Abstract
Aging is a significant risk factor for cardiovascular disease. Despite the fact that endothelial cells play critical roles in cardiovascular function and disease, the molecular impact of aging on this cell population in many organ systems remains unknown. In this study, we sought to determine age-associated transcriptional alterations in cardiac endothelial cells. Highly enriched populations of endothelial cells (ECs) isolated from the heart, brain, and kidney of young (3 mo) and aged (24 mo) C57/BL6 mice were profiled for RNA expression via bulk RNA sequencing. Approximately 700 cardiac endothelial transcripts significantly differ by age. Gene set enrichment analysis indicated similar patterns for cellular pathway perturbations. Receptor-ligand comparisons indicated parallel alterations in age-affected circulating factors and cardiac endothelial-expressed receptors. Gene and pathway enrichment analyses show that age-related transcriptional response of cardiac endothelial cells is distinct from that of endothelial cells derived from the brain or kidney vascular bed. Furthermore, single-cell analysis identified nine distinct EC subtypes and shows that the Apelin Receptor-enriched subtype is reduced with age in mouse heart. Finally, we identify age-dysregulated genes in specific aged cardiac endothelial subtypes.
Keywords: aging, endothelial, heart, transcription
INTRODUCTION
Age is a well-established risk factor for cardiovascular disease and is associated with endothelial cell (EC) dysfunction (1,2). The optimal function of all organ systems is dependent on a robust vascular network to circulate blood throughout the body (3,4). However, the process of aging results in a deterioration of vascular function characterized by age-related EC dysfunction. ECs comprise the largest organ in the body and are essential for the protection and proper function of all other organs and physiological systems where they act as barriers, filters, and mediators of intercellular communication and humoral homeostasis. Consistent with these essential roles, endothelial dysfunction is increasingly implicated as a major driver of various age-related cardiovascular diseases. Although the effects of aging on vascular and endothelial functions have been well documented, the molecular changes underlying adaptation to age-related challenges in any given organ have only been explored to a limited degree.
Most efforts geared toward understanding age-related cellular and transcriptional alterations have used whole organs (5–7) rather than parsing the constituent cell types in these organs. Recent studies have begun to pursue a more delimited, cell-type specific approach (8–11), including a study of aged brain ECs that found the upregulation of Vascular Cell Adhesion Molecule 1 (VCAM1) expression contributes to age-related changes in brain (12). Yet, whether VCAM1 or other molecular alterations exist in aged cardiac ECs has yet to be elucidated.
The cardiac endothelium comprises a heterogeneous population of cells (13), and the advent of single-cell RNA sequencing (scRNA-seq) technologies now permits the investigation of how genetic and environmental challenges such as aging impact the cellular and molecular diversity in the cardiac endothelium. Defining the diversity of aging responses among the distinct endothelial subtypes within the heart is a key hurdle to elucidating the complex responses to cardiac aging.
Here, we have undertaken a bulk mRNA cell transcriptomic analysis of cardiac ECs to understand how gene expression is altered in the aging cardiac endothelium. We also tested if age-induced dysregulated genes/pathways in cardiac ECs are shared by aged ECs from two other vascular beds with distinct functional requirements: the brain and kidney. In addition, we applied scRNA-seq to determine if aging alters endothelial subtype composition in heart. We identified endothelial subtypes responsible for contributing specific transcriptional changes in the bulk RNA-seq data set by integrating it with scRNA-seq from young and aged cardiac ECs. This study provides insights into various aspects of cardiac endothelial aging in at least two ways. First, it identifies aging-associated transcriptional signatures that are common or unique among endothelial cell populations in the heart and other organs systems and second, it pinpoints altered gene expression in specific subtypes of cardiac ECs between young and aged hearts.
METHODS
Mice
Male C57/B6 young (3 mo, 13 animals) and aged (24 mo, 13 animals) mice (Hilltop Laboratories) were used in this study. Animals were housed in an AAALAC-accredited animal facility at Oregon Health and Science University. All experiments (n = 3 animals per age) were performed with the approval of the Institutional Animal Care and Use Committee at Oregon Health and Science University. Sex-specific differences clearly exist through the aging process; however, budgetary constraints forced this study to be limited to males. Future studies would incorporate both sexes for comparison.
Antibodies
Antibody information can be found in Supplemental Table S1.
Isolation of Adult ECs from Mouse Heart, Brain, or Kidney
Mice were euthanized by cervical dislocation following isoflurane induction. Organs were dissected, minced, and digested at 37°C for 30 min in DMEM media containing (1 mg/mL) Collagenase II (Worthington) and DNAase I (40 U/mL). The digest was triturated using a 14 gauge canula with a 10-mL syringe and filtered through a 70-µm cell strainer. Dissociation was slowed by dilution in 1 volume of endothelial dissociation buffer (EDB) (PBS containing 0.5% BSA, 2 mM EDTA and 50 mg Heparin) followed by pelleting at 1,200 rpm for 10 min and resuspension in 100 µL of EDB.
To enrich for ECs, resuspended cells were incubated with 10 µL of mouse anti-CD31 magnetic beads (Miltenyi Biotec) at 4°C for 15 min followed by a 300 g spin for 10 min to remove unbound beads. The cellular pellet was resuspended in EDB. ECs bound to anti-CD31 beads were isolated using an AutoMACS sorter (Miltinyi Biotec).
EC purity was further increased by fluorescent-activated cell sorting (FACS) using a Cell Sorter SH800 (Sony). Anti-GP38 and anti-CD45 were used to exclude lymphatic ECs and immune cells respectively and subsequent selection of ECs using anti-CD31 (GP38-, CD45-, CD31+ cells). Selection against GP38 ensured that transcripts found our bulk RNAseq analysis would reflect the state of vascular endothelial cells rather than the very similar lymphatic endothelium. Purified ECs for bulk RNA-seq were sorted into buffer RLT from Qiagen and stored at −80°C degrees until total RNA extraction.
Following this protocol, we isolated an average of 101,133 cells from the young heart, 89,604 cells from the aged heart, 65,208 cells from young kidney, 98,850 cells from aged kidney, 72,612 cells from the young brain, and 45,523 cells from the aged brain. For scRNA-seq, ECs were sorted directly into PBS buffer and immediately loaded onto a Chromium Controller (10× Genomics).
Quantification of VCAM-Positive ECs in Young and Aged Mouse Heart, Brain, and Kidney
Levels of VCAM expression on the ECs from young and aged mouse tissues were simultaneously quantified with flow cytometry using PE conjugated anti-VCAM1 antibody. Representative FCS files from each cell sort were loaded into FlowJo and ECs (GP38-, CD45-, CD31+) from each organ were gated followed by quantification of the frequency and intensity of VCAM1 signal.
Bulk RNA Sequencing
RNA extraction, library preparation, and sequencing.
Total RNA was extracted from cells and stored in buffer RLT using the RNAeasy Micro Kit (Qiagen). Quality and quantity of extracted RNA were assessed using the Agilent 2100 BioAnalyzer RNA 6000 Pico chip with each sample averaging 6 ng of RNA and all samples having an RNA integrity number (RIN) >7. We prepared cDNA libraries using SmartSeq V4 chemistry (Takara) and sequenced the libraries on an HiSeq 2500 (Illumina). The average read count per sample was 52,622,953 (range 47,074,089 to 73,493,567). On average, 89% of these reads were uniquely mapped, resulting in 20,069 transcripts detected in heart endothelium, 20,342 transcripts detected for brain endothelium, and 22,113 transcripts detected for kidney endothelium. Transcripts with an adjusted P value [false discovery rate (FDR)] less than 0.05 were considered differentially expressed.
Mapping of sequenced reads and transcript quantification.
Tophat2 with default parameters was used to align the sequenced reads against the mouse genome using the GRCm38. HTSeq python software with default parameters was used to quantify the transcripts of the aligned reads using the corresponding GRCm38 gene annotation model from Ensembl.
Differential gene analysis.
The count data generated above was then used as an input for the EBseq differential gene analysis (EBseq version 1.26.0 and R version 3.6.1; Leng, John Dawson, and Christina Kendziorski, EBSeq: An R package for differential expression analysis using RNA-seq data, Bioconductor) was used. For targeted differential gene analysis of MHC II and Serpin gene families, we extracted all members of the gene families from each tissue data set. Heatmap was subsequently used to display log2 fold changes for each gene family across each tissue and age.
Pathway analysis.
The Gene Set Enrichment Analysis (GSEA) package (14) was used to identify cellular pathways dysregulated in aged ECs. The dysregulated pathways for each tissue were visualized using dot plots generated by ggplot2 in R.
Receptors and ligands analysis.
