ABSTRACT
Osteoporosis is a global health issue among the aging population. The effect of the acid or base interventions on bone health remains controversial. This study performed a systematic review and meta-analysis to investigate effects of acidic diets and alkaline supplements on bone health simultaneously. We conducted a comprehensive literature search in 5 available databases and 1 registered clinical trial system to identify randomized controlled trials (RCTs) that assessed effects of the acid-base intervention on bone health. Depending on heterogeneity across studies, the pooled effects were calculated by fixed-effects or random-effects models. The present study included 13 acidic diet intervention studies and 13 alkaline supplement studies for final quantitative assessments. The meta-analysis showed that acidic diets significantly increased net acid excretion [NAE; standardized mean difference (SMD) = 2.99; P = 0.003] and urinary calcium excretion (SMD = 0.47, P < 0.00001) but had no significant effect on bone turnover markers and bone mineral density (BMD). On the other hand, alkaline supplement intervention significantly reduced NAE (SMD = −1.29, P < 0.00001), urinary calcium excretion (SMD = −0.44, P = 0.007), bone resorption marker aminoterminal cross-linking telopeptide (NTX; SMD = −0.29, P = 0.003), and bone formation marker osteocalcin (OC; SMD = −0.23, P = 0.02), but did not affect the other bone turnover markers. Furthermore, alkaline supplements significantly increased BMD in femoral neck [mean difference (MD) = 1.62, P < 0.00001, I2 = 0%], lumbar spine (MD = 1.66, P < 0.00001, I2 = 87%), and total hip (MD = 0.98, P = 0.02, I2 = 99%). Subsequently, meta-regression analyses identified 1 study that substantially contributed to the high heterogeneity of BMD in the latter 2 sites, but sensitivity analysis suggested that this study did not affect the significant pooled effects. Despite that, the results should be interpreted with caution and need to be further validated by a larger RCT. In summary, through integrating evidence from RCTs, the present meta-analysis initially suggests that alkaline supplements may be beneficial to bone metabolism and acidic diets may not be harmful to bone health. This work may be clinically useful for both clinicians and patients with osteoporosis.
Keywords: acidic diets, alkaline supplements, bone mineral density, bone turnover markers, bone health
Introduction
Osteoporosis is an important health problem for the aging population (1, 2). With increasing age, the body's ability to maintain blood pH declines. The most likely cause of this situation is that kidney function declines with age, thereby reducing the kidney's ability to excrete noncarbonic acid. In the past, high protein intake was thought to induce chronic metabolic acidosis, which may lead to hypercalcemia and accelerated mineral dissolution, thereby damaging bone health (3). Acid-producing foods (cereals or animal proteins) are rich in compounds that contain sulfuric acid, hydrochloric acid, phosphoric acid, or nitric acid residues. These compounds could increase the net acid content in the body and could only be excreted through the urine of the kidneys (4). Due to the limited acidification ability of the kidneys, a large amount of nonoxidizable dietary acids need to be neutralized by blood and extracellular pH buffering agents, such as the alkali released from the bone matrix. Therefore, acidic diets may cause the loss of bone matrix (5). Slight metabolic acidosis can reduce the reabsorption of calcium by the renal tubules, and also strongly stimulate the activity of osteoclasts and inhibit the activity of osteoblasts (6). A 60-d human acidic diet intervention study suggested that elevated renal net acid excretion (NAE) was associated with increased serum parathyroid hormone (PTH), bone resorption, and calcium losses (7).
Accumulating evidence implied that adequate protein intake may be beneficial to bone health via providing substantial amino acids, increasing calcium absorption (8) and improving circulating insulin-like growth factor I (IGF-I) (9), which could improve bone metabolism for the elderly. Moreover, most studies in elderly subjects showed that relatively high protein intake was associated with a reduction in bone loss (10) and hip fracture risk (11). However, several randomized controlled diet studies demonstrated that high-protein, acid-producing diets have no significant effect on bone turnover markers and bone mineral density (BMD) (12, 13).
On the other hand, whether supplementing alkali salts can promote bone health by reducing renal acid excretion and bone calcium loss is a clinically meaningful question but without conclusive evidence. A previous meta-analysis by Lambert et al. (14) suggested that supplementation with oral alkaline potassium can reduce renal calcium loss and bone resorption but has no significant effect on BMD. Darling et al. (15) conducted a meta-analysis studying the effect of protein supplementation on bone metabolism, and they did not find a significant effect of any form of protein on bone health. In addition, they included studies without a control group for confounding factors, which may overestimate or underestimate the effect of the diet intervention on the outcome indicators. Those confounding factors mainly include consuming both alkaline and high-salt diets or diuretics at the same time (16, 17) and exercising and losing weight (18, 19).
Although several relevant review articles have been published, these studies (1, 6, 14) only conducted narrative reviews or investigated the effect of acidic diets or alkaline supplements alone on bone health. Moreover, they may include low-quality studies, which refer to articles that contain some confounding factors (such as studying obese patients and restricting calorie intake) that may exert an adverse effect on the pooled effects. The present study performed a comprehensive literature search to select those high-quality randomized controlled trials (RCTs) only for meta-analysis. Meanwhile, this study also excluded those reports involving supplements in combination with other forms of diet or drugs, as well as those studies with changes in nutrients that affect bone metabolism and those studies on specific protein types. These 2 strategies could substantially avoid the adverse effect from confounding factors. In addition, the current study carried out meta-analyses of the effect of acidic diets and alkaline supplements on bone health simultaneously. With regard to outcome indicators, this study not only explored the effect on bone metabolism markers but also on BMD at 2 available body sites.
Methods
Search strategy
As of May 2020, we systematically searched through 5 mainstream databases: PubMed, Embase, Web of Science, Scopus, and Cochrane Library. To avoid missing any other relevant studies, we also searched other sources (Figure 1), including conference proceedings, reference lists of relevant reviews, and 1 clinical trial registration system (http://www.ClinicalTrials.gov). The literature search was limited to English-language articles. We searched for the effect of the acid-base intervention on bone metabolism in 2 parts. The electronic search strategy for acidic diets on bone health was “acid” or “protein” and “diet” and “bone” or “osteoporosis” or “bone mineral density” or “bone turnover marker” or “fracture.” For the other part, the search strategy for the effect of alkali salts supplements on bone health was “alkali” or “potassium citrate” or “potassium bicarbonate” and “bone” or “osteoporosis” or “bone mineral density” or “bone turnover marker” or “fracture.” All of the studies were limited through “randomized clinical trials” and “human.” Two authors independently screened whether the articles met the inclusion criteria. The screening was done by reviewing the titles and abstracts of the included articles (Figure 1). After that, the full texts were assessed for inclusion as the final eligible articles. The authors discussed and resolved inconsistencies to reach consensus, and finally identified potentially included articles.
FIGURE 1.
The PRISMA flow diagram for selection procedures of studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Eligibility criteria
Inclusion criteria
The inclusion criteria were as follows:
RCTs (including parallel or crossover design).
Only adult studies were included.
The intervention was an acidic diet or high-protein diet or alkaline supplement. For the intervention of high-protein diets, when the protein intake exceeds the current RDA at 0.8 g protein / kg of body weight, it was considered as a study of acid intervention. (1). The wide definition of acidic diets allows different types of food to be studied in the included articles. For example, some studies defined high-protein diet as acidic diet, while other studies did not emphasize protein intake volume but defined acidic or nonacidic diets by comparing the NAE between the intervention group and control group.
Outcome indicators were urinary calcium excretion, NAE, bone resorption and formation markers, bone mineral density, fractures.
The included subjects had no previous history of bone disease, kidney disease, and other medical illnesses, and did not used drugs for osteoporosis.
Exclusion criteria
The exclusion criteria were as follows:
Nonoriginal article (narrative reviews, editorials).
Nonhuman research.
Cell research.
Research without control groups.
Subjects in a state that may alter the effect of exposure on outcomes (e.g., pregnancy, breastfeeding women, weight loss, bed rest).
Supplements are used in combination with other forms of diet or drugs (such as high salt intake or diuretic administration, taking birth control pills).
The experimental and control groups in the study also included changes in nutrients (such as calcium, sodium, potassium, magnesium, and/or phosphate) that would affect bone metabolism.
Research on specific protein types (such as isoflavones in soy protein, which have estrogen effects) that have potential to affect bone metabolism.
Raw data cannot be obtained.
