ABSTRACT
Elevated circulating trimethylamine N-oxide (TMAO) concentrations have been observed in patients with chronic kidney disease (CKD). We aimed to systematically estimate and quantify the association between TMAO concentrations and kidney function. The PubMed, EMBASE, Cochrane Library, Scopus, and Web of Science databases were systematically searched from 1995 to 1 June, 2020, for clinical studies on circulating TMAO concentrations and kidney function indicators. We used R software to conduct meta-analyses of the extracted data. A cumulative meta-analysis was applied to test whether health status affected the pooled effect value. Meta-regression and subgroup analyses were performed to identify possible sources of heterogeneity. Ultimately, we included a total of 32 eligible clinical studies involving 42,062 participants. In meta-analyses of continuous-outcome variables, advanced CKD was associated with a 67.9 μmol/L (95% CI: 52.7, 83.2; P < 0.01) increase in TMAO concentration, and subjects with high concentrations of TMAO had a 12.9 mL/(min·1.73 m2) (95% CI: −16.6, −9.14; P < 0.01) decrease in glomerular filtration rate (GFR). In meta-analyses of the correlations, TMAO was strongly inversely correlated with GFR [Fisher's z-transformed correlation coefficient (ZCOR): −0.45; 95% CI: −0.58, −0.32; P < 0.01] and positively associated with the urine albumin-to-creatinine ratio (UACR; ZCOR: 0.26; 95% CI: 0.08, 0.43; P < 0.01), serum creatinine (sCr; ZCOR: 0.43; 95% CI: 0.28, 0.58; P < 0.01), urine albumin excretion rate (UAER; ZCOR: 0.06; 95% CI: 0.04, 0.09; P < 0.01), blood urea (ZCOR: 0.50; 95% CI: 0.29, 0.72; P < 0.01), blood uric acid (ZCOR: 0.32; 95% CI: 0.25, 0.38; P < 0.01), and serum cystatin C (CysC; ZCOR: 0.47, 95% CI: 0.44, 0.51; P < 0.01). This is the first systematic review and meta-analysis to reveal a negative association between circulating TMAO concentrations and kidney function.
Keywords: TMAO, trimethylamine N-oxide, gut microbiome, kidney function, kidney impairment, chronic kidney disease, glomerular filtration rate, meta-analysis
This is the first systematic review and meta-analysis to reveal a negative association between circulating trimethylamine N-oxide (TMAO) concentrations and kidney function.
Introduction
Increasing evidence suggests that the gut microbiome is associated with homeostasis, health, and multiple diseases (1, 2). Dietary intake is considered to be the greatest source of human exposure to the environment and, in turn, exerts important influences on the composition and metabolism of the gut microbiota (3, 4). As living organisms, bacteria thrive by taking nutrients and energy from the host's diet. They also function as a living barrier against ingested noxious substances and liberate otherwise inaccessible nutrients or even potentially harmful metabolic by-products for systemic absorption. Patients with kidney disease are at a high risk of accumulating all kinds of gut microbial metabolites, owing to a decreased capability for systemic metabolite clearance. Gut microbiota-derived uremic toxins, in turn, have been implicated in the progression of chronic kidney disease (CKD) due to their promoting adverse pathophysiological changes in the kidneys, including fibrosis (5), loss of kidney tubular function (6, 7), and reduction in glomerular filtration rate (GFR) (8). Impaired kidney function seriously affects many aspects of human health. It has been reported as an independent risk factor for cardiovascular disease (CVD) (9, 10). In 2017, impaired kidney function resulted in ∼61.3 million disability-adjusted life-years and 1.4 million CVD-related deaths (11). Therefore, early identification and effective control of risk factors for kidney impairment are crucial.
Trimethylamine N-oxide (TMAO) is a gut microbiota-derived metabolite that is formed in the liver from trimethylamine (TMA), which is derived from trimethyl-alkyl ammonium compounds (e.g. choline and carnitine) by the gut microbiota (12) and excreted through the kidneys during urination (13, 14). The formation of the TMAO precursor TMA is inseparable from intestinal flora, and its substrate metabolites are abundant in foods rich in choline and carnitine, including dairy products, egg yolks, organ and muscle meats, and seafoods such as fish and crustaceans (15, 16). Over the last 10 y, TMAO has attracted a great deal of attention after a series of reports revealed its important roles in the occurrence and development of CVD (17) and other diseases such as heart failure (18, 19), incident atrial fibrillation (20), hypertension (21), and diabetes (22). A metabolomic study of 1434 Framingham Heart Study participants with an estimated baseline GFR (eGFR) of ≥60 mL/(min·1.73 m2) showed that 9 metabolites predicted the development of CKD and that choline, the precursor of TMAO, was 1 of 3 markers that remained significant after adjustments for confounders (23). Mammals cannot metabolize TMAO, and ≤95% of total TMAO in the body is excreted unchanged by the kidneys through glomerular filtration and tubular secretion (24, 25). Studies have shown that TMAO concentrations are elevated in patients with end-stage kidney disease (ESKD) (26–30), and an ∼30-fold increase in TMAO concentrations has been reported in hemodialysis patients versus individuals with normal kidney function (24, 27, 31, 32). In addition, among CKD subjects [GFR <60 mL/(min·1.73 m2)], Tang et al. (26) observed that high concentrations of TMAO accelerated the decline of kidney function and were associated with a 2.8-fold increased mortality risk. In a linear-regression analysis, TMAO was an independent predictor of carotid atherosclerosis burden (33). Mouse studies mimicking long-term exposure to elevated TMAO concentrations have revealed that TMAO might contribute to progressive tubulointerstitial fibrosis, collagen deposition, and kidney function impairment (29). These data suggest a significant association between TMAO and kidney function and the possibility that TMAO could function as a biomarker of kidney disease.
To better understand the relation between circulating TMAO concentrations and kidney function, we performed the current systematic review and meta‐analyses to quantify the correlation between circulating TMAO concentrations and kidney function impairment.
Methods
The prospectively developed protocol of the present study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (No. CRD42020191612). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (34) were followed throughout this study.
Data sources and searches
We systematically searched the PubMed, EMBASE, Cochrane Library, Scopus, and Web of Science databases from 1995 to 1 June, 2020. Medical Subject Headings (MeSH) and free-text terms were combined to identify relevant articles, with no language restrictions. Additionally, we searched clinicaltrials.gov and manually searched the references in the selected trials and meta-analyses to identify additional eligible studies. Details of the search terms are provided in Supplemental Table 1. We contacted the study authors for additional information when necessary to ensure that all relevant articles were included in the search. Two reviewers (YZ and MG) developed the selection criteria and screened the titles and abstracts of the searched articles for relevance after removal of duplicates. Two authors (YZ and XF) independently assessed the full texts of the remaining articles to determine their eligibility for inclusion based on predetermined criteria. The reviewers resolved any disagreement through discussion or, if required, adjudication by a third reviewer.
Study selection
Referring to the recommendations of the Global Burden of Diseases, Injuries, and Risk Factors Study (11) and the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines (35), we used the terms “CKD,” “ESKD,” and “advanced CKD” (CKD5 or ESKD) to refer to different stages or degrees of kidney function impairment (Table 1). We used the term “impaired kidney function” to refer to all stages of CKD and the indicators of kidney function damage, including decreased GFR and increased urine albumin-to-creatinine ratio (UACR), serum creatinine (sCr), urine albumin excretion rate (UAER), blood urea nitrogen (BUN), blood urea, blood uric acid, and serum cystatin C (CysC).
TABLE 1.
GFR categories in CKD1
| CKD stage | Definition | Description of kidney function |
|---|---|---|
| CKD12 | GFR ≥90 mL/(min·1.73 m2) and UACR ≥30 mg/g | Normal or high |
| CKD22 | GFR 60–89 mL/(min·1.73 m2) | Mildly decreased |
| CKD3a2 | GFR 45–59 mL/(min·1.73 m2) | Mildly to moderately decreased |
| CKD3b2 | GFR 30–44 mL/(min·1.73 m2) | Moderately to severely decreased |
| CKD42 | GFR 15–29 mL/(min·1.73 m2) | Severely decreased |
| CKD53 | GFR <15 mL/(min·1.73 m2) | Kidney failure (advanced CKD) |
| ESKD | Kidney transplant recipients and patients treated by dialysis | Kidney failure (advanced CKD) |
GFR is the total amount of fluid filtered through all functioning nephrons per unit of time, which is the best available indicator of overall kidney function (36). The Kidney Disease Improving Global Outcomes (KDIGO) initiative classifies an individual as having CKD if abnormalities of kidney structure or function persist for >3 mo. Criteria included reduced GFR, presence of albuminuria, or abnormalities of kidney structure (35). Notably, in the absence of evidence of kidney damage, neither the CKD1 nor the CKD2 GFR category fulfilled the criteria for CKD (35). CKD, chronic kidney disease; ESKD: end-stage kidney disease; GFR, glomerular filtration rate; UACR: urine albumin-to-creatinine ratio.
Not including kidney transplant recipients.
Not including kidney transplant recipients or patients treated by dialysis.
We included observational studies with cohort, cross-sectional, or case-control designs if they: 1) used serum or plasma samples from participants, 2) used MS for analysis, 3) reported TMAO concentrations in healthy subjects and patients with advanced CKD, 4) reported the values of kidney function indicators in ≥2 TMAO categories, 5) reported continuous-outcome variables (TMAO concentrations, values of kidney function indicators) as mean (SD) or median (IQR), 6) reported the Pearson correlation coefficient (r) or Spearman rank correlation coefficient (rho) between TMAO concentrations and kidney function indicators, and 7) involved subjects aged >18 y. Studies that included pregnant women, participants with any other diseases such as cancer or immune-related disease, or participants who took nephrotoxic drugs were excluded.
