Abstract
Black Americans have an earlier onset, higher average blood pressure, and higher rates of hypertension-related mortality and morbidity, compared to whites. The racial difference may be related to microvasculature, the major regulatory site of blood pressure. The goal of this study was to compare the response of resistance vessels to high intraluminal pressure between black and white participants. A total of 38 vessels were obtained from human fat samples [21 black, 17 white; mean age 32 ± 12 yr and body mass index (BMI) 26.9 ± 4.9; between-group P ≥ 0.05] and included in this study. Internal diameter was measured in response to the flow induced by various pressure gradients (Δ10, Δ20, Δ40, Δ60, and Δ100 cmH2O), and flow-induced dilation (FID) was calculated before and after high intraluminal pressure (150 cmH2O). Before high intraluminal pressure, FID was not different between blacks and whites (P = 0.112). After exposure to high intraluminal pressure, FID was reduced at every pressure gradient in vessels from blacks (P < 0.001), whereas FID did not change in white participants except at Δ100 cmH2O. When incubated with the hydrogen peroxide (H2O2) scavenger polyethylene glycol-catalase (PEG-catalase), the FID response in vessels from black, but not white, individuals was significantly reduced and the magnitude was higher at normal pressure relative to high pressure. Our findings suggest that the vessels from self-identified black individuals are more susceptible to microvascular dysfunction following transient periods of high intraluminal pressure compared to whites and show greater dependence on H2O2 as a main contributor to FID at normal pressures.
NEW & NOTEWORTHY Microvascular function regulates blood pressure and may contribute to racial differences in the incidence and prevalence of hypertension and other cardiovascular diseases. Here, we show that using an ex vivo model of resistance arterioles isolated from human gluteal fat tissue, flow-induced dilation is not different between black and white participants. However, when exposed to transient increases in intraluminal pressure, the flow-induced dilation in resistance arterioles from black participants demonstrated greater reductions relative to their white counterparts, indicating a higher sensitivity to pressure change in the microvasculature.
Keywords: endothelium, hypertension, microcirculation, racial differences, resistance vessels
INTRODUCTION
Racial differences in the incidence and prevalence of hypertension (HTN) have been widely reported, with rates being much higher in blacks compared to whites (1, 2). It has also been demonstrated that blacks have exaggerated blood pressure (BP) responses to exercise compared to whites (3). Multiple mechanistic factors have been suggested to explain this disparity, including sympathetic overactivity (4), oxidative stress (5), arterial stiffness (6), and impaired endothelial function (7–9). Endothelial function is an essential component of healthy arterial function, and measures of endothelial function are important prognostic indicators of future disease, including HTN (10–12). The microcirculation is responsible for total peripheral resistance and plays an important role in regulating BP. Dysfunctional endothelium may limit the ability of the microvasculature to respond during exercise and other physiological stressors, such as a transient increase in pressure (13–15), leading to the increases in the total peripheral resistance and eventually elevations in BP and the development of HTN.
The ex vivo model of the isolated resistance arterioles has been developed to investigate endothelial function in the microvasculature without being confounded by other systemic neurohormonal factors. In the resistance arterioles isolated from the human fat tissue, studies have shown that endothelial function, measured as flow-induced dilation (FID), was reduced following a transient (16) and long-term increase in pressure (17). The primary mediator of FID in human resistance arterioles is nitric oxide (NO), and reduced bioavailability of NO contributes to a reduction in FID (18). To preserve or maintain FID following the high pressure related to reduced bioavailability of NO, a unique compensatory mechanism in the microvasculature may take place and FID becomes hydrogen peroxide (H2O2) dependent (13, 16). However, it remains unclear whether there are any racial differences in arteriolar FID, FID response following high pressure, and vasodilatory mechanisms.
As such, using the ex vivo model of resistance arterioles isolated from the human fat tissue, the purpose of this study was to determine if any baseline differences in FID are present between black and white individuals as well as to determine FID responses following exposure to high intraluminal pressure between the two groups. We hypothesized that resistance vessels obtained from black participants would show reduced FID compared to their white counterparts at rest (normal pressure) and after exposure to high intraluminal pressure. We also hypothesized that FID would be H2O2 dependent in blacks compared to whites. This information will allow for enhanced understanding of endothelial function in the microvasculature and how the microcirculation responds to elevated pressure in both racial groups.
METHODS
Study Participants
Participants aged between 18 and 55 yr, self-identified as either black or white, and sedentary to moderately physically active were recruited. Exclusion criteria included a history of cardiovascular disease, diabetes mellitus, thyroid dysfunction, cancer, alcohol or illicit drug abuse, pregnancy, tobacco use in the past 6 mo, and regular exercise training (defined as ≥3 days of moderate- or high-intensity exercise per week). The study protocol was approved by the Institutional Review Board at the University of Illinois at Chicago. All participants provided written informed consent before participation.
Study Procedures
Participants were asked to fast for 10–12 h before the study visit and refrain from caffeine and physical exercise for 24 h before the visit. After a rest period of 10 min, seated systolic BP (SBP) and diastolic BP (DBP) were measured twice using an automated oscillometric device, and then the average of the two readings (1 min apart) was calculated. If the BP readings varied by >5 mmHg, a third reading was collected, and the average of the closest two measurements was used. Anthropometric measurements collected included height, weight, and waist measurements. Plasma samples were collected for the measurement of total cholesterol, triglycerides, HDL-C, LDL-C, and glucose levels. Physical activity was assessed using the international physical activity questionnaire (IPAQ) (19). In a subgroup of participants (15 black and 12 white), peak oxygen consumption (V̇o2peak), a measure of cardiorespiratory fitness, was assessed using maximal graded cardiopulmonary treadmill exercise tests (Parvo Medics True One 2400, Sandy, UT) (20). Test speed was individualized to a comfortable fast-walking pace during the warm-up, followed by grade increments of 2% every 2 min.
Resistance Artery Flow-Induced Dilation
As previously described, measurements of FID were performed in resistance arterioles obtained from the human fat tissues (15, 21). All participants underwent an anesthetized subcutaneous gluteal fat biopsy. Approximately 1 mL of fat tissue was removed and placed in (4°C) HEPES buffer solution. Resistance arterioles were then dissected from the extracted tissue and cleaned of fat and connective tissue. Vessels were cannulated to glass micropipettes with internal diameters of 30–50 mm in an organ chamber filled with Krebs solution, on the stage of an inverted video microscope. Both ends were secured with surgical ties on the micropipette, connected to a Krebs-filled reservoir. Vessels were continuously perfused at 40 mL/min (MasterFlex pump, Cole Parmer). The organ chamber was aerated with a gas mixture of 21% O2, 5% CO2, and N2 balanced and maintained at 37°C using a Langerdorff perfusion system connected to a thermostat (PC200, Thermo Scientific, NC). Internal diameters of the vessel were measured using a real-time video-measuring device (Boeckeler; model VIA-100).
