Abstract
Extracellular matrix deposition characterizes idiopathic pulmonary fibrosis (IPF) and is orchestrated by myofibroblasts. The lung mesenchyme is an essential source of myofibroblasts in pulmonary fibrosis. Although the transcription factor serum response factor (SRF) has shown to be critical in the process of myofibroblast differentiation, its role in development of pulmonary fibrosis has not been determined in vivo. In this study, we observed that SRF expression localized to mesenchymal compartments, areas of dense fibrosis, and fibroblastic foci in human (IPF and normal) and bleomycin-treated mouse lungs. To determine the role of mesenchymal SRF in pulmonary fibrosis, we utilized a doxycycline-inducible, Tbx4 lung enhancer (Tbx4LE)-driven Cre-recombinase to disrupt SRF expression in the lung mesenchyme in vivo. Doxycycline-treated Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f (and controls) were treated with a single intratracheal dose of bleomycin to induce pulmonary fibrosis and examined for lung mesenchymal expansion, pulmonary fibrosis, and inflammatory response. Bleomycin-treated Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f mice showed decreased numbers of Tbx4LE-positive lung mesenchymal cells (LMCs) and collagen accumulation (via hydroxyproline assay) compared with controls. This effect was associated with SRF-null LMCs losing their proliferative and myofibroblast differentiation potential compared with SRF-positive controls. Together, these data demonstrate that SRF plays a critical role in LMC myofibroblast expansion during bleomycin-induced pulmonary fibrosis. This sets the stage for pharmacological strategies that specifically target SRF in the lung mesenchyme as a potential means of treating pulmonary fibrosis.
Keywords: fibroblast, lung mesenchymal cell, pulmonary fibrosis, SRF, Tbx4
INTRODUCTION
Pulmonary fibrosis, such as idiopathic form of pulmonary fibrosis (IPF), is characterized by excessive extracellular matrix (ECM) accumulation that is driven by myofibroblasts. Selectively targeting the expansion and differentiation of these cells during tissue fibrogenesis is a potential strategy to disrupt tissue fibrosis. The development of cell- and ligand-specific therapeutics now enables highly directed therapeutic approaches (1–3). Thus, identification of critical cell lineage-specific mediators of in vivo lung fibrosis is essential to optimize therapeutic strategies.
We have previously observed that the transcription factor serum response factor (SRF) is critical in the process of myofibroblast differentiation in isolated human lung fibroblasts in ex vivo cultures (4). SRF is a member of the MCM1, Agamous, Deficiens, and SRF (MADS) box group of transcription factors that bind at CC[A/T]6GG (CArG) DNA sequences. SRF is activated by rho-associated kinase (ROCK) signaling and actin dynamics (5) to induce the antiapoptotic and contractile gene expression that characterizes myofibroblast differentiation (4, 6–8). Furthermore, increased SRF expression has been observed in the lung during bleomycin-induced fibrosis (9). However, determining the in vivo role of SRF during lung fibrosis has been challenging because its germline deletion results in embryonic lethality, due to the requirement for SRF in mesodermal differentiation (10), and conditional deletion of SRF using the myofibroblast marker transgelin (TGLN) results in disruption of enteric smooth muscle cell homeostasis and subsequent organ dysfunction (11).
We have previously shown that a transcriptional coactivator of SRF, myocardin-related transcription factor-A (MRTFA), is required for the development of bleomycin-induced pulmonary fibrosis (12). However, germline deletion of MRTFA modifies the early neutrophilic inflammation in response to bleomycin (12), and MRTFA and SRF have nonredundant functions. For example, ternary complex factors (TCF) under the control of mitogen-activated protein (MAP) kinases can compete with MRTFA for binding to SRF to induce immediate early response genes during proliferation (5, 13, 14). Thus, SRF may have pleotropic effects that are relevant to the lung mesenchyme’s response to tissue injury, and as such, its in vivo role in the expansion of the myofibroblast population and development of pulmonary fibrosis is not known.
The lung mesenchyme is an essential source of myofibroblasts in pulmonary fibrosis (15–17). Specification of the lung mesenchyme is enabled by the activity of a previously characterized 5.5 kb enhancer for the T-box 4 transcription factor (Tbx4) (18, 19). Coupling of this enhancer to a TetO/Cre system enables lineage-specific genetic modifications in the lung mesenchyme (19). In this study, we harnessed this approach to create a transgenic mouse that enables doxycycline-inducible, Cre-recombinase-mediated deletion of SRF in lung mesenchymal cells (LMCs). This approach allowed us to determine the mesenchymal-specific role of SRF in mediating myofibroblast expansion and differentiation from native LMC precursors, and the resulting effect on the development of bleomycin-induced pulmonary fibrosis.
METHODS
Mice
Animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Wisconsin-Madison and done in accordance with the National Institutes of Health and Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines. All animals were bred and housed in specific pathogen-free level 3 and animal biosafety level 1 facility. Animals were housed in static cages with Diamond Dri paper pellet bedding (Innovive, San Diego, CA) under controlled temperature (∼74°F), humidity (∼30%), and illumination (12-h light/12-h dark cycle), with unlimited access to laboratory chow and water. Animals were cared for by trained veterinarians and veterinary technicians and received daily health checks and weekly cage changes. Whenever possible, mice were housed in groups, with groups not exceeding more than five mice per cage. At experimental end point, mice were anesthetized with intraperitoneal injection of ketamine (200 mg/kg; Zoetis Inc., Parsippany-Troy Hills, NJ) and xylazine (20 mg/kg; Akorn Pharmaceuticals, Lake Forest, IL), exsanguinated, and perfused with 0.9% NaCl Irrigation (NS; Baxter, Madison, WI) and lungs collected for postprocessing.
Transgenic Mouse Strains
Tbx4LE-rtTA-LacZ mouse line was developed and described by Dr. Wei Shi (University of Southern California) (19). TetO-Cre mouse line was originally developed by Dr. Jeffrey Whitsett (Cincinnati Children’s Hospital) (20). tdTomato (tdTom) reporter mice were first developed by Dr. Hongkui Zeng (Allen Institute for Brain Science) (21), whereas membrane-targeted tdTomato/membrane-targeted enhanced green fluorescent protein (mT/mG) mice were developed by Dr. Liqun Luo (Stanford University) (22). SRF-flox (SRFf,f) mouse line was developed and shared by Dr. Joseph Miano (University of Rochester) (23). All of the mouse lines were bred on the C57BL/6J background, except for the mT-mG, which was on the stock background.
Tbx4LE-rtTA-LacZ, TetO-Cre, and tdTom (and SRFf,f) were crossed to obtain Tbx4LE-rtTA/TetO-Cre/tdTom and Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f mouse lines (see Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.12622037). mT-mG reporter was used for reporter studies (immunofluorescence) in Tbx4LE-rtTA/TetO-Cre/mT-mG and Tbx4LE-rtTA/TetO-Cre/mT-mG/SRFf,f colonies. All TetO-Cre-based colonies were regulated using the tetracycline analog, doxycycline (Sigma Aldrich, St. Louis, MO), in water (2 mg/mL + 50 mg/mL sucrose, Sigma Aldrich), replaced twice weekly. Sham control consisted of 50 mg/mL sucrose in water.
Bleomycin Treatment
Male and female mice (12 ± 3 wk of age) were anesthetized with intraperitoneal administration of ketamine (100 mg/kg) and xylazine (15 mg/kg) before delivering a single dose of intratracheal bleomycin (1 U/kg, Teva Pharmaceutical Industries, Israel) dissolved in 50-μL NS via endotracheal intubation. Control animals were treated with a single intratracheal dose of 50-μL NS and kept in cages separate from animals treated with bleomycin. When possible, littermate controls were utilized. When divergent genotype comparisons were required, age-matched, contemporaneous controls were utilized. Mouse weight and survival following treatment were recorded daily until end point (up to 21 days). One-way ANOVA with Holm–Šídák test (P < 0.05) was used for statistical analysis of weight loss. Log rank test (P < 0.05) was used for statistical analysis of survival.
Hydroxyproline Assay
Hydroxyproline assay was performed similarly as before (12, 24–26). Briefly, whole lungs were homogenized into double distilled H2O (ddH2O, Sigma Aldrich) by dounce homogenization, and subsequently hydrolyzed in 6 N HCl for 3 h at 120°C. After cooling to room temperature (RT), samples were centrifuged to remove debris, aliquoted in triplicates into 96-well optical plate (Greiner bio-one, 655101) and dried at 65°C for 45 min. Samples were then incubated with chloramine T solution at room temperature (RT) for 20 min before adding Ehrlich’s solution and incubating at 65°C for 15 min. After cooling, sample and standard absorbance were measured using a colormetric plate reader at 550 nm. Standard curve generated from trans-4-Hydroxy-l-proline (Sigma-Aldrich) was utilized to calculate hydroxyproline content. One-way ANOVA with Holm–Šídák test (P < 0.05) was used for statistical analyses.
Histology
Deidentified human tissue specimens of normal (nonfibrotic) and fibrotic (IPF) lungs were obtained from thoracic surgical resection samples at the Carbone Cancer Center Translational Science BioCore at the University of Wisconsin-Madison (IRB Approval No. 2011-0840). Lung samples were histologically assessed to ensure either normal tissue architecture and absence of occult disease for nonfibrotic tissue, or a usual interstitial pneumonia (UIP) pattern of disease for fibrotic tissue. Normal and fibrotic tissues were subject to paraffin embedding, sectioning, and immunostaining against SRF and smooth muscle α actin (αSMA) antibodies with horseradish peroxidase (HRP)-labeled secondary antibodies and ChromoMap 3,3-diaminobenzidine (DAB) detection step. Harris hematoxylin counterstain was performed for nuclear labeling. As negative controls, appropriate immunoglobulins along with matching secondary antibodies and detection steps were applied. Stained slides were scanned using Aperio Digital Pathology Slide Scanner System and snapshots acquired in Aperio ImageScope (Leica Biosystems, Wetzlar, Germany).
Mouse right lungs were inflated using 10% neutral buffered formalin (Fisherbrand, Pittsburg, PA) and fixed for 24–48 h before being submerged in 70% ethanol. After paraffin embedding and sectioning, lungs were subject to Masson’s trichrome staining and immunostaining against SRF, αSMA, red fluorescent protein (RFP), mCherry, Desmin, prosurfactant protein C (Pro-SPC), neural/glial antigen 2 chondroitin sulfate proteoglycan (NG2), green fluorescence protein (GFP), or cleaved caspase 3. As negative controls, appropriate immunoglobulins along with matching secondary antibodies were applied. Immunofluorescence images (against αSMA, RFP, mCherry, Desmin, Pro-SPC, NG2; Fig. 3C) were acquired contemporaneously by blinded observers using an Olympus 1X71 fluorescent microscope (Shinjuku City, Tokyo, Japan) and Qimaging Retiga 2000 R camera (Tucson, AZ). GFP fluorescence images [at least 10 fields of view (FOV) at ×20 magnification per mouse and 3 mice per group] were captured by blinded observers with a Nikon Ti Eclipse microscope (Minato City, Tokyo, Japan) and Hamamatsu C11440 digital camera (Hamamatsu City, Japan). Relevant groups of fluorescence images (including immunoglobulin and NS controls) were acquired and postprocessed using identical exposure times and thresholds on two occasions immediately following immunofluorescence staining. Parenchymal regions were isolated and integrated density measured in each image using ImageJ (27). Mean values from each animal in the group (n = 3/condition) were combined and compared using Unpaired Student’s t test (P < 0.05).
