Abstract
The measurement of vascular function in isolated vessels has revealed important insights into the structural, functional, and biomechanical features of the normal and diseased cardiovascular system and has provided a molecular understanding of the cells that constitutes arteries and veins and their interaction. Further, this approach has allowed the discovery of vital pharmacological treatments for cardiovascular diseases. However, the expansion of the vascular physiology field has also brought new concerns over scientific rigor and reproducibility. Therefore, it is appropriate to set guidelines for the best practices of evaluating vascular function in isolated vessels. These guidelines are a comprehensive document detailing the best practices and pitfalls for the assessment of function in large and small arteries and veins. Herein, we bring together experts in the field of vascular physiology with the purpose of developing guidelines for evaluating ex vivo vascular function. By using this document, vascular physiologists will have consistency among methodological approaches, producing more reliable and reproducible results.
Keywords: arteries, contraction, methods, relaxation, veins
INTRODUCTION
Overview of the Consensus Guidelines
Since the publication of the historical book titled, On the Motion of the Heart and Blood in Animals, from William Harvey in 1628 (1), our understanding of the vascular regulation of blood flow and perfusion in the peripheral circulation has grown dramatically. Driven by a search for the truth, Harvey applied the scientific method and deductive logic to show to the world that vessels are functional, connected to tissues, including the lungs, and are essential for the circulation of blood (1). As with many new theories, Harvey's revolutionary idea was received with a great deal of pessimism among his colleagues (2). Personal resentments, professional “territorialism,” religious, mystical, and philosophical arguments were among the several reasons for rejecting the circulation theory. Nonetheless, the controversy was fundamental because it established the use of the scientific method (2).
As modern‐day scientists and clinicians, we are facing a new paradox: an increasing concern about irreproducible scientific results. The lack of guidelines for experimental design, inappropriate or nonrandomization methods, and inconsistencies in methodological approaches between studies contribute to unreliable data. Best practices in common experimental approaches are needed to ensure robust and unbiased methods, analysis, and interpretation of the results. Accordingly, funding agencies, reputable journals, including AJP-Heart and Circulatory Physiology, and scientists have taken substantive steps to improve scientific rigor, reproducibility, and transparency among publications. The Guidelines for Measurement of Vascular Function and Structure in Isolated Arteries and Veins is a comprehensive document detailing best practices and pitfalls for measurement of function and structure in large (conduit) and small (resistance) arteries, and veins. This document brought together experts in the field of vascular physiology with the purpose of developing a consensus guide for ex vivo vascular function. By following these recommendations, scientists will have consistency among methodological approaches, and consequently the results will be reproduced and validated by other vascular physiologists. Therefore, by using these guidelines, researchers will “get rigorous” in their scientific approach (3), collect reliable data, and enhance peer and public perception of science.
GENERAL CONSIDERATIONS AND COMMON CHARACTERISTICS OF STRUCTURE AND FUNCTION OF THE VESSELS
Contraction and Relaxation of Conduit and Resistance Blood Vessels
The vasculature performs multiple roles in the body, including transferring nutrients and mediators to the essential organs, exchanging them across the vascular walls, and blood pressure regulation. Large arteries serve as conduits that carry the oxygenated blood in to the smaller arteries. On the other hand, vessels that, when relaxed, measure <250 μm in lumen diameter, act as the major site of vascular resistance and include a network of resistance arteries (lumen diameter ∼150 to 250 μm) and arterioles (<150 μm). Capillaries are the major sites of exchange across the vascular walls. Capillaries consist of a single layer of endothelial cells and lack the contractile vascular smooth muscle cells (VSMC). In contrast with arteries, veins are exposed to lower intraluminal pressures, carry deoxygenated blood back to the heart, and have valves that prevent backflow. Vasoconstriction is a direct result of contraction of VSMC in the vascular wall. Therefore, contractility and relaxation assays have been used in large arteries, resistance arteries and arterioles, venules and veins to obtain information on: 1) the contractile state of VSMC under basal conditions (i.e., myogenic tone); 2) increase in VSMC contractility in response to a treatment; 3) decrease in VSMC contractility in response to a treatment; and 4) endothelial function. The ultimate functional response is a consequence of VSMC signaling and intercellular communication among VSMC. The latter enables the neighboring VSMC to share small metabolites, ions, and second messengers.
The contractile state of small arteries and arterioles defines vascular resistance. Since arterial pressure is a product of cardiac output and total peripheral resistance, resistance arteries and arterioles are key determinants of blood pressure. Conduit arteries have less influence on blood pressure directly and function to convey blood flow in to the resistance vessels controlling blood flow to downstream organs and tissues. Contractility studies have been performed routinely in conduit arteries. However, the contractility of conduit arteries does not directly alter the vascular resistance. A major functional difference between conduit and resistance arteries is that the resistance arteries display pressure-induced or myogenic constriction, which is an inherent property of VSMC (4). Myogenic constriction is a physiological autoregulatory mechanism that maintains vascular resistance under resting conditions and prevents the buildup of excessive hydrostatic pressure in the capillaries. Structurally, conduit arteries such as the aorta have 10–15 layers of VSMC. In contrast, resistance arteries have one or two VSMC layers. The extracellular matrix composition of the vascular wall also differs between resistance and conduit arteries. Elastin is the main component of the extracellular matrix that allows large arteries (elastic reservoirs) to expand and relax with every cardiac cycle (5). Resistance arteries have more collagen than conduit arteries, and arteries collectively have more collagen and elastin than veins (6).
Endothelial cells line the lumen of all blood vessels, and they release mediators that relax or contract VSMC, including nitric oxide (NO), K+ (7), prostaglandins, and other hyperpolarizing factors. Endothelium-dependent vasodilation relaxes VSMC and lowers vascular resistance whereas endothelium-dependent vasoconstriction increases these parameters. The endothelial cell layer is separated from the surrounding VSMC layer by the internal elastic lamina. In resistance arteries, endothelial cells send projections to the VSMC layer through the internal elastic lamina, called myoendothelial projections (8). Myoendothelial projections electrically connect the endothelial cells and VSMC layers via myoendothelial gap junctions (MEGJs) that facilitate the communication between the two cell layers (9). Endothelial cell hyperpolarization is the predominant mechanism for endothelium-dependent vasodilation of resistance arteries (10). Endothelial cell hyperpolarization is transmitted to VSMC via MEGJs, thereby relaxing VSMC. Myoendothelial projections/junctions are either absent or found at a very low number in conduit arteries. Endothelium-dependent relaxation of conduit arteries occurs predominantly through NO release from endothelial cells and its diffusion to VSMC.
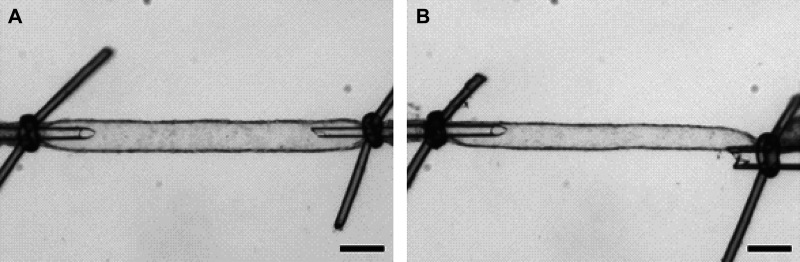
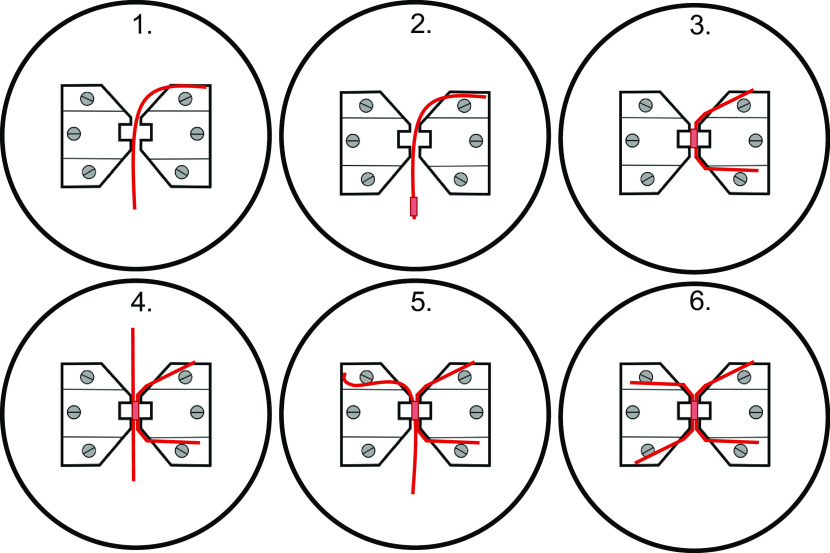
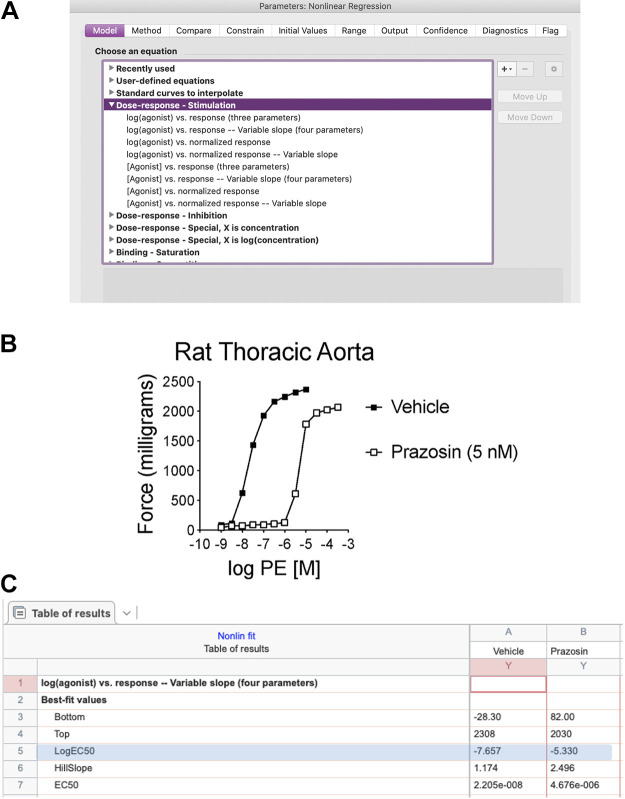
Studying vascular contractility: pressure versus wire myography.
VSMC are effectors and integrators of multiple inputs (pressure, nerves, endothelial cells, vasoactive substances in blood, autacoids). A myograph is any type of device used to measure the force produced by a muscle during contraction due to the different inputs described above. The force generated by the VSMC in the walls of blood vessels is typically studied using pressure, wire, or pin myography (Fig. 1). Pressure myography and wire myography are the two most common ex vivo methods for studying vascular contractility. There is a consensus that pressure myography more closely mimics physiological conditions than wire myography, since vessels exits under pressure in vivo, not stretched between wires. Pressure myography involves cannulation of the blood vessel at one or both ends. The intraluminal pressure is set at a physiologically relevant value through the inflow cannula. It should be noted that distinct vascular beds are exposed to different intraluminal pressures in the body (e.g., 40–60 mmHg in cerebral arteries, 60–80 mmHg in mesenteric resistance arteries, 10–15 mmHg in pulmonary arteries and veins). The ability to manipulate intraluminal pressure enables the studies of the myogenic response with pressure myography. Additionally, pressure myography allows manipulation of intraluminal flow/shear stress, which is a crucial regulator of vascular contractility. Flow/shear stress can be increased by varying the pressure difference between inflow and outflow cannula to yield the appropriate shear (3–20 dyn/cm2) without changing the intraluminal pressure. Alternatively, shear stress can be elevated by increasing the buffer viscosity with dextran (11, 12). For further information about flow, please see section pharmacological and physiological assessment (Flow-Mediated Dilation) below.
Figure 1.
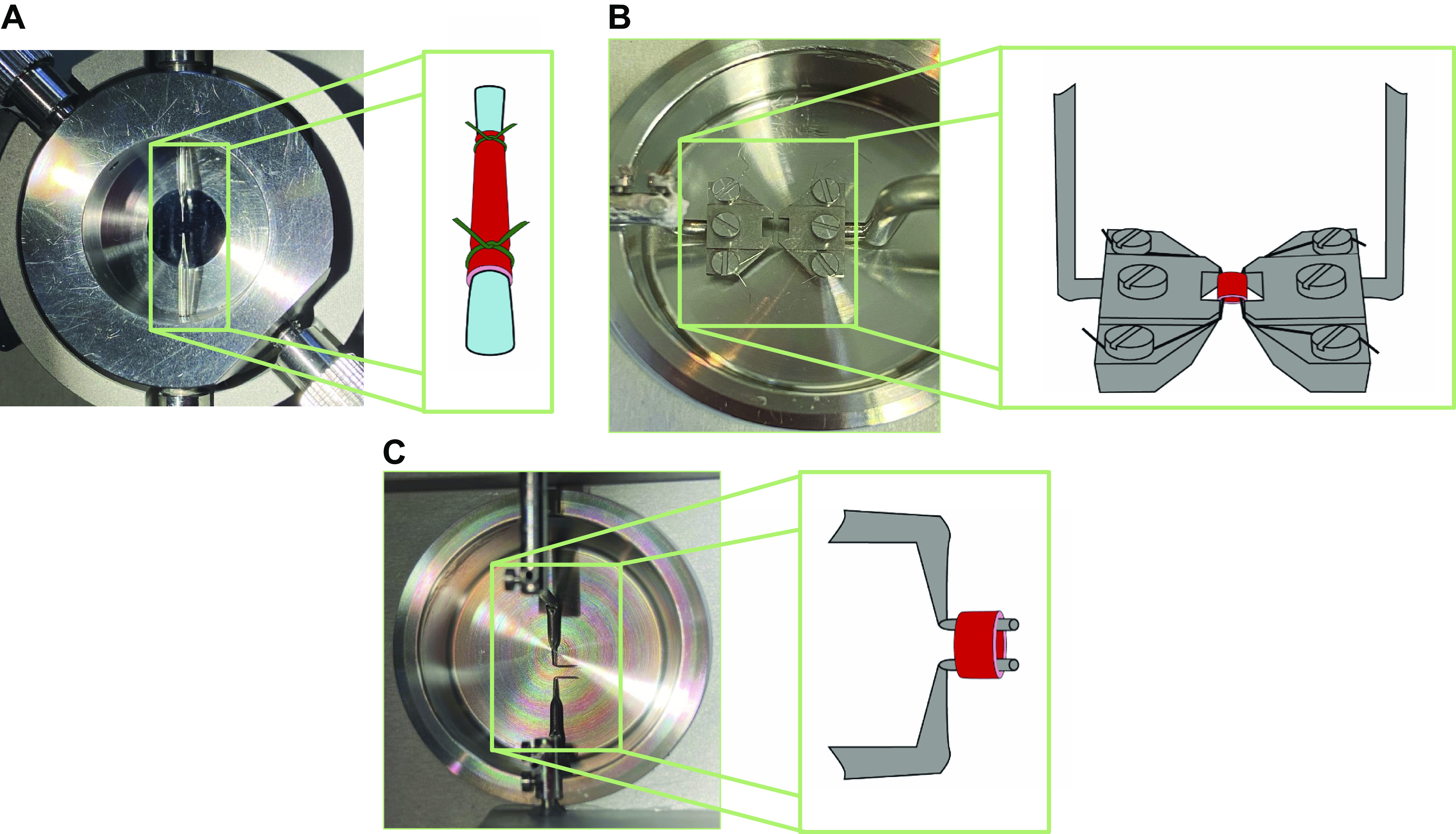
In a pressure myograph system, blood vessel segments are cannulated with glass pipettes (A) to regulate the intraluminal pressure. In isometric force myograph systems, wires (B) or pins (C) are passed through the lumen of a blood vessel and is stretched to level that approximates physiological conditions.
Wire myography involves mounting a blood vessel on fine, steel wires and recording the tension generated by the vascular wall. Because wire myography does not involve pressurizing a blood vessel, flow/shear stress effects cannot be assessed using a wire-myography setup. Although, myogenic response can be assessed using wire myograph, pressure myograph is more reliable and sensitive to evaluate this parameter. In most cases, wire myography experiments require pharmacological constriction of mounted arteries with a physiological or synthetic vasoconstrictor to generate increased tension. The main strength of the method is that we can discern the contribution of different mechanisms to vascular reactivity by studying segments of the same artery and same animal. This is a strong experimental design that can reduce variability. Additionally, wire myography is a good alternative to pressure myography in instances where the blood vessel’s length is insufficient for pressure myography.
Studies suggest that the conclusions based on pressure myography data could be different from those from wire myography data. For example, the concentration-response curves for phenylephrine-induced constriction of mesenteric resistance arteries show higher sensitivity and lower EC50 values in pressure myography than wire myography (13, 14). The use of a pharmacological constrictor versus pressure for generating baseline tension could itself be a source of variability. Pharmacological constrictors may trigger VSMC signaling pathways that are different from intraluminal pressure, thereby altering the functional outcome. In this regard, pharmacological constrictors (e.g., phenylephrine) have been associated with a negative feedback activation of dilatory endothelial pathways that attempt to limit the constriction (15, 16). Moreover, endothelial pathways elicited in pressure myography experiments could differ from those elicited in wire myography experiments (14). In resistance arteries with myogenic response, endothelial cell-derived hyperpolarization plays a more prominent role than NO in vasodilation. On the contrary, in wire myography experiments in the presence of a pharmacological constrictor, NO appears to be the predominant mechanism for endothelium-dependent relaxation. Therefore, the technique used (pressure vs. wire) and the method used for generating baseline constriction (pressure vs. pharmacological constrictor) warrant particular attention when interpreting and comparing data from myography experiments.
Both pressure and wire myography have been used to determine the effect of endothelial and VSMC signaling mechanisms on vascular diameter. The effect of endothelium on VSMC contractility is studied either as endothelium-dependent vasodilation or a decrease in vascular contractility. The endothelium-dependent nature of dilation/relaxation can be confirmed by its absence when the endothelium is denuded. Endothelial denudation can be accomplished by physically disrupting the endothelial cell layer, as described in section mounting and normalization. Acetylcholine and bradykinin are the classical agonists used for eliciting endothelium-dependent vasodilation. Conclusions on VSMC contractility can be drawn by comparing the contractions in response to increased or decreased luminal pressure (pressure myography only), or a vasoconstrictor (phenylephrine, serotonin, thromboxane A2 receptor activation). High concentrations of potassium chloride (KCl, ≥60 mM) have also been used in myography studies either as a pharmacological constrictor and to determine maximal contractility. High concentrations of KCl depolarize VSMC membrane, resulting in the activation of voltage-gated calcium channels and vasoconstriction. The presence of KCl, however, can influence the myography results. High concentrations of extracellular K+ (≥ 60 mM) can prevent endothelial hyperpolarization and a vasodilatory response. Moreover, KCl-induced membrane depolarization can alter dilator or constrictor responses in VSMC. Finally, intraluminal pressure is another important source of variability in pressure myography experiments. Both VSMC and EC responses are altered by changes in intraluminal pressure (17, 18). In this case, the best practice is to use a physiologically relevant pressure for the blood vessel type and size.
Overall, pressure and wire vascular myography techniques have distinct advantages and disadvantages. Pressure myography more closely mimics physiological conditions and allows the relationship between intraluminal pressure and VSMC contractility to be evaluated directly. Wire myography allows better access to the vascular endothelium, and isometric preparation is useful for simultaneous recording of contractile force and VSMC membrane potential with intracellular electrodes because electrodes are not displaced by movement of the tissue.
Important Considerations
Variability in myography results is a significant concern in vascular biology. In this regard, the choice of myography technique appears to have a telling effect on the results and conclusions. As discussed above, the discrepancy in vascular contractility studies could be attributed to one or more of the following variables:
Myography technique (pressure vs. wire)
Vascular bed
Size of the artery (resistance vs. conduit)
Myogenic constriction vs. pharmacological constrictor
Presence or absence of endothelium
Intraluminal pressure used in pressure myography experiments.
Therefore, it is imperative that detailed descriptions of the above parameters accompany the presentation of vascular contractility data. Moreover, conclusions derived from vascular relaxation and contractility studies should always consider the above parameters.
Methodological Consideration before Starting
a. Myographs.
In a pressure myography system, segments of isolated blood vessels are cannulated at one end. More commonly, both ends are mounted and tied onto glass pipettes, and the intraluminal pressure is regulated by controlling the pressure at one of the cannulas (Fig. 2A). The downstream cannula may be closed off to create a “blind sack” preparation or left open for flow-through applications (Fig. 2B). It is important to cannulate branches of a sufficient length (≥ 2–3 mm) to have an undamaged segment in between the two cannulas. However, in some cases, 2–3 mm in length without branching is not possible (e.g., coronaries). Therefore, in this situation, it is important to cannulate vessels of sufficient length (at least 2–3× the luminal diameter) to have an undamaged segment in between the two cannulas. The preparation is typically superfused with physiological saline solution warmed to 37°C and aerated with an appropriate gas mixture to maintain proper oxygen levels and pH. Chemically buffered solutions (i.e., HEPES buffered saline) are occasionally used in particular circumstances (please see Composition of the physiological salt solutions section). Drugs and other substances are usually applied to the preparation in the superfusing bath. Administration of substances to the vascular lumen through one of the cannulas is also feasible but less common, as flow rates through small-diameter vessels are slow. Blood vessel diameter is commonly recorded using video microscopy and specialized software that tracks the relative positions of the inner and/or outer walls. These data can be expressed as inner (luminal) or outer diameter, and wall thickness can be calculated. The contractility of VSMC is often expressed as the active diameter normalized to the passive diameter recorded in a calcium (Ca2+)-free solution to prevent contractile responses. Preparations can be viable for hours (∼6–9 h) after they are cannulated, depending on the vessel type used. Appropriate time control experiments should be performed to ascertain this information.
Figure 2.
Blood vessel segments can be cannulated at both ends for flow-through applications (A), or tied off and secured to the cannula at one end to create a “blind sack” (B).
During wire myography, blood vessel segments are threaded onto a pair of pins, for large arteries, or wires for muscular and resistance-sized arteries (Fig. 1, B and C). The tissue is prestretched or normalized to a level that approximates physiological conditions. The segments are bathed in warmed and aerated physiological saline to maintain appropriate pH and oxygenation levels. Drugs can be applied directly to the organ bath chamber and removed by rapid washes. The mounting wires are connected to force transducers, and isometric force generated by contracting VSMC is directly recorded. Contractility data are most frequently normalized to the force generated when the preparation is exposed to a high concentration (i.e., ≥60 mM) of KCl to directly depolarize the plasma membrane of VSMC. Preparations can be viable for hours (∼6–9 h) after they are mounted, but appropriate time control experiments should be performed to ascertain this information.
b. Dissecting microscopes.
