Abstract
Objectives
COVID-19 has had devastating effects on long-term care homes across much of the world, and especially within Canada, with more than 50% of the mortality from COVID-19 in 2020 in these homes. Understanding the way in which the virus spreads within these homes is critical to preventing further outbreaks.
Design
Retrospective chart review.
Settings and Participants
Long-term care home residents and staff in Ontario, Canada.
Methods
We conducted a longitudinal study of a large long-term care home COVID-19 outbreak in Ontario, Canada, using electronic medical records, public health records, staff assignments, and resident room locations to spatially map the outbreak through the facility.
Results
By analyzing the outbreak longitudinally, we were able to draw 3 important conclusions: (1) 84.5% had typical COVID-19 symptoms and only 15.5% of residents had asymptomatic infection; (2) there was a high attack rate of 85.8%, which appeared to be explained by a high degree of interconnectedness within the home exacerbated by staffing shortages; and (3) clustering of infections within multibedded rooms was common.
Conclusion and Implications
Low rates of asymptomatic infection suggest that symptom-based screening in residents remains very important for detecting outbreaks, a high degree of interconnectedness explains the high attack rate, and there is a need for improved guidance for homes with multibedded rooms on optimizing resident room movement to mitigate spread of COVID-19 in long-term care homes.
Keywords: COVID-19, SARS-CoV-2, nursing home, long-term care
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly spread around the world with over 1.5 million deaths in 2020.1 The virus has had a particularly significant impact on long-term care (LTC) homes. Recent estimates suggest LTC homes account for approximately 50% of all COVID-19 mortality,2 and estimates from Canada have been as high as 80%.3 Residents’ older age, high rates of comorbidities, dependence on staff members (staff) for care, difficulties practicing hand hygiene and physical distancing owing to cognitive impairment, and crowded environment have all been implicated in increased risk of outbreaks and high mortality.4 Ontario LTCs in particular have high rates of multibedded rooms, and this may have further exacerbated outbreaks compared with other countries.
There remains significant debate about why some LTC homes have had such large COVID-19 outbreaks, whereas others have not, leaving uncertainty around effective policies to both prevent and manage outbreaks. Previous research related to LTC outbreaks have predominantly examined homes cross-sectionally,5, 6, 7, 8 or by using aggregate data9, 10, 11; although these studies have provided important information, they cannot provide the granularity of detail that an in-depth longitudinal study can provide.
To address the important gaps that remain in our understanding of LTC COVID-19 outbreaks, we conducted an in-depth longitudinal, spatial analysis of an LTC home in Ontario, Canada, followed for the entirety of their large outbreak (March 1–May 21, 2020), to understand the clinical characteristics of cases, including percentage of asymptomatic cases, and the mechanisms of spread within the home. We hypothesized that there would be a relatively low rate of asymptomatic disease within the home, and that spread in multibedded rooms would play a significant role in transmission.
Description of the Outbreak
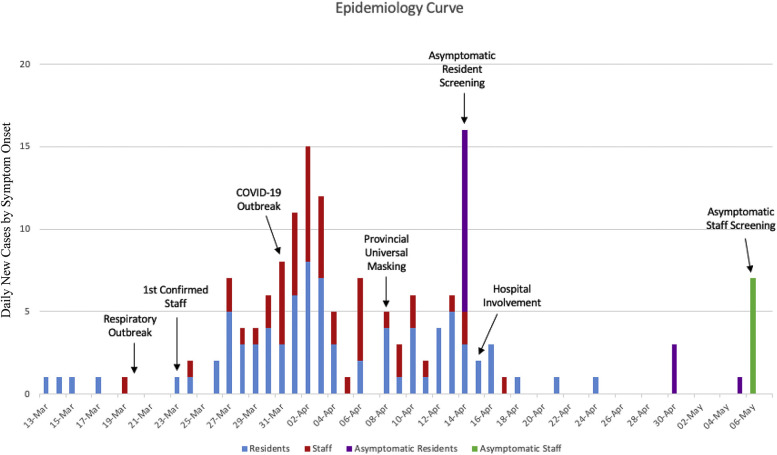
At the start of the outbreak, there were more than 110 residents living in the LTC home. The first resident developed symptoms March 13, 2020, and by March 20 a respiratory cluster was identified by the home. There were 6 symptomatic residents, and 2 were tested for SARS-CoV-2, in accordance with existing provincial LTC respiratory outbreak guidelines at the time.12 Both tested negative, resulting in the declaration of a non–COVID-19 respiratory outbreak by the local public health authority. The first identified staff developed symptoms on March 19, testing positive for SARS-CoV-2 on March 23. Owing to a lack of other confirmed cases, a COVID-19 outbreak was not declared until March 31, when a second case was confirmed, as the provincial definition of an outbreak required 2 confirmed cases at the time. At that point, based on retrospective review, there were already 26 symptomatic residents and 13 symptomatic staff. Ontario provincial recommendations recommended against staff from working in more than 1 facility and from family visitation on March 22,13 recommended resident and staff symptom screening on March 30, and universal masking of staff on April 8.14 Droplet and Contact Precautions for all residents were implemented on March 31 when a COVID-19 outbreak was declared. Prior to the above dates, precautions were only being used for symptomatic residents who had been recognized and placed on Droplet and Contact Precautions. The epidemiologic curve is shown in Figure 1 .