Before the FID measurements, vessels were stabilized at an intraluminal pressure of 60 cmH2O (∼45 mmHg) for 30 min. Vessels were then preconstricted by 40%–60% with endothelin-1 (ET-1; final concentrations of 100–200 pmoL) and exposed to the flow generated by a series of pressure gradients (Δ10, Δ20, Δ40, Δ60, and Δ100 cmH2O). Pressure gradients were created by moving the reservoirs in equal but opposite directions. Internal diameters were measured before preconstriction (baseline), after preconstriction, and after 3 min of exposure to each pressure gradient. Maximal vessel diameter was determined with papaverine (100 mM) at the end of each protocol. FID was calculated as the percent change from the ET-1-constricted diameter relative to baseline diameter preconstruction. To induce high intraluminal pressure, both reservoirs were raised to 150 cmH2O (∼110 mmHg pressure) for 45 min and then brought down to 60 cmH2O for 15 min for re-equilibration and flow experiments were repeated as above (13, 21, 22). For a number of vessels, FID measurement was repeated in the presence of 1) nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 100 mM) to determine the contribution of nitric oxide (NO) to FID and 2) H2O2 scavenger polyethylene glycol-catalase (PEG-catalase; 500 U/mL) to determine the contribution of H2O2 on FID.
Statistics
All data are expressed as means ± SD unless otherwise stated. SPSS statistical software, version 26.0 (SPSS Inc.), was used for all analyses. To compare participant characteristics between two groups, continuous variables were analyzed by using independent t tests whereas categorical data were analyzed using χ2 tests. When comparing differences in FID between black and white participants, a mixed-model ANOVA for group and time effects was used, where group was the fixed variable (race) and time was the repeated measure (pressure gradient). To test for differences in FID within a racial group in regard to different experimental conditions (e.g., normal pressure vs. high pressure), a two-way repeated measures ANOVA followed by pairwise comparisons using Bonferroni’s adjustment (treatment × time) was conducted. Significant interactions were followed up using simple main effects. The α was set at <0.05.
RESULTS
Participant Characteristics
Three participants were excluded due to nonresponding resistance arterioles, defined as failure to achieve at least 40% vasoconstriction induced by ET-1. Final analysis included 38 participants [21 black (55.3%), 17 white (44.7%)]. Participants had similar age, body mass index, BP, lipid profile, and glucose (Table 1; P ≥ 0.05). Activity levels were not different between groups (black: 2,466 ± 1,066 MET-min/wk; white: 2,489 ± 868 MET-min/wk; P = 0.972). In a subgroup of participants (15 blacks, 12 whites), V̇o2peak was not different between groups (black: 31.3 ± 9.9 mLO2·kg−1·min−1; white: 38.9 ± 12.9 mLO2·kg−1·min−1; P = 0.098).
Table 1.
Participant characteristics
| Variable | Blacks | Whites | P Value |
|---|---|---|---|
| n | 21 | 17 | |
| Age, yr | 33 ± 12 | 30 ± 12 | 0.5 |
| Sex, men/women | 12/9 | 11/6 | 0.18 |
| Height, cm | 170 ± 10 | 171 ± 10 | 0.79 |
| Weight, kg | 78.3 ± 13.1 | 78.8 ± 20.4 | 0.931 |
| BMI, kg/m2 | 27.2 ± 4.9 | 26.6 ± 5.0 | 0.669 |
| Waist circumference, cm | 88.7 ± 14.1 | 87.3 ± 16.6 | 0.776 |
| SBP, mmHg | 118 ± 10 | 118 ± 7 | 0.982 |
| DBP, mmHg | 74 ± 9 | 70 ± 10 | 0.208 |
| Pulse pressure, mmHg | 44 ± 8 | 48 ± 7 | 0.113 |
| Total cholesterol, mg/dL | 163 ± 31 | 169 ± 41 | 0.583 |
| HDL-C, mg/dL | 54 ± 14 | 58 ± 12 | 0.402 |
| LDL-C, mg/dL | 92 ± 28 | 89 ± 35 | 0.712 |
| Triglycerides, mg/dL | 80 ± 62 | 111 ± 92 | 0.213 |
| Glucose, mg/dL | 96 ± 9 | 93 ± 8 | 0.465 |
Values are means ± SD along with results of independent t tests or χ2 test when applicable. BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
FID before High Intraluminal Pressure in Black versus White Participants
There was no significant interaction between racial groups and pressure gradient on FID (Fig. 1A), indicating no differences in FID before high intraluminal pressure between groups. Incubation of l-NAME significantly reduced FID in both black (P = 0.044) and white participant groups (P < 0.001) (Fig. 1, B and C). Incubation with PEG-catalase significantly reduced FID at every pressure gradient in black participants (P = 0.001; Fig. 1D), but it did not have effects on FID in white participants (Fig. 1E).
Figure 1.
