Abstract
We assessed the efficacy of oral supplementation with the flavanoid apigenin on arterial function during aging and identified critical mechanisms of action. Young (6 mo) and old (27 mo) C57BL/6N mice (model of arterial aging) consumed drinking water containing vehicle (0.2% carboxymethylcellulose; 10 young and 7 old) or apigenin (0.5 mg/mL in vehicle; 10 young and 9 old) for 6 wk. In vehicle-treated animals, isolated carotid artery endothelium-dependent dilation (EDD), bioassay of endothelial function, was impaired in old versus young (70% ± 9% vs. 92% ± 1%, P < 0.0001) due to reduced nitric oxide (NO) bioavailability. Old mice had greater arterial reactive oxygen species (ROS) production and oxidative stress (higher nitrotyrosine) associated with greater nicotinamide adenine dinucleotide phosphate oxidase (oxidant enzyme) and lower superoxide dismutase 1 and 2 (antioxidant enzymes); ex vivo administration of Tempol (antioxidant) restored EDD to young levels, indicating ROS-mediated suppression of EDD. Old animals also had greater aortic stiffness as indicated by higher aortic pulse wave velocity (PWV, 434 ± 9 vs. 346 ± 5 cm/s, P < 0.0001) due to greater intrinsic aortic wall stiffness associated with lower elastin levels and higher collagen, advanced glycation end products (AGEs), and proinflammatory cytokine abundance. In old mice, apigenin restored EDD (96% ± 2%) by increasing NO bioavailability, normalized arterial ROS, oxidative stress, and antioxidant expression, and abolished ROS inhibition of EDD. Moreover, apigenin prevented foam cell formation in vitro (initiating step in atherosclerosis) and mitigated age-associated aortic stiffening (PWV 373 ± 5 cm/s) by normalizing aortic intrinsic wall stiffness, collagen, elastin, AGEs, and inflammation. Thus, apigenin is a promising therapeutic for arterial aging.
NEW & NOTEWORTHY Our study provides novel evidence that oral apigenin supplementation can reverse two clinically important indicators of arterial dysfunction with age, namely, vascular endothelial dysfunction and large elastic artery stiffening, and prevents foam cell formation in an established cell culture model of early atherosclerosis. Importantly, our results provide extensive insight into the biological mechanisms of apigenin action, including increased nitric oxide bioavailability, normalization of age-related increases in arterial ROS production and oxidative stress, reversal of age-associated aortic intrinsic mechanical wall stiffening and adverse remodeling of the extracellular matrix, and suppression of vascular inflammation. Given that apigenin is commercially available as a dietary supplement in humans, these preclinical findings provide the experimental basis for future translational studies assessing the potential of apigenin to treat arterial dysfunction and reduce cardiovascular disease risk with aging.
Keywords: aortic pulse wave velocity, atherosclerosis, endothelium-dependent dilation, nutraceutical, reactive oxygen species
INTRODUCTION
Cardiovascular diseases (CVDs) are the leading cause of mortality in the developed world, and advanced age is by far the strongest risk factor for CVDs (1). Arterial dysfunction with aging, characterized by impaired endothelial function and aortic stiffening, are key antecedents to the development of age-related CVDs (2).
Age-related endothelial dysfunction, as indicated by reduced endothelium-dependent dilation (EDD), is mediated primarily by reduced nitric oxide (NO) bioavailability (2–5). This impairment in NO-mediated endothelial function is largely due to excessive production of reactive oxygen species (ROS), which react with NO, reducing its bioavailability and resulting in arterial oxidative stress (6). Importantly, these events can initiate the early stages of atherosclerosis, including promoting foam cell formation (7).
Age-related aortic stiffening is evident in vivo by increases in aortic pulse wave velocity (PWV) (8, 9) and is driven largely by increased aortic intrinsic mechanical wall stiffness. This stiffening of the aortic wall occurs mainly as a result of changes in the extracellular matrix, featuring increased collagen deposition (fibrosis), elastin degradation and cross linking of these structural proteins by advanced glycation end products (AGEs) (6, 10).
Stimulated primarily by oxidative stress, the above age-related arterial changes are reinforced by the development of chronic low-grade vascular inflammation, as indicated by increased arterial abundance of proinflammatory cytokines (6). Thus, lifestyle and pharmacological therapies aimed at reducing vascular oxidative stress and inflammation show promise for improving arterial function during aging (11–13).
Apigenin is a natural flavonoid found primarily in cabbage, celery, parsley, and chamomile (14). There is some evidence that higher natural intake and/or supplementation of these foods may have selective health benefits, and no adverse effects have been reported (14). However, the therapeutic potential of apigenin for improving arterial health with aging has not been investigated and is unknown. This lack of information is likely due in part to the fact that apigenin content in food is highly variable, making feeding studies in humans inexact and difficult to conduct. Rather, increasing apigenin intake via concentrated dietary supplements may be a more promising approach. Concerning the latter, cell culture findings suggest that apigenin may have antioxidant and/or anti-inflammatory properties (15, 16), but these potential effects have not been documented in vivo. To determine whether apigenin exerts antioxidant and/or anti-inflammatory vascular effects, initial studies are needed in animal models, which provide access to arterial tissue samples for biochemical analyses.
In the present study, we tested the integrative hypothesis that 1) in old, but not young mice, oral dietary supplementation with the natural compound apigenin would improve vascular endothelial function by increasing NO bioavailability as a result of reducing arterial ROS production and oxidative stress; 2) apigenin would prevent oxidized lipid-induced foam cell formation (initiating step of atherosclerosis) in vitro; and 3) oral supplementation with apigenin would reduce in vivo aortic stiffness due to favorable effects on extracellular matrix composition and inhibition of vascular inflammation in old but not young mice.
METHODS
Ethical Approval
The study and all procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Colorado Boulder (Protocol No. 2618). All procedures adhered to the guidelines set forth by the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (17).
Animals
Young adult (6 mo, n = 20) and old (27 mo, n = 20) male C57BL/6 mice were obtained from the National Institute of Aging colony (maintained by Charles River, Wilmington, MA). As reported, C57BL/6 mice are a model of age-related arterial dysfunction, including arterial stiffening and vascular endothelial dysfunction (3, 18–21). Mice were allowed to acclimate to our facilities for 4 wk before beginning the study. For the duration of the study, mice were single housed at the University of Colorado Boulder with a 12-h:12-h light-dark cycle and allowed ad libitum access to normal rodent chow (Envigo 7917).
Apigenin Intervention
For the intervention period, young and old mice consumed either control [0.2% carboxymethylcellulose (Sigma-Aldrich, St. Louis, MO; Cat. No. C5013)-enriched drinking water] or apigenin (0.5 mg/mL in control drinking water; Fisher Scientific; Cat. No. 501449769; young; n = 10; old: n = 9) for 6 wk. Throughout the intervention period, four old mice died (vehicle: n = 3; apigenin: n = 1) as a result of typical age-related attrition, which resulted in a final sample size of young control (YC), n = 10; old control (OC), n = 7; young apigenin (YA), n = 10; and old apigenin (OA), n = 9. Water and energy intake (grams of food consumed × kcals/gram) were assessed every other day throughout the duration of the intervention. Treatment groups were matched for baseline body weight and aortic PWV.
Administration of apigenin via oral gavage at a dose of 50 mg/kg/day for 6 wk was shown to improve aortic EDD in a rat model of type 2 diabetes, with no further improvement at a dose of 100 mg/kg/day (22). To avoid the stress associated with daily oral gavage in old mice, as well as to increase the translational potential of our expected results, we administered apigenin orally in the drinking water for the first time. To accomplish this, we first suspended apigenin in 0.2% carboxymethylcellulose [similar to previous reports using oral gavage (22, 23), as apigenin is minimally soluble in water], and then diluted it into the mouse drinking water that had been supplemented with 0.2% carboxymethylcellulose.