Fig. 1.
Epidemiologic curve.
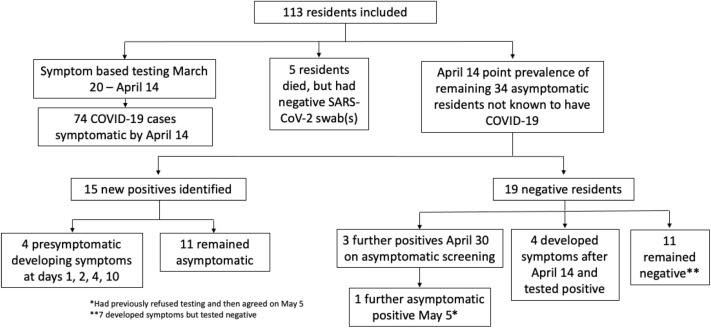
By April 14, when asymptomatic testing was recommended by the province for outbreak facilities, there were 74 residents with COVID-19 who had already developed symptoms, 5 had died but had negative SARS-CoV-2 testing, and 4 refused testing; thus, the remaining 34 asymptomatic residents were tested (Figure 2 ). This identified an additional 16 asymptomatic positive residents, 5 of which were presymptomatic and 11 remained asymptomatic. Of the 18 remaining uninfected residents, 3 developed symptoms over the next 14 days and had positive testing. On April 15, a partnership was established between LTC homes and hospitals15 with the goal to support homes through the COVID-19 pandemic through additional specialized knowledge, personal protective equipment, access to testing, etc.
Fig. 2.
Flowchart of testing.
On April 30, an additional round of asymptomatic resident testing was conducted of the remaining 15 negative residents, and this resulted in 3 additional positive results. On May 5, 1 additional asymptomatic positive resident was identified (had refused April 30 testing). These 4 positive residents did not develop symptoms. On May 6, an additional 7 staff were identified on asymptomatic screening. On May 21, 2 weeks after the last identified case, the outbreak was declared over.
Analysis of this outbreak as it pertains to the sensitivity of testing for SARS-CoV-2 has previously been published.16
Methods
We performed a retrospective chart review of all the residents in the LTC home from March 1, 2020, 14 days before the first symptomatic resident, till May 21, 2020, the day the outbreak was declared over. Data were extracted from the home’s electronic medical record system. Certain aspects of the home have been removed to maintain confidentiality of the home. The article was reviewed and approved by the board of directors of the home.
Extraction of Resident Data
Resident age, gender, comorbidities, the rooms they resided in and for which dates, their symptoms, the timing of symptom onset, and their final outcome were extracted. Residents were considered to have symptoms based on provincial symptom guidance documents including both typical (fever, cough, and dyspnea) and atypical symptoms (sore throat, rhinorrhea, nasal congestion, decreased smell or taste, nausea and vomiting, diarrhea, abdominal pain, chills, headache, conjunctivitis, fatigue, malaise, myalgias, anorexia, exacerbation of chronic conditions, tachycardia, hypotension, hypoxia, delirium, falls, functional decline).17 Staff symptom onset and SARS-CoV-2 testing results were captured by outbreak line lists. Residents and staff were assumed to be infectious from 2 days prior to symptom onset until day 8 after symptom onset, based on studies looking at isolation of live virus18, 19, 20 and transmission modeling studies.21 , 22 Incubation period for COVID-19 was assumed to be 2-14 days, with the majority of cases manifesting 3-7 days after exposure.23, 24, 25 Given the evidence of reduced transmission of true asymptomatic disease,26 , 27 only presymptomatic and symptomatic residents were considered to be potentially infectious.
Reverse Transcription–Polymerase Chain Reaction Testing
Until April 13, 2020, testing was performed using a published laboratory developed test targeting the E gene and RdRp of SARS-CoV-2.28 From April 14 onward, reverse transcription–polymerase chain reaction (RT-PCR) was performed using the Allplex 2019-nCoV Assay, which targets the E, N, and RdRp genes of SARS-CoV-2.