Data extraction and quality assessment
Data were independently extracted by a single author (YZ) using a standardized and pilot-tested form before being checked by a second author (XF) for accuracy. In the event of discrepancies, all authors discussed the results to establish a consensus. The extracted data included information on trial characteristics (first author, publication year, area, sample size, and study design), baseline information of participants (mean age and/or age range, sex, and underlying disease), TMAO characteristics [sample source of TMAO (serum or plasma) and TMAO analysis method], and the main results of the study. For the meta-analysis of continuous-outcome variables, if the original study grouped variables by kidney function, we extracted the number of participants/cases in each stage of CKD and the reported mean (SD) or median (IQR) of TMAO concentrations across CKD stages. If the original study grouped variables by TMAO concentration, we extracted the number of participants/cases in each category of TMAO and the reported mean (SD) or median (IQR) of each kidney function indicator across TMAO categories. For the meta-analysis of the correlations, we extracted the r or rho values between TMAO concentration and each kidney function indicator. Additionally, we did not place excessive restrictions on the measurement of any indicator. For example, we reported both actually measured GFR (28, 31) and eGFR as GFR in this study.
Two researchers (YZ and MG) independently assessed the quality of the studies, and all discrepancies were resolved through discussion and the involvement of 2 additional researchers (XF and MW). The Newcastle–Ottawa Scale (NOS) (37) was used to assess the quality assessment of cohort and case-control studies based on study group selection, group comparability, and ascertainment of outcome/exposure with 8 detailed questions. Studies with ≥6 stars were deemed to be of high quality. We used the Agency for Healthcare Research and Quality (AHRQ) checklist (38) for cross-sectional studies. Items were scored “1” if the answer was “yes” and “0” if the answer was “no” or “unclear.” Final quality assessment scores were as follows: low quality, 0–3; moderate quality, 4–7; high quality, ≥8. Details of quality assessment are presented in Supplemental Tables 2 and 3.
Data synthesis and analysis
We performed 3 meta-analyses to further analyze the relation between TMAO and kidney function. All data were analyzed using R software version 4.0.0, and P values <0.05 were considered statistically significant unless otherwise specified.
First, we performed a meta-analysis of circulating TMAO concentrations in patients with advanced CKD compared with healthy subjects. Second, we conducted meta-analyses of kidney function indicators in subjects with high concentrations of TMAO versus those with low concentrations of TMAO. In this analysis, if the original study reported circulating TMAO concentrations as quartiles or tertiles, the high concentration of TMAO included the highest layer of TMAO concentration, and the other layers were classified into the low concentration of TMAO. For continuous-outcome variables, we analyzed differences using mean differences (MDs) with 95% CIs; when variables were presented as median values, mean (SD) was estimated according to the method described by Wan et al. (39). If the original study did not specify the number of individuals in each TMAO category, we assumed that equal numbers of participants were enrolled in each group. Third, we performed meta-analyses of correlations between circulating TMAO concentrations and kidney function indicators by combining the correlation coefficients obtained in the individual studies. The following procedures were performed to ensure that an unbiased estimate was calculated: we extracted r values from each study and calculated 95% CIs after applying Fisher's z transformation. The latter was presented as the outcome variable (40–42). In addition, if the original study reported rho values, we converted the data to the corresponding r values for further calculations (43).
We evaluated heterogeneity between studies using the P value of the Q test and the I2 statistic, with significance established at P <0.1 or an I2 statistic of >50%, which was considered representative of statistical heterogeneity. The random-effects model was applied when heterogeneity was high (i.e. I2 >50%). We performed meta-regression and subgroup analyses to identify possible sources of heterogeneity according to study location, design, disease status, sample size, and sample source for TMAO measurement. A cumulative meta-analysis was applied to test whether health status affected the pooled effect value. Possible publication bias was examined using Begg's and Egger's tests. To ensure the stability of the results, we performed sensitivity analysis by removing 1 study at a time from the primary analysis.
Results
Literature search and study characteristics
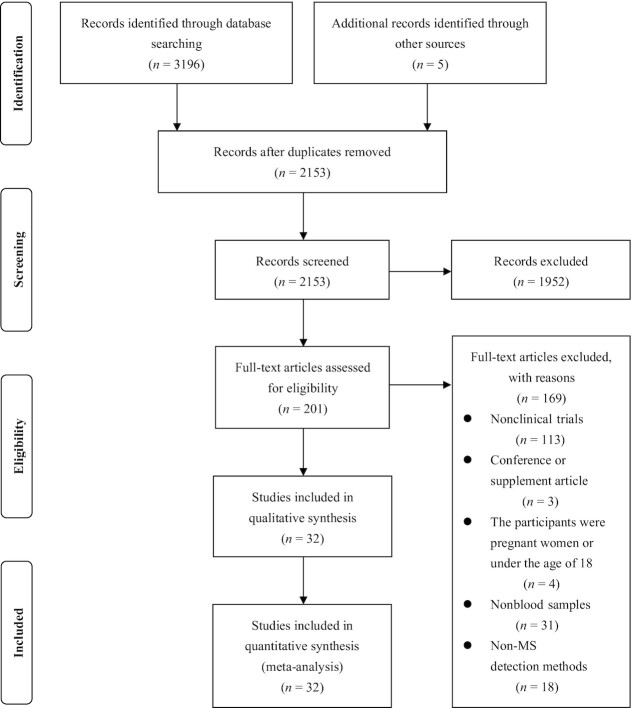
Base on the above-mentioned inclusion criteria, we identified 3201 reports and screened their summaries for eligibility after removing duplicates. Of the 201 full-text articles assessed, we selected 32 for data synthesis. A flow chart of the literature search and study selection is presented in Figure 1. Of these articles, 9 (24, 27–29, 31, 32, 44–46) investigated TMAO concentrations on different CKD stages (Table 2, Supplemental Tables 4 and 5). Seventeen studies (17, 19–21, 27, 47–58) reported the values of kidney function indicators in different TMAO categories (Table 3, Supplemental Table 6). Fifteen studies (18, 19, 26, 28, 31, 32, 45, 50, 51, 54, 58–62) reported the correlation coefficient between TMAO concentration and kidney function indicators (Table 4, Supplemental Table 7). Based on the original data, we included 6 articles (24, 27–29, 31, 32) for meta-analysis of the circulating TMAO concentrations in patients with advanced CKD compared with healthy subjects and 16 articles (17, 19–21, 27, 47–51, 53–58) for meta-analysis of the GFR in subjects with high concentrations of TMAO versus those with low concentrations of TMAO. With respect to the meta-analyses of the correlations between TMAO and kidney function indicators, we included 13 articles for GFR (18, 19, 26, 28, 45, 50, 51, 54, 58–62), 3 for sCr (19, 32, 45), 3 for CysC (26, 45, 59), 2 for blood urea (19, 51), 1 for plasma uric acid (31), 1 for serum uric acid (50), 1 for UACR (31), and 1 for UAER (31). Of note, the studies by Svingen et al. (20) and Lever et al. (62) were each included as 2 independent studies.
FIGURE 1.
Flow chart of the literature search and study selection process.
TABLE 2.
Details of the selected studies and baseline characteristics of the participants in the comparisons of circulating TMAO concentrations between patients with CKD and healthy subjects1
| First author | Year | Journal | Country | Design | Subjects, n | Age,2 y | Male, % | Disease status | Sample | Assay | Main results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kim et al. (45) | 2016 | Kidney Int | Canada | Cohort | 2529 | 68.2 ± 12.7 | 62.5 | CKD | Plasma | LC-MS/MS | Stage 4 patients with severe CKD had higher TMAO concentrations than stage 3b patients with moderate CKD (P <0.001) |
| Missailidis et al. (28) | 2016 | PLoS One | Sweden | Cohort | 259 | 57.2 ± 13.8 | 66.8 | CKD and healthy subjects | Plasma | LC-MS/MS | Plasma TMAO concentrations increased with decreasing kidney function. CKD5 patients had a 13-fold increase in TMAO compared with healthy subjects (P < 0.001) |
| Xu et al. (44) | 2017 | Sci Rep | China | Cross-sectional | 64 | 54.2 ± 12.5 | 50 | CKD and healthy subjects | Plasma | LC-MS/MS | Patients with CKD had obviously higher TMAO concentrations than healthy subjects (P < 0.001) |
| Stubbs et al. (27) | 2016 | J Am Soc Nephrol | USA | Cohort | 104 | 57.6 ± 14.1 | 50 | CKD, ESKD, and healthy subjects | Serum | UHPLC-MS/MS | Serum TMAO concentrations increased with advancing CKD stage, with median concentrations in dialysis-dependent patients with ESKD ∼30-fold higher than in controls (P < 0.001) |
| Kaysen et al. (32) | 2015 | J Ren Nutr | USA | Cohort | 235 | 61.8 ± 14.2 | 55.3 | ESKD and healthy subjects | Plasma | UPLC-MS | Serum TMAO concentrations were elevated in patients undergoing hemodialysis compared with individuals with normal or near-normal kidney function (P < 0.05) |
| Pelletier et al. (31) | 2019 | Toxins | France | Cross-sectional | 124 | ≥18 | 54 | CKD, ESKD, and healthy subjects | Plasma | LC-MS/MS | Plasma TMAO concentrations were increased in patients with CKD (P < 0.05) |
| Bain et al. (29) | 2006 | Nephrol Dial Trans | USA | Cross-sectional | 20 | 45.5 ± 17.3 | 60 | ESKD and healthy subjects | Plasma | GC-MS | Plasma TMAO concentrations in predialysis patients were significantly higher than corresponding concentrations in healthy subjects (P < 0.05) |
| Hai et al. (24) | 2015 | PLoS One | USA | Cross-sectional | 13 | ≥18 | NR | ESKD and healthy subjects | Plasma | LC-MS/MS | Patients with ESKD had much higher peak predialysis plasma TMAO concentrations (P < 0.05) |
| Al-Obaide et al. (46) | 2017 | J Clin Med | USA | Cross-sectional | 40 | 59.35 ± 5.8 | NR | CKD and healthy subjects | Serum | LC-MS | TMAO concentrations in the serum of patients with type 2 DM and CKD were higher than those in healthy subjects (P < 0.0001) |
CKD, chronic kidney disease; DM, diabetes mellitus; ESKD, end-stage kidney disease; LC-MS/MS, LC-tandem MS; NR, not reported; TMAO, trimethylamine N-oxide; UHPLC, ultra-high-performance LC; UPLC, ultra-performance LC.