Whether using a wire or a pressure myograph, the successful mounting or cannulation of isolated vessels often requires the use of a dissecting, or stereo microscope, particularly for smaller arteries and arterioles. Most major microscope manufacturers make dissecting microscopes, and the types and features of them are almost limitless. The successful mounting or cannulation of blood vessels does not require expensive add-on features, although they can be very useful, depending on the application. Although some larger blood vessels (e.g., conduit arteries) can be cannulated and mounted without the use of a dissecting microscope, we recommend using dissecting microscopes whenever possible so that the investigator is able to clearly visualize the vessel and prevent damage. The successful mounting/cannulation of resistance arteries and arterioles of the microcirculation (e.g., <250 µm diameter) requires the use of a dissecting microscope with at least ×5 magnification.
For the purposes of isolating blood vessels, we recommend using binocular or trinocular microscopes. Both types of microscope has two eyepieces for viewing the vessels and provide depth perception. A trinocular microscope also has a third eye tube for connecting a camera, which makes it useful for teaching purposes. The trinocular setup on dissecting microscopes is optimal when teaching how to successfully isolate different blood vessels. Some binocular or trinocular microscopes are situated over a weighted base on which tissue dissections can be performed. The use of these types of microscopes are probably okay for larger vessels that require microscopy for cannulation (e.g., aorta or similar sized vessels >500 µm); however, we recommend using dissecting microscopes that are connected to a weighted base via a boom arm (Fig. 3). This allows one to stabilize their hands on the laboratory bench for easier isolation and mounting/cannulation.
Figure 3.
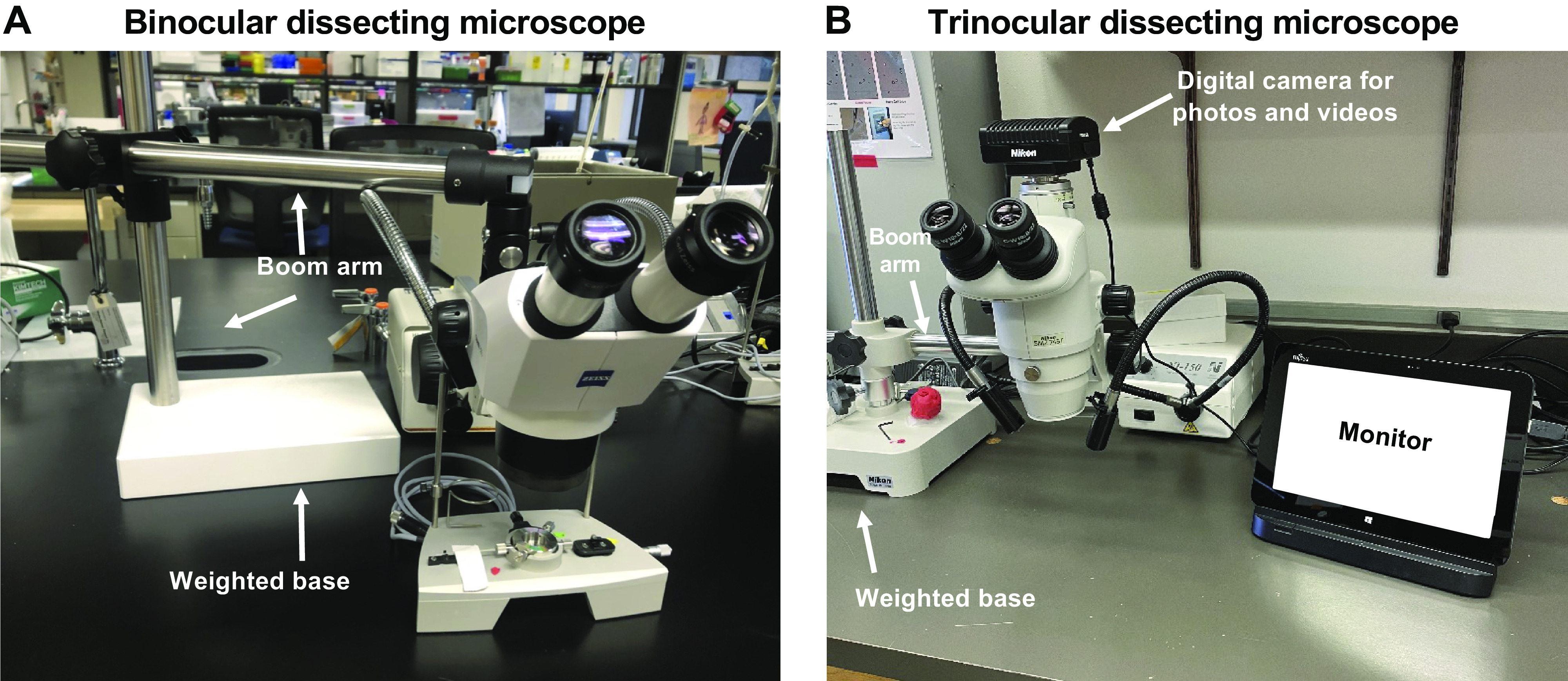
Binocular (A) and a trinocular (B) dissecting microscope connected to a weighted base via a boom arm.
c. Tools.
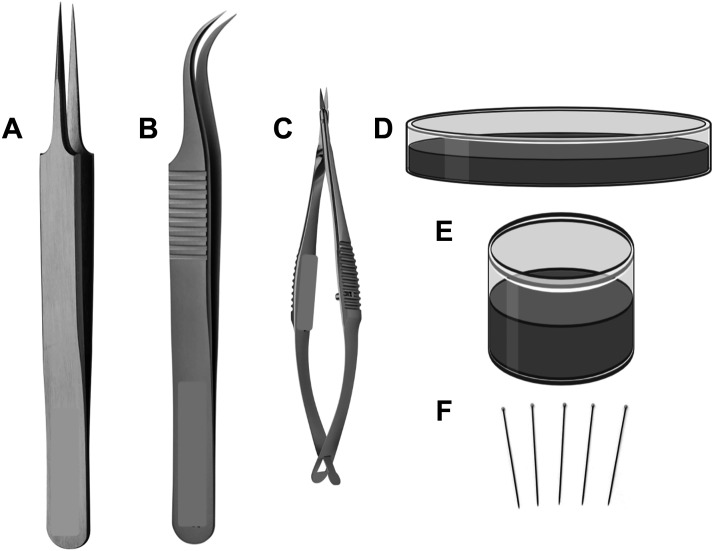
Sharp and well-maintained tools are essential for careful dissection of macro- and microvessels from surrounding adipose and connective tissues before ex vivo experiments. Vannas spring scissors, glass-dissecting dishes with a smooth layer of silicone elastomer (e.g., Sylgard 184) on the bottom, and insect pins (or ∼28 gauge needles) are necessary for vessel dissection and cleaning, whereas forceps with fine tips are required for vessel dissection but also for vessel mounting and cannulation (Fig. 4). The length of the cutting edge of the Vannas scissors and the width of the tips of the forceps vary with vessel type and size. Typically, scissors with a cutting edge of 2.5 mm length are appropriate for dissection of small vessels (e.g., rodent uterine arteries, mesenteric arteries and veins, coronary arteries), whereas scissors with a longer cutting edge (∼4–5 mm) may be used for larger arteries (e.g., aorta). Vannas scissors may be made from titanium or stainless steel. Forceps with fine tips are made for high precision laboratory work under the microscope and are extremely delicate and fragile. Thus, care is required in handling these instruments. Popular styles of dissection forceps are the Dumont No. 5 (straight forceps, tip dimensions: 0.05 × 0.01 mm) and Dumont No. 7 (curved forceps, tip dimensions: 0.07 × 0.03 mm). Forceps with fine tips may be made by several materials (i.e., titanium, stainless steel, carbon). The material used determines the durability, corrosion resistance, resistance to mineral and organic acids, and ability to autoclave these instruments. Glass-dissecting dishes with silicone elastomer can be purchased by various vendors or prepared by the investigator. We recommend using black silicone elastomer to improve contrast and visualization. Availability of dissecting dishes of various sizes and depths is important to accommodate different vessels and dissection procedures. For example, a shallow dish can be used for the dissection of mesenteric arteries, whereas a deep dissection dish can be used for isolation of cerebral vessels. Insect pins are used to secure the tissue in the dissecting dish.
Figure 4.
Essential tools for dissecting macro- and microvessels for ex vivo experiments. Straight forceps with fine tips (A), curved forceps with fine tips (B), Vannas spring scissors with straight cutting edge (C) (curved scissors are also available, not shown), shallow and deep glass dissecting dishes with black silicone elastometer on the bottom (D, E), and insect pins (F). Created with Biorender.com.
d. Composition of the physiological salt solutions.
The ideal buffer for all vascular function is one that maintains pH within acceptable levels (resting intracellular pH in VSMC ranges from 7.1–7.3 regardless of vascular bed) (19) and mimics the in vivo concentration of circulating ions. Regardless of which buffer recipe is used, whether it be one described here, or another one of the many adaptations found in the literature, standardization of the buffer to your laboratory’s preparation and consistency across experiments are the most important principles to follow for scientific rigor and reproducibility of data.
Isometric force.
Presented in Table 1 are the concentration of compounds that make up classical Krebs-Henseleit buffer and an adapted recipe that we recommend for isometric vascular preparations. The recipe has been modified from the classical Krebs-Henseleit buffer in the following ways:
Table 1.
Concentrations of compounds that make up the classical Krebs-Henseleit buffer and the adapted physiological salt solution that we recommend for isometric force measurement on wire and pin myographs
| Compound (Mol wt), g/Mol | Classical Krebs-Henseleit Buffer, mM | Vascular Adaptation for Isometric Force Studies, mM |
|---|---|---|
| NaCl (58.44) | 118.0 | 130.0 |
| KCl (74.55) | 4.7 | 4.7 |
| KH2PO4 (136.09) | 1.2 | 1.2 |
| MgSO4 (246.28) | 1.2 | 1.2 |
| NaHCO3 (84.00) | 24.9 | 14.9 |
| Glucose (180.16) | 10.0 | 5.6 |
| EDTA (292.15) | - | 0.03 |
| CaCl2 (147.02) | 2.5 | 1.6 |
Less bicarbonate, which has been shown not to affect contractile responses (20).
Less glucose, because glucose concentrations approaching 10 mM are considered prediabetic.
Includes EDTA, which is added to the buffer before the addition of CaCl2 to chelate any Ca2+ that may have inadvertently ended up in the buffer.
Less CaCl2 because 1.6 mM is closer to the concentration of circulating “free” ionized calcium which could contribute to vascular contraction. Although 2.5 mM is the total concentration of circulating Ca2+, as much as ∼40% of this Ca2+ is bound to proteins (e.g., albumin) or complexed with anions (e.g., bicarbonate or phosphate) and is unavailable to contribute to contraction.
Several commonly used artificial buffers have been suggested to attenuate VSMC contractile responses (21, 22). Therefore, we recommend optimizing your preparation if you wish to incorporate an artificial buffer such as Tris, HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], MOPS [3-morpholinopropane-1-sulfonic acid], BICINE [N,N-bis(2-hydroxyethyl)glycine], or PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] in isometric preparations (wire or pin myograph).
Isobaric conditions (myogenic tone).
Special considerations in preparing buffers for studies of myogenic tone are to maintain pH and osmolality near physiological levels. The small vessels studied are more sensitive to osmotic and pH shock than larger, more robust arteries. Some laboratories add glycol or albumin to the solutions to maintain buffer viscosity, but this is not an absolute requirement. The physiological saline solutions we recommend is 5% CO2–21% O2 bubbled bicarbonate buffer (see Table 2A for recipe) for superfused preparations and a 21% O2 bubbled HEPES buffer (Table 2B) for nonsuperfused preparations. In isobaric preparations, we also recommend using a buffer with a greater calcium chloride concentration (≥ 2–2.5 mM), as this enhances myogenic tone.
Table 2.
Concentrations of compounds and that make up the bicarbonate buffer, the HEPES buffer, and the calcium (Ca2+)-free bicarbonate buffer
| Compound (Mol wt), g/Mol | Bicarbonate Buffer, mM | HEPES Buffer, mM | Ca2+-Free Bicarbonate Buffer, mM |
|---|---|---|---|
| NaCl (58.44) | 130.0 | 134.0 | 130.0 |
| KCl (74.55) | 5.4 | 6.0 | 5.4 |
| NaH2PO4 (119.98) | 0.5 | - | 0.5 |
| MgSO4 (246.28) | 0.8 | - | 0.8 |
| MgCl2 (95.21) | - | 1.0 | - |
| NaHCO3 (84.00) | 19.0 | - | 19.0 |
| Glucose (180.16) | 5.5 | 10.0 | 5.5 |
| EDTA (292.15) | - | 0.03 | 4.8 |
| CaCl2 (147.02) | 1.8 | 2.0 | - |
| HEPES (238.30) | - | 10.0 | - |
To evaluate myogenic tone, it is also important to isolate and cannulate arteries quickly, avoid stretching or damaging the arteries and to prevent “cold shock” by not using ice-cold buffers. If performed quickly, the vessels can be isolated, cleaned, and mounted at room temperature. However, for the coronary circulation, the heart will continue to beat unless placed in ice-cold solution. Thus, in the heart, room temperature dissection is not recommended, even if studying the myogenic response. Please see Coronary arteries and microcirculation described below for more information. For isolation and cannulation, we use the HEPES buffer to also avoid the high pH common in bicarbonate buffer and is not constantly bubbled with a CO2 solution.
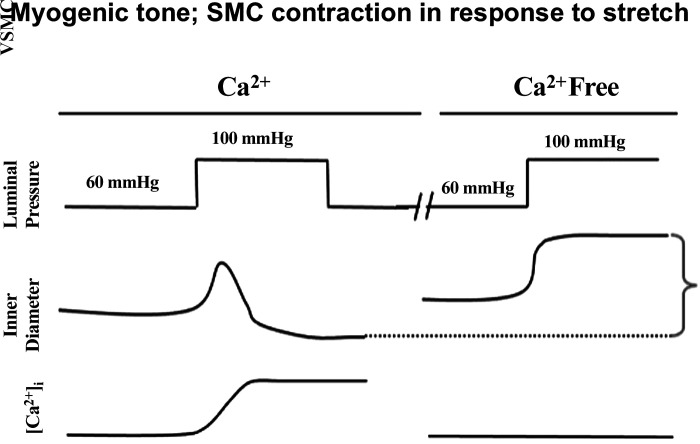
For the recording of myogenic tone and/or passive vascular measurements, it is also necessary to prepare a Ca2+-free buffer (Table 2C) to assess the passive properties of the vessel. The protocol we use to record myogenic tone is illustrated in Fig. 5.
Figure 5.
Diagram showing method to evaluate myogenic tone in an isolated, pressurized artery. VSMC, vascular smooth muscle cell.
VASCULAR FUNCTION IN ISOLATED VESSELS
Dissection and Isolation of the Vessels
Important Considerations:
Anesthesia
Kill the animal humanely using a method recommended by the Panel on Euthanasia from the American Veterinary Medical Association (e.g., overdose of general anesthesia isoflurane via a nose cone and thoracotomy and exsanguination via cardiac puncture).Differentiating arterioles from veins
Veins have thinner wall, more branches, and are more easily compressed and distorted by minor pressure while dissecting.Vessel dissection and cleaning
Isolating arteries and veins that remain viable throughout an experiment is a skill with a steep (weeks to months) learning curve. Even a modicum of stretching during dissection can damage a vessel sufficiently to alter normal vasomotor responses.
Dissecting time varies between vascular bed, and extricating vessels from some tissues (e.g., heart or skeletal muscle) is much more difficult than from other tissues (e.g., subcutaneous adipose). However, a general rule of thumb is that the longer you clean, the greater chance of unintended vessel damage. Many of the vascular beds described in this article can be efficiently dissected in ∼15–25 min.
“Over trimming” the adventitia and excessively “cleaning” of the perivascular adipose tissue often elicits unintended traumatic injury to the underlying media and intima.Species considerations
When working with human tissue, it is recommended to leave intact a modest layer of adventitia surrounding the vessel (enough so that the luminal edges are still easily detectable).Vessel viability and integrity testingExperimental demonstration of intact responses to known vasomotor agents is important.
With a delicate and efficient dissection, vessels can easily remain viable for the entirety of an experiment [i.e., multiple hours (∼6–9 h)].
a. Aorta.
The aorta is the largest artery in the body. The aortic arch rises from the heart's left ventricle and oxygen-rich blood flows down the thoracic cavity, through the diaphragm, and into the abdomen. Many smaller arteries branch from it, including the renal arteries and organs of the digestive system. The abdominal aorta subsequently divides into the iliac arteries.
The aorta not only serves as a conduit during systole but also acts as a reservoir for blood. Its elastic properties allow the aorta to store half of the ejected stroke volume. Aortic recoil during diastole pushes the remaining stored volume forward into the peripheral circulation. This phenomenon is known as the Windkessel function (23). This elasticity allows the aorta to absorb the force of the blood as it is pumped from the heart and subsequently propelling it to downstream organs. In some diseases however (e.g., hypertension), this elasticity is lost due in large part to aortic stiffening. Aortic stiffening is defined as decreased compliance because of elastic fiber degradation, increased fibrosis, and in the case of hypertension, increased distending pressures. Aortic stiffening can have deleterious hemodynamic consequences for delicate downstream organs and increases the risk for other terminal cardiovascular diseases (e.g., myocardial infarction, heart failure, and stroke).
Although the aorta is the largest, and arguably the most durable artery in the body, care needs to be applied during its isolation and cleaning to avoid mechanical damage. Generally, the thoracic aorta, as opposed to the abdominal aorta, is used in isolated vascular function studies as the thoracic aorta is primarily responsible for the propelling blood flow downstream. Increased contractility and endothelial dysfunction of the thoracic aorta are indicative of enhanced stiffness (24).
Practical tips: aorta.
Promptly following thoracotomy and exsanguination, move the dead animal into a position so that the chest cavity can be opened, allowing as much room as possible.
Next, remove the lungs and pulmonary artery by gently pulling the lungs up and opening the fibrous sheath between the pulmonary artery (which will be pulled up) and the thoracic aorta which will be running directly adjacent to the spine. The lungs can be carefully removed by cutting the pulmonary artery perpendicularly at both ends.
At this point, if exsanguination via cardiac puncture was performed, there is probably a pool of blood obscuring the view of the thoracic aorta. This blood can be gently wiped and absorbed using gauze (be careful not to touch the aorta).
Next is the extraction of the aorta itself, which can be performed with or without a dissecting microscope. First, secure the trachea or aortic arch with forceps and gently lift the aorta up. Begin cutting distally toward the abdomen, in parallel to the aorta and spine. If the surgical scissors are gently pressed against the spine of the animal, the cutting will always avoid the aortic wall and will be only cutting the surrounding perivascular adipose tissue (PVAT). The PVAT can be fully removed at a later step.
Continue cutting from the aortic arch down toward the abdomen, always maintaining slack on the aorta. A common beginner error is to pull the aorta too forcefully during this procedure and damaging it from excessive stretch. Eventually, the view of the thoracic aorta will become obscured by the liver and a perpendicular cut can be performed to remove it entirely.
Secure the aorta a Petri dish filled with ice cold physiological salt solution by pinning the PVAT at both ends. Ice-cold physiological salt solution is acceptable for the aorta, as it is less-sensitive to “cold shock” to evaluate myogenic tone in resistance arteries and arterioles. If the research question does not involve understanding intact aortic PVAT, we recommended dissecting it off at this point as a standard operating procedure due the influences of PVAT on vascular contractility (25)
Regardless of whether the PVAT is maintained or removed, using a dissecting microscope, cut the thoracic aorta into 2-mm segments for functional assessment if studying rats and 3-mm segments if studying mice.
b. Mesenteric resistance arteries.
As described in the buffer section, small arteries should be treated with care to prevent damage (<250 µm). The entire mesentery should be removed from the animal within minutes of euthanasia and transferred to a dissection chamber containing pH 7 cold physiological saline solution (e.g., Krebs, Table 1; as described above, cold solution should not be used for evaluating myogenic tone) or HEPES buffered physiological saline solution (Table 2B). The buffer should be changed as necessary to remove fecal material and adipose tissue that is released into the solution.
The mesentery can be gently pinned out to allow easier isolation of the individual branches. Isolation of resistance mesenteric arteries requires a dissecting scope with good optics (preferably ×10 magnification) and sharp, high quality microdissection tools (please see Fig. 4), as discussed earlier. For rat mesenteric resistance arteries, usually a 6th to 7th order artery (lumen diameter <150 µm) is required to achieve the desired size to evaluate myogenic tone using pressure myograph. For wire myograph studies, a third-fifth order artery (lumen diameter 150–250 µm) can be used to evaluate resistance artery function. For mouse mesenteric arteries, a fifth order artery (lumen diameter <150 µm) is usually the appropriate size to evaluate myogenic tone, and third-fourth order (lumen diameter 150–250 µm) to evaluate vascular function. Figure 6 illustrates the method we recommend to identify and isolate mesenteric resistance arteries.
Figure 6.