Data extraction
One researcher extracted data from all studies and another researcher reviewed and confirmed the data. Information extracted from eligible studies included the following: first author, year of publication, study design (parallel and crossover experiments), age range of participants, interventions, exposure assessment, and outcome indicators. For studies using different doses of supplements, the results of the highest dose were extracted; for studies measuring results at multiple time points, data from the final time point were used. If studies had ≥1 common intervention groups, we only selected 2 groups that met the inclusion criteria for analysis. Primary outcome indicators were reported as measured values after intervention or differences in changes from baseline (also called a change score). Both the final value and the change score were included in the analysis. For those data displayed in the article as a figure, we used professional software to obtain an estimate of the original data (GetData Graph Digitizer version 2.26; http://getdata-graph-digitizer.com/index.php). The outcome indicators included the mean, SD, and number of participants for the 2 compared groups.
Quality analysis
The quality of the included articles was evaluated by 2 independent authors based on Cochrane Collaboration standards. Disagreements were resolved through discussion or consultation with a third author. The standards contain 6 aspects: random-sequence generation, allocation concealment, blind evaluation of results, incomplete results data, selective reports, and other sources of bias.
Meta-analysis
Review Manager (version 5; Cochrane Collaboration) was used for all statistical analyses, and P values <0.05 were considered to be statistically significant. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (20). Results were pooled using the inverse variance method. If the measurement unit of each study is different across studies, results were displayed as standardized mean difference (SMD). Alternatively, results were showed as mean difference (MD). All reported CIs of MD and SMD were 95% limits. Higgins et al. (21) suggested that I2 values of 25%, 50%, and 75% stand for low, moderate, and high thresholds of heterogeneity, respectively. As reported, a meta-analysis with little heterogeneity indicated little variability between studies that cannot be explained by chance. Moreover, a meta-analysis with a low heterogeneity (25% ≤ I2 < 50%) implied that the variability had only a small effect. Therefore, the meta-analysis was initially performed within a fixed-effects framework for those studies without heterogeneity or with a low heterogeneity, while a random-effects framework was instead used in case of a moderate or high heterogeneity.
Publication bias and heterogeneity analysis
Sensitivity analysis was performed via omitting 1 study each time from the overall analysis using the “meta” package in R programming language (R Foundation for Statistical Computing) (22). Funnel plots were generated to assess whether there was any potential publication bias. Moreover, Egger's test was applied to determine the extent of publication bias (23). P values >0.1 indicated no publication bias.
According to the recommendations of the Cochrane System Intervention Review Manual, a subgroup analysis of outcome indicators could be performed to find heterogeneous sources if the overall analysis included >8 studies. Meta-regression analysis was used to investigate potential sources of heterogeneity if moderate or high heterogeneity was present in meta-analysis. In the analysis, we also used the method by Hartung and Knapp to adjust test statistics and CIs (24). Moreover, a Baujat plot was plotted to estimate each study's contribution to the overall heterogeneity across studies.
Results
Summary of included studies
The PRISMA flowchart shows the main research process for selecting eligible studies for meta-analysis (Figure 1). Finally, a total of 26 studies were screened out for final quantitative synthesis from the initially retrieved 2159 articles. Among those, 13 acidic diet interventions were included (Table 1), of which 5 used a parallel design (7, 12, 13, 25, 26) and 8 used a crossover design (4, 27–33). Two of the 13 studies enrolled postmenopausal female subjects (Table 1); some of the subjects in 1 study were postmenopausal subjects. Additionally, the intervention duration in 3 studies was ≥6 mo. The alkali-salt supplements intervention also included 13 studies (Table 2), of which 10 followed a parallel design (34–43) and 3 followed a crossover design (44–46). In addition, 6 studies enrolled postmenopausal female subjects (Table 2) and 7 studies had an intervention duration >6 mo.
TABLE 1.
Included randomized controlled trials for studying the effect of acidic diet on bone health1
| Cochrane risk-of-bias assessment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year (reference) | Design | Participants | Intervention | Diet period (washout)2 | Exposure quantified | Outcomes | Sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective outcome reporting | Other sources of bias |
| Jajoo, 2006 (7) | Parallel | 40 Healthy older men and women (age >50 y) | Cereal or fruit + vegetable | 60 d | NAE, renal titratable acidity | Calcium excretion and BTM | Unclear | Unclear | Low | Low | Low | Low |
| Bulló, 2009 (25) | Parallel | 238 Old men and women (age 60–80 y) | Mediterranean diet with mixed nuts or control diet | 12 mo | NEAP, PRAL | Calcium excretion and BTM | Low | Unclear | Low | Low | High | Low |
| Cao, 2011 (30) | Crossover | 16 Postmenopausal women (age 40–75 y) | High protein (118 g/d) or low protein (61 g/d) | 7 wk (1 wk) | PRAL | Calcium excretion and BTM | Low | Unclear | High | Low | Low | Low |
| Kerstetter, 2015 (12) | Parallel | 208 Older women and men (age >60 y) | 45 g/d Whey protein or 45 g/d isocaloric maltodextrin | 18 mo | g Protein | Calcium excretion and BTM and BMD | Low | Low | Low | Low | High | Low |
| Zhu, 2011 (26) | Parallel | 219 Healthy women (age 70–80 y) | High-protein drink (30 g/d whey protein) or placebo drink | 2 y | g Protein | Calcium excretion and BTM and BMD | Low | Low | Low | Low | Low | Low |
| Cao, 2014 (13) | Parallel | 39 Healthy young adults (age 20–22 y) | Protein (2.4 g/kg/d) or protein (0.8 g/kg/d) | 31 d | g Protein, PRAL | Calcium excretion and BTM and BMD | Unclear | Unclear | High | Low | Low | Low |
| Roughead, 2003 (31) | Crossover | 15 Postmenopausal women (age 50–75 y) | High meat (20% of energy) or low meat (12% of energy) | 8 wk (4 wk) | Renal titratable acidity, urine PH | Calcium excretion and BTM | Low | Unclear | High | Low | Low | Low |
| Buclin, 2001 (3) | Crossover | 8 Healthy male volunteers (age 22–31 y) | Diet A (acid-forming nutrients) or diet B (base-forming nutrients) | 4 d (3 d) | Urine PH | Calcium excretion and BTM | Low | Unclear | High | Low | Low | Low |
| Kerstetter, 2005 (32) | Crossover | 13 Healthy women (age 20–40 y) | High protein (2.1 g/kg/d) or moderate protein (1.0 g/kg/d) | 10 d (2 wk) | g Protein, NAE | Calcium excretion and BTM | Low | Unclear | High | Low | Low | Low |
| Kerstetter, 2006 (33) | Crossover | 20 Women (12 young, 20–38 y;8 postmenopausual, 53–66 y) | High-protein meat-based or low-protein meat-based | 4 d (2 wk) | g Protein, NAE | Calcium excretion and BTM | Low | Unclear | High | Low | Low | Low |
| Dahl, 1995 (28) | Crossover | 9 Healthy men (age 19–38 y) | Control diet plus 130 g/d dry lentils or control diet | 3 wk (1 wk) | g Protein, NAE | Calcium excretion | Low | Unclear | High | Low | Low | Low |
| Kerstetter, 1999 (29) | Crossover | 16 Healthy women (age 20–40 y) | High protein (2.1 g/kg/d) or low protein (0.7 g/kg/d) | 4 d (2 wk) | g Protein | Calcium excretion and BTM | Low | Unclear | High | Low | Low | Low |
| Breslau, 1988 (27) | Crossover | 15 women and men (age 23–46 yr) | Animal protein or vegetarian protein | 6 d (6 d) | NAE, urine pH | Calcium excretion | Low | Unclear | High | Low | Low | Low |
BMD, bone mineral density; BTM, bone turnover marker; crossover, random crossover study; NAE, net acid excretion; NEAP, net endogenous acid; parallel, random parallel trial; PRAL, potential renal acid load.
“period” indicates diet intervention time (washout period).
TABLE 2.
