Keywords: globin, heme, hypoxia, neovascularization, nitric oxide
Abstract
Myoglobin (Mb) is a regulator of O2 bioavailability in type I muscle and heart, at least when tissue O2 levels drop. Mb also plays a role in regulating cellular nitric oxide (NO) pools. Robust binding of long-chain fatty acids and long-chain acylcarnitines to Mb, and enhanced glucose metabolism in hearts of Mb knockout (KO) mice, suggest additional roles in muscle intermediary metabolism and fuel selection. To evaluate this hypothesis, we measured energy expenditure (EE), respiratory exchange ratio (RER), body weight gain and adiposity, glucose tolerance, and insulin sensitivity in Mb knockout (Mb−/−) and wild-type (WT) mice challenged with a high-fat diet (HFD, 45% of calories). In males (n = 10/genotype) and females (n = 9/genotype) tested at 5–6, 11–12, and 17–18 wk, there were no genotype effects on RER, EE, or food intake. RER and EE during cold (10°C, 72 h), and glucose and insulin tolerance, were not different compared with within-sex WT controls. At ∼18 and ∼19 wk of age, female Mb−/− adiposity was ∼42%–48% higher versus WT females (P = 0.1). Transcriptomics analyses (whole gastrocnemius, soleus) revealed few consistent changes, with the notable exception of a 20% drop in soleus transferrin receptor (Tfrc) mRNA. Capillarity indices were significantly increased in Mb−/−, specifically in Mb-rich soleus and deep gastrocnemius. The results indicate that Mb loss does not have a major impact on whole body glucose homeostasis, EE, RER, or response to a cold challenge in mice. However, the greater adiposity in female Mb−/− mice indicates a sex-specific effect of Mb KO on fat storage and feed efficiency.
NEW & NOTEWORTHY The roles of myoglobin remain to be elaborated. We address sexual dimorphism in terms of outcomes in response to the loss of myoglobin in knockout mice and perform, for the first time, a series of comprehensive metabolic studies under conditions in which fat is mobilized (high-fat diet, cold). The results highlight that myoglobin is not necessary and sufficient for maintaining oxidative metabolism and point to alternative roles for this protein in muscle and heart.
INTRODUCTION
One of the most abundant proteins in heart and oxidative skeletal muscle is myoglobin (Mb). A traditional view for myoglobin’s role in skeletal and cardiac muscle is to serve as an O2 reservoir, responding to reductions in partial pressures of tissue O2 () by releasing O2 in support of mitochondrial oxidative phosphorylation. Under well-oxygenated conditions, Mb is primarily in the saturated, oxygenated form but under hypoxia or during exercise, progressive Mb desaturation is observed (e.g., Refs. 1–6). Yet, the importance of Mb to support oxidative metabolism and muscle function is probably context specific and the evidence is equivocal. In one study, Mb knockout mice (Mb−/−) displayed more rapid onset fatigue (by ∼6 min) and lower distance run (by ∼120 m) in a treadmill paradigm (7), but no differences in performance were observed before or after a voluntary wheel-running paradigm (8). Fatigue was indistinguishable between WT and Mb−/− following ex vivo stimulation of extensor digitorum longus or soleus (100 Hz) in normoxic or hypoxic conditions (9), but at a slower frequency (40 Hz), soleus force was eventually lowered by ∼12% in muscle preparations from Mb−/− mice in a normoxic 60-min protocol (10). Thus, the roles of Mb in support of working muscle are not fully established, and knowledge gaps remain in terms of Mb function in tissue oxidative metabolism of fuels.
Another proposed role for Mb is regulation of nitric oxide (NO) pools by varying levels of this signaling molecule (11). As reviewed by Hendgen-Cotta et al. (12) and Clanton (13), at levels above the P50 for O2 binding to Mb, oxy-Mb acts as a NO scavenger: the oxy-Mb reacts with NO to produce nitrate (NO3−) plus met-Mb. During exercise or exposure to hypoxic conditions as and pH are reduced, this scavenging role is attenuated and deoxy-Mb reacts with nitrite (NO2−) and H+ to form met-Mb, NO, and OH−. Since NO is a powerful inhibitor of mitochondrial electron transport chain (ETC) complex IV cytochrome c oxidase, Mb might serve regulate oxidative phosphorylation in part through modifying NO pools, as suggested by Shiva et al. (14) and Rassaf et al. (15). If true, such activity could alter cellular oxidative versus nonoxidative metabolism and hence fuel selection.
Myoglobin may have an additional role beyond intracellular O2 and NO homeostasis. Early reports showing that freshly prepared rat heart Mb or chicken gizzard oxy-Mb can bind long-chain fatty acids (LCFAs) (16, 17) raised the possibility that Mb regulates lipid metabolism, prompting Jue and colleagues (8, 18–20) to hypothesize that Mb participates in lipid trafficking by serving as a physiological fatty acid-binding protein. Although untested, this proposal seems plausible considering that Mb tissue concentrations can reach ∼360–800 µM in muscle [e.g., horse quadriceps muscle or human muscle (21–23)) and ∼150–270 µM in heart (e.g., murine and human heart (2, 23, 24)] (assumes in some cases 1 mL/g tissue and 70% tissue hydration). By leveraging sequence homologies to known fatty acid-binding proteins and molecular modeling, we refined and identified a specific LCFA binding pocket near the heme group of oxy-Mb (25). This site in oxy-Mb (but not deoxy-Mb) was also discovered to be an avid binding site for acylcarnitine LCFA derivatives (26). In spite of specific binding of LCFAs and acylcarnitines of >12 carbon length to Mb, evidence supporting a role for Mb in regulating fat metabolism and tissue fuel selection is mixed and sometimes contrary. Glucose uptake and metabolism in heart were substantially increased (27, 28), and heart membrane GLUT4 protein was ∼60% higher (27) in Mb−/− mice. Flux of palmitate carbon to tricarboxylic acid-generated glutamate in heart was reduced in one case (27), but palmitate flux to acetyl-CoA was unchanged in a different study (28). One group found threefold higher triacylglyceride (TAG) and diacylglyceride levels in Mb−/− heart (29), in contrast to a report indicating a ∼30% TAG reduction (8). Thus, there is evidence that in the absence of Mb, glucose metabolism in cardiac muscle is increased, but the effect of Mb deficiency in lipid trafficking and LCFA combustion remains an open question.
Notably, excessive accumulation of LCFA-derived metabolites (including certain medium- to long-chain acylcarnitines) is hypothesized to contribute to lipotoxicity, for example, those associated with cardiac ischemia damage, skeletal muscle insulin resistance, and episodic myopathies characteristic of fatty acid oxidation disorders (reviewed in: Ref. 30). It follows that upon deoxygenation, Mb off-loading of acylcarnitines and LCFAs would cause an increase in intracellular free concentrations and bioavailabilities of lipids, with potential implications for lipotoxicity (26). In spite of the critical importance of skeletal muscle to whole body glucose disposal and the potential for myocellular lipotoxicities associated with perturbed fat metabolism, the role of muscle Mb on glucose homeostasis and insulin action has not been addressed.
With these knowledge gaps in mind, a thorough evaluation of the roles of Mb in regulating whole body fat oxidation, fuel selection, blood sugar control, and energy balance is clearly needed. Previous studies have mostly focused on heart (patho)physiology and have been almost entirely conducted in male Mb−/− mice. To address whether Mb plays a role in regulation of whole body intermediary metabolism and to assess the impact of Mb−/− in both sexes, female and male Mb−/− mice were used to examine metabolic phenotypes in response to high-fat diet and cold exposure challenges that significantly engage fat metabolism. We hypothesized that if Mb plays a significant role in modulating fat oxidation and maintaining normal skeletal and cardiac muscle lipid content, then cold- or high-fat diet-exposed Mb−/− mice would display higher respiratory exchange ratios, altered glucose tolerance (perhaps improved due to glucose as a preferred fuel), insulin resistance (due to lipotoxicity), and greater adiposity. In addition, considering previous reports that Mb−/− mice have adaptive increases in vascularity (10, 28, 31) and type I to type II fiber-type switching (10), we assessed these factors in the heart and skeletal muscle.
METHODS
Animals
All studies were completed by the University of California (UC) Davis Mouse Biology Program and the Energy Balance, Exercise and Behavior Core of the UC Davis Mouse Metabolic Phenotyping Center. Experiments were approved by the UC Davis Institutional Animal Care and Use Committee (Protocol #20396).
Mouse Generation
We used clustered, regularly interspaced short palindromic repeat (CRISPR) technology to derive a null Mb allele in mice. Briefly, selection of guide RNA (gRNA) used the online tool ChopChop (chopchop.cbu.uib.no) to assess optimal gRNA candidates targeting the Mb gene (32). The following guide sequences were selected and synthesized as crRNA for use as an Alt-R two-part guide system with tracrRNA and crRNA provided separately (Integrated DNA Technologies, Coralville, IA): GAACTTGTCAAACTTATCCA and CGGTGCAACCATGCTTCTTC (Fig. 1A). gRNAs were validated using a PCR-amplified target sequence in vitro to ensure targeted and efficient cleavage activity. The CRISPR preparation was assembled on the day of electroporation of C57BL/6N [wild-type (WT)] mouse zygotes following the CRISPR-EZ protocol (33). Ribonucleic protein (RNP) was mixed at a 1:1.5 molar ratio of Cas9 protein to pgRNA and complexed at 37°C for 10 min at a concentration of 16 µM. Before electroporation, the 16 µM RNP was equally mixed with Opti-MEM reduced serum medium (Thermo Fisher Scientific, 31985070, Waltham, MA) yielding a final concentration of 8 µM RNP. Electroporation was performed as previously described (33). Embryos were transferred in groups of 10 per oviduct into 0.5-day postcoitum pseudopregnant CD1 recipient females. Tail snips (∼3 mm) were sampled from mice at 2 wk of age and genomic DNA was extracted using Agencourt DNAdvance magnetic beads (Beckman-Coulter, A48706, Brea, CA) on an automated and LIMS-tracked Microlab STAR (Hamilton Robotics, Reno, NV). DNA samples were screened using a quantitative PCR (qPCR) relative Ct method using Mb sequence-specific primers/probe: forward primer ACGGGTCTCACTCCATCTCTTC, reverse primer CCAGGGCTGTGAGCACG, probe 6FAM- ACCTGAAGGCGCATGGTTGCAC-BHQ1 (LGC, Biosearch Technologies, Novato, CA), and quantified next to an endogenous T-cell receptor δ (Tcrd) reference target amplified using the following primers/probe: forward primer CAGACTGGTTATCTGCAAAGCAA, reverse primer TCTATGCCAGTTCCAAAAAACATC, probe VIC- ATTATAACGTGCTCCTGG-MGB (Thermo Fisher Scientific, Waltham, MA) (34). Gene-specific PCR was performed using GoTaq G2 kit (Promega, M7433, Madison, WI) and a touchdown thermal-cycling on a BioRad Tetrad 2 (Hercules, CA). Conditions were as follows: an initial denaturing at 94°C for 5 min; 10 cycles of 94°C for 15 s, 65°C–55°C for 30 s (lowering by 1°C/cycle), 72°C for 40 s; 30 cycles of 94°C for 15 s, 55°C for 30 s, 72°C for 40 s; final extension of 72°C for 5 min and maintained at 4°C. PCR reactions included a nontemplate control (NTC) and a negative wild-type control (B6). The following primers, each beyond ssODN homology arms, were used: forward primer TCAGCATCTTCAGGCAGGAAGC, reverse primer GGATGCTGTGTGGCTCCTCAG with subsequent amplicons resolved on a Qiaxcel Advance (Qiagen, Hilden, Germany) capillary system. The expected 544 bp amplicons from the qPCR-screened Mb deletion founders described below were purified using Qiaquick Gel extraction kit following manufacturer’s instructions (Qiagen, 28506, Hilden, Germany). PCR amplicons for each founder animal were then sequenced by standard Sanger sequencing methods and analyzed using Sequencher 5.0 software (Gene Codes corporation, Ann Arbor, MO). Sequence-confirmed founder mice were subsequently backcrossed to wild-type C57BL/6Ncrl to transmit through the germline, followed by sequence confirmation of the desired alleles. Seven potential off-target sites, consisting of three mismatches each, were assessed in the first four germline N1 heterozygous animals used to propagate the line. Each potential off-target region was PCR amplified and sequenced using Sanger sequencing methods in which we confirmed that there were no off-target effects in the potential candidate regions.
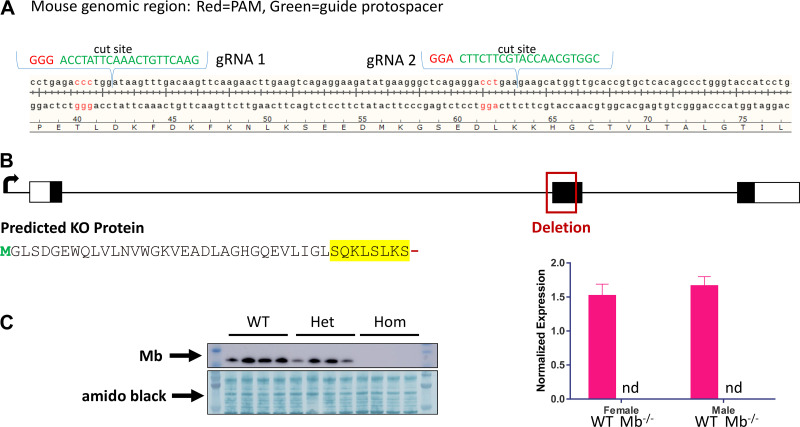
Figure 1.