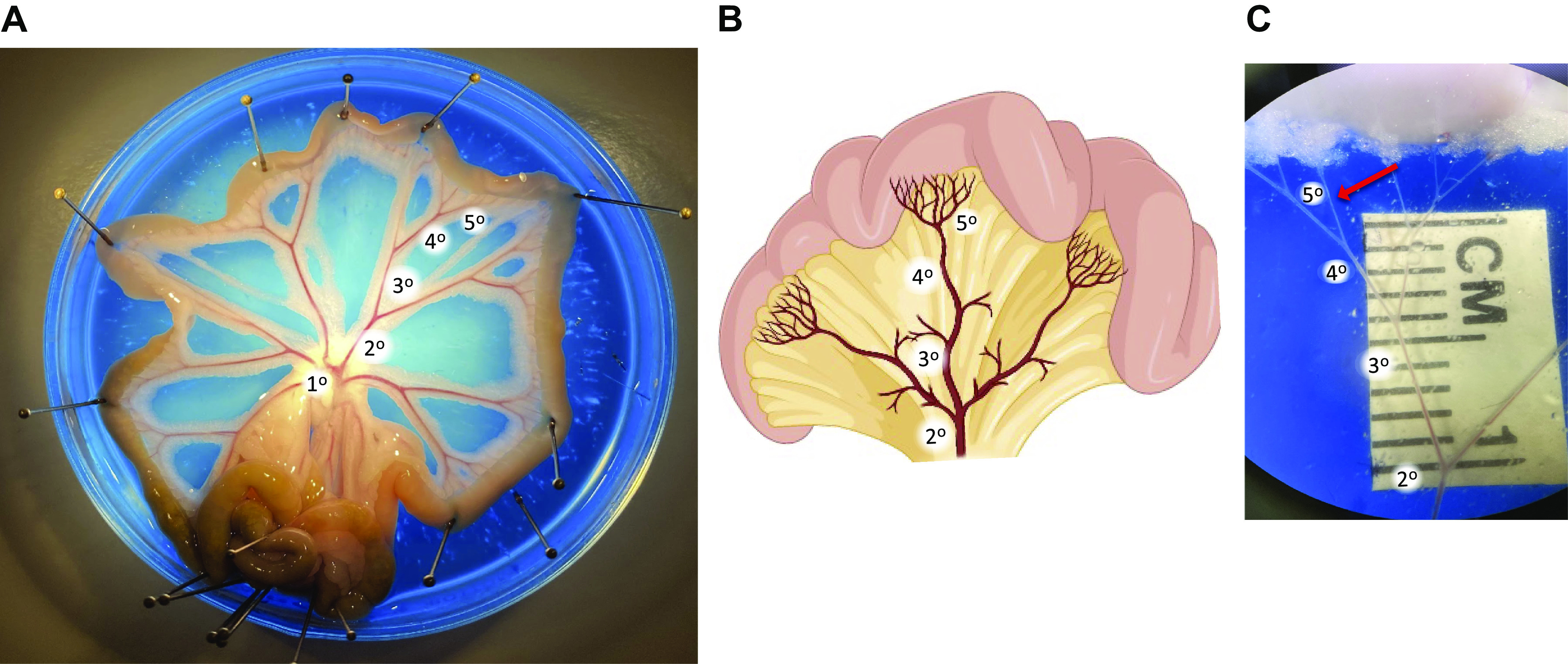
Isolation of small mesenteric arteries for vascular studies. Isolated rat mesenteric bed that is pinned out in a petri dish containing colored silicon elastometer and filled with physiological salt solution (A and B). C is magnified to illustrate the 5th order artery selected for cannulation after removal of the adipose, veins, lymph vessels, and connective tissue. Figure provided by Perenkita Mendiola at the Dept. of Cell Biology and Physiology, Univ. of New Mexico, Albuquerque, NM.
Practical tips: mesenteric resistance arteries.
The first step is to identify a vascular tree with appropriate small vessels entering the gut wall. Care should be taken to consistently isolate either jejunal arcades (proximal to the stomach) or ileal arcades (proximal to the colon), as there are subtle differences in vascular function between the different sections. Both form anastomotic arcades within the mesentery from which the small straight arteries (arteriae recta, AR) branch to enter the intestinal wall. The jejunal arteries have a slightly greater diameter than the ileal arteries of the same branches, whereas the ileal arteries have a greater number of arcades.
A 2010 study suggested that, in human mesenteric arteries (26), the small AR are more muscular than their feed arteries but that the muscularity does not differ significantly between the jejunal and ileal sections.
Because the AR are longer in the jejunal region, we recommend using this region to collect arteries for isolated artery studies using either isometric or isobaric preparations. It is also important to note that there have been conflicting reports of the degree of myogenic tone present in this bed (27–29). The endothelium provides a strong suppression of myogenic tone, and significant myogenic tone occurs after removal of the endothelium or after inhibition of endothelial dilator pathways (NO synthase, cystathionine gamma lyase, cyclooxygenase). The presence or absence of an active endothelium is therefore important to define in the methods of any study of myogenic tone in the mesentery. Please see mounting and normalization.
c. Coronary arteries and microcirculation.
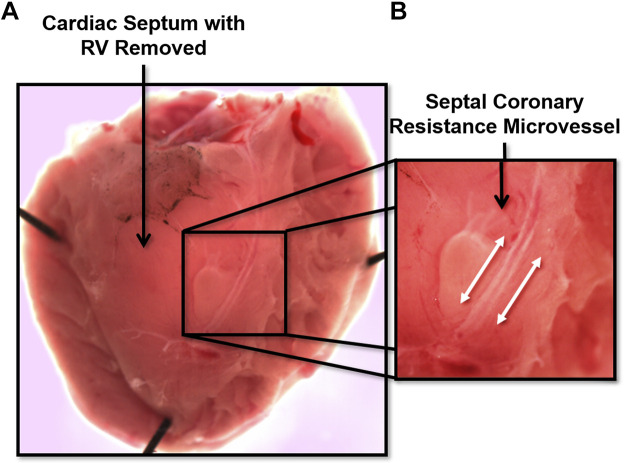
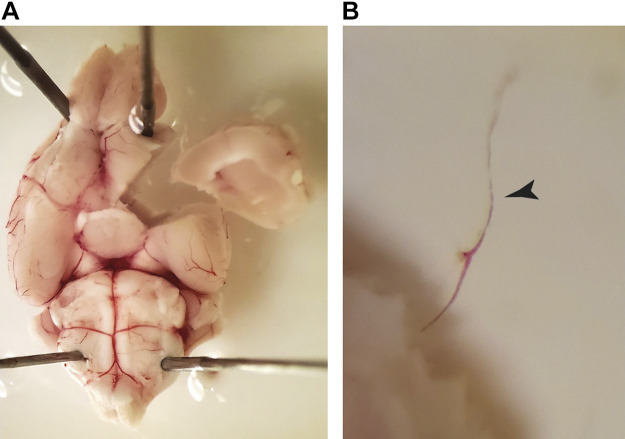
The coronary circulation supplies blood flow to the myocardium. It is unique because is the only vascular bed to flow primarily during diastole, owing primarily to extravascular compression during cardiac systole. Similar to the other vascular beds, the regulation of coronary blood flow is governed by a myriad of factors, including local metabolites, coronary perfusion pressure, myogenic tone, neurohormonal influences, VSMC and endothelial cells, structure, and biomechanics (30, 31). Much of the data gathered to date has been collected on left anterior descending (LAD) coronary arteries, left circumflex coronary artery, right coronary artery, and the arterioles and microcirculation that branches from these upstream arteries. The successful dissection and isolation of these vessels depends on the size, with varying approaches for each, mostly due to the large variation in the amount of connective tissue that must be dissected in order to isolate the vessels. In large species (e.g., pigs and humans), the LAD and other commonly used arteries are considered epicardial conduit arteries with diameters between 2–3 mm. To delicately isolate coronary arteries, we recommend a combination of curved forceps with a ∼0.5-mm tip width and Dumont SS forceps with a 45° angle to bluntly dissect large arteries free from myocardial tissue in large species that have well-developed connective tissue and PVAT (e.g., pig). During the dissection and isolation of large coronary arteries, branches must be cut with small Vannas spring scissors. If the desired experiment is pressure myography, one must leave sufficient length on branches such that they can be tied closed with suture. This is not a requirement if the vessel will be mounted in a wire or pin myograph. In rodents, these vessels are considerably smaller (∼250–400 µm in diameter). Arterioles within the coronary microcirculation are important regulators of coronary blood flow. An example is the mouse septal coronary arteriole (Fig. 7), which can be exposed by cutting off the right ventricle with a pair of Vannas spring scissors (4–5 mm cutting edge) and exposing the interventricular septum. Once exposed, this rodent vessel, and others of similar size (e.g., LAD), can be isolated using a pair of Dumont 55 or 55SF (“superfine”) forceps and removed from the tissue using very fine Vannas spring scissors (2 mm cutting edge) for mounting on a myograph of choice. In larger species such as the pig, coronary arterioles are richest in the endocardial and apical regions of the myocardium. We recommend to cut a manageable slice of myocardium (2 cm × 2 cm × 5–10 mm thick) using a razor blade or scalpel to keep it pinned to a dish, immersed in cold buffer or physiological saline solution, and to more easily identify vessels of interest. This is also true when isolating larger epicardial conduit coronary arteries.
Figure 7.
Image shows a cardiac septum with right ventricle (RV) removed (A), and an amplified image for a septal coronary resistance microvessel (B).
Practical tips: coronary vessels.
Coronary arteries and arterioles are embedded within a dynamic muscle, and as such, it can take significant effort and practice to isolate them from surrounding tissue that could influence the resultant data. Significant practice is critical in the proper assessment of coronary structure, function, and biomechanics, and it is somewhat of an art to balance dissecting with risk for damaging the vessel. These practical tips can help investigators be successful in dissecting and isolating coronary vessels:
Tools: For the dissection and isolation of coronary arterioles (regardless of species), we recommend using Dumont 55 or 55SF forceps that have maintained a sharp tip. Be aware of the location of the tip at all times because it only takes one slip-up to ruin a good pair of forceps! For excision, a good pair of Vannas spring scissors with a 2-mm cutting edge work well.
Dissecting Dish: a low-profile glass dissecting dish of choice filled with an ∼5-mm-thick layer of silicone elastomer (e.g., Sylgard) on the bottom and filled with ice-cold buffer or physiological salt solution sufficient to cover the myocardial tissue from which coronary arteries or arterioles will be isolated. This setup allows one to secure either slices of tissue (in the case of large animal coronary dissection) or rodent hearts for dissection using tissue pins.
Dissection of coronary arteries: It is important for coronary arteries and arterioles to be devoid of surrounding tissue. This is best accomplished, particularly for smaller arterioles, when the vessels are still connected within the tissue. This method allows the investigator to clean the vessels from all angles (top, bottom, sides) without having to hold or secure it in place as would be the case if it was excised.
d. Skeletal muscle arteries.
Supply of oxygenated blood to skeletal muscle is essential to maintain skeletal muscle metabolic activity in a range of functional events from exercise, to contraction of the diaphragm during breathing. Indeed, active hyperemia in skeletal muscle can cause blood flow to dramatically increase from resting levels of ∼1 mL/min, up to ∼75 mL/min (dependent on muscle type). As one might expect then, the arteries in the skeletal muscle must be capable of rapid dilation and constriction many times the resting tone. Thus, skeletal muscle arteries respond to a variety of stimuli, including shear stress, pressure, hormonal, metabolic molecules, oxygen, neuronal, and blood volume.
Skeletal muscle arteries can be directly dissected from an anesthetized animal (i.e., muscles will not need to be separately dissected) or first dissecting out the muscles. This will depend on the muscle of interest as to how the resistance arteries can be visualized for dissection and removal. However, because of the large variety and location of skeletal muscle, the removal of the skeletal muscle artery requires some preplanning to ensure the animal is in the proper position for the skeletal bed desired, as well as what size artery will need to be removed. Begin at the feed artery of the skeletal muscle bed and work down as far as desired. Generally, the smaller arteries near terminal arterioles (lumen diameter <100 µm) are difficult to obtain for ex vivo preparations due to the amount of dissection of skeletal muscle required.
Practical tips: skeletal muscle arteries.
Three variables will almost always determine the efficacy of your dissected skeletal muscle arteries: time of dissection, stretch of the artery, and dampness of preparation.
Once the arteries are determined (Fig. 8), gently remove the adventitia of the muscle around the artery. Finding the artery on muscle can be difficult due to translucent nature of vessels, but the remaining blood in the artery can help orientate the branch order.
Keep muscle damp to every degree possible. This can be done simply by pipetting buffer on the muscle regularly or having a regular drip system on the muscle.
Next cut the desired artery using fine tip scissors and very gently lift with fine tip forceps. Cut the connective tissue along the length of the artery to remove from the muscle and ensure it is of proper length for cannulation or mounting before making the final cut.
With the fine tip forceps, place the artery in buffer, being careful to note the direction of blood (so that directionality can be obtained if desired).
Figure 8.
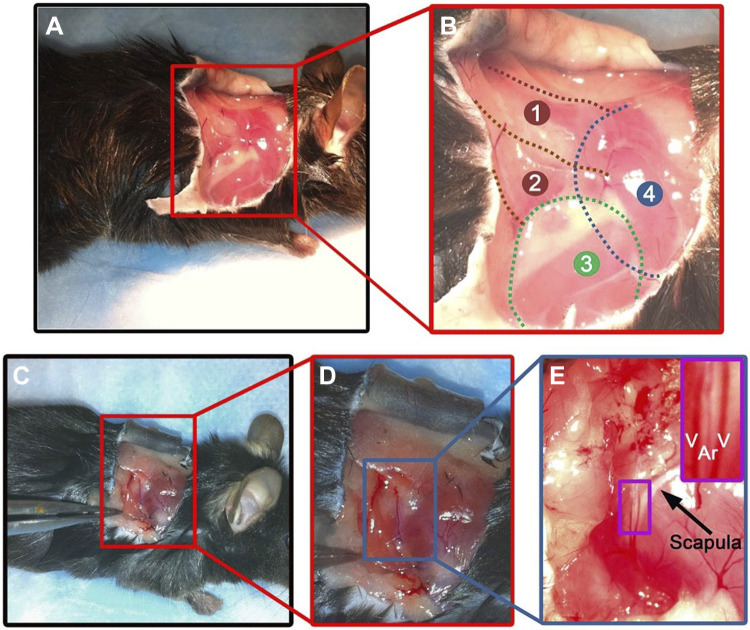
Image shows a muscle artery predissection. Thoracodorsal artery (TDA; ∼200 µm in lumen diameter) from mouse lays on top of the spinotrapezius muscle. Lateral view of the right shoulder of a mouse (A). Enlargement of the red box shown in A describing the anatomy of the superficial muscles of the right shoulder of a mouse: 1: spinotrapezius muscle; 2: latissimus dorsi muscle; 3: triceps brachii muscle (forelimb); 4: position of the scapula (B). Lateral view of the right shoulder of a mouse with a forceps pulling the latissimus dorsi muscle, revealing the TDA (C). Enlargement of the red box shown in C (D). Enlargement of the blue box in D (E). The purple box on the upper right shows a zoom in view of the TDA (Ar) surrounded by two veins (V) located on the caudal boarder of the scapula (designated by the arrow). Used with permission from Billaud et al. (32).
e. Uterine arteries.
The uterine arteries (UA) are the main conduits of blood to the uterus. During pregnancy, the UA ultimately branch into specialized vessels called spiral arteries, which carry maternal blood to the placenta to support fetal development. To meet the needs of the developing fetus, the UA must undergo dynamic changes to increase blood flow to the placenta. Multiple factors regulate the remodeling of the UA and a more thorough description of species-specific remodeling has been reviewed (33, 34).
The uterine artery is located along the medial margin of the uterus (Fig. 9). Mice and rats have a bicornate uterus with the main utero-ovarian (or parametrial) arteries running parallel to, but distant from the uterine wall within a sheet of connective tissue called the mesometrium. Isolation and dissection of these vessels is dependent on multiple factors: the estrous cycle (if isolating vessels in nonpregnant animals); the degree of branching of smaller arcuate arteries; the presence of the endothelium, which has a significant role in the adaptation of the UA during pregnancy; and most notably the stage of pregnancy. During pregnancy, the diameter of the main uterine artery increases 2–3-fold, with or without changes in vascular wall thickness. Experiments on the UA will require that the readers understand how UA respond to pregnancy in the studied model.
Figure 9.
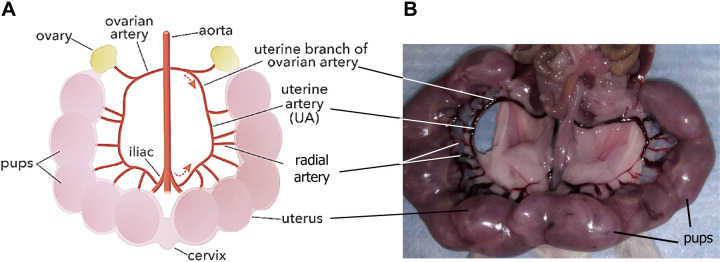
Schematic of uterine artery bed from mice (A) (created with Biorender.com). Image shows uterine artery bed predissection (B). Lines indicate uterus, cervix, ovary, pups, and different arterial segments branched from ovarian arteries (A and B). Figure provided by Dr. Ramon A. Lorca at the Univ. of Colorado-Anschutz Medical Campus.
For the mouse model, it is recommended isolating arteries in ice-cold physiological salt solution or phosphate buffered saline using surgical tools (see description below). Although nonpregnant vessels are typically ∼250 µm in lumen diameter, UA are ∼600 µm in lumen diameter at day 18 of pregnancy (day 19.5 is day of delivery in C57BL/6 mice). For isolation of the UA, mice should be euthanized by using volatile anesthetics, or CO2 inhalation and cervical dislocation as a secondary euthanasia method. In pregnant animals, pups should be euthanized by decapitation, as they are more resistant to volatile anesthetics. After cleaning and disinfecting the abdominal skin, a midline laparotomy is performed to expose the uterus using surgical scissors. A fat pad lies along each uterine horn which contains the UA and uterine veins. Before dissecting out the UA, we identify the ovarian and cervical ends within the fat. Both UA and vein are dissected with the fat pad and placed in a petri dish with ice-cold physiological salt solution or phosphate buffered saline. To avoid damaging the vessel, pin down the vessels with two needles and using the fat tissue on each end as the anchor. Under a dissecting microscope and with the help of small forceps, the uterine veins and arteries can be identified. Generally, arteries have smaller diameters than veins and show a distinctive “white wall” surrounding them, due to the thicker VSMC layer present in the arteries. Both vessels should still contain blood in their lumen; however, only the blood in the vein should easily dissipate by gently pushing the vessel with the forceps. The distinction between uterine artery and vein may be harder in nonpregnant than pregnant animals due to the small size of the vessel. Once secured by the pins, the fat surrounding the UA can be carefully dissected from the blood vessels. Once the fat is removed, the vein can be easily separated. The UA can be cleaned by pulling and cutting away the remaining fat and connective tissue, the latter of which appears as a translucent membrane. It is recommended cleaning the entirety of the isolated UA (up to each anchoring pin) to ensure there is sufficient length for each study. Once cleaned and while still pinned down, the UA can be cut in small pieces according to their subsequent use. For pressure myography, it is recommended to cut ∼2–3-mm segments avoiding areas that contain branches of the arcuate arteries. If avoiding branches is not possible, the branches should be sutured, which could be challenging to accomplish in smaller vessels. For wire myography, 2-mm segments can be used and branches do not need to be sutured.
To study the role of endothelial factors, the endothelium can be removed before each experiment by passing air bubbles through the lumen and then flushing with Krebs buffer. Endothelial removal should be confirmed by a major loss, defined as less than 15%–10%, of vasorelaxation to 10 µM acetylcholine in vessels contracted with 15 mM KCl or 1–3 µM phenylephrine.
Practical tips: uterine arteries.
UA are highly dynamic and undergo vast changes in size and properties as pregnancy develops. Additionally, due to the role of the UA in supplying blood to the uterus, even in nonpregnant animals, it is subject to changes during the estrous cycle. Thus, if investigating the UA function in nonpregnant animals, please record the stage of estrus to better control the studies. If studies are performed on UA isolated during pregnancy, the stage of pregnancy should be recorded, as the baseline diameter will change due to the high degree of remodeling that occurs.
Tools: For dissection of uterine arteries, it is recommended to use sharp Dumont No. 5 forceps and 2-mm cutting edge Vannas spring scissors. Two ∼28–31-gauge needles are used to pin down the vessels into the dissection dish.
Dissection Dish: Due to the size of the UA, we recommend a low-profile plastic/glass petri dish with black Sylgard on the bottom. The dish should be filled with physiological salt solution at 4°C that covers the vessel. As described above, the vessels should be pinned down by the fat pad as close to the end of the vessels as possible.
Vessel Integrity: Due to the vast changes in the UA during pregnancy, great caution has to be taken to not stretch the UA or damage them by excessive manipulation.
Vessel Location during Dissection: The UA span the entirety of the uterus, and they are surrounded by fat. A helpful method of localizing them is to gently push the fat underneath the uterine horn, exposing the vessels (uterine veins will most likely be visible as they are bigger). Grab the fat and vessels with forceps and cut as close to the uterus as possible, from the top near the ovarian end of the uterus toward the cervical end, or vice versa, depending on the uterine side (left or right) and whether the researcher is right- or left-handed.
f. Pulmonary arteries.
Pulmonary arteries are structurally and functionally very different from systemic arteries. They carry deoxygenated blood at a low intraluminal pressure of 10–15 mmHg. Because of the low intraluminal pressures, pulmonary arteries exhibit minimal myogenic constriction under normal conditions. As a result, most of the contractility studies on pulmonary arteries use pharmacological constrictors to generate constriction. Pulmonary arteries from rodent models of pulmonary hypertension, however, display robust myogenic constrictions. Arteries with an internal diameter of ∼50–100 μm are thought to be resistance-sized in the pulmonary circulation. In a mouse lung, this corresponds to fourth- or higher-order arteries (Fig. 10A). Most contractility studies on pulmonary arteries have been performed using wire myography. Pressure myography of pulmonary arteries is technically demanding due to several reasons. First, resistance pulmonary arteries are so well-embedded into the lung parenchyma that dissecting them out is challenging. The presence of blood inside the arteries makes it easier to visualize them. It is recommended that the heart remains connected to the lungs during the dissection. In case, your dissecting scope stage has a bottom illumination, you should turn it off to improve visibility of pulmonary arteries; only use the top illumination. The walls of resistance pulmonary arteries are thin. Therefore, the connective tissue around the arterial walls must be cleared very carefully to prevent damage to the arterial walls. To locate pulmonary arteries in a mouse lung, we first cut open the veins on the surface. This exposes the airway, which is directly underneath the veins and can be identified by air bubbles inside. Pulmonary arteries run alongside the airway. Cutting open the airway facilitates visualization of pulmonary arteries. Second, the extensive branching of pulmonary arteries means that only a short length of the artery is available for cannulation and pressurization without leaks. In a mouse lung, this corresponds to ∼200 microns between two branching points. Some of the branching points are too small to be seen during dissection. Therefore, as a standard practice, we recommend cannulating the arteries first, pressurize them to 15 mmHg (Fig. 10B), and then attempt to visualize any leaks due to branching points. After locating the leak points with this approach, the position of the ties on cannula can be adjusted to bypass the branching point.
Figure 10.