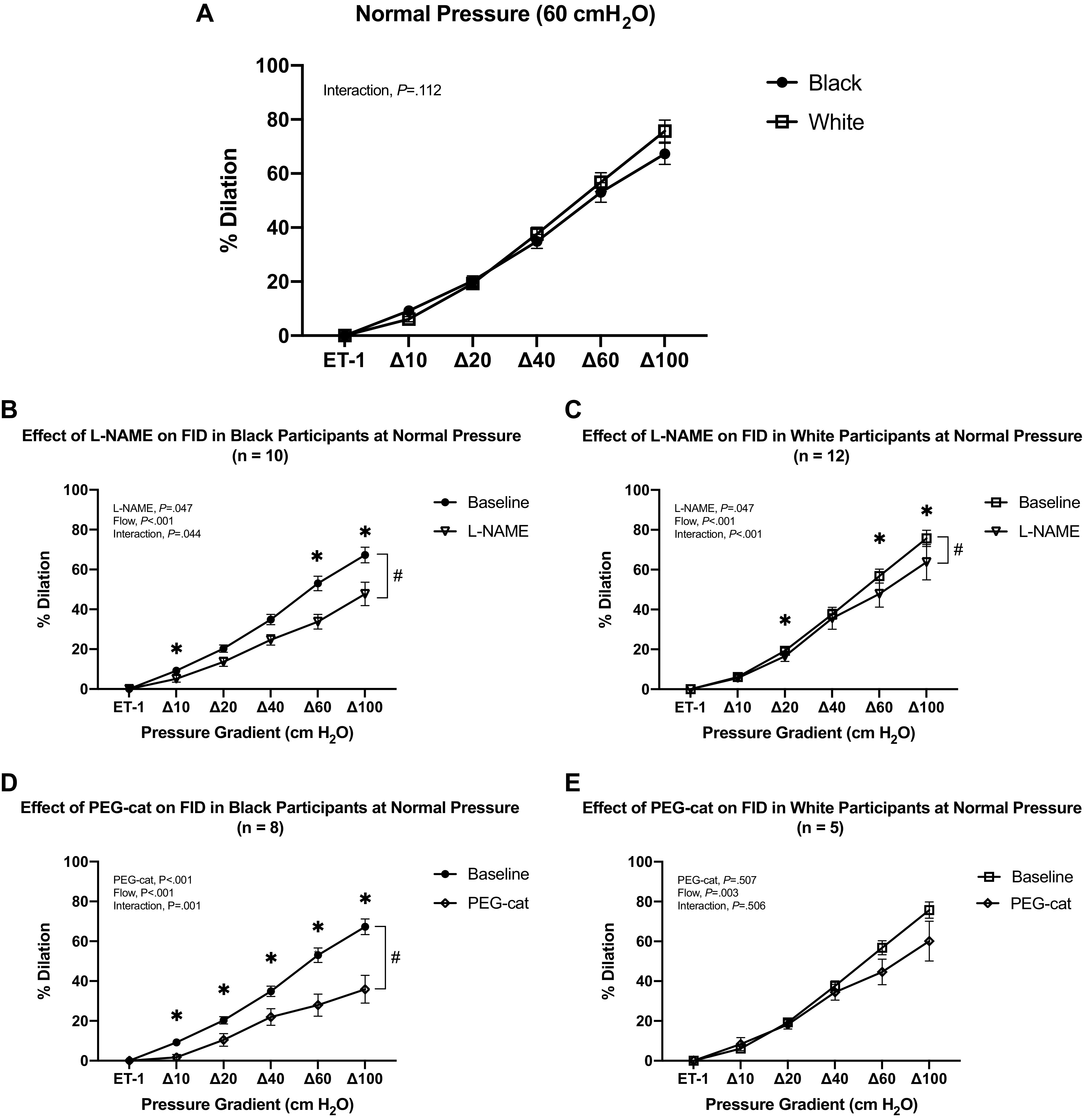
Resistance vessel FID responses between black and white participants at normal intraluminal pressure (60 cmH2O). A: comparing FID responses in vessels from black and white participants at normal intraluminal pressure (60 cmH2O). B: comparing FID responses in resistance vessels from black participants before and after incubation with l-NAME at normal intraluminal pressure (60 cmH2O) (*, #P < 0.05). C: comparing FID responses in resistance vessels from white participants before and after incubation with l-NAME at normal intraluminal pressure (60 cmH2O) (*, #P < 0.05). D: comparing FID responses in resistance vessels from black participants before and after incubation with PEG-cat at normal intraluminal pressure (60 cmH2O) (*, #P < 0.05). E: comparing FID responses in resistance vessels from white participants before and after incubation with PEG-cat at normal intraluminal pressure (60 cmH2O). Data are means ± SE. FID, flow-induced dilation; l-NAME, NG-nitro-l-arginine methyl ester; PEG-cat, polyethylene glycol-catalase.
Effects of High Intraluminal Pressure on FID in Black versus White Participants
After exposure to high pressure (150 cmH2O), FID was significantly reduced at every pressure gradient in black participants (P = 0.002 at Δ10 cmH2O and < 0.001 at Δ20, 40, 60, and 100 cmH2O; Fig. 2A), whereas in white participants, FID was reduced only at Δ100 cmH2O pressure gradient (P = 0.004; Fig. 2B). Compared to white participants, FID following high intraluminal pressure was lower at Δ20, Δ40, Δ60, and Δ100 cmH2O pressure gradient in black participants (P = 0.046, 0.03, 0.01, and 0.013, respectively; Fig. 3A). In both black and white participants, l-NAME incubation did not have any significant effects on FID following high intraluminal pressure (Fig. 3, B and C). Incubation with PEG-catalase further reduced overall FID following high intraluminal pressure in the black participant group (Fig. 3D), whereas it did not have effects in the white participant group (Fig. 3E). Figure 4 represents change in percent dilation at maximal pressure gradient (Δ100 cmH2O) with and without inhibitors at normal and high intraluminal pressures between groups. The reduction with PEG-catalase in FID was more pronounced at normal pressure relative to the high pressure in blacks (Fig. 4, C and D).
Figure 2.
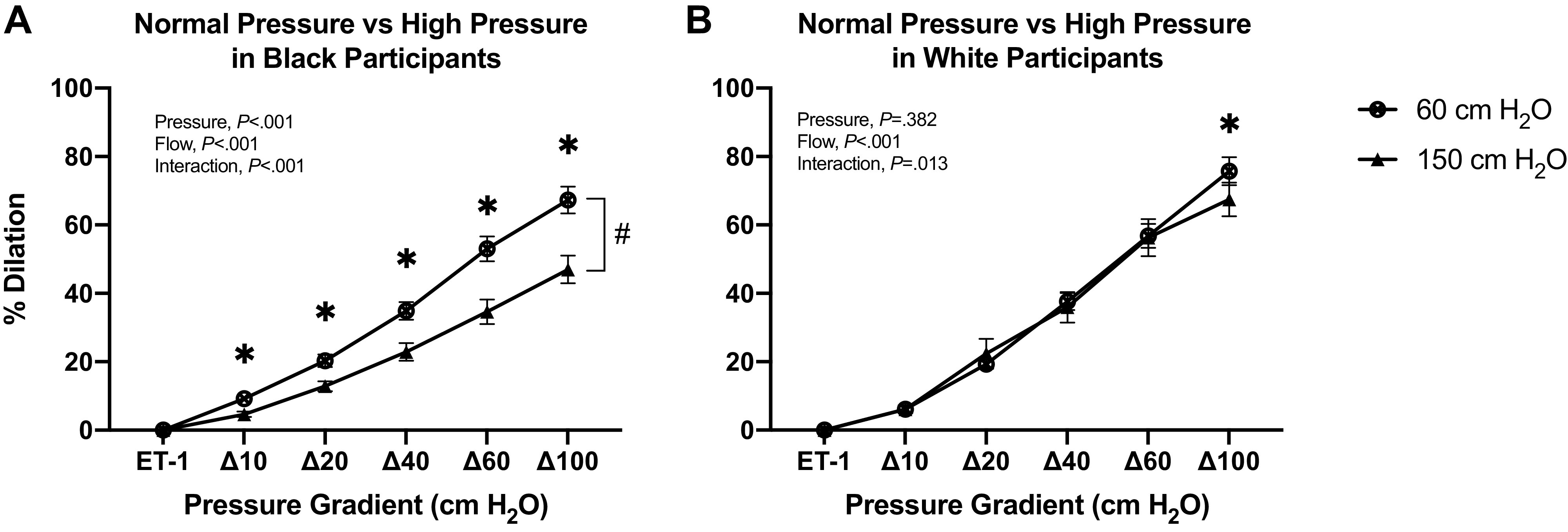
Resistance vessel FID responses at normal pressure (60 cmH2O) and after exposure to high intraluminal pressure (150 cmH2O). A: comparing FID responses in resistance vessels from black participants before and after exposure to high intraluminal pressure (60 vs. 150 cmH2O; *, #P < 0.05). B: comparing FID responses in resistance vessels from white participants before and after exposure to high intraluminal pressure (60 vs. 150 cmH2O; *P < 0.05). Data are means ± SE. ET-1, endothelin-1; FID, flow-induced dilation.
