ABSTRACT
The excess consumption of added sugar is consistently found to be associated with weight gain, and a higher risk of type 2 diabetes mellitus, coronary heart disease, and stroke. In an effort to reduce the risk of cardiometabolic disease, sugar is frequently replaced by low- and null-calorie sweeteners (LCSs). Alarmingly, though, emerging evidence indicates that the consumption of LCSs is associated with an increase in cardiovascular mortality risk that is amplified in those who are overweight or obese. Sucralose, a null-caloric high-intensity sweetener, is the most commonly used LCS worldwide, which is regularly consumed by healthy individuals and patients with metabolic disease. To explore a potential causal role for sucralose in increased cardiovascular risk, this present review summarizes the preclinical and clinical data from current research detailing the effects of sucralose on systems controlling food intake, glucose homeostasis, and gut microbiota.
Keywords: sucralose, low-calorie sweetener, sweet and bitter taste receptor, taste signaling cascade, cardiovascular health, glucose metabolism
Introduction
Low-calorie sweeteners and cardiometabolic risk
The burden of obesity and cardiometabolic disease has increased worldwide over recent decades (1–3). Cardiovascular events remain the single leading cause of death, accounting for ∼31% of global mortality rate in people with obesity and/or type 2 diabetes (4, 5). Although the development of metabolic abnormalities, including obesity and insulin resistance (6), is driven by a series of complex physiological and lifestyle interactions, excess added sugar consumption has long been considered a prominent dietary mediating factor (7).
In recognition of the adverse health impact of high-energy added sugars, low-calorie sweeteners (LCSs) have become more commonly used in foods and beverages. By providing consumers with products that have a sweet taste and low energy content, LCSs appear to be the silver bullet in enhancing weight loss and reducing cardiometabolic disease rates (8–11). However, meta-analyses of observational studies reveal an increase in body weight, BMI, and the incidence of type 2 diabetes in people consuming LCS beverages (12). Additionally, 3 large cohort studies from Europe and the United States demonstrate an increase in all-cause mortality in those consuming ≥2 glasses of LCS beverages per day (13–15). This appears to be mainly driven by cardiovascular deaths, as confirmed in a very recent meta-analysis (16), especially in participants with either a BMI ≥25 (13) or ≥30 kg/m2 (15). However, the interpretation of these findings is complicated, mainly due to the uncontrolled nature of the observational study design and/or by potential reverse causation (i.e., LCSs are typically consumed by individuals already suffering from obesity or cardiometabolic conditions). In addition, reports of exposure to LCSs could be incomplete and/or inaccurate; and the nature of the foods and beverages containing LCSs, as well as LCSs themselves, might dramatically change over the period of follow-up of these cohorts. Finally, most studies focused on the consumption of beverages, which is not the only source of LCSs. Despite these limitations, the epidemiological evidence is consistent across studies and, thus, the commonly held belief that LCSs are physiologically neutral can be challenged.
The recognition of sucralose as a major player among LCSs
LCSs are categorized into different groups according to their origin (e.g., natural or artificial), their sweetness potency, and their nutritional value (caloric or noncaloric; and digestibility) (17, 18). Natural sweeteners include the monk fruit (Siraitia grosvenorii), swingle fruit extract, stevia, and the sweet-tasting protein, thaumatin. The most common artificial sweeteners are acesulfame potassium (acesulfame-K), advantame, aspartame, neotame, saccharin, and sucralose. The latter is the most commonly used artificial sweetener worldwide, accounting for 30% of the US$2.29 billion global LCS market in 2016 (19). Although aspartame has been widely used in LCS beverages, more and more diet drinks now contain sucralose in their recipe, with an increase of 10% in sucralose-sweetened diet beverages between 2008 and 2016 (20). Sucralose is a semisynthetic sweetener that is derived from a chemical modification of sucrose, whereby the 3 hydroxyl groups are substituted by chlorine atoms (at the 4′-, 1′-, and 6′-positions). Sucralose is ∼600 times sweeter than sucrose, with a quality and temporal profile very close to that of sucrose (18). The FDA and European Food Safety Authority have both approved sucralose, setting an acceptable limit of consumption [e.g., acceptable daily intake (ADI) of 5 and 15 mg/kg/d, respectively], for use in a variety of foods including baked goods, beverages, chewing gum, gelatins, and frozen dairy desserts. Thus, sucralose is considered safe for human consumption (21), partly because of its zero-calorie characteristic, which means that blood glucose concentrations are not significantly altered after consumption (22). The consumption of LCSs has even been recommended by health authorities as part of a weight loss strategy in overweight and obese individuals, as well as in people with glucose intolerance or diabetes and in those who are pregnant (23, 24). Moreover, sucralose is also consumed indirectly in pharmaceutical drugs and is even detected in the breast milk of lactating women, even among women who had not reported any LCS consumption (25). Despite the apparent neutral effects of sucralose on blood glucose and/or body weight (23, 26), consideration of the evidence from the recent observational and epidemiological studies described above, as well as the statistics demonstrating that sucralose consumption is increasing, warrants a need to question the physiological inactivity of sucralose and the potential long-term effects it might have on cardiovascular and metabolic human health (27–30).
Thus, the purpose of this review is: 1) to summarize the current understanding of sucralose molecular sensors; and 2) to review recent clinical and preclinical data related to sucralose-mediated effects on glucose metabolism and the cardiovascular system, exploring a potential causal role of sucralose consumption in the increase in cardiovascular risk.
Sucralose: Its Receptors and Signaling Pathways
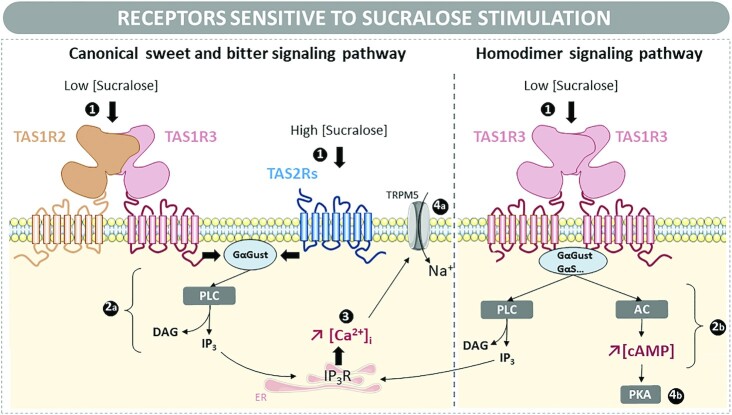
The most evident effect of sucralose is its generation of a sweet sensation via stimulation of the mechanisms for sweetness perception. In the early 2000s, starting with a discovery by Nelson and collaborators (31), several studies reported that sweet-tasting compounds (e.g., natural sugars, artificial and natural null-caloric sweeteners) were detected by a single sweet-taste receptor expressed on the apical membranes of the taste receptor cells residing in the taste buds of the oral cavity (Figure 1). The sweet-taste receptor is a heterodimeric class C G-protein–coupled receptor composed of TAS1R2 (taste receptor type 1, member 2) and TAS1R3 (taste receptor type 1, member 3) subunits (32). Knockout mice studies and cellular assays have established that the heterodimeric TAS1R2/TAS1R3 is the primary sweet-taste receptor (31, 33, 34). These proteins consist of a 7-transmembrane-helix connected by a short cysteine-rich linker to a Venus flytrap domain. Natural monosaccharides and disaccharides, as well as artificial sweeteners such as sucralose, bind to the Venus flytrap domain of the TAS1R2 and/or TAS1R3 subunits (17, 35). Although glucose can activate TAS1R2/TAS1R3 at very high concentrations (>300 mM), most LCSs are much more powerful agonists with lower concentrations (<100 μM) being sufficient for activation of these taste receptors (36). Indeed, solutions containing concentrations of sucralose in the micromolar range [half-effective concentration (EC50) = 39 μM)] are sufficient to activate TAS1R2/TAS1R3 (37). In taste bud cells, the signaling cascade involves the activation of the heterotrimeric G-protein, α-gustducin, and subsequent stimulation of phospholipase C (PLC) β2. This leads to a calcium-dependent activation of the transient receptor potential cation channel M5, which results in membrane depolarization, opening of Pannexin-1 and, ultimately, a release of ATP. In turn, extracellular ATP excites primary afferent sensory fibers, stimulating them to send a sweet taste signal to the brain (36, 38–40).
FIGURE 1.
The effect of sucralose on sweet and bitter taste receptors with the associated signaling pathways. Description of the signaling pathways being activated following the binding of sucralose to bitter receptors (TAS2Rs), sweet taste receptors, or the TAS1R3 homodimer. (1) The interaction between sucralose and its receptor is associated with the activation of (2) signaling cascades involving (2a) PLC activation or (2b) both PLC and AC activation. PLC activation leads to an increase in intracellular IP3, which will interact with its receptor, IP3R, localized in the ER. It also induces (3) a release of intracellular calcium, which (4a) activates TRPM5. The subsequent entrance of sodium to the cell via TRPM5 allows membrane depolarization and nervous perception of a bitter or sweet taste. Alternatively, activation of AC increases cAMP production and PKA activation with (4b) various consequences for the cell type in which the process occurs. AC, adenylate cyclase; [Ca2+]i, intracellular calcium; DAG, diacylglycerol; ER, endoplasmic reticulum; GαGust, G-protein α-gustducin; GαS, G-protein αs subunit; IP3, inositol triphosphate; IP3R, inositol triphosphate receptor; PKA, protein kinase A; PLC, phospholipase C; TAS1R2/3 and TAS1R3/3, sweet taste receptor; TAS2R, bitter taste receptor; TRPM5, transient receptor potential cation channel subfamily M member 5.