No data in humans were available to guide dosing in the present study. Accordingly, we chose a concentration of apigenin in the drinking water of 0.5 mg/mL because our laboratory previously showed that old mice on average drink 3–4 mL of water per day and weigh ∼30 g (3), which equates to 1.5–2 mg/day of apigenin, a dose similar to that received by a 30 g mouse when administered 50 mg/kg/day by oral gavage. Overall, this method of apigenin administration was effective and did not influence overall water intake within each age group.
Vascular endothelial function: Endothelium-dependent dilation, nitric oxide-mediated EDD, and tonic reactive oxygen species suppression of EDD
Upon completion of in vivo measures, mice were euthanized via cardiac exsanguination under maintained inhaled isoflurane anesthesia and carotid arteries were immediately excised. Vascular endothelial function was measured via ex vivo carotid artery EDD and endothelium-independent dilation (EID) in response to increasing doses of acetylcholine (ACh) and sodium nitroprusside (SNP), respectively, as described previously (24, 25). In brief, after vessels were preconstricted with phenylephrine (PE; 2 µM; Sigma-Aldrich, Cat. No. P6126), EDD was assessed by measuring increases in inner luminal diameter in response to increasing concentrations of acetylcholine (ACh; 1 × 10−9 to 1 × 10−4 M; Sigma-Aldrich, Cat. No. A6625) with and without coadministration of the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 0.1 mM, 30-min preincubation; Sigma-Aldrich, Cat. No. N5751) or ROS scavenger Tempol (1 mM, 60 min incubation; Sigma-Aldrich, Cat. No. 2226-96-2). NO-mediated dilation was then calculated as the difference between maximum EDD to ACh alone and in the presence of ACh and l-NAME:
NO-mediated dilation (%) = Maximum dilationACh − Maximal dilationACh+L-NAME
Following EDD, EID was assessed by measuring the increase in luminal diameter in response to increasing concentrations of SNP, an exogenous NO donor (1 × 10−10 to 1 × 10−4 M; Sigma-Aldrich, Cat. No. 13755-38-9). All dose response data are presented as percent dilation relative to baseline diameter to account for differences in baseline vessel diameter.
Foam Cell Formation Cell Culture Experiments
Cell culture.
RAW 264.7 murine macrophage cells (American Type Culture Collection, Manassas, VA) were cultured and passaged (17 passages maximum) at 37°C and 5% CO2 to ∼80% confluency in high-glucose (4.5 g/L) DMEM (Gibco, Gaithersburg, MD) supplemented with 4 mM l-alanyl-l-glutamine (Gibco), 10 mM HEPES (Gibco), 10% qualified fetal bovine serum (FBS, Gibco), 100 U/mL penicillin G, and 100 μg/mL streptomycin (GeminiBio, Sacramento, CA). Upon reaching confluency, cells were washed with Hanks’ buffered salt solution (HBSS) and gently lifted by scraping into fresh supplemented Dulbecco’s modified Eagle’s medium (DMEM). Cells were counted and diluted to 9 × 103 cells/mL (designed to result in 60%–80% confluency over a 48-h incubation) in supplemented DMEM. In all, 100 μL of RAW 264.7 cell suspension was added to each well of a 96-well black polystyrene imaging plate with 0.170 ± 0.005 mm No. 1.5 cover glass bottom (Cellvis, Mountain View, CA). After a 24-h incubation, attached cells were washed once with HBSS and incubated with supplemented DMEM for a second 24-h period. During the second incubation, four conditions were created by adding the following to wells in quadruplicate on two separate days (N = 8 for each condition): 1) no further addition (supplemented DMEM only); 2) 50 µg/mL high-oxidized low-density lipoprotein (LDL) (Kalen Biomedical, Germantown, MD) and 0.5 µg/mL fluorescent DiI (Dye aye)-labeled high-oxidized LDL (Kalen Biomedical); 3) 50 µg/mL high-oxidized LDL (Kalen Biomedical) and 0.5 µg/mL fluorescent DiI-labeled high-oxidized LDL (Kalen Biomedical) with 1 µM apigenin (TCI, Portland, OR); and 4) 50 µg/mL high-oxidized LDL (Kalen Biomedical) and 0.5 µg/mL fluorescent DiI-labeled high-oxidized LDL (Kalen Biomedical) with 2 µM apigenin. An apigenin concentrate was added to media in dimethyl sulfoxide (DMSO; Thermo Fisher Scientific, Eugene, OR) such that the DMSO concentration did not exceed 0.06% vol/vol (8.5 mM).
Foam cell visualization.
After the second 24-h incubation, attached cells were washed three times with HBSS and left in 100 μL of HBSS for microscopic imaging along with 1.8 µg/mL Hoechst 33342 fluorescent nuclear probe. After a 10-min incubation at 37°C and 5% CO2, cells were imaged on a CellVoyager CV1000 Confocal Scanner System (Yokogawa, Sugar Land, TX) equipped with dual microlens-enhanced Nipkow scanning disks. Cells were imaged at ×10 and ×20 magnification and were selected for further analysis according to cell morphology in bright-field operating mode and nuclear morphology via Hoeschst 33342 fluorescence. Intact cells were evaluated for accumulation of cytoplasmic fluorescent DiI-oxidized LDL, indicative of foam cell formation. Degree of fluorescent DiI accumulation was determined using Fiji, the open-source processing program distribution of ImageJ (National Institutes of Health, Bethesda, MD) (26).
In Vivo Aortic Stiffness and Arterial Blood Pressure
Aortic stiffness was assessed in vivo using the gold standard measure, aortic PWV, as we previously described (18, 19). Briefly, mice were placed under light isoflurane anesthesia (1.5%–2.0%) and positioned supine on a warmed heat pad. Front- and hind-limb paws were then secured to corresponding ECG electrodes. Two Doppler probes were then placed on the skin at the transverse aortic arch and the abdominal aorta. Once clear R-waves were registered, three repeated 2-s ultrasound tracings were recorded and average pre-ejection time (i.e., time between the R-wave of the ECG to the foot of the Doppler signal) was determined for each location. To calculate aortic PWV, the distance between the two probes was divided by the difference between the thoracic and abdominal aorta pre-ejection times (timeabdominal − timethoracic) and is reported as centimeters/second (cm/s). To examine the potential contribution of changes in arterial blood pressure to any treatment-related differences in aortic PWV, systolic and diastolic blood pressure were assessed using a CODA noninvasive tail-cuff system (Kent Scientific, Torrington, CT), as we have described previously (3, 27). Briefly, the pressure measurements from 20 collection cycles (following 5 acclimation cycles) each of 3 consecutive days were averaged for each mouse at each timepoint.
Aortic Intrinsic Mechanical Wall Stiffness (Elastic Modulus)
Aortas were promptly excised from the mice following carotid artery excision, rinsed with cold physiological saline solution and cleared of any remnant perivascular connective tissue. To measure ex vivo aortic stiffness, two thoracic aorta samples (∼1 mm in length) were cut and used to determine intrinsic mechanical stiffness via wire myography as previously described by us (18, 19). In short, aorta samples were placed in heated (37°C) baths filled with calcium-free, phosphate-buffered saline. The samples were then mounted on two wire prongs, followed by three rounds of prestretching. Once prestretching was complete, aortic ring diameter was increased until ∼1 mN of force was reached and incrementally increased by 50 µM every 3 min thereafter until failure. The force corresponding to each stretching interval was recorded and used to calculate stress and strain. A stress-strain curve was then generated using the following equations:
where d is diameter and di is initial diameter.
where L is one-dimensional load, H is wall thickness, and D is vessel length.