CRISPR-Cas9 strategy to generate myoglobin (Mb) mutations, resulting in a Mb knockout mouse line. A: example CRISPR-Cas9 design for targeting Mb mutations. B: genomic and translational aspects of the Mb knockout line used for metabolic phenotyping studies, illustrating deletion of much of exon 2 leading to a predicted truncation and amino acid sequence change, shown in yellow (see methods). C: confirmation of Mb null in a subset of mice by Western blot (subsample of whole gastrocnemius from 12-wk-old males and females, n = 2 ea per genotype, chow diet). Also shown are results from qPCR demonstrating no detectable Mb mRNA in gastrocnemius samples collected at the end of the metabolic phenotyping study (females, n = 9/genotype; males, n = 10/genotype). For detection of myoglobin expression, a PrimeTime STD qPCR Assay (Integrated DNA Technologies, IDT) was designed using IDT’s Realtime PCR Tool. The assay was designed to cover the mouse genomic region containing the insertion/deletion region of the myoglobin gene using forward primer: 5′- CCTCTGAGCCCTTCATATCTTC-3′; reverse primer: 5′- GAAGTCCTCATCGGTCTGTTT-3′; probe: 5′-/56-FAM/ AAGACTCAC/ZEN/ CCTGAGACCCTGGAT/3IABkFQ/-3′. WT, wild type; Het, heterozygous; Hom, homozygous knockout.
A Mb frameshift null allele was identified and confirmed as a result of postcleavage repair via nonhomologous end joining. Specifically, sequencing of founder mouse no. 14 revealed deletion of the splice acceptor and a majority of the second coding exon on chr15:76901822–76902020 (GRCm39) (deleted sequence: tccatctcttcttgtcccacagtctgtttaagactcaccctgagaccctggataagtttgacaagttcaagaacttgaagtcagaggaagatatgaagggctcagaggacctgaagaagcatggttgcaccgtgctcacagccctgggtaccatcctgaagaagaagggacaacatgctgccgagatccagcctctagc). During RNA processing, the frameshift induced by fusion of exon 1 to exon 3 results in a premature stop codon and eight nonsensical residues (SQKLSLKS) following the normal residue 33 lysine (Fig. 1B). Mb protein deficiency was confirmed by Western blot analyses of gastrocnemius muscle tissue from 12-wk-old male and female mice and quantitative PCR of muscle collected at the end of the metabolic phenotyping study (Fig. 1C). In certain cancers, alternative Mb transcripts can be generated (35); however, since these differ only in their 5' noncoding regions, the coding region is the same and the variants would all include the deletion region. For Western blot, pulverized tissue was lysed in RIPA buffer (Thermo Fisher Scientific) containing PMSF, HALT protease inhibitors, and HALT phosphatase inhibitors (Thermo Fisher Scientific). Protein was quantified using BCA assay (Thermo Fisher Scientific), and samples containing ∼25–30 µg of protein were separated by a 10% SDS-PAGE and transferred to polyvinyl difluoride membranes (Thermo Fisher Scientific). Membranes were blocked in ×1 TBS (Bio-Rad, Hercules, CA) and 0.1% (vol/vol) Tween 20 (Santa Cruz Biotechnology, Dallas, TX) (TBST) solution containing 5% (wt/vol) dry milk. Membranes were probed for 1–3 h at room temperature or overnight at 4°C with antimyoglobin antibody (ab77232, Abcam). Membranes were then incubated with horseradish peroxidase-conjugated secondary antibody (Southern Biotech, Birmingham, AL) at a 1:10,000 dilution in TBST + 2.5% dry milk for 1 h at room temperature. Bands were visualized using Clarity Western ECL reagent (Bio-Rad) or Pierce ECL 2 Western Blotting Substrate (Thermo Fisher Scientific) and imaged on an Amersham Imager 600 (GE Healthcare, Piscataway, NJ). We have observed no negative effect of the homozygous Mb knockout genotype (Mb−/−) on fecundity or survival: for 103 litters from heterozygous matings to date, there have been 181 Mb−/− and 160 WT pups born, and no differences in the very rare occurrences of mouse mortality through weaning or through the young adult age range of the current study.
Mouse Metabolic Phenotyping
All phenotyping studies were conducted in Mb−/− mice and whenever possible, their littermate WT controls. Mice were individually housed in duplex cages (allowing visual and scent cues to the neighboring mouse). Two independent cohorts were tested, totaling 10 male and 9 female Mb−/− mice and their corresponding age- and sex-matched WT controls. Unless otherwise noted, mice were housed in a vivarium maintained at 20°C–24°C and 30%–40% relative humidity with a 12-h light/12-h dark cycle (lights on, 06:00) under specific pathogen-free housing conditions with water and food ad libitum. The basic study schematic is provided in Fig. 2A, illustrating diet intervention and physiological measurements.
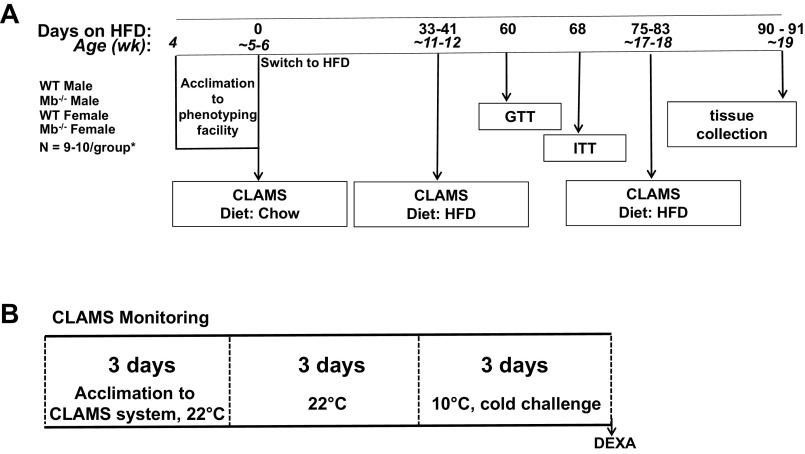
Figure 2.
Metabolic phenotyping study schematic. A: design of the ∼13 wk feeding study comparing male and female wild-type (WT) and myoglobin knockout (Mb KO) mice. Food intake and body weights were measured three times/wk unless mice were being evaluated in the Comprehensive Lab Animal Monitoring System (CLAMS). B: CLAMS indirect calorimetry and temperature challenge study design; data from the final 48 h within each temperature were used for analyses and results herein. GTT, glucose tolerance test; ITT, insulin tolerance test; HFD, high-fat diet (45% of energy from fat). n = 10 males/genotype; n = 9 females/genotype. Mice were weaned at 3 wk of age, then at 4 wk of age moved into the phenotyping housing area for acclimation, prior to commencement of the first 9 d CLAMS protocol starting at 5 wk of age.
Mice were weaned at 3 wk of age. At ∼4 wk of age, mice were acclimated in the facility for 1 wk before undergoing baseline CLAMS (Comprehensive Lab Animal Monitoring System, Columbus Instruments, Columbus, OH) measurements starting at 5 wk of age while being fed a standard chow diet (Teklad 2918, Envigo, Livermore, CA). After being transferred back to their home cages, mice were switched to a high-fat diet (HFD): the diet (Teklad 08511, Envigo, Livermore, CA) provided 45% of energy from fat and has previously been shown to induce obesity and insulin resistance in male C57BL/6J mice (36) and male C57BL/6N mice (37). Food and body weights were measured three times a week (MWF) at the same time of day (∼10:00). A high number of mice in this study crumbled and spilled their food into their bedding, making energy intake quantification difficult. A period of 21 days was identified as low spillage days and food intake is reported during this time (see results).
Temperature challenge and indirect calorimetry.
Energy expenditure by indirect calorimetry and ambulation (activity) were evaluated using the automated CLAMS system. The CLAMS system software (see manufacturer’s technical document, Oxymax for Windows, appendix a) uses O2 consumption (V̇o2) and CO2 production (V̇co2) to calculate the respiratory exchange ratio (RER: V̇co2/V̇o2) and to calculate energy expenditure (heat production) using an equation based on RER-associated kilocalories per liter of consumed O2: “calorific values” from Lusk (38). Acclimation, measurement, and housing protocols were based on our previously published studies examining the effects of temperature on gas exchange in HFD-fed mice (37). Briefly, to minimize variance due to transition of animals to the calorimetry environment and caging, mice were placed in acclimation cages (calorimeter chambers not connected to the system) and housed in the calorimeter room at 22°C for 48 h. They were then transferred to calorimetry chambers inside a 22°C temperature-controlled cabinet and further acclimated for 24 h before the start of the calorimetry measurements taken at 22°C and then 10°C. The input weight for the CLAMS energy expenditure calculation was the weight of each mouse at the start of each temperature period. The final 48 h of measurement at each temperature was used to calculate and report energy expenditure and energy intake. At the conclusion of the 10°C CLAMS period, body composition was assessed by DEXA under isoflurane anesthesia using a Lunar PIXImus II Densitometer, using the manufacturer’s standard protocol that excludes the head region (GE Medical Systems, Chalfont St. Giles, UK). The mice were then returned to their home cages. Food and water intakes were measured by the automated CLAMS system but are not reported herein as for unknown reasons there was excessive food and water spillage for the mice in the current experiments. Energy expenditure results are reported as “adjusted energy expenditure,” using body weight as a covariate.
Glucose and insulin tolerance tests.
Glucose and insulin tolerance tests (GTT and ITT, respectively) were administered at 8.5 wk and 9.5 wk on the HFD. Glucose (2 mg/g of body wt, Hospira, Inc., Lake Forest, IL) or insulin (0.75 U/kg of body wt, Humulin, Lilly USA, LLC) was administered via IP injection. Whole blood glucose was measured at 0-, 15-, 30-, 60-, and 120-min postinjection using an Accu-Chek Aviva meter (Roche Diabetes Care, Inc., Basel, Switzerland). Mice were fasted overnight for the GTT and 4 h in the morning (starting at ∼06:00) for the ITT.
Tissue collection and end-of-study body composition.
Starting at ∼06:00, mice were briefly fasted (∼4–6 h, depending on a given animal’s place in the queue). While still under isoflurane, mice were euthanized via cardiac puncture coincident with cardiac blood collection. EDTA plasma, adipose tissues [subcutaneous, inguinal, retroperitoneal, perigonadal, and interscapular brown adipose tissue (BAT), left and right pads] were weighed, and samples were snap frozen in liquid nitrogen and stored at −70°C. Adipose tissue masses are reported as the sum of the right and left side. Heart and left-side skeletal muscles [gastrocnemius, soleus, plantaris, extensor digitorum longus (EDL)] were weighed and snap frozen in liquid nitrogen. As described below, the right-side skeletal muscles and top half of the heart were fixed in optimal cutting temperature (OCT) cryosection media. Only the left-side muscle masses are reported herein. The order of the tissue and blood collection process for each mouse was randomized across experimental groups. Nonesterified fatty acids (NEFAs) and triglycerides were measured using HR Series NEFA-HR(2) and L-Type TG M reagents and the microtiter procedure supplied by the manufacturer (Wako Chemical USA, Richmond, VA).
Muscle Fiber Typing and Vascularity
The triceps surae [gastrocnemius (GA), soleus (SOL), and plantaris (PLT)] muscles and extensor digitorum longus (EDL) muscle were excised from both legs. Muscles from the left leg were immediately frozen in liquid N2 for later molecular analyses.
Histology preparation.
From the right leg, the midbelly of each muscle was quickly and carefully excised and placed on a cork (with tissue mounting medium) and then flash frozen in isopentane-cooled liquid N2. The heart was cut across the middle of the left and right ventricles, placed on a cork (with tissue mounting medium), and then flash frozen in isopentane-cooled liquid N2. All samples were then stored at –80°C until processed for molecular or morphological analyses.
Muscle fiber typing and capillarity.
Frozen tissue was cut using a –20°C cryomicrotome (Jung-Reichert Cryocut 1800; Cambridge Instruments, Nussloch, Germany) to yield 8-μm-thick transverse sections. Great care was taken to ensure that the cryosectioned muscles were cut along the transverse plane. All tissues were stained for fiber type using the lead nitrate-ATPase method described by Rosenblatt et al. (39) allowing for direct assessment of lightly stained (type II) fibers, and darkly stained (type I) fibers. Capillarity assessment was done using FITC-labeled Griffonia isolectin B4, as previously reported (40, 41). Here, muscle sections on the slides were fixed in 4% paraformaldehyde and stained with FITC-conjugated Griffonia simplicifolia I isolectin (1:100; Vector Labs) for 30–60 min. Light or fluorescent microscopy (using ×40 objective) was used to digitally acquire images of sectioned muscle regions of interest, from each muscle type. Capillary and myofiber counting were performed by a single individual who was blinded to the identity of each of the samples. For the gastrocnemius and heart muscles, we obtained images in a checkerboard fashion across the entire muscle (endocardial area for heart). For the other muscles, full-screen nonoverlapping images were obtained and analyzed. Counting was performed by visualization from acquired images using NIH Image J software to visually mark/count the capillaries and fibers on each image. Capillary-to-fiber ratio (C:F; number of capillaries/number muscle fibers), capillary density (CD; number of capillaries/muscle fiber area), and fiber cross-sectional area (FCSA) were measured and calculated.
