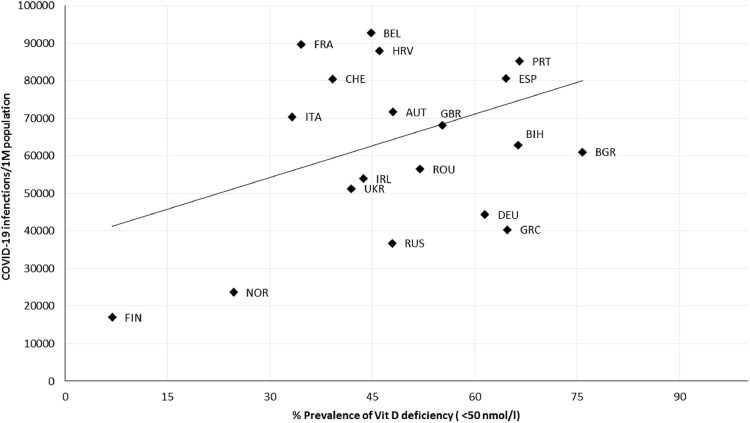
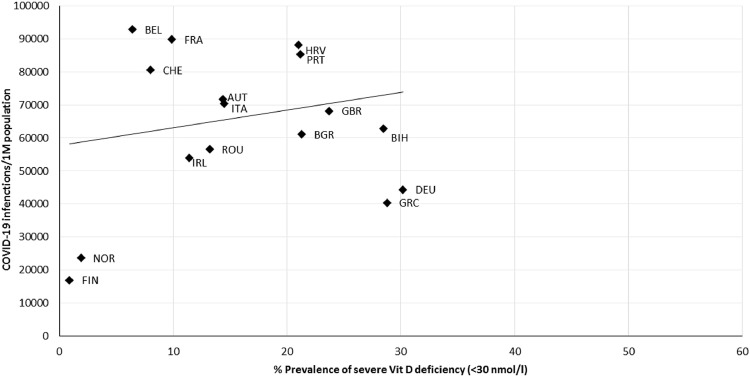
Cases of COVID-19 infection per million people displayed a non-significant, positive correlation (r = 0.363, P = 0.116) with the prevalence of Vit D deficiency (<50 nmol/L; Fig. 1 ). A non-significant correlation (r = 0.215, P = 0.392) was also observed between COVID-19 infections and severe Vit D deficiency (<30 nmol/L; Fig. 2 ).
Fig. 1.
Scatter diagram of the prevalence of vitamin D deficiency (<50 nmol/L) versus COVID-19 infections per million people, as of June 22, 2021 (r = 0.363, P = 0.166). AUT, Austria; BEL, Belgium; BGR, Bulgaria; BIH, Bosnia and Herzegovina; CHE, Switzerland; DEU, Germany; ESP, Spain; FIN, Finland; FRA, France; GBR, United Kingdom; GRC, Greece; HRV, Croatia; IRL, Ireland; ITA, Italy; NOR, Norway; PRT, Portugal; ROU, Romania; RUS, Russia; SVN, Slovenia; UKR, Ukraine.
Fig. 2.
Scatter diagram of the prevalence of severe vitamin D deficiency (<30 nmol/L) versus COVID-19 infections per million people, as of June 22, 2021 (r = 0.215, P = 0.392). AUT, Austria; BEL, Belgium; BGR, Bulgaria; BIH, Bosnia and Herzegovina; CHE, Switzerland; DEU, Germany; GBR, United Kingdom; GRC, Greece; HRV, Croatia; FIN, Finland; FRA, France; IRL, Ireland; ITA, Italy; NOR, Norway; PRT, Portugal; ROU, Romania; SVN, Slovenia; UKR, Ukraine.