Included randomized controlled trials for studying the effect of alkaline supplements on bone health1
| Cochrane risk-of-bias assessment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year (reference) | Design | Participants | Intervention | Diet period (washout)2 | Exposure quantified | Outcomes | Sequence generation | Allocation concealment | Blinding | Selective outcome reporting | Other sources of bias |
| Macdonald, 2008 (34) | Parallel | 188 Postmenopausal women (aged 55–65 y) | K+ citrate (55.5 mEq/d) or placebo | 2 y | NEAP, urinary pH | BMD, urinary calcium, BTM | Low | Unclear | Low | Low | Low |
| Jehle, 2006 (42) | Parallel | 161 Postmenopausal women (age <70 y) | K+ citrate (30 mEq/d) or K+ chloride (30 mEq/d) | 1 y | NAE | Calcium excretion, BMD, BTM | Low | Low | Low | Low | Low |
| Granchi, 2018 (36) | Parallel | 40 Postmenopausal women (age >55 y) | K+ citrate (30 mEq/d) or placebo | 6 mo | PRAL, urinary pH | Calcium excretion, BTM | Low | Low | Low | Low | Low |
| Gregory, 2015 (38) | Parallel | 83 Postmenopausal women (age 65.6 ± 6.5 y) | K+ citrate (40 mEq/d) or placebo | 1 y | Urinary pH | Calcium excretion, BMD, BTM | Low | Unclear | Low | Unclear | Low |
| Sakhaee, 2005 (45) | Crossover | 18 Postmenopausal women (age 48–76 y) | K+ citrate (40 mmol/d) or placebo | 2 wk (2 wk) | Urinary pH | Calcium excretion, BTM | Low | Low | Low | Low | Low |
| Jehle, 2013 (35) | Parallel | 201 Healthy men and women (>65 y) | K+ citrate (60 mEq/d) or placebo | 1 y | NAE | Calcium excretion, BMD, BTM | Low | Unclear | Low | Low | Low |
| Dawson-Hughes, 2015 (37) | Parallel | 233 Healthy men and women (age >50 y) | K+ bicarbonate (1 mmol/kg/d) or (1.5 mmol/kg/d) | 3 mo | NAE, urinary pH | Calcium excretion, BTM | Low | Unclear | Low | Low | Low |
| Dawson-Hughes, 2009 (40) | Parallel | 171 Healthy men and women (>50 y) | KHCO3/NaHCO3 or KCl/placebo (67.5 mmol/d) | 3 mo | NAE | Calcium excretion, BTM | Low | Unclear | Low | Low | Low |
| Ceglia, 2009 (41) | Parallel | 19 Healthy subjects (age 54–82 y) | K+ bicarbonate (90 mmol/d) or placebo | 25 d | NAE | Calcium balance, BTM | Low | Unclear | Low | Low | Low |
| Frassetto, 2005 (43) | Parallel | 170 Postmenopausal women (age 61 ± 7 y) | K+ bicarbonate (90 mmol/d) or placebo | 36 mo | Urinary pH | Calcium excretion | Low | Unclear | High | Low | Low |
| He, 2010 (44) | Crossover | 42 Individuals with untreated mildly raised blood pressure | K+ bicarbonate or placebo (64 mmol/d) | 4 wk | Urinary pH | Calcium excretion, BTM | Low | Unclear | Low | Low | Low |
| Moseley, 2013 (39) | Parallel | 52 Healthy men and women (age 65.2 ± 6.2 y) | K+ citrate (90 mmol/d) or placebo | 6 mo | NAE | Calcium balance, BTM | Low | Unclear | Low | Low | Low |
| Sakhaee, 1983 (46) | Crossover | 5 Patients with documented uric acid lithiasis (age 42–69 y) | K+ chloride (60 mEq/d) or placebo | 4 wk (3 wk) | Urinary pH | Calcium excretion | Low | Unclear | Low | Low | Low |
1BMD, bone mineral density; BTM, bone turnover marker; crossover, random crossover study; NAE, net acid excretion; NEAP, net endogenous acid production; parallel, random control trial; PRAL, potential renal acid load.
“period” indicates means diet intervention time (washout period).
As shown in Table 1, 11 of the 13 acidic diet intervention studies explored urinary calcium outcomes, 5 studies for NAE outcomes, 8 studies for PTH, 6 studies for aminoterminal cross-linking telopeptide (NTX), 4 studies for IGF-I, and 3 studies for osteocalcin (OC), bone-specific alkaline phosphatase (BAP), and tartrate-resistant acid phosphatase (TRAP), respectively. There were 2 studies reporting BMD in the hip and femoral neck. With regard to studies on alkaline supplement (Table 2), there were 8 studies with urinary calcium outcome indicators; 6 studies with PTH; and 5 studies with IGF-I, carboxyterminal cross-linking telopeptide (CTX), BAP, and NTX; 4 studies with OC and NAE; and 3 studies with an outcome of BMD in the lumbar spine and hip, 2 studies involving BMD in the femoral neck.
Quality evaluation
According to the Cochrane systematic review manual, we performed quality assessment for all of the included studies. For the acidic diet interventions, 4 articles used blinding strategies and the rest of the studies did not use blinding methods (Table 1). However, whether the blinding method was adapted or not has little effect on the pooled results. Additionally, 11 studies described the method of random-sequence generation, whereas 2 of them only mentioned random grouping (Table 1). There were 2 studies that carefully described the random-sequence assignment by third parties in the assignment concealment (26, 32), but the remaining studies did not have relevant descriptions. There were differences in the number of losses to follow-up between the 2 articles (26, 32) in the experimental group and the control group, and the reasons for the losses to follow-up were uneven, which may lead to the possibility of selective reporting of the collected data. We did not find sources of bias in the other studies.
Thirteen studies were included in the intervention study of alkali salts supplements (Table 2). Random-sequence generation, selective reports, and other sources of bias did not report bias. The allocation concealment was described in 3 studies, and the remaining studies were unclear. Twelve articles were blinded, and only 1 article did not describe that strategy. In 1 article, the number of subjects who were lost to follow-up was uneven between the 2 groups, but the reasons for the losses to follow-up were similar. Due to the small number of the included studies, the information of quality evaluation was used to guide interpretation of the results rather than to exclude the study itself.
Risk of publication bias
We assessed potential publication bias for the meta-analyses of effect of acidic diet and alkaline supplement on urinary calcium excretion as the 2 analyses included the largest number of studies in the 2 interventions, respectively [11 studies for acidic diet (Table 3); 8 studies for alkaline supplement (Table 4)]. The funnel chart was basically symmetrical (Supplemental Figure 1). Egger's test was also used to detect the publication bias. Both the P values for acidic diet (P = 0.278) and alkali salt supplementation (P = 0.413) were >0.1, suggesting that there was no publication bias. Since there was a small number of included studies for the other meta-analyses, it was not suitable to conduct publication bias analysis. Alternatively, we performed sensitivity analyses and meta-regression analyses to justify the stability of pooled effects for those meta-analyses with a moderate or high heterogeneity, as stated below.
TABLE 3.
Meta-analysis of effects of acidic diet on bone metabolism markers and BMD1
| Outcome type and indicators | Studies, n | P | SMD (95% CI) | I 2 , % |
|---|---|---|---|---|
| Markers of mineral metabolism | ||||
| NAE | 5 | 0.003 | 2.99 (1.01, 4.96) | 94 |
| Calcium excretion | 11 | <0.00001 | 0.47 (0.30, 0.64) | 47 |
| Hormone-regulating mineral metabolism | ||||
| IGF-I | 4 | 0.21 | 0.14 (−0.08, 0.35) | 0 |
| PTH | 8 | 0.35 | −0.26 (−0.81, 0.29) | 85 |
| Bone resorption marker | ||||
| NTX | 6 | 0.30 | 0.15 (−0.13, 0.43) | 27 |
| TRAP | 3 | 0.95 | −0.01 (−0.34, 0.32)2 | 0 |
| Bone formation marker | ||||
| BAP | 3 | 0.38 | 0.13 (−0.16, 0.42) | 35 |
| OC | 3 | 0.31 | −0.15 (−0.44, 0.14) | 0 |
| BMD | ||||
| Total hip BMD | 2 | 0.95 | 0.01 (−0.21, 0.22) | 0 |
| Femoral neck BMD | 2 | 0.18 | −0.14 (−0.36, 0.07) | 0 |
1BAP, bone-specific alkaline phosphatase; BMD, bone mineral density; IGF-I, insulin-like growth factor I; NAE, renal net acid excretion; NTX, aminoterminal cross-linking telopeptide of bone collagen; OC, osteocalcin; PTH, parathyroid hormone; SMD, standardized mean difference; TRAP, tartrate-resistant acid phosphatase.
Values are mean differences.
TABLE 4.