The elastic modulus of the stress-strain curve was determined as the slope of the linear regression fit to the final four points of the stress-strain curve, as previously reported by our laboratory (3, 21). Aortic wall thickness and diameter were assessed as we have described previously (3, 27). Briefly, aortic rings (1 mm) were frozen in optimal cutting temperature solution and stored at −80°C until the time of sectioning. Aortic sectioning was performed on a Cryostat (Leica Biosystems, Wetzlar, Germany) at −22°C and 100 µM sections were visualized, and images were captured with a bright-field microscope. Aortic wall thickness and diameter were calculated using ImageJ software (26).
Aortic ROS Production
Aortic ROS production was assessed by electron paramagnetic resonance (EPR) spectrometry using the spin probe 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH; Enzo Life Sciences), as we have previously described (3, 24). Two 1-mm aortic rings were washed in warm physiological saline solution and incubated in Krebs/HEPES buffer, consisting of 99 mM NaCl, 4.7 mM KCl, 1.87 mM CaCl2, 1.2 mM MgSO4, 25 mM NaHCO3, 1.03 mM KH2PO4, 20 mM Na-HEPES, 11.1 mM glucose, 0.1 mM diethylenetriaminepenta-acetic acid, 0.0035 mM sodium diethyldithiocarbamate, and Chelex (Sigma-Aldrich), containing 0.5 mM CMH at 37°C for 60 min. Samples were analyzed using a MS300 Xband EPR spectrometer (Magnettech, Berlin, Germany) with the following instrument parameters: B0-Field, 3,350 G; sweep, 80 G; sweep time, 60 s; modulation, 3,000 mG; MW atten, 7 dB; gain, 5 × e^1.
Aortic Protein Abundance
Protein abundance was measured in segments of thoracic aorta following mechanical homogenization in radio-immunoprecipitation assay lysis buffer supplemented with protease and phosphatase inhibitors [1 mMol/L sodium orthovanadate, 1X complete mini protease inhibitor cocktail tablet (Roche, Mannheim, Germany), 1 mMol/L phenylmethylsulfonyl fluoride, 1:100 Phosphatase Inhibitor Cocktail (Sigma-Aldrich), 5 mMol/L sodium fluoride, and 5 mMol/L sodium pyrophosphate]. Total protein content was quantified using a bicinchoninic acid assay (Thermo Fisher Scientific, Eugene, OR). Next, abundance of nitrotyrosine (anti-goat; 1:100; Abcam, Cambridge, MA, Cat. No. ab7048), nicotinamide adenine dinucleotide phosphate oxidase (anti-goat, 1:200, BD Biosciences, San Jose, CA, Cat. No. 610912), superoxide dismutase (SOD) 1 (anti-goat, 1:500, R&D Sytems, Minneapolis, MN, Cat. No. AF3787), SOD2 (anti-goat; 1:50; R&D Systems, Cat. No. AF3419), collagen-1 (anti-rabbit; 1:10; Sigma-Aldrich, St. Louis, MO, Cat. No. 234167), α-elastin (anti-rabbit; 1:20; Invitrogen, Eugene, OR, Cat. No. PA572440), and advanced glycation end products (AGEs) (anti-rabbit; 1:250; Abcam, Cat. No. ab23722) were determined by loading 20 ng/mL of aortic protein per capillary in a 25-lane (capillary) automated Western blot quantitative analyzer (WES, ProteinSimple, San Jose, CA), according to the manufacturer’s guidelines, as described previously (28), following the validation of these antibodies in test aorta lysates. Anti-goat and anti-rabbit secondary antibodies were provided by the manufacturer and used according to the manufacturer’s guidelines. A grayscale analysis of the band intensities was then performed to quantify protein abundance using Compass software (ProteinSimple), with target proteins expressed relative to a loading control (GAPDH; 1:200; Cell Signaling, Cat. No. 2118). Aortic protein abundance of inflammatory cytokines was determined using a custom multiplex ELISA (Ciraplex, Aushon Biosystems, Billerica, MA), as previously described (3, 21). The multiplex plates were custom designed (custom Ciraplex, Aushon Biosystems) for detection of the following murine proinflammatory cytokines, namely, interleukin-1β (IL-1β), interleukin-6 (IL-6), interferon γ (IFNγ), and tumor necrosis factor α (TNFα). In all, 15 µg of aortic lysate were loaded into microplate wells, and an assay was performed according to the manufacturer’s instructions. Images were captured using Cirascan software (Aushon Biosystems). If levels of a given cytokine were undetectable (e.g., fell below the limit of detection of the assay), samples were excluded from the analysis.
Statistical Analyses
Statistical analyses were conducted in Prism, version 8 (GraphPad Software, Inc., La Jolla, CA). Data were first assessed for outliers (ROUT method, Q = 1%) and normality (Shapiro–Wilk normality test, P > 0.05) within groups. Two-way mixed ANOVAs were used to determine differences in aortic PWV, systolic and diastolic blood pressure [group × time (pre-/posttreatment)]. Differences in EDD and EID, both of which are terminal measurements obtained upon euthanasia, were also assessed using a two-way mixed-design ANOVA (group × dose). Differences across animal groups in morphological and artery characteristics, water intake, energy intake, NO-mediated dilation, foam cell formation, ROS production, immunoblot markers, elastic modulus, and inflammatory cytokines were assessed using a two-way ANOVA. When significant main effects were detected, pairwise comparisons were made using the Holm–Sidak post hoc test. Significance was set to α = 0.05. Unless otherwise noted, data are presented as means ± SE.
RESULTS
Animal Characteristics
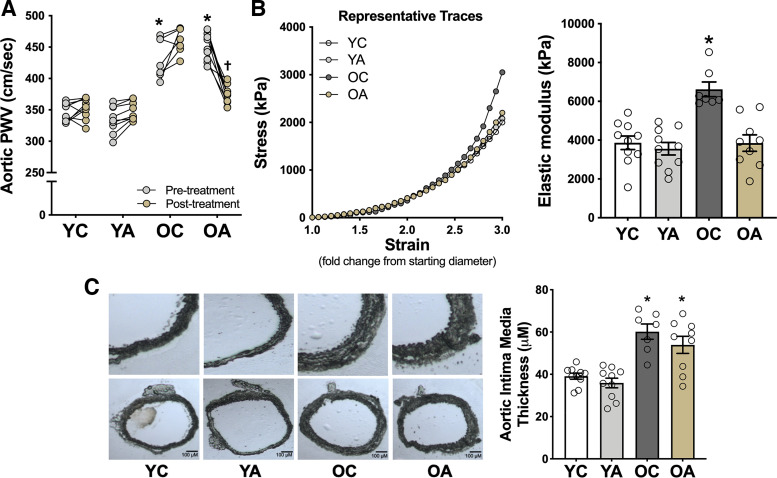
Water and energy intake, total body mass, mass of select organs/tissues and aorta, and carotid artery characteristics at time of euthanasia in the four groups are presented in Table 1. The old mice had higher (P < 0.0001) water intake relative to young mice, as we have observed previously (3). The presence of apigenin in the drinking water did not alter water intake in either age group, and energy intake and body mass did not differ across age or treatment groups. However, old mice (independent of group) had higher heart mass (P = 0.002) and lower quadricep (P < 0.0001) and visceral adipose (P < 0.0001) tissue mass, relative to young mice, which is similar to previous reports (3, 29, 30). Furthermore, relative to young animals, old mice had greater maximal carotid artery diameter (P = 0.02) and intima medial aortic wall thickness (P < 0.0001; see also Fig. 4C), as described previously (3), but there were no effects of apigenin supplementation.
