Keywords: aldosterone-sensitive distal nephron, distal convoluted tubule, epithelial Na+ channel, K+ excretion, Na+ transport
Abstract
High-dietary K+ (HK) intake inhibits basolateral Kir4.1/Kir5.1 activity in the distal convoluted tubule (DCT), and HK-induced inhibition of Kir4.1/Kir5.1 is essential for HK-induced inhibition of NaCl cotransporter (NCC). Here, we examined whether neural precursor cell expressed developmentally downregulated 4-2 (Nedd4-2) deletion compromises the effect of HK on basolateral Kir4.1/Kir5.1 and NCC in the DCT. Single-channel recording and whole cell recording showed that neither HK decreased nor low-dietary K+ (LK) increased basolateral Kir4.1/Kir5.1 activity of the DCT in kidney tubule-specific Nedd4-2 knockout (Ks-Nedd4-2 KO) mice. In contrast, HK inhibited and LK increased Kir4.1/Kir5.1 activity in control mice [neural precursor cell expressed developmentally downregulated 4-like (Nedd4l)flox/flox]. Also, HK intake decreased the negativity of K+ current reversal potential in the DCT (depolarization) only in control mice but not in Ks-Nedd4-2 KO mice. Renal clearance experiments showed that HK intake decreased, whereas LK intake increased, hydrochlorothiazide-induced renal Na+ excretion only in control mice, but this effect was absent in Ks-Nedd4-2 KO mice. Western blot analysis also demonstrated that HK-induced inhibition of phosphorylated NCC (Thr53) and total NCC was observed only in control mice but not in Ks-Nedd4-2 KO mice. Furthermore, expression of all three subunits of the epithelial Na+ channel in Ks-Nedd4-2 KO mice on HK was higher than in control mice. Thus, plasma K+ concentrations were similar between Nedd4lflox/flox and Ks-Nedd4-2 KO mice on HK for 7 days despite high NCC expression. We conclude that Nedd4-2 plays a role in regulating HK-induced inhibition of Kir4.1/Kir5.1 and NCC in the DCT.
NEW & NOTEWORTHY Basolateral Kir4.1/Kir5.1 in the distal convoluted tubule plays an important role as a “K+ sensor” in the regulation of renal K+ excretion after high K+ intake. We found that neural precursor cell expressed developmentally downregulated 4-2 (Nedd4-2) a role in mediating the effect of K+ diet on Kir4.1/Kir5.1 and NaCl cotransporter because high K+ intake failed to inhibit basolateral Kir4.1/Kir5.1 and NaCl cotransporter in kidney tubule-specific Nedd4-2 knockout mice.
INTRODUCTION
Basolateral K+ channels in the distal convoluted tubule (DCT) are composed of Kir4.1 and Kir5.1 (1–6); the Kir4.1/Kir5.1 heterotetramer forms a 40-pS inwardly rectifying K+ channel (3, 7, 8). Kir4.1 provides the K+ conductance for the heterotetramer, as Kir4.1 deletion almost completely abolishes basolateral K+ conductance in the DCT (1, 9). On the other hand, Kir5.1 is an important regulatory subunit for the Kir4.1/Kir5.1 heterotetramer (6, 10–13). We have previously demonstrated that neural precursor cell expressed developmentally downregulated 4-2 (Nedd4-2) was associated with Kir5.1 at the COOH-terminus and that it regulated Kir4.1 ubiquitination in the presence of Kir5.1, thereby inhibiting Kir4.1 activity (13). The role of Nedd4-2 in regulating Kir4.1 activity of the DCT was also strongly suggested by the finding that deletion of Nedd4-2 increased Kir4.1 expression and hyperpolarized the DCT membrane (14).
Several studies have demonstrated that dietary K+ intake regulates NaCl cotransporter (NCC) expression/activity in the DCT, such that low K+ (LK) intake stimulates NCC expression/activity, whereas high K+ (HK) intake inhibits NCC expression/activity (9, 15–21). Previous studies have also shown that LK-induced stimulation of NCC was associated with increased Kir4.1/Kir5.1 activity, whereas HK-induced inhibition of NCC activity was associated with diminished Kir4.1/Kir5.1 activity (9, 22). The notion that basolateral Kir4.1 and Kir5.1 in the DCT are responsible for the effect of dietary K+ intake on NCC is strongly indicated by the finding that the deletion of either Kir4.1 or Kir5.1 abolishes the effect of HK or LK on NCC expression or activity (9, 22). Since Nedd4-2 is expressed in the DCT and plays a role in regulating the abundance of NCC and Kir4.1 in the DCT (14, 23), the aim of the present study was to examine whether deletion of Nedd4-2 compromises the effect of HK intake on basolateral K+ channel activity of the DCT and NCC expression/activity.
METHODS
Animals
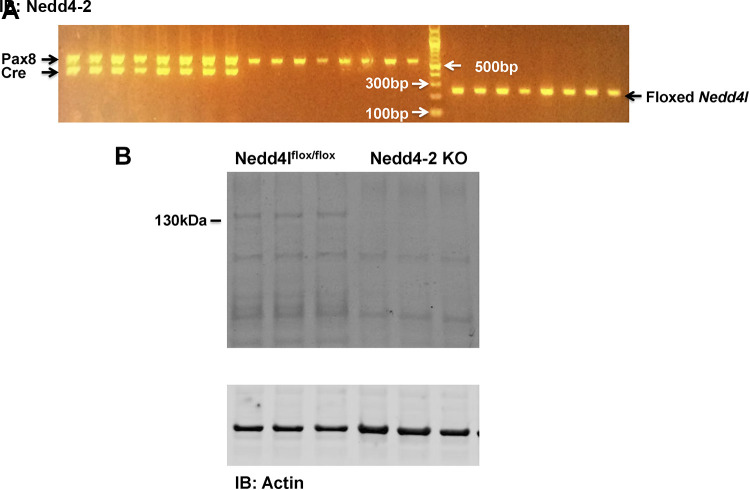
Floxed neural precursor cell expressed developmentally downregulated 4-like (Nedd4lfl/fl) mice (control) and kidney tubule-specific Nedd4-2 knockout (Ks-Nedd4-2 KO) mice were used in our study (23), and the method for generation of animals has been previously described (14). Briefly, mice expressing Pax8-rtTA and tet-on LC-1, which drive Pax8 and Cre expression under tetracycline-dependent induction, were crossed with Nedd4lfl/fl mice to generate inducible Ks-Nedd4-2 KO mice. For genotyping, we amplified tail DNA by the PCR method. For Nedd4l, the forward and reverse primers were 5′- TGAGCTCATTGCTTCACTTCC-3′ and 5′- TTCATGCTCGAAGCCTTAGC-3′, respectively (230 bp for floxed Nedd4l and 150 bp for wild-type). For Pax8-rtTA, the forward and reverse primers were 5′- CCATGTCTAGACTGGACAAGA-3′ and 5′- CTCCAGGCCACATATGATTAG-3′, respectively (a 650-bp product). For LC1-CRE, the primers were forward 5′- TCGCTGCATTACCGGTCGATGC-3′ and reverse 5′- CCATGAGTGAACGAACCTGGTCG-3′, respectively (a 430-bp product). Figure 1A shows an agarose gel demonstrating the genotype results. We fed 10- to 12-wk-old Pax8-cre-Nedd4lfl/fl mice with doxycycline (2 mg in 2% sucrose solution) for 2 wk, and mice were then kept for an additional 2 wk without doxycycline. We randomly selected three mice from each group of peers to conduct Western blot analysis to confirm the deletion of Nedd4-2. Figure 1B shows a Western blot demonstrating Nedd4-2 expression in wild-type and Ks-Nedd4-2 KO mice. We also fed Nedd4lfl/fl mice with doxycycline at the beginning of the study as the control. Since doxycycline treatment did not have effect on Kir4.1/Kir5.1 activity and NCC expression, we then used Nedd4lflox/flox mice treated with only 2% sucrose for 2 wk as control mice. Animals were housed in the New York Medical College animal facility with lights on at 7:00 AM and off at 7:00 PM. Mice had unlimited access to water and rodent chow. Mice were fed with the control diet (0.8% K+ and 0.4% Na+), HK diet (5% K+ and 0.4% Na+), or LK diet (0.01−0.02% K+ and 0.4% Na+) for 7 days. HK diet (Cat. No. TD110866) and LK diet (Cat. No. TD 120441) were purchased from Envigo-Teklad Diets (Madison, WI). All procedures were reviewed and approved by the Institutional Animal Care and Use Committee.