To identify receptors and ligands dysregulated in the aging endothelium, we downloaded a database of mammalian receptors and ligands from https://baderlab.org/CellCellInteractions. Intersecting this database with the differential gene list identified receptors or ligands dysregulated in the aging endothelium and displayed with a heatmap. A published data set of age-dysregulated ligands in the circulatory system (15) were then mapped to cardiac endothelial receptors dysregulated in our analysis to identify receptor-ligand interactions that were both dysregulated by age. Mapping and visualization of dysregulated receptor-ligand pair were generated using the R package CCInx (https://github.com/BaderLab/CCInx).
Quantitative reverse transcription PCR.
RNA extracted from purified mouse heart, brain and kidney ECs were converted to cDNA using the High Capacity RNA-to-cDNA kit (Applied Biosystems). TaqMan Pre-amplification master mix kit (ThermoFisher) was used to pre-amplify genes of interest and the resulting cDNA was examined using appropriate TaqMan probes from Applied Biosystems detailed in the Major Resources Table in the supplemental materials. Finally, quantitative real-time PCR was performed to quantify the levels of genes of interest from the pre-amplified cDNA using TaqMan Gene Expression Master Mix (ThermoFisher). All experiments were done in triplicates. Gene expression for target genes was normalized to 18S RNA. Statistical analysis of young and aged samples used an unpaired t-test.
Single-cell RNA-seq library prep and sequencing.
Single-cell suspensions enriched for adult cardiac ECs from FACS were loaded into 10× Chromium platform (10× Genomics) with an estimation of ∼1,000 single cell capture. Indexed sequencing libraries of captured single cells were generated using the V2 barcoding chemistry of 10× Genomic library kit in accordance with the manufacturer’s protocol. Library quality was checked on a 2200 TapeStation (Agilent). Libraries were sequenced on a HiSeq2500 (Illumina) using 10× Genomics’ recommended sequencing protocol with a target of 50,000 reads per cell.
Processing of raw single-cell RNA-seq data.
Sample demultiplexing was done using Bcl2Fastq (Illumina), followed by single analysis using Cellranger (10× Genomics). The reads were then aligned to mouse reference genome mm10 with Cellranger 3.0.0.using Cell Ranger Suite default parameters. Supplemental Table S2 contains: the total number of cells analyzed for each sample as well as both median genes and mean reads per cell.
Single-Cell Gene Expression Data Analysis and Identification of Endothelial Subtypes
The raw gene expression data generated for each single cell from CellRanger was used as input to the Seurat R single cell package (Seurat 2.3.4 and R v. 3.4.2).
Cell filtration step.
The CreateSeuratObject function was used to create a Seurat object from the raw gene expression data with genes expressed in <3 cells, as well as cells with <200 detected genes excluded from the object. In addition, the FilterCells function was used to filter out cells with an abnormal unique gene count (<200 or >3,000) and also cells with high mitochondrial gene expression with the high threshold for the percent.mito set at 0.1.
Normalizing gene expression data.
Data were normalized by log transformation of raw count data using the default parameters of the NormalizeData function. After normalization, variable genes underlying data set heterogeneity were identified using the FindVariable gene function with default parameters. ScaleData function was then used to remove variations due to number of detected molecules as well as percentage of mitochondrial gene content.
Cluster identification, visualization, and marker identification.
Linear dimensional reduction using principal component analysis on the variable genes from the scaled data was followed by the FindCluster function which implements a graph-based clustering approach to identify clusters using the first ten principal components as input. Identified clusters were visualized using the RunUMAP function to implement the UMAP algorithm. Top markers for each cluster were identified using the FindAllMarkers function and visualized using the VlnPlot and the FeaturePlot function. A top 10 marker heatmap for each cluster was generated with the DoHeatmap function.
Differential gene analysis between corresponding clusters in young and aged data sets.
Canonical correlation analysis was used to merge single-cell data set of young ECs and aged ECs using the RunCCA function in Seurat. Identification of clusters from the integrated data set was then performed as described previously (see Cluster identification, visualization and marker identification section). Differential gene analysis was performed between young and aged ECs within each cluster with the Seurat FindMarkers function using the Wilcoxon rank sum test for statistical significance.
Immunostaining.
Mice were first sacrificed using cervical dislocation and the hearts were first perfused with Dulbecco’s phosphate buffered saline to remove blood. The perfused hearts were dissected out, perfused with Optimal Cutting Temperature (OCT) compound and immersed in OCT for storage at −80°C until sectioned at 20 µm. For immunostaining, frozen sections were equilibrated at 4°C for 5 min and fixed for 1 h at 4°C with 4% PFA. Fixed tissues were then washed with 1×PBS, blocked with Fish Skin Blocking Buffer (FSSB, 1% BSA, 0.5% TritonX-100, 1 mM TBS 0.1% Cold fish skin gelatin) for 1 h at room temperature (RT) before overnight incubation at 4°C with primary antibody diluted in FSSB. After the overnight incubation, sections were washed 3 times using 1× PBS at RT and subsequently incubated for 2 h at RT with secondary antibodies in FSSB.
RESULTS
Aged Cardiac ECs Do Not Upregulate VCAM1 Expression
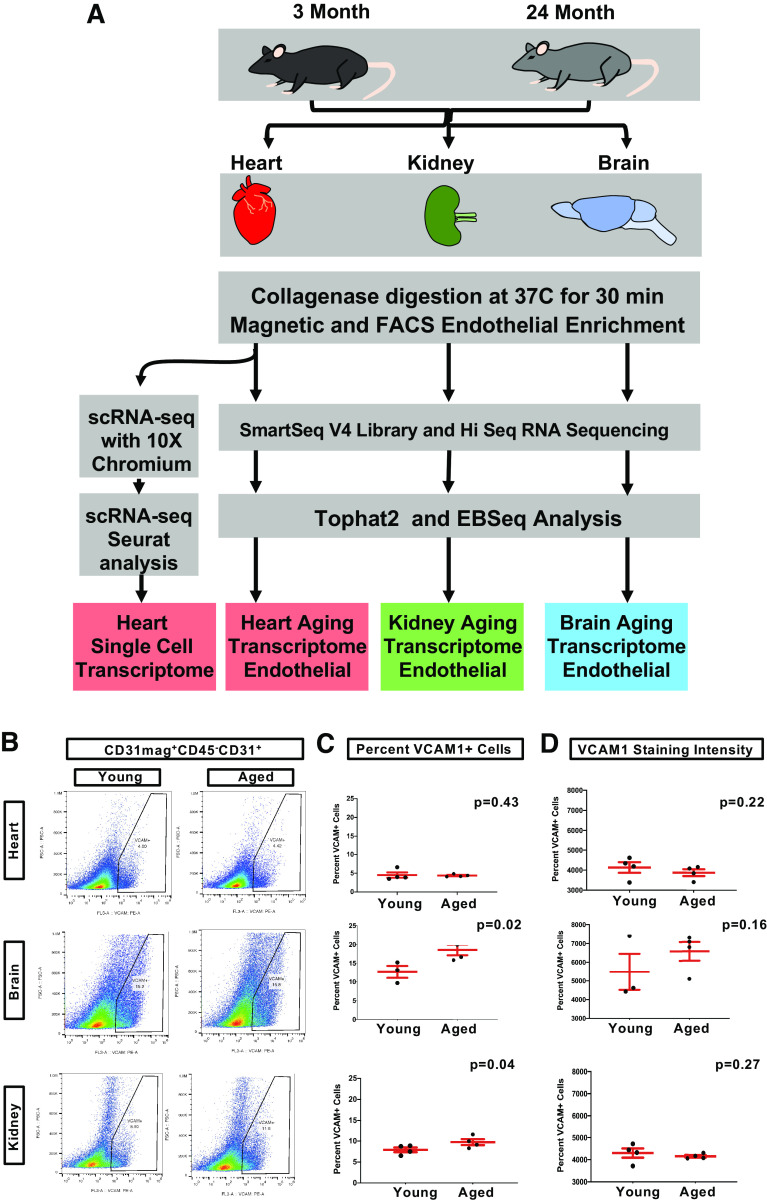
VCAM1 upregulation is reported to promote age-related phenotypes in brain ECs (12). We tested if aged cardiac endothelial cells (cEC) also exhibit an upregulation of VCAM1 expression using flow cytometry. Briefly, ECs were purified from 3 mo (young) and 24 mo (aged) mouse hearts, brains, and kidneys using a combination of magnetic and fluorescence-activated cell sorting (FACS). Fluorescently-labeled VCAM1 antibodies were used to quantify VCAM1 expression levels on ECs from each organ. (Figs. 1, A and B, and Supplemental Fig. S1).
Figure 1.
Purification and analysis of vascular endothelium from heart, brain, and kidney. A: purification workflow to generate enriched endothelial populations using a combination of collagenase digestion, magnetic sorting, and fluorescence activated cell sorting (FACS). B: FACS plots of indicating gating of VCAM1+ cells. C: quantification of the percentage of VCAM1+ endothelial cells from each young and aged organ, n = 4 for heart and n = 3 for brain and kidney. D: quantification of the mean intensity per cell detected during FACS, n = 4. Unpaired t test was applied in both C and D. VCAM1, vascular cell adhesion molecule 1.