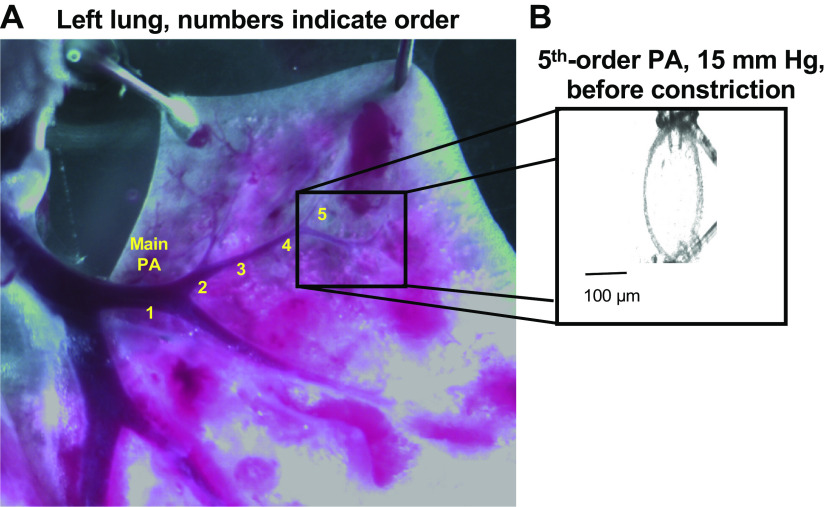
Image shows left lung and numbers indicate pulmonary artery (PA) divided into five branches (A). Please note that the lumen diameter of the fifth artery branch is ∼ 50 µm before cannulation and pressurization (A). Amplified image for a fifth-order PA cannulated at both ends (B).
Important considerations.
Resistance pulmonary arteries in pressure myography experiments display little to no dilation in response to the classical endothelium-dependent vasodilators acetylcholine and bradykinin. Moreover, they have shown significant differences in the localization patterns of endothelial vasodilator proteins in systemic and pulmonary arteries. Nitric oxide is the primary mediator of endothelium-dependent dilation in resistance pulmonary arteries. In systemic resistance arteries, endothelial nitric oxide synthase is present close to a NO scavenging protein hemoglobin α, limiting the role of NO in systemic resistance arteries. In the endothelium from pulmonary arteries, however, hemoglobin α is not observed. Moreover, endothelial calcium-activated potassium channels (KCa) are present at myoendothelial projections underling endothelium-derived hyperpolarization leading to vasodilation in systemic resistance arteries but not in pulmonary resistance arteries. This signaling arrangement explains the predominant role of NO in endothelium-dependent vasodilation in pulmonary resistance arteries but not in systemic resistance arteries.
g. Cerebral arteries.
The cerebral circulation is highly specialized to support the metabolic demands of the brain. The arterial blood supply originates from the left and right vertebral arteries, and the left and right internal carotid arteries. The vertebral arteries join to form the basilar artery which supplies the cerebellum and brain stem. The basilar artery connects with the two internal carotids via intermediate communicating arteries to form a complete ring at the base of the brain known as the circle of Willis. Three pairs of major arteries arise from the circle of Willis to supply different parts of the cerebrum: the anterior, middle, and posterior cerebral arteries. These vessels further divide into progressively smaller arteries along the surface of the brain. Collectively, the surface vessels are termed pial arteries (Fig. 11). Smaller pial arteries eventually penetrate into the brain within the Virchow–Robin space to form parenchymal arterioles that feed the subsurface microcirculation. Parenchymal arterioles are a key component of the neurovascular unit, a structure that locally controls perfusion to match the metabolic activity of neurons. Parenchymal arterioles are structurally and functionally distinct from surface pial arteries. First, pial arteries contain approximately two to three layers of circumferentially oriented VSMC but parenchymal arterioles only have one layer. Functionally, parenchymal arterioles have greater myogenic reactivity but are unresponsive to certain contractile agonists (e.g., norepinephrine). Second, pial arteries are innervated by perivascular nerves of the peripheral nervous system, known as “extrinsic” innervation, whereas parenchymal arterioles are “intrinsically” innervated by surrounding neurons. Arterioles are also encased by astrocytic processes which can regulate blood flow. Lastly, unlike the surface pial arteries, parenchymal arterioles do not have a collateral system where an occlusion can result in significant reduction in blood flow and tissue damage.
Figure 11.
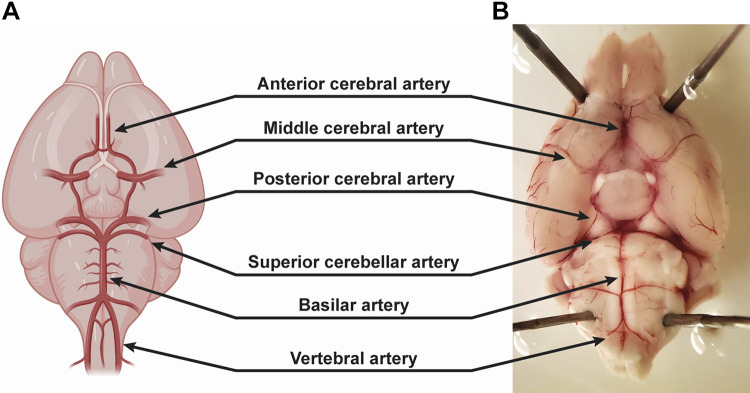
Anatomy of surface pial arteries (A and B). Created with Biorender.com.
Practical tips: cerebral arteries.
Most surface pial arteries are easy to remove and clean from the surrounding parenchyma. Although arteries can be peeled off the surface of the brain, this approach overstretches and damages vessels impacting the vascular response. We recommend using sharp Vannas spring scissors to cut the arteries away from the surrounding tissue, then carefully removing any excess remaining tissue. Unlike other pial arteries, the basilar artery and its branches are surrounded by an extensive amount of connective tissue and additional care is required to remove it.
Parenchymal arterioles are loosely connected to the surrounding tissue and are easy to separate from the parenchyma. For performing experiments on parenchymal arterioles arising from the middle cerebral artery, it is recommended to cut a section of the brain surrounding the middle cerebral artery (e.g., 3 mm W × 5 mm L × 3 mm D of a mouse brain), after use forceps to blunt dissect the parenchyma until the arterioles are identified and can be removed (Fig. 12). These vessels are small in diameter (∼20–70 µm) and may be difficult to identify at first. Blood trapped within the lumen can help visualize arterioles.
A separate method for isolating parenchymal arterioles is to gently peel away large surface arteries pulling the arterioles along with it. However, this method may overstretch and damage arterioles.
Figure 12.
A section of the brain cut around the middle cerebral artery and parenchymal arterioles are carefully dissected from this section (A). Image of a parenchymal arteriole (arrow) that is embedded within the parenchyma (B).
Brain slice preparation.
The use of brain slices for the study of neurovascular coupling has advanced the field by allowing investigators to address dynamic signaling events at the level of the neurovascular unit. In addition to providing direct visualization of blood vessels, this ex vivo preparation enables the use of fluorescence imaging and electrophysiological recordings, making it a powerful approach for assessing intercellular communication in health and disease. Here, we describe the steps used to assess parenchymal arteriole vascular reactivity in brain slices, focusing on the cannulated parenchymal arteriole preparation. Where applicable, we also highlight limitations of the technique.
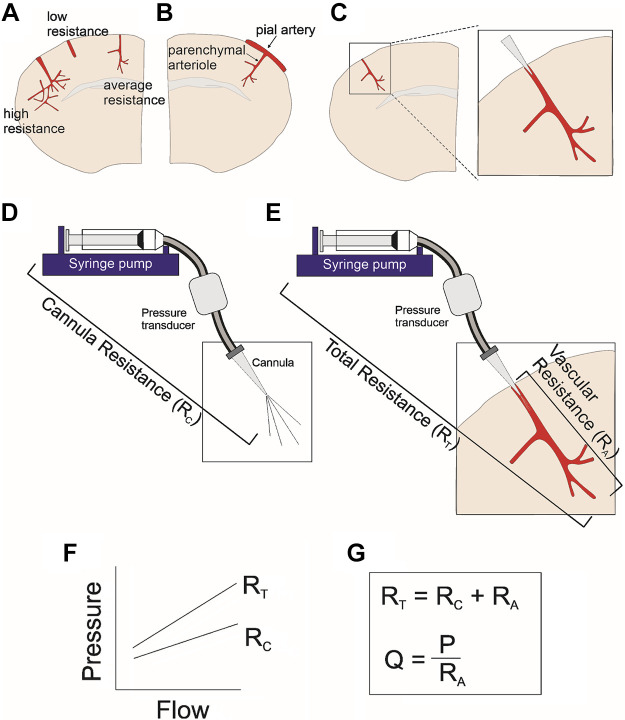
The first studies addressing the properties of parenchymal arterioles in a brain slice were reported by Lovick et al. (35). These authors described an experimental approach in which a glass cannula was introduced into the lumen of small parenchymal arterioles, allowing them to be perfused (35). The system also included perfused arterioles with open ends. The authors concluded that the technique was challenging and cited the buildup of pressure within the perfused vascular network as a potential problem (35). Over the years, some of the limitations of the original technique by Lovick’s work were addressed. However, there are still challenges with the approach, and understanding its limitations has helped fine-tune the types of questions that can be explored using it. Below is a step-by-step description of the approach (Fig. 13), considerations that influence the success of the procedure, and discussion of data interpretation.
Figure 13.
Representative illustrations from a brain slice cannulation technique. A coronal brain slice with three parenchymal arteriole examples corresponding to a highly vascularized downstream network (high resistance), a short parenchymal arteriole (low resistance), and an averaged length and branched parenchymal arteriole (A). Coronal brain slice showing a pial arteriole with a branching downstream parenchymal arteriole not suitable for cannulation (B). The expanded imaged of an ideal cannulated parenchymal arteriole (C). The perfusion system which includes the syringe pump, pressure transducer, and cannula (D). Complete system which includes the perfusion system plus the vascular network (E). Example of the pressure-flow relationship corresponding to the experimental steps used to determine the resistance of the cannula (RC) as well as the resistance of the entire system (F). Equations used to determine the resistance of the vascular network (RA) as well as the equation used to determine the flow rate (Q) needed to bring the perfused system to a desired pressure (P) (G).
Brain slice cutting.
Brain slices from a region of interest must be cut in a manner that yields the highest number of arterioles. Thus, an understanding of the structural organization of the vascular bed within the brain region of interest before cutting is important. The orientation—coronal, horizontal, or sagittal—used for cutting should yield arterioles that run longitudinally with the tissue. Some of the work has mainly been performed in cortical (36–38) and hypothalamic (39) brain slices; thus, the following account focuses on cortical coronal brain slices.
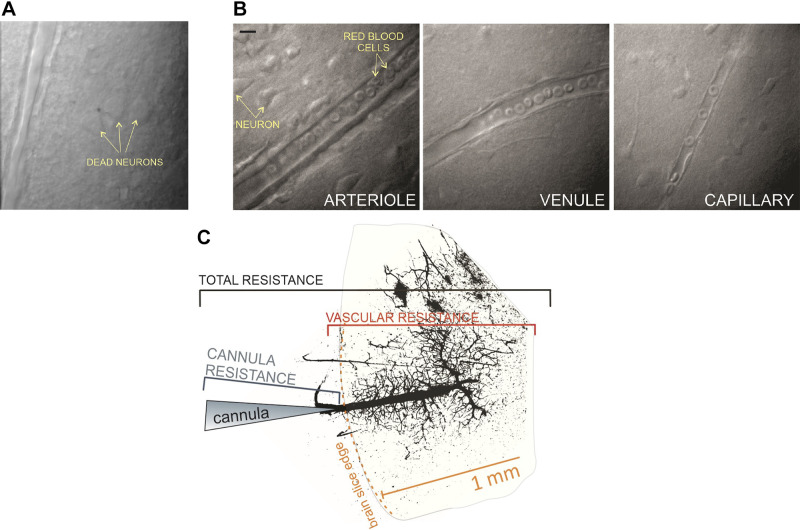
Brain slices are cut in ice-cold artificial cerebral spinal fluid (aCSF) (38) (Table 3) and placed in a chamber gassed with 95% O2-5% CO2. Immediately after cutting, slices are incubated for ∼30 min in 32–34°C gassed aCSF; this step aids in the recovery of neuronal membranes. Some laboratories use alternative solutions during cutting and/or recovery phase (40, 41). In any case, the details of these solutions should be clearly reported in each study to ensure the reproducibility of reported results. Slices are kept in the gassed chamber at room temperature (∼22°C) until they are used. For the experienced experimenter, the tissue remains viable for ∼6–9 h. Time is required to obtain good quality tissue; thus, the importance of this step should not be underestimated. Tissue viability can be determined by assessing the slice for the presence of dead neurons, which are clearly visible in the slice (Fig. 14A). Although it is normal to have a few dead cells at the surface, neurons one or more layers deeper into the tissue should appear healthy. We emphasize the importance of making these observations as the signaling modalities under investigation can be altered by the viability of the tissue. Appropriate timing of subsequent experimental assessments is also important. Most laboratories conducting brain slice experiments are equipped with an electrophysiological setup. Thus, an optimal way to determine the viability of the tissue is through neuronal electrophysiological recordings. Alternative methods could include measuring field potential in response to a known stimulus or measuring neuronal responses using imaging approaches. Although these techniques require considerable instrumentation, these positive control experiments can ensure that the tissue used throughout the experiment is viable.
Table 3.
Concentrations of compounds that make up aCSF that we recommend for the brain slice preparation
| Compound (Mol wt), g/Mol | aCSF, mM |
|---|---|
| NaCl (58.44) | 120.0 |
| KCl (74.55) | 3.0 |
| NaH2PO4 (119.98) | 1.25 |
| MgCl2 (95.21) | 1.0 |
| NaHCO3 (84.00) | 26.0 |
| Glucose (180.16) | 10.0 |
| CaCl2 (147.02) | 2.0 |
| l-Ascorbic acid (176.12) | 0.4 |
aCSF, artificial cerebrospinal fluid.
Figure 14.
Representative image from a cortical brain slice showing the presence of dead neurons (A), and arteriole, venule, and a capillary (B). Calibration bar, 10 μm. Image shows cannulated arteriole labeled with FITC (postexperiment) to define the downstream vascular network and the factors that contribute to the total resistance of the system (C).
Temperature.
Temperature is an essential variable for physiological and homeostatic processes, including the development of tone (42); thus, room temperature experiments are discouraged. Ideally, experiments should be conducted at physiological temperatures, but this consideration is balanced against the fact that the viability of the metabolically active brain slice decreases with increasing temperature. Because a typical experimental protocol may last 1–2 h, running experiments at a slightly lower temperature (∼33 ± 1°C) can help preserve tissue viability.
Tissue oxygenation.
The use of hyperoxic conditions for experiments using brain slice preparations has been a continuing subject of discussion in the field (43–45). Notably, however, the metabolic requirements of the brain slice are different from those in vivo (44). The high PO2 used in brain slice experiments (95% O2) has been shown to maintain oxidative metabolism and thus satisfy the metabolic needs of activated neuronal populations (44). The lack of alternative energy substrates (e.g., lactate) in glucose-containing aCSF and possible alterations in the condition of the tissue and arteriolar tone due to vasoactive signals (e.g., adenosine) have proven to be important considerations in determining the role of neurons in neurovascular function. Thus, the metabolic state of the tissue, which may significantly differ from those observed in vivo, is important.
Calibration of the cannula.
Before cannulation of a parenchymal arteriole in a brain slice, the glass cannula needs to be calibrated by determining the pressure-flow relationship (Fig. 13D). The cannula is then submerged under the aCSF solution (at the microscope chamber) and subjected to stepwise increases in flow rate (four steps are recommended). The corresponding pressure at each flow rate is recorded and tracked with an acquisition system (e.g., pClamp). Successful myogenic responses are achieved when the cannula resistance (RC) is low [<25 arbitrary units (AU)]. Low cannula resistance, however, implies a larger tip opening, which makes introduction of the cannula into the small arteriole lumen (<30 µm) challenging. The success rate can be increased by beveling cannulas, and variability across experiments can be minimized by keeping RC within a narrow range (20 to 25 AU) (46).
Identification of an optimal arteriole for cannulation.
Once cannulas are calibrated, the brain slice is brought to the temperature-controlled microscope chamber. Arterioles in the slice are clearly distinguishable from venules by virtue of their single, circumferentially oriented VSMC layer. Venules and capillaries are characterized by their thin walls and considerably smaller diameters, respectively (Fig. 14B) (46). The ideal arteriole for cannulating has an opening just at the edge of the slice. Also, it is important that the parenchymal arteriole does not branch from a surface pial artery (Fig. 13B). Although it is considerably easier to cannulate the larger pial artery, the problem here is the significant decrease in flow at the distal end of these vessels, which causes a large pressure drop and thus prevents branching parenchymal arterioles from experiencing sufficient pressure to develop myogenic tone. Furthermore, an ideal arteriole is one that is long enough to cross all cortical layers, has considerable length in the same focal plane, and gives rise to a capillary network. It is also possible to visualize post-capillary venules. However, as discussed below, vascular network density/resistance considerations are important. Assuming a healthy tissue, a long arteriole and the presence of a downstream capillary network, the next step is introduction of the cannula into the lumen of the parenchymal arteriole. This is a delicate step requiring minimal arteriole manipulation. The edge of the arteriole is sticky, and the abluminal side of the vessel wall sometimes becomes stuck to the cannula; pulling the cannula away from the vessel in this situation may cause stretching of the arteriole wall and damage to the vessel. To achieve minimal contact with the vessel opening, it is helpful to provide a small amount of positive flow when approaching the arteriole opening with the cannula. When the positive flow causes the opening of the arteriole to enlarge, the tip of the cannula is moved from side to side at the open end, thereby aligning the cannula with the arteriole. At this point, the cannula is slowly introduced into the arteriole.
Determining the resistance of the vascular network.
Following the successful introduction of the cannula into the parenchymal arteriole (Fig. 13C), the next step is to determine the total resistance (RT) of the perfused system, comprising resistance of the cannula perfusion system (syringe pump + tubing + cannula), denoted RC, plus the resistance of the arteriolar network (arteriole + downstream capillaries), denoted RA (Fig. 13E). A second pressure-flow relationship is experimentally established using at least three flow rates. This step is essential because if arterioles are too short, they will not be able to maintain intravascular pressure and thus will not develop tone. This latter situation would be evidenced by a drop in pressure over time under constant flow rate conditions. On the other hand, if the cannulated arteriole has an extensive downstream vascular network, it may result in a high RT. Although arterioles with an extensive downstream vascular network are ideal, they are relatively uncommon. Thus, when such extreme conditions are encountered, it is best to continue looking for another arteriole with an RT value closer to average. An average RT value is ∼70 AU, whereas an RT value > 100 AU may indicate an extensive downstream vascular network [please see Fig. 2, A and B, in Kim et al. (46)]. Also, as noted by Lovick et al. (35), cases in which resistance progressively increases may indicate fluid buildup within the perfused network (35). Once the RT is determined and found to lie within an appropriate range, the arteriole is equilibrated to physiological pressure (∼30–40 mmHg). This step requires calculating the flow rate needed to bring the vascular network to the estimated desired pressure.
Arteriole pressurization.
After determining RC and RT, the next step is determining the resistance of the RA, defined as RT – RC. RA, calculated from Ohm’s law (P = Q·R), is used to determine the flow rate needed to bring arterioles to the desired intravascular pressure, where P is the desired pressure, RA is the resistance of the perfused vascular network, and Q is the flow rate. The same approach is used to achieve lower or higher intravascular pressure values. The flow rate is then set to the value needed and the vessel is allowed to equilibrate. Following equilibration, determined by establishment of a plateau pressure and thus diameter, the experiment can be started.
As noted above, an important limitation of the system is that the distal ends of the perfused network are open. Thus, at equilibrium, the plateau reflects the combined effects of inflow, outflow and leaks from the distal ends of the perfused network. Manipulating the intravascular flow rate will cause the pressure to fluctuate until a new equilibrium is achieved. Notably, the system cannot be used at very high flow rates because this will cause distal openings to expand, resulting in greater efflux and a drop in pressure.
Determining passive diameters.
A final step in the cannulation technique is determining the passive diameter of the cannulated arteriole. Because various flow rates may have been used depending on the protocol, at the end of the experiment, passive arteriole diameters are measured in the presence of zero Ca2+ plus papaverine at each flow rate used during the experiment. Of note, papaverine causes vasodilation for passive diameter measurement by nonspecific phosphodiesterase inhibition (47).
Data analysis.
Images are acquired through video microscopy, typically at 1 frame/s. Diameter changes are extracted by processing image sequences using automated image software, such as Image J, for which share codes are now available. Automated approaches minimize experimenter bias potentially introduced during manual measurements of small diameter changes (e.g., 10%). Diameters can be measured at multiple points along the vessel wall and then averaged. The same approach is used to measure passive diameters. The passive diameter value at a given flow rate is then used to determine the active diameter or tone of the arteriole. A caveat here is that the intravascular pressures reported using this approach are estimates and not measured values (36). Equal flow rates (and not exact pressure values) are used to determine active and passive arteriole diameters. Thus, in the presence of Ca2+, the tone of the arteriole is expected to result in a slightly higher intravascular pressure than that generated by the same flow rate in zero Ca2+.
Exclusion criteria.
Given the challenges and limitations of the brain slice preparation, which on average yields one successful experiment per day per experimenter, it is essential that technical variabilities be minimized. For example, if the perfused arteriole network has above average RT reflecting a connected dense capillary network or below average RT owing to a short arteriole, the recommendation is to stop and look for another arteriole to cannulate before conducting the experiment. The experienced experimenter should track the length of the vessel and visually estimate the density of the perfused vascular network. Alternatively, a fluorescent dye (e.g., Alexa 488) can be added to the intraluminal solution, and the extent of the vessel network estimated based on visualization (Fig. 14C). It is important to avoid short vessels. Here, the efflux of the fluorescent solution is evident. Also, it is best to avoid arterioles with a dense vascular network as these are the exception. For quantification purposes, at the end of the experiment, the used slice with the corresponding stained perfused vasculature can be fixed, and the vascular density measured using image software (e.g., Image J, Vesselucida). Likewise, if RC is too high, the cannula would need to be changed. Conducting an experiment with a high RC requires high flow rates, which can significantly increase shear stress. In addition, in cases where RC is the main contributor to RT, the vessel might not develop tone at flow rates comparable to those that promote tone in other vessels. Thus, because many factors can result in outlier values that could significantly alter experimental conditions, care must be taken during the experiment and subsequent data interpretation. In summary, comparable RC and RT values between experiments and an RA contribution to RT greater than that of RC (preferably >50%) is recommended.