RNA Seq, Sequence Alignment, and Data Analysis
RNA was isolated from a single gastrocnemius and a single soleus muscle per mouse to evaluate transcriptomics patterns in each tissue. Gastrocnemius samples were cryopulverized using a Spex geno grinder 2010 (Metuchen, NJ). Gastrocnemius powder (∼25 mg) or whole soleus were homogenized in Tri Reagent (Invitrogen, Thermo Fisher Scientific) using a Precellys 24 homogenizer at 5,000 rpm for 20 s and 6,700 rpm for 28 s, two times, respectively. Homogenates were incubated at room temperature for 5 min before being frozen at −80°C. Once thawed, 1-bromo-3-chloropropane (BCP, Sigma) was added (1:100 BCP:Tri Reagent). Samples were vortexed at maximum speed, incubated at room temperature for 15 min, and centrifuged at 20,800 g for 15 min at 4°C. The aqueous phase was mixed with equal parts of 70% ethanol and applied to RNeasy mini spin columns (Qiagen). The spin columns were washed once with half the recommended volume of RW1 buffer followed by a DNase treatment (15 min, Qiagen). The columns were washed with half the recommended volume of RW1 buffer. The remaining steps were done per the manufacturer’s protocol. RNA concentrations and 260/280 ratios were determined via UV spec. RNA quality was assessed using Agilent TapeStation system as per manufacturer’s instructions (Santa Clara, CA).
For transcriptomics analyses of mixed muscle (gastrocnemius) and type I, Mb-rich muscle (SOL), next generation sequencing and RNA-seq library preparation was performed by the UAMS Genomics Core Facility located in the Winthrop P. Rockefeller Cancer Institute at the University of Arkansas for Medical Sciences. RNA-seq libraries were prepared using the Illumina TruSeq Stranded total RNA Library Prep Kit (San Diego, CA) with TruSeq Unique Dual Indexed adapters. Prepared libraries were assessed for mass with the Qubit 3.0 fluorometer using the Qubit 1× dsDNA High-Sensitivity Assay Kit (Thermo Fisher Scientific), for functionality with the KAPA Library Quantification Kit (Roche Sequencing and Life Science, Wilmington, MA), and for fragment size with the Agilent Fragment Analyzer using the High-Sensitivity NGS Gel Kit (Santa Clara, CA). Molarity was calculated for libraries and then diluted and denatured according to Illumina recommendations for clustering on a cBot and paired-end sequencing on a HiSeq 3000 with a 150-cycle SBS kit for 2 × 75 reads. Alignment to mouse (GRCm38) genome was carried out using STAR (42). All the aligned reads were exported in BAM format and subsequent data analysis was performed in the SeqMonk software package (Babraham Bioinformatics, Cambridge, UK). Differentially expressed genes between samples from Mb−/− and WT mice were analyzed using iDEP version 0.92 (43) (http://bioinformatics.sdstate.edu/idep/). Individual contrasts were within-sex and within-muscle group (e.g., comparing male Mb−/− soleus to male WT soleus). The “DEG1” feature of the program was used to generate transcript FDR-adjusted P value and fold-difference lists, which were exported to Excel and sorted by fold- and P values to visualize differentially expressed genes.
Statistical Analysis
Repeated-measures linear Mixed-Effects Models were fit to the primary study responses to assess the differences between experimental groups over the course of the longitudinal feeding study, specifically for periods between the CLAMS data collection windows (R, lme4 package). If interaction terms were significant, the groups were appropriately subdivided by sex, then by temperatures. Two tailed t tests were conducted when genotype was the only comparison: for example, for parameters that were not longitudinal, such as starting and terminal body weights within sex. When multiple comparisons were made among experimental group means, the Tukey multiple comparison procedure was employed.
RESULTS
Body Mass, Adiposity, Tissue Weights, and Glucose Homeostasis
Although there was a trend toward higher body weight in female Mb−/− mice, whole body deletion of Mb did not significantly affect body weight changes of male or female mice fed HFD (Fig. 3A). Terminal body weight was also not significantly affected by genotype (Table 1). As reported in methods, mice in this study had excessive food spillage, thus we cannot accurately report energy intake data for the entire study period. A 21-day period was identified where excess food spillage was reduced, and during this time window energy intake was not different between genotypes or between sexes (Fig. 3B). Interestingly, females regardless of genotype gained less weight then males despite equivalent food intake, reflective of sex differences in feed efficiency (lower in females). The nonsignificant increase in body weight of female Mb−/− mice was also reflected in increases (P = 0.1) for masses of certain fat depots (inguinal and perigonadal) and the total adiposity (sum of all collected fat depots) (Table 1). Fat mass as measured by DEXA following the CLAMS protocol showed similar trends, with higher adiposity of female Mb−/− mice versus WT mice at ages 12 wk and 18 wk, but these differences did not reach statistical significance (Table 2). Fat mass of male mice did not differ by genotype, as assessed by tissue weights (Table 3) or DEXA results (Table 4). Skeletal muscle masses did not differ by genotype, and the masses of several other tissues did not differ by genotype or sex (Tables 1 and 3).
Figure 3.
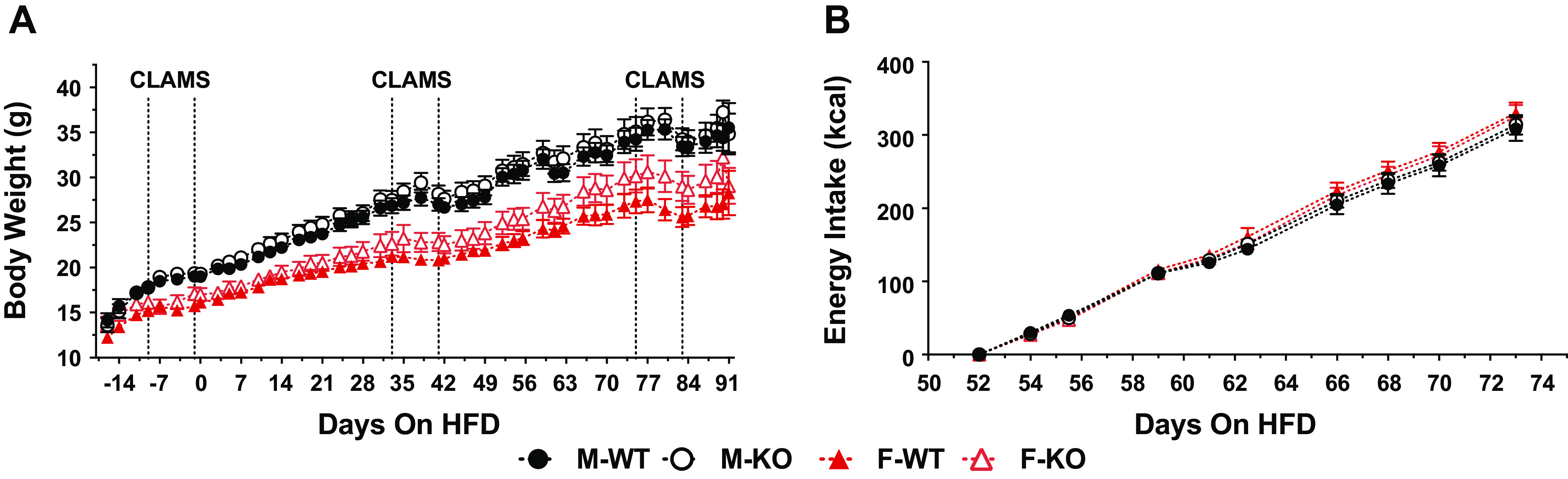
Body weight and energy intake patterns of male and female wildt-ype (WT) and myoglobin (Mb) KO mice fed a high-fat diet (45% of energy) for ∼13 wk. A: body weights of mice over the course of the study. Dotted vertical lines indicate when respiratory exchange ratio (RER) and energy expenditure were measured using an indirect calorimetry CLAMS instrument. B: cumulative energy intake (kcal) of mice during a time period that had the lowest number of excess spillage days (many mice in the study spilled food for much of the time frame, allowing only a subset of days to be analyzed with accuracy). Values depict means ± SE. Body weight: males, n = 10 per group; females, n = 9 per group. Energy intake: males, n = 9–10 per group; females n = 7–9 per group. HFD, high-fat diet (45% of energy).
Table 1.
Female body weight, tissue masses (in grams), and postabsorptive blood plasma lipids in wild-type and myoglobin knockout mice following ∼13 wk of feeding a high-fat diet (45% of energy)
| WT | Mb−/− | t Test P Valuea | |
|---|---|---|---|
| Body weight | 26.5 ± 1.3 | 29.7 ± 1.7 | 0.16 |
| Brain | 0.496 ± 0.012 | 0.473 ± 0.005 | 0.12 |
| Liver | 1.030 ± 0.022 | 1.030 ± 0.048 | 0.91 |
| Cecumb | 0.160 ± 0.015 | 0.166 ± 0.013 | 0.77 |
| Adiposec | |||
| Total adipose | 2.383 ± 0.467 | 3.548 ± 0.453 | 0.09 |
| Subcutaneous | 0.785 ± 0.153 | 1.088 ± 0.128 | 0.15 |
| Inguinal | 0.210 ± 0.039 | 0.300 ± 0.03 | 0.09 |
| Prigonadal | 0.971 ± 0.218 | 1.590 ± 0.248 | 0.08 |
| Retroperitoneal | 0.248 ± 0.055 | 0.371 ± 0.045 | 0.10 |
| Brown adipose tissue | 0.168 ± 0.017 | 0.198 ± 0.015 | 0.20 |
| Muscled | |||
| Heart | 0.128 ± 0.002 | 0.130 ± 0.003 | 0.64 |
| Left gastrocnemius | 0.113 ± 0.002 | 0.113 ± 0.003 | 0.99 |
| Left plantaris | 0.014 ± 0.0005 | 0.014 ± 0.0007 | 0.53 |
| Left soleus | 0.008 ± 0.0003 | 0.008 ± 0.0004 | 0.55 |
| Left extensor digitorum longus | 0.008 ± 0.0004 | 0.008 ± 0.0004 | 0.85 |
| Plasma lipids | |||
| Triglycerides (mg/dL) | 23.3 ± 1.3 | 22.6 ± 1.4 | 0.69 |
| Nonesterified fatty acids (μM) | 356 ± 16 | 322 ± 13 | 0.12 |
Two-tailed t test; n = 9/genotype.
Cecum was not emptied.
Masses of left and right fat pads; “total adipose” is the summed weight of all measured white adipose pads.
Mass of the left-side muscle only. Mb−/−, myoglobin knockout; WT, wild type.
Table 2.
Female lean and fat masses (grams) in wild-type and myoglobin knockout mice, measured by DEXA following CLAMS studies at different ages
| WT | Mb−/− | t Test P Valuea | |
|---|---|---|---|
| 6 wk* | |||
| Lean mass | 12.486 ± 0.405 | 13.342 ± 0.565 | 0.24 |
| Fat mass | 2.032 ± 0.120 | 2.315 ± 0.129 | 0.13 |
| 12 wk* | |||
| Lean mass | 16.038 ± 0.313 | 16.694 ± 0.464 | 0.26 |
| Fat mass | 3.611 ± 0.477 | 5.061 ± 0.684 | 0.10 |
| 18 wk* | |||
| Lean mass | 17.041 ± 0.298 | 17.579 ± 0.475 | 0.35 |
| Fat mass | 7.172 ± 1.208 | 10.206 ± 1.328 | 0.11 |
Approximate ages when mice were removed from the CLAMS instrument and DEXA measurements were taken, with preceding 3 days at 10°C, and before placement back into home cages. Mb−/−, myoglobin knockout; WT, wild type.
Two-tailed t test; n = 9/genotype.
Table 3.
Male body weight, tissue masses (in grams), and postabsorptive blood plasma lipids in wild-type and myoglobin knockout mice following ∼13 wk of feeding a high-fat diet (45% of energy)
| WT | Mb−/− | t Test P Valuea | |
|---|---|---|---|
| Body weight | 33.8 ± 1.26 | 34.8 ± 1.45 | 0.63 |
| Brain | 0.462 ± 0.011 | 0.473 ± 0.011 | 0.47 |
| Liver | 1.230 ± 0.041 | 1.330 ± 0.078 | 0.29 |
| Cecumb | 0.187 ± 0.018 | 0.195 ± 0.013 | 0.74 |
| Adiposec | |||
| Total adipose | 4.116 ± 0.284 | 3.900 ± 0.201 | 0.54 |
| Subcutaneous | 1.083 ± 0.091 | 1.062 ± 0.082 | 0.87 |
| Inguinal | 0.258 ± 0.023 | 0.245 ± 0.014 | 0.65 |
| Perigonadal | 1.917 ± 0.134 | 1.742 ± 0.094 | 0.30 |
| Retroperitoneal | 0.545 ± 0.039 | 0.514 ± 0.022 | 0.50 |
| Brown adipose tissue | 0.314 ± 0.020 | 0.337 ± 0.028 | 0.50 |
| Muscled | |||
| Heart | 0.139 ± 0.003 | 0.149 ± 0.005 | 0.08 |
| Left gastrocnemius | 0.124 ± 0.003 | 0.130 ± 0.003 | 0.23 |
| Left plantaris | 0.018 ± 0.0005 | 0.017 ± 0.0007 | 0.35 |
| Left soleus | 0.009 ± 0.001 | 0.009 ± 0.0004 | 0.76 |
| Left extensor digitorum longus | 0.009 ± 0.0005 | 0.009 ± 0.0005 | 0.42 |
| Plasma Lipids | |||
| Triglycerides (mg/dL) | 23.4 ± 1.1 | 23.1 ± 0.5 | 0.86 |
| Nonesterified fatty acids (μM) | 332 ± 8 | 341 ± 19 | 0.65 |
Two-tailed t test; n = 10/genotype.