Values are mean ± SD or range.
TABLE 3.
Details of the selected studies and baseline characteristics of the participants in meta-analyses of kidney function indicators in subjects with high concentrations of TMAO versus subjects with low concentrations of TMAO1
| First author | Year | Journal | Country | Design | Subjects, n | Age,2 y | Male, % | Disease status | Sample | Assay | TMAO range,2 μmol/L | Main results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tang et al. (17) | 2013 | New Engl J Med | USA | Cohort | 4007 | 63 ± 11 | 65 | CAG | Plasma | LC-MS/MS | 3.7 [2.4–6.2] | eGFR was lower in the high-concentration than in the low-concentration TMAO group (P < 0.001) |
| Tang et al. (50) | 2014 | J Am Coll Cardiol | USA | Cohort | 720 | 66 ± 10 | 59 | Patients with a history of HF | Plasma | LC-MS/MS | 5.0 [3.0–8.5] | eGFR was lower in the high-concentration than in the low-concentration TMAO group (P < 0.001) |
| Tang et al. (49) | 2017 | Clin Chem | USA | Cohort | 1216 | 64.4 ± 10.2 | 58 | Type 2 DM | Plasma | LC-MS/MS | 4.4 [2.8–7.7] | eGFR was lower in T3 of TMAO than in T1 (P < 0.001) |
| Stubbs et al. (27) | 2016 | J Am Soc Nephrol | USA | Cohort | 220 | 69.7 ± 10.3 | 42.7 | CKD | Serum | UHPLC-MS/MS | 6.9 [4.8–10.9] | eGFR was lower in T3 of TMAO than in T1 and T2 (P < 0.001) |
| Stubbs et al. (52) | 2019 | Clin J Am Soc Nephrol | Global | RCT | 1243 | 54 ± 14 | 60 | ESKD | Serum | UPLC-MS | 0.91–7.01 | No significant difference in BUN was observed between groups |
| Nie et al. (21) | 2018 | Stroke | China | Case-control | 1244 | 45–75 | 47 | Hypertensive | Serum | LC-MS/MS | T1 <1.79, T3 ≥3.19 | eGFR was lower in T3 of TMAO than in T1 (P < 0.001) |
| Gruppen et al. (58) | 2017 | Sci Rep | Netherlands | Cohort | 5469 | 53.5 ± 12 | 48.7 | Healthy adults | Plasma | LC-MS/MS | 3.2 [1.70–5.70] | eGFR was lower and UAER was higher in Q4 of TMAO than in Q1 (P < 0.001, both) |
| Svingen et al. (20) | 2018 | Int J Cardiol | Norway | Cohort | 4141 | 51–73 | 72 | Suspected stable angina | Plasma | LC-MS/MS | 9.25 [2.2–23.5] | eGFR was lower in Q4 of TMAO than in Q1 (P < 0.0001) |
| Svingen et al. (20) | 2018 | Int J Cardiol | Norway | Cohort | 3143 | 72 [71–73] | 43 | Healthy adults | Plasma | LC-MS/MS | 8.82 [2.6–31.6] | eGFR was lower in Q4 of TMAO than in Q1 (P < 0.0001) |
| Randrianarisoa et al. (54) | 2016 | Int J Cardiol | Germany | Cross-sectional | 220 | 46 ± 11 | 41 | Healthy adults | Serum | LC-MS/MS | T1: 2.13 ± 0.94, T3: 3.84 ± 2.06 | No significant difference in eGFR was observed between groups |
| Winther et al. (48) | 2019 | Diabetes Care | Denmark | Cohort | 1159 | 46 ± 13 | 58 | Type 1 DM | Plasma | LC-MS/MS | 5.7 [3.8–9.9] | eGFR was lower and UAER was higher in Q4 of TMAO than in Q1 (P < 0.001, both) |
| Krüger et al. (57) | 2017 | Mol Nutr Food Res | Germany | Cross-sectional | 297 | 47.5 ± 17.2 | 57.6 | Healthy adults | Plasma | LC-MS/MS | Q1 <2.91, Q4 >6.02 | eGFR was lower in Q4 of TMAO than in Q1 (P < 0.0001) |
| Meyer et al. (55) | 2016 | J Am Heart Assoc | USA | Cohort | 817 | 33–55 | 43 | Healthy adults | Plasma | HPLC-MS/MS | 2.6 [1.8–4.2] | eGFR was lower in Q4 of TMAO than in Q1 (P = 0.002); no difference in UACR was observed between groups (P = 0.554) |
| Suzuki et al. (19) | 2016 | Heart | England | Cohort | 972 | 78 [69–84] | 61 | AHF | Plasma | UPLC-MS/MS | 5.6 [3.4–10.5] | eGFR was lower and blood urea and sCr were higher in the high-concentration than in the low-concentration TMAO group (P < 0.0005, all) |
| Suzuki et al. (51) | 2017 | Clin Chem | England | Cohort | 1079 | 65 [57–77] | 72 | Acute MI | Plasma | UPLC-MS/MS | 3.7 [4.6–6.4] | eGFR was lower and blood urea was higher in T3 of TMAO than in T1 (P < 0.0005, both) |
| Senthong et al. (53) | 2016 | J Am Heart Assoc | USA | Cohort | 2235 | 63 ± 11 | 71 | Stable CAD | Plasma | HPLC-MS/MS | 3.8 [2.5–6.5] | eGFR was lower in Q4 of TMAO than in other quartiles (P < 0.001) |
| Matsuzawa et al. (56) | 2019 | Sci Rep | Japan | Cross-sectional | 112 | 63 [56–71] | 88 | STEMI | Plasma | LC-MS/MS | 6.76 [3.82–12.5] | No significant difference in eGFR was observed between groups (P = 0.11) |
| Zhou et al. (47) | 2020 | ESC Heart Fail | China | Cohort | 1208 | 73 [64–80] | 68.5 | CHF patients after MI | Plasma | UHPLC | lower <4.5, higher ≥4.5 | eGFR was lower in the high-concentration than in the low-concentration TMAO group (P < 0.001) |
In this analysis, if the original study reported circulating TMAO concentrations as quartiles or tertiles, the high concentration of TMAO included the highest layer of TMAO concentration, and the other layers were classified into the low concentration of TMAO. AHF, acute heart failure; BUN, blood urea nitrogen; CAD, coronary-artery disease; CAG, coronary angiography; CHF, chronic heart failure; CKD, chronic kidney disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HF, heart failure; LC-MS/MS, LC-tandem MS; MI, myocardial infarction; Q1, quartile 1; Q4, quartile 4; RCT, randomized controlled trial; sCr, serum creatinine; STEMI, ST-segment elevation myocardial infarction; T1, tertile 1; T2, tertile 2; T3, tertile 3; TMAO, trimethylamine N-oxide; UACR, urine albumin-to-creatinine ratio; UAER, urine albumin excretion rate; UHPLC, ultra-high-performance LC; UPLC, ultra-performance LC.
Values are mean ± SD, range, or median [IQR].
TABLE 4.
