Keywords: G protein-coupled receptors NANC relaxation, neurotrophic factor, rectoanal incontinence, RhoA/ROCK, smooth muscle tone
Abstract
Aging can lead to rectoanal incontinence due to internal anal sphincter (IAS) dysfunction, which is characterized by a decrease in IAS tone and contractility and an increase in nonadrenergic noncholinergic (NANC) relaxation. We aimed to determine whether brain-derived neurotropic factor (BDNF) rescues this aging-associated IAS dysfunction (AAID). To do so, we studied the effects of BDNF on the basal and G protein-coupled receptors (GPCR)-stimulated IAS smooth muscle tone and on NANC relaxation in Fischer 344 rats representing different age groups [26-mo-old (aging) vs. 6-mo-old (young)], before and after tyrosine kinase receptor B (TrkB) antagonist K252a. We also used isolated smooth muscle cells (SMCs) to determine the effects of BDNF before and after different agonists. For some studies, we monitored NO release using smooth muscle perfusates. BDNF reversed AAID by rescuing the basal IAS tone and agonists [thromboxane A2 analog (U46619) and angiotensin II (ANG II)]-induced contractility, and NANC relaxation. These rescue effects of BDNF were selective as K252a attenuated the changes in the IAS without modifying the effects of K+depolarization. Because of the direct association between the basal and GPCR-stimulated IAS tone and RhoA/ROCK activation, we speculate that this pathway in the rescue effects of BDNF. Conversely, our data suggest that aging-associated increased NANC relaxation is reversed by decreased release of NO and decrease in the sensitivity of the released inhibitory neurotransmitter. In summary, BDNF rescue of AAID involves RhoA/ROCK and inhibitory neurotransmission. These data have direct implications for the role of BDNF in the pathophysiology and therapeutic targeting of aging-associated rectoanal motility disorders.
NEW & NOTEWORTHY These studies demonstrate that brain-derived neurotropic factor (BDNF) rescues the aging-associated internal anal sphincter (IAS) dysfunction, characterized by a decrease in IAS tone, and increase in non-adrenergic noncholinergic relaxation. We determined the effects of BDNF on the basal and GPCR (TXA2 and ANG II)-stimulated IAS tone, and on NANC relaxation, before and after TrkB inhibitor K252a. BDNF may have an important role in the pathophysiology and therapeutic targeting of certain rectoanal motility disorders.
INTRODUCTION
The internal anal sphincter (IAS) plays a major role in rectoanal continence by regulating the basal IAS tone and rectoanal inhibitory reflex (RAIR) (1–6). The basal tone is primarily myogenic in nature via the molecular control of RhoA/ROCK within the smooth muscle cells (SMCs) (7–10). Conversely, the RAIR is neurogenic primarily via the release of nitric oxide (NO, nitrergic relaxation) by nonadrenergic, noncholinergic (NANC) nerve stimulation (11–14). The latter can be mimicked in vitro using appropriate parameters of electrical field stimulation (EFS) in muscle bath studies (12, 14). Studies have indicated that RhoA/ROCK in the IAS SMCs may be G protein-coupled receptors (GPCR)-regulated via the renin angiotensin system (RAS) and arachidonic acid (AA) pathways via activation of AT1-R by angiotensin II (ANG II) and TXA2-R by thromboxane A2 (TXA2), respectively (8, 10, 15, 16). Because of the transient effects of TXA2, a synthetic analog (U46619) has been developed that mimics its endogenous effect but is more long-lasting (15).
Aging can lead to rectoanal incontinence (RI) related to IAS dysfunction (AAID), which is characterized by a decrease in IAS tone and augmented response to RAIR (1, 4, 16, 17). In spite of significant progress understanding the molecular control mechanisms of the basal IAS tone and nitrergic relaxation, specific therapeutic modalities for RI that are free of side effects are limited. In that regard, because of the dual beneficial effects (on the basal IAS tone and nitrergic relaxation), an endogenous agent such as brain-derived neurotrophic factor (BDNF) (18) may be of significant interest in the AAID.
Initially, BDNF, an important member of the neurotrophin family, was shown to be present and released primarily in the CNS (19–22). Since then, BDNF has also been shown to be present and released in the peripheral nervous system, especially in the enteric nervous system (ENS) and in the smooth muscles of different organ systems including the gastrointestinal tract (23–28). Using different systems, BDNF has been shown to augment GPCR-coupled smooth muscle contractility and relaxation (24, 29–32). Recent studies using adult rats have shown that BDNF in the IAS, besides being present in the ENS, is also present and released by the SMCs (33). In addition, BDNF has been shown to augment basal IAS tone, GPCR-mediated contractility, and NANC nerve-mediated smooth muscle relaxation (18). However, the therapeutic potential of BDNF in the AAID has not been explored.
Therefore, the purpose of the present studies was to investigate the rescuing potential of BDNF in the aging IAS, primarily its effect on the basal tone, GPCR-mediated contractility (by U46619 and ANG II) and relaxation (by NO releaser sodium nitroprusside or SNP), and NANC nerve stimulation. Our studies showed that in the aging group, BDNF rescues AAID by increase in the IAS tone and by attenuation of the increased NANC relaxation.
MATERIALS AND METHODS
Selection of the Animals for Aging Studies
Male Fischer 344 rats of two distinct age groups [6-mo-old (young group) and 26-mo-old (old group)] were obtained from Charles River Laboratories through the National Institute of Aging (Bethesda, MD). The reason for selecting 6- and 26-mo-old rats to represent young and aging animals, respectively, for the present studies was based on our earlier studies showing discrete differences in the molecular and functional parameters in these age groups after examining a wider spectrum of age groups (17). The reason for using male animals for the present project was to minimize the variables associated with hormonal changes in aging-related differences responsible for the IAS functions. The issues of sexual differences with regard to aging-associated changes in the IAS need to be addressed separately. The total number of animals used for the present studies was 24 (6 for the smooth muscle strips and 6 for the SMC studies, for each age group). All animals were in-housed under controlled environment conditions (maintained at a regular 12/12-h light/dark cycle) with free access to food and water. Animal care and handling were in accordance with and approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University, following the recommendations of the American Association for the Accreditation of Laboratory Animal Care.
Preparation of IAS SM Strips
The animals were euthanized using decapitation under isoflurane anesthesia. The anorectal tissue was surgically removed and transferred to oxygenated (95% O2/5% CO2) ice cold 4°C Krebs physiological solution (KPS) with the following composition (in mM): 118.07 NaCl, 4.69 KCl, 2.52 CaCl2, 1.16 MgSO4, 1.01 NaH2PO4, 25 NaHCO3, and 11.1 glucose. The IAS smooth muscle (SM) strips prepared from the lowermost part of the anorectum were employed for the recording of force, as previously described (16–18). Briefly, the IAS strips (∼1 × 10 mm) were isolated from the circular SM layer of the IAS after the adventitious and connective tissues were cleaned off using sharp dissection (under the dissection microscope). The SM strips thus prepared were mounted in 2 mL organ baths (Radnoti, Monrovia, CA) pre-filled with oxygenated KPS at 37°C and 7.4 pH. The IAS SM strips were stretched by 1 g tension, followed by an equilibration period of 60 min under constant oxygenation.
Measurement of Isometric Tension of IAS SM Strips
During the equilibration period, the SM strips were repeatedly washed with fresh oxygenated KPS buffer (37°C, 7.4 pH) every 15 min (16–18). The IAS SM strips were functionally verified by the development of spontaneous tone and by the demonstration of relaxation in response to EFS under NANC conditions. The basal tone and its changes in response to different stimuli were monitored using high sensitivity force transducers (FORT10; WPI, Sarasota, FL). All data were recorded using Chart v4.1.2 via a PowerLab/8SP data acquisition system (ADInstruments Inc., Colorado Springs, CO). The spontaneously developed active basal IAS tone was quantified by the relaxation following zero Ca2+ response. This formulated the basis for the basal tone (the developed tone minus the passive tone remaining following zero Ca2+), and for the calculation of percentage maximal relaxation or decrease in the IAS in each smooth muscle strip. Following this, the SM strips were replenished with normal KPS, and their response to 10−4 M bethanechol (which is known to be the most potent agonist for producing an increase in the rat IAS tone, as compared with other agonists) was used to calculate the percentage maximal contraction (or increase in the IAS tone) (15, 34, 35). After washing was completed, we performed concentration-dependent response studies with U46619, ANG II, and KCl using appropriate washing in between before and after either BDNF, K252a, or K252a+BDNF. Such protocols were repeated in six different animals. The effect of different contractile agonists and BDNF, K252a, or K252a+BDNF was reproducible after thorough washing of the smooth muscle strips with normal KPS. This was ascertained by the return of the basal tone and by the concentration-dependent responses comparable with the naïve (control) levels, and by the responses to 0Ca2+, and 10−4 M bethanechol at the end of each experiment.
Measurement of NANC Relaxation by Electrical Field Stimulation
Electrical field stimulation (EFS) (10 V, 0.5 ms pulse duration, 200–400 mA, 4 s train at 0.5–20 Hz) was delivered using S11Grass stimulator (Grass Instruments, Quincy, MA) connected in series to a Med-Lab Stimuli-Splitter II (Loveland, CO) (18, 36). The electrodes used for the EFS were a pair of platinum wires built into the organ baths, oriented parallel to the SM strips. These experiments were performed under NANC conditions (in the presence of guanethidine and atropine, both at a concentration of 1 μM).