Our results indicate that aged cardiac endothelia do not display a significant difference in either percentage of VCAM1+ cells (Fig. 1C) or VCAM1 surface expression levels (Fig. 1D) relative to young cardiac endothelia. However, both aged kidney and brain ECs display increased numbers of VCAM+ positive ECs (Fig. 1B), the latter being consistent with observations from Yousef et al. (12). Thus, aging does not promote VCAM1 upregulation in cardiac ECs in contrast to kidney and brain endothelium, implying that unique age-induced molecular alterations exist in cardiac endothelium distinct from those found in brain and kidney ECs. Bulk RNA-seq was next employed to identify additional age-linked molecular changes that might exist in the cardiac endothelium relative to other vascular beds.
Aged Cardiac Endothelium Exhibits Common and Distinct Transcriptional Alterations Compared with Aged Brain and Kidney Endothelia
Transcriptional differences between the cardiac endothelium of young and aged mice were identified using bulk mRNA, and endothelia from aged brain and kidney were similarly processed to distinguish alterations unique to cardiac ECs from those shared with other vascular ECs. Sequential magnetic sorting and FACS analysis were used to purify ECs from each organ (Fig. 1A) that were subsequently processed for bulk transcriptomic sequencing (see methods). A survey of the transcriptome datasets for endothelial and non-endothelial cell type-enriched genes indicated that our endothelial enrichment strategy was very effective (Supplemental Fig. S2, A and B). Furthermore, each sorted endothelial population showed specificity for previously published endothelial markers for their respective organs: Dickkopf WNT signaling pathway inhibitor 2 (Dkk2) for kidney (16), Mesenchyme homeobox 2 (Meox2) for heart (17), and zinc finger protein of the cerebellum 3 (Zic3) for brain (18) (Supplemental Fig. S2C).
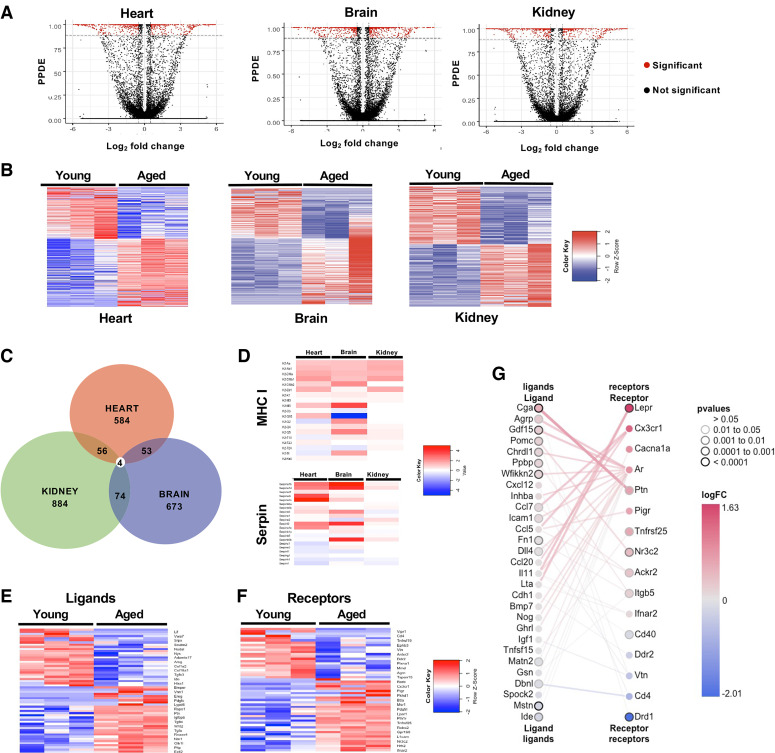
The EBSeq2 differential gene analysis package (19) was used to compare the transcriptomes of young and aged vascular endothelial of each organ and identify genes dysregulated by aging. Volcano plots and heat maps display the differential gene analysis (Fig. 2, A and B, Supplemental Tables S2, S4, and S5). Quantitative PCR (qPCR) validation of the top six altered genes for each tissue indicates a strong concordance between RNA-seq differential gene expression analysis and qPCR measurements in all cases (Supplemental Fig. S2E, Supplemental Table S6). A comparison of differentially expressed genes identified from the aged cardiac endothelium with those found from aged brain and kidney endothelia highlights unique and shared age-induced transcriptional programs (Fig. 2C, Supplemental Table S7). This analysis identified four genes with altered expression across all three vascular endothelial beds [1700034H15Rik, Obscurin-like 1 (Obsl1), MHC class I H2-Q7/Q9, and SerpinA1e; Fig. 2C), and we performed qPCR validation for H2-Q7/Q9 (Supplemental Fig. S2E). Of these four genes, MHC class I H2-Q7/Q9 and SerpinA1e belong to larger gene families, and querying our transcriptome data sets indicates increased MHC class I and Serpina1 gene family members are a property shared by all three vascular beds (Fig. 2D). We also identified common genes shared between only two of the three tissues using a pairwise comparison approach (Fig. 2C, Supplemental Table S7). Interestingly, cardiac endothelial age-affected transcripts were largely unique compared with changes in the brain or kidney endothelium.
Figure 2.
Gene expression comparisons of young and aged endothelial for each organ. A: volcano plots indicating the number and distribution of genes that exhibit both a ≥2 log2-fold change and a posterior probability of differential expression (PPDE) ≥90 (red). B: heat map of differentially expressed genes between young and aged endothelia of each vascular bed (upregulated, red; downregulated, blue). C: Venn diagram indicating gene expression changes shared by one or more endothelial beds. D: heat maps of differential gene expression patterns for the MHC I and Serpin gene family. E and F: heat maps of secreted ligands and receptors altered in aging cardiac endothelium indicating their logFC. G: receptor-ligand pair matrix of age dysregulated genes expressed in endothelial cells. logFC, logarithmic fold change.
A recent study has highlighted the role of brain ECs as sensors of the aging serum milieu (20). To identify age-modified expression of cardiac endothelial receptors and ligands, we screened our cardiac differentially expressed gene set against a database of mammalian receptors and ligands (15). This approach identified a number of endothelial receptors and ligands dysregulated during aging (Fig. 2F). We next compared our list of altered receptor transcripts in cardiac ECs to previously reported age-dependent changes in the circulating proteome (15). Briefly, we identified receptors dysregulated in our aging cardiac data set and mapped them to a published data set of age-dysregulated ligands in the circulatory system(15) using the R package CCInx (https://github.com/BaderLab/CCInx). This analysis identified cases where both ligand and receptor were up- or down-regulated (Fig. 2G), representing one mechanism by which aging may shape the sensitivity of cardiac ECs to circulating factors.
Unique and Shared Genetic Pathways Affected by Aging Are Revealed by GSEA Analysis
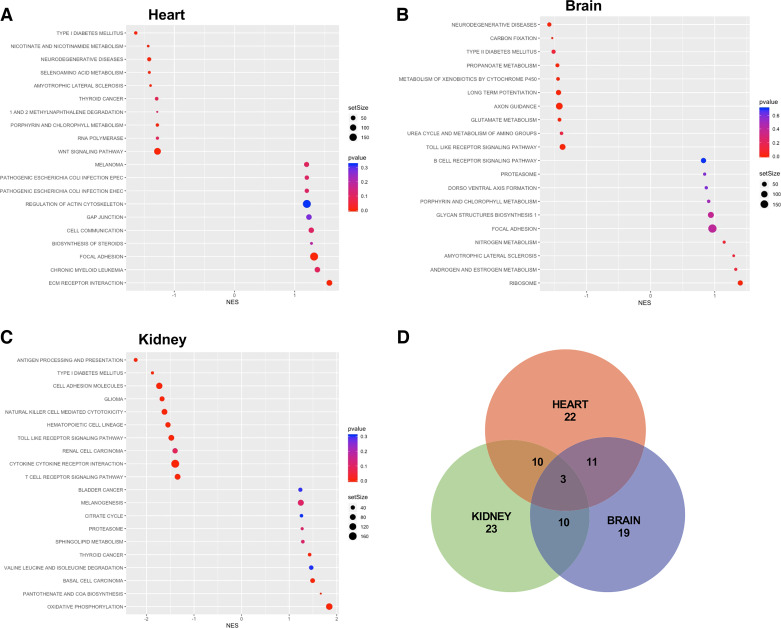
Gene-level analysis indicates most age-induced transcriptional alterations in the cardiac endothelium are largely distinct from those found in either the brain or kidney endothelium. However, this individual gene analysis does not exclude age-induced pathways being shared between endothelial beds. We performed Gene Set Enrichment Analysis (GSEA) to identify enriched pathways in aged ECs from each organ (Fig. 3 and Supplemental Fig. S3). In the cardiac endothelium, GSEA shows that aging downregulates pathways promoting endothelial barrier function (ECM receptor interaction, focal adhesion and gap junction genes) and calcium signaling uptake, while the upregulating pathways are related to Type 1 diabetes and nicotinamide metabolism (Fig. 3A). We compared our GSEA findings in the cardiac endothelium to the pathways identified in either the aged brain (Fig. 3B) or the kidney (Fig. 3C) endothelia. Similar to our observations for individual genes, cardiac ECs share only a limited number of age dysregulated cellular pathways with the brain (∼33%) and kidney (∼35%) ECs (Fig. 3D), underscoring the largely distinct cardiac endothelial cell response to aging relative to the age-related responses of either brain or kidney ECs.