Details of the selected studies and baseline characteristics of the participants in meta-analyses of the correlations between circulating TMAO concentrations and kidney function indicators1
| First author | Year | Journal | Country | Design | Subjects, n | Age,2 y | Male, % | Disease status | Sample | Assay | Main results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kim et al. (45) | 2016 | Kidney Int | Canada | Cohort | 2529 | 68.2 ± 12.7 | 62.5 | CKD | Plasma | LC-MS/MS | TMAO concentrations correlated with eGFR (rho = −0.40, P < 0.001), CysC (rho = 0.42, P < 0.001), and sCr (rho = 0.40, P < 0.001) |
| Missailidis et al. (28) | 2016 | PLoS One | Sweden | Cohort | 179 | 55 ± 14 | 65 | CKD | Plasma | LC-MS/MS | TMAO concentrations correlated with actually measured GFR (rho = −0.69, P < 0.0001) |
| Tang et al. (50) | 2014 | J Am Coll Cardiol | USA | Cohort | 720 | 66 ± 10 | 59 | Patients with a history of HF | Plasma | LC-MS/MS | TMAO concentrations correlated with eGFR (rho = −0.55, P < 0.001) and serum uric acid (rho = 0.29, P < 0.001) |
| Tang et al. (59) | 2015 | J Card Fail | USA | Cohort | 112 | 57 ± 14 | 75 | CHF | Plasma | LC-MS/MS | TMAO concentrations correlated with eGFR (rho = −0.36, P < 0.001) and CysC (rho = 0.40, P < 0.0001) |
| Tang et al. (26) | 2015 | Circ Res | USA | Cohort | 521 | 70 ± 10 | 48 | CKD | Plasma | LC-MS/MS | TMAO concentrations correlated with eGFR (rho = −0.48, P < 0.001) and CysC (rho = 0.46, P < 0.001) |
| Kaysen et al. (32) | 2015 | J Ren Nutr | USA | Cohort | 235 | 61.8 ± 14.2 | 55.3 | CKD, ESKD, and healthy subjects | Plasma | UPLC-MS | TMAO concentrations correlated with sCr (rho = 0.21, P = 0.002) |
| Pelletier et al. (31) | 2019 | Toxins | France | Cross-sectional | 124 | ≥18 | 54 | CKD, ESKD, and healthy subjects | Plasma | LC-MS/MS | TMAO concentrations correlated with UACR (rho = 0.24, P < 0.005) and plasma uric acid (rho = 0.31, P < 0.005) |
| Gruppen et al. (58) | 2017 | Sci Rep | Netherlands | Cohort | 5469 | 53.5 ± 12 | 48.7 | Healthy adults | Plasma | LC-MS/MS | TMAO concentrations correlated with eGFR (r = −0.095, P < 0.001) and UAER (r = 0.062, P < 0.001) |
| Randrianarisoa et al. (54) | 2016 | Sci Rep | Germany | Cross-sectional | 220 | 46 ± 11 | 41 | Healthy adults | Serum | LC-MS/MS | TMAO concentrations correlated with eGFR (r = −0.14, P = 0.04) |
| Lever et al. (62) (CVD with DM) | 2014 | PLoS One | New Zealand | Cohort | 79 | 74 [47–87] | 73.4 | CVD with DM | Plasma | UPLC-MS | TMAO concentrations correlated with eGFR (rho = −0.31, P < 0.001) |
| Lever et al. (62) (CVD without DM) | 2014 | PLoS One | New Zealand | Cohort | 396 | 68 [55–93] | 72.7 | CVD without DM | Plasma | UPLC-MS | TMAO concentrations correlated with eGFR (rho = −0.39, P < 0.001) |
| Suzuki et al. (19) | 2016 | Heart | UK | Cohort | 972 | 78 [69–84] | 61 | AHF | Plasma | UPLC-MS | TMAO concentrations correlated with eGFR (rho = −0.528, P < 0.0005), blood urea (rho = 0.529, P < 0.0005), and sCr (rho = 0.515, P < 0.0005) |
| Suzuki et al. (51) | 2017 | Clin Chem | UK | Cohort | 1079 | 65 [57–77] | 72 | CAD | Plasma | UPLC-MS | TMAO concentrations correlated with eGFR (rho = −0.371, P ≤0.035) and blood urea (rho = 0.358, P ≤0.035) |
| Mueller et al. (61) | 2015 | Atherosclerosis | Austria | Cohort | 339 | 63 [55–71] | 68 | Diagnostic CAG | Plasma | LC-HRMS | TMAO concentrations correlated with eGFR (r = −0.281, P < 0.01) |
| Roncal et al. (60) | 2019 | Sci Rep | Spain | Cohort | 262 | 70 ± 11 | 87 | Peripheral-artery disease | Plasma | UHPLC-MS/MS | TMAO concentrations correlated with eGFR (r = −0.40, P < 0.001) |
| Trøseid et al. (18) | 2015 | J Intern Med | Norway | Cohort | 155 | 57 ± 11 | 83 | CHF | Plasma | LC-MS/MS | TMAO concentrations correlated with eGFR (r = −0.54, P < 0.001) |
AHF, acute heart failure; BUN, blood urea nitrogen; CAD, coronary-artery disease; CAG, coronary angiography; CHF, chronic cardiac failure; CKD, chronic kidney disease; CVD, cardiovascular disease; CysC, serum cystatin C, DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HF, heart failure; LC-HRMS, LC-high-resolution MS; LC-MS/MS, LC-tandem MS; r, Pearson correlation coefficient; rho, Spearman rank correlation coefficient; sCr, serum creatinine; TMAO, trimethylamine N-oxide; UACR, urine albumin-to-creatinine ratio; UAER, urine albumin excretion rate; UHPLC, ultra-high-performance LC; UPLC, ultra-performance LC.
Values are mean ± SD, range, or median [IQR].
The general characteristics of the studies included in the meta-analyses and the demographic and mean baseline parameters of the enrolled patients are summarized in Tables 2–4 and Supplemental Tables 4–7. These studies were published between 2006 and 2020. A total of 42,062 participants were included in the final data synthesis. The design of these studies was mainly cohort (17–20, 26–28, 32, 45, 47–53, 55, 58–62) or cross-sectional (24, 29, 31, 44, 46, 54, 56, 57); only 1 study had a case-control design (21). Of all articles, 3 reported serum-derived TMAO concentrations (21, 27, 54), and the rest reported plasma-derived TMAO concentrations (17–20, 24, 26, 28, 29, 31, 32, 44–53, 55–62). These clinical studies were conducted in different countries worldwide: 13 were from the USA (17, 24, 26, 27, 29, 32, 46, 49, 50, 53, 55, 56, 59); 3 were from China (21, 44, 47); 2 were from Norway (18, 20); 2 were from the UK (19, 51); 2 were from Germany (54, 57); 1 was from each of Canada (45), Denmark (48), Spain (60), France (31), Sweden (28), Netherlands (58), Austria (61), and New Zealand (62); and 1 was a global clinical trial (52). Study participants differed by disease status: 11 studies examined patients with CKD (24, 26–29, 31, 32, 44–46, 52), 9 examined patients with CVD (18–20, 47, 50, 51, 53, 56, 62), 3 examined patients with diabetes mellitus (DM) (48, 49, 62), 2 examined patients undergoing diagnostic coronary angiography (CAG) (17, 61), 1 examined patients with peripheral-artery disease (60), 1 examined patients with hypertension (21), and 5 examined healthy disease-free subjects (20, 54, 55, 57, 58).
Meta-analysis of the circulating TMAO concentrations in patients with advanced CKD compared with healthy subjects
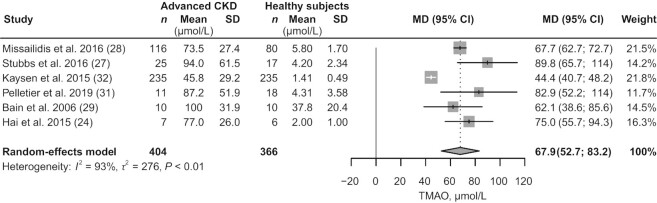
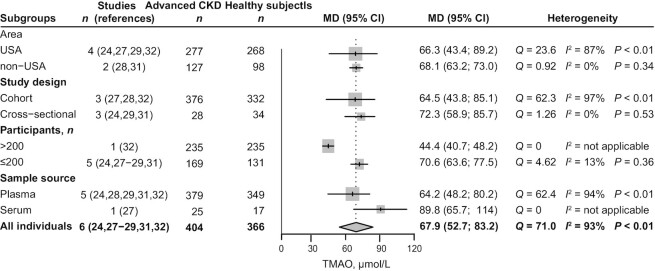
Six articles (24, 27–29, 31, 32) were included for comparison of circulating TMAO concentrations in patients with advanced CKD (404 participants) versus healthy subjects (366 participants). TMAO accumulated in all stages of CKD and increased with the aggravation of kidney function damage (Table 2, Supplemental Table 4). Advanced CKD was associated with a 67.9 μmol/L (95% CI: 52.7, 83.2; P < 0.01; I2 = 93%; Figure 2) increase in circulating TMAO concentrations, compared with concentrations in healthy subjects. Results of the subgroup analyses are shown in Figure 3. In a sample size of ≤200 participants, heterogeneity was significantly reduced (from 93% to 13%).
FIGURE 2.
Meta-analysis of the circulating TMAO concentrations in patients with advanced CKD compared with healthy subjects. CKD, chronic kidney disease; MD, mean difference; TMAO, trimethylamine N-oxide.
FIGURE 3.
Subgroup analyses in meta-analysis of the circulating TMAO concentrations in patients with advanced CKD compared with healthy subjects. CKD, chronic kidney disease; MD, mean difference; TMAO, trimethylamine N-oxide.