Bright-field images of Masson’s trichrome staining were acquired using Aperio Digital Pathology Slide Scanner System and snapshots were taken in Aperio ImageScope. To quantify fibrosis, modified Ashcroft scoring was done by blinded observers from Masson’s trichrome stains (at least 20 FOV at ×20 magnification per mouse from at least 4 animals/condition), as previously described (28). The data were analyzed by Mixed Model ANOVA (P < 0.05) with a random intercept being allowed using a random statement in SAS v9.4 proc mixed with all other default settings applied.
Bronchoalveolar Lavage
Bronchoalveolar lavage (BAL) fluid was extracted from mouse lungs 5 days after doxycycline and 3 days after intratracheal bleomycin (or NS) treatment. Collection of BAL fluid was done as before (12). Briefly, mice were anesthetized, exsanguinated (as described in section Mice), and a catheter was inserted into the exposed trachea to intubate the mice. Lungs were lavaged five times using 0.8–1 mL of Dulbecco's phosphate-buffered saline (DPBS; Corning Inc., Corning, NY), BAL cells isolated, counted, and cytospins prepared. Cytospins were stained using Wring Giemsa stain (Fisher Scientific, Hampton, NH), counted by blinded observers to determine the ratio of neutrophils, macrophages, lymphocytes, and eosinophils out of a total of 200 cells. One-way ANOVA with Holm–Šídák test (P < 0.05) was used for statistical analysis.
Isolation of Mouse Lung Fibroblasts
Mouse lung fibroblasts (MLFs) were derived, as described previously (4, 12). Briefly, extracted lungs were minced, washed in cell culture medium, composed of Dulbecco’s modified Eagle medium (DMEM) + l-glutamine (Corning Inc.), 100 U/mL streptomycin, 250 ng/mL amphotericin B, 100 U/mL penicillin (PSA, Mediatech, Herndon, VA), 10 μg/mL ciprofloxacin (Corning Inc.), and 2 mM l-glutamine (Thermo Scientific, Waltham, MA) supplemented with 20% fetal bovine serum (FBS, HyClone, Buckinghamshire, UK), and plated in collagen-coated [0.1 mg/mL PureCol Bovine Collagen Solution, Type 3 (Advanced Biomatrix, Carlsbad, CA)] cell culture plastic dishes to attach. The cell culture media supplemented with 20% FBS was replaced twice weekly until fibroblasts reached a confluence of 80%–90%. At this point, the cells were lifted using Trypsin (Sigma-Aldrich) and passaged or utilized for further studies.
β-Galactosidase Staining
Mouse lungs were inflated with 2% low-melt agarose at 37°C, extracted from the chest cavity, and immediately placed in phenol-red-free cell culture medium, as described in section Isolation of Mouse Lung Fibroblasts, supplemented with 10% FBS. Still on ice, the lungs were subsequently sliced to 150 μm thickness using Vibratome (Leica VT1200 S) at the speed of 0.5 mm/s. Sliced tissue was then washed in DPBS, followed by a wash in X-gal buffer (5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2; all from Sigma Aldrich), and finally incubated in X-gal, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (1:50 in X-gal buffer, Promega Corp., Madison, WI), at 37°C for 24 h. After this time, the lungs were washed in X-gal buffer, followed by DPBS, and imaged under ×40 magnification using Olympus IX70 microscope and DP21 camera.
Fluorescence-Activated Cell Sorting
For whole lung fluorescence activated cell sorting (FACS), mice were anesthetized, exsanguinated (as described in section on Mice), and intubated by placing a catheter into the exposed trachea. Lungs were lavaged once with 1 mL of DPBS (lavage was discarded) and inflated with 1 mL of digest medium, which consisted of cell culture medium (described in Isolation of Mouse Lung Fibroblasts) supplemented with 10% FBS, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, Cellgro, Mediatech, Inc. Manassas, VA), 250 U/mL Collagenase Type I (Worthington Biochemical Corp., Lakewood, NJ), and 50 U/mL DNase (Worthington Biochemical Corp.). Lungs were then submerged in 4-mL digest medium, minced and incubated at 37°C for 15 min before quenching the reaction with 2 mM sterile ethylenediaminetetraacetic acid (EDTA, Sigma Aldrich) at 37°C for 15 min. Lung pieces were passed through a 70-µm cell strainer (Fisher Scientific), rinsed with FACS buffer (25 mM HEPES and 2% FBS in DPBS), buffers removed after centrifugation (1,200 rpm for 5 min at 4°C), and red blood cells lysed with 1 mL of Red Blood Cell Lysing Buffer (Sigma Aldrich) for 5 min at RT. Subsequently, buffers were removed by centrifugation, cells counted, FACS buffer volume adjusted to 1–10 million cells/mL, cells strained and 0.05 µg/mL of 4′,6-diamidino-2-phenylindole (DAPI, Sigma Aldrich) was added to eliminate dead cells, and enable gating during FACS.
For MLFs FACS, MLFs were lifted off plastic dishes using Accutase (Sigma Aldrich) supplemented with 10 mM EDTA for 10 min at 37°C, centrifuged (1,200 rpm for 5 min at RT), counted, resuspended in FACS buffer at a concentration of 1–10 million cells/mL, strained, and treated with 0.05 µg/mL DAPI.
FACS was performed to isolate tdTom-positive and tdTom-negative cells with BD FACS AriaII BSL-2 Cell Sorter using 130 µm 14 pounds per square inch (psi) nozzle. Same lot of rainbow beads (Spherotech, Lake Forest, IL) was used to set system voltages based on consistent mean fluorescence intensity (MFI) and provide uniform cutoff between tdTom-positive and tdTom-negative fluorescence for sorts that were not performed on the same day. TdTom-positive gates were set using a negative control. All cells were kept at 4°C during the sort. Sorted MLFs were seeded onto collagen-coated plates with maintenance medium for further passaging. Cells from whole lung and MLF sorts were centrifuged (1,200 rpm for 10 min at 4°C), supernatants removed, cells resuspended in 1-mL RNA Stat-60 (Amsbio, Abingdon, UK) in RNase-free tubes and immediately frozen at −80°C.
Reverse Transcription Quantitative Real-Time PCR
Sorted cells were lysed for RNA in RNA STAT-60 either immediately upon FACS or within nine passages postsorting as before (29). Approximately 1 µg of total RNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time PCR analysis was performed using iTaq SYBR Green supermix (Bio-Rad) in an ABI 7500 multicolor real-time PCR detection system (Applied Biosystems, Foster City, CA). PCR primers were Srf: AGACGGGCATCATGAAGAAG (forward), GGGTGGCAAAGGTATACACA (reverse); Actin alpha 2 (Acta2): AAACAGGAATACGACGAAG (forward), CAGGAATGATTTGGAAAGGA (reverse); Collagen 1A2: GAACGGTCCACGATTGCATG (forward), GGCATGTTGCTAGGCACGAAG (reverse); c-fos: GGGGACAGCCTTTCCTA (forward), CTGTCACCGTGGGGATAAAG (reverse); Glucuronidase β (Gusb): CCGACCTCTCGAACAACCG (forward), GCTTCCCGTTCATACCACACC (reverse); Glyceraldehyde 3-phosphate dehydrogenase (Gapdh): GCCCAAGATGCCCTTCAGTG (forward), CATCCACTGGTGCTGCCAAG (reverse). Data are expressed as fold change using comparative cycle threshold (ΔΔCT) method. Unpaired or paired Student’s t test (P < 0.05) was utilized for assessment of statistical significance, as appropriate (detailed in figure legends).
Proliferation Assay
MLFs proliferation assay was performed as before (30). Briefly, 1 × 104 MLFs were plated into wells of 96-well plate in 100 µL maintenance medium supplemented with 20% FBS. After 6 h, medium was replaced with maintenance medium supplemented with bovine serum albumin (0.1%; Sigma Aldrich), overnight. The following day, cells were incubated in maintenance medium supplemented with 1% FBS for 30 min before incubating in Bromodeoxyuridine (BrdU) for 24 h and performing BrdU cell proliferation assay (Calbiochem, EDM Millipore Corp., San Diego, CA), as per manufacturer direction. Dual wavelength (450–550 nM) absorbance measurements were made using a spectrophotometric plate reader. Every condition was repeated in triplicates, and control conditions treated with no BrdU were subtracted from measured absorbance. Paired Student’s t test (P < 0.05) was utilized for assessment of statistical significance between tdTom-positive and tdTom-negative FAC-sorted samples.
Western Blotting
Sorted cells were lysed for protein either immediately after FACS or within five passages of the sort in radioimmunoprecipitation assay (RIPA) buffer containing 20 mM Tris HCl (Sigma Aldrich), 150 mM NaCl (Sigma Aldrich), 1% Triton-X 100 (Fisher Scientific), 0.1% SDS (Fisherbrand), 2 mM EDTA, 2 mM egtazic acid (EGTA, Sigma Aldrich), 10% glycerol (Fisherbrand), 0.25% Deoxycholate (FisherBrand), 200 µM phenylmethylsulfonyl fluoride (PMSF, Sigma Aldrich), 200 μM Na-orthovanadate (ICN Biomedical Inc., Costa Mesa, CA), 1 mM Na-phyrophosphate (Sigma Aldrich), and protease inhibitor cocktail (Thermo-Scientific) for 10 min on ice. The cells were then lifted, sonicated, and centrifuged at 21.1 g for 10 min at 4°C. The supernatants were mixed with Laemmli buffer, boiled for 5 min, electrophoresed on polyacrylamide gels at 150 V for 1 h, and transferred at 100 V for 1 h, as described previously (12). Western blot (WB) was run with indicated primary and corresponding HRP-conjugated secondary antibodies and developed by an enhanced chemiluminescence (ECL) reaction (GE, Boston, MA). Images were obtained using GE LAS4000 chemiluminescence imager. Densitometry of selected blots was analyzed via ImageJ (27). Unpaired Student’s t test (P < 0.05) was utilized to assess statistical significance.
Antibodies
Rabbit polyclonal antibody against αSMA (ab5694; RRID AB_2223021; used for mouse IHC) (31), rabbit monoclonal antibodies against GFP (ab183734; RRID AB_2732027) (32), and desmin (ab32362; RRID AB_731901) (33) were from Abcam (Cambridge, UK). Rabbit polyclonal antibodies against cleaved caspase 3 (9661; RRID AB_331595) (34) and α/β Tubulin (2148; RRID AB_2288042) were from Cell Signaling (Danvers, MA). Rabbit polyclonal antibodies against Pro-SPC (AB3786; RRID AB_91588) (35) and NG2 (AB5320; RRID AB_91789) (36) were from Millipore (Burlington, MA). Goat polyclonal antibody against mCherry (AB0081-20; RRID AB_2333094) (37) was from SicGen Antibodies (Carcavelos, Portugal). Rabbit polyclonal antibody against SRF (HPA001819; RRID AB_1079928; used for human and mouse IHC) (38) and mouse monoclonal antibody against αSMA (A5228; RRID AB_262054; used for human IHC, mouse immunofluorescence staining, WB) (39) were from Sigma Aldrich. Rabbit polyclonal antibody against RFP (600-401-379; RRID AB_2209751) (40) was from Rockland Immunochemicals (Limerick, PA).
