Abstract
Background and study aims
Gastrointestinal manifestations are common during coronavirus disease (COVID-19) infection. They can occur before respiratory symptoms, resulting in a diagnostic delay and an increased risk of disease transmission. The current study reports major gastrointestinal manifestations as initial symptoms of COVID-19.
Patients and methods
This prospective, descriptive, cross-sectional, and single-center study of 713 cases was conducted in a field hospital in Morocco over a 5-week period from June 21 to July 25, 2020.
Results
The average age of our patients was 31.95 years. Clinically, on admission, anorexia was the main symptom, present in 32.3% of patients. Gastrointestinal manifestations were present in 14.9% of patients, including watery diarrhea in 8.6% of cases, nausea and/or vomiting in 4.6% of cases, and abdominal pain in 1.6% of cases.
Six hundred thirty-two patients were treated in accordance with one of the two therapeutic protocols recommended by the National Ministry of Health. The treatment-related effects that occurred in 61.4% of patients were primarily digestive in 55.3% of cases.
In multivariate analysis, following adjustment of the studied parameters, only the presence of gastrointestinal manifestations (odds ratio [OR]: 1.478 confidence interval [CI]: 1.286–1.698; p < 0.001) and treatment side effects (OR = 1.069, CI: 1.020–1.119, p = 0.005) altered the rate of negative polymerase chain reaction (PCR) tests on day 10.
Conclusion
Gastrointestinal manifestations are common during COVID-19 and seem to be linked to a longer duration of disease. SARS-CoV-2 (the causative virus of COVID-19) can persist in the digestive tract, with the possibility of fecal–oral transmission. Therefore, hygiene is extremely important, especially handwashing and strict precautions when performing gastrointestinal endoscopy and handling stools from infected patients.
Keywords: SARS-CoV-2, COVID-19, Digestive symptoms, Gastrointestinal manifestations
Introduction
Initially reported in China in December 2019, coronavirus disease (COVID-19) was quickly declared a public health emergency on January 30, 2020, and then a pandemic on March 11, 2020, by the World Health Organization. In Morocco, the first case of COVID-19 was announced on March 2, 2020, and as of today, Morocco has reported more than 200,000 infected people, with a death toll exceeding 4000 people and numbers continuing to rise.
Respiratory tract manifestations are the most commonly reported symptoms in COVID-19, which is indicative of droplet transmission and contact transmission. However, emerging data suggest that the gastrointestinal tract might also be affected by SARS-CoV-2, on the basis that gastrointestinal epithelial cells express angiotensin-converting enzyme 2 (ACE2), the major receptor of SARS-CoV-2. The most commonly reported gastrointestinal symptoms associated with COVID-19 are anorexia, diarrhea, nausea or vomiting, and abdominal pain. Gastrointestinal manifestations of SAR-CoV-2 not only pose an important diagnostic challenge to clinicians when dealing with patients with mild COVID-19 symptoms on initial presentation but also signify potential fecal transmission of this virus. With the increasing number of reported cases of COVID-19, there is an urgent need to systemically summarize the gastrointestinal manifestations of COVID-19 and the temporal profile of fecal shedding of the SARS-CoV-2 virus, particularly for gastroenterologists and endoscopists who may not be familiar with this disease.
In the current study, we aim to report the major gastrointestinal manifestations that occur as initial symptoms of COVID-19, and when to suggest the diagnosis of COVID-19 in patients at risk, who may have been exposed to SARS-CoV-2 and presenting with digestive symptoms, even in the absence of respiratory symptomatology.
Patients and methods
Study design, setting, and participants
This prospective descriptive, cross-sectional, and single-center study of 713 cases was conducted in the Sidi Yahya El Gharb Field Hospital in Sidi Slimane Province, Rabat-Salé-Kénitra Region, Morocco, over a 5-week period from June 21 to July 25, 2020.
The study included all patients with confirmed COVID-19 by a polymerase chain reaction (PCR) on nasopharyngeal swab, irrespective of whether patients were symptomatic, and without any contraindications for the medical treatment provided. Patients who did not meet the above inclusion criteria were excluded from the study.