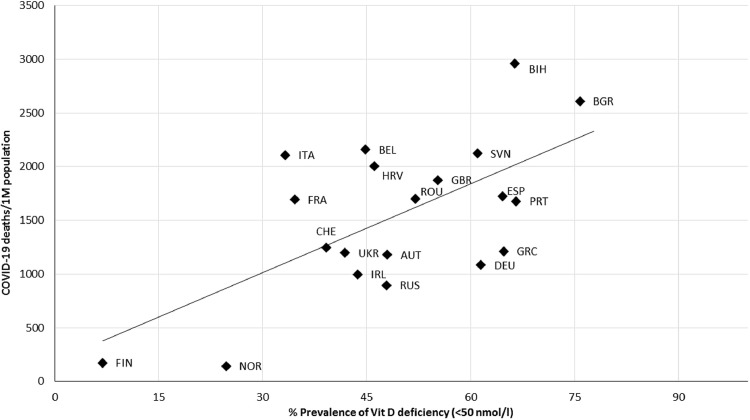
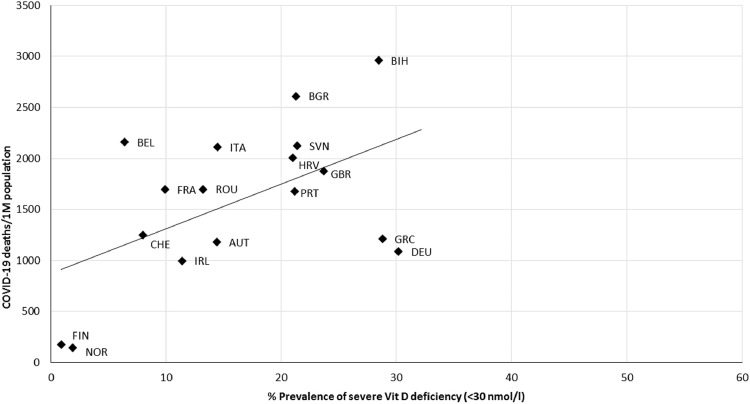
As illustrated in Figure 3 , COVID-19 mortality per million people was correlated with the prevalence of Vit D deficiency (<50 nmol/L; r = 0.634, P = 0.003), and similarly, a positive correlation was found between the prevalence of severe Vit D deficiency (<30 nmol/L; r = 0.538, P = 0.021) and COVID-19 mortality rates, as can be seen in Figure 4 .
Fig. 3.
Scatter diagram of the prevalence of vitamin D deficiency (<50 nmol/L) versus COVID-19 deaths per million people, as of June 22, 2021 (r = 0.634, P = 0.003). AUT, Austria; BEL, Belgium; BGR, Bulgaria; BIH, Bosnia and Herzegovina; CHE, Switzerland; DEU, Germany; ESP, Spain; FIN, Finland; FRA, France; GBR, United Kingdom; GRC, Greece; HRV, Croatia; IRL, Ireland; ITA, Italy; NOR, Norway; PRT, Portugal; ROU, Romania; RUS, Russia; SVN, Slovenia; UKR, Ukraine.
Fig. 4.
Scatter diagram of the prevalence of severe vitamin D deficiency (<30 nmol/L) versus COVID-19 deaths per million people, as of June 22, 2021 (r = 0.538, P = 0.021). AUT, Austria; BEL, Belgium; BGR, Bulgaria; BIH, Bosnia and Herzegovina; CHE, Switzerland; DEU, Germany; FIN, Finland; FRA, France; GBR, United Kingdom; GRC, Greece; HRV, Croatia; IRL, Ireland; ITA, Italy; NOR, Norway; PRT, Portugal; ROU, Romania; SVN, Slovenia; UKR, Ukraine.
As for recovered COVID-19 cases per million people, non-significant correlations with the prevalence of both Vit D deficiency and severe deficiency can be observed in Supplementary File 1 (respectively, r = 0.388, P = 0.091, and r = 0.242, P = 0.332).