Figure 1.
A: agarose gel showing the results of genotyping Pax8, Cre, and floxed neural precursor cell expressed developmentally downregulated 4-like (Nedd4l). B: Western blot showing the expression of neural precursor cell expressed developmentally downregulated 4-2 (Nedd4-2) in control and kidney tubule-specific Nedd4-2 knockout (Ks-Nedd4-2 KO) mice. Tissues of the kidney cortex including the proximal tubule, distal convoluted tubule, and cortical collecting duct were used for immunoblots (IB; n = 3 animals).
Preparation of the DCT
We used 14- to 16-wk-old male and female Nedd4lflox/flox and Ks-Nedd4-2 KO mice for dissecting the renal DCT, harvesting renal tissues, and collecting plasma samples. For collecting plasma samples through cardiac puncture, mice were anesthetized with isoflurane inhalation. Mice were then euthanized by cervical dislocation for the purpose of harvesting or perfusing the kidneys. For perfusion, the abdomen was opened to expose the left kidney, which was then perfused with 2-mL L-15 medium (Life Technology) containing collagenase type 2 (250 U/mL). After removal of the collagenase-perfused kidney, the renal cortex was separated and further cut into small pieces for additional incubation in collagenase-containing L-15 media for 30–50 min at 37°C. The tissue was then washed three times with fresh L-15 medium and transferred to an ice-cold chamber for dissection. The isolated DCT tubules were placed on a small cover glass coated with polylysine, and the cover glass was placed on a chamber mounted on an inverted microscope.
Single-Channel Recording
We used freshly isolated DCTs of both male and female mice for conducting single K+ channel experiments. Single-channel patch-clamp experiments for studying the 40-pS K+ channel (Kir4.1/Kir5.1) were performed in the basolateral membrane of both the early DCT (DCT1) and late DCT (DCT2). Single K+ currents (IK) were recorded with an Axon200B amplifier (Axon), low-pass filtered at 1 kHz, and digitized by an Axon interface (Digidata 1332) with a sampling rate of 4 kHz. The pipette solution for the single-channel recording contained (in mM) 135 KCl, 2 MgCl2, 1 EGTA, and 10 HEPES (titrated with KOH to pH 7.4), and the bath solution contained (in mM) 135 NaCl, 5 KCl, 2 MgCl2, 1.8 CaCl2, 5 glucose, and 5 HEPES (titrated with NaOH to pH 7.4). For the calculation of channel numbers, we selected a channel recording at least 10 min long. We determined the channel open probability (Po) from the channel number (N) and NPo (the product of N and Po), which was calculated from data samples of 60-s duration in the steady-state. NPo was determined using the following equation:
where ti is the fractional open time spent at each of the observed current levels. Channel conductance was determined by measuring the current amplitudes over several voltages.
Whole Cell Recording
We measured whole cell IK and IK reversal potential only in the DCT1 of male and female mice with an Axon 200 A amplifier. To measure IK reversal potential, the tip of the pipette was filled with pipette solution containing (in mM) 140 KCl, 2 MgCl2, 1 EGTA, and 10 HEPES (pH 7.4) and was then back filled with pipette solution containing amphotericin B (20 μg/0.1 mL). The bath solution was the same as those we used for single-channel recordings. For the measurement of whole cell Ba2+-sensitive IK, the bath solution contained (in mM) 140 KCl, 2 MgCl2, 1.8 CaCl2, and 10 HEPES (pH 7.4). After a high-resistance seal had been formed, membrane capacitance was monitored until the whole cell patch configuration was formed. We measured whole cell currents twice (before and after 0.1 mM Ba2+), and Ba2+-sensitive IK was obtained by subtracting Ba2+-insensitive currents from total currents. The currents were low-pass filtered at 1 kHz and digitized by an Axon interface with a sampling rate of 4 kHz (Digidata 1440 A). Data were analyzed using the pClamp software system 9.0 (Axon).
Measurement of Hydrochlorothiazide-Induced Natriuresis
Animals were anesthetized with a peritoneal injection of Inactin at 100 mg/kg, and mice were placed on a heated small blanket to maintain body temperature at 37°C. The trachea was cannulated to clear mucus, and a carotid artery was catheterized with PE-10 tubing for blood collection. Jugular vein was also cannulated for intravenous infusion, and the bladder was exposed for urine collections (catheterized via a suprapubic incision with a 10-cm piece of PE-10 tubing). After completion of surgery, isotonic saline was given intravenously for 4 h (0.25−0.3 mL/1 h and total 1.0−1.2 mL of 0.9% saline) to replace surgical fluid losses and to maintain hemodynamics. Urine collections started 1 h after infusion of 0.3-mL saline, and a total of six collections (every 30 min) were performed [2 before and 4 after hydrochlorothiazide (HCTZ) at 30 mg/kg body wt]. Plasma and urine Na+ and K+ concentrations were measured using a dual-channel flame photometer with an internal lithium standard (Cole-Parmer Instrument, Vernon Hills, IL).
Immunoblot Analysis
Tissue of the renal cortex (male mice) was homogenized in buffer containing 250 mM sucrose, 50 mM Tris·HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 1 mM DTT plus phosphatase inhibitor cocktails 1 and 2 (catalog nos. P2850 and P5726) and protease inhibitor cocktails (catalog no. P8340, Sigma). Quick Start Bradford Protein Assay Kit (Cat. No. 5000202) from Bio-Rad was used with a 250-µL microplate assay to determine the protein concentrations. Protein (40–60 µg) was separated on 4–12% (wt/vol) Tris-glycine gels (Thermo Fisher Scientific) and transferred to nitrocellulose membrane. Membranes were incubated for 1 h with LI-COR blocking buffer (PBS) and then incubated overnight at 4°C with primary antibodies of NCC, phosphorylated NCC (pNCC), Nedd4-2, and the following epithelial Na+ channel subunits: ENaCα, ENaCβ, and ENaCγ (Table 1). After being washed three times, the membranes were then incubated with secondary antibody from LI-COR (IRDye 800CW donkey antirabbit IgG, P/N: 926–32213). An Odyssey infrared imaging system (LI-COR) was used to capture the images at a wavelength of 680 or 800 nm.
Table 1.
Information and references of antibodies used for Western blots
| Antibody | Species | Dilution for Western Blot | Source |
|---|---|---|---|
| pT53-NCC (Cat. No. p1311-53) | Rabbit | 1:3,000 | PhosphoSolutions (14) |
| NCC (Cat. No. AB 3553) | Rabbit | 1:2,000 | Millipore (14) |
| ENaCα (Cat. No. SPC-4030) | Rabbit | 1:1,000 | StressMarq (24) |
| ENaCβ (Cat. No. SPC-4040) | Rabbit | 1:1,000 | StressMarq (24) |
| ENaCγ (Cat. No. SPC-4050) | Rabbit | 1:1,000 | StressMarq (24) |
| Nedd4-2 (Cat. No. 40135) | Rabbit | 1:1,000 | Cell Signaling (14) |
| Actin (Cat. No. 4970 L) | Rabbit | 1:1,000 | Cell Signaling (22) |
| GAPDH (Cat. No. 2118 L) | Rabbit | 1:1,000 | Cell Signaling (22) |
ENaC, epithelial Na+ channel; NCC, NaCl cotransporter; Nedd4-2, neural precursor cell expressed developmentally downregulated 4-2; pT53-NCC, phospho-Thr53 NaCl cotransporter.
Materials
Inactin and HCTZ were purchased from Sigma-Aldrich (St. Louis, MO). We purchased NCC antibody from Millipore and pNCC (Thr53) antibody from PhosphoSolutions. Antibodies for ENaCα, ENaCβ, and ENaCγ were obtained from StressMarq. Table 1 shows information regarding the catalog number, dilution, and corresponding literature.