Statistics
Methods of statistical analysis are listed under each assay and in figure legends.
RESULTS
SRF Expression Is Increased in Fibrotic Areas of Human and Mouse Lung
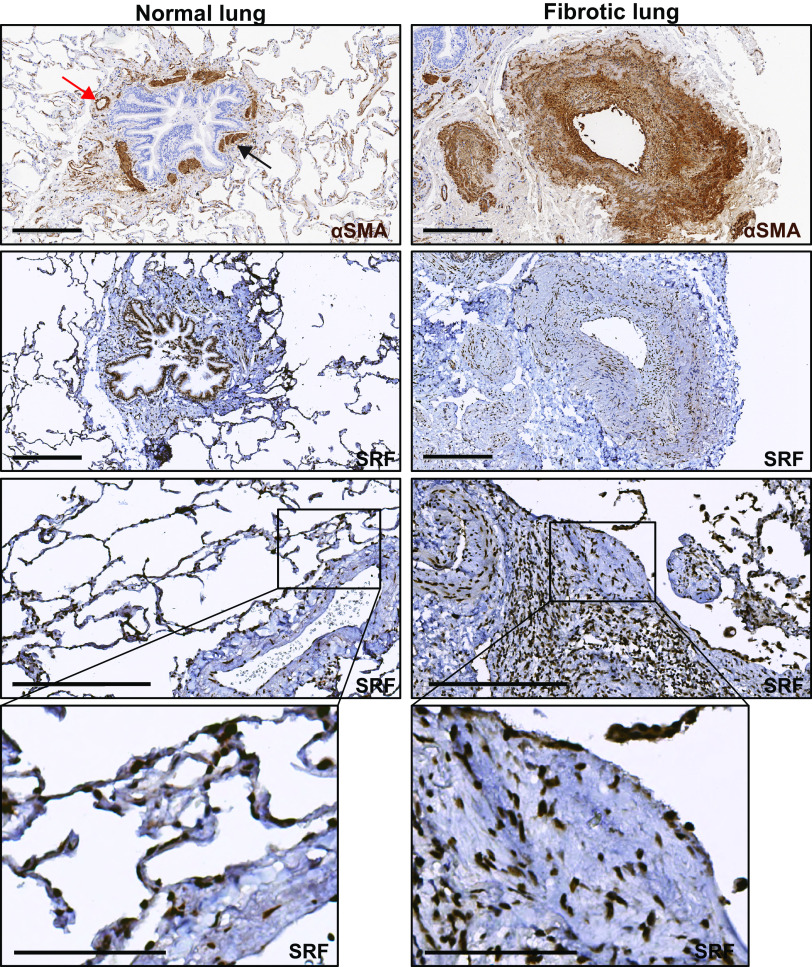
SRF is a ubiquitous, nuclear-localized transcription factor. However, differential expression and intensity of nuclear staining are apparent in individual tissue compartments. We examined the immunohistochemical staining of SRF in normal and fibrotic lung. In normal lung, there is intense nuclear staining of SRF in smooth muscle α actin (αSMA, encoded by Acta2 genes)-positive smooth muscle cell bands surrounding the airways and vasculature (Fig. 1, left column, row 1 and 2). There is also a more faint, diffuse cytoplasmic staining of pseudostratified columnar epithelium that has been described (https://www.proteinatlas.org/ENSG00000112658-SRF/tissue/bronchus) but may be nonspecific. In addition, there is faint nuclear staining in the cells of the alveolar compartment of the lung (Fig. 1, left column, row 3 and 4). In fibrotic human lung, similar to normal lung, there are areas of intense nuclear SRF staining in the smooth muscle layer of the airway and pulmonary arterial walls. In these areas, there is expansion of the αSMA-positive layer around multiple vessels corresponding with intense SRF staining (Fig. 1, right column, row 1 and 2). In addition, we observed strong staining for SRF in the nuclei of cells in structures morphologically consistent with fibroblastic foci (Fig. 1, right column, row 3 and 4). In fibrotic lung, less intense nuclear SRF staining is also apparent in the scattered collections of mixed cell population residing in the fibrotic lung interstitium (Fig. 1, right column, row 3).
Figure 1.
Serum response factor (SRF) expression is increased in fibrotic regions of human idiopathic pulmonary fibrosis (IPF). Immunohistochemical staining of normal and IPF lung samples (n = 3 subjects for each condition) against smooth muscle α actin (αSMA) and SRF. First row: normal (left) and fibrotic (right) lung samples were stained against αSMA. Normal lung (first row, left) shows increased αSMA stain predominantly in the muscularized bands of large airway (black arrow) and vessel (red arrow). SRF staining of normal samples (left column, second through fourth rows) shows localization of SRF staining to the same airway muscle bands (second row), vessel walls, and some nuclei of the alveolar walls (third and fourth rows). Right column shows increased αSMA stain (first row) in the fibrotic interstitium and in the expanded interstitium around a large vessel. SRF staining of fibrotic samples (right column, second through fourth rows) shows localization of increased nuclear SRF staining in the areas of αSMA stain in the fibrotic interstitium and vessel wall. Areas that are morphologically consistent with a fibroblastic focus (third and fourth row) show SRF staining both within and in the surrounding interstitium. Harris hematoxylin counterstain was performed for nuclear labeling in all stains. Scale bar = 300 μm (rows 1–3). Fourth row: digitally zoomed regions of increased expression of SRF in the blood vessel (left, normal) and fibroblastic focus (right, fibrotic). Scale bar = 100 μm (row 4).
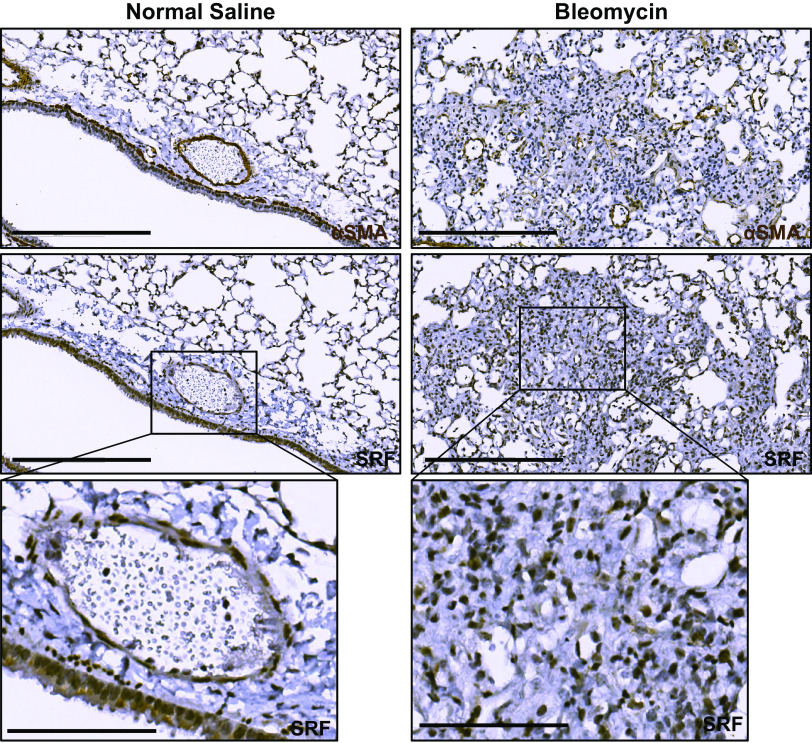
In the murine lung, previous investigators have identified upregulation of Srf gene expression in the bleomycin model of pulmonary fibrosis (9). Similar to human lung, immunohistochemical staining of uninjured adult murine lung demonstrate localization of SRF to the αSMA-positive smooth muscle layer of vascular and airway structures (Fig. 2, left column). However, in adult mouse lungs that were isolated 14 days after the administration of intratracheal bleomycin, we also observed nuclear SRF staining in cells located in fibrotic areas, where αSMA staining was increased (Fig. 2, right column). This suggests that there is expansion of an SRF-positive population of cells during the development of bleomycin-induced fibrosis. In light of these observations, previous studies showing upregulation of SRF in the murine bleomycin model (9), and our previous in vitro investigations demonstrating both upregulation of SRF expression and activity in human lung fibroblasts during myofibroblast differentiation (4, 7), we hypothesized that targeted disruption of SRF expression in a critical myofibroblast precursor cell lineage would modify the global fibrotic response in the lung after bleomycin-induced injury.
Figure 2.
Serum response factor (SRF) expression is increased in the fibrotic regions of bleomycin-induced pulmonary fibrosis. Wild-type mice were intratracheally treated with 0.9% normal saline (left column) or 1 U/kg bleomycin (right column) (n = 3 animals for each condition). Fourteen days later, mice were euthanized and isolated lungs were fixed with 10% neutral buffered formalin, paraffin embedded, and subject to immunohistochemical staining against smooth muscle α actin (αSMA) and SRF. Left column: normal saline-treated, uninjured mouse lungs stained against αSMA (first row) showed increased expression surrounding airways and blood vessels. Matching histologic areas demonstrate increased SRF staining in the smooth muscle layer of blood vessels and airways (matching αSMA expressing cells), as well as faint staining in the columnar epithelium of the airway (row two and three). In fibrotic mouse lung (right column), there is increased αSMA staining of consolidated and fibrotic lung parenchyma (first row). SRF immunostaining of matched histologic samples shows increased staining in the same areas of the fibrotic parenchyma (second and third rows). Harris hematoxylin counterstain was performed for nuclear labeling in all stains. Scale bar = 300 μm (rows 1 and 2). Third row: digitally zoomed regions of increased expression of SRF in the blood vessel and part of airway (left, normal) and fibroblastic focus (right, fibrotic). Scale bar = 100 μm (row 3).
Lung Mesenchymal Cell Populations Expand in Response to Bleomycin
Myofibroblast populations in the murine lung during bleomycin-induced fibrosis originate from various lung mesenchymal sources including pericytes, lipofibroblasts, and resident interstitial fibroblasts (15, 41, 42). The Tbx4 lung enhancer (Tbx4LE), a 5.5-kb region in the Tbx4 gene locus, enables lineage marking of the lung mesenchyme (18, 19), which includes interstitial lung fibroblasts, lipofibroblasts, pericytes, and vascular smooth muscle cells (41). The Tbx4LE lung mesenchyme is expanded in response to bleomycin, representing a promising target cell population to modify the fibrotic response (17).
