Abstract
In vitro biomarkers to assess cystic fibrosis transmembrane conductance regulator activity are desirable for precision modulator selection and as a tool for clinical trials. Here, we describe an organoid swelling assay derived from human nasal epithelia using commercially available reagents and equipment and an automated imaging process. Cells were collected in nasal brush biopsies, expanded in vitro, and cultured as spherical organoids or as monolayers. Organoids were used in a functional swelling assay with automated measurements and analysis, whereas monolayers were used for short-circuit current measurements to assess ion channel activity. Clinical data were collected from patients on modulators. Relationships between swelling data and short-circuit current, as well as between swelling data and clinical outcome measures, were assessed. The organoid assay measurements correlated with short-circuit current measurements for ion channel activity. The functional organoid assay distinguished individual responses as well as differences between groups. The organoid assay distinguished incremental drug responses to modulator monotherapy with ivacaftor and combination therapy with ivacaftor, tezacaftor, and elexacaftor. The swelling activity paralleled the clinical response. In conclusion, an in vitro biomarker derived from patients’ cells can be used to predict responses to drugs and is likely to be useful as a preclinical tool to aid in the development of novel treatments and as a clinical trial outcome measure for a variety of applications, including gene therapy or editing.
Keywords: drug response, elexacaftor, ivacaftor, organoids, tezacaftor
INTRODUCTION
Cystic fibrosis (CF) is a genetic disorder with significant morbidity, especially in the lung, that limits life span (1, 2). CF transmembrane conductance regulator (CFTR) modulators, including correctors targeted at repairing misprocessed proteins and potentiators intended to restore CFTR gating mutations at the cell surface, have increasingly transformed the care of patients with CF (3–5). Four modulator therapies are in clinical use, expanding modulator therapy to ∼90% of patients: monotherapy with ivacaftor (6–11) and combination therapy with lumacaftor/ivacaftor (12–14), tezacaftor/ivacaftor (15–18), or elexacaftor/tezacaftor/ivacaftor (19, 20). The development of new therapies for CF (www.cff.org/trials/pipeline) will benefit from well-characterized preclinical tools.
Intestinal organoids have shown reproducible responses to CFTR-targeted therapies, including correlation with measures of clinical response at both individual and group levels (21–25). However, in the United States and elsewhere, this assay is not universally available, whereas many CF centers in the United States have sampled human nasal epithelial cells (HNEs). HNEs are obtained inexpensively and safely and require no specialized equipment other than a cytology brush used similarly to a viral nasal swab. Nasal brush biopsies can be used to culture epithelial monolayers for short-circuit current (Isc) measurements, historically the standard in vitro test for CFTR activity (24, 26). However, monolayers require a relatively large amount of material, and the automation is difficult. Furthermore, measurement of the downstream effects of CFTR (fluid and mucus transport), rather than ion transport alone, is crucial to estimating drug response and provides additional mechanistic insights (27–32).
In this study, we describe further development of an in vitro HNE organoid swelling assay (33) as a functional tool and associations with clinical response to modulators, including the recently approved triple-combination CFTR modulator therapy. This assay combines the advantages of intestinal organoid models with nasal cell culture: tissue that is easily, safely, and inexpensively obtained in a reproducible, automated assay that recapitulates the mutation phenotype of the individual. This assay significantly differs from prior publications (34–36) that describe terminally differentiated epithelial cells that self-organize into free-floating spheres with the apical membrane exposed to the media bath, with limited applications. Another model (37) is similar but with significant differences in methodology and analysis and a lack of evaluation against clinical outcomes. Here, we describe an assay that could be useful as a biomarker for CFTR function not only for precision selection of drug therapy for individual patients but also as a patient-derived model in clinical studies, as a surrogate biomarker for clinical trials, and as a new model for understanding CF pathophysiology. Some of the results of these studies have been previously reported in the form of an abstract (38).
