The COVID-19 pandemic has disrupted the provision of health services. Until June 19th, 2021, over one year after the World Health Organization (WHO) declared COVID-19 a pandemic, 178,331,044 cases and 3,862,251 deaths had been reported globally (1). In Mexico, up to that same date, 2.6 million cases and 243,728 deaths have been confirmed, taking the fourth position in the world in terms of mortality (2).
SARS-CoV-2produces a clinical presentation closely resembling Severe Acute Respiratory Syndrome (SARS). COVID-19 disease, resulting from SARS-CoV-2 infection, has strained health systems worldwide, and despite efforts to curve spread and mortality, most nations have faced two or three different waves with devastating outcomes.
Although COVID-19 affects a wide variety of individuals, several factors increase the risk for severe COVID-19 presentation and mortality, including advanced age, obesity, diabetes, cardiovascular illness, and immunosuppression (3). Cancer is a frequently recognized comorbidity, and patients diagnosed with cancer have an increased risk of death from COVID-19 (RR 1.8) (4), particularly patients with hematological malignancies (RR 2.5), and patients with a recent diagnosis (5,6). Patients with cancer have diverse immune alterations, affecting both innate and adaptive immune responses. As such, patients might be immunocompromised as a direct consequence of the neoplastic process, or as an effect of anti-tumor treatment, including the use of immune checkpoint inhibitors and supportive medication such as steroids. Furthermore, patients with cancer are often diagnosed at an advanced age and in the context of other comorbidities which might also drive poor COVID-19 outcomes, including smoking, metabolic comorbidities, and poor functional status (5). They also more frequently attend hospital settings and are in contact with healthcare providers in closed spaces. Additionally, several sociodemographic factors have been associated with worse COVID-19 outcomes in Mexico, including living in socially deprived areas andhigh-density urban dwellings (7).
Social distancing measures have reduced COVID-19 spread, though they have been insufficient to stop the pandemic (8,9). Fortunately, several vaccines have been developed in a short time span and proven to have a high efficacy for reducing the risk of COVID-19 illness and serious outcomes. To date, data on 22 vaccines have been submitted for evaluation and approval by WHO, among which eight have completed the process (10). In Mexico, the Federal Commission for Protection against Sanitary Risks (COFEPRIS) has granted emergency use approval to seven vaccines. Up to June 18th, 2021, 39.62 million people in Mexico had received at least one vaccine dose, with most of the health personnel and older adults in the country having a complete vaccination schedule (11).
Among studies exploring the efficacy of the seven vaccines approved for emergency use in Mexico, only one provides data regarding the number of adults with cancer included (12), illustrating the scarce participation of patients with cancer in vaccine clinical trials. Current COFEPRIS-approved vaccines include diverse platforms: one mRNA vaccine, two inactivated virus vaccines, and four adenoviral vector-based vaccines, none of which have the potential to cause active infections in immunocompromised patients. Although some local and systemic side effects associated with inflammation can be observed, symptoms mostly self-limit in 3–5 d following vaccination, there have been no additional safety signals or serious adverse events among adults with cancer which could justify differing or limiting vaccine rollout to this patient subgroup, and COVID-19 vaccines appear to be both effective and safe among people with active cancer and cancer survivors (13). As in patients without cancer, the only absolute contraindication for receiving a COVID-19 vaccine is a history of allergic reactions to vaccine components.
Adults with COVID-19 and solid tumors seem to have an adequate immune response post-infection, though it may be hindered in patients with hematologic tumors (14). Additionally, although antineoplastic therapy may blunt lymphocyte proliferation, suppression is not complete, and immune responses can be elicited even while receiving cytotoxic therapy (13). However, the SOAP-vaccine trial, which measured the immune response following administration of the Pfizer-BioNTech vaccine in adults with cancer, showed that 95% of study participants developed antibodies against SARS-CoV-2 after two vaccine doses, although response was low following one dose (38% of participants with solid tumors and 18% of participants with hematological malignancies developed anti-SARS-CoV-2 antibodies, compared with 94% of participants without known malignancies). This highlights the importance of a timely administration of both vaccine doses for individuals receiving mRNA vaccines, particularly adults with cancer (15). Results from the VOICE trial are pending, which will prospectively evaluate safety and efficacy of COVID-19 vaccination in 627 adults with cancer undergoing cytotoxic chemotherapy, immunotherapy, and combination schemes (16). Although targeted therapies and immunotherapy may inhibit antigen presenting functions, T-cell activation, and B-cell signaling, data from previous studies with other types of vaccines shows that patients treated with these medications still generate protective responses (13).