Staff Data Extraction
We obtained staffing records from the LTC home from March 1 until May 21 to extract the days staff worked, their role, and the residents they were assigned. For personal support worker (PSW) analysis, direct contact was equivalent to one 8-hour shift of direct care. Work assignments were not available from the home for 3 PSW staff who developed COVID-19.
Mapping Reconstruction
Virtual reconstruction and mapping of the outbreak was done in partnership with an architectural firm (Montgomery Saison). Floor plans were obtained from the home, and diagrams were created in Adobe Illustrator and animations in Adobe Photoshop (Adobe Inc, San Jose, CA).
Cohorting Calculations
Residents were considered incorrectly cohorted if they were symptomatic and not in isolation, or if they had had a high-risk exposure to a confirmed case (within 14 days) and were not isolated. The percentage of correct cohorting was calculated as the inverse of those incorrectly cohorted on a given day. We also examined the percentage of remaining susceptible individuals who were sharing a room with a potentially infectious individual on each day of the outbreak.
Statistical Analysis and Ethics
Results were summarized using descriptive statistics, including calculations for mean and attack rate (percentage positive divided by overall resident numbers). Graphing was done through Microsoft Excel. P value was calculated for symptom onset on each floor using R-studio. Research Ethics Board approval for the study was obtained from our institution (Sinai Health, REB 20-0188-C, May 19, 2020).
Results
Characteristics of the Home and Residents
The LTC home is a for-profit entity, and has a capacity of more than 110 residents with 51.6% residing in 3- or 4-bedded rooms. The mean age of the residents was 78.3 years (range 51-101), 45.5% were male, and the rates of comorbidities were as follows: 59.0% hypertension, 47.9% cognitive impairment, 30.0% cardiovascular disease, 29.0% diabetes, 28.0% previous stroke, and 24.0% chronic lung disease.
Clinical Outcomes of the Residents
Four residents refused SARS-CoV-2 testing throughout the entire outbreak. At the end of the outbreak there were a total of 85.8% residents with confirmed COVID-19, 19.6% being admitted to hospital and 21.6% mortality. Only 3.5% of the residents did not test positive for SARS-CoV-2 and did not have symptoms.
Symptoms of the Residents
Of the 97 residents with positive RT-PCR for SARS-CoV-2 (attack rate of 85.8%), 84.5% developed symptoms and 15.5% remained asymptomatic. In addition, 68.0% had a fever and 52.6% had a cough. In total, 83.5% of those with symptoms developed either fever, cough, or dyspnea during their illness (Table 1 ).
Table 1.
COVID-19 Resident Symptoms
| Symptoms | % (n) (n = 97) |
|---|---|
| Fever | 68.0 (66) |
| Cough | 52.6 (51) |
| Dyspnea or hypoxia | 9.3 (9) |
| Sore throat or rhinorrhea | 7.2 (7) |
| Asymptomatic | 15.5 (15) |
| Fever, cough, or dyspnea and hypoxia | 83.5 (81) |
On April 14 when facility-wide testing of asymptomatic residents occurred, there were 40 residents with positive SARS-CoV-2 RT-PCR who neither had symptoms nor developed symptoms in the following 14 days. Through retrospective chart review, 62.5% (25/40) of these residents had experienced recent symptoms compatible with COVID-19,17 but had recovered from their illness at the time of testing.
Infected Staff Exposures
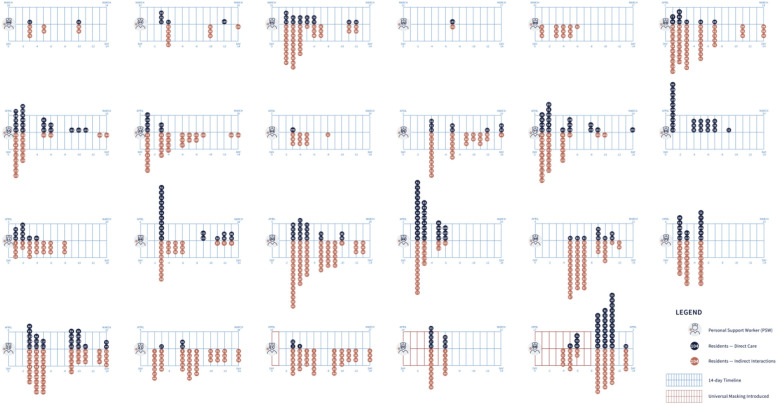
There were 130 staff who worked at the home during the outbreak, of which 55 (42.3%) developed confirmed SARS-CoV-2 infection during the outbreak. To determine possible cases of staff infections acquired from residents or other staff, we analyzed the exposures of all 23 PSWs with confirmed symptomatic COVID-19 infection for whom staffing assignments were available (Figure 3 ). On average, each PSW had 5.6 direct contacts (range 1-15) within the preceding 3-7 days. The final symptomatic staff case developed symptoms on April 17, 9 days after implementing universal masking.