METHODS
Participants
Patients with CF (n = 18; Supplemental Table S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.13497210.v1) and non-CF subjects (n = 5) volunteered for the donation of nasal epithelial biopsy (University of Alabama at Birmingham Institutional Review Board No. 151030001), as previously described (33). Written informed consent was obtained from all human subjects. Patient information was extracted from medical records.
Culture of Organoids and Monolayers
Brush biopsies were processed, expanded using conditional reprogramming culture, and seeded as monolayers or organoids, as previously described (33, 39). Because repeated passaging can reduce CFTR expression, we did not use cells higher than passage 3 (40). Briefly, for organoid formation and culture, cells were seeded as a single-cell suspension at a concentration of 500 cells/µL in 20% Matrigel (minimum protein concentration of 9 mg/mL, Corning, New York, NY) into 15-well angiogenesis slides (ibidi USA, Fitchburg, WI) precoated with 100% Matrigel. After 1 h of consolidation of the 20% Matrigel cell suspension in the culture incubator at 37°C, 50 µL of Ultroser-G-based cell culture medium was exchanged in each well every other day to maintain and differentiate the organoids for 14–21 days (41).
Forskolin-Induced Swelling Assays
Forskolin (FSK)-induced swelling (FIS) assay methods were modified from previously described protocols (21, 33). With the exception of the FSK dose-response curves shown in Fig. 1, organoids were pretreated for 48 h with vehicle (DMSO), tezacaftor (3 µM, Selleck Chemicals, Houston, TX), and/or elexacaftor (3 µM, Cystic Fibrosis Foundation Research Laboratory, Bethesda, MD); incubated in media containing DAPI (Invitrogen, Waltham, MA) with or without 10 µM CFTR inhibitor 172 (INH172; Selleck Chemicals) for 1 h; and treated with a stimulation cocktail containing FSK (10 µM) and 1-methyl-3-isobutylxanthine (IBMX; 100 µM) with or without ivacaftor (1 µM, Selleck Chemicals) diluted in PBS and media (1:1). Due to the concern for detrimental effects on CFTR with chronic administration of ivacaftor (42–46), as well as previously published data that measured (47) or estimated (44) in vivo concentrations, we applied 1 µM ivacaftor compared with commonly used 10 µM ivacaftor in the Isc assay. We define baseline CFTR function as the swelling response from the stimulation by FSK and IBMX (FI) in the absence of any CFTR modulators. Organoids were imaged in an automated inverted microscope (Lionheart FX, Biotek, Winooski, VT) at 37°C with 5% CO2 every 15–20 min over 8 h.
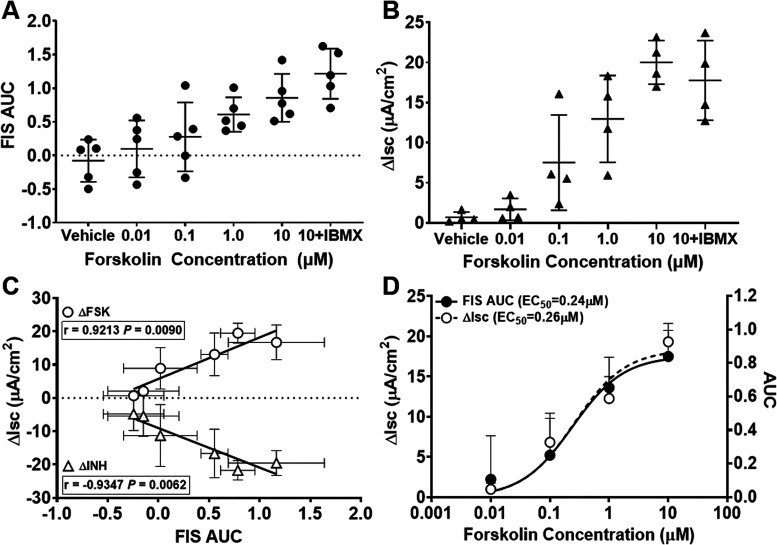
Figure 1.
