ABSTRACT
A meta-analysis of prospective studies was conducted to examine the association of total, supplemental, and dietary magnesium intakes with risk of all-cause, cancer, and cardiovascular disease (CVD) mortality and identify the dose–response relations involved in these association. We performed a systematic search of PubMed, Scopus, Google Scholar, and ISI Web of Knowledge up to April 2020. Prospective cohort studies that reported risk estimates for the association between total, supplemental, and dietary magnesium intakes and risk of mortality were included. Random effects models were used. Nineteen publication with a total of 1,168,756 participants were included in the current meta-analysis. In total, 52,378 deaths from all causes, 23,478 from CVD, and 11,408 from cancer were identified during the follow-up period of 3.5 to 32 years. Dietary magnesium intake was associated with a lower risk of all-cause [pooled effect size (ES): 0.87; 95% CI: 0.79, 0.97; P = 0.009; I2 = 70.7%; P < 0.001] and cancer mortality (pooled ES: 0.80; 95% CI: 0.67, 0.97; P = 0.023; I2 = 55.7%; P = 0.027), but not with CVD mortality (pooled ES: 0.93; 95% CI: 0.82, 1.07; P = 0.313; I2 = 72.3%; P < 0.001). For supplemental and total magnesium intakes, we did not find any significant associations with risks of all-cause, CVD, and cancer mortality. However, linear dose–response meta-analysis indicated that each additional intake of 100 mg/d of dietary magnesium was associated with a 6% and 5% reduced risk of all-cause and cancer mortality, respectively. In conclusion, higher intake of dietary magnesium was associated with a reduced risk of all-cause and cancer mortality, but not CVD mortality. Supplemental and total magnesium intakes were not associated with the risk of all-cause, CVD, and cancer mortality. These findings indicate that consumption of magnesium from dietary sources may be beneficial in reducing all-cause and cancer mortality and thus have practical importance for public health.
Keywords: mortality, death, magnesium, diet, cancer, cardiovascular disease
This study was conducted to examine the association of total, supplemental, and dietary magnesium intakes with risk of all-cause, cancer, and CVD mortality.
Introduction
Magnesium is involved in several physiological functions in the body (1). It plays a key role in DNA synthesis, energy production, protein synthesis, muscle and nerve function, blood glucose control, active transmembrane transports of ions, oxidative phosphorylation, and glycolysis (1–3). Moreover, it is a cofactor of hundreds of enzymes involved in essential reactions in the body (1, 3).
Despite the abundant distribution of magnesium in foods (1, 3), studies in several parts of the world have indicated its deficient intakes, such that in US adults the dietary magnesium intake is ∼70% lower than the dietary reference intake (DRI) (4–6). Magnesium deficiency has been associated with an increased risk of metabolic syndrome (7), type 2 diabetes (8), cardiovascular disease (CVD) (9), and colorectal cancer (10, 11). Magnesium deficiency has also been associated with insulin resistance, inflammation, and elevated blood pressure and coagulation (3, 12–14). Although several studies have examined the association between dietary and supplemental magnesium intake and all-cause mortality, findings are controversial. While some prospective cohort studies reported an inverse association between dietary and supplemental magnesium intake and risk of cardiovascular (9, 15, 16), cancer (15, 17), and all-cause mortality (15, 18), others failed to demonstrate such associations (19–23). In a 2016 meta-analysis, dietary magnesium intake was investigated in relation to risk of all-cause mortality (24) and the investigators reported increase in magnesium intake was associated with a reduced risk of all-cause mortality. However, these investigators did not examine supplemental and total magnesium intakes with risk of mortality. In addition, they did not investigate the association with cancer and CVD mortality. Furthermore, ≥6 additional cohort studies (16–18, 25–27) have been published since the release of that meta-analysis, which highlights the necessity of an updated and comprehensive meta-analysis. This study was therefore performed to systematically review the available literature on the association of dietary, supplemental, and total magnesium intakes with risk of all-cause, CVD, and cancer mortality and to quantify the probable association through a dose–response meta-analysis of prospective cohort studies.
Methods
The present meta-analysis was designed and reported based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (28).
Search strategy
We systematically searched online databases, including Clarivate Web of Science, PubMed, Scopus, and Google Scholar up to April 2020. The following keywords were used in the systematic search: (“magnesium” OR “dietary magnesium intake” OR “magnesium supplementation” OR “magnesium supplements”) AND (“mortality” OR “survival” OR “death” OR “fatal”) AND (“human” OR “humans”). Full information about the search terms is provided in Supplemental Table 1. We did not restrict the time of publication or language in our search. Duplicate citations were deleted.
Eligibility criteria
We included studies that met the following criteria: 1) observational cohort studies or longitudinal RCTs that were conducted on human adults, 2) studies that provided HR or RR or ORs with 95% CIs for the association between dietary, supplemental, or total intakes of magnesium as the exposures of interest and mortality from all causes, CVD, and cancer considered as the outcomes of interest.
Excluded studies
We excluded comments, letters, short communications, reviews, meta-analyses, ecologic studies, and animal studies during the screening of publications. Also, studies conducted on children, adolescents, pregnant women, chronic kidney disease patients, hemodialysis patients, end-stage cancer patients, and critically ill patients were excluded. Moreover, studies that considered plasma magnesium or intravenous infusion of magnesium as the exposure, and those that assessed the association of magnesium intake with incidence of chronic diseases, rather than mortality, were excluded. A flow diagram of the study selection process is shown in Supplemental Figure 1.
Data extraction
Two independent researchers (AB and OS) extracted the following information: first author's name, publication year, study design, location of study, patient mean age and/or age range, gender, sample size of the cohort, incidence of death, duration of follow-up, exposure, method of assessment of exposure, mean and/or range of magnesium intake, comparison categories and relevant effect sizes of comparison categories along with 95% CI, and covariates adjusted for in the statistical analysis. When the data were reported separately for men and women, each section was considered a separate study.
Risk of bias assessment
A version of the Newcastle Ottawa Scale (NOS) designed for nonrandomized studies was used to assess the quality of prospective studies (29). Studies were categorized into low, moderate, and high quality (or risk of bias) based on the scores 0–3, 4–6, and 7–9, respectively. The principal investigator (AE) was consulted for in case of any discrepancies.
Statistical analysis
All reported RRs and HRs (and their 95% CIs) for comparison of the highest compared with lowest categories of total, dietary, and supplemental magnesium intakes were used to calculate the log RR and its SE. Because a random-effects model can take between-study variations into account (30), this model was used to calculate the overall effect size. Heterogeneity between studies was evaluated using Cochran's Q test and the I2 statistic, and I2 values >50% were considered to have significant between-study heterogeneity (31). In studies that reported RR or HR for the lowest category of magnesium intake compared with the highest category, we computed the highest compared with the lowest estimates using a method suggested by Orsini et al (32). If a significant between-study heterogeneity was determined, we conducted subgroup analysis to find out the possible sources of heterogeneity. These analyses were performed according to the duration of the study, geographical area, gender and number of participants, type of FFQ collection, mean or median BMI (kg/m2) of study participants, and statistical controlling for total energy intake. The fixed-effects model was performed to assess between-subgroup heterogeneity. Sensitivity analysis was conducted to determine if any single study contributed more to the heterogeneity than another. Publication bias was examined through visual inspection on Begg's funnel plots. Formal statistical assessment of funnel plot asymmetry was performed using Egger's regression asymmetry test.
For the dose–response meta-analysis, we used a method suggested by Greenland (33) and Orsini (32) to compute the trend of RR and HR estimates and their 95% CIs across categories of magnesium intake. The total number of deaths and the total number of participants for each category and the HRs and RRs with CIs for ≥3 quantitative categories of exposure were extracted. We considered the midpoint of dietary magnesium intake in each category. For studies that reported the magnesium intake as a range, we estimated the midpoint in each category by calculating the mean of the lower and upper bound. When the highest and lowest categories were open ended, the lengths of these open-ended intervals were assumed to be the same as those of the adjacent intervals. A 2-stage random-effects dose–response meta-analysis was applied to examine a possible nonlinear association between magnesium intake and mortality. We performed this analysis through modelling of magnesium intake and restricted cubic splines with 4 knots at fixed percentiles of 5, 35, 65, and 95% of the distribution. According to the Orsini method (32), restricted cubic spline models were calculated using a generalized least-squares trend estimation method, that consider the correlation within each set of specified RRs and HRs. Then, we combined all the study-specific estimates by the use of the restricted maximum likelihood method in a multivariate random-effects meta-analysis (34). A probability value for nonlinearity was estimated using null hypothesis testing in which the coefficient of the second spline was considered equal to 0. The 2-stage generalized least-squares trend estimation method was used to examine a linear dose–response association of an additional 100 mg/d from magnesium with mortality. We estimated study-specific slope lines, and then we combined these lines to obtain a total average slope (32). Finally, we combined study-specific slope lines through a random-effects model. All statistical analyses were applied using STATA version 14 (STATA Corp.). P values < 0.05 were considered statistically significant for all tests including Cochran's Q test.