Mice harboring a transgene containing the 5.5 kb Tbx4LE in frame with an Internal Ribosome Entry Site (IRES)-LacZ sequence and a reverse tetracycline transactivator (Tbx4LE-rtTA) cassette have been described (19). When coupled with a transgene specifying Tet operon-driven Cre-recombinase (TetO-Cre), this transgenic setup enables inducible Cre-expression in LMCs (19). To enable lineage tracing of LMCs in vivo and assess the response of this population during lung injury and fibrosis, we crossed Tbx4LE-rtTA/TetO-Cre mice with either Rosa-tandem dimer Tomato (tdTom) or membrane-targeted tdTomato/membrane-targeted enhanced green fluorescent protein (mT/mG), resulting in Tbx4LE-rtTA/TetO-Cre/tdTom or Tbx4LE-rtTA/TetO-Cre/mTmG mouse lineages, respectively (Supplemental Fig. S1A).
To determine how the LMC population responds to bleomycin, we leveraged the IRES-LacZ sequence in line with Tbx4LE-rtTA (Supplemental Fig. S1A). We subjected 150 µM precision-cut lung slices (PCLS) from uninjured mice to a β-galactosidase assay to determine the basal Tbx4LE activity in Tbx4LE-rtTA adult mice. As shown in Fig. 3A (middle), X-gal staining demonstrates Tbx4LE activity in a high percentage of cells in normal adult lung consistent with mesenchymal localization, similar to what has been previously reported (19). Eight days after inducing injury with intratracheal bleomycin (1 U/kg) in mice, we observed a notable increase in the density of X-gal-positive cells in PCLS when compared with NS-treated control lungs (Fig. 3A, middle and right). This finding is consistent with previously reported expansion of LMCs in response to injury (17).
Figure 3.
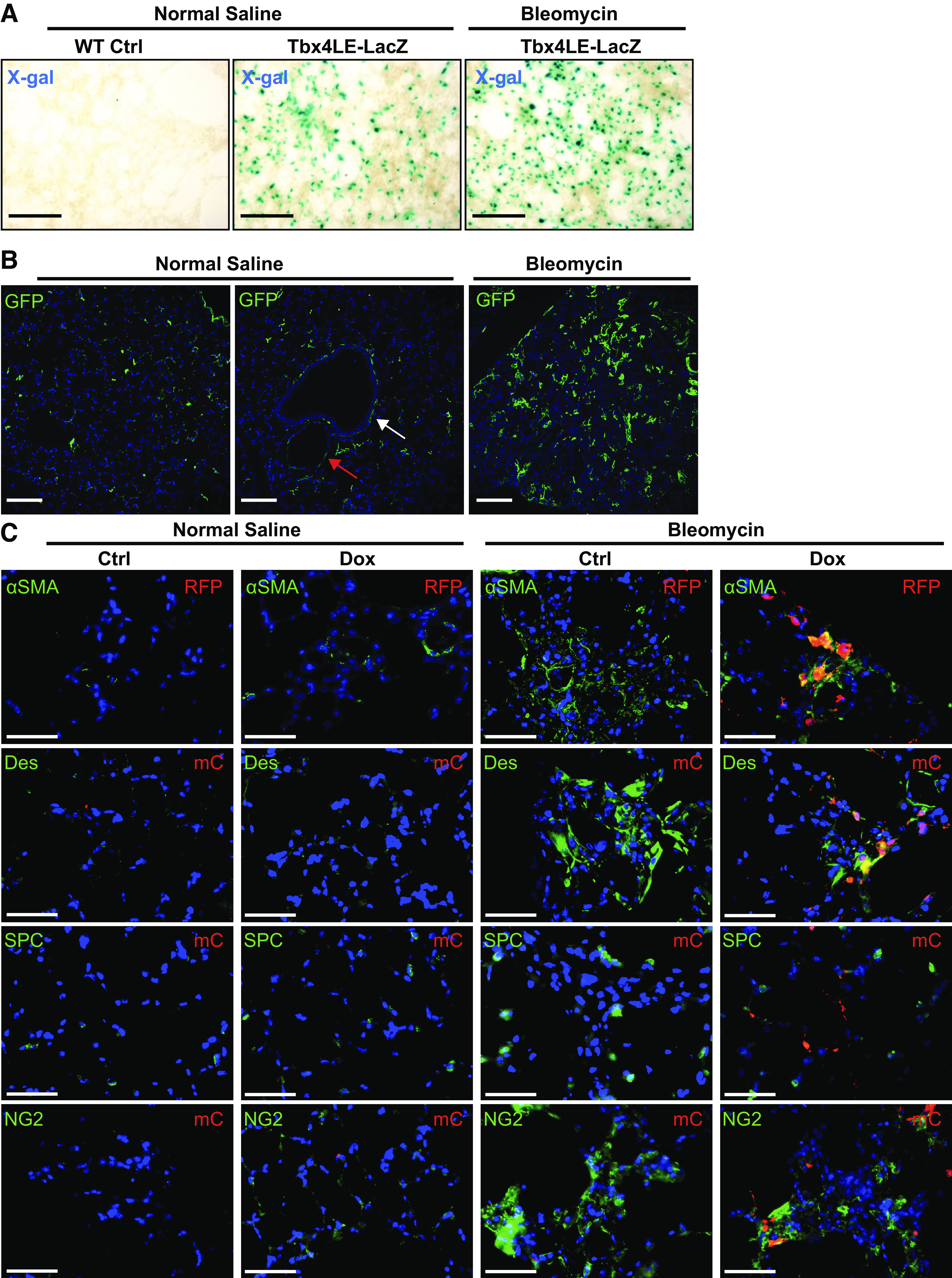
Lung mesenchymal cells expand and express myofibroblast markers postbleomycin treatment. A: Tbx4LE-rtTA-LacZ-expressing mice or wild-type (WT) controls were subject to intratracheal bleomycin (1 U/kg suspended in 0.9% normal saline, NS) or NS treatment. Eight days after bleomycin treatment, 150-μm-thick precision cut lung slices were stained with X-gal and imaged. Representative images are displayed for each condition. B: Tbx4LE-rtTA/TetO-Cre/mTmG mice were treated with doxycycline (2 mg/mL + 50 mg/mL sucrose in water), and 2 days later, subject to intratracheal bleomycin or NS treatment. Fourteen days after bleomycin instillation, lungs were fixed with 10% neutral buffered formalin, paraffin embedded, and stained against green fluorescent protein (GFP). Red arrow points to a blood vessel and white arrow points to an airway (middle). C: Tbx4LE-rtTA/TetO-Cre/tdTom mice were given doxycycline (dox) in water (2 mg/mL + 50 mg/mL sucrose) or sham (50 mg/mL sucrose) as a control starting 2 days before a single intratracheal bleomycin or NS treatment. Fourteen days after bleomycin instillation, mouse lungs were fixed with 10% neutral buffered formalin, paraffin embedded, and subject to immunofluorescence double staining against red fluorescent protein (RFP) or mCherry (mC) to enhance tdTomato signal and smooth muscle α actin (αSMA), Desmin (Des), prosurfactant protein C (SPC), or neural/glial antigen 2 chondroitin sulfate proteoglycan (NG2). All fluorescence images are counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). In all images, scale bar = 100 µm.
To further investigate the expansion and lineage specificity of the Tbx4LE-positive cells in response to bleomycin injury, we utilized Tbx4LE-rtTA/TetO-Cre/tdTom and Tbx4LE-rtTA/TetO-Cre/mTmG mice (Supplemental Fig. S1A), enabling fluorescent reporter marking of Tbx4LE-positive cells upon treatment with doxycline. Immunofluorescence stains of lung specimens from untreated Tbx4LE-rtTA/tetO-Cre/mTmG mice had essentially no detectable GFP expression, indicating a very low level of basal leak in these animals (Supplemental Fig. S2A; see https://doi.org/10.6084/m9.figshare.12622010). In doxycycline-treated Tbx4LE-rtTA/tetO-Cre/mTmG mice without injury, some GFP-positive cells are found in the alveolar parenchyma (Fig. 3B, left) along with focal localization surrounding airways and vasculature (Fig. 3B, middle). This is consistent with previously observed increased presence of the LMC lineage in alveolar fibroblasts, along with airway and vascular smooth muscle cells (17, 19). This distribution is also similar to that of SRF seen in normal human and mouse lung (Figs. 1 and 2). Immunostaining of doxycycline-treated Tbx4LE-rtTA/tetO-Cre/mTmG mice 14 days after injury with bleomycin shows increased numbers of GFP-positive LMCs in the parenchymal spaces, consistent with previously identified expansion of the lung mesenchyme in response to bleomycin injury (Fig. 3B, right) (17). Importantly, using Tbx4LE-rtTA/TetO-Cre/tdTom, we identified that upon bleomycin treatment, LMCs colocalize with the myofibroblast marker, αSMA (Fig. 3C, top row, far right column), and the mesenchymal marker desmin (Fig. 3C, second row, far right column), indicating that upon expansion, LMCs differentiate into myofibroblasts. Conversely, tdTom expression has no colocalization with pro-SPC, an alveolar epithelial cell marker (Fig. 3C, third row, far right column) and demonstrates some colocalization with NG2, a marker of pericytes (Fig. 3C, bottom row, far right column). These results suggest that the LMC population is a relevant target as a progenitor population of myofibroblasts, consistent with previous report (17). Furthermore, the overlapping spatial distribution of SRF expression with LMC markers suggests that SRF could play a role in the development of the myofibroblast population from LMC in vivo.
Inducible Knockdown of SRF in the Lung Mesenchyme
To test this hypothesis, we sought to enable conditional deletion of SRF in LMC in a murine model. To facilitate this, we crossed the Tbx4LE-rtTA/TetO-Cre/tdTom and Tbx4LE-rtTA/TetO-Cre/mTmG lines with a SRFf,f mouse line, containing LoxP sites flanking exon 1 of the Srf gene, which included sequences encoding the DNA-binding region (23). The resulting mice, Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f or Tbx4LE-rtTA/TetO-Cre/mTmG/SRFf,f, enabled LMC-specific deletion of SRF upon treatment with doxycyline (Supplemental Fig. S1B).