Fig. 3.
Personal support worker (PSW) exposures: Exposures for the 23 symptomatic PSWs who contracted COVID-19. Black dots represent direct care for a potentially infectious resident and orange dots represent residents who were potentially infectious on the same floor as the staff during their shift. Three PSWs tested positive, but details of their work schedule were not available and are not included.
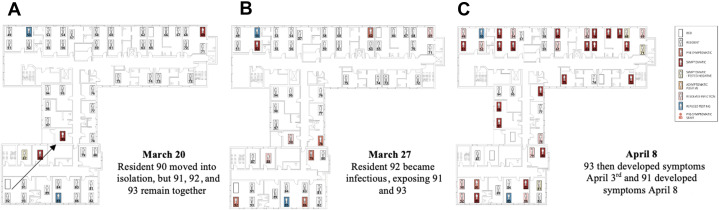
Mapping of the Outbreak
Using the resident room records and staffing assignment records, the spread of the virus throughout the home was mapped based on symptom onset date (Figure 4 ). There was a trend toward earlier symptom onset of residents in multibedded rooms, averaging 2 days earlier; however, these results were not significant.
Fig. 4.
Outbreak mapping and room moves, second floor: Residents are mapped based on their location on each day during the outbreak and their symptom onset. Potentially infectious staff are also shown when providing direct patient care. Select images are shown below (for full video, see online version). (A) Symptomatic resident moved out, but residents 91, 92, and 93 remain. (B) Resident 92 became symptomatic exposing residents 91 and 93, who then develop COVID-19 in panel C.
Room Movement
By visualizing the outbreak over time, we found multiple examples of clustered transmission within rooms (Figure 4). Symptomatic residents were initially managed within their shared room (as recommended in provincial respiratory outbreak guidelines12). As recommendations changed to recommend private rooms for symptomatic residents, this process of room movement was especially challenging as by April 1, 4 cleaning staff from the home had become ill (Supplementary Table 1) and external cleaning services had to be hired.
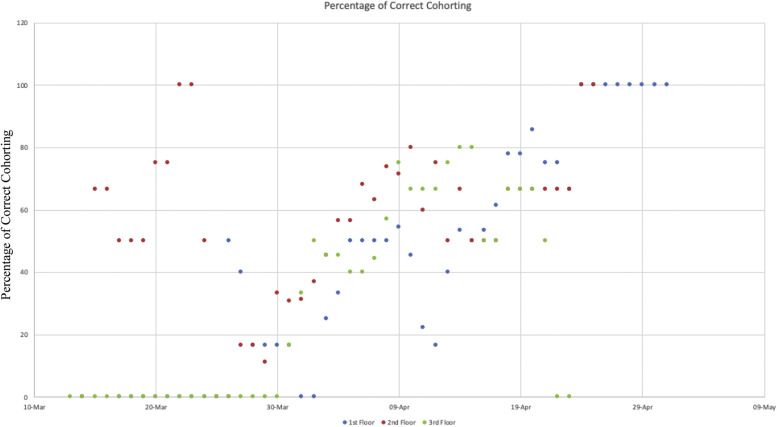
Over the course of the outbreak, the percentage of correctly cohorted symptomatic individuals averaged 43.7%. The degree of correct cohorting improved over time (Supplementary Figure 1). Symptomatic residents were isolated or cohorted with other positive cases, but the remaining asymptomatic residents were not separated from each other, despite their exposure, and there were transmission clusters around these events. Examples of this can be seen in transmission series shown in Figure 4A–C and accompanying Videos 1–3 (Video 1: transmission from resident 103 to 105 to 106, second 0:28 to 1:01; and from 115 to 118 to 116, second 0:45 to 1:05).
Supplementary Fig. 1.
Percentage of correct cohorting by floor. Considered correctly cohorted if asymptomatic residents remained together, symptomatic resident were in private room, or confirmed COVID-19 residents cohorted together.