Meta-analysis of effects of alkaline supplements on bone metabolism markers and BMD1
| Outcome type and indicators | Studies, n | P | SMD (95% CI) | I 2, % |
|---|---|---|---|---|
| Markers of mineral metabolism | ||||
| NAE | 4 | <0.00001 | −1.29 (−1.52, −1.07) | 37 |
| Calcium excretion | 8 | 0.007 | −0.44 (−0.76, −0.12) | 63 |
| Hormone-regulating mineral metabolism | ||||
| IGF-I | 5 | 0.13 | −0.15 (−0.34, 0.04) | 0 |
| PTH | 6 | 0.25 | −2.292 (−6.22, 1.65) | 57 |
| Bone resorption marker | ||||
| NTX | 5 | 0.003 | −0.29 (−0.48, −0.10) | 0 |
| CTX | 5 | 0.17 | −0.13 (−0.33, 0.06) | 0 |
| Bone formation marker | ||||
| BAP | 5 | 0.76 | −0.03 (−0.24, 0.18) | 0 |
| PINP | 5 | 0.13 | −0.15 (−0.34, 0.04) | 0 |
| OC | 4 | 0.02 | −0.23 (−0.42, −0.03) | 0 |
| BMD | ||||
| Total hip BMD | 3 | 0.02 | 0.982 (0.13,1.83) | 99 |
| Femoral neck BMD | 2 | <0.00001 | 1.622 (1.50,1.74) | 0 |
| Lumbar spine BMD | 3 | <0.00001 | 1.662 (1.33,1.99) | 87 |
BAP, bone-specific alkaline phosphatase; BMD, bone mineral density; CTX, carboxyterminal cross-linking telopeptide; IGF-I, insulin-like growth factor I; NAE, renal net acid excretion; NTX, aminoterminal cross-linking telopeptide of bone collagen; OC, osteocalcin; PINP, serum aminoterminal propeptide of type I procollagen; PTH, parathyroid hormone; SMD, standardized mean difference.
Values are mean differences.
Main results of meta-analysis
Acidic dietary intervention
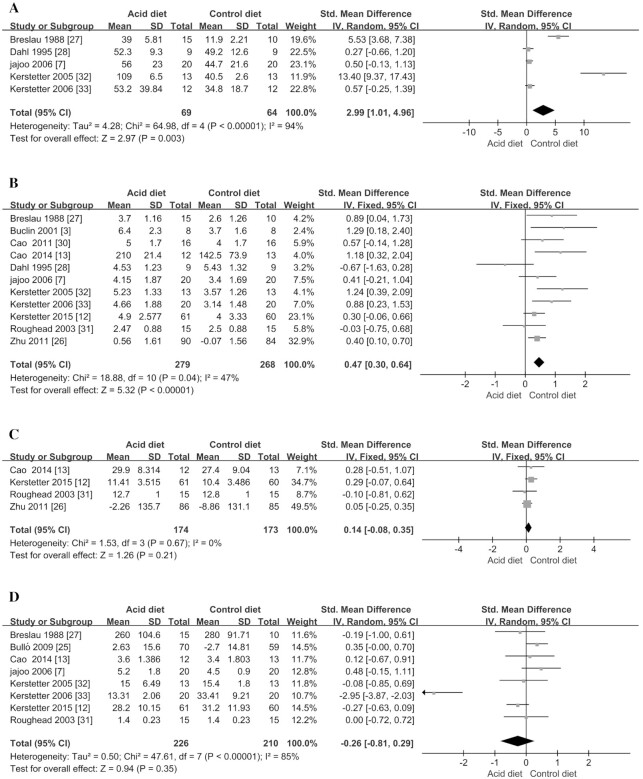
In the meta-analysis, we observed that acidic diet could significantly increase urinary NAE (SMD = 2.99; 95% CI: 1.01, 4.96; P = 0.003) (Figure 2A, Table 3) and calcium excretion (SMD = 0.47; 95% CI: 0.30, 0.64; P < 0.00001) (Figure 2B, Table 3). However, we did not find any significant difference for bone metabolism hormones, which include IGF-I and PTH, between the intervention group and the control group (P > 0.21; Figure 2C, D; Table 3), indicating that bone metabolism–related hormones did not significantly differ between the 2 groups.
FIGURE 2.
Meta-analysis of effect of acidic diet on mineral metabolism markers. (A) renal net acid excretion; (B) urinary calcium excretion; (C) insulin-like growth factor I; (D) parathyroid hormone. IV, inverse variance; Std., standardized.
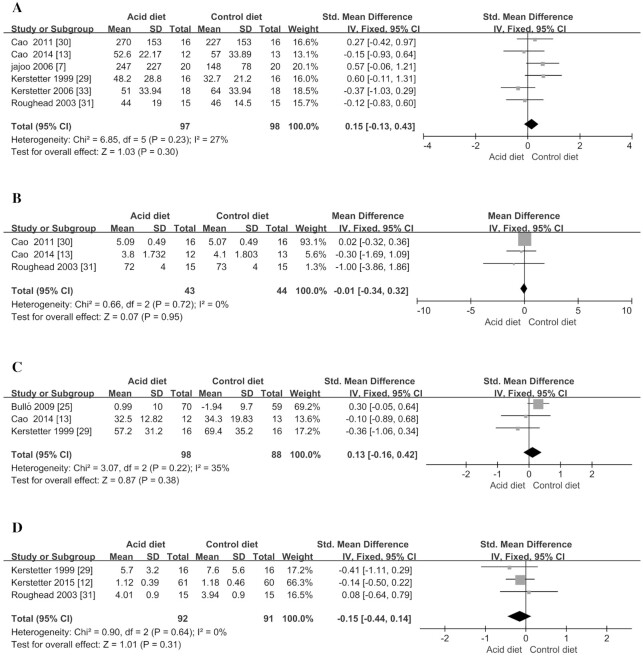
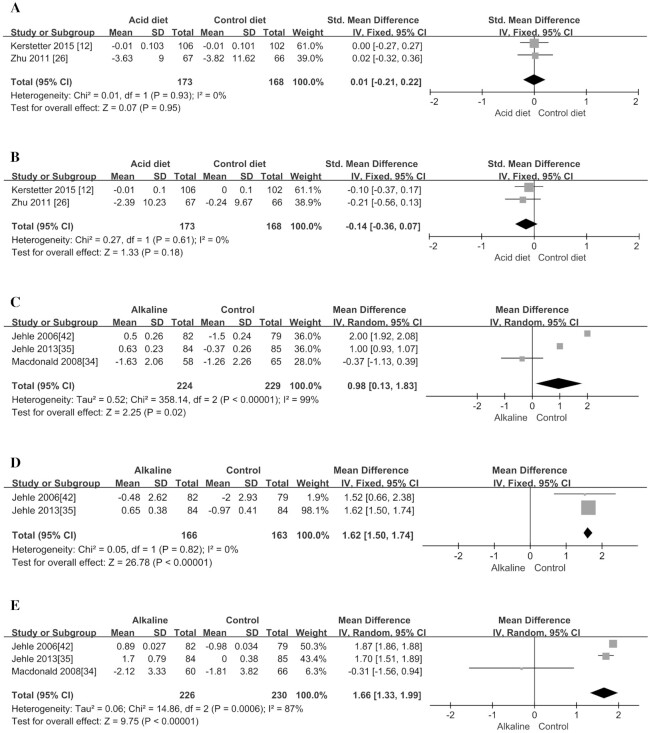
Bone formation markers of BAP and OC were included in the study for meta-analysis. Compared with the control group, acidic dietary intervention did not significantly change the 2 markers (P > 0.31; Figure 3C, D; Table 3). In addition, the acidic diet also did not significantly affect 2 kinds of bone resorption markers including NTX and TRAP (P > 0.30; Figure 3A, B; Table 3), suggesting that the acidic diet would not cause significant changes in bone resorption markers. In addition, the meta-analyses also explored the effects of acidic diet on BMD but did not find any significant decrease in BMD in the total hip and femoral neck (P > 0.18; Figure 4A, B; Table 3).
FIGURE 3.
Meta-analysis of effect of acidic diet on bone resorption and formation markers. (A) aminoterminal cross-linking telopeptide of bone collagen; (B) tartrate-resistant acid phosphatase; (C) bone-specific alkaline phosphatase; (D) osteocalcin. IV, inverse variance; Std., standardized.