Isolation of Smooth Muscle Cells
The IAS smooth muscle cells (SMCs) were isolated per our previously described method (6, 16, 18, 37) with a few modifications. The IAS tissues were cut into 2-mm cubes and incubated in oxygenated KPS containing 0.2% collagenase and 0.02% trypsin inhibitor, into a 15-mL sterile centrifuge tube on a gentle rocking at 37°C for 1 h. During the incubation, the buffer was replenished every 20 min (3 times in total) with fresh prewarmed buffer at 37°C. The cells’ suspension was centrifuged at 350 g for 10 min, followed by washing with fresh oxygenated KPS (without Ca2+ and Mg2+), which was then filtered through a sterile cell strainer (100 µm) in a 15-mL sterile centrifuge tube. The mixture was again centrifuged at 350 g for 10 min at RT. The cells pellet was transferred onto collagen-coated plates in DMEM [with 5% FBS, 5% pen-strep (1:1), 50 µg/mL gentamicin, 0.2 µg/mL amphotericin B, and 50 µg/mL sodium ascorbate] in a 75-cm2 tissue culture flask (Thermo Fisher Scientific Nunc Cat. No. 156472) and incubated at 37°C and 5% CO2 with regulated humidity (for the maximum adherence of the cells) for 24 h, followed by 50% media change as needed. These SMCs were used for the morphometric analyses as follows.
Measurements of SMC length (digital morphometric analyses).
Lengths of freshly isolated SMCs were measured by morphometric analysis as described previously (16, 18, 38). The cells’ pellet was resuspended in oxygenated fresh KPS (at 37°C) at a cell density of 3 × 104 cells/ml. The slides were monitored periodically by examining 10-μL aliquots of the mixture under the phase-contrast microscopy. Then aliquots of the cells were divided into different agonist treatments. Each 30 μL of the IAS SMCs aliquots was treated for 5 min with different agonists; bethanechol, KCl, ANG II, U46619, and sodium nitroprusside (SNP) at different concentrations. After these treatments, the SMCs were fixed with 0.1% acrolein (final concentration), and the slides were cover slipped using media mountant. The length of the cells was measured before and after different treatments, using randomly selected 50 SMCs using digital micrometry under phase-contrast microscopy. The basal cell lengths (before any treatment) were used to determine percentage maximal shortening (percentage maximal decrease in cell lengths or contraction) or percentage maximal lengthening (percentage maximal increase in cell lengths or relaxation). To examine the specificity of the effects of BDNF on the high-affinity receptor of BDNF (TrkB) in causing contraction of SMCs, we determined the effect of K252a (a specific antagonist of TrkB) added 15 min before the addition of the agonists. This study was repeated in six animals using 50 SMCs selected at random from each group.
Measurement of Nitric Oxide Release from the Muscle Bath Perfusates
In the IAS muscle bath perfusates using fresh smooth muscle strips, NO levels were monitored using previously established protocol from our laboratory (18, 39–41), with a few modifications. We used a highly sensitive chemiluminescence NO detector (Zysense 280i Nitric Oxide Analyzer NOA, Zysense Instruments, Fredrick, CO) coupled with a purge vessel for reaction chamber, based on a gas-phase (NO) and ozone-chemiluminescence technology using liquid analyze software. This equipment was connected with gas (95% O2/5% CO2) supply to the ozone generator at a flow rate of 30 mL/min at 6 psi) via Teflon tubing and Swagelok bulkhead connector. Then, 200 μL of 50 mM of potassium iodide (KI) solution (freshly prepared in ddH2O) was mixed with 10 mL of glacial acetic acid. Then, we filled the reaction chamber and waited for threshold stabilization at check cell pressure of 4.0–8.0 ± 0.1, supply pressure of 5.9 psi O2, and cooler temperature of –12°C. Then, we opened the gas chamber and waited until –12°C and 4 mV calibration point at 0–100 mV set scale range. After establishing the threshold and calibration, we injected 50 μL of each sample from the muscle bath perfusates collected from different experimental protocols into the purge vessel (before and after NANC stimulation were collected in the absence and presence of BDNF, K252a, or BDNF + K252a from both age groups). Normal KPS served as a negative control in these experiments. Test samples were tested in triplicate under identical conditions of dark and O2-free environment. For the NO standard curve, we used different concentrations of freshly prepared sodium nitrite (NaNO2) in Milli-Q water.
Drugs and Chemicals
Angiotensin II, bethanechol chloride, glacial acetic acid, sodium nitroprusside (SNP), and potassium iodide (KI) were purchased from Sigma Aldrich (St. Louis, MO). BDNF, K252a, and U46619 were purchased from Tocris Bioscience (Minneapolis, MN). Sodium nitrate (NaNO3) and potassium chloride (KCl) were purchased from Thermo Fischer Scientific (Pittsburgh, PA). All other chemicals and reagents were purchased from Sigma Aldrich (St. Louis, MO).
Statistical Analysis
Data were analyzed and graphed using GraphPad Prism 9 (San Diego, CA) as means ± SE of multiple experiments, and a P value < 0.05 was considered statistically significant. For comparisons between two groups, Student’s t test was performed. The concentration-response curves (CRC) were compared and analyzed using one-way ANOVA method.
RESULTS
Effect of BDNF on the Basal IAS Tone in Young Versus Aging Rats
Figure 1A provides details of the time-course data for BDNF (1 nM)-induced increase in the IAS tone in the young group and the aging group. These data show that significant increase in the IAS tone following BDNF was sustained for 1 h in both age groups (*P < 0.05, n = 10 observations in 6 animals). Figure 1B provides typical tracings representing the effects of BDNF and K252a (100 nM) on the IAS tone in both age groups. Figure 1C summarizes these data at the end of 1 h following BDNF and K252a in both age groups. Of importance, BDNF significantly (*P < 0.05) reversed the IAS tone in the aging group to values not significantly different from those in the young control group (P > 0.05).
Figure 1.
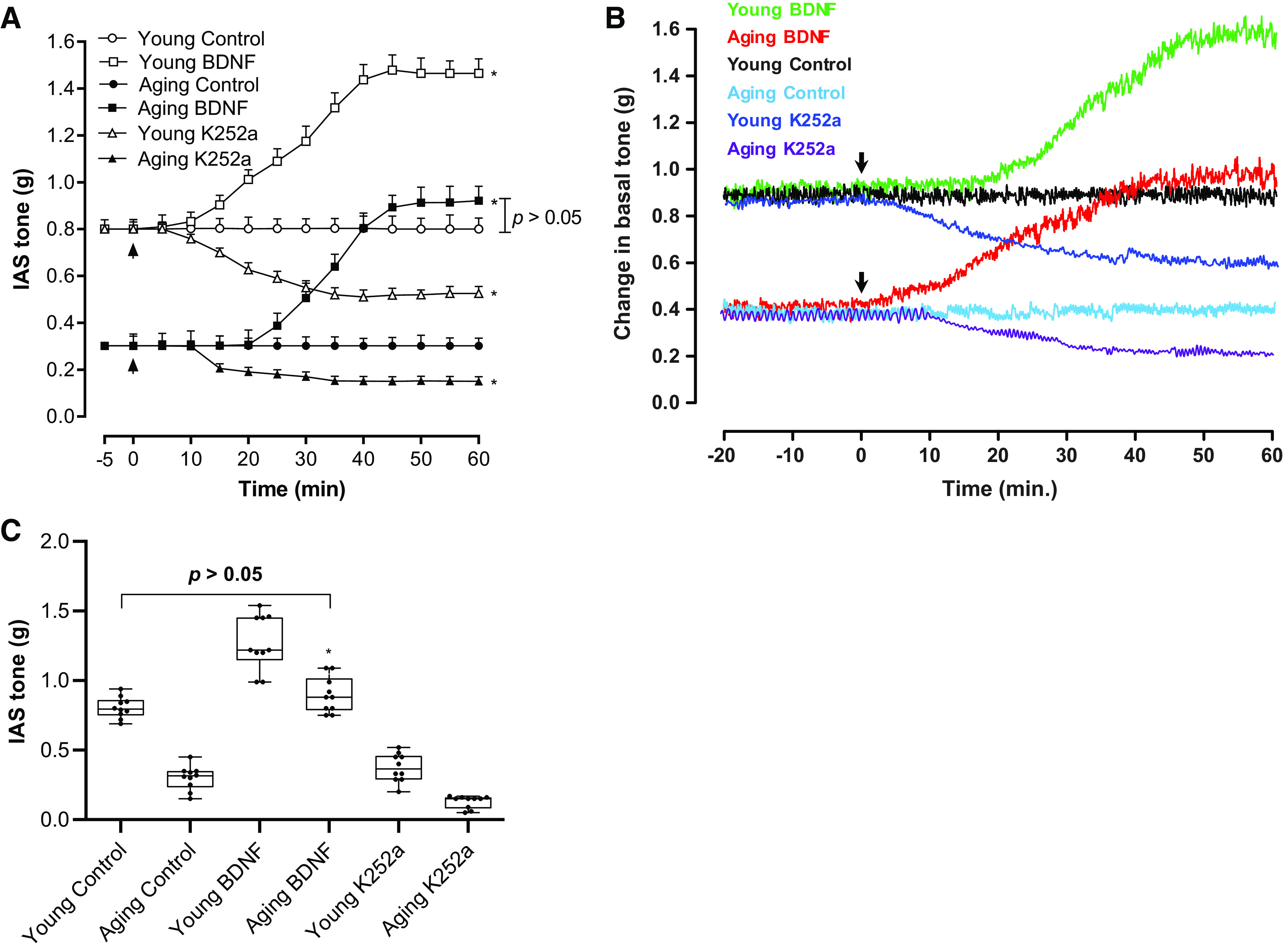
A: time-course effect of BDNF on the basal IAS tone showing significant (t test; *P < 0.05; n = 10 observations from 6 animals) increase in young and aging rats. Addition of BDNF or K252a has been denoted by the arrows in the respective experiments. The respective controls represent steady basal tone in the young and aging groups, without the addition of any agent. B: typical tracings showing the effects of BDNF and K252a on the basal IAS tone in both age groups of animal. C: whisker plot showing that BDNF produces a significant (one-way ANOVA; *P < 0.05; n = 10 observations from 6 animals) and sustained increase in the established basal IAS tone in young and aging rats, determined at the end of 1 h from the beginning of the treatment. These data show that BDNF rescues the aging-associated decrease in the basal IAS tone to the levels not significantly different from that of the young group (P > 0.05). BDNF, brain-derived neurotropic factor; IAS, internal anal sphincter; K252a, TrkB inhibitor.