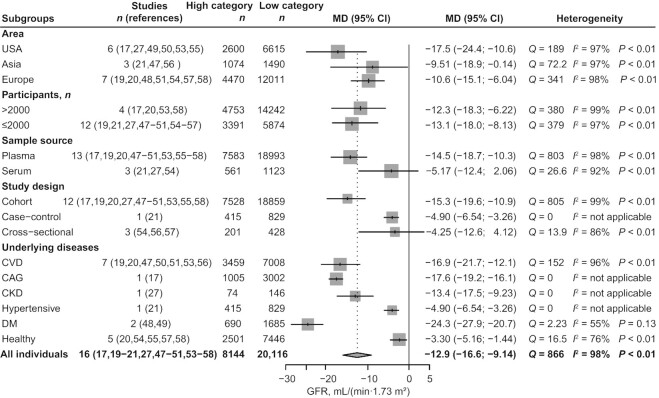
Meta-analysis of the GFR in subjects with high concentrations of TMAO compared with subjects with low concentrations of TMAO
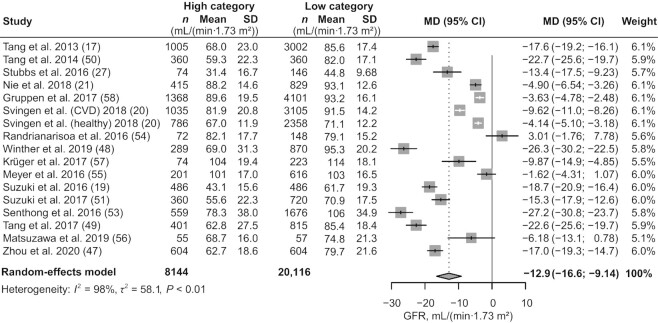
We included 16 studies (17, 19–21, 27, 47–51, 53–58) with 28,260 participants for GFR comparison in the high- and low-TMAO categories, with the study by Svingen et al. (20) included as 2 independent studies. Subjects with high concentrations of TMAO had a 12.9 mL/(min 1.73 m2) (95% CI: −16.6, −9.14; P < 0.01; I2 = 98%; Figure 4) decrease in GFR level. Meta-regression analysis revealed age (tau2 reduced from 0.119 to 0.108; P = 0.0186) and health status (tau2 reduced from 0.119 to 0.0428; P < 0.0001) of participants as possible sources of heterogeneity (Supplemental Table 8). To further investigate the factors affecting the strength of the association between GFR and TMAO, we performed a series of subgroup analyses (Figure 5) based on study location, health status, sample size, and sample source. A greater association between TMAO and GFR was found in studies conducted in the USA and Europe than in those performed in Asia [USA (MD: −17.5; 95% CI: −24.4, −10.6; I2 = 97%), Europe (MD: −10.6; 95% CI: −15.1, −6.04; I2 = 98%), Asia (MD: −9.51; 95% CI: −18.9, −0.41; I2 = 97%)]. Compared with healthy individuals, patients with underlying diseases had a greater effect size {CVD [MD: −16.9; 95% CI: −21.7, −12.1; I2 = 96%], CAG [MD: −17.6; 95% CI: −19.2, −16.1; I2 = not applicable (N/A)], CKD [MD: −13.4; 95% CI: −17.5, −9.23; I2 = N/A], hypertension [MD: −4.90; 95% CI: −6.54, −3.26; I2 = N/A], DM [MD: −24.3; 95% CI: −27.9, −20.7; I2 = 55%], health status [MD: −3.30; 95% CI: −5.16, −1.44; I2 = 76%]}. This finding was consistent with the results of our cumulative analysis, which showed a progressive increase in pooled effect size according to health status in individual studies (Supplemental Figure 1; k1–k5: healthy disease-free subjects, k6–k17: patients with underlying disease). GFR decreased in participants with elevated TMAO concentrations regardless of sample size [size >2000 (MD: −12.3; 95% CI: −18.3, −6.22; I2 = 99%), size ≤2000 (MD: −13.1; 95% CI: −18.0, −8.13; I2 = 97%)]. This finding was applicable to plasma samples (MD: −14.5; 95% CI: −18.7, −10.3; I2 = 98%) but not to serum samples (MD: −5.17; 95% CI: −12.4, 2.06; I2 = 92%). We did not observe decreased GFR with elevated TMAO concentrations in cross-sectional studies (MD: −4.25; 95% CI: −12.6, 4.12; I2 = 86%), cohort studies (MD: −15.3; 95% CI: −19.6, −10.9; I2 = 99%), or the 1 case-control study (MD: −4.90; 95% CI: −6.54, −3.26; I2 = N/A). We conducted no meta-analyses of kidney function indicators other than GFR, although a general description of the indicators is provided in the characteristics table due to the small number of studies (Table 3, Supplemental Table 6).
FIGURE 4.
Meta-analysis of the GFR in subjects with high concentrations of TMAO compared with subjects with low concentrations of TMAO. In this analysis, if the original study reported circulating TMAO concentrations as quartiles or tertiles, the high concentration of TMAO included the highest layer of TMAO concentration, and the other layers were classified into the low concentration of TMAO. High category: subjects with high concentrations of TMAO. Low category: subjects with low concentrations of TMAO. GFR, glomerular filtration rate; MD, mean difference; TMAO, trimethylamine N-oxide.
FIGURE 5.
Subgroup analyses in meta-analysis of the GFR in subjects with high concentrations of TMAO compared with subjects with low concentrations of TMAO. In this analysis, if the original study reported circulating TMAO concentrations as quartiles or tertiles, the high concentration of TMAO included the highest layer of TMAO concentration, and the other layers were classified into the low concentration of TMAO. High category: subjects with high concentrations of TMAO. Low category: subjects with low concentrations of TMAO. CAG, coronary angiography; CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus; GFR, glomerular filtration rate; MD, mean difference; TMAO, trimethylamine N-oxide.
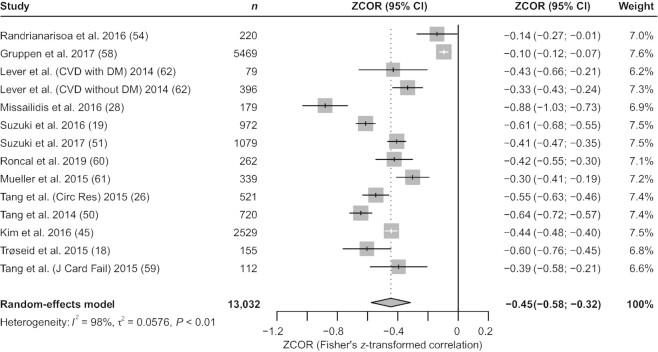
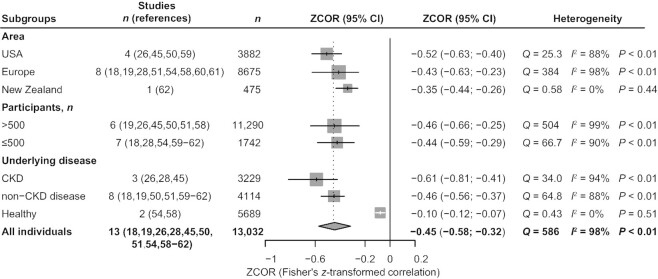
Meta-analysis of the correlations between circulating TMAO concentrations and GFR
We included 13 studies (18, 19, 26, 28, 45, 50, 51, 54, 58–62) with 13,032 participants in our analysis and synthesis of the correlation between TMAO and GFR; the study by Lever et al. (62) was included as 2 independent studies. TMAO was inversely related to GFR [Fisher's z-transformed correlation coefficient (ZCOR): −0.45; 95% CI: −0.58, −0.32; P < 0.01; I2 = 98%; Figure 6]. Meta-regression analysis revealed healthy subjects (tau2 reduced from 0.0576 to 0.0136; P < 0.0001) as the source of heterogeneity (Supplemental Table 8). Results of the subgroup analyses are shown in Figure 7. Similarly, studies conducted in the USA and Europe showed a stronger correlation between TMAO and GFR than studies conducted in other areas [USA (ZCOR: −0.52; 95% CI: −0.63, −0.40; I2 = 88%), Europe (ZCOR: −0.43; 95% CI: −0.63, −0.23; I2 = 98%), New Zealand (ZCOR: −0.35; 95% CI: −0.44, −0.26; I2 = 0%)]. The negative correlation between TMAO and GFR was significantly stronger among patients with CKD than among those with non-CKD diseases and among healthy participants [CKD (ZCOR: −0.61; 95% CI: −0.81, −0.41; I2 = 94%), non-CKD (ZCOR: −0.46; 95% CI: −0.56, −0.37; I2 = 88%), healthy subjects (ZCOR: −0.10; 95% CI: −0.12, −0.07; I2 = 0%)]. This finding was consistent with the results of cumulative analysis (Supplemental Figure 2) and with those of the above-mentioned meta-analysis of TMAO concentration comparisons. Additional subgroup analysis demonstrated that this correlation existed regardless of sample size [size >500 (ZCOR: −0.46; 95% CI: −0.66, −0.25; I2 = 99%), size ≤500 (ZCOR: −0.44; 95% CI: −0.59, −0.29; I2 = 90%)]. As only 1 study (54) had a cross-sectional design and used only serum samples, we performed no subgroup analyses of study type and sample source. TMAO concentration was positively associated with UACR, sCr, UAER, blood urea, blood uric acid, and CysC [UACR (ZCOR: 0.26; 95% CI: 0.08, 0.43; I2 = N/A), sCr (ZCOR: 0.43; 95% CI: 0.28, 0.58; I2 = 94%), UAER (ZCOR: 0.06; 95% CI: 0.04, 0.09; I2 = N/A), blood urea (ZCOR: 0.50; 95% CI: 0.29, 0.72; I2 = 96%), blood uric acid (ZCOR: 0.32; 95% CI: 0.25, 0.38; I2 = 0%), CysC (ZCOR: 0.47; 95% CI: 0.44, 0.51; I2 = 0%); all P values <0.01; Supplemental Figure 3]. Again, all indicators are generally described in the characteristics table (Table 4). No further analyses of other indicators except GFR were performed, owing to the small number of included studies.
FIGURE 6.
Meta-analysis of the correlations between circulating TMAO concentrations and GFR. CVD, cardiovascular disease; DM, diabetes mellitus; GFR, glomerular filtration rate; TMAO, trimethylamine N-oxide; ZCOR, Fisher's z-transformed correlation coefficient.
FIGURE 7.
Subgroup analyses in meta-analysis of the correlations between circulating TMAO concentrations and GFR. CKD, chronic kidney disease; GFR, glomerular filtration rate; TMAO, trimethylamine N-oxide; ZCOR, Fisher's z-transformed correlation coefficient.
Study quality assessment
We used the NOS (37) to assess the quality of the cohort and case-control studies; most of the included studies of these types were found to have moderate-to-high quality, with NOS scores of 5–9 (Supplemental Table 2). For cross-sectional studies, we used the AHRQ checklist; all included studies of this type were judged to have “good” or “fair” quality (Supplemental Table 3).
Publication bias
We examined publication bias using Begg's and Egger's regression tests. No evidence of publication bias was found in the meta-analysis of GFR in subjects with high concentrations of TMAO compared with subjects with low concentrations of TMAO (z = 0, P = 1; t = −0.886, P = 0.390; Supplemental Table 9). Publication bias was observed in the meta-analysis of correlations between circulating TMAO concentrations and GFR (z = 0.711, P = 0.477; t = −2.28, P = 0.041; Supplemental Table 9).