Figure 3.
GSEA pathways analysis of transcriptional changes for each endothelial bed. Top 10 pathways affected by aging with gene expression changes resulting in either up- or downregulation based on Normalized Enrichment Score (NES) relative to expression in young endothelia of the heart (A), brain (B), or kidney (C) with negative scores reflecting pathway enrichment in aged samples and positive scores with reductions. D: Venn diagram of overlapping pathways between organs. GSEA, Gene Set Enrichment Analysis.
Single-Cell Transcriptome Sequencing of Young and Aged Cardiac Endothelium
Our bulk RNA-seq studies identified genes and cellular pathways that are altered in the aged cardiac endothelium, and it is possible that these age-related transcriptional changes are produced by specific endothelial subtypes as the cardiac vascular bed is made up of a heterogenous endothelium (13,21–26). Equally unclear is whether aging alters the homeostatic composition of endothelial subtypes in the cardiac vasculature. We addressed these questions using scRNA-seq (Fig. 1A) analysis to compare the subtype composition and molecular profile of purified ECs in the young and aged cardiac endothelium. Triplicate harvests of each age group (Supplemental Fig. S4A) yielded a total of 13,800 young and 12,940 aged single cardiac ECs. A single-cell transcriptome data set was generated by integrated experimental replicates into a single manifold using Seurat and a single cell data integration anchoring strategy (27), then visualized using the uniform manifold approximation and projection algorithm (UMAP) (28) (Supplemental Fig. S4B).
Classification of Cardiac ECs into Distinct Subtypes
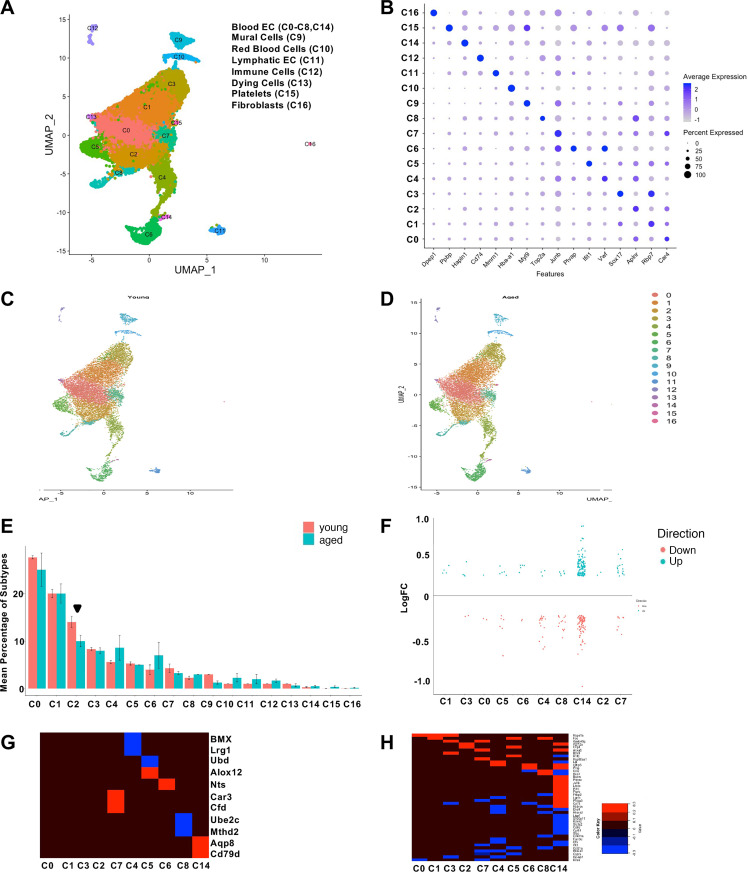
Our clustering analysis identified seventeen distinct cell clusters (Fig. 4, A and B, Supplemental Fig. S5A, Supplemental Table S8). Five clusters consist of nonendothelial cell types based on gene expression patterns: mural cells (C9) expressing myosin light chain 9 (Myl9) (29), red blood cells (C12) identified by expression of hemoglobin (Hba-a1, C10) (30), immune cells as identified by CD74 antigen (Cd74) (31), megakaryocytes/platelets as identified by pro-platelet basic protein (Ppbp) expression (32) (C15), fibroblasts by dipeptidase 1 (Dpep1) (33) (C16) as well as lymphatic ECs identified by multimerin 1 (Mmrn1) expression (34) (C11; Fig. 4B, Supplemental Fig. S5B). Clustering analysis also revealed a relatively small group of compromised/low quality cells as identified by high levels of mitochondrial gene expression (C13; Fig. 4A).
Figure 4.
Single-cell analysis of cardiac endothelial cells and age-dependent shift in endothelial subtype assignment. A: UMAP plot of cluster analysis using Seruat2. B: Dot plot of genes defining one or more cluster. C and D: UMAP plots of young (C) and aged (D) cell distributions in the combined manifold. E: bar graph indicating the percentage of each endothelial population in young (red) and aged (blue) data sets, the significantly different cluster, C2, is denoted by an arrowhead. F: strip chart indicating the logarithmic fold change (logFC) of genes upregulated (blue) or downregulated (red) in each endothelial subtype. G: heat map displaying genes altered in only one cluster and the magnitude of their logFC up- or downregulation, red and blue, respectively (blue). H: heat maps displaying logFC of altered genes detected in multiple endothelial populations. EC, endothelial cell.
The remaining 10 cell clusters exhibited gene expression patterns consistent with ECs including expression of CD31 (PECAM1, platelet/endothelial cell adhesion molecule 1), VEGFR2 (vascular endothelial growth receptor 2)/Kdr and Cadherin 5 (Cdh5) (data not shown). Beyond shared endothelial markers, these remaining cells diverged into ten classes based on marker genes that included carbonic anhydrase 4 (Car4)-enriched ECs (C0), SRY-box 17 (Sox17)-enriched ECs (C1 and C3), and Apelin receptor (Alpr)-enriched ECs (C2 and C7). Similarly, plasmalemma vesicle associated protein (Plvap) expression (35) was associated with endocardial ECs (C6, Fig. 4B, Supplemental Fig. S5B). We also observed a subset of ECs that were positive for transcripts associated with extracellular matrix genes hyaluronan and proteoglycan link protein 1 (Hapln1) (C14, Fig. 4B, Supplemental Fig. S5B), whereas other cells were enriched in proinflammatory genes such as Von Willebrand factor (Vwf) (C4), those enriched for cytokine/interferon signaling genes such as interferon-induced protein with tetratricopeptide repeats 1 (Ifit1) (C5), and cells enriched for proliferation-associated genes [topoisomerase 2a (Top2a), C8] (Fig. 4, A and B, Supplemental Fig. S5B). A subset of these endothelial subtypes were validated in sections of young mouse heart tissue. Ki67 staining confirmed a rare population of proliferating cardiac ECs (Supplemental Fig. S6, A and B), and Plvap in vivo expression confirmed its restriction to the adult endocardium (Supplemental Figs. S5B and S6C), while Vcam1 and Vwf expression were also restricted to certain cardiac ECs in both the myocardium and endocardium (Supplemental Fig. S6, C and D). Sox17- and Car4-positive endothelial populations were also detected using immunehistochemistry (Supplemental Fig. S6, E and F). In summary, our scRNA-seq analysis demonstrates a high degree of cellular diversity among cardiac ECs and can be transcriptionally classified into multiple subtypes typified by proinflammatory, proliferative, and cytokine gene sets.
Specific Cardiac Endothelial Subpopulations Display Age-Induced Compositional Alterations
Our findings highlight the diversity of endothelial subtypes within the heart and raise the possibility that these populations respond distinctly to aging. The population assignments generated from our adult murine cardiac vasculature datasets allowed us to determine if the composition of each cell type cluster is altered by aging. We first split the data set by age (Fig. 4, C and D) and calculated the percent contribution of each cluster to the total cell count for each time-point. Comparison of percentage of cells found in each cluster between young and aged samples reveals a reduction in the proportion of Cluster 2 cells from the aged cardiac endothelium (Fig. 4E, arrowhead). We consistently observe these population-level alterations across our replicate datasets indicating that these findings do not reflect batch artifacts (Supplemental Fig. S7).
