Abstract
Mouse models of cardiac disease have become essential tools in the study of pathological mechanisms, but the small size of rodents makes it challenging to quantify heart function with noninvasive imaging. Building off recent developments in high-frequency four-dimensional ultrasound (4DUS) imaging, we have applied this technology to study cardiac dysfunction progression in a murine model of metabolic cardiomyopathy. Cardiac knockout of carnitine palmitoyltransferase 2 (Cpt2M−/−) in mice hinders cardiomyocyte bioenergetic metabolism of long-chain fatty acids, and leads to progressive cardiac hypertrophy and heart failure. The proposed analysis provides a standardized approach to measure localized wall kinematics and simultaneously extracts metrics of global cardiac function, LV morphometry, regional circumferential strain, and regional longitudinal strain from an interpolated 4-D mesh of the endo- and epicardial boundaries. Comparison of metric changes due to aging suggests that circumferential strain at the base and longitudinal strain along the posterior wall are most sensitive to disease progression. We further introduce a novel hybrid strain index (HSI) that incorporates information from these two regions and may have greater utility to characterize disease progression relative to other extracted metrics. Potential applications to additional disease models are discussed that could further demonstrate the utility of metrics derived from 4DUS imaging and strain mapping.
NEW & NOTEWORTHY High-frequency four-dimensional ultrasound can be used in conjunction with standardized analysis procedures to simultaneously extract left-ventricular global function, morphometry, and regional strain metrics. Furthermore, a novel hybrid strain index (HSI) formula demonstrates greater performance compared with all other metrics in characterizing disease progression in a model of metabolic cardiomyopathy.
Keywords: contractility, four-dimensional ultrasound, hypertrophy, left ventricle, strain mapping
INTRODUCTION
Due to the heart’s central role in the distribution of oxygen, nutrients, signaling factors, and heat throughout the body, progressive cardiac dysfunction can not only compromise an individual’s quality-of-life, but also lead to systemic organ failure, morbidity, and mortality (1). Although cardiac hypertrophy is often a nonpathogenic adaptation to cardiovascular stress (e.g., aerobic exercise), when stressors persist or cardiomyocyte adaptation is compromised, the heart may progress to hypertrophic cardiomyopathy (2–4). Conditions such as metabolic disease and hypertension can introduce these persistent stressors on the heart by interrupting normal fatty acid oxidative metabolism in the myocardium, limiting energy substrate use in cardiomyocytes (5, 6). This predisposition to metabolic inflexibility leading to maladaptive pathologic remodeling will be characterized herein as metabolic cardiomyopathy (7–11).
Establishing a better understanding of the pathological mechanisms and progression of metabolic cardiomyopathy using murine models allows for controlled manipulations of disease-linked factors and aging-based monitoring of cardiac function (12–17). Herein we focus on one such model, in which metabolic cardiomyopathy is induced by a cardiac homozygous knockout of the carnitine palmitoyltransferase 2 (Cpt2) gene in the heart. We have demonstrated that deficiency of CPT2, a required enzyme for mitochondrial fatty acid oxidation of long-chain fatty acids, causes cardiomyocyte metabolic inflexibility and leads to progressive myocardial hypertrophy (18, 19).
Complementary to such murine models of cardiac disease, high-frequency ultrasound has become one of the most commonly used imaging tools to serially and noninvasively monitor heart function. Traditional ultrasound transducers for small animals only allow for planar imaging, thus reported metrics of global cardiac function (e.g., ejection fraction, stroke volume, cardiac output) heavily rely on predictions of the idealized geometries of the left ventricle (LV) (20–23). Fortunately, measurements of regional left-ventricular strain can be derived directly from planar imaging views and demonstrate greater sensitivity to myocardial function compared with ejection fraction (24). Still, collection of multiple nonparallel views of the left-ventricle requires physical repositioning of the ultrasound probe, introducing potential misalignment errors when comparing values within a mouse or across animals. Recent advancements in high-frequency four-dimensional ultrasound (4DUS) imaging mitigates these issues, providing gated volumetric data of the entire murine left-ventricle, with faster acquisitions and higher spatiotemporal resolution compared with high-field cine-MRI (25–27).
Using serial 4DUS data collected from both Cpt2M−/− mice and littermate controls, we describe here for the first time a standardized method for extracting regional kinematics information from the endo- and epicardial boundaries of the LV myocardium and investigate how cardiac strain metrics derived from these kinematic data can be used to characterize disease progression. Furthermore, we propose a hybrid strain index (HSI) metric which combines basal circumferential and posterior free-wall (FW) longitudinal strains, and demonstrate its superior utility in characterizing metabolic cardiomyopathy progression.
METHODS
Animal Models and Study Timeline
Heart and skeletal muscle (Cpt2M−/−) CPT2 conditionally deficient C57BL/6 female mice were generated using the MCK-Cre mice (Jackson Laboratories Stock No. 006475) as described (19). In that study, earlier lethality was shown in the male Cpt2M−/− mice compared with Cpt2M−/− females, and thus we focused our study on female mice to better investigate changes in cardiac function through aging. Littermates lacking Cre expression were used as controls. Mice were given free access to water and standard chow (PicoLab 5053, Lab Diets), in pathogen-free housing under 12-h light-dark cycles. A total of 26 mice were used for this study, comprising of 12 Cpt2M−/− mice (47 total 4DUS datasets) and 14 littermate controls (41 total 4DUS datasets), each serially imaged at least twice between 4 and 18 wk of age.
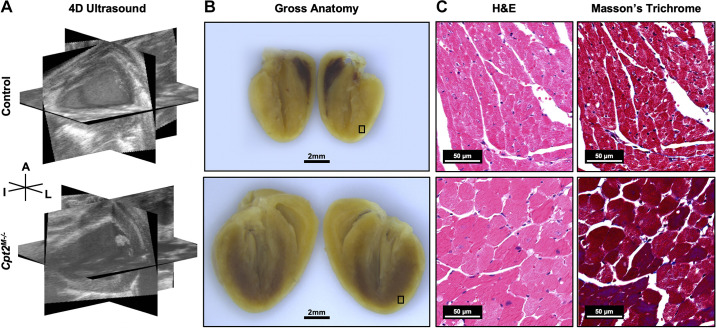
Figure 1 provides example of 4DUS images, gross sections, and histology from a representative control and Cpt2M−/− mouse, highlighting the increased cardiomyocyte size reported in prior literature (19). Cpt2M−/− and littermate control mice were euthanized at week 18 and had hearts freshly isolated and similarly prepared for histology (hematoxylin-eosin, H&E and Masson’s trichrome). If all Cpt2M−/− mice in a cohort died before week 18, the corresponding littermate controls were euthanized. All animal experiments were approved by the Purdue Animal Care and Use Committee.
Figure 1.
Cpt2M−/− induced hypertrophy compared with control. Representative control (top) and Cpt2M−/− (bottom) mouse hearts at 15 wk of age visualized with three orthogonal views from four-dimensional ultrasound (4DUS) data at end-diastole (A), gross anatomical sections post-fixation (B), and hematoxylin-eosin (H&E) and Masson’s trichrome histology from selected box regions (C). Ultrasound and gross section scale bar = 2 mm. Histology scale bar = 50 μm. Orientation is designated with labels for the anterior (A), inferior (I), and left (L) sides of the heart.
Ultrasound Imaging
4DUS data were collected using the Vevo2100 high-frequency ultrasound system (FUJIFILM VisualSonics Inc., Toronto, ON, Canada), a 40-MHz center frequency transducer (MS550D), and a translating linear step motor. Mice were weighed before imaging and then anesthetized using a low-flow vaporizer (SomnoSuite, Kent Scientific, Torrington, CT) with ∼2.5% isoflurane at 250 mL/min (28). Mice were then secured to a heated stage with gold-plated electrodes and had ventral thorax hair removed using depilatory cream. Serial short-axis ECG-gated kilohertz visualization (EKV) cine loops were acquired across the full left-ventricle (i.e., inferior to the epicardial apex through superior to the aortic valve), with step sizes of ∼200 µm and gating by cardiac- and respiratory-signals (25, 26). Extracted ultrasound data were then spatiotemporally compiled into a 4DUS dataset in MATLAB (MathWorks, Inc., Natick, MA).