Discussion
Our analysis concludes that available data on the prevalence of Vit D deficiency among the European population do not allow for concluding that it constitutes a strong risk factor in the COVID-19 epidemic. However, these findings are not in line with outcomes of similar research works published recently [10,22,23,45].
According to the outcomes of our study, in several of the European countries included in this analysis, more than 50% of the adult population was Vit D deficient, which constitutes a factor that should not be disregarded in the planning of public health preventive measures [27,28,32,33,37,38,40,42,44]. Factors that can influence Vit D concentration include the fluctuations in sunlight exposure across the seasons [46], especially the negligible amount of sunlight during winter and cloud cover during summer—which could potentially reduce the cutaneous synthesis of Vit D in some countries located less to the south [47,48]—as well as the extent of clothing coverage and sunscreen use [49,50]. Furthermore, dietary sources of Vit D are limited and the obesity epidemic in the Europe, which is also related to poorer dietary choices, can worsen its deficiency [51]. Additionally, vegetarianism and veganism [52] and chronic diseases such as kidney disease [53,54], liver disease [55], malignancies [56], and genetic and epigenetic factors [57] can influence Vit D concentration.
The results of our analysis show that the prevalence of Vit D deficiency and severe deficiency are not associated with COVID-19 infections. The fact that these results differ from those of previous similar published studies [10,22,23] can be attributed to our alternative methodological approach, which we think is correct. In our study, only prevalence of Vit D deficiency for each European country was used, instead of mean Vit D concentration for each country (which was used in those other studies). A mean value cannot be representative of the Vit D concentration of a whole country, because it is influenced by outliers and skewed populations. Therefore, we think that in the light of the most recent evidence of the COVID-19 pandemic, and using more updated information on the prevalence of Vit D deficiency for each country included (with measurements completed not earlier than 2010), we think that we have ended up with more accurate conclusions.
We need to underline the fact that COVID-19 mortalities seem to be correlated with the prevalence of Vit D deficiency (<50 nmol/L and <30 nmol/L). However, for a variety of reasons, this is not enough to allow for a conclusion that Vit D concentration can be associated with the COVID-19 epidemic. First of all, COVID-19 infection precedes mortality, and the former was found to be correlated with the prevalence of neither mild nor severe deficiency. Moreover, underlying conditions, such as diabetes mellitus, cancer, cardiovascular diseases, autoimmune disorders, and infectious diseases, which are related with Vit D deficiency [58,59] can be co-factors of COVID-19–specific mortality [60,61]. Furthermore, recent analyses show that high ultraviolet A radiation exposure could be associated with lower COVID-19–specific mortality, which could be an effect independent of Vit D [62], [63], [64].
Undoubtedly, Vit D deficiency observed in several European countries is considered an important factor that should be treated—frequently under medical supervision. Such a deficiency cannot be always tackled by advising enhanced dietary intake [65,66], nor should an often-unjustified (over)use of Vit D supplementation be used as a method to lower the risk for COVID-19 infection.
Numerous preprints regarding Vit D status and its association with COVID-19 infection, recovery, and mortality can be found in relevant databases (e.g., medRxiv), but these preprints have not been peer-reviewed and therefore should not be used as clinical practice guidance; additionally, such fast-track publications constitute a common risk for low-quality information and should not be considered of paramount importance during the COVID-19 pandemic. An editorial in Lancet is equally skeptical of findings regarding Vit D supplementation in people with COVID-19, until more solid data become available [67]. In addition, Szeto et al. examined the association between Vit D prehospitalized concentrations and COVID-19 clinical outcomes, and the data could not support any relationship [68]. Moreover, also outside the scope of COVID-19, evidence on associations of Vit D with any outcome seems not to be convincing, despite the great number of systematic reviews and meta-analyses that have been published [69].