Figure 3.
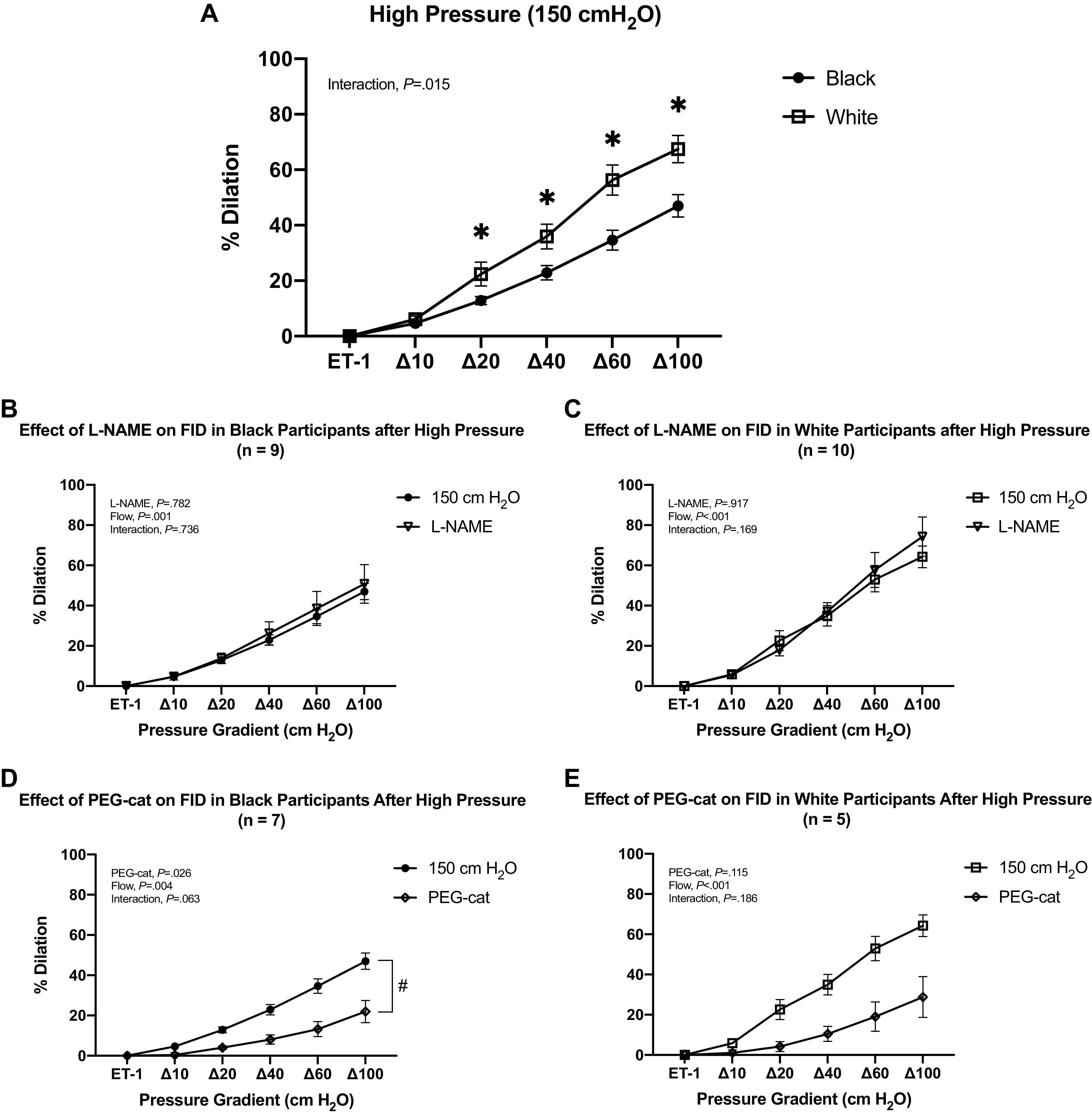
Resistance vessel FID responses between black and white participants at high intraluminal pressure (150 cmH2O). A: comparing FID responses in vessels from black and white participants after 45-min exposure to high intraluminal pressure (150 cmH2O; *P < 0.05). B: comparing FID responses in resistance vessels from black participants before and after incubation with l-NAME after exposure to high intraluminal pressure (150 cmH2O). C: comparing FID responses in resistance vessels from white participants before and after incubation with l-NAME after exposure to high intraluminal pressure (150 cmH2O). D: comparing FID responses in resistance vessels from black participants before and after incubation with PEG-cat after exposure to high intraluminal pressure (150 cmH2O; #P < 0.05). E: comparing FID responses in resistance vessels from white participants before and after incubation with PEG-cat after exposure to high intraluminal pressure (150 cmH2O). Data are means ± SE. ET-1, endothelin-1; FID, flow-induced dilation; l-NAME, NG-nitro-l-arginine methyl ester; PEG-cat, polyethylene glycol-catalase.
Figure 4.