Boundary Definition and Kinematics Analysis
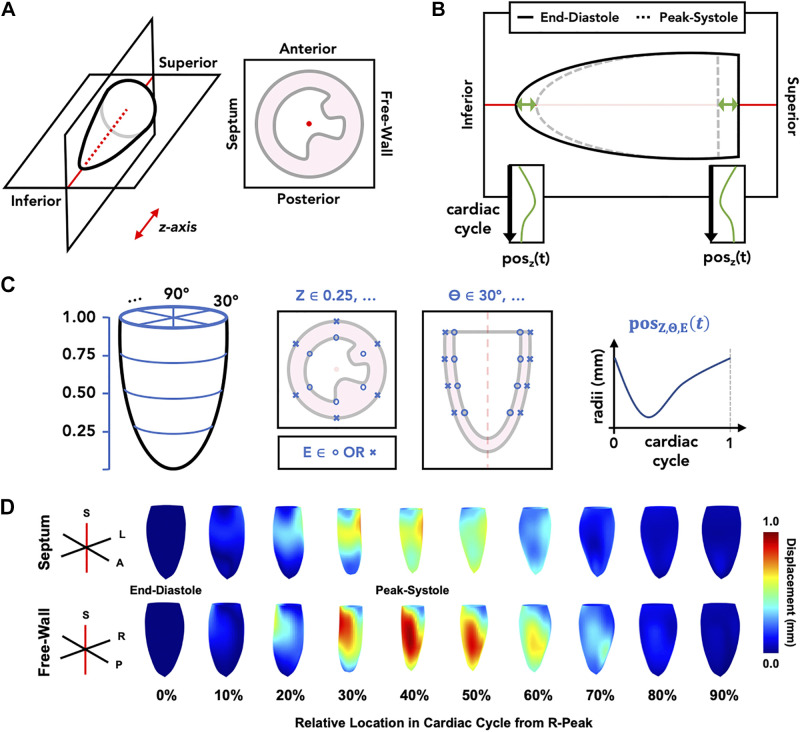
An in-house MATLAB graphical user interface (GUI) was used to visualize 4DUS data and track LV kinematics along the endo- and epicardial borders. Figure 2 shows a schematic representation of the standardized analysis procedure:
Figure 2.
Schematic for standardized four-dimensional ultrasound (4DUS) analysis procedure. The outlined steps include: reorientation of data to defined axes (A), tracking z-axis position (posz) of the left-ventricular base and apex across relative time (t) in the cardiac cycle (B), defining and tracking local wall motion (posZ,Θ,E) at a grid of endo- (E = o) and epicardial (E = x) points equally spaced across four short-axis slices (Z) and six rotations (Θ) around the left ventricle (C), and quantification of regional kinematics from which strain metrics are calculated (D). Meshes in section (D) are oriented such that the viewer is looking at the septal wall (top) or the free-wall (bottom), at 10% increments throughout a representative cardiac cycle. Red axes denote the same central “z-axis” that the data is oriented to in the first step.
Spatially reorient volumes to a standard set of x, y, and z axes (i.e., the apex-to-baseline defines a central z-axis, the anterior and posterior walls lie perpendicular to the y-axis, and the free-wall and septum lie perpendicular to the x-axis);
Track the translation of the LV chamber base and apex along the z-axis throughout the cardiac cycle;
Using a grid of automatically defined endo- and epicardial boundary points, track local wall motion throughout the cardiac cycle using the distance between each point and the central z-axis as reference; and
Using the tracked grid positions, interpolate a final 4-D mesh of each boundary, sampled uniformly at 60 rotations around the z-axis, 60 slices from base to apex, and at 60 time points across the cardiac cycle.
The grid defined in step C is structured such that points lie along four parallel short-axis slices (i.e., 25%, 50%, 75%, and 100% from the apex to base) and at six rotations around the kinematic axis (i.e., 30°, 90°, 150°, 210°, 270° and 330° from the free-wall oriented axis). As the base and apex positions are time dependent, tracked in step B, the z-positions of grid points are subsequently dependent on the base and apex positions at any given point across the cardiac cycle.
Using the derived 4-D meshes of each LV boundary, measurements of global cardiac function (e.g., ejection fraction, stroke volume), LV morphometry (e.g., end diastolic volume, left-ventricular mass), and regional strain were calculated, similar to those previously reported (29–31).
Derivation of Regional Cardiac Strain
We calculated the circumferential component of the Green–Lagrange strain tensor to estimate cyclic strain (Eθθ), assuming a circular cross-section at each short-axis slice location (32):
| (1) |
where C represents the relative circumference at short-axis slice z and time t in the cardiac cycle; CD is the circumference at end-diastole (i.e., t = 0). Curves of Eθθ were derived for slices corresponding to the basal, mid-ventricular, and apical regions of the left ventricle, from which peak strain, early systolic strain rate, late systolic strain rate, early diastolic strain rate, and late diastolic strain rate were extracted.
Complementary measurements of longitudinal (ELL) strain were calculated using the engineering linear small strain approximation:
| (2) |
where L represents the apex-to-base length along the boundary at rotation θ and time t in the cardiac cycle, and LD is the respective length at end-diastole. Metrics of peak-strain, systolic strain rate, early diastolic strain rate, and late diastolic strain rate were similarly extracted for each of the strain curves corresponding to the anterior free-wall, anterior, anterior septum, posterior septum, posterior, and posterior free-wall sections of the heart.
Hybrid Strain Index
Since the circumferential and longitudinal reference frames reflect the two conventional views of the LV (i.e., short-axis and long-axis), we sought to investigate whether a combination of the most sensitive location-specific metric in each respective reference frame could create an even better marker of cardiac dysfunction progression. Thus, we propose here a novel metric, the hybrid strain index (HSI):
| (3) |
computed as the L2 norm of the peak circumferential strain value at the base of the heart (Eθθ,Base) and peak longitudinal strain value along the posterior wall (ELL,Posterior), which were identified as the most sensitive metrics in the circumferential and longitudinal reference frames, respectively.
Aging-Based Metric Analysis
To assess the rate of change in each studied metric through time, a linear regression was fit to values from the same animal against age at the time of imaging. Herein, we refer to the slope derived from linear regression as the metric’s “trend.” In addition, to assess changes as a function of genotype and age only, a linear regression was fit to a pool of all values from each cohort against the age at imaging, treating all data as independent. Using 95% confidence intervals (CIs) associated with linear regression from each cohort, the earliest age at which the CIs no longer overlap was used as a marker for how soon the respective metric might be able to differentiate Cpt2M−/− mice from controls.
Receiver Operating Characteristic Analysis
To quantify the degree to which each metric can differentiate data from each cohort, using either the metric value or aging-based trends, we calculated area under the curve (AUC) values for each respective receiver operating characteristics (ROC) curve. Although disease progression in the Cpt2M−/− cohort was variable (i.e., some mice died prematurely while others reached 18 wk of age to be euthanized), all Cpt2M−/− mice were considered to represent progressive cardiac dysfunction.
Statistics
All statistical tests were performed in Prism (GraphPad Software, San Diego, CA). Cohort differences in metric trends were investigated using a nonparametric Mann–Whitney test, and P < 0.05 was considered statistically significant. All cohort-specific metric trend summaries are reported as median [interquartile range]. To further assess the relative variability of metric trends in the Cpt2M−/− cohort compared with control, an interquartile range (IQR) ratio is computed:
| (4) |
where IQRCpt2 is the interquartile range of the Cpt2M−/− cohort values, and IQRcontrol is the respective interquartile range of the control cohort values. Metric summary information, Mann–Whitney test P values, IQR ratio, AUC values, and 95% CI intersections for all computed metrics, except strain rate, are reported in Table 1. All comparable information for strain rate metrics is reported in Supplemental Table S1 (all Supplemental material is available at https://doi.org/10.6084/m9.figshare.c.5425209).
Table 1.