Variables
We extracted the epidemiological history (i.e., contact history), demographic data (such as age, sex, race, smoking status, and body mass index), comorbidities (history of hypertension, diabetes, dyslipidemia, coronary artery disease, chronic obstructive pulmonary disease, and asthma), clinical characteristics (including digestive symptoms [anorexia, nausea/vomiting, diarrhea, or abdominal pain], and other symptoms [fever, cough, expectoration, and dyspnea]) on admission, treatment programs, and evolutionary data.
Statistical analysis
Descriptive data are presented as means (±standard deviation [SD]) for normally distributed continuous variables. Categorical variables were presented as counts and percentages.
No imputation was made for missing data. Because the study cohort was not derived from random selection, all statistics are presumed to be descriptive only.
To investigate the association between the presence of gastrointestinal manifestations and the rate of negative PCR tests on day 10, we performed logistic regression with adjustment for potential confounders to calculate odds ratios (ORs) and 95% confidence intervals (CIs) to identify risk factors for these outcomes.
A two-tailed P-value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 22.0 program.
Results
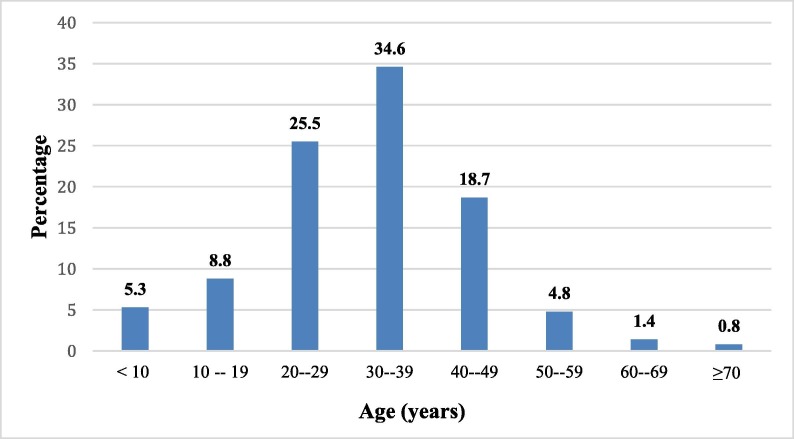
The average age of our patients was 31.95 ± 12.95 years, with extremes ranging from 11 days to 80 years, and the predominant age range was from 30 to 39 years (Fig. 1 ).
Fig. 1.
Distribution of patients by age group.
Our series was characterized by a clear female predominance estimated at 88.9%, compared to 11.1% in men, with a sex ratio of 0.12. This female predominance was linked with our medical facility’s patient population, mostly comprised a COVID-19 cluster which included women working in strawberry packing plants in Lalla Mimouna region.
On admission, the degree of severity of COVID-19 was classified as mild in all patients (Appendix 1) [1].
The case history revealed pre-exiting conditions (diabetes and/or high blood pressure) in 2.1% (n = 15). Clinically, on admission, anorexia was the main symptom and was present in 32.3% of patients (n = 230). Gastrointestinal manifestations were present in 14.9% of patients (n = 106), including watery diarrhea in 8.6% of cases, nausea and/or vomiting in 4.6% of cases, and abdominal pain in 1.6% of cases. A total of 632 patients were treated in accordance with one of the two therapeutic protocols recommended by the National Ministry of Health, which are based on chloroquine (Nivaquine) (500 mg × 2 per day for 10 days) in combination with azithromycin (500 mg on day 1 and then 250 mg per day from days 2 to 7), vitamin C (1 g/12 h), and zinc (100 mg/day) for the first protocol, which was used to treat 51.15% of cases (n = 364), and the second protocol consisted of hydroxychloroquine sulfate (Plaquenil) (200 mg × 3 per day for 10 days) in combination with azithromycin (500 mg on day 1 and then 250 mg per day from days 2 to 7), vitamin C (1 g/12 h), and zinc (100 mg per day). This second protocol was adopted to treat 268 patients (37.6% of cases).