Statistical Analysis
We used SigmaPlot software for the statistical analysis. To analyze the values between two groups, we used a t test; for comparisons of the values within the same group, we used a paired t test. We used one-way or two-way ANOVA to analyze the results of more than two groups, and a Holm–Šídák test was used as the post hoc analysis. P values of <0.05 were considered statistically significant. Data are presented as means ± SE.
RESULTS
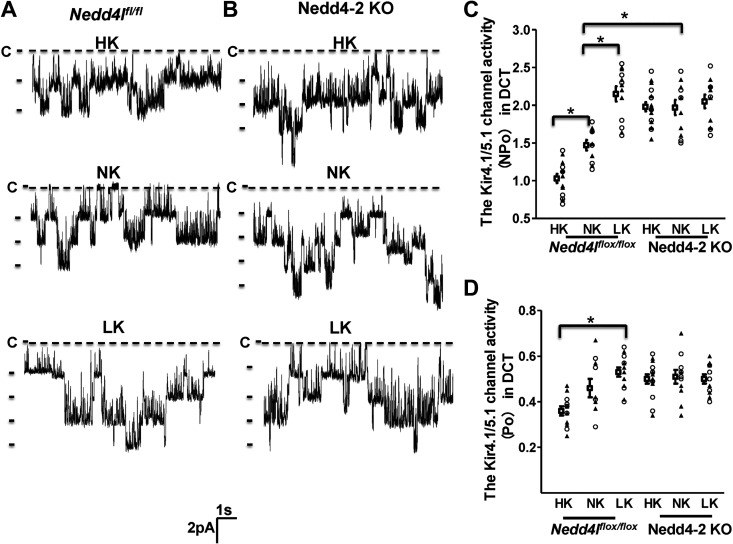
We first used the single-channel recording to examine the effect of HK or LK (7 days) on the basolateral 40-pS K+ channel (Kir4.1/Kir5.1 heterotetramer) in the DCT of Nedd4lfl/fl (control) (Fig. 2A) and Ks-Nedd4-2 KO mice (Fig. 2B). We confirmed the previous finding that HK decreased 40-pS K+ channel activity defined by NPo (1.03 ± 0.06, n = 13) and LK increased NPo (2.15 ± 0.10, n = 13) compared with normal K+ (NK; 1.47 ± 0.07, n = 10; Fig. 2C) (9, 22). Analysis of single-channel recording also demonstrated that channel Po of the 40-pS K+ channel in control mice on LK (0.53 ± 0.02) was significantly higher than on HK (0.36 ± 0.02; Fig. 2D). Deletion of Nedd4-2 significantly increased NPo of the 40-pS K+ channel (1.97 ± 0.10, n = 11) compared with control mice on NK. Moreover, HK intake failed to inhibit 40-pS K+ channel activity of the DCT in Ks-Nedd4-2 KO mice (1.98 ± 0.06, n = 17), whereas LK intake had no significant effect on 40-pS K+ channel activity (2.05 ± 0.09, n = 11).
Figure 2.
Deletion of neural precursor cell expressed developmentally downregulated 4-2 (Nedd4-2) abolishes the effect of dietary K+ intake on the basolateral 40-pS K+ channel in the distal convoluted tubule (DCT). Representative single-channel recordings show basolateral K+ channel activity in the DCT of control [neural precursor cell expressed developmentally downregulated 4-like (Nedd4l)fl/fl mice; A] and kidney tubule-specific Nedd4-2 knockout (Ks-Nedd4-2 KO) mice (B) on normal K+ (NK), high K+ (HK), or low K+ (LK) diets for 7 days, respectively. Two scatter graphs summarize the results of experiments in which 40-pS K+ channel activity (NPo; C) and single-channel open probability (Po; D) were measured in both male mice (triangles, n = 7/5/7 patches from 4/3/4 control mice on HK/NK/LK and n = 11/6/6 patches from 6/4/4 Ks-Nedd4-2KO mice on HK/NK/LK) and female mice (circles, n = 6/5/6 patches from 4/3/4 control mice on HK/NK/LK and n = 6/5/5 patches from 4/3/3 Ks-Nedd4-2 KO mice on HK/NK/LK). Experiments were performed in cell-attached patches at a holding potential of 0 mV. The DCT was bathed in a solution containing 140 mM NaCl/5 mM KCl and the pipette solution contained 145 mM K+. *Significant difference (P < 0.05). Significance was determined by two-way ANOVA. C, closed.
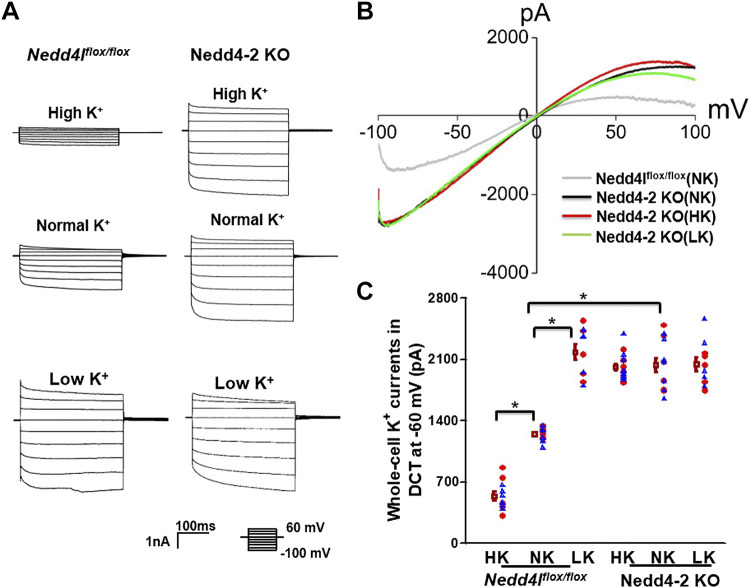
We next used perforated whole cell recording to examine Ba2+-sensitive IK in the DCT1. Since Kir4.1/Kir5.1 is the only type of K+ channel in the DCT1 (8), whole cell IK represent total Kir4.1/Kir5.1 activity. Figure 3A shows a set of traces demonstrating Ba2+-sensitive IK measured with a step protocol from −100 to 60 mV in control and Ks-Nedd4-2 KO mice on HK, NK, and LK for 7 days. Figure 3B shows a set of traces of whole cell Kir4.1/Kir5.1 currents measured with a ramp protocol from −100 to 100 mV in the DCT of Ks-Nedd4-2 KO mice on different K+ diets for 7 days compared with control mice on NK. Figure 3C shows a scatterplot summarizing the results measured at −60 mV in the DCT1 of mice on different K+ diets for 7 days. It was apparent that LK increased whole cell Kir4.1/Kir5.1 currents of the DCT1 (2,180 ± 90 pA, n = 10) and HK decreased Kir4.1/Kir5.1 currents (540 ± 50 pA, n = 11) compared with NK (1,250 ± 30 pA, n = 11) in control mice. However, the effect of dietary K+ intake on Kir4.1/Kir5.1 of the DCT was absent in Ks-Nedd4-2 KO mice (HK: 2,020 ± 40 pA, n = 15; NK: 2,040 ± 80 pA, n = 13; and LK: 2,050 ± 80 pA, n = 10). Thus, deletion of Nedd4-2 abolished HK-induced inhibition of Kir4.1/Kir5.1 in the DCT.
Figure 3.