The basal IAS SM tone was significantly lower in the aging rats as compared with the younger rats (0.3 ± 0.03 vs. 0.80 ± 0.02 g; *P < 0.05). BDNF rescued the aging IAS tone to 0.89 ± 0.04 g, a value not significantly different from the young group values (P > 0.05). In the IAS from the young group, BDNF caused a significant increase in the tone (*P < 0.05). K252a by itself produced a small but significant decrease in the IAS tone in both the young and aging groups (*P < 0.05). The concentrations of BDNF (1 nM) and K252a (100 nM) used for the present investigations were based on previously published optimization studies (18).
Effect of BDNF Before and After K252a on GPCR-Agonists (U46619, ANG II) Versus KCl-Induced Increase in the IAS Tone
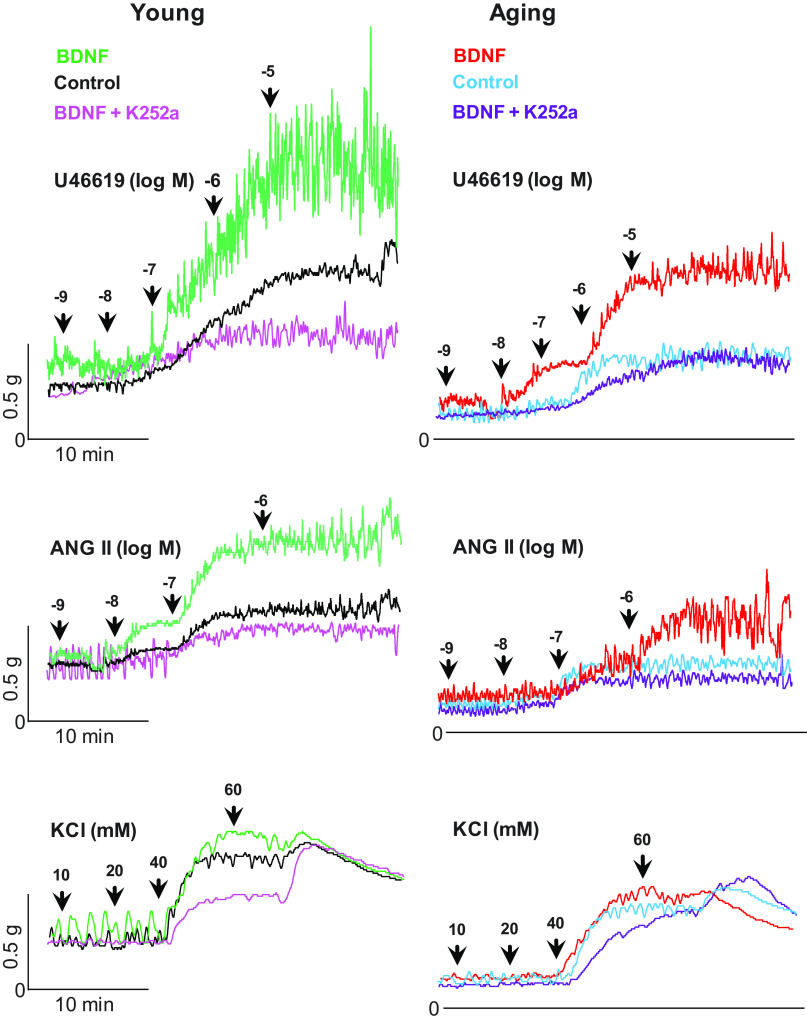
BDNF (1 nM) produced a significant increase in the contractile effects of U46619 and ANG II (*P < 0.05; n = 10 observations from 6 animals; Fig. 2, A and B), as shown by the significant leftward shifts in their control concentration-response curves in young and aging rats. It is noteworthy that BDNF significantly (*P < 0.05, n = 10 observations from 6 animals) reversed the aging-associated decrease in the GPCR-activation CRC to the levels not significantly different from those in the young control group (P > 0.05). In contrast, the effects KCl CRCs were not significantly affected by any of the maneuvers (P > 0.05; n = 10 observations from 6 different animals; Fig. 2C). Effects of BDNF, K252s, and different agonists were reproducible following washout as validated by the return of the basal tone and by the agonists’ responses to the control levels. Typical tracings of experiments with U46619, ANG II, and KCl in young and aging rats, before or after BDNF or BDNF + K252a, are shown in Fig. 3.
Figure 2.
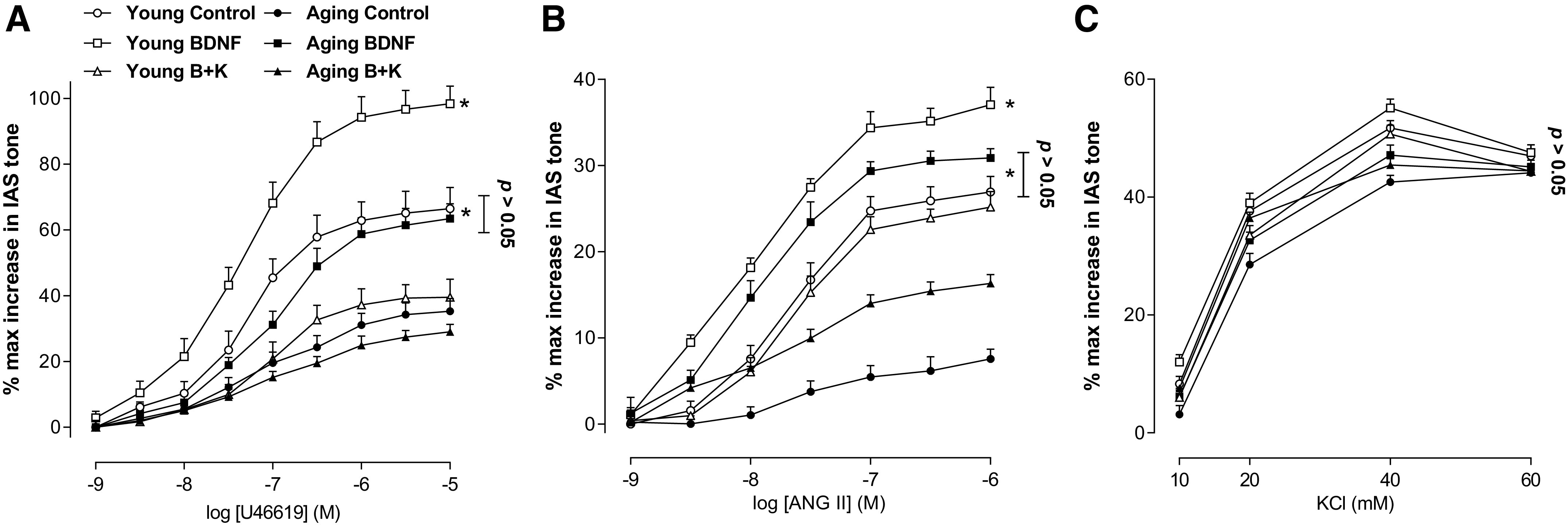
These data show that BDNF augments concentration-response curves for U46619 (A) and ANG II (B) in both age groups as shown by the significant leftward shifts in the GPCR-mediated (t test; *P < 0.05; n = 10 observations from 6 animals). In contrast, BDNF has no significant effect on the KCl-induced IAS contractility (C) (one-way ANOVA; P > 0.05). Data show that BDNF rescues the aging-associated decrease in the IAS contractility to the levels not significantly different from the young group (t test; P > 0.05; n = 10 observations from 6 animals). These effects of BDNF were blocked by K252a (100 nM). ANG II, angiotensin II; BDNF, brain-derived neurotropic factor; GPCR, G protein-coupled receptors; IAS, internal anal sphincter; KCl, potassium chloride; K252a, TrkB inhibitor; U46619, thromboxane A2 analog.
Figure 3.