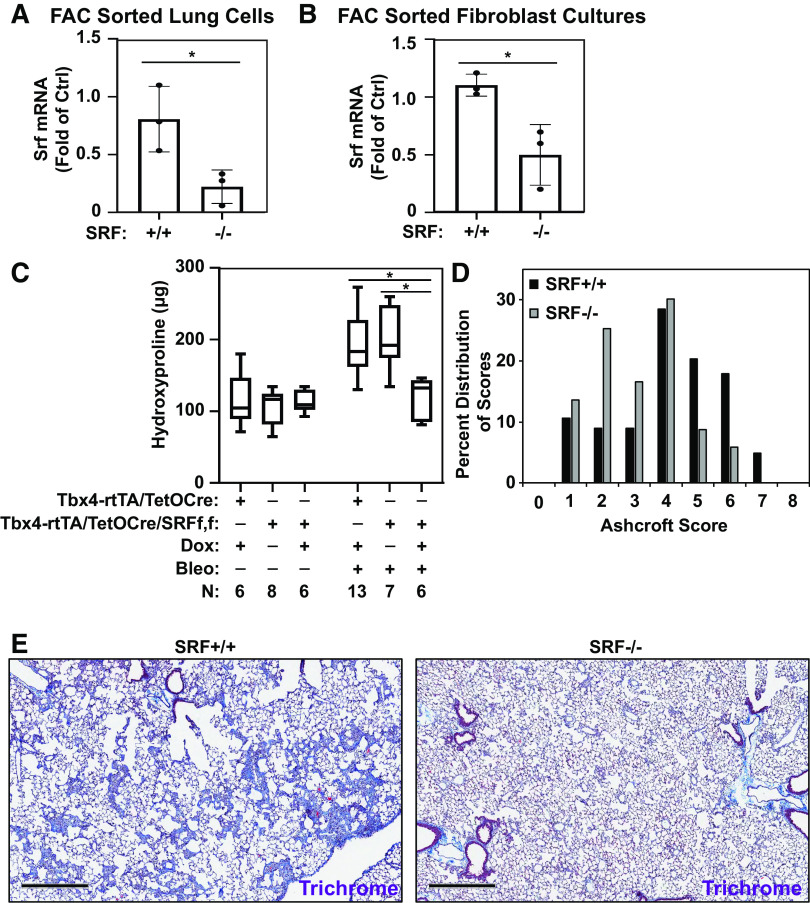
Subsequently, we assessed the efficiency of SRF deletion in LMC. First, Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f mice were treated with doxycycline from day 2 until end point (to induce deletion of SRF in LMC), as well as 1 U/kg of bleomycin instilled intratracheally at day 0 (to induce LMC expansion). Eight days after bleomycin treatment (10 days after doxycycline treatment), whole lung cell populations were sorted by fluorescence-activated cell sorting (FACS) against expression of tdTom to isolate LMCs from murine lungs. RT-PCR analysis of the sorted cells revealed an 80% reduction in SRF mRNA expression in LMCs from Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f (SRF−/−) lung compared with LMCs from Tbx4LE-rtTA/TetO-Cre/tdTom (SRF+/+) controls, establishing the efficiency of SRF deletion in this cell population (Fig. 4A).
Figure 4.
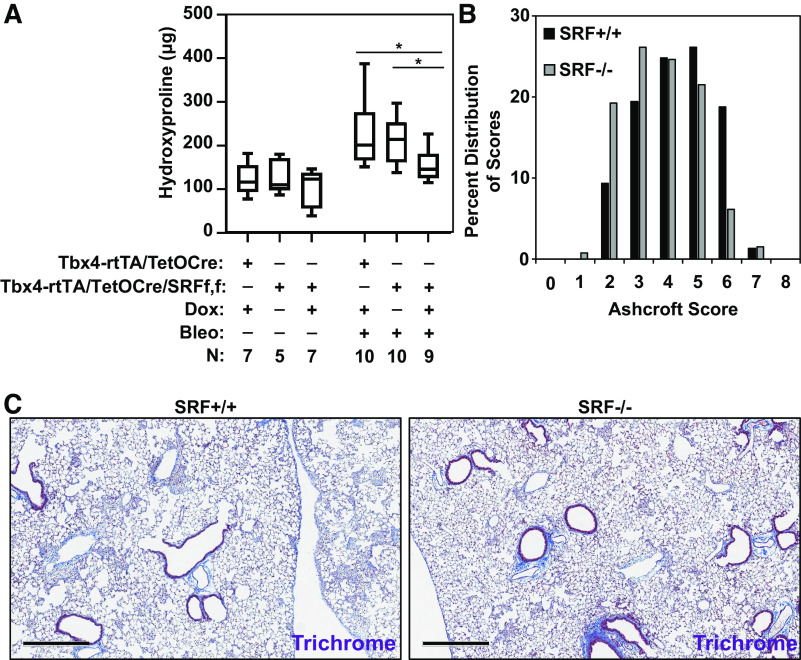
Loss of SRF in lung mesenchymal cells attenuates bleomycin-induced fibrosis. Tbx4LE-rtTA/TetO-Cre/tdTom or Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f were given doxycycline (2 mg/mL + 50 mg/mL sucrose) or sham control (50 mg/mL sucrose) in water 2 days before intratracheal treatment with bleomycin (1 U/kg suspended in normal saline, NS) or NS control, as indicated. A: 8 days after bleomycin (and 10 days after start of doxycycline) treatment, Tbx4LE-rtTA/TetO-Cre/tdTom (SRF+/+) or Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f (SRF−/− in LMC) mouse lungs were digested to single-cell suspension and subjected to fluorescence-activated cell sorting (FACS) for expression of tdTomato, separating lung mesenchymal cells (LMCs) from the rest of the cell populations. tdTom-positive LMCs were immediately lysed for RNA and subject to RT-PCR against Srf, along with a housekeeping gene (n = 3 for each condition). B: 5 days after start of doxycycline treatment, Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f primary mouse lung fibroblasts (MLFs) were derived. Following expansion, MLFs were subjected to FACS against tdTomato. tdTom-positive LMCs (SRF−/−) and tdTom-negative control fibroblasts (SRF+/+) were lysed for RNA and subject to RT-PCR for expression of Srf and housekeeping gene (n = 3 for each condition). Column bars are representing means ± SE, and Unpaired Student’s t test (*P < 0.05) was used for statistical analyses in A and B. C: quantification of collagen accumulation was done 14 days after bleomycin or NS installation (and 16 days after the start of doxycycline/sham treatment) via hydroxyproline assay of total lung homogenates (both lungs) in indicated groups (C; N indicated in figure). One-way ANOVA with Holm–Šídák test was used for statistical analyses (*P < 0.05). D: lung fibrosis was semiquantitatively assessed via lung fixation, paraffin embedding, and Ashcroft fibrosis scoring of Masson’s trichrome stains in Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f mice treated with doxycycline (SRF−/− in LMC) or sham control (SRF+/+) and intratracheal bleomycin (n = 5 for each group). Mixed model ANOVA (*P < 0.05) was used for statistical analysis. E: a set of representative images of data from D are displayed (scale bar = 500 µm). Bleo, bleomycin; dox, doxycycline; SRF, serum response factor.
Second, we isolated a cell population from Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f mouse lungs 5 days after doxycycline treatment via the explant method to isolate a mouse “fibroblast” population (43). This expanded, spindle-shaped cell population was subsequently sorted via FACS against expression of tdTom. tdTom-positive and tdTom-negative sorted cell populations were lysed for RNA, showing 60% decrease in SRF mRNA expression in tdTom-positive cells compared with tdTom-negative control fibroblasts (Fig. 4B).
Loss of SRF in the Lung Mesenchyme Decreases Lung Fibrosis after Intratracheal Bleomycin
After confirming efficient inducible deletion of SRF in LMC in this transgenic mouse model, we sought to determine the effect of SRF loss in the lung mesenchyme on the fibrotic response to bleomycin-induced injury. To do so, we examined the fibrotic response of mice 14 days after bleomycin instillation (and 16 days after onset of doxycycline treatment). Assessment of lung collagen content by hydroxyproline assay shows that doxycycline-treated Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f animals have significantly less collagen accumulation in their lungs compared with bleomycin and sham-treated Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f or bleomycin and doxycycline-treated Tbx4LE-rtTA/TetO-Cre/tdTom controls (both of which retained expression of SRF in their LMCs) (Fig. 4C) and that there is no apparent sex-dependence of these results (Supplemental Fig. S3, A and B; see https://doi.org/10.6084/m9.figshare.12622058). Semiquantitative assessment of the histologic severity of fibrosis using modified Ashcroft scoring shows a significant leftward shift in the distribution of fibrosis severity scores in mice with loss of SRF expression in LMC (doxycline-treated Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f) compared with sham-treated controls, confirming a reduction in the severity of histologically apparent fibrosis (Fig. 4, D and E). Furthermore, we found an improvement in survival of Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f animals treated with doxycycline to delete SRF in LMC compared with sham-treated controls (Supplemental Fig. S3, C–E). Together, these data demonstrate that SRF expression in LMCs is essential for the full development of the fibrotic response after bleomycin-induced lung injury.
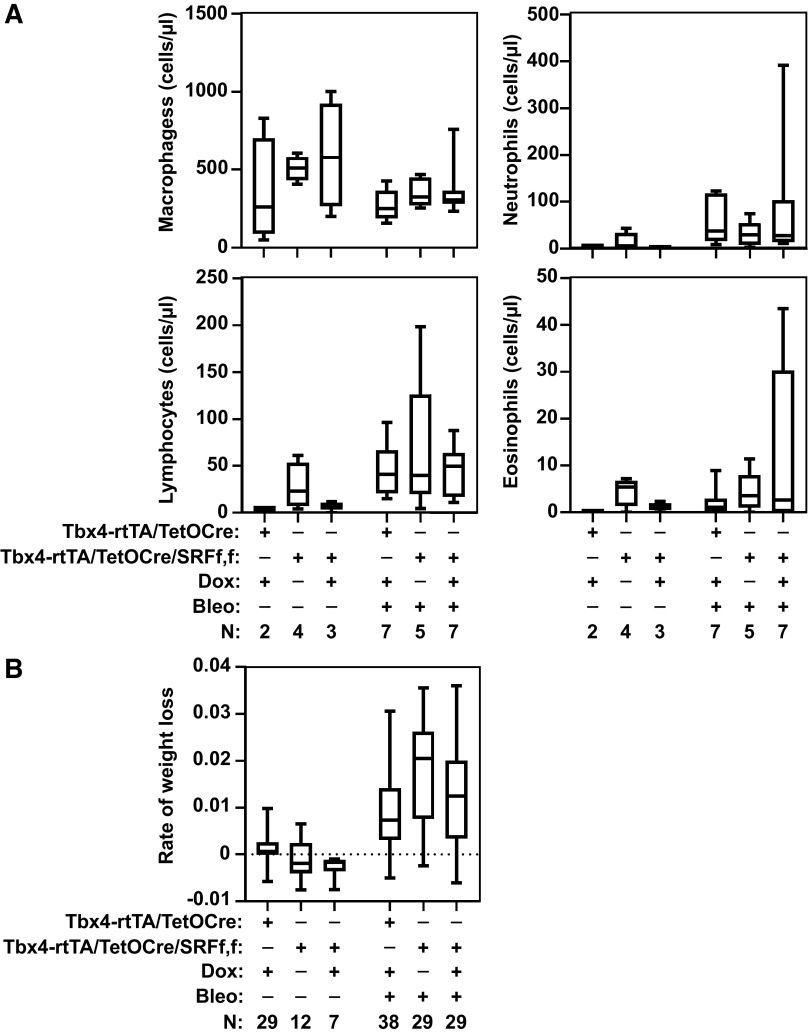
Loss of SRF in LMCs Does Not Affect Markers of Early Injury or Inflammation
The initial response to intratracheal treatment with bleomycin is characterized by alveolar epithelial cell injury and subsequent inflammatory response. To determine whether loss of SRF in LMCs modified the early inflammatory response, we analyzed BAL fluid 3 days after bleomycin injury. We observed no differences in the neutrophilic response between bleomycin-treated mice lacking SRF in their LMCs and SRF expressing controls (Fig. 5A). There were also no differences in macrophage, lymphocyte, or eosinophil counts at day 3. In addition, given that weight loss is associated with the onset and severity of the inflammatory response, we compared the rate of weight loss between bleomycin-treated animals that were SRF-null in LMCs and controls and found no significant change difference (Fig. 5B). Overall, these data suggest that loss of SRF in LMCs does not have an appreciable effect on the early bleomycin-induced inflammatory response.