Results
Literature search
We found 12,222 articles in our systematic search, plus 6 articles through hand searching. Of these, 2805 articles were excluded due to duplication and another 9357 papers that were not relevant were excluded. After reviewing the full text of the 60 remaining articles, 40 additional papers were excluded for the following reasons: 20 articles did not consider magnesium intake and risk of mortality, 3 reported studies that used intravenous or dialysate magnesium, 2 assessed plasma magnesium, 3 reported studies conducted in ICU patients, 1 reported a study conducted in pediatric patients, 2 were just commentary, 3 were on multivitamin intake and mortality, 1 was on vitamin D and mortality, and 5 had abstracts without reports of effect size (35–39). Finally, 20 reports of cohort studies were included in this systematic review (9, 15–23, 25–27, 40–46) and 19 were qualified for the meta-analysis (9, 15–23, 25–27, 40, 41, 43–46); 19 studies had reported effect sizes for all-cause mortality (9, 15–23, 25–27, 40, 41, 43–46), 12 studies for CVD mortality (9, 15, 16, 18, 19, 21, 22, 27, 41, 43, 44, 46), and 8 papers for cancer mortality (15, 17, 18, 20–22, 40, 43). A flow diagram of the study selection is provided in Supplemental Figure 1. From these 20 included publications in the systematic review, the studies by Mursu et al. (22) and Inoue-Choi et al. (42) were conducted on the same dataset, the Iowa Women's Health Study. The study by Mursu et al. (22) was included in our main analysis because of its comprehensiveness. Moreover, the study of Li et al. (16) was excluded from the dose–response analysis due to lack of reporting number of cases. Studies on supplemental magnesium intake had reported the effect sizes for only 2 categories of taking compared with not taking the supplements. Because we needed the effect sizes for ≥3 categories to do the dose–response analysis, we were not able to use these studies in the dose–response analysis.
Characteristics of included studies
Characteristics of included prospective cohort studies are presented in Tables 1–3. The numbers of participants in these cohort studies ranged from 1169 to 153,569 people, with an age range between 20 and 89 y. A total of 1,168,756 participants were included in these 19 publications. Follow-up duration ranged from 4.7 to 26 y. In total, 52,378 deaths from all causes, 23,478 from CVD, and 11,408 from cancer were identified in these studies. Dietary magnesium intake ranged from 126 to 523 mg/d, and range of total magnesium intake was 185 to 469 mg/d. Mean or range of supplemental magnesium intake had not been reported in the included studies. As mentioned above, when 2 studies were published based on similar datasets, we calculated the sample size from the most comprehensive one. Ten publications had reported effect size for women (9, 16, 17, 19, 22, 23, 40, 43, 45, 46), 3 for men (9, 21, 43), and 7 for both genders (15, 17, 18, 20, 26, 27, 41). In total, 11 studies were conducted in the United States (16–19, 22, 23, 25, 40, 44–46), and 8 in non-US countries (9, 15, 20, 21, 26, 27, 41, 43). Of these publications, 15 had reported effect sizes for dietary magnesium (9, 15–18, 20, 21, 23, 26, 27, 40, 41, 43, 44, 46), 8 for total magnesium (17–19, 23, 25, 26, 40, 45), and 3 for supplemental magnesium (17, 18, 22). To assess magnesium intake, 15 reported studies had used an FFQ (9, 15–17, 19–23, 26, 27, 40, 42–44, 46) and 4 had used dietary records or recalls (18, 25, 41, 45). All publications reported studies with consideration of baseline data of magnesium intake in their analysis (single measurement), whereas 1 study had used the average of repeated measurements of magnesium intake as the main exposure (44). In most reported studies cohort analyses were controlled for some conventional risk factors, including energy intake (n = 17) and BMI (n = 18). According to the NOS, 16 articles had a low risk of bias.
TABLE 1.
Characteristics of included studies on the association between magnesium intake and all-cause mortality in adults aged >18 y1
| Author, y (ref) | Country | Age (y) | Sample size (n) | Follow-up2 (y) | Cases (n) | Exposure | Exposure assessment | FCT used | NOS score | MG mean (mg/d) | MG range (mg/d) | MG intake comparison (mg/d) | ES (95% CI)3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dai et al. 2013 (43) | China | 40–74 | M: 61,414, | 13 | M: 3806, | DMG | 81-item FFQ | Chinese food composition | 9 | NR | 232–347 | ≥320 vs. <251 | HR: 0.87 (0.71, 1.07) |
| F: 73,232 | F:2418 | 258–367 | HR: 1.09 (0.94, 1.27) | ||||||||||
| Guasch-Ferré et al. 2014 (15) | Spain | 55–80 | Both:7216 | 4.8 | 323 | DMG | 137-item FFQ | Spanish food composition | 8 | NR | 312–442 | >391 vs. <326 | HR: 0.63 (0.46, 0.86) |
| Kaluza et al. 2010 (21) | Swedish | 45–79 | M: 23,366 | 10 | 2358 | DMG | 96-item FFQ | Swedish National Food Administration Database | 8 | NR | 387–523 | ≥481 vs. <426 | HR: 1.06 (0.91, 1.24) |
| Tao et al. 2016 (40) | US | 35–79 | F: 1170 | 7.3 | 170 | DMG | 121-item FFQ | US food composition | 7 | 241 | 156–306 | ≥268 vs. <193 | HR: 0.5 (0.28, 0.9) |
| TMG | 295 | 185–381 | ≥332 vs. <234 | HR: 0.58 (0.31, 1.08) | |||||||||
| Chen et al. 2019 (18) | US | >20 | B:27,725 | 6.1 | 2845 | DMG | Food recall | US food composition | 7 | 294 | NR | >EAR vs. <EAR | RR: 0.78 (0.65, 0.93) |
| B:27,725 | 3613 | TMG | 338 | NR | >EAR vs. <EAR | RR: 0.85 (0.74, 0.98) | |||||||
| B:27,725 | 3613 | SMG | 147 | NR | User vs. nonuser | RR: 0.97 (0.86, 1.09) | |||||||
| Huang et al. 2015 (41) | Tiwan | 65–97 | Both: 1400 | 8.7 | 475 | DMG | Food recall | Taiwanese food composition | 7 | 227 | 126–325 | ≥265 vs. <155 | HR: 1.05 (0.74, 1.49) |
| Levitan et al. 2013 (23) | US | 50–79 | F: 3340 | 4.6 | 1433 | DMG | 122-item FFQ | Minnesota Nutrition Data System | 6 | 247 | 187–309 | >285 vs. <207 | HR: 0.84 (0.63, 1.12) |
| TMG | NR | 199–408 | >408 vs. <199 | HR: 1.07 (0.88, 1.3) | |||||||||
| Wesselink et al. 2020 (26) | Netherlands | 61.7–72.9 | B: 1407 | 4.7 | 174 | DMG | 204-item FFQ | Dutch food composition | 6 | 318 | 242–432 | >400 vs. <282 | HR: 0.59 (0.29, 1.2) |
| B: 1169 | 191 | TMG | NR | 255–469 | >431 vs. <286 | HR: 0.65 (0.35, 1.21) | |||||||
| Saquib et al. 2011 (45) | US | 18–70 | F: 3081 | 9 | 412 | TMG | Food recall | Minnesota Nutrition Data System | 6 | NR | NR | Above RDA vs. below RDA | HR: 0.98 (0.66, 1.45) |
| Mursu et al. 2011 (22) | US | 55–69 | F: 38,772 | 19 | 15,594 | SMG | 127-item FFQ | USDA sources | 8 | NR | NR | User vs. nonuser | HR: 1.08 (1.01, 1.15) |
| Chiuve et al. 2011 (46) | US | 30–55 | F: 88,375 | 26 | 505 | DMG | FFQ | USDA sources | 8 | 305 | 235–383 | >345 vs. <261 | RR: 0.66 (0.46, 0.95) |
| Zhang et al. 2012 (9) | Japan | 40–79 | M: 23,083 | 14.7 | 1343 | DMG | 33-item FFQ | Japan food tables | 8 | NR | 173–294 | >257 vs. <190 | HR: 1.02 (0.85, 1.22) |
| F: 35,533 | 1347 | 174–274 | >241 vs. <190 | HR: 1.26 (0.9, 1.75) | |||||||||
| Chiuve et al. 2013 (44) | US | 30–55 | F: 86,323 | 28 | 1103 | DMG | FFQ | USDA sources | 8 | NR | 231–360 | >342 vs. <246 | RR: 0.64 (0.46, 0.87) |
| Li et al. 2020 (16) | US | 50–79 | F:153,569 | 10.5 | 3,277 | DMG | 122-item FFQ | Minnesota Nutrition Data System | 8 | 244 | 189–330 | >289 vs. <197 | HR: 0.84 (0.78, 0.9) |
| Talaei et al. 2019 (27) | China | 45–74 | B: 57,078 | 17.2 | 4871 | DMG | 165-item FFQ | Singapore Food Composition Database | 8 | NR | 205–290 | 290 vs. 205 | HR: 1.06 (0.94, 1.19) |
| Song et al. 2005 (19) | US | 39–89 | F: 35,601 | 10 | 120 | TMG | 131-item FFQ | USDA sources | 8 | NR | 255–433 | >409 vs. <268 | RR: 1.32 (0.71, 2.47) |
| Li et al. 2011 (20) | German | 35–64 | B: 24,323 | 11 | 513 | DMG | 158-item FFQ | German Dietary Nutrient Database | 8 | NR | 261–381 | >354 vs. <280 | HR: 1.04 (0.79, 1.36) |
| Zhong et al. 2020 (17) | US | 55–74 | B:104,025 | 11.5 | 100 | DMG | 137-item FFQ | USDA sources | 8 | NR | 205–408 | >358 vs. <256 | HR: 0.34 (0.18, 0.66) |
| B:104,025 | 100 | TMG | 301–413 | >413 vs. <301 | HR: 0.37 (0.19, 0.71) | ||||||||
| SMG | NR | HR: 1.12 (0.76, 1.67) | |||||||||||
| Wu et al. 2017 (25) | US | 20–74 | B: 13,504 | 14.6 | NR | TMG | Food recall | USDA sources | 8 | 321 | 309–332 | Per every 100-mg increase | HR: 0.51 (0.26, 1.01) |
B, both male and female; DMG, dietary magnesium; EAR, estimated average requirement; ES, effect size; F, female; FCT, food composition table; M, male; MG, magnesium; NOS, Newcastle Ottawa Scale; NR, not reported; SMG, supplemental magnesium; TMG, total magnesium; ref, reference.