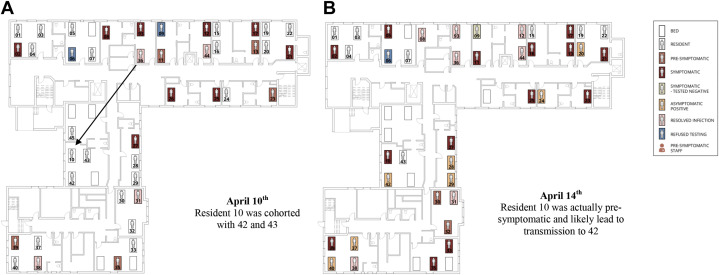
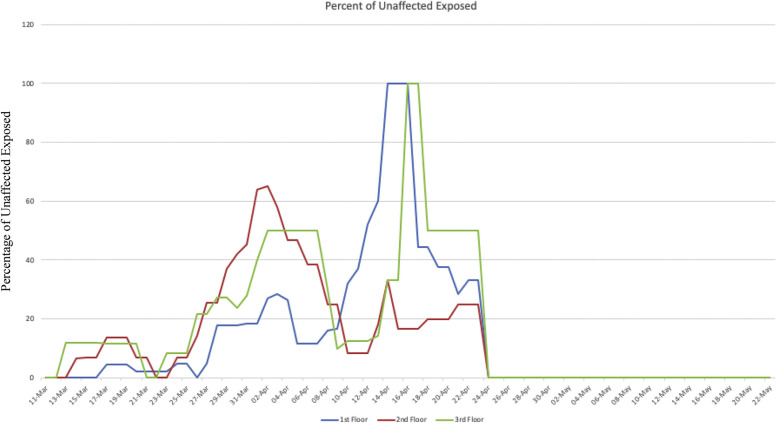
There were also examples where asymptomatic residents were cohorted together after a high-risk exposure from a previous symptomatic roommate. This can be seen through possible transmission events due to incorrect cohorting of asymptomatic residents shown in Supplementary Figure 2A and B. There was a high degree of roommate exposures, with all floors having more than 50% of unaffected residents, being exposed to a SARS-CoV-2 roommate at the peak of the outbreak (Supplementary Figure 3).
Supplementary Fig. 2.
Outbreak mapping and room moves, first floor. Residents are mapped based on their locations on each day during the outbreak and their symptom onset. Potentially infectious staff are also shown when providing direct patient care. Select images are shown below (for full video see online version). Supplementary Figure 2A resident 10 who was asymptomatic but had a high risk exposure was cohorted with residents 42 and 42 leading to COVID-19 in resident 42 shown in Supplementary Figure 2B.
Supplementary Fig. 3.
Percentage of unaffected exposed residents (negative SARS-CoV-2 testing and asymptomatic) that had direct roommate exposures to COVID-19.
Discussion
Spatial analysis of outbreaks have been used previously for other infectious diseases, but represent a minority of outbreak analysis, and is much more commonly done for water/sanitation outbreaks.29 Our study provides multiple valuable insights about LTC home outbreaks and also highlights some possible pitfalls of previous research investigating COVID-19 outbreaks in LTC settings.
First, the vast majority of residents (83.5%) with positive RT-PCR for SARS-CoV-2 had typical COVID-19 symptoms and there was a relatively low proportion of asymptomatic infection of only 15.5%. This is in contrast to multiple other reports5, 6, 7 , 11 of high proportion of asymptomatic cases in LTC homes, ranging from 56.5%6 to 87%.11 The most likely explanation for the difference is the longitudinal nature of our study allowed us to identify both presymptomatic residents and those with recovered illness that may be missed in point prevalence studies. In our study, 62.5% of residents who were asymptomatic on the day of testing actually had recently resolved symptoms, compatible with a COVID-19 illness, and thus may not have had asymptomatic SARS-CoV-2-–positive results, but in fact resolved symptomatic illness. By identifying these presumed resolved symptomatic illness, through retrospective chart review, the rate of asymptomatic infection fell from 41.2% to 15.5%. This is especially relevant in older adults, who can shed virus for many weeks after recovering.30 A similar study found low rates of asymptomatic disease (12%) in older patients when examined longitudinally.31 Our results suggest that resources be focused on vigilant symptom surveillance, coupled with broad testing of asymptomatic residents in facilities when symptomatic cases are identified.
Second, there was a high degree of interconnectedness within the home, which we define as either direct care of a resident by a staff member, or through residents sharing a room. Despite the very high attack rate in the home, these direct connections can explain the majority of transmission, without the need to evoke aerosolization transmission, as has been previously suggested.32 Our findings instead align with another recent study using whole genome sequencing to examine an LTC home outbreak and found that most cases were related to direct patient care rather than aerosolizing events.33 The interconnectedness we observed in the home is highlighted by PSWs having on average 5.9 direct prolonged exposures to potentially infectious residents18, 19, 20 during the 3-7 days21, 22, 23 preceding their own symptom onset. Given severe restrictions to personal protective equipment in March and early April, presymptomatic transmission, and late introduction of universal masking, many of these exposures occurred without personal protective equipment. There were no further symptomatic staff cases after April 17, 14 days after universal masking was implemented, offering additional support for the role of universal masking in preventing further staff infections.34 This interconnectedness was exacerbated as staff shortages worsened during the outbreak from other sick staff, especially with shortages in housekeeping staff on April 1 (Supplementary Table 1). Although not all transmission can be explained through our direct connections observed in our analysis, there were many possible direct connections that were not able to be captured through documented electronic medical records and staffing records, and this could explain further transmission events.