Measurements of metric trends through aging
| Metric | Control | Cpt2−/− | P Value | IQR Ratio | Trend AUC | 95% CI Interval | Metric AUC |
|---|---|---|---|---|---|---|---|
| n | 14 | 12 | |||||
| End-diastolic volume | 0.711 [0.187, 1.194] | 5.826 [2.537, 16.037] | 0.0001 | 13.40 | 0.946 | 8.96 | 0.720 |
| Peak-systolic volume | 0.265 [0.048, 0.603] | 5.768 [1.850, 16.034] | 0.0000 | 25.57 | 0.988 | 8.64 | 0.789 |
| Stroke volume | 0.397 [0.037, 0.802] | 0.281 [−0.086, 0.687] | 0.6620 | 1.01 | 0.554 | – | 0.536 |
| Ejection fraction | −0.205 [−0.741, 0.216] | −3.364 [−6.559 ,−1.633] | 0.0000 | 5.15 | 1.000 | 7.11 | 0.839 |
| Left ventricle mass | 2.078 [1.299, 2.525] | 9.302 [8.599, 25.402] | 0.0000 | 13.71 | 0.988 | DNO | 0.949 |
| LV wall thickness | 0.007 [0.003, 0.010] | 0.024 [0.002, 0.034] | 0.0760 | 4.85 | 0.708 | DNO | 0.981 |
| Peak Eθθ | |||||||
| Base | 0.106 [−0.093, 0.404] | 1.586 [1.065, 3.148] | 0.0000 | 4.19 | 0.988 | 6.52 | 0.861 |
| Mid-LV | 0.181 [−0.157, 0.435] | 1.712 [0.670, 3.346] | 0.0002 | 4.52 | 0.929 | 8.30 | 0.730 |
| Apical | 0.007 [−0.126, 0.338] | 1.787 [0.414, 2.840] | 0.0037 | 5.22 | 0.839 | 9.77 | 0.652 |
| Peak ELL | |||||||
| Ant. FW | 0.168 [−0.006, 0.429] | 0.898 [0.547, 1.118] | 0.0002 | 1.31 | 0.929 | 4.58 | 0.903 |
| Anterior | 0.129 [−0.043, 0.396] | 0.893 [0.496, 1.089] | 0.0002 | 1.35 | 0.940 | 6.06 | 0.860 |
| Ant. Sep. | 0.160 [−0.026, 0.361] | 0.845 [0.509, 1.115] | 0.0001 | 1.57 | 0.952 | 6.91 | 0.821 |
| Post. Sep | 0.062 [−0.034, 0.263] | 1.068 [0.641, 1.314] | 0.0000 | 2.27 | 0.976 | 6.17 | 0.880 |
| Posterior | 0.069 [−0.064, 0.427] | 1.223 [0.708, 1.684] | 0.0000 | 1.99 | 0.982 | 5.27 | 0.913 |
| Post. FW | 0.155 [−0.041, 0.478] | 1.002 [0.594, 1.254] | 0.0001 | 1.27 | 0.952 | 4.86 | 0.909 |
| Hybrid strain index | −0.100 [−0.445, 0.180] | −1.893 [−3.723, −1.261] | 0.0000 | 3.94 | 1.000 | 5.72 | 0.907 |
For each cohort, medians [interquartile ranges] are provided for control (n = 14) and Cpt2M−/− (n = 12) mice, along with P values from Mann–Whitney tests, ratio of interquartile ranges (IQR) in Cpt2M−/− and control trends, area under curve (AUC) values, and the 95% confidence interval (CI) intersection locations. Metrics where 95% confidence intervals overlapped for the entire age range of the study were noted with “–”, and metrics where 95% confidence intervals did not overlap are noted with “DNO.” FW, free-wall; LV, left ventricle.
RESULTS
Global Function and LV Morphometry
Focusing on aging-based changes in each animal, Fig. 3, B, D, and E, shows significant trend differences in ejection fraction (EF), end-diastolic volume (EDV), and left-ventricular mass (LVM), respectively. No significant trend differences were observed for stroke volume (SV; Fig. 3A) or wall thickness (Fig. 3F). Figure 3C demonstrates with EF the use of linear regression 95% confidence intervals to identify the earliest age at which the confidence intervals no longer overlap; subsequent measurements for all metrics are shown in Table 1. It is notable that stroke volume showed no separation based on 95% CI, suggesting that regardless of age no significant differences in stroke volume would be observed. Furthermore, both left-ventricular mass and wall thickness had no overlapping regions of 95% CI, suggesting that significant differences between control and Cpt2M−/− mice were present even before 4 wk of age. Gravimetric analysis of 4DUS-derived left-ventricular mass against heart mass at euthanasia (Supplemental Fig. S1) showed an R2 value of 0.87, comparable with correlations reported in literature (20). EF demonstrated the highest AUC value (AUC = 1.000) among all global function trends, with an 95% CI intersection at 7.11 wk of age (Table 1); however, produced a lower AUC (AUC = 0.839) compared with left-ventricular mass (AUC = 0.949) and wall thickness (AUC = 0.981) when comparing metric values independent of age.
Figure 3.
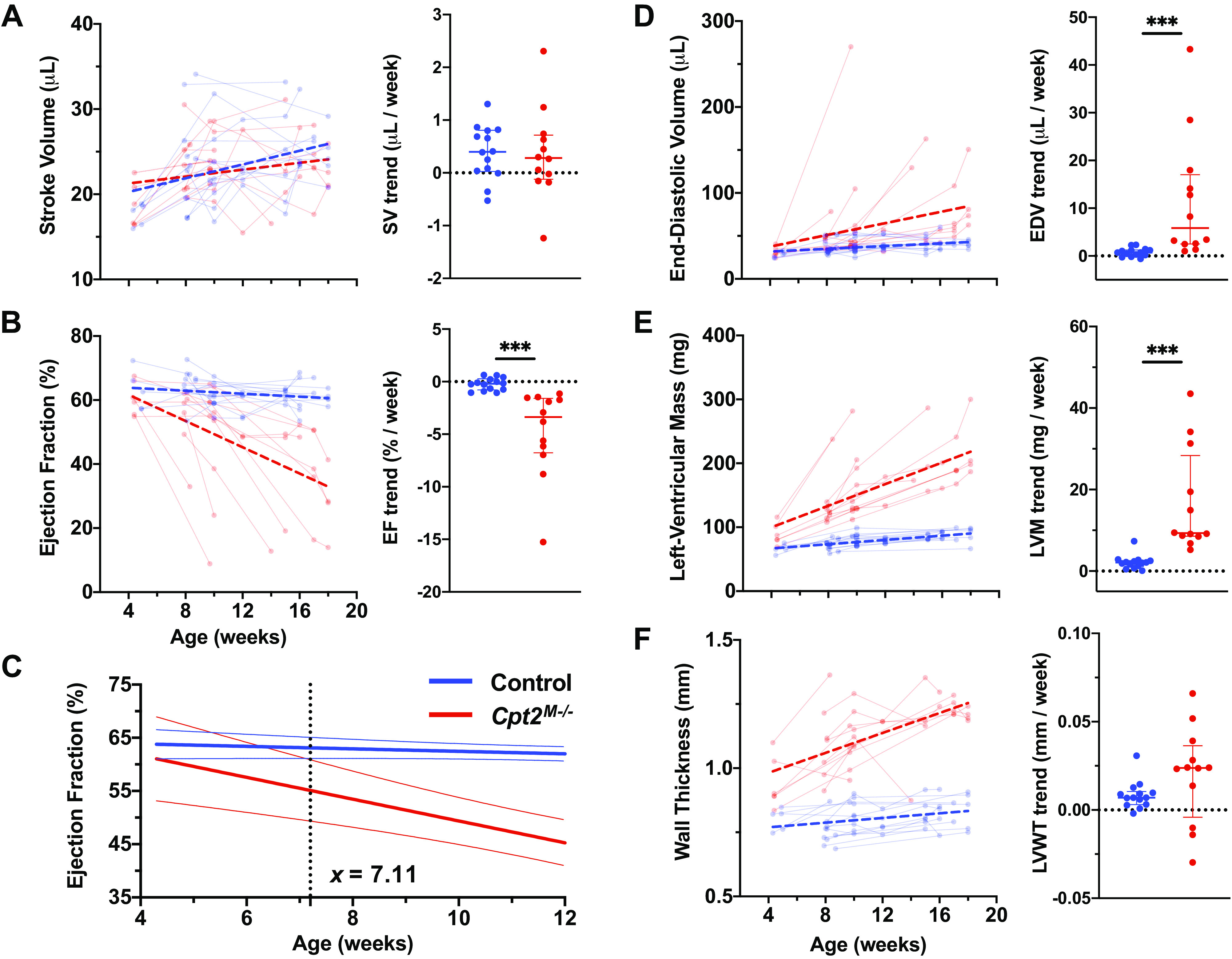
Global function and morphometric quantification of hypertrophy-driven dysfunction through aging. Global function and anatomy metrics derived from endo- and epicardial surfaces at both end-diastole and peak-systole are investigated, including stroke volume (SV) (A), ejection fraction (EF) (B), end-diastolic volume (EDV) (D), left-ventricular mass (LVM) (E), and wall thickness (LVWT) (F). On each scatter plot of serially collected measurements, data from control mice (n = 14) are shown in blue and Cpt2M−/− mice (n = 12) are shown in red. Points from the same mouse are shown connected, and linear regression performed on across each cohort is visualized with thick dashed lines. To assess the earliest age in which metrics from a Cpt2M−/− mouse might deviate from the control population, the cumulative linear regression lines with 95% confidence intervals (C) for ejection fraction were plotted and the age at which the confidence intervals no longer overlap was identified (i.e., 7.11 wk for EF). In addition, dot plots are shown for each metric comparing slopes derived from mouse-specific linear regression, with horizontal lines designating median and interquartile ranges. Markers above each comparison plot indicate significance levels from nonparametric Mann–Whitney tests (***P < 0.001).