Additional potential sources for exclusion are large deviations in pressure readings resulting from a blockage in the cannula. This can occur during longer (>1.5 h) experiments. Attention to pressure readings during the experiment can help prevent this problem from becoming unmanageable. If a large increase in pressure is noted, it could indicate blockage of the tip of the cannula with debris; if so, the tip needs to be visualized, and the debris needs to be removed. Once this is achieved, the previously calibrated cannula can be reintroduced into the vessel. In cases where this step is not possible and data obtained before the obstruction includes both active and passive diameter recordings, the data can be used up until the point where the cannula became clogged.
Although myogenic responses might be reduced under disease conditions, it is possible that an observed lack of tone could result from a damaged arteriole, caused by distension of the arteriole during dissection or the cannulation procedure itself. Testing the reactivity of the arteriole to a bath-applied vasoconstrictive agonist at the end of the experiment can be a good positive control for distinguishing between artifactual and disease-related changes in tone.
Important considerations.
It is important to mention that a conventional, nonpressurized, nonperfused brain slice preparation has been a widely used approach for addressing intercellular communication at the neurovascular unit in brain slices. This includes studies in which stimulus-evoked diameter changes are measured in constricted vessels with pharmacological agonists. Although this experimental approach is capable of higher throughput and can provide insights into mechanisms driving vascular responses, it also comes with notable limitations. In this approach, parenchymal arterioles are constricted with an agonist, most commonly the thromboxane A2 (TXA2) analog U46619, included in the bath; thus, the entire slice is exposed to the agonist (e.g., see Refs. 35, 44, 48, and 49). Thus, if cells other than VSMC express the TXA2 receptor, their activity can also be affected. The same would apply to alternative agonists. In addition, the lack of fluid flow within the arteriole lumen eliminates mechanical stimuli important in the regulation of intracellular Ca2+ and membrane potential of vascular cells, thereby eliminating the biomechanical integration with mechanosensitive ion channels and receptors, which are critical determinants of vascular tone (50). Accordingly, lack of perfusion and flow within the parenchymal arterioles in a slice may also preclude the assessment of recovery processes upon termination of the stimulus. For example, it is difficult for vessels to relax to baseline levels after having been exposed to increasing concentrations of a vasoconstrictive agent. Likewise, the degree to which vessels dilate to vasodilators may be an underestimation compared with values that would be obtained if vessels were fluid-filled.
h. Veins.
Veins complement arterial function, with the two circulation types working together to enable cardiovascular homeostasis. In large part, arteries are paired with a vein, with the artery and vein lying next to one another and seemingly in close contact. A good example of this is the abdominal aorta and inferior vena cava. The necessity of such pairing is because of their individual functions. The arterial circulation is responsible for carrying oxygen-laden and nutrient rich blood from the heart to tissues and works at a relatively high pressure. By contrast, the venous circulation brings deoxygenated, waste-carrying blood back to the heart at a pressure roughly 10% that of the arterial circulation. Veins also serve a capacitance function, being a reservoir of blood. Though the veins have the similar basic components as do arteries (a tunica intima, tunica media, tunica adventitia and perivascular adipose tissue), several properties of veins make them distinct from arteries. First, veins possess fewer layers of VSMC, as compared to an artery with a similar diameter. Figure 15A shows VSMC alpha actin staining in the rat thoracic aorta versus vena cava (above the liver). Though similar in diameter, the aorta has 9–10 concentric layers of VSMC, the vena cava contains one. Thus, the ability to change caliber of size is greater in the artery versus veins and lends to the substantial control that arteries have versus veins over determining total peripheral resistance. Second, veins have valves (Fig. 15B). These valves promote the one-way movement of blood, from tissues to the heart. This is essential because of the low pressure of the venous circulation. The function of the valves is assisted by the muscle surrounding it, which serve to pump the veins such that blood moves, step by step, back to the heart. Third, venous structure enables blood to pool. The best example to relate this idea is gravity-induced venous pooling in the extremities with inactivity. Physiologically, pooling specifically in the splanchnic circulation allows storage of an inactive component of blood that can be called into action in times of need, such as hemorrhage (52).
Figure 15.
A: three colors stain (Trichrome) and vascular smooth muscle cells (VSMC) alpha actin staining in the rat thoracic aorta vs. vena cava (above the liver). L, lumen. B: veins have valves to promote the one-way movement of blood from tissues back to the heart. Used with permission from Hartmannsgruber et al. (51).
Practical tips: veins.
The differences in structure make working with veins more challenging than most arteries. Their greatest difficulty has been in tearing the venous wall during dissection from the body and in cleaning. Veins can be wrinkly and are not smooth walled as is an artery. This occurs more when pooling of blood occurs before dissection.
One way to reduce this is to exsanguinate the animal before dissection. Although veins may be less visible with this (e.g., not pooled), this reduces the likelihood of clot formation, the removal of which can rip the vein.
When appropriate, try to remove the vein away from the artery first, by cannulating the artery on a wire to carefully dissect off the vein as a whole. Two examples of this are the superior mesenteric vein and abdominal vena cava.
Another technique that can be used to reduce tearing is to refrigerate (4°C) the veins and/or dissection in a cold physiological salt solution for about 30 min, followed by a warming step, also around 30 min, once tissues are mounted in their platform. Adoption of this depends on whether or not this would introduce a new variable into your experiment.
Consideration of perivascular adipose tissue.
PVAT surrounds most arteries and veins and has distinct phenotypic and genotypic characteristics when compared to other adipose depots (53–55). Due to its intimate interaction with its adjacent vascular cells, and its paracrine properties, PVAT can affect the production and release of endothelium-derived vasoactive factors and the contraction and relaxation responses of VSMC (56). In addition, PVAT influences vascular mechanical properties and integrity and cellular composition of the vascular wall. These vasoactive actions of PVAT have been confirmed in many vascular beds and in various species including humans (57–59), rodents (60–63), mice (64–67), and swine (68–71).
The vasoactive properties of PVAT vary by pathological and physiological states, vascular bed, and type of vascular preparation, and these parameters should be considered when PVAT is examined (62, 71–73). For example, PVAT-mediated anticontractile effects are suppressed or lost in disease conditions, such as obesity and hypertension (74, 75). When comparing actions of PVAT among different vascular beds, coronary PVAT has procontractile (71) and antidilatory (70) effects on coronary arteries from healthy swine, whereas aortic PVAT has anticontractile effects in healthy rat (76). Although uterine PVAT had antidilatory effects on isolated uterine arteries, removal of PVAT from uterine arteries in anesthetized pregnant rats reduced in vivo uterine artery blood flow, indicating a prodilatory effect in vivo and antidilatory effect in vitro (62). Regardless of the exact effect of PVAT on vascular responses (e.g., procontractile vs. anticontractile), there is consensus that in addition to inside-out vascular signaling, molecular signals can be directed from outside-in (i.e., PVAT-to-VSMC) to determine vascular reactivity. Recently, a novel function of PVAT was discovered. Watts et al. provided evidence that aortic PVAT supported stress-induced relaxation in rat aorta, and this effect was independent of structural connections between the aortic vascular cells and their adjacent PVAT (77). Thus, PVAT has paracrine effects on its neighboring vessel, but it can also sense and respond to mechanical stimuli.
Despite the large number of published scientific reports providing evidence of its vasoactive role, PVAT is generally still removed as a standard procedure, before ex vivo vascular experiments in most studies. Figure 15 presents examples of protocols that can be used to assess the vasoactive effects of PVAT on isolated vessels. This illustration depicts experiments using wire myography; however, similar protocols can be used in pressure myography (77). In protocol A (Fig. 16A), two segments from the same vessel are tested, with one segment being PVAT-denuded and the other PVAT-intact. Strengths of this preparation are that no vascular damage is issued by removing PVAT and the interaction between PVAT and its neighboring vessel in this ex vivo experiment mimics in vivo conditions. Protocol B allows for crossover experiments (62, 71) (Fig. 16B), whereas protocol C can be used to determine whether the effects of PVAT on vascular reactivity are due to PVAT-derived factors (62, 71, 73) (Fig. 16C).
Figure 16.
Experimental protocols assessing the vasoactive effects of perivascular adipose tissue (PVAT). Two segments of the same vessel are tested, with one segment being PVAT-denuded and the other PVAT-intact (A). A vascular segment denuded from PVAT is mounted into a wire myograph and is tested in the absence of PVAT (left). Subsequently, the same vascular segment is tested in the presence of its neighboring PVAT (right), which had been initially removed and stored at 4°C physiological salt solution until experimentation. In this protocol, PVAT from comparing groups and conditions can be used to determine whether the observed effect is specific to (patho)physiological state (B). A vascular segment is cleaned from its PVAT, which is incubated in physiological salt solution at 37°C to create PVAT-conditioned media. The PVAT solution is filtered and added into the wire myograph chamber. Concentration-response curves to selected stimuli are performed in the absence and presence of PVAT-conditioned media (C). Created with Biorender.com.
MOUNTING AND NORMALIZATION
a. Pin and Wire Myograph
Before performing a set of experiments, myographs should always be calibrated according to the manufacturer’s instructions. It is not necessary to calibrate every day, and we recommend calibrating for every new set of experiments. Before mounting isolated vessels on pin and wire myographs, please turn on myograph and heat settings (37°C) for at least 15 min in advance of mounting. The data acquisition system (e.g., The PowerLab) and software (e.g., LabChart) can be also turned on at this point. Fill the myograph chambers with the physiological salt solution appropriate for isometric procedures and start the flow of gas (either 95% O2/5% CO2 or blood gas mixture), which is important for both oxygenation of the isolated vessels and pH control. Unplug an individual pin myograph chamber and bring it close to the silicon petri dish that holds the isolated segments and the dissecting microscope. Using the microscope and fine forceps, carefully transfer the isolated vessel to the myograph chamber.
Large conduct arteries and veins.
For transferring large conduit arteries and veins, it is recommended that a protruding branch of the segment (if possible), or a piece of PVAT, be held with fine forceps so that mechanical damage does not occur to the part of the segment that will be functionally tested. The isolated segment can be then slid onto the two stainless steel myograph pins that should be close together, but not touching one another. Before letting go of the isolated segment, open the pins with the micrometer so the pins are touching the walls of the vessel, but not generating any appreciable tension or force (this is to stop the isolated vessel from floating off the pins due to buffer movement from gassing). Next, the myograph and data acquisition software should be reset to read zero (0) mN force. Then, passive force can be applied by opening the pins with the micrometer. Table 4 presents recommended passive forces applied to commonly studied conduit artery and venous segments (78–80). The rationale for setting these basal passive forces is that vessels produce maximal active response in accordance with the actin-myosin interactions for the specific vessel type. The actin-myosin interface is optimal when the vessel is stretched to an extent where the actin and myosin filaments have maximal contact but with enough of a baseline stretch to provide room to contract (81). However, if not enough stretch is applied, the opportunity for contraction is reduced, and conversely, too great a stretch both damages the tissue and diminishes the actin-myosin interaction (81). Finally, allow the vessel to “rest” for at least 30 min to equilibrate. Change the buffer every 15 min and reapply passive force if vessel loses tone.
Table 4.
Recommended passive forces, for optimal length-tension relationships, from commonly studied conduit artery and venous segments on pin myographs
| Species | Vascular Bed | Force, mN |
|---|---|---|
| Mouse | Aorta | 5.0 |
| Rat | Aorta | 30.0 or 40.0 |
| Rat | Carotid artery | 10.0 |
| Rat | Superior mesenteric artery | 15.0 |
| Rat | Vena cava | 10.0 |
| Rat | Superior mesenteric vein | 1.0 |
To understand the contribution of endothelium-derived factors on vascular contraction and/or relaxation, the endothelium can be denuded. To denude conduit vessels, before mounting, place vessel on a medium-sized pipette tip (i.e., ∼200 µL) and gently role the vessel segment on the pipette tip across a gloved fore finger or palm ∼10 times. The absence or reduction of relaxation (less than 15–10%) to an endothelium-dependent vasodilator (e.g., acetylcholine, 3–10 μM) will confirm successful denudation.
Small vessels.
For transferring small resistance arteries and veins, it is recommended that the vessel be lifted using either two forceps or forceps and dissecting scissors, as physically holding the vessel will likely induce damage that will likely impede or “kill” vessel function. Next, place the vessel on the jaw of the myograph that is connected to the micrometer. At this point, the vessel can be trimmed to the required 2 mm length. If needed, adjust the dissecting microscope to maximum magnification. Secure the stainless-steel wires. Stainless-steel wires are generally 25 or 40 µm in diameter. Although there is no specific criteria on which wire diameter to use, choice is generally relative to vessel diameter, and consistency need to be maintained across all vessels and animals within an experiment, as wire diameter is an important variable in the normalization process (please see below). Vessels can be mounted as indicated in Fig. 17. Below is a step-by-step description of the procedure:
Figure 17.
Step-by-step description of mounting small resistance arteries and veins on the wire myograph.
Bring the jaws together to gently “hold” the first wire in position and then secure the “top-end” of the wire on the micrometer-side jaw. It should be noted that both ends of both wires are secured in a clockwise direction when the artery is finally mounted. Of note, in some classical myographs systems, (e.g., Living Systems Instrumentation), jaws have to have rubber blocks put under them so jaws and transducer aren’t pressed down and broken during the mounting procedure.
With fine forceps, delicately pick up the vessel at its tip and move it to the end of the wire at the unsecured end. Use the wire to gently open the lumen, if necessary. Blood streaming out of the vessel is a good sign the lumen is opened.
Once the vessel is on the wire, slowly push or pull the vessel up to the space between the two myograph jaws. A common error that is likely to denude the endothelium or kill the vessel is pulling the vessel so it stretches too much, or pushing so it becomes scrunched. This is a slow and controlled procedure. Once the vessel is between the two jaws, secure the “bottom-end” of the wire (again, clockwise) on the micrometer-side jaw.
Gently thread the second wire through the lumen of the vessel in one motion using the previously secured wire as a guide. Avoid rubbing the endothelium. After enough wire has been threaded through the vessel, bring the jaws together to gently “hold” the second wire in place. Do not worry if the tips of the vessel are pinched in the myograph jaws.
Secure the “top-end” of the wire on the force-transducer-side jaw. Extra care should be applied to securing wires on the force transducer-side jaw.
Straighten the second wire being careful not to accidently stretch the vessel, and secure the “bottom-end” of the wire on the force transducer-side jaw. Maneuver the wires with forceps so that they are parallel and adjust the micrometer to that the wires are close and not generating any appreciable tension or force. After mounting, allow vessels 20–30 min “rest” before proceeding with normalization.
To denude small vessels, after mounting, gently open the wires and vessel using the micrometer and rub the lumen with a hair shaft (82). Err on the side of caution regarding the vigorousness of the rubbing, as this can potentially damage the vessel, especially if it gets scrunched during this process. Contraction to a vasoconstrictor agonist will confirm that the vessel was not killed during this procedure, and the absence or reduction of relaxation (less than 15%–10%) to an endothelium-dependent vasodilator (e.g., acetylcholine, 3–10 μM) will confirm successful denudation.
Normalization.
As with all experimental protocols, consistent baseline conditions are necessary to obtain reproducible data. Normalization is the process of standardizing the baseline experimental conditions for vessel function measurements assessed via wire myography (83). The initial, passive conditions at which the vessels are set are necessary for the execution of rigorous vascular function studies as this:
Standardizes experimental conditions, allowing for comparisons between vessels.
Optimizes vessel response (e.g., actin-myosin cross-bridge interaction).
Reduces a potential source of error.
Generally, the normalization procedure, as described below, is only applied to small vessels such as resistance arteries and arterioles. The “normalization procedure” for conduit vessels such as the aorta is the application of a consistent passive force (e.g., 5 mN for mice and 30 mN for rats) and this was described above.
In general, each individual vessel is stretched to internal circumference that reproduces the wall force exerted on the vessel at a suitable resting transmural pressure. In other words, it mimics the natural in vivo state of the vessel in terms of pressure and circumference. The transmural pressure generally adopted in most vascular function studies is 100 mmHg or 13.3 kPa. Most modern myographs have normalization software that allows the user to construct a passive length/tension graphs based on tension (mN/mm) relative to the change internal circumference (IC; μm) of a vessel of a known length (e.g., 2 mm). Each incremental force measurement taken at a known circumference, the corresponding transmural pressure is calculated using the Law of LaPlace {pressure = wall tension/[internal circumference/(2 x π)]}. This process is repeated, with incrementally smaller applied changes in internal circumference, until 13.3 kPa (100 mmHg) transmural pressure is reached or passed. The IC100 that is determined, however, may not produce the optimal pretension to be applied to the vessel; as noted previously, the reason for this is related to actin-myosin cross bridge interactions. Therefore, the optimal lumen diameter can be set by adjusting the micrometer position to the IC1 in which the IC100 is corrected (multiplied) by a normalization factor. The normalization factor (also known as the IC1/IC100 ratio or factor k) for commonly studied arteries from rats are indicated in Table 5 (84–87). If you are studying a novel vessel with an unknown normalization factor, we recommend that you experimentally determine this value to optimal results. We refer readers to the Danish Myo Technology (DMT) Normalization Guide for full mathematical and biophysical rationale of the normalization procedure.
Table 5.
Normalization factor values (also known as the IC1/IC100 ratio or factor k), for optimal length-tension relationships, from commonly studied rat artery beds on wire myographs
| Vascular Bed | Normalization Value |
|---|---|
| Mesenteric artery | 0.9 |
| Femoral artery | 1.1 |
| Basilar artery | 0.9 |
| Coronary artery | 0.9 |
b. Pressure Myograph
Arteries, arterioles, and veins can be mounted in-between two opposite glass cannulas in the pressure myograph chamber. Pressure can be provided through a pressure servo-controlled system or an inexpensive hydrostatic gravity column, both of which are connected to a pressure transducer to precisely monitor the pressure being applied to vessels. For understanding how to generate and control flow, please see Flow-Mediated Dilation section described below.
The pressure transducer should be calibrated regularly, as changes in barometric pressure can affect pressures since atmospheric pressure is assumed to be “0” in experimental protocols. The diameter of the cannula should be a sufficient size to fit the vessel of interest, but large enough that it does not impede solutions to flow through. Cannulas are prepared by pulling borosilicate glass capillaries using a micropipette puller to generate a cannula with a long and thin tip at one end. Break the tip of the cannula to the desired size under a dissection microscope. The nontip end should be fire polished before inserting it into the feeding tube to ensure that it does not scrape against the tubing and potentially block the cannula. Myograph vendors typically sell cannulas in various sizes as well. The cannula is filled with oxygenated physiological salt solution using a syringe attached with an attached 0.2-µm diameter filter. This will prevent the cannula from being blocked by artefacts. Ensure no bubbles are in the cannula or feeding line as this may pass through the vessel and strip the endothelium. Mounting the vessel onto the cannula can be difficult and requires practice and patience. Below is provided a step-by-step description of the procedure:
If the lumen of the vessel is open, use two fine forceps to hold onto the opening of the vessel and slide it over the cannula. If the lumen is closed, attempt to open it by using the forceps or the cannula tip. Although this method is easy to use for large diameter arteries and veins, it can be difficult to cannulate small diameter arterioles as it is difficult to grip onto the ends and open the lumen. An alternate approach is to force the cannula through the vessel wall at a branch point or cannulate a larger artery and advance the vessel along the cannula until the target area is reached. Alternatively, particularly for very small arterioles where a larger feed branch isn’t available, one may also leave a very small amount of connective tissue only at the ends of the vessel to grip to facilitate cannulation. If using this method, it is important that the bulk of the vessel be as clean as possible for measurements.
The vessel is secured to the cannula using thin (12-0) nylon suture thread. Sutures with a loose knot (half-hitch knots or similar) should be prepared so that they tighten once the ends are pulled together. It is useful when securing large arteries to add an extra loop to the knot so that the tie does not loosen over time.
Once one end is secured to the cannula, residual blood within the lumen can be removed by gently flowing solution through the lumen. This will also help open the distal end of the vessel if it is closed. The distal end can be cannulated and secured for flow-through applications, such as intraluminal administration of compounds.
If the experiment is to examine the effects of pressure alone, the vessel can be tied off and secured to the cannula to create a “blind sack” (Fig. 2B).
Once mounted, the chamber is placed onto a microscope with the recording equipment. Changes in diameter, wall thickness, and other parameters are recorded using video microscopy and specialized software. Settings should be adjusted to visualize the clearest image possible.
Vessels are aligned and stretched to an approximate physiological level by straightening the vessel at a high intraluminal pressure. This will also ensure that the vessel will remain within the focal plane when adjusting pressures.
The presence of leaks should be evaluated as this will influence vasodilation and contraction responses. Leaks can arise due to small branches or holes in the vessel, or if the preparation is not secured to the cannulas correctly. The presence of leaks can be tested for in vessels are pressurized at a high intraluminal pressure and closed to external pressure. The diameter should not change in a closed pressure system once pressure is reintroduced (Fig. 18).
Mounted preparations should be allowed to equilibrate at a low intraluminal pressure, after which the intraluminal pressure is set to a physiological level by increasing the pressure in a step-wise manner until the target pressure is achieved. The target intraluminal pressure is dependent on factors such as the vessel type, experimental design, and research question. For example, mesenteric arterioles are typically pressurized to 60 mmHg, but a lower pressure may be used to prevent the development of myogenic tone if investigating the effects of contractile agonists.
Similar to the pin and wire myograph, the endothelium can be denuded on the pressure myograph after cannulating the artery securing its proximal end. Passing bubbles through the artery or rubbing the lumen with the tip of a hair shaft (e.g., moose mane, which can be found at fly-tying shops) are two potential methods. If done correctly, small pieces of cellular debris should be visible after flushing the artery with fresh buffer and the constricted artery should not demonstrate vasodilation in response to acetylcholine (less than 15%–10% dilation).
Figure 18.
Example traces demonstrating loss of lumen diameter in a pressurized vessel with a leak. Representative traces showing (A) lumen diameter is stable in vessel without a leak and (B) is reduced in a preparation with a leak (either due to a branch/hole in the vessel or it was not tied to the cannulas correctly) in preparations pressurized to 100 mmHg and closed to external pressure. Lumen diameter is restored in the leaky vessel once pressure is reintroduced.