There was also a high degree of interconnectedness among the residents. This was predominantly due to the high degree of multibedded rooms (51.6%) and the fact that less than 50% of symptomatic residents were in private rooms during the peak of the outbreak. This likely played a strong role in transmission, especially early in the outbreak. As a result, more than 50% of residents had a clear COVID-19 exposure from a roommate prior to developing COVID-19 themselves. Given the distress to residents of moving rooms, residents were initially placed in precautions at the bedside, but as the outbreak progressed it became clear that residents would need to be moved. This process was further challenged because of significant cleaning staff shortages at the height of the outbreak (Supplementary Table 1). On August 28, 2020, the Ontario government took steps to restrict new admission to rooms with a maximum of 2 residents,14 but this does not address the issue of needing available private rooms to move symptomatic or exposed residents. LTC homes with multibedded rooms should ensure they have dedicated space available to quickly separate these residents when needed.
As cohorting of residents began to occur, there was very limited guidance available to the homes on how to safely do this,14 and as a result, there were instances where asymptomatic residents were not separated after high-risk exposures, and in some cases these residents were cohorted with uninfected residents while they were in fact presymptomatic, unintentionally increasing exposure of infected residents. There remains significant uncertainty about the best way to cohort in COVID-19 outbreaks,35 and our study demonstrates that further work is needed to ensure that cohorting is done in a safe manner to prevent transmission. A rough guide has been published previously.36
Last, the high degree of interconnectedness and direct connections, seen through examination of both staff and resident cases, suggests that even in a large rapid outbreak like the one in this home, droplet spread through direct connections could explain most transmission in the absence of aerosolized spread as previously hypothesized.37 , 38 The absence of evidence for aerosolized spread certainly does not eliminate it as a possibility, but we would argue that the major driver of transmission in this home remained close prolonged contact and associated droplet spread. Of note, there were no aerosol-generating medical procedures done in the home throughout the course of the outbreak.
There are multiple limitations to our study that should be noted. First, this was only a single-center study, meaning there should be caution in extrapolating our findings more broadly, especially in homes that do not have multibedded rooms. The retrospective nature of our study means that those with mild symptoms may have been missed and labeled as asymptomatic. Furthermore, over the course of the outbreak, there were many significant changes in the access to testing, personal protective equipment, staffing etc that may have had changes in the outbreak, but given the retrospective nature of the study, only major changes could be captured, potentially missing smaller, but significant, events. The very late asymptomatic staff screening meant that there were likely many staff with mild or asymptomatic disease earlier in the outbreak that might have been missed, especially given that documentation in this home is unstructured (with the exception of temperature checks). Finally, the study was only able to capture connections through direct resident care, but inevitably missed many staff-to-staff connections both in and outside of the home, as well as the possible role of fomite transmission. Visitors were restricted after March 22, but before this there was no record and may have also played a role in transmission that was not captured. Whole genome sequencing was not available to us at the time for outbreak management, but would have strengthened the study to confirm that all cases were acquired at the home.
Conclusions and Implications
In this large outbreak, we found a relatively low percentage of asymptomatic residents. Despite the high attack rate and rapid spread, we found that this could be explained by droplet and contact spread due to a high degree of interconnectedness in the home. Our study also reemphasizes the role for universal masking for staff within LTC homes. Finally, there is a need for clear policies and direction on how to safely move both symptomatic residents and asymptomatic exposed residents within the home to prevent transmission.
Footnotes
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.jamda.2021.07.021.
Supplementary Data
First floor outbreak: Video shows what rooms each resident on the first floor were over time when they became potentially infectious (pre-symptomatic) and then symptomatic. Also shown are the potentially infectious (pre-symptomatic staff) and the residents they worked with in the 48 hours before symptom onset.
Second floor outbreak: Video shows where residents on the second floor were over time when they became potentially infectious and then symptomatic, as well as potentially infectious staff.
Third floor outbreak: Video shows where residents on the third floor were over time when they became potentially infectious and then symptomatic, as well as potentially infectious staff.
Supplementary Table 1.