Considering the absence of robust data, the Mexican Society of Oncology (SMEO) formed a multidisciplinary group to draft recommendations regarding COVID-19 vaccination in adults with cancer. These recommendations are based on the best available evidence and a thorough revision of guidelines released by other international societies (17., 18., 19.). A literature search was performed in Pubmed utilizing the Medical Subject Headings (MeSH) terms “Neoplasms”, “COVID-19”, and “COVID-19 Vaccines”. Available data on the use of COVID-19 vaccines in patients with cancer were reviewed by the expert panel, and resulting recommendations were graded according to the University of Oxford Centre for Evidence-Based Medicine methodology (20).
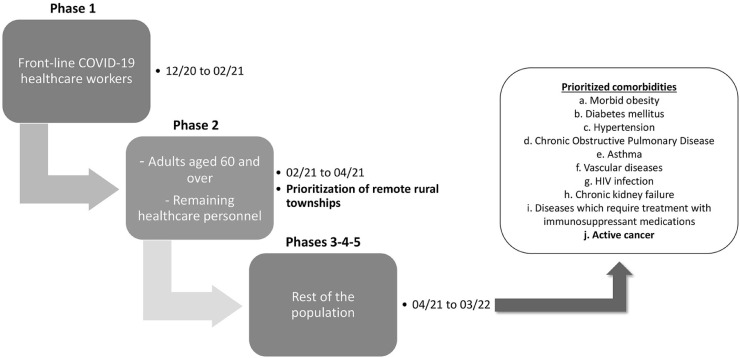
These recommendations are in line and complementary to the National Vaccine Rollout Policy against SARS-CoV-2 for the Prevention of COVID-19 released by the Mexican Government (Figure 1 ) (21,22). This policy prioritizes vaccination of older adults, which represents the most practical approach to immunize the population most at risk for serious and fatal outcomes, given that the prevalence of high-risk comorbidities in older adults reaches 66% (23). Adults <60 years of age with comorbidities, including cancer, will be next in priority (21). The complete recommendations are shown in Table 1 .
Figure 1.
Representative scheme of the different stages delineated in the National Vaccine Rollout Policy against SARS-CoV-2 for the Prevention of COVID-19 in Mexico (21).
Table 1.
Recommendations on COVID-19 vaccine rollout for adults with cancer in Mexico.
| Topic | Recommendation |
|---|---|
| Vaccination in adults with cancer | SMEO recommends that all adults with cancer in Mexico be vaccinated in strict accordance with the National Vaccine Rollout Policy, using any of the currently available vaccines authorized by COFEPRIS (21). (Level of evidence 1b, grade of recommendation B). |
| Prioritization of adults with cancer in vaccination campaigns | Adults living with active cancer and undergoing antineoplastic treatment or who are planning to initiate treatment, especially for hematological neoplasms, should be priority for vaccine rollout, particularly considering limited vaccine availability (17,19,24). (Level of evidence 2b, grade of recommendation B). |
| Vaccination in patients receiving cytotoxic chemotherapy | Vaccination should not be delayed (Level of evidence 2b, grade of recommendation B). If possible, and if this does not hinder timely access to the vaccine, vaccination should take place two weeks before initiating treatment, or when the absolute neutrophil count is within normal ranges, particularly for adults with hematological malignancies (13,19). Updated bloodwork is not needed before vaccination. (Level of evidence 5, grade of recommendation D). |
| Vaccination in patients receiving immunotherapy or targeted therapy | Vaccination should not be delayed. Previous studies with other vaccines have concluded that vaccination does not increase the risk for immune-related adverse events (25,26). A study in Israel among adults who received the Pfizer-BioNTech vaccine showed that vaccination was not associated with a higher risk for immune-related adverse events (27). (Level of evidence 2b, grade of recommendation C). |
| Vaccination in patients after hematopoietic stem cell transplantation | Among patients who undergo hematopoietic stem cell transplantation, vaccination should be delayed 3-6 months post-transplant (28., 29., 30.). If vaccination is feasible before transplant, a 2–4-week term is recommended between both events, if possible (31). (Level of evidence 2b, grade of recommendation C). |
| Vaccination in patients receiving radiotherapy | Vaccination should not be delayed. Each case should be evaluated by the attending radio-oncologist for specific recommendations (32). (Level of evidence 5, grade of recommendation D). |
| Vaccination in patients treated surgically | Vaccination should take place at least 7-10 d after the surgical procedure. This should avoid any vaccine-related adverse events (fever, fatigue, etc.) being incorrectly attributed as surgical complications (19). Additionally, this would allow for adjuvant treatment initiation in candidate patients (33). (Level of evidence 5, grade of recommendation D). |
| Number of vaccine doses | Among adults with cancer who receive two-dose vaccines, administration of the second dose not be delayed under any circumstance, since this could lead to a hindered response and insufficient protection (15). (Level of evidence 2b, grade of recommendation C). |