Number of years that individuals were followed in the prospective cohort studies.
These effect sizes are for the highest compared with lowest comparison.
TABLE 2.
Characteristics of included studies on the association between magnesium intake and CVD mortality in adults aged >18 y1
| Author, y (ref) | Country | Age (y) | Sample size (n) | Follow-up2 (y) | Cases (n) | Exposure | Exposure assessment | FCT used | NOS score | MG mean (mg/d) | MG range (mg/d) | MG intake comparison (mg/d) | ES (95% CI)3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chiuve et al. 2011 (46) | US | 30–55 | F: 88,375 | 26 | 505 | DMG | FFQ | USDA sources | 8 | 305 | 235–383 | >345 vs. <261 | RR: 0.66 (0.46, 0.95) |
| Zhang et al. 2012 (9) | Japan | 40–79 | M: 23,083 | 14.7 | 1343 | DMG | 33-item FFQ | Japan Food Tables | 8 | NR | 173–294 | >257 vs. <190 | HR: 1.02 (0.85, 1.22) |
| F: 35,533 | 1347 | 174–274 | >241 vs. <190 | HR: 1.26 (0.9, 1.75) | |||||||||
| Chiuve et al. 2013 (44) | US | 30–55 | F: 86,323 | 28 | 1103 | DMG | FFQ | USDA sources | 8 | NR | 231–360 | >342 vs. <246 | RR: 0.64 (0.46, 0.87) |
| Dai et al. 2013 (43) | China | 40–74 | M: 61,414, | 13 | M:800 | DMG | 81-item FFQ | Chinese food composition | 9 | NR | 232–347 | ≥320 vs. <251 | HR: 0.84 (0.58, 1.21) |
| F: 73,232 | F:1147 | 258–367 | HR: 1.35 (1.03, 1.77) | ||||||||||
| Guasch-Ferré et al. 2014 (15) | Spain | 55–80 | B: 7216 | 4.8 | 81 | DMG | 137-item FFQ | Spanish food composition | 8 | NR | 312–442 | >391 vs. <326 | HR: 0.53 (0.28, 0.99) |
| Kaluza et al. 2010 (21) | Swedish | 45–79 | M: 23,366 | 10 | 819 | DMG | 96-ietm FFQ | Swedish National Food Administration Database | 8 | NR | 387–523 | ≥481 vs. <426 | HR: 1.25 (0.96, 1.61) |
| Li et al. 2020 (16) | US | 50–79 | F:153,569 | 10.5 | 3,277 | DMG | 122-item FFQ | Minnesota Nutrition Data System | 8 | 244 | 189–330 | >289 vs. <197 | HR: 0.84 (0.78, 0.9) |
| Talaei et al. 2019 (27) | China | 45–74 | B: 57,078 | 17.2 | 4871 | DMG | 165-item FFQ | Singapore Food Composition Database | 8 | NR | 205–290 | 290 vs. 205 | HR: 1.06 (0.94, 1.19) |
| Chen et al. 2019 (18) | US | >20 | B:27,725 | 6.1 | 724 | DMG | Food recall | US food composition | 7 | 294 | NR | >EAR vs. <EAR | RR: 0.73 (0.51, 1.03) |
| B:27,725 | 724 | TMG | 338 | NR | >EAR vs. <EAR | RR: 0.83 (0.63, 1.11) | |||||||
| B:27,725 | 701 | SMG | 147 | NR | user vs. nonuser | RR: 0.93 (0.75, 1.14) | |||||||
| Huang et al. 2015 (41) | Tiwan | 65–97 | B: 1400 | 8.7 | 124 | DMG | Food recall | Taiwanese food composition | 7 | 227 | 126–325 | ≥265 vs. <155 | HR: 1.24 (0.59, 2.6) |
| Song et al. 2005 (19) | US | 39–89 | F: 35,601 | 10 | 120 | TMG | 131-item FFQ | USDA sources | 8 | NR | 255–433 | >409 vs. <268 | RR: 1.32 (0.71, 2.47) |
| Mursu et al. 2011 (22) | US | 55–69 | F: 38,772 | 19 | 5721 | SMG | 127-item FFQ | USDA sources | 8 | NR | NR | User vs. nonuser | HR: 1.16 (1.01, 1.34) |
B, both male and female; DMG, dietary magnesium; EAR, estimated average requirement; ES, effect size; F, female; FCT, food composition table; M, male; MG, magnesium; NOS, Newcastle Ottawa Scale; NR, not reported; SMG, supplemental magnesium; TMG, total magnesium; ref, reference.
Number of years that individuals were followed in the prospective cohort studies.
These ESs are for the highest compared with lowest comparison.
TABLE 3.
Characteristics of included studies on the association between magnesium intake and cancer mortality in adults aged >18 y1
| Author, year (ref) | Country | Age (y) | Sample size (n) | Follow-up2 (y) | Cases (n) | Exposure | Exposure assessment | FCT used | NOS score | MG mean (mg/d) | MG range (mg/d) | MG intake comparison (mg/d) | ES (95% CI)3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dai et al. 2013 (43) | China | 40–74 | M:61,414 | 13 | M:1616 | DMG | 81-item FFQ | Chinese food composition | 9 | NR | 232–347 | ≥320 vs. <251 | HR: 0.93 (0.68, 1.29) |
| F: 73,232 | F: 1050 | 258–367 | HR: 0.89 (0.7, 1.14) | ||||||||||
| Guasch-Ferré et al. 2014 (15) | Spain | 55–80 | B: 7216 | 4.8 | 130 | DMG | 137-item FFQ | Spanish food composition | 8 | NR | 312–442 | >391 vs. <326 | HR: 0.55 (0.33, 0.91) |
| Kaluza et al. 2010 (21) | Swedish | 45–79 | M: 23,366 | 10 | 738 | DMG | 96-ietm FFQ | Swedish National Food Administration Database | 8 | NR | 387–523 | ≥481 vs. <426 | HR: 0.98 (0.75, 1.28) |
| Tao et al. 2016 (40) | US | 35–79 | F: 1170 | 7.3 | 100 | DMG | 121-item FFQ | US food composition | 7 | 241 | 156–306 | ≥268 vs. <193 | HR: 0.7 (0.33, 1.49) |
| 70 | TMG | 295 | 185–381 | ≥332.2 vs. <234.2 | HR: 0.74 (0.33, 1.64) | ||||||||
| Li et al. 2011 (20) | German | 35–64 | B: 24,323 | 11 | 513 | DMG | 122-item FFQ | Minnesota Nutrition Data System | 8 | 244 | 189–330 | >354 vs. <280 | HR: 1.04 (0.79, 1.36) |
| Zhong et al. 2020 (17) | US | 55–74 | B:104,025 | 11.5 | 100 | DMG | 137-item FFQ | USDA sources | 8 | NR | 205–408 | >358.1 vs. <256 | HR: 0.34 (0.18, 0.66) |
| B:104,025 | 100 | TMG | 301–413 | >413.2 vs. <301 | HR: 0.37 (0.19, 0.71) | ||||||||
| B:104,025 | 100 | SMG | NR | User vs. nonuser | HR: 1.12 (0.76, 1.67) | ||||||||
| Chen et al. 2019 (18) | US | >20 | B: 27,725 | 6.1 | 666 | DMG | Food recall | US food composition | 7 | 294 | NR | >EAR vs. <EAR | RR: 0.71 (0.55, 0.99) |
| B: 27,725 | 666 | TMG | 338 | NR | >EAR vs. <EAR | RR: 0.97 (0.74, 1.28) | |||||||
| B: 27,725 | 636 | SMG | 147 | NR | User vs. nonuser | RR: 1.04 (0.82, 1.32) | |||||||
| Mursu et al. 2011 (22) | US | 55–69 | F: 38,772 | 19 | 4923 | SMG | 127-item FFQ | USDA sources | 8 | NR | NR | User vs. nonuser | HR: 1.14 (0.98, 1.33) |
B, both male and female; DMG, dietary magnesium; EAR, estimated average requirement; ES, effect size; F, female; FCT, food composition table; M, male; MG, magnesium; NOS, Newcastle Ottawa Scale; NR, not reported; SMG, supplemental magnesium; TMG, total magnesium; ref, reference.