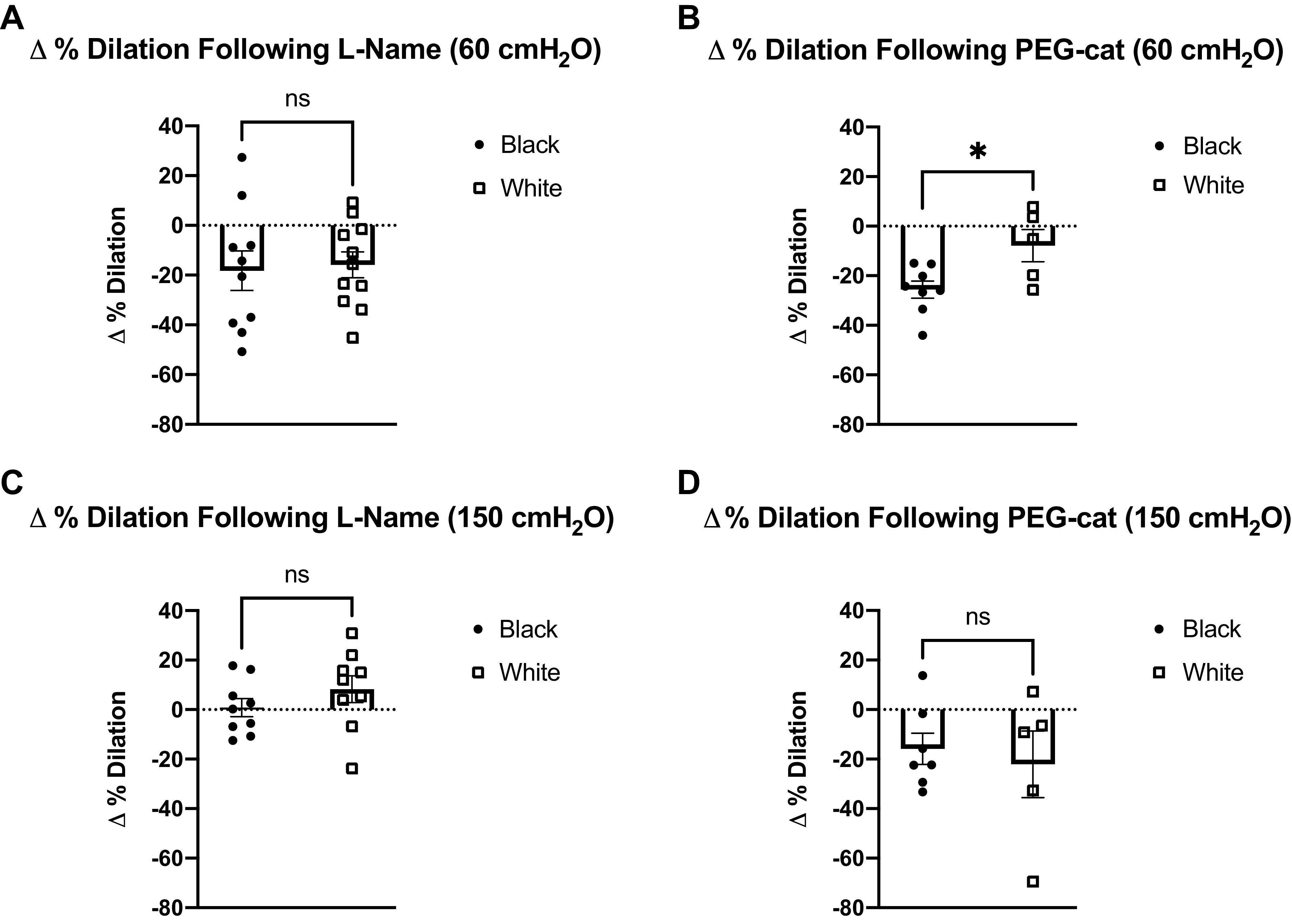
Change in percent dilation at maximal pressure gradient (Δ100 cmH2O) with and without inhibitors at normal and high intraluminal pressures (% dilation without inhibitor at Δ100 cmH2O − % dilation with inhibitor at Δ100 cmH2O). A: Δ% dilation before and after incubation with l-NAME at normal intraluminal pressure (60 cmH2O; ns, not significant). B: Δ% dilation before and after incubation with l-NAME at high intraluminal pressure (150 cmH2O; ns, not significant). C: Δ% dilation before and after incubation with PEG-cat at normal intraluminal pressure (60 cmH2O; *P < 0.05). D: Δ% dilation before and after incubation with PEG-cat at high intraluminal pressure (150 cmH2O; ns, not significant). Data are means ± SE. l-NAME, NG-nitro-l-arginine methyl ester; ns, not significant; PEG-cat, polyethylene glycol-catalase.
Baseline Diameter and Endothelium-Independent Vasodilation
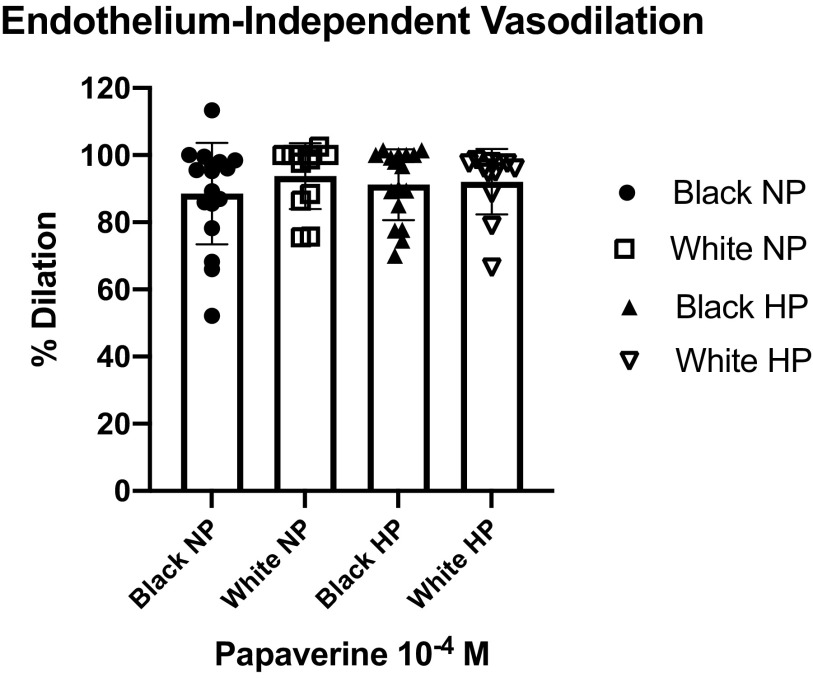
There were significant between-group differences in baseline internal diameter before and following high pressure, with black participants having lower mean diameters compared to whites (before: 97.6 ± 48.4 vs. 151.7 ± 75.3 µm, P = 0.023; following high pressure: 97.3 ± 50 vs. 152.6 ± 76.8 µm, P = 0.013). Considering vasodilation in responses to papaverine, there were no statistically significant differences between blacks and whites before (91.2 ± 15% vs. 95.5 ± 9.5%, P = 0.311) and after high intraluminal pressure (92.5 ± 12.5% vs. 90.1 ± 11.2%, P = 0.546). Additionally, high intraluminal pressure did not influence the vasodilation response to papaverine in all participants (see appendix).