According to our knowledge, this is the first review examining not only the prevalence of Vit D deficiency (<50 nmol/L) but also the prevalence of severe Vit D deficiency (<30 nmol/L). Although only 20 European countries satisfied our inclusion criteria, the analysis included a significant part of the European population [70]. Therefore, the results of our study could be generalized to most of the excluded European countries too. Moreover, along with the majority of high-income countries, upper-middle income countries such as Bosnia and Herzegovina were also included in the analysis [71], reflecting the effect of economic status in the outcomes.
Among the limitations of our study is that the data on the prevalence of Vit D deficiency in the countries included was not generated from national-level surveys. Therefore, very recently published studies with the most representative sample for each country's population were carefully selected for our analysis. As described in our Methods, screened studies were limited to adults (ages ≥ 18 y), as the severity of COVID-19 infections in children has been rather mild [72]. More detailed data regarding either COVID-19 infection rates by age or age distribution for each country was not available, and therefore correlations for these subgroups could not be performed. The fact that data on the prevalence of Vit D deficiency were not reported over the same period of the COVID-19 pandemic could have an effect on the accuracy of our results. Moreover, seasonal values for Vit D concentrations were not available in the majority of the studies included, and therefore only annual averaged rates were used.
Governments should implement proper preventive measures to increase awareness among the population of the risk of Vit D deficiency rather than of its role during the COVID-19 pandemic. Vit D supplementation should be advised only for those in a high-risk group for deficiency, such as newborns, toddlers, people who are pregnant, older people, and non-Western immigrants [73], and always under medical supervision, not as a preventive factor against COVID-19 infection, because the long-term effects of such an approach are unknown. There might be several ongoing randomized controlled trials examining Vit D supplementation in people with COVID-19 [74], but until solid data are available from well-designed randomized controlled trials that will allow us to take relevant clinical decisions, the supplementation of Vit D as a way to prevent infection or improve recovery cannot be suggested based on evidence.
Conclusion
An absence of correlation was found between the total numbers of the COVID-19 epidemic and country-specific prevalence of Vit D deficiency and severe deficiency in 20 European countries. Our different methodological approach and the updated data regarding Vit D prevalence in each country included led to different results from those of previous published studies, and this should be considered for clinical practice.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Neither author has a conflict of interest to disclose regarding this study.
Author contributions—D.-R. B.: conceptualization, methodology, validation, data curation, preparation of the original draft. M. C.: conceptualization, methodology, validation, supervision, reviewing, and editing.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.nut.2021.111441.
Appendix. Supplementary materials
References
- 1.Worldometer, COVID-19 Coronavirus Pandemic 2020. Available at: https://www.worldometers.info/coronavirus/coronavirus-cases/. Accessed June 23rd, 2021.