Deletion of neural precursor cell expressed developmentally downregulated 4-2 (Nedd4-2) eliminates the effect of dietary K+ intake on basolateral K+ conductance in the distal convoluted tubule (DCT). A: a set of traces showing whole cell Ba2+-sensitive K+ currents measured with a step protocol from –100 to 60 mV in the DCT of control mice and kidney tubule-specific Nedd4-2 knockout (Ks-Nedd4-2 KO) mice on normal K+ (NK), high K+ (HK), and low K+ (LK) diets for 7 days, respectively. B: a set of traces showing whole cell Ba2+-sensitive K+ currents measured with a ramp protocol (from −100 to 100 mV) in Ks-Nedd4-2 KO mice on NK, HK, and LK diets for 7 days. The DCT was bathed in 140 mM KCl-containing solution and the pipette solution also contained 140 mM KCl. C: scatter graph summarizing K+ currents measured at –60 mV in the DCT of male mice (blue triangles; n = 6/6/5 patches from 4/4/3 control mice on HK/NK/LK and n = 9/7/5 patches from 6/5/3 Ks-Nedd4-2 KO mice on HK/NK/LK) and female mice (red circles; n = 5/5/5 patches from 3/3/3 control mice on HK/NK/LK and n = 6/6/5 patches from 4/4/3 Ks-Nedd4-2 KO mice on HK/NK/LK). Mean values and SEs are shown on the left of each column. *Significant difference (P < 0.05). Significance was determined by two-way ANOVA. Neddlflox/flox, floxed neural precursor cell expressed developmentally downregulated 4-like mice.
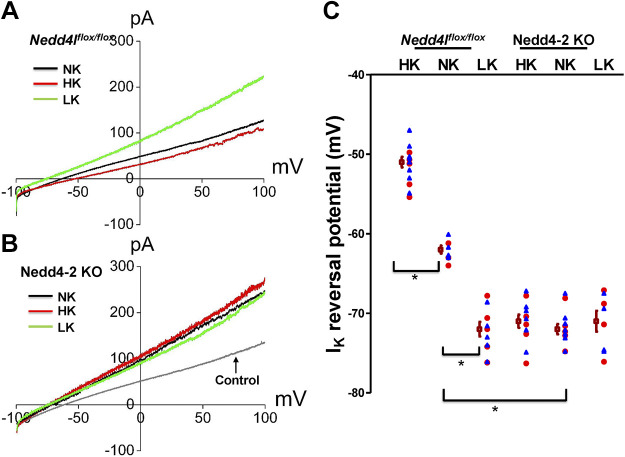
We then used whole cell recording to measure IK reversal potential (as an index of the membrane potential) of the DCT. Figure 4, A and B, shows two sets of typical traces demonstrating IK reversal potential of the DCT1 in control and Ks-Nedd4-2 KO mice on HK, NK, and LK for 7 days, respectively. The experimental results were summarized in a scatterplot (Fig. 4C). We confirmed that HK decreased (−51 ± 1 mV, n = 13), whereas LK increased (−72 ± 1 mV, n = 10), the negativity of IK reversal potential of the DCT1 in control mice compared with NK (−62 ± 0.5 mV, n = 7) (9). Deletion of Nedd4-2 increased the negativity of IK reversal potential (−72 ± 1 mV, n = 12) compared with control mice on NK. Moreover, neither HK decreased (−71 ± 1 mV, n = 12) nor LK increased (−71 ± 1.3 mV, n = 8) the negativity of IK reversal potential in Ks-Nedd4-2 KO mice. Thus, the electrophysiological experiments strongly suggested that Nedd4-2 plays a role in mediating the effect of HK intake on Kir4.1/Kir5.1 activity in the DCT.
Figure 4.
Deletion of neural precursor cell expressed developmentally downregulated 4-2 (Nedd4-2) abolishes the effect of dietary K+ intake on distal convoluted tubule (DCT) membrane potential. A: perforated whole cell recording showing K+ current (IK) reversal potential in the DCT of control mice on normal K+ (NK), high K+ (HK), and low K+ (LK) diets for 7 days, respectively. B: perforated whole cell recording showing IK reversal potential in the DCT of kidney tubule-specific Nedd4-2 knockout (Ks-Nedd4-2 KO) mice on NK, HK, and LK diets for 7 days, respectively (a recording for control mice on NK is indicated). C: scatter graph summarizing the results of experiments in which IK reversal potential was measured in both male mice (blue triangles; n = 8/4/5 patches from 5/3/4 control mice on HK/NK/LK and n = 7/7/4 patches from 4/4/3 Ks-Nedd4-2 KO mice on HK/NK/LK) and female mice (red circles; n = 5/3/5 patches from 4/3/4 control mice on HK/NK/LK and n = 5/5/4 from 4/4/3 Ks-Nedd4-2 KO mice on HK/NK/LK). Mean values and SEs are shown on the left of each column. For the measurement of IK reversal potential, the bath solution contained 140 mM NaCl and 5 mM KCl and the pipette solution has 140 mM KCl. *Significant difference (P < 0.05). Significance was determined by two-way ANOVA. Neddlflox/flox, floxed neural precursor cell expressed developmentally downregulated 4-like mice.
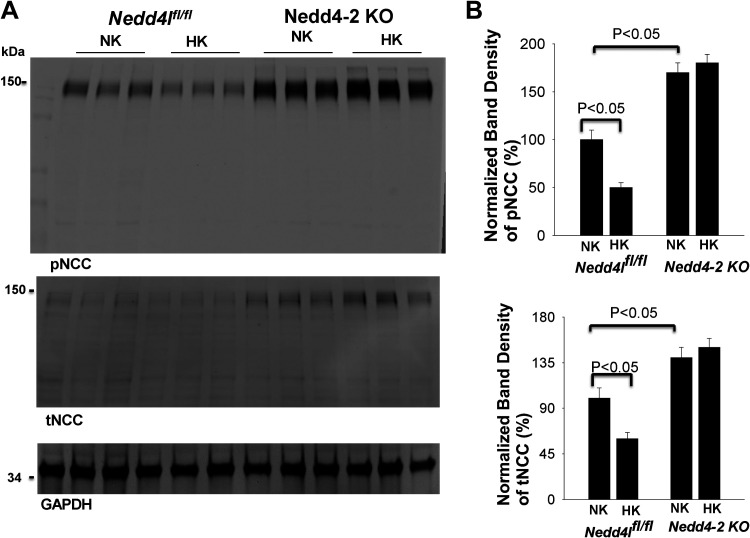
Figure 6.
Deletion of neural precursor cell expressed developmentally downregulated 4-2 (Nedd4-2) abolishes the effect of high K+ (HK) intake on the NaCl cotransporter (NCC). A: Western blots showing the expression of phosphorylated NCC (pNCC; at Thr53) and total NCC (tNCC) in three male control [floxed neural precursor cell expressed developmentally downregulated 4-like (Nedd4fl/fl)] mice and kidney tubule-specific Nedd4-2 knockout (Ks-Nedd4-2 KO) mice on normal K+ (NK) and HK for 7 days. B: two bar graphs summarizing the normalized band intensity of the above experiments for pNCC and tNCC (n = 3 male mice for each NK/HK/LK group of control and Ks-Nedd4-2 KO mice).
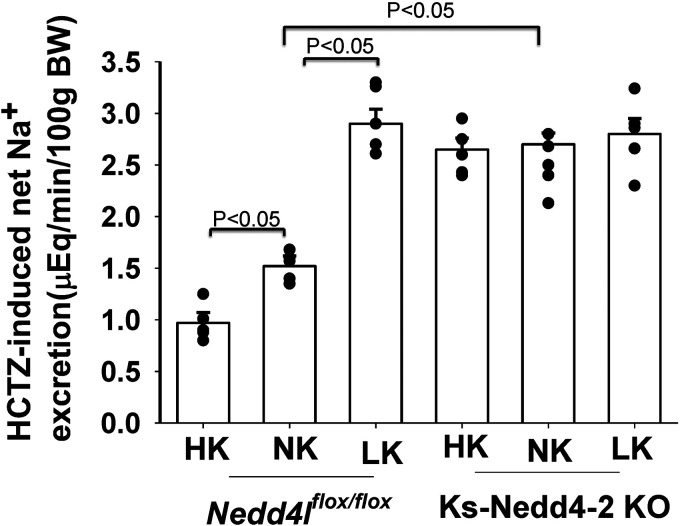
A large body of evidence has indicated that Kir4.1/Kir5.1 activity is closely associated with NCC expression/activity such that high Kir4.1/Kir5.1 activity in the DCT is associated with increased NCC function, whereas low Kir4.1/Kir5.1 activity is related to decreased NCC expression/activity (1, 9, 25, 26). Figure 5 shows a bar graph summarizing the results of experiments in which we examined the effect of HCTZ (30 mg/kg body wt) on urinary Na+ excretion using the renal clearance method in control and Ks-Nedd4-2 KO mice on a NK, HK, or LK diet for 7 days. It was apparent that LK intake significantly increased HCTZ-induced net natriuresis in control mice (2.61 ± 0.09 µeq/min/100 g body wt, n = 4 male mice), whereas HK intake decreased HCTZ-induced net natriuresis (0.82 ± 0.07 µeq/min/100 g body wt, n = 4 male mice) compared with NK (1.23 ± 0.07 µeq/min/100 g body wt, n = 7 male mice). HCTZ-induced net natriuresis was significantly larger in Ks-Nedd4-2 KO mice (2.46 ± 0.14 µeq/min/100 g body wt, n = 8 male mice) compared with control mice on NK, indicting increased NCC activity. Moreover, neither HK (2.31 ± 0.11 µeq/min/100 g body wt, n = 6 male mice) nor LK (2.41 ± 0.15 µeq/min/100 g body wt, n = 7 male mice) had a significant effect on HCTZ-induced net natriuresis in Ks-Nedd4-2 KO mice.