The remainder of the untreated patients included 17 pregnant women (2.4%) and 64 children (9%). All cases were monitored twice daily to detect early signs of worsening; this allowed us to identify adverse treatment-related effects in 61.4% of patients (n = 438), which were primarily digestive in 55.3% of cases (watery diarrhea in 43% of patients, abdominal pain in 16.6% of patients, and nausea and/or vomiting in 13.1% of patients) managed by symptomatic treatment and neurological treatment in 6.1% of patients (insomnia in 9% of cases, dizziness in 9.9% of cases, and hallucinations in only one patient), which were reversible upon discontinuation of treatment. PCR analysis on day 10 of treatment was negative in 75.6% of patients.
In a multivariate analysis following adjustment of the study parameters, namely, age, the presence of associated morbidities, anorexia, and the adopted therapeutic protocol, only the presence of gastrointestinal manifestations (OR: 1.478, CI: 1.286–1.698, p < 0.001) and treatment side effects (OR = 1.069, CI: 1.020–1.119, p = 0.005) altered the rate of negative PCR tests on day 10.
Digestive symptoms appeared to be linked to a longer disease duration; 82.2% of patients without digestive symptoms recovered on the 10th day of treatment, whereas only 37.7% of patients with digestive symptoms recovered on the same date of treatment. Similarly, but to a lesser degree, the presence of therapeutic adverse effects prolongs the duration of the disease; 82.2% of patients without therapeutic adverse effects recovered on the 10th day of treatment, whereas 71.46% of patients with therapeutic adverse effects recovered on the same day of treatment. This could be explained by poor adherence to treatment caused by poor treatment tolerance in the patients. No cases of death were recorded in our series, and the PCR performed on day 14 of admission came back negative for the remaining 24.4% of patients.
Discussion
COVID-19 may be responsible for a polymorphic digestive symptomatology, which may precede the onset of respiratory symptoms. The mechanism of action of the virus is similar to that of SARS-CoV, as it uses ACE2 as a functional receptor for cell entry. ACE2 is found on cell membranes primarily belonging to pneumocytes and other body cells including gastrointestinal epithelial cells [2].
The gastrointestinal digestive manifestations of COVID-19 were described in the United States [3], in a 35-year-old man, who had a history of nausea and vomiting 2 days before his admission, followed by diarrhea and abdominal discomfort on the second day of hospitalization. The incidence of gastrointestinal manifestations during COVID-19 infection varies between 12% and 61% [4], [5], [6] depending on the study (Table 1 ). These manifestations may be associated with prolonged disease duration, but they have not been associated with increased mortality [5], [7]. In our series, gastrointestinal manifestations were present in 14.9% of patients, which is consistent with data from the literature.
Table 1.
Gastrointestinal symptoms in COVID-19-positive patients in different studies.
| Study | All patients | Diarrhea (%) | Nausea and/or vomiting (%) | Anorexia (%) | Abdominal pain (%) |
|---|---|---|---|---|---|
| Luo et al. [10] | 1141 | 5.96 | 18.93 | 15.77 | 3.94 |
| Guan et al. [9] | 1099 | 3.73 | 5 | — | — |
| Jin et al. [11] | 651 | 8.14 | 2.61 | — | — |
| Redd WD et al. [7] | 318 | 33.7 | 26.4 | 34.8 | |
| Pan L et al. [6] | 204 | 34 | 3.9 | 78.6 | 1.9 |
| Zhang et al. [3] | 139 | 12.95 | 17.26 | 12.23 | 5.75 |
| Wang et al. [17] | 138 | 10.14 | 10.14 | 39.85 | 2.17 |
| Our study | 713 | 8.6 | 4.6 | 32.3 | 1.6 |
Sometimes, various gastrointestinal symptoms, including epigastric pain, constipation, diarrhea, nausea, vomiting, muscle pain, and melena, which are therapy resistant, may be the only symptoms in patients suspected to have COVID-19. Timely diagnosis and isolation of these patients can guarantee population to avoid the spread of this highly contagious infection [8], [9].
In a cohort conducted in 552 Chinese hospitals with 1099 patients published in the New England Journal of Medicine, the authors observed nausea or vomiting in 5% of cases and diarrhea in 3.8% of cases [10]. In a meta-analysis including 35 studies of 6686 patients with COVID-19, of which 29 studies (n = 6064) reported gastrointestinal symptoms at the time of diagnosis, with a prevalence of 15%, the symptoms included anorexia in 21% of cases, diarrhea in 9% of cases, nausea and/or vomiting in 6% of cases, and abdominal pain in 3% of cases [7].