Finally, we identified age-related transcriptional changes in each endothelial subtype by performing differential gene analysis between young and aged endothelial subtypes in our data set. The results of this analysis indicate that ECs from the cluster enriched for extracellular matrix transcripts (C14) possess the largest number of differential genes among all cardiac endothelial subtypes (Fig. 4F and Supplemental Table S9). We extended our analysis by identifying age-dysregulated genes that were unique to a particular endothelial cluster (Fig. 4G, Supplemental Table S11) as well as age-dysregulated genes shared across two or more endothelial clusters (Fig. 4H, Supplemental Table S12). In summary, our analysis demonstrates that the aged cardiac vasculature exhibits reduced cell numbers for the endothelial subtype found in Cluster 2.
Integrating Bulk and Single-Cell RNA-Seq Data Sets Reveals Sources of Transcriptional Changes
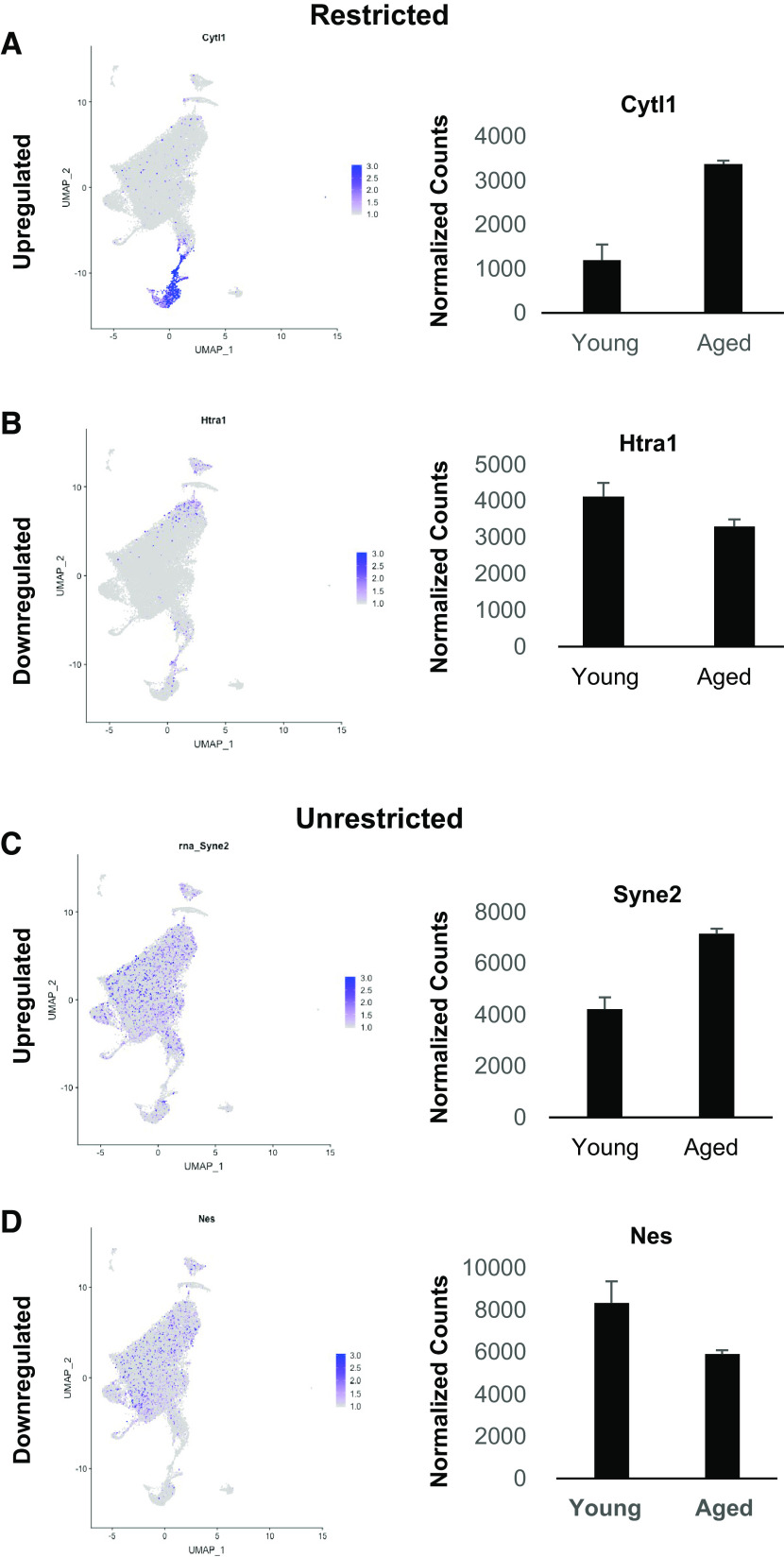
This single-cell analysis of the aged cardiac endothelium provides the opportunity to distinguish the source of transcriptional alterations identified in the bulk aging cardiac endothelial cell data set (Figs. 1–3). We accomplished this convergence by mapping differentially expressed genes identified from the bulk RNA-seq data (mean normalized counts ≥500, fold change ≥2) onto the joint manifold of the single-cell data set. This analysis stratified the age-altered transcripts from the bulk RNA-seq analysis into two groups: genes with expression restricted to a distinct endothelial subtype as exemplified in Fig. 5, A and B, and genes with broad expression across all endothelial cell subtypes as exemplified in Fig. 5, C and D (Supplemental Tables S13, S14, S15, and S16, Supplemental Fig. S7). Interestingly, this combined approach indicates that most of the upregulated genes with restricted expression are localized to clusters 0,1, 4 and 6, whereas the downregulated genes with restricted expression in ECs were observed predominately in clusters 1 through 4 (Supplemental Tables S13 and S14). Using this approach, we show that aging affects the function of the cardiac vasculature by dysregulating unique subsets of genes in distinct endothelial subtypes. We next validated a subset of genes found to be significantly affected in both the bulk and single-cell datasets with quantitative PCR using four independent replicates of young and aged mouse hearts (Fig. 6).
Figure 5.
Examples of cluster mapping of significantly altered gene from bulk RNAseq. A and B: cluster maps and bulk expression for those in subsets of clusters for genes upregulated, (A) Cytokine like 1 (Cytl1) and downregulated (B) Htra serine peptidase (Htra). C and D: other gene sets display a more broad expression for those upregulated such as Spectrin repeat containing, nuclear envelope 2 (Syne2) (C) and downregulated genes such as Nestin (Nes) (D). RNA-seq, RNA sequencing.
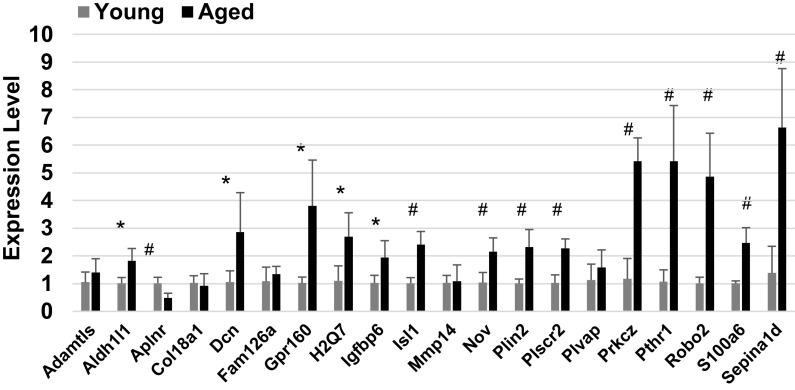
Figure 6.
Quantitative PCR analysis of cardiac endothelial transcripts altered in both bulk RNAseq and Sciseq data sets. Unpaired t test was applied to each gene comparison. *P ≤ 0.05, #P ≤ 0.01, n = 3 per gene per age.
DISCUSSION
In this study, we have defined the changes in the transcriptional profile of aged cardiac ECs relative to other endothelial populations in order to identify messages that are affected similarly between tissues and those alterations uniquely associated with heart endothelia. We accomplished this by leveraging the coverage of bulk RNA-seq and determined the aging response of specific sub-populations of cardiac ECs using the population sampling power of single-cell profiling. Our bulk RNA-seq analysis revealed that a small set of transcripts are significantly affected in all three tissues and a slightly larger number of transcripts were shared between the heart and either the brain or kidney, but not both. However, the vast majority of altered transcripts identified in the heart and other tissues were unique to each organ’s endothelial bed. These observations are consistent with the notion that individual organs have distinct functional demands that are reflected in broad-spectrum gene expression changes to maintain organ-specific vascular homeostasis.