Typical tracings showing the effects of U46619, ANG II, and KCl on the basal IAS tone, before and after BDNF and K252a, in the young and aging groups of rats. The tracing show that BDNF enhances the effects of U46619 and ANG II, which are minimized by the combined use of BDNF and K252a, in both age groups. It is noteworthy that in the aging group, BDNF rescues the agonists’ effects approximately to the young control levels. Conversely, neither BDNF nor BDNF+K252a affect the KCl-induced smooth muscle contractility in any of the age groups. ANG II, angiotensin II; BDNF, brain-derived neurotropic factor; IAS, internal anal sphincter; KCl, potassium chloride; K252a, TrkB inhibitor; U46619, thromboxane A2 analog
Effect of BDNF, Before and After K252a, on NANC Relaxation in IAS SM Strips from Aging Versus Young Animals
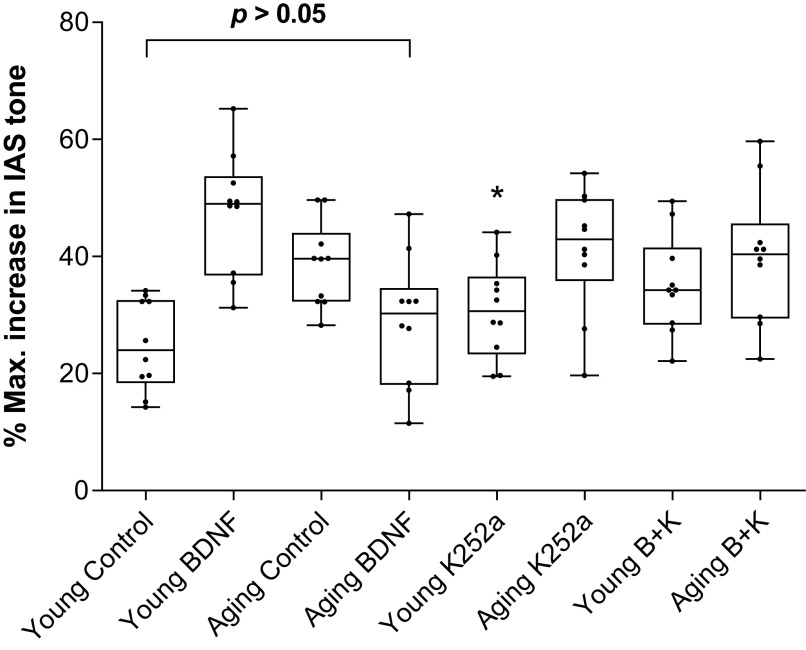
BDNF produced a significant (*P < 0.05; n = 10 observations from 6 animals; Fig. 4A) rescue of the aging-associated increase in the NANC relaxation in the IAS to the values not significantly different (P > 0.05; n = 10 observations from 6 animals; Fig. 4A) from the control young group. Conversely, in the IAS from young animals, BDNF augmented the NANC relaxation. K252a (B + K), significantly attenuated BDNF-produced changes in the NANC relaxation (i.e., decrease in the aging group, and increase in the young group). A summary of such data using EFS at 10 Hz (the maximal effective frequency) appears in Fig. 4B.
Figure 4.
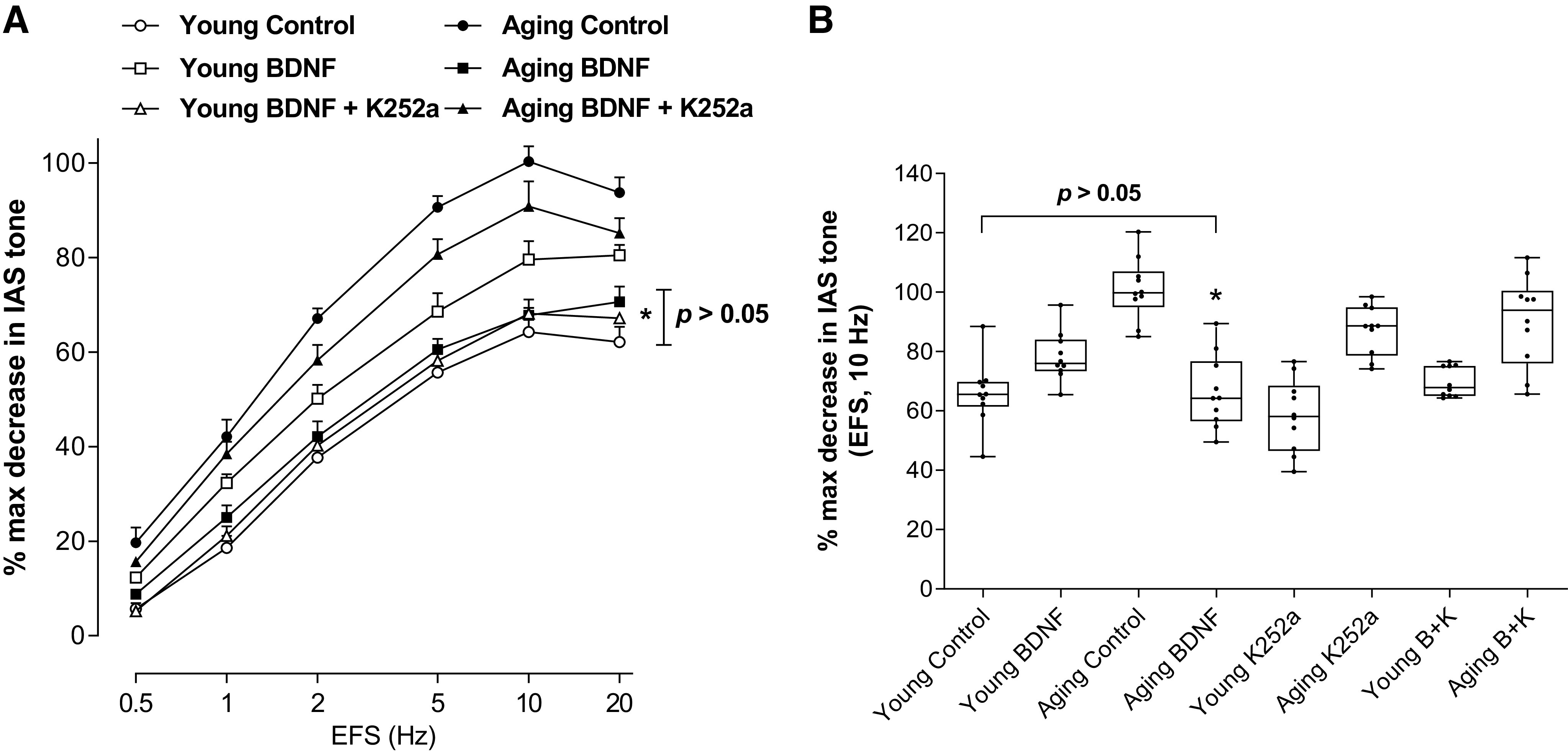
A: data show that BDNF significantly (t test; *P < 0.05; n = 10 observations from 6 animals) rescues the aging-associated increase in the NANC relaxation in the IAS, at different frequencies to the levels not significantly (P > 0.05; n = 10 observations from 6 animals) different from the control young group. B: summarized data for 10 EFS show significant rescue of the aging-associated increase in the NANC relaxation in the IAS by BDNF (B) to the levels not significantly (one-way ANOVA; P > 0.05; n = 10 observations from six animals) different from the control young group. BDNF, brain-derived neurotropic factor; EFS, electrical field stimulation; IAS, internal anal sphincter; NANC, nonadrenergic noncholinergic.
Effect of BDNF on NO Release following NANC Stimulation, Before and After K252a, in the Aging Versus Young Animals
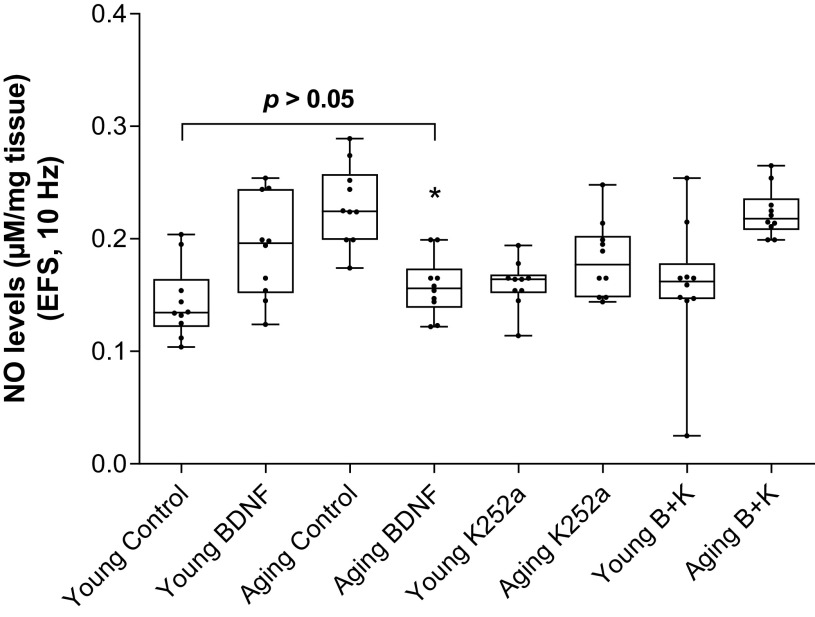
These experiments were carried out to determine the role of NO release in BDNF-induced rescuing effect of augmented NANC relaxation during aging. Data revealed a significant increase (*P < 0.05; n = 10 observations from 6 animals; Fig. 5) in NO release from the IAS smooth muscle bath perfusates taken immediately after EFS in the aging versus the young group. BDNF significantly (*P < 0.05; n = 10 observations from 6 animals) rescued aging-associated increased NANC relaxation by decreasing the amount of NO release to levels not significantly different from the control young group (P > 0.05). In these experiments, there was a correlation between the degree of IAS relaxation and the levels of NO release. K252a (B + K) blocked the BDNF-induced changes in the NANC relaxation and in the levels of NO release. K252a by itself affected neither the NANC relaxation nor the amount of NO release in any of the groups. The exact mechanism involved in these BDNF-associated rescue events remains to be determined.
Figure 5.
BDNF significantly (one-way ANOVA; *P < 0.05) rescues (by attenuating) the NANC nerve stimulation-induced increased release of NO in the aging groups to the levels not significantly different from the control young group. Conversely, however, in the young group, BDNF augments the NANC relaxation in parallel with the increased levels of NO release. All BDNF-induced changes in the NANC relaxation and NO release were attenuated by K252a (B + K) (one-way ANOVA; *P < 0.05; n = 10 observations from 6 animals), whereas K252a by itself has no significant effect on these parameters (P > 0.05). BDNF, brain-derived neurotropic factor; K252a, TrkB inhibitor; NANC, nonadrenergic noncholinergic; NO, nitric oxide.