Number of years that individuals were followed in the prospective cohort studies.
These ESs are for the highest compared with lowest comparison.
Findings from the systematic review
Out of 19 studies about all-cause mortality, 15 studies had considered dietary magnesium intake (9, 15–18, 20, 21, 23, 26, 27, 40, 41, 43, 44, 46); of these studies, 7 (15–18, 40, 44, 46) had demonstrated significant inverse association and 8 (9, 20, 21, 23, 26, 27, 41, 43) no significant association between dietary magnesium intake and risk of all-cause mortality. Three studies (17, 18, 22) had reported effect sizes for supplemental magnesium intake: 2 studies (17, 18) had reported no significant association with all-cause mortality, and 1 reported a significant increased risk (22). In total, 8 studies (17–19, 23, 25, 26, 40, 45) reported total magnesium intake in relation to risk of all-cause mortality: 2 (17, 18) had reached an inverse association, whereas 6 others (19, 23, 25, 26, 40, 45) did not find any link between total magnesium intake and all-cause mortality.
Out of 12 publications on CVD mortality (9, 15, 16, 18, 19, 21, 22, 27, 41, 43, 44, 46), 10 studies had considered dietary magnesium intake (9, 15, 16, 18, 21, 27, 41, 43, 44, 46): 4 studies (15, 16, 44, 46) revealed a protective association, while 6 others (9, 18, 21, 27, 41, 43) reported no significant association. Out of 2 studies (18, 22) about supplemental magnesium intake and CVD mortality, 1 study (22) had reported an increased risk, while the other study revealed no significant association (18). None of 2 studies about total magnesium intake and CVD mortality reported a significant association (18, 19).
Out of 8 studies that reported effect size for cancer mortality (15, 17, 18, 20–22, 40, 43), 7 studies had assessed dietary magnesium intake: 3 had reported a decreased risk (15, 17, 18), while 4 other studies did not find a significant association (20, 21, 40, 43). Out of 3 publications about supplemental magnesium intake and cancer mortality (17, 18, 22), none had reported a significant association. Out of 3 publications about total magnesium intake and cancer mortality (17, 18, 40), 2 studies (18, 40) had reported no significant association, while the study of Zhong et al. (17) reported a protective association.
Findings from the meta-analysis on magnesium intake and all-cause mortality
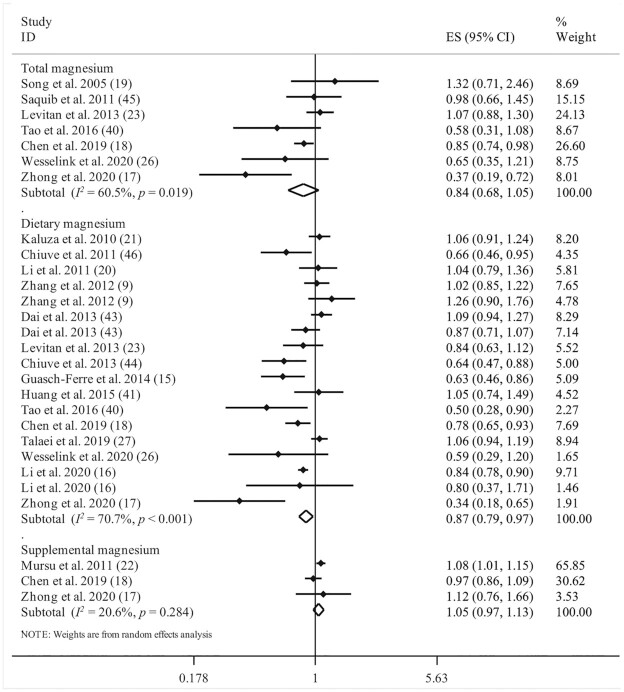
Investigating the association between dietary magnesium intake and risk of all-cause mortality in 15 articles (9, 15–18, 20, 21, 23, 26, 27, 40, 41, 43, 44, 46) that involved a total of 900,825 participants with 27,132 deaths, we found a significant protective association between dietary magnesium intake and risk of all-cause mortality [pooled effect size (ES) comparing the highest and lowest intakes: 0.87; 95% CI: 0.79, 0.97; P = 0.009), with a high heterogeneity among the studies (I2 = 70.7%; P < 0.001) (Figure 1).
FIGURE 1.
Forest plot for association between total, dietary, and supplemental magnesium intakes and risk of all-cause mortality. Diamonds represent pooled estimates from random-effects analysis.
Three studies, with a total of 170,522 participants and 19,307 cases of death, were included in the analysis of supplemental magnesium intake and risk of all-cause mortality (17, 18, 22). We found that supplemental magnesium intake was not associated with increased risk of all-cause mortality (pooled ES: 1.05; 95% CI: 0.97, 1.13; P = 0.502; I2 = 20.6%; P = 0.284) (Figure 1).
With regard to total magnesium intake, based on 7 studies with a total of 176,111 participants and 6,039 deaths (17–19, 23, 26, 40, 45), we found no significant association with risk of all-cause mortality (pooled ES: 0.84; 95% CI: 0.68, 1.05; P = 0.12). However, significant between-study heterogeneity was observed (I2 = 60.5%; P = 0.01) (Figure 1).
Findings from the meta-analysis on magnesium intake and CVD mortality
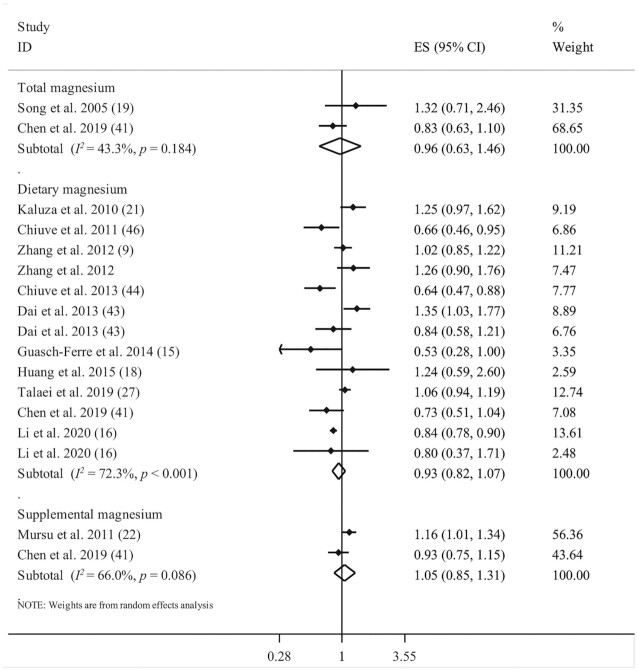
Ten studies, with a total of 791,883 participants and 16,212 deaths, had assessed the association between dietary magnesium intake and risk of CVD mortality (9, 15, 16, 18, 21, 27, 41, 43, 44, 46). The summary effect size for CVD mortality comparing the highest and lowest categories of dietary magnesium intake was 0.93 (95% CI: 0.82, 1.07; P = 0.313), indicating no significant association. Significant between-study heterogeneity was documented (I2 = 72.3%; P < 0.001) (Figure 2).
FIGURE 2.