In a Chinese retrospective series of 1141 confirmed cases of COVID-19, 16% of patients had isolated digestive symptoms [11]. These included anorexia in 98% of cases, followed by nausea in 73%, vomiting in 65%, diarrhea in 37%, and abdominal pain in 25% of cases.
A higher prevalence of gastrointestinal symptoms evaluated at 61.3% was reported in a multicentric cohort study conducted in the United States by Walker D. Redd on 318 patients with symptoms, including anorexia in 34.8% of patients, diarrhea in 33.7% of patients, and nausea in 26.4% of cases [6].
In another multicentric study of 204 patients, 51% of patients had digestive involvement, including diarrhea in 34% of cases [5]. Patients with digestive symptoms had a longer period of hospitalization than had patients without digestive symptoms. The authors also observed a correlation between the severity of respiratory involvement and the intensity of digestive symptoms. These elements were confirmed by a study on 651 patients, of whom 11.4% had at least one digestive symptom. Patients with digestive symptoms had more severe disease (p < 0.001) [12].
Anorexia during COVID-19 virus infection appears to be common and is reported to occur in 40% to 50% of cases. Although the onset mechanism of anorexia in patients with COVID-19 remains uncertain, it appears to be associated with dysgeusia in 88% of cases and anosmia in 85.6%, which may be partially explained by its mechanism of onset [13]. In our study, anorexia was the main symptom on admission and was present in 32.3% of patients.
With regard to the characteristics of diarrhea, a single-center study conducted on gastrointestinal manifestations of COVID-19 in Wuhan showed that the onset of diarrhea was 1–8 days after the onset of COVID-19 (with a median duration of 3.3 days). In 34.3% of cases, the stools were liquid, and their analysis showed abnormal results in 6.9% of cases, specifically the presence of white blood cells in 5.2% of cases and a positive Hemoccult test in 1.7% of patients, with the absence of red blood cells [14]. This was consistent with the characteristics of diarrhea, which were non-bloody, usually associated with vomiting, and may have been accompanied by nausea, abdominal cramps, and fever [5]. Generally, diarrhea was short term (i.e., not exceeding three to four stools per day) [15], [16] and had to be distinguished from diarrhea related to the administered drugs (hydroxychloroquine associated or not with azythromycin) or concomitant infections (especially Clostridium difficile). In our study, diarrhea was watery and was present in 8.6% of cases.
SARS-CoV-2 RNA was first detected in a stool sample from the first case of COVID-19 reported in the United States (US) [3]. In another Chinese cohort consisting of 73 hospitalized patients infected with SARS-CoV-2, viral RNA was detected in the stools of 53.42% of patients, and it remained positive for 17 patients (23.29%), even after the virus became undetectable in the airways [17]. Meanwhile, SARS-CoV-2 was also detected in stool samples from patients without gastrointestinal symptoms [18].
A recent study proved the possibility of a prolonged duration of fecal viral shedding up to 5 weeks after patients’ respiratory samples turned negative for detecting SARS-CoV-2 RNA [19].
A pediatric study also confirmed that most children demonstrated viral RNA in their stools even after nasopharyngeal swabs came back negative [20]. These data suggest that the virus could remain viable in the environment for days, with a potential risk of fecal–oral transmission, but further direct evidence of fecal transmission of COVID-19 has yet to be identified.
In light of these data, several recommendations regarding the organization and practice of procedures in endoscopy departments have been implemented to avoid any transmission and can be summarized as follows:
-
-
Before the procedure:
All patients should go through triage and risk assessment, and all suspected patients should undergo a nasopharyngeal swab with PCR analysis before any endoscopic procedure [21].
For these suspected or confirmed COVID-19 patients, the clinical indication for endoscopy should be reassessed, and only urgent life-threatening cases should benefit from endoscopy [21].