Figure 5.
Loss of serum response factor (SRF) in lung mesenchymal cells does not affect the early inflammatory response to bleomycin injury. Tbx4LE-rtTA/TetO-Cre/tdTom and Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f were given doxycycline (dox, 2 mg/mL + 50 mg/mL sucrose) or control (50 mg/mL sucrose) in water 2 days before an intratracheal instillation of 1 U/kg bleomycin (Bleo) or normal saline. A: 3 days after bleomycin treatment, bronchoalveolar lavage cells were collected and cell differential counts obtained. B: animals were weighed daily until end point (day 14) to compare rates of weight loss between groups. N for each group is indicated in the figure. One-way ANOVA with Holm–Šídák test (P < 0.05) was used for statistical analyses.
SRF Is Essential for LMC Expansion during Pulmonary Fibrosis
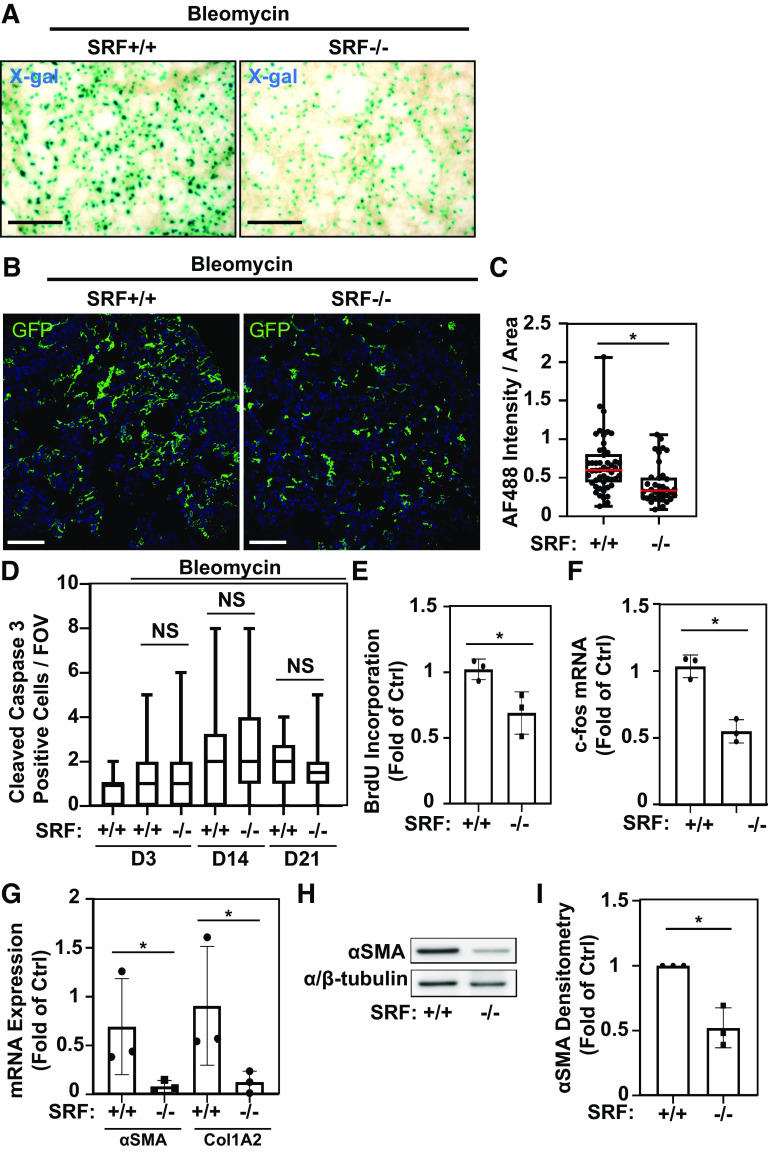
The LMC population considerably expands after bleomycin injury (Fig. 3) (17), and we sought to determine how loss of SRF affected LMC number after bleomycin-induced injury. To assess the LMC population under deletion of SRF, we utilized doxycycline-treated Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f mice (or sham for control) followed by intratracheal treatment with bleomycin as we did previously in Fig. 3. We then assessed the LMC population upon loss of SRF via X-gal staining of PCLS, which demonstrated a noticeable decrease in X-gal-positive cells (Fig. 6A). To enable quantitative assessment, we used a similar approach in doxycycline and bleomycin-treated Tbx4LE-rtTA/TetO-Cre/mTmG/SRFf,f and Tbx4LE-rtTA/TetO-Cre/mTmG control mice and performed immunofluorescence staining against GFP 14 days after bleomycin treatment (Fig. 6B and Supplemental Fig. S2B for negative control). We observed a significant decrease in GFP staining in mice lacking SRF in LMCs compared with bleomycin-injured controls (Fig. 6C), suggesting that loss of SRF inhibits expansion of the LMC population, thereby limiting the overall fibrotic response.
Figure 6.
Serum response factor (SRF) is essential for lung mesenchymal cell expansion, proliferation, and myofibroblast differentiation in bleomycin-induced pulmonary fibrosis. A: Tbx4LE-rtTA-LacZ/TetO-Cre/SRFf,f were administered doxycycline (2 mg/mL + 50 mg/mL sucrose in water), resulting in SRF knockdown in LMC (SRF−/−), or sham (50 mg/mL sucrose in water; SRF+/+) starting 2 days before intratracheal treatment with 1 U/kg bleomycin. Eight days after bleomycin treatment, 150-μm-thick living lung slices were stained with X-gal and imaged (representative images are displayed). B: Tbx4LE-rtTA/TetO-Cre/mTmG (SRF+/+) and Tbx4LE-rtTA/TetO-Cre/mTmG/SRFf,f (SRF−/− in LMC) mice were treated with doxycycline, and 2 days later subject to intratracheal bleomycin. Fourteen days after bleomycin instillation, lungs were fixed with 10% neutral buffered formalin, paraffin embedded, and stained against green fluorescent protein (GFP). Fluorescence images were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). C: total GFP expression per field of view (FOV, ×20 magnification) in at least 10 FOV/mouse and three mice/condition was quantified via alexa fluor 488 (AF488) quantification in the mouse lung parenchyma using ImageJ (red bar represents median value). D: Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f or Tbx4LE-rtTA/TetO-Cre/tdTom controls (SRF+/+) were administered either doxycycline (SRF−/− in LMC) or sham (SRF+/+) starting 2 days before (days 3 and 14 end points) or 5 days after (day 21 end point) intratracheal bleomycin treatment. Three, 14, or 21 days after bleomycin treatment, mouse lungs were fixed with 10% neutral buffered formalin, paraffin embedded, and subject to immunohistochemical staining against cleaved caspase 3. Cleaved caspase 3-positive cells were manually counted per FOV (×40 magnification) in at least 10 FOV/mouse and three mice/condition. E and F: Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f were administered doxycycline starting 2 days before intratracheal bleomycin treatment. Three days after bleomycin treatment, mouse lungs were minced to derive mouse lung fibroblasts (MLFs) cultures and subsequently fluorescence-activated cell sorted (FACS) against tdTom expression (to isolate tdTom-positive LMCs). tdTom-positive LMC (SRF−/−) and tdTom-negative fibroblasts (SRF+/+) were then subject to either bromodeoxyuridine (BrdU) cell proliferation assay in triplicate (E, n = 3 for each condition) or RT-PCR against c-fos and housekeeping gene (F, n = 3 including two biological replicates and one technical replicate: one cell line FAC sorted, cultured, and RT-PCR analyzed separately). Paired Student’s t test (*P < 0.05) was utilized to assess statistical significance in E and F. G: Tbx4LE-rtTA/TetO-Cre/tdTom (SRF+/+) or Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f (SRF−/− in LMC) were given doxycycline in water 2 days before intratracheal treatment with bleomycin, as indicated. Eight days after bleomycin (and 10 days after start of doxycycline) treatment, mouse lungs were digested to single-cell suspension and subjected to FACS for expression of tdTom, separating LMC from the rest of the cell populations. tdTom-positive LMCs from the two mouse groups were immediately lysed for RNA and subject to RT-PCR against smooth muscle α actin (αSMA, encoded by Acta2 gene), Collagen 1A2 (Col1A2), and housekeeping gene (n = 3 for each condition). H: Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f were treated as in E and F. Following FAC sorting, tdTom-positive LMCs and tdTom-negative fibroblast lysates were analyzed by Western blot with indicated antibodies. I: densitometry of Western blots (n = 3 for each condition) was done using ImageJ. Unless otherwise noted, unpaired Student’s t test (*P < 0.05) was utilized to assess statistical significance between groups. In all images, scale bar = 100 µm. All bar graphs represent means ± SD.
SRF is also implicated in the regulation of apoptotic genes such as B-cell lymphoma 2 (Bcl2) (8, 44), which could account for the observed differences in LMC presence in Fig. 6, A–C. We examined the overall level of apoptosis in lungs harboring SRF-null LMC. Quantification of cleaved caspase-3 staining from doxycycline-treated Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f (SRF−/− in LMC) mice compared with WT (SRF+/+) controls showed that there was no change in the overall number of apoptotic cells 3, 14, or 21 days after the onset of bleomycin injury (Fig. 6D). This suggests that variable rates of apoptosis do not account for the observed differences in LMC numbers at day 14. In contrast, examination of isolated FAC-sorted fibroblasts from doxycycline-treated Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f mouse lungs (3 days postbleomycin treatment) shows that SRF-null (tdTom-positive) LMCs have a decreased proliferative potential compared with SRF-expressing (tdTom-negative) fibroblasts (Fig. 6E). Consistent with this, FAC-sorted SRF-null (tdTom-positive) LMCs have decreased RNA expression of FBJ osteosarcoma oncogene c-fos, a transcription factor involved in early growth response, compared with SRF-expressing (tdTom-negative) fibroblasts (Fig. 6F). Altogether, these data reveal that SRF is essential for the expression of early growth response genes and proliferative capacity in the LMC compartment (13, 45, 46).
SRF Modifies Myofibroblast-Associated Genes in LMC
Given the established role of SRF in myofibroblast differentiation in ex vivo cell cultures (4, 7), we hypothesized that SRF-null LMC lose the capacity to differentiate into myofibroblasts during bleomycin-induced pulmonary fibrosis, thereby contributing to the diminished fibrotic response. To determine this, FACS-sorted LMC from doxycycline-treated Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f and Tbx4LE-rtTA/TetO-Cre/tdTom mice were isolated after bleomycin treatment. In this population, we found decreased expression of αSMA mRNA and Collagen 1A2 mRNA in SRF-null LMC (Fig. 6G), known SRF target genes (47). Similarly, we found a decrease in αSMA protein levels in SRF-null LMC compared with SRF-expressing fibroblasts. (Fig. 6, H and I). Altogether, these data demonstrate that the SRF plays a central role in the process of LMC differentiation toward the myofibroblast phenotype in vivo, thereby increasing the fibrotic response in bleomycin-induced pulmonary fibrosis.