- 2.Zhu H, Wei L, Niu P. The novel coronavirus outbreak in Wuhan. China. Glob Health Res Policy. 2020;5:6. doi: 10.1186/s41256-020-00135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudhry R, Dranitsaris G, Mubashir T, Bartoszko J, Riazi S. A country level analysis measuring the impact of government actions, country preparedness and socioeconomic factors on COVID-19 mortality and related health outcomes. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fedele D, De Francesco A, Riso S, Collo A. Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: an overview. Nutrition. 2020;81 doi: 10.1016/j.nut.2020.111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8:e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Söderström L, Rosenblad A, Adolfsson ET, Bergkvist L. Malnutrition is associated with increased mortality in older adults regardless of the cause of death. Br J Nutr. 2017;117:532–540. doi: 10.1017/S0007114517000435. [DOI] [PubMed] [Google Scholar]
- 7.Jayawardena R, Sooriyaarachchi P, Chourdakis M, Jeewandara C, Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab Syndr. 2020;14:367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wacker M, Holick MF. Vitamin D—effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111–148. doi: 10.3390/nu5010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J Infect Public Health. 2020;13:1373–1380. doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergman P. The link between vitamin D and COVID-19: distinguishing facts from fiction. J Intern Med. 2021;289:131–133. doi: 10.1111/joim.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murdaca G, Pioggia G, Negrini S. Vitamin D and COVID-19: an update on evidence and potential therapeutic implications. Clin Mol Allergy. 2020;18:23. doi: 10.1186/s12948-020-00139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cereda E, Bogliolo L, Lobascio F, Barichella M, Zecchinelli AL, Pezzoli G, et al. Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy. Italy. Nutrition. 2021;82 doi: 10.1016/j.nut.2020.111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meltzer DO, Best TJ, Zhang H, Vokes T, Arora VM, Solway J. Association of vitamin D levels, race/ethnicity, and clinical characteristics with COVID-19 test results. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui C, Xu P, Li G, Qiao Y, Han W, Geng C, et al. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: role of renin-angiotensin system. Redox Biol. 2019;26 doi: 10.1016/j.redox.2019.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Zeng Z, Cao Y, Liu Y, Ping F, Liang M, et al. Angiotensin-converting enzyme 2 prevents lipopolysaccharide-induced rat acute lung injury via suppressing the ERK1/2 and NF-κB signaling pathways. Sci Rep. 2016;6:27911. doi: 10.1038/srep27911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhodes JM, Subramanian S, Laird E, Griffin G, Kenny RA. Perspective: vitamin D deficiency and COVID-19 severity—plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med. 2021;289:97–115. doi: 10.1111/joim.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin–angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89–90:387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, et al. SARS-CoV2: should inhibitors of the renin–angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020;41:1801–1803. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpagnano GE, Di Lecce V, Quaranta VN, Zito A, Buonamico E, Capozza E, et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Invest. 2021;44:765–771. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin Exp Res. 2020;32:2141–2158. doi: 10.1007/s40520-020-01677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh S, Kaur R, Singh RK. Revisiting the role of vitamin D levels in the prevention of COVID-19 infection and mortality in European countries post infections peak. Aging Clin Exp Res. 2020;32:1609–1612. doi: 10.1007/s40520-020-01619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32:1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ISO 3166 Country Codes. The International Standard for country codes and codes for their subdivisions: International Organization for Standardization. 2021 Available at: https://www.iso.org/iso-3166-country-codes.html. Accessed June 23rd, 2021
- 25.Elmadfa I, Meyer AL, Wottawa D, Wagner K, Hasenegger V. Vitamin D intake and status in Austria and its effects on some health indicators. Austin J Nutr Metab. 2017;4:1050. [Google Scholar]