Regional Circumferential Strain
Locations at which circumferential strain (Eθθ) metrics were derived are shown in Fig. 4A, alongside a representative strain curve with markings for peak strain, systolic strain rate, early diastolic strain rate, and late diastolic strain rate. Similar to those shown for global function metrics, aging-based changes in peak Eθθ at the base, mid-ventricle (Mid-LV), and apical regions are plotted in Fig. 4B. Comparisons of peak Eθθ trends show statistically significant differences between control and Cpt2M−/− mice at each location, with P values given in Table 1. Computed AUC values suggest that the greatest trend differences were observed at the base (AUC = 0.988), followed by Mid-LV (AUC = 0.929), and then apical regions (AUC = 0.839). Furthermore, although IQR ratios are similar between all three regions (i.e., 4.19, 4.52, and 5.22 respectively), trends at the base observed the earliest 95% CI intersection at 6.52 wk of age. When comparing metric values independent of age, peak Eθθ at the base still showed the highest AUC (AUC = 0.861) compared with the Mid-LV (AUC = 0.730) and apical regions (AUC = 0.652).
Figure 4.
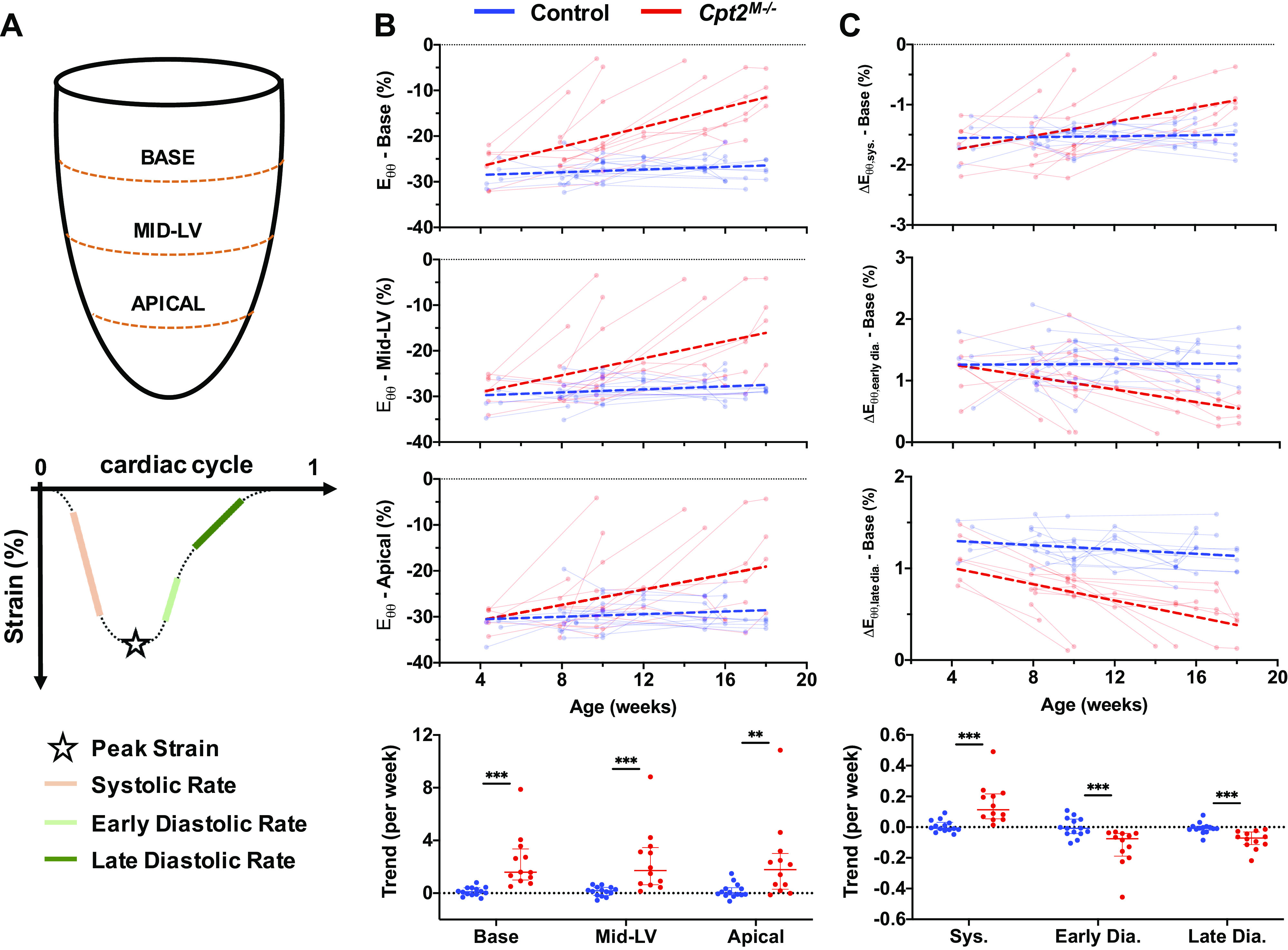
Regional circumferential strain measurements. A: schematic of where across the left ventricle circumferential strain was measured, and how peak strain (Eθθ), systolic strain rate (ΔEθθ,sys), early diastolic strain rate (ΔEθθ,early dia), and late diastolic strain rate (ΔEθθ,late dia) were defined on a representative strain curve. B: serially measured peak circumferential strain is shown for the base, mid-ventricle (Mid-LV), and apical slices, with data from control mice (n = 14) shown in blue and Cpt2M−/− mice (n = 12) shown in red. Points from the same mouse are shown connected, and linear regression performed on across each cohort is visualized with thick dashed lines. Comparison plots of strain trends from each mouse and region are shown (bottom), with horizontal lines designating median and interquartile ranges. Significance markers above each comparison plot indicate P value level from nonparametric Mann–Whitney tests (***P < 0.001; **P < 0.01). In addition, example plots of aging-based measurements, and trend comparisons, are provided for systolic (sys.), early diastolic (early dia.), and late diastolic (late dia.) strain rates at the base of the heart (C).
Complementary to peak strain values, example plots of aging-based metric are shown in Fig. 4C for systolic strain rate (ΔEθθ,sys), early diastolic strain rate (ΔEθθ,early dia), and late diastolic strain rate (ΔEθθ,late dia) at the base of the heart. Supplemental Fig. S2 provides aging-based and trend comparison plots of all strain rate metrics at the base, mid-LV, and apical regions. Comparisons of individual trends, with values at the base shown in Fig. 4C, indicate significant differences between control and Cpt2M−/− mice for all ΔEθθ metrics. Despite these significant differences, no ΔEθθ metric had an AUC > 0.988, suggesting that peak Eθθ at the base still demonstrates the best performance among circumferential strain metrics in differentiating trends from Cpt2M−/− mice. Of note, ΔEθθ,lat dia at the base did not show any overlap in 95% CIs, suggesting that ventricular compliance and/or filling impairments at the base may be compromised even before 4 wk of age.