Figure 5.
Deletion of neural precursor cell expressed developmentally downregulated 4-2 (Nedd4-2) abolishes the effect of K+ diets on hydrochlorothiazide (HCTZ)-induced natriuresis. Shown is HCTZ-induced net renal Na+ excretion in control and kidney tubule-specific Nedd4-2 knockout (Ks-Nedd4-2 KO) mice on low K+ (LK), normal K+ (NK), and high K+ (HK) diets for 7 days. Individual data points are indicated by black circles. Significance was determined by two-way ANOVA. BW, body weight; Neddlflox/flox, floxed neural precursor cell expressed developmentally downregulated 4-like mice.
The notion that deletion of Nedd4-2 may compromise the effect of HK on NCC function was also indicated by experiments in which immunoblot analysis was performed to examine the effect of HK (7 days) on the expression of pNCC and total NCC in three male control and Ks-Nedd4-2 KO mice. Figure 6A shows a Western blot demonstrating that deletion of Nedd4-2 increased the expression of pNCC (170 ± 10% of control mice on NK) and total NCC (140 ± 10% of control). The data are completely consistent with previous report showing that NCC expression is increased in Nedd4-2-deficient mice (14, 23). Moreover, Although HK decreased the expression of pNCC by 50 ± 5% and total NCC by 40 ± 6% in control mice (compared with NK), HK intake failed to inhibit the expression of pNCC and total NCC in Ks-Nedd4-2 KO mice (Fig. 6, A and B). Thus, Nedd4-2 deletion abolished the inhibitory effect of HK on pNCC and total NCC expression. Taken together, the data indicate that Nedd4-2 is required for the inhibitory effect of HK intake on NCC expression/activity.
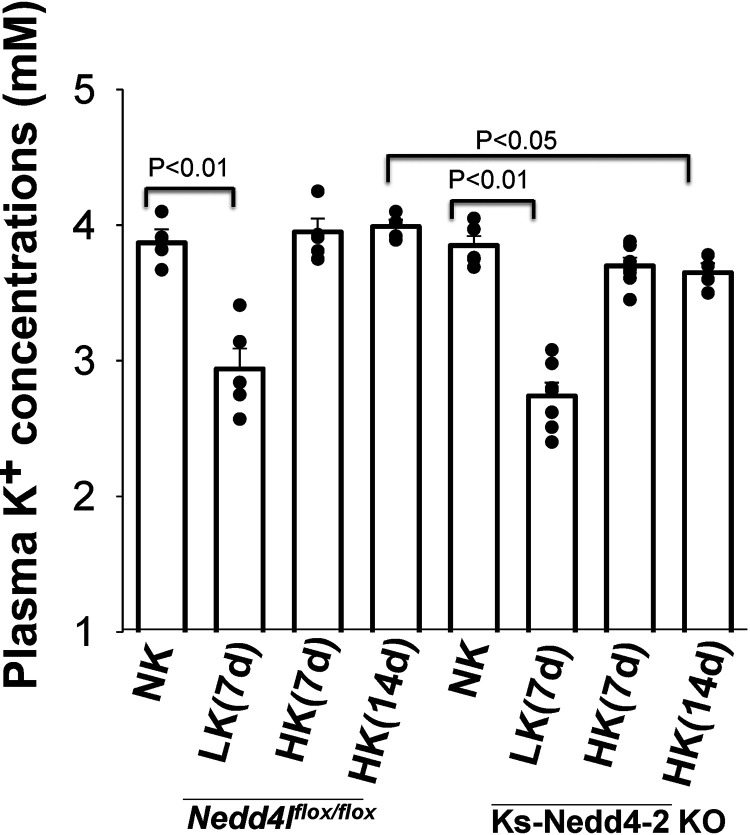
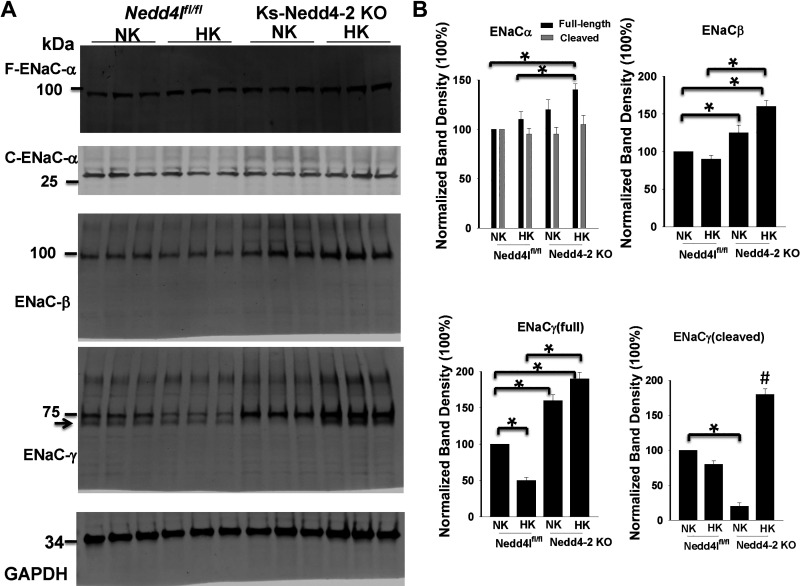
Although HK-induced inhibition of pNCC and total NCC expression were compromised in Ks-Nedd4-2 KO mice, we confirmed a previous report showing that plasma K+ concentrations in Ks-Nedd4-2 KO mice (3.70 ± 0.12 mM, n = 7) on HK diet for 7 days were not significantly different from control mice on HK (3.95 ± 0.13 mM, n = 5; Fig. 7) (27). Moreover, increased HK intake for 14 days did not significantly alter plasma K+ concentrations (3.65 ± 0.07 mM) in Ks-Nedd4-2 KO mice compared with NK and HK for 7 days (n = 4), and the plasma K+ level in Ks-Nedd4-2 KO mice was even lower than that of control mice on HK for 14 days (3.99 ± 0.05 mM, n = 4). Our previous study demonstrated that ENaC activity in Ks-Nedd4-2 KO mice was significantly augmented compared with the corresponding control mice (28). Thus, we speculate that deletion of the Nedd4-2-induced increase in ENaC function may compensate for abnormal high NCC expression/activity during increasing dietary K+ intake in Ks-Nedd4-2 KO mice. We next used Western blot analysis to examine the expression of ENaC in three male control and Ks-Nedd4-2 KO mice on NK and HK for 7 days. As shown in Fig. 8A, it was apparent that the expression of full-length ENaCα, ENaCβ, full-length ENaCγ, and cleaved ENaCγ (but not cleaved ENaCα) were all enhanced in Ks-Nedd4-2 KO mice on HK diet for 7 days compared with control mice on HK. Figure 8B shows a bar graph summarizing the results demonstrating that the expression of full-length ENaCα in Ks-Nedd4-2 KO mice on HK was 140 ± 6% of control mice on NK. HK intake also increased expression of ENaCβ (160 ± 8%), full-length ENaCγ (190 ± 9%), and cleaved ENaCγ (180 ± 8%) in Ks-Nedd4-2 KO mice compared with control mice on NK. In contrast, HK intake only increased the ratio between cleaved ENaCγ and full-length ENaCγ but did not stimulate the expression of ENaCα, ENaCβ, and full-length ENaCγ compared with NK in control mice. Thus, the data indicate that ENaC expression was increased in Nedd4-2 KO mice on a HK diet.