Effect of BDNF, Before and After K252a, on SNP-Induced IAS Relaxation in the Aging Versus Young Animals
BDNF significantly (*P < 0.05; n = 10 observations from 6 animals; Fig. 6) rescued (by decreasing) aging-associated increase in the sensitivity to SNP-induced relaxation to levels not significantly (P > 0.05) different from that observed in the young control group. On the contrary, BDNF augmented SNP-induced IAS relaxation in the young group. K252a (B + K) blocked BDNF-induced changes in the SNP effects in the IAS from the young and aging animals.
Figure 6.
Data show that BDNF significantly (one-way ANOVA; *P < 0.05; n = 10 observations from 6 animals) rescues aging-associated increase in the IAS relaxation in response to SNP (100 nM) to the levels not significantly (P > 0.05) different from the control young group (P > 0.05). BDNF-induced changes in SNP responses were significantly (*P < 0.05; n = 10 observations from 6 animals) attenuated by K252a (B + K) (*P < 0.05), which by itself has no significant effect (P > 0.05). BDNF, brain-derived neurotropic factor; IAS, internal anal sphincter; K252a, TrkB inhibitor; SNP, sodium nitroprusside.
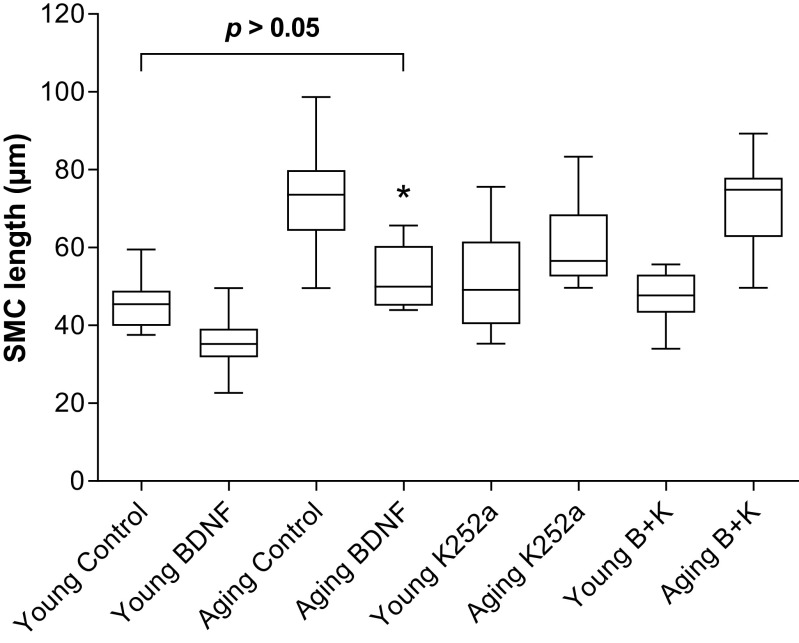
Effect of BDNF, Before and After K252a, on the Basal IAS SMC Lengths and Contractility in the Aging Versus Young Animals
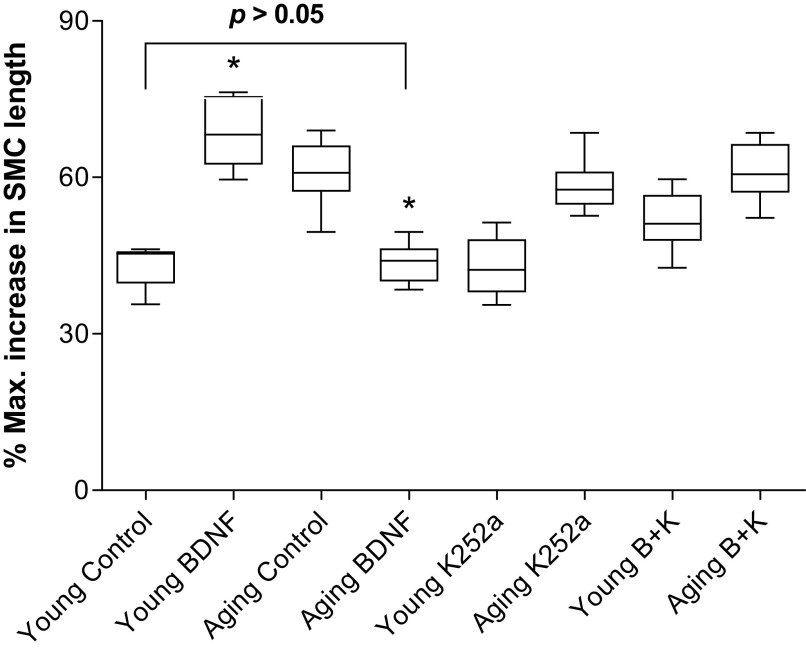
Data show that the SMCs from aging animals’ IAS were significantly longer than from the young animals. BDNF produced a significant rescue of the increased cell length in the aging group from 72.7 ± 4.1 to 45.4 ± 2.0 μm (*P < 0.05; n = 50 cells from 6 animals; Fig. 7). The rescued cell lengths in the older group were not significantly different from the basal IAS SMCs lengths from those in the young control group (45.4 ± 2.0 μm) (P > 0.05). K252a (B + K) blocked the BDNF effects on the basal SMC length.
Figure 7.
Data show that BDNF significantly (one-way ANOVA; *P > 0.05; n = 50 cells from 6 animals) rescues the aging-associated increase in the IAS SMC length in the basal state, to the levels not significantly different from the control values in young group (P > 0.05). The effect of BDNF was reversed by K252a (B + K). BDNF, brain-derived neurotropic factor; IAS, internal anal sphincter; K252a, TrkB inhibitor; SMC, smooth muscle cell.
BDNF also significantly rescued (*P < 0.05; n = 50 cells from 6 animals; Fig. 8, A and B) the aging-associated decrease in the IAS SMC contractility caused by the GPCR-agonists U46619 and ANG II to the levels not significantly (P > 0.05) different from those in young control group. The effects of BDNF were blocked by K252a. These effects of BDNF and K252a were found to be selective since these agents failed to modify the KCl-induced SMC contractility in any of the age groups (P > 0.05; n = 50 cells from 6 animals; Fig. 8C).
Figure 8.
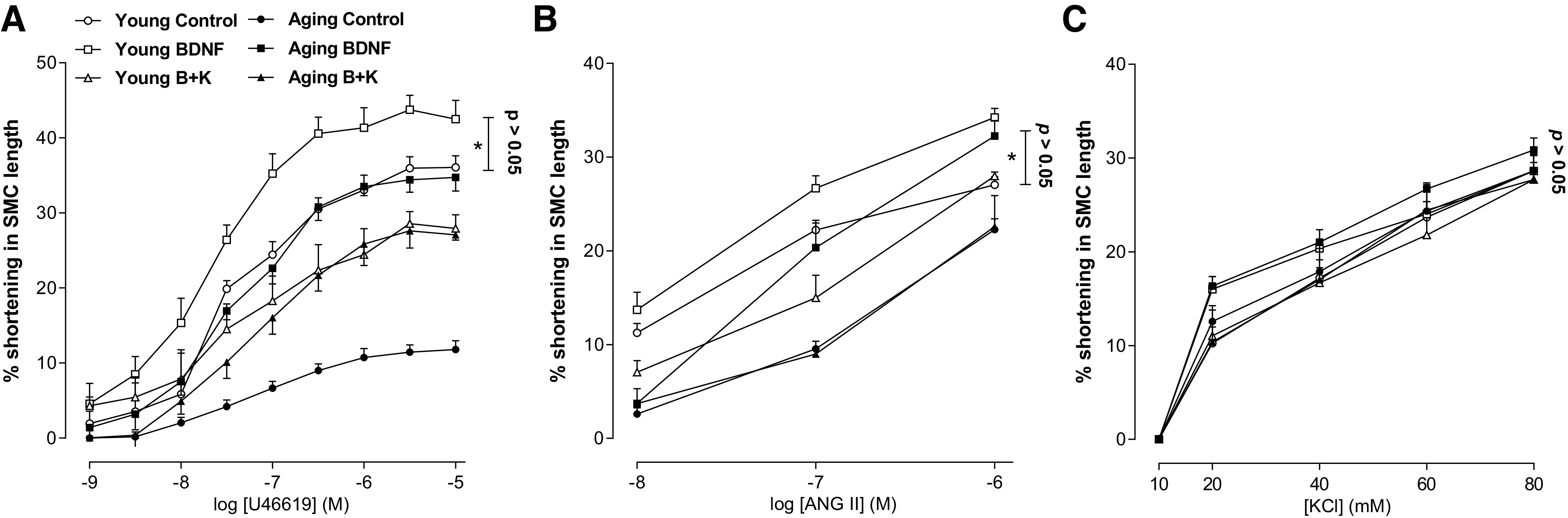
Data show that BDNF significantly rescues (t test; *P > 0.05; n = 50 cells from 6 animals) the aging-associated reduced SMC contractility in response to U46619 (A) and ANG II (B), to the levels not significantly different from the control values in young group (P > 0.05). This effect of BDNF is attenuated by K252a, which by itself has no significant effect (P > 0.05). C: conversely, BDNF failed to modify the effects of KCl. ANG II, angiotensin II; BDNF, brain-derived neurotropic factor; KCl, potassium chloride; K252a, TrkB inhibitor; SMC, smooth muscle cell; U46619, thromboxane A2 analog.