Regional Longitudinal Strain
Locations at which circumferential strain (ELL) metrics were derived are schematically shown in Fig. 5A. Aging-based changes in peak ELL along the posterior septum, posterior, posterior free-wall, anterior free-wall, anterior, and anterior septum regions are plotted in Fig. 5B. In addition, comparisons of peak ELL trends show statistically significant differences between control and Cpt2M−/− mice at each location. Computed AUC values are comparable across all regions (AUC = 0.929–0.982), though greatest performance is observed along the posterior wall (AUC = 0.982). Computed 95% CI intersections show earlier separation for peak ELL along the posterior wall at 5.27 wk of age, though the earliest intersection is seen at 4.58 wk of age along the anterior free-wall. When comparing metric values independent of age, peak ELL along the posterior wall still showed the highest AUC (AUC = 0.913) compared with all other regions (AUC = 0.821–0.909).
Figure 5.
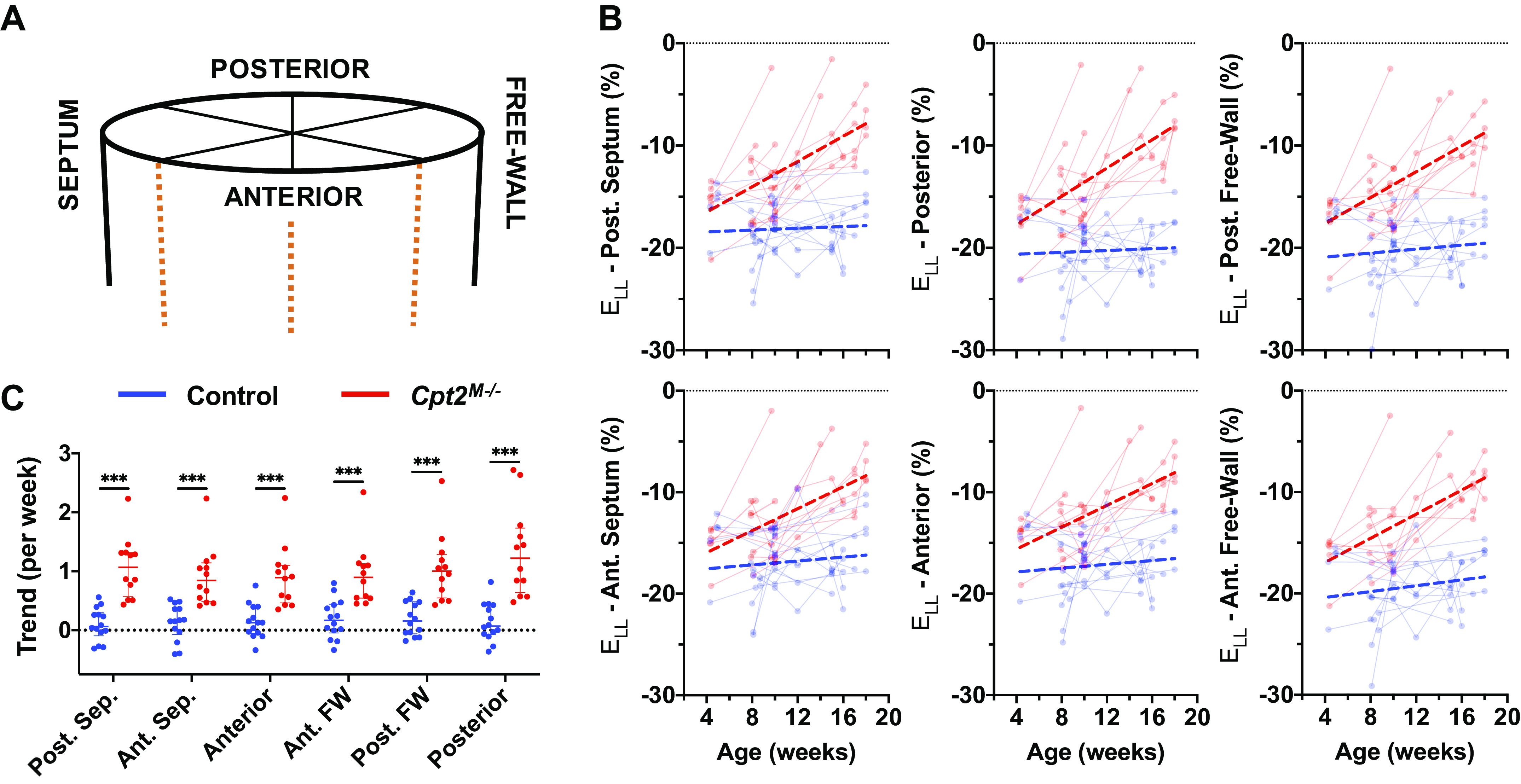
Regional longitudinal strain measurements. A: schematic localizing the area from which the left ventricle longitudinal strain (ELL) was measured. B: peak longitudinal strain measured across time is shown for rotations around the central z-axis corresponding to the posterior septum (Post. Sep.), posterior, posterior free-wall (Post. FW), anterior free-wall (Ant. FW), anterior, and anterior septum (Ant. Sep.) regions of the heart. Data from control mice (n = 14) shown in blue and Cpt2M−/− mice (n = 12) shown in red. Points from the same mouse are shown connected, and linear regression performed on across each cohort is visualized with thick dashed lines. Comparison plots (C) of strain trends from each mouse and region are shown (bottom), with horizontal lines designating median and interquartile ranges. Significance markers above each comparison plot indicate P value level from nonparametric Mann–Whitney tests (***P < 0.001).
Aging-based and trend comparison plots of systolic strain rate (ΔELL,sys), early diastolic strain rate (ΔELL,early dia), and late diastolic strain rate (ΔELL,late dia) for each respective region are provided in Supplemental Fig. S3. All ΔELL trend differences were statistically significant, except for ΔELL,late dia along the posterior free-wall. The highest computed AUC value for ΔELL trends was for ΔELL,sys along the posterior wall (AUC = 0.893), suggesting that peak ELL along the posterior wall still demonstrates the best performance in differentiating trends from Cpt2M−/− mice among all longitudinal strain metrics. Of note, ΔELL,late dia along the anterior free-wall, posterior wall, and posterior free-wall did not show any overlap in 95% CIs, suggesting that similar to findings with ΔEθθ,late dia, myocardial relaxation along the entire free-wall may be compromised even before 4 wk of age.
Hybrid Strain Index
Plotting peak posterior wall ELL values along the against peak base Eθθ values, as shown in Fig. 6A, we can visualize the potential utility of using both metrics simultaneously to differentiate data from control and Cpt2M−/− mice. Taking the L2 norm of these two values—effectively the distance from (0,0) to any given point—we compute the proposed hybrid strain index (HSI). Aging-based plots of HSI values and associated comparison of HSI trends are provided in Fig. 6B, demonstrating a significant difference in trends between control and Cpt2M−/− mice (P < 0.001).
Figure 6.