PHARMACOLOGICAL AND PHYSIOLOGICAL ASSESSMENT
a. Concentration Response Curves
Concentration response curves, or the graded biological response to a drug from a low to a high concentration, can be constructed in two ways in both isolated and in situ vessels. A cumulative concentration response curve increases the concentration of drug in an additive fashion. The lowest concentration of drug is added, and then drug is added step by step, waiting for each response to plateau before the next addition (Fig. 19A). By contrast, a noncumulative curve tests the biological effect of each drug concentration separately, with tissues being washed in between (Fig. 19B).
Figure 19.
A cumulative concentration response curve (A) and noncumulative curve (B) using increasing concentrations of serotonin (5-HT, 5-hydroxytryptamine).
Important Considerations
Both approaches have advantages and limitations. For a cumulative curve, advantages are that this can be done faster and with less buffer. A disadvantage is that this approach assumes that there is little to no tachyphylaxis/desensitization to the drug being given. If this were so, the maximum response caused by the drug would be underestimated. For a noncumulative curve, the advantage is that one more assuredly avoids the problem of desensitization. However, this approach can take a long time and a tissue can change during the course of an experiment. As such, the choice of most pharmacologists is a cumulative response curve. It is important that the method you use for constructing a pharmacological curve be communicated for just these reasons.
b. Pressure-Response Curve
Vascular pressure-response curves may be obtained using in situ preparations (88) or isolated vessels mounted on pressure myographs (89, 90). These curves are commonly generated to determine the myogenic capacity of a specific vessel. The myogenic capacity or myogenic response represents the ability of a blood vessel, in particular a resistance artery or an arteriole, to undergo vasoconstriction or vasodilation as intraluminal pressure increases or decreases, respectively. For comprehensive reviews on the physiological roles and the mechanisms that underlie these phenomena, please see Refs. 18, 91, and 92.
Due to its similarity to in vivo conditions, assessment of pressure-response curves in isolated and cannulated resistance arteries and arterioles is the most common means to determine myogenic phenomena in blood vessels. The level of myogenic tone in a vessel is the magnitude of vasoconstriction present at a specific intraluminal pressure with respect to the internal diameter measured under passive conditions at the same pressure. To achieve passive conditions, vessels are usually incubated in Ca2+-free buffer in the presence of a Ca2+-chelator, such as EGTA [ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid], and a vasodilator, such as adenosine or sodium nitroprusside (90, 93). Incubation in such a Ca2+-free buffer for 5 min is sufficient to deplete VSMC from their capacity to undergo pressure-dependent or agonist-induced contractions (94). However, a brief exposure to caffeine or an agonist-induced vasoconstrictor, such as norepinephrine, while under Ca2+-free conditions may be used to ensure that all intracellular Ca2+ stores are completely depleted (95, 96).
Intraluminal pressure steps should range from a minimal pressure that does not allow the vessel to collapse (e.g., 3–5 mmHg or 0 mmHg in the case of coronary arterioles) to that representing 1.2- to 1.5-times the mean in vivo intravascular pressure of the vessel. The latter may refer to published pressures obtained by others or to estimated pressures based on the branch order of the arterial tree to which the vessel being tested belongs. At a minimum, the range in pressure should encompass a low pressure point before myogenic tone is present and a high pressure that achieves maximal passive diameter, which is usually also the pressure of maximal myogenic tone. Care should be taken to ensure that the maximal pressure used does not reach the pressure at which vessels experience forced dilation and presumably mechanical disruption of VSMC cytoskeletal structures and the cell’s contractile apparatus (97). The steps in pressure are commonly 10 or 20 mmHg in size and 2–10 min in duration, and may be achieved via an abrupt or a ramped change in pressure (98, 99). It is also important to corroborate that vessels do not bend or bow at high pressures. This may be accomplished by extending the cannulated vessel to its original in situ length, when the vessel comes from nondistensible tissues (e.g., cerebral vessels). However, if the vessel comes from distensible tissues (e.g., mesenteric resistance arteries), it is best to extend it under cold (∼4°C) or passive (Ca2+-free) conditions until it is straight at the maximal pressure to be used in the curve.
Myogenic responses may be reported in different numerical expressions. The most common ones include: 1) percent myogenic tone (100, 101), and 2) slope of the myogenic response (90, 102). Percent myogenic tone is obtained by the equation:
| (1) |
where MT is percent myogenic tone, PD is the passive diameter (in Ca2+-free solution), and AD is the active diameter (in Ca2+-containing solution). Diameters usually refer to luminal diameters, but external diameters may also be used. To obtain the slope of the myogenic response, a linear regression of either the percent myogenic tone or percent initial diameter (i.e., the diameter at the lowest pressure in which myogenic tone was detected) should be obtained. That slope is derived from the equation:
| (2) |
where y is a specific internal diameter of the vessel, α is the intercept of the projected line, β represents the slope of the pressure to luminal diameter line, and x is a specific intraluminal pressure. The slope may be easily used to compare the magnitude of the myogenic response across experimental conditions but has the caveat of only working best for the linear portion of the pressure-response curve. Data obtained from pressure-response curves may be presented in many different graphical forms. It is recommended that the plot clearly shows the main characteristic of the curve that represents the focus of the study or experimental series, e.g., the degree of myogenic responsiveness or the range of pressures showing myogenic response. Although vasodilation in response to reductions in intraluminal pressure is part of the myogenic phenomena associated with pressure-response curves, this vasomotor characteristic has not been thoroughly studied. It is usually assumed that the diameter change observed upon increasing intraluminal pressure in a myogenic responsive vessel would be similar, in most of its characteristics, to that observed when the same level of pressure is reduced.
c. Flow-Mediated Dilation
Blood, or any physiological liquid traversing a vessel, creates shear on the vessel luminal wall, the apical side of the endothelium. The magnitude of generated shear stress depends on the rate of flow, the laminarity of flow, and on fluid viscosity. Endothelial cells have a variety of mechanisms to sense and respond to shear stress. One of the best studied effects of shear on endothelial cells is vasodilation (flow-mediated dilation, FMD), which results from the release of dilator factors including, but not limited to NO, arachidonic acid metabolites, reactive oxygen species, and from activation of electrical connections with underlying VSMC. FMD is one of the most physiologically important forms of endothelium-dependent dilation (103, 104) and has been studied in health and disease. Virtually all arteries and veins studied from conduit to resistance vessels, and across species and vascular beds, demonstrate an endothelium-dependent FMD with rare exception (105).
The fundamental procedures for ex vivo study of microvascular function were described in a methods paper published in AJP almost 40 years ago by Duling et al. (106). His technique, however, would not have allowed for the assessment of FMD since only one side of a studied vessel was cannulated, but many of the general technical considerations are relevant today. In addition to general technical issues associated with imaging of pressurized arterioles in vitro, there are distinct technical considerations when studying FMD. Both are reviewed below.
Air bubbles.
It is critical to avoid trapping air bubbles in the line during cannulation. Once flow is generated even small amounts of air passing through the vessel can damage endothelial function. Extraordinary care must be taken to remove air within tubing before initiating the experiment. This includes insuring that the tubing and buffer are warmed to the same temperature, filling the reservoirs with buffer using the distal end of the tubing, and tapping the lines and stopcocks before connecting the tubing to the cannula. There are no hard and fast rules for effectively avoiding air bubbles, but preventive measures (full flushing of all lines before cannulation) are necessary. It is also critical to seek and remove existing air bubbles within the vessel or glass pipettes before initiating flow.
Mounting a vessel onto a pipette.
The goal of vessel cannulation is to mimic the degree of baseline circumferential (intraluminal pressure) and longitudinal (stretch) stress that the vessel experienced in vivo. The former is achieved by raising pressure of the perfusing fluid to estimated physiological levels, typically between 20–60 mmHg (107). The amount of pressure used will influence the magnitude of vasomotor responses. Pipettes should be positioned so that the cannulated vessel is not stretched beyond its pre-cannulation state but is not so loose as to create curves in the vessel segment.
Measuring vessel diameter.
Not all segments of a cannulated vessel respond in unison to applied vasomotor stimuli. It is important to find a small region of the vessel and consistently record changes in diameter from that area throughout the experiment. It is customary to find a segment with easily delineated internal borders that is not within 1 mm of either pipette tip (i.e., nearest the center of the vessel). If vessels are too short (< 2 mm in length) not enough shear is generated on the endothelial wall to cause dilation. Manual assessment of vascular diameter typically uses electronic video calipers overlaid on the vessel image. This provides an accurate and easily obtained measurement at the same place in a given vessel over time and avoids parallax associated with mechanical calipers. By convention vessel diameter is measured as internal diameter of arterioles based on the darker shadow delineating the vessel edge as it curves perpendicular to the plane of view. A single measurement can be made in the center of the vessel, or multiple equally spaced measurements can be averaged. Imaging software for automatic tracking of vessel diameter is available but accuracy varies depending on how cleanly the adventitia was isolated especially for those systems which measure outside diameters. Note that for flow studies in conduit vessels, monitoring outer diameter is necessary since inner diameter is typically not discernable.
Dealing with vessel side-branches.
To generate flow through a vessel, it is important to have a closed system, so fluids do not escape from the vessel or tubing. The most common reason for a breach in continuity is presence of vessel side branches which result in lower intraluminal pressures rendering diameter measurements suspect. The presence of one small side-branch can be tied off with 10-0 silk suture using a square knot (also used for securing the vessel to the pipette). If multiple side-branches, side-branches with short stalks, or holes in the vessel are present, it is necessary to begin with a new vessel. This issue of leaky vessels is more thoroughly discussed above (mounting and normalization; b. Pressure Myograph: Flow-Mediated Dilation, Item No. 7)
Generating flow.
The investigator has a choice of methods for generating flow through the vessel. The goal is to do this without changing intraluminal pressure. Commercial systems can be obtained that generate variable amounts of flow by modulating inflow and outflow rates. The advantage is knowing the flow rate and inflow and outflow pressures, but disadvantages are cost and having to calculate estimated shear stress, the stimulus for FMD, based on vessel diameter.
An alternative approach harnesses gravity to generate a pressure gradient across the vessel. To do this, two columns of fluid are used, one connected to each cannulating pipette. Initially both columns are equilibrated (∼60 mmHg). To generate flow and maintain near constant intraluminal pressure, one cannula reservoir is raised 10 mmHg and the other is lowered by the same amount. This adjustment creates a pressure gradient across the vessel with minimal impact on intraluminal pressure. Based on the pressure gradient and the calculated resistance from minimal pipette internal diameter, one can estimate flow rates and shear stress. Alternatively, commercial flow meters (e.g., see Livingsystems Instrumentation: livingsys.com; or Flowmeter—micro from Corsolutions; ElveFlow.com) can be put in line with the reservoir column to measure flow directly. Advantages are simplicity and direct assessment of flow. Disadvantages include additional expense for measuring flow and the need to calculate flow velocity.
Matching pipette impendence.
Whichever system is used to generate flow, it is important to use pipettes matched for impedance. This can be estimated by severing the tip of each pipette to create an orifice of ∼30 µm (for a 100–150 µm vessel). Using pipettes of similar resistance from one experiment to the next will help ensure similar degrees of flow at a given pressure gradient. Having pipettes of similar resistance within an experiment also ensures maintenance of pressure inside the vessel when adjusting flow. At the end of each flow experiment, it is recommended that pipette resistance matching be confirmed. To do this, vessels are first rendered passive by incubating with papaverine, nitroprusside, and/or Ca2+-free buffer. In this way the vessel acts as a pressure sensor, dilating if flow is initiated anterograde through the larger diameter pipette and collapsing if flow is generated from the reservoir attached to the smaller tipped pipette. Next 100 mmHg gradient should be imposed in one direction, recording diameter, and again in the opposite direction with recording of diameter. If pipettes are matched the diameters at peak flow forward and in reverse will vary by < 5 μm. A larger discrepancy would be indicative of mismatched pipette resistances.
Important Considerations
Physiological administration of agonists and interventions.
One advantage of a cannulated arteriolar system is the ability to expose vessels to exogenous agents either intraluminally or extraluminally, something not possible in wire tension myography. Thus, autonomic neurotransmitters or parenchymal tissue metabolites can be delivered from the physiologically relevant adventitial side of the vessel, whereas hormonal or paracrine compounds, as well as systemic pharmacological agents, can be delivered intraluminally as would occur in vivo.
There is some evidence that short-term (hours) exposure of certain compounds intraluminally affect primarily endothelial cells, whereas exposure through the circulating bath primarily affects VSMC (108). Therefore, it might be possible to apply distinct treatments to endothelial cells and/or vascular VSMC.
There are a number of laboratories that routinely use pressure myography for assessing microvascular function, including FMD. It is recommended that in-person training be considered when establishing this as a new methodology in a laboratory.
SOFTWARE AND ANALYSIS
a. Software
IonOptix, Living Systems, and DMT are all examples of companies that have commonly used proprietary software that can be used to measure vasoreactivity of isolated arteries. Recently, open-source software from VasoTracker (109) have also been developed that is free of charge and is conceptually able to work in conjunction with any microscopy system. It is out of the purview of this review to provide instructions on how to use the individual software components from every source, but in general they should all do similar tasks. For wire myography, the most common data acquisition used is PowerLab (https://www.adinstruments.com/products/labchart) developed by ADinstruments. PowerLab detects and converts analog force signals to digital data in user-determined units (such as grams or milli-Newtons). LabChart is a physiological data analysis software that creates a platform for recording devices to work together. It allows to acquire biological signals from multiple sources simultaneously and apply advanced calculations and plots. For optimization and analysis of wire myography experiments, it is possible to use specialized software modules, including the DMT normalization, peak analysis, and concentration-response modules.
For pressure myography, the software should accurately and rapidly (as close to real time as possible) measure the inner diameter of the isolated arteries using digital calipers. The calipers should always measure more than one spot of the isolated artery at any one time with concurrent data being obtained from each location the calipers are being used. The actual values of the inner diameters that are obtained at every specific time point needs to be able to be easily removed and placed into a graphical program for analysis. These numbers from the digital calipers are just as important for analysis as the ability to easily obtain and remove representative traces of the isolated arteries responding to particular stimuli; it is helpful to provide these with the quantified data of diameter changes for publication. The digital video calipers in whichever program is to be used are generally set using a hemocytometer or micrometer scale, and the accurate calibration of these will be essential to accurate digital caliper measurements. There is a plethora of other software components that can be integrated with measuring artery diameter that could include temperature, or flow rate (and other readouts). Depending on your experimental design, budget, and other laboratory materials, one should consider the utility of adding these to the diameter measurements by the software.
b. Pharmacological Analysis
Whichever way concentration-response curves are constructed, analysis provides important parameters that define a response. Figure 20 is an example of a cumulative concentration response curve to the agonist serotonin (5-HT, 5-hydroxytryptamine) in the isolated abdominal vena cava, reported as a percentage of an initial challenge to the adrenergic agonist norepinephrine. This sigmoidal curve is defined primarily by three parameters. The threshold is the lowest concentration which causes a biological response. The maximum is the maximum biological response. The potency of an agonist, as this graph shares, can be found by determining the maximum response, finding the value for a half-maximal (50% of its own maximum) response, and drawing a line (dashed black line) from this level to where it meets the concentration-response curve. Drop down from this point on the curve to the x-axis, and the value on the x-axis is the EC50 value (effective concentration that causes a 50% response). In Fig. 20, the threshold is just around 1 × 10−7 M and EC50 value 1 × 10−6 M.
Figure 20.
Cumulative concentration response curve to the agonist serotonin (5-HT, 5-hydroxytryptamine) in the isolated abdominal vena cava, reported as a percentage of an initial challenge to the adrenergic agonist norepinephrine (NE). CRC, concentration response curve.
Practical Tips: Pharmacological Analysis
Analyses can be calculated by hand, and this is a useful exercise to do when first learning pharmacology (Figs. 20 and 21).
Figure 21.
GraphPad Prism software allows a number of different methods for analyzing sigmoidal pharmacological curve (A). Concentration-response curve to phenylephrine (PE) in the presence or absence of Prazosin (alpha-1 antagonist, 5 nM) in rat thoracic aorta (B). GraphPad Prism software presents the option of Log (agonist) vs. response—variable slope (four parameters) (C), to calculate data that are derived from the antagonist experiment in B.
That said, a number of programs exist that do much of this work for you. In particular, GraphPad Prism (https://www.graphpad.com/scientific-software/prism/) was designed with pharmacologists in mind. We have no financial or other associations with the company that owns this program, founded by Dr. Harvey Motulsky. This is a valuable program.
Figure 21A shows the choices one has for analyzing a sigmoidal pharmacological curve, under Analysis. In Fig. 21, B and C, we use the option of Log (agonist) vs. response—variable slope (four parameters) to calculate data within GraphPad Prism (Fig. 21C) that are derived from the antagonist experiment in Fig. 20B. In this experiment, we wanted to calculate the pKB or apparent antagonist dissociation constant for the antagonist prazosin against the alpha-1 adrenergic receptor agonist phenylephrine. The EC50 value for phenylephrine (shaded in blue) shifted from 2.2 × 10−8 M to 4.67 × 10−6 M in the presence of prazosin (Fig. 21C).
c. Mechanical Analysis
Analysis of pressure-diameter curves obtained under passive conditions in isolated vessels mounted on pressure myographs is the most common ex vivo means to determine the mechanical properties of the vascular wall (110–112). Preparation of cannulated vessels for mechanical analyses in Ca2+-free solution should follow the same principles as described in the section Pressure-Response Curve. Also, the pressure steps and pressure range used to build the pressure-diameter curves should follow the rationale mentioned in that section. It must be reemphasized that the maximal intraluminal pressure used should not reach the point of force dilation or rupture, as the quantification of stiffness requires the intraluminal pressure to be limited to the elasto-plastic domain. This should allow for maintenance of the intracellular (cytoskeletal) and extracellular matrix structures that affect the mechanical properties of the vascular wall. Indeed, it has been shown that cytoskeletal structures, such as F-actin stress fibers, as well as extracellular matrix components, such as elastin and collagen, participate in providing mechanical strength to the vascular wall under passive conditions, albeit each at different intravascular pressures (113–120). In addition, to avoid a major involvement of the axial component in the strain and stress assessment, vessels should be extended to eliminate any bending or bowing at the maximal intraluminal pressure used.
For the appropriate acquisition of vascular wall mechanical parameters, it is essential that measurements of wall thicknesses and internal diameters be accurate. Vascular wall thickness may be measured with the use of manual, electronic or computer-based video dimension analyses, as described above in the section Software. A manual system allows for visual measurement of the vascular wall as projected in a television screen from the input of a camera capturing the image generated by a microscopy system. This analysis may be accurate, but introduces the potential of individual bias as well as inter-observer variability. Electronic systems consist, for the most part, of commercially available video-dimension analyzers that automatically detect changes in light density and thus measure the luminal size as well as the wall thickness of the vessel being imaged. This system eliminates bias, but depends on the capacity of the apparatus to faithfully detect changes in light density and as such may introduce inaccurate measurements, particularly in images obtained when the vessel has fat or connective tissue still attached or is constricted and the edges of the vascular wall become more difficult to detect. Computerized systems use software to detect vessel wall thickness and allow for the development of programs that introduce premises that attempt to avoid bias as well as inconsistencies dependent on the capacity to detect changes in light density. These systems do so by assuming that the wall is incompressible and therefore preserves its volume across intraluminal pressures. Based on that principle, it is also possible to measure wall thickness at the intraluminal pressure that provides the best image and use the parameters obtained to calculate wall thickness at different intraluminal diameters. However, this latter method of assessing vascular wall thickness is seldom used.
Practical Tips: Mechanical Analysis
Wall thickness is most commonly measured at each different intravascular pressure and used in the calculation of a variety of vascular wall structural and mechanical parameters (111, 112, 121, 122). These parameters include:
- Wall to lumen ratio (W/L), commonly used for assessment of structural remodeling, calculated with the equation:
where τ is wall thickness and ID is internal diameter at a given pressure P.(3) - Wall cross-sectional area (CSA), which is calculated using the equation:
where ED and ID represent the external and internal vascular diameters at intraluminal pressure P, respectively.(4) - Circumferential Strain (ϵ), calculated with the equation:
where ID represents the internal vascular diameter measured at a specific intraluminal pressure P and ID0 represents the internal vascular diameter measured at the lowest intraluminal pressure used to build the pressure-diameter curve.(5) - Circumferential Stress (σ), calculated with the equation:
(6)
where ID and τ represent the internal diameter and wall thickness measured at a specific intraluminal pressure P.
The relationship between strain and stress is commonly used to assess the stiffness of a vessel (111). For example, a rightward shift on the strain versus stress plot is considered to represent a decrease in stiffness. It is also common to use the strain and stress data to determine stiffness via calculation of the Young’s (E) and/or the incremental moduli of elasticity (Einc), where higher modulus values indicate the vessel is stiffer. It is important to note that the Young’s modulus of elasticity should be used only when the strain versus stress relationship is linear. However, for blood vessels, this relationship is mostly curvilinear.
The incremental modulus of elasticity (Einc) is calculated with the equation:
| (7) |
where Δσ and Δϵ represent the changes in circumferential stress and circumferential strain at two consecutive incremental changes in intraluminal pressure.
The Einc is better suited to assess stiffness at high pressures (e.g., above 70 mmHg). Therefore, it is recommended to complement this parameter with the cross-sectional compliance (CSC) quantifying vascular stiffness at low pressures (e.g., below 40 mmHg).
The CSC is calculated by the equation:
| (8) |
where ΔA and ΔP represent the change in internal lumen cross-sectional area at a specific change in two consecutive intraluminal pressures, respectively.
Having two measures of vascular stiffness at complementary regions of the pressure-diameter curve allows for determination of the partial contribution that elements such as elastin and collagen play in the mechanical properties of the vascular wall (122). At low pressures, elastin is considered to be the dominant element, whereas at higher pressures collagen is dominant (114, 115). Therefore, calculation of the low modulus of elasticity (Elow, also known as Eelastin) at low pressures, and the high modulus of elasticity (Ehigh, also known as Ecollagen) at higher pressures, estimates the partial contribution of elastin and collagen to the mechanical properties of the vessel wall.