Table 1.
Animal characteristics
| Young |
Old |
|||
|---|---|---|---|---|
| Characteristics | Vehicle | Apigenin | Vehicle | Apigenin |
| n | 10 | 10 | 7 | 9 |
| Body weight, g | 31.1 ± 1.5 | 30.6 ± 1.1 | 30.9 ± 1.3 | 29.7 ± 1.1 |
| Energy intake, kcals/day | 15.3 ± 0.3 | 15.8 ± 0.2 | 15.3 ± 0.9 | 14.4 ± 0.6 |
| Water intake, mL/day | 4.3 ± 0.1 | 4.0 ± 0.1 | 5.4 ± 1.5* | 5.1 ± 1.8* |
| Apigenin intake, mg/day | 2.0 ± 0.1 | 2.5 ± 0.9 | ||
| Heart mass, mg | 146.0 ± 5.9 | 133.7 ± 4.8 | 163.3 ± 12.1* | 166.0 ± 7.8* |
| Quadriceps mass, mg | 312.5 ± 12.1 | 336.9 ± 16.1 | 240.0 ± 10.1* | 251.7 ± 15.5* |
| Visceral adipose mass, g | 1.1 ± 0.2 | 1.0 ± 0.1 | 0.3 ± 0.1* | 0.2 ± 0.1* |
| Carotid artery | ||||
| Resting diameter, µM | 361 ± 5 | 357 ± 6 | 370 ± 8 | 386 ± 10 |
| Maximal diameter, µM | 402 ± 6 | 410 ± 9 | 437 ± 11* | 440 ± 8* |
| Aorta | ||||
| Diameter, µM | 613 ± 8 | 635 ± 19 | 636 ± 8 | 646 ± 21 |
| Intima media thickness, µM | 39 ± 1 | 36 ± 2 | 60 ± 4* | 54 ± 4* |
| Systolic blood pressure, mm/Hg | ||||
| Pre | 106 ± 2 | 101 ± 3 | 96 ± 6 | 98 ± 6 |
| Post | 100 ± 4 | 97 ± 3 | 98 ± 4 | 96 ± 3 |
| Diastolic blood pressure, mm/Hg | ||||
| Pre | 79 ± 4 | 75 ± 4 | 77 ± 3 | 75 ± 6 |
| Post | 75 ± 2 | 78 ± 3 | 76 ± 4 | 74 ± 5 |
Values are means ± SE; n, number of mice.
*P < 0.05 young vehicle vs. old vehicle; young apigenin vs. old apigenin.
Apigenin Improves Endothelial Function in Old Mice by Restoring NO Bioavailability and Abolishing Oxidative Stress and Tonic Suppression of EDD
EDD.
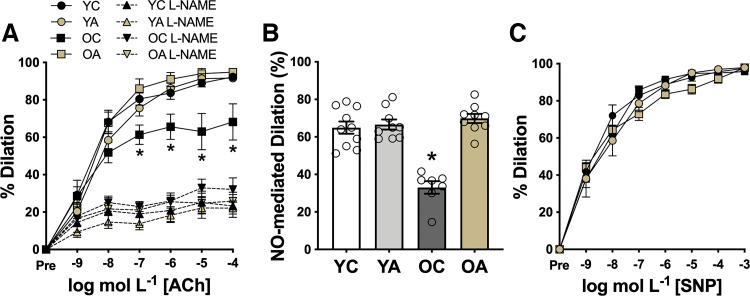
Vascular endothelial function was assessed ex vivo in carotid arteries as EDD to increasing doses of ACh (Fig. 1). Peak EDD was 26% lower (P = 0.006) in OC than in YC control mice (peak EDD: YC, 94% ± 1%; OC, 70% ± 9%; Fig. 1A). Oral supplementation with apigenin for 6 wk completely restored EDD in old mice (OA, 95% ± 2%) to levels of young animals without affecting EDD in young mice (YA vs. YC, P = 0.9). These observations indicate that apigenin selectively enhances EDD in old animals, effectively ameliorating age-related vascular endothelial dysfunction.
Figure 1.
Apigenin selectively enhances endothelial function in old mice by increasing nitric oxide (NO) bioavailability. A: carotid artery dose responses to the endothelium-dependent dilator acetylcholine (ACh) in young and old controls (YC and OC) and young and old apigenin-supplemented (YA and OA) mice (n = 7–10/group) with and without coadministration of the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME). B: NO-mediated dilation [peak dilation of ACh (−) peak dilation of ACh + l-NAME] (n = 7–10/group). C: dose responses to the endothelium-independent dilator sodium nitroprusside (SNP) (n = 7–10/group). Values are means ± SE; n, number of mice/group. *P < 0.05 vs. all other groups (under ACh-only conditions in A).
NO bioavailability.
Next, we assessed the effects of aging and apigenin on NO-mediated EDD. Inhibition of NO production by administration of the NO synthase inhibitor l-NAME abolished group differences in EDD (P = 0.46) (Fig. 1A), implicating differences in NO bioavailability as the underlying mechanism. Consistent with this idea, peak NO-mediated dilation [peak EDD to ACh alone (−) peak EDD to ACh + l-NAME] was ∼50% lower (P = 0.01) in old versus young controls (YC, 63% ± 7%; OC, 32% ± 6%). Importantly, apigenin completely restored NO-mediated dilation in old mice (OA, 69% ± 4%) to young animal levels (Fig. 1B). To determine the mechanisms by which apigenin restores EDD in old mice, we measured endothelium-independent dilation as the response to SNP, a NO donor. We found no differences among groups (P = 0.25), indicating that apigenin does not improve EDD in old animals by increasing vascular smooth muscle sensitivity to NO (Fig. 1C). Together, these data indicate that oral supplementation with apigenin reverses age-related endothelial dysfunction by restoring NO-mediated EDD.
Oxidative stress.