Cecum was not emptied.
Masses of left and right fat pads; “total adipose” is the summed weight of all measured white adipose pads.
Mass of the left-side muscle only. Mb−/−,myoglobin knockout; WT, wild-type.
Table 4.
Male lean and fat masses (grams) in wild-type and myoglobin knockout mice, measured by DEXA following CLAMS studies at different ages
| WT | Mb−/− | t Test P Valuea | |
|---|---|---|---|
| 6 wk* | |||
| Lean mass | 15.365 ± 0.356 | 15.426 ± 0.381 | 0.910 |
| Fat mass | 2.419 ± 0.132 | 2.671 ± 0.153 | 0.230 |
| 12 wk* | |||
| Lean mass | 18.932 ± 0.326 | 19.188 ± 0.418 | 0.640 |
| Fat mass | 6.916 ± 0.480 | 7.796 ± 0.709 | 0.320 |
| 18 wk* | |||
| Lean mass | 20.454 ± 0.251 | 20.644 ± 0.598 | 0.770 |
| Fat mass | 11.828 ± 0.866 | 12.082 ± 0.830 | 0.830 |
Approximate ages when mice were removed from the CLAMS instrument and DEXA measurements were taken, with preceding 3 days at 10°C, and before placement back into home cages. Mb−/−, myoglobin knockout; WT, wild type.
Two-tailed t test; n = 10/genotype.
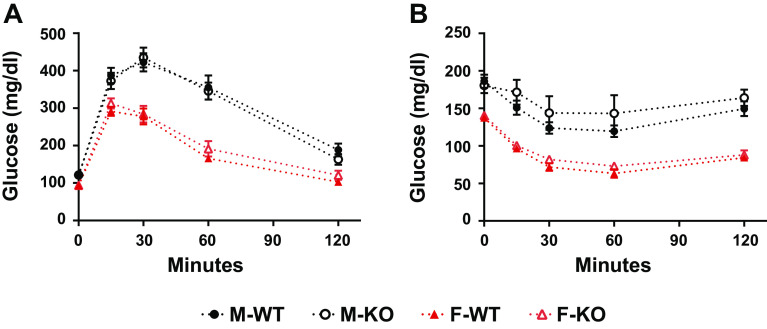
Skeletal muscle is a primary site of postprandial glucose disposal or in response to insulin, and lipid-induced insulin resistance can manifest in muscle. The potential roles of Mb to support muscle fuel selection and to limit inordinate accumulation of tissue lipids prompted an assessment of glucose tolerance and insulin action at weeks 8.5 and 9.5 of the HFD challenge. Females displayed substantially better profiles of glucose disposal and lower postabsorptive blood glucose concentrations (e.g., time zero of the ITT) when compared with males (Fig. 4). However, the deletion of Mb had no significant effect on glucose tolerance or insulin-associated blood glucose reductions (Fig. 4).
Figure 4.
Myoglobin knockout (Mb KO) in male and female mice had no impact on glucose and insulin tolerance when compared with wild-type (WT) controls. A: glucose tolerance test (GTT): the glucose response of male and female, WT and Mb KO mice to intraperitoneal injection of 2 mg/kg of glucose. Mice were fasted overnight before the injection. B: insulin tolerance test (ITT): the glucose response of male and female, WT, and Mb KO mice to intraperitoneal injection of 0.75 U/kg of Humulin insulin. Mice were fasted for 4 h before the injection. Values are means ± SE; males, n = 10 per group; females, n = 9 per group.
CLAMS Experiments: Metabolic Rate and Whole Body Fuel Oxidation
Should Mb serve to regulate muscle and heart fat oxidation under basal conditions or when challenged by conditions promoting fatty acid oxidation (e.g., cold exposure), this should be reflected in changes to the respiratory exchange ratio (RER). However, the lack of Mb did not affect the RER significantly at any age, diet, or temperature (Fig. 5). As expected, the HFD itself led to lower total daily average, light cycle, and dark cycle RERs in all mice when compared with the chow diet, indicating higher reliance on fat as a fuel source during HFD feeding. HFD-fed male C57BL/N males housed at 10°C had a reduced RER compared with males housed at 22°C (see ages 11–12 wk and 17–18 wk in Fig. 5). Ambient temperature had no impact on RER of female mice. Energy expenditure was not affected by Mb−/−. As anticipated, the cold temperature (10°C) increased energy expenditure and this effect was similar regardless of age, genotype, sex, or diet (Fig. 6). The energy expenditure as calculated from indirect calorimetry was not different between males and females (Fig. 6), which is surprising in light of disparate body weight gains (Fig. 3).
Figure 5.
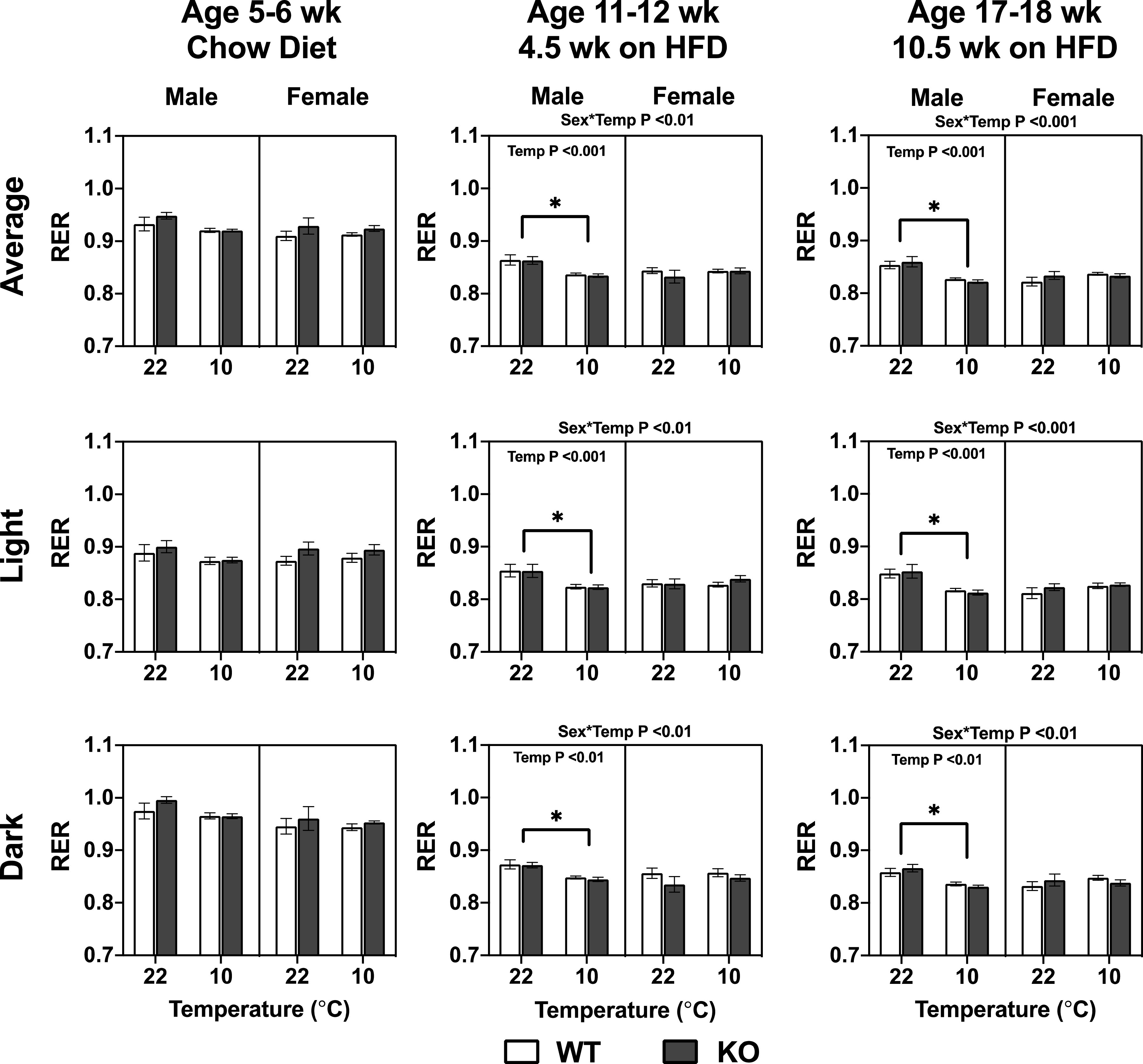
The lack of myoglobin (Mb) in male and female mice had no impact on RER when mice are fed a chow diet or high-fat diet (HFD, 45% energy) at different ambient temperatures. The results were calculated from two light and dark cycles per temperature, following an initial 24-h acclimation within each temperature in an indirect calorimetry CLAMS instrument. Ages indicate approximate weeks when animals were tested under the 9 d CLAMS protocol. Three-way repeated measures ANOVA assessed differences between genotype (Geno), sex, temperature (Temp), and interactions. The interactions and P values displayed on the top of the graphs are from a three-way repeated measures ANOVA testing for effects of genotype (Geno), sex, temperature (Temp), and interactions. The P values displayed within the graphs are from a two-way repeated measures ANOVA testing for Geno and Temp effects for each sex independently, and using a Tukey’s post hoc test. *Significant differences for the temperature main effect, at P < 0.05. Values are means ± SE; males, n = 10 per group; females, n = 9 per group.
Figure 6.
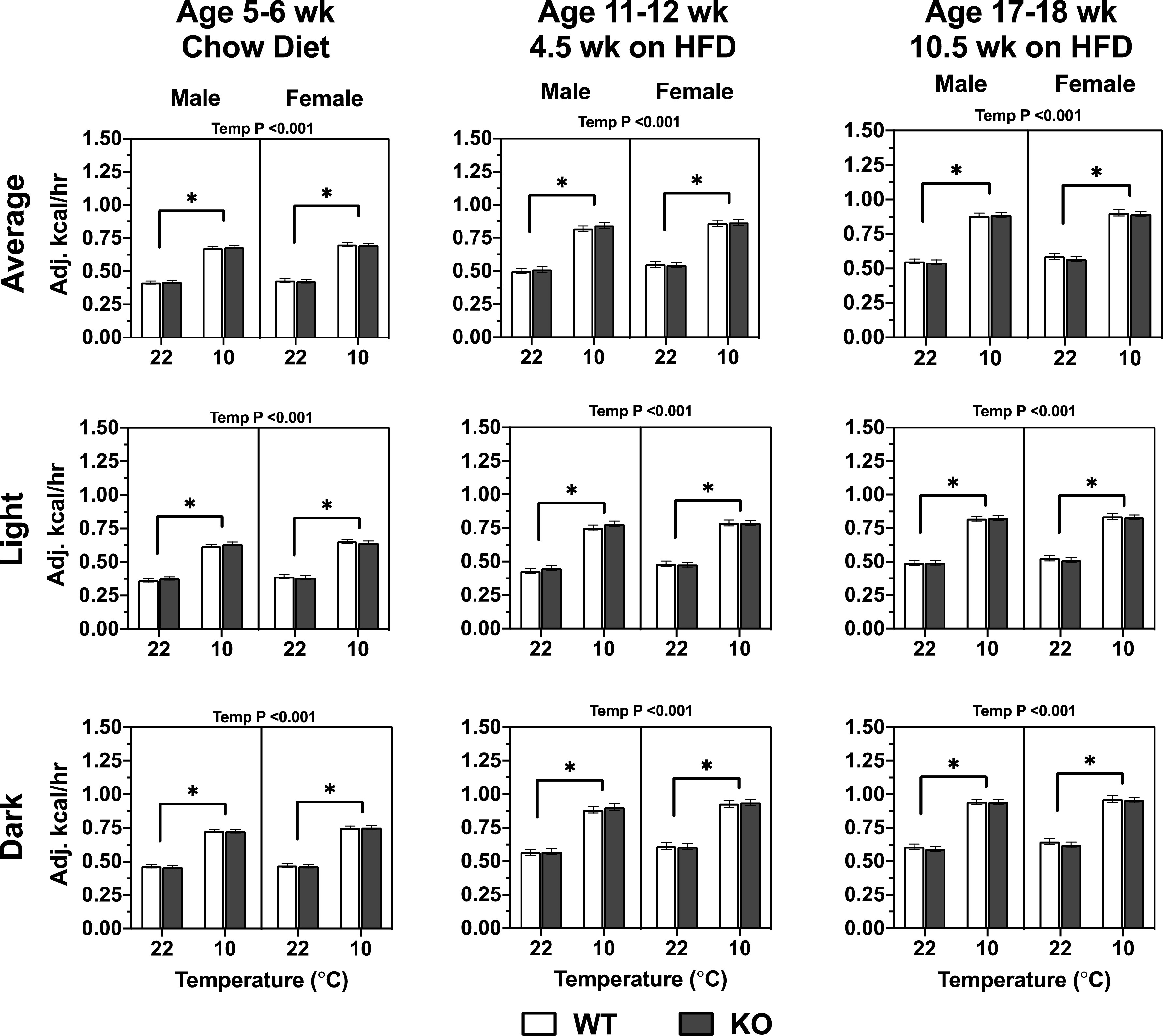
The lack of myoglobin (Mb) in male and female mice had no impact on body weight covariate-adjusted energy expenditure when mice are fed chow or high-fat diet (HFD) at different ambient temperatures. The results were calculated from two light and dark cycles per temperature, following an initial 24-h acclimation within each temperature (see methods and Fig. 1). Ages indicate approximate weeks when animals were tested under the 9 d CLAMS protocol. Three-way repeated measures ANOVA assessed differences between genotype (Geno), sex, temperature (Temp), and interactions. The interactions and P values displayed on the top of the graphs are from a three-way repeated measures ANOVA testing for effects of genotype (Geno), sex, temperature (Temp), and interactions. The P values displayed within the graphs are from a two-way repeated measures ANOVA testing for Geno and Temp effects for each sex independently, and using a Tukey’s post hoc test. *Differences for the temperature main effect, at P < 0.05. Values are means ± SE; males, n = 10 per group; females, n = 9 per group.