| Socioeconomic factors and vaccination | Given the observed associations between poverty, overcrowded living conditions, and worse COVID-19 related outcomes, adults with cancer from rural and marginalized areas should be considered a priority in vaccine rollout strategies (7). (Level of evidence 2c, grade of recommendation B). |
| Social distancing after vaccination | Social distancing and mask wearing strategies are highly encouraged even after vaccination for adults with cancer, given the lack of robust evidence regarding the immune response in patients with cancer, particularly those undergoing active treatment. (Level of evidence 5, grade of recommendation D). |
| Vaccination-related research | We encourage research centers to design and implement studies aimed at measuring the immune response and prevalence of adverse events following vaccination against COVID-19 in patients with cancer (17). (Level of evidence 5, grade of recommendation D). |
| Strategies to decrease vaccine hesitance | Government instances, medical societies, patient advocacy organizations and all medical practitioners should actively participate in conveying information via traditional and social media regarding the safety and efficacy of COVID-19 vaccination, with the objective of generating public confidence and increasing vaccine uptake (17,34). (Level of evidence 5, grade of recommendation D). |
| Cancer registries and vaccination | We recommend strengthening national cancer registries to facilitate the identification of patients at higher risk for worse outcomes in the event of future pandemics (35). (Level of evidence 5, grade of recommendation D). |
| Vaccination of healthcare providers | We recommend all healthcare personnel involved in the care and treatment of patients with cancer be vaccinated according to the strategies proposed in the National Vaccine Rollout Policy (21). (Level of evidence 5, grade of recommendation D). |
Through these recommendations, SMEO joins the international effort to prioritize patients who are at risk of worse outcomes from COVID-19. We believe these recommendations can be useful not only for oncologists and other physicians practicing in Mexico but also in countries across Latin America with similar issues regarding access to vaccines. It is in the best interest of our societies to achieve the primary goal of reducing severe and fatal cases through the prioritization of vulnerable populations, including patients with cancer, who require the highest protection against this devastating disease.
Conflict of Interest
Oscar Arrieta has received personal fees from Pfizer, grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, personal fees from Lilly, grants and personal fees from Merck, grants and personal fees from Bristol Myers Squibb, grants and personal fees from Roche. Yanin Chávarri-Guerra has received research grants from Roche and travel support from Pfizer. All other authors have no conflict of interest to declare related to the publication of this manuscript.
References
- 1.CSSE – Center for Systems Science and Engineering at JHU. COVID-19 Map - Johns Hopkins Coronavirus Resource Center. Available online at: https://coronavirus.jhu.edu/map.html. (Accessed June 20, 2021).
- 2.Consejo Nacional de Ciencia y Tecnología. COVID-19 México, Información General. Available online at: https://datos.covid-19.conacyt.mx/. (Accessed June 20, 2021).
- 3.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. New Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Chai P, Yu J, et al. Effects of cancer on patients with COVID-19: a systematic review and meta-analysis of 63,019 participants. Cancer Biol Med. 2021;18:298–307. doi: 10.20892/j.issn.2095-3941.2020.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonio-Villa NE, Fernández-Chirino L, Pisanty-Alatorre J, et al. Comprehensive evaluation of the impact of sociodemographic inequalities on adverse outcomes and excess mortality during the COVID-19 pandemic in Mexico City. medRxiv. 2021:2021.03.11.21253402. (Accessed June 20, 2021). [DOI] [PubMed]
- 8.Courtemanche C, Garuccio J, Le A, et al. Strong Social Distancing Measures In The United States Reduced The COVID-19 Growth Rate. Health Affairs. 2020;39:1237–1246. doi: 10.1377/hlthaff.2020.00608. [DOI] [PubMed] [Google Scholar]
- 9.Thu TPB, Ngoc PNH, Hai NM, et al. Effect of the social distancing measures on the spread of COVID-19 in 10 highly infected countries. Sci Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process. Available online at: https://extranet.who.int/pqweb/sites/default/files/documents/Status_of_COVID-19_Vaccines_within_WHO_EUL-PQ_evaluation_process-16June2021_Final.pdf. (Accessed June 20, 2021).
- 11.Our World in Data. Coronavirus (COVID-19) Vaccinations. Available online at: https://ourworldindata.org/covid-vaccinations?country=∼MEX. (Accessed June 20, 2021).