Effect of BDNF, Before and After K252a, on SNP-Induced IAS SMC Relaxation in the Aging Versus Young Animals
BDNF significantly (*P < 0.05; n = 50 cells from 6 animals; Fig. 9) rescued the aging-associated increase in sensitivity to NO donor SNP in causing the IAS SMC relaxation to a level not different (P > 0.05) from the young control group. In the young group, BDNF produced an increase rather than a decrease in the SMC relaxation in response to SNP. K252a significantly attenuated these BDNF (B + K) responses of either rescuing (in the aging group) or of increasing (in the young group) (*P < 0.05; Fig. 9), while having no significant effect by itself. The BDNF effects on SNP-induced smooth muscle and SMCs relaxation suggest postsynaptic effect in addition to its presynaptic effect reflected by the NO release.
Figure 9.
Data show that BDNF significantly (one-way ANOVA; *P < 0.05; n = 50 cells from 6 animals) rescues the aging-associated increase in the IAS SMC relaxation in response to SNP (100 nM) to the levels not significantly (P > 0.05) different from the control young group (P > 0.05). The BDNF-induced changes in SNP responses were significantly (*P < 0.05) attenuated by K252a (B + K) (*P < 0.05). BDNF, brain-derived neurotropic factor; IAS, internal anal sphincter; K252a, TrkB inhibitor; SMC, smooth muscle cell; SNP, sodium nitroprusside.
DISCUSSION
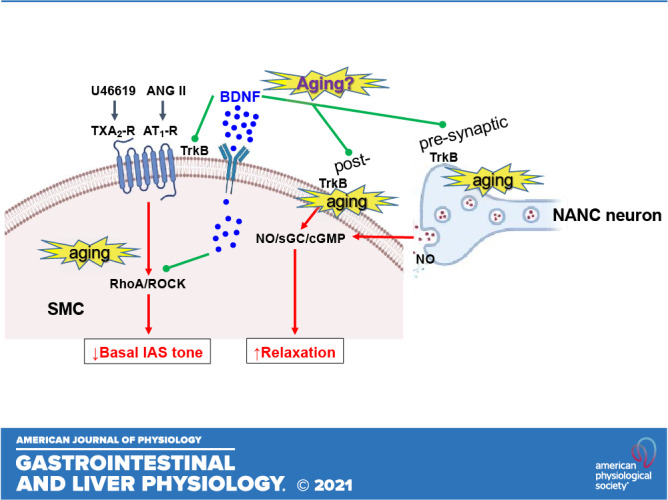
Present studies show for the first time that BDNF rescues the aging-associated IAS dysfunction (AAID), characterized by a decrease in the IAS tone and contractility and increase in the neurogenic NANC relaxation. These events compromise the final gate keeper of colorectal contents, thus making the exodus of the waste products easier and more frequent resulting in rectoanal incontinence (RI). Data show that BDNF rescues AAID by increasing the basal IAS tone and agonists-stimulated contractility, and by decreasing the autonomic IAS relaxation via NANC nerve stimulation (that mimics RAIR).
BDNF increases the basal and agonist-stimulated IAS tone in the young group and the aging group. Interestingly, BDNF elevates the compromised IAS tone in the aging group to the levels not significantly different from those in the young group. In addition, BDNF significantly increases the aging-associated suppressed IAS contractile responses to GPCR-coupled agonists (U46619 and ANG II). The purpose of using U6619 and ANG II in these studies was twofold: 1) to determine the effect of stimulation of the IAS smooth muscle by the autocrine agonists TXA2 and ANG II, shown to be produced by the arachidonic acid (AA) and renin-angiotensin (RAS) pathways within the IAS SMCs, respectively (6, 8, 10, 15, 34, 42); and 2) to determine the mechanism underlying the rescuing effect of BDNF in the IAS tone in the aging group.
Previous studies have established that AA and RAS are primary contributors to the myogenic tone in the IAS via RhoA/ROCK/p-MYPT1/p-MLC20 signaling cascade (7, 8, 10, 16, 43, 44) triggered by GPCR-coupled activation by U46619 and ANG II. Present data show that these effects of BDNF in rescuing the basal and agonist-stimulated IAS tone are specifically reversed by TrkB antagonist K252a, as reported in other systems (21, 24, 28, 30, 45). These findings combined with the lack of influence of these agents on increase in the IAS tone by K+depolarization suggest the specificity of effects of BDNF and the antagonist in the IAS of both the young and aging groups. Based on the present findings along with the critical role of RhoA/ROCK in the effects of BDNF in the IAS tone (18), we speculate that the rescuing effect of BDNF in the IAS during aging involves an upregulation of RhoA/ROCK signaling.
We also demonstrated that BDNF rescues the AAID via a decrease in the NANC relaxation in the aging group, in contrast with augmenting NANC relaxation in the young group. The augmented NANC relaxation in the IAS of the young group of F344R (used in the present studies) was similar to that in the adult Sprague-Dawley rats, as shown previously (18). Importantly, these changes in the NANC relaxation by BDNF in both age groups were blocked by TrkB antagonist K252a, suggesting the specificity of actions of the neurotrophin in these experiments.
It is noteworthy that unexpected BDNF-induced decrease in the IAS relaxation by NANC nerve stimulation in the aging group was in sharp contrast with the augmented relaxation in the young group. These findings, however, were in parallel to the decreased amount of NO release and to the decreased sensitivity to the IAS relaxation by NO donor SNP. These BDNF effects were blocked by K252a. Collectively, these data suggest that the mechanism underlying the rescuing effect of aging in the IAS relaxation by BDNF involves the decrease in the release of the inhibitory neurotransmitter as well as decreased sensitivity to the released NO during NANC nerve stimulation. These effects suggest both pre- and post-synaptic effects of BDNF in the NANC relaxation. However, the mechanisms underlying these BDNF-induced changes in the nitrergic signaling in the aging versus young remain to be determined.
With regard to inhibitory neurotransmission, the main focus of the present studies was on the effect of BDNF on the nitrergic relaxation. This focus was based on previously published reports that in different species (only a few cited here in the following review articles) (5, 46, 47) and in humans (48–50) that the major inhibitory neurotransmitter for the NANC relaxation in the IAS is NO. Some of these studies simultaneously examined the effect of NOS inhibitor, NO scavenger, and guanylate inhibitor on the IAS relaxation and the NO release (12, 13, 51–55). Data showed that these inhibitors nearly obliterated the NANC relaxation in correlation with the corresponding decrease in NO levels. However, present studies do not rule out a partial contribution of other mediators, such as the purinergic inhibitory neurotransmission via ATP (56, 57), in the effect of BDNF in the rat IAS.
The effect of aging in the enhanced NANC-mediated nitrergic relaxation and that of SNP observed in the present studies contrasts with an earlier report by Fidalgo and colleagues in mice (58), which showed a decrease in the NANC relaxation, and no effect on SNP effect, with aging. The exact reason for these discrepant effects is not known. Possibilities include species differences and the high intensity stimulus parameter for the EFS in the mice studies (70 V vs. 10 V in our studies). Additionally, the mice studies reported no definite age-dependent trend for the changes in these relaxation responses. A plausible explanation for the lack of increase in SNP effect in the aging mice may be the use of carbachol (muscarinic agonist) in those studies, which may affect the final outcome in the smooth muscle responses. Other than these differences, the mice data are consistent with our data in showing an aging-associated decrease in the IAS tone.
Present studies have significant implications for RI during aging. Although RI is multifactorial, AAID characterized by the decrease in the basal IAS tone and augmented relaxation are some of the major components (1, 3–5, 58, 59). These findings suggest that BDNF may serve as an important therapeutic target for the RI involving AAID. Whether BDNF has similar effects on the other factors involved in RI, such as external anal sphincter and puborectalis skeletal muscles and colorectal contractility (60, 61), remains to be determined.
It is possible that the effects of BDNF in different smooth muscle systems involve the SMCs, myenteric and submucosal neurons, enterochromaffin cells, and the epithelial cells (23, 29, 31, 62, 63). Recent studies from different laboratories have shown that BDNF is not only present in and released from the enteric neurons but also is present and released from the SMCs (24, 25, 29, 33). However, the effects of BDNF via enteric neurons and glial cells may be ruled out by the lack of significant influence of TTX and gliotoxin on BDNF effects (18, 33). Additional data monitoring the SMCs’ contractility and relaxation further document the direct effects of BDNF in the IAS SMCs. This was shown by the similarities of the effects of BDNF on the basal cell lengths, and SMCs’ shortening by U46619 and ANG II in the young and aging groups, and by the findings that K252a blocked these effects of BDNF. It is of interest that the effects of SNP (NO releaser) in the IAS SMCs were in congruence with those in the IAS muscle strips from the young group and the aging group, before and after BDNF alone and in the presence of K252a. These studies further strengthen the case for decreased release of NO, and sensitivity to the effects of the released inhibitory neurotransmitter NO following NANC nerve stimulation by the EFS, following BDNF in the aging IAS smooth muscle.
Our data, in principle, agree with those of the previously published reports in different systems showing that BDNF augments the smooth muscle contractility via TrkB signaling (24, 29–31, 63–66). Present data agree with the earlier reports showing that in the young group of rats and guinea pigs, BDNF improves NANC relaxation in the IAS (18, 67). The guinea pigs data suggest that BDNF may augment the IAS relaxation because of an increase in the neurogenesis (67), perhaps of NANC neurons. It is of further interest that, in support of our hypothesis for the role of RhoA/ROCK in the BDNF-induced increase in the basal and stimulated IAS tone, there is some data to suggest that this pathway is also involved in the increased neurogenesis (68). However, the role of BDNF/TrkB signaling in the pathophysiological and therapeutic targeting of rectoanal motility disorders involving AAID had not been previously addressed before now. Collectively, the present studies suggest that RhoA/ROCK signaling and nitrergic inhibitory neurotransmission are involved in the rescuing effect of BDNF in the AAID.