Additionally, sensory studies conducted in humans and preference tests conducted in rodents have shown that sucralose has a bitter taste quality (41). Cellular assays using human embryonic kidney 293 cells have demonstrated that this off-taste is due to the capacity of sucralose to activate several human bitter taste receptors named TAS2Rs (taste receptors type 2). The human TAS2R family, which also belongs to the G-protein–coupled receptor superfamily, encompasses ∼25 members possessing different ligand profiles. TAS1Rs and TAS2Rs share the same signaling cascade. Sucralose has been shown to activate the human TAS2R1, 10, 31, 44, and 46 (EC50 = 16–60 mM) (42).
Since first being discovered in the mouth, taste receptors have subsequently been demonstrated to be expressed in numerous nongustatory systems. In some tissues (e.g., gut, pancreas, brain, and adipose tissues), both TAS1R2 and TAS1R3 are functionally expressed. However, in some other tissues (e.g., the stomach, liver, lymphocytes, kidney, lung, and, probably, the endothelium), TAS1R3 are only expressed as the monomeric subunit, most likely in a homodimeric form (31, 43). Cellular assays have shown that TAS1R3 alone (i.e., in absence of TAS1R2) has a low sensitivity to monosaccharides and disaccharides (43). Despite a lack of evidence, it has been thought that the TAS1R3 homodimer could be involved in the detection of sucralose. Additionally, numerous TAS2Rs, including several sucralose-sensitive isoforms, have also been found in other tissues including the heart and vascular wall (44–46).
In these other tissues, stimulation of the sweet taste receptor, TAS1R2/TAS1R3, and bitter taste receptor, TAS2R, by sucralose or any other LCS has been largely associated with activation of the canonical pathway, initially identified in the taste buds (i.e., Gα-gustducin/PLCβ2/inositol triphosphate pathway) (Figure 1, left panel). However, recent literature also suggests that taste receptors can trigger alternative signaling pathways. In this context, Nakagawa and collaborators (47, 48) provided evidence that LCSs act as biased agonists toward TAS1R3 (i.e., they can activate distinct signaling pathways) in mouse insulinoma 6 cell types (Figure 1, right panel). For example, in these cells, the interaction of sucralose, acesulfame-K, or Na-saccharin with TAS1R3 results in a distinct activation of the PLC and/or the adenylate cyclase pathways and, thus, in a different pattern of response for changes in intracellular calcium and/or cAMP concentrations.
Beyond the oral cavity and the initiation of sweet sensation, there is accumulating evidence that taste receptors act as a nutrient sensor and have a role in metabolic control. Findings that detail evidence for their role in glucose intestinal absorption, metabolism, and cardiovascular functions (44–46) have provided the basis for developing new therapeutic strategies (49) and/or for clarifying the aforementioned concerns associated with the habitual consumption of LCSs (which will be partly discussed in the next sections). However, because most of the effects of LCSs have been observed in vitro, it is not completely clear whether their action on taste receptors translates into physiological modifications in habitual consumers. Indeed, there are reasons to doubt that sucralose activates taste receptors in certain organs. Although the pharmacokinetics of sucralose have scarcely been explored, previous research has indicated that its oral bioavailability is very low, close to 14% (50). This seems to be specific to the sucralose molecule, because other LCSs commonly used in the food and beverage industry, such as acesulfame-K or saccharin, are absorbed at a rate of 80–100% into the plasmatic compartment (51) and, thus, are more likely to amount to a plasmatic concentration adequate for the activation of TAS1Rs. However, sucralose plasma concentration is largely dependent on gastrointestinal permeability (52–56), which could be increased in conditions such as obesity and diabetes (57, 58). Finally, little is known about the distribution of sucralose in nondigestive tissues, a gap in the knowledge that needs to be addressed to better understand the effect of sucralose consumption on human health (Supplemental Tables 1–3).
The Effects of Sucralose on Glucose Metabolism and the Cardiovascular System
Appetite and taste preferences
Multiple systems contribute to the control over what and how much we eat. Looking at the interaction of LCSs with these systems could thus provide an entry into understanding whether and, eventually how, each LCS affects eating behaviors. Unsurprisingly, many research efforts have been made to characterize the impact of LCSs on taste perception and, of course, how to refine it. It is, indeed, commonly accepted that the sense of taste guides essential appetitive behaviors through its action on neural mechanisms that elaborate reward and aversion. Findings obtained using laboratory rodent models demonstrate that by manipulating the brain fields that represent sweet taste, it is possible to directly control the animal's internal representation, sensory perception, and behavioral actions (i.e., sugar appetency) (59). In humans it is thought that chronic stimulation of similar reward-related systems by highly palatable food, such as sugar, can override homeostatic signals and eventually lead to overeating and subsequent obesity (60). In line with this theory, behavioral tests show that when given the choice between a sugar solution and water, rodent models, even when replete, increase their fluid intake by consuming almost exclusively the sugar solution (61). Interestingly, when sugar is substituted with an LCS, comportment and attraction to sweeter elements is similar (62). However, discrepancies in perceived taste are reported between each LCS and thus different hedonic responses are induced. Most LCSs interact with TAS2R and elicit a bitter side taste that is particularly evident in high concentrations. Several clinical reports indicate that sucralose has a relatively low bitterness compared with saccharin or acesulfame-K and thus provides a more acceptable sucrose-like taste quality (63, 64). As previously mentioned, sweet taste perception is peripherally mediated by TAS1R2/TAS1R3, which recognize both sugar and sweeteners. Surprisingly, ageusic animals—in which the sweet receptors have been inhibited—still develop a preference for sugar over time, but not for sweeteners, indicating that sugar can recruit additional reward mechanisms (33, 65, 66). Consistent with this, clinical brain imaging data also indicate that sucrose and sucralose both activate taste-reward circuits, but responses to sucrose appear to be greater in intensity and involve additional brain areas related to pleasure (67). The available data suggest that an attraction and preference for sweeter elements involves the integration of both orosensory and postingestion signals. Recently, Zuker's team (68) identified a gut-to-brain neuronal circuit that communicates the presence of sugar to the brain and drives the preference for sugar. This postoral recognition system relies on the sodium-glucose cotransporter (SGLT), which only reacts with some hexoses (e.g., glucose, galactose), but does not recognize any of the LCSs. This last observation might explain why sucrose and glucose are more “rewarding” than sucralose (or other sweeteners) after prolonged exposure (68–70). The dominant paradigm in the regulation of feeding suggests that 2 parallel systems interact to influence food consumption, the previously cited hedonic system and the homeostatic system. The homeostatic system relies on the activity of several hormonal regulators such as leptin, ghrelin, insulin, glucagon-like peptide type 1 (GLP-1), and peptide tyrosine-tyrosine (PYY), which inform the brain about peripheral energy levels. When ingested, carbohydrates stimulate the secretion of these hormones in the gut and thereby send a satiety signal. Several in vitro studies have reported that GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) secretions in the enterocyte are controlled by the sweet receptor TAS1R2/TAS1R3 and, correspondingly, that sucralose is a potent stimulus for their secretion (71, 72). Although animal and clinical studies reported no change in leptin concentration after sucralose consumption, ghrelin seems to be affected by this molecule. In vivo and clinical studies, however, provide contrasting results (73–76). The significance of such mechanisms for appetite and the regulation of food consumption warrants further investigation.
Body composition
The use of LCSs is often proposed as a strategy to promote weight loss or for weight maintenance. However, as recently reviewed by Hunter et al. (23), a meta-analysis of epidemiological data mostly reported a positive or a lack of association between the consumption of LCSs and body weight (12, 77, 78). In contrast, meta-analyses of randomized controlled trials tend to show no, or only a small, negative association between LCS consumption and body weight (12, 77–80), but there are no available secondary analyses of the specific effect of sucralose. One of the major arguments proposed to explain these epidemiological findings is the possibility of reverse causation (81). Interventional research also provides mixed findings. It is likely that this results from the fact that studies differ largely in duration and in the population studied (e.g., by age, gender, health status, and weight). Also, as pointed out by Higgins et al. (8), LCSs are often studied in combination (differing in composition between studies), despite the fact that LCSs might not all be equal when it comes to weight management. To address this point, Higgins and collaborators conducted a randomized controlled trial to compare the effects on body weight of 4 LCSs with sucrose. After 12 wk, sucrose and saccharin consumption yielded a significant and comparable increase in body weight, whereas there was no change in body weight in those consuming aspartame, rebaudioside A, and sucralose. The body weight of those consuming sucralose even tended to decrease and was significantly lower than that in those consuming any other sweetener. Hence, this trial suggests that sucralose could be one of the most efficient LCSs for weight control. Another study reported that the consumption of diet beverages containing sucralose and acesulfame-K reduced weight gain in children compared with those who consumed sugar-sweetened beverages (10). In an overweight population, the replacement of sugar-sweetened beverages by diet beverages tended to induce greater weight loss than water (82). However, these results have been obtained using a limited number of participants. Indeed, larger longitudinal studies are required to draw firm conclusions. In animal models focusing on the effects of sucralose, some studies provided evidence that its consumption was associated with weight gain when compared with water (83–86) or both water and sucrose consumption (87), whereas in other studies no change was reported (88, 89). Nevertheless, even when body weight is not altered, visceral fat can be accumulated, increasing the risk of metabolic syndrome (90). In fact, after a median follow-up of 10 y, Chia et al. (91) reported that LCS consumers had a higher risk of developing abdominal obesity than nonconsumers. To better understand the underlying mechanisms that promote an increase in adipose tissue, a recent study conducted in normal-weight rats drinking sucralose for 10 wk showed no increase in body weight, but did exhibit changes in the distribution of fat pads when compared with their control counterparts (89). The rearrangement of fat compartments leads to metabolic adaptations in this tissue, affecting its metabolic status. Dysregulations in this system can be associated with increased cardiometabolic risk. It has been shown that exposing adipose tissue derived from human mesenchymal stromal cells to LCSs can lead to an increase in adipogenesis. In the same study, the authors demonstrated that this phenomenon was also observed in human adipocytes in individuals with obesity who are known to consume LCSs (92). In rodents, Sánchez-Tapia et al. (84) demonstrated that rats fed with sucrose or sucralose have a significantly larger area of adipocytes compared with those drinking water. Furthermore, the authors describe that the rats fed with sucralose presented with the biggest brown adipocyte size, compared with those fed with water or sucrose. Consistent with the adipogenesis described in human adipocytes, this rodent study demonstrated that sucralose consumption promotes hyperinsulinemia and significantly increases lipogenesis mediated by sterol regulatory element-binding protein 1 (SREBP-1). Interestingly, it has been reported that sucralose is metabolized by rats into compounds that are less polar and more lipophilic than the parent compound. Those compounds remained in adipose tissue 2 wk after the last treatment, even when they were not detected in urine and feces (84, 86). Whether the sequestration of sucralose or its secondary metabolites in adipose tissue influences its metabolism remains to be demonstrated. Given that modifications in body composition are known to be associated with cardiovascular impairment, exploration of this phenomenon is of interest to determine if sucralose is associated with cardiovascular dysregulation.