CLAMS Experiments: Activity Patterns
The subset of behaviors reflective of X dimension ambulatory activity (XAMB, movement down the length of the cage) at 5–6 wk of age was lower at 10°C than at 22°C, regardless of genotype or sex (Fig. 7A). At 11–12 wk of age, female Mb−/− mice had reduced XAMB beam breaks compared with female WT mice regardless of temperature (genotype < 0.05). The effect of genotype showed a similar trend in 17- to 18-wk-old females, but this was not statistically significant. The opposite pattern was seen in males: male Mb−/− mice had significantly increased XAMB beam breaks at 17–18 wk of age and a similar trend at 11–12 wk of age. Total X dimension beam breaks (XTOT) represent total beam breaks down the length of the cage (XAMB breaks due to ambulatory movement, plus any other beam breaks that can occur in a more stationary condition [e.g., grooming with repeated single beam break]). Chow-fed 5- to 6-wk-old mice had reduced XTOT at 10°C compared with 22°C (Fig. 7B). This did not differ by genotype or sex. At 11–12 and 17–18 wk of age, there were genotype by sex interactions; on average, female Mb−/− mice had lower XTOT movement compared with WT and the opposite was seen for male Mb−/− compared with their WT counterparts. At 11–12 wk of age, female Mb−/− mice had significantly reduced XTOT movement at 10°C (genotype by temperature interaction, P < 0.05; t test, P < 0.05). Total movements detected in the Z dimension (ZTOT) are due to beam breaks lengthwise down the cage and higher up, thus reflective of rearing or jumping-type activity. At 5–6 wk of age, mice had reduced ZTOT beam breaks at 10°C compared with 22°C regardless of sex or genotype (Fig. 7C). Differences between female Mb−/− and WT mice were not statistically significant despite higher average values for female Mb−/− mice. At 11–12 wk of age, female mice exhibited reduced ZTOT beam breaks in response to cold temperature, with no effect in males. By 17–18 wk of age, there was a sex by genotype by temperature interaction, with no significant effect of genotype.
Figure 7.
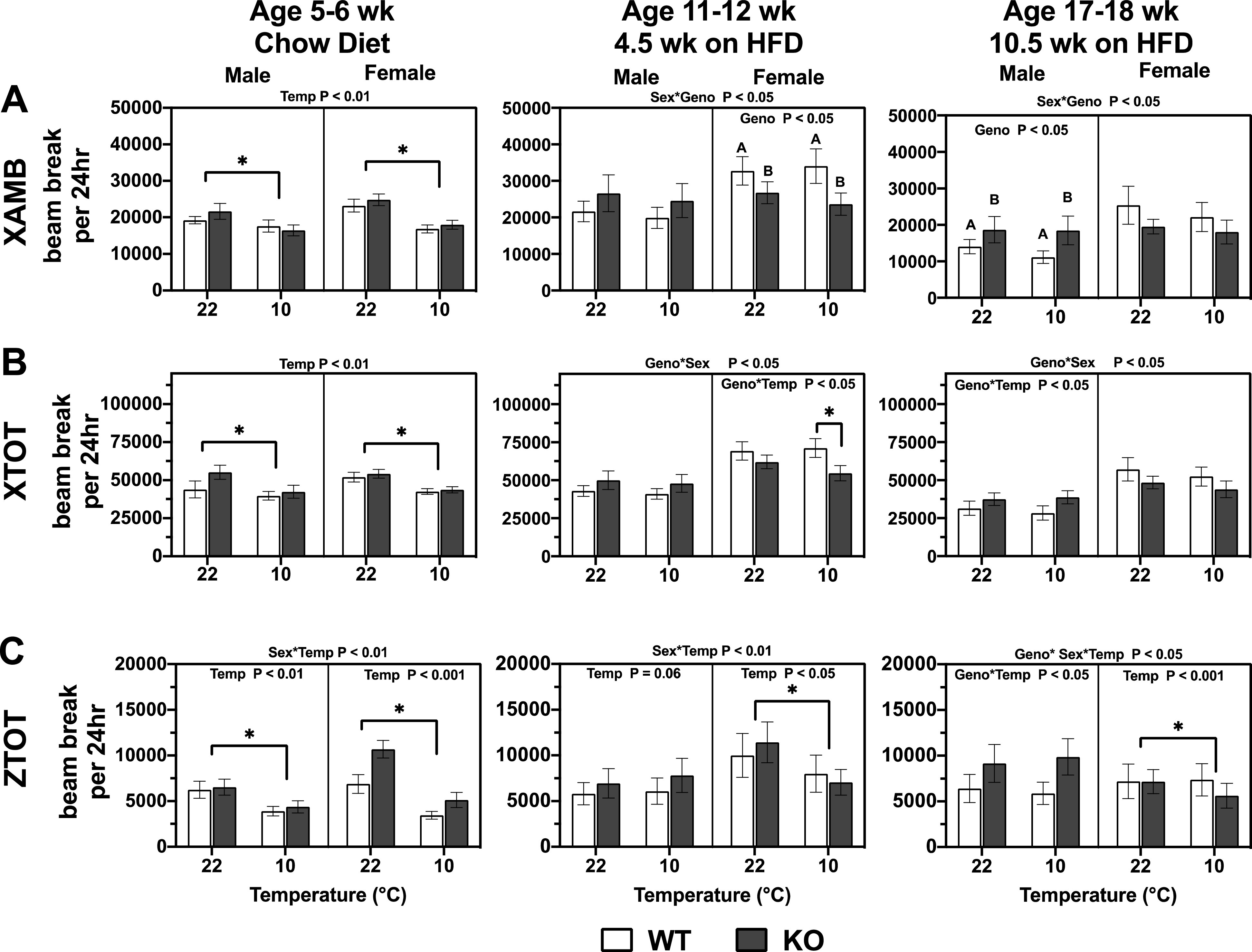
Activity patterns—XAMB (A), XTOT (B), and ZTOT (C)—in male and female, wild-type (WT), and myoglobin knockout (Mb-KO) mice at different ambient temperatures. The results were calculated from two light and dark cycles per temperature, following an initial 24-h acclimation within each temperature (see METHODS and Fig. 1). Ages indicate approximate weeks when animals were tested under the 9 d CLAMS protocol. Three-way repeated measures ANOVA assessed differences between genotype (Geno), sex, temperature (Temp), and interactions. The interactions and P values displayed on the top of the graphs are from a three-way repeated measures ANOVA testing for effects of genotype (Geno), sex, temperature (Temp), and interactions. The P values displayed within the graphs are from a two-way repeated measures ANOVA testing for Geno and Temp effects for each sex independently, and using a Tukey’s post hoc test. *Significant differences for the temperature main effect, at P < 0.05. Values are means ± SE; males, n = 7–8 per group; females, n = 7–8 per group [due to outlier analysis (Rout test)]. XAMB, X dimension ambulatory activity; XTOT, total X dimension beam breaks; ZTOT, Total movements detected in the Z dimension.
Muscle Fiber and Capillary Phenotyping: Genotype and Sex Effects
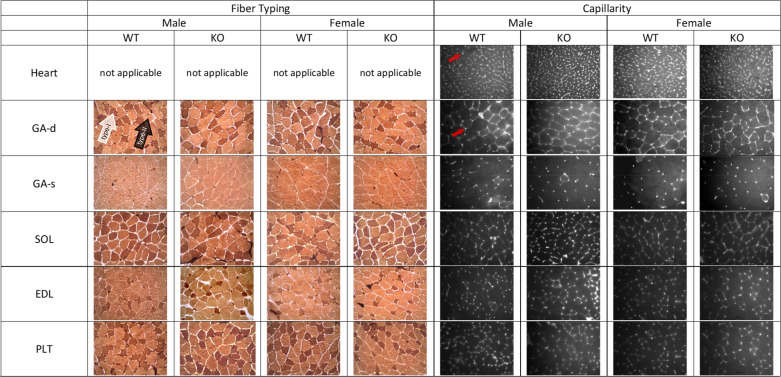
Considering the role of Mb in O2 management, it is possible that loss of the protein could upregulate pathways regulating neovascularization and/or muscle fiber type switching (i.e., to favor type II fibers over more oxidative type I fibers). With this in mind, we quantified indices of capillarity and fiber phenotypes in heart and multiple muscle groups [gastrocnemius-superficial (GA-s), gastrocnemius-deep (GA-d), soleus (SOL), extensor digitorum longus (EDL), and plantaris (PLT)]. Representative histology and histochemical images may be found in Fig. 8. Using females and males together in the initial statistical analyses (Table 5), several points are noteworthy with respect to the effect of Mb knockout. First, capillary density (number of capillaries per area) and the ratio of capillary numbers to fiber numbers (C:F ratio) were significantly increased in the deep gastrocnemius and soleus of Mb−/− mice, but not in other muscle regions. Second, heart capillarity (capillary density) had a significant sex x genotype interaction, with a modestly increased capillary number in males but a modestly lower number in females (see Tables 6 and 7). Third, there was no effect of genotype on mean fiber area in any muscle group, which also supports the finding of a specific increase in capillarity as seen via capillary density (in GA-d and SOL). Fourth, although some trends were seen, there was a lack of significant genotype effects on fiber type percent in any muscle group; in other words, no strong evidence for a “fiber type switch” associated with loss of muscle Mb.
Figure 8.
Representative images for fiber typing (stained with lead nitrate-ATPase method described by Rosenblatt) and capillarity (stained with FIT-C labeled Griffonia isolectin B4) in mouse skeletal muscle. For fiber typing, darkly stained fibers are type I (as shown by the white arrow) and lightly stained are type II (as shown by the black arrow). Red arrows show examples of capillaries in lectin stained images. GA-d, gastrocnemius muscle deep; GA-s, gastrocnemius muscle superficial; SOL, soleus; EDL, extensor digitorum longus; Mb−/−, myoglobin knockout; PLT, plantaris; WT, wild type.
Table 5.
Capillary, muscle and heart phenotypes in wild-type and myoglobin knockout mice following ∼13 wk feeding of a high-fat diet (45% of calories)
| Genotype |
Sex |
Genotype × Sex |
|||
|---|---|---|---|---|---|
| WT | Mb−/− | P Value | P value | P Value | |
| C:F (#capillaries/#fibers) | |||||
| Heart | na | na | |||
| GA-d | 1.60 ± 0.06 | 1.84 ± 0.05*† | <0.01 | <0.05 | ns |
| GA-s | 1.33 ± 0.05 | 1.39 ± 0.06† | ns | <0.001 | ns |
| SOL | 1.92 ± 0.07 | 2.16 ± 0.05* | <0.01 | ns (0.10) | ns |
| EDL | 1.45 ± 0.06 | 1.46 ± 0.05 | ns | <0.05 | ns |
| PLT | 2.07 ± 0.07 | 1.97 ± 0.07 | ns | ns | ns (0.06) |
| Capillary density (#/mm2) | |||||
| Heart | 2,423 ± 62 | 2,408 ± 66‡ | ns | ns (0.08) | <0.01 |
| GA-d | 740 ± 27 | 872 ± 38* | <0.01 | ns | ns |
| GA-s | 463 ± 17 | 475 ± 12† | ns | <0.001 | ns (0.072) |
| SOL | 920 ± 30 | 1,078 ± 36*† | <0.001 | <0.05 | ns |
| EDL | 764 ± 23 | 797 ± 34 | ns | ns (0.06) | ns |
| PLT | 925 ± 27 | 866 ± 45 | ns | ns | ns |
| Mean fiber area (μm2) | |||||
| Heart | na | na | |||
| GA-d | 2,051 ± 48 | 2,103 ± 84 | ns | ns | ns |
| GA-s | 2,472 ± 49 | 2,460 ± 70 | ns | ns | ns (0.072) |
| SOL | 2,029 ± 68 | 2,041 ± 68 | ns | ns | ns |
| EDL | 1,900 ± 77 | 1,782 ± 49 | ns | ns | ns |
| PLT | 2,228 ± 102 | 2,295 ± 140 | ns | ns | ns |
| Type 1 fibers (%) | |||||
| Heart | na | na | |||
| GA-d | 32.2 ± 0.01 | 34.7 ± 0.01 | ns (0.057) | ns | ns |
| GA-s | 0.3 ± 0.1 | 0.3 ± 0.1 | ns | ns | ns |
| SOL | 48.3 ± 1.3 | 49.6 ± 2.3† | ns | <0.01 | ns |
| EDL | 14.9 ± 0.9 | 14.4 ± 0.07† | ns | <0.05 | ns (0.069) |
| PLT | 40.6 ± 1.3 | 37.1 ± 1.3 | ns (0.068) | ns | ns |
| Type 2 fibers (%) | |||||
| Heart | na | na | |||
| GA-d | 67.6 ± 0.1 | 65.3 ± 0.1 | ns (0.067) | ns | ns |
| GA-s | 99.7 ± 0.1 | 99.8 ± 0.3 | ns | ns | ns |
| SOL | 52.2 ± 1.3 | 50.7 ± 2.3† | ns | <0.01 | ns |
| EDL | 85.1 ± 0.1 | 85.5 ± 0.1† | ns | <0.05 | ns (0.053) |
| PLT | 59.4 ± 1.3 | 62.9 ± 1.3 | ns (0.068) | ns | ns |
Means ± SE. na, not analyzed. Significant difference by *genotype, †sex, ‡genotype × sex interaction; ns, not statistically significant (trends at P < 0.1 in parentheses); n = 16–20/genotype. EDL, extensor digitorum longus; GA-d, gastrocnemius muscle deep; GA-s, gastrocnemius muscle superficial; Mb−/−, myoglobin knockout; PLT, plantaris; SOL, soleus; WT, wild type.
