Abstract
Many cancer cells, regardless of their tissue origin or genetic landscape, have increased expression or activity of the plasma membrane Na-H exchanger NHE1 and a higher intracellular pH (pHi) compared with untransformed cells. A current perspective that remains to be validated is that increased NHE1 activity and pHi enable a Warburg-like metabolic reprogramming of increased glycolysis and decreased mitochondrial oxidative phosphorylation. We tested this perspective and find it is not accurate for clonal pancreatic and breast cancer cells. Using the pharmacological reagent ethyl isopropyl amiloride (EIPA) to inhibit NHE1 activity and decrease pHi, we observe no change in glycolysis, as indicated by secreted lactate and intracellular pyruvate, despite confirming increased activity of the glycolytic enzyme phosphofructokinase-1 at higher pH. Also, in contrast to predictions, we find a significant decrease in oxidative phosphorylation with EIPA, as indicated by oxygen consumption rate (OCR). Decreased OCR with EIPA is not associated with changes in pathways that fuel oxidative phosphorylation or with mitochondrial membrane potential but occurs with a change in mitochondrial dynamics that includes a significant increase in elongated mitochondrial networks, suggesting increased fusion. These findings conflict with current paradigms on increased pHi inhibiting oxidative phosphorylation and increased oxidative phosphorylation being associated with mitochondrial fusion. Moreover, these findings raise questions on the suggested use of EIPA-like compounds to limit metabolic reprogramming in cancer cells.
Keywords: cancer metabolism, intracellular pH, lactate, mitochondria, NHE1, oxidative phosphorylation
INTRODUCTION
Metabolic reprogramming is considered a hallmark of cancer cells that often includes increased glycolysis and decreased mitochondrial oxidative phosphorylation (1, 2) to generate biomass for fueling rapid proliferation. Although it is widely accepted that cancer cells predominantly convert glucose to lactate, either due to increased expression of the converting enzyme lactate dehydrogenase A (LDHA) (3, 4) or an impaired mitochondrial pyruvate import carrier (5), the functional status of mitochondria in cancer cells remains controversial. Cancer cells are reported to have dysfunctional mitochondria (6–8) but in contrast, the TCA cycle in mitochondria often remains a major source for ATP and functional mitochondria are essential for cancer cells to survive during tumor progression (2, 9, 10).
Another distinctive feature of most cancer cells is a constitutively higher intracellular pH (pHi) compared with untransformed cells (11–14). Although initially considered a conundrum based on metabolic acids produced by rapidly proliferating cancer cells, the higher pHi of cancer cells is now known to be in-part determined by increased activity or expression of acid extruding plasma membrane ion transporters, including the Na-H exchanger, NHE1, monocarboxylate transporters (MCTs), Na+/HCO3− co-transporters, and V-ATPases (11, 12, 15, 16). The higher pHi of cancer cells is suggested to enable metabolic reprogramming and a shift from reliance on oxidative phosphorylation to glycolysis or glutaminolysis (11–14); however, this has not been conclusively determined. In addition, metabolic (17–19) and pHi (20, 21) heterogeneity occurs in cancers but whether or how these parameters might be linked in determining their heterogeneity has received limited attention (20, 22). These considerations are important because tumor heterogeneity is a challenge for effective cancer therapies.
We addressed a number of questions on pHi dynamics and cancer cell metabolism in studies using human clonal pancreatic cancer BxPC3 cells and breast cancer MDA-MB-157 cells. We focused on these cell types because our initial studies indicated that BxPC3 but not MDA-MB-157 cells show a metabolic reprogramming of increased glycolysis and decreased oxidative phosphorylation compared with untransformed tissue-matched clonal epithelial cells, which give us models with contrasting metabolic profiles. In addition, emerging findings indicate that features of metabolic reprogramming are different across cancer subtypes (23). We found that pharmacologically inhibiting NHE1 and lowering pHi has no effect on glycolysis but markedly decreases oxidative phosphorylation and increases mitochondrial fusion to a more tubular network. These effects are seen in both clonal cancer cells as well as in clonal untransformed pancreatic and mammary epithelial cells. Our findings challenge two current views; first, that increased activity of NHE1 and a higher pHi enable glycolysis and limit oxidative phosphorylation in cancer cells, and second, that mitochondrial fusion is associated with increased oxidative phosphorylation.
MATERIALS AND METHODS
Cell Lines
Clonal BxPC3 human pancreatic cancer cells obtained from Rushika Perera (UCSF) and H1299 human lung cancer cells obtained from ATCC were maintained in RPMI-1640 medium supplemented with 10% FBS, PANC-1 human pancreatic cancer cells obtained from Rushika Perera were maintained in DMEM supplemented with 10% FBS, and untransformed human pancreatic ductal epithelial (HPDE) cells, also obtained from Rushika Perera, were maintained in keratinocyte medium (Thermo Fisher, 37010022) supplemented with 10% FBS. MDA-MB-157 and MDA-MB-453 triple negative human breast cancer cells obtained from ATCC were maintained in Leibovitz L15 medium supplemented with 15% FBS in the absence of CO2, and MCF10A untransformed human mammary epithelial cells obtained from Jay Debnath (UCSF) were maintained as previously described (24). Cell lines were confirmed to be mycoplasma free as tested by RT-PCR (Mycoplasma Detection Kit abm No. G238) and were authenticated commercially by IDEXX BioAnalytics.
Mitochondrial Stress and Fuel Assays
Mitochondrial oxygen consumption rate (OCR) was determined using a mito-stress assay and measured with an XF24 flux analyzer (Agilent Technologies). Cells were seeded at densities optimized to obtain a linear basal OCR between 100 and 300 pmol/min. Effects of the selective NHE1 inhibitor 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) on OCR was determined after maintaining cells in growth medium containing 10 μM EIPA for 18–20 h. Growth medium was removed and washed with nonbuffered mito-stress test assay medium (DMEM basal medium supplemented where indicated with glucose, glutamine, and sodium pyruvate). MDA-MB-157 cells, grown in atmospheric conditions were washed and maintained in assay medium for 2 h before measuring OCR. Basal OCR was measured, followed by measurements with the addition of a series of mito-stress compounds as indicated by Agilent Technologies specifications (https://www.agilent.com/en/support/cell-analysis/mitochondrial-respiration-xf-cell-mito-stress-test), including successively oligomycin (1 μM), FCCP (1 μM), rotenone (1 μM), and antimycin (1 μM). OCR was expressed relative to the number of cells in each well determined at the time of the assay. Cellular ATP was determined by a luminescence assay (Cayman Chemicals Detection Assay 700410) according to manufacturer’s specifications.
Intracellular pH and NHE1 Activity
Steady-state pHi was measured in cells plated for 48 h in 24-well plates and loaded with the pH-sensitive dye 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF; Invitrogen) in a HCO3-containing buffer as previously described (25). Ratios of BCECF fluorescence at Ex490/Em530 (pH-sensitive) and Ex440/Em530 (pH-insensitive) were measured using a SpectraMax plate reader (Molecular Devices) and calibrated to pHi by treating cells at the end of each measurement in a potassium phosphate buffer containing the protonophore nigericin (10 μM) at pH 6.5 and 7.5, as previously described (25). To experimentally lower steady-state pHi, cells were incubated in growth medium containing 10 μM EIPA for 18–20 h. Loss of NHE1 activity with EIPA was confirmed by recording pHi recovery in a HEPES buffer after an NH4Cl-induced acid load as previously described (26).