Glucose Metabolism
Fasting glycemia, insulinemia, and glucose regulation
Findings detailing the effect of sucralose consumption on glucose regulation have been contradictory. Variations in the results depend not only on differences in the matrix containing sucralose, but also whether sucralose is ingested alone or if its consumption is followed by a caloric/glucose load to promote changes in glucose and insulin concentrations (22, 27, 93). In their systematic review of studies published from 1996 to 2012, Romo-Romo et al. (94) reported that sucralose consumption was not associated with changes in fasting glucose, insulin, or glycated hemoglobin (HbA1c) in humans; it should be noted, however, that most of the studies they reviewed had evaluated only acute effects of a single exposure to sucralose. The doses of sucralose used in these acute exposure studies were from 60 mg to 1000 mg and were administered via capsules or by using pure sucralose, commercial granular sucralose, or diet soda containing sucralose. These interventions were given alone or before a standard breakfast or an oral-glucose-tolerance test (OGTT) or via intragastric and intraduodenal infusions (74–76, 95–98). With regard to the effects of habitual sucralose consumption, 2 human studies from the same research group reported that 12 and 13 wk of sucralose consumption had no effect on HbA1c, fasting glucose, insulin, and C-peptide. It should be noted, however, that sucralose was administered daily using capsules containing 333 and 667 mg, respectively, in those studies (99, 100).
Four studies have shown a significant reduction in insulin sensitivity after sucralose exposure. Pepino et al. (101) found in 17 participants with morbid obesity that, compared with water consumption, a single exposure to 48 mg sucralose produced higher glucose, insulin, and C-peptide concentrations at specific time-points and a 23 ± 20% (P = 0.01) decrease in insulin sensitivity during a 5-h OGTT. Nichol et al. (102) also showed that an acute exposure to 48 mg of sucralose induced a 30 ± 10% higher glucose AUC (P = 0.03) after an OGTT compared with water in both normal-weight participants and in those with obesity. Moreover, insulin secretion was decreased 20–40 min after the beginning of the OGTT in normal-weight people, whereas it was increased at 90–120 min in individuals with obesity (P < 0.05). Interestingly, this latter work showed that, in both normal-weight and obese participants, only sucralose ingestion but not sucralose tasting and expectoration increased glucose AUC during OGTT. Yet, oral sensation on its own could also have metabolic consequences because sucralose tasting alone, before a glucose drink, significantly dampened the plasma insulin rise. Regarding the chronic effects, when healthy participants consumed daily capsules containing 200 mg of sucralose over a 4-wk period (103), there was a significant (P < 0.01) reduction in insulin sensitivity measured with the Matsuda index and the homeostasis model assessment of insulin sensitivity (HOMA-%S) and an increase in both the homeostasis model assessment of β-cell function (HOMA-%B) and the insulinogenic index during an OGTT. A decrease in insulin sensitivity was also demonstrated after an intravenous glucose tolerance test when healthy lean individuals were asked to consume sucralose (15% of the recommended ADI) over a period of 14 d (104). Finally, Dalenberg et al. (105) provided evidence that consumption of beverages containing 60 mg of sucralose and 31.83 g of maltodextrins over a period of 2 wk significantly increased the AUC of insulin (P < 0.01), but not with beverages containing only sucralose or only maltodextrins. This study was performed in adults and adolescents. However, the trial was suspended for the adolescents due to an important change in the HOMA-IR in the sucralose/maltodextrin group (from <3.5 to >12.9).
Different mechanisms explaining how sucralose could have an impact on glucose and insulin concentrations have been described, such as an increase in the activation of glucose transporters, the stimulation of incretin release via interaction with sweet taste receptors located in the intestine and in the pancreas, as well as modifications in the gut microbiota (106). Interestingly, sweet taste receptors are also found in pancreatic β cells (107). In vitro studies have shown that LCSs such as sucralose, acesulfame-K, and saccharin activate the TAS1R2/TAS1R3, thus stimulating insulin secretion (40). Although the interaction between LCS and pancreatic β cells does not generate ATP to depolarize the cell membrane and promote insulin secretion, activation of the sweet taste receptors induces this effect via cytoplasmic calcium and cAMP-dependent mechanisms. In studies with isolated pancreatic islets from mice, it has been observed that sucralose can potentiate the release of insulin in the presence of glucose (107). However, many studies have reported little or no absorption of sucralose in humans (50, 108). So, there is a low probability that sucralose interacts with the pancreatic β cells and its metabolic effects are most likely produced exclusively in the gastrointestinal tract (51).
Margolskee et al. (71) provided evidence in animal models that a low-carbohydrate diet supplemented with sucralose, as well as other LCSs (e.g., acesulfame-K and saccharin, but not aspartame), generated a greater expression of SGLT1 in enteroendocrine cells. This increased expression appears to be the consequence of the LCS interaction with the sweet taste receptors, TAS1R3 and α-gustducin, because this effect was not replicated in TAS1R3 and α-gustducin knockout mice. Mace et al. (39) found that LCS stimulated the apical expression of glucose transporter 2, regulated by the sweet taste receptors TAS1R2, TAS1R3, and α-gustducin, in the small intestines of rats. The greatest increase in glucose absorption caused by LCS was observed with acesulfame-K, followed by sucralose and, to a lesser extent, saccharin. These studies suggest that LCSs can promote both active and passive transport of glucose in the enterocyte.
A recent study performed in male Wistar rats reported that sucralose consumption over a period of 4 mo improved glycemic and insulinemic responses compared with sucrose-consuming rats. These effects were accompanied by reduced insulin receptor substrate 1 and protein kinase B phosphorylation and decreased expression of glucose transporter 4 and SREBP-1 in the basal situation (84).
For many years, including present times, sucralose has been considered not to alter glucose metabolism (26, 109). However, considering recent findings, there is controversy regarding the methodologies that have been used in these studies, which are not sensitive enough (i.e., fasting glucose and insulin, HbA1c, or OGTT) to guarantee that LCSs have a neutral effect on glucose homeostasis. In addition, many of these studies have been crossover trials with a single exposure to sucralose (28). There is a need to perform parallel-randomized clinical trials using more precise techniques such as the hyperinsulinemic euglycemic glucose clamp to identify early changes in variables like insulin sensitivity, which could predispose an individual to developing significant disturbances in glucose tolerance with a longer exposure to sucralose.
Incretin secretion: GLP-1 and GIP
Incretins are intestinal peptides that act as hormones, accounting for ∼50% of the postprandial insulin release in a glucose-dependent manner. The main incretins are GLP-1 and GIP. There is evidence that incretins have roles in neogenesis and prevention of β-cell apoptosis in the pancreas (110–112). The administration of GLP-1 in humans reduces food consumption by increasing the feeling of fullness, promoting weight loss in the long term. The mechanisms driving this effect include delayed gastric emptying and the regulation of food intake by the central nervous system (113–115).
Several in vitro and animal studies have indicated that sucralose specifically stimulates the secretion of GLP-1 and GIP. This increase in incretins is induced by the interaction between the sweet taste receptors TAS1R2, TAS1R3, and α-gustducin, located in enteroendocrine L and K cells (71, 72, 84, 116). Nonetheless, it could also be mediated by sucralose-stimulated increase in the expression of the SGLT1 transporter, as previously mentioned (71, 117). Some studies performed in small intestinal tissue of mice suggested that the effect of sucralose on incretins is dose-dependent (116), but other studies reported that higher amounts of sucralose do not potentiate the secretion of incretins (27, 72).
Several studies in humans have evaluated the effects of sucralose on the release of incretins, with contradictory results. In all studies that reported no effect on GLP-1 and GIP secretion, sucralose was consumed alone. The doses used in these trials ranged from 40 to 960 mg. All of them were crossover studies and they evaluated the effects of sucralose following a single dose of the sweetener (74–76, 97, 118). However, studies that performed an OGTT after sucralose consumption found higher GLP-1 concentrations, suggesting that the combination of this LCS with glucose potentiates the secretion of incretins, when compared with sucralose alone. The doses used in these trials ranged from 24 to 200 mg and each study was also a crossover design (73, 98, 103). It is important to note, however, that 2 studies performed an OGTT after sucralose consumption and found no change in GLP-1 and GIP concentrations (26, 101).