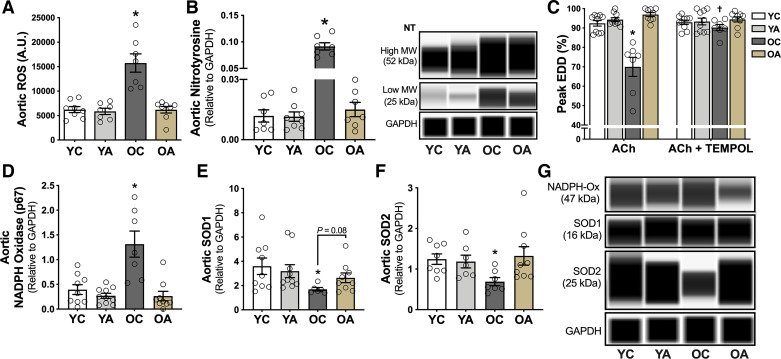
To determine whether these favorable effects of apigenin on endothelial function in old animals were mediated by reduced oxidative stress, we assessed ROS production in dissected aortic rings using electron paramagnetic resonance spectroscopy, as we have previously described (18, 24, 25). Old mice had 2.6-fold higher aortic ROS production relative to young mice (P = 0.01, OC vs. YC; Fig. 2A). Oral apigenin supplementation normalized the age-related increase in aortic ROS to levels observed in young animals, without affecting aortic ROS production in the other groups. To determine whether these effects of age and apigenin treatment on aortic ROS were associated with molecular evidence of differences in oxidative stress (oxidant modification of macromolecules), we assessed the abundance of nitrotyrosine, an established marker of endogenous oxidative protein modification (31), via a capillary electrophoresis immunoprobe method. Aortic nitrotyrosine (sum of the 25 and 52 kDa bands) was 8.3-fold higher in old relative to young mice (P = 0.02, OC vs. YC). Consistent with the effects on ROS production, apigenin largely reversed the age-related increase in aortic nitrotyrosine (P = 0.04, OA vs. OC), such that nitrotyrosine abundance was not significantly different in old apigenin-treated animals compared with the young groups, which were unaffected by the treatment (Fig. 2B). Next, we determined whether these age- and apigenin-related differences in ROS and oxidative stress causally influenced group differences in endothelial function. We used a well-established bioassay to assess carotid artery EDD with and without prior incubation with the ROS scavenger Tempol (24, 29) (Fig. 2C). Tempol administration restored peak EDD selectively in OC mice (P = 0.02, with vs. without Tempol) to levels not different (P = 0.9) from young controls, without affecting EDD in the young animals. Thus, the impaired EDD in OC mice is mediated by excess vascular ROS, as we previously showed (32, 33). Particularly, preincubation with Tempol did not further improve EDD in old mice that received oral apigenin supplementation (OA: ACh alone vs. ACh + Tempol, P = 0.92), suggesting that apigenin improved endothelial function in old mice by ameliorating tonic ROS-related suppression of EDD. Finally, we aimed to determine the potential mechanisms of the age-related arterial oxidative stress observed with regard to changes in expression of oxidant and/or antioxidant enzymes and whether apigenin supplementation mitigated these changes. We found that NADPH oxidase, a major ROS producing enzyme (34), was approximately threefold higher in the old than in young control groups (P = 0.003, OC vs. YC), which is consistent with previous studies in our laboratory (13, 35). This age-related difference was ameliorated with apigenin supplementation (P = 0.001, OC vs. OA; Fig. 2, D and G). We also found not only a lack of normal oxidative stress-induced compensatory upregulation of arterial antioxidant defense proteins, such as SOD1 and SOD2 with aging, but actually lower abundance of both of these enzymes (P = 0.03 and 0.006, respectively, OC vs. YC; Fig. 2, E–G), as we have previously observed (12). In old mice, apigenin supplementation was associated with arterial SOD1 and SOD2 abundance that was not different from expression levels observed in young animals (Fig. 2, E–G).
Figure 2.
Apigenin supplementation ameliorates age-related vascular oxidative stress. A: aortic reactive oxygen species (ROS) production [electron paramagnetic resonance spectroscopy absorbance units (AU)] in young and old controls (YC and OC) and young and old apigenin-supplemented (YA and OA) mice (n = 7–10/group). B: aortic nitrotyrosine (NT) abundance and representative images in YC, YA, OC, and OA mice (n = 7–10/group). Data are the sum of the high and low molecular weight bands normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). C: maximal carotid artery dose response to the endothelium-dependent dilator acetylcholine (ACh) in YC, YA, OC, and OA mice in the presence or absence of the ROS scavenger Tempol (n = 7–10/group). Aortic abundance and representative images of NADPH oxidase (D and G); superoxide dismutase (SOD) 1 (E and G); and SOD2 (F and G), all of which are normalized to GAPDH. Values are means ± SE; n, number of mice/group. *P < 0.05 vs. all other groups; †P < 0.05 ACh vs. ACh + Tempol. EDD, endothelium-dependent dilation; MW, molecular weight; NADPH-ox, nicotinamide adenine dinucleotide phosphate oxidase.
Apigenin Prevents Foam Cell Formation in Cultured RAW 264.7 Macrophages
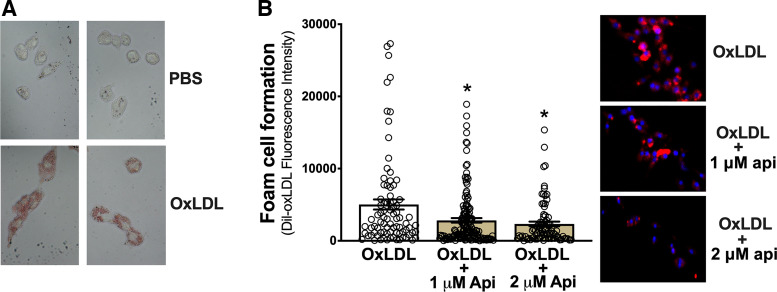
Vascular endothelial dysfunction featuring reduced NO bioavailability is a key antecedent to the development of atherosclerosis and clinical CVD. Foam cell formation in response to NO deficiency and associated endothelial dysfunction is, in turn, considered the initial step in the pathogenesis of atherosclerosis (7, 36). Because dietary apigenin supplementation reversed age-related endothelial dysfunction by restoring NO bioavailability, we asked whether apigenin also suppressed foam cell formation. To initially address this question, we used a RAW 264.7 macrophage cell line grown in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with phosphate-buffered saline (PBS) or oxidized human low-density lipoprotein (oxLDL) to qualitatively determine proof-of-concept that oxLDL increases foam cell formation (Oil Red O uptake)—an established cell culture model of atherosclerosis-associated foam cell development (37, 38) (Fig. 3A). Next, to quantitatively assess foam cell formation, we cultured RAW 264.7 macrophage cells with fluorescently labeled DiI-oxLDL and assessed the increase in fluorescent lipid uptake, which is also an established model of foam cell development (36, 37). To determine the potential anti-atherogenic effects of apigenin, we selected a 1 µM dose because pharmacokinetic studies show that 1 µM is the average plasma concentration of apigenin 24 h following an oral bolus (a dose similar to that used in the present study) in humans (39). Because apigenin is nondetectable in plasma 28 h following ingestion (40), our approach essentially modeled the anti-atherogenic influence of daily apigenin administration. Apigenin at 1 µM reduced (P = 0.02 vs. oxidized LDL alone) uptake of fluorescent DiI-oxLDL in response to oxidized LDL, whereas 2 µM apigenin tended to further reduce (nonsignificantly) fluorescent DiI-oxLDL (Fig. 3B). These results provide the first evidence that a physiologically relevant dose of apigenin inhibits experimental foam cell formation induced by oxidized LDL in a macrophage cell culture model of atherosclerosis.
Figure 3.
Apigenin prevents oxidized low-density lipoprotein (oxLDL)-induced foam cell formation in cultured RAW 264.7 macrophages. A: qualitative proof of concept images that oxLDL stimulates foam cell formation as defined by greater Oil Red O uptake in Raw 264.7 macrophages when exposed to oxLDL [in phosphate-buffered saline (PBS) vs. PBS alone]. B: foam cell formation, quantified as fluorescent DiI-oxLDL incorporation into Raw 264.7 macrophages, following incubation with 0.5 µg/mL DiI-oxLDL + 50 µg/mL oxLDL; 0.5 µg/mL DiI-oxLDL + 50 µg/mL oxLDL + 1 µM apigenin (api); or 0.5 µg/mL DiI-oxLDL + 50 µg/mL oxLDL + 2 µM api. Representative images are provided on the right. Each condition was tested in quadruplicate on two separate days, with n = 8/condition/day. Values are means ± SE; n, number of imaged wells/condition per day. *P < 0.05 vs. oxLDL.
Apigenin Reduces Aortic Stiffness in Old Mice: Effects on Intrinsic Mechanical Wall Stiffness, Structural Proteins, and AGEs
Aortic PWV.