Cellular Lactate and Pyruvate Measurements
Extracellular lactic acid was measured by a modification of previously described methods (27). In brief, cells were plated in 24-well plates and grown for 48 h including treatment with EIPA for the last 20 h. For each measurement in triplicate, cells were washed and incubated for 2 h in 0.5 mL FBS- and glutamine-free medium, 50 µL of medium was collected, centrifuged to remove debris, and lactic acid in the supernatant was measured using an enzyme-linked assay in a hydrazine-glycine buffer with NAD+ as a cofactor (27). Protein concentration was measured from lysed cells remaining in the wells used to collect medium and extracellular lactic acid that was expressed relative to cell total protein for each sample. Intracellular lactate and pyruvate were measured according to manufacturer’s instructions (Cayman chemicals Nos. 700510 and 700470, respectively) in cells plated in 24-well plates. Briefly, cells were grown for 48 h as described for extracellular lactate measurements and collected after trypsinizing. Cell counts were determined, and then cells were washed with 1 mL of PBS, and collected by centrifugation. The cell pellet was treated on ice for 5 min with 0.25 M of metaphosphoric acid (MPA) to deproteinate the cells. The supernatant was collected by centrifugation, neutralized with potassium carbonate, and subjected to another centrifugation. The supernatant was immediately used for assays, which were performed in 96-well black plates as indicated by assay instructions, with fluorescence signals measured at Ex 530–540 nm and Em 585–595 nm. The determined intracellular lactate and pyruvate were represented relative to cell number.
Recombinant Human Phosphofructokinase-1 and Lactate Dehydrogenase A Expression, Purification, and Activity
Recombinant phosphofructokinase-1 (PFK1) muscle (PFKM) was expressed and purified as previously described (27, 28). The activity of recombinant PFKM was determined at 0.5 mM fructose 6-phosphate and 4 mM ATP using an auxiliary enzyme assay linking F1,6bP production to NADH oxidation as previously reported (27). MOPS and HEPES buffers were used for pH 6.8–7.6 and 7.4–8.2, respectively. Activity was determined at pH 7.4 and 7.6 in both MOPS and HEPES buffer to confirm the buffer system does not affect activity.
hLDHA cDNA with an NH2-terminal His tag was cloned into pFastbac HTa vector containing a baculovirus-specific promoter by using the Gibson Assembly method. Baculovirus was produced using the Escherichia coli strain DH10Bac containing a baculovirus shuttle vector and helper plasmid that allow the generation of bacmid according to Bac-to-Bac Baculovirus expression system (Invitrogen Life Technologies). Insect Spodoptera frugiperda Sf21 cells were infected with bacmid using Cellfectin II (Invitrogen) and supernatant was collected (P1) and amplified (P2), with 200 mL of P3 supernatant used for protein expression. Post 48-h infection, Sf21 cells were collected by centrifugation, lysed in a 10 mM potassium phosphate buffer containing 0.1% Triton-X100 at pH 7.5 and protein was purified using cobalt resin, as we previously described (27). The activity of recombinant hLDHA was determined by using 0.2 mg of purified enzyme with 0.2 mM NADH and 0.3 mM pyruvate as substrates in 100 mM potassium phosphate buffer of pH 6.8–7.6 by measuring the time-dependent decrease of NADH at 340 nm.
Mitochondrial Membrane Potential
Mitochondrial membrane potential was measured as previously described (29). In brief, cells seeded on 35-mm MatTek dishes (MatTek Corporation) and maintained for 48 h were washed and incubated with the methyl ester form of the cationic dye tetramethylrhodamine at a final concentration of 25 nM for 15 min. Unincorporated dye was removed by washing with a HEPES buffer, and basal rhodamine fluorescence intensity was acquired by spinning disk confocal imaging using an environmentally controlled inverted microscope stand (Nikon TI) equipped with a motorized TIRF illuminator (Nikon), an iXon electron-multiplying CCD camera (Andor), and a ×100 1.49 numerical aperture CFI objective (Nikon) controlled by NIS-Elements software 4.3 (Nikon). The mitochondrial membrane potential (Δψm) was then quenched with FCCP (10 μM) and then images were again acquired using identical field of views as controls. Data were expressed as the ratio of basal/FCCP fluorescent intensity, with image processing and analysis performed using NIS-Elements software.
Immunoblotting for AMPK and Measurement of Cellular ATP
Proteins in 20–30 µg of total cell lysate were separated by 10% SDS, transferred to Imobilon membranes, and immunoblotted as previously described (30). Membranes were probed with antibodies to phosphorylated AMPK and total AMPK (1:1,000; Cell Signaling Technology No. 2535 and No. 2532, respectively). Cellular ATP was determined using a luminescence assay according to manufacturer’s instructions (Cayman No. 700410) and cells were plated in 24-well plates. Data were normalized to cell number determined in parallel samples and expressed as cellular ATP with EIPA relative to control.
Mitochondrial Morphology
For imaging, mitochondria were stained using Mitotracker DeepRed (Life Technologies No. D12345) according to the manufacturer’s specifications. In brief, cells were seeded at ∼25% confluence in 35-mm glass bottom MatTek dishes, maintained for 24 h in growth medium, and then live cells were stained with 25 nM Mitotracker DeepRed for 15 min. Excess stain was washed and Z-stack images of 0.1 mm were obtained with a ×100 objective and spinning disk confocal microscope as described for Δψm. Reconstructions of images were generated and mitochondrial length quantified by using Imaris image analysis software with surface function and default thresholding settings.
Data Presentation and Statistical Analysis
Box- and whisker plots were generated using Analyse-It for Microsoft Excel and show median, first, and third quartile, observations within 1.5 times the interquartile range, and all individual data points. Significance of multiple comparisons was calculated by Tukey–Kramer honest significant difference (HSD) test in Analyse-It for Microsoft. Bar graphs were generated using Excel or Prism software with significance determined using Student’s paired t test. Figures were assembled in Adobe Illustrator CS5.
RESULTS
EIPA Decreases Basal and Maximal Respiratory Capacity of Cells
In cancer cells, increased NHE1 activity is predicted to enable metabolic reprogramming by increasing glycolysis and suppressing mitochondrial oxidative phosphorylation (11–14). To our knowledge, however, this prediction has not been experimentally verified. To address this prediction, we tested a series of clonal cancer cells and chose to focus further on BxPC3 human pancreatic ductal adenocarcinoma cells and MDA-MB-157 human triple-negative breast cancer cells. Our criteria for selecting these cells included published data on differences in metabolic profiles of clonal cancer cells, using cancer cells with established tissue-matched untransformed cells (HPDE for BxPC3 and MCF10A for MDA-MB-157) and our data on pHi in the absence and presence of EIPA (Supplemental Fig. S1A; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.14633286). For example, although BxPC3 and PANC-1 pancreatic ductal adenocarcinoma cells have distinct metabolic profiles (31) and a higher pHi than untransformed HPDE cells (Supplemental Fig. S1A), EIPA significantly decreased pHi in BxPC3 cells but not PANC-1 cells. In addition, EIPA had no effect on pHi of MDA-MB-453 human breast cancer cells or H1299 human lung cancer cells (Supplemental Fig. S1A); hence we did not include these cells for further study.