When sucralose was consumed habitually (200 mg/d for 4 wk), higher concentrations of GLP-1 were detected (103). However, it was recently reported that regular sucralose consumption in healthy people for a period of 2 wk did not modify the fasting plasma concentrations of appetite-regulating hormones like GLP-1, ghrelin, leptin, and PYY (119). Two of the studies that reported an effect of sucralose on GLP-1 secretion included participants with type 1 or type 2 diabetes mellitus. Higher concentrations of GLP-1 were detected only in participants with type 1 diabetes and in healthy participants, but not in those with type 2 diabetes (73, 98). Consequently, the increase in GLP-1 secretion could be beneficial for health in patients with type 2 diabetes due to its effect on appetite and insulin release. There have been no reports of an effect of sucralose on GIP in humans.
Leptin and ghrelin secretion
It is also suspected that sucralose alters leptin and ghrelin hormones, which have been recognized to have a major influence on energy balance. Leptin is a hormone produced mainly by the white adipose tissue and is stimulated by insulin. It is involved in appetite regulation and energy expenditure, stimulating the expression of anorexigenic neurons in the hypothalamus in response to increased fat mass (120–122). Individuals with obesity are characterized by hyperleptinemia (123). However, it has also been proposed that leptin signaling through its receptor could be impaired in obesity (124), especially when it is associated with metabolic syndrome (125).
Ghrelin is an orexigenic peptide produced in the stomach, mainly in the gastric fundus. It increases food consumption and promotes weight gain by decreasing fatty acid oxidation (120, 126). The concentrations of these 2 hormones rise during fasting or in a state of negative energy balance and fall with increases in food consumption or obesity, correlating negatively with BMI (122, 127).
An animal study and a clinical trial reported that sucralose consumption over a period of 12 and 2 wk, respectively, did not change leptin concentrations (26, 87). An in vitro study reported that ghrelin concentration is decreased in the presence of sugar, but increased when consumed with sucralose, without being mediated by sweet taste receptors or glucose transporters (128). Two clinical trials have reported that a single exposure of sucralose has no effect on ghrelin concentrations using doses of 62 and 420 mg (74, 96). A study in male C57BL/6 wild-type mice found no changes in ghrelin concentrations after 8 wk of exposure to sucralose (88).
Nevertheless, 3 studies in mice have found a significant increase in body weight after sucralose consumption compared with the control group, although the food intake was decreased in the animals that consumed this LCS (83, 84, 87). One of these studies reported an increase in the adipocyte size and in leptin expression, in addition to a decrease in the expression of adiponectin and the uncoupling protein 1, after 4 mo of exposure to sucralose; the same effects were observed with the high-caloric sweeteners sucrose, glucose, and fructose (84).
Gut microbiota
The gut microbiota is a collection of microorganisms that codevelop with the host from birth according to the host's intrinsic (genetics, health status, etc.) and environmental factors. It is clear that diet itself has an important influence on the composition of gut microbiota (129). The growing appreciation of the relation between gut microbiota and human health over recent years has also raised questions about the impact of habitual LCS consumption on gut microbiota. A primary in vitro observation is that several LCSs, including sucralose, exert strong bacteriostatic effects on the metabolism of many bacterial species found in the gastrointestinal tract (130–132). From an in vivo perspective, a second important consideration is the probability of the LCS interacting with gut microbiota. In this context, it is known that acesulfame-K and saccharin are quickly and readily absorbed into the systemic circulation, which greatly limits their interaction with the colonic microbiome. Sucralose, however, being poorly absorbed, moves through the gastrointestinal tract unchanged, making it a potentially more potent perturbing agent (50, 51, 86). However, this has not been confirmed. It should also be noted that so far no study has compared the relative in vivo impact of each sweetener on the microbiota.
To date, we have identified 5 preclinical studies assessing gut microbiota changes in response to the habitual consumption of pure sucralose or in the form of commercial sachets (Splenda) (30, 85, 133–135). All of these studies have been conducted on mice or rats, with exposure ranging from 1.1 to 15 mg/kg/d over 6 wk to 6 mo. Together, these studies provide convincing in vivo evidence that habitual sucralose consumption alters the gut microbiota composition (i.e., dysbiosis). In healthy models, the shortest exposures were associated with slight changes, whereas the study with the longest exposure was associated with more bacterial genera being impacted; suggesting that significant, observable microbiome remodeling in response to sucralose exposure can take time to occur. Changes in microbiome are mostly observed at the genus level with the relative presence of some species being up- or downregulated instead of a whole phylum being altered. In this context, all studies also concurrently reported dysbiosis after several weeks of sucralose exposure. However, data are inconsistent when it comes to the genera being impacted by sucralose consumption. Indeed, some studies even reported opposite variations for the same genus (i.e., Bifidobacterium) (85, 135). Yet, it is also unclear whether changes in microbiota induced by sucralose have any direct consequences on the host's health. Because many previous studies have indicated that dysbiosis is associated with propensity to metabolic diseases such as obesity and type 2 diabetes (136–138), there is hope for information indicating whether the sucralose impact on the microbiome would be expected to translate into a metabolic disorder or not. Most of the studies cited reported little to no blunting of the body mass gain in healthy or in diet-induced obese rodents. One study, however, reported an increase in body weight after the supplementation was delivered. Interestingly, in 2 studies, several microbiota metabolites were significantly different between the control mice and those receiving sucralose. Beyond its effect on the composition of the microbiome, there are data indicating that sucralose alters bacterial metabolism in vivo, with possible consequences for cholesterol metabolism and an increase in inflammation (133, 134). In contrast, only 1 study assessed the effects of sucralose consumption on the gut microbiota in humans. This randomized controlled trial included 34 participants receiving either sucralose capsules (780 mg/d) or a placebo over a period of 7 d. The gut microbiome was evaluated before and after the intervention, but there was no significant change following sucralose exposure. Nevertheless, changes were only evaluated at the phylum level (109). As a whole, there is accumulating preclinical evidence that long-term consumption of sucralose, even at levels within the ADI, can affect the gut microbiome. It is, however, important to note that data obtained in rodent models could lack clinical relevance because bacterial genera and species differ greatly between mice and humans. There is currently insufficient clinical evidence to exclude any impact of sucralose on the human microbiota. Future studies will have to clarify the effect of sucralose on the balance and diversity of the gut microbiota, as well as the potential functional consequences on bile acids and, thus, cholesterol metabolism, glucose metabolism, and overall health. Demonstration of causality between constituents of the microbiota and specific diseases remains to be determined.
Blood pressure, heart function, and circulation
Several recent epidemiological studies conducted within large US and European cohorts reported an association between habitual and high consumption of LCSs and a higher risk of hypertension, vascular heart diseases, and death from circulatory diseases (13–15). Despite these concerning findings, few studies have provided mechanistic or causal evidence for a direct participatory role of acute and, more importantly, long-term LCS consumption in the impairment of cardiovascular function. Yet, only a few trials with a very limited number of participants have been conducted. We identified only 3 studies aimed at testing the potential hemodynamic consequences of sucralose consumption in humans. One was a tolerance study in which sucralose was administered at doses ≤10 mg/kg/d. After 17 d, there was no change in blood pressure (139). A second study was designed to investigate the acute impact of sucralose in postprandial hypotension, a condition frequently associated with increased mortality in elders. The authors demonstrated that whereas glucose intraduodenal infusion induces a massive increase in superior mesenteric artery blood flow and a decrease in blood pressure, sucralose and saline infusion (4 mM) had no significant effect. Their results, therefore, suggest that it might be beneficial for elders with postprandial hypotension to replace glucose with sucralose (140). However, it is not known whether this effect is specific to sucralose or whether it could be extrapolated to other LCSs. A third study provided similar findings, reporting that flow-mediated dilatation of the brachial artery was not affected after acute consumption of sucralose (141). However, in this latter study, the dose of sucralose that was administered was not mentioned. Considered together, the evidence from these studies is insufficient to rule out the potential association between the habitual consumption of sucralose and an increase in cardiovascular risk. As mentioned before, more long-term and large-scale randomized controlled trials in humans are required. This need for a greater understanding of the interaction between sucralose consumption and cardiovascular health is also emphasized by some preclinical studies. For example, we reported marked vascular endothelial dysfunction in healthy rats after consumption of water sweetened with acesulfame-K and sucralose over a period of 10 wk at doses in line with the ADI (15 mg/kg/d) (89). Although this latter observation is based on the use of a cocktail, preventing any conclusion about the specific effect of sucralose, it is still substantial because of the pivotal role of endothelial dysfunction in atherosclerosis and its adverse complications. Similar concerns have also been recently raised by Chichger's group (142, 143), providing convincing in vitro evidence that sucralose can modulate various endothelial cell properties such as vascular permeability, proliferation, migration, adhesion, and tube formation via activation of TAS1R3. There is also a growing body of evidence that TAS2Rs, which can be stimulated by sucralose, are expressed and have a specific function in the cardiovascular system. Notably, Foster and Xin (44, 45, 144) have shown that activation of TAS2Rs results in negative inotropic and vasodilator effects. There is, thus, another possibility that by interacting with TAS2Rs, sucralose can alter the cardiovascular system and play a role in the development of cardiometabolic diseases. Yet the significance of the interaction between sucralose and the taste receptors remains to be determined in vivo and at the clinical level. Overall, there is a need to conduct additional well-designed, interventional studies to allow us to better understand epidemiological findings and clarify the long-term impact of consuming LCSs on blood pressure and vascular health, in consumers with or without cardiometabolic risk factors.