DISCUSSION
This is the first study, using an ex vivo model of human resistance arterioles, to examine whether there are racial differences in microvascular function and the response to high intraluminal pressure in asymptomatic individuals without overt diseases. The main findings are 1) before high intraluminal pressure, arteriolar FID was not different between black and white participants, and 2) after the 45-min exposure to high intraluminal pressure (150 cmH2O or 110 mmHg), arteriolar FID in black participants was reduced, whereas vessels from white participants appeared to be protected from these adverse effects. These findings suggest that the response of resistance arterioles after acute exposures to high intraluminal pressure is different between black and white individuals. Furthermore, this difference may be related to a maintained H2O2-dependent vasodilatory mechanism in black participants as evident by significant reductions in FID with a H2O2 scavenger, PEG-catalase, both before and after high intraluminal pressure.
Compared to whites, blacks are at higher risk of developing cardiovascular diseases, including HTN (1, 2). The development of HTN is associated with reduced microvascular endothelial function (18), and patients with HTN also demonstrate decreased FID due to reduced bioavailability of NO (17), the potent vasodilator. To preserve or maintain FID in the state of reduced bioavailability of NO, a unique compensatory mechanism in the microvasculature may take place and FID becomes H2O2 dependent (13). Before high intraluminal pressure, our results show that FID was similar between black and white participants, and FID in both groups was reduced by the eNOS inhibitor, l-NAME, suggesting that FID is NO dependent. However, following the incubation with H2O2 scavenger, PEG-catalase, FID was reduced only in black participants. This H2O2-dependent vasodilatory mechanism appears not to be induced by age-related pathology and is rather specific to coronary artery disease (23, 24), related to flow-induced release of superoxide from the mitochondria (25, 26). Although our black participants had relatively normal BP (118 ± 10 mmHg) and were asymptomatic, and a younger cohort without any overt diseases, H2O2-dependent vasodilation was observed in blacks even at rest. Although the clinical relevance of this result is not readily clear, this finding may suggest a higher risk of cardiovascular diseases in black individuals before the development of cardiovascular risk factors. In addition, the result may suggest that the compensatory dilator H2O2 mechanism to flow is available in blacks before it is available in whites.
The reduction in microvascular function after a transient increase in BP has been reported in vivo (27) and ex vivo as evident by reductions in FID after exposure to high intraluminal pressure (13, 15, 21, 28). In this current study, after the 45-min exposure to high intraluminal pressure, ex vivo arteriolar FID in black participants was reduced whereas FID in white participants did not change overall. These findings suggest that the response of resistance arterioles after acute exposures to high intraluminal pressure is different between black and white individuals, with microvascular function in blacks being more sensitive to pressure change. Although the ex vivo model of isolated arterioles was used, our findings may provide clinical implications to situations of exercise training and exercise-based rehabilitation. The effects of transient increases in BP have also been reproduced by using acute exercise, such as weightlifting, and the effect on both acetylcholine-induced vasodilation in isolated arterioles (13) and brachial artery flow-mediated dilation (FMD) was examined (13, 15). Buchanan et al. (29) further demonstrated that high intraluminal pressure is a negative stimulus to endothelial function postacute exercise and protecting vessels from this high pressure can preserve brachial artery FMD. On the other hand, repeated exposure to transient levels of high pressure induced by exercise may lead to positive adaptations in the microvasculature and improve the overall vascular response to high pressure in blacks.
Other factors known to influence microvascular function and response include cardiorespiratory fitness and physical activity levels. Using resistance arterioles isolated from subcutaneous gluteal fat tissues in humans, Durand et al. (13) found that acetylcholine-induced vasodilation was reduced following high intraluminal pressure in resistance arterioles from sedentary individuals, while it maintained in trained individuals. Findings in murine models by Robinson et al. (21) also demonstrated that aerobic exercise training prevented the reductions in FID after exposure to high intraluminal pressure. In the current study, blacks had ∼20% lower V̇o2peak on an average compared to their white counterparts. However, only a subgroup of participants received cardiorespiratory fitness assessment in this study, and we found no statistical differences in V̇o2peak between the two groups even though there was a trend toward reduction. Additionally, self-reported physical activity levels were also similar between groups. The differences in V̇o2peak are thus not believed to be a major contributing factor to the differences in FID responses to high intraluminal pressure between blacks and whites. Future studies should include cardiorespiratory fitness assessments and objective measurements of physical activity (e.g., accelerometer data) to better define the role of cardiorespiratory fitness and physical activity in racial differences in microvascular function.
Our study also found that the incubation with l-NAME did not change FID following high pressure in all participants, indicating that vasodilation was mediated by other non-NO-mediated pathways. A transient increase in BP has been shown to increase sympathetic nervous system activity (30), angiotensin II sensitivity (22), and oxidative stress (28, 31), causing reduced bioavailability of NO and may explain the shift toward non-NO-mediated vasodilation post high pressure. One of the predominant vasodilatory mechanisms following high pressure is via H2O2, which has been reported as a protective vasodilatory mechanism to maintain FID after acute exercise and after increases in high intraluminal pressure in exercise-trained individuals (13, 15). In this study, FID was already H2O2 dependent in black participants before the exposure to high pressure. This may cause an insufficient compensatory mechanism to maintain FID and explain why FID was reduced by high pressure in black participants. Interestingly, although FID did not change following high pressure in white participants, the vasodilation appears to be unrelated to H2O2, as evident by unchanged FID with the incubation of PEG-catalase. Other nonvasodilatory pathways, such as prostacyclin (18, 32, 33), may be contributing to the different vasodilatory mechanisms between blacks and whites.
Neurovascular interaction is other potential mechanism behind the racial differences in microvascular endothelial function and response (22, 30). It has been reported that black individuals tend to have lower activation levels of renin-angiotensin system (RAS) compared to whites, evident by lower levels of plasma renin and reduced response to angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers (2, 34). However, lower renin levels may actually be a result of increased renin-angiotensin system activity and angiotensin II concentrations (2, 35) and were shown to be inversely associated with BP (36). It may be possible that an increased role of tissue renin-angiotensin system in response to high pressure is exaggerated in black individuals relative to their white counterparts thus leading to more pronounced reductions in FID. Vranish et al. (37) demonstrated that healthy young black men had a higher vasoconstriction in response to spontaneous muscle sympathetic nerve activity compared to their white counterparts. The augmented sympathetic vascular transduction in blacks, combined with increase in local tissue RAS activation, may partially explain the significant differences in FID following high pressure compared to whites
Study Limitations
There are a number of limitations to this study. First, our sample size was relatively small, and we do not have sufficient power to investigate any potential sex differences. In addition, due to a small sample size, our findings may be influenced by outliers as observed in the PEG-catalase experiment in white participants (n = 5; Fig. 4D). We isolated arterioles from the subcutaneous adipose tissue of participants. The unique ex vivo model of isolated arterioles allowed us to perform various protocols and dissect mechanisms while minimizing subject participation time and related risks. Although previous studies have shown agreement between in vivo and ex vivo measurements of vascular function (13), future studies should include in vivo measurements of microvascular or macrovascular endothelial function, such as brachial artery FMD, to comprehensively examine racial differences in vascular function. Furthermore, adipokines are secreted by fat tissue and influence vascular health (38) and therefore arterioles isolated from fat tissue may be directly impacted by adipokine profiles. The phenotype of subcutaneous fat tissues can be examined to determine if adipokines mediated our study findings. In addition, the structural property or stiffness of arterioles (39) may influence our study findings, especially at higher pressures. Finally, it is important to note that race is a social construct and the physiological differences between races observed in our study cannot be simply explained by biological or genetic variation but are indeed complicated by multiple socioeconomic and other environmental factors (40–42). These factors include socioeconomic status, access to medical care, cultural and language differences, and others that have all shown to lead to significant health disparities (7, 41, 43). Despite the overall decline in cardiovascular disease mortality, racial differences and disparities still exist, stressing the need for continued research and policy change to address the impact of societal factors on microvascular endothelial function and the responses of the circulation to stressors such as high pressure in black young adults.