To determine whether oral supplementation with apigenin selectively reduces aortic stiffness in old mice, we noninvasively assessed in vivo aortic PWV, an established translational model of carotid-femoral PWV in humans (11, 32), at baseline and after treatment in each of the four groups of mice. Aortic PWV was 25% greater in old than in young mice at baseline (P < 0.0001), consistent with age-related aortic stiffening in humans (41). Apigenin supplementation in old mice (OA group) eliminated 70% of this age-related difference in aortic PWV (P < 0.0001 vs. OC), whereas no significant changes were observed over time within the YC, YA, or OC groups (Fig. 4A). These effects occurred independently of changes in systolic or diastolic blood pressure, as measured noninvasively in vivo using the tail-cuff method (Table 1), suggesting a direct influence of apigenin on reducing in vivo aortic stiffness rather than a secondary effect via reductions in blood pressure.
Figure 4.
Apigenin reverses age-related aortic stiffening. A: aortic pulse wave velocity (PWV) in young and old controls (YC and OC) and young and old apigenin-supplemented (YA and OA) mice (n = 7–10/group). B: representative stress-strain curve of an aorta ring from YC, YA, OC, and OA mice for determination of ex vivo intrinsic mechanical wall stiffness. Aortic elastic modulus (calculated as the slope of the final four points in the stress-strain curve) in YC, YA, OC, and OA mice. C: intima-media thickness of the aorta with representative images of whole aortic sections (bottom) and enlargements of the same sections (top) included to the left of the mean data (n = 7–10/group). Values are means ± SE; n, number of mice/group. *P < 0.05 vs. all other groups (aortic PWV, pretreatment); †P < 0.05 pretreatment vs. posttreatment within group.
Intrinsic mechanical wall stiffness.
To determine whether structural changes to the arterial wall might contribute to the apigenin-induced decrease in aortic PWV, we measured the elastic modulus of aorta rings isolated from YC, YA, OC, and OA mice. Elastic modulus is defined as the association between the change in stress on the arterial wall before and after a given strain (stretch) and is indicative of the intrinsic mechanical stiffness of the aortic wall (21, 42). Figure 4B shows a representative stress-strain curve of the aortic elastic modulus from OC and OA mice. Aortic elastic modulus was 67% greater in OC versus YC mice (P < 0.0001), consistent with our previous studies (18, 19). Apigenin supplementation markedly reduced aortic elastic modulus in old mice, but had no effect in young mice, thus ameliorating the age-related increase in intrinsic mechanical wall stiffness (OA vs. OC, P = 0.0005; Fig. 4C).
Aortic wall structural proteins and AGEs.
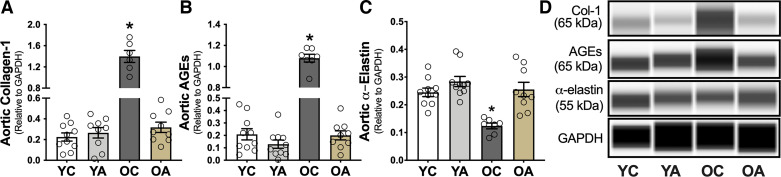
Age-related increases in aortic PWV and intrinsic mechanical wall stiffness are mediated in part by increased collagen deposition and cross linking of collagen fibers by AGEs in the aortic wall (6). Elastin fragmentation and degradation can also contribute to age-related aortic stiffening (43). To determine the mechanisms contributing to age- and apigenin treatment-associated effects on aortic stiffness, we assessed the abundance of collagen, AGEs, and elastin in aortic lysates from YC, YA, OC, and OA mice by a capillary electrophoresis immunoprobe. Aortic collagen (Fig. 5A) and AGEs (Fig. 5B) were 6.3- and 4.9-fold higher in OC versus YC, respectively (P = 0.009 and 0.03), consistent with previous reports (3, 18, 21). Apigenin supplementation prevented the age-related increase in collagen (P = 0.01, OA vs. OC; Fig. 5A) and AGEs (P = 0.04; Fig. 5B). Aortic elastin (Fig. 5C) was twofold lower in OC versus YC mice (P = 0.03), as we previously showed (3, 21). Apigenin largely prevented the age-related loss of aortic elastin (P = 0.03, OA vs. OC); there was no difference between YA and OA (P = 0.56). Thus, apigenin “destiffened” the aorta of old mice by mitigating the structural changes that occur in the arterial wall with aging.
Figure 5.
Apigenin supplementation reverses age-related increases in aortic collagen and advanced glycation end products (AGEs) and prevents the age-related reduction in aortic elastin. A and D: aortic abundance of collagen-1 (Col-1) in young and old controls (YC and OC) and young and old apigenin-supplemented (YA and OA) mice (n = 7–10/group). B and D: aortic abundance of AGEs in YC, YA, OC, and OA mice. C and D: aortic abundance of α-elastin in YC, YA, OC, and OA mice. Data are normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Values are means ± SE; n, number of mice/group. *P < 0.05 vs. all other groups.
Apigenin Attenuates Age-Related Vascular Inflammation
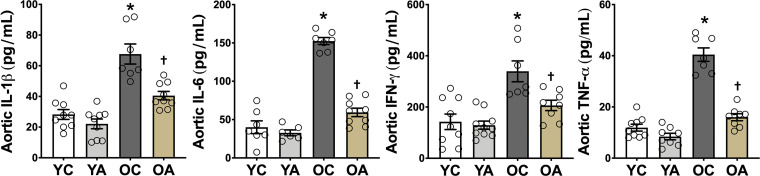
Oxidative stress and inflammation are closely related and mutually reinforcing; therefore, we asked whether suppression of vascular oxidative stress with apigenin also reduced vascular inflammation. We found that the abundance of the proinflammatory cytokines interleukin-1β (IL-1β), interleukin-6 (IL-6), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) in aortic lysates was 2.4–3.7-fold higher in OC relative to YC mice (P < 0.0001), as we have reported previously (3, 21) (Fig. 6). Oral apigenin supplementation reduced the aortic abundance of these proinflammatory cytokines in OA by 68%–83% relative to OC (P = 0.04–0.006; Fig. 6). Thus, apigenin exerts significant anti-inflammatory effects in aorta of old mice.
Figure 6.
Apigenin supplementation attenuates age-related vascular inflammation. Aortic abundance of interleukin-1β (IL-1β), interleukin-6 (IL-6), interferon γ (IFNγ), and tumor necrosis factor α (TNFα) in young and old controls (YC and OC) and young and old apigenin-supplemented (YA and OA) mice (n = 7–10/group). Values are means ± SE; n, number of mice/group. *P < 0.05 vs. all other groups; †P < 0.05 vs. all other groups.
DISCUSSION
Our results demonstrate for the first time that oral supplementation with the natural compound apigenin ameliorates age-related vascular endothelial dysfunction and aortic stiffening, and in vitro, apigenin inhibits foam cell formation—the critical initiating event in atherosclerosis. Our findings also provide insight into the cellular and molecular mechanisms underlying these effects. The latter include complete restoration of NO bioavailability, amelioration of arterial oxidative stress associated with favorable modulation of key oxidant and antioxidant enzymes, normalization of aortic intrinsic mechanical wall stiffness by reversing adverse extracellular matrix remodeling, and suppression of vascular inflammation.
Endothelial dysfunction is the major antecedent to atherosclerosis as a consequence of reduced NO bioavailability and increased ROS, which activate proinflammatory pathways. These events enhance oxidation of LDL, which is incorporated into macrophages to create foam cells that form atherosclerotic plaques (7). In our study, 6 wk of apigenin supplementation completely restored NO-mediated dilation in old animals, similar to what we have previously shown with other natural compounds (12, 44–46).