Non-cystic fibrosis (non-CF) forskolin (FSK) dose response. A: area under the curve (AUC) of non-CF subjects (n = 5 subjects) responding to FSK dose. One-way ANOVA indicated that the AUC was significantly increased in the FSK [1 µM, 10 µM, and 10 µM + 1-methyl-3-isobutylxanthine (IBMX)]-treated group compared with vehicle treatment (P < 0.0001). B: comparison of short-circuit current (ΔIsc) due to FSK treatment for non-CF subjects (n = 4 subjects). One-way ANOVA indicated that ΔIsc was significantly increased in the FSK (1 µM, 10 µM, and 10 µM + IBMX)-treated group compared with vehicle treatment (P < 0.0001). C: Pearson’s correlation of ΔIsc for FSK and CF transmembrane conductance regulator inhibitor-172 (INH) vs. FSK-induced swelling (FIS) AUC for the same individuals and concentrations used in B. Each dot represents the mean of those individuals at the different FSK concentrations. D: FSK dose-response curves (three parameters) of the data shown in C.
Isc Measurements
Isc measurements of HNE monolayers were performed as previously described (48) and were treated with tezacaftor (3 µM), elexacaftor (3 µM), and their combination for 48 h before stimulation. After baseline Isc stabilized, amiloride (100 µM) was used to inhibit apical epithelial Na+ channels, a Cl− gradient was created by replacement of apical Ringer’s solution with low-Cl− Ringer’s solution and amiloride, the cAMP agonist FSK (10 µM) was used to stimulate cAMP-sensitive CFTR channels, ivacaftor (10 µM, unless otherwise stated) was used to potentiate CFTR activity, and the CFTR-specific INH172 was added at 10 µM.
FIS Analysis
Organoid analysis was performed using Gen5 ImagePrime software (Biotek). Organoids were masked using bright-field or DAPI channels. Automated total surface area of the organoids was measured for each well. Quality control exclusion criteria for masking included organoid masks smaller than 90 µm or larger than 500 µm, masking of bubbles, masking of cellular debris, and whole organoid not in frame for the entirety of the 8 h. The change in sum total surface area for all organoids in each well from 0 to 8 h was graphed over time, and the average fractional change (AFC) was calculated as the total surface area at the end of the indicated imaging time divided by the total surface area at beginning of imaging. The net area under the curve (AUC) was calculated for the full 8 h using GraphPad Prism 8 (baseline = 1 and accounting for peaks that went below baseline, reducing AUC, and representing fluid transport out of the lumen). Because there are substantial technical challenges with the analysis of similar assays (49), we incorporated additional quality control measures. To standardize quality control screening of individual wells, results for each condition that were more than 1 SD from the mean of that condition (n = 5 replicate wells) were excluded to account for inevitable technical errors (e.g., debris, introduction of bubbles, and evaporation of media from wells). A subset of wells was manually checked in every experiment to ensure any exclusions were consistently due to such technical errors. On average, approximately one of five wells was excluded due to these technical errors. The sum area of an average of 40 organoids is measured per well, and five replicate wells were treated for every condition. To be included in the final analysis, a minimum of three replicate wells meeting all quality control criteria was required. Unless otherwise specified in the text, each data point for each condition from a single donor represents the mean of three to five replicate wells. Baseline lumen ratio, a measurement of the lumen compared with total surface area in the absence of any treatments, was calculated as previously described (33). The percentage of non-CF [wild-type (WT)] control in Figs. 4, 5, and 6 was calculated using the mean FIS AUC and ΔIsc values for the non-CF (n = 4) individuals in Fig. 2B.
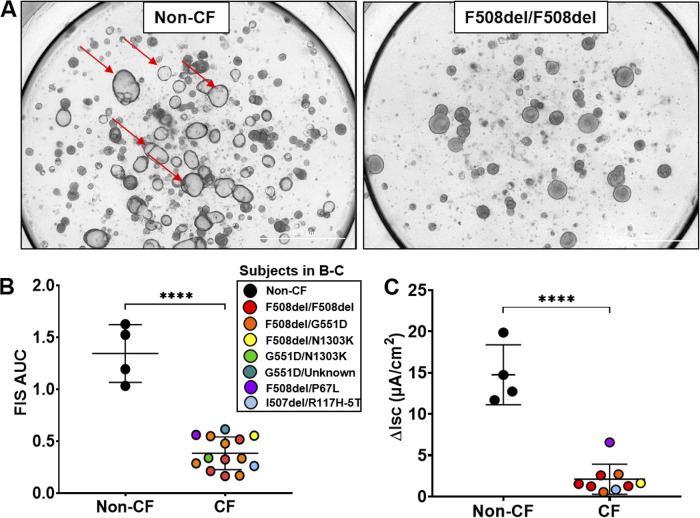
Figure 2.