Conclusions
In conclusion, our results demonstrate that microvascular endothelial function, assessed by FID, was not different between black and white participants. However, when exposed to transient increases in intraluminal pressure, resistance arterioles from black participants demonstrated greater reductions in FID relative to their white counterparts, indicating a higher sensitivity to pressure changes. We also show that the contribution of NO to FID was significant at normal pressure, but this significance was lost after transient exposure to high intraluminal pressures. Furthermore, vessels from black participants show dependence on H2O2 as a main contributor to FID at baseline, but this mechanism is significantly reduced after exposure to high intraluminal pressure.
GRANTS
This study was supported in part by National Institutes of Health Grants T32-HL-139439 (to A. Sabbahi), K99-AA-028537 (to C.-L. Hwang), R01-HL-130513 (to S. A. Phillips), and UL1-TR-002003.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S. and S.A.P. conceived and designed research; A.S., A.E., and C-L.H. performed experiments; A.S. analyzed data; A.S., C-L.H., and S.A.P. interpreted results of experiments; A.S. prepared figures; A.S. drafted manuscript; A.S., C-L.H., and S.A.P. edited and revised manuscript; A.S., A.E., C-L.H., and S.A.P. approved final version of manuscript.
BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
ACKNOWLEDGMENTS
We acknowledge significant support from Maryann Holtcamp and the rest of the staff at the Center for Clinical and Translational Science.
APPENDIX
Endothelium-independent vasodilation as assessed with papaverine showed no statistically significant differences between blacks and whites both before (91.2 ± 15% vs. 95.5 ± 9.5%, P = 0.311) and after high intraluminal pressure (92.5 ± 12.5% vs. 90.1 ± 11.2%, P = 0.546). Additionally, high intraluminal pressure did not influence the vasodilation response to papaverine in all participants (Fig. A1).
Figure A1.
Endothelium-independent vasodilation assessed by response to papaverine before and after high intraluminal pressure. HP, high pressure; NP, normal pressure. Data are means ± SD.
REFERENCES
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137: e67–e492, 2018[Erratum inCirculation137: e493, 2018]. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Flack JM, Sica DA, Bakris G, Brown AL, Ferdinand KC, Grimm RH, Hall WD, Jones WE, Kountz DS, Lea JP, Nasser S, Nesbitt SD, Saunders E, Scisney-Matlock M, Jamerson KA. Management of high blood pressure in blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 56: 780–800, 2010. doi: 10.1161/HYPERTENSIONAHA.110.152892. [DOI] [PubMed] [Google Scholar]
- 3.Sabbahi A, Arena R, Kaminsky LA, Myers J, Fernhall B, Sundeep C, Phillips SA. Characterization of the blood pressure response during cycle ergometer cardiopulmonary exercise testing in black and white men: data from the Fitness Registry and Importance of Exercise: a national database (FRIEND). J Hum Hypertens, 2020. doi: 10.1038/s41371-020-00411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, Howell-Stampley T, Vongpatanasin W, Victor RG. Overweight and sympathetic overactivity in black Americans. Hypertension 38: 379–383, 2001. doi: 10.1161/01.HYP.38.3.379. [DOI] [PubMed] [Google Scholar]
- 5.Feairheller DL, Park J-Y, Sturgeon KM, Williamson ST, Diaz KM, Veerabhadrappa P, Brown MD. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clin Transl Sci 4: 32–37, 2011. doi: 10.1111/j.1752-8062.2011.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan H, Ranadive SM, Heffernan KS, Lane AD, Kappus RM, Cook MD, Wu P-T, Sun P, Harvey IS, Woods JA, Wilund KR, Fernhall B. Hemodynamic and arterial stiffness differences between African-Americans and Caucasians after maximal exercise. Am J Physiol Heart Circ Physiol 306: H60–H68, 2014. doi: 10.1152/ajpheart.00710.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HAJ, Willis M, Yancy CW; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation 136: e393–e423, 2017. doi: 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 8.Duck MM, Hoffman RP. Impaired endothelial function in healthy African-American adolescents compared with Caucasians. J Pediatr 150: 400–406, 2007. doi: 10.1016/j.jpeds.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vita JA. Nitric oxide and vascular reactivity in African American patients with hypertension. J Card Fail 9: S199–S204, 2003. Discussion S205–S209. doi: 10.1054/S1071-9164(03)00588-8. [DOI] [PubMed] [Google Scholar]
- 10.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 23: 168–175, 2003. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 11.SchäChinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101: 1899–1906, 2000. doi: 10.1161/01.CIR.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 12.Widmer RJ, Lerman A. Endothelial dysfunction and cardiovascular disease. Glob Cardiol Sci Pract 2014: 291–308, 2014. doi: 10.5339/gcsp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durand MJ, Dharmashankar K, Bian J-T, Das E, Vidovich M, Gutterman DD, Phillips SA. Acute exertion elicits a H2O2-dependent vasodilator mechanism in the microvasculature of exercise-trained but not sedentary adults. Hypertension 65: 140–145, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips SA, Das E, Wang J, Pritchard K, Gutterman DD. Resistance and aerobic exercise protects against acute endothelial impairment induced by a single exposure to hypertension during exertion. J Appl Physiol (1985) 110: 1013–1020, 2011. doi: 10.1152/japplphysiol.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson AT, Franklin NC, Norkeviciute E, Bian JT, Babana JC, Szczurek MR, Phillips SA. Improved arterial flow-mediated dilation after exertion involves hydrogen peroxide in overweight and obese adults following aerobic exercise training. J Hypertens 34: 1309–1316, 2016. doi: 10.1097/HJH.0000000000000946. [DOI] [PubMed] [Google Scholar]
- 16.Beyer AM, Durand MJ, Hockenberry J, Gamblin TC, Phillips SA, Gutterman DD. An acute rise in intraluminal pressure shifts the mediator of flow-mediated dilation from nitric oxide to hydrogen peroxide in human arterioles. Am J Physiol Hear Circ Physiol 307: H1587–H1593, 2014. doi: 10.1152/ajpheart.00557.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paniagua OA, Bryant MB, Panza JA. Role of endothelial nitric oxide in shear stress–induced vasodilation of human microvasculature. Circulation 103: 1752–1758, 2001. doi: 10.1161/01.CIR.103.13.1752. [DOI] [PubMed] [Google Scholar]