Table 6.
Muscle capillary and fiber type phenotypes in male wild-type and myoglobin knockout mice
| Male | WT | Mb−/− |
|---|---|---|
| C:F (#Capillaries/#Fibers) | ||
| Heart | na | na |
| GA-d | 1.71 ± 0.05 | 1.91 ± 0.05 |
| GA-s | 1.49 ± 0.07 | 1.55 ± 0.07 |
| SOL | 1.98 ± 0.08 | 2.24 ± 0.06 |
| EDL | 1.59 ± 0.07 | 1.49 ± 0.03 |
| PLT | 1.94 ± 0.07 | 2.08 ± 0.07 |
| Capillary density (#/mm2) | ||
| Heart | 2,357 ± 101 | 2,551 ± 68 |
| GA-d | 764 ± 22 | 895 ± 49 |
| GA-s | 520 ± 9 | 502 ± 15 |
| SOL | 957 ± 44 | 1,133 ± 53 |
| EDL | 799 ± 16 | 839 ± 43 |
| PLT | 912 ± 48 | 880 ± 73 |
| Mean fiber area (μm2) | ||
| Heart | na | na |
| GA-d | 2,028 ± 79 | 2,071 ± 60 |
| GA-s | 2,422 ± 49 | 2,517 ± 83 |
| SOL | 2,103 ± 95 | 2,034 ± 114 |
| EDL | 2,032 ± 142 | 1,784 ± 77 |
| PLT | 2,188 ± 105 | 2,295 ± 183 |
| Type 1 fibers (%) | ||
| Heart | na | na |
| GA-d | 32.2 ± 1.3 | 33.3 ± 1.1 |
| GA-s | 0.2 ± 0.1 | 0.1 ± 0.1 |
| SOL | 52.1 ± 1.3 | 52.7 ± 4.1 |
| EDL | 14.8 ± 1.3 | 12.4 ± 0.1 |
| PLT | 40.2 ± 1.7 | 38.1 ± 1.3 |
| Type 2 fibers (%) | ||
| Heart | na | na |
| GA-d | 67.6 ± 1.3 | 66.7 ± 1.1 |
| GA-s | 99.8 ± 0.1 | 99.9 ± 0.6 |
| SOL | 48.3 ± 1.1 | 47.3 ± 4.1 |
| EDL | 85.2 ± 1.3 | 87.7 ± 0.8 |
| PLT | 59.8 ± 1.7 | 61.9 ± 1.3 |
GA-d, deep gastrocnemius; GA-s, superficial gastrocnemius; SOL, soleus; EDL, extensor digitorum longus; Mb−/−,myoglobin knockout; PLT, plantaris; WT, wild type.
n = 8–10 per genotype; statistics for genotype effects provided in Table 5.
Table 7.
Muscle capillary and fiber type phenotypes in female wild-type and myoglobin knockout mice
| Female | WT | Mb−/− |
|---|---|---|
| C:F (#Capillaries/#Fibers) | ||
| Heart | na | na |
| GA-d | 1.49 ± 0.09 | 1.77 ± 0.08 |
| GA-s | 1.17 ± 0.02 | 1.24 ± 0.06 |
| SOL | 1.87 ± 0.10 | 2.07 ± 0.08 |
| EDL | 1.33 ± 0.8 | 1.42 ± 0.10 |
| PLT | 2.07 ± 0.11 | 1.86 ± 0.98 |
| Capillary density (#/mm2) | ||
| Heart | 2,482 ± 76 | 2,203 ± 81 |
| GA-d | 716 ± 50 | 850 ± 59 |
| GA-s | 407 ± 20 | 445 ± 15 |
| SOL | 888 ± 40 | 1,018 ± 42 |
| EDL | 733 ± 39 | 751 ± 53 |
| PLT | 938 ± 27 | 850 ± 52 |
| Mean fiber area (μm2) | ||
| Heart | na | na |
| GA-d | 2,169 ± 130 | 2,036 ± 109 |
| GA-s | 2,561 ± 114 | 2,347 ± 64 |
| SOL | 2,128 ± 84 | 2,091 ± 85 |
| EDL | 1,782 ± 54 | 1,781 ± 60 |
| PLT | 2,269 ± 145 | 2,294 ± 224 |
| Type 1 fibers (%) | ||
| Heart | na | na |
| GA-d | 32.3 ± 1.2 | 36.1 ± 1.4 |
| GA-s | 0.4 ± 0.2 | 0.4 ± 0.2 |
| SOL | 44.8 ± 1.5 | 46.2 ± 1.5 |
| EDL | 15.0 ± 1.3 | 16.6 ± 0.1 |
| PLT | 40.9 ± 2.1 | 36.0 ± 2.4 |
| Type 2 fibers (%) | ||
| Heart | na | na |
| GA-d | 67.7 ± 1.2 | 63.9 ± 1.4 |
| GA-s | 99.6 ± 0.2 | 99.6 ± 0.2 |
| SOL | 55.6 ± 1.6 | 54.5 ± 1.1 |
| EDL | 85.0 ± 1.3 | 83.1 ± 0.8 |
| PLT | 59.1 ± 2.1 | 64.0 ± 2.4 |
EDL, extensor digitorum longus; GA-d, deep gastrocnemius; GA-s, superficial gastrocnemius; Mb−/−, myoglobin knockout; SOL, soleus; PLT, plantaris; WT, wild type.
n = 8–10 per genotype; statistics for genotype effects provided in Table 5.
There were several significant findings with respect to sexual dimorphism of muscle phenotype. A full evaluation of this phenomenon is beyond the scope of the current report; this is the specific focus of a separate paper (in preparation) that will include larger numbers of males and females.
Muscle Transcriptomics
The loss of myoglobin function may cause adaptative changes in signals that regulate gene expression, which could in theory be specific to muscle type. For this reason, a large-scale transcriptomics analysis was conducted in whole gastrocnemius (mixed fiber type) and type I-rich soleus muscle. Considering the sex X genotype and sex effects on capillary and fiber type phenotypes (see above), we compared within-sex transcriptomics patterns of Mb−/− mice and WT mice. As expected, the most significant transcript comparing gastrocnemius and soleus muscles between genotypes was Mb (see Supplemental Material S1; all Supplemental material is available at https://doi.org/10.15482/USDA.ADC/1520663). Some residual Mb mRNA reads could be detected, which is due to the presence of truncated transcript (see Fig. 1A).
Gastrocnemius
In gastrocnemius, apart from Mb itself, genotype differences in gene expression were unremarkable, with few discernable changes in specific transcript abundances comparing WT to Mb−/− female mice (Table 8; see Supplemental Material S1 for the complete data sets). The modest but statistically significant reductions in phosphatase, orphan 1 (Phospho1; also known as phosphoethanolamine/phosphocholine phosphatase 1 in humans) and heat shock 105 kDa/110 kDa protein 1 (Hsph1) mRNAs in female Mb−/− mice were not seen in males, and these transcripts did not differ by genotype in soleus.
Table 8.
Skeletal muscle transcript abundances that differed significantly when comparing wild-type and myoglobin knockout mice following ∼13 wk of feeding a high-fat diet (45% of energy)
| Mb−/− as a % of WTa | FDR-Adjusted P Valueb | |
|---|---|---|
| Female gastrocnemiusc,d | ||
| Phospho1 | 88% | 0.0006 |
| Hsph1 | 77% | 0.013 |
| Female soleus | ||
| Tfrc | 80% | 0.0002 |
| Pygo1 | 111% | 0.002 |
| Fn1 | 91% | 0.0085 |
| Adamts9 | 72% | 0.037 |
| Serpinh1 | 92% | 0.037 |
| Mitf | 108% | 0.043 |
| Myadm | 88% | 0.045 |
| Male soleuse | ||
| Dapp1 | 144% | 0.001 |
| Tm6sf1 | 125% | 0.0018 |
| Arpp21 | 119% | 0.002 |
| St3gal2 | 90% | 0.0027 |
| Gm15543 | 142% | 0.007 |
| Sorl1 | 111% | 0.010 |
| Plce1 | 111% | 0.0125 |
| Catsper4 | 71% | 0.0125 |
| Armh4 | 80% | 0.015 |
| Obsl1 | 89% | 0.020 |
| Fam198b | 120% | 0.020 |
| Kcnq4 | 110% | 0.020 |
| Acan | 197% | 0.020 |
| Barx2 | 113% | 0.020 |
| Aldh1b1 | 141% | 0.020 |
| Ssx2ip | 115% | 0.020 |
| Gm5105 | 148% | 0.020 |
| Mfsd4a | 146% | 0.020 |
| Lyrm9 | 119% | 0.020 |
| Tfrc | 82% | 0.022 |
| Chrna4 | 312% | 0.022 |
| Zfp750 | 192% | 0.022 |
| Lrtm1 | 113% | 0.028 |
| Igfbp2 | 218% | 0.030 |
| Adcy2 | 110% | 0.033 |
| Ern1 | 86% | 0.034 |
| AI838599 | 76% | 0.045 |
| 1700025GRik | 89% | 0.045 |
Excluding myoglobin transcript and including only transcripts with FDR-corrected P ≤ 0.05. Mb−/−,myoglobin knockout; WT, wildtype.
Calculated as: % of WT = 2^(log2 fold change) × 100.
False discovery rate-adjusted pvalue, from iDEP output.
Females: results derived from n = 9 WT and n = 9 Mb−/−, except soleus where n = 8 Mb−/− due to outlier removal (detected by principal component analysis).
There were no statistically significant differences in male gastrocnemius.
Males: results derived from n = 10 WT and n = 10 Mb−/−.
Soleus
In soleus muscle, where Mb is typically far more abundant than mixed gastrocnemius, there were more transcripts with differential abundance comparing WT to Mb−/− mice (Table 8; see Supplemental Material S1 for the complete data sets). Not including Mb, in females, there were 7 transcripts changed significantly in knockout animals, and in Mb−/− males, there were 28 transcript differences. Only one statistically significant genotype-associated mRNA pattern was shared in soleus of both sexes—transferrin receptor (Tfrc), which was decreased by ∼20% in Mb−/−animals.
DISCUSSION
We and others have speculated that LCFA binding to Mb signals is an important role for Mb to regulate fatty acid oxidation (FAO), and/or trafficking and sequestration of long-chain fatty acids (LCFAs) or derivatives such as long-chain acylcarnitines (LCACs). The premise of this concept stems from observations of robust binding of LCFAs to oxygenated Mb (oxy-Mb) (8, 16–20, 25), and the identification of a candidate region of oxy-Mb that accommodates specific LCFA- and LCAC binding (25,26, 44). Yet, evidence linking Mb to FAO has been mixed. In an isolated mouse heart model in which perfusate contained a mix of stable isotope-labeled substrates, the relative contribution of LCFA carbon to the acetyl-CoA pool was not different comparing WT to Mb−/− mice, despite an increase in flux from the lactate pool (28). In contrast, the proportion of U-13C-labeled palmitate that ended up in 13C-glutamate (as a measure of entry of fat carbon into the tricarboxylic acid cycle following β-oxidation) was lowered by ∼34% in perfused hearts of Mb−/− mice in a different study, and labeled glucose flux was increased (27). Thus, in heart, the loss of Mb seems to promote increased reliance on carbohydrate oxidation, with effects on FAO less certain. An important caveat to interpreting LCFA flux studies involves O2 management; if loss of Mb impacts O2 bioavailability, it follows that oxidative metabolism of LCFA substrates would be reduced regardless of the putative role of LCFA-Mb binding in FAO.