Forskolin-induced swelling (FIS) assessment of baseline cystic fibrosis (CF) transmembrane conductance regulator activity. A: representative images of a non-CF subject and a CF subject (F508del/F508del) after 8 h of FIS. Red arrows point to a few of the many fluid-filled lumens in non-CF organoids (left) after 8 h of imaging compared with no fluid-filled lumens in the CF organoids on the right. B: comparison of non-CF (n = 4) and CF (n = 14) baseline 8-h FIS responses using an unpaired t test. ****P < 0.0001. C: comparison of non-CF (n = 4) and CF (n = 9) baseline changes in short-circuit current (ΔIsc) using an unpaired t test. ****P < 0.0001. For this study, not all samples available for the area under the curve (AUC) were used for subsequent Isc testing (B and C). Each symbol represents a different subject, with colored symbols representing subjects with CF and black circles representing subjects without CF (middle key).
Statistical Analysis
Results are presented as means ± SD or SE where noted. Analysis was performed using Pearson’s correlation, one-way ANOVA (with Tukey’s multiple-comparisons test when appropriate), or Student’s t test, as indicated using GraphPad Prism 8. Due to this study’s small sample size, normality was assumed where the quantiles of the residuals were similar to quantiles for a normal distribution (50). If the assumption of normality was not reasonable on visual assessment, then the values were log-transformed and reassessed. All datasets were normally or log normally distributed; therefore, all comparisons were made using parametric analysis. P values of <0.05 were considered significant.
RESULTS
HNE Organoid Swelling Assay
We have previously optimized and characterized the differentiated HNE organoid model (33). To improve the dynamic range and discriminating capacity, we extended the length of the assay to 8 h (33) in contrast to the prior similar organoid study (37), which enabled us to clearly distinguish differences between conditions that were not always apparent at earlier time points (Supplemental Fig. S1). The maximal stimulatory FSK dose in non-CF HNE organoids was determined using increasing doses (0.01–10 µM) as well as IBMX (100 µM) to maintain the concentrations of cAMP during the lengthened assay (Fig. 1). Total surface area was measured by automated image analysis and plotted over time to determine AFC (Supplemental Fig. S2A). The AUC was used as the primary measure for detecting differences between conditions. The magnitude of swelling was significantly increased in a linear fashion as FSK concentration increased (Fig. 1A). There was a nonsignificant increase in swelling when IBMX was added to the maximal stimulating FSK concentration (10 µM; Fig. 1A). Given the length of the assay and the larger response when used, we opted to include it in further experiments.
Isc is a standard measure of CFTR activity and has been shown to correlate with clinical responses (51), but it requires a large amount of cell material, is laborious, and is difficult to fully automate. Because the FIS assay is automated and requires far fewer cells per condition, we assessed whether the FIS assay may substitute for Isc. Isc was measured across HNE monolayers concurrently performed in cells from the same biopsy from four non-CF subjects to assess the correlation of swelling with Isc in response to increasing FSK concentrations, as illustrated by the representative Isc tracings (Supplemental Fig. S2B) and the summary change in current (ΔIsc; Fig. 1B). There was a significant correlation between mean ΔIsc and AUC in response to increasing concentrations of FSK (r = 0.921, P = 0.009) as well as specific inhibition of CFTR by INH172 (r = −0.935, P = 0.006), as shown in Fig. 1C (n = 3 subjects). AUC and ΔIsc data were used to generate FSK dose-response curves for each and were nearly identical, showing EC50 values of 0.24 and 0.26 µM, respectively (Fig. 1D).