We show here that apigenin normalized aortic ROS production, nitrotyrosine, NADPH oxidase, and SOD1 and SOD2 to levels not different from those observed in young mice, which abolished tonic ROS-associated suppression of EDD, while mitigating vascular inflammation in the old animals. Importantly, apigenin prevented oxidized LDL-mediated foam cell formation in cultured macrophages. Taken together, our findings suggest that apigenin supplementation may hold promise for attenuating the early stages of atherosclerosis in older adults. However, future studies are needed to determine whether apigenin can attenuate foam cell formation and atherosclerosis in vivo as well as the mechanism(s) by which those benefits are conferred.
Stiffening of the aorta occurs with advancing age, as indicated by an increase in aortic PWV, and is a major independent risk factor for age-associated cardiovascular (CV) events, clinical CVD, kidney dysfunction, and cognitive impairment (47–49). Aortic stiffening reduces the ability to buffer increases in pressure produced by systolic ejection of blood into the large elastic arteries with each contraction of the left ventricle. This reduced ability increases systolic blood pressure and arterial pulse pressure (the difference between systolic and diastolic blood pressure), as well as the “pulsatility” of blood flow, which is transmitted to the delicate microvasculature of vulnerable high-flow organs (e.g., kidney and brain), causing end-organ damage and other pathologies (2, 50, 51). We show that 6 wk of apigenin supplementation reverses the age-associated increase in aortic PWV, an established preclinical surrogate of the gold standard clinical measure of aortic stiffness in humans, that is, carotid-femoral PWV (9, 50). This finding is line with previous reports from our laboratory demonstrating that oral supplementation with natural compounds can reverse age-related aortic stiffening (12, 44–46). As such, our findings have potential clinical significance for mitigating aortic stiffening and its pathological sequelae in humans because presently there are no Food and Drug Administration (FDA)-approved pharmacological treatments for age-associated arterial stiffening.
The mechanisms by which the aorta stiffens with age are incompletely understood, but the primary event involved is believed to be increased intrinsic stiffness of the aortic wall, as indicated by an increase in the elastic modulus of segments of the proximal aorta on stress-strain analysis (18, 19, 42). The cellular and molecular mechanisms underlying increased intrinsic aortic wall stiffness with age involve remodeling of the composition of structural molecules within the extracellular matrix of the aortic wall. Collagen (type I) is the primary load-bearing protein in the aortic wall, and its abundance increases with advancing age, conferring increased mechanical stiffness (3, 10, 18). In contrast, elastin, the main structural protein conferring elasticity in the aorta, becomes fragmented and undergoes degradation with aging, thus reducing its abundance relative to collagen (3, 21, 29). Moreover, with advancing age the formation and accumulation of AGEs, which cross link structural proteins, increases in the aortic wall, further increasing stiffness (52). We have previously showed in mice that oxidative stress plays an important role in these age-associated structural changes within large elastic arteries (11).
Here, we demonstrate that 6 wk of apigenin supplementation normalized the intrinsic mechanical stiffness of the aortic wall of old mice, as indicted by a reduction in the elastic modulus to levels similar to those observed in young controls and apigenin-treated animals. The normalization of intrinsic wall stiffness by apigenin treatment in the old mice was associated with a remarkable remodeling of the extracellular matrix of the aortic wall. Specifically, apigenin reversed the increases in collagen type-I deposition and AGEs formation with age, while preventing most of the age-related degradation of elastin. Collectively, these results argue that apigenin supplementation reduces age-related aortic wall stiffening at least, in part, by ameliorating adverse structural changes. However, future pharmacokinetic studies are needed to determine the optimal time window during which oral supplementation with apigenin can elicit the observed improvements in arterial function.
Conclusions
In summary, our results provide the first evidence that apigenin supplementation reverses two clinically important indicators of arterial dysfunction with age, namely, vascular endothelial dysfunction and large elastic artery stiffening, and prevents foam cell formation in an established cell culture model of the initial phase of atherosclerotic plaque development. Moreover, our results provide insight into the biological mechanisms of apigenin action, including increased NO bioavailability, normalization of age-related increases in arterial ROS production, oxidative stress, oxidant and antioxidant enzyme expression, reversal of age-associated aortic intrinsic mechanical wall stiffening and adverse remodeling of the extracellular matrix, and suppression of vascular inflammation. Given that apigenin is commercially available as a dietary supplement in humans, these preclinical findings provide the experimental basis for future translational studies assessing the potential of apigenin to treat arterial dysfunction during aging and to potentially reduce CVD risk in humans.
GRANTS
This work was supported by National Institutes of Health Grants R01-AG055822 (to D.R.S., J.C., and S.M.), T32-DK007135 (to Z.S.C.), F32-HL151022 (to Z.S.C.), and AG055822-02S1 (to D.R.S. and Z.S.C.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.S.C., M.J.R., J.C., S.M., and D.R.S. conceived and designed research; Z.S.C., D.A.H., V.E.B., N.S.V., B.P.Z., A.G.C., N.T.G., and A.N.M. performed experiments; Z.S.C., D.A.H., B.P.Z., and A.N.M. analyzed data; Z.S.C., D.A.H., V.E.B., B.P.Z., and D.R.S. interpreted results of experiments; Z.S.C. prepared figures; Z.S.C. drafted manuscript; Z.S.C., D.A.H., V.E.B., N.S.V., B.P.Z., A.G.C., N.T.G., M.J.R., J.C., S.M., and D.R.S. edited and revised manuscript; Z.S.C., D.A.H., N.S.V., B.P.Z., A.G.C., N.T.G., M.J.R., J.C., S.M., and D.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Anthony Sun, John Van Hecke, Jill Miyamoto-Ditmon, and Zachary Cook for technical assistance.