BxPC3 but not MDA-MB-157 cells showed decreased oxidative phosphorylation compared with tissue-matched untransformed epithelial cells (Fig. 1, A and E). We determined oxidative phosphorylation by measuring oxygen consumption rate (OCR) in growing cells using the well-established mitochondrial stress test and a Seahorse XF Analyzer (32). After measuring basal OCR, a series of mitochondrial membrane transport inhibitors were injected, including oligomycin (1 µM) to determine ATP-linked respiration, FCCP (1 µM) to determine maximal respiration, and antimycin (1 µM) + rotenone (1 µM) to determine spare respiratory capacity (Fig. 1A). We used the mitochondrial stress test with untreated control cells and with cells treated with EIPA (10 µM) for 18–20 h to selectively inhibit activity of NHE1, a H+ extruder. We confirmed treatment with EIPA completely inhibited NHE1 activity in BxPC3 and MDA-MB-157 cells, determined by loss of pHi recovery from an acid load (Supplemental Fig. S1, B and C), and decreased pHi in all cells used in our study (Supplemental Fig. S1A). Our pHi measurements indicate that control pHi in BxPC3 and MDA-MB-157 cells was significantly higher than tissue-matched nontransformed cells and also that NHE1 activity contributes substantially to steady-state pHi in these cells. We chose to pharmacologically inhibit NHE1 activity rather than genetically silence NHE1 because the exchanger has functions in addition to ion transport, including its C-terminal cytoplasmic domain functioning as a scaffold by binding signaling proteins and anchoring actin filaments to the plasma membrane (33, 34). In addition, long-term loss of NHE1 activity is synthetic lethal with activated oncogenes (35).
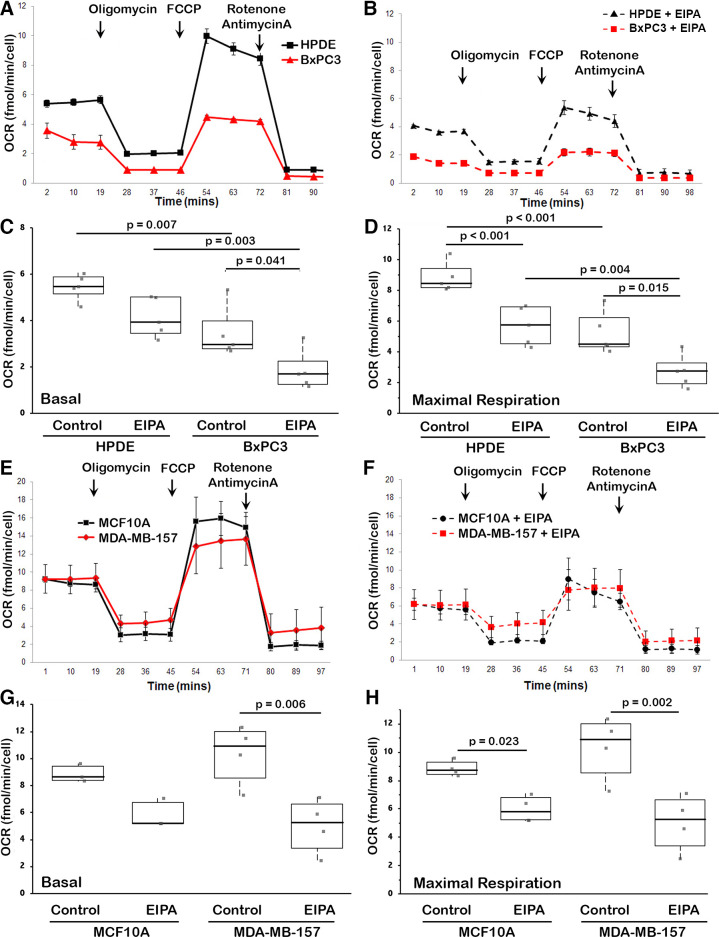
Figure 1.
Oxygen consumption rates (OCR) for the indicated cell types and conditions. A and B: mitochondrial stress test with human pancreatic ductal epithelial (HPDE) and BxPC3 cells for control (A) and with ethyl isopropyl amiloride (EIPA) (10 µM) (B). As labeled in A, shown is basal OCR before sequentially adding mitochondrial transporter inhibitors at 1 µM, including oligomycin for ATP-linked respiration, FCCP for maximal respiration, and antimycin + rotenone for spare respiratory capacity. Data are means ± SE of five separate cell preparations. C and D: basal (C) and maximal (D) OCR for HPDE and BxPC3 cells in the absence (control) and presence of EIPA. Data are from five separate cell preparations. Box plots show median, first, and third quartile, with whiskers extending to observations within 1.5 times the interquartile range, and all individual data points. Statistical analysis by Tukey–Kramer honest significant difference (HSD) test. E and F: mitochondrial stress test as in (A) with MCF10A and MDA-MB-157 cells for control (E) and with EIPA (F). Data are means ± SE of five separate cell preparations. G and H: basal (G) and maximal (H) OCR for HPDE and BxPC3 cells in the absence (control) and presence of EIPA. Data are from n = 5 separate experiments for HPDE and BxPC3 cells and n = 4 separate experiments for MCF10A and MDA-MB-157 cells with Box plots and statistical analysis as described for C and D.
We found that BxPC3 cells had a lower OCR compared with untransformed HPDE human normal pancreatic ductal epithelial cells (Fig. 1A), including basal OCR (Fig. 1C) and maximal respiration (Fig. 1D). Although OCR with BxPC3 cells is reported to be markedly lower compared with a number of other pancreatic ductal adenocarcinoma cells, including PANC-1 and Mia-PaCa2 (31); this report did not include OCR for untransformed human pancreatic ductal epithelial cells. In contrast to the prediction of increased NHE1 activity suppressing oxidative phosphorylation, we found that EIPA decreased OCR in both BxPC3 and HPDE cells, with significantly decreased basal OCR (Fig. 1C) and maximal respiration (Fig. 1D).
In contrast to BxPC3 and HPDE cells, the OCR for MDA-MB-157 breast cancer cells and untransformed MCF10A human mammary epithelial cells was not different (Fig. 1E), including no difference in basal OCR (Fig. 1G) and maximal respiration (Fig. 1H). However, in both cell types, EIPA decreased OCR, including basal OCR and maximal respiration (Fig. 1, F–H). These data indicate that BxPC3 cells have a higher pHi and lower OCR than HPDE cells, and that MDA-MB-157 cells also have a higher pHi but not different basal OCR or maximal respiration compared with MCF10A cells. However, in contrast to the prediction that increased NHE1 activity and pHi suppress oxidative phosphorylation, inhibiting NHE1 activity with EIPA decreased OCR in all four cell types.
EIPA Does Not Change Glycolysis despite pH-Regulated Activity of Phosphofructokinase-1
Consistent with BxPC3 cells showing metabolic reprograming with decreased oxidative phosphorylation, these cells also had increased secreted lactate compared with untransformed HPDE cells (Fig. 2A), suggesting increased glycolysis. For MDA-MB-157 cells, although OCR is not different compared with untransformed MCF10A cells, secreted lactate is significantly less (Fig. 2B), suggesting a lower glycolytic rate. However, despite EIPA decreasing OCR in all of these cells, it had no effect on amounts of secreted lactate (Fig. 2, A and B). We also measured intracellular lactic acid in MDA-MB-157 cells, which was not different in the absence compared with the presence of EIPA (Supplemental Fig. S2A).