Assessment of Baseline CFTR Function in the Organoid Swelling Assay
We evaluated baseline CFTR function in the FIS assay from patients with CF in addition to those without CF. We noted significant differences in FIS between non-CF and CF organoids of varying CF genotype after 8 h of FI stimulation (Fig. 2). Figure 2A shows example images of a non-CF subject and a subject with CF (F508del/F508del) after 8 h of FI stimulation. The AUC after 8 h of swelling was significantly lower in subjects with CF compared with non-CF subjects (P < 0.0001; Fig. 2B). ΔIsc of monolayers from the same subjects paralleled the response of swelling assays (P < 0.0001; Fig. 2C). Due to a paucity of swelling values in the intermediate range, correlation analysis was not performed.
Individual CFTR Modulator Response
HNEs have been used to assess responses to different CFTR modulators on an individual basis (26, 37, 51). To assess this capacity in our assay, we conducted a series of experiments in individual patients with different mutation combinations. We used three key modulator treatments that are currently in clinical use: ivacaftor alone, tezacaftor/ivacaftor, and elexacaftor/tezacaftor/ivacaftor. A sample of individual patient results is shown in Fig. 3 and provided in Supplemental Fig. S1 for three patients with different CFTR genotypes (F508del/F508del, F508del/G551D, and F508del/P67L). In this assessment, we showed representative experiments including summary 8 h FIS AUC (Fig. 3, A–C, left), summary ΔIsc responses (Fig. 3, A–C, right), AFC (Supplemental Fig. S1, A–C, left), and representative Isc tracings (Supplemental Fig. S3). These results demonstrate the ability of this assay to distinguish an individual response to various modulator combinations. Both FIS and Isc generally agreed as to the pattern of modulator response, which was consistent with clinically observed findings. However, in the case of ivacaftor versus tezacaftor/ivacaftor shown in Fig. 3B, the swelling results were more reflective of the subject’s clinical response (subject A; Supplemental Table S2).
Figure 3.
Theratyping by forskolin-induced swelling (FIS) and short-circuit current (Isc) responses to cystic fibrosis transmembrane conductance regulator (CFTR) modulators in patients with CF. F508del/F508del (A), F508del/G551D (B), and F508del/P67L (C) individual areas under the curve (AUCs; left) over 8 h for each condition and changes in Isc (ΔIsc) under each condition (right). All conditions were compared using one-way ANOVA with Tukey’s multiple-comparisons test, although not all comparisons are shown for simplicity. **P < 0.01; ***P < 0.001; ****P < 0.0001. AUC, area under the curve; elex, elexacaftor; INH172, CFTR inhibitor-172; iva, ivacaftor; ns, not significant; tez, tezacaftor.
Overall CF Modulator Swelling Response Correlates with Isc Measurements
Baseline organoid FIS and ΔIsc were highly correlated in non-CF, as shown in Fig. 1. Although fluid transport is a complex process incorporating both CFTR activation and other pathways, and the modulator response may not be as simple as restoring CFTR alone, we hypothesized that the response to modulators in organoid swelling should have a similar response to change in current, even if the swelling assay does not always fully reflect the modulator effects, as shown in Fig. 3. A prior study of such correlations included non-CF subjects in similar assessments (37). We assessed whether the FIS assay would correlate with the change in current in the absence of non-CF responses, which would increase sample size at the high end of the range and potentially confound the association. We found significant correlations between baseline and modulator-treated AUC and ΔIsc as percentages of non-CF (WT) control, when combination treatments were evaluated separately and also for all treatments together (Fig. 4, A–D). Treatment with ivacaftor alone did not show a significant correlation with Isc, possibly as a result of the small, homogeneous sample. We also analyzed correlation by sequentially removing the baseline activity of all participants, leaving only the response to modulators in the correlation analysis (r = 0.5515, P = 0.0177; Fig. 4D).
Figure 4.