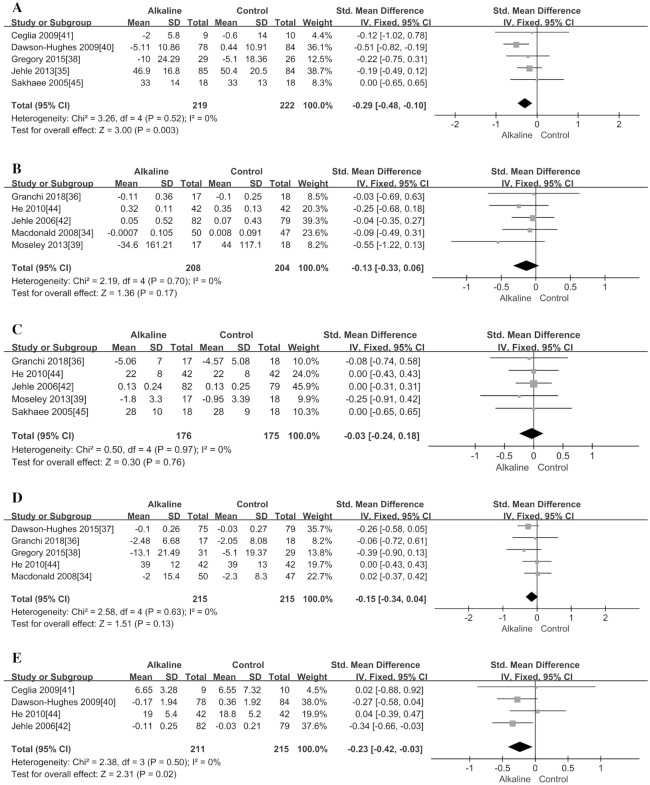
FIGURE 6.
Meta-analysis of effect of alkaline supplementation on bone resorption and formation markers. (A) aminoterminal cross-linking telopeptide of bone collagen; (B) carboxyterminal cross-linking telopeptide; (C) bone-specific alkaline phosphatase; (D) serum aminoterminal propeptide of type I procollagen; (E) osteocalcin. IV, inverse variance; Std., standardized.
Alkaline supplement intervention
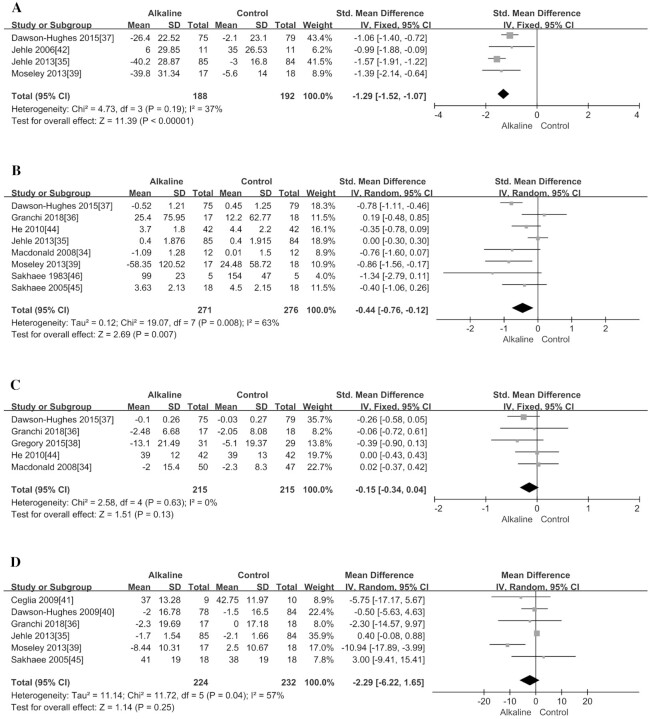
As shown in Table 4, supplementing with alkali salts could significantly reduce the urinary NAE (SMD = −1.29; 95% CI: −1.52, −1.07; P < 0.00001) (Figure 5A) and calcium excretion (SMD = −0.44; 95% CI: −0.76, −0.12; P = 0.007) (Figure 5B). However, we did not observe any significant change in 2 types of hormone-regulating bone metabolism markers (i.e., PTH and IGF-I; P > 0.13; Figure 5C, D).
FIGURE 4.
Meta-analysis of effect of acidic diet and alkaline supplementation on bone mineral density at 2 different body sites. (A) acidic diet—total hip; (B) acidic diet—femoral neck; (C) alkaline supplementation—total hip; (D) alkaline supplementation—femoral neck; (E) alkaline supplementation—lumbar spine. IV, inverse variance; Std., standardized.
With regard to BMD, this meta-analysis investigated if alkali salt supplementation had a substantial impact on it, and found that supplementation could significantly increase BMD in the lumbar spine (MD = 1.66; 95% CI: 1.33, 1.99; P < 0.00001) (Figure 4E), total hip (MD = 0.98; 95% CI: 0.13, 1.83; P = 0.02) (Figure 4C), and femoral neck (MD = 1.62; 95% CI: 1.50, 1.74; P < 0.00001) (Figure 4D) from baseline values (Table 4). In addition, we also found that supplementation could significantly reduce the concentration of the bone formation marker OC (SMD = −0.23; 95% CI: −0.42, −0.03; P = 0.02; Figure 6E), but it did not have an obvious effect on the other 2 formation markers, BAP and serum amino-terminal propeptide of type I procollagen (P1NP) (P > 0.13; Figure 6C, D). Moreover, the supplementation could significantly reduce the bone resorption marker NTX (SMD = −0.29; 95% CI: −0.48, −0.10; P = 0.003) (Figure 6A), but it did not have a substantial effect on another resorption marker, CTX (P = 0.17; Figure 6B).
FIGURE 5.
Meta-analysis of effect of alkaline supplementation on mineral metabolism markers. (A) renal net acid excretion; (B) urinary calcium excretion; (C) insulin-like growth factor I; (D) parathyroid hormone. IV, inverse variance; Std., standardized.
Sensitivity and meta-regression analyses
For those meta-analyses with a moderate or high level of heterogeneity (I2 > 50%), we further performed subgroup analyses to find the potential source of heterogeneity based on intervention duration (< or ≥6 mo), study design (Parallel or Crossover), and characteristics of participants (enrolled postmenopausal women or not). Moreover, both sensitivity analysis and meta-regression analysis were also carried out to find the potential source of heterogeneity and identify those studies having a substantial contribution to overall heterogeneity.
Acidic diet intervention
Among the outcome indicators of acidic diet intervention, both meta-analyses for NAE (I2 = 94%) and PTH (I2 = 85%) showed high heterogeneity (Table 3). We divided the included studies into subgroups in terms of intervention duration, participants, and research design and explored the potential sources of heterogeneity. The subgroup analyses suggested that heterogeneity in meta-analysis for PTH was not induced by any of the 3 factors (Supplemental Figure 2A–C;P > 0.17 for subgroup difference tests). In contrast, meta-regression analysis implied that the study performed by Kerstetter et al. (33) contributed much to the overall heterogeneity (Supplemental Figure 6B), which was reduced to a lower level (26%) if we excluded this study. However, the pooled results did not become significant when the study was omitted (SMD: 0.07; 95% CI: −0.18, 0.32) (Supplemental Figure 6A). With regard to the meta-analysis for NAE, the subgroup analysis implied that research design (RCT or RCO) and characteristics of studied subjects (enrolled postmenopausal women or not) would cause a high heterogeneity (both P values are 0.02 for subgroup difference tests; Supplemental Figure 3A, B). Furthermore, meta-regression analysis suggested that Kerstetter et al's study (32) contributed the largest proportion to the high heterogeneity (Supplemental Figure 7B). However, this study could not change the significant pooled results, although the pooled effect size was reduced to a lower level (SMD = 1.44; Supplemental Figure 7A) compared with the original one (SMD = 2.99). The overall heterogeneity for the other outcome indicators is small (urinary calcium excretion, BAP, and NTX; Table 3) or as low as zero (IGF-I, OC, TRAP, BMD in total hip and femoral neck; Table 3).
Alkaline supplement intervention
With regard to alkaline supplementation, heterogeneities for meta-analyses of BMD in the total hip and lumbar spine were at a high level (I2 = 99% and 87%, respectively; Table 4). The very small number of included studies (3 articles for both) is not suitable to perform subgroup analysis, but the small number may partially explain the high heterogeneity. Alternatively, meta-regression analysis for the pooled results of BMD in the total hip implied that the included 3 studies would be divided into 2 clusters (upper right corner and lower left corner in Supplemental Figure 8B), indicating that the study by Macdonald et al. (34) may induce the high level of heterogeneity. However, omitting this study from the meta-analysis did not change the significance and direction of effect (MD = 1.50; 95% CI: 0.52, 2.48) (Supplemental Figure 8A). Sensitivity and meta-regression analysis for pooled results of BMD in the lumbar spine generated a similar outcome—that is, the Macdonald et al. study induced the high heterogeneity but did not change the significant pooled effect (Supplemental Figure 9A, B).