Sucralose Consumption During Pregnancy and Lactation
Sucralose persists in the bloodstream for >18 h after ingestion (50); during pregnancy, it can thus reach the placenta and developing fetus for prolonged periods. Sucralose and other LCSs have also been detected in breast milk (25). Together, these findings raise questions regarding the potential health impacts of maternal sucralose consumption during gestation and breastfeeding on the health of the fetus and infant, respectively. Indeed, exposure to LCSs during gestation or infancy has been suspected to induce long-term effects, with predisposition to metabolic disease being acquired or “programmed” early in life (65). In humans, regular consumption of LCSs by pregnant women has been associated with an elevation in BMI in their children (145). More specifically, the authors described how sucralose consumption by pregnant dams induced subsequent elevations in body weight, adiposity, and insulin resistance in their offspring, especially in males. Notably, in this same study, data from both a culture model of adipocyte differentiation (3T3-L1) and adipose tissue from offspring revealed upregulation of several proadipogenic regulators (e.g., CCAAT/enhancer binding protein alpha, fatty acid-binding protein 4, and fatty acid synthase) in conditions that had been exposed to sucralose, suggesting that sucralose could directly target adipose tissue. These morphological and metabolic disturbances induced by early-life sucralose exposure could potentially explain microbiota disturbances in the offspring. Indeed, 2 recent studies described microbiota dysbiosis induced by maternal sucralose consumption, one focusing on the effect of sucralose alone (146) and the other on sucralose mixed with another popular high-intensity sweetener, acesulfame-K (147). The first study demonstrated that maternal sucralose consumption significantly inhibited intestinal development and disrupted barrier function in 3-wk-old offspring. Furthermore, a high-fat diet combined with sucralose consumption exacerbated hepatic steatosis, disturbed fatty acid biosynthesis and metabolism, and induced gut dysbiosis in the progeny. Finally, maternal sucralose consumption promoted low-grade intestinal inflammation and significantly changed the compositions and diversity of the gut microbiota, including a reduction in butyrate-producing bacteria and cecal butyrate production with downregulation of G-protein-coupled receptor 43, an important regulator of inflammatory responses (146). These results were consistent with the second study, which showed a downregulation of hepatic detoxification mechanisms and changes in bacterial metabolites. Indeed, the microbiome profiling confirmed a significant increase in firmicutes and a striking decrease of Akkermansia muciniphila (147). The growing body of evidence surrounding the long-term effects of LCS consumption during pregnancy and throughout the postpartum period on the offspring requires further experimental and clinical studies. The findings from these studies could better inform not only concerned women, but also health organizations, dietary guidelines, and practitioners.
Conclusions
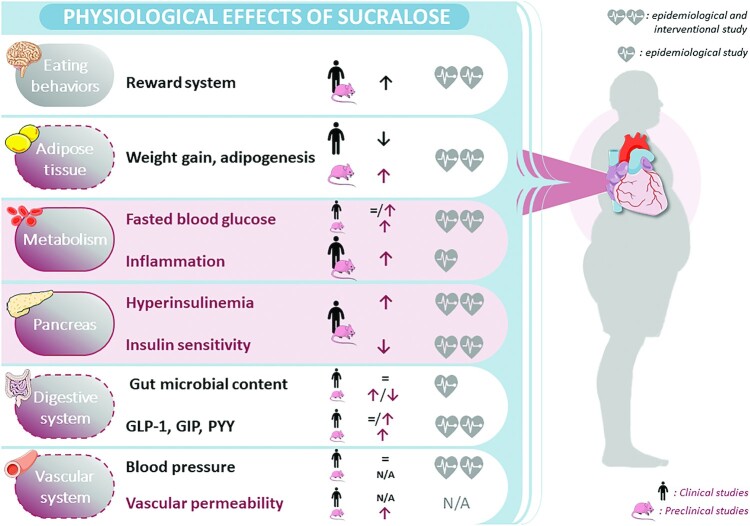
Due to several challenges in separately examining the mechanisms and impact of specific LCSs, most of the scientific literature, especially epidemiological studies, treats LCSs as a large family of molecules. Nevertheless, it is mandatory to remember that each variety of LCS differs markedly in its chemical structure, pharmacokinetic profile, and, more importantly, in its pharmacological activity. Because sucralose is one of the most widely used LCSs in food and drinks worldwide, this review focused on the specific effect of habitual sucralose consumption on cardiometabolic health (Figure 2). Although LCSs, including sucralose, target the same cellular receptors (i.e., TAS1R2/TAS1R3, TAS1R3/TAS1R3, and TAS2Rs), they have different affinities to those receptors, and an individual LCS such as sucralose can also behave as a biased agonist. Thus, sucralose is a specific pharmacological agent inducing its own physiological and pathological responses. Although, as noted earlier, sucralose has been detected in human plasma and breast milk, little is currently known about the distribution of sucralose in nondigestive tissues. This remains a key question to be addressed to better understand the effect of sucralose consumption on human health. Consequently, further research is needed to clarify what concentration of sucralose can be consumed before it has the potential to reach the bloodstream and thus directly act on the cardiovascular system. Noting this, prospective blinded clinical trials and preclinical studies are important to decipher the underlying mechanisms explaining the conflicting results regarding the metabolic and cardiovascular effects of sucralose consumption observed in animal and human studies. Altogether, the available data identified in this present review raise serious concerns, and strongly suggest that more research is needed to assess the potential adverse health impacts of habitual sucralose consumption and its role in increasing lifelong cardiometabolic disease risk.
FIGURE 2.
The effect of sucralose on digestive, cardiovascular, and metabolic physiology and its potential link with cardiovascular risk. The purple shading represents the effects of sucralose that could potentially explain the increase in total cardiovascular mortality risk. The level of proof is indicated with 1 or 2 hearts for epidemiological only or epidemiological and interventional studies, respectively. GIP, glucose-dependent insulinotropic peptide; GLP-1, glucagon-like peptide-1; N/A, data not available; PYY: peptide tyrosine-tyrosine.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—GW: developed the scientific question; SR, SB, and AR-R: performed the extraction and analysis of the data; SR, SB, AR-R, MR, and GW: interpreted results, prepared tables and figures, and drafted and finalized the manuscript; and all authors: read and approved the final manuscript.
Notes
This work was supported by Agence National de la Recherche (ANR #19-CE21-0003-01, to GW), Fondation Francophone pour la Recherche sur le Diabète (FFRD, to GW), sponsored by Fédération Française des Diabétiques (FFD), Abbott, AstraZeneca, Eli Lilly, Merck Sharp & Dohme (MSD) et Novo Nordisk , Avignon Université (to GW), and Conseil Regional Provence-Alpes-Côte d'Azur (to SR).
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
SR, SB, and AR-R contributed equally to this work.
Abbreviations used: acesulfame-K, acesulfame potassium; ADI, acceptable daily intake; EC50, half-effective concentration; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide 1; HbA1c, glycated hemoglobin; HOMA-%B, homeostasis model assessment of β-cell function; HOMA-%S, homeostasis model assessment of insulin sensitivity; LCS, low-calorie sweetener; OGTT, oral-glucose-tolerance test; PLC, phospholipase C; PYY, peptide tyrosine tyrosine; SGLT-1, sodium glucose cotransporter 1; SREBP-1, sterol regulatory element-binding protein 1; TAS1R, taste receptor type 1; TAS2R, taste receptor type 2.
Contributor Information
Sydney Risdon, Avignon University, LAPEC EA4278, Avignon, France.
Sylvain Battault, Avignon University, LAPEC EA4278, Avignon, France.
Alonso Romo-Romo, Department of Endocrinology and Metabolism, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, México City, México.
Matthieu Roustit, Université Grenoble Alpes, Inserm U1042, Grenoble, France; Grenoble Alpes University Hospital, Clinical Pharmacology, Inserm CIC1406, Grenoble, France.
Loic Briand, AgroSup Dijon, INRAE, Université de Bourgogne Franche-Comté, CNRS, Centre des Sciences du Goût et de l'Alimentation, Dijon, France.
Grégory Meyer, Avignon University, LAPEC EA4278, Avignon, France.
Paloma Almeda-Valdes, Department of Endocrinology and Metabolism, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, México City, México.
Guillaume Walther, Avignon University, LAPEC EA4278, Avignon, France.