Delayed Deletion of SRF in LMC Attenuates Collagen Deposition in Response to Bleomycin
Based on our observations that SRF-null LMC are deficient in markers of myofibroblasts, including collagen production, we hypothesized that delayed deletion of SRF in LMC may also impact the development of pulmonary fibrosis, including collagen deposition, in response to bleomycin. Five days after bleomycin treatment, Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f and Tbx4LE-rtTA/TetO-Cre/tdTom controls were treated with doxycycline (or sham). Twenty-one days after bleomycin treatment, isolated lungs from these animals were subjected to hydroxyproline assay, revealing a decrease in collagen content in the mice lacking SRF in LMC compared controls (Fig. 7A). Fibrosis scoring of trichrome-stained histologic samples from the same time point showed a trend toward a decrease in fibrotic extent (P = 0.0586 using Mixed Model ANOVA), concordant with the observed improvement in collagen content (Fig. 7, B and C). Collectively, these data further demonstrate that expression of SRF in the lung mesenchyme is required to allow for full development of the fibrotic response even after the onset of lung injury.
Figure 7.
Delayed deletion of serum response factor (SRF) in lung mesenchymal cells results in decreased collagen deposition in response to bleomycin injury. Tbx4LE-rtTA/TetO-Cre/tdTom and Tbx4LE-rtTA/TetO-Cre/tdTom/SRFf,f were treated with 1 U/kg bleomycin (Bleo) or normal saline, followed by delayed commencement of doxycycline (dox) treatment in water (2 mg/mL + 50 mg/mL sucrose), or sham control (50 mg/mL sucrose), five days later to induce deletion of SRF. Twenty-one days after bleomycin instillation, mouse lung homogenates (both lungs) were assessed for total collagen content via hydroxyproline assay (A, N indicated in figure), while another set of lungs were fixed with 10% formalin, paraffin embedded, and stained using Masson’s trichrome staining for Modified Ashcroft scoring (B, n = 4 for each group). C: representative Masson’s trichrome-stained samples are shown (scale bar = 500 µm). One-way ANOVA with Holm–Šídák test (*P < 0.05) was used for statistical analyses in A. Mixed model ANOVA was used for statistical analysis (P = 0.0586) in B.
DISCUSSION
The lung mesenchyme plays an important role in remodeling by expanding and contributing to collagen-producing myofibroblasts (17). Similar to the work by Xie et al., we observed robust expansion of the Tbx4LE LMC population in response to intratracheal bleomycin injury, suggesting that this cell population plays an important role in development of the fibrotic response. We also observed a strong similarity in the spatial distribution of SRF, a myogenic transcription factor that controls myofibroblast differentiation in vitro (4), and LMC. Thus, in this study, we harnessed the Tbx4LE to drive Cre-recombinase and target SRF in the lung mesenchyme.
Our work shows that SRF plays a role in fibrotic development in the lung mesenchyme throughout the evolution of experimental fibrosis. Our primary finding is that loss of SRF in LMC is sufficient to disrupt the development of pulmonary fibrosis. Specifically, when targeting SRF during the early phase of bleomycin lung injury, we observed a decrease in LMC expansion and diminished collagen deposition and lung fibrosis scores at day 14. Importantly, loss of SRF was associated with decreased c-fos expression and proliferative capacity in LMC, but not changes in markers of apoptosis in vivo, suggesting that the primary defect in LMC expansion was related to proliferation rather than accelerated LMC apoptosis. In addition, we also observed loss of the expression of myofibroblast-associated mRNA, αSMA and Collagen 1A2, in the lung mesenchyme, consistent with a role for SRF in driving a myofibroblast phenotype. Overall, our work suggests that SRF plays a two-part role in LMC biology, one by controlling proliferation during mesenchymal expansion, via regulation of early growth response genes, and the second in the regulation of myofibroblast genes. While we and others have previously demonstrated that both SRF and its coactivator MRTFA play a role in controlling myofibroblast differentiation (4, 7, 9, 12), SRF also contributes to cell proliferation through MRTFA-independent MAPK/ELK1 signaling mechanism (5, 13, 14). Our present studies support this concept where we observe loss of LMC proliferative capacity and decreased expansion capacity upon SRF knockdown in bleomycin-induced lung injury.
Our study builds upon previous work that has demonstrated that disruption of LMC via diphtheria toxin-mediated ablation of LMCs (Tbx4LE cell population) was sufficient to inhibit the development of pulmonary fibrosis (17). However, this approach is not likely translatable to a human therapeutic. In our study, we instead targeted an endogenously expressed transcription factor, SRF, in the lung mesenchyme (4). This is a relatively understudied area, with only one publication specifically targeting an intracellular signaling pathway in the Tbx4LE LMC population during fibrosis (16). Thus, we view these findings as an important starting point, given the central role of the lung mesenchyme in orchestrating ECM deposition during fibrosis and the importance of identifying key molecules that drive fibrogenesis. Still, we acknowledge that further studies are required in order to assess the role of SRF in lung mesenchymal cells during established fibrosis, as our study addressed the early stage of disease development.
The bleomycin murine lung fibrosis model has inherent limitations, which can restrict its applicability to the idiopathic form of human pulmonary fibrosis, as it is characterized by a pronounced early inflammatory response before the onset of fibrosis. We addressed this limitation in two ways. First, when utilizing an early knockdown strategy, we showed that loss of SRF in LMC did not impact the bleomycin-initiated inflammatory response. Second, we utilized a delayed treatment with doxycycline to target SRF deletion during the early fibrotic phase, and still observed a decrease in collagen deposition in the lung. We also acknowledge that it is possible that targeting SRF after bleomycin-induced lung fibrosis has fully developed may result in differential effects on LMC proliferation, survival, and fibrotic resolution. However, these questions were outside of the primary focus and scope of this study.
We view the use of our transgenic model as a major strength, as it allowed us to probe LMC-specific biological effects during fibrosis. SRF plays a role in neutrophil migration and lymphocyte maturation (48, 49), and our previous work demonstrated that MRTF-A mediates the bleomycin-induced neutrophilic response in the lung 3 days after the onset of injury (12). Thus, broadly targeting this signaling axis (MRTFA/SRF) in the bleomycin lung fibrosis model could be confounded by effects on the inflammatory response to injury. Finally, our use of a temporally controlled system enabling genetic alterations only in adult mice allowed us to avoid the potential developmental changes and compensatory effects that can occur with germline deletions and can alter biologic responses. This approach allowed us to confidently conclude that isolated targeting of SRF in the mesenchyme is sufficient to alter tissue fibrogenesis.
Conclusions
Together, these data demonstrate that LMCs are a critical source of myofibroblasts that expand during bleomycin-induced pulmonary fibrosis and that loss of SRF in LMC attenuates the development of bleomycin-induced pulmonary fibrosis. This work provides the first proof of principle that targeting a key transcription factor essential for differentiation into myofibroblasts solely in the lung mesenchyme is sufficient to attenuate fibrotic progression. These findings are relevant for the development of targeted therapeutics for pulmonary fibrosis, given the increasing feasibility of utilizing drug delivery systems to localize pharmaceutical payloads to cell- and ligand-specific targets.
SUPPLEMENTAL DATA
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.12622037.
Supplemental Fig. S2: https://doi.org/10.6084/m9.figshare.12622010.
Supplemental Fig. S3: https://doi.org/10.6084/m9.figshare.12622058.
GRANTS
This work was supported by American Heart Association Postdoctoral Fellowship (to K. Bernau), the National Institutes of Health/National Heart, Lung, and Blood Institute Award K08 HL093367 (to N. Sandbo), R03HL136795 (to N. Sandbo), R01HL146402 (to N. Sandbo), and American Thoracic Society/Pulmonary Fibrosis Foundation/Coalition for Pulmonary Fibrosis Research Award (to N. Sandbo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.B. and N.S. conceived and designed research; K.B., J.P.L., E.M.B., A.J.T., T.Z., and N.S. performed experiments; K.B., J.P.L., and N.S. analyzed data; K.B., J.P.L., and N.S. interpreted results of experiments; K.B. and N.S. prepared figures; K.B. and N.S. drafted manuscript; K.B., J.P.L., E.M.B., and N.S. edited and revised manuscript; K.B., J.P.L., E.M.B., A.J.T., T.Z., and N.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Wei Shi and Xin Sun for sharing the Tbx4LE/TetO-Cre mouse line and Dr. Joseph Miano for sharing the SRFf,f mouse line. We also thank Kristine Lee for help with Ashcroft scoring analysis. We acknowledge the contribution of the University of Wisconsin Carbone Cancer Center (UWCCC) Translational Science BioCore (Biobanking and Histology Services) to our work under the support of the UWCCC NIH Grant P30 CA014520. We acknowledge the UWCCC Flow Cytometry Laboratory and the Multi-color Benchtop Flow Cytometer Project (Grant No.: 1S10RR025483-01) used to purchase BD FACS AriaII BSL-2 Cell Sorter (“Jill”). In addition, we acknowledge the contribution of the UW-Madison Department of Surgery Histology Core facility and the University of Wisconsin Translational Research Initiatives in Pathology laboratory (TRIP), supported by the UW Department of Pathology and Laboratory Medicine, UWCCC (P30 CA014520) and the Office of The Director- NIH (S10OD023526), for use of facilities and services. We acknowledge that a portion of this work was presented at the American Thoracic Society’s annual meeting on May 22, 2017 (50).
Present address of A. J. Tubbs: Madison Emergency Physicians, 700 S Park St. Madison, WI 53715.