Date of Staff Illness
| Symptom Onset | Position | ||||||
|---|---|---|---|---|---|---|---|
| March 19 | Housekeeping | ||||||
| March 20-23 | |||||||
| March 24 | Maintenance | ||||||
| March 25-26 | |||||||
| March 27 | Housekeeping | Laundry | |||||
| March 28 | PSW | ||||||
| March 29 | Nursing | ||||||
| March 30 | PSW | Administration | |||||
| March 31 | Housekeeping | PSW | PSW | PSW | PSW | ||
| April 1 | Housekeeping | PSW | PSW | PSW | Nursing | ||
| April 2 | PSW | PSW | PSW | PSW | Nursing | Nursing | Dietary |
| April 3 | PSW | PSW | PSW | PSW | PSW | ||
| April 4 | PSW | PSW | |||||
| April 5 | Administration | ||||||
| April 6 | PSW | Nursing | Nursing | Administration | Administration | ||
| April 7 | |||||||
| April 8 | PSW | ||||||
| April 9 | PSW | Nursing | |||||
| April 10 | PSW | Nursing | |||||
| April 11 | Housekeeping∗ | ||||||
| April 12 | |||||||
| April 13 | Nursing | ||||||
| April 14 | PSW | Dietary | |||||
| April 15-16 | |||||||
| April 17 | PSW | ||||||
New hire.
References
- 1.COVID-19 Dashboard by the Centre for Systems Science and Engineering (CSSE) at John Hopkins University. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Available at:
- 2.Comas-Herrera A., Zalakaín J., Litwin C. June 26, 2020. Mortality associated with COVID-19 outbreaks in care homes: Early international evidence. LTCcovid.org, International Long-Term Care Policy Network, CPEC-LSE. [Google Scholar]
- 3.Public Health Ontario Daily Epidemiologic Summary: COVID-19 in Ontario. https://www.ontario.ca/page/how-ontario-is-responding-covid-19#section-1 Available at:
- 4.Fisman D., Bogoch I., Lapointe-Shaw L. Risk factors associated with mortality among residents with coronavirus disease 2019 (COVID-19) in long-term care facilities in Ontario, Canada. JAMA Network Open. 2020;3:e2015957. doi: 10.1001/jamanetworkopen.2020.15957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh S., O’Laughlin K., Ehrlich H.Y. Point prevalence testing of residents for SARS-CoV-2 in a subset of Connecticut nursing homes. JAMA. 2020;324:1101–1103. doi: 10.1001/jama.2020.14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dora A.V., Winnett A., Jatt L.P. Universal and serial laboratory testing for SARS-CoV-2 at a long-term care skilled nursing facility for Veterans—Los Angeles, California, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:651–655. doi: 10.15585/mmwr.mm6921e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoxha A., Wyndham-Thomas C., Klamer S. Asymptomatic SARS-CoV-2 infection in Belgian long-term care facilities. Lancet Infect Dis. 2021;21:e67. doi: 10.1016/S1473-3099(20)30560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimball A., Hatfield K.M., Arons M. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stall N.M., Jones A., Brown K.A. For-profit long-term care homes and the risk of COVID-19 outbreaks and resident deaths. CMAJ. 2020;192:E946–E955. doi: 10.1503/cmaj.201197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown K.A., Jones A., Daneman N. Association between nursing home crowding and COVID-19 infection and mortality in Ontario, Canada. JAMA Intern Med. 2021;181:229–236. doi: 10.1001/jamainternmed.2020.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bigelow B.F., Tang O., Barshick B. Outcomes of universal COVID-19 testing following detection of incident cases in 11 long-term care facilities. JAMA Intern Med. 2021;181:127–129. doi: 10.1001/jamainternmed.2020.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ministry of Health and Long-term Care Control of respiratory infection outbreaks in long-term care homes, 2018. http://www.health.gov.on.ca/en/pro/programs/publichealth/oph_standards/docs/reference/RESP_Infectn_ctrl_guide_LTC_2018_en.pdf Available at: Published November 2018. Accessed July 24, 2020.
- 13.Chief Medical Officer of Health COVID-19 directive #3 for long-term care homes under the Long-Term Care Homes Act, 2007. https://www.rhra.ca/wp-content/uploads/2020/03/CMOH-Directive-3-Long-Term-Care-Homes.pdf Available at: Published March 22, 2020. Accessed July 24, 2020.