- 26.Hoge A, Donneau A-F, Streel S, Kolh P, Chapelle J-P, Albert A, et al. Vitamin D deficiency is common among adults in Wallonia (Belgium, 51°30′ North): findings from the Nutrition, Environment and Cardio-Vascular Health study. Nutr Res. 2015;35:716–725. doi: 10.1016/j.nutres.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Sokolovic S, Alimanovic-Alagic R, Dzananovic L, Cavaljuga S, Beslic N, Ferhatbegovic-Opankovic E. Vitamin D status in Bosnia and Herzegovina: the cross-sectional epidemiological analysis. Osteoporos Int. 2017;28:1021–1025. doi: 10.1007/s00198-016-3831-0. [DOI] [PubMed] [Google Scholar]
- 28.Borissova A-M, Shinkov A, Vlahov J, Dakovska L, Todorov T, Svinarov D, et al. Vitamin D status in Bulgaria—winter data. Arch Osteoporos. 2013;8:133. doi: 10.1007/s11657-013-0133-4. [DOI] [PubMed] [Google Scholar]
- 29.Colić Barić I, Keser I, Bituh M, Rumbak I, Rumora Samarin I, Beljan K, et al. Book of Abstracts of 4th International Congress of Nutritionists Zadar, Hrvatska. 2016. Vitamin D status and prevalence of inadequacy in Croatian population; p. 97. [Google Scholar]
- 30.Adebayo FA, Itkonen ST, Lilja E, Jääskeläinen T, Lundqvist A, Laatikainen T, et al. Prevalence and determinants of vitamin D deficiency and insufficiency among three immigrant groups in Finland: evidence from a population-based study using standardised 25-hydroxyvitamin D data. Public Health Nutr. 2020;23:1254–1265. doi: 10.1017/S1368980019004312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souberbielle J-C, Massart C, Brailly-Tabard S, Cavalier E, Chanson P. Prevalence and determinants of vitamin D deficiency in healthy French adults: the VARIETE study. Endocrine. 2016;53:543–550. doi: 10.1007/s12020-016-0960-3. [DOI] [PubMed] [Google Scholar]
- 32.Rabenberg M, Scheidt-Nave C, Busch MA, Rieckmann N, Hintzpeter B, Mensink GBM. Vitamin D status among adults in Germany—results from the German Health Interview and Examination Survey for Adults (DEGS1) BMC Public Health. 2015;15:641. doi: 10.1186/s12889-015-2016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimakopoulos I, Magriplis E, Mitsopoulou A-V, Karageorgou D, Bakogianni I, Micha R, et al. Association of serum vitamin D status with dietary intake and sun exposure in adults. Clin Nutr ESPEN. 2019;34:23–31. doi: 10.1016/j.clnesp.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Cashman KD, Kiely M, Kinsella M, Durazo-Arvizu RA, Tian L, Zhang Y, et al. Evaluation of Vitamin D Standardization Program protocols for standardizing serum 25-hydroxyvitamin D data: a case study of the program's potential for national nutrition and health surveys. Am J Clin Nutr. 2013;97:1235–1242. doi: 10.3945/ajcn.112.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giuliani S, Barbieri V, Di Pierro AM, Rossi F, Widmann T, Lucchiari M, et al. LC-MS/MS based 25(OH)D status in a large Southern European outpatient cohort: gender- and age-specific differences. Eur J Nutr. 2019;58:2511–2520. doi: 10.1007/s00394-018-1803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrenya N, Lamberg-Allardt C, Melhus M, Broderstad AR, Brustad M. Vitamin D status in a multi-ethnic population of northern Norway: the SAMINOR 2 Clinical Survey. Public Health Nutr. 2020;23:1186–1200. doi: 10.1017/S1368980018003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duarte C, Carvalheiro H, Rodrigues AM, Dias SS, Marques A, Santiago T, et al. Prevalence of vitamin D deficiency and its predictors in the Portuguese population: a nationwide population-based study. Arch Osteoporos. 2020;15:36. doi: 10.1007/s11657-020-0695-x. [DOI] [PubMed] [Google Scholar]
- 38.Niculescu DA, Capatina CAM, Dusceac R, Caragheorgheopol A, Ghemigian A, Poiana C. Seasonal variation of serum vitamin D levels in Romania. Arch Osteoporos. 2017;12:113. doi: 10.1007/s11657-017-0407-3. [DOI] [PubMed] [Google Scholar]