In summary, the data presented here suggest that BDNF rescues AAID via an increase in the basal and GPCR-coupled agonist-stimulated IAS tone by activation of RhoA/ROCK in the SMCs, and via decrease in the nitrergic NANC inhibitory neurotransmission. These studies however, do not rule out a role of other structures in the IAS such as the interstial cells of Cajal. These findings have significant implications for BDNF in the pathophysiological and therapeutic targeting for a number of rectoanal motility disorders, especially those involving AAID.
GRANTS
This work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases Grant RO1DK035385, an institutional grant from Thomas Jefferson University, Philadelphia, Pennsylvania, and a TJU SKCC Core facility grant NCI 5 P30 CA-56036.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S. and S.R. conceived and designed research; A.S. performed experiments; A.S. analyzed data; A.S. and S.R. interpreted results of experiments; A.S. prepared figures; A.S. and S.R. drafted manuscript; A.S. and S.R. edited and revised manuscript; A.S. and S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jennifer Wilson for critical proofreading and Dr. Yajing Wang for assistance in NO measurements.
REFERENCES
- 1.Bharucha AE, Dunivan G, Goode PS, Lukacz ES, Markland AD, Matthews CA, Mott L, Rogers RG, Zinsmeister AR, Whitehead WE, Rao SS, Fa H. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol 110: 127–136, 2015. doi: 10.1038/ajg.2014.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharucha AE, Rao SSC. An update on anorectal disorders for gastroenterologists. Gastroenterology 146: 37–45, 2014. doi: 10.1053/j.gastro.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heyt GJ, Oh MK, Alemzadeh N, Rivera S, Jimenez SA, Rattan S, Cohen S, DiMarino AJ. Impaired rectoanal inhibitory response in scleroderma (systemic sclerosis): an association with fecal incontinence. Dig Dis Sci 49: 1040–1045, 2004. doi: 10.1023/B:DDAS.0000034569.85066.69. [DOI] [PubMed] [Google Scholar]
- 4.Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology 126: S14–S22, 2004. doi: 10.1053/j.gastro.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Rattan S, Singh J. Basal internal anal sphincter tone, inhibitory neurotransmission, and other factors contributing to the maintenance of high pressures in the anal canal. Neurogastroenterol Motil 23: 3–7, 2011. doi: 10.1111/j.1365-2982.2010.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Godoy MAF, Dunn SR, Rattan S. Evidence for the role of angiotensin II biosynthesis in the rat internal anal sphincter tone. Gastroenterology 127: 127–138, 2004. doi: 10.1053/j.gastro.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 7.Rattan S. Ca2+/calmodulin/MLCK pathway initiates, and RhoA/ROCK maintains, the internal anal sphincter smooth muscle tone. Am J Physiol Gastrointest Liver Physiol 312: G63–G66, 2017. doi: 10.1152/ajpgi.00370.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rattan S, Benjamin P, Maxwell PJ IV.. RhoA/ROCK-kinase: pathophysiologic and therapeutic implications in gastrointestinal smooth muscle tone and relaxation. Gastroenterology 138: 13–18, 2010. doi: 10.1053/j.gastro.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rattan S, De Godoy MAF, Patel CA. Rho kinase as a novel molecular therapeutic target for hypertensive internal anal sphincter. Gastroenterology 131: 108–116, 2006. doi: 10.1053/j.gastro.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 10.Rattan S, Singh J, Kumar S, Phillips B. Nature of extracellular signal that triggers RhoA/ROCK activation for the basal internal anal sphincter tone in humans. Am J Physiol Gastrointest Liver Physiol 308: G924–G933, 2015. [Erratum in Am J Physiol Gastrointest Liver Physiol 309: G132, 2015] doi: 10.1152/ajpgi.00017.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rattan S, Sarkar A, Chakder S. Nitric oxide pathway in rectoanal inhibitory reflex of opossum internal anal sphincter. Gastroenterology 103: 43–50, 1992. doi: 10.1016/0016-5085(92)91093-J. [DOI] [PubMed] [Google Scholar]
- 12.Rattan S, Chakder S. Role of nitric oxide as a mediator of internal anal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol 262: G107–G112, 1992. doi: 10.1152/ajpgi.1992.262.1.G107. [DOI] [PubMed] [Google Scholar]
- 13.Rattan S, Regan RF, Patel CA, De Godoy MAF. Nitric oxide not carbon monoxide mediates nonadrenergic noncholinergic relaxation in the murine internal anal sphincter. Gastroenterology 129: 1954–1966, 2005. doi: 10.1053/j.gastro.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 14.Terauchi A, Kobayashi D, Mashimo H. Distinct roles of constitutive nitric oxide synthases and interstitial cells of Cajal in rectoanal relaxation. Am J Physiol Gastrointest Liver Physiol 289: G291–G299, 2005. doi: 10.1152/ajpgi.00005.2005. [DOI] [PubMed] [Google Scholar]
- 15.De Godoy MAF, Rattan N, Rattan S. Arachidonic acid metabolites follow the preferential course of cyclooxygenase pathway for the basal tone in the internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 296: G727–G734, 2009. doi: 10.1152/ajpgi.90707.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohanty I, Singh J, Rattan S. Downregulation of thromboxane A2 and angiotensin II type 1 receptors associated with aging-related decrease in internal anal sphincter tone. Sci Rep 9: 6759, 2019. doi: 10.1038/s41598-019-42894-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh J, Boopathi E, Addya S, Phillips B, Rigoutsos I, Penn RB, Rattan S. Aging-associated changes in microRNA expression profile of internal anal sphincter smooth muscle: role of microRNA-133a. Am J Physiol Gastrointest Liver Physiol 311: G964–G973, 2016. doi: 10.1152/ajpgi.00290.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh A, Mohanty I, Singh J, Rattan S. BDNF augments rat internal anal sphincter smooth muscle tone via RhoA/ROCK signaling, and nonadrenergic noncholinergic relaxation via increased NO release. Am J Physiol Gastrointest Liver Physiol 318: G23–G33, 2020. doi: 10.1152/ajpgi.00247.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hempstead BL. Brain-derived neurotrophic factor: three ligands, many actions. Trans Am Clin Climatol Assoc 126: 9–19, 2015. [PMC free article] [PubMed] [Google Scholar]
- 20.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29: 507–538, 2006. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 21.Skaper SD. The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol 846: 1–12, 2012. doi: 10.1007/978-1-61779-536-7_1. [DOI] [PubMed] [Google Scholar]
- 22.Thomas K, Davies A. Neurotrophins: a ticket to ride for BDNF. Curr Biol 15: R262–R264, 2005. doi: 10.1016/j.cub.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Hoehner JC, Wester T, Pahlman S, Olsen L. Localization of neurotrophins and their high-affinity receptors during human enteric nervous system development. Gastroenterology 110: 756–767, 1996. doi: 10.1053/gast.1996.v110.pm8608885. [DOI] [PubMed] [Google Scholar]
- 24.Prakash YS, Martin RJ. Brain-derived neurotrophic factor in the airways. Pharmacol Ther 143: 74–86, 2014. doi: 10.1016/j.pharmthera.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Britt RD Jr, Thompson MA, Wicher SA, Manlove LJ, Roesler A, Fang YH, Roos C, Smith L, Miller JD, Pabelick CM, Prakash YS. Smooth muscle brain-derived neurotrophic factor contributes to airway hyperreactivity in a mouse model of allergic asthma. FASEB J 33: 3024–3034, 2019. doi: 10.1096/fj.201801002R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S. Neurotrophic factors in enteric physiology and pathophysiology. Neurogastroenterol Motil 30: e13446, 2018. doi: 10.1111/nmo.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donovan MJ, Miranda RC, Kraemer R, McCaffrey TA, Tessarollo L, Mahadeo D, Sharif S, Kaplan DR, Tsoulfas P, Parada L. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. Am J Pathol 147: 309–324, 1995. [PMC free article] [PubMed] [Google Scholar]
- 28.Aravamudan B, Thompson MA, Pabelick CM, Prakash YS. Mechanisms of BDNF regulation in asthmatic airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 311: L270–L279, 2016. doi: 10.1152/ajplung.00414.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Qudah M, Alkahtani R, Akbarali HI, Murthy KS, Grider JR. Stimulation of synthesis and release of brain-derived neurotropic factor from intestinal smooth muscle cells by substance P and pituitary adenylate cyclase-activating peptide. Neurogastroenterol Motil 27: 1162–1174, 2015. doi: 10.1111/nmo.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Qudah M, Anderson CD, Mahavadi S, Bradley ZL, Akbarali HI, Murthy KS, Grider JR. Brain-derived neurotrophic factor enhances cholinergic contraction of longitudinal muscle of rabbit intestine via activation of phospholipase C. Am J Physiol Gastrointest Liver Physiol 306: G328–G337, 2014. doi: 10.1152/ajpgi.00203.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grider JR, Piland BE, Gulick MA, Qiao LY. Brain-derived neurotrophic factor augments peristalsis by augmenting 5-HT and calcitonin gene-related peptide release. Gastroenterology 130: 771–780, 2006. doi: 10.1053/j.gastro.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 32.Chen FX, Yu YB, Yuan XM, Zuo XL, Li YQ. Brain-derived neurotrophic factor enhances the contraction of intestinal muscle strips induced by SP and CGRP in mice. Regul Pept 178: 86–94, 2012. doi: 10.1016/j.regpep.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Singh A, Singh J, Rattan S. Evidence for the presence and release of BDNF in the neuronal and non-neuronal structures of the internal anal sphincter. Neurogastroenterol Motil 33: e14099, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Godoy MAF, Rattan S. Angiotensin-converting enzyme and angiotensin II receptor subtype 1 inhibitors restitute hypertensive internal anal sphincter in the spontaneously hypertensive rats. J Pharmacol Exp Ther 318: 725–734, 2006. doi: 10.1124/jpet.106.103366. [DOI] [PubMed] [Google Scholar]