Metabolic studies in vivo reflect integrated regulation by endocrine, biochemical and bioenergetics systems, and should give a true sense of the physiological relevance of Mb knockout on whole body FAO and energy balance. Herein, we provide the most comprehensive assessment of these parameters to date, under basal conditions and after challenges that promote FAO and higher muscle and heart exposures to LCFAs (cold, high-fat feeding). In both females and males, almost equivalent respiratory exchange ratio (RER) patterns in WT and Mb−/− mice indicated that there is no convincing, robust effect of Mb loss on whole body FAO. However, it may be argued that the high-fat diet masked genotype effects. Although we cannot discount this possibility, it was notable that no RER differences (or EE differences) were observed when comparing 5- to 6-wk old WT and Mb−/− mice fed a chow diet or upon a cold challenge at any age. These null findings for genotype effects on RER and EE outcomes were seen in both females and males. In a study of chow-fed male Mb−/− mice, Merx et al. (7) reported a significantly higher RER (by ∼0.05) compared to WT mice at baseline and during an acute exercise bout: this suggests that knockout mice in that study relied slightly more on carbohydrates and proteins versus oxidative combustion of fat. However, the mice were only acclimated to indirect calorimetry cages for 1 h, studies were conducted over just 4 h, and it was not clear what feeding status or light:dark cycle timing was in play. Although we do not reject the idea that effects of Mb on FAO regulation may be context- or tissue-specific, the results from the current experiments do not support the idea that has a major impact on whole body fuel selection or energy expenditure in mice.
The latter point is further supported by body weight trajectories, which were not statistically significantly different when comparing WT with Mb−/− mice, especially clear in male mice. Garry et al. (9) also reported no differences in body weight gain in chow-fed male Mb−/− mice, and there were no differences in terminal body weight in chow fed Mb−/− male mice in the study of Flogel et al. (27). However, adiposity was not determined in either of those studies. In female knockouts, we observed a modest trend toward higher weight gain but a more robust increase in adiposity (e.g., ∼48% higher total summed fat pad weight, ∼42% higher fat mass by DEXA toward the end of the study). The fact that ∼40%+ higher body fat was found by two independent methods suggests that Mb knockout increases fat stores and feed efficiency (energy gain:energy intake) in female mice specifically. The mechanisms for the adipose phenotype in females remain to be elaborated but might involve differences in bioenergetic efficiencies in muscle and/or adaptive thermogenesis in brown adipose tissue (BAT). The premise for the latter is that Mb is expressed in BAT (45–47; also see 53) and thus might have some sort of functional role in that tissue.
A lack of Mb may in theory lead to a switch in substrate use that favors nonoxidative metabolism of glucose. This manifested in Mb−/− mice as higher in vivo uptake of [18F]fluorodeoxyglucose into heart, increased flux of isotope-labeled glucose into the tricarboxylic acid cycle in isolated hearts, and an increase in heart GLUT4 protein expression (27). Should these outcomes present in skeletal muscle, it would impact glucose tolerance. Furthermore, a dysregulation of FAO or lipid metabolism could alter insulin sensitivity. Some diacylglycerides (DAGs), and lipotoxicity in general, have been implicated in muscle insulin resistance [e.g., see Szendroedi et al. (48)], which implies that differences in muscle lipid accumulation in Mb−/− mice could yield differences in insulin action in vivo. In one study, triacylglycerides (TAGs) and DAGs were more prevalent in hearts from Mb−/− mice (29). In contrast, Jue et al. (8) reported lower TAGs in heart, gastrocnemius, and soleus of Mb−/− mice. There were no effects of Mb−/− on plasma nonesterified fatty acid concentration in the current study. Furthermore, we did not observe any differences in postabsorptive blood glucose in HFD-fed male or female Mb knockout mice, similar to reports using different lines of Mb null male mice-fed chow (27, 29). There were also no effects of Mb knockout on glucose patterns during a GTT or ITT in our studies of HFD-fed mice, with the interpretive caveat that mice were overnight-fasted for the GTT and hence in a catabolic state upon challenge. However, taken together and in light of the fact that the ITT was conducted in mice that had underwent only a brief fast, the data do not support a critical role for muscle Mb in regulating whole body glucose disposal or blood sugar control.
In addition to metabolic phenotypes, we comprehensively assessed skeletal muscle fiber type and muscle/heart capillarity in myoglobin knockout mice. This effort stemmed from some reports using male mice indicating that loss of Mb increases fatigue in vivo using a treadmill test (7) or ex vivo in soleus muscle contractions in response to 40-Hz stimulation (10), each of which may reflect a transition away from more oxidative, slow twitch type I fibers in Mb−/− mice. Grange et al. (10) reported significantly reduced type I fiber type and increased capillarity in male Mb−/− soleus muscle (with no change in type II-rich EDL). Heart capillarity was increased by ∼30% in male mice (28). These changes may reflect an adaptation toward greater O2 delivery and lower reliance on oxidative metabolism in these tissues. Consistent with the literature, we found that capillary density and the capillary:fiber ratios in Mb−/− mice were increased in muscle beds typically enriched with Mb and type I fibers (soleus and deep gastrocnemius), but this difference was not apparent at other sites more enriched in type II fibers. In males, heart capillarity was modestly increased, but this was not the case in females. In contrast to Grange et al. (10), however, there was no clear evidence in our study for a switch in fiber types in Mb−/− mice. Based on our results and the extant literature, the loss of Mb in oxidative muscle regions and cardiomyocytes triggers signals that enhance capillary networks in the surrounding tissue. For instance, mRNAs encoding hypoxia-inducible factor-1 (HIF-1) and vascular endothelial growth factor (VEGF) were reportedly higher in soleus muscle of Mb knockouts (10); however, our RNASeq results did not identify these transcripts as modified in Mb−/− gastrocnemius or soleus muscle. Thus, the specific factors that regulate capillarity and neovascularization in Mb−/− conditions remain to be fully elaborated.
To our knowledge, this study is the first to conduct a comprehensive transcriptomics analysis of skeletal muscle comparing WT and Mb−/− mice. Remarkably, most expression differences were modest in magnitude and few patterns were shared between female and male mice. This suggests that loss of muscle Mb has only a mild effect on global gene expression in murine muscle, at least under the experimental conditions herein. Only one gene transcript, Tfrc (a.k.a. Tfrc1), was significantly reduced (by ∼20%, in soleus only) in both male and female mice. The physiological relevance of this observation is not clear, and we acknowledge that protein expression and activity of the receptor may not track mRNA. Transferrin receptor protein is an endosomal protein, colocated with a subfraction of GLUT4; the amount of colocated transferrin receptor and GLUT4 proteins increases and decreases with insulin stimulation or contractions, respectively (49). The protein’s primary role is to interact with transferrin as part of a system that manages cellular iron through endocytosis (important to heme-containing proteins such as Mb), a process that is upregulated in part by LCFA [palmitate (50)]. That such a gene would be regulated by changes in Mb status in soleus muscle may be logical based on these interconnected pathways, but functional implications are not known.
An interesting observation, unrelated to the question of myoglobin knockout, was that female mice challenged with the high-fat diet (HFD) displayed far less weight gain compared with males, despite equivalent energy intake. This is similar to results from a recent report comparing male and female C57BL/6J mice fed a similar HFD diet over 4 wk (51). Sex differences in bioenergetics are rarely considered but based on the current results and those of Huang et al. (51), it appears that female mice maintain a higher EE and/or reduced efficiency of fuel conversion to ATP. In our study, there were no discernable sex differences in body weight covariate-adjusted EE calculated from indirect calorimetry at 22°C or 10°C. Huang et al. reported a modest but significantly higher lean mass-adjusted light cycle EE in females consuming a HFD, but no differences on a low-fat diet despite females still appearing to have lower feed efficiency (weight gain relative to energy intake) compared with males. We speculate that there are innate sex differences in energy conversion efficiencies (e.g., leading to differential ATP:O ratios), and this contributes to reduced weight gain in females. This is difficult to fully evaluate using indirect calorimetry alone; we recently highlighted concerns with standard indirect calorimetry equations for EE, which do not account for differences in tissue-level heat:V̇o2 relationships that may exist due to sex, age, diet, genotype, and other factors (37). These concepts will require further evaluation in prospective studies that compare direct and indirect calorimetry results across sexes and diets types.
In conclusion, our results concur with certain aspects of previously published work in male Mb−/− lines, including evidence for increased capillarity in some muscle beds and heart, and no effect on fasting glucose or body weight in males. Major strengths of the study are the dual assessment of both males and females, and a first-ever comprehensive whole body metabolic phenotyping under conditions that challenge the fat metabolism machinery. There were several sex-specific outcomes including a modest increase in weight and a large but variable increase in adiposity in female Mb−/− compared with WT female mice. The mechanisms driving this sex difference remain to be identified. Whole body metabolic phenotypes, including RER, EE, and glucose or insulin tolerance measures, were unchanged in Mb−/− mice of either sex, indicating that Mb is not required to maintain whole body metabolic homeostasis (at least under the conditions tested). We cannot exclude the possibilities that Mb knockout leads to more subtle phenotypes or outcomes not measured herein, or that more obvious Mb−/− effects would manifest whether phenotypes were evaluated on different genetic backgrounds or diets. It is also possible that compensatory adaptations countered the loss of Mb in knockout animals; for example, cytoglobin is expressed in the same tissues as Mb [see http://biogps.org/#goto=genereport&id=114886 (47)] and shares at least some biochemical activities including regulation of NO metabolism (reviewed in Ref. 52). Thus, future studies addressing the potential functional overlap among the globin family members and the physiological function of Mb may require double-knockout strategies and in-depth assessments of NO homeostasis. The results support the idea that Mb is not necessary and sufficient to support oxidative phosphorylation in muscle and heart under normoxic, nonexercising conditions, and alternative physiological roles should be considered.
SUPPLEMENTAL DATA
Supplemental Material: https://doi.org/10.15482/USDA.ADC/1520663.
GRANTS
This research was supported, in part, by the Arkansas Children’s Research Institute and the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000. Additional support was from USDA-ARS Project 6026–51000-012-06S. The University of California, Davis Mouse Metabolic Phenotyping Center (MMPC) is supported, in part, by NIH-NIDDK U2CDK092993 and the UC Davis Mouse Biology Program. We also acknowledge. West Virginia University, School of Medicine, and the NIH U54 GM104942 with support to the West Virginia Clinical and Translational Science Institute.
DISCLOSURES
S.H. Adams is founder and principal of XenoMed, LLC, which is focused on research and discovery unrelated to the studies herein.
AUTHOR CONTRIBUTIONS
K.D.O.-M., I.M.O., J.M.R., S.V.C., B.J.W., M.L.B., K.C.K.L., and S.H.A. conceived and designed research; K.D.O.-M., I.M.O., J.M.R., S.V.C., B.J.W., M.L.B., D.K.W., J.O., T.T. performed experiments; K.D.O.-M., I.M.O., J.M.R., S.V.C., B.J.W., M.L.B., D.K.W., J.O., T.T., K.C.K.L., and S.H.A. analyzed data; K.D.O.-M., I.M.O., J.M.R., S.V.C., B.J.W., M.L.B., D.K.W., J.O., K.C.K.L., and S.H.A., interpreted results of experiments; K.D.O.-M., I.M.O., J.M.R., B.J.W., M.L.B., D.K.W., J.O. and S.H.A. prepared figures; K.D.O.-M., I.M.O., J.M.R., B.J.W., M.L.B., S.H.A. drafted manuscript; K.D.O.-M., K.C.K.L., I.M.O., J.M.R., S.V.C., B.J.W., M.L.B., D.K.W., K.C.K.L. and S.H.A., edited and revised manuscript; K.D.O.-M., K.C.K.L., I.M.O., J.M.R., S.V.C., B.J.W., M.L.B., D.K.W., J.O., T.T., and S.H.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful for technical support and services provided by the University of California, Davis Mouse Metabolic Phenotyping Center (MMPC-University of California Davis, RRID:SCR_015357) and Energy Balance, Exercise and Behavior Core (MMPC-University of California Davis Energy Balance Exercise, and Behavior Core, RRID:SCR_015364). We are appreciative of the collaboration with the UC Davis Mouse Biology Laboratory for generation of myoglobin mutant mice used in this project.