- 39.Karonova T, Andreeva A, Nikitina I, Belyaeva O, Mokhova E, Galkina O, et al. Prevalence of vitamin D deficiency in the north-west region of Russia: a cross-sectional study. J Steroid Biochem Mol Biol. 2016;164:230–234. doi: 10.1016/j.jsbmb.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 40.Hribar M, Hristov H, Gregorič M, Blaznik U, Zaletel K, Oblak A, et al. Nutrihealth study: seasonal variation in vitamin D status among the Slovenian adult and elderly population. Nutrients. 2020;12:1838. doi: 10.3390/nu12061838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakem B, Nock C, Stanga Z, Medina P, Nydegger UE, Risch M, et al. Serum concentrations of 25-hydroxyvitamin D and immunoglobulins in an older Swiss cohort: results of the Senior Labor Study. BMC Med. 2013;11:176. doi: 10.1186/1741-7015-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aspell N, Laird E, Healy M, Shannon T, Lawlor B, O'Sullivan M. The prevalence and determinants of vitamin D status in community-dwelling older adults: results from the English Longitudinal Study of Ageing (ELSA) Nutrients. 2019;11:1253. doi: 10.3390/nu11061253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shchubelka K. Vitamin D status in adults and children in Transcarpathia, Ukraine in 2019. BMC Nutr. 2020;6:48. doi: 10.1186/s40795-020-00380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Díaz-López A, Paz-Graniel I, Alonso-Sanz R, Marqués Baldero C, Mateos Gil C, Arija Val V. Vitamin D deficiency in primary health care users at risk in Spain. Nutr Hosp. 2021:03565. doi: 10.20960/nh.03565. [DOI] [PubMed] [Google Scholar]
- 45.Kazemi A, Mohammadi V, Aghababaee SK, Golzarand M, Clark CCT, Babajafari S. Association of vitamin D status with SARS-CoV-2 infection or COVID-19 severity: a systematic review and meta-analysis [e-pub ahead of print] Adv Nutr. 2021 doi: 10.1093/advances/nmab012. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schramm S, Lahner H, Jöckel K-H, Erbel R, Führer D. Moebus S; on behalf of the Heinz Nixdorf Recall Study Group. Impact of season and different vitamin D thresholds on prevalence of vitamin D deficiency in epidemiological cohorts—a note of caution. Endocrine. 2017;56:658–666. doi: 10.1007/s12020-017-1292-7. [DOI] [PubMed] [Google Scholar]
- 47.Webb AR, Kift R, Durkin MT, O'Brien SJ, Vail A, Berry JL, et al. The role of sunlight exposure in determining the vitamin D status of the U.K. white adult population. Br J Dermatol. 2010;163:1050–1055. doi: 10.1111/j.1365-2133.2010.09975.x. [DOI] [PubMed] [Google Scholar]
- 48.Mendes MM, Darling AL, Hart KH, Morse S, Murphy RJ, Lanham-New SA. Impact of high latitude, urban living and ethnicity on 25-hydroxyvitamin D status: a need for multidisciplinary action? J Steroid Biochem Mol Biol. 2019;188:95–102. doi: 10.1016/j.jsbmb.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 49.Mishal AA. Effects of different dress styles on vitamin D levels in healthy young Jordanian women. Osteoporos Int. 2001;12:931–935. doi: 10.1007/s001980170021. [DOI] [PubMed] [Google Scholar]
- 50.Neale RE, Khan SR, Lucas RM, Waterhouse M, Whiteman DC, Olsen CM. The effect of sunscreen on vitamin D: a review. Br J Dermatol. 2019;181:907–915. doi: 10.1111/bjd.17980. [DOI] [PubMed] [Google Scholar]
- 51.Snijder MB, van Dam RM, Visser M, Deeg DJH, Dekker JM, Bouter LM, et al. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab. 2005;90:4119–4123. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- 52.Bakaloudi DR, Halloran A, Rippin HL, Oikonomidou AC, Dardavesis TI, Williams J, et al. Intake and adequacy of the vegan diet: a systematic review of the evidence. Clin Nutr. 2020;40:3503–3521. doi: 10.1016/j.clnu.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 53.Li M, Li Y. Prevalence and influencing factors of vitamin D deficiency in chronic kidney disease: a cross-sectional study. Int J Clin Pharmacol Ther. 2020;58:595–600. doi: 10.5414/CP203737. [DOI] [PubMed] [Google Scholar]