Figure 7.
Plasma K+ concentrations in kidney tubule-specific neural precursor cell expressed developmentally downregulated 4-2 knockout (Ks-Nedd4-2 KO) mice and floxed neural precursor cell expressed developmentally downregulated 4-like (Nedd4fl/fl) mice on different K+ diets. The bar graph shows plasma K+ concentrations in control mice [normal K+ (NK): n = 5 mice (3 male/2 female); low K+ (LK): n = 5 mice (3 male/2 female); high K+ (HK) for 7 days: n = 5 mice (3 male/2 female); and HK for 14 days: n = 4 mice (2 male/2 female)] and Ks-Nedd4-2 KO mice [NK: n = 5 mice (3 male/2 female); LK: n = 7 mice (4 male/3 female); HK for 7 days: n = 7 mice (4 male/3 female); and HK for 14 days: n = 4 mice (2 male/2 female)]. Individual data points are indicated by black circles. Significance was determined by two-way ANOVA.
Figure 8.
Epithelial Na+ channel (ENaC) expression is stimulated in kidney tubule-specific neural precursor cell expressed developmentally downregulated 4-2 knockout (Ks-Nedd4-2 KO) mice on high K+ (HK). A: expression of full-length (F-)ENaCα, cleaved (C-)ENaCα, ENaCβ, and ENaCγ in three control (male) and three Ks-Nedd4-2 KO (male) mice on normal K+ (NK) or HK for 7 days. The arrow indicates cleaved formed ENaCγ. B: bar graph showing the normalized band density of ENaCα (full-length and cleaved forms), ENaCβ, F-ENaCγ, and C-ENaCγ. *Significant difference (P < 0.05); #significant difference compared with the other groups. Significance was determined by two-way ANOVA. Neddlflox/flox, floxed neural precursor cell expressed developmentally downregulated 4-like mice.
DISCUSSION
The first main finding of the present study is that the dietary K+ intake fails to regulate basolateral Kir4.1/Kir5.1 activity in the DCT of Ks-Nedd4-2 KO mice. Three lines of evidence have strongly suggested that Nedd4-2 plays a role in mediating the inhibitory effect of HK on basolateral K+ channel activity in the DCT: 1) the HK intake-induced decrease in whole cell Kir4.1/Kir5.1 currents of the DCT was abolished in Nedd4-2-deficient mice; 2) HK intake failed to decrease the negativity of the membrane potential of the DCT in Ks-Nedd4-2 KO mice, whereas it depolarized the DCT membrane in control mice; and 3) HK intake did not decrease basolateral 40-pS K+ channel activity (NPo) in Ks-Nedd4-2 KO mice. In contrast, we confirmed the previous finding that HK inhibits, whereas LK stimulates, basolateral 40-pS K+ channel activity in the DCT (9). Although we did not observe obvious difference regarding Kir4.1/Kir5.1 K+ channel activity between male and female mice, previous studies have convincingly demonstrated that the expression of thiazide-sensitive NCC was higher in female mice than in male mice (29, 30). It was demonstrated that NCC density was higher in the DCT of female mice than in male mice. Thus, our data suggest that the higher NCC expression in female mice compared with male mice is unlikely the result of different Kir4.1/Kir5.1 expression/activity between the two sexes.
The basolateral 40-pS K+ channel in the DCT is composed of Kir4.1 and Kir5.1. Although the conductance of the 40-pS K+ channel is provided by Kir4.1 (1, 5, 9), Kir5.1 is a regulatory subunit for the heterotetramer (6, 10–13). Our previous in vitro experiments have shown that Nedd4-2 binds to Kir5.1 and that coexpression of Kir5.1 and Nedd4-2 promotes ubiquitination of Kir4.1 (13). The notion that Kir5.1 and Nedd4-2 play a role in the regulation of basolateral K+ conductance of the DCT has been strongly suggested by the finding that deletion of either Nedd4-2 or Kir5.1 increased basolateral K+ conductance in the DCT (14, 22). Our previous study also demonstrated that high Kir4.1/Kir5.1 activity in the DCT was partially responsible for Nedd4-2 deletion-induced high NCC expression/activity (14). Although conditional Ks-Nedd4-2 KO mice have higher NCC and ENaC expression, we and others have demonstrated that conditional Ks-Nedd4-2 KO mice were normokalemic under control conditions (23). In contrast, Henshall et al. (31) reported that constitutive deletion of Nedd4-2 in the renal tubules caused hypokalemia. We believe that because conditional deletion of Nedd4-2 in the renal tubules was performed in adult animals, Nedd4-2-deficient mice were able to compensate for the stimulatory effect of high ENaC expression/activity on renal K+ excretion under control conditions. However, the ability of maintaining K+ homeostasis in conditional Ks-Nedd4-2 KO mice is limited, as evidenced by the fact that these mice could not effectively inhibit renal K+ excretion during long-term K+ restriction (27).
It is well established that increased dietary K+ intake inhibits basolateral Kir4.1/Kir5.1 currents in the DCT under control condition (9, 22). We have previously demonstrated that the inhibitory effect of HK intake on basolateral K+ conductance of the DCT was absent in Kir5.1-deficient mice (22), suggesting the role of Kir5.1 in mediating the effect of HK on basolateral K+ conductance of the DCT. Our present observation that HK failed to inhibit Kir4.1/Kir5.1 in Ks-Nedd4-2 KO mice also suggests that Nedd4-2 is required to mediate the inhibitory effect of HK on Kir4.1/Kir5.1 in the DCT. Further experiments are required to explore whether HK intake-induced inhibition of basolateral K+ channel activity in the DCT is due to increase Kir4.1 ubiquitination by a Kir5.1-Nedd4-2-dependent mechanism.
Our present experiments also demonstrated that HK-induced inhibition of NCC activity/expression was absent in Ks-Nedd4-2 KO mice, suggesting the role of Nedd4-2 in mediating the inhibitory effect of HK intake on NCC. Although it is possible that Nedd4-2 may directly be involved in the regulation of NCC expression (23), we believe that lack of HK-induced inhibition of Kir4.1/Kir5.1 activity in the DCT should be partially responsible for abolishing HK-induced inhibition of NCC in Nedd4-2 KO mice. This view is supported by the finding that lack of Kir4.1 attenuated Nedd4-2 deletion-induced stimulation of pNCC and total NCC expression (14). The possible mechanism by which basolateral Kir4.1/Kir5.1 channel activity in the DCT mediates the effect of HK on NCC activity depends on with no lysine kinase (WNK), a key kinase regulating NCC expression/activity (32–38). HK-induced inhibition of Kir4.1/Kir5.1 activity is expected to depolarize the DCT membrane. Since Cl− exit through basolateral ClC-Kb is electrogenic, a decrease in the DCT basolateral membrane negativity should inhibit Cl− movement across the basolateral membrane and increase intracellular Cl− concentrations, thereby suppressing WNK activity (39). Consequently, HK should also inhibit STE20/SPS1-related proline-alanine-rich kinase (SPAK) or oxidative stress responsive kinase-1 (OSR1), which is responsible for activating NCC by phosphorylation (34, 40–42). Also, Nedd4-2 deletion-induced stimulation of NCC may be induced by activating WNK1 expression (27, 43). Figure 9 shows a scheme illustrating the possible role of Nedd4-2 in mediating the effect of HK intake on Kir4.1/Kir5.1 and NCC. We speculate that HK intake inhibits Kir4.1/Kir5.1 activity by enhancing Nedd4-2-dependent Kir4.1 ubiquitination. A decrease in Kir4.1/Kir5.1 activity should inhibit WNK/SPAK/OSR pathway, thereby inhibiting NCC. In addition, Nedd4-2 can also directly modulate WNK expression and NCC ubiquitination in response to HK intake.
Figure 9.