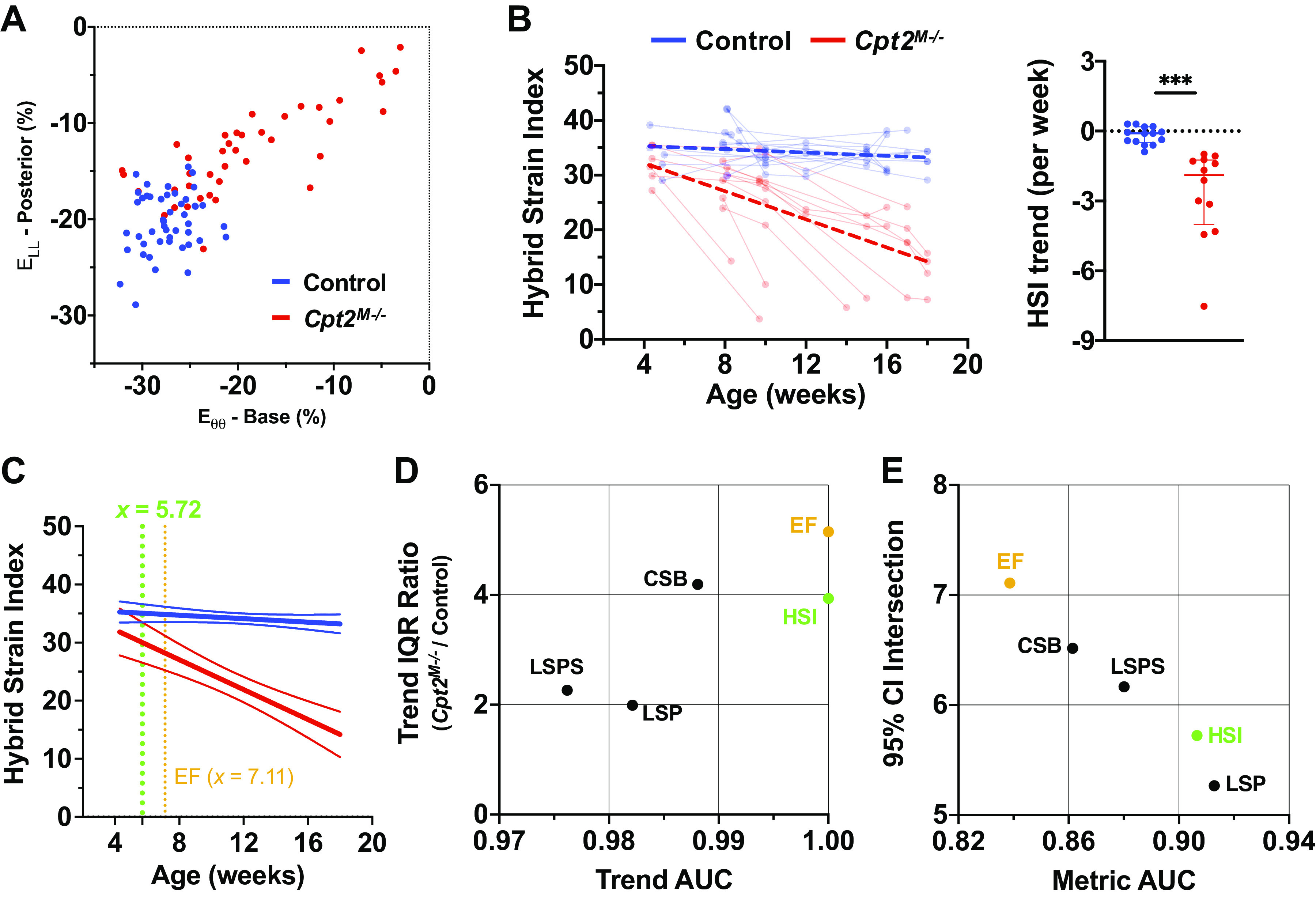
Hybrid strain index (HSI). A: scatter plot of the basal peak circumferential strain (Eθθ) value against posterior peak longitudinal strain (ELL) value for each four-dimensional ultrasound (4DUS) dataset, with data from control mice (n = 14) shown in blue and Cpt2M−/− mice (n = 12) shown in red, visualizes the relationship between the two components incorporated into the HSI metric. Similar to those shown for circumferential and longitudinal strain, HSI measurements across time (B) are overlaid with cumulative linear regression results as thick dashed lines, as well as a comparison plot of HSI trends for individual mice. Significance markers indicate P value level from nonparametric Mann–Whitney tests (***P < 0.001). To assess the earliest age in which the HSI value from a Cpt2M−/− mouse might deviate from the control mice, the cumulative linear regression lines with 95% confidence intervals (C) for HSI were plotted and the age at which the confidence intervals no longer overlap was identified (i.e., 5.72 wk for against 7.11 wk for EF). Further demonstrating of the use of HSI in better characterizing disease progression, a scatter plot (D) of area under the curve (AUC) values from receiver operating characteristics (ROC) analysis against the ratio of trend standard deviations is provided, with HSI (green) providing the maximum area under curve (AUC) value alongside ejection fraction (EF; orange), yet with a lower interquartile range (IQR) ratio. Other shown metrics include: peak circumferential strain at the base (CSB), and peak longitudinal strain at the posterior wall (LSP), and posterior septum (LSPS). Metric utility is further explored by plotting 95% confidence interval intersections against age-independent metric AUC values (E), demonstrating with a higher AUC and earlier intersection that HSI could also be a more sensitive diagnostic marker of disease than ejection fraction.
Comparing the relative utility of all computed metrics in differentiating disease progression in Cpt2M−/− mice against natural aging in control mice, Fig. 6C shows trend IQR ratios plotted against AUC values. Axes are zoomed to show metrics for which AUC > 0.97 and IQR < 6, respectively. Not only does HSI have the highest achievable AUC value (AUC = 1.00), but it has a lower IQR ratio (3.94) than EF (AUC = 1.00; IQR ratio = 5.15) and peak base Eθθ (AUC = 0.988; IQR ratio = 4.19). Although lower IQR ratios are observed in peak ELL along the posterior wall (1.99) and posterior septum (2.27), neither of these metrics show trend AUC values > 0.985. Figure 6D further highlights the age-independent utility of HSI by plotting 95% confidence interval intersections against metric AUC values.
DISCUSSION
In this study, we demonstrate how high-frequency 4DUS and derived metrics can be used to comprehensively study cardiac dysfunction progression in a murine model of metabolic cardiomyopathy. We specifically outline a standardized procedure for analysis of left-ventricular 4DUS data. This approach was taken to try and mitigate noted issues in reproducibility during standard echocardiographic data acquisition due to physical manipulation of the ultrasound probe (33–35). This is accomplished by leveraging the relatively high spatial resolution of 4DUS to allow post-acquisition alignment of data to a standard set of axes, based on landmarks common to left-ventricular anatomy. Following reorientation, tracking of base and apex motion along the central z-axis allows us to compensate for the longitudinal contraction of the heart when extracting short-axis views across the cardiac cycle, effectively minimizing the effects of through-plane motion common to cardiac imaging. Finally, the proposed 24-point grid structure from which endocardial and epicardial 4-D meshes are interpolated provides a uniformly distributed subset of spatial locations around the left-ventricle, which also simplifies wall motion tracking to a single axis (i.e., radius between each point and the center z-axis).
Applying these methods to the Cpt2M−/− mouse model, measurements of LV morphometry suggest that hallmarks of metabolic cardiomyopathy, namely LV mass and wall thickness are significantly increased even before the earliest imaging time points of 4 wk of age. Observed significant increases in end-diastolic volume and decreases in EF provide additional evidence that Cpt2M−/− mice exhibit hallmarks of progressive LV chamber dilatation and cardiac dysfunction leading to heart failure, respectively. This is further supported by the fact that six of the twelve Cpt2M−/− mice (50%) died premature to the planned final time point. Predicting mouse survival is not possible without establishing cardiac parameters that are directly related to the progression and severity of the cardiomyopathy. Hence, the presentation of variable rates of disease progression in this Cpt2M−/− model provided us with the power to correlate imaging data to the progression toward heart failure. This approach provides insights into highly predictive correlates with the potential for use in both research and clinical applications.
Looking to better understand regional drivers of cardiac dysfunction, we investigated circumferential and longitudinal strain metrics from select regions around the heart. As recent literature has pointed to regional strain being a more sensitive metric to cardiac dysfunction than EF (24), we focused here on identifying which regions and strain metrics that were most sensitive to disease progression. In these regard, peak Eθθ at the base and peak ELL along the posterior wall were identified as two important regional metrics. Although the identification of peak ELL along the posterior wall may reflect the overall reduction of LV contractility, as this is where the highest magnitude ELL values are observed around the LV, the disproportionately large reduction in peak Eθθ at the base may hint at effects of diastolic dysfunction, which is a reported pathophysiology associated with hypertrophic cardiomyopathy (36–39). Significantly different aging-based trends in early and late diastolic strain rates, shown in Supplemental Figs. S2 and S3, also support the presence of diastolic dysfunction within the Cpt2M−/− mice; however, the lack of associated pulse wave Doppler data through the mitral valve in our studied population limit our ability to confirm the presence and quantify the level of diastolic dysfunction. Follow-up studies acquiring both 4DUS and pulse-wave Doppler information will allow us to more directly investigate the relationship between early and late diastolic strain rate metrics derived from 4DUS data and diastolic hemodynamic function (e.g., E/A wave ratio).