Discussion
This study is the first to examine the relation between circulating TMAO concentration and kidney function indicators using data from 32 studies with 42,062 participants. We extracted multiple clinical kidney function indicators from these studies and performed multiple statistical analyses to assess the association of circulating TMAO concentration with kidney function. First, we found that TMAO accumulated in all stages of CKD and increased with the aggravation of kidney function damage, especially in advanced CKD, with TMAO concentrations showing an increase of 67.9 μmol/L (95% CI: 52.7, 83.2; P < 0.01) in patients with advanced CKD compared with healthy subjects. Second, GFR decreased in subjects with high TMAO concentrations. Concentrations of sCr, UAER, and blood urea were greater in subjects with high TMAO concentrations, whereas BUN and UACR concentrations showed no significant difference between the 2 categories. For BUN, as all participants in the original study (52) were undergoing maintenance hemodialysis, no significant difference existed because the indicators were excessively elevated in both groups. For UACR, as all participants in the original study (55) were healthy individuals, this indicator showed no obvious changes; however, when TMAO concentrations were high, the changes in all other indicators suggested kidney impairment. Third, meta-analyses of correlations showed that TMAO was inversely associated with GFR and positively associated with UACR, sCr, UAER, blood urea, blood uric acid, and CysC, with respective correlation coefficients of −0.45, 0.26, 0.43, 0.06, 0.50, 0.32, and 0.47. Fourth, in subgroup analyses, patients with underlying diseases (especially CKD) had a greater effect size for the relation between circulating TMAO concentrations and kidney function, compared with healthy individuals. Cumulative meta-analyses according to healthy status also showed the impact of underlying diseases on the merger effect (Supplemental Figures 1 and 2). Taken together, these data showed a significant negative correlation between circulating TMAO concentrations and kidney function.
The mechanisms underlying the potential relation between TMAO and kidney function require further investigation. Previous studies have reported that atherosclerosis, caused by the accumulation of cholesterol-laden macrophages in the arterial wall, is the underlying cause of most CVDs and many kidney diseases (63). TMAO has been found to accelerate atherosclerotic progression in animal models via mechanisms that include decreasing reverse cholesterol transport, increasing macrophage activation, and increasing the expression of Cluster of Differentiation 36 (CD36) and scavenger receptor A (15, 64–66). Therefore, TMAO-mediated atherosclerosis could be an important cause of heart and kidney diseases (12). Kidney fibrosis is a common outcome of almost all progressive CKDs and one of the main causes of ESKD (67). Activation of Smad3, which is an important component of the transforming growth factor-β (TGF-β) signaling pathway, plays a key profibrotic role in mouse models of tissue fibrosis (68–70). Tang et al. (26) provided evidence that the TMAO pathway likely contributes to the progression of kidney disease. Their study showed that dietary exposure to either choline or TMAO led to a significant increase in TMAO concentrations and corresponding increases in tubulointerstitial fibrosis, collagen deposition, and the ratio of phosphorylated Smad3 to total Smad3. Furthermore, increased expression of kidney injury marker-1 (KIM-1) and CysC suggested causality in that experimental mouse model. In a recently published mouse CKD model (71), iodomethyl choline, the gut microbial choline trimethylamine-lyase inhibitor, suppressed choline diet-induced kidney tubulointerstitial fibrosis and profibrotic-gene expression. This implies that the TMAO pathway contributes to the progression of “fibrotic” kidney disease and that inhibition of this pathway might prevent kidney function impairment and kidney injury. Current evidence indicates that reducing circulating TMAO concentrations could be a potential therapeutic approach to the treatment of kidney impairment.
As previously reported, the circulating TMAO concentration largely depends on habitual dietary patterns or the dominant species in the intestinal microbiota of mice and humans, which was believed to have little genetic influence (72). As TMAO is a small-molecule (75.1 Da) uremic toxin, its clearance from the circulation requires good kidney function and is largely dependent on urinary excretion (17, 27). Our meta-analyses showed that health status has a key influence on the association between TMAO concentration and kidney function damage. Healthy subjects seemingly had adequate TMAO excretion and likely experienced less impact on their kidney function. However, subjects with underlying diseases, particularly CKD, likely experienced poor kidney function leading to lower TMAO excretion and accumulation, which in turn might have exacerbated kidney damage. Studies have shown that even modest impairment of kidney function, to an eGFR of 66–78 mL/(min·1.73 m2), significantly raises plasma TMAO concentration (73). Accordingly, in patients with kidney impairment, reducing the production of TMAO by adjusting dietary patterns seems to be a necessary intervention.
Choline and l-carnitine are respectively the greatest and second-greatest precursors of TMAO (15, 17, 66). According to the USDA database, which lists the choline content of common foods (74), the food richest in total choline (choline + phosphatidylcholine + phosphocholine + sphingomyelin + glycerophosphocholine) is egg (yolk: ∼600 mg/100 g; whole: ∼250 mg/100 g), followed by the livers of various animals (∼200–400 mg/100 g), instant coffee and cake (∼100 mg/100 g), red meat and fish (∼80 mg/100 g), cereal grains (∼40 mg/100 g), legumes and legume products (∼30 mg/g), vegetables and fruits (∼20 mg/100 g), milk (∼10/100 g), and fats and oils (∼5 mg/100 g). Consumption of choline-rich diets can increase circulating TMAO concentrations (15, 17, 66, 75). A linear dose-response relation exists between TMAO concentration and egg consumption: 2 eggs more than double TMAO concentrations, and even 1 egg nearly doubles the plasma TMAO concentration (76). l-carnitine, the second-greatest precursor of TMAO, is abundantly present in red meat (15). In a recent study (77) of the health effects of dietary risks, both the USA and Europe were found to be areas of high red meat consumption. In our current analysis, it seemed that pooled effect values in those 2 regions were greater than those in any other region. The increased sources of TMAO contributed by Western dietary patterns can partially explain the discrepancies in data from different areas. Given the limitation that most of the original studies were conducted in the USA and Europe, despite potential differences in dietary habits between these 2 regions, the different geographic locations of participants did not ultimately yield different results. However, previous studies have shown that TMAO concentration in both plasma and urine was greater in omnivores and lower in vegans or vegetarians (15) and that red meat consumption significantly increased plasma and urinary TMAO concentrations (75). Notably, the increase in plasma TMAO concentrations was, on average, 3-fold higher compared with either white meat or nonmeat sources (75). In that study, switching from red to white meat or to nonmeat protein markedly reduced plasma TMAO concentrations. In addition, fish and other seafoods contain large amounts of both TMAO and TMA (13, 78). For example, 100 g of cod contains >300 mg TMAO and ∼3 mg TMA, whereas 100 g of beef or egg both contain <1 mg TMAO and <0.1 mg TMA; meanwhile, fruits showed no detectable concentrations of these metabolites (78). The specific contribution of the TMAO content of food itself to human circulating TMAO concentrations is currently unknown and needs further investigation. However, in healthy young men, fish consumption yielded an increase in plasma TMAO concentrations ∼50-fold higher than that yielded by either egg or beef consumption (78). Generally, reducing the consumption of TMAO-rich and TMAO precursor-rich foods seems necessary for patients with impaired kidney function.
However, as fish consumption is well known to have cardioprotective properties (79, 80) and association with a lower risk of depression and mortality, it could be considered a healthy animal-based source of dietary protein (80). Therefore, caution is warranted when proposing dietary recommendations that restrict the consumption of fish. Furthermore, choline is a semiessential nutrient that is important for phospholipid synthesis in cell membranes, methyl metabolism, acetylcholine synthesis, and cholinergic neurotransmission in humans (74). In 1998, the US Institute of Medicine (Food and Nutrition Board) (81) estimated the adequate consumption of choline to be 550 mg/d for men and 425 mg/d for women. Therefore, foods containing choline should not be completely excluded from the diet. Taken together, these findings indicate that a more feasible way of reducing TMAO concentrations in patients with kidney impairment is to first control the consumption of l-carnitine by introducing a diet low in red meat, and then subsequently limit the intake of choline-rich foods such as egg yolk while increasing the intake of plant-based foods, which are associated with lower plasma TMAO concentrations. Additionally, choline content varies by cooking method. Raw or fried food retains more choline (74); for example, 100 g of whole egg contains 225, 251, and 273 mg of total choline when hard boiled, raw, and fried, respectively (74). Therefore, to reduce choline intake, boiling, rather than frying, can be adopted as the cooking method.
The sensitivity analyses showed that all results in this meta-analysis study were stable (Supplemental Figures 4–6). However, the variability in the range of TMAO concentrations across the studies due to different sample sources, multiple measurement methods of TMAO and kidney function indicators, and the presence of several different populations of subjects contributed to the high heterogeneity we observed. Meta-regression analyses revealed participant age and health status as the possible sources of heterogeneity in the meta-analysis of GFR comparison. Additionally, health status was also a possible source of heterogeneity in the meta-analysis of correlation coefficients.
Study limitations
Several major limitations in the current study warrant consideration. First, although we attempted to investigate the sources of heterogeneity, we failed to explain all possible heterogeneities because the inherent differences in characteristics, definitions of the included studies, and TMAO concentrations in the general population are currently unknown. Second, most study participants were from Western countries, which limited the applicability of the results to other ethnic groups such as Asians and Africans. Third, because dietary intakes were not assessed in all included studies, we failed to investigate the potential role thereof in the association between intestinal microbial metabolites and outcomes. Fourth, publication bias was observed in the meta-analysis of correlation coefficients. Despite detailed article retrieval, we failed to extract sufficient available data due to the limited number of original studies that reported correlation coefficients, which could have allowed the possibility of reporting biases. Furthermore, we extracted only r and rho. Other evaluation indicators such as regression coefficients were not included in the current analysis, which might have resulted in selection bias. Therefore, the results should be interpreted with caution and considered as hypothesis generating.