- 14.Chief Medical Officer of Health COVID-19 directive #3 for long-term care homes under the Long-Term Care Homes Act, 2007. https://www.rhra.ca/wp-content/uploads/2020/04/CMOH-Directive-3-Long-Term-Care-Homes-April-8-2020.pdf Published April 8, 2020. Available at:
- 15.Stall N.M., Farquharson C., Fan-Lun C. A hospital partnership with a nursing home experiencing a COVID-19 outbreak: Description of a multiphase emergency response in Toronto. Canada. J Am Geriatr Soc. 2020;68:1376–1381. doi: 10.1111/jgs.16625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kain D., McCreight E., Mazzulli T. Diagnostic sensitivity of nasopharyngeal RT-PCR in a long-term care home outbreak. J Am Med Dir Assoc. 2020;21:1570–1572.e1. doi: 10.1016/j.jamda.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ontario Ministry of Health . May 14, 2020. COVID-19 reference document for symptoms. Version 4.0. [Google Scholar]
- 18.Zou L., Ruan F., Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wölfel R., Corman V.M., Guggemos W. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 20.Bullard J., Dust K., Funk D. Predicting infectious SARS-CoV-2 from diagnostics samples. Clin Infect Dis. 2020;71:2663–2666. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He X., Lau E.H.Y., Wu P. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 22.Du Z., Xu X., Wu Y. Serial interval of COVID-19 among publicly reported confirmed cases. Emerg Infect Dis. 2020;26:1341–1343. doi: 10.3201/eid2606.200357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L., Dai J., Zhao J. Estimation of incubation period and serial interval of COVID-19: Analysis of 178 cases and 131 transmission chains in Hubei province, China. Epidemiol Infect. 2020;148:1–6. doi: 10.1017/S0950268820001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He W., Yi G.E., Zhu Y. Estimation of the basic reproductive number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID-19: Meta-analysis and sensitivity analysis. J Med Virol. 2020;92:2543–2550. doi: 10.1002/jmv.26041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Böhmer M.M., Buchholz U., Corman V. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: A case series. Lancet Infect Dis. 2020;20:920–928. doi: 10.1016/S1473-3099(20)30314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sayampanathan A.A., Heng C.S., Pin H.P. Infectivity of asymptomatic versus symptomatic COVID-19. Lancet. 2021;397:93–94. doi: 10.1016/S0140-6736(20)32651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y., Bloxham C.J., Hulme K.D. A meta-analysis on the role of children in severe acute respiratory syndrome coronavirus 2 in household transmission clusters. Clin Infect Dis. 2021;72:e1146–e1153. doi: 10.1093/cid/ciaa1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corman V.M., Landt O., Kaiser M. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith C.M., Le Comber S.C., Fry H. Spatial methods for infectious disease outbreak investigations: systematic literature review. Euro Surveill. 2015;20 doi: 10.2807/1560-7917.ES.2015.20.39.30026. [DOI] [PubMed] [Google Scholar]
- 30.Cevik M., Tate M., Lloyd O. SARS-CoV-2 viral load dynamics, duration of viral shedding and infectiousness: A living systematic review and meta-analysis. Lancet Microbe. 2021;2:e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livingstone G., Rostamipour H., Gallagher P. Prevalence, management, and outcomes of SARS-CoV-2 infections in older people and those with dementia in mental health wards in London, UK: A retrospective observational study. Lancet Psychiatry. 2020;7:1054–1063. doi: 10.1016/S2215-0366(20)30434-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMichael T.M., Currie D.W., Clark S. Epidemiology of COVID-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucey M., Macori G., Mullane N. Whole-genome sequencing to track SARS-CoV-2 transmission in nosocomial outbreaks. Clin Infect Dis. 2021;72:e727–e735. doi: 10.1093/cid/ciaa1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X., Ferro E.G., Zhou G. Association between universal masking in a health care system and SARS-CoV-2 positivity among health care workers. JAMA. 2020;324:703–704. doi: 10.1001/jama.2020.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Collaborating Centre for Methods and Tools Rapid review: What is the effectiveness of cohorting virus-positive residents to shared rooms in care facilities? http://www.nccmt.ca/knowledge-repositories/covid-19-evidence-reviews Available at: Published 2020. Accessed January 8, 2021.
- 36.Kain D., McCreight L., Johnstone J. Dealing with coronavirus disease 2019 (COVID-19) outbreaks in long-term care homes: A protocol for room moving and cohorting. Infect Control Hosp Epidemiol. 2020 Oct 28. doi: 10.1017/ice.2020.1302. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamner L., Dubbel P., Capron I. High SARS-CoV-2 attack rate following exposure at a choir practice – Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606–610. doi: 10.15585/mmwr.mm6919e6. [DOI] [PubMed] [Google Scholar]
- 38.Park S.Y., Kim Y.M., Yi S. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26:1666–1670. doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
First floor outbreak: Video shows what rooms each resident on the first floor were over time when they became potentially infectious (pre-symptomatic) and then symptomatic. Also shown are the potentially infectious (pre-symptomatic staff) and the residents they worked with in the 48 hours before symptom onset.
Second floor outbreak: Video shows where residents on the second floor were over time when they became potentially infectious and then symptomatic, as well as potentially infectious staff.
Third floor outbreak: Video shows where residents on the third floor were over time when they became potentially infectious and then symptomatic, as well as potentially infectious staff.