Forest plot for association between total, dietary, and supplemental magnesium intakes and risk of cardiovascular mortality. Diamonds represent pooled estimates from random-effects analysis.
For supplemental magnesium intake and CVD mortality, 2 published studies, which included a total of 66,497 participants with 6422 deaths, were considered (18, 22). Combining information from these 2 studies, we did not find any significant association (pooled ES comparing supplement use with nonuse: 1.05; 95% CI: 0.85, 1.31; P = 0.63; I2 = 66%; P = 0.08) (Figure 2).
Analyzing the association of total magnesium intake and risk of CVD mortality, based on 2 studies that included a total of 63,326 participants with 844 deaths (18, 19), we found no significant association (pooled ES comparing the highest and lowest intakes: 0.96; 95% CI: 0.63, 1.46; P = 0.84; I2 = 43.3%; P = 0.18) (Figure 2).
Findings from the meta-analysis on magnesium intake and cancer mortality
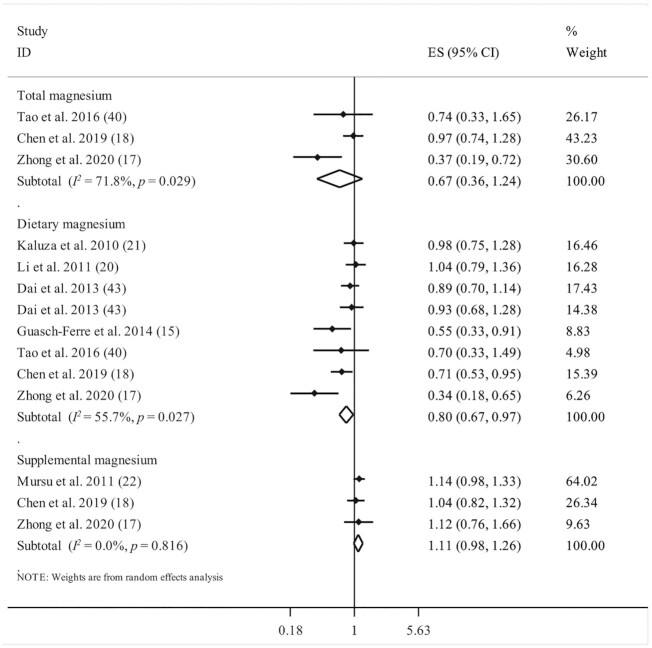
Seven studies reported data for dietary magnesium intake and cancer mortality (15, 17, 18, 20, 21, 40, 43), with a total of 322,471 participants and 4913 cancer deaths. The summary effect size for cancer mortality comparing the highest with the lowest categories of dietary magnesium intake was 0.80 (95% CI: 0.67, 0.97; P = 0.023), indicating a significant inverse association between dietary magnesium intake and risk of cancer mortality. There was a significant heterogeneity among studies (I2 = 55.7%; P = 0.027) (Figure 3).
FIGURE 3.
Forest plot for association between total, dietary, and supplemental magnesium intakes and risk of cancer mortality. Diamonds represent pooled estimates from random-effects analysis.
Considering the supplemental magnesium intake, we found a nonsignificant positive association between taking supplemental magnesium and risk of cancer mortality, based on 3 publications (17, 18, 22) with a total of 170,522 participants and 5659 cancer deaths (pooled ES: 1.11; 95% CI: 0.98, 1.26; P = 0.092), with no significant between-study heterogeneity (I2 = 0%; P = 0.81) (Figure 3).
Total magnesium intake was investigated in 3 studies with a total of 132,920 participants and 836 cancer deaths (17, 18, 40). We did not find any significant association between total magnesium intake and risk of cancer mortality (ES: 0.67; 95% CI: 0.36, 1.24; P = 0.206); however, between-study heterogeneity was significant (I2 = 71.8%; P = 0.02) (Figure 3).
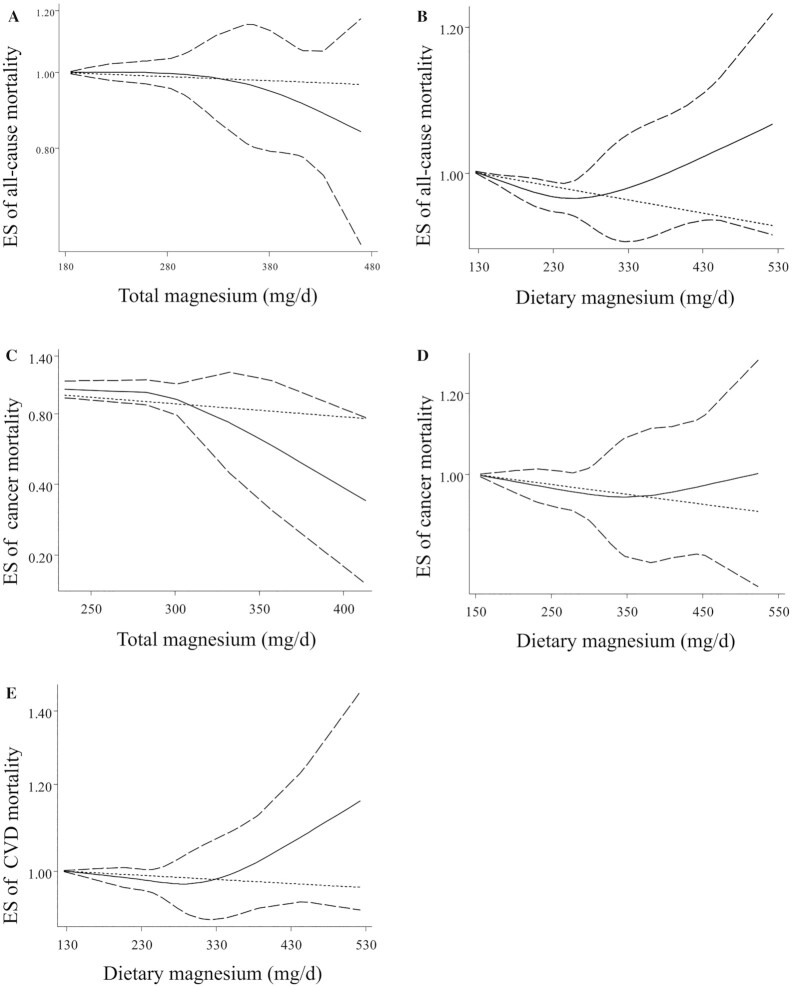
Findings from linear and nonlinear dose–response analysis
Out of 19 articles, 13 studies (9, 15, 17, 20, 21, 23, 26, 27, 40, 41, 43, 44, 46) were included in the dose–response analysis on the association between dietary magnesium intake and all-cause mortality (Figure 4). We found a significant nonlinear association (P-nonlinearity = 0.01). Moreover, linear dose–response meta-analyses demonstrated that each additional intake of 100 mg/d of dietary magnesium was associated with a 6% reduced risk of all-cause mortality (pooled ES: 0.94; 95% CI: 0.91, 0.97; P < 0.001) (Supplemental Figure 2). In the dose–response analysis of dietary magnesium intake and CVD mortality, based on 8 publications (9, 15, 21, 27, 41, 43, 44, 46), out of 10 papers, we did not find a significant nonlinear association (P-nonlinearity = 0.34) (Figure 4). According to linear dose–response analysis, an additional 100 mg/d dietary magnesium intake was not associated with a risk of CVD mortality (pooled ES: 0.97; 95% CI: 0.94, 1.01; P = 0.12) (Supplemental Figure 3). We included 6 publications (15, 17, 20, 21, 40, 43) out of 7 papers including analysis of the nonlinear dose–response association of dietary magnesium intake and mortality from cancer, where we found no significant association (P-nonlinearity = 0.08). However, the linear dose–response analysis demonstrated that each 100-mg/d additional intake of dietary magnesium was associated with a 5% decreased risk of cancer mortality (pooled ES: 0.95; 95% CI: 0.91, 0.99; P = 0.03) (Supplemental Figure 4).
FIGURE 4.
Nonlinear dose–response results. (A) Total magnesium intake and all-cause mortality. (B) Dietary magnesium intake and all-cause mortality. (C) Total magnesium intake and cancer mortality. (D) Dietary magnesium intake and cancer mortality. (E) Dietary magnesium intake and cardiovascular mortality. Dietary and total magnesium intakes were modeled with restricted cubic splines in a multivariate random-effects dose–response model. It should be noted that the number of ESs for conducting nonlinear association between total magnesium intake and risk of cardiovascular mortality was inadequate; therefore, the analysis for this was not done. The black line indicates the linear model; the solid black line indicates the spline model and the dotted lines represent 95% CIs. ES, effect size.