As pulse wave velocity (PWV) is considered the in vivo gold-standard measurement of arterial stiffness, estimations of this parameter may be calculated using mechanical parameters obtained from pressure-diameter curves in isolated and cannulated vessels. This calculated incremental PWV (cPWVinc) is obtained using the Moens–Korteweg equation:
| (9) |
where Einc is the incremental modulus of elasticity, τ and D represent wall thickness and internal diameter measured at a specific intraluminal pressure, and is the density of the intraluminal buffer (usually ∼ 1,005 kg/m3) used in the experiments.
d. Statistical Comparisons of Vascular Function
Regardless of the scientific method used to evaluate ex vivo vascular function, several factors should be considered when conducting statistical comparisons, such as confidence levels and margins of error, power, and effect sizes. For instance, the sample size determines accuracy or level of confidence in a true biological effect. It is an important factor in the majority of the biomedical research, given that it allows the scientist to make inference about a population or phenomenon from a sample. Sample size may differ between studies, and it can be determined based on several aspects, such as time, cost, and accessibility of collecting the data. However, the most important determinate of the appropriate sample size is adequate statistical power. Statistical power is the probability that the experiment correctly rejects the null hypothesis. The greater the statistical power, the higher probability of observing a true effect, and the lower probability of making a type II (false negative) error. It is common to design vascular experiments with a statistical power of 80% or greater. Therefore, choosing an adequate sample size is important for accuracy and scientific rigor, since too small of a sample size may lead to a misinterpretation of the true biological effect in the experiment (123). On the other hand, a sample size that is too large may lead to unnecessary wasting of resources and animals (123). As described by Charan et al. (123), it is possible to use a simple formula for sample size calculation [sample size = 2 SD2 (Zα/2 + Zβ)2/d2], where standard deviation (SD) is found in previous studies; Z values are from the Z table at type 1 error of 5% and at 80% power, respectively; d represents effect size or difference between mean values. Software and online (free) calculators are also available for sample size determination before an experiment. For further information on methods of sample size calculations for animal studies, we recommend the following reference (Ref. 123).
In a hypothetical situation, a vascular biologist aims to determine whether the absence of microbiota leads to changes in vascular structure. For preliminary data, she collected mesenteric resistance arteries from germ-free rats and germ-full rats. She observed that the absence of microbiota induced vascular atrophy or hypothrophic remodeling when compared to the germ-full rat. Specifically, the vascular wall thickness of the third branch of the mesenteric resistance arteries from male germ-free rat (∼7 wk old) was ∼20 μm at 60 mmHg. On the other hand, vascular wall thickness from germ-full rats was 35 μm. The standard deviation (SD) was 8 μm. Based on these data, the vascular biologist hypothesized that reintroduction of microbiota will normalize vascular wall thickness in arteries from the germ-free rat. For her next experiments, the vascular biologist selected the desired power of 0.80 with a probability of a Type I error of 0.05. For statistical testing, she used the two tailed unpaired t-test. The size effect in this situation will be 35 − 20 = 15. Therefore, the sample size will be: Sample size = 2 (8)2 (1.96 + 0.842)2/(15)2 = 4.46. Based on this calculation, the vascular biologist will need at least five animals per group. However, given that isolation of resistance arteries is a delicate process and may lead to vascular damage, the vascular biologist would expect that 10% of arteries will be lost. Therefore, she may adjust this attrition in calculated sample size (123). For this, the sample size should be divided by 0.9 to have the real sample size. As a result, including 10% attrition, she will use six rats per group (4.46/0.9 = 5.56 or ∼6 rats) in her next experiment to test her hypothesis.
For vascular function, the use of descriptive statistics is very common. Accordingly, for the ratio and interval data following the normal distribution, the most common descriptive statistics is mean and standard deviation (SD). For data not following the normal distribution, it is median and range (124). Nonetheless, several studies also use mean and standard error of mean (SEM) to describe the variability within the sample to summarize the variability in their data because it is less than the SD (125). Using SEM versus SD has to mostly do with data presentation and what message the investigator wants to carry. Presenting all individual data is the best approach especially for the small sample sizes we use in vascular experiments.
The main specific statistical analyses used to compare changes in vascular function in different conditions are: 1) Student’s t test (Student was the pen name of W.S. Gosset) to compare the means between two samples (unpaired in the case of independent samples, paired in the case of dependent samples), such as changes in efficacy (Emax) or potency (EC50), or vascular lumen diameter, etc.; 2) one-way or two-way analysis of variance (ANOVA) to compare more than two conditions and concentration response curves respectively; 3) Tukey’s and the Bonferroni correction are commonly used as post hoc analyses in one-way ANOVA and two-way ANOVAs, respectively. Note that repeated measures (RM) two-way ANOVA is acceptable and normally used to compare concentration-response or time-course curves. In general, RM two-way ANOVA compares curves without the need to fit a model with nonlinear regression, and is useful when you either have too few concentrations (or time points) to fit curves or curves that are nonsigmoidal in shape (e.g., relaxation to low concentrations of acetylcholine and contraction to high concentrations of acetylcholine) (126, 127). Importantly, all these tests are parametric stats. If your data are not normally distributed, they should not be used unless they are transformed to make it fit a Gaussian distribution. Transforming data is a method of changing the distribution by applying a mathematical function to each data value. However, a common reason that a data sample is non-Gaussian is because the size of the data sample is too small. Therefore, we recommend to increase the sample size prior transform the data to fit a Gaussian distribution. Please see GraphPad Prism guidelines to further information (https://www.graphpad.com).
Therefore, for evaluating concentration-response curves, it is preferable to evaluate potency (EC50) and/or efficacy or maximum response (Emax) values (Figs. 20 and 21). As described in the section above (Pharmacological Analyses), concentration-response curves are classically graphed with the concentration or concentration function (e.g., log10) on the x-axis and the measured effect, such as contraction, relaxation and tension, on the y-axis. The response to the drug is due to a function of concentration and time, but the graph illustrates the concentration-response relationship independent of time. When the concentration-response curve is plotted in a semilog scale (logarithm of drug concentration versus response), the relationship becomes sigmoidal (S-shaped). This is the most used method to evaluate EC50 values. Using a semilog scale, it is also possible to determine efficacy or Emax. Concentration-response curves are generally performed with concentrations that are equally spaced on a log scale and are usually fit to find the best-fit value of the logEC50. In general, a logistic function (logit) is used for fitting concentration-response curves. It is easy to visualize how the values are “stretched” at the lower and upper extremes to linearize the sigmoid curve. Essentially, this weights the entire curve rather than just using the linear portion of the S-curve to predict the EC50 value (Fig. 22). For further information about fitting concentration-response curves, please see Ref. 128.
Figure 22.
Logistic function (logit) is used for fitting sigmoidal concentration-response curves.
RECOMMENDATION AND CONCLUSION
Significant advances have been made in our understanding of the mechanisms underlying vascular function, structure, and more recently biomechanics, in physiology and diseases. Despite these advances, many questions still remain to be answered, and many controversies need to be resolved. In addition, many concerns are being raised about scientific results that cannot be reproduced. Differences in species, vascular bed, vessel size, and method of study contribute to the variability in many of the results described in the vascular biology literature. Therefore, by using these guidelines, scientists will increase scientific rigor (3) and collect reliable data that can be reproduced and validated by other vascular biologists.
Regardless of the scientific method used to evaluate ex vivo vascular function, such as pin, wire, or pressure myograph, the minimum recommendation is that scientists consider following, in every experiment (3):
Valid and reliable techniques.
Sex, age, and other relevant biological variables.
Authenticated models and others as appropriate to the field. If possible, adherence to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines which are intended to improve the reporting of research using experimental animals (129).
Sample size calculations.
Randomization of animals/samples to experimental conditions.
Sample collection should be performed at consistent times per day.
Tissue collection should be anatomically standardized when harvesting.
Vascular function should be performed in freshly harvested tissues.
Lumen diameter should be standardized within each vascular bed. For example, mesenteric resistance arteries: ∼3rd-4th order branches should be used for vascular function, and 4th-6th order for vascular mechanics; aortic sections should be from the thoracic aorta and excluding the aortic arch; segments should be consistent in their length, e.g., cut in 2-mm segments.
Proper negative and positive controls (e.g., loss of dilation to acetylcholine while maintaining constriction to phenylephrine).
Appropriate replicates within experiments (as well as repeat experiments).
Blinded analyses when feasible.
Measures to control sampling bias and controlling for interoperator variability.
Robust and precise statistics. For example, for concentration-response curves, maximum response (efficacy or Emax) should be calculated to show the highest point on the curve. Half maximal effective concentration (EC50) should only be calculated to evaluate potency of the drug when no differences were observed in Emax. If multiple groups are being compared, repeated measures ANOVA with a post hoc test incorporating correction for multiple comparisons (e.g., Bonferonni) should be used.
Inclusion/exclusion criteria and all data should be reported.
If outliers are removed, a justification should be provided and a method of outlier determination and removal should be described.
Therefore, by following these recommendations, experimental conditions should be standardized in a manner that is optimal for investigating physiological and pharmacological differences in arteries and veins. Nevertheless, it is ultimately up to the individual scientist to perform experiments in a rigorous and ethical manner (3).
GRANTS
This work was supported by National Institutes of Health Grants R00GM118885 and R01HL149762 (to C.F.W.); K99HL151889 (to C.G.M.); R01HL091905, R01HL137852, R01HL139585, R01HL146054, RF1NS110044, R61NS115132, and P20GM130459 (to S.E.); R01HD037831, R01HD096737, and R01HD088097 (to S.K.E.); R01NS082521 (to J.A.F.); R01HL146562 (to S.G.); R01HL135901 (to D.D.G.); R01HL088554 and R01HL137112 (to B.E.I.); R01HL123301, R01HL121871, and R01ES014639 (to N.L.K.); R01 HL088105 (to L.A.M-L.); R01HL142808 and R01HL146914 (to S.K.S); R00HL116769, R21EB026518, and S10OD023438 (to A.J.T.); R01HL151413 (to S.W.W.); and P01HL134604, DK076169, and DK115255 (to R.C.W.) and The Abigail Wexner Research Institute at Nationwide Children’s Hospital (to A.J.T.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.F.W. and R.C.W. conceived and designed research; C.F.W., L.A.M-L., S.K.S., P.T., A.J.T., S.W.W., R.C.W., C.G.M., S.E., S.K.E., J.A.F., S.G., D.D.G., B.E.I., and N.L.K. prepared figures; C.F.W., L.A.M-L., S.K.S., P.T., A.J.T., S.W.W., R.C.W., C.G.M., S.E., S.K.E., J.A.F., S.G., D.D.G., B.E.I., and N.L.K. drafted manuscript; C.F.W., L.A.M-L., S.K.S., P.T., A.J.T., S.W.W., R.C.W., C.G.M., S.E., S.K.E., J.A.F., S.G., D.D.G., B.E.I., and N.L.K. edited and revised manuscript; C.F.W., L.A.M-L., S.K.S., P.T., A.J.T., S.W.W., R.C.W., C.G.M., S.E., S.K.E., J.A.F., S.G., D.D.G., B.E.I., and N.L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Irving Zucker, former Editor-In-Chief of the American Journal of Physiology–Heart and Circulatory Physiology, for providing us with the opportunity to write these guidelines.
We thank Dr. Zdravka Daneva at the University of Virginia, Dr. Ramon A. Lorca at the University of Colorado–Anschutz Medical Campus, Dr. Francisco I. Ramirez-Perez at the Dalton Cardiovascular Research Center, Departments of Biomedical, Biological and Chemical Engineering, and Drs. Beyer A, Freed J, Durand M, Abu-hatoum O at the Departments of Medicine, Anesthesiology, and Physical Medicine and Rehabilitation, Cardiovascular Center, Medical College of Wisconsin, Milwaukee, WI, and Department of Surgery, HaEmek Medical Center, Technion Medical School, Haifa, Israel, for providing valuable feedback on these guidelines.
REFERENCES
- 1.Aird WC. Discovery of the cardiovascular system: from Galen to William Harvey. J Thromb Haemost 9 Suppl 1: 118–129, 2011. doi: 10.1111/j.1538-7836.2011.04312.x. [DOI] [PubMed] [Google Scholar]
- 2.Lubitz SA. Early reactions to Harvey's circulation theory: the impact on medicine. Mt Sinai J Med 71: 4: 274–280, 2004. [PubMed] [Google Scholar]
- 3.Hofseth LJ. Getting rigorous with scientific rigor. Carcinogenesis 39: 21–25, 2018. doi: 10.1093/carcin/bgx085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol 28: 220–231, 1902. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocciolone AJ, Hawes JZ, Staiculescu MC, Johnson EO, Murshed M, Wagenseil JE. Elastin, arterial mechanics, and cardiovascular disease. Am J Physiol Heart Circ Physiol 315: H189–H205, 2018. doi: 10.1152/ajpheart.00087.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Shi GP. Vascular wall extracellular matrix proteins and vascular diseases. Biochim Biophys Acta 1842: 2106–2119, 2014. doi: 10.1016/j.bbadis.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 8.Shu X, Ruddiman CA, KellerTCS , 4th, Keller AS, Yang Y, Good ME, Best AK, Columbus L, Isakson BE. Heterocellular contact can dictate arterial function. Circ Res 124: 1473–1481, 2019. doi: 10.1161/CIRCRESAHA.118.313926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res 86: 341–346, 2000. doi: 10.1161/01.RES.86.3.341. [DOI] [PubMed] [Google Scholar]
- 10.Hwa JJ, Ghibaudi L, Williams P, Chatterjee M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am J Physiol Heart Circ Physiol 266: H952–H958, 1994. doi: 10.1152/ajpheart.1994.266.3.H952. [DOI] [PubMed] [Google Scholar]
- 11.Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Kohler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One 2: e827, 2007. doi: 10.1371/journal.pone.0000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol 298: H466–H476, 2010. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buus NH, VanBavel E, Mulvany MJ. Differences in sensitivity of rat mesenteric small arteries to agonists when studied as ring preparations or as cannulated preparations. Br J Pharmacol 112: 579–587, 1994. doi: 10.1111/j.1476-5381.1994.tb13114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falloon BJ, Stephens N, Tulip JR, Heagerty AM. Comparison of small artery sensitivity and morphology in pressurized and wire-mounted preparations. Am J Physiol Heart Circ Physiol 268: H670–H678, 1995. doi: 10.1152/ajpheart.1995.268.2.H670. [DOI] [PubMed] [Google Scholar]
- 15.Garland CJ, Bagher P, Powell C, Ye X, Lemmey HAL, Borysova L, Dora KA. Voltage-dependent Ca(2+) entry into smooth muscle during contraction promotes endothelium-mediated feedback vasodilation in arterioles. Sci Signal 10: eaal3806, 2017. doi: 10.1126/scisignal.aal3806. [DOI] [PubMed] [Google Scholar]
- 16.Hong K, Cope EL, DeLalio LJ, Marziano C, Isakson BE, Sonkusare SK. TRPV4 (transient receptor potential vanilloid 4) channel-dependent negative feedback mechanism regulates Gq protein-coupled receptor-induced vasoconstriction. Arterioscler Thromb Vasc Biol 38: 542–554, 2018. doi: 10.1161/ATVBAHA.117.310038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci USA 109: 18174–18179, 2012. doi: 10.1073/pnas.1211946109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 19.Smith GL, Austin C, Crichton C, Wray S. A review of the actions and control of intracellular pH in vascular smooth muscle. Cardiovasc Res 38: 316–331, 1998. doi: 10.1016/s0008-6363(98)00020-0. [DOI] [PubMed] [Google Scholar]
- 20.Turlapaty PD, Altura BT, Altura BM. Interactions of Tris buffer and ethanol on agonist-induced responses of vascular smooth muscle and on calcium-45 uptake. J Pharmacol Exp Ther 211: 59–67, 1979. [PubMed] [Google Scholar]
- 21.Altura BM, Altura BT, Carella A, Turlapaty PD. Adverse effects of artificial buffers on contractile responses of arterial and venous smooth muscle. Br J Pharmacol 69: 207–214, 1980. doi: 10.1111/j.1476-5381.1980.tb07892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altura BM, Carella A, Altura BT. Adverse effects of Tris. HEPES and MOPS buffers on contractile responses of arterial and venous smooth muscle induced by prostaglandins. Prostaglandins Med 5: 123–130, 1980. doi: 10.1016/0161-4630(80)90099-3. [DOI] [PubMed] [Google Scholar]
- 23.Sethi S, Rivera O, Oliveros R, Chilton R. Aortic stiffness: pathophysiology, clinical implications, and approach to treatment. Integr Blood Press Control 7: 29–34, 2014. doi: 10.2147/IBPC.S59535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chamiot-Clerc P, Renaud JF, Safar ME. Pulse pressure, aortic reactivity, and endothelium dysfunction in old hypertensive rats. Hypertension 37: 313–321, 2001. doi: 10.1161/01.hyp.37.2.313. [DOI] [PubMed] [Google Scholar]
- 25.Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci (Lond) 122: 1–12, 2012. doi: 10.1042/CS20110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conley D, Hurst PR, Stringer MD. An investigation of human jejunal and ileal arteries. Anat Sci Int 85: 23–30, 2010. doi: 10.1007/s12565-009-0047-9. [DOI] [PubMed] [Google Scholar]
- 27.Earley S, Walker BR. Endothelium-dependent blunting of myogenic responsiveness after chronic hypoxia. Am J Physiol Heart Circ Physiol 283: H2202–H2209, 2002. doi: 10.1152/ajpheart.00125.2002. [DOI] [PubMed] [Google Scholar]
- 28.Jackson-Weaver O, Paredes DA, Gonzalez Bosc LV, Walker BR, Kanagy NL. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca(2+)-activated potassium channels. Circ Res 108: 1439–1447, 2011. doi: 10.1161/CIRCRESAHA.110.228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jelinic M, Leo CH, Marshall SA, Senadheera SN, Parry LJ, Tare M. Short-term (48 hours) intravenous serelaxin infusion has no effect on myogenic tone or vascular remodeling in rat mesenteric arteries. Microcirculation 24: e12371, 2017. doi: 10.1111/micc.12371. [DOI] [PubMed] [Google Scholar]
- 30.Goodwill AG, Dick GM, Kiel AM, Tune JD. Regulation of coronary blood flow. Compr Physiol 7: 321–382, 2017. doi: 10.1002/cphy.c160016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCallinhart P, Scandling BW, Trask AJ. Coronary remodeling and biomechanics: are we going with the flow in 2020? Am J Physiol Heart Circ Physiol 320: H584–H592, 2020. doi: 10.1152/ajpheart.00634.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Billaud M, Lohman AW, Straub AC, Parpaite T, Johnstone SR, Isakson BE. Characterization of the thoracodorsal artery: morphology and reactivity. Microcirculation 19: 360–372, 2012. doi: 10.1111/j.1549-8719.2012.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osol G, Ko NL, Mandala M. Plasticity of the maternal vasculature during pregnancy. Annu Rev Physiol 81: 89–111, 2019. doi: 10.1146/annurev-physiol-020518-114435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24: 58–71, 2009. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovick TA, Brown LA, Key BJ. Neuronal activity-related coupling in cortical arterioles: involvement of astrocyte-derived factors. Exp Physiol 90: 131–140, 2005. doi: 10.1113/expphysiol.2004.028811. [DOI] [PubMed] [Google Scholar]
- 36.Kim KJ, Filosa JA. Advanced in vitro approach to study neurovascular coupling mechanisms in the brain microcirculation. J Physiol 590: 1757–1770, 2012. doi: 10.1113/jphysiol.2011.222778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim KJ, Iddings JA, Stern JE, Blanco VM, Croom D, Kirov SA, Filosa JA. Astrocyte contributions to flow/pressure-evoked parenchymal arteriole vasoconstriction. J Neurosci 35: 8245–8257, 2015. doi: 10.1523/JNEUROSCI.4486-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim KJ, Ramiro Diaz J, Iddings JA, Filosa JA. Vasculo-neuronal coupling: retrograde vascular communication to brain neurons. J Neurosci 36: 12624–12639, 2016. doi: 10.1523/JNEUROSCI.1300-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du W, Stern JE, Filosa JA. Neuronal-derived nitric oxide and somatodendritically released vasopressin regulate neurovascular coupling in the rat hypothalamic supraoptic nucleus. J Neurosci 35: 5330–5341, 2015. doi: 10.1523/JNEUROSCI.3674-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buskila Y, Breen PP, Tapson J, van Schaik A, Barton M, Morley JW. Extending the viability of acute brain slices. Sci Rep 4: 5309, 2014. doi: 10.1038/srep05309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ting JT, Lee BR, Chong P, Soler-Llavina G, Cobbs C, Koch C, Zeng H, Lein E. Preparation of acute brain slices using an optimized N-Methyl-D-glucamine protective recovery method. J Vis Exp 132: e53825, 2018. doi: 10.3791/53825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogura K, Takayasu M, DaceyRG , Jr.. Effects of hypothermia and hyperthermia on the reactivity of rat intracerebral arterioles in vitro. J Neurosurg 75: 433–439, 1991. doi: 10.3171/jns.1991.75.3.0433. [DOI] [PubMed] [Google Scholar]