References
- 1. WHO . Obesity and overweight. [Internet]. [cited 2017 Jul 6]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Google Scholar]
- 2. NCD Risk Factor Collaboration (NCD-RisC) . Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nwaneri C, Cooper H, Bowen-Jones D. Mortality in type 2 diabetes mellitus: magnitude of the evidence from a systematic review and meta-analysis. Br J Diabetes Vasc Dis. 2013;13:192–207. [Google Scholar]
- 5. WHO . Cardiovascular diseases (CVDs). [Internet]. [cited 2017 Jul 6]. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). [Google Scholar]
- 6. Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J, American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism and the Council on Epidemiology and Prevention, Dietary sugars intake and cardiovascular health. Circulation. 2009;120:1011–20. [DOI] [PubMed] [Google Scholar]
- 7. Malik V S, Popkin B M, Bray G A, J-P, Hu F B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Higgins KA, Mattes RD. A randomized controlled trial contrasting the effects of 4 low-calorie sweeteners and sucrose on body weight in adults with overweight or obesity. Am J Clin Nutr. 2019;109:1288–301. [DOI] [PubMed] [Google Scholar]
- 9. Vos M B, Kaar J L, Welsh J A, Van Horn L V, Feig D I, Anderson C AM, Patel M J, J C, Krebs N F, Xanthakos S Aet al. Added sugars and cardiovascular disease risk in children: a scientific statement from the American Heart Association. Circulation. 2017;135:e1017–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367:1397–406. [DOI] [PubMed] [Google Scholar]
- 11. Reid AE, Chauhan BF, Rabbani R, Lys J, Copstein L, Mann A, Abou-Setta AM, Fiander M, MacKay DS, McGavock Jet al. Early exposure to nonnutritive sweeteners and long-term metabolic health: a systematic review. Pediatrics. 2016;137(3):e20153603. [DOI] [PubMed] [Google Scholar]
- 12. Azad MB, Abou-Setta AM, Chauhan BF, Rabbani R, Lys J, Copstein L, Mann A, Jeyaraman MM, Reid AE, Fiander Met al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189:E929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malik VS, Li Y, Pan A, De Koning L, Schernhammer E, Willett WC, Hu FB. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139:2113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mullee A, Romaguera D, Pearson-Stuttard J, Viallon V, Stepien M, Freisling H, Fagherazzi G, Mancini FR, Boutron-Ruault M-C, Kühn Tet al. Association between soft drink consumption and mortality in 10 European countries. JAMA Intern Med. 2019;179:1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mossavar-Rahmani Y, Kamensky V, Manson JE, Silver B, Rapp SR, Haring B, Beresford SAA, Snetselaar L, Wassertheil-Smoller S. Artificially sweetened beverages and stroke, coronary heart disease, and all-cause mortality in the Women's Health Initiative. Stroke. 2019;50:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yin J, Zhu Y, Malik V, Li X, Peng X, Zhang FF, Shan Z, Liu L. Intake of sugar-sweetened and low-calorie sweetened beverages and risk of cardiovascular disease: a meta-analysis and systematic review. Adv Nutr. [Internet]2020;nmaa084. doi:10.1093/advances/nmaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Belloir C, Neiers F, Briand L. Sweeteners and sweetness enhancers. Curr Opin Clin Nutr Metab Care. 2017;20:279–85. [DOI] [PubMed] [Google Scholar]
- 18. Laffitte A, Neiers F, Briand L. Characterization of taste compounds: chemical structures and sensory properties. In: Guichard E, Salles C, Morzel M, Le Bon A-M, editors. Flavour: from food to perception. Oxford (UK): John Wiley & Sons, Ltd; 2016. p. 154–91. [Google Scholar]
- 19. Business Wire . Global zero-calorie sweetener market projected to be worth USD 2.84 billion by 2021 [Internet]. Technavio; 2019; [cited 2020 Oct 2]. Available from: https://web.archive.org/web/20190328081134mp_/https://www.businesswire.com/news/home/20170331005203/en/ [Google Scholar]
- 20. Anses (Agence Nationale de Sécurité Sanitaire de L'alimentation, de L'environnement et du Travail). Rapport Oqali: Bilan et évolution de l'utilisation des additifs dans les produits transformés. [Internet]. [cited 2020 Oct 20]. Available from: https://www.anses.fr/fr/content/rapport-oqali-bilan-et-%C3%A9volution-de-lutilisation-des-additifs-dans-les-produits-transform%C3%A9s [Google Scholar]
- 21. Maragkoudakis P. Sugars and sweeteners. [Internet]. EU Science Hub; 2017; [cited 2019 Mar 25]. Available from: https://ec.europa.eu/jrc/en/health-knowledge-gateway/promotion-prevention/nutrition/sugars-sweeteners [Google Scholar]
- 22. Tucker RM, Tan S-Y. Do non-nutritive sweeteners influence acute glucose homeostasis in humans? A systematic review. Physiol Behav. 2017;182:17–26. [DOI] [PubMed] [Google Scholar]
- 23. Hunter SR, Reister EJ, Cheon E, Mattes RD. Low calorie sweeteners differ in their physiological effects in humans. Nutrients. 2019;11:2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palatnik A, Moosreiner A, Olivier-Van Stichelen S. Consumption of non-nutritive sweeteners during pregnancy. Am J Obstet Gynecol. 2020;223:211–8. [DOI] [PubMed] [Google Scholar]
- 25. Sylvetsky AC, Gardner AL, Bauman V, Blau JE, Garraffo HM, Walter PJ, Rother KI. Non-nutritive sweeteners in breast milk. J Toxicol Environ Health, Part A. 2015;78:1029–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahmad SY, Friel JK, MacKay DS. The effect of the artificial sweeteners on glucose metabolism in healthy adults: a randomized double-blinded crossover clinical trial. Appl Physiol Nutr Metab. 2020;45(6):606–12. [DOI] [PubMed] [Google Scholar]
- 27. Pepino MY. Metabolic effects of non-nutritive sweeteners. Physiol Behav. 2015;152:450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Risdon S, Roustit M, Meyer G, Walther G. Is fasting blood glucose a reliable parameter to investigate the effect of non-nutritive sweeteners on glucose metabolism?. Eur J Clin Nutr. 2019;73:331–2. [DOI] [PubMed] [Google Scholar]
- 29. Magnuson BA, Roberts A, Nestmann ER. Critical review of the current literature on the safety of sucralose. Food Chem Toxicol. 2017;106:324–55. [DOI] [PubMed] [Google Scholar]
- 30. Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger Aet al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–6. [DOI] [PubMed] [Google Scholar]
- 31. Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJP, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–90. [DOI] [PubMed] [Google Scholar]
- 32. Behrens M, Briand L, de March CA, Matsunami H, Yamashita A, Meyerhof W, Weyand S. Structure-function relationships of olfactory and taste receptors. Chem Senses. 2018;43:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–3. [DOI] [PubMed] [Google Scholar]
- 34. Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99:4692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kojima I, Nakagawa Y, Ohtsu Y, Hamano K, Medina J, Nagasawa M. Return of the glucoreceptor: glucose activates the glucose-sensing receptor T1R3 and facilitates metabolism in pancreatic β-cells. J Diabetes Invest. 2015;6:256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang F, Klebansky B, Fine RM, Liu H, Xu H, Servant G, Zoller M, Tachdjian C, Li X. Molecular mechanism of the sweet taste enhancers. Proc Natl Acad Sci U S A. 2010;107:4752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Servant G, Tachdjian C, Tang X-Q, Werner S, Zhang F, Li X, Kamdar P, Petrovic G, Ditschun T, Java Aet al. Positive allosteric modulators of the human sweet taste receptor enhance sweet taste. Proc Natl Acad Sci U S A. 2010;107:4746–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Depoortere I. Taste receptors of the gut: emerging roles in health and disease. Gut. 2014;63:179–90. [DOI] [PubMed] [Google Scholar]
- 39. Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2: sweet taste receptors regulate apical GLUT2. J Physiol. 2007;582:379–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Santa-Cruz Calvo S, Egan JM. The endocrinology of taste receptors. Nat Rev Endocrinol. 2015;11:213–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Torregrossa A-M, Loney GC, Smith JC, Eckel LA. Examination of the perception of sweet- and bitter-like taste qualities in sucralose preferring and avoiding rats. Physiol Behav. 2015;140:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brune NEI, Slack JP, Ungureanu IM, Gray K, Simons CT, Pennimpede JEE. Methods to identify modulators using TAS2R bitter taste receptor. [Internet]. US patent US8597896B2. 2013 [cited 2020 Oct 20]. Available from: https://patents.google.com/patent/US8597896/en [Google Scholar]
- 43. Ariyasu T, Matsumoto S, Kyono F, Hanaya T, Arai S, Ikeda M, Kurimoto M. Taste receptor T1R3 is an essential molecule for the cellular recognition of the disaccharide trehalose. In Vitro Cell Dev Biol Anim. 2003;39:80–8. [DOI] [PubMed] [Google Scholar]
- 44. Foster SR, Porrello ER, Purdue B, Chan H-W, Voigt A, Frenzel S, Hannan RD, Moritz KM, Simmons DG, Molenaar Pet al. Expression, regulation and putative nutrient-sensing function of taste GPCRs in the heart. PLoS One. 2013;8:e64579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xin W, Chen Q. The cellular mechanism of bitter taste receptor mediated relaxation of rat aorta. FASEB J. 2017;31:672.5–672.5. [Google Scholar]
- 46. Xin W, Wang T, Jing Y, Fernandes VSL. The novel mechanism of bitter taste receptors attenuating rat ventricular contractility. FASEB J. 2018;32:839.10–839.10. [Google Scholar]
- 47. Nakagawa Y, Nagasawa M, Mogami H, Lohse M, Ninomiya Y, Kojima I. Multimodal function of the sweet taste receptor expressed in pancreatic β-cells: generation of diverse patterns of intracellular signals by sweet agonists. Endocr J. 2013;60:1191–206. [DOI] [PubMed] [Google Scholar]
- 48. Kojima I, Nakagawa Y, Ohtsu Y, Medina A, Nagasawa M. Sweet taste-sensing receptors expressed in pancreatic β-cells: sweet molecules act as biased agonists. Endocrinol Metab. 2014;29:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roura E, Foster S, Winklebach A, Navarro M, Thomas W, Campbell K, Stowasser M. Taste and hypertension in humans: targeting cardiovascular disease. Curr Pharm Des. 2016;22:2290–305. [DOI] [PubMed] [Google Scholar]