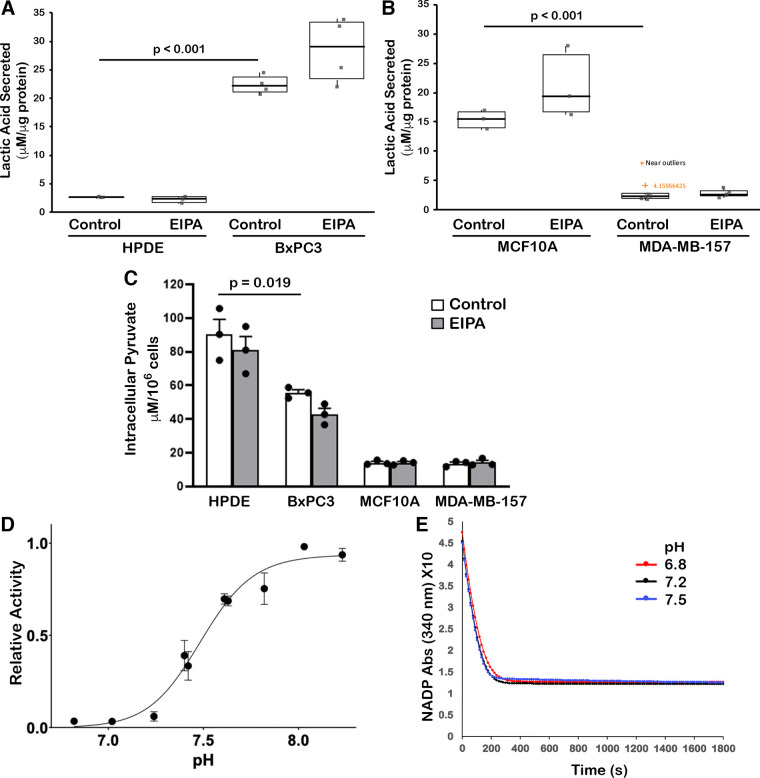
Figure 2.
Glycolysis and activity of the glycolytic enzymes phosphofructokinase-1 (PFK1) and lactate dehydrogenase A (LDHA). A and B: lactic acid secreted from human pancreatic ductal epithelial (HPDE) and BxPC3 cells (A) and MCF10A and MDA-MB-157 cells (B) in the absence (control) and presence of ethyl isopropyl amiloride (EIPA) (10 µM). Data are from four (A) and three (B) separate cell preparations. Box plots show median, first, and third quartile, with whiskers extending to observations within 1.5 times the interquartile range, and all individual data points. Statistical analysis by Tukey–Kramer honest significant difference (HSD) test. C: intracellular pyruvate for the indicated cell types and conditions. Data are means ± SE of three separate cell preparations, with statistical analysis using Student’s paired t test. D: activity of PFK1 muscle (PFKM), expressed relative to maximal activity at 4 mM ATP and 0.5 mM fructose 6-phosphate. Data are means ± SE of seven separate measurements with two different protein preparations. E: activity, expressed as decreased NADH over time, of recombinant purified LDHA at buffer pH 6.8, 7.2, and 7.5. Data are means of three separate measurements with two different protein preparations.
To further test glycolysis we measured intracellular pyruvate, a metabolite upstream of lactate, which was significantly lower in BXPC3 compared with HPDE cells (Fig. 2C), suggesting BxPC3 cells may be more effectively converting pyruvate to lactate. In addition, despite MCF10A cells having more secreted lactic acid than MDA-MB-157 cells, levels of intracellular pyruvate were not different in these two cell types (Fig. 2C). However, in all four cell types EIPA has no effect on secreted lactate or intracellular pyruvate, which strongly suggests it does not alter glycolytic flux.
The prediction that increased NHE1 activity and pHi in cancer cells increases glycolysis to enable metabolic reprogramming is in part based on previous work showing that activity of phosphofructokinase-1 (PFK1), which catalyzes the rate-limiting step committing glucose to breakdown, is pH sensitive with increased activity at higher pH (36, 37). In addition, lactate dehydrogenase A (LDHA), an enzyme catalyzing the conversion of pyruvate to lactate is reported to be pH sensitive, with yeast LDH having increased activity at higher pH (38, 39) and the M but not H isoform of recombinant human muscle LDH having increased activity with increasing pH above 7.0 (40). In contrast, recombinant human LDH was recently found to have promiscuous reduction of α-keto-glutarate to 2-hydroxyglutarate at acidic pH (41, 42).
To further reconcile our findings on glycolysis with reported effects of pH on activity of PFK1 and LDHA, we generated and purified recombinant human enzymes (Supplemental Fig. S2, B and C) and determined the effect of pH on activity of the enzymes. Although all three PFK1 isoforms, muscle, platelet, and liver, have increased activity at higher pH (37, 43, 44), the muscle isoform, PFKM, is most sensitive to pH and has pH-dependent relief of allosteric inhibition by ATP. Using recombinant human PFKM generated in a baculovirus expression system to enable post-translational modifications that might affect activity, we found that PFKM activity was pH-dependent with increased activity at high pH (Fig. 2D). The data indicated a distinct linear increase in activity from ∼7.2 to ∼7.7, which is very consistent with findings using purified native PFKM (36, 37). In contrast, activity of recombinant purified human LDHA, also generated using a baculovirus expression system, was not different within the range of pH 6.8–7.5 (Fig. 2E). Our finding of increased PFKM with higher pHi does coincide with no change in glycolysis when pHi is decreased with EIPA. However, we cannot rule out possible effects of pHi on PFK1 activity in cells that could be mediated by other isoforms, changes in substrate, or the activity of other glycolytic enzymes.
EIPA Does Not Change Fatty Acid, Glutamine- or Pyruvate-Fueled OCR and Has No Effect on Mitochondrial Membrane Potential
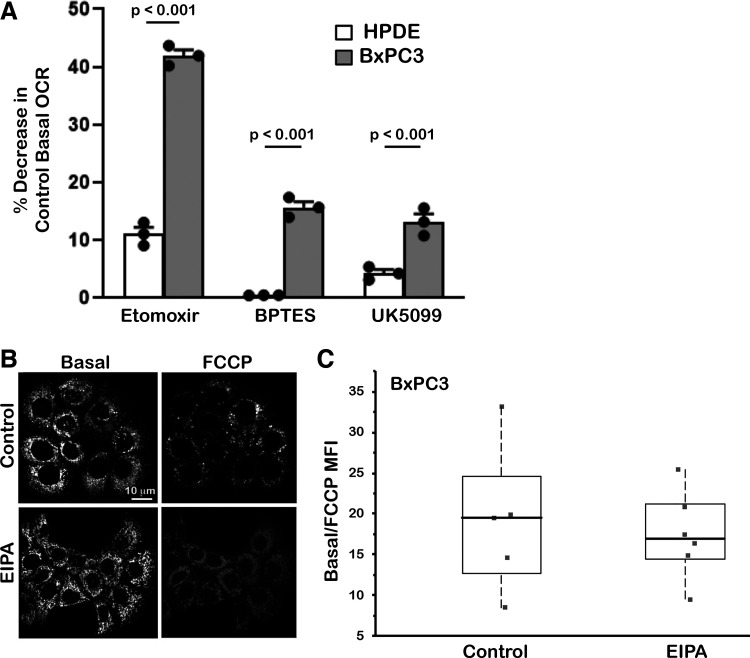
Mitochondrial respiration is regulated by metabolites from fatty acid oxidation (FAO), glutaminolysis, and glycolysis. To determine how EIPA might decrease basal OCR, we tested whether it limits how these pathways fuel mitochondrial respiration. We quantified the contribution of each pathway by measuring basal OCR in the absence and presence of selective inhibitors of each pathway, including Etomoxir for FAO, BPTES for glutaminolysis, and UK5099 for the mitochondrial pyruvate carrier. We focused on HPDE and BxPC3 cells because in control conditions they had distinct OCR values but MCF10 and MDA-MB-157 cells did not (Fig. 1, A and E). In addition to HPDE and BxPC3 cells having different basal and maximal respiration, they also had differences in the pathways that fuel OCR. We found that dependence on FAO was ∼9% in HPDE cells but ∼40% for BxPC3 cells, dependence on glutaminolysis was nearly undetectable in HPDE cells but ∼16% in BxPC3 cells, and dependence on pyruvate was ∼4% in HPDE cells but ∼14% in BxPC3 cells (Fig. 3A). The significantly higher contributions from FAO and glutaminolysis in BxPC3 cells compared with HPDE cells is consistent with upregulated FAO in many cancer types (23) and pancreatic cancers being reliant on glutaminolysis pathways (45). However, although EIPA lowered basal OCR in HPDE and BxPC3 cells, it had no effect on the relative contribution of FAO (Supplemental Fig. S3A), glutaminolysis, or pyruvate (data not shown) we observe in control cells. Oxidative phosphorylation is also regulated by phosphorylated AMP kinase (pAMPK), an indicator of cellular ATP levels and bioenergetics, and by mitochondrial membrane potential (Δψm). Although EIPA decreased ATP-linked OCR, determined as basal OCR minus OCR with oligomycin (Supplemental Fig. S3B), it had no effect on pAMPK in BxPC3 cells, determined by immunoblotting (Supplemental Fig. S3C) or cellular ATP concentration in the four cell lines we studied (Supplemental Fig. S3D). EIPA also had no effect on Δψm, determined by quantifying confocal images of BxPC3 cells loaded with the methyl ester of the cationic dye tetramethylrhodamine (Fig. 3, B and C). Hence, although inhibiting NHE1 activity with EIPA decreased basal and maximal OCR in BxPC3 cells, it had no marked effect on the contribution of pathways fueling OCR or bioenergetics level as indicated by pAMPK, cellular ATP, or Δψm.