- 54.El Din US, Fayed A, El Nokeety MM, Abdulazim DO, Salem MM. Vitamin-D deficiency is encountered in almost all Egyptian stage 3–5 chronic kidney disease patients in spite of the sunny weather. Saudi J Kidney Dis Transpl. 2019;30:1389–1397. doi: 10.4103/1319-2442.275483. [DOI] [PubMed] [Google Scholar]
- 55.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624–2628. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 56.Gupta D, Vashi PG, Trukova K, Lis CG, Lammersfeld CA. Prevalence of serum vitamin D deficiency and insufficiency in cancer: review of the epidemiological literature. Exp Ther Med. 2011;2:181–193. doi: 10.3892/etm.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bahrami A, Sadeghnia HR, Tabatabaeizadeh S-A, Bahrami-Taghanaki H, Behboodi N, Esmaeili H, et al. Genetic and epigenetic factors influencing vitamin D status. J Cell Physiol. 2018;233:4033–4043. doi: 10.1002/jcp.26216. [DOI] [PubMed] [Google Scholar]
- 58.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:8S. doi: 10.1093/ajcn/80.6.1678S. 1678S–8. [DOI] [PubMed] [Google Scholar]
- 59.Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16:261–266. doi: 10.1016/j.tem.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parohan M, Yaghoubi S, Seraji A, Javanbakht MH, Sarraf P, Djalali M. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male. 2020;2020:1416–1424. doi: 10.1080/13685538.2020.1774748. [DOI] [PubMed] [Google Scholar]
- 62.Cherrie M, Clemens T, Colandrea C, Feng Z, Webb DJ, Weller RB, et al. Ultraviolet A radiation and COVID-19 deaths in the USA with replication studies in England and Italy [e-pub ahead of print] Br J Dermatol. 2021;185:363–370. doi: 10.1111/bjd.20093. Epub 2021 May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Isaia G, Diémoz H, Maluta F, Fountoulakis I, Ceccon D, di Sarra A, et al. Does solar ultraviolet radiation play a role in COVID-19 infection and deaths? an environmental ecological study in Italy. Sci Total Environ. 2021;757 doi: 10.1016/j.scitotenv.2020.143757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gorman S, Weller RB. Investigating the potential for ultraviolet light to modulate morbidity and mortality from COVID-19: a narrative review and update. Front Cardiovasc Med. 2020;7 doi: 10.3389/fcvm.2020.616527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kasapidou E, Oikonomidou A, Chourdakis M. Vitamin D status among Mediterranean regions. Hippokratia. 2018;22:191. [PMC free article] [PubMed] [Google Scholar]
- 66.van Schoor N, Lips P. In: Vitamin D, Vol. 2: health, disease and therapeutics. 4th ed. Hewison M, Bouillon R, Giovannucci E, Goltzman D, editors. Elsevier; 2018. Worldwide vitamin D status; pp. 15–40. Hardcover ISBN: 9780128099636. [Google Scholar]
- 67.The Lancet Diabetes & Endocrinology. Vitamin D and COVID-19: why the controversy? Lancet Diabetes Endocrinol. 2021;9:53. doi: 10.1016/S2213-8587(21)00003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szeto B, Zucker JE, LaSota ED, Rubin MR, Walker MD, Yin MT, et al. Vitamin D status and COVID-19 clinical outcomes in hospitalized patients. Endocr Res. 2021;46:66–73. doi: 10.1080/07435800.2020.1867162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JPA. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.WHO Coronavirus (COVID-19) Dashboard. Situation by WHO Region, Country, Territory & Area. Available at: https://covid19.who.int Accessed June 23rd, 2021
- 71.Stafford N. Covid-19: why Germany's case fatality rate seems so low. BMJ. 2020;369:m1395. doi: 10.1136/bmj.m1395. [DOI] [PubMed] [Google Scholar]
- 72.Brodin P. Why is COVID-19 so mild in children? Acta Paediatr. 2020;109:1082–1083. doi: 10.1111/apa.15271. [DOI] [PubMed] [Google Scholar]
- 73.Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180:23–54. doi: 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
- 74.Annweiler C, Beaudenon M, Gautier J, Simon R, Dubée V, Gonsard J, et al. COvid-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): study protocol for a randomized controlled trial. Trials. 2020;21:1031. doi: 10.1186/s13063-020-04928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.