A number of studies have applied transcriptomic profiling to explore aging in various organs systems either in broad surveys of whole tissue transcriptomes (5–7) or focusing on a single cell type via selective purification (10), or utilizing high throughput single-cell techniques (8). These studies have identified key gene expression patterns associated with aging, including those reflecting the challenges faced by the vasculature during natural aging such as increased blood pressure, tissue remodeling/fibrosis, inflammatory challenge/burden and metabolic stress. Our analysis revealed four genes that were shared by all three vascular beds: 1700034H15Rik, Obsl1, MHC Class I H2-Q7, and SerpinA1e. 1700034H15Rik is a broadly expressed, long non-coding RNA of unknown function. OBSL1 is a cytoskeletal protein that is downregulated in endothelial transcriptomes from all three tissues which would have an impact on both microtubule and ultimately genome stability (36). Endothelial H2-Q7 expression has been reported to be upregulated in response to infection (37) and is observed to be increased in aged pneumocytes (38), perhaps reflecting pathogenic challenges across life span. The functional significance of MHC Class I upregulation in aged endothelia could relate to antigen presentation as seen in transplant rejection (39), and autoimmunity (40) or immune cell extravasation. Previous studies have found the MHC engagement elicits endothelial contraction that modifies barrier functions, facilitating diapedesis, tissue surveillance or immune responses (41,42). This potentially decreased barrier function may represent compensation for reduced immune system function in advanced age (43) as well as altered expression of circulating immune modulators as described below. Interestingly, a recent study of whole organ aging has also reported immune response genes being upregulated in aging (44).
The SerpinA1e gene is part of a large gene duplication region in mice that produced six highly homologous proteins, all similar to human SerpinA1 (Alpha-1 Antitrypsin), an established antiangiogenic protease inhibitor (45). Age-dependent emphysema has been noted in mice lacking the Serpins A1a-e (46); however, no other overt longevity or vascular phenotypes were observed. MHCI H2-Q7 and SeprinA1E are members of larger gene families whose members are fairly consistently up or down regulated across tissues with aged endothelium (Fig. 2E), with the most robust responses observed in cardiac and brain endothelia. Human genetic studies have linked increased life span with loss of another member of the Serpin family, SerpinE1, but the mechanisms underlying this effect remain unclear (47). Although we do not detect significant changes of SerpinE1 in aged cardiac ECs, a recent report indicates that fibroblasts upregulate both Serpin E1 and E2 in older hearts (48). These observations may represent cell type specific responses to aging via a common mechanism of protease inhibition. Interestingly, the upregulated Serpin family of genes in cardiac ECs corresponds to those associated with antiangiogenic activity and those downregulated with either pro-angiogenic or functions linked to other vascular events (49). These findings may imply a coordinated suppression of angiogenesis in aging vascular endothelia that correlates with previously described reductions in angiogenesis with age (50). However, it will be important to determine in future studies whether each Serpin is released locally to affect extra-cellular remodeling or into the bloodstream with distant effects on vasculature function.
Although our findings clearly demonstrate common gene expression patterns for aging ECs between one or more vascular beds, they also highlight the unique nature of cardiac endothelial cell aging. It is well established that age is a significant risk factor for cardiac disease (51), but the contribution of the individual cellular components of the heart to age-associated risk is only beginning to be understood. This study defined a set of age-sensitive transcripts that may be unique to cardiac endothelia, which manifest in pathway analysis as unique differences between the heart and other vascular beds, thus revealing potential targets for interventions to slow the progression of age-related decline in endothelial function.
ECs are ideally positioned to constantly sample the circulation, serving as sensitive detectors of changes occurring in the circulating serum proteome. Our results indicate that not only are the soluble ligands produced by ECs altered by aging, but so are receptors sensitive to the age-altered blood factors described by others, including compositional alterations in both aged rodents (15,52,53) and humans (15,54). These studies establish clear signatures of aging in the circulating proteome and an additional study has found that brain ECs are also responsive to these alterations in blood content (20). Conceptually, our findings could reflect a synergy between these age-related changes in circulating factors and receptor repertoire alterations occurring in cardiac ECs driving the cells further from functional homeostasis. Future studies will be required to understand specifically how these age-dysregulated cardiac endothelial ligands and receptors may contribute to age-associated cardiovascular diseases.
Finally, we used single-cell transcriptome sequencing to profile endothelial cells from young and aged mouse hearts. Our data set represents, to date, the largest number of cardiac ECs profiled by scRNA-seq, allowing a number of observations regarding the effects of aging on endothelial cell subtypes. First, we were able to distinguish 9 distinct cardiac endothelial cell subtypes, providing clear markers for each of these populations. Second, we did not observe any divergence in endothelial identity between young and aged hearts. Third, despite this maintenance of identity clustering, we find that the number of cells in Aplnr positive clusters decreased in the aged samples. Fourth, we also identified age-related alterations that were distinct to each cluster, which indicates that beyond tissue-specific endothelial age-dependent alterations found in our bulk RNA-seq, cardiac endothelial subtypes mount distinct responses to aging. Interestingly, our single cell data highlighted gene expression changes associated with specific cardiac endothelial sub-populations that could be detected in our bulk RNA-seq data. Similarly, we were able to map transcripts found in our bulk RNA-seq to individual cardiac endothelial clusters. This indicates that relatively small numbers of cells can undergo significant transcriptional changes during aging that might otherwise be considered a global endothelial response, highlighting the power of combining high coverage RNA-seq with single transcriptomics.
Organisms from the fly (55) and mouse (9,44,56) to humans (57–59) have been surveyed for age-related gene expression changes in the heart, with most studies focused on transcriptional alterations in the whole organ. Our findings offer unique insights into transcriptional signatures of endothelial aging, and future increases in cellular throughput and transcript coverage will undoubtedly provide more clarity as to the molecular forces driving cardiovascular decline with advancing age. Here, we have attempted to define cardiac aging in the context of the endothelial transcriptome to produce a clearer picture of how this specific cell population within the heart adapts to the evolving challenges of aging. This work contributes to efforts aimed at understanding the cellular factors undermining each organ system during aging. It reveals genes potentially responsible for these physiologic compromises that could be future targets for slowing or reversing the endothelial aging process.
DATA AVAILABILITY
All data and materials that support this study have been made publicly available at NCBI and can be accessed at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE163823
SUPPLEMENTAL DATA
Supplemental Figs. S1–S7: https://doi.org/10.6084/m9.figshare.14589597.v1.
Supplemental Tables S1–S16: https://doi.org/10.6084/m9.figshare.14402486.v1.
GRANTS
This work was funded by the Knight Cardiovascular Institute, National Institute of General Medical Sciences Grant R35GM124704 and National Institute on Aging Grant 1RF1AG058273-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
U.E., J.W.N., N.J.A., S.K., A.C.A., and A.P.B. conceived and designed research; U.E., J.W.N., and A.P.B. performed experiments; U.E., J.W.N., A.C.A., and A.P.B. analyzed data; U.E., J.W.N., and A.P.B. interpreted results of experiments; U.E., J.W.N., and A.P.B. prepared figures; U.E., J.W.N., and A.P.B. drafted manuscript; U.E., J.W.N., N.J.A., S.K., and A.P.B. edited and revised manuscript; U.E., J.W.N., N.J.A., S.K., A.C.A., and A.P.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The bulk RNA sequencing and single-cell RNA sequencing were performed by the Oregon Health and Science University Massively Parallel Sequencing Shared Resource. We thank Dr. Sarah Santiago, PhD, for her assistance in graph generation, Dr. Helen Liu, D.V.M., of the Association for Assessment and Accreditation of Laboratory Animal Care Histology Core for her assistance in sectioning and mounting cryo-preserved heart tissue sections for staining, and Dr. Paul Muller for his advice on cardiac tissue preparation and immunostaining.