Correlation of short-circuit current (Isc) and forskolin-induced swelling (FIS) area under the curve (AUC) in response to modulator treatment as a percentage of the non-cystic fibrosis response [wild type (WT)]. A: baseline and ivacaftor (iva) responses (n = 10; subjects A, B, G, K, and M). B: baseline and tezacaftor/ivacaftor (tez/iva) responses (n = 14; subjects A, B, C, G, H, J, and L). C: baseline and elexacaftor/tezacaftor/ivacaftor (elex/tez/iva) responses (n = 12; subjects A, B, C, G, H, and J). D: baseline and responses for all modulator therapies (n = 27; subjects A, B, C, G, H, J, K, L, and M). All error bars are ±SE. Letters and corresponding colors match the subject codes provided in Supplemental Table S1.
Comparison of Organoid Swelling with Clinical Measurements in Patients
Ultimately, the goal of this in vitro assay was to attempt to predict the in vivo clinical response to a therapeutic intervention. In Fig. 5, we assessed baseline sweat Cl− (SwCl−) of individual patients with their own baseline HNE organoid FIS AUC (Fig. 5A) and their baseline lumen ratio (Fig. 5B). Baseline SwCl− was not available for non-CF healthy volunteers. Therefore, we used the mean SwCl− value from available clinical trial data with non-CF participants coupled with the mean FIS response or baseline lumen ratio for our non-CF participants. A significant correlation was seen between baseline SwCl− and the FIS response (r = −0.7478, P = 0.0081; Fig. 5A). In the absence of the mean non-CF value, the correlation was lost, similar to that reported by other investigators (23, 37, 52), in part likely due to an absence of more intermediate values. We have previously reported that lumen formation, as measured by baseline lumen ratio, was correlated with CFTR activity, as measured by Isc (33). Baseline lumen ratio was also significantly correlated with baseline SwCl− (r = −0.7625, P = 0.0015; Fig. 5B) and was persistent when the non-CF value was excluded (r = 0.6060, P = 0.0280). Paired pre- and postmodulator treatment SwCl− values were not available for enough patients to analyze, since all clinical data were obtained retrospectively and posttreatment SwCl− is not routinely obtained in our center.
Figure 5.
Correlations of the forskolin-induced swelling (FIS) response with clinical data. A: patient baseline sweat Cl− (SwCl−) values (n = 11) compared with baseline FIS area under the curve (AUC) as a percentage of the non-cystic fibrosis (non-CF) response [wild type (WT)]. B: baseline SwCl− values (n = 10) compared with baseline lumen ratio. C: patients’ absolute change in percent predicted forced expiratory volume in 1 s (ΔppFEV1) with the organoid response as a percentage of WT (n = 10). Dashed rectangle represents the in vitro threshold of the response (10% of WT) to yield a clinically relevant response (5%). D: organoid swelling due to modulators as a percentage of WT using the subjects shown in C to compare responders and nonresponders using an unpaired t test. **P < 0.01. The baseline SwCl− values for the mean non-CF control average value in A and B are from a clinical trial healthy control group (PROSPECT; NCT02477319). Letters and corresponding colors match the subject codes provided in Supplemental Table S1. Data from C and D can be found in Supplemental Table S2. †These subjects switched CF transmembrane conductance regulator modulators during their treatment course, so in vitro and in vivo data were included for both.
For lung function assessment, our cohort (n = 8 subjects) produced at least one value for percent predicted forced expiratory volume in 1 s (ppFEV1) in the year preceding and following modulator initiation. Only values in the absence of a pulmonary exacerbation were included in the analysis. If more than one value meeting that criterion was available, the average of these values was used as the baseline. We found a significant correlation between each individual’s in vitro organoid response (AUC as a percentage of WT) and their in vivo response (absolute ΔppFEV1 as a percentage of WT) to modulator treatment (r = 0.7778, P = 0.0081; Fig. 5C). Because of the small sample size, we also evaluated patient response as a categorical variable. Patients were categorized as responders or nonresponders (nonresponder: <5% absolute ΔppFEV1), with 5% being the threshold accounting for the technical variability of FEV1 and a perceived improvement by the patient (53). When classified in this manner, a significant difference (P = 0.0016) was seen for the FIS AUC drug response between responders and nonresponders (Fig. 5D). As shown in Fig. 5C, all subjects (within the dashed rectangle) categorized as nonresponders also had a <10% of WT organoid swelling response (dashed line, Fig. 5, C and D). This 10% of WT CFTR function was considered clinically significant, due to its correlation with mild disease (i.e., higher rates of pancreatic sufficiency, milder respiratory manifestations, and lower SwCl− values) (54, 55).