Conclusions
The results of the current study suggest that the toxin TMAO accumulated in patients with CKD, TMAO concentration was significantly greater in patients with advanced CKD, and GFR was decreased in individuals with high TMAO concentrations. Moreover, a strong inverse correlation exists between TMAO and GFR, and a positive correlation exists between TMAO and UACR, sCr, UAER, blood urea, blood uric acid, and Cys. Our meta-analyses revealed a positive association between circulating TMAO concentrations and kidney function impairment. Taken together, these findings suggest that a high circulating TMAO concentration could be an effective biomarker of kidney function impairment; however, further interventional studies with prospective longitudinal designs are needed to better understand this relation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—YZ, YX, and YL: participated in the study conception and design; YZ, MG, and XF: performed the systematic literature search and extracted the data; YZ, MG, and XT: analyzed the data; YZ, XL, and MW: checked for statistical consistency; YZ, MG, and XF contributed to data interpretation and drafted the manuscript; FT: conducted the statistical review; YX and YL: critically reviewed the manuscript and assumed primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
This work was supported by grants from Luzhou-Southwest Medical University Cooperation Project (No. 2018LZXNYD-PT01) and Sichuan Science and Technology Program (Nos. 2019YFS0537 and 2020YFS0456).
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–9 and Supplemental Figures 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
YZ and MG contributed equally to this work.
Abbreviations used: AHRQ, Agency for Healthcare Research and Quality; BUN, blood urea nitrogen; CAG, coronary angiography; CKD, chronic kidney disease; CVD, cardiovascular disease; CysC, serum cystatin C; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; GFR, glomerular filtration rate; MD, mean difference; N/A, not applicable; NOS, Newcastle–Ottawa Scale; r, Pearson correlation coefficient; rho, Spearman rank correlation coefficient; sCr, serum creatinine; TMA, trimethylamine; TMAO, trimethylamine N-oxide; UACR, urine albumin-to-creatinine ratio; UAER, urine albumin excretion rate; ZCOR, Fisher's z-transformed correlation coefficient.
Contributor Information
Yan Zeng, Department of Endocrinology and Metabolism, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Luzhou Key Laboratory of Cardiovascular and Metabolic Diseases, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Sichuan Clinical Research Center for Nephropathy, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China.
Man Guo, Department of Endocrinology and Metabolism, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Luzhou Key Laboratory of Cardiovascular and Metabolic Diseases, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Sichuan Clinical Research Center for Nephropathy, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China.
Xia Fang, Department of Endocrinology and Metabolism, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Luzhou Key Laboratory of Cardiovascular and Metabolic Diseases, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Sichuan Clinical Research Center for Nephropathy, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China.
Fangyuan Teng, Department of Endocrinology and Metabolism, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Luzhou Key Laboratory of Cardiovascular and Metabolic Diseases, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Sichuan Clinical Research Center for Nephropathy, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Experimental Medicine Center, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China.
Xiaozhen Tan, Department of Endocrinology and Metabolism, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Luzhou Key Laboratory of Cardiovascular and Metabolic Diseases, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Sichuan Clinical Research Center for Nephropathy, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China.
Xinyue Li, Department of Endocrinology and Metabolism, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Luzhou Key Laboratory of Cardiovascular and Metabolic Diseases, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Sichuan Clinical Research Center for Nephropathy, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China.
Mei Wang, Department of Endocrinology and Metabolism, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Luzhou Key Laboratory of Cardiovascular and Metabolic Diseases, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Sichuan Clinical Research Center for Nephropathy, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China.
Yang Long, Department of Endocrinology and Metabolism, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Luzhou Key Laboratory of Cardiovascular and Metabolic Diseases, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Sichuan Clinical Research Center for Nephropathy, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Experimental Medicine Center, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China.
Yong Xu, Department of Endocrinology and Metabolism, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Luzhou Key Laboratory of Cardiovascular and Metabolic Diseases, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China; Sichuan Clinical Research Center for Nephropathy, the Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China.
References
- 1. Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–79. [DOI] [PubMed] [Google Scholar]
- 3. Graf D, Di Cagno R, Fåk F, Flint HJ, Nyman M, Saarela M, Watzl B. Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis. 2015;26:26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron Net al. . Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–8. [DOI] [PubMed] [Google Scholar]
- 5. Sun C-Y, Chang S-C, Wu M-S. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS One. 2012;7(3):e34026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watanabe H, Miyamoto Y, Honda D, Tanaka H, Wu Q, Endo M, Noguchi T, Kadowaki D, Ishima Y, Kotani Set al. . p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int. 2013;83(4):582–92. [DOI] [PubMed] [Google Scholar]
- 7. Sun C-Y, Chang S-C, Wu M-S. Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int. 2012;81(7):640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meijers BKI, Claes K, Bammens B, de Loor H, Viaene L, Verbeke K, Kuypers D, Vanrenterghem Y, Evenepoel P. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. CJASN. 2010;5(7):1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJet al. . Kidney disease as a risk factor for development of cardiovascular disease. Circulation. 2003;108(17):2154–69. [DOI] [PubMed] [Google Scholar]
- 10. Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GWD, Muntner Pet al. . Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. The Lancet Diabetes & Endocrinology. 2015;3(7):514–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chronic Kidney Disease Collaboration GBD. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomlinson JAP, Wheeler DC. The role of trimethylamine N-oxide as a mediator of cardiovascular complications in chronic kidney disease. Kidney Int. 2017;92(4):809–15. [DOI] [PubMed] [Google Scholar]
- 13. De La Huerga J, Popper H, Steigmann F. Urinary excretion of choline and trimethylamines after intravenous administration of choline in liver diseases. J Lab Clin Med. 1951;38(6):904–10. [PubMed] [Google Scholar]
- 14. Bell JD, Lee JA, Lee HA, Sadler PJ, Wilkie DR, Woodham RH. Nuclear magnetic resonance studies of blood plasma and urine from subjects with chronic renal failure: identification of trimethylamine-N-oxide. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1991;1096(2):101–7. [DOI] [PubMed] [Google Scholar]
- 15. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li Let al. . Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hazen SL, Brown JM. Eggs as a dietary source for gut microbial production of trimethylamine-N-oxide. Am J Clin Nutr. 2014;100(3):741–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trøseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, Aakhus S, Gude E, Bjørndal B, Halvorsen Bet al. . Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277(6):717–26. [DOI] [PubMed] [Google Scholar]
- 19. Suzuki T, Heaney LM, Bhandari SS, Jones DJL, Ng LL. Trimethylamine N-oxide and prognosis in acute heart failure. Heart. 2016;102(11):841–8. [DOI] [PubMed] [Google Scholar]
- 20. Svingen GFT, Zuo H, Ueland PM, Seifert R, Løland KH, Pedersen ER, Schuster PM, Karlsson T, Tell GS, Schartum-Hansen Het al. . Increased plasma trimethylamine-N-oxide is associated with incident atrial fibrillation. Int J Cardiol. 2018;267:100–6. [DOI] [PubMed] [Google Scholar]
- 21. Nie J, Xie L, Zhao BX, Li Y, Qiu B, Zhu F, Li GF, He M, Wang Y, Wang Bet al. . Serum trimethylamine N-oxide concentration is positively associated with first stroke in hypertensive patients. Stroke. 2018;49(9):2021–8. [DOI] [PubMed] [Google Scholar]
- 22. Dambrova M, Latkovskis G, Kuka J, Strele I, Konrade I, Grinberga S, Hartmane D, Pugovics O, Erglis A, Liepinsh E. Diabetes is associated with higher trimethylamine N-oxide plasma levels. Exp Clin Endocrinol Diabetes. 2016;124(4):251–6. [DOI] [PubMed] [Google Scholar]
- 23. Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, Yang Q, Cheng S, Pierce K, Deik Aet al. . A combined epidemiologic and metabolomic approach improves CKD prediction. JASN. 2013;24(8):1330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hai X, Landeras V, Dobre MA, DeOreo P, Meyer TW, Hostetter TH. Mechanism of prominent trimethylamine oxide (TMAO) accumulation in hemodialysis patients. PLoS One. 2015;10(12):e0143731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Al-waiz M, Mitchell SC, Idle JR, Smith RL. The metabolism of 14C-labelled trimethylamine and its N-oxide in man. Xenobiotica. 1987;17(5):551–8. [DOI] [PubMed] [Google Scholar]
- 26. Tang WHW, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TDet al. . Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. JASN. 2016;27(1):305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Missailidis C, Hällqvist J, Qureshi AR, Barany P, Heimbürger O, Lindholm B, Stenvinkel P, Bergman P. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. 2016;11(1):e0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant. 2006;21(5):1300–4. [DOI] [PubMed] [Google Scholar]