Five publications reported adequate data for the dose–response analysis on the association between total magnesium intake and all-cause mortality (17, 19, 23, 26, 40). We found no significant nonlinear associations (P-nonlinearity = 0.508) (Figure 4). Also, linear dose–response analysis indicated no significant association between an additional intake of 100 mg/d of total magnesium and risk of all-cause mortality (pooled ES: 0.95; 95% CI: 0.87, 1.03; P = 0.22) (Supplemental Figure 2).
A protective significant nonlinear association was observed between total magnesium intake and risk of cancer mortality based on 2 publications (17, 40) (P-nonlinearity = 0.01) (Figure 4). However, linear dose–response analysis revealed that each 100 mg/d additional intake of total magnesium was not associated with a risk of mortality from cancer (pooled ES: 0.88; 95% CI: 0.77, 1.01; P = 0.071) (Supplemental Figure 4).
Findings from subgroup and sensitivity analyses and publication bias
Subgroup analyses were performed to examine the robustness of the results and explore the potential source of heterogeneity across studies. These analyses were conducted based on predefined criteria, including gender, study location, duration of follow-up, number of participants, dietary assessment based on the interview, mean BMI of study participants, and statistical adjusting for total energy intake. Supplemental Table 2 shows findings from the different subgroups. A significant inverse association was seen between dietary magnesium intake and all-cause mortality in women and in studies conducted in the United States and Western countries and those performed on a population with a mean BMI ≥25. With regard to dietary magnesium intake and risk of CVD mortality, a significant protective association was observed in women and in studies performed in the United States and Western countries, studies that applied self-reported FFQs, studies with >15,000 participants, studies with a baseline mean BMI ≥25, and studies that controlled for energy intake. With regard to dietary magnesium intake and cancer mortality, a significant inverse association was observed in studies with participants of both genders, those conducted in the United States and Western countries, those with a follow-up duration <10 y, those that applied interviewer-based FFQ, and those with a mean BMI ≥25. Concerning total magnesium intake and risk of all-cause mortality, the significant protective association persisted across most of the subgroups, including studies conducted in the United States, studies with participants of both genders, studies without adjustment for BMI, studies with >15,000 participants, studies with interviewer-based FFQ, and studies with a mean BMI ≥25.
Based on sensitivity analysis, we found that the obtained combined effect sizes for magnesium intake and risk of mortality did not depend on a particular study. Moreover, visual inspection of funnel plots revealed no evidence of publication bias in the performed analyses.
Discussion
In this systematic review and meta-analysis of prospective cohort studies, a significant inverse association was observed between dietary magnesium intake and risk of mortality from all causes and cancer; however, no significant association was seen between supplemental and total magnesium intakes and risk of all-cause, CVD, as well as cancer mortality. In addition, dietary magnesium intake was not associated with CVD mortality. On the other hand, a dose–response analysis revealed that an additional intake of 100 mg/d dietary magnesium was significantly associated with 6% and 5% decreased risks of all-cause and cancer mortality, respectively. To the best of our knowledge, this is the most comprehensive systematic review and meta-analysis of prospective cohort studies to investigate the association of total, supplemental, and dietary magnesium intakes and risk of all-cause, CVD, and cancer mortality.
In the current meta-analysis, we found that only dietary magnesium intake was associated with decreased risk of all-cause mortality. Nevertheless, there is no clear association between dietary, supplemental, and total magnesium intakes and risk of mortality from CVD. In line with our findings, a meta-analysis of cohort studies published in 2016 reported that increased dietary magnesium intake was associated with a reduced risk of all-cause mortality, but not with incident coronary artery disease or total CVD events (24). Unlike our findings, in a meta-analysis in 2016 with 449,748 participants and 10,313 CVD deaths, an inverse association was seen between dietary magnesium intake and risk of CVD death (47). However, in that study the total numbers of participants and CVD deaths included were lower than those included in our meta-analysis. Another meta-analysis was conducted in 2019 to evaluate the association between serum and dietary magnesium and risk of CVD and coronary artery disease events; in which the investigators reported that high dietary magnesium intake or serum magnesium concentrations were inversely associated with a risk of total CVD and coronary artery disease incidence (48). It is worth mentioning that we also observed an inverse association between dietary magnesium intake and CVD mortality in most subgroups. Overall, it seems that further well-design studies are required to clarify the association between dietary magnesium intake and risk of CVD mortality.
In the present meta-analysis, dietary magnesium intake was protectively associated with the risk of cancer mortality. Moreover, our dose–response analysis indicated that each additional 100 mg/d increase in dietary magnesium intake was associated with a decreased risk of mortality from cancer. To our knowledge, there is no earlier meta-analysis investigating dietary magnesium intake in relation to the risk of cancer mortality. Studies conducted on the association between dietary magnesium intake and risk of colorectal cancer suggested an inverse correlation between increasing dietary magnesium intake and risk of colorectal cancer (10, 49, 50). Moreover, a meta-analysis in 2019 indicated that higher magnesium intake might have a protective role in cancer, especially colorectal cancer (51).
Concerning supplemental magnesium intake, a systematic review and meta-analysis in 2017 showed that, like the findings of the current study, supplemental magnesium intake was not associated with CVD events or risk of all-cause and cancer mortality (52). Despite several health benefits of supplemental magnesium demonstrated in earlier studies (53), it must be noted that we performed the analysis on only 3 studies to find the association of supplemental magnesium intake with all-cause and cancer mortality and only 2 studies about CVD mortality. This information is too limited to come to a conclusion, and further studies are needed in this area. Compared to supplemental magnesium, dietary magnesium intake may gradually increase magnesium concentrations, and it is safe and effective (24). Moreover, foods rich in magnesium, including whole grains, green vegetables, nuts, and beans, provide a wide range of healthy components (e.g., chlorophyll, fiber, and antioxidants) for the human body (3).
Subgroup analyses of dietary magnesium intake and mortality showed that the protective association remained significant in most subgroups. For instance, we observed that study location might be an explanatory factor for between-study heterogeneity. This finding might be due to the differences in genetics, dietary patterns, and study quality as well as characteristics of study participants. Mean BMI of study participants was another factor that was associated with between-study heterogeneity. As we all know, nutrient requirements increase with increasing body weight (54). Therefore, in overweight and obese people obtaining an appropriate amount of magnesium is important given the role of magnesium in several health conditions. Currently, the recommended dietary intake of magnesium in overweight and obese people is not different from that in normal-weight individuals. Given that obesity per se is associated with increased risk of mortality and the protective association between dietary magnesium intake and mortality we found in the current study, the inclusion of high amounts of magnesium in the diet of overweight and obese individuals might result in longer life in these people. Therefore, revising the requirements for magnesium in these people is suggested. Although we did not find any significant association between dietary magnesium intake and risk of CVD mortality, this association was protective in most subgroups. For instance, this protective association was seen in studies in which the analysis was controlled for total energy intake. Given the important role of total energy intake on diet–disease associations, it seems that the association between dietary magnesium intake and risk of CVD mortality was masked when this important factor was not taken into account. Generally speaking, it seems that in the studies in which all the covariates were taken into account dietary magnesium intake was found to be a possible protective factor against mortality.
Several potential mechanisms might explain the inverse association of magnesium intake with all-cause and cancer mortality. Magnesium has several crucial roles in human health, including its antiplatelet effect (55), maintaining glucose and insulin homeostasis (56, 57), improving lipid metabolism (58, 59) and endothelial function (60), and enhancing vascular and myocardial contractility (60, 61). Therefore, these proposed mechanisms may play a role in its effect on risk of death. Magnesium has essential roles in DNA synthesis and repair and the maintenance of genomic stability (62, 63). So, inadequate magnesium intake may induce tumor progression and angiogenesis as well as genetic instability (64). Furthermore, magnesium deficiency is related to chronic inflammation, which may be reflected in increased concentrations of C-reactive protein, IL-6, fibrinogen, total homocysteine, and NF-κB (65–67). Overall, it seems that magnesium can affect the risk of mortality through exerting direct and indirect effects on gene stability and expression and controlling inflammation.
The current meta-analysis has several strengths. First, the large number of participants and deaths in the included studies allowed us to detect a more reliable association between magnesium intake and risk of mortality. Second, we performed a dose–response analysis to assess the linear and nonlinear correlations. Third, the effect of recall and selection bias is negligible due to the inclusion of prospective cohort studies in the current meta-analysis. Finally, to our knowledge these data provide the most comprehensive insight to date into the association between total, supplemental, and dietary magnesium intakes and risk of mortality. However, as with all studies, this study has some limitations as well. Due to the observational nature of the included studies, residual confounding may have affected the association of magnesium intake and mortality. Although several potential confounders were adjusted for in most studies, some reports did not consider other components of diet, including some nutrients or total energy intake as covariates. Lack of adjustment for dietary calcium and potassium intake, which coexist in most food sources of magnesium, might affect the independent association between magnesium intake and mortality. In addition, we were unable to include some studies in the dose–response analysis due to lack of sufficient information. Due to the limited number of effect sizes available regarding supplemental magnesium intake and mortality, our findings should be interpreted cautiously. Measurement errors in the included studies, due to their observational nature, might also attenuate the association of dietary magnesium intake with risk of mortality. As most studies included are from Western countries, the possible extrapolation of these findings to other populations should be done cautiously.