Correlation of Organoid Swelling with Clinical Measurements from Clinical Trials
To assess clinical correlations at a group level as seen with clinical trial cohorts for CFTR modulator studies, we extracted mean ΔSwCl− (Fig. 6A) and mean absolute ΔppFEV1 (Fig. 6B) from clinical trial results (Fig. 6C) for which our subjects would have been eligible to participate, similar to previous assessments for intestinal organoids (56). FIS drug response data for specific genotype-treatment pairs were averaged and compared with respective clinical trial data (Supplemental Table S2). Correlations for both mean ΔSwCl− (r = −0.8056, P = 0.0287; Fig. 6A) and absolute ΔppFEV1 (r = 0.9574, P = 0.0007; Fig. 6B) are shown.
Figure 6.
Mean organoid responses correlated with clinical trial data. Pearson’s correlation of the organoid swelling response to the clinical trial mean change in sweat Cl− (ΔSwCl−; A) and to the clinical trial matched absolute change in percent predicted forced expiratory volume in 1 s (ΔppFEV1; B). The dashed vertical line represents 10% modulator improvement from baseline as a percentage of the non-cystic fibrosis (non-CF) response [wild type (WT)]. C: clinical trial details corresponding to the numbers above each data point in A and B. The forskolin-induced swelling (FIS) area under the curve (AUC) percent in the WT column corresponds to the mean (SD) drug response of the subjects listed in the last column. These subject codes correspond with the subject codes provided in Supplemental Table S1. CFTR, CF transmembrane conductance regulator; elex/tez/iva, elexacaftor/tezacaftor/ivacaftor; iva, ivacaftor; tez/iva, tezacaftor/ivacaftor.
DISCUSSION
Our results show that baseline CFTR function of the HNE organoid model and response to modulators for different mutation combinations correlate significantly with Isc. This illustrates that the organoid model parallels measures of monolayer culture for CFTR ion channel activity, while providing advantages over the more resource-consuming assay. At the same time, the correlation with clinical data suggests that our assay is a candidate ex vivo biomarker that may predict clinical responses to therapeutic interventions for either individuals or groups.
In many ways, our HNE organoid model is similar to the intestinal organoid system that correlates well with clinical phenotype and drug response (23, 56–63). However, rectal biopsy is not easily obtainable in all centers for assessment of CFTR activity. The HNE organoid model in the present study recapitulates many of the desirable characteristics of the intestinal organoid assay but instead uses a tissue type that is easily accessible and acts as a surrogate for the lower airway. Because of the extensive efforts of the Cystic Fibrosis Foundation (CFF) in multiple observational trials that procured nasal cells, the nasal brush biopsy technique is well known across most centers and can be performed in infants, children, and adults without sedation in an outpatient examination room with simple, inexpensive brushes. We have also paid particular attention to using materials that are widely available, including well-published methodology for culturing and commercially available culture dishes, reagents, equipment, and analysis software. Our intent is to allow expansion across centers, using an automated imaging system that helps reduce subjective bias from measurements.