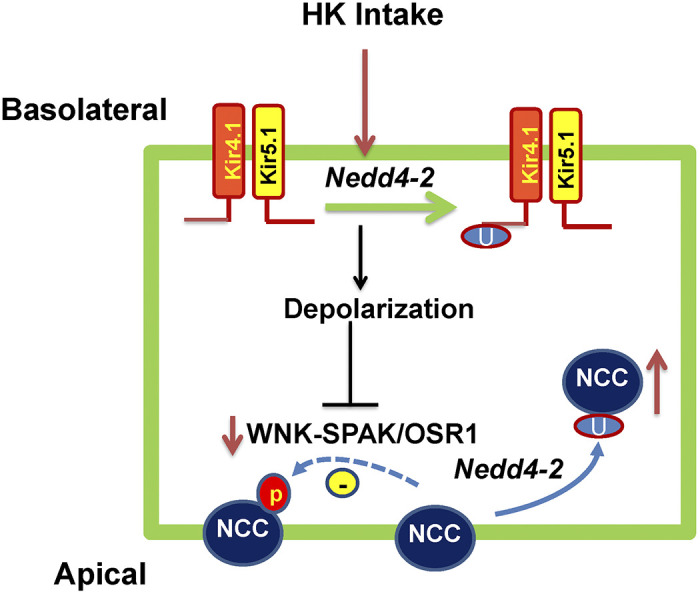
Cell scheme illustrating the role of neural precursor cell expressed developmentally downregulated 4-2 (Nedd4-2) in mediating the effect of high K+ (HK) intake on Kir4.1/Kir5.1 in the distal convoluted tubule and NaCl cotransporter (NCC). The dotted line and solid line represent diminished or enhanced effects. OSR1, oxidative stress responsive kinase-1; SPAK, STE20/SPS1-related proline-alanine-rich kinase; WNK, with no lysine kinase.
HK-induced inhibition of NCC expression/activity plays an important role in regulating renal K+ excretion by increasing Na+ and fluid volume delivery to the aldosterone-sensitive distal nephron, thereby stimulating renal outer medullary K+ channel-dependent or Ca2+-activated large-conductance K+ channel-dependent renal K+ excretion (44, 45). However, we and others have shown that Ks-Nedd4-2 KO mice are not hyperkalemic during HK loading despite high expression of pNCC and total NCC (27). The observation that plasma K+ concentrations were slightly but significantly lower in Ks-Nedd4-2 KO mice on HK for 14 days compared with control mice suggests that high ENaC activity can effectively offset the inhibitory effect of high NCC activity on net renal K+ excretion. This notion is also supported by our unpublished patch-clamp data showing that HK further increased amiloride-sensitive Na+ currents in the cortical collecting duct of Ks-Nedd4-2 KO mice (W.-H. Wang, unpublished observations). Presumably, long-term HK intake should increase aldosterone secretion, thereby further increasing ENaC activity in the cortical collecting duct, thereby enhancing ENaC-dependent renal K+ excretion. On the other hand, it is unlikely that lack of a HK effect on Kir4.1/Kir5.1 of the DCT and NCC was due to renal K+ wasting in Ks-Nedd4-2 KO mice. This view is also supported by our recent study showing that the failure of high salt-induced inhibition of NCC and Kir4.1/Kir5.1 of the DCT was still observed in Ks-Nedd4-2 KO mice on high salt plus K+ supplement to offset renal K+ wasting (28). We believe that the lack of the HK effect on Kir4.1/Kir5.1 and NCC in the DCT of Nedd4-2 KO mice is due to the fact that Nedd4-2 plays a role in regulating basolateral Kir4.1/Kir5.1 channel and NCC expression during K+ loading.
In summary, our present study has two novel aspects for the role of Nedd4-2 in the kidney: 1) deletion of Nedd4-2 abolished the inhibitory effect of chronic HK intake on NCC expression/activity and Kir4.1/Kir5.1 in the DCT and 2) HK intake robustly increased ENaC expression in Nedd4-2 KO mice. We conclude that Nedd4-2 plays an important role in mediating the effect of HK intake on basolateral Kir4.1/Kir5.1 activity and NCC expression/activity. Also, lack of HK-induced inhibition of Kir4.1/Kir5.1 activity in the DCT is partially responsible for high NCC expression/activity in Nedd4-2 KO mice.
GRANTS
The work is supported by National Institutes of Health Grant DK115366 (to D.-H.L.) and DK54983 (to W.-H.W.). Y.X. is supported by Education Department of Heilongjiang Grant 2016-KYYWF-0850. X.-P.D. is supported by China National Natural Science Foundation Grant 81900648 and Natural Science Foundation of Jiangsu High Education Institute Grant 19KJB310019.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W-H.W. and D-H.L. conceived and designed research; Y.X., X-P.D., D-D.Z., W-H.W., and D-H.L. performed experiments; Y.X., X-P.D., D-D.Z., W-H.W., and D-H.L. analyzed data; Y.X., X-P.D., D-D.Z., W-H.W., and D-H.L. interpreted results of experiments; Y.X., X-P.D., D-D.Z., W-H.W., and D-H.L. prepared figures; X-P.D., W-H.W., and D-H.L. drafted manuscript; W-H.W. and D-H.L. edited and revised manuscript; Y.X., X-P.D., D-D.Z., W-H.W., and D-H.L. approved final version of manuscript.
REFERENCES
- 1.Cuevas CA, Su XT, Wang MX, Terker AS, Lin DH, McCormick JA, Yang C-L, Ellison DH, Wang WH. Potassium sensing by renal distal tubules requires Kir4.1. J Am Soc Nephrol 28: 1814–1825, 2017. doi: 10.1681/ASN.2016090935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Sindic A, Hill CE, Hujer KM, Chan KW, Sassen M, Wu Z, Kurachi Y, Nielsen S, Romero MF, Miller RT. Interaction of the Ca2+-sensing receptor with the inwardly rectifying potassium channels Kir4.1 and Kir4.2 results in inhibition of channel function. Am J Physiol Renal Physiol 292: F1073–F1081, 2007. doi: 10.1152/ajprenal.j00269.2006. [DOI] [PubMed] [Google Scholar]
- 3.Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J. An inward rectifier K. + channel at the basolateral membrane of the mouse distal convoluted tubles: similarites with Kir4-Kir5.1 heteromeric channels. J Physiol 538: 391–404, 2002. doi: 10.1113/jphysiol.2001.012961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik S, Lambert E, Zhang J, Wang T, Clark HL, Cypress M, Goldman BI, Porter GA, Pena S, Nino W, Gray DA. Potassium conservation is impaired in mice with reduced renal expression of Kir4.1. Am J Physiol Renal Physiol 315: F1271–F1282, 2018. doi: 10.1152/ajprenal.00022.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Pochynyuk OM, Jacob HJ, Geurts AM, Hodges MR, Staruschenko A. Essential role of Kir5.1 channels in renal salt handling and blood pressure control. JCI Insight 2: 92331, 2017. doi: 10.1172/jci.insight.92331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tucker SJ, Imbrici P, Salvatore L, D'Adamo MC, Pessia M. pH dependence of the inwardly rectifying potassium channel, Kir5.1, and localization in renal tubular epithelia. J Biol Chem 275: 16404–16407, 2000. doi: 10.1074/jbc.C000127200. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Wang L, Thomas S, Wang K, Lin DH, Rinehart J, Wang WH. Src-family protein tyrosine kinase regulates the basolateral K channel in the distal convoluted tubule (DCT) by phosphorylation of KCNJ10. J Biol Chem 288: 26135–26146, 2013. doi: 10.1074/jbc.M113.478453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C, Wang L, Zhang J, Su X-T, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci USA 111: 11864–11869, 2014. doi: 10.1073/pnas.1411705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M-X, Cuevas CA, Su X-T, Wu P, Gao Z-X, Lin D-H, McCormick JA, Yang C-L, Wang W-H, Ellison DH. Potassium (K+) intake modulates NCC activity via the K+ channel. Kidney Int 93: 893–902, 2018. doi: 10.1016/j.kint.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casamassima M, D'Adamo MC, Pessia M, Tucker SJ. Identification of a heteromeric interaction that influences the rectification, gating, and pH sensitivity of Kir4.1/Kir5.1 potassium channels. J Biol Chem 278: 43533–43540, 2003. doi: 10.1074/jbc.M306596200. [DOI] [PubMed] [Google Scholar]