-
-
During the procedure:
Use of personal protective equipment (PPE), including a filtering facepiece respirator (N95, FFP2, or FFP3), a disposable waterproof gown, two pairs of gloves, a cap, and a face shield or goggles [22], [23], [24], should be done according to the risk level. All staff members should be trained on how to dress and undress from the PPE. The procedure should be performed in a negative pressure room when available [25]. However, because of the lengthy time requirement needed and the strong possibility of aspiration of oral and fecal material during endoscopy, we should limit this procedure to emergency patients only. In these patients (e.g., those with cholangitis, cholangiosepsis, or active gastrointestinal bleeding), we should try performing procedures with the patient completely sedated while preserving a minimum safe distance. In the case of some procedures, including endoscopic retrograde cholangiopancreatography, endosonography, or therapeutic endoscopy for patients with gastrointestinal bleeding, it may be better if patients are intubated to reduce gastro-esophageal reflux [26].
-
-
After the procedure:
After an endoscopy, rooms without negative pressure should be considered contaminated for at least 1 h; otherwise, a new patient may be admitted to the endoscopy room after 30 to 60 min [27], although no case of transmission of COVID-19 following endoscopy has been reported. Once environmental surfaces are cleaned, disinfection with ethanol 70%–90%, chlorine-based products (sodium hypochlorite, for example) or hydrogen peroxide, reduces coronavirus concentrations with a contact time of at least 1 min [28], [29].
Further, all entrance doors to the endoscopy and colonoscopy buildings should be sterilized after completing the medical care of individual patients [26]. For the time being, no changes regarding the sterilization of gastrointestinal endoscopes have been recommended in COVID-19-positive patients [27]. Finally, it is strongly recommended to insist on universal handwashing and use of alcohol-based disinfectants when entering and leaving the endoscopy rooms.
In an Iranian study conducted on 200 patients, followed up for both inflammatory bowel disease and auto-immune hepatitis with a history of receiving immunosuppressive drugs for treating gastrointestinal disorders, it was found that these patients exhibited low potential for infection, and also surprisingly COVID-19 confirmed cases presented with mild symptoms and showed minimal lung involvement in chest CT scan image. Furthermore, all symptoms and radiologic findings disappeared sooner in immunocompromised patients compared to immunocompetent cases. Therefore, these findings further validated immunosuppressive drug benefits, especially the IL-6 receptor antagonist for emergency use in critical infected COVID-19 patients [30].
A single autopsy report with details of gastrointestinal pathology has been published on an 85-year-old man with COVID-19, who had shown alternating segmental dilations and heil stenosis [31]. However, more studies are needed to clarify whether this finding is secondary to COVID-19 or a pre-existing gastrointestinal comorbidity.
Our study has some limitations to consider. First, it was conducted within a single center. Second, we did not test for RNA of SARS-CoV-2 in fecal specimens of patients with COVID-19, to correlate digestive symptom prevalence and severity with the presence of viral RNA in stool specimens. Third, our study was characterized by a clear female predominance, which could be a source of selection bias. Finally, given the dynamic character of the current COVID-19 pandemic, the correlation between these patient prognosis and digestive symptoms is yet to be confirmed in the future with more data worldwide.
In conclusion, gastrointestinal manifestations are common during COVID-19, and investigating them should be systematically performed during history taking. These manifestations can be the first symptoms that occur before respiratory symptoms and thus result in a potential factor for diagnostic delay with an increased risk of disease transmission. The existence of digestive symptoms may be correlated with more severe respiratory involvement. SARS-CoV-2 can persist longer in the digestive tract than in the respiratory system with the possibility of fecal–oral transmission, thereby underscoring the extreme importance of hygiene, especially handwashing. Strict precautions must be observed when performing gastrointestinal endoscopy and handling stool from patients infected with the COVID-19 virus. The question thus remains whether a rectal swab test before discharging patients with gastrointestinal manifestations should integrate the algorithm for COVID-19 therapeutic management.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix 1. COVID-19 mild disease severity based on the WHO clinical management of COVID-19 updated on May 27, 2020 (1)
Symptomatic patients presenting with fever, cough, fatigue, anorexia, and myalgias; other non-specific symptoms, such as sore throat, nasal congestion, headache, diarrhea, nausea, and vomiting; loss of smell (anosmia) or loss of taste (ageusia) preceding the onset of respiratory symptoms without evidence of viral pneumonia or hypoxia.