- 43.Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456: 745–749, 2008. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ivanov A, Zilberter Y. Critical state of energy metabolism in brain slices: the principal role of oxygen delivery and energy substrates in shaping neuronal activity. Front Neuroenergetics 3: 9, 2011. doi: 10.3389/fnene.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishra A, Hamid A, Newman EA. Oxygen modulation of neurovascular coupling in the retina. Proc Natl Acad Sci USA 108: 17827–17831, 2011. doi: 10.1073/pnas.1110533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim KJ, Diaz JR, Presa JL, Muller PR, Brands MW, Khan MB, Hess DC, Althammer F, Stern JE, Filosa JA. Decreased parenchymal arteriolar tone uncouples vessel-to-neuronal communication in a mouse model of vascular cognitive impairment. Geroscience, 2021. doi: 10.1007/s11357-020-00305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol 147 Suppl 1: S252–S257, 2006. doi: 10.1038/sj.bjp.0706495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iddings JA, Kim KJ, Zhou Y, Higashimori H, Filosa JA. Enhanced parenchymal arteriole tone and astrocyte signaling protect neurovascular coupling mediated parenchymal arteriole vasodilation in the spontaneously hypertensive rat. J Cereb Blood Flow Metab 35: 1127–1136, 2015. doi: 10.1038/jcbfm.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenegger DG, Tran CH, Wamsteeker Cusulin JI, Gordon GR. Tonic local brain blood flow control by astrocytes independent of phasic neurovascular coupling. J Neurosci 35: 13463–13474, 2015. doi: 10.1523/JNEUROSCI.1780-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Longden TA, Hill-Eubanks DC, Nelson MT. Ion channel networks in the control of cerebral blood flow. J Cereb Blood Flow Metab 36: 492–512, 2016. doi: 10.1177/0271678X15616138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rondelli CM, Szasz IT, Kayal A, Thakali K, Watson RE, Rovner AS, Eddinger TJ, Fink GD, Watts SW. Preferential myosin heavy chain isoform B Expression may contribute to the faster velocity of contraction in veins versus arteries. J Vasc Res 44: 264–272, 2007. doi: 10.1159/000100991. [DOI] [PubMed] [Google Scholar]
- 52.Fink GD. Arthur C. Corcoran Memorial Lecture. Sympathetic activity, vascular capacitance, and long-term regulation of arterial pressure. Hypertension 53: 307–312, 2009. doi: 10.1161/HYPERTENSIONAHA.108.119990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun 5: 4099, 2014. doi: 10.1038/ncomms5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun C, Berry WL, Olson LE. PDGFRalpha controls the balance of stromal and adipogenic cells during adipose tissue organogenesis. Development 144: 83–94, 2017. doi: 10.1242/dev.135962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye M, Ruan CC, Fu M, Xu L, Chen D, Zhu M, Zhu D, Gao P. Developmental and functional characteristics of the thoracic aorta perivascular adipocyte. Cell Mol Life Sci 76: 777–789, 2019. doi: 10.1007/s00018-018-2970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A 13: 277–296, 1991. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 57.Ahmadieh S, Kim HW, Weintraub NL. Potential role of perivascular adipose tissue in modulating atherosclerosis. Clin Sci (Lond) 134: 3–13, 2020. doi: 10.1042/CS20190577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernandez-Alfonso MS, Gil-Ortega M, Aranguez I, Souza D, Dreifaldt M, Somoza B, Dashwood MR. Role of PVAT in coronary atherosclerosis and vein graft patency: friend or foe? Br J Pharmacol 174: 3561–3572, 2017. doi: 10.1111/bph.13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao YJ, Zeng ZH, Teoh K, Sharma AM, Abouzahr L, Cybulsky I, Lamy A, Semelhago L, Lee RM. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg 130: 1130–1136, 2005. doi: 10.1016/j.jtcvs.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 60.Ahmad MF, Ferland D, Ayala-Lopez N, Contreras GA, Darios E, Thompson J, Ismail A, Thelen K, Moeser AJ, Burnett R, Anantharam A, Watts SW. Perivascular adipocytes store norepinephrine by vesicular transport. Arterioscler Thromb Vasc Biol 39: 188–199, 2019. doi: 10.1161/ATVBAHA.118.311720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fontes MT, Paula SM, Lino CA, Senger N, Couto GK, Barreto-Chaves MLM, Mill JG, Rossoni LV. Renin-angiotensin system overactivation in perivascular adipose tissue contributes to vascular dysfunction in heart failure. Clin Sci (Lond) 134: 3195–3211, 2020. doi: 10.1042/CS20201099. [DOI] [PubMed] [Google Scholar]
- 62.Osikoya O, Ahmed H, Panahi S, Bourque SL, Goulopoulou S. Uterine perivascular adipose tissue is a novel mediator of uterine artery blood flow and reactivity in rat pregnancy. J Physiol 597: 3833–3852, 2019. doi: 10.1113/JP277643. [DOI] [PubMed] [Google Scholar]
- 63.Restini CBA, Fink GD, Watts SW. Vascular reactivity stimulated by TMA and TMAO: are perivascular adipose tissue and endothelium involved? Pharmacol Res 163: 105273, 2021. doi: 10.1016/j.phrs.2020.105273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bar A, Kieronska-Rudek A, Proniewski B, Suraj-Prazmowska J, Czamara K, Marczyk B, Matyjaszczyk-Gwarda K, Jasztal A, Kus E, Majka Z, Kaczor A, Kurpinska A, Walczak M, Pieterman EJ, Princen HMG, Chlopicki S. In vivo magnetic resonance imaging-based detection of heterogeneous endothelial response in thoracic and abdominal aorta to short-term high-fat diet ascribed to differences in perivascular adipose tissue in mice. J Am Heart Assoc 9: e016929, 2020. doi: 10.1161/JAHA.120.016929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boucher JM, Ryzhova L, Harrington A, Davis-Knowlton J, Turner JE, Cooper E, Maridas D, Ryzhov S, Rosen CJ, Vary CPH, Liaw L. Pathological conversion of mouse perivascular adipose tissue by notch activation. Arterioscler Thromb Vasc Biol 40: 2227–2243, 2020. doi: 10.1161/ATVBAHA.120.314731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang L, Xiong W, Zhao X, Fan Y, Guo Y, Garcia-Barrio M, Zhang J, Jiang Z, Lin JD, Chen YE. Bmal1 in perivascular adipose tissue regulates resting-phase blood pressure through transcriptional regulation of angiotensinogen. Circulation 138: 67–79, 2018. doi: 10.1161/CIRCULATIONAHA.117.029972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saxton SN, Whitley AS, Potter RJ, Withers SB, Grencis R, Heagerty AM. Interleukin-33 rescues perivascular adipose tissue anticontractile function in obesity. Am J Physiol Heart Circ Physiol 319: H1387–H1397, 2020. doi: 10.1152/ajpheart.00491.2020. [DOI] [PubMed] [Google Scholar]
- 68.Ahmad AA, Randall MD, Roberts RE. Sex differences in the role of phospholipase A2 -dependent arachidonic acid pathway in the perivascular adipose tissue function in pigs. J Physiol 595: 6623–6634, 2017. doi: 10.1113/JP274831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donovan J, Wong PS, Garle MJ, Alexander SPH, Dunn WR, Ralevic V. Coronary artery hypoxic vasorelaxation is augmented by perivascular adipose tissue through a mechanism involving hydrogen sulphide and cystathionine-beta-synthase. Acta Physiol (Oxf) 224: e13126, 2018. doi: 10.1111/apha.13126. [DOI] [PubMed] [Google Scholar]
- 70.Noblet JN, Owen MK, Goodwill AG, Sassoon DJ, Tune JD. Lean and obese coronary perivascular adipose tissue impairs vasodilation via differential inhibition of vascular smooth muscle K+ channels. Arterioscler Thromb Vasc Biol 35: 1393–1400, 2015. doi: 10.1161/ATVBAHA.115.305500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Owen MK, Witzmann FA, McKenney ML, Lai X, Berwick ZC, Moberly SP, Alloosh M, Sturek M, Tune JD. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: influence of obesity. Circulation 128: 9–18, 2013. doi: 10.1161/CIRCULATIONAHA.112.001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ayala-Lopez N, Thompson JM, Watts SW. Perivascular adipose tissue's impact on norepinephrine-induced contraction of mesenteric resistance arteries. Front Physiol 8: 37, 2017. doi: 10.3389/fphys.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J 16: 1057–1063, 2002. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 74.da Costa RM, da Silva JF, Alves JV, Dias TB, Rassi DM, Garcia LV, Lobato NS, Tostes RC. Increased O-GlcNAcylation of endothelial nitric oxide synthase compromises the anti-contractile properties of perivascular adipose tissue in metabolic syndrome. Front Physiol 9: 341, 2018. doi: 10.3389/fphys.2018.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galvez B, de Castro J, Herold D, Dubrovska G, Arribas S, Gonzalez MC, Aranguez I, Luft FC, Ramos MP, Gollasch M, Fernandez Alfonso MS. Perivascular adipose tissue and mesenteric vascular function in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 26: 1297–1302, 2006. doi: 10.1161/01.ATV.0000220381.40739.dd. [DOI] [PubMed] [Google Scholar]
- 76.Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol 286: H1107–H1113, 2004. doi: 10.1152/ajpheart.00656.2003. [DOI] [PubMed] [Google Scholar]
- 77.Watts SW, Flood ED, Garver H, Fink GD, Roccabianca S. A new function for perivascular adipose tissue (PVAT): assistance of arterial stress relaxation. Sci Rep 10: 1807–1020, 2020. doi: 10.1038/s41598-020-58368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCarthy CG, Wenceslau CF, Goulopoulou S, Ogbi S, Matsumoto T, Webb RC. Autoimmune therapeutic chloroquine lowers blood pressure and improves endothelial function in spontaneously hypertensive rats. Pharmacol Res 113: 384–394, 2016. doi: 10.1016/j.phrs.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tykocki NR, Thompson JM, Jackson WF, Watts SW. Ryanodine receptors are uncoupled from contraction in rat vena cava. Cell Calcium 53: 112–119, 2013. doi: 10.1016/j.ceca.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wenceslau CF, McCarthy CG, Szasz T, Calmasini FB, Mamenko M, Webb RC. Formyl peptide receptor-1 activation exerts a critical role for the dynamic plasticity of arteries via actin polymerization. Pharmacol Res 141: 276–290, 2019. doi: 10.1016/j.phrs.2019.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ 27: 201–206, 2003. doi: 10.1152/advances.2003.27.4.201. [DOI] [PubMed] [Google Scholar]
- 82.McCarthy CG, Wenceslau CF, Ogbi S, Szasz T, Webb RC. Toll-like receptor 9-dependent AMPKalpha activation occurs via TAK1 and contributes to RhoA/ROCK signaling and actin polymerization in vascular smooth muscle cells. J Pharmacol Exp Ther 365: 60–71, 2018. doi: 10.1124/jpet.117.245746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mulvany MJ, Halpern W. Mechanical properties of vascular smooth muscle cells in situ. Nature 260: 617–619, 1976. doi: 10.1038/260617a0. [DOI] [PubMed] [Google Scholar]
- 84.Mulvany MJ, Nyborg N. An increased calcium sensitivity of mesenteric resistance vessels in young and adult spontaneously hypertensive rats. Br J Pharmacol 71: 585–596, 1980. doi: 10.1111/j.1476-5381.1980.tb10977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ping NN, Cao L, Xiao X, Li S, Cao YX. The determination of optimal initial tension in rat coronary artery using wire myography. Physiol Res 63: 143–146, 2014. doi: 10.33549/physiolres.932631. [DOI] [PubMed] [Google Scholar]
- 86.Slezak P, Waczulikova I, Balis P, Puzserova A. Accurate normalization factor for wire myography of rat femoral artery. Physiol Res 59: 1033–1036, 2010. doi: 10.33549/physiolres.932043. [DOI] [PubMed] [Google Scholar]
- 87.Xiao X, Ping NN, Li S, Cao L, Cao YX. An optimal initial tension for rat basilar artery in wire myography. Microvasc Res 97: 156–158, 2015. doi: 10.1016/j.mvr.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 88.Hill MA, Davis MJ, Meininger GA. Cyclooxygenase inhibition potentiates myogenic activity in skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 258: H127–H133, 1990. doi: 10.1152/ajpheart.1990.258.1.H127. [DOI] [PubMed] [Google Scholar]
- 89.Hong K, Zhao G, Hong Z, Sun Z, Yang Y, Clifford PS, Davis MJ, Meininger GA, Hill MA. Mechanical activation of angiotensin II type 1 receptors causes actin remodelling and myogenic responsiveness in skeletal muscle arterioles. J Physiol 594: 7027–7047, 2016. doi: 10.1113/JP272834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. Alphavbeta3- and alpha5beta1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol 289: H322–H329, 2005. doi: 10.1152/ajpheart.00923.2003. [DOI] [PubMed] [Google Scholar]
- 91.Davis MJ. Perspective: physiological role(s) of the vascular myogenic response. Microcirculation 19: 99–114, 2012. doi: 10.1111/j.1549-8719.2011.00131.x. [DOI] [PubMed] [Google Scholar]
- 92.Hong KS, Kim K, Hill MA. Regulation of blood flow in small arteries: mechanosensory events underlying myogenic vasoconstriction. J Exerc Rehabil 16: 207–215, 2020. doi: 10.12965/jer.2040432.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinez-Lemus LA. Persistent agonist-induced vasoconstriction is not required for angiotensin II to mediate inward remodeling of isolated arterioles with myogenic tone. J Vasc Res 45: 211–221, 2008. doi: 10.1159/000112513. [DOI] [PubMed] [Google Scholar]
- 94.Uchida E, Bohr DF. Myogenic tone in isolated perfused resistance vessels from rats. Am J Physiol 216: 1343–1350, 1969. doi: 10.1152/ajplegacy.1969.216.6.1343. [DOI] [PubMed] [Google Scholar]
- 95.Coats P, Johnston F, MacDonald J, McMurray JJ, Hillier C. Signalling mechanisms underlying the myogenic response in human subcutaneous resistance arteries. Cardiovasc Res 49: 828–837, 2001. doi: 10.1016/S0008-6363(00)00314-X. [DOI] [PubMed] [Google Scholar]
- 96.Frisbee JC. Remodeling of the skeletal muscle microcirculation increases resistance to perfusion in obese Zucker rats. Am J Physiol Heart Circ Physiol 285: H104–H111, 2003. doi: 10.1152/ajpheart.00118.2003. [DOI] [PubMed] [Google Scholar]
- 97.Osol G, Brekke JF, McElroy-Yaggy K, Gokina NI. Myogenic tone, reactivity, and forced dilatation: a three-phase model of in vitro arterial myogenic behavior. Am J Physiol Heart Circ Physiol 283: H2260–H2267, 2002. doi: 10.1152/ajpheart.00634.2002. [DOI] [PubMed] [Google Scholar]
- 98.Halvorson BD, Whitehead SN, McGuire JJ, Wiseman RW, Frisbee JC. Endothelium-dependent impairments to cerebral vascular reactivity with type 2 diabetes mellitus in the Goto-Kakizaki rat. Am J Physiol Regul Integr Comp Physiol 317: R149–R159, 2019. doi: 10.1152/ajpregu.00088.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zou H, Ratz PH, Hill MA. Role of myosin phosphorylation and [Ca2+]i in myogenic reactivity and arteriolar tone. Am J Physiol Heart Circ Physiol 269: H1590–H1596, 1995. doi: 10.1152/ajpheart.1995.269.5.H1590. [DOI] [PubMed] [Google Scholar]
- 100.Kotecha N, Hill MA. Myogenic contraction in rat skeletal muscle arterioles: smooth muscle membrane potential and Ca(2+) signaling. Am J Physiol Heart Circ Physiol 289: H1326–H1334, 2005. doi: 10.1152/ajpheart.00323.2005. [DOI] [PubMed] [Google Scholar]
- 101.Syed AU, Reddy GR, Ghosh D, Prada MP, Nystoriak MA, Morotti S, Grandi E, Sirish P, Chiamvimonvat N, Hell JW, Santana LF, Xiang YK, Nieves-Cintron M, Navedo MF. Adenylyl cyclase 5-generated cAMP controls cerebral vascular reactivity during diabetic hyperglycemia. J Clin Invest 129: 3140–3152, 2019. doi: 10.1172/JCI124705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frisbee JC, Roman RJ, Falck JR, Krishna UM, Lombard JH. 20-HETE contributes to myogenic activation of skeletal muscle resistance arteries in Brown Norway and Sprague-Dawley rats. Microcirculation 8: 45–55, 2001. [PubMed] [Google Scholar]
- 103.Koller A, Sun D, Huang A, Kaley G. Corelease of nitric oxide and prostaglandins mediates flow-dependent dilation of rat gracilis muscle arterioles. Am J Physiol Heart Circ Physiol 267: H326–H332, 1994. doi: 10.1152/ajpheart.1994.267.1.H326. [DOI] [PubMed] [Google Scholar]
- 104.Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol 250: H1145–H1149, 1986. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- 105.Clifford PS, Madden JA, Hamann JJ, Buckwalter JB, Valic Z. Absence of flow-mediated vasodilation in the rabbit femoral artery. Physiol Res 59: 331–338, 2010. doi: 10.33549/physiolres.931672. [DOI] [PubMed] [Google Scholar]
- 106.Duling BR, Gore RW, DaceyRG , Jr., Damon DN. Methods for isolation, cannulation, and in vitro study of single microvessels. Am J Physiol Heart Circ Physiol 241: H108–H116, 1981. doi: 10.1152/ajpheart.1981.241.1.H108. [DOI] [PubMed] [Google Scholar]
- 107.Chilian WM, Layne SM, Klausner EC, Eastham CL, Marcus ML. Redistribution of coronary microvascular resistance produced by dipyridamole. Am J Physiol Heart Circ Physiol 256: H383–H390, 1989. doi: 10.1152/ajpheart.1989.256.2.H383. [DOI] [PubMed] [Google Scholar]
- 108.Marrelli SP, O'neil RG, Brown RC, BryanRM , Jr.. PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol Heart Circ Physiol 292: H1390–H1397, 2007. doi: 10.1152/ajpheart.01006.2006. [DOI] [PubMed] [Google Scholar]
- 109.Lawton PF, Lee MD, Saunter CD, Girkin JM, McCarron JG, Wilson C. VasoTracker, a low-cost and open source pressure myograph system for vascular physiology. Front Physiol 10: 99, 2019. doi: 10.3389/fphys.2019.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McCallinhart PE, Sunyecz IL, Trask AJ. Coronary microvascular remodeling in type 2 diabetes: synonymous with early aging? Front Physiol 9: 1463, 2018. doi: 10.3389/fphys.2018.01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morales-Quinones M, Ramirez-Perez FI, Foote CA, Ghiarone T, Ferreira-Santos L, Bloksgaard M, Spencer N, Kimchi ET, Manrique-Acevedo C, Padilla J, Martinez-Lemus LA. LIMK (LIM Kinase) inhibition prevents vasoconstriction- and hypertension-induced arterial stiffening and remodeling. Hypertension 76: 393–403, 2020. doi: 10.1161/HYPERTENSIONAHA.120.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Padilla J, Ramirez-Perez FI, Habibi J, Bostick B, Aroor AR, Hayden MR, Jia G, Garro M, DeMarco VG, Manrique C, Booth FW, Martinez-Lemus LA, Sowers JR. Regular exercise reduces endothelial cortical stiffness in Western diet-fed female mice. Hypertension 68: 1236–1244, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Anghelescu M, Tonniges JR, Calomeni E, Shamhart PE, Agarwal G, Gooch KJ, Trask AJ. Vascular mechanics in decellularized aortas and coronary resistance microvessels in type 2 diabetic db/db mice. Ann Biomed Eng 43: 2760–2770, 2015. doi: 10.1007/s10439-015-1333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bloksgaard M, Leurgans TM, Spronck B, Heusinkveld MHG, Thorsted B, Rosenstand K, Nissen I, Hansen UM, Brewer JR, Bagatolli LA, Rasmussen LM, Irmukhamedov A, Reesink KD, De Mey JGR. Imaging and modeling of acute pressure-induced changes of collagen and elastin microarchitectures in pig and human resistance arteries. Am J Physiol Heart Circ Physiol 313: H164–H178, 2017. doi: 10.1152/ajpheart.00110.2017. [DOI] [PubMed] [Google Scholar]
- 115.Bloksgaard M, Thorsted B, Brewer JR, De Mey MGR. Assessing collagen and elastin pressure-dependent microarchitectures in live, human resistance arteries by label-free fluorescence microscopy. J Vis Exp 134: e57451, 2018. doi: 10.3791/57451. [29683445] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Castorena-Gonzalez JA, Staiculescu MC, Foote CA, Polo-Parada L, Martinez-Lemus LA. The obligatory role of the actin cytoskeleton on inward remodeling induced by dithiothreitol activation of endogenous transglutaminase in isolated arterioles. Am J Physiol Heart Circ Physiol 306: H485–H495, 2014. doi: 10.1152/ajpheart.00557.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Katz PS, Trask AJ, Souza-Smith FM, Hutchinson KR, Galantowicz ML, Lord KC, StewartJA, Jr., Cismowski MJ, Varner KJ, Lucchesi PA. Coronary arterioles in type 2 diabetic (db/db) mice undergo a distinct pattern of remodeling associated with decreased vessel stiffness. Basic Res Cardiol 106: 1123–1134, 2011. doi: 10.1007/s00395-011-0201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McCallinhart PE, Cho Y, Sun Z, Ghadiali S, Meininger GA, Trask AJ. Reduced stiffness and augmented traction force in type 2 diabetic coronary microvascular smooth muscle. Am J Physiol Heart Circ Physiol 318: H1410–H1419, 2020. doi: 10.1152/ajpheart.00542.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Staiculescu MC, Galinanes EL, Zhao G, Ulloa U, Jin M, Beig MI, Meininger GA, Martinez-Lemus LA. Prolonged vasoconstriction of resistance arteries involves vascular smooth muscle actin polymerization leading to inward remodelling. Cardiovasc Res 98: 428–436, 2013. doi: 10.1093/cvr/cvt034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Trask AJ, Katz PS, Kelly AP, Galantowicz ML, Cismowski MJ, West TA, Neeb ZP, Berwick ZC, Goodwill AG, Alloosh M, Tune JD, Sturek M, Lucchesi PA. Dynamic micro- and macrovascular remodeling in coronary circulation of obese Ossabaw pigs with metabolic syndrome. J Appl Physiol (1985) 113: 1128–1140, 2012. doi: 10.1152/japplphysiol.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aroor AR, Das NA, Carpenter AJ, Habibi J, Jia G, Ramirez-Perez FI, Martinez-Lemus L, Manrique-Acevedo CM, Hayden MR, Duta C, Nistala R, Mayoux E, Padilla J, Chandrasekar B, DeMarco VG. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc Diabetol 17: 108, 2018. doi: 10.1186/s12933-018-0750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pennington KA, Ramirez-Perez FI, Pollock KE, Talton OO, Foote CA, Reyes-Aldasoro CC, Wu HH, Ji T, Martinez-Lemus LA, Schulz LC. Maternal hyperleptinemia is associated with male offspring's altered vascular function and structure in mice. PLoS One 11: e0155377, 2016. doi: 10.1371/journal.pone.0155377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother 4: 303–306, 2013. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jaykaran. “Mean +/- SEM” or “Mean (SD)”? Indian J Pharmacol 42: 329, 2010. doi: 10.4103/0253-7613.70402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nagele P. Misuse of standard error of the mean (SEM) when reporting variability of a sample. A critical evaluation of four anaesthesia journals. Br J Anaesth 90: 514–516, 2003. doi: 10.1093/bja/aeg087. [DOI] [PubMed] [Google Scholar]
- 126.Edwards JM, McCarthy CG, Wenceslau CF. The obligatory role of the acetylcholine-induced endothelium-dependent contraction in hypertension: can arachidonic acid resolve this inflammation? Curr Pharm Des 26: 3723–3732, 2020. doi: 10.2174/1381612826666200417150121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension 8: 344–348, 1986. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- 128.Motulsky H, Christopoulos A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting. San Diego, CA: GraphPad Software Inc., 2003. [Google Scholar]
- 129.Percie Du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Wurbel H. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Physiol 598: 3793–3801, 2020. doi: 10.1113/JP280389. [DOI] [PMC free article] [PubMed] [Google Scholar]