There was a medium level of heterogeneity in the meta-analyses of urinary calcium excretion and PTH (I2 = 63% and 57%, respectively; Table 4). Subgroup analysis suggested that the intervention duration may be the potential source of heterogeneity for the meta-analysis of urinary calcium excretion (P = 0.03; Supplemental Figure 4C). Meta-regression analysis implied that the studies by Jehle et al. (35) and Dawson-Hughes et al. (37) contributed much to the overall heterogeneity (Supplemental Figure 10B), but each of the 2 studies did not change the significance of the pooled effect, respectively (Supplemental Figure 10A). With regard to the meta-analysis of PTH, subgroup analysis did not identify any affecting factor (Supplemental Figure 5A–C). Meta-regression analysis found a substantial role of the study by Moseley et al. (39) in inducing the overall heterogeneity (Supplemental Figure 11B). However, this study did not affect the nonsignificant pooled effect (Supplemental Figure 11A). The overall heterogeneity for most of the other outcome indicators is as low as zero (IGF-I, CTX, OC, BAP, NTX, PINP, and BMD in femoral neck; Table 4).
Discussion
Our meta-analysis showed that an acidic diet intervention increased NAE and urinary calcium excretion but had no significant effect on bone metabolism markers and BMD. On the other hand, an alkaline supplement intervention was found to significantly decrease NAE and urinary calcium excretion. Moreover, the alkaline supplement intervention could reduce the bone resorption marker NTX and the bone formation marker OC but had no obvious effect on the other bone turnover markers. Notably, the present meta-analysis showed that the supplementation of alkaline salt could significantly increase BMD in the femoral neck, lumbar spine, and total hip. Changes in these indicators suggested that an acid-base intervention have a beneficial effect on bone metabolism.
Urinary calcium excretion related to the acid-base balance
Acidic intervention increases the excretion of urinary calcium and NAE, while alkaline supplementation can reduce urinary calcium and NAE, indicating that the acid-base balance is related to calcium excretion. Food consumption causes an increase in net acid load, which would cause a low-grade acidosis. To prevent the unstoppable accumulation of acid in the body and gradually increase the degree of metabolic acidosis, the body will mobilize the bone system that contains a large amount of calcium and alkaline salts to buffer (47). Cellular studies have shown that a substantial increase in systemic acid concentrations would first activate osteoclasts, and then increase bone calcium flux and promote bone dissolution (48). The adverse effect could be rescued by administration of bicarbonate ions (48). However, it is worth noting that urinary calcium loss does not necessarily mean calcium imbalance or bone loss. One previous study showed that high protein intake would increase intestinal calcium absorption (49), and this increase might offset the urinary calcium excretion that is caused by the protein-load acid. Thus, the increased urinary calcium excretion may be related to the increase in intestinal calcium absorption, not necessarily due to the increased bone calcium loss that is caused by the increase in bone resorption. However, other studies did not report a significant increase in calcium absorption (12, 25, 28, 32, 40, 42, 46), and more evidence is therefore needed to support whether urinary calcium excretion is related to the decrease in bone density that is caused by acidic diets.
Effect of acidic and alkaline interventions on bone metabolism markers
Several previous studies performed meta-analyses to investigate the effect of acidic diet or alkaline salt supplements on bone health. Fenton et al. (50) conducted a systematic review and meta-analysis on the effects of dietary acids on bone health with the application of Hill's causality criteria. They reviewed 238 studies and included 55 studies, among which 22 were randomized intervention trials. In the end, they concluded that the existing evidence does not support the causal relation between dietary acid load and osteoporosis, and that there is not enough evidence to support a beneficial effect of alkaline supplements on bone health. However, that study did not conduct any further statistical analysis but only applied the Hill standard review to retrospectively describe the relation between acid load and bone metabolism. Moreover, the selected 13 randomized controlled studies not only included the measurement of bone turnover markers but also the measurement of BMD (12, 25, 26, 32). Unlike the study by Fenton et al., the present study performed meta-analysis and meta-regression analysis and found that acidic diets were not harmful to bone health, but alkaline supplementation had a beneficial effect on various bone metabolism markers and BMD. This result may be related to the fact that protein supplements in food offset the adverse effects of the acid load. Cao et al.’s (6) latest review of the impact of changes in renal acid load on bone health suggests that proteins can not only provide amino acid precursors for the synthesis and maintenance of the bone structure but also increase IGF-I secretion to stimulate bone formation. Therefore, it can partially or completely compensate for the negative effect of increased acid load on the musculoskeletal system.
Groenendijk et al. (1) conducted a meta-analysis on the effects of high protein supplementation on bone health. They found that protein intake exceeding the current RDA may reduce the risk of hip fracture, but their research mainly included cohort studies and lacked support from randomized intervention studies. Moreover, Darling et al. (15) reviewed and meta-analyzed the evidence released over the past 40 y to assess the association between dietary protein and bone health throughout the life course. They did not find a significant effect of any form of protein on bone health.
There is still no strong evidence on whether protein can offset the adverse effects of acid on bones. Due to limitations of experimental designs and ethical issues, it is not possible to assess the effect of excessive acid load on human bone health. Lambert et al. (14) conducted a meta-analysis of 14 intervention studies on the effects of alkaline potassium supplementation on calcium metabolism and bone health. They found that supplementation with potassium alkali could significantly reduce the excretion of the bone resorption marker NTX and urinary calcium and acids but did not affect the concentrations of bone formation markers. What differs from their results is that we found that supplementation with alkali salts not only reduced the bone resorption marker NTX but also reduced the bone formation marker OC. The inclusion criteria in our study are similar to those of Lambert et al.’s study. The main difference is that Lambert et al.’s study also included supplementations that were combined with other forms of dietary or pharmaceutical manipulation, such as a high-protein or -salt intake or diuretic administration, while we did not include those studies in order to avoid the potential interaction effect between 2 simultaneous interventions.
Beneficial effect of alkaline supplements on BMD
In terms of BMD, we added several newly published reports to the meta-analysis and discovered the potential beneficial effect of alkali salt supplementation on BMD. Sromicki et al. (5) conducted a prospective study of 183 patients, and the results showed that 23% of patients with osteopenia/osteoporosis had abnormal renal tubular acidification. According to the conventional treatment of osteoporosis, long-term alkaline treatment could improve the bone density of the lumbar spine and other bone parts (5). Although we did not observe a decrease in BMD in human acidic diet intervention studies, animal experiments have confirmed that a more acidic environment may decouple the bone-remodeling process. Stimulation of osteoblasts by a low HCO3 environment can enhance the autocrine signaling of prostaglandin E2 and the function of E2 (7, 12, 13), which leads to the activation of osteoclasts by increasing the secretion of the receptor activator of the nuclear factor-B ligand. It has been observed in rat osteoblast cultures that, without any detectable reduction in collagen synthesis, the induction of a physiological degree of metabolic acidosis leads to a significant reduction in matrix mineralization (42). And exogenous potassium citrate supplementation can improve bone mineralization within a period of time after alkalization (8, 48). Our results show that both bone resorption markers and bone turnover markers are reduced, and there seems to be no significant beneficial changes in bone turnover markers. Moreover, the intervention with an alkaline supplement seems to cause a decreasing process of bone resorption and bone formation at the same time. Whether the increase in bone density is due to a weaker bone resorption activity is still unknown. It is even possible that changes in bone density do not depend on changes in the above-mentioned biomarkers. Previous studies of bisphosphonates suggested that, regardless of the cell-mediated changes in bone markers, the increase in mineralization of bone matrix caused by alkali would continue to increase BMD (6, 9). The beneficial effect, however, should be treated with caution as there is a high level of heterogeneity in meta-analyses of BMD in the total hip and lumbar spine. The very small number of studies included in the 2 meta-analyses may partially explain the high heterogeneity. In addition, the meta-regression analysis implied that the study by Macdonald et al. (34) may cause the high heterogeneity, although omitting that study did not affect the significance and direction of effect. This study did not emphasize vitamin D and calcium supplementation, but the other 2 studies had adequate supplementation of vitamin D and calcium (35, 42). This large difference may contribute much to the high heterogeneity. Among the outcome indexes of acidic diet intervention, the heterogeneity for meta-analyses of NAE and PTH was also high. It is suspected that the heterogeneity mainly comes from the different acid diet composition and the level of intake.