As an extension information provided by the outlined 4DUS analysis methods, our proposed novel hybrid strain index (HSI) draws from the best performing circumferential and longitudinal strain metrics to provide a more sensitive marker of disease progression than all other computed function metrics. The relative performance of all metrics was quantified using both metric and trend AUC values, IQR ratio, and 95% CI intersection, from which HSI demonstrates the best overall utility in identifying Cpt2M−/− mice against controls. Although both EF and HSI provide maximal trend AUC values, thus suggesting similar utility in describing disease progression, HSI provides both a higher metric AUC and earlier 95% CI intersection than EF, indicating that it might be more sensitive to mild cardiac dysfunction and would decrease before EF. This seems intuitive as HSI reflects the contractility of two orthogonal reference frames (i.e., circumferential and longitudinal) that together cover the extent of the LV, yet isolates the metric in each frame that demonstrates the greatest sensitivity to cardiac dysfunction. With this in mind, further studies utilizing the HSI metric will be needed to investigate its wider usage, and potentially different variations could be formulated that are specific to various cardiomyopathies including myocardial infarction where the kinetic abnormalities are likely more prominent in the apical region.
Finally, although the imaging time points of the mice in this study were inconsistent, a variety of ages, disease states, and follow-up scan timings are commonplace in the clinic. As numerous factors might influence the frequency by which patients have echocardiographic assessments, the focus on changes in measurements over several imaging sessions is more informative to potential declines in cardiac function than an isolated measurement. Although further validation work is needed, our results suggest that in this paradigm HSI could be a complementary marker to help characterize cardiac dysfunction progression and disease progression. In particular, the use of HSI could prove useful in the characterization of heart failure with preserved ejection fraction (HFpEF) where a more sensitive marker to cardiac dysfunction could help in diagnosis and to better assess patient responses to subsequent treatment (40–42).
Limitations
Despite the demonstrated promise in the proposed analysis and results, several limitations still exist. The use of ultrasound technology for cardiac imaging inherently presents issues with both shadowing artifacts from the ribcage and mirror artifacts from the surrounding lungs. Their impact can be mitigated during imaging with proper probe placement, and during 4DUS analysis by using multiplanar views to estimate borders through impacted regions. Second, the calculation of strain in both the circumferential and longitudinal frames is assumed to be uniform throughout the cardiac cycle. Although this assumption allows the tracking of regional wall motion to be simplified to the change in radius of local grid points, relative to the center z-axis, recent literature has suggested this assumption may oversimplify more complex disease models, such as myocardial infarction where focal akinetic regions of the heart are observed (43, 44). Finally, one component of cardiac kinematics that has not been addressed in this analysis is myocardial twist. Although it is known that the rotation of the base and apex of the heart through the cardiac cycle can be impacted in various cardiomyopathies, the ability to quantify rotation and twist has not yet been integrated into the current analysis. Future work will be needed to both incorporate rotational information into the creation of LV 4-D meshes and quantify its impact in various disease models.
Conclusion
We demonstrate here for the first time the application of high-frequency 4DUS imaging to a murine model of metabolic cardiomyopathy. The proposed analysis provides a standardized approach to measure localized wall kinematics and simultaneously extract metrics of global cardiac function, LV morphometry, regional circumferential strain, and regional longitudinal strain from an interpolated 4-D mesh of the endo- and epicardial boundaries. We further propose a hybrid strain index (HSI), composed of peak circumferential strain at the base and longitudinal strain along the posterior wall, which provides greater utility to characterizing disease progression than all other extracted metrics. Future studies could be used to apply these methods to additional disease models and further validate the utility of metrics derived from the 4DUS imaging and strain mapping.
SUPPLEMENTAL DATA
Supplemental Figs. S1–S3 and Supplemental Table S1: https://doi.org/10.6084/m9.figshare.c.5425209.
GRANTS
This work was supported by National Institutes of Health Grants F30-HL145980 (to F. W. Damen) and T32-DK101001 and R01-DK125812-01 (to J. M. Ellis) and the Leslie A. Geddes Endowment at Purdue University (to C. J. Goergen).
DISCLAIMERS
FUJIFILM VisualSonics Inc. had no role in the design, execution, interpretation, or writing of the study.
DISCLOSURES
The authors declare that C. J. Goergen serves on the scientific advisory board for FUJIFILM VisualSonics Inc. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
F.W.D. and A.S.P. conceived and designed research; F.W.D., J.P.S., and A.S.P. performed experiments; F.W.D. and J.P.S. analyzed data; F.W.D., A.S.P., J.M.E., and C.J.G. interpreted results of experiments; F.W.D. prepared figures; F.W.D. drafted manuscript; F.W.D., J.P.S., A.S.P., J.M.E., and C.J.G. edited and revised manuscript; F.W.D., J.P.S., A.S.P., J.M.E., and C.J.G. approved final version of manuscript.
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135: e146–e603, 2017. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier/Saunders, 2015. [Google Scholar]
- 3.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB; American Heart Association; Council on Clinical Cardiology, Heart Failure Transplantation Committee; Quality of Care and Outcomes Reaserch and Functional Genomics and Translational Biology Interdisciplinary Working Group, Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113: 1807–1816, 2006. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 4.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 93: 841–842, 1996. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 5.Levelt E, Mahmod M, Piechnik SK, Ariga R, Francis JM, Rodgers CT, Clarke WT, Sabharwal N, Schneider JE, Karamitsos TD, Clarke K, Rider OJ, Neubauer S. Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes 65: 44–52, 2016. doi: 10.2337/db15-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murdolo G, Angeli F, Reboldi G, Di Giacomo L, Aita A, Bartolini C, Vedecchia P. Left ventricular hypertrophy and obesity: only a matter of fat? High Blood Press Cardiovasc Prev 22: 29–41, 2015. doi: 10.1007/s40292-014-0068-x. [DOI] [PubMed] [Google Scholar]
- 7.Cox KB, Liu J, Tian L, Barnes S, Yang Q, Wood PA. Cardiac hypertrophy in mice with long-chain acyl-CoA dehydrogenase or very long-chain acyl-CoA dehydrogenase deficiency. Lab Invest 89: 1348–1354, 2009. doi: 10.1038/labinvest.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis JM, Mentock SM, Depetrillo MA, Koves TR, Sen S, Watkins SM, Muoio DM, Cline GW, Taegtmeyer H, Shulman GI, Willis MS, Coleman RA. Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs fatty acid oxidation and induces cardiac hypertrophy. Mol Cell Biol 31: 1252–1262, 2011. doi: 10.1128/MCB.01085-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haynie KR, Vandanmagsar B, Wicks SE, Zhang J, Mynatt RL. Inhibition of carnitine palymitoyltransferase1b induces cardiac hypertrophy and mortality in mice. Diabetes Obes Metab 16: 757–760, 2014. doi: 10.1111/dom.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houten SM, Violante S, Ventura FV, Wanders RJ. The biochemistry and physiology of mitochondrial fatty acid beta-oxidation and its genetic disorders. Annu Rev Physiol 78: 23–44, 2016. doi: 10.1146/annurev-physiol-021115-105045. [DOI] [PubMed] [Google Scholar]