REFERENCES
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137: e67–e492, 2018. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 3.Brunt VE, Gioscia-Ryan RA, Richey JJ, Zigler MC, Cuevas LM, Gonzalez A, Vázquez-Baeza Y, Battson ML, Smithson AT, Gilley AD, Ackermann G, Neilson AP, Weir T, Davy KP, Knight R, Seals DR. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J Physiol 597: 2361–2378, 2019. doi: 10.1113/JP277336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Assar M, Angulo J, Vallejo S, Peiró C, Sánchez-Ferrer CF, Rodríguez-Mañas L. . Mechanisms involved in the aging-induced vascular dysfunction. Front Physiol 3: 132, 2012. doi: 10.3389/fphys.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497, 2003. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 7.Tabas I, García-Cardeña G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol 209: 13–22, 2015. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke 34: 1203–1206, 2003. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 9.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55: 1318–1327, 2010. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 10.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 25: 932–943, 2005. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 11.Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11: 269–276, 2012. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M, Seals DR. Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol 48: 269–276, 2013. doi: 10.1016/j.exger.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead Box O phosphorylation. J Gerontol A Biol Sci Med Sci 66: 409–418, 2011. doi: 10.1093/gerona/glq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A, Lucarini M, Santini A, Souto EB, Novellino E, Antolak H, Azzini E, Setzer WN, Martins N. The therapeutic potential of apigenin. Int J Mol Sci 20: 1305, 2019. doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin W, Ren B, Wang S, Liang S, He B, Shi X, Wang L, Liang J, Wu F. Apigenin and naringenin ameliorate PKCβII-associated endothelial dysfunction via regulating ROS/caspase-3 and NO pathway in endothelial cells exposed to high glucose. Vascul Pharmacol 85: 39–49, 2016. doi: 10.1016/j.vph.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Q, Cheng KW, Gong J, Li ETS, Wang M. Apigenin and its methylglyoxal-adduct inhibit advanced glycation end products-induced oxidative stress and inflammation in endothelial cells. Biochem Pharmacol 166: 231–241, 2019. doi: 10.1016/j.bcp.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 17.The guide for the care and use of laboratory animals. ILAR J 57: NP, 2016. doi: 10.1093/ilar/ilw049. [DOI] [Google Scholar]
- 18.Gioscia-Ryan RA, Clayton ZS, Fleenor BS, Eng JS, Johnson LC, Rossman MJ, Zigler MC, Evans TD, Seals DR. Late-life voluntary wheel running reverses age-related aortic stiffness in mice: a translational model for studying mechanisms of exercise-mediated arterial de-stiffening. Geroscience 43: 423–432, 2020. doi: 10.1007/s11357-020-00212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gioscia-Ryan RA, Clayton ZS, Zigler MC, Richey JJ, Cuevas LM, Rossman MJ, Battson ML, Ziemba BP, Hutton DA, VanDongen NS, Seals DR. Lifelong voluntary aerobic exercise prevents age- and Western diet- induced vascular dysfunction, mitochondrial oxidative stress, and inflammation in mice. J Physiol 599: 911–925, 2020. doi: 10.1113/JP280607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunt VE, Gioscia-Ryan RA, Casso AG, VanDongen NS, Ziemba BP, Sapinsley ZJ, Richey JJ, Zigler MC, Neilson AP, Davy KP, Seals DR. Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension 76: 101–112, 2020. doi: 10.1161/HYPERTENSIONAHA.120.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol (1985) 124: 1194–1202, 2018. doi: 10.1152/japplphysiol.00670.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren B, Qin W, Wu F, Wang S, Pan C, Wang L, Zeng B, Ma S, Liang J. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur J Pharmacol 773: 13–23, 2016. doi: 10.1016/j.ejphar.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Li D, Ma J, Wang L, Xin S. Apigenin prevent abdominal aortic aneurysms formation by inhibiting the NF-κB signaling pathway. J Cardiovasc Pharmacol 75: 229–239, 2020. doi: 10.1097/FJC.0000000000000785. [DOI] [PubMed] [Google Scholar]
- 24.Clayton ZS, Brunt VE, Hutton DA, VanDongen NS, D’Alessandro A, Reisz JA, Ziemba BP, Seals DR. Doxorubicin-induced oxidative stress and endothelial dysfunction in conduit arteries is prevented by mitochondrial-specific antioxidant treatment. JACC CardioOncol 2: 475–488, 2020. doi: 10.1016/j.jaccao.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gioscia-Ryan RA, Clayton ZS, Zigler MC, Richey JJ, Cuevas LM, Rossman MJ, Battson ML, Ziemba BP, Hutton DA, VanDongen NS, Seals DR. Lifelong voluntary aerobic exercise prevents age- and Western diet- induced vascular dysfunction, mitochondrial oxidative stress and inflammation in mice. J Physiol 599: 911–925, 2021. doi: 10.1113/JP280607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clayton ZS, Brunt VE, Hutton DA, Casso AG, Ziemba BP, Melov S, Campisi J, Seals DR. Tumor necrosis factor alpha-mediated inflammation and remodeling of the extracellular matrix underlies aortic stiffening induced by the common chemotherapeutic agent doxorubicin. Hypertension 77: 1581–1590, 2021. doi: 10.1161/HYPERTENSIONAHA.120.16759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballak DB, Brunt VE, Sapinsley ZJ, Richey JJ, Zigler MC, Johnson LC, Gioscia-Ryan RA, Culp-Hill R, Eisenmesser EZ, D’Alessandro A, Dinarello CA, Seals DR. Short-term interleukin-37 treatment improves vascular endothelial function, endurance exercise capacity, and whole-body glucose metabolism in old mice. Aging Cell. Nov 19: e13074, 2019. doi: 10.1111/acel.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15: 522–530, 2016. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol 592: 2549–2561, 2014. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol Cell Physiol 271: C1424–C1437, 1996. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 32.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10: 429–437, 2011. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lesniewski LA, Seals DR, Walker AE, Henson GD, Blimline MW, Trott DW, Bosshardt GC, LaRocca TJ, Lawson BR, Zigler MC, Donato AJ. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell 16: 17–26, 2017. doi: 10.1111/acel.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181: 1129–1139, 2008. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587: 3271–3285, 2009. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cahill PA, Redmond EM. Vascular endothelium - gatekeeper of vessel health. Atherosclerosis. 248: 97–109, 2016. doi: 10.1016/j.atherosclerosis.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi H, Mao X, Zhong Y, Liu Y, Zhao X, Yu K, Zhu R, Wei Y, Zhu J, Sun H, Mao Y, Zeng Q. Lanatoside C promotes foam cell formation and atherosclerosis. Sci Rep 6: 20154, 2016. doi: 10.1038/srep20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu S, Huang Y, Xie Y, Lan T, Le K, Chen J, Chen S, Gao S, Xu X, Shen X, Huang H, Liu P. Evaluation of foam cell formation in cultured macrophages: an improved method with Oil Red O staining and DiI-oxLDL uptake. Cytotechnology 62: 473–481, 2010. doi: 10.1007/s10616-010-9290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding SM, Zhang ZH, Song J, Cheng XD, Jiang J, Jia XB. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int J Nanomedicine 9: 2327–2333, 2014. doi: 10.2147/IJN.S60938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao J, Zhang Y, Chen W, Zhao X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br J Nutr 103: 249–255, 2010. doi: 10.1017/S000711450999170X. [DOI] [PubMed] [Google Scholar]
- 41.Seals DR, Edward F. Adolph distinguished lecture: the remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol (1985) 117: 425–439, 2014. doi: 10.1152/japplphysiol.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butlin M, Tan I, Spronck B, Avolio AP. Measuring arterial stiffness in animal experimental studies. Arterioscler Thromb Vasc Biol 40: 1068–1077, 2020. doi: 10.1161/ATVBAHA.119.313861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res 5: 264–273, 2012. doi: 10.1007/s12265-012-9349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol 590: 3305–3316, 2012. doi: 10.1113/jphysiol.2012.229690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaRocca TJ, Hearon CM, Henson GD, Seals DR. Mitochondrial quality control and age-associated arterial stiffening. Exp Gerontol 58: 78–82, 2014. doi: 10.1016/j.exger.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LaRocca TJ, Gioscia-Ryan RA, Hearon CM, Seals DR. The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev 134: 314–320, 2013. doi: 10.1016/j.mad.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reddy AK, Li YH, Pham TT, Ochoa LN, Trevino MT, Hartley CJ, Michael LH, Entman ML, Taffet GE. Measurement of aortic input impedance in mice: effects of age on aortic stiffness. Am J Physiol Heart Circ Physiol 285: H1464–H1470, 2003. doi: 10.1152/ajpheart.00004.2003. [DOI] [PubMed] [Google Scholar]
- 48.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A; Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 111: 3384–3390, 2005. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 49.Fleenor BS. Large elastic artery stiffness with aging: novel translational mechanisms and interventions. Aging Dis 4: 76–83, 2013. [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121: 505–511, 2010. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell GF. Arterial stiffness and hypertension. Hypertension . 64: 13–18, 2014. doi: 10.1161/HYPERTENSIONAHA.114.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 21: 3–12, 2003. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]