REFERENCES
- 1.Chung Y, Mole PA, Sailasuta N, Tran TK, Hurd R, Jue T. Control of respiration and bioenergetics during muscle contraction. Am J Physiol Cell Physiol 288: C730–C738, 2005. doi: 10.1152/ajpcell.00138.2004. [DOI] [PubMed] [Google Scholar]
- 2.Merx MW, Flogel U, Stumpe T, Godecke A, Decking UK, Schrader J. Myoglobin facilitates oxygen diffusion. FASEB J 15: 1077–1079, 2001. doi: 10.1096/fj.00-0497fje. [DOI] [PubMed] [Google Scholar]
- 3.Mole PA, Chung Y, Tran TK, Sailasuta N, Hurd R, Jue T. Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. Am J Physiol Regul Integr Comp Physiol 277: R173–R180, 1999. doi: 10.1152/ajpregu.1999.277.1.R173. [DOI] [PubMed] [Google Scholar]
- 4.Ponganis PJ, Kreutzer U, Stockard TK, Lin PC, Sailasuta N, Tran TK, Hurd R, Jue T. Blood flow and metabolic regulation in seal muscle during apnea. J Exp Biol 211: 3323–3332, 2008. doi: 10.1242/jeb.018887. [DOI] [PubMed] [Google Scholar]
- 5.Richardson RS, Leigh JS, Wagner PD, Noyszewski EA. Cellular PO2 as a determinant of maximal mitochondrial O2 consumption in trained human skeletal muscle. J Appl Physiol (1985) 87: 325–331, 1999. doi: 10.1152/jappl.1999.87.1.325. [DOI] [PubMed] [Google Scholar]
- 6.Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest 96: 1916–1926, 1995. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merx MW, Godecke A, Flogel U, Schrader J. Oxygen supply and nitric oxide scavenging by myoglobin contribute to exercise endurance and cardiac function. FASEB J 19: 1015–1017, 2005. doi: 10.1096/fj.04-2886fje. [DOI] [PubMed] [Google Scholar]
- 8.Jue T, Simond G, Wright TJ, Shih L, Chung Y, Sriram R, Kreutzer U, Davis RW. Effect of fatty acid interaction on myoglobin oxygen affinity and triglyceride metabolism. J Physiol Biochem 73: 359–370, 2016. [Erratum in J Physiol Biochem 73: 623, 2017]. doi: 10.1007/s13105-017-0559-z. [DOI] [PubMed] [Google Scholar]
- 9.Garry DJ, Ordway GA, Lorenz JN, Radford NB, Chin ER, Grange RW, Bassel-Duby R, Williams RS. Mice without myoglobin. Nature 395: 905–908, 1998. doi: 10.1038/27681. [DOI] [PubMed] [Google Scholar]
- 10.Grange RW, Meeson A, Chin E, Lau KS, Stull JT, Shelton JM, Williams RS, Garry DJ. Functional and molecular adaptations in skeletal muscle of myoglobin-mutant mice. Am J Physiol Cell Physiol 281: C1487–C1494, 2001. doi: 10.1152/ajpcell.2001.281.5.C1487. [DOI] [PubMed] [Google Scholar]
- 11.Flogel U, Merx MW, Godecke A, Decking UK, Schrader J. Myoglobin: A scavenger of bioactive NO. Proc Natl Acad Sci USA 98: 735–740, 2001. [Erratum in Proc Natl Acad Sci USA 98: 4276, 2001]. doi: 10.1073/pnas.011460298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendgen-Cotta UB, Kelm M, Rassaf T. Myoglobin functions in the heart. Free Radic Biol Med 73: 252–259, 2014. doi: 10.1016/j.freeradbiomed.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Clanton TL. Managing the power grid: how myoglobin can regulate PO2 and energy distribution in skeletal muscle. J Appl Physiol (1985) 126: 787–790, 2019. doi: 10.1152/japplphysiol.00614.2018. [DOI] [PubMed] [Google Scholar]
- 14.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res 100: 654–661, 2007. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 15.Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res 100: 1749–1754, 2007. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 16.Gloster J, Harris P. Fatty acid binding to cytoplasmic proteins of myocardium and red and white skeletal muscle in the rat. A possible new role for myoglobin. Biochem Biophys Res Commun 74: 506–513, 1977. doi: 10.1016/0006-291X(77)90333-3. [DOI] [PubMed] [Google Scholar]
- 17.Gotz FM, Hertel M, Groschel-Stewart U. Fatty acid binding of myoglobin depends on its oxygenation. Biol Chem Hoppe Seyler 375: 387–392, 1994. doi: 10.1515/bchm3.1994.375.6.387. [DOI] [PubMed] [Google Scholar]
- 18.Shih L, Chung Y, Sriram R, Jue T. Interaction of myoglobin with oleic acid. Chem Phys Lipids 191: 115–122, 2015. doi: 10.1016/j.chemphyslip.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shih L, Chung Y, Sriram R, Jue T. Palmitate interaction with physiological states of myoglobin. Biochim Biophys Acta 1840: 656–666, 2014. doi: 10.1016/j.bbagen.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sriram R, Kreutzer U, Shih L, Jue T. Interaction of fatty acid with myoglobin. FEBS Lett 582: 3643–3649, 2008. doi: 10.1016/j.febslet.2008.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong RB, Essen-Gustavsson B, Hoppeler H, Jones JH, Kayar SR, Laughlin MH, Lindholm A, Longworth KE, Taylor CR, Weibel ER. O2 delivery at VO2max and oxidative capacity in muscles of standardbred horses. J Appl Physiol (1985) 73: 2274–2282, 1992. doi: 10.1152/jappl.1992.73.6.2274. [DOI] [PubMed] [Google Scholar]
- 22.Bekedam MA, van Beek-Harmsen BJ, van Mechelen W, Boonstra A, van der Laarse WJ. Myoglobin concentration in skeletal muscle fibers of chronic heart failure patients. J Appl Physiol (1985) 107: 1138–1143, 2009. doi: 10.1152/japplphysiol.00149.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sylven C, Jansson E, Book K. Myoglobin content in human skeletal muscle and myocardium: relation to fibre size and oxidative capacity. Cardiovasc Res 18: 443–446, 1984. doi: 10.1093/cvr/18.7.443. [DOI] [PubMed] [Google Scholar]
- 24.Masuda K, Truscott K, Lin PC, Kreutzer U, Chung Y, Sriram R, Jue T. Determination of myoglobin concentration in blood-perfused tissue. Eur J Appl Physiol 104: 41–48, 2008. doi: 10.1007/s00421-008-0775-x. [DOI] [PubMed] [Google Scholar]
- 25.Chintapalli SV, Bhardwaj G, Patel R, Shah N, Patterson RL, van Rossum DB, Anishkin A, Adams SH. Molecular dynamic simulations reveal the structural determinants of fatty acid binding to oxy-myoglobin. PLoS One 10: e0128496, 2015. doi: 10.1371/journal.pone.0128496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chintapalli SV, Jayanthi S, Mallipeddi PL, Gundampati R, Suresh Kumar TK, van Rossum DB, Anishkin A, Adams SH. Novel molecular interactions of acylcarnitines and fatty acids with myoglobin. J Biol Chem 291: 25133–25143, 2016. doi: 10.1074/jbc.M116.754978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flogel U, Laussmann T, Godecke A, Abanador N, Schafers M, Fingas CD, Metzger S, Levkau B, Jacoby C, Schrader J. Lack of myoglobin causes a switch in cardiac substrate selection. Circ Res 96: e68–e75, 2005. doi: 10.1161/01.RES.0000165481.36288.d2. [DOI] [PubMed] [Google Scholar]
- 28.Meeson AP, Radford N, Shelton JM, Mammen PP, DiMaio JM, Hutcheson K, Kong Y, Elterman J, Williams RS, Garry DJ. Adaptive mechanisms that preserve cardiac function in mice without myoglobin. Circ Res 88: 713–720, 2001. doi: 10.1161/hh0701.089753. [DOI] [PubMed] [Google Scholar]
- 29.Hendgen-Cotta UB, Esfeld S, Coman C, Ahrends R, Klein-Hitpass L, Flogel U, Rassaf T, Totzeck M. A novel physiological role for cardiac myoglobin in lipid metabolism. Sci Rep 7: 43219, 2017. doi: 10.1038/srep43219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCoin CS, Knotts TA, Adams SH. Acylcarnitines—old actors auditioning for new roles in metabolic physiology. Nat Rev Endocrinol 11: 617–625, 2015. doi: 10.1038/nrendo.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godecke A, Flogel U, Zanger K, Ding Z, Hirchenhain J, Decking UK, Schrader J. Disruption of myoglobin in mice induces multiple compensatory mechanisms. Proc Natl Acad Sci USA 96: 10495–10500, 1999. doi: 10.1073/pnas.96.18.10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labun K, Montague TG, Krause M, Torres Cleuren YN, Tjeldnes H, Valen E. CHOPCHOP v3: expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res 47: W171–W174, 2019. doi: 10.1093/nar/gkz365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modzelewski AJ, Chen S, Willis BJ, Lloyd KCK, Wood JA, He L. Efficient mouse genome engineering by CRISPR-EZ technology. Nat Protoc 13: 1253–1274, 2018. doi: 10.1038/nprot.2018.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tesson L, Heslan JM, Menoret S, Anegon I. Rapid and accurate determination of zygosity in transgenic animals by real-time quantitative PCR. Transgenic Res 11: 43–48, 2002. doi: 10.1023/a:1013928600442. [DOI] [PubMed] [Google Scholar]
- 35.El-Tohamy R, Elkholi I, Elsherbiny ME, Magdy M, Hammam O, Allalunis-Turner J, Emara M. Myoglobin variants are expressed in human glioblastoma cellshypoxia effect? Oncol Rep 43: 975–985, 2020. doi: 10.3892/or.2020.7479. [DOI] [PubMed] [Google Scholar]
- 36.Thomas AP, Dunn TN, Oort PJ, Grino M, Adams SH. Inflammatory phenotyping identifies CD11d as a gene markedly induced in white adipose tissue in obese rodents and women. J Nutr 141: 1172–1180, 2011. doi: 10.3945/jn.110.127068. [DOI] [PubMed] [Google Scholar]
- 37.Ono-Moore KD, Rutkowsky JM, Pearson NA, Williams DK, Grobe JL, Tolentino T, Lloyd KCK, Adams SH. Coupling of energy intake and energy expenditure across a temperature spectrum: impact of diet-induced obesity in mice. Am J Physiol Endocrinol Metab 319: E472–E484, 2020. doi: 10.1152/ajpendo.00041.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lusk G. Animal calorimetry: twenty-fourth paper. Analysis of the oxidation of mixtures of carbohydrate and fat. J Biol Chem 59: 41–42, 1924. https://www.semanticscholar.org/paper/ANIMAL-CALORIMETRY-Twenty-Fourth-Paper.-ANALYSIS-OF-Lusk/5aa8cd9c79aa783b9e8c645c08012e969e13a846. [Google Scholar]
- 39.Rosenblatt JD, Kuzon WM, Plyley MJJr, Pynn BR, McKee NH. A histochemical method for the simultaneous demonstration of capillaries and fiber type in skeletal muscle. Stain Technol 62: 85–92, 1987. doi: 10.3109/10520298709107973. [DOI] [PubMed] [Google Scholar]
- 40.Gorman JL, Liu ST, Slopack D, Shariati K, Hasanee A, Olenich S, Olfert IM, Haas TL. Angiotensin II evokes angiogenic signals within skeletal muscle through co-ordinated effects on skeletal myocytes and endothelial cells. PLoS One 9: e85537, 2014. doi: 10.1371/journal.pone.0085537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchida C, Nwadozi E, Hasanee A, Olenich S, Olfert IM, Haas TL. Muscle-derived vascular endothelial growth factor regulates microvascular remodelling in response to increased shear stress in mice. Acta Physiol (Oxf) 214: 349–360, 2015. doi: 10.1111/apha.12463. [DOI] [PubMed] [Google Scholar]
- 42.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ge SX, Son EW, Yao R. iDEP: an integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinformatics 19: 534, 2018. doi: 10.1186/s12859-018-2486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chintapalli SV, Anishkin A, Adams SH. Exploring the entry route of palmitic acid and palmitoylcarnitine into myoglobin. Arch Biochem Biophys 655: 56–66, 2018. doi: 10.1016/j.abb.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fournier B, Murray B, Gutzwiller S, Marcaletti S, Marcellin D, Bergling S, Brachat S, Persohn E, Pierrel E, Bombard F, Hatakeyama S, Trendelenburg AU, Morvan F, Richardson B, Glass DJ, Lach-Trifilieff E, Feige JN. Blockade of the activin receptor IIb activates functional brown adipogenesis and thermogenesis by inducing mitochondrial oxidative metabolism. Mol Cell Biol 32: 2871–2879, 2012. doi: 10.1128/MCB.06575-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe M, Yamamoto T, Kakuhata R, Okada N, Kajimoto K, Yamazaki N, Kataoka M, Baba Y, Tamaki T, Shinohara Y. Synchronized changes in transcript levels of genes activating cold exposure-induced thermogenesis in brown adipose tissue of experimental animals. Biochim Biophys Acta 1777: 104–112, 2008. doi: 10.1016/j.bbabio.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW 3rd, Su AI. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol 10: R130, 2009. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szendroedi J, Yoshimura T, Phielix E, Koliaki C, Marcucci M, Zhang D, Jelenik T, Muller J, Herder C, Nowotny P, Shulman GI, Roden M. Role of diacylglycerol activation of PKCtheta in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci USA 111: 9597–9602, 2014. doi: 10.1073/pnas.1409229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ploug T, van Deurs B, Ai H, Cushman SW, Ralston E. Analysis of GLUT4 distribution in whole skeletal muscle fibers: identification of distinct storage compartments that are recruited by insulin and muscle contractions. J Cell Biol 142: 1429–1446, 1998. doi: 10.1083/jcb.142.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui R, Choi SE, Kim TH, Lee HJ, Lee SJ, Kang Y, Jeon JY, Kim HJ, Lee KW. Iron overload by transferrin receptor protein 1 regulation plays an important role in palmitate-induced insulin resistance in human skeletal muscle cells. FASEB J 33: 1771–1786, 2019. doi: 10.1096/fj.201800448R. [DOI] [PubMed] [Google Scholar]
- 51.Huang KP, Ronveaux CC, Knotts TA, Rutkowsky JR, Ramsey JJ, Raybould HE. Sex differences in response to short-term high fat diet in mice. Physiol Behav 221: 112894, 2020. doi: 10.1016/j.physbeh.2020.112894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rochon ER, Corti P. Globins and nitric oxide homeostasis in fish embryonic development. Mar Genomics 49: 100721, 2020. doi: 10.1016/j.margen.2019.100721. [DOI] [PubMed] [Google Scholar]
- 53.Blackburn ML, Wankhade UD, Ono-Moore KD, Chintapalli SV, Fox R, Rutkowsky JM, Willis BJ, Tolentino T, Lloyd KCK, Adams SH. On the potential role of globins in brown adipose tissue: a novel conceptual model and studies in myoglobin knockout mice. Am J Physiol Endocrinol Metab. in press, doi: 10.1152/ajpendo.00662.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]