- 11.Knottnerus SJG, Bleeker JC, Ferdinandusse S, Houtkooper RH, Langeveld M, Nederveen AJ, Strijkers GJ, Visser G, Wanders RJA, Wijburg FA, Boekholdt SM, Bakermans AJ. Subclinical effects of long-chain fatty acid beta-oxidation deficiency on the adult heart: a case-control magnetic resonance study. J Inherit Metab Dis 43: 969–980, 2020. doi: 10.1002/jimd.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Ceholski DK, Liang L, Fish K, Hajjar RJ. Variability in coronary artery anatomy affects consistency of cardiac damage after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 313: H275–H282, 2017. doi: 10.1152/ajpheart.00127.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James JF, Hewett TE, Robbins J. Cardiac physiology in transgenic mice. Circ Res 82: 407–415, 1998. doi: 10.1161/01.res.82.4.407. [DOI] [PubMed] [Google Scholar]
- 14.Lindsey ML, Kassiri Z, Virag JAI, de Castro Bras LE, Scherrer-Crosbie M. Guidelines for measuring cardiac physiology in mice. Am J Physiol Heart Circ Physiol 314: H733–H752, 2018. doi: 10.1152/ajpheart.00339.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McConnell BK, Fatkin D, Semsarian C, Jones KA, Georgakopoulos D, Maguire CT, Healey MJ, Mudd JO, Moskowitz IP, Conner DA, Giewat M, Wakimoto H, Berul CI, Schoen FJ, Kass DA, Seidman CE, Seidman JG. Comparison of two murine models of familial hypertrophic cardiomyopathy. Circ Res 88: 383–389, 2001. doi: 10.1161/01.RES.88.4.383. [DOI] [PubMed] [Google Scholar]
- 16.Muthuramu I, Lox M, Jacobs F, De Geest B. Permanent ligation of the left anterior descending coronary artery in mice: a model of post-myocardial infarction remodelling and heart failure. J Vis Exp 94: e52206, 2014. doi: 10.3791/52206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patten RD, Hall-Porter MR. Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail 2: 138–144, 2009. doi: 10.1161/CIRCHEARTFAILURE.108.839761. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Ellis JM, Wolfgang MJ. Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress-induced inflammation. Cell Rep 10: 266–279, 2015. doi: 10.1016/j.celrep.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereyra AS, Hasek LY, Harris KL, Berman AG, Damen FW, Goergen CJ, Ellis JM. Loss of cardiac carnitine palmitoyltransferase 2 results in rapamycin-resistant, acetylation-independent hypertrophy. J Biol Chem 292: 18443–18456, 2017. doi: 10.1074/jbc.M117.800839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardin JM, Siri FM, Kitsis RN, Edwards JG, Leinwand LA. Echocardiographic assessment of left ventricular mass and systolic function in mice. Circ Res 76: 907–914, 1995. doi: 10.1161/01.res.76.5.907. [DOI] [PubMed] [Google Scholar]
- 21.Hartley CJ, Taffet GE, Reddy AK, Entman ML, Michael LH. Noninvasive cardiovascular phenotyping in mice. ILAR J 43: 147–158, 2002. doi: 10.1093/ilar.43.3.147. [DOI] [PubMed] [Google Scholar]
- 22.Pistner A, Belmonte S, Coulthard T, Blaxall B. Murine echocardiography and ultrasound imaging. J Vis Exp 42: e2100, 2010. doi: 10.3791/2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiller NB, Foster E. Analysis of left ventricular systolic function. Heart 75: 17–26, 1996. doi: 10.1136/hrt.75.6_suppl_2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, Edvardsen T, Ew R. Geometry as a confounder when assessing ventricular systolic function: comparison between ejection fraction and strain. J Am Coll Cardiol 70: 942–954, 2017. doi: 10.1016/j.jacc.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 25.Damen FW, Berman AG, Soepriatna AH, Ellis JM, Buttars SD, Aasa KL, Goergen CJ. High-frequency 4-dimensional ultrasound (4DUS): a reliable method for assessing murine cardiac function. Tomography 3: 180–187, 2017. doi: 10.18383/j.tom.2017.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soepriatna A, Damen FW, Vlachos PP, Goergen CJ. Cardiac and respiratory-gated volumetric murine ultrasound. Int J Cardiovas Imaging 34: 713–724, 2018. doi: 10.1007/s10554-017-1283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soepriatna AH, Yeh AK, Clifford AD, Bezci SE, O'Connell GD, Goergen CJ. Three-dimensional myocardial strain correlates with murine left ventricular remodelling severity post-infarction. J R Soc Interface 16: 20190570, 2019. doi: 10.1098/rsif.2019.0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damen FW, Adelsperger AR, Wilson KE, Goergen CJ. Comparison of traditional and integrated digital anesthetic vaporizers. J Am Assoc Lab Anim 54: 756–762, 2015. [PMC free article] [PubMed] [Google Scholar]
- 29.Abduch MC, Alencar AM, Mathias W Jr, Vieira ML. Cardiac mechanics evaluated by speckle tracking echocardiography. Arq Bras Cardiol 102: 403–412, 2014. doi: 10.5935/abc.20140041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer M, Cheng S, Jain M, Ngoy S, Theodoropoulos C, Trujillo A, Lin FC, Liao R. Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res 108: 908–916, 2011. doi: 10.1161/CIRCRESAHA.110.239574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muraru D, Niero A, Rodriguez-Zanella H, Cherata D, Badano L. Three-dimensional speckle-tracking echocardiography: benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc Diagn Ther 8: 101–117, 2018. doi: 10.21037/cdt.2017.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison TM, Choi G, Zarins CK, Taylor CA. Circumferential and longitudinal cyclic strain of the human thoracic aorta: age-related changes. J Vasc Surg 49: 1029–1036, 2009. doi: 10.1016/j.jvs.2008.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grune J, Blumrich A, Brix S, Jeuthe S, Drescher C, Grune T, Foryst-Ludwig A, Messroghli D, Kuebler WM, Ott C, Kintscher U. Evaluation of a commercial multi-dimensional echocardiography technique for ventricular volumetry in small animals. Cardiovasc Ultrasound 16: 10, 2018. doi: 10.1186/s12947-018-0128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran CM, Thomson AJW. Preclinical ultrasound imaging—a review of techniques and imaging applications. Front Phys 8: 124, 2020. doi: 10.3389/fphy.2020.00124. [DOI] [Google Scholar]
- 35.Rutledge C, Cater G, McMahon B, Guo L, Nouraie SM, Wu Y, Villanueva F, Kaufman BA. Commercial 4-dimensional echocardiography for murine heart volumetric evaluation after myocardial infarction. Cardiovasc Ultrasound 18: 9, 2020. doi: 10.1186/s12947-020-00191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abraham TP, Jones M, Kazmierczak K, Liang HY, Pinheiro AC, Wagg CS, Lopaschuk GD, Szczesna-Cordary D. Diastolic dysfunction in familial hypertrophic cardiomyopathy transgenic model mice. Cardiovasc Res 82: 84–92, 2009. doi: 10.1093/cvr/cvp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obokata M, Reddy YNV, Borlaug BA. Diastolic dysfunction and heart failure with preserved ejection fraction: understanding mechanisms by using noninvasive methods. JACC Cardiovasc Imaging 13: 245–257, 2020. doi: 10.1016/j.jcmg.2018.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsson MC, Palmer BM, Leinwand LA, Moore RL. Gender and aging in a transgenic mouse model of hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol 280: H1136–H1144, 2001. doi: 10.1152/ajpheart.2001.280.3.H1136. [DOI] [PubMed] [Google Scholar]
- 39.Westermann D, Knollmann BC, Steendijk P, Rutschow S, Riad A, Pauschinger M, Potter JD, Schultheiss HP, Tschope C. Diltiazem treatment prevents diastolic heart failure in mice with familial hypertrophic cardiomyopathy. Eur J Heart Fail 8: 115–121, 2006. doi: 10.1016/j.ejheart.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Hiebert JB, Vacek J, Shah Z, Rahman F, Pierce JD. Use of speckle tracking to assess heart failure with preserved ejection fraction. J Cardiol 74: 397–402, 2019. doi: 10.1016/j.jjcc.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu JJ, Ziaeian B, Fonarow GC. Heart failure with mid-range (borderline) ejection fraction: clinical implications and future directions. JACC Heart Fail 5: 763–771, 2017. doi: 10.1016/j.jchf.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lekavich CL, Barksdale DJ, Neelon V, Wu JR. Heart failure preserved ejection fraction (HFpEF): an integrated and strategic review. Heart Fail Rev 20: 643–653, 2015. doi: 10.1007/s10741-015-9506-7. [DOI] [PubMed] [Google Scholar]
- 43.Amzulescu MS, De Craene M, Langet H, Pasquet A, Vancraeynest D, Pouleur AC, Vanoverschelde JL, Gerber BL. Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. Eur Heart J Cardiovasc Imaging 20: 605–619, 2019. doi: 10.1093/ehjci/jez041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torres WM, Jacobs J, Doviak H, Barlow SC, Zile MR, Shazly T, Spinale FG. Regional and temporal changes in left ventricular strain and stiffness in a porcine model of myocardial infarction. Am J Physiol Heart Circ Physiol 315: H958–H967, 2018. doi: 10.1152/ajpheart.00279.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]