- 50. Roberts A, Renwick AG, Sims J, Snodin DJ. Sucralose metabolism and pharmacokinetics in man. Food Chem Toxicol. 2000;38:31–41. [DOI] [PubMed] [Google Scholar]
- 51. Magnuson BA, Carakostas MC, Moore NH, Poulos SP, Renwick AG. Biological fate of low-calorie sweeteners. Nutr Rev. 2016;74:670–89. [DOI] [PubMed] [Google Scholar]
- 52. Meddings JB, Gibbons I. Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology. 1998;114:83–92. [DOI] [PubMed] [Google Scholar]
- 53. Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Teixeira TFS, Collado MC, Ferreira C, Bressan J, Peluzio M do CG. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res. 2012;32:637–47. [DOI] [PubMed] [Google Scholar]
- 55. Turner JR, Cohen DE, Mrsny RJ, Madara JL. Noninvasive in vivo analysis of human small intestinal paracellular absorption: regulation by Na+-glucose cotransport. Dig Dis Sci. 2000;45:2122–6. [DOI] [PubMed] [Google Scholar]
- 56. Valle-Pinero AYD, Deventer HEV, Fourie NH, Martino AC, Patel NS, Remaley AT, Henderson WA. Gastrointestinal permeability in patients with irritable bowel syndrome assessed using a four probe permeability solution. Clin Chim Acta. 2013;418:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Frazier TH, DiBaise JK, McClain CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J Parenter Enteral Nutr. 2011;35:14S–20S. [DOI] [PubMed] [Google Scholar]
- 58. Jayashree B, Bibin YS, Prabhu D, Shanthirani CS, Gokulakrishnan K, Lakshmi BS, Mohan V, Balasubramanyam M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol Cell Biochem. 2014;388:203–10. [DOI] [PubMed] [Google Scholar]
- 59. Peng Y, Gillis-Smith S, Jin H, Tränkner D, Ryba NJP, Zuker CS. Sweet and bitter taste in the brain of awake behaving animals. Nature. 2015;527:512–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Beilharz JE, Maniam J, Morris MJ. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain Behav Immun. 2014;37:134–41. [DOI] [PubMed] [Google Scholar]
- 61. Yin K-J, Xie D-Y, Zhao L, Fan G, Ren J-N, Zhang L-L, Pan S-Y. Effects of different sweeteners on behavior and neurotransmitters release in mice. J Food Sci Technol. 2020;57:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schiffman SS, Booth BJ, Losee ML, Pecore SD, Warwick ZS. Bitterness of sweeteners as a function of concentration. Brain Res Bull. 1995;36:505–13. [DOI] [PubMed] [Google Scholar]
- 64. Wiet SG, Beyts PK. Sensory characteristics of sucralose and other high intensity sweeteners. J Food Sci. 1992;57:1014–9. [Google Scholar]
- 65. de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–41. [DOI] [PubMed] [Google Scholar]
- 66. Sclafani A, Marambaud P, Ackroff K. Sucrose-conditioned flavor preferences in sweet ageusic T1r3 and Calhm1 knockout mice. Physiol Behav. 2014;126:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Frank GKW, Oberndorfer TA, Simmons AN, Paulus MP, Fudge JL, Yang TT, Kaye WH. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage. 2008;39:1559–69. [DOI] [PubMed] [Google Scholar]
- 68. Tan H-E, Sisti AC, Jin H, Vignovich M, Villavicencio M, Tsang KS, Goffer Y, Zuker CS. The gut–brain axis mediates sugar preference. Nature. 2020;580:511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sclafani A, Zukerman S, Ackroff K. Postoral glucose sensing, not caloric content, determines sugar reward in C57BL/6J mice. Chem Senses. 2015;40:245–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Margolskee RF, Dyer J, Kokrashvili Z, Salmon KSH, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jang H-J, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim B-J, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova Met al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Temizkan S, Deyneli O, Yasar M, Arpa M, Gunes M, Yazici D, Sirikci O, Haklar G, Imeryuz N, Yavuz DG. Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in patients with type 2 diabetes. Eur J Clin Nutr. 2015;69:162–6. [DOI] [PubMed] [Google Scholar]
- 74. Steinert RE, Frey F, Töpfer A, Drewe J, Beglinger C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br J Nutr. 2011;105:1320–8. [DOI] [PubMed] [Google Scholar]
- 75. Wu T, Zhao BR, Bound MJ, Checklin HL, Bellon M, Little TJ, Young RL, Jones KL, Horowitz M, Rayner CK. Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am J Clin Nutr. 2012;95:78–83. [DOI] [PubMed] [Google Scholar]
- 76. Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, Bloom SR. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr. 2011;65:508–13. [DOI] [PubMed] [Google Scholar]
- 77. Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr. 2014;100:765–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rogers PJ, Hogenkamp PS, de Graaf C, Higgs S, Lluch A, Ness AR, Penfold C, Perry R, Putz P, Yeomans MRet al. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes. 2016;40:381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Laviada-Molina H, Molina-Segui F, Pérez-Gaxiola G, Cuello-García C, Arjona-Villicaña R, Espinosa-Marrón A, Martinez-Portilla RJ. Effects of nonnutritive sweeteners on body weight and BMI in diverse clinical contexts: systematic review and meta-analysis. Obes Rev. 2020;21(7):e13020. [DOI] [PubMed] [Google Scholar]
- 80. Toews I, Lohner S, Gaudry DK, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ. 2019;364:k4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Malik VS. Non-sugar sweeteners and health. BMJ. 2019;364:k5005. [DOI] [PubMed] [Google Scholar]
- 82. Tate DF, Turner-McGrievy G, Lyons E, Stevens J, Erickson K, Polzien K, Diamond M, Wang X, Popkin B. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial1234. Am J Clin Nutr. 2012;95:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Barrios-Correa AA, Estrada JA, Martel C, Olivier M, López-Santiago R, Contreras I. Chronic intake of commercial sweeteners induces changes in feeding behavior and signaling pathways related to the control of appetite in BALB/c mice. Biomed Res Int. 2018;2018:3628121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sánchez-Tapia M, Martínez-Medina J, Tovar AR, Torres N. Natural and artificial sweeteners and high fat diet modify differential taste receptors, insulin, and TLR4-mediated inflammatory pathways in adipose tissues of rats. Nutrients. 2019;11:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, McLendon RE, Schiffman SS. Splenda alters gut microflora and increases intestinal P-glycoprotein and cytochrome P-450 in male rats. J Toxicol Environ Health A. 2008;71:1415–29. [DOI] [PubMed] [Google Scholar]
- 86. Bornemann V, Werness SC, Buslinger L, Schiffman SS. Intestinal metabolism and bioaccumulation of sucralose in adipose tissue in the rat. J Toxicol Environ Health A. 2018;81:913–23. [DOI] [PubMed] [Google Scholar]
- 87. Rosales-Gómez CA, Martínez-Carrillo BE, Reséndiz-Albor AA, Ramírez-Durán N, Valdés-Ramos R, Mondragón-Velásquez T, Escoto-Herrera JA. Chronic consumption of sweeteners and its effect on glycaemia, cytokines, hormones, and lymphocytes of GALT in CD1 mice. Biomed Res Int. 2018;2018:1345282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Steensels S, Cools L, Avau B, Vancleef L, Farré R, Verbeke K, Depoortere I. Supplementation of oligofructose, but not sucralose, decreases high-fat diet induced body weight gain in mice independent of gustducin-mediated gut hormone release. Mol Nutr Food Res. 2017;61:1600716. [DOI] [PubMed] [Google Scholar]
- 89. Risdon S, Meyer G, Marziou A, Riva C, Roustit M, Walther G. Artificial sweeteners impair endothelial vascular reactivity: preliminary results in rodents. Nutr Metab Cardiovasc Dis. 2020;30:843–6. [DOI] [PubMed] [Google Scholar]
- 90. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. [DOI] [PubMed] [Google Scholar]
- 91. Chia CW, Shardell M, Tanaka T, Liu DD, Gravenstein KS, Simonsick EM, Egan JM, Ferrucci L. Chronic low-calorie sweetener use and risk of abdominal obesity among older adults: a cohort study. PLoS One. 2016;11:e0167241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kundu N, Domingues CC, Patel J, Aljishi M, Ahmadi N, Fakhri M, Sylvetsky AC, Sen S. Sucralose promotes accumulation of reactive oxygen species (ROS) and adipogenesis in mesenchymal stromal cells. Stem Cell Res Ther. 2020;11:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chan CB, Hashemi Z, Subhan FB. The impact of low and no-caloric sweeteners on glucose absorption, incretin secretion, and glucose tolerance. Appl Physiol Nutr Metab. 2017;42:793–801. [DOI] [PubMed] [Google Scholar]
- 94. Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez Díaz RA, Vilchis Valentín D, Almeda-Valdes P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: systematic review of observational prospective studies and clinical trials. PLoS One. 2016;11:e0161264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mezitis NH, Maggio CA, Koch P, Quddoos A, Allison DB, Pi-Sunyer FX. Glycemic effect of a single high oral dose of the novel sweetener sucralose in patients with diabetes. Diabetes Care. 1996;19:1004–5. [DOI] [PubMed] [Google Scholar]
- 96. Brown AW, Bohan Brown MM, Onken KL, Beitz DC. Short-term consumption of sucralose, a nonnutritive sweetener, is similar to water with regard to select markers of hunger signaling and short-term glucose homeostasis in women. Nutr Res. 2011;31:882–8. [DOI] [PubMed] [Google Scholar]
- 97. Ma J, Bellon M, Wishart JM, Young R, Blackshaw LA, Jones KL, Horowitz M, Rayner CK. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2009;296:G735–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Brown RJ, Walter M, Rother KI. Effects of diet soda on gut hormones in youths with diabetes. Diabetes Care. 2012;35:959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, Trout JR, Pi-Sunyer FX. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Assoc. 2003;103:1607–12. [DOI] [PubMed] [Google Scholar]
- 100. Grotz VL, Pi-Sunyer X, Porte D, Roberts A, Trout JR. A 12-week randomized clinical trial investigating the potential for sucralose to affect glucose homeostasis. Regul Toxicol Pharmacol. 2017;88:22–33. [DOI] [PubMed] [Google Scholar]