Figure 3.
Contributions to mitochondrial respiration and mitochondrial membrane potential. A: for human pancreatic ductal epithelial (HPDE) and BxPC3 cells, oxygen consumption rates (OCR) with inhibitors, each at 1 µM for pathways fueling mitochondrial respiration, including etomoxir for fatty acid oxidation (FAO), BPTES for glutaminolysis, and UK5099 for the mitochondrial pyruvate carrier. Data are expressed as percent decrease relative to control OCR and are means ± SE of three cell preparations with statistical analysis by Student’s paired t test. B: representative confocal images of BxPC3 cells loaded with the methyl ester of the cationic dye tetramethylrhodamine that indicates mitochondrial membrane potential (Δψm) for control and with FCCP in the absence and presence of ethyl isopropyl amiloride (EIPA) (10 µM). C: quantification of tetramethylrhodamine staining expressed as control values relative to values with FCCP from five field-of-views in two cell preparations. Box plots show median, first, and third quartile, with whiskers extending to observations within 1.5 times the interquartile range, and all individual data points.
EIPA Induces Tubular Mitochondrial Structure
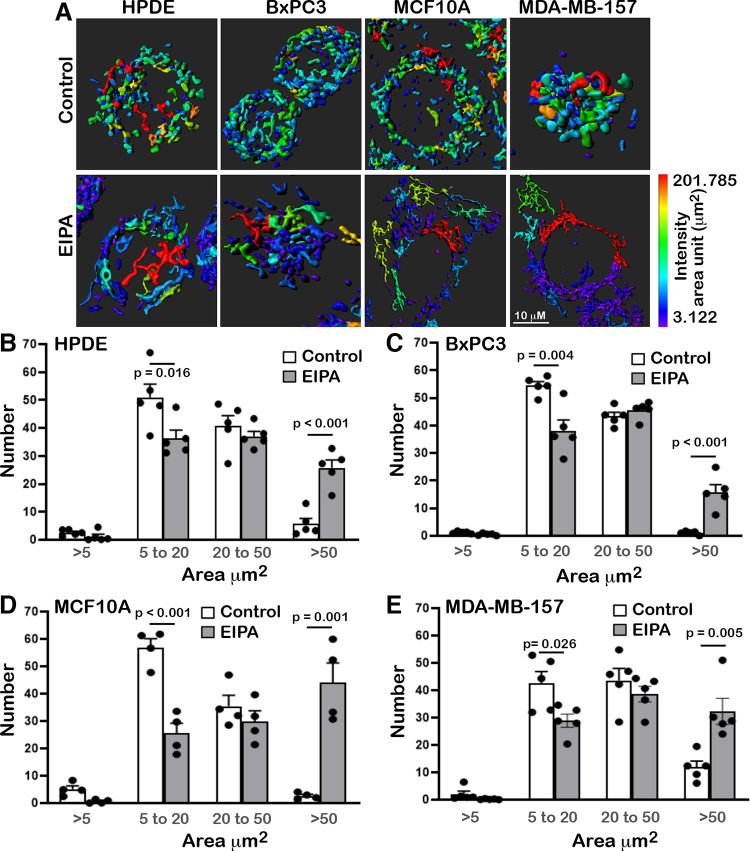
Yet another regulator of OCR is mitochondrial morphology, which we found is markedly changed with EIPA. Changes in mitochondrial morphology, termed mitochondrial dynamics, include the processes of fission, or the division or fragmentation of individual mitochondrion, and fusion, or the union of mitochondria generally into a tubular network. We used images of live cells stained with Mitotracker DeepRed and imaged by spinning disk confocal microscopy to generate reconstructions of mitochondrial morphology (Fig. 4A) and to quantify mitochondrial lengths with Imaris software (Fig. 4, B–E). We binned mitochondrial lengths into four categories, 0–5 μm, 5–20 μm, 20–50 μm, and >50 μm, and expressed data as a percent of mitochondria within each length range. Quantification indicated that mitochondria morphology of control cells was predominantly in the intermediate range of 5–50 μm, with BxPC3 having substantially fewer mitochondria of >50 μm, with EIPA, however, all four cell types had significantly fewer mitochondria of 5–20 μm length and more >50 μm, indicating an increased tubular network (Fig. 4, C–F). Visually, control cells appear to have a more compact mitochondrial network, and with EIPA cells are more spread with a less compact network (Fig. 4A). This observation was most pronounced for MDA-MBA-157 cells, despite OCR not being different in MDA-MB-157 and MCF10A cells. A current view is that elongated mitochondrial networks are associated with increased oxidative phosphorylation (46). However, our findings indicate that EIPA changes mitochondrial dynamics to a more elongated tubular network, suggesting increased fusion, despite EIPA decreasing OCR.
Figure 4.
Ethyl isopropyl amiloride (EIPA) changes mitochondrial morphology. A: Imaris reconstructions of confocal images of the indicated cell types stained with Mitotracker DeepRed in the absence (control) or presence of EIPA (10 µM). Heat map of mitochondrial lengths and scale bar are included at bottom right. B–E: for each indicated cell line, quantified mitochondrial lengths measured from confocal imagines using Imaris software, as indicated in Materials and Methods, and binned using Excel software into four categories, 0–5 μm, 5–20 μm, 20–50 μm, and >50 μm, with data expressed as the number of mitochondrial structures within each category. Data are means ± SE of five fields of view from two cell preparations with statistical analysis by Student’s paired t test.
DISCUSSION
Our study tests a commonly conveyed prediction that in cancer cells increased activity of NHE1 and a higher pHi enable metabolic reprogramming by suppressing mitochondrial oxidative phosphorylation and increasing glycolysis (12–14). Contrary to this prediction, however, we found that inhibiting NHE1 activity and decreasing pHi with EIPA decreased oxidative phosphorylation in clonal cancer and untransformed cells, determined by measuring oxygen consumption rates, and had no effect on glycolysis, determined by measuring extracellular lactate and intracellular pyruvate. In HEK cells, the NHE1 inhibitor cariporide also has no effect on steady-state lactate production (47) and to our knowledge the MCT lactate-H+ transporters are not sensitive to EIPA. We confirmed that recombinant PFKM generated to include post-translational modifications has increased activity at higher pH, as previously reported for native enzyme isolated from muscle (36, 37). The lack of an effect of decreased pHi on glycolysis despite pH-dependent PFKM activity could reflect the relative expression of muscle compared with platelet and liver isoforms. However, the activity of these isoforms also is greater at higher pH, albeit with less sensitivity than PFKM (37, 43, 44). We found no effect of pH within the physiological range on activity of purified recombinant LDHA, despite conflicting findings that yeast (38, 39) and human (40) LDH have increased activity at higher pH and that recombinant mammalian LDH has promiscuous increased activity at more acidic pH (41, 42). Moreover, our findings contrast to an NHE1-dependent increase in glycolysis induced by human carcinogens (48).
Although EIPA decreased oxygen consumption rate in cancer and untransformed cells, it had no effect on contributions of distinct metabolic pathways to mitochondrial respiration, including FAO, glutaminolysis, and glycolysis, or on mitochondrial membrane potential. However, EIPA did induce marked changes in mitochondrial morphology, increasing mitochondria lengths and a more tubular network in the cancer as well as nontransformed cells we studied. Our findings are in contrast to a current view that elongated mitochondrial networks are associated with increased oxidative phosphorylation (46). However, extracellular acidosis, a feature of tumor-like increased pHi, promotes mitochondria fusion, and limits fragmentation (49). Moreover, Nehrke and coworker report links between a Na-H exchanger, pHi, and mitochondrial morphology in Caenorhabditis elegans, with chronic mitochondrial fragmentation lowering pHi (50) and in C. elegans and mammalian cells a lower pHi inhibits mitochondrial fusion with effects mediated by reactive oxygen species (ROS) (51). Although increased ROS inhibits NHE1 expression and/or activity and lowers pHi (52, 53), whether EIPA regulates ROS abundance remains unclear. EIPA inhibits increases in ROS (54, 55), which can be mediated by decreased NHE1 activity. In contrast, however, cell injury stress is recently reported to increase redox signaling and mitochondrial fission (56), although this study does not include effects on mitochondrial respiration. Hence, there remains caution on an oversimplified assumption that mitochondrial fusion is associated with increased oxidative phosphorylation, and as suggested, correlations between mitochondrial dynamics and metabolism remain unclear (57).
Our experimental design does not rule out a direct effect of EIPA on mitochondrial morphology; although if this occurs it is not likely mediated by a Na-H exchanger. A mitochondrial localized NHE isoform distinct from plasma membrane NHE1 remains controversial. A mitochondrial cation/proton exchange by NHA2 (SLC9B2) is reported (58, 59); however, in isolated mitochondria, EIPA has no effect on H+ transport (60) or respiration (61) at concentrations up to 100 µM, compared with the 10 µM we used in our study. A recent study shows that long-term (5 days) but not short-term (1 day) treatment with EIPA has cytotoxic effects independent of NHE1 (62).
In addition to possible bidirectional effects of EIPA on oxidative phosphorylation and mitochondrial morphology, the decreased OCR we observed with EIPA could reflect decreased micropinocytosis because this cellular uptake process is inhibited by amiloride (63) and EIPA (64), with effects of EIPA shown using BxPC3 cells as we used in our study. An additional and not mutually exclusive mechanism mediating decreased OCR with EIPA could be effects on intracellular Ca2+, which is lowered by EIPA (65, 66), although possibly secondary to lowering pHi. In addition, decreasing Ca2+ entry by the Trpm2 channel decreases OCR (67).
Further studies are needed to resolve the effects of EIPA on mitochondrial respiration and morphology and whether they are mediated by decreased NHE1 activity and lower pHi. Our findings that a lower pHi is associated with decreased OCR raise questions on the prevailing prediction that the higher pHi of cancer cells enables metabolic reprogramming by suppressing oxidative phosphorylation and also for lowering pHi as a therapeutic strategy to limit cancer progression by reversing metabolic reprogramming.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S3: https://doi.org/10.6084/m9.figshare.14633286.
GRANTS
This work was supported by National Institutes of Health Grants CA937855 (to D. L. Barber) and CA197855-04S1 (to K. Kisor). Seahorse Instrumentation was available through the UCSF Mouse Metabolism Core within the Nutrition Obesity Center that is supported by National Institutes of Health grant 1P30DK098722.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S.M. and D.L.B. conceived and designed research; S.S.M., K.K., B.A.W., and D.L.B. performed experiments; S.S.M., K.K., B.A.W., and D.L.B. analyzed data; S.S.M., K.K., B.A.W., and D.L.B. interpreted results of experiments; S.S.M., K.K., and D.L.B. prepared figures; S.S.M. and D.L.B. drafted manuscript; S.S.M., K.K., B.A.W., and D.L.B. edited and revised manuscript; S.S.M., K.K., B.A.W., and D.L.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Rushika Perera for providing BxPC3 and HPDE cells and Jay Debnath for providing MCF10A cells. Seahorse Instrumentation was available through the UCSF Mouse Metabolism Core within the Nutrition Obesity Center.
REFERENCES
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 144: 646–674, 2011. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21: 297–308, 2012. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9: 425–434, 2006. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Xiao X, Huang X, Ye F, Chen B, Song C, Wen J, Zhang Z, Zheng G, Tang H, Xie X. The miR-34a-LDHA axis regulates glucose metabolism and tumor growth in breast cancer. Sci Rep 6: 21735, 2016. doi: 10.1038/srep21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schell JC, Olson KA, Jiang L, Hawkins AJ, Van Vranken JG, Xie J, Egnatchik RA, Earl EG, DeBerardinis RJ, Rutter J. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell 56: 400–413, 2014. doi: 10.1016/j.molcel.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arora R, Schmitt D, Karanam B, Tan M, Yates C, Dean-Colomb W. Inhibition of the Warburg effect with a natural compound reveals a novel measurement for determining the metastatic potential of breast cancers. Oncotarget 6: 662–678, 2015. doi: 10.18632/oncotarget.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boland ML, Chourasia AH, Macleod KF. Mitochondrial dysfunction in cancer. Front Oncol 3: 292, 2013. doi: 10.1177/1535370216641787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelicano H, Zhang W, Liu J, Hammoudi N, Dai J, Xu RH, Pusztai L, Huang P. Mitochondrial dysfunction in some triple-negative breast cancer cell lines: role of mTOR pathway and therapeutic potential. Breast Cancer Res 16: 434, 2014. doi: 10.1186/s13058-014-0434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace DC. Mitochondria and cancer. Nat Rev Cancer 12: 685–698, 2012. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, Sudderth J, Calvaruso MA, Lumata L, Mitsche M, Rutter J, Merritt ME, DeBerardinis RJ. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell 56: 414–424, 2014. doi: 10.1016/j.molcel.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damaghi M, Wojtkowiak JW, Gillies RJ. pH sensing and regulation in cancer. Front Physiol 4: 370, 2013. doi: 10.3389/fphys.2013.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parks SK, Chiche J, Pouyssegur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer 13: 611–623, 2013. doi: 10.1038/nrc3579. [DOI] [PubMed] [Google Scholar]
- 13.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 11: 671–677, 2011. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 14.White KA, Grillo-Hill BK, Barber DL. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J Cell Sci 130: 663–669, 2017. doi: 10.1242/jcs.195297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stock C, Pedersen SF. Roles of pH and the Na+/H+ exchanger NHE1 in cancer: from cell biology and animal models to an emerging translational perspective? Semin Cancer Biol 43: 5–16, 2017. doi: 10.1016/j.semcancer.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Swietach P, Vaughan-Jones RD, Harris AL, Hulikova A. The chemistry, physiology and pathology of pH in cancer. Philos Trans R Soc Lond B Biol Sci 369: 20130099, 2014. doi: 10.1098/rstb.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J, Jiang L, Ko B, Skelton R, Loudat L, Wodzak M, Klimko C, McMillan E, Butt Y, Ni M, Oliver D, Torrealba J, Malloy CR, Kernstine K, Lenkinski RE, DeBerardinis RJ. Metabolic heterogeneity in human lung tumors. Cell 164: 681–694, 2016. doi: 10.1016/j.cell.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanning NJ, Castle JP, Singh SJ, Leon AN, Tovar EA, Sanghera A, MacKeigan JP, Filipp FV, Graveel CR. Metabolic profiling of triple-negative breast cancer cells reveals metabolic vulnerabilities. Cancer Metab 5: 6, 2017. doi: 10.1186/s40170-017-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tasdogan A, Faubert B, Ramesh V, Ubellacker JM, Shen B, Solmonson A, Murphy MM, Gu Z, Gu W, Martin M, Kasitinon SY, Vandergriff T, Mathews TP, Zhao Z, Schadendorf D, DeBerardinis RJ, Morrison SJ. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 577: 115–120, 2020. doi: 10.1038/s41586-019-1847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbet C, Feron O. Tumour acidosis: from the passenger to the driver's seat. Nat Rev Cancer 17: 577–593, 2017. doi: 10.1038/nrc.2017.77. [DOI] [PubMed] [Google Scholar]
- 21.Korenchan DE, Flavell RR. Spatiotemporal pH heterogeneity as a promoter of cancer progression and therapeutic resistance. Cancers (Basel) 11: 1026, 2019. doi: 10.3390/cancers11071026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobo RC, Hubbard NE, Damonte P, Mori H, Penzvalto Z, Pham C, Koehne AL, Go AC, Anderson SE, Cala PM, Borowsky AD. Glucose uptake and intracellular pH in a mouse model of ductal carcinoma in situ (DCIS) suggests metabolic heterogeneity. Front Cell Dev Biol 4: 93, 2016. doi: 10.3389/fcell.2016.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, DeBerardinis RJ. Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metab 30: 434–446, 2019. doi: 10.1016/j.cmet.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30: 256–268, 2003. doi: 10.1016/S1046-2023(03)00032-X. [DOI] [PubMed] [Google Scholar]
- 25.Grillo-Hill BK, Webb BA, Barber DL. Ratiometric imaging of pH probes. Methods Cell Biol 123: 429–448, 2014. doi: 10.1016/B978-0-12-420138-5.00023-9. [DOI] [PubMed] [Google Scholar]
- 26.Meima ME, Webb BA, Witkowska HE, Barber DL. The sodium-hydrogen exchanger NHE1 is an Akt substrate necessary for actin filament reorganization by growth factors. J Biol Chem 284: 26666–26675, 2009. doi: 10.1074/jbc.M109.019448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webb BA, Forouhar F, Szu FE, Seetharaman J, Tong L, Barber DL. Structures of human phosphofructokinase-1 and atomic basis of cancer-associated mutations. Nature 523: 111–114, 2015. doi: 10.1038/nature14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webb BA, Dosey AM, Wittmann T, Kollman JM, Barber DL. The glycolytic enzyme phosphofructokinase-1 assembles into filaments. J Cell Biol 216: 2305–2313, 2017. doi: 10.1083/jcb.201701084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sukumar M, Liu J, Mehta GU, Patel SJ, Roychoudhuri R, Crompton JG, Klebanoff CA, Ji Y, Li P, Yu Z, Whitehill GD, Clever D, Eil RL, Palmer DC, Mitra S, Rao M, Keyvanfar K, Schrump DS, Wang E, Marincola FM, Gattinoni L, Leonard WJ, Muranski P, Finkel T, Restifo NP. Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell Metab 23: 63–76, 2016. doi: 10.1016/j.cmet.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White KA, Grillo-Hill BK, Esquivel M, Peralta J, Bui VN, Chire I, Barber DL. β-Catenin is a pH sensor with decreased stability at higher intracellular pH. J Cell Biol 217: 3965–3976, 2018. doi: 10.1083/jcb.201712041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovalenko I, Glasauer A, Schockel L, Sauter DR, Ehrmann A, Sohler F, Hagebarth A, Novak I, Christian S. Identification of KCa3.1 channel as a novel regulator of oxidative phosphorylation in a subset of pancreatic carcinoma cell lines. PLoS One 11: e0160658, 2016. doi: 10.1371/journal.pone.0160658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eriksson M, Ambroise G, Ouchida AT, Lima Queiroz A, Smith D, Gimenez-Cassina A, Iwanicki MP, Muller PA, Norberg E, Vakifahmetoglu-Norberg H. Effect of mutant p53 proteins on glycolysis and mitochondrial metabolism. Mol Cell Biol 37: e00328-17, 2017. doi: 10.1128/MCB.00328-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumgartner M, Patel H, Barber DL. Na+/H+ exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am J Physiol Cell Physiol 287: C844–C850, 2004. doi: 10.1152/ajpcell.00094.2004. [DOI] [PubMed] [Google Scholar]
- 34.Meima ME, Mackley JR, Barber DL. Beyond ion translocation: structural functions of the sodium-hydrogen exchanger isoform-1. Curr Opin Nephrol Hypertens 16: 365–372, 2007. doi: 10.1097/MNH.0b013e3281bd888d. [DOI] [PubMed] [Google Scholar]
- 35.Grillo-Hill BK, Choi C, Jimenez-Vidal M, Barber DL. Increased H(+) efflux is sufficient to induce dysplasia and necessary for viability with oncogene expression. eLife 4: e03270, 2015. doi: 10.7554/eLife.03270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobson GP, Yamamoto E, Hochachka PW. Phosphofructokinase control in muscle: nature and reversal of pH-dependent ATP inhibition. Am J Physiol Regul Integr Comp Physiol 250: R71–R76, 1986. doi: 10.1152/ajpregu.1986.250.1.R71. [DOI] [PubMed] [Google Scholar]
- 37.Trivedi B, Danforth WH. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem 241: 4110–4112, 1966. doi: 10.1016/S0021-9258(18)99819-4. [DOI] [PubMed] [Google Scholar]
- 38.Gaspar P, Neves AR, Shearman CA, Gasson MJ, Baptista AM, Turner DL, Soares CM, Santos H. The lactate dehydrogenases encoded by the ldh and ldhB genes in Lactococcus lactis exhibit distinct regulation and catalytic properties—comparative modeling to probe the molecular basis. FEBS J 274: 5924–5936, 2007. doi: 10.1111/j.1742-4658.2007.06115.x. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki H, Ogura Y. Effect of pH on the kinetic parameters of yeast l(+)-lactate dehydrogenase (cytochrome b2). J Biochem 67: 291–295, 1970. doi: 10.1093/oxfordjournals.jbchem.a129252. [DOI] [PubMed] [Google Scholar]
- 40.Read JA, Winter VJ, Eszes CM, Sessions RB, Brady RL. Structural basis for altered activity of M- and H-isozyme forms of human lactate dehydrogenase. Proteins 43: 175–185, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 41.Intlekofer AM, Wang B, Liu H, Shah H, Carmona-Fontaine C, Rustenburg AS, Salah S, Gunner MR, Chodera JD, Cross JR, Thompson CB. L-2-Hydroxyglutarate production arises from noncanonical enzyme function at acidic pH. Nat Chem Biol 13: 494–500, 2017. doi: 10.1038/nchembio.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nadtochiy SM, Schafer X, Fu D, Nehrke K, Munger J, Brookes PS. Acidic pH is a metabolic switch for 2-hydroxyglutarate generation and signaling. J Biol Chem 291: 20188–20197, 2016. doi: 10.1074/jbc.M116.738799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akkerman JW, Gorter G, Sixma JJ, Staal GE. Human platelet 6-phosphofructokinase. Purification, kinetic parameters and the influence of sulphate ions on enzyme activity. Biochim Biophys Acta 370: 102–112, 1974. doi: 10.1016/0005-2744(74)90036-9. [DOI] [PubMed] [Google Scholar]
- 44.Reinhart GD. Influence of pH on the regulatory kinetics of rat liver phosphofructokinase: a thermodynamic linked-function analysis. Biochemistry 24: 7166–7172, 1985. doi: 10.1021/bi00346a022. [DOI] [PubMed] [Google Scholar]
- 45.Bott AJ, Shen J, Tonelli C, Zhan L, Sivaram N, Jiang YP, Yu X, Bhatt V, Chiles E, Zhong H, Maimouni S, Dai W, Velasquez S, Pan JA, Muthalagu N, Morton J, Anthony TG, Feng H, Lamers WH, Murphy DJ, Guo JY, Jin J, Crawford HC, Zhang L, White E, Lin RZ, Su X, Tuveson DA, Zong WX. Glutamine anabolism plays a critical role in pancreatic cancer by coupling carbon and nitrogen metabolism. Cell Rep 29: 1287–1298.e6, 2019. doi: 10.1016/j.celrep.2019.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol 212: 379–387, 2016. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rotte A, Pasham V, Eichenmuller M, Bhandaru M, Foller M, Lang F. Upregulation of Na+/H+ exchanger by the AMP-activated protein kinase. Biochem Biophys Res Commun 398: 677–682, 2010. doi: 10.1016/j.bbrc.2010.06.135. [DOI] [PubMed] [Google Scholar]
- 48.Hardonniere K, Huc L, Sergent O, Holme JA, Lagadic-Gossmann D. Environmental carcinogenesis and ph homeostasis: not only a matter of dysregulated metabolism. Semin Cancer Biol 43: 49–65, 2017. doi: 10.1016/j.semcancer.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Khacho M, Tarabay M, Patten D, Khacho P, MacLaurin JG, Guadagno J, Bergeron R, Cregan SP, Harper ME, Park DS, Slack RS. Acidosis overrides oxygen deprivation to maintain mitochondrial function and cell survival. Nat Commun 5: 3550, 2014. doi: 10.1038/ncomms4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson D, Nehrke K. Mitochondrial fragmentation leads to intracellular acidification in Caenorhabditis elegans and mammalian cells. Mol Biol Cell 21: 2191–2201, 2010. doi: 10.1091/mbc.e09-10-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson D, Allman E, Nehrke K. Regulation of acid-base transporters by reactive oxygen species following mitochondrial fragmentation. Am J Physiol Cell Physiol 302: C1045–C1054, 2012. doi: 10.1152/ajpcell.00411.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akram S, Teong HF, Fliegel L, Pervaiz S, Clement MV. Reactive oxygen species-mediated regulation of the Na+-H+ exchanger 1 gene expression connects intracellular redox status with cells' sensitivity to death triggers. Cell Death Differ 13: 628–641, 2006. doi: 10.1038/sj.cdd.4401775. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura U, Iwase M, Uchizono Y, Sonoki K, Sasaki N, Imoto H, Goto D, Iida M. Rapid intracellular acidification and cell death by H2O2 and alloxan in pancreatic β cells. Free Radic Biol Med 40: 2047–2055, 2006. doi: 10.1016/j.freeradbiomed.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 54.Baldini PM, De VP, Martino A, Fraziano M, Grimaldi C, Luly P, Zalfa F, Colizzi V. Differential sensitivity of human monocytes and macrophages to ANP: a role of intracellular pH on reactive oxygen species production through the phospholipase involvement. J Leukoc Biol 73: 502–510, 2003. doi: 10.1189/jlb.0702377. [DOI] [PubMed] [Google Scholar]
- 55.Dailianis S, Piperakis SM, Kaloyianni M. Cadmium effects on ros production and DNA damage via adrenergic receptors stimulation: role of Na+/H+ exchanger and PKC. Free Radic Res 39: 1059–1070, 2005. doi: 10.1080/10715760500243765. [DOI] [PubMed] [Google Scholar]
- 56.Horn A, Raavicharla S, Shah S, Cox D, Jaiswal JK. Mitochondrial fragmentation enables localized signaling required for cell repair. J Cell Biol 219: e201909154, 2020. doi: 10.1083/jcb.201909154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen H, Chan DC. Mitochondrial dynamics in regulating the unique phenotypes of cancer and stem cells. Cell Metab 26: 39–48, 2017. doi: 10.1016/j.cmet.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Battaglino RA, Pham L, Morse LR, Vokes M, Sharma A, Odgren PR, Yang M, Sasaki H, Stashenko P. NHA-oc/NHA2: a mitochondrial cation-proton antiporter selectively expressed in osteoclasts. Bone 42: 180–192, 2008. doi: 10.1016/j.bone.2007.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fuster DG, Zhang J, Shi M, Bobulescu IA, Andersson S, Moe OW. Characterization of the sodium/hydrogen exchanger NHA2. J Am Soc Nephrol 19: 1547–1556, 2008. doi: 10.1681/ASN.2007111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kapus A, Lukács GL, Cragoe EJ, Ligeti E, Fonyó A. Characterization of the mitochondrial Na+-H+ exchange. The effect of amiloride analogues. Biochim Biophys Acta 944: 383–390, 1988. doi: 10.1016/0005-2736(88)90509-3. [DOI] [PubMed] [Google Scholar]
- 61.Dlaskova A, Hlavata L, Jezek J, Jezek P. Mitochondrial Complex I superoxide production is attenuated by uncoupling. Int J Biochem Cell Biol 40: 2098–2109, 2008. doi: 10.1016/j.biocel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 62.Rolver MG, Elingaard-Larsen LO, Andersen AP, Counillon L, Pedersen SF. Pyrazine ring-based Na+/H+ exchanger (NHE) inhibitors potently inhibit cancer cell growth in 3D culture, independent of NHE1. Sci Rep 10: 5800, 2020. doi: 10.1038/s41598-020-62430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, Alexander T, Touret N, Hahn KM, Grinstein S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol 188: 547–563, 2010. [Erratum in J Cell Biol 189: 385, 2010]. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, Metallo CM, Vander Heiden MG, Bar-Sagi D. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497: 633–637, 2013. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldman A, Chen H, Khan MR, Roesly H, Hill KA, Shahidullah M, Mandal A, Delamere NA, Dvorak K. The Na+/H+ exchanger controls deoxycholic acid-induced apoptosis by a H+-activated, Na+-dependent ionic shift in esophageal cells. PLoS One 6: e23835, 2011. doi: 10.1371/journal.pone.0023835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thibonnier M, Bayer AL, Simonson MS, Douglas JG. Effects of amiloride analogues on AVP binding and activation of V1-receptor-expressing cells. Am J Physiol Endocrinol Metab 262: E76–E86, 1992. doi: 10.1152/ajpendo.1992.262.1.E76. [DOI] [PubMed] [Google Scholar]
- 67.Hoffman NE, Miller BA, Wang J, Elrod JW, Rajan S, Gao E, Song J, Zhang XQ, Hirschler-Laszkiewicz I, Shanmughapriya S, Koch WJ, Feldman AM, Madesh M, Cheung JY. Ca2+ entry via Trpm2 is essential for cardiac myocyte bioenergetics maintenance. Am J Physiol Heart Circ Physiol 308: H637–H650, 2015. doi: 10.1152/ajpheart.00720.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]