In conclusion, we found a significant inverse association between dietary magnesium intake and risk of mortality from all causes and cancer; In contrast, we observed no significant association of supplemental and total magnesium intake with all-cause, CVD, and cancer mortality. Practically, 100 mg/d additional intake of dietary magnesium was associated with 6% and 5% lower risks of all-cause and cancer mortality, respectively. These findings have practical importance for public health, supporting the consumption of magnesium from dietary sources as being beneficial in reducing all-cause and cancer mortality.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—AB, SN, and OS: contributed to literature search, data extraction, and data analysis; AB: wrote the manuscript; BL: contributed to study conception, manuscript drafting, and data analysis; AE: contributed in study conception, manuscript drafting, data analysis, and approving the final manuscript and is the guarantor; and all authors: read and approved the final manuscript.
Notes
The authors report no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–4 and Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CVD, cardiovascular disease; DRI, dietary reference intake; ES, effect size; NOS, Newcastle Ottawa Scale
Contributor Information
Amir Bagheri, Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
Sina Naghshi, Department of Clinical Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
Omid Sadeghi, Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
Bagher Larijani, Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran.
Ahmad Esmaillzadeh, Professor of Nutrition, Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran; Obesity and Eating Habits Research Center, Endocrinology and Metabolism Molecular-Cellular Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran; Department of Community Nutrition, Isfahan University of Medical Sciences, Isfahan, Iran.
References
- 1. Raymond JL, Mahan LK. Krause and Mahan’s food and the nutrition care process. 15th Edition. Health sciences division: Elsevier; 2020. [Google Scholar]
- 2. Veronese N, Zanforlini BM, Manzato E, Sergi G. Magnesium and healthy aging. Magnes Res. 2015;28(3):112–5. [DOI] [PubMed] [Google Scholar]
- 3. Volpe SL. Magnesium in disease prevention and overall health. Adv Nutr. 2013;4(3):378S–83S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosanoff A, Weaver CM, Rude RK. Suboptimal magnesium status in the United States: are the health consequences underestimated? Nutr Rev. 2012;70(3):153–64. [DOI] [PubMed] [Google Scholar]
- 5. Rosanoff A. Rising Ca: Mg intake ratio from food in USA adults: a concern?. Magnes Res. 2010;23(4):S181–93. [DOI] [PubMed] [Google Scholar]
- 6. Agarwal S, Reider C, Brooks JR, Fulgoni III VL. Comparison of prevalence of inadequate nutrient intake based on body weight status of adults in the United States: an analysis of NHANES 2001–2008. J Am Coll Nutr. 2015;34(2):126–34. [DOI] [PubMed] [Google Scholar]
- 7. McKeown NM, Jacques PF, Zhang XL, Juan W, Sahyoun NR. Dietary magnesium intake is related to metabolic syndrome in older Americans. Eur J Nutr. 2008;47(4):210–6. [DOI] [PubMed] [Google Scholar]
- 8. Dong J-Y, Xun P, He K, Qin L-Q. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Dia Care. 2011;34(9):2116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang W, Iso H, Ohira T, Date C, Tamakoshi A, Group JS. Associations of dietary magnesium intake with mortality from cardiovascular disease: the JACC study. Atherosclerosis. 2012;221(2):587–95. [DOI] [PubMed] [Google Scholar]
- 10. Chen G, Pang Z, Liu Q. Magnesium intake and risk of colorectal cancer: a meta-analysis of prospective studies. Eur J Clin Nutr. 2012;66(11):1182–6. [DOI] [PubMed] [Google Scholar]
- 11. Wark PA, Lau R, Norat T, Kampman E. Magnesium intake and colorectal tumor risk: a case-control study and meta-analysis. Am J Clin Nutr. 2012;96(3):622–31. [DOI] [PubMed] [Google Scholar]
- 12. Song Y, Liu S. Magnesium for cardiovascular health: time for intervention. Am J Clin Nutr. 2012;95(2):269–70. [DOI] [PubMed] [Google Scholar]
- 13. Nielsen FH. Magnesium deficiency and increased inflammation: current perspectives. JIR. 2018;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cruz KJC, de Oliveira ARS, Pinto DP, Morais JBS, da Silva Lima F, Colli C, Torres-Leal FL, do Nascimento Marreiro D. Influence of magnesium on insulin resistance in obese women. Biol Trace Elem Res. 2014;160(3):305–10. [DOI] [PubMed] [Google Scholar]
- 15. Guasch-Ferré M, Bulló M, Estruch R, Corella D, Martínez-González MA, Ros E, Covas M, Arós F, Gómez-Gracia E, Fiol M. Dietary magnesium intake is inversely associated with mortality in adults at high cardiovascular disease risk. J Nutr. 2014;144(1):55–60. [DOI] [PubMed] [Google Scholar]
- 16. Li J, Hovey KM, Andrews CA, Quddus A, Allison MA, Van Horn L, Martin LW, Salmoirago-Blotcher E, Song Y, Manson JE. Association of dietary magnesium intake with fatal coronary heart disease and sudden cardiac death. J Womens Health. 2020;29(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhong GC, Peng Y, Wang K, Wan L, Wu YQL, Hao FB, Hu JJ, Gu HT. Magnesium intake and primary liver cancer incidence and mortality in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int J Cancer. 2020, 147(6):1577–86. [DOI] [PubMed] [Google Scholar]
- 18. Chen F, Du M, Blumberg JB, Chui KKH, Ruan M, Rogers G, Shan Z, Zeng L, Zhang FF. Association among dietary supplement use, nutrient intake, and mortality among US adults: a cohort study. Ann Intern Med. 2019;170(9):604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song Y, Manson JE, Cook NR, Albert CM, Buring JE, Liu S. Dietary magnesium intake and risk of cardiovascular disease among women. Am J Cardiol. 2005;96(8):1135–41. [DOI] [PubMed] [Google Scholar]
- 20. Li K, Kaaks R, Linseisen J, Rohrmann S. Dietary calcium and magnesium intake in relation to cancer incidence and mortality in a German prospective cohort (EPIC-Heidelberg). Cancer Causes Control. 2011;22(10):1375–82. [DOI] [PubMed] [Google Scholar]
- 21. Kaluza J, Orsini N, Levitan EB, Brzozowska A, Roszkowski W, Wolk A. Dietary calcium and magnesium intake and mortality: a prospective study of men. Am J Epidemiol. 2010;171(7):801–7. [DOI] [PubMed] [Google Scholar]
- 22. Mursu J, Robien K, Harnack LJ, Park K, Jacobs DR. Dietary supplements and mortality rate in older women: the Iowa Women's Health Study. Arch Intern Med. 2011;171(18):1625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levitan EB, Shikany JM, Ahmed A, Snetselaar LG, Martin LW, Curb JD, Lewis CE. Calcium, magnesium and potassium intake and mortality in women with heart failure: the Women's Health Initiative. Br J Nutr. 2013;110(1):179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fang X, Wang K, Han D, He X, Wei J, Zhao L, Imam MU, Ping Z, Li Y, Xu Y. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: a dose–response meta-analysis of prospective cohort studies. BMC Med. 2016;14(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu L, Zhu X, Fan L, Kabagambe EK, Song Y, Tao M, Zhong X, Hou L, Shrubsole MJ, Liu J. Magnesium intake and mortality due to liver diseases: results from the Third National Health and Nutrition Examination Survey Cohort. Sci Rep. 2017;7(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wesselink E, Kok DE, Bours MJ, de Wilt JH, van Baar H, van Zutphen M, Geijsen AM, Keulen ET, Hansson BM, van den Ouweland J. Vitamin D, magnesium, calcium, and their interaction in relation to colorectal cancer recurrence and all-cause mortality. Am J Clin Nutr. 2020;111(5):1007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Talaei M, Koh WP, Yuan JM, van Dam RM. DASH dietary pattern, mediation by mineral intakes, and the risk of coronary artery disease and stroke mortality. JAHA. 2019;8(5):e011054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Panic N, Leoncini E, De Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. Eur J Public Health. 2013;23(suppl_1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 30. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 31. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. 2nd Edition. Chichester (UK): John Wiley & Sons; 2019. [Google Scholar]