- 30. Mafune A, Iwamoto T, Tsutsumi Y, Nakashima A, Yamamoto I, Yokoyama K, Yokoo T, Urashima M. Associations among serum trimethylamine-N-oxide (TMAO) levels, kidney function and infarcted coronary artery number in patients undergoing cardiovascular surgery: a cross-sectional study. Clin Exp Nephrol. 2016;20(5):731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pelletier CC, Croyal M, Ene L, Aguesse A, Billon-Crossouard S, Krempf M, Lemoine S, Guebre-Egziabher F, Juillard L, Soulage CO. Elevation of trimethylamine-N-oxide in chronic kidney disease: contribution of decreased glomerular filtration rate. Toxins. 2019;11(11):635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaysen GA, Johansen KL, Chertow GM, Dalrymple LS, Kornak J, Grimes B, Dwyer T, Chassy AW, Fiehn O. Associations of trimethylamine N-oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J Ren Nutr. 2015;25(4):351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bogiatzi C, Gloor G, Allen-Vercoe E, Reid G, Wong RG, Urquhart BL, Dinculescu V, Ruetz KN, Velenosi TJ, Pignanelli Met al. . Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis. 2018;273:91–7. [DOI] [PubMed] [Google Scholar]
- 34. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JPA, Straus S, Thorlund K, Jansen JPet al. . The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. [DOI] [PubMed] [Google Scholar]
- 35. KDIGO . KDIGO 2012 Clinical Practice Guideline for the evaluation and management of chronic kidney disease. [Internet]. New York (NY): KDIGO; 2013. [Cited 2020 Dec 31]. Available from: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf. [DOI] [PubMed] [Google Scholar]
- 36. Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313(8):837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Internet]. Ottawa: Ottawa Hospital Research Institute; 2000. [Cited 2020 Dec 31]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 38. Rostom A, Dubé C, Cranney A, Saloojee N, Sy R, Garritty C, Sampson M, Zhang L, Yazdi F, Mamaladze Vet al. . Celiac disease (evidence reports/technology assessments, No. 104). Appendix D. Quality assessment forms. [Internet]. Rockville (MD): Agency for Healthcare Research and Quality; 2004. [Cited 2020 Dec 31]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK35156/. [PMC free article] [PubMed] [Google Scholar]
- 39. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tsiligianni I, Kocks J, Tzanakis N, Siafakas N, van der Molen T. Factors that influence disease-specific quality of life or health status in patients with COPD: a review and meta-analysis of Pearson correlations. Prim Care Respir J. 2011;20(3):257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cooper H, Hedges LV, editors. Handbook of research synthesis. New York (NY): Russell Sage Foundation; 1994. [Google Scholar]
- 42. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009. [Google Scholar]
- 43. Rupinski MT, Dunlap WP. Approximating Pearson product-moment correlations from Kendall's Tau and Spearman's Rho. Educational and Psychological Measurement. 1996;56(3):419–29. [Google Scholar]
- 44. Xu K-Y, Xia G-H, Lu J-Q, Chen M-X, Zhen X, Wang S, You C, Nie J, Zhou H-W, Yin J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci Rep. 2017;7:1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim RB, Morse BL, Djurdjev O, Tang M, Muirhead N, Barrett B, Holmes DT, Madore F, Clase CM, Rigatto Cet al. . Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016;89(5):1144–52. [DOI] [PubMed] [Google Scholar]
- 46. Al-Obaide MAI, Singh R, Datta P, Rewers-Felkins KA, Salguero MV, Al-Obaidi I, Kottapalli KR, Vasylyeva TL. Gut microbiota-dependent trimethylamine-N-oxide and serum biomarkers in patients with T2DM and advanced CKD. JCM. 2017;6(9):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhou X, Jin M, Liu L, Yu Z, Lu X, Zhang H. Trimethylamine N-oxide and cardiovascular outcomes in patients with chronic heart failure after myocardial infarction. ESC Heart Fail. 2020;7(1):188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Winther SA, Øllgaard JC, Tofte N, Tarnow L, Wang Z, Ahluwalia TS, Jorsal A, Theilade S, Parving H-H, Hansen TWet al. . Utility of plasma concentration of trimethylamine N-oxide in predicting cardiovascular and renal complications in individuals with type 1 diabetes. Dia Care. 2019;42(8):1512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tang WHW, Wang Z, Li XS, Fan Y, Li DS, Wu Y, Hazen SL. Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. 2017;63(1):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tang WHW, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64(18):1908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suzuki T, Heaney LM, Jones DJL, Ng LL. Trimethylamine N-oxide and risk stratification after acute myocardial infarction. Clin Chem. 2017;63(1):420–8. [DOI] [PubMed] [Google Scholar]
- 52. Stubbs JR, Stedman MR, Liu S, Long J, Franchetti Y, West RE, Prokopienko AJ, Mahnken JD, Chertow GM, Nolin TD. Trimethylamine N-oxide and cardiovascular outcomes in patients with ESKD receiving maintenance hemodialysis. CJASN. 2019;14(2):261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Senthong V, Wang Z, Li XS, Fan Y, Wu Y, Tang WHW, Hazen SL. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. JAHA. 2016;5(6):e002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Randrianarisoa E, Lehn-Stefan A, Wang X, Hoene M, Peter A, Heinzmann SS, Zhao X, Königsrainer I, Königsrainer A, Balletshofer Bet al. . Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci Rep. 2016;6:26745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meyer KA, Benton TZ, Bennett BJ, Jacobs DR Jr, Lloyd-Jones DM, Gross MD, Carr JJ, Gordon-Larsen P, Zeisel SH. Microbiota-dependent metabolite trimethylamine N-oxide and coronary artery calcium in the coronary artery risk development in young adults study (CARDIA). JAHA. 2016;5(10):e003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Matsuzawa Y, Nakahashi H, Konishi M, Sato R, Kawashima C, Kikuchi S, Akiyama E, Iwahashi N, Maejima N, Okada Ket al. . Microbiota-derived trimethylamine N-oxide predicts cardiovascular risk after STEMI. Sci Rep. 2019;9:11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Krüger R, Merz B, Rist MJ, Ferrario PG, Bub A, Kulling SE, Watzl B. Associations of current diet with plasma and urine TMAO in the KarMeN study: direct and indirect contributions. Mol Nutr Food Res. 2017;61(11):1700363. [DOI] [PubMed] [Google Scholar]
- 58. Gruppen EG, Garcia E, Connelly MA, Jeyarajah EJ, Otvos JD, Bakker SJL, Dullaart RPF. TMAO is associated with mortality: impact of modestly impaired renal function. Sci Rep. 2017;7:13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tang WHW, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, Klein AL, Hazen SL. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21(2):91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roncal C, Martínez-Aguilar E, Orbe J, Ravassa S, Fernandez-Montero A, Saenz-Pipaon G, Ugarte A, Estella-Hermoso de Mendoza A, Rodriguez JA, Fernández-Alonso Set al. . Trimethylamine-N-oxide (TMAO) predicts cardiovascular mortality in peripheral artery disease. Sci Rep. 2019;9:15580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mueller DM, Allenspach M, Othman A, Saely CH, Muendlein A, Vonbank A, Drexel H, von Eckardstein A. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis. 2015;243(2):638–44. [DOI] [PubMed] [Google Scholar]
- 62. Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, McEntyre CJ, Elmslie JL, Atkinson W, Molyneux SLet al. . Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS One. 2014;9(12):e114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-Pet al. . Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–360. [DOI] [PubMed] [Google Scholar]
- 64. Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki Tet al. . A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386(6622):292–6. [DOI] [PubMed] [Google Scholar]
- 65. Matsumoto Y, Sato M, Ohashi H, Araki H, Tadokoro M, Osumi Y, Ito H, Morita H, Amano I. Effects of L-carnitine supplementation on cardiac morbidity in hemodialyzed patients. Am J Nephrol. 2000;20(3):201–7. [DOI] [PubMed] [Google Scholar]
- 66. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung Y-Met al. . Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang P, Luo M-L, Song E, Zhou Z, Ma T, Wang J, Jia N, Wang G, Nie S, Liu Yet al. . Long noncoding RNA lnc-TSI inhibits renal fibrogenesis by negatively regulating the TGF-β/Smad3 pathway. Sci Transl Med. 2018;10(462):eaat2039. [DOI] [PubMed] [Google Scholar]
- 68. Zimmerman CM, Padgett RW. Transforming growth factor β signaling mediators and modulators. Gene. 2000;249(1–2):17–30. [DOI] [PubMed] [Google Scholar]
- 69. Qu X, Li X, Zheng Y, Ren Y, Puelles VG, Caruana G, Nikolic-Paterson DJ, Li J. Regulation of renal fibrosis by Smad3 Thr388 phosphorylation. Am J Pathol. 2014;184(4):944–52. [DOI] [PubMed] [Google Scholar]
- 70. Kretzschmar M, Massagué J. SMADs: mediators and regulators of TGF-β signaling. Current Opinion in Genetics & Development. 1998;8(1):103–11. [DOI] [PubMed] [Google Scholar]
- 71. Gupta N, Buffa JA, Roberts AB, Sangwan N, Skye SM, Li L, Ho KJ, Varga J, DiDonato JA, Tang WHWet al. . Targeted inhibition of gut microbial trimethylamine N-oxide production reduces renal tubulointerstitial fibrosis and functional impairment in a murine model of chronic kidney disease. ATVB. 2020;40(5):1239–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hartiala J, Bennett BJ, Tang WHW, Wang Z, Stewart AFR, Roberts R, McPherson R, Lusis AJ, Hazen SL, Allayee Het al. . Comparative genome-wide association studies in mice and humans for trimethylamine N-oxide, a proatherogenic metabolite of choline and L-carnitine. Arterioscler Thromb Vasc Biol. 2014;34(6):1307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pignanelli M, Bogiatzi C, Gloor G, Allen-Vercoe E, Reid G, Urquhart BL, Ruetz KN, Velenosi TJ, Spence JD. Moderate renal impairment and toxic metabolites produced by the intestinal microbiome: dietary implications. J Ren Nutr. 2019;29(1):55–64. [DOI] [PubMed] [Google Scholar]
- 74. Patterson KY, Bhagwat S, Williams JR, Howe JC, Holden JM, Zeisel SH, Dacosta KA, Mar M-H. USDA database for the choline content of common foods, release 2. Beltsville (MD): USDA; 2008. 10.15482/USDA.ADC/1178141. [DOI] [Google Scholar]
- 75. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHWet al. . Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40(7):583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Miller CA, Corbin KD, da Costa K-A, Zhang S, Zhao X, Galanko JA, Blevins T, Bennett BJ, O'Connor A, Zeisel SH. Effect of egg ingestion on trimethylamine-N-oxide production in humans: a randomized, controlled, dose-response study. Am J Clin Nutr. 2014;100(3):778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. GDB 2017 Diet Collaborators . Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet North Am Ed. 2019;393(10184):1958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J, Sutter JL, Caudill MA. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. 2017;61(1):1600324. [DOI] [PubMed] [Google Scholar]
- 79. Galli C, Risé P. Fish consumption, omega 3 fatty acids and cardiovascular disease: the science and the clinical trials. Nutr Health. 2009;20(1):11–20. [DOI] [PubMed] [Google Scholar]
- 80. Jayedi A, Shab-Bidar S. Fish consumption and the risk of chronic disease: an umbrella review of meta-analyses of prospective cohort studies. Adv Nutr. 2020;11(5):1123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Institute of Medicine . Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press; 1998. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.