- 35.Krishna CV, Singh J, Kumar S, Rattan S. Heme oxygenase-1 upregulation modulates tone and fibroelastic properties of internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 307: G595–G601, 2014. doi: 10.1152/ajpgi.00159.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel CA, Rattan S. Cellular regulation of basal tone in internal anal sphincter smooth muscle by RhoA/ROCK. Am J Physiol Gastrointest Liver Physiol 292: G1747–G1756, 2007. doi: 10.1152/ajpgi.00438.2006. [DOI] [PubMed] [Google Scholar]
- 37.Singh J, Mohanty I, Addya S, Phillips B, Mee YH, An SS, Penn RB, Rattan S. Role of differentially expressed microRNA-139-5p in the regulation of phenotypic internal anal sphincter smooth muscle tone. Sci Rep 7: 1477–1487, 2017. doi: 10.1038/s41598-017-01550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Godoy MAF, Rattan N, Rattan S. COX-1 vs. COX-2 as a determinant of the basal tone in the internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 296: G219–G225, 2009. doi: 10.1152/ajpgi.90485.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakder S, Rathi S, Ma X, Rattan S. Heme oxygenase inhibitor zinc protoporphyrin IX causes an activation of nitric oxide synthase in the rabbit internal anal sphincter. J Pharmacol Exp Ther 277: 1376–1382, 1996. [PubMed] [Google Scholar]
- 40.Banwait KS, Rattan S. Role of nitric oxide in β3-adrenoceptor activation on basal tone of internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 285: G547–G555, 2003. doi: 10.1152/ajpgi.00545.2002. [DOI] [PubMed] [Google Scholar]
- 41.Endo Y, Shirai T, Akamatsu T, Asada K. Comparison of fractional exhaled nitric oxide levels measured using the NIOX VERO and NOA 280i. Ann Allergy Asthma Immunol 119: 383–385, 2017. doi: 10.1016/j.anai.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 42.De Godoy MAF, Rattan S. Autocrine regulation of internal anal sphincter tone by renin-angiotensin system: comparison with phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol 289: G1164–G1175, 2005. doi: 10.1152/ajpgi.00115.2005. [DOI] [PubMed] [Google Scholar]
- 43.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 44.Harnett KM, Cao W, Biancani P. Signal-transduction pathways that regulate smooth muscle function I. Signal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles. Am J Physiol Gastrointest Liver Physiol 288: G407–G416, 2005. doi: 10.1152/ajpgi.00398.2004. [DOI] [PubMed] [Google Scholar]
- 45.Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol 11: 272–280, 2001. doi: 10.1016/S0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 46.Rattan S. The internal anal sphincter: regulation of smooth muscle tone and relaxation. Neurogastroenterol Motil 17: 50–59, 2005. doi: 10.1111/j.1365-2982.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 47.Said SI, Rattan S. The multiple mediators of neurogenic smooth muscle relaxation. Trends Endocrinol Metab 15: 189–191, 2004. doi: 10.1016/j.tem.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 48.O'Kelly T, Brading A, Mortensen N. Nerve mediated relaxation of the human internal anal sphincter: the role of nitric oxide. Gut 34: 689–693, 1993. doi: 10.1136/gut.34.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glavind EB, Forman A, Madsen G, Tottrup A. Effects of transmural field stimulation in isolated smooth muscle of human rectum and internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 272: G1075–G1082, 1997. doi: 10.1152/ajpgi.1997.272.5.G1075. [DOI] [PubMed] [Google Scholar]
- 50.Burleigh DE. Ng-Nitro-L-arginine reduces nonadrenergic, noncholinergic relaxations of human gut. Gastroenterology 102: 679–683, 1992. doi: 10.1016/0016-5085(92)90120-N. [DOI] [PubMed] [Google Scholar]
- 51.Chakder S, Rattan S. Release of nitric oxide by activation of nonadrenergic noncholinergic neurons of internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 264: G7–G12, 1993. doi: 10.1152/ajpgi.1993.264.1.G7. [DOI] [PubMed] [Google Scholar]
- 52.Chakder S, Rattan S. Involvement of cAMP and cGMP in relaxation of internal anal sphincter by neural stimulation, VIP, and NO. Am J Physiol Gastrointest Liver Physiol 264: G702–G707, 1993. doi: 10.1152/ajpgi.1993.264.4.G702. [DOI] [PubMed] [Google Scholar]
- 53.Rattan S, Rosenthal GJ, Chakder S. Human recombinant hemoglobin (rHb1.1) inhibits nonadrenergic noncholinergic (NANC) nerve-mediated relaxation of internal anal sphincter. J Pharmacol Exp Ther 272: 1211–1216, 1995. [PubMed] [Google Scholar]
- 54.Rattan S, Al Haj R, De Godoy MAF. Mechanism of internal anal sphincter relaxation by CORM-1, authentic CO, and NANC nerve stimulation. Am J Physiol Gastrointest Liver Physiol 287: G605–G611, 2004. doi: 10.1152/ajpgi.00070.2004. [DOI] [PubMed] [Google Scholar]
- 55.Chakder S, Rattan S. Neurally mediated relaxation of opossum internal anal sphincter: Influence of superoxide anion generator and the scavenger. J Pharmacol Exp Ther 260: 1113–1118, 1992. [PubMed] [Google Scholar]
- 56.Opazo A, Lecea B, Gil V, Jimenéz M, Clavé P, Gallego D. Specific and complimentary roles of nitric oxide and ATP in the inhibitory motor pathways to rat internal anal sphincter. Neurogastroenterol Motil 23: e11–e25, 2011. doi: 10.1111/j.1365-2982.2010.01602.x. [DOI] [PubMed] [Google Scholar]
- 57.Duffy AM, Cobine CA, Keef KD. Changes in neuromuscular transmission in the W/Wv mouse internal anal sphincter. Neurogastroenterol Motil 24: e42–e55, 2012. doi: 10.1111/j.1365-2982.2011.01806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fidalgo S, Patel BA, Ranson RN, Saffrey MJ, Yeoman MS. Changes in murine anorectum signaling across the life course. Neurogastroenterol Motil 30: e13426, 2018. doi: 10.1111/nmo.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mandaliya R, DiMarino AJ, Moleski S, Rattan S, Cohen S. Survey of anal sphincter dysfunction using anal manometry in patients with fecal incontinence: a possible guide to therapy. Ann Gastroenterol 28: 469–474, 2015. [PMC free article] [PubMed] [Google Scholar]
- 60.Jonker JE, Trzpis M, Broens PMA. Fecal continence for solid and liquid stool: the function of the anal-external ephincter continence reflex and the puborectal continence reflex. Dis Colon Rectum 63: 1419–1426, 2020. doi: 10.1097/DCR.0000000000001615. [DOI] [PubMed] [Google Scholar]
- 61.Rajasekaran MR, Kanoo S, Fu J, Nguyen ML, Bhargava V, Mittal RK. Age-related external anal sphincter muscle dysfunction and fibrosis: possible role of Wnt/beta-catenin signaling pathways. Am J Physiol Gastrointest Liver Physiol 313: G581–G588, 2017. doi: 10.1152/ajpgi.00209.2017. [DOI] [PubMed] [Google Scholar]
- 62.Lommatzsch M, Braun A, Mannsfeldt A, Botchkarev VA, Botchkareva NV, Paus R, Fischer A, Lewin GR, Renz H. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived neurotrophic functions. Am J Pathol 155: 1183–1193, 1999. doi: 10.1016/S0002-9440(10)65221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Esteban I, Hannestad J, Levanti B, Del Valle ME, Naves FJ, Vega JA. Neurotrophin receptor proteins immunoreactivity in human gastrointestinal endocrine cells. Brain Res Bull 38: 539–543, 1995. doi: 10.1016/0361-9230(95)02025-9. [DOI] [PubMed] [Google Scholar]
- 64.Coulie B, Szarka LA, Camilleri M, Burton DD, McKinzie S, Stambler N, Cedarbaum JM. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology 119: 41–50, 2000. doi: 10.1053/gast.2000.8553. [DOI] [PubMed] [Google Scholar]
- 65.Fu Y, Lin YM, Winston JH, Radhakrishnan R, Huang LM, Shi XZ. Role of brain-derived neurotrophic factor in the pathogenesis of distention-associated abdominal pain in bowel obstruction. Neurogastroenterol Motil 30: e13373, 2018. doi: 10.1111/nmo.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen F, Yu Y, Wang P, Dong Y, Wang T, Zuo X, Li Y. Brain-derived neurotrophic factor accelerates gut motility in slow-transit constipation. Acta Physiol (Oxf) 212: 226–238, 2014. doi: 10.1111/apha.12374. [DOI] [PubMed] [Google Scholar]
- 67.Katsui R, Kojima Y, Kuniyasu H, Shimizu J, Koyama F, Fujii H, Nakajima Y, Takaki M. A new possibility for repairing the anal dysfunction by promoting regeneration of the reflex pathways in the enteric nervous system. Am J Physiol Gastrointest Liver Physiol 294: G1084–G1093, 2008. doi: 10.1152/ajpgi.00345.2007. [DOI] [PubMed] [Google Scholar]
- 68.Zhou XP, Wu KY, Liang B, Fu XQ, Luo ZG. TrkB-mediated activation of geranylgeranyltransferase I promotes dendritic morphogenesis. Proc Natl Acad Sci USA 105: 17181–17186, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]