- 18.Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM. The human microcirculation. Circ Res 118: 157–172, 2016. doi: 10.1161/CIRCRESAHA.115.305364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35: 1381–1395, 2003. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, Gulati M, Madan K, Rhodes J, Thompson PD, Williams MA; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 128: 873–934, 2013. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 21.Robinson AT, Fancher IS, Sudhahar V, Bian JT, Cook MD, Mahmoud AM, Ali MM, Ushio-Fukai M, Brown MD, Fukai T, Phillips SA. Short-term regular aerobic exercise reduces oxidative stress produced by acute in the adipose microvasculature. Am J Physiol Heart Circ Physiol 312: H896–H906, 2017. doi: 10.1152/ajpheart.00684.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durand MJ, Phillips SA, Widlansky ME, Otterson MF, Gutterman DD. The vascular renin-angiotensin system contributes to blunted vasodilation induced by transient high pressure in human adipose microvessels. Am J Physiol Heart Circ Physiol 307: H25–H32, 2014. doi: 10.1152/ajpheart.00055.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allaqaband H, Gutterman DD, Kadlec AO. Physiological consequences of coronary arteriolar dysfunction and its influence on cardiovascular disease. Physiology (Bethesda) 33: 338–347, 2018. doi: 10.1152/physiol.00019.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beyer AM, Zinkevich N, Miller B, Liu Y, Wittenburg AL, Mitchell M, Galdieri R, Sorokin A, Gutterman DD. Transition in the mechanism of flow-mediated dilation with aging and development of coronary artery disease. Basic Res Cardiol 112: 5–12, 2017. doi: 10.1007/s00395-016-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bubolz AH, Mendoza SA, Zheng X, Zinkevich NS, Li R, Gutterman DD, Zhang DX. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. Am J Physiol Heart Circ Physiol 302: H634–H642, 2012. doi: 10.1152/ajpheart.00717.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 27.Millgard J, Lind L. Acute hypertension impairs endothelium-dependent vasodilation. Clin Sci (Lond) 94: 601–607, 1998. doi: 10.1042/cs0940601. [DOI] [PubMed] [Google Scholar]
- 28.Robinson AT, Fancher IS, Mahmoud AM, Phillips SA. Microvascular vasodilator plasticity following acute exercise. Exerc Sport Sci Rev 46: 48–55, 2017. doi: 10.1249/JES.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchanan CE, Kadlec AO, Hoch AZ, Gutterman DD, Durand MJ. Hypertension during weight lifting reduces flow-mediated dilation in nonathletes. Med Sci Sport Exerc 49: 669–675, 2017. doi: 10.1249/MSS.0000000000001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkinson CL, Lewis NCS, Carter HH, Thijssen DHJ, Ainslie PN, Green DJ. Impact of sympathetic nervous system activity on post-exercise flow-mediated dilatation in humans. J Physiol 593: 5145–5156, 2015. doi: 10.1113/JP270946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rush JW, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol 279: H2068–H2076, 2000. doi: 10.1152/ajpheart.2000.279.5.H2068. [DOI] [PubMed] [Google Scholar]
- 32.Mahmoud AM, Hwang CL, Szczurek MR, Bian JT, Ranieri C, Gutterman DD, Phillips SA. Low-fat diet designed for weight loss but not weight maintenance improves nitric oxide-dependent arteriolar vasodilation in obese adults. Nutrients 11: 1339, 2019. doi: 10.3390/nu11061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell JA, Ali F, Bailey L, Moreno L, Harrington LS. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol 93: 141–147, 2008. doi: 10.1113/expphysiol.2007.038588. [DOI] [PubMed] [Google Scholar]
- 34.Spence JD, Rayner BL. Hypertension in blacks individualized therapy based on renin/aldosterone phenotyping. Hypertension 72: 263–269, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11064. [DOI] [PubMed] [Google Scholar]
- 35.Price DA, Fisher NDL. The renin-angiotensin system in blacks: active, passive, or what? Curr Hypertens Rep 5: 225–230, 2003. doi: 10.1007/s11906-003-0025-x. [DOI] [PubMed] [Google Scholar]
- 36.Michel FS, Norton GR, Majane OHI, Badenhorst M, Vengethasamy L, Paiker J, Maseko MJ, Sareli P, Woodiwiss AJ. Contribution of circulating angiotensinogen concentrations to variations in aldosterone and blood pressure in a group of African ancestry depends on salt intake. Hypertension 59: 62–69, 2012. doi: 10.1161/HYPERTENSIONAHA.111.181230. [DOI] [PubMed] [Google Scholar]
- 37.Vranish JR, Holwerda SW, Young BE, Credeur DP, Patik JC, Barbosa TC, Keller DM, Fadel PJ. Exaggerated vasoconstriction to spontaneous bursts of muscle sympathetic nerve activity in healthy young black men. Hypertension 71: 192–198, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ntaios G, Gatselis NK, Makaritsis K, Dalekos GN. Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis 227: 216–221, 2013. doi: 10.1016/j.atherosclerosis.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 39.Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension 36: 312–318, 2000. doi: 10.1161/01.HYP.36.3.312. [DOI] [PubMed] [Google Scholar]
- 40.Gravlee CC. How race becomes biology: embodiment of social inequality. Am J Phys Anthropol 139: 47–57, 2009. doi: 10.1002/ajpa.20983. [DOI] [PubMed] [Google Scholar]
- 41.Johnson A. Understanding why black patients have worse coronary heart disease outcomes: does the answer lie in knowing where patients seek care? J Am Heart Assoc 8: 1–3, 2019. doi: 10.1161/JAHA.119.014706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keita SOY, Kittles RA, Royal CDM, Bonney GE, Furbert-Harris P, Dunston GM, Rotimi CN. Conceptualizing human variation. Nat Genet 36: s17–s20, 2004. doi: 10.1038/ng1455. [DOI] [PubMed] [Google Scholar]
- 43.Havranek EP, Mujahid MS, Barr DA, Blair I V, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW, Rosal M, Yancy CW; American Heart Association Council on Quality of Care and Outcomes Research, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, and Stroke Council. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation 132: 873–898, 2015. doi: 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]