- 12.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang JK, Zhang T, Wang AZ, et al. COVID-19 vaccines for patients with cancer: benefits likely outweigh risks. J Hematol Oncol. 2021;14:38. doi: 10.1186/s13045-021-01046-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdul-Jawad S, Baù L, Alaguthurai T, et al. Acute Immune Signatures and Their Legacies in Severe Acute Respiratory Syndrome Coronavirus-2 Infected Cancer Patients. Cancer Cell. 2021;39:257–275. doi: 10.1016/j.ccell.2021.01.001. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Veldt AAM, Oosting SF, Dingemans A-MC, et al. COVID-19 vaccination: the VOICE for patients with cancer. Nat Med. 2021;27:568–569. doi: 10.1038/s41591-021-01240-w. [DOI] [PubMed] [Google Scholar]
- 17.Garassino MC, Vyas M, de Vries EGE, et al. The ESMO Call to Action on COVID-19 vaccinations and patients with cancer: Vaccinate. Monitor. Educate. Ann Oncol. 2021;32:579–581. doi: 10.1016/j.annonc.2021.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mislang AR, Soto-Perez-de-Celis E, Russo C, et al. The SIOG COVID-19 working group recommendations on the rollout of COVID-19 vaccines among older adults with cancer. J Geriatr Oncol. 2021;12:848–850. doi: 10.1016/j.jgo.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network. Recommendations of the NCCN COVID-19 Vaccination Advisory Committee Version 2.0 03/10/2021. Available online at: https://www.nccn.org/covid-19/pdf/COVID-19_Vaccination_Guidance_V2.0.pdf. (Accessed June 20, 2021).
- 20.Oxford Centre for Evidence-Based Medicine: Levels of Evidence . March 2009. Available online at.https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (Accessed June 20, 2021) [Google Scholar]
- 21.Gobierno de México. Política Nacional de Vacunación contra el virus SARS-CoV-2, para la prevención de la COVID-19 en México. Documento Rector. Available online at: https://coronavirus.gob.mx/wp-content/uploads/2021/01/PolVx_COVID_-11Ene2021.pdf. (Accessed March 13, 2021).
- 22.Grupo Técnico Asesor de Vacunación C Priorización inicial y consecutiva para la vacunación contra SARS-CoV-2 en la población mexicana. Salud Publica Mex. 2020;63:288–309. doi: 10.21149/12399. Recomendaciones preliminares. [DOI] [PubMed] [Google Scholar]
- 23.Clark A, Jit M, Warren-Gash C, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8:e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribas A, Sengupta R, Locke T, et al. Priority COVID-19 Vaccination for Patients with Cancer while Vaccine Supply Is Limited. Cancer Discov. 2021;11:233. doi: 10.1158/2159-8290.CD-20-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Failing JJ, Ho TP, Yadav S, et al. Safety of Influenza Vaccine in Patients With Cancer Receiving Pembrolizumab. JCO Oncol Pract. 2020;16:e573–e580. doi: 10.1200/JOP.19.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Shakankery KH, Kefas J, Miller R. COVID-19, the Future Vaccine and What It Means for Cancer Patients on. Immunotherapy. Front Oncol. 2021;10 doi: 10.3389/fonc.2020.631611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waissengrin B, Agbarya A, Safadi E, et al. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Hematology Association. Recommendations for COVID-19 vaccination in patients with hematologic cancer. Available online at: https://ehaweb.org/covid-19/eha-statement-on-covid-19-vaccines/recommendations-for-covid-19-vaccination-in-patients-with-hematologic-cancer/. (Accessed March 13, 2021).
- 30.American Society of Hematology. ASH-ASTCT COVID-19 Vaccination for HCT and CAR T Cell Recipients: Frequently Asked Questions. Available online at: https://www.hematology.org/covid-19/ash-astct-covid-19-vaccination-for-hct-and-car-t-cell-recipients. (Accessed March 13, 2021).
- 31.American Society of Hematology. COVID-19 and Vaccines for the Immunocompromised: Frequently Asked Questions. Available online at: https://www.hematology.org/covid-19/ash-astct-covid-19-and-vaccines. (Accessed March 13, 2021).
- 32.American Society for Radiation Oncology. ASTRO Recommendation on COVID-19 Vaccination for Cancer Patients Receiving Radiation Therapy. Available online at: https://www.astro.org/Daily-Practice/COVID-19-Recommendations-and-Information/Clinical-Guidance. (Accessed March 20, 2021).
- 33.Silvestris N, Brunetti O, Bernardini R, et al. COVID Vaccination in Cancer Patients: What Vaccination Priority Strategies Should There Be? Front Oncol. 2021;11 doi: 10.3389/fonc.2021.641388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waisbord S, Larson H. 2005. Why Invest in Communication for Immunization: Evidence and Lessons Learned. A joint publication of the Health Communication Partnership based at Johns Hopkins Bloomberg School of Public Health/Center for Communication Programs (Baltimore) and the United Nations Children's Fund (New York) [Google Scholar]
- 35.European Center for Disease Prevention and Control. Technical Report: COVID-19 vaccination and prioritization strategies in the EU/EEA. Available on line at: https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-vaccination-and-prioritisation-strategies.pdf. (Accessed March 13, 2021).