References
- 1.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrera MD, Mingorance C, Rodriguez-Rodriguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res Rev 9: 142–152, 2010. doi: 10.1016/j.arr.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Hill CE, Phillips JK, Sandow SL. Heterogeneous control of blood flow amongst different vascular beds. Med Res Rev 21: 1–60, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 4.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 91: 3527–3561, 1998. [PubMed] [Google Scholar]
- 5.Yu Y, Fuscoe JC, Zhao C, Guo C, Jia M, Qing T, Bannon DI, Lancashire L, Bao W, Du T, Luo H, Su Z, Jones WD, Moland CL, Branham WS, Qian F, Ning B, Li Y, Hong H, Guo L, Mei N, Shi T, Wang KY, Wolfinger RD, Nikolsky Y, Walker SJ, Duerksen-Hughes P, Mason CE, Tong W, Thierry-Mieg J, Shi L, Wang C. A rat RNA-Seq transcriptomic BodyMap across 11 organs and 4 developmental stages. Nat Commun 5: 3230, 2014. doi: 10.1038/ncomms4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shavlakadze T, Morris M, Fang J, Wang SX, Zhu J, Zhou W, Tse HW, Mondragon-Gonzalez R, Roma G, Glass DJ. Age-related gene expression signature in rats demonstrate early, late, and linear transcriptional changes from multiple tissues. Cell Rep 28: 3263–3273.e3, 2019. doi: 10.1016/j.celrep.2019.08.043. [DOI] [PubMed] [Google Scholar]
- 7.Zahn JM, Poosala S, Owen AB, Ingram DK, Lustig A, Carter A, Weeraratna AT, Taub DD, Gorospe M, Mazan-Mamczarz K, Lakatta EG, Boheler KR, Xu X, Mattson MP, Falco G, Ko MS, Schlessinger D, Firman J, Kummerfeld SK, Wood WH 3rd, Zonderman AB, Kim SK, Becker KG. AGEMAP: a gene expression database for aging in mice. PLoS Genet 3: e201, 2007. doi: 10.1371/journal.pgen.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaum N, Lehallier B, Hahn O, Palovics R, Hosseinzadeh S, Lee SE, Sit R, Lee DP, Losada PM, Zardeneta ME, Fehlmann T, Webber JT, McGeever A, Calcuttawala K, Zhang H, Berdnik D, Mathur V, Tan W, Zee A, Tan M, Tabula Muris C, Pisco AO, Karkanias J, Neff NF, Keller A, Darmanis S, Quake SR, Wyss-Coray T; Tabula Muris Consortium. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 583: 596–602, 2020. doi: 10.1038/s41586-020-2499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimmel JC, Penland L, Rubinstein ND, Hendrickson DG, Kelley DR, Rosenthal AZ. Murine single-cell RNA-seq reveals cell-identity- and tissue-specific trajectories of aging. Genome Res 29: 2088–2103, 2019. doi: 10.1101/gr.253880.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Liu M, Li Q, Shen B, Hu C, Fu R, Liu M, Deng J, Cao Q, Wang Y, Wang Y. Identification of differential gene expression in endothelial cells from young and aged mice using RNA-Seq technique. Am J Transl Res 11: 6553–6560, 2019. [PMC free article] [PubMed] [Google Scholar]
- 11.Tabula Muris C. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583: 590–595, 2020. doi: 10.1038/s41586-020-2496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yousef H, Czupalla CJ, Lee D, Chen MB, Burke AN, Zera KA, Zandstra J, Berber E, Lehallier B, Mathur V, Nair RV, Bonanno LN, Yang AC, Peterson T, Hadeiba H, Merkel T, Korbelin J, Schwaninger M, Buckwalter MS, Quake SR, Butcher EC, Wyss-Coray T. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat Med 25: 988–1000, 2019. doi: 10.1038/s41591-019-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med 2: a006429, 2012. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550, 2005. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehallier B, Gate D, Schaum N, Nanasi T, Lee SE, Yousef H, Moran Losada P, Berdnik D, Keller A, Verghese J, Sathyan S, Franceschi C, Milman S, Barzilai N, Wyss-Coray T. Undulating changes in human plasma proteome profiles across the lifespan. Nat Med 25: 1843–1850, 2019. doi: 10.1038/s41591-019-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O, Rafii S. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 26: 204–219, 2013. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppiello G, Collantes M, Sirerol-Piquer MS, Vandenwijngaert S, Schoors S, Swinnen M, Vandersmissen I, Herijgers P, Topal B, van Loon J, Goffin J, Prosper F, Carmeliet P, Garcia-Verdugo JM, Janssens S, Penuelas I, Aranguren XL, Luttun A. Meox2/Tcf15 heterodimers program the heart capillary endothelium for cardiac fatty acid uptake. Circulation 131: 815–826, 2015. doi: 10.1161/CIRCULATIONAHA.114.013721. [DOI] [PubMed] [Google Scholar]
- 18.Hupe M, Li MX, Kneitz S, Davydova D, Yokota C, Kele J, Hot B, Stenman JM, Gessler M. Gene expression profiles of brain endothelial cells during embryonic development at bulk and single-cell levels. Sci Signal 10: eaag2476, 2017. doi: 10.1126/scisignal.aag2476. [DOI] [PubMed] [Google Scholar]
- 19.Leng N, Dawson JA, Thomson JA, Ruotti V, Rissman AI, Smits BM, Haag JD, Gould MN, Stewart RM, Kendziorski C. EBSeq: an empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 29: 1035–1043, 2013. [Erratum in Bioinformatics 29: 2073, 2013]. doi: 10.1093/bioinformatics/btt087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen MB, Yang AC, Yousef H, Lee D, Chen W, Schaum N, Lehallier B, Quake SR, Wyss-Coray T. Brain endothelial cells are exquisite sensors of age-related circulatory cues. Cell Rep 30: 4418–4432.e4, 2020. doi: 10.1016/j.celrep.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Q, Eichten A, Parveen A, Adler C, Huang Y, Wang W, Ding Y, Adler A, Nevins T, Ni M, Wei Y, Thurston G. Single-cell transcriptome analyses reveal endothelial cell heterogeneity in tumors and changes following antiangiogenic treatment. Cancer Res 78: 2370–2382, 2018. doi: 10.1158/0008-5472.CAN-17-2728. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Solomonidis EG, Meloni M, Taylor RS, Duffin R, Dobie R, Magalhaes MS, Henderson BEP, Louwe PA, D'Amico G, Hodivala-Dilke KM, Shah AM, Mills NL, Simons BD, Gray GA, Henderson NC, Baker AH, Brittan M. Single-cell transcriptome analyses reveal novel targets modulating cardiac neovascularization by resident endothelial cells following myocardial infarction. Eur Heart J 40: 2507–2520, 2019. doi: 10.1093/eurheartj/ehz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng W, Chen L, Nguyen PK, Wu SM, Li G. Single cell analysis of endothelial cells identified organ-specific molecular signatures and heart-specific cell populations and molecular features. Front Cardiovasc Med 6: 165, 2019. doi: 10.3389/fcvm.2019.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Litviňuková M, Talavera-López C, Maatz H, Reichart D, Worth CL, Lindberg EL, Kanda M, Polanski K, Heinig M, Lee M, Nadelmann ER, Roberts K, Tuck L, Fasouli ES, DeLaughter DM, McDonough B, Wakimoto H, Gorham JM, Samari S, Mahbubani KT, Saeb-Parsy K, Patone G, Boyle JJ, Zhang H, Zhang H, Viveiros A, Oudit GY, Bayraktar OA, Seidman JG, Seidman CE, Noseda M, Hubner N, Teichmann SA. Cells of the adult human heart. Nature 588: 466–472, 2020. doi: 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jambusaria A, Hong Z, Zhang L, Srivastava S, Jana A, Toth PT, Dai Y, Malik AB, Rehman J. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. eLife 9: e51413, 2020. doi: 10.7554/eLife.51413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paik DT, Tian L, Williams IM, Rhee S, Zhang H, Liu C, Mishra R, Wu SM, Red-Horse K, Wu JC. Single-cell RNA sequencing unveils unique transcriptomic signatures of organ-specific endothelial cells. Circulation 142: 1848–1862, 2020. doi: 10.1161/CIRCULATIONAHA.119.041433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, Hao Y, Stoeckius M, Smibert P, Satija R. Comprehensive integration of single-cell data. Cell 177: 1888–1902.e21, 2019. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becht E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG, Ginhoux F, Newell EW. Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol 37: 38–44, 2019. doi: 10.1038/nbt.4314. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Chen W, Li W, Li Y, Priest JR, Zhou B, Wang J, Zhou Z. Single-cell RNA-seq of the developing cardiac outflow tract reveals convergent development of the vascular smooth muscle cells. Cell Rep 28: 1346–1361.e4, 2019. doi: 10.1016/j.celrep.2019.06.092. [DOI] [PubMed] [Google Scholar]