We have shown a significant correlation between HNE monolayer current and the HNE organoid swelling assay. Our data provide evidence that this organoid swelling assay can serve as a substitute for or an adjunct to HNE monolayers. Several studies have shown substantial utility of HNE monolayers for not only theratyping (52, 64, 65) but also studies of the pathophysiology of CFTR mutations, phenotype of CF, and impact of various potential therapeutic approaches, among other uses. We have also shown a correlation between FIS in organoids treated with CFTR modulators and the Isc response of the same cells. The HNE organoid assay itself requires less material (∼5,000 cells/replicate) and less labor than traditional current measurements due to automation of the analysis. It is further amenable to additional automation and higher throughput approaches than shown here, as the imaging slides used come as 96-well plates and the imaging systems have options to use cell-culture robotics to treat and image many plates. Most importantly, the swelling response in our assay is attributable to CFTR activation and correlates well with clinical responses. This is in contrast to a previously published model that did not correlate the in vitro CFTR modulator response with in vivo measures of the clinical response (37). This is significant for the HNE organoid assay to be used as both a model for precision medicine approaches as well as generalizing drug response to broader CF patient cohorts.
This study is limited by the retrospective nature of the clinical observations and a small sample size. A prospective, larger theratyping study should be performed to confirm reproducibility of the assay and correlations with clinical measures. The small sample size enabled optimization of the assay and concurrent monolayer studies. Future studies will focus on developing this model using a larger sample size into a biomarker that can ultimately be implemented as a theranostic, prognostic, and/or diagnostic tool in clinical laboratories.
In conclusion, we describe here an HNE organoid assay that recapitulates many characteristics of the intestinal organoid system but may be feasible for more laboratories to complete. In addition to individual theratyping, it may be useful for the screening of novel compounds that improve CFTR response to non-CF levels, for assessing additive effects of additional modulators such as those in development, and for the screening of drugs that address nonsense and splice mutations in CFTR. It has further utility as a clinical trial biomarker for patients already on a modulator to assess for ex vivo responses to novel interventions, including gene therapy. Correlations of baseline CFTR activity could be useful for predicting manifestation of CFTR dysfunction in individuals who have novel mutations with unknown clinical significance or for those with inconclusive diagnoses. Strong correlations between organoid response to modulators and clinical responses are essential to use this model as a clinical decision tool or as a trial biomarker.
SUPPLEMENTAL DATA
Supplemental Tables S1 and S2 and Supplemental Figs. S1–S3: https://doi.org/10.6084/m9.figshare.13497210.v1.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant K23HL143167 (to J.S.G.), Cystic Fibrosis Foundation (CFF) Grants GUIMBE18A0-Q and GUIMBE1710 (to J.S.G.), the Gregory Fleming James Cystic Fibrosis Center [NIH Grants R35HL135816 and DK072482 and the CFF University of Alabama at Birmingham (UAB) Research and Development Program (Rowe19RO)], and the UAB Center for Clinical and Translational Science (NIH Grant UL1TR001417).
DISCLAIMERS
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
DISCLOSURES
J.S.G. is listed as an inventor on a patent application 20170242033 from the University of North Carolina (UNC) that describes a similar model. When licensed technology from UNC produces royalties, the inventors receive a share of the revenue. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
J.S.G. conceived and designed research; J.D.A., Z.L., and L.K. performed experiments; J.D.A., Z.L., L.V.O., and J.S.G. analyzed data; J.S.G. interpreted results of experiments; J.D.A. and Z.L. prepared figures; J.D.A., Z.L., and J.S.G. drafted manuscript; J.D.A., Z.L., and J.S.G. edited and revised manuscript; J.D.A., Z.L., L.V.O., L.K., and J.S.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Steven M. Rowe, Director of the Gregory Fleming James Cystic Fibrosis Research Center, for critical review of this manuscript, support, and funding the automated live cell microscope without which this work would not have been possible. We also thank Dr. Sarah Guadiana (Biotek) for technical assistance and training. We thank Dr. William T. Harris for critical review of the manuscript and Jennifer Natt for assistance in preparing the manuscript. Elexacaftor (VX-445) was provided as a generous gift from Dr. Martin Mense (CFF Research Laboratory).
REFERENCES
- 1.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med 173: 475–482, 2006. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 2.Lubamba B, Dhooghe B, Noel S, Leal T. Cystic fibrosis: insight into CFTR pathophysiology and pharmacotherapy. Clin Biochem 45: 1132–1144, 2012. doi: