Abstract
In whole cell patch clamp recordings, it was discovered that normal human adrenal zona glomerulosa (AZG) cells express members of the three major families of K+ channels. Among these are a two-pore (K2P) leak-type and a G protein-coupled, inwardly rectifying (GIRK) channel, both inhibited by peptide hormones that stimulate aldosterone secretion. The K2P current displayed properties identifying it as TREK-1 (KCNK2). This outwardly rectifying current was activated by arachidonic acid and inhibited by angiotensin II (ANG II), adrenocorticotrophic hormone (ACTH), and forskolin. The activation and inhibition of TREK-1 was coupled to AZG cell hyperpolarization and depolarization, respectively. A second K2P channel, TASK-1 (KCNK3), was expressed at a lower density in AZG cells. Human AZG cells also express inwardly rectifying K+ current(s) (KIR) that include quasi-instantaneous and time-dependent components. This is the first report demonstrating the presence of KIR in whole cell recordings from AZG cells of any species. The time-dependent current was selectively inhibited by ANG II, and ACTH, identifying it as a G protein-coupled (GIRK) channel, most likely KIR3.4 (KCNJ5). The quasi-instantaneous KIR current was not inhibited by ANG II or ACTH and may be a separate non-GIRK current. Finally, AZG cells express a voltage-gated, rapidly inactivating K+ current whose properties identified as KV1.4 (KCNA4), a conclusion confirmed by Northern blot. These findings demonstrate that human AZG cells express K2P and GIRK channels whose inhibition by ANG II and ACTH is likely coupled to depolarization-dependent secretion. They further demonstrate that human AZG K+ channels differ fundamentally from the widely adopted rodent models for human aldosterone secretion.
Keywords: ACTH, angiotensin II, human adrenal glomerulosa, KCNJ5, TREK-1
INTRODUCTION
In mammals, adrenal zona glomerulosa (AZG) cells of the outer cortex secrete the mineralocorticoid aldosterone, which acts on distal tubule cells of the kidney, increasing Na+ resorption and K+ secretion, thereby regulating water and electrolyte balance and blood pressure (1). Angiotensin II (ANG II) and elevated serum K+ serve as the principal physiological stimuli for aldosterone secretion, whereas ACTH plays a lesser role (1–4).
Although AZG cells lack voltage-gated Na+ channels and do not store aldosterone in secretory vesicles, the ionic mechanisms that regulate hormone synthesis and secretion share similarities with traditional excitable secretory cells including neurons and neuroendocrine cells. Specifically, AZG cells from several species, including bovine and murine, maintain negative resting potentials determined by the membrane K+ permeability and distribution across the cell membrane, as predicted by the Nernst equation (3, 5–7). Further, aldosterone secretion in response to ANG II, K+, or ACTH is linked to membrane depolarization through the activation of voltage-gated Ca2+ channels (3, 5, 6, 8, 9).
The membrane depolarization itself may be mediated by ANG II or ACTH through the inhibition of specific K+ channels that set the resting potential of the cell (3, 5, 10). Increasing external [K+] in turn depolarizes AZG cells by shifting the Nernst equilibrium potential in the positive direction. Although depolarization-dependent aldosterone secretion appears to be coupled to the coordinated activity of Ca2+ and K+ channels, major questions remain unanswered. First, the molecular identities of many ion channels that regulate secretion by AZG cells of various species have not been identified. In this regard, although mammalian AZG cells may express members of each of the three major families of K+ channels, the specific channels could vary widely among species, conferring parallel differences in their electrical and secretory properties (3, 10–12).
Although systematic patch voltage clamp studies describing K+ currents in mammalian AZG cells are lacking, existing reports on mouse, rat, and bovine cells produced using a variety of techniques, indicate a clear difference in the types of channels expressed. In this regard, variability in the expression of specific K2P and KIR channels may be of particular significance. With regard to K2P channels, in whole cell patch clamp recordings, we discovered that bovine AZG cells robustly express TREK-1 K2P channels (3). In contrast, TREK-1 mRNA and protein are absent in murine AZG, where TASK-1 and TASK-3 are the predominant K2P channels (10–13).
K2P K+ currents have not yet been detected in whole cell recordings from normal human AZG cells. In the only whole cell patch clamp study of K+ currents in human AZG cells completed more than 25 yr ago, only two K+ currents were observed. Both of these were voltage gated and included a rapidly inactivating A-type current and a slowly activating, delayed rectifier (14).
Mammalian AZG cells may also differ in their expression of KIR channels. Although normal human AZG cells express mRNA and protein for the G protein-coupled KCNJ5 (KIR3.4) K+ channel, these are absent in murine AZG cells (10, 11, 15). Although these studies suggest that KCNJ5 channels function in the regulation of aldosterone secretion in humans but not in rats or mice, it is important to note that no KIR currents have ever been reported in whole cell recordings from AZG cells of any species. However, unidentified KIR channels have been reported in single-channel recordings from rat and bovine AZG cells (16).
Relatedly, it has been shown that a mutated form of KCNJ5 expressed in human adrenal adenomas induces a severe form of primary aldosteronism and associated hereditary hypertension (15). However, even though mRNA for these channels, which display enhanced Na+ permeability, is highly expressed in the adenomas, no KCNJ5 current has been recorded from the cells.
Overall, although murine AZG cells have been widely adopted as convenient models for the physiology of human aldosterone secretion, studies to date suggest major differences in the types of K+ channels found in mouse and rat, compared with human AZG cells. Consequently, because of their pivotal role in the physiology and pathophysiology of human aldosterone secretion, it will be important to identify the specific K+ channels expressed by human AZG cells and to determine how each functions in controlling membrane potential, Ca2+ entry, and secretion.
In whole cell patch clamp recordings, it was discovered that normal human AZG cells express members of each of the three major K+ channel families. The identity of several of these was established based on their biophysical and pharmacological properties, in conjunction with Northern blot analysis.
MATERIALS AND METHODS
Materials
Tissue culture media, antibiotics, fibronectin, and fetal bovine sera (FBS) were obtained from Invitrogen (Carlsbad, CA). Coverslips were from Bellco (Vineland, NJ). Phosphate-buffered saline (PBS), 1,2-bis-(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), MgATP, collagenase (C0130), DNase, forskolin, ACTH (1–24), ANG II, and arachidonic acid (AA) were obtained from Sigma-Aldrich (St. Louis. MO). Cinnamyl 1–3,4-dihydroxy-α-cyano-cinnamate (CDC) was purchased from Enzo Life Sciences (Farmingdale, NY). Tertiapin Q was purchased from Tocris, a Bio-Techne Corporation (Minneapolis, MN).
Isolation and Culture of AZG Cells
Human adrenals were obtained from 11 deceased organ donors (age 10–62 yr, male and female, of either White or Black race) through The Ohio State University Department of Transplant Surgery and Lifeline of Ohio within 3 h of organ removal from donor. Institutional Review Board and ethical consenting practices for donor tissue were strictly followed. Organs were kept in cold saline, on wet ice, until available to be collected. Several adrenal cell isolations (3/11) yielded cells that could not be used for our experiments, as evidenced by extremely fragile membranes and lack of recordable ion currents. However, we were able to develop a method for isolating and storing human AZG cells from a majority of the glands received (8/11) wherein they retained their biochemical and electrophysiological properties for a minimum of 18 mo. Briefly, glands with some surrounding fat were submerged in cold PBS, kept on ice, and transported within 3 h of removal from the donor to the laboratory. Fat was removed, and thin tissue slices were obtained using a Stadie-Riggs tissue slicer. The first slice containing mainly adrenal capsule and zona glomerulosa was used for the isolation of adrenal glomerulosa (AZG) cells. Subsequent slices were designated as fasciculata and were used to prepare adrenal zona fasciculata (AZF) cells. In 7 of 11 isolations, the adrenal medulla could be easily identified and manually dissected away from the cortex. In the remaining cases, the medulla, as observed under a dissecting microscope, appeared to infiltrate the cortex, and was thus more difficult to separate from the outer layers of the adrenal cortex. This characteristic was not related to age, sex, or race of the donor. Cortical slices were finely chopped into 1- to 2-mm fragments, followed by two incubation periods of 60 min in DMEM/F12 containing collagenase (Sigma C0140, 2 mg/mL) and DNase (Sigma DN25, 0.5 mg/mL). Cells were then disrupted by gentle aspiration with a sterile, fire-polished Pasteur pipette before being filtered using a cell dissociation sieve (Sigma CD-1, #60 mesh) and centrifuged for 5 min at 100 g. The cell pellet was washed twice with DMEM/F12; resuspended in DMEM/F12 (1:1), 10% FBS, 100 U/mL penicillin, 0.1 mg/mL streptomycin, antioxidants α-tocopherol (1 μM), 20 nM selenite and 100 μM ascorbic acid (DMEM/F12+); and either plated for immediate use or re-suspended in FBS/5% DMSO, divided into 1-mL aliquots, and frozen (initially at −80°C for 24 h, then at −196°C in a Thermolyne Locator Plus liquid nitrogen canister) for future use. To ensure cell attachment when culturing cells, glass coverslips (Bellco, 9 × 9 mm) in 35 mm dishes were treated with fibronectin (10 μg/mL) at 37°C for 30 min then rinsed with warm, sterile PBS immediately before adding cells. Cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Measurement of Ion Channel mRNA
RNeasy columns (Qiagen, Valencia, CA) that had been treated with RNase-free DNase (Qiagen, Valencia, CA) to remove genomic contamination were used to extract total RNA from AZG cells that had been isolated and cultured in DMEM/F12+, as described above, for 24 h. Ten micrograms of total RNA/lane was separated on an 8% formaldehyde, 1.0% agarose gel, then transferred to a nylon membrane (Gene Screen Plus, NEN). RNA was fixed to the membrane by UV-crosslinking. Northern blots were prehybridized for 2 h at 42°C in ULTRAhyb (Ambion, Austin, TX), hybridized with each [α 32P]dCTP-labeled probe for 18 h, and washed, as previously described (17). Specific probes as follows: 1.3-kb PVUII fragment for the KCNA4 probe (coding for the Kv1.4 K+ channel), obtained as previously described (18); 700 bp KCNK2 probe for the TREK-1 channel, obtained by ECOR1 digest of the full-length KCNK2 cDNA as previously described (19); KCNK9 probe for the TASK-3 channel, obtained from EcoR1 digest of purchased KCNK9 cDNA clone (MGC:103976 IMAGE:30915383, Open Biosystems/Thermo Fisher Scientific); KCNJ5 probe (for the GIRK4 channel), obtained from the ECOR1 digest of KCNJ5 cDNA clone (MGC:104097 IMAGE:30915550, Open Biosystems/Thermo Fisher Scientific); specific ∼1 Kb probes for KCNJ3 (for the GIRK1 channel) and KCNK3 (TASK-1 channel) were obtained from their respective cDNAs (#SC332750 and SC118713, OriGene Technologies, Inc.). Northern autoradiograms were imaged using a Typhoon 9200 variable mode phosphorimager after either 4-h or 72-h exposure to phosphorimaging screen (GE Lifecare Health Science).
Patch Clamp Experiments
Cellular identification for patch clamp experiments.
Precautions were taken to ensure that the cells selected for recording were AZG cells. First, within the AZG fraction, we selected only smaller cells (Cp < 15 pF), as AZG cells are typically smaller with capacitance <20 pF. Second, in whole cell patch voltage clamp recordings, we found that human subcapsular cells from the AZG fraction expressed a distinctive inwardly rectifying K+ current, which was not present in the great majority of AZF cells, as we have previously reported (20).
K+ currents.
Patch clamp recordings of K+ channel currents were made in the whole cell configuration from human subcapsular adrenocortical cells. The standard external solution consisted of 141.5 mM NaCl, 5 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 10 mM HEPES, and 5 mM glucose, with pH adjusted to 7.3 using NaOH. The standard pipette solution consisted of 120 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 11 mM BAPTA, 10 mM HEPES, 5 mM ATP, and 200 µM GTP, with pH titrated to 6.8–7.2 using KOH. All solutions were filtered through 0.22 µM cellulose acetate filters.
Recording conditions and electronics.
Cells were used for patch clamp experiments 2–48 h after plating. Coverslips were transferred from 35-mm culture dishes to the recording chamber (volume: 1.5 mL) that was continuously perfused by gravity at a rate of 3–5 mL/min. For whole cell recordings, patch electrodes with resistances of 1.0–2.0 MΩ were fabricated from Corning 0010 glass (World Precision Instruments, Sarasota, FL). These electrodes routinely yielded access resistances of 1.5–4.0 MΩ and voltage-clamp time constants of <100 µs. K+ currents were recorded at room temperature (22°–25°C) according to the procedure of Hamill et al. (21) using a List EPC-7 patch clamp amplifier. To minimize series resistance errors, lower resistance electrodes (<1.5 MΩ) were chosen for measurement of voltage-dependent gating and kinetic properties.
Pulse generation and data acquisition were done using a personal computer and PCLAMP software with Digidata 1200 interface (Axon Instruments, Inc., Burlingame, CA). Currents were digitized at 2–50 KHz after filtering with an 8-pole Bessel filter (Frequency Devices, Haverhill, MA). Linear leak and capacity currents were subtracted from current records using summed scaled hyperpolarizing steps of 1/4 or 1/5 pulse amplitude applied from a potential of −60 mV. Data were analyzed using CLAMPFIT 9.2 (Molecular Devices, Sunnyvale, CA) and SigmaPlot (version 11.0) software. Data in the Results are expressed as means ± SE. Drugs were applied by bath perfusion, controlled manually by a six-way rotary valve.
RESULTS
Human AZG Cells Express Multiple K+ Channels
In whole cell patch clamp recordings from human adrenocortical subcapsular cells, multiple types of K+ currents were detected in nearly every cell. These currents displayed properties representing each of the three major K+ channel families. Importantly, included among these was an inwardly rectifying current. Until now, KIR currents had not been reported in whole cell recordings from adrenocortical cells of any species. Furthermore, in a previous study, we discovered that KIR currents were not detectable in the great majority of human AZF cells (20). Therefore, the robust expression of inward rectifier K+ currents in human subcapsular adrenocortical cells indicates that these channels may be a hallmark of human AZG cells.
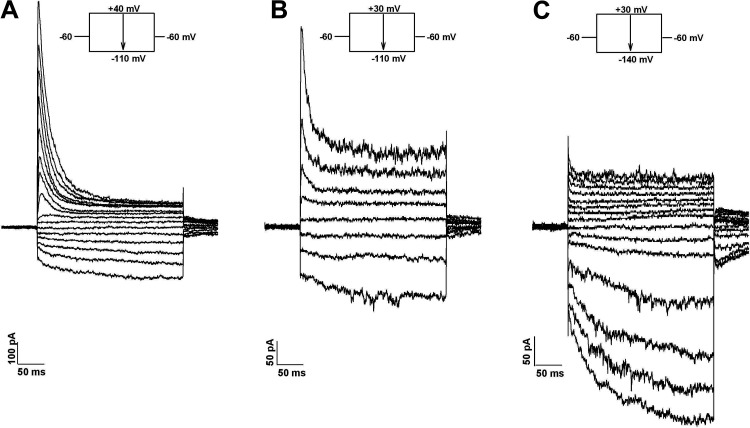
When current-voltage relationships were obtained from AZG cells at test potentials between −140 and +40 mV from a holding potential of −60 mV, each of three distinct K+ currents was typically present in varying amounts, as illustrated in Fig. 1. The traces in Fig. 1A were obtained from a cell expressing large voltage-gated A-type and a KIR current that included instantaneous and time-dependent components.
Figure 1.
K+ currents of human AZG cells. I-V relationships. Whole cell K+ currents were activated at 30 s intervals by voltage steps of 300 ms duration in 10 or 20 mV increments to test potentials between +40 mV and −140 mV from a holding potential of −60 mV. A: current traces at potentials between +40 and −110 mV in 10 mV increments. B: current traces at potentials between +30 and −110 in 20 mV increments. C: current traces at potentials between +30 mV and −140 mV in 10 mV increments. Number of independent experiments: n = 33.
By comparison, the traces in Fig. 1B were recorded from a cell that, in addition to A-type and KIR currents, also expressed a prominent noninactivating, outwardly rectifying current resembling TREK-1 of human and bovine AZF and bovine AZG cells (3, 19, 20, 22). Figure 1C shows traces from a cell expressing a very large KIR and smaller leak and voltage-gated currents. The time-dependent component of the KIR current at −140 mV was fit with a single exponential time constant of 71.3 ms.
A series of experiments was done to further characterize each of these K+ currents with respect to their voltage-dependent gating, kinetics, rectification, and modulation by pharmacological agents and peptide hormones, including ANG II and ACTH, which physiologically regulate aldosterone secretion. These studies were valuable in establishing the molecular identity of these channels and their possible role in aldosterone secretion.
Voltage-Gated A-Type K+ Current
When K+ currents were activated by depolarizing voltage steps applied from a holding potential of −80 mV, a rapidly inactivating K+ current was observed in nearly every cell at test potentials positive to −50 mV (Fig. 2A). This current could be isolated by recording currents immediately upon obtaining the whole cell configuration, before the outwardly rectifying noninactivating leak-type current developed or by recording from cells where the leak-type current was absent. Alternatively, the rapidly inactivating A-type K+ current was recorded from cells in which the outwardly rectifying, noninactivating current had been completely inhibited by ACTH. As we previously observed in human AZF cells, and as we now report for AZG cells (see Fig. 9), ACTH had no effect on this transient K+ current (20). Overall, the A-type K+ current density recorded at a test potential of +20 mV was 87.2 ± 8.5 pA/pF (n = 100). Experiments were done to characterize the voltage-dependent gating and kinetic properties of this current.
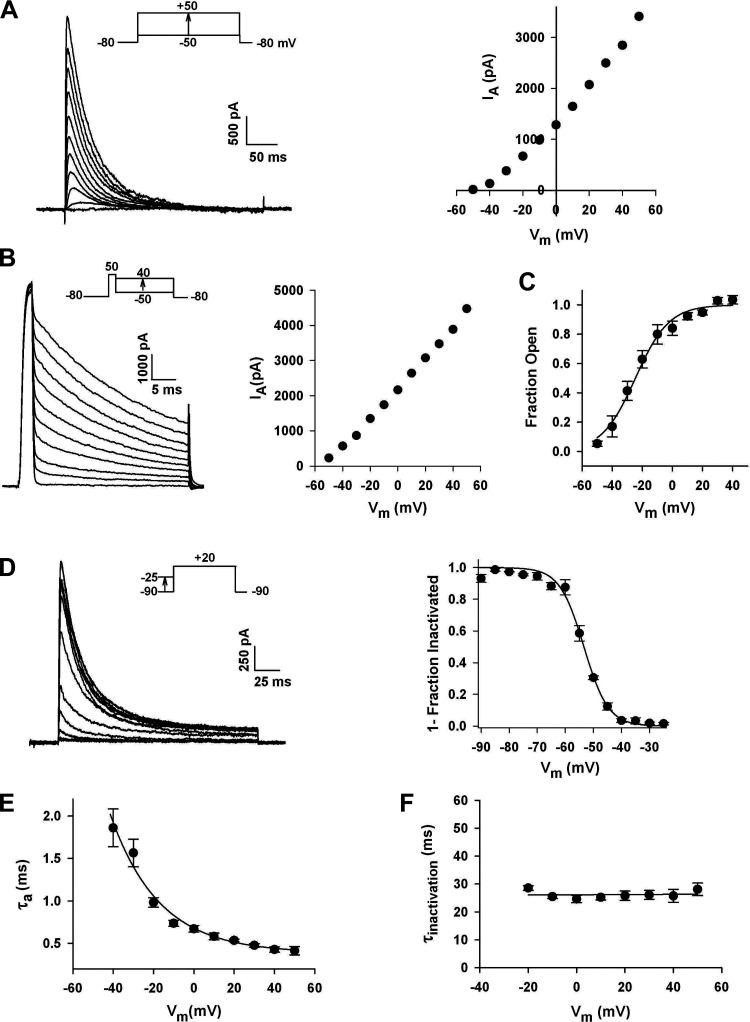
Figure 2.
Voltage-dependent gating and kinetics of A-type K+ currents in human adrenal zona glomerulosa (AZG) cells. A: I-V relationship: whole-cell K+ currents were activated by voltage steps to various test potentials applied at 30 s intervals from a holding potential of −80 mV. Peak current amplitudes are plotted against test potential from corresponding current traces at left. B: open channel I-V relationship: after activating IA with a 3-ms voltage step to +50 mV from a holding potential of −80 mV, membrane potential was stepped to various values between +40 and −70 mV where decaying currents were recorded. C: activation: current amplitudes from the II-V are plotted against test voltage. The voltage-dependence of IA channel activation was determined by dividing peak current amplitudes derived from the steady-state I-V by corresponding values from the II-V. These values were plotted as the fraction of open channels against voltage and fit by a Boltzmann function of the form: Fraction Open = 1/[1+exp(v½ − v)/k] where v½ is the voltage at which one half of the channels are in the open conformation and k is the slope factor. Curve was fit to data points at test potentials between −60 and +40 mV for n = 5. D: inactivation: the voltage-dependence of steady-state inactivation was measured by applying 10 s prepulses to potentials between −90 and −25 mV. Normalized current (means ± SE) for four cells was plotted against conditioning voltage and fitted with the equation I/IMAX = 1/[1 + exp(v − v½)/k] where IMAX is the current activated from a holding potential of −90 mV and v½ is the potential at which half of the channels are inactivated. E: voltage-dependent activation kinetics of IA: ascending portions of current traces from I-V protocols were fit with an equation of the form I = I∞[1 − exp(−Τ/τa)]N[exp(−Τ/τi)] where τa is the activation time constant, τi is the inactivation time constant, and N is an integer between 1 and 5. τa values at potentials from −40 to +50 mV are plotted as means ± SE for seven cells. Points relating τa and membrane voltage were fit with a single exponential. F: inactivation kinetics. IA currents were activated by voltage of varying size from a holding potential of −80 mV. Inactivation time constants (τi) were determined at each test potential by fitting the decaying phase of each current with a single exponential. τi values averaged from five cells are plotted against test voltage as means ± SE. Data were analyzed with least squares linear regression analysis to yield a line with slope of zero and intercept of 26.1 ± 0.4 ms. Number of independent experiments: n = 87 (A); n = 5 (B); n = 8 (C).
The voltage-dependence of IA activation was determined by dividing the peak amplitudes, measured in the steady-state I-V relationship by corresponding amplitudes from the instantaneous I-V (II-V) relationship, as previously described (20, 23). The II-V, or open channel I-V, provides a measure of membrane conductance at different potentials, after all available channels have been activated by a brief, strong depolarization. The II-V for IA was obtained by activating channels with 3 ms depolarizing steps to +50 mV, after which the membrane potential was stepped to new levels between +40 and −50 mV. The K+ current at each test voltage was then measured after 1 ms before a significant change in the number of open channels occurred (Fig. 2B).
Values calculated by dividing peak current amplitudes from the steady-state I-V by corresponding amplitudes from the II-V were plotted as the fraction of open channels against test potential and fit by a Boltzmann function of the form:
where v½ is the voltage at which half of the channels are in the open conformation, and k is the slope factor (Fig. 2C). Curves were fit to data points acquired at test potentials between −50 and +50 mV. The activation function had a midpoint of −24.6 mV, and a k of 11.66 ± 1.25 mV per e-fold change in the fraction open (Fig. 2C).
The voltage-dependence of steady-state inactivation of the A-type K+ current was determined by applying 10 s conditioning pulses to potentials between −90 and −25 mV in 5 mV increments, followed by activating voltage steps to +20 mV. The normalized current was plotted as a function of the conditioning voltage and fitted with the equation:
where IMAX is the current activated from a holding potential of −90 mV. IA inactivation varied as a steep function of voltage with v½ equal to −53.4 ± 0.47 mV (n = 4), and a slope factor of 4.34 ± 0.40 mV (n = 4) per e-fold change in the fraction inactivated (Fig. 2D).
The voltage-dependent kinetics of activation and inactivation of the human AZG cell IA current were measured in whole cell recordings. For activation, current traces from I-V protocols were fit with an equation of the form:
where τa and τi are activation and inactivation time constants and N is an integer between 1 and 5. IA activation kinetics were sigmoidal, voltage dependent, and accelerated by stronger depolarizations. In Fig. 2E, τa is plotted against voltage at 10 different test potentials. Onset kinetics was best fit by integer values of N of 4 or 5. τa varied from 1.86 ± 0.22 to 0.41 ± 0.05 ms (n = 7) at test potentials of −40 mV and +50 mV, respectively. The function relating τa and membrane voltage potential could be expressed as a single exponential with an e-fold change per 24.4 mV and a voltage-independent offset of 0.37 ms.
Rapid inactivation is a signature property of A-type K+ currents. Inactivation kinetics of the human AZG IA current could be fit with a single voltage-independent time constant (τi). In Fig. 2F, averaged τis, determined from measurements from five separate cells, are plotted against test voltages from −20 to +50 mV. Least squares linear regression analysis of these data yielded a line with a slope not significantly different from zero, and an intercept of 26.1 ms. Overall, with respect to voltage-dependent gating and kinetics, the A-type current in human AZG cells was nearly identical to the KV1.4 (KCNA4) current of bovine and human AZF cells (20, 24). Accordingly, Northern blot analysis showed that Kv1.4 (hKCNA4) mRNA was highly expressed in the human AZG (Fig. 12).
K2P K+ Channels
In addition to the voltage-gated KV1.4 current, we found that AZG cells express K2P leak-type K+ channels. When K+ currents were recorded in response to voltage steps to +20 mV from a holding potential of −80 mV, a noninactivating current was present in a majority of cells and typically grew to a maximum value over a period of 10–20 min (Fig. 3A). The noninactivating component of K+ current could be measured near the end of a 300-ms test pulse when KV1.4 had completely inactivated. A spontaneously increasing, noninactivating component of K+ current appeared in 70.1% (61/87) of human AZG cells.
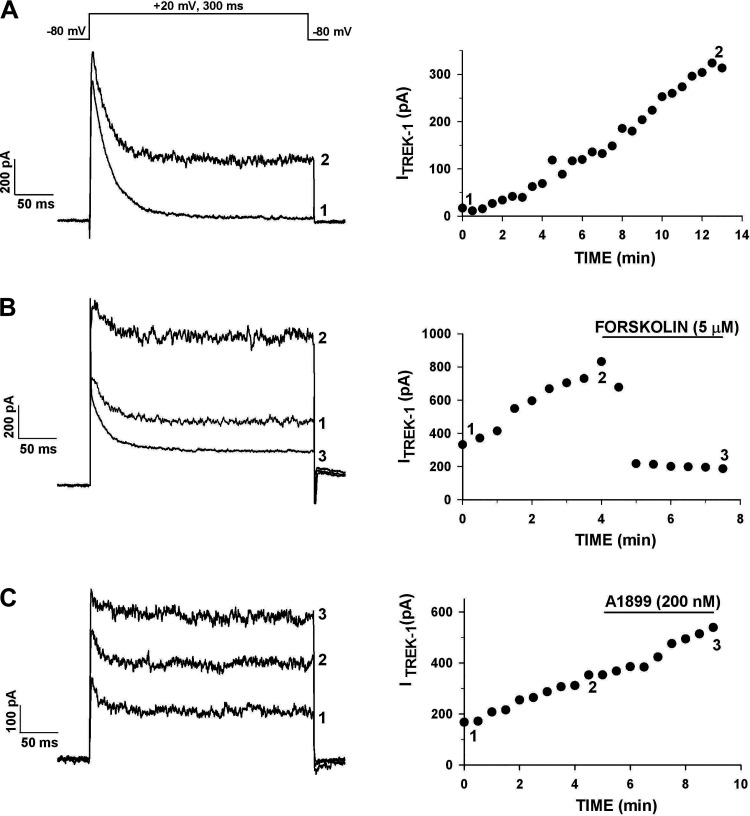
Figure 3.
Human AZG cells express a noninactivating K+ current inhibited by forskolin. K+ currents were activated by voltage steps to +20 mV applied at 30 s intervals from a holding potential of −80 mV. Current amplitudes are plotted against time at right. Numbers on graph correspond to those on traces at left. A: currents in control saline. B: forskolin inhibition: cell was superfused with 5 µM forskolin, as indicated. C: effect of A1899. Cell was superfused with 200 nM A1899, as indicated. Number of independent experiments: n = 6 (A); n = 6 (B); n = 8 (C).
Experiments were done to determine the molecular identity of the K2P channel(s) that produced the noninactivating K+ current in human AZG cells. This current resembled the TREK-1 current of human AZF cells and bovine AZF and AZG cells (3, 19, 20, 22). Importantly, TREK-1 and TREK-2 (KCNK10) are the only members of the 15-channel member K2P family that are inhibited by cAMP (25). Although the TREK-1 gene is highly expressed in the human adrenal cortex, TREK-2 is nearly undetectable (26). Northern blot analysis showed that TREK-1 mRNA was readily detected in AZG cells (Fig. 12).
The diterpine adenylate cyclase-activator forskolin effectively inhibited the noninactivating K+ current in human AZG cells but had no effect on KV1.4 (Fig. 3B). In six AZG cells, forskolin inhibited the K2P current by 81.8% ± 8.0%. The specific inhibition of a large fraction of the noninactivating K+ current by forskolin indicated that it was largely composed of TREK-1.
TASK-1 (KCNK3) has been reported to be strongly expressed in the human adrenal (11, 26). Accordingly, we found that TASK-1 mRNA was expressed in the human AZG (Fig. 12). TASK-1 currents have not been described in human AZG cells. The specific TASK-1 K+ channel antagonist A1899 (IC50 = 35 nM) was used to determine to what extent, if any, these channels contributed to the non-inactivating K+ current in human AZG (27). This agent when applied at 200 nM, significantly inhibited the noninactivating K+ current in only two of eight AZG cells. Overall, in these eight cells A1899 inhibited this current by only 12.2% ± 3.6% (Table 1). In the experiment illustrated in Fig. 3C, A1899 (200 nM) failed to alter the growing, noninactivating K+ current. Experiments with forskolin and A1899 indicated that TREK-1 is the dominant K2P current in human AZG cells.
Table 1.
Modulation of AZG K+ currents
| Percent Inhibition |
||
|---|---|---|
| K2P | KIR | |
| ANG II (2–10 nM) | 70.0 ± 8.9 (n = 6) | 89.4 ± 6.5 (n = 4) |
| Adrenocorticotrophic hormone (500 pM–1 nM) | 89.9 ± 5.1 (n = 5) | 61.9 ± 7.8 (n = 4) |
| Forskolin (5 µM) | 81.8 ± 8.0 (n = 6) | 74.1 ± 9.8 (n = 3) |
| A1899 (200 nM) | 12.2 ± 3.6 (n = 8) | |
| Tertiapin Q (500 nM) | 43.9 ± 6.6 (n = 4)* | |
| Percent Increase |
|
|---|---|
| K2P | |
| Arachidonic acid (20 µM) | 4,320 ± 937 (n = 6) |
| Cinnamyl 1-3,4-dihydroxy-α-cyano-cinnamate (10 or 20 µM) | 724 ± 163 (n = 6) |
Values are means ± SE. Shown are the inhibition and increase of two-pore K+ (K2P) and inwardly rectifying K+ (KIR) currents in human adrenal zona glomerulosa (AZG) cells. *KIR instantaneous component.
In 21/87 AZG cells, the noninactivating K+ current failed to spontaneously increase with time and was nearly undetectable. Of the 15 K2P channels, TREK and TRAAK are the only ones that are activated by arachidonic acid (AA) (25, 28). AA (10–20 µM) produced remarkable increases in the noninactivating K+ current in human AZG cells, even when this current was undetectable under control conditions. In the experiment illustrated in Fig. 4A, only the KV1.4 current was activated in response to depolarizing steps in control saline. Superfusing the cell with AA (20 µM) rapidly inhibited the KV1.4 current and induced a slower increase in the noninactivating K+ current, which reached an amplitude of 3.5 nA after 15 min. Overall, in six human AZG cells, AA (20 µM) increased this current by an average of 4,320% (Table 1).
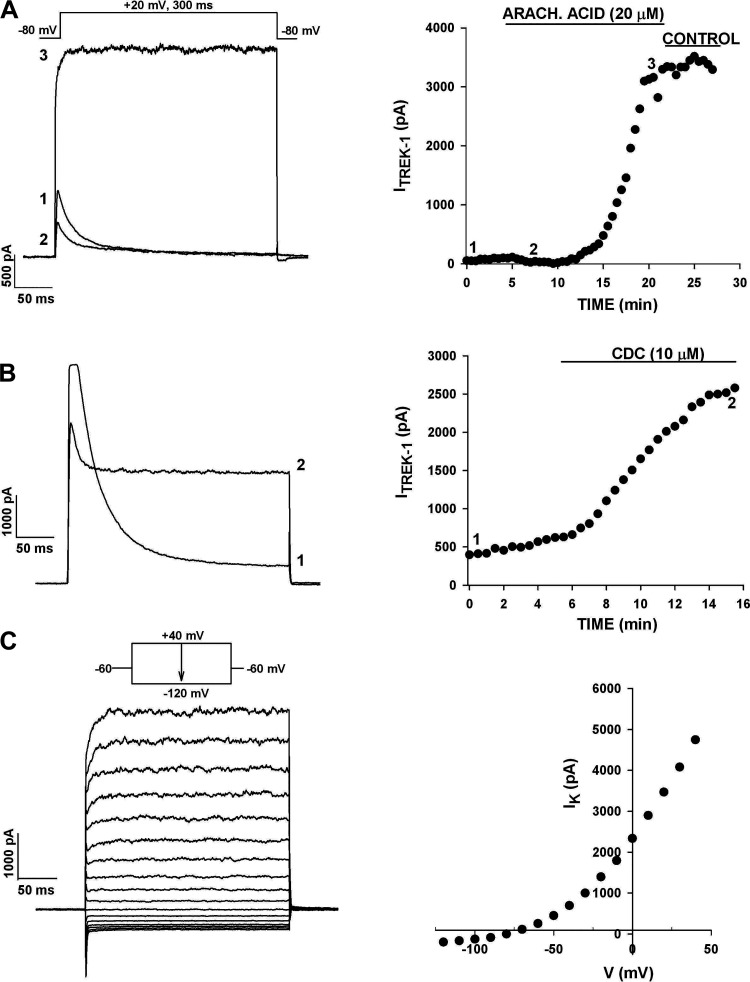
Figure 4.
Arachidonic acid (AA) and cinnamyl 1–3,4-dihydroxy-α-cyano-cinnamate (CDC) activate the leak-type K+ current in human AZG cells. Whole cell recordings of K+ currents were made from human AZG cells that had been in culture for up to 48 h. After recording K+ currents in standard saline, cells were superfused with AA or CDC. A: effect of AA. K+ currents were activated by voltage steps to +20 mV applied at 30 s intervals from a holding potential of −80 mV. After 5 min, the cell was superfused with 20 µM AA. Numbers on current traces correspond to those in the plot of current amplitudes against time at right. B: effect of CDC. K+ currents were recorded, as described in A. After 6 min, the cell was superfused with 10 µM CDC. Numbers on current traces correspond to those on plot of current amplitudes at right. C: CDC and I-V. Whole cell K+ currents were recorded from the same cell as in B by applying voltage steps of 300-ms duration in 10 mV increments to test potentials between +40 and −110 mV from a holding potential of −60 mV. Current amplitudes measured at the midpoint of the traces at left are plotted against test potential at right. Number of independent experiments: n = 6 (A); n = 6 (B and C).
The caffeic acid derivative cinnamyl 1–3,4-dihydroxy-α-cyanocinnamate (CDC) activates TREK-1 channels in bovine AZF and AZG and human AZF cells (3, 20, 29). It was discovered that CDC induced large increases in the noninactivating K+ current of human AZG cells, while also inhibiting the KV1.4 current. In the experiment illustrated in Fig. 4B, superfusing the cell for 10 min with CDC (10 µM) increased the noninactivating component of K+ current from a control value of 560–2,530 pA. A current-voltage relationship obtained from the same cell at test potentials between −120 and +40 mV revealed that the CDC-induced, outwardly rectifying current was indistinguishable from the TREK-1 current activated by CDC in the abovementioned adrenocortical cells (Fig. 4C). Overall, in six human AZG cells, CDC (10 μM) increased this current by an average of 724% (Table 1). Experiments with AA and CDC indicated that TREK-1 K+ channels are highly expressed in human AZG cells and that these channels may exist in an inactive state when currents are recorded at room temperature.
Inwardly Rectifying K+ Currents
Inwardly rectifying K+ currents (KIR) have not previously been reported to be present in whole cell patch clamp recordings from AZG cells, nor have they been recorded from human H295-R cells, a cell line often used as a model for human AZG cells (30). We found that KIR currents were robustly expressed in 92.9% (91/98) of normal human adrenal subcapsular cells. The size of the inwardly rectifying current increased with the [K+]O. Furthermore, the reversal potential of this current varied with [K+]O as predicted by the Nernst equation for a channel selectively permeable to K+ (Fig. 5, A and B). In a previous study, we found that KIR currents were not detectable in the great majority of human AZF cells under identical recording conditions (20). In a random sample of cells from the AZF fraction, we now report that KIR currents were present in only 8 of 56 cells. These results indicate that KIR channels are a prominent, and perhaps identifying, feature of human AZG cells.
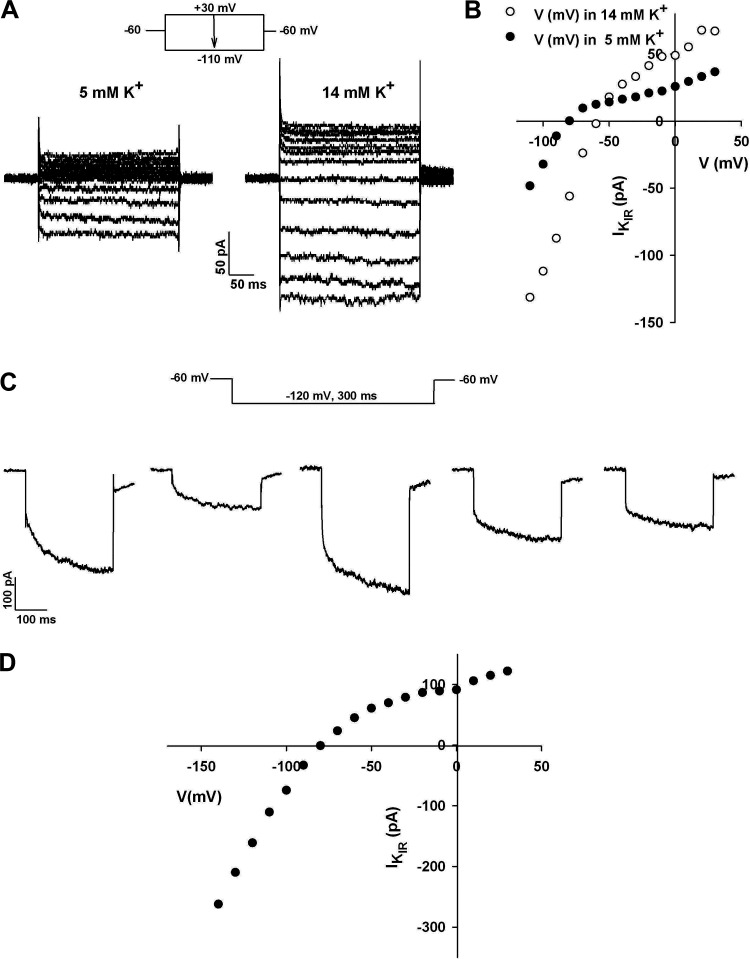
Figure 5.
KIR of human adrenal zona glomerulosa (AZG) cells. A: K+ selectivity. I-V relationships were obtained for the inwardly rectifying current of human AZG cells in external solutions containing 5 mM or 14 mM K+. Current traces in response to voltage steps of 300-ms duration applied at 30 s intervals from a holding potential of −60 mV to test potentials from +30 to −110 mV in 5 mM or 14 mM K+. B: current amplitudes recorded in 5 mM and 14 mM K+ are plotted against test potential for the traces in A. C: instantaneous and time-dependent components. KIR current traces from five separate cells activated by test potentials to −110 mV from a holding potential of −60 mV. D: current-voltage relationship. Current amplitudes are plotted against test potential for traces in Fig. 1C at voltages from −140 to +30 mV. Number of independent experiments: = 5 (A and B); n = 5 (C and D).
In most cells, the KIR current included both instantaneous and time-dependent components, which were present in varying proportions. Figure 5C shows KIRs recorded from five different human AZG cells at a test potential of −120 mV. For each cell, the time-dependent component of KIR could be fit with a single exponential time constant (τa), which averaged 75.7 ± 3.5 ms (n = 5).
Recordings of I-V relationships from cells expressing large time-dependent, inwardly rectifying currents also displayed reversal potentials expected for channels selectively permeable to K+. Figure 5D shows that a plot of current amplitudes against voltages for traces from the first cell in Fig. 5C exhibited a reversal potential of −80 mV.
Although the molecular identity of the KIR current in human AZG cells has not been determined, KCNJ5 mRNA and protein have been reported to be expressed in the adrenal cells (15, 26). Accordingly, we found that KCNJ5 mRNA is robustly expressed in human subcapsular adrenal cells obtained from kidney donors (Fig. 12). In other cell types, such as those of the sinoatrial node, KIR channels are composed of KCNJ3 (GIRK1) and KCNJ5 (GIRK4) heteromultimers (31, 32). However, in Northern blot analysis, we found that KCNJ3 mRNA was undetectable in human AZG mRNA (Fig. 12). Moreover, in a study of the human adrenal gland proteome, KCNJ5 (KIR3.4) was the only member of the G protein-coupled, inwardly rectifying potassium channel subfamily whose mRNA and protein were expressed in the human adrenal gland (26).
Inhibition of K2P and KIR Currents by ANG II
Since the K+ channels that we have identified in human AZG cells may set the resting membrane potential and couple the activation of ANG II and ACTH receptors to electrical events leading to depolarization-dependent Ca2+ entry and aldosterone secretion, it was important to determine whether these peptide hormones control the activity of specific K+ channels. It was discovered that ANG II inhibited at least two types of K+ currents in human AZG cells.
In whole cell recordings, ANG II (2–10 nM) inhibited the outwardly rectifying leak-type K+ current, but had no effect on the voltage-gated KV1.4 current. In the experiment illustrated in Fig. 6, A and B, the non-inactivating component of K+ current in an AZG cells grew from near zero to ∼600 pA over a period of 10 min, after which superfusion of 2 nM ANG II inhibited this current almost completely within 5 min. In contrast, KV1.4 was not altered. Overall, in a total of six experiments, ANG II (2–10 nM) inhibited the outwardly rectifying leak-type K+ current by 70.0 ± 8.9% (n = 6) (Table 1). Inhibition of this current was poorly reversible. In three cells, washing for 20 min with control saline reversed the ANG II -mediated block by only 15.8 ± 6.2% The ANG II -inhibited current strongly resembled the TREK-1 current in other bovine and human adrenocortical cells (3, 19, 20, 22). Since TREK-1 appears to be the predominant K2P current expressed by human AZG cells, it is likely to have been the major current inhibited by ANG II.
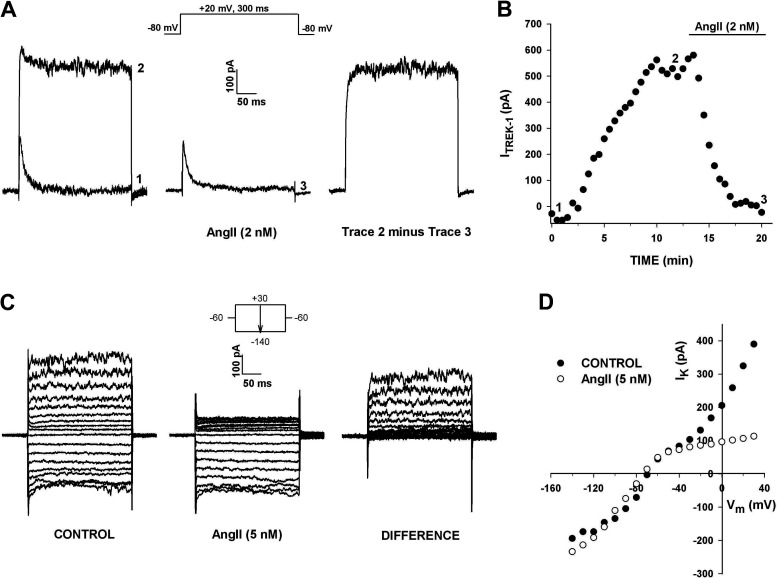
Figure 6.
Selective inhibition of K2P current by ANG II. Whole cell K+ currents were recorded in response to voltage steps to +20 mV applied at 30 s intervals from a holding potential of −80 mV before and after superfusing cell with 2 nM ANG II. A: current traces recorded immediately after initiating recording (1), when K+ current reached maximum amplitude (2), and after inhibition by 2 nM ANG II (3). Digital subtraction of trace 3 from trace 2 shows the ANG II -inhibited current. B: plot of current amplitudes, measured near the end of test pulse, against time. Numbers on graph correspond to those on traces in A. C and D: selective inhibition of K2P current. Effect of ANG II on K+ current I-Vs. K+ currents were recorded from human adrenal zona glomerulosa (AZG) cell in response to voltage steps between +40 and −140 mV, applied from a holding potential of −60 mV in 10 mV increments. C: traces show currents in standard saline (control), 5 nM ANG II, and the difference obtained by digitally subtracting currents in ANG II from control currents. D: I-V plots in control saline and after 5 nM ANG II. Current amplitudes before and after ANG II are plotted against test voltage. Number of independent experiments: n = 6 (A and B); n = 3 (C and D).
ANG II also had an inhibitory effect on the KIR current of AZG cells. However, this inhibition was selective for the time-dependent component of KIR. The experiment illustrated in Fig. 6, C and D, shows I-V relationships obtained from a cell that expressed only the instantaneous component of KIR, in addition to the outwardly rectifying K2P current. The current traces and associated I-V plots show that superfusion of the cells with ANG II (5 nM) produced near-complete inhibition of the K2P current at every test potential. By comparison, KIR was nearly unaffected at any voltage.
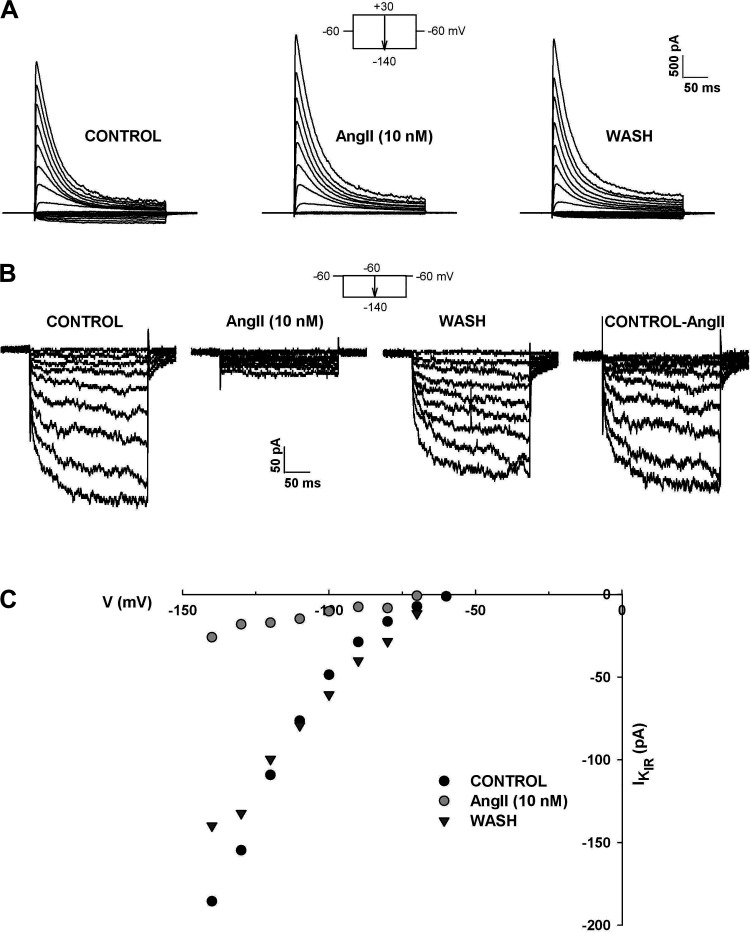
In contrast to its ineffectiveness as an inhibitor of the instantaneous component of KIR, ANG II potently and effectively inhibited the time-dependent component of this current. The experiment illustrated in Fig. 7, A and B, shows current traces from a cell that expressed a large voltage-gated A-type K+ current and an inward rectifier. The KIR current included a large time-dependent component that could be fit with a single exponential time constant of 68.2 ms at a test potential of −120 mV (Fig. 7B). Current-voltage relationships obtained from a holding potential of −60 mV showed that, while ANG II failed to alter the voltage-gated A-type current, it blocked the time-dependent component of KIR at every test potential (Fig. 7, A–C). The inhibition of KIR by ANG II was rapidly reversible, in contrast to its poorly reversible effect on K2P currents in these cells (Fig. 7, B and C). This difference in reversibility suggests that inhibition of the two currents occurs through different mechanisms. In four cells, ANG II (10 nM) inhibited the time-dependent component of KIR by 89.4% ± 6.5% (Table 1).
Figure 7.
Selective inhibition of KIR by ANG II. Whole cell K+ currents were recorded in response to voltage steps to test potentials from +30 to −140 mV applied at 30 s intervals from a holding potential of −60 mV. I-Vs were recorded in standard external solution and in 10 nM ANG II and after washing in control saline. A: I-V traces in control saline, 10 nM ANG II and after washing, as indicated. B: amplified KIR traces at test potentials from −60 mV to −140 mV from the same cells as in (A). currents labeled Control-ANG II show KIR blocked by ANG II. C: KIR IVs. Current amplitudes for KIR current shown in B are plotted against test potential under the three conditions indicated. Number of independent experiments: n = 4.
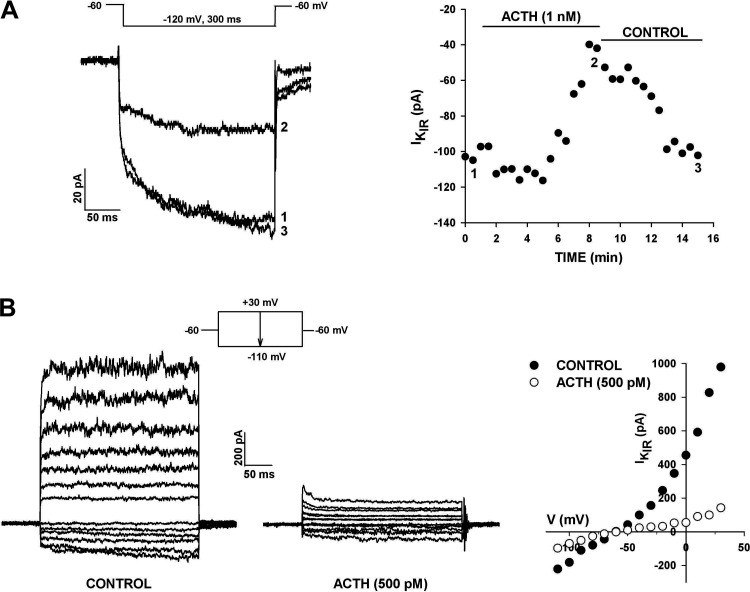
Inhibition of K+ Currents by ACTH and Forskolin
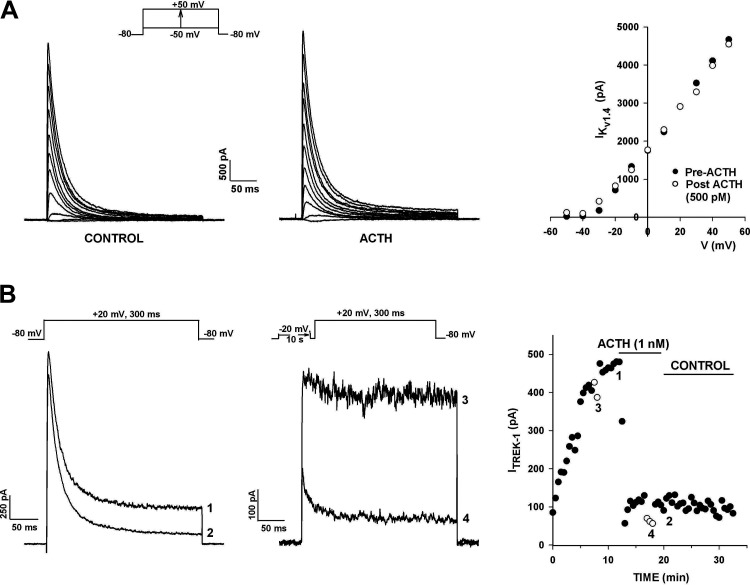
ACTH inhibited multiple K+ channels in human AZG cells. However, similar to ANG II, ACTH had no effect on the KV1.4 K+ current. Accordingly, current-voltage relationships for KV1.4 recorded in the absence and presence of ACTH (500 pM) were nearly superimposable (Fig. 8A).
Figure 8.
Effect of ACTH on human adrenal zona glomerulosa (AZG) K+ currents. A: A-type K+ currents. K+ currents were activated by voltage steps to test potentials from −50 to +40 mV, applied at 30 s intervals from a holding potential of −80 mV. Current traces were recorded before and after superfusing cell with ACTH. Peak current amplitudes in the absence and presence of 500 pM ACTH are plotted against test potential. B: K2P current. K+ currents were recorded in response to voltage steps to +20 mV applied at 30 s intervals from a holding potential of −80 mV with or without 10 s prepulses to −20 mV. Noninactivating current amplitudes with (open circles) or without (solid circles) prepulses are plotted against time. Numbers on plot correspond to those on traces before and after 1 nM ACTH. Number of independent experiments: n = 4 (A); n = 5 (B).
In contrast to its lack of effect on the KV1.4 current, ACTH potently inhibited the outwardly rectifying leak-type K+ current. In the experiment illustrated in Fig. 8B, ACTH (1 nM) inhibited the noninactivating current, recorded using two separate voltage protocols, by 81.0%. In one of these, KV1.4 was inactivated by a 10-s prepulse to −20 mV. Inhibition of the leak-type current was not reversed by washing for 10 min with control saline. In similar experiments, ACTH (500 or 1 nM) inhibited this current by 89.9 ± 5.1% (n = 5) by a mechanism that was poorly reversible (Table 1). Since ACTH activates adenylate cyclase, it is highly likely that the ACTH-sensitive current in these experiments was TREK-1.
ACTH also preferentially inhibited the time-dependent component of KIR in AZG cells. However, in contrast to its effect on K2P current, the inhibition of KIR by ACTH was rapidly reversible (Fig. 9A). These results suggest that the inhibition of K2P and KIR currents in human AZG cells by ACTH occur through different mechanisms, as is the case for ANG II. In this same cell, the KIR current was also reversibly inhibited by ANG II, demonstrating that individual AZG cells express both ACTH and ANG II receptors, each of which when activated reversibly inhibits the same KIR current (data not shown).
Figure 9.
Effect of ACTH on human AZG KIR. A: KIR current. K+ currents were recorded in response to voltage steps to −120 mV applied at 30 s intervals from a holding potential of −60 mV. KIR current amplitudes are plotted against time. Numbers on plot correspond to those on current traces in the absence and presence of 1 nM ACTH. B: combined K2P and KIR currents. K+ currents were recorded in response to voltage steps to test potentials between +30 mV and −110 mV in 10 mV increments at 30 s intervals from a holding potential of −60 mV. Current amplitudes at test potentials in the absence and presence of 500 pM ACTH are plotted against time at right. Number of independent experiments: n = 3 (A and B).
ACTH inhibited both K2P and KIR K+ currents in the same cell over a wide range of test potentials. In the experiment illustrated in Fig. 9B, ACTH (500 pM) inhibited the leak-type current by 96%–100% at test potentials between 0 and +40 mV. ACTH also inhibited the KIR current in this cell by 52%–49% at test potentials between −110 and −80 mV. In four cells, ACTH (500 pM or 1 nM) inhibited the time-dependent component of KIR by 61.9 ± 7.8% (n = 4) (Table 1).
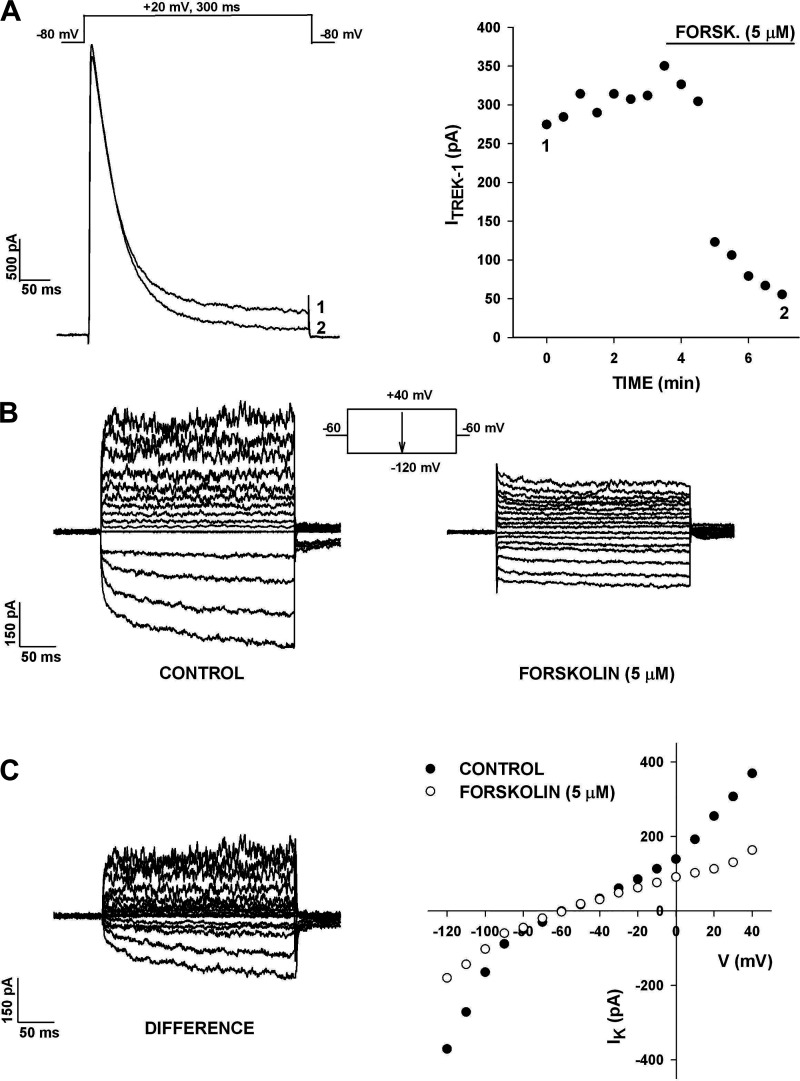
The adenylate cyclase activator forskolin had effects on each of the K+ currents in human AZG cells that were similar to those of ACTH. The experiment illustrated in Fig. 10A shows that forskolin inhibited the noninactivating K2P current by 79%, without altering KV1.4 current amplitude or inactivation kinetics. In six cells, forskolin (5 μM) inhibited this current by 81.8 ± 8.0% (Table 1).
Figure 10.
Effect of forskolin on human AZG K+ currents. A: inhibition of TREK-1. K+ currents were recorded in response to voltage steps to +20 mV applied at 30 s intervals from a holding potential of −80 mV. TREK-1 amplitudes are plotted against time. Numbers on plot correspond to those on traces before and after 5 µM forskolin. B: inhibition of TREK-1 and KIR by forskolin. K+ currents were recorded in response to voltage steps to test potentials from +40 to −120 mV applied at 30 s intervals from a holding potential of −60 mV. Traces show IVs in control saline and in 5 µM forskolin. C: inhibited K+ currents and I-Vs. Difference current traces obtained by digital subtraction of K+ currents in forskolin from control currents. Current amplitudes from B are plotted against test potential. Number of independent experiments: n = 6 (A); n = 2 (B and C).
Forskolin also selectively inhibited the time-dependent component of the KIR current. The experiment illustrated in Fig. 10, B and C, shows the current-voltage relationship recorded from a holding potential of −60 mV at test potentials between +40 and −120 mV. In this cell, both the outwardly rectifying and inwardly rectifying K+ currents were significantly inhibited at every test potential (Fig. 10, B and C). The time-dependent component of KIR was nearly totally inhibited by forskolin, whereas the instantaneous fraction remained intact. In three cells, forskolin (5 µM) inhibited the time-dependent component of KIR by 74.1 ± 9.8% (Table 1).
Overall, these results show that human AZG cells express K2P and KIR K+ currents, both of which are inhibited by ACTH and forskolin. In addition to indicating that both of these K+ currents are inhibited by cAMP, these results also identify the major fraction of the K2P current as TREK-1.
The inhibition of the KIR current by Gs- and Gq-coupled receptors and forskolin identifies it as a member of the GIRK subfamily of K+ currents. The failure of ACTH or ANG II to inhibit the quasi-instantaneous component of KIR indicated that this current was not a GIRK current. Tertiapin Q, a stable derivative of a bee venom peptide, inhibits GIRK1/4 with an IC50 <10 nM (33). In four AZG cells that expressed only the rapidly activating component of KIR, Tertiapin Q (500 nM) inhibited this current by only 43.9 ± 6.6%, further evidence that this was not a GIRK current (Table 1).
K2P Channels and the Control of Membrane Potential
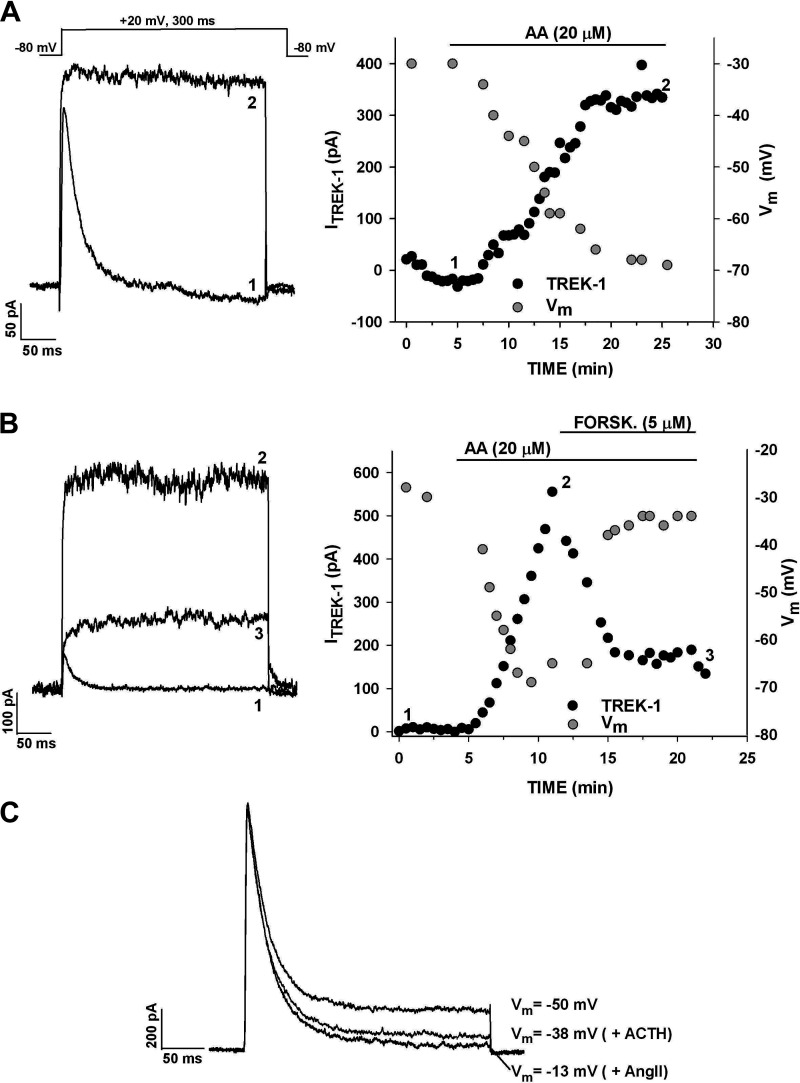
TREK-1 appears to be the major K2P K+ channel expressed in human AZG cells. If so, the activity of these channels could function in establishing the AZG cell membrane potential. Accordingly, it was discovered that the activation or inhibition of the K2P current by AA and forskolin, respectively, hyperpolarized and depolarized AZG cells. In the experiments illustrated in Fig. 11A, superfusion of the cell with AA (20 µM) increased the noninactivating K+ current from nearly 0–335 pA. The membrane potential, monitored by switching to current clamp, increased from−-30 to −69 mV over the same 20-min time interval. Overall, in addition to producing large increases in the presumed TREK-1 current, AA also hyperpolarized cells by 32.0 ± 6.1 mV (n = 4).
Figure 11.
Effect of arachidonic acid and forskolin on TREK-1 and membrane potential. K+ currents were recorded in response to voltage steps to +20 mV applied at 30 s intervals from a holding potential of −80 mV. Membrane potential was recorded by switching to current clamp. A: current traces recorded before (1) and 20 min after (2) superfusing cell with 20 µM arachidonic acid (AA). Current amplitudes (black circles) and membrane potential (gray circles) are plotted against time. Numbers on plot correspond to those on current traces. B: current traces recorded in control saline (1), after 10-min exposure to 20 µM AA (2) and after 10-min exposure to 20 µM AA plus 5 µM forskolin (3). Current amplitudes (black circles) and membrane potential (gray circles) are plotted against time. Numbers on plot correspond to those on current traces. C: effect of ACTH and ANG II on K+ current and membrane potential. K+ currents were recorded in response to voltage steps to +20 mV from a holding potential of −80 mV. Cell was superfused with 500 pM ACTH followed by 5 nM ANG II. Current traces and corresponding membrane potential are shown. Number of independent experiments: n = 4 (A); n = 4 (B); n = 3 (C).
In other experiments, forskolin was found to inhibit TREK-1 current activated by AA, and this inhibition was tightly coupled to membrane depolarization. In the experiment illustrated in Fig. 11B, AA increased the TREK-1 amplitude from near 0–550 pA and hyperpolarized the cell by 41 mV. The subsequent superfusion of forskolin inhibited TREK-1 by 72% and depolarized the cell by 35 mV. Similar results were obtained in each of three experiments. Overall, the respective activation and inhibition of the K2P current by AA and forskolin, in combination with the tightly coupled membrane hyperpolarization and depolarization, further establish this channel’s identity as TREK-1 and demonstrate its ability to set the human AZG cell membrane potential.
The inhibition of K2P K+ currents by ACTH and ANG II was accompanied by depolarization of AZG cells. In the experiment illustrated in Fig. 11C, ACTH (1 nM) inhibited the K2P current by 73% and depolarized the cell by 12 mV. Subsequent superfusion of ANG II (2 nM) inhibited the current by an additional 43% and depolarized the cell further by 25 mV. Overall, when applied independently, ACTH (500 pM) inhibited the K2P current by 79.3 ± 10.1% and depolarized AZG cells by 17.5 ± 2.7 mV (n = 4). ANG II (5 nM) inhibited this current by 66 ± 14% and depolarized AZG cells by 24 ± 7.0 mV (n = 3).
Northern Blot Analysis of Human AZG K+ Channel mRNAs
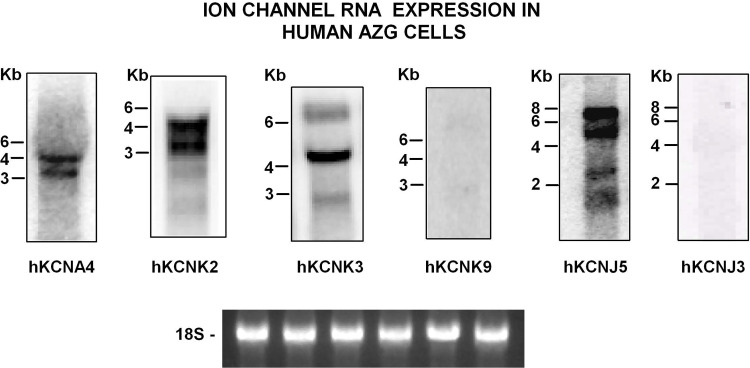
As illustrated in Table 2, whole cell patch clamp experiments indicated that a large majority of human AZG cells express members of each of the three major families of K+ channels. Accordingly, Northern blot analysis showed that mRNA transcripts coding for hKCNA4, hKCNJ5, hKCNK2, and hKCNK3 were clearly expressed by human subcapsular adrenal cells, whereas hKCNJ3 and KCNK9 were not detectable (Fig. 12).
Table 2.
Human AZG K+ current expression
| Percentage | Number of Cells | |
|---|---|---|
| KV1.4 | 97.0% | 97/100 |
| K2P | 70.1% | 61/87 |
| KIR | 92.9% | 97/98 |
Shown are percentage and number of human adrenal zona glomerulosa (AZG) cells expressing K+ channel subtypes in recordings made in control saline. K2P, two-pore K+ current; KIR, inwardly rectifying K+ current.
Figure 12.
Ion channel gene expression in human adrenal zona glomerulosa cells. Northern autoradiogram with total RNA isolated from human adrenal zona glomerulosa (AZG) cells that were isolated and cultured for 24 h, as described in the materials and methods. Northern blot membrane was separated into six equal lanes, each containing 10 µg of total RNA for hybridization with specific channel probes, as described the materials and methods.. Northern autoradiograms were imaged after 4 h (hKCNA4, hKCNJ5, hKCNK2, hKCNK3) or 18-h (hKCNK3, hKCNJ3) exposure to imaging screen. 18 S lanes are shown as evidence of even loading. Bands correspond to transcripts of ∼4.0 and 3.2 Kb for hKCNA4, ∼3.6 and 4.2 Kb for hKCNK2, ∼5.0, 6.5, and a weaker band at ∼2.5 Kb for hKCNK3, no discernible bands for hKCNK9, ∼6.8, 5.2, and weaker bands at ∼2.4 and 1.6 Kb for KCNJ5, and no discernible bands for hKCNJ3. Number of independent experiments: n = 2.
DISCUSSION
In a whole cell patch clamp study, we discovered that individual, normal human AZG cells expressed multiple K+ currents representing each of the major K+ channel families. These currents differed significantly from those previously described in AZG cells of any species, including the single study of human AZG cells completed over 25 years ago (14). Importantly, we showed that human AZG cells express KIR current(s) that appear as two kinetically distinct components, one of which is a GIRK channel inhibited by both ANG II and ACTH. These AZG cells also robustly express TREK-1 K+ channels whose inhibition by ANG II and ACTH is coupled to membrane depolarization. Overall, these findings suggest a model wherein specific ion channels control membrane potential, Ca2+ entry, and aldosterone secretion by human AZG cells. The K+ channels in these human cells differ fundamentally from those of the widely adopted murine models, suggesting an evolutionary divergence resulting in parallel species differences in the physiology of aldosterone secretion. These human AZG K+ channels including their identity and modulation are discussed below.
Voltage-Gated A-Type K+ Current
The A-type current in human AZG cells was indistinguishable from the Kv1.4 current of bovine and human AZF cells with respect to voltage-dependent gating and kinetics (20, 23). It was also similarly insensitive to ANG II and ACTH. We and others have shown that the Kv1.4 (KCNA4) gene is highly expressed in the human adrenal, including zona fasciculata cells (15, 20). In the present study, we presented data demonstrating that human AZG cells express Kv1.4 mRNA and functional channels. It has also been shown that KCNA4 is the only member of the KCNA K+ channel gene family that is highly expressed in the human adrenal gland (26). The function of Kv1.4 channels in human AZG physiology has not been determined.
K2P Currents
Patch clamp and Northern blot experiments indicated that human AZG cells expressed KCNK2 (TREK-1) and KCNK3 (TASK-1) K2P channels. Accordingly, previous studies showed that these were two of the most highly expressed K+ channel mRNAs in the human adrenal whose transcripts were present at levels far in excess of any other member of the KCNK family (15, 26). Further, KCNK2 is one of only 37 genes that display a markedly greater expression level in the adrenal compared with 31 other normal human tissues (26).
Analysis of the human adrenal transcriptome and proteome in combination with our patch clamp studies provide convincing evidence that TREK-1 is the dominant K2P channel of human AZG cells. Specifically, the current identified was indistinguishable from the K+ current that we first discovered in patch voltage clamp recording, and later identified with molecular cloning in bovine AZF cells (19, 22). TREK-1 was also found to be the dominant K2P current in bovine AZG and human AZF cells (3, 20). Similar to the TREK-1 current in these three cell types, the K2P current in human AZG cells often grew spontaneously upon initiating whole cell recording under control conditions.
Furthermore, of the 15 K2P channels, only TREK-1 and TREK-2 are inhibited by cAMP (25, 34). Since only TREK-1 mRNA and protein are expressed in the human adrenal, the inhibition of the K2P current in human AZG cells by ACTH and forskolin confirm its molecular identity (26). Similarly, among all the K2P channels, AA activates only the mechanogated TRAAK (KCNK4), TREK-1, and TREK-2 channels (25, 35). Of these three, only TREK-1 transcripts and protein are expressed in the human adrenal (26). Therefore, the leak type K+ current activated by AA is almost certainly TREK-1.
The difference in K2P current densities that we observed could reflect the several fold larger unitary conductance of TREK-1 channels, compared with TASK-1 (25). Because of the extreme temperature sensitivity of TREK-1, the difference in current density would have been greater if the experiments had been done at 37°C (36).
We cannot explain why a K+ current resembling TREK-1 was not detected in the previously mentioned study of human AZG cells obtained from kidney transplant patients (14). It is important to point out that in our experiments, many otherwise functional TREK-1 channels existed in a dormant state until activated by AA or CDC. In addition, the expression of TREK-1 currents depended critically on the condition of the donated adrenal and the resulting viability of the isolated cells.
The inhibition of TREK-1 in human AZG cells and the associated membrane depolarization produced by ANG II and ACTH resemble the effects of these two peptides in bovine AZF and AZG cells and human AZF cells (3, 18–20). In these previous studies, it was discovered that inhibition of TREK-1 by either peptide is mediated through multiple signaling pathways. TREK-1 inhibition by ANG II in the bovine adrenal occurs through a Gq-coupled AT1 receptor and is mediated through separate Ca2+- and ATP hydrolysis-dependent signaling pathways (3, 37, 38). In the present study, only the ATP pathway was available because [Ca2+]i was strongly buffered to 22 nM with 11 mM BAPTA (37).
In contrast to the Ca2+ pathway, the ATP-dependent inhibition of TREK-1 is mediated through a novel mechanism that does not require PLC activation (38). This distinctive difference may underlie the observed disparity in reversibility of ANG II inhibition of TREK-1 and KIR in these cells. It is possible that a minor fraction of the K2P current inhibited by ANG II was TASK-1. These channels are inhibited through Gq-coupled pathways by multiple signaling mechanisms (12, 39).
The effective inhibition of the K2P current in human AZG cells by ACTH and forskolin identified it as TREK-1. Inhibition of TREK-1 in bovine AZF cells occurs through both PKA and EPAC2, a guanine nucleotide exchange protein activated by cAMP and highly expressed in the adrenal cortex (34, 40, 41). It is likely that ACTH and cAMP function similarly in human AZG cells.
Overall, as the dominant K2P channel in human AZG cells, TREK-1 may act pivotally in establishing the resting membrane potential of these cells and in coupling both ANG II and ACTH receptor activation to depolarization-dependent Ca2+ entry and aldosterone secretion. Importantly, TREK-1 channels appear to function in a similar capacity in human AZF cells, as well as in bovine AZF and AZG cells (3, 19, 20, 42).
KIR Currents
Although the molecular identities of the KIR channels underlying the K+ currents that we recorded from human AZG cells have not been determined with certainty, the results from our study, in combination with those of others, indicate that two specific K+ channels are responsible. Importantly, one of these, a slowly activating current inhibited by ANG II and ACTH, is likely produced by a KCNJ5 homomultimer, the dominant GIRK channel expressed in these cells.
The inhibition of the time-dependent component of the KIR current by activation of Gq- and Gs-coupled ANG II and ACTH receptors indicate that this is a GIRK current. Accordingly, we found that KCNJ5 mRNA is robustly expressed in human adrenal subcapsular cells. Similarly, others have reported that KCNJ5 mRNA and protein are present at high levels in the human adrenal, and further, that these transcripts are expressed predominantly in the human AZG (11, 15, 26, 43). Similarly, our patch clamp experiments showed that a GIRK K+ current is expressed in a great majority of human subcapsular adrenocortical cells, whereas it is detectable in only a small fraction of AZF cells. It isn’t known whether KCNJ5 is expressed exclusively in aldosterone-secreting human adrenal cells.
In other tissues such as sinoatrial nodal cells, GIRK1/GIRK4 heteromultimeric channels produce KIR currents resembling those present in human AZG cells (31, 32, 44). However, in agreement with a previous study, our results indicate that KCNJ3 mRNA and protein are absent from the human adrenal (26). Similarly, the remaining two members of the GIRK subfamily, GIRK2 (KCNJ6) and GIRK3 (KCNJ9), are also undetectable or expressed at levels at least 100-fold lower than KCNJ5 in the human adrenal (26). Overall, these results indicate that GIRK4 (KCNJ5) is the dominant and likely the only GIRK channel expressed in human AZG cells, and further, that the GIRK4 homomultimer generates the time-dependent KIR current inhibited by ANG II and ACTH in these cells.
In this regard, it is known that GIRK4 confers appropriate processing and cell surface localization to G protein-gated potassium channels (45). Further, GIRK4 homotetramers form functional channels that reside in macromolecular signaling complexes (44, 46). Within these complexes, regulators of G protein signaling (RGS) proteins confer slow relaxation kinetics to the homotetramers (47).
The failure of ACTH and ANG II to inhibit the instantaneous component of KIR indicates that it is not a GIRK family current. Accordingly, Tertiapin Q, which inhibits GIRK1/4 channels with an IC50 of <10 nM (33), was more than 50-fold less potent as an inhibitor of the instantaneous KIR. Of the 15 genes coding for KIR channels, the only one, other than KCNJ5, expressed at significant levels in the human adrenal is KCNJ8 (KIR 6.1), a member of the KATP subfamily of K+ channels (15, 26). The distribution of KCNJ8 within the human adrenal hasn’t been determined. However, in studies of human adrenal chromaffin cell K+ currents, we have detected no KIR current, indicating that KCNJ8 may be expressed only in the adrenal cortex (manuscript in preparation). It will be important to determine whether the quasi-instantaneous KIR current in human AZG cells is KIR 6.1.
Our results show that both ANG II and ACTH inhibit an inwardly rectifying K+ current in AZG cells and that this current is likely KIR3.4, the only GIRK channel expressed in these cells. However, the inhibition of GIRK channels, including KIR3.4, through the activation of G protein-coupled receptors is not well understood. It is known that GIRK channels, including KIR3.4, are inhibited by Gq-coupled receptors and that this inhibition is mediated through PLC-dependent depletion of phosphatidylinositol 4,5-bisphosphate (PIP2) or protein kinase C activation (31). GIRK channels, including KIR3.4, are activated by PIP2, which is required for these channels to remain active (48, 49). Accordingly, these K+ channels are inhibited by PLC-coupled receptors that deplete PIP2, including the AT1 receptor (50, 51). PKC inhibits GIRK4 channels through phosphorylation of a specific serine residue (52). These results suggest plausible mechanisms by which ANG II inhibits the KIR current in human AZG cells. However, because the Gq-coupled AT1 receptor in these cells activates multiple signaling pathways, the mechanisms mediating inhibition of GIRK and K2P channels remain to be discovered (37, 38, 51).
The inhibition of the time-dependent component of the KIR current in AZG cells by both the Gs-coupled ACTH receptor and forskolin indicated that this was GIRK4, and furthermore, that this KIR3.4 current can be inhibited by cAMP. The signaling pathways by which cAMP inhibits this current have not been identified. In bovine AZF cells, ACTH and cAMP inhibit TREK-1 by both PKA- and exchange protein activated by cyclic-AMP (EPAC2)-dependent mechanisms (34). EPAC2 and PKA are both highly expressed in the bovine and human adrenal cortex (26, 41). Although the specific inhibition of KIR3.4 by cAMP has not been previously reported, cAMP may inhibit this current in human AZG cells by PKA or EPAC2. Accordingly, cAMP inhibits a neuronal GIRK current by PKA- and EPAC-dependent mechanisms (53).
In contrast to TREK-1 inhibition by ANG II and ACTH in human AZG cells, which was poorly reversible, the inhibition of GIRK by either peptide was rapidly reversed. This disparity indicates that ANG II and ACTH each inhibit TREK-1 and GIRK channels through separate signaling pathways. The relevance of these differences in reversibility to the physiology of aldosterone secretion is unknown.
In summary, we have attempted to identify and characterize each of the K+ currents expressed by human AZG cells and to describe the modulation of these currents by ANG II and ACTH. It was discovered that these cells express Kv1.4, TREK-1, and a GIRK channel that is very likely KIR3.4. Of these three currents, it was discovered that TREK-1 and the GIRK current were each inhibited by both ANG II and ACTH.
Importantly, these results show that the K+ channels of the human AZG differ fundamentally from those of murine cells, which serve as the standard model for aldosterone secretion. Specifically, although systematic studies of K+ currents in murine AZG cells using whole cell patch clamp are lacking, combined biochemical and electrophysiological evidence indicate that neither mouse nor rat cells express Kv1.4, TREK-1, or GIRK4 channels (10, 11, 13).
In conclusion, we have found that normal human AZG cells express specific K2P and GIRK K+ channels whose open probability is controlled by the peptide hormones that regulate membrane potential and aldosterone secretion. These findings establish these channels as pivotal control points where hormonal and biochemical signals are integrated and transduced to ion conductance changes and membrane depolarization. They provide the foundation for a model describing the electrical events that underlie secretion by human AZG cells.
GRANTS
This work was supported in part by Award Number R56DK047875 (to J. J. Enyeart) from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.J.E. conceived and designed research; J.J.E. and J.A.E. performed experiments; J.J.E. and J.A.E. analyzed data; J.J.E. interpreted results of experiments; J.A.E. prepared figures; J.J.E. and J.A.E. edited and revised manuscript; J.J.E. and J.A.E. approved final version of manuscript.
REFERENCES
- 1.Quinn SJ, Williams GH. Regulation of aldosterone secretion. Annu Rev Physiol 50: 409–426, 1988. doi: 10.1146/annurev.ph.50.030188.002205. [DOI] [PubMed] [Google Scholar]
- 2.Williams GH. Aldosterone biosynthesis, regulation, and classical mechanism of action. Heart Fail Rev 10: 7–13, 2005. doi: 10.1007/s10741-005-2343-3. [DOI] [PubMed] [Google Scholar]
- 3.Enyeart JA, Danthi SJ, Enyeart JJ. TREK-1 K+ channels couple angiotensin II receptors to membrane depolarization and aldosterone secretion in bovine adrenal glomerulosa cells. Am J Physiol Endocrinol Metab 287: E1154–E1165, 2004. doi: 10.1152/ajpendo.00223.2004. [DOI] [PubMed] [Google Scholar]
- 4.Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol 350: 151–162, 2012. doi: 10.1016/j.mce.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn SJ, Cornwall MC, Williams GH. Electrophysiological responses to angiotensin II of isolated rat adrenal glomerulosa cells. Endocrinology 120: 1581–1589, 1987. doi: 10.1210/endo-120-4-1581. [DOI] [PubMed] [Google Scholar]
- 6.Natke EJ, Kabela E. Electrical responses in cat adrenal cortex: possible relation to aldosterone secretion. Am J Physiol Endocrinol Metab 237: E158–E162, 1979. doi: 10.1152/ajpendo.1979.237.2.E158. [DOI] [PubMed] [Google Scholar]
- 7.Quinn SJ, Cornwall MC, Williams GH. Electrical properties of isolated rat adrenal glomerulosa and fasciculata cells. Endocrinology 120: 903–914, 1987. doi: 10.1210/endo-120-3-903. [DOI] [PubMed] [Google Scholar]
- 8.Uebele VN, Nuss CE, Renger JJ, Connolly TM. Role of voltage-gated calcium channels in potassium-stimulated aldosterone secretion from rat adrenal zona glomerulosa cells. J Steroid Biochem Mol Biol 92: 209–218, 2004. doi: 10.1016/j.jsbmb.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Barrett PQ, Guagliardo NA, Klein PM, Hu C, Breault DT, Beenhakker MP. Role of voltage-gated calcium channels in the regulation of aldosterone production from zona glomerulosa cells of the adrenal cortex. J Physiol 594: 5851–5860, 2016. doi: 10.1113/JP271896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandulik S, Tauber P, Lalli E, Barhanin J, Warth R. Two-pore domain potassium channels in the adrenal cortex. Pflugers Arch 467: 1027–1042, 2015. doi: 10.1007/s00424-014-1628-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen AX, Nishimoto K, Nanba K, Rainey WE. Potassium channels related to primary aldosteronism: expression similarities and differences between human and rat adrenals. Mol Cell Endocrinol 417: 141–148, 2015. doi: 10.1016/j.mce.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czirjak G, Fischer T, Spat A, Lesage F, Enyedi P. TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol Endocrinol 14: 863–874, 2000. doi: 10.1210/me.14.6.863. [DOI] [PubMed] [Google Scholar]
- 13.Czirják G, Enyedi P. TASK-3 dominates the background potassium conductance in rat adrenal glomerulosa cells. Mol Endocrinol 16: 621–629, 2002. doi: 10.1210/me.16.3.621. [DOI] [PubMed] [Google Scholar]
- 14.Payet MD, Durroux T, Bilodeau L, Guillon G, Gallo-Payet N. Characterization of K+ and Ca2+ ionic currents in glomerulosa from human adrenal glands. Endocrinology 134: 2589–2598, 1994. doi: 10.1210/endo.134.6.7515004. [DOI] [PubMed] [Google Scholar]
- 15.Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Akerstrom G, Wang W, Carling T, Lifton RP. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 331: 768–772, 2011. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassilev PM, Kanazirska MV, Quinn SJ, Tillotson DL, Williams GH. K+ channels in adrenal zona glomerulosa cells. I. Characterization of distinct channel types. Am J Physiol Endocrinol Metab 263: E752–E759, 1992. doi: 10.1152/ajpendo.1992.263.4.E752. [DOI] [PubMed] [Google Scholar]
- 17.Enyeart JA, Danthi SJ, Enyeart JJ. Corticotropin induces the expression of TREK-1 mRNA and K+ current in adrenocortical cells. Mol Pharmacol 64: 132–142, 2003. doi: 10.1124/mol.64.1.132. [DOI] [PubMed] [Google Scholar]
- 18.Enyeart JA, Xu L, Enyeart JJA. Bovine adrenocortical Kv1.4 K+ channel whose expression is potently inhibited by ACTH. J Biol Chem 275: 34640–34649, 2000. doi: 10.1074/jbc.M004214200. [DOI] [PubMed] [Google Scholar]
- 19.Enyeart JJ, Xu L, Danthi S, Enyeart JA. An ACTH- and ATP-regulated background K+ channel in adrenocortical cells is TREK-1. J Biol Chem 277: 49186–49199, 2002. doi: 10.1074/jbc.M207233200. [DOI] [PubMed] [Google Scholar]
- 20.Enyeart JJ, Enyeart JA. Ca2+ and K+ channels of normal human adrenal zona fasciculata cells: properties and modulation by ACTH and AngII. J Gen Physiol 142: 137–155, 2013. doi: 10.1085/jgp.201310964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391: 85–100, 1981. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 22.Mlinar B, Biagi BA, Enyeart JJ. A novel K+ current inhibited by ACTH and Angiotensin II in adrenal cortical cells. J Biol Chem 268: 8640–8644, 1993. Nodoi: 10.1016/S0021-9258(18)52922-7. [DOI] [PubMed] [Google Scholar]
- 23.Mlinar B, Enyeart JJ. Voltage-gated transient currents in bovine adrenal fasciculata cells II: A-type K+ current. J Gen Physiol 102: 239–255, 1993. doi: 10.1085/jgp.102.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mlinar B, Biagi BA, Enyeart JJ. Voltage-gated transient currents in bovine adrenal fasciculata cells I: T-type Ca2+ current. J Gen Physiol 102: 217–237, 1993. doi: 10.1085/jgp.102.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enyedi P, Czirják G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90: 559–605, 2010. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 26.Bergman J, Botling J, Fagerberg L, Hallström B, Djureinovic D, Uhlén M, Pontén F. The human adrenal gland proteome defined by transcriptomics and antibody-based profiling. Endocrinology 158: 239–251, 2017. doi: 10.1210/en.2016-1758. [DOI] [PubMed] [Google Scholar]
- 27.Streit AK, Netter MF, Kempf F, Walecki M, Rinne S, Bollepalli MK, Preisig-Muller R, Renigunta V, Daut J, Baukrowitz T, Sansom MS, Stansfeld PJ, Decher N. A specific two-pore domain potassium channel blocker defines the structure of the TASK-1 open pore. J Biol Chem 286: 13977–13984, 2011. doi: 10.1074/jbc.M111.227884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danthi S, Enyeart JA, Enyeart JJ. Modulation of native TREK-1 and Kv1.4 channels by polyunsaturated fatty acids and lysophospholipids. J Membr Biol 195: 147–164, 2003. doi: 10.1007/s00232-003-0616-0. [DOI] [PubMed] [Google Scholar]
- 29.Danthi S, Enyeart JA, Enyeart JJ. Caffeic acid esters activate TREK-1 potassium channels and inhibit depolarization-dependent secretion. Mol Pharmacol 65: 599–512, 2004. doi: 10.1124/mol.65.3.599. [DOI] [PubMed] [Google Scholar]
- 30.Bird IM, Hanley NA, Word RA, Mathis JM, McCarthy JL, Mason JI, Rainey WE. Human NCI-H295 adrenocortical carcinoma cells: a model for angiotensin-II-responsive aldosterone secretion. Endocrinology 133: 1555–1561, 1993. doi: 10.1210/endo.133.4.8404594. [DOI] [PubMed] [Google Scholar]
- 31.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90: 291–366, 2010. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 32.Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature 374: 135–141, 1995. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- 33.Jin W, Lu Z. Synthesis of a stable form of Tertiapin: a high-affinity inhibitor for inward rectifier K+ channels. Biochemistry 38: 14286–14293, 1999. doi: 10.1021/bi991205r. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Enyeart JA, Enyeart JJ. ACTH inhibits bTREK-1 K+ channels through multiple cAMP-dependent signaling pathways. J Gen Physiol 132: 279–294, 2008. doi: 10.1085/jgp.200810003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noël J, Sandoz G, Lesage F. Molecular regulations governing TREK and TRAAK channel functions. Channels (Austin) 5: 402–409, 2011. doi: 10.4161/chan.5.5.16469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagriantsev SN, Clark KA, Minor DL Jr.. Metabolic and thermal stimuli control K(2P)2.1 (TREK-1) through modular sensory and gating domains. EMBO J 31: 3297–3308, 2012. doi: 10.1038/emboj.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Enyeart JJ, Danthi SJ, Liu H, Enyeart JA. Angiotensin II inhibits bTREK-1 K+ channels in adrenocortical cells by separate Ca2+- and ATP hydrolysis-dependent mechanisms. J Biol Chem 280: 30814–30828, 2005. doi: 10.1074/jbc.M504283200. [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Enyeart JA, Enyeart JJ. Angiotensin II inhibits native bTREK-1 K+ channels through a PLC-, kinase C-, and PIP2-independent pathway requiring ATP hydrolysis. Am J Physiol Cell Physiol 293: C682–C695, 2007. doi: 10.1152/ajpcell.00087.2007. [DOI] [PubMed] [Google Scholar]
- 39.Wilke BU, Lindner M, Greifenberg L, Albus A, Kronimus Y, Bünemann M, Leitner MG, Oliver D. Diacylglycerol mediates regulation of TASK potassium channels by Gq-coupled receptors. Nat Commun 5: 1–11, 2014. doi: 10.1038/ncomms6540. [DOI] [PubMed] [Google Scholar]
- 40.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474–477, 1998. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 41.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science 282: 2275–2279, 1998. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 42.Enyeart JJ, Mlinar B, Enyeart JA. T-type Ca2+ channels are required for ACTH-stimulated cortisol synthesis by bovine adrenal zona fasciculata cells. Mol Endocrinol 7: 1031–1040, 1993. doi: 10.1210/mend.7.8.8232302. [DOI] [PubMed] [Google Scholar]
- 43.Monticone S, Hattangady NG, Nishimoto K, Mantero F, Rubin B, Cicala MV, Pezzani R, Auchus RJ, Ghayee HK, Shibata H, Kurihara I, Williams TA, Giri JG, Bollag RJ, Edwards MA, Isales CM, Rainey WE. Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells. J Clin Endocrinol Metab 97: E1567–E1572, 2012. doi: 10.1210/jc.2011-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Touhara KK, Wang W, MacKinnon R. The GIRK1 subunit potentiates G protein activation of cardiac GIRK1/4 hetero-tetramers. eLife 5: 1–12, 2016. doi: 10.7554/eLife.15750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kennedy ME, Nemec J, Corey S, Wickman K, Clapham DE. GIRK4 confers appropriate processing and cell surface localization to G-protein-gated potassium channels. J Biol Chem 274: 2571–2582, 1999. doi: 10.1074/jbc.274.4.2571. [DOI] [PubMed] [Google Scholar]
- 46.Lüscher C, Slesinger P. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci 11: 301–315, 2010. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inanobe A, Fujita S, Makino Y, Matsushita K, Ishii M, Chachin M, Kurachi Y. Interaction between the RGS domain of RGS4 with G protein alpha subunits mediates the voltage-dependent relaxation of the G protein-gated potassium channel. J Physiol 535: 133–143, 2001. doi: 10.1111/j.1469-7793.2001.t01-1-00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang C-L, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbg. Nature 391: 803–806, 1998. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 49.Whorton MR, MacKinnon R. X-ray structure of the mammalian GIRK2-bg G-protein complex. Nature 498: 190–197, 2013. doi: 10.1038/nature12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho H, Lee D, Lee SH, Ho W-K. Receptor-induced depletion of phosphatidylinositol 4,5-bisphosphate inhibits inwardly rectifying K+ channels in a receptor-specific manner. Proc Natl Acad Sci USA 102: 4643–4648, 2005. doi: 10.1073/pnas.0408844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol 20: 953–970, 2006. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 52.Mao J, Wang X, Chen F, Wang R, Rojas A, Shi Y, Piao H, Jiang C. Molecular basis for the inhibition of G protein-coupled inward rectifier K+ channels by protein kinase C. Proc Natl Acad Sci USA 101: 1087–1092, 2004. doi: 10.1073/pnas.0304827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Witkowski G, Szulczyk P. Effect of cyclic adenosine monophosphate on the G protein-dependent inward rectifier K+-like channel current in medial prefrontal cortex pyramidal neurons. J Physiol Pharmacol 63: 457–462, 2012. [PubMed] [Google Scholar]