References
- 1.World Health Organization. Clinical management of COVID-19: interim guidance. 2020 May 27. (https://www.who.int/publications/i/item/clinical-management-of-covid-19).
- 2.Zhang H., Kang Z., Gong H., et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69(6):1010–1018. [Google Scholar]
- 3.Holshue M.L., DeBolt C., Lindquist S., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan L., Mu M., Yang P., et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redd W.D., Zhou J.C., Hathorn K.E., et al. Prevalence and characteristics of gastrointestinal symptoms in patients with SARS-CoV-2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020;159(2) doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao R., Qiu Y., He J.S., et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hormati A., Shahhamzeh A., Afifian M., et al. Can COVID-19 present unusual GI symptoms? J Microbiol Immunol Inf. 2020;53(3):384–385. doi: 10.1016/j.jmii.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hormati A., Shahhamzeh A., Aminnejad R., et al. Gastrointestinal presentation in a patient with COVID-19 without respiratory tract symptoms: A case report From Qom, Iran. Jundishapur J Microbiol. 2020;13(5):e102844. [Google Scholar]
- 10.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [PMID: 32109013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo S., Zhang X., Xu H. Do not overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19) Clin Gastroenterol Hepatol. 2020;18(7):1636–1637. doi: 10.1016/j.cgh.2020.03.043. [PMID: 32205220] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin X., Lian J.S., Hu J.H., et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [PMID: 32213556] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID- 19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [PMID: 32253535] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang D., Ma J.D., Guan J.L., et al. Manifestations of digestive system in hospitalized patients with novel coronavirus pneumonia in Wuhan, China: a single-center, descriptive study. Chin J Dig. 2020;40(03):151–156. [Google Scholar]
- 15.Wong S.H., Lui R.NS., Sung J.JY. COVID-19 and the digestive system. J Gastroenterol Hepatol. 2020;35(5):744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 16.Tian Y., Rong L., Nian W., et al. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51(9):843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao F., Tang M., Zheng X., et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W., Xu Y., Gao R., et al. Detection of SARS- CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [PMID: 32159775] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y., Guo C., Tang L., et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y., Li X., Zhu B., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu P.W.Y., Ng S.C., Inoue H., et al. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements) Gut. 2020;69(6):991–996. doi: 10.1136/gutjnl-2020-321185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ASGE Quality Assurance in Endoscopy Committee, Calderwood A.H., Day L.W., et al. ASGE guideline for infection control during Gi endoscopy. Gastrointest Endosc. 2018;87(5):1167–1179. doi: 10.1016/j.gie.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Beilenhoff U., Biering H., Blum R., et al. Reprocessing of edible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) - Update 2018. Endoscopy. 2018;50:1205–1234. doi: 10.1055/a-0759-1629. [DOI] [PubMed] [Google Scholar]
- 24.Kilinc F.S. A review of isolation gowns in healthcare: fabric and gown properties. J Eng Fiber Fabr. 2015;10(3):180–190. [PMID: 26989351] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinonquel P., Roelandt P., Demedts I., et al. COVID-19 and gastrointestinal endoscopy: What should be taken into account? Dig Endosc. 2020;32(5):723–731. doi: 10.1111/den.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hormati A., Ghadir M.R., Zamani F., et al. Preventive strategies used by GI physicians during the COVID-19 pandemic. New Microbe New Infect. 2020;35:100676. doi: 10.1016/j.nmni.2020.100676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Repici A., Maselli R., Colombo M., et al. Coronavirus (COVID-19) outbreak: What the department of endoscopy should know. Gastrointest Endosc. 2020;92(1):192–197. doi: 10.1016/j.gie.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kampf G., Todt D., Pfaender S., et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutala W.A., Weber D.J. Best practices for disinfection of noncritical environmental surfaces and equipment in healthcare facilities: A bundle approach. Am J Infect Control. 2019;47:A96–A105. doi: 10.1016/j.ajic.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Hormati A., Ghadir M.R., Zamani F., et al. Are there any association between COVID-19 severity and immunosuppressive therapy? Immunol Lett. 2020;224:12–13. doi: 10.1016/j.imlet.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X., Wang R.S., Qu G.Q., et al. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36(1):21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [PMID: 32198987] [DOI] [PubMed] [Google Scholar]