- 101. Pepino MY, Tiemann CD, Patterson BW, Wice BM, Klein S. Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care. 2013;36:2530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nichol AD, Salame C, Rother KI, Pepino MY. Effects of sucralose ingestion versus sucralose taste on metabolic responses to an oral glucose tolerance test in participants with normal weight and obesity: a randomized crossover trial. Nutrients. 2019;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lertrit A, Srimachai S, Saetung S, Chanprasertyothin S, Chailurkit L-O, Areevut C, Katekao P, Ongphiphadhanakul B, Sriphrapradang C. Effects of sucralose on insulin and glucagon-like peptide-1 secretion in healthy subjects: a randomized, double-blind, placebo-controlled trial. Nutrition. 2018;55-56:125–30. [DOI] [PubMed] [Google Scholar]
- 104. Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez-Díaz RA, Almeda-Valdes P. Sucralose decreases insulin sensitivity in healthy subjects: a randomized controlled trial. Am J Clin Nutr. 2018;108:485–91. [DOI] [PubMed] [Google Scholar]
- 105. Dalenberg JR, Patel BP, Denis R, Veldhuizen MG, Nakamura Y, Vinke PC, Luquet S, Small DM. Short-term consumption of sucralose with, but not without, carbohydrate impairs neural and metabolic sensitivity to sugar in humans. Cell Metab. 2020;31:493–502.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Romo-Romo A, Aguilar-Salinas CA, Gómez-Díaz RA, Brito-Córdova GX, Gómez-Velasco DV, López-Rocha MJ, Almeda-Valdés P. Non-nutritive sweeteners: evidence on their association with metabolic diseases and potential effects on glucose metabolism and appetite. Rev Invest Clin. 2017;69:129–38. [DOI] [PubMed] [Google Scholar]
- 107. Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y, Kojima I. Sweet taste receptor expressed in pancreatic β-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One. 2009;4:e5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rother KI, Sylvetsky AC, Walter PJ, Garraffo HM, Fields DA. Pharmacokinetics of sucralose and acesulfame-potassium in breast milk following ingestion of diet soda. J Pediatr Gastroenterol Nutr. 2018;66:466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Thomson P, Santibañez R, Aguirre C, Galgani JE, Garrido D. Short-term impact of sucralose consumption on the metabolic response and gut microbiome of healthy adults. Br J Nutr. 2019:122:856–62. [DOI] [PubMed] [Google Scholar]
- 110. Gromada J, Holst JJ, Rorsman P. Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide 1. Pflugers Arch. 1998;435:583–94. [DOI] [PubMed] [Google Scholar]
- 111. Campioni M, Toffolo G, Shuster LT, Service FJ, Rizza RA, Cobelli C. Incretin effect potentiates β-cell responsivity to glucose as well as to its rate of change: OGTT and matched intravenous study. Am J Physiol Endocrinol Metab. 2007;292:E54–60. [DOI] [PubMed] [Google Scholar]
- 112. Garber AJ. Incretin effects on β-cell function, replication, and mass. Diabetes Care. 2011;34:S258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gutzwiller J, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, Winterhalder R, Conen D, Beglinger C. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Asmar M. New physiological effects of the incretin hormones GLP-1 and GIP. Dan Med Bull. 2011;58:B4248. [PubMed] [Google Scholar]
- 115. Shah M, Vella A. Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord. 2014;15:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Daly K, Al-Rammahi M, Arora DK, Moran AW, Proudman CJ, Ninomiya Y, Shirazi-Beechey SP. Expression of sweet receptor components in equine small intestine: relevance to intestinal glucose transport. Am J Physiol Regul Integr Comp Physiol. 2012;303:R199–208. [DOI] [PubMed] [Google Scholar]
- 117. Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard Ret al. Na+-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ma J, Chang J, Checklin HL, Young RL, Jones KL, Horowitz M, Rayner CK. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br J Nutr. 2010;104:803–6. [DOI] [PubMed] [Google Scholar]
- 119. Romo-Romo A, Aguilar-Salinas CA, López-Carrasco MG, Guillén-Pineda LE, Brito-Córdova GX, Gómez-Díaz RA, Gómez-Pérez FJ, Almeda-Valdes P. Sucralose consumption over 2 weeks in healthy subjects does not modify fasting plasma concentrations of appetite-regulating hormones: a randomized clinical trial. J Acad Nutr Diet. 2020;120:1295–304. [DOI] [PubMed] [Google Scholar]
- 120. Williams J, Mobarhan S. A critical interaction: leptin and ghrelin. Nutr Rev. 2003;61:391–3. [DOI] [PubMed] [Google Scholar]
- 121. Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18:158–68. [DOI] [PubMed] [Google Scholar]
- 122. Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57:359–72. [DOI] [PubMed] [Google Scholar]
- 123. Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan Set al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–61. [DOI] [PubMed] [Google Scholar]
- 124. Morris A. Mechanisms of leptin resistance revealed. Nat Rev Endocrinol. 2018;14:628. [DOI] [PubMed] [Google Scholar]
- 125. Paz-Filho GJ, Volaco A, Suplicy HL, Radominski RB, Boguszewski CL. Decrease in leptin production by the adipose tissue in obesity associated with severe metabolic syndrome. Arq Bras Endocrinol. Metabol. 2009;53:1088–95. [DOI] [PubMed] [Google Scholar]
- 126. Côté CD, Zadeh-Tahmasebi M, Rasmussen BA, Duca FA, Lam TKT. Hormonal signaling in the gut. J Biol Chem. 2014;289:11642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ikezaki A, Hosoda H, Ito K, Iwama S, Miura N, Matsuoka H, Kondo C, Kojima M, Kangawa K, Sugihara S. Fasting plasma ghrelin levels are negatively correlated with insulin resistance and PAI-1, but not with leptin, in obese children and adolescents. Diabetes. 2002;51:3408–11. [DOI] [PubMed] [Google Scholar]
- 128. Steensels S, Vancleef L, Depoortere I. The sweetener-sensing mechanisms of the ghrelin cell. Nutrients. 2016;8:795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Graf D, Di Cagno R, Fåk F, Flint HJ, Nyman M, Saarela M, Watzl B. Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis. 2015;26:26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Omran A, Ahearn G, Bowers D, Swenson J, Coughlin C. Metabolic effects of sucralose on environmental bacteria. J Toxicol. 2013;2013:372986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pfeffer M, Ziesenitz SC, Siebert G. Acesulfame K, cyclamate and saccharin inhibit the anaerobic fermentation of glucose by intestinal bacteria. Z Ernahrungswiss. 1985;24:231–5. [DOI] [PubMed] [Google Scholar]
- 132. Prashant GM, Patil RB, Nagaraj T, Patel VB. The antimicrobial activity of the three commercially available intense sweeteners against common periodontal pathogens: an in vitro study. J Contemp Dent Pract. 2012;13:749–52. [DOI] [PubMed] [Google Scholar]
- 133. Uebanso T, Ohnishi A, Kitayama R, Yoshimoto A, Nakahashi M, Shimohata T, Mawatari K, Takahashi A. Effects of low-dose non-caloric sweetener consumption on gut microbiota in mice. Nutrients. 2017;9:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bian X, Chi L, Gao B, Tu P, Ru H, Lu K. Gut microbiome response to sucralose and its potential role in inducing liver inflammation in mice. Front Physiol. 2017;8:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wang Q-P, Browman D, Herzog H, Neely GG. Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. PLoS One. 2018;13:e0199080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen Det al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. [DOI] [PubMed] [Google Scholar]
- 137. Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NMet al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Baird IM, Shephard NW, Merritt RJ, Hildick-Smith G. Repeated dose study of sucralose tolerance in human subjects. Food Chem Toxicol. 2000;38(Suppl 2):123. [DOI] [PubMed] [Google Scholar]
- 140. Pham HT, Stevens JE, Rigda RS, Phillips LK, Wu T, Hausken T, Soenen S, Visvanathan R, Rayner CK, Horowitz Met al. Effects of intraduodenal administration of the artificial sweetener sucralose on blood pressure and superior mesenteric artery blood flow in healthy older subjects. Am J Clin Nutr. 2018;108:156–62. [DOI] [PubMed] [Google Scholar]
- 141. Memon MQ, Simpson EJ, Macdonald IA. Effect of fructose and sucralose on flow-mediated vasodilatation in healthy, white European males. J Pak Med Assoc. 2014;64:743–7. [PubMed] [Google Scholar]
- 142. Harrington EO, Vang A, Braza J, Shil A, Chichger H. Activation of the sweet taste receptor, T1R3, by the artificial sweetener sucralose regulates the pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2018;314:L165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lizunkova P, Enuwosa E, Chichger H. Activation of the sweet taste receptor T1R3 by sucralose attenuates VEGF-induced vasculogenesis in a cell model of the retinal microvascular endothelium. Graefes Arch Clin. Exp Ophthalmol. 2019;257:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Foster SR, Blank K, See Hoe LE, Behrens M, Meyerhof W, Peart JN, Thomas WG. Bitter taste receptor agonists elicit G-protein-dependent negative inotropy in the murine heart. FASEB J. 2014;28:4497–508. [DOI] [PubMed] [Google Scholar]
- 145. Azad MB, Archibald A, Tomczyk MM, Head A, Cheung KG, de Souza RJ, Becker AB, Mandhane PJ, Turvey SE, Moraes TJet al. Nonnutritive sweetener consumption during pregnancy, adiposity, and adipocyte differentiation in offspring: evidence from humans, mice, and cells. Int J Obes. 2020;44:2137–48. [DOI] [PubMed] [Google Scholar]
- 146. Dai X, Guo Z, Chen D, Li L, Song X, Liu T, Jin G, Li Y, Liu Y, Ajiguli Aet al. Maternal sucralose intake alters gut microbiota of offspring and exacerbates hepatic steatosis in adulthood. Gut Microbes. 2020;11:1043–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Olivier-Van Stichelen S, Rother KI, Hanover JA. Maternal exposure to non-nutritive sweeteners impacts progeny's. metabolism and microbiome. Front Microbiol. 2019;10:1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.