The main limitation of this study is that most studies use changes in urinary calcium excretion and bone metabolism markers as evidence of bone health effects, while there is a lack of studies with fracture risk as the main endpoint to directly illustrate the long-term effect of the acid-base intervention on bone health. However, although osteoporosis is the leading cause of fracture risk, there are many other possible fracture risk factors such as vision problems, dementia, and increased risk for falls. These risk factors prevent scientists from drawing a conclusion as to whether there is a direct effect of acid-base intervention on fracture risk. It is therefore not surprising that the included RCTs did not assess the effect of acid-base intervention on fracture risk. Second, the heterogeneities for several meta-analyses were at a high level, which may weaken the reliability of the pooled effects. However, the small number of included studies may partially explain the high heterogeneity and the meta-regression analysis further ensures the stability of the results. In fact, we used 2 vital strategies to guarantee the homogeneity of these meta-analyses. The first is that all of the included studies are high-quality RCTs. In addition, we applied strict inclusion and exclusion criteria to exclude studies on the use of supplements in combination with other forms of diet or drugs, as well as studies with changes in nutrients that affect bone metabolism and those studies on specific protein types.
Another limitation of this study is that the pooled effects did not consider the age-related decline in kidney function, which was reported to have a substantial effect on renal NAE that contributed the largest amount to the elimination of noncarbonic acid. Theoretically, a sensitivity analysis should be conducted to investigate the age-related decline in renal function on the pooled effects. Unfortunately, most of the included articles did not provide an accurate value of the estimated glomerular filtration rate (eGFR) or the creatinine clearance rate (3, 7, 13, 25–28, 30, 35, 36, 38–41, 43–45). Thus, it is difficult to conduct a stratified verification analysis to verify the potential role of the age-related renal function on the pooled effects, which should be interpreted with caution. In addition, the acid ash hypothesis proposes that diets with a high phosphate content are acidic and phosphate supplementation involves different levels of calcium and phosphorus interventions, so this study also did not include phosphate research due to the possible complication caused by the calcium supplementation.
The acid ash hypothesis produced the concept that excessive acid in the diet may cause several diseases in modern society and the “alkaline diet” could prevent and cure these diseases. This study showed that it is not necessary to emphasize the adverse effect of acidic diets on bone health. Future research should focus more on whether urinary calcium excretion is related to bone calcium loss that is caused by an acidic diet and whether protein can offset the adverse effects of acid on bones. More research is also needed to further verify whether alkaline supplementation combined with sufficient vitamin D and calcium is beneficial to bone health.
In conclusion, the present meta-analysis of the latest randomized intervention studies demonstrated that acid intervention may not be harmful to bone health. On the other side, alkaline supplementation could significantly reduce the excretion of urinary calcium and renal NAE, indicating a potential benefit to BMD, which was further validated by meta-analyses of BMD at 2 available body sites. It is initially suggested that supplementation with alkali salts may be beneficial to bone health. However, the finding should be interpreted with caution and needs support from more clinical evidence.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Prof. Mary Waye for her great help in revising the whole manuscript. The authors’ responsibilities were as follows—YC and LY: designed the project; YH, LL, MA, and SR: conducted research and analyzed data; YH, MA, and SR: wrote the manuscript; and all the authors: read and approved the final manuscript.
Notes
The study was supported by grants from the National Natural Science Foundation of China (grant 81500679); the Natural Science Foundation of Guangdong Province, China (grant 2018A030313609); Science and Technology Plan of Guangdong Province, China (grant 2017A020215045); and the Guangdong Provincial Department of Education High-level University Construction Funds Southern Medical University Clinical Research Startup Project, China (grant LC2016YM007).
Supplemental Figures 1–11 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
YH , MA, and YL contributed equally to this work.
Abbreviations used, BAP, bone-specific alkaline phosphatase; BMD, bone mineral density; CTX, carboxyterminal cross-linking telopeptide; IGF-I, insulin-like growth factor I; MD, mean difference; NAE, net acid excretion; NTX, aminoterminal cross-linking telopeptide; OC, osteocalcin; PINP, serum aminoterminal propeptide of type I procollagen; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PTH, parathyroid hormone; RCT, randomized controlled trial; SMD, standardized mean difference; TRAP, tartrate-resistant acid phosphatase.
Contributor Information
Yibing Han, Department of Endocrinology, Zhujiang Hospital of Southern Medical University, Guangzhou City, Guangdong Province, China.
Min An, Department of Endocrinology, Zhujiang Hospital of Southern Medical University, Guangzhou City, Guangdong Province, China.
Li Yang, Department of Endocrinology, Zhujiang Hospital of Southern Medical University, Guangzhou City, Guangdong Province, China.
Liuran Li, Department of Endocrinology, Zhujiang Hospital of Southern Medical University, Guangzhou City, Guangdong Province, China.
Shitao Rao, School of Medical Technology and Engineering, Fujian Medical University, Fuzhou City, Fujian Province, China; School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Shatin City, New Territories, Hong Kong Special Administrative Region, China.
Yanzhen Cheng, Department of Endocrinology, Zhujiang Hospital of Southern Medical University, Guangzhou City, Guangdong Province, China.
References
- 1. Groenendijk I, den Boeft L, van Loon LJC, de Groot L. High versus low dietary protein intake and bone health in older adults: a systematic review and meta-analysis. Comput Struct Biotechnol J. 2019;17:1101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang YB, Zhong ZM, Hou G, Jiang H, Chen JT. Involvement of oxidative stress in age-related bone loss. J Surg Res. 2011;169(1):e37–42. [DOI] [PubMed] [Google Scholar]
- 3. Buclin T, Cosma M, Appenzeller M, Jacquet AF, Decosterd LA, Biollaz J, Burckhardt P. Diet acids and alkalis influence calcium retention in bone. Osteoporos Int. 2001;12:493–9. [DOI] [PubMed] [Google Scholar]
- 4. Lennon EJ, Lemann J, Litzow JR. The effects of diet and stool composition on the net external acid balance of normal subjects. J Clin Invest. 1966;45(10):1601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sromicki JJ, Hess B. Abnormal distal renal tubular acidification in patients with low bone mass: prevalence and impact of alkali treatment. Urolithiasis. 2017;45(3):263–9. [DOI] [PubMed] [Google Scholar]
- 6. Cao JJ, Nielsen FH. Acid diet (high-meat protein) effects on calcium metabolism and bone health. Curr Opin Clin Nutr Metab Care. 2010;13(6):698–702. [DOI] [PubMed] [Google Scholar]
- 7. Jajoo R, Song L, Rasmussen H, Harris SS, Dawson-Hughes B. Dietary acid-base balance, bone resorption, and calcium excretion. J Am Coll Nutr. 2006;25:224–30. [DOI] [PubMed] [Google Scholar]
- 8. Kerstetter JE, O'Brien KO, Insogna KL. Dietary protein, calcium metabolism, and skeletal homeostasis revisited. Am J Clin Nutr. 2003;78:584S–92S. [DOI] [PubMed] [Google Scholar]
- 9. Schurch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:801–9. [DOI] [PubMed] [Google Scholar]
- 10. Devine A, Dick IM, Islam AF, Dhaliwal SS, Prince RL. Protein consumption is an important predictor of lower limb bone mass in elderly women. Am J Clin Nutr. 2005; 81:1423–8. [DOI] [PubMed] [Google Scholar]
- 11. Wengreen HJ, Munger RG, West NA, Cutler DR, Corcoran CD, Zhang J, Sassano NE. Dietary protein intake and risk of osteoporotic hip fracture in elderly residents of Utah. J Bone Miner Res. 2004;19:537–45. [DOI] [PubMed] [Google Scholar]