- 32. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6(1):40–57. [Google Scholar]
- 33. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. [DOI] [PubMed] [Google Scholar]
- 34. Jackson D, Riley R, White IR. Multivariate meta-analysis: potential and promise. Statist Med. 2011;30(20):2481–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sei M, Nakamura H, Miyoshi T. Nutritional epidemiological study on mineral intake and mortality from cardiovascular disease. Tokushima J Exp Med. 1993;40(3-4):199–207. [PubMed] [Google Scholar]
- 36. Elwood PC, Fehily A, Ising H, Poor D, Pickering J, Kamel F. Dietary magnesium does not predict ischaemic heart disease in the Caerphilly cohort. Eur J Clin Nutr. 1996;50(10):694–7. [PubMed] [Google Scholar]
- 37. Singh R, Singh N, Niaz M, Sharma J. Effect of treatment with magnesium and potassium on mortality and reinfarction rate of patients with suspected acute myocardial infarction. Int J Clin Pharmacol Ther. 1996;34(5):219–25. [PubMed] [Google Scholar]
- 38. Singh R. Effect of dietary magnesium supplementation in the prevention of coronary heart disease and sudden cardiac death. Magnes Trace Elem. 1990;9(3):143–51. [PubMed] [Google Scholar]
- 39. Kałuza J, Dołowa J, Roszkowski W, Brzozowska A. [Survival and habitual nutrient intake among elderly men]. Rocz Panstw Zakl Hig. 2005;56(4):361. In Polish. [PubMed] [Google Scholar]
- 40. Tao M-H, Dai Q, Millen AE, Nie J, Edge SB, Trevisan M, Shields PG, Freudenheim JL. Associations of intakes of magnesium and calcium and survival among women with breast cancer: results from Western New York Exposures and Breast Cancer (WEB) Study. Am J Cancer Res. 2016;6(1):105–13. [PMC free article] [PubMed] [Google Scholar]
- 41. Huang Y-C, Wahlqvist ML, Kao M-D, Wang J-L, Lee M-S. Optimal dietary and plasma magnesium statuses depend on dietary quality for a reduction in the risk of all-cause mortality in older adults. Nutrients. 2015;7(7):5664–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Inoue-Choi M, Greenlee H, Oppeneer SJ, Robien K. The association between postdiagnosis dietary supplement use and total mortality differs by diet quality among older female cancer survivors. Cancer Epidemiol Biomarkers Prev. 2014;23(5):865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dai Q, Shu X-O, Deng X, Xiang Y-B, Li H, Yang G, Shrubsole MJ, Ji B, Cai H, Chow W-H. Modifying effect of calcium/magnesium intake ratio and mortality: a population-based cohort study. BMJ. 2013;3(2):e002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chiuve SE, Sun Q, Curhan GC, Taylor EN, Spiegelman D, Willett WC, Manson JE, Rexrode KM, Albert CM. Dietary and plasma magnesium and risk of coronary heart disease among women. JAHA. 2013;2(2):e000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saquib J, Rock CL, Natarajan L, Saquib N, Newman VA, Patterson RE, Thomson CA, Al-Delaimy WK, Pierce JP. Dietary intake, supplement use, and survival among women diagnosed with early-stage breast cancer. Nutr Cancer. 2011;63(3):327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chiuve SE, Korngold EC, Januzzi Jr JL, Gantzer ML, Albert CM. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am J Clin Nutr. 2011;93(2):253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fang X, Liang C, Li M, Montgomery S, Fall K, Aaseth J, Cao Y. Dose-response relationship between dietary magnesium intake and cardiovascular mortality: a systematic review and dose-based meta-regression analysis of prospective studies. J Trace Elem Med Biol. 2016;38:64–73. [DOI] [PubMed] [Google Scholar]
- 48. Zhao L, Hu M, Yang L, Xu H, Song W, Qian Y, Zhao M. Quantitative association between serum/dietary magnesium and cardiovascular disease/coronary heart disease risk: a dose–response meta-analysis of prospective cohort studies. J Cardiovasc Pharmacol. 2019;74(6):516–27. [DOI] [PubMed] [Google Scholar]
- 49. Qu X, Jin F, Hao Y, Zhu Z, Li H, Tang T, Dai K. Nonlinear association between magnesium intake and the risk of colorectal cancer. European J Gastroenterol Hepatol. 2013;25(3):309–18. [DOI] [PubMed] [Google Scholar]
- 50. Meng Y, Sun J, Yu J, Wang C, Su J. Dietary intakes of calcium, iron, magnesium, and potassium elements and the risk of colorectal cancer: A meta-analysis. Biol Trace Elem Res. 2019;189(2):325–35. [DOI] [PubMed] [Google Scholar]
- 51. Ko HJ, Youn CH, Kim HM, Cho YJ, Lee GH, Lee WK. Dietary magnesium intake and risk of cancer: a meta-analysis of epidemiologic studies. Nutr Cancer. 2014;66(6):915–23. [DOI] [PubMed] [Google Scholar]
- 52. Schwingshackl L, Boeing H, Stelmach-Mardas M, Gottschald M, Dietrich S, Hoffmann G, Chaimani A. Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: a systematic review and meta-analysis of primary prevention trials. Adv Nutr. 2017;8(1):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Simental-Mendía LE, Sahebkar A, Rodríguez-Morán M, Guerrero-Romero F. A systematic review and meta-analysis of randomized controlled trials on the effects of magnesium supplementation on insulin sensitivity and glucose control. Pharmacol Res. 2016;111:272–82. [DOI] [PubMed] [Google Scholar]
- 54. Liu J, Zhu X, Fulda KG, Chen S, Tao M-H. Comparison of dietary micronutrient intakes by body weight status among mexican-american and non-Hispanic Black women aged 19–39 years: an analysis of NHANES 2003–2014. Nutrients. 2019;11(12):2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chakraborti S, Chakraborti T, Mandal M, Mandal A, Das S, Ghosh S. Protective role of magnesium in cardiovascular diseases: a review. Mol Cell Biochem. 2002;238(1–2):163–79. [DOI] [PubMed] [Google Scholar]
- 56. Nadler JL, Buchanan T, Natarajan R, Antonipillai I, Bergman R, Rude R. Magnesium deficiency produces insulin resistance and increased thromboxane synthesis. Hypertension. 1993;21(6 pt 2):1024–9. [DOI] [PubMed] [Google Scholar]
- 57. Paolisso G, Ravussin E. Intracellular magnesium and insulin resistance: results in Pima Indians and Caucasians. J Clin Endocrinol Metab. 1995;80(4):1382–5. [DOI] [PubMed] [Google Scholar]
- 58. Itoh K, Kawasaki T, Nakamura M. The effects of high oral magnesium supplementation on blood pressure, serum lipids and related variables in apparently healthy Japanese subjects. Br J Nutr. 1997;78(5):737–50. [DOI] [PubMed] [Google Scholar]
- 59. Rasmussen HS, Aurup P, Goldstein K, McNair P, Mortensen PB, Larsen O, Lawaetz H. Influence of magnesium substitution therapy on blood lipid composition in patients with ischemic heart disease: a double-blind, placebo controlled study. Arch Intern Med. 1989;149(5):1050–3. [PubMed] [Google Scholar]
- 60. Barbagallo M, Dominguez LJ, Galioto A, Ferlisi A, Cani C, Malfa L, Pineo A, Paolisso G. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Aspects Med. 2003;24(1–3):39–52. [DOI] [PubMed] [Google Scholar]
- 61. Touyz R. Role of magnesium in the pathogenesis of hypertension. Mol Aspects Med. 2003;24(1–3):107–36. [DOI] [PubMed] [Google Scholar]
- 62. Hartwig A. Role of magnesium in genomic stability. Mutation research/fundamental and molecular mechanisms of mutagenesis. 2001;475(1–2):113–21. [DOI] [PubMed] [Google Scholar]
- 63. Wolf FI, Trapani V. Cell (patho) physiology of magnesium. Clin Sci. 2008;114(1):27–35. [DOI] [PubMed] [Google Scholar]
- 64. Trapani V, Wolf FI, Scaldaferri F. Dietary magnesium: the magic mineral that protects from colon cancer?. Magnes Res. 2015;28(3):108–11. [DOI] [PubMed] [Google Scholar]
- 65. Kim DJ, Xun P, Liu K, Loria C, Yokota K, Jacobs DR, He K. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care. 2010;33(12):2604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. de Oliveira Otto MC, Alonso A, Lee D-H, Delclos GL, Jenny NS, Jiang R, Lima JA, Symanski E, Jacobs Jr DR, Nettleton JA. Dietary micronutrient intakes are associated with markers of inflammation but not with markers of subclinical atherosclerosis. J Nutr. 2011;141(8):1508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shahi A, Aslani S, Ataollahi M, Mahmoudi M. The role of magnesium in different inflammatory diseases. Inflammopharmacology. 2019;27(4):649–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.