REFERENCES
- 1.Iqbal J, Anwar F, Afridi S. Targeted drug delivery systems and their therapeutic applications in cancer and immune pathological conditions. Infect Disord Drug Targets 17: 149–159, 2017. doi: 10.2174/1871526517666170606102623. [DOI] [PubMed] [Google Scholar]
- 2.Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. Liver fibrosis: direct antifibrotic agents and targeted therapies. Matrix Biol 68–69: 435–451, 2018. doi: 10.1016/j.matbio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasarao M, Low PS. Ligand-targeted drug delivery. Chem Rev 117: 12133–12164, 2017. doi: 10.1021/acs.chemrev.7b00013. [DOI] [PubMed] [Google Scholar]
- 4.Sandbo N, Kregel S, Taurin S, Bhorade S, Dulin NO. Critical role of serum response factor in pulmonary myofibroblast differentiation induced by TGF-β. Am J Respir Cell Mol Biol 41: 332–338, 2009. doi: 10.1165/rcmb.2008-0288OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gineitis D, Treisman R. Differential usage of signal transduction pathways defines two types of serum response factor target gene. J Biol Chem 276: 24531–24539, 2001. doi: 10.1074/jbc.M102678200. [DOI] [PubMed] [Google Scholar]
- 6.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 47: 340–348, 2012. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandbo N, Lau A, Kach J, Ngam C, Yau D, Dulin NO. Delayed stress fiber formation mediates pulmonary myofibroblast differentiation in response to TGF-β. Am J Physiol Lung Cell Mol Physiol 301: L656–L666, 2011. doi: 10.1152/ajplung.00166.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sisson TH, Ajayi IO, Subbotina N, Dodi AE, Rodansky ES, Chibucos LN, Kim KK, Keshamouni VG, White ES, Zhou Y, Higgins PD, Larsen SD, Neubig RR, Horowitz JC. Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis. Am J Pathol 185: 969–986, 2015. doi: 10.1016/j.ajpath.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Zhe X, Phan SH, Ullenbruch M, Schuger L. Involvement of serum response factor isoforms in myofibroblast differentiation during bleomycin-induced lung injury. Am J Respir Cell Mol Biol 29: 583–590, 2003. doi: 10.1165/rcmb.2002-0315OC. [DOI] [PubMed] [Google Scholar]
- 10.Arsenian S, Weinhold B, Oelgeschlager M, Ruther U, Nordheim A. Serum response factor is essential for mesoderm formation during mouse embryogenesis. EMBO J 17: 6289–6299, 1998. doi: 10.1093/emboj/17.21.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angstenberger M, Wegener JW, Pichler BJ, Judenhofer MS, Feil S, Alberti S, Feil R, Nordheim A. Severe intestinal obstruction on induced smooth muscle-specific ablation of the transcription factor SRF in adult mice. Gastroenterology 133: 1948–1959, 2007. doi: 10.1053/j.gastro.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 12.Bernau K, Ngam C, Torr EE, Acton B, Kach J, Dulin NO, Sandbo N. Megakaryoblastic leukemia-1 is required for the development of bleomycin-induced pulmonary fibrosis. Respir Res 16: 45, 2015. doi: 10.1186/s12931-015-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell 73: 381–393, 1993. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 428: 185–189, 2004. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- 15.Li R, Bernau K, Sandbo N, Gu J, Preissl S, Sun X. Pdgfra marks a cellular lineage with distinct contributions to myofibroblasts in lung maturation and injury response. eLife 7: e3685, 2018. doi: 10.7554/eLife.36865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Y, Xu W, Chen H, Warburton D, Dong R, Qian B, Selman M, Gauldie J, Kolb M, Shi W. A novel profibrotic mechanism mediated by TGF-beta-stimulated collagen prolyl hydroxylase expression in fibrotic lung mesenchymal cells. J Pathol 236: 384–394, 2015. doi: 10.1002/path.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie T, Liang J, Liu N, Huan C, Zhang Y, Liu W, Kumar M, Xiao R, D'Armiento J, Metzger D, Chambon P, Papaioannou VE, Stripp BR, Jiang D, Noble PW. Transcription factor TBX4 regulates myofibroblast accumulation and lung fibrosis. J Clin Invest 126: 3626, 2016. doi: 10.1172/JCI89968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menke DB, Guenther C, Kingsley DM. Dual hindlimb control elements in the Tbx4 gene and region-specific control of bone size in vertebrate limbs. Development 135: 2543–2553, 2008. doi: 10.1242/dev.017384. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Menke DB, Jiang M, Chen H, Warburton D, Turcatel G, Lu CH, Xu W, Luo Y, Shi W. Spatial-temporal targeting of lung-specific mesenchyme by a Tbx4 enhancer. BMC Biol 11: 111, 2013. doi: 10.1186/1741-7007-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA 99: 10482–10487, 2002. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng HA. robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 23.Miano JM, Ramanan N, Georger MA, de Mesy Bentley KL, Emerson RL, Balza RO, Xiao Q Jr, Weiler H, Ginty DD, Misra RP. Restricted inactivation of serum response factor to the cardiovascular system. Proc Natl Acad Sci USA 101: 17132–17137, 2004. doi: 10.1073/pnas.0406041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kach J, Sandbo N, La J, Denner D, Reed EB, Akimova O, Koltsova S, Orlov SN, Dulin NO. Antifibrotic effects of noscapine through activation of prostaglandin E2 receptors and protein kinase A. J Biol Chem 289: 7505–7513, 2014. doi: 10.1074/jbc.M113.546812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kach J, Sandbo N, Sethakorn N, Williams J, Reed EB, La J, Tian X, Brain SD, Rajendran K, Krishnan R, Sperling AI, Birukov K, Dulin NO. Regulation of myofibroblast differentiation and bleomycin-induced pulmonary fibrosis by adrenomedullin. Am J Physiol Lung Cell Mol Physiol 304: L757–L764, 2013. doi: 10.1152/ajplung.00262.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woessner JF Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93: 440–447, 1961. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 27.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubner RH, Gitter W, El Mokhtari NE, Mathiak M, Both M, Bolte H, Freitag-Wolf S, Bewig B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 44: 507–507, 2008. doi: 10.2144/000112729. [DOI] [PubMed] [Google Scholar]
- 29.Sandbo N, Ngam C, Torr E, Kregel S, Kach J, Dulin N. Control of myofibroblast differentiation by microtubule dynamics through a regulated localization of mDia2. J Biol Chem 288: 15466–15473, 2013. doi: 10.1074/jbc.M113.464461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esnault S, Torr EE, Bernau K, Johansson MW, Kelly EA, Sandbo N, Jarjour NN. Endogenous semaphorin-7A impedes human lung fibroblast differentiation. PLoS One 12: e0170207, 2017. doi: 10.1371/journal.pone.0170207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang YH, Kuo HC, Yang YL, Wang FS. MicroRNA-29a is a key regulon that regulates BRD4 and mitigates liver fibrosis in mice by inhibiting hepatic stellate cell activation. Int J Med Sci 16: 212–220, 2019. doi: 10.7150/ijms.29930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang P, Liu X, Kong C, Liu X, Teng Z, Ma Y, Yong L, Liang C, He G, Lu S. Potential role of the IL17RC gene in the thoracic ossification of the posterior longitudinal ligament. Int J Mol Med 43: 2005–2014, 2019. doi: 10.3892/ijmm.2019.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niu F, Liao K, Hu G, Sil S, Callen S, Guo ML, Yang L, Buch S. Cocaine-induced release of CXCL10 from pericytes regulates monocyte transmigration into the CNS. J Cell Biol 218: 700–721, 2019. doi: 10.1083/jcb.201712011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nazim UM, Yin H, Park SY. Neferine treatment enhances the TRAILinduced apoptosis of human prostate cancer cells via autophagic flux and the JNK pathway. Int J Oncol 56: 1152–1161, 2020. doi: 10.3892/ijo.2020.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X, Huang L, Futtner C, Schwab B, Rampersad RR, Lu Y, Sporn TA, Hogan BL, Onaitis MW. The cell of origin and subtype of K-Ras-induced lung tumors are modified by Notch and Sox2. Genes Dev 28: 1929–1939, 2014. doi: 10.1101/gad.243717.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.di Tomaso E, London N, Fuja D, Logie J, Tyrrell JA, Kamoun W, Munn LL, Jain RK. PDGF-C induces maturation of blood vessels in a model of glioblastoma and attenuates the response to anti-VEGF treatment. PLoS One 4: e5123, 2009. doi: 10.1371/journal.pone.0005123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marin-Mogollon C, Salman AM, Koolen KMJ, Bolscher JM, van Pul FJA, Miyazaki S, Imai T, Othman AS, Ramesar J, van Gemert GJ, Kroeze H, Chevalley-Maurel S, Franke-Fayard B, Sauerwein RW, Hill AVS, Dechering KJ, Janse CJ, Khan SM. A P. falciparum NF54 reporter line expressing mCherry-luciferase in gametocytes. Front Cell Infect Microbiol 9: 96, 2019. doi: 10.3389/fcimb.2019.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiao J, Liu Z, Yang C, Gu L, Deng D. SRF promotes gastric cancer metastasis through stromal fibroblasts in an SDF1-CXCR4-dependent manner. Oncotarget 7: 46088–46099, 2016. doi: 10.18632/oncotarget.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith KA, Zhou B, Avdulov S, Benyumov A, Peterson M, Liu Y, Okon A, Hergert P, Braziunas J, Wagner CR, Borok Z, Bitterman PB. Transforming growth factor-beta1 induced epithelial mesenchymal transition is blocked by a chemical antagonist of translation factor eIF4E. Sci Rep 5: 18233, 2015. doi: 10.1038/srep18233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hannafon BN, Gin AL, Xu YF, Bruns M, Calloway CL, Ding WQ. Metastasis-associated protein 1 (MTA1) is transferred by exosomes and contributes to the regulation of hypoxia and estrogen signaling in breast cancer cells. Cell Commun Signal 17: 13, 2019. doi: 10.1186/s12964-019-0325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 188: 820–830, 2013. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li A, Ma S, Smith SM, Lee MK, Fischer A, Borok Z, Bellusci S, Li C, Minoo P. Mesodermal ALK5 controls lung myofibroblast versus lipofibroblast cell fate. BMC Biol 14: 19, 2016. doi: 10.1186/s12915-016-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keerthisingam CB, Jenkins RG, Harrison NK, Hernandez-Rodriguez NA, Booth H, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am J Pathol 158: 1411–1422, 2001. doi: 10.1016/S0002-9440(10)64092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schratt G, Philippar U, Hockemeyer D, Schwarz H, Alberti S, Nordheim A. SRF regulates Bcl-2 expression and promotes cell survival during murine embryonic development. EMBO J 23: 1834–1844, 2004. doi: 10.1038/sj.emboj.7600188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johansen FE, Prywes R. Two pathways for serum regulation of the c-fos serum response element require specific sequence elements and a minimal domain of serum response factor. Mol Cell Biol 14: 5920–5928, 1994. doi: 10.1128/mcb.14.9.5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werth D, Grassi G, Konjer N, Dapas B, Farra R, Giansante C, Kandolf R, Guarnieri G, Nordheim A, Heidenreich O. Proliferation of human primary vascular smooth muscle cells depends on serum response factor. Eur J Cell Biol 89: 216–224, 2010. doi: 10.1016/j.ejcb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Luchsinger LL, Patenaude CA, Smith BD, Layne MD. Myocardin-related transcription factor-A complexes activate type I collagen expression in lung fibroblasts. J Biol Chem 286: 44116–44125, 2011. doi: 10.1074/jbc.M111.276931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleige A, Alberti S, Grobe L, Frischmann U, Geffers R, Muller W, Nordheim A, Schippers A. Serum response factor contributes selectively to lymphocyte development. J Biol Chem 282: 24320–24328, 2007. doi: 10.1074/jbc.M703119200. [DOI] [PubMed] [Google Scholar]
- 49.Taylor A, Tang W, Bruscia EM, Zhang PX, Lin A, Gaines P, Wu D, Halene S. SRF is required for neutrophil migration in response to inflammation. Blood 123: 3027–3036, 2014. doi: 10.1182/blood-2013-06-507582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bernau K, Leet JP, Aoki J, Torr EE, Sandbo N. Loss of serum response factor in lung mesenchymal cells ameliorates pulmonary fibrosis. American Thoracic Society Conference Oral presentation. May 22, 2017, p. A4665.