- 12. Kerstetter JE, Bihuniak JD, Brindisi J, Sullivan RR, Mangano KM, Larocque S, Kotler BM, Simpson CA, Cusano AM, Gaffney-Stomberg Eet al. . The effect of a whey protein supplement on bone mass in older caucasian adults. J Clin Endocrinol Metab. 2015;100:2214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao JJ, Pasiakos SM, Margolis LM, Sauter ER, Whigham LD, McClung JP, Young AJ, Combs GJ. Calcium homeostasis and bone metabolic responses to high-protein diets during energy deficit in healthy young adults: a randomized controlled trial. Am J Clin Nutr. 2014;99:400–7. [DOI] [PubMed] [Google Scholar]
- 14. Lambert H, Frassetto L, Moore JB, Torgerson D, Gannon R, Burckhardt P, Lanham-New S. The effect of supplementation with alkaline potassium salts on bone metabolism: a meta-analysis. Osteoporos Int. 2015;26(4):1311–8. [DOI] [PubMed] [Google Scholar]
- 15. Darling AL, Manders RJF, Sahni S, Zhu K, Hewitt CE, Prince RL, Millward DJ, Lanham-New SA. Dietary protein and bone health across the life-course: an updated systematic review and meta-analysis over 40 years. Osteoporos Int. 2019;30(4):741–61. [DOI] [PubMed] [Google Scholar]
- 16. Buehlmeier J, Frings-Meuthen P, Remer T, Maser-Gluth C, Stehle P, Biolo G, Heer M. Alkaline salts to counteract bone resorption and protein wasting induced by high salt intake: results of a randomized controlled trial. J Clin Endocrinol Metab. 2012;97(12):4789–97. [DOI] [PubMed] [Google Scholar]
- 17. Frassetto LA, Nash E, Morris RC, Sebastian A. Comparative effects of potassium chloride and bicarbonate on thiazide-induced reduction in urinary calcium excretion. Kidney Int. 2000;58(2):748–52. [DOI] [PubMed] [Google Scholar]
- 18. Zha DS, Zhu QA, Pei WW, Zheng JC, Wu SH, Xu ZX, Li T, Chen JT. Does whole-body vibration with alternative tilting increase bone mineral density and change bone metabolism in senior people?. Aging Clin Exp Res. 2012;24(1):28–36. [DOI] [PubMed] [Google Scholar]
- 19. Weaver AA, Houston DK, Shapses SA, Lyles MF, Henderson RM, Beavers DP, Baker AC, Beavers KM. Effect of a hypocaloric, nutritionally complete, higher-protein meal plan on bone density and quality in older adults with obesity: a randomized trial. Am J Clin Nutr. 2019;109(2):478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Irwig L, Macaskil P, Berry G, Glasziou P. Bias in meta-analysis detected by a simple, graphical test: graphical test is itself biased. BMJ. 1998;316(7129):470; author reply: 470–1. [PMC free article] [PubMed] [Google Scholar]
- 24. Viechtbauer W. Conducting meta-analyses in R with the Metafor package. J Stat Soft. 2010;36:1–48. [Google Scholar]
- 25. Bull, M, Amigo-Correig P, Marquez-Sandoval F, Babio N, Martinez-Gonzalez MA, Estruch R, Basora J, Sola R, Salas-Salvado J. Mediterranean diet and high dietary acid load associated with mixed nuts: effect on bone metabolism in elderly subjects. J Am Geriatr Soc. 2009;57:1789–98. [DOI] [PubMed] [Google Scholar]
- 26. Zhu K, Meng XQ, Kerr DA, Devine A, Solah V, Binns CW, Prince RL. The effects of a two-year randomized, controlled trial of whey protein supplementation on bone structure, IGF-1, and urinary calcium excretion in older postmenopausal women. J Bone Miner Res. 2011; 26:2298–306. [DOI] [PubMed] [Google Scholar]
- 27. Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab. 1988;66:140–6. [DOI] [PubMed] [Google Scholar]
- 28. Dahl WJ, Whiting SJ, Stephen AM. Dietary lentils and calcium balance in adult men. Nutr Res. 1995;15:1587. [Google Scholar]
- 29. Kerstetter JE, Mitnick ME, Gundberg CM, Caseria DM, Ellison AF, Carpenter TO, Insogna KL. Changes in bone turnover in young women consuming different levels of dietary protein. J Clin Endocrinol Metab. 1999;84:1052–5. [DOI] [PubMed] [Google Scholar]
- 30. Cao JJ, Johnson LK, Hunt JR. A diet high in meat protein and potential renal acid load increases fractional calcium absorption and urinary calcium excretion without affecting markers of bone resorption or formation in postmenopausal women. J Nutr. 2011;141(3): 391–7. [DOI] [PubMed] [Google Scholar]
- 31. Roughead ZK, Johnson LK, Lykken GI, Hunt JR. Controlled high meat diets do not affect calcium retention or indices of bone status in healthy postmenopausal women. J Nutr. 2003;133:1020–6. [DOI] [PubMed] [Google Scholar]
- 32. Kerstetter JE, O'Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab. 2005;90:26–31. [DOI] [PubMed] [Google Scholar]
- 33. Kerstetter JE, Wall DE, O'Brien KO, Caseria DM, Insogna KL. Meat and soy protein affect calcium homeostasis in healthy women. J Nutr. 2006;136:1890–5. [DOI] [PubMed] [Google Scholar]
- 34. Macdonald HM, Black AJ, Aucott L, Duthie G, Duthie S, Sandison R, Hardcastle AC, Lanham NS, Fraser WD, Reid DM. Effect of potassium citrate supplementation or increased fruit and vegetable intake on bone metabolism in healthy postmenopausal women: a randomized controlled trial. Am J Clin Nutr. 2008;88:465–74. [DOI] [PubMed] [Google Scholar]
- 35. Jehle S, Hulter HN, Krapf R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2013;98:207–17. [DOI] [PubMed] [Google Scholar]
- 36. Granchi D, Caudarella R, Ripamonti C, Spinnato P, Bazzocchi A, Massa A, Baldini N. Potassium citrate supplementation decreases the biochemical markers of bone loss in a group of osteopenic women: the results of a randomized, double-blind, placebo-controlled pilot study. Nutrients. 2018;10(9):1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dawson-Hughes B, Harris SS, Palermo NJ, Gilhooly CH, Shea MK, Fielding RA, Ceglia L. Potassium bicarbonate supplementation lowers bone turnover and calcium excretion in older men and women: a randomized dose-finding trial. J Bone Miner Res. 2015;30:2103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gregory NS, Kumar R, Stein EM, Alexander E, Christos P, Bockman RS, Rodman JS. Potassium citrate decreases bone resorption in postmenopausal women with osteopenia: a randomized,double-blind clinicaltrial. Endocr Pract. 2015;21:1380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moseley KF, Weaver CM, Appel L, Sebastian A, Sellmeyer DE. Potassium citrate supplementation results in sustained improvement in calcium balance in older men and women. J Bone Miner Res. 2013;28:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dawson-Hughes B, Harris SS, Palermo NJ, Castaneda-Sceppa C, Rasmussen HM, Dallal GE. Treatment with potassium bicarbonate lowers calcium excretion and bone resorption in older men and women. J Clin Endocrinol Metab. 2009;94:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ceglia L, Harris SS, Abrams SA, Rasmussen HM, Dallal GE, Dawson-Hughes B. Potassium bicarbonate attenuates the urinary nitrogen excretion that accompanies an increase in dietary protein and may promote calcium absorption. J Clin Endocrinol Metab. 2009;94:645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jehle S, Zanetti A, Muser J, Hulter HN, Krapf R. Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J Am Soc Nephrol. 2006;17(11):3213–22. [DOI] [PubMed] [Google Scholar]
- 43. Frassetto L, Morris RC, Sebastian A. Long-term persistence of the urine calcium-lowering effect of potassium bicarbonate in postmenopausal women. J Clin Endocrinol Metab. 2005;90:831–4. [DOI] [PubMed] [Google Scholar]
- 44. He FJ, Marciniak M, Carney C, Markandu ND, Anand V, Fraser WD, Dalton RN, Kaski JC, MacGregor GA. Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension. 2010;55:681–8. [DOI] [PubMed] [Google Scholar]
- 45. Sakhaee K, Maalouf NM, Abrams SA, Pak CY. Effects of potassium alkali and calcium supplementation on bone turnover in postmenopausal women. J Clin Endocrinol Metab. 2005;90:3528–33. [DOI] [PubMed] [Google Scholar]
- 46. Sakhaee K, Nicar M, Hill K, Pak CY. Contrasting effects of potassium citrate and sodium citrate therapies on urinary chemistries and crystallization of stone-forming salts. Kidney Int. 1983;24:348–52. [DOI] [PubMed] [Google Scholar]
- 47. Krieger NS, Frick KK, Bushinsky DA. Mechanism of acid-induced bone resorption. Curr Opin Nephrol Hypertens. 2004;13(4):423–36. [DOI] [PubMed] [Google Scholar]
- 48. Kraut JA, Mishler DR, Singer FR, Goodman WG. The effects of metabolic acidosis on bone formation and bone resorption in the rat. Kidney Int. 1986;30(5):694–700. [DOI] [PubMed] [Google Scholar]
- 49. Hunt JR, Johnson LK, Fariba Roughead ZK. Dietary protein and calcium interact to influence calcium retention: a controlled feeding study. Am J Clin Nutr. 2009;89(5):1357–65. [DOI] [PubMed] [Google Scholar]
- 50. Fenton TR, Tough SC, Lyon AW, Eliasziw M, Hanley DA. Causal assessment of dietary acid load and bone disease: a systematic review & meta-analysis applying Hill's epidemiologic criteria for causality. Nutr J. 2010;10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.