- 30.Saha D, Patgaonkar M, Shroff A, Ayyar K, Bashir T, Reddy KV. Hemoglobin expression in nonerythroid cells: novel or ubiquitous? Int J Inflam 2014: 803237, 2014. doi: 10.1155/2014/803237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeiner PS, Preusse C, Blank AE, Zachskorn C, Baumgarten P, Caspary L, Braczynski AK, Weissenberger J, Bratzke H, Reiss S, Pennartz S, Winkelmann R, Senft C, Plate KH, Wischhusen J, Stenzel W, Harter PN, Mittelbronn M. MIF receptor CD74 is restricted to microglia/macrophages, associated with a M1-polarized immune milieu and prolonged patient survival in gliomas. Brain Pathol 25: 491–504, 2015. doi: 10.1111/bpa.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C, Thornton MA, Kowalska MA, Sachis BS, Feldman M, Poncz M, McKenzie SE, Reilly MP. Localization of distal regulatory domains in the megakaryocyte-specific platelet basic protein/platelet factor 4 gene locus. Blood 98: 610–617, 2001. doi: 10.1182/blood.v98.3.610. [DOI] [PubMed] [Google Scholar]
- 33.Forte E, Skelly DA, Chen M, Daigle S, Morelli KA, Hon O, Philip VM, Costa MW, Rosenthal NA, Furtado MB. Dynamic interstitial cell response during myocardial infarction predicts resilience to rupture in genetically diverse mice. Cell Rep 30: 3149–3163.e6, 2020. doi: 10.1016/j.celrep.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang M, Grosso RA, Takeda A, Pan J, Bekkhus T, Brulois K, Dermadi D, Nordling S, Vanlandewijck M, Jalkanen S, Ulvmar MH, Butcher EC. A single-cell transcriptional roadmap of the mouse and human lymph node lymphatic vasculature. Front Cardiovasc Med 7: 52, 2020. doi: 10.3389/fcvm.2020.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrnberger L, Seitz R, Kuespert S, Bosl MR, Fuchshofer R, Tamm ER. Lack of endothelial diaphragms in fenestrae and caveolae of mutant Plvap-deficient mice. Histochem Cell Biol 138: 709–724, 2012. doi: 10.1007/s00418-012-0987-3. [DOI] [PubMed] [Google Scholar]
- 36.Jackson PK. Regulating microtubules and genome stability via the CUL7/3M syndrome complex and CUL9. Mol Cell 54: 713–715, 2014. doi: 10.1016/j.molcel.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 37.Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J Immunol 178: 6017–6022, 2007. doi: 10.4049/jimmunol.178.10.6017. [DOI] [PubMed] [Google Scholar]
- 38.Angelidis I, Simon LM, Fernandez IE, Strunz M, Mayr CH, Greiffo FR, Tsitsiridis G, Ansari M, Graf E, Strom TM, Nagendran M, Desai T, Eickelberg O, Mann M, Theis FJ, Schiller HB. An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat Commun 10: 963, 2019. doi: 10.1038/s41467-019-08831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walch JM, Zeng Q, Li Q, Oberbarnscheidt MH, Hoffman RA, Williams AL, Rothstein DM, Shlomchik WD, Kim JV, Camirand G, Lakkis FG. Cognate antigen directs CD8+ T cell migration to vascularized transplants. J Clin Invest 123: 2663–2671, 2013. doi: 10.1172/JCI66722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savinov AY, Wong FS, Stonebraker AC, Chervonsky AV. Presentation of antigen by endothelial cells and chemoattraction are required for homing of insulin-specific CD8+ T cells. J Exp Med 197: 643–656, 2003. doi: 10.1084/jem.20021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma L, Cheung KC, Kishore M, Nourshargh S, Mauro C, Marelli-Berg FM. CD31 exhibits multiple roles in regulating T lymphocyte trafficking in vivo. J Immunol 189: 4104–4111, 2012. doi: 10.4049/jimmunol.1201739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheung KCP, Fanti S, Mauro C, Wang G, Nair AS, Fu H, Angeletti S, Spoto S, Fogolari M, Romano F, Aksentijevic D, Liu W, Li B, Cheng L, Jiang L, Vuononvirta J, Poobalasingam TR, Smith DM, Ciccozzi M, Solito E, Marelli-Berg FM. Preservation of microvascular barrier function requires CD31 receptor-induced metabolic reprogramming. Nat Commun 11: 3595, 2020. doi: 10.1038/s41467-020-17329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikolich-Zugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol 19: 10–19, 2018. doi: 10.1038/s41590-017-0006-x. [DOI] [PubMed] [Google Scholar]
- 44.Bartling B, Niemann K, Pliquett RU, Treede H, Simm A. Altered gene expression pattern indicates the differential regulation of the immune response system as an important factor in cardiac aging. Exp Gerontol 117: 13–20, 2019. doi: 10.1016/j.exger.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Huang H, Campbell SC, Nelius T, Bedford DF, Veliceasa D, Bouck NP, Volpert OV. Alpha1-antitrypsin inhibits angiogenesis and tumor growth. Int J Cancer 112: 1042–1048, 2004. doi: 10.1002/ijc.20494. [DOI] [PubMed] [Google Scholar]
- 46.Borel F, Sun H, Zieger M, Cox A, Cardozo B, Li W, Oliveira G, Davis A, Gruntman A, Flotte TR, Brodsky MH, Hoffman AM, Elmallah MK, Mueller C. Editing out five Serpina1 paralogs to create a mouse model of genetic emphysema. Proc Natl Acad Sci USA 115: 2788–2793, 2018. doi: 10.1073/pnas.1713689115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan SS, Shah SJ, Klyachko E, Baldridge AS, Eren M, Place AT, Aviv A, Puterman E, Lloyd-Jones DM, Heiman M, Miyata T, Gupta S, Shapiro AD, Vaughan DE. A null mutation in SERPINE1 protects against biological aging in humans. Sci Adv 3: eaao1617, 2017. doi: 10.1126/sciadv.aao1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vidal R, Wagner JUG, Braeuning C, Fischer C, Patrick R, Tombor L, Muhly-Reinholz M, John D, Kliem M, Conrad T, Guimaraes-Camboa N, Harvey R, Dimmeler S, Sauer S. Transcriptional heterogeneity of fibroblasts is a hallmark of the aging heart. JCI Insight 4: e131092, 2019. doi: 10.1172/jci.insight.131092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cierniewski CS, Boncela J. Serpins in angiogenesis. In: Angiogenesis and Vascularisation, edited by Dulak A J., Józkowicz A.. Vienna, Australia: ŁobodaSpringer-Verlag Wien, 2013, p. 101–118. doi: 10.1007/978-3-7091-1428-5_5. [DOI] [Google Scholar]
- 50.Lahteenvuo J, Rosenzweig A. Effects of aging on angiogenesis. Circ Res 110: 1252–1264, 2012. doi: 10.1161/CIRCRESAHA.111.246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiao YA, Rabinovitch PS. The aging heart. Cold Spring Harb Perspect Med 5: a025148, 2015. doi: 10.1101/cshperspect.a025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding J, Kopchick JJ. Plasma biomarkers of mouse aging. Age (Dordr) 33: 291–307, 2011. doi: 10.1007/s11357-010-9179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bell-Temin H, Yousefzadeh MJ, Bondarenko A, Quarles E, Jones-Laughner J, Robbins PD, Ladiges W, Niedernhofer LJ, Yates NA. Measuring biological age in mice using differential mass spectrometry. Aging (Albany, NY) 11: 1045–1061, 2019. doi: 10.18632/aging.101810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka T, Biancotto A, Moaddel R, Moore AZ, Gonzalez-Freire M, Aon MA, Candia J, Zhang P, Cheung F, Fantoni G, Consortium CHI, Semba RD, Ferrucci L; CHI consortium. Plasma proteomic signature of age in healthy humans. Aging Cell 17: e12799, 2018. doi: 10.1111/acel.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cannon L, Zambon AC, Cammarato A, Zhang Z, Vogler G, Munoz M, Taylor E, Cartry J, Bernstein SI, Melov S, Bodmer R. Expression patterns of cardiac aging in Drosophila. Aging Cell 16: 82–92, 2017. doi: 10.1111/acel.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee CK, Allison DB, Brand J, Weindruch R, Prolla TA. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc Natl Acad Sci USA 99: 14988–14993, 2002. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang J, Huang T, Petralia F, Long Q, Zhang B, Argmann C, Zhao Y, Mobbs CV, Schadt EE, Zhu J, Tu Z, Consortium GT; GTEx Consortium. Synchronized age-related gene expression changes across multiple tissues in human and the link to complex diseases. Sci Rep 5: 15145, 2015. [Erratum in Sci Rep 6: 19384, 2016]. doi: 10.1038/srep15145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng L, Yang J, Peng S, Zhu J, Zhang B, Suh Y, Tu Z. Transcriptome analysis reveals the difference between “healthy” and “common” aging and their connection with age-related diseases. Aging Cell 19: e13121, 2020. doi: 10.1111/acel.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asp M, Giacomello S, Larsson L, Wu C, Furth D, Qian X, Wardell E, Custodio J, Reimegard J, Salmen F, Osterholm C, Stahl PL, Sundstrom E, Akesson E, Bergmann O, Bienko M, Mansson-Broberg A, Nilsson M, Sylven C, Lundeberg J. A spatiotemporal organ-wide gene expression and cell atlas of the developing human heart. Cell 179: 1647–1660.e19, 2019. doi: 10.1016/j.cell.2019.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials that support this study have been made publicly available at NCBI and can be accessed at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE163823