- 11.Pessia M, Imbrici P, D'Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1. J Physiol 532: 359–367, 2001. doi: 10.1111/j.1469-7793.2001.0359f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanemoto M, Kittaka N, Inanobe A, Kurachi Y. In vivo formation of a proton-sensitive K+ channel by heteromeric subunit assembly of Kir5.1 with Kir4.1. J Physiol 525 Pt 3: 587–592, 2000. doi: 10.1111/j.1469-7793.2000.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang MX, Su XT, Wu P, Gao ZX, Wang WH, Staub O, Lin DH. Kir5.1 regulates Nedd4-2-mediated ubiquitination of Kir4.1 in distal nephron. Am J Physiol Renal Physiol 315: F986–F996, 2018. doi: 10.1152/ajprenal.00059.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu P, Su XT, Gao ZX, Zhang DD, Duan XP, Xiao Y, Staub O, Wang WH, Lin DH. Renal tubule Nedd4-2 deficiency stimulates Kir4.1/Kir5.1 and thiazide-sensitive NaCl cotransporter in distal convoluted tubule. J Am Soc Nephrol 31: 1226–1242, 2020. doi: 10.1681/ASN.2019090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castaneda-Bueno M, Cervantes-Perez LG, Rojas-Vega L, Arroyo-Garza I, Vazquez N, Moreno E, Gamba G. Modulation of NCC activity by low and high K+ intake: insights into the signaling pathways involved. Am J Physiol Renal Physiol 306: F1507–F1519, 2014. doi: 10.1152/ajprenal.00255.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA. Increasing plasma K+ by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306: F1059–F1068, 2014. [Erratum in Am J Physiol Renal Physiol 310: F688, 2016]. doi: 10.1152/ajprenal.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 18.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–134, 2016. doi: 10.1038/ki.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terker A-S, Zhang C, McCormick J-A, Lazelle R-A, Zhang C, Meermeier N-P, Siler D-A, Park H-J, Fu Y, Cohen D-M, Weinstein A-M, Wang WH, Yang CL, Ellison D-H. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AHJ, Fenton RA, Zietse R, Hoorn EJ. K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+ -Cl- cotransporter. Am J Physiol Renal Physiol 305: F1177–F1188, 2013. doi: 10.1152/ajprenal.00201.2013. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Xu S, Guo X, Uchida S, Weinstein AM, Wang T, Palmer LG. Regulation of renal Na transporters in response to dietary K. Am J Physiol Renal Physiol 315: F1032–F1041, 2018. doi: 10.1152/ajprenal.00117.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu P, Gao ZX, Zhang DD, Su XT, Wang WH, Lin DH. Deletion of Kir5.1 impairs renal ability to excrete potassium during increased dietary potassium intake. J Am Soc Nephrol 30: 1425–1438, 2019. doi: 10.1681/ASN.2019010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ronzaud C, Loffing-Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler-Jaeger N, Boase NA, Perrier R, Maillard M, Yang B, Stokes JB, Koesters R, Kumar S, Hummler E, Loffing J, Staub O. Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Invest 123: 657–665, 2013. doi: 10.1172/JCI61110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu P, Gao Z, Zhang D, Duan X, Terker AS, Lin D, Ellison DH, Wang W. Effect of angiotensin II on ENaC in the distal convoluted tubule and in the cortical collecting duct of mineralocorticoid receptor deficient mice. J Am Heart Assoc 9: e014996, 2020. doi: 10.1161/JAHA.119.014996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su XT, Ellison DH, Wang WH. Kir4.1/Kir5.1 in the DCT plays a role in the regulation of renal K+ excretion. Am J Physiol Renal Physiol 316: F582–F586, 2019. doi: 10.1152/ajprenal.00412.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu P, Gao ZX, Su XT, Wang MX, Wang WH, Lin DH. Kir4.1/Kir5.1 activity is essential for dietary sodium intake-induced modulation of Na-Cl cotransporter. J Am Soc Nephrol 30: 216–227, 2019. doi: 10.1681/ASN.2018080799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Qusairi L, Basquin D, Roy A, Rajaram RD, Maillard MP, Subramanya AR, Staub O. Renal tubular uUbiquitin-protein ligase NEDD4-2 is required for renal adaptation during long-term potassium depletion. J Am Soc Nephrol 28: 2431–2442, 2017. doi: 10.1681/ASN.2016070732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang DD, Duan XP, Xiao Y, Wu P, Gao Z-X, Wang W-H, Lin D-H. Deletion of renal Nedd4-2 abolishes the effect of high sodium intake (HS) on Kir4.1, ENaC and NCC, and causeds hypokalemia during HS. Am J Physiol Renal Physiol 320: F883–F896, 2021. doi: 10.1152/ajprenal.00555.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tahaei E, Coleman R, Saritas T, Ellison DH, Welling PA. Distal covoluted tubule sexual dimorphism revealed by advanced 3D imaging. Am J Physiol Renal Physiol 319: F754–F764, 2020. doi: 10.1152/ajprenal.00441.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol 28: 3504–3517, 2017. doi: 10.1681/ASN.2017030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henshall TL, Manning JA, Alfassy OS, Goel P, Boase MA, Kawabe H, Kumar S. Deletion of Nedd4-2 results in progressive kidney disease in mice. Cell Death Differ 24: 2150–2160, 2017. doi: 10.1038/cdd.2017.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, Xie J, Wu T, Truong T, Auchus RJ, Huang CL. Downregulation of NCC and NKCC2 cotransporters by kidney-specific WNK1 revealed by gene disruption and transgenic mouse models. Hum Mol Genet 20: 855–866, 2011. doi: 10.1093/hmg/ddq525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang C-L, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH. A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14: 352–364, 2011. doi: 10.1016/j.cmet.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277: 50812–50819, 2002. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- 36.Rodan AR. WNK-SPAK/OSR1 signaling: lessons learned from an insect renal epithelium. Am J Physiol Renal Physiol 315: F903–F907, 2018. doi: 10.1152/ajprenal.00176.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subramanya AR, Yang CL, McCormick JA, Ellison DH. WNK kinases regulate sodium chloride and potassium transport by the aldosterone-sensitive distal nephron. Kidney Int 70: 630–634, 2006. doi: 10.1038/sj.ki.5001634. [DOI] [PubMed] [Google Scholar]
- 38.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21: 1868–1877, 2010. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piala AT, Moon TM, Akella R, He HX, Cobb MH, Goldsmith EJ. Chloride sensing by WNK1 involves inhibition of autophosphosphorylation. Sci Signal 7: ra41, 2014. doi: 10.1126/scisignal.2005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castaneda-Bueno M, Cervantes-Perez LG, Vazquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+:Cl−cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA 109: 7929–7934, 2012. doi: 10.1073/pnas.1200947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grimm PR, Coleman R, Delpire E, Welling PA. Constitutively active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J Am Soc Nephrol 28: 2555–2563, 2017. doi: 10.1681/ASN.2016090948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thastrup J, Rafiqi F, Vitari A, Pozo-Guisado E, Deak M, Mehellou Y, Alessi D. SPAK/OSR1 regulate NKCC1 and WNK activity: analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation. Biochem J 441: 325–337, 2012. doi: 10.1042/BJ20111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roy A, Al-Qusairi L, Donnelly BF, Ronzaud C, Marciszyn AL, Gong F, Chang YPC, Butterworth MB, Pastor-Soler NM, Hallows KR, Staub O, Subramanya AR. Alternatively spliced proline-rich cassettes link WNK1 to aldosterone action. J Clin Invest 125: 3433–3448, 2015. doi: 10.1172/JCI75245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frindt G, Palmer LG. K+ secretion in the rat kidney: Na+ channel-dependent and -independent mechanisms. Am J Physiol Renal Physiol 297: F389–F396, 2009. doi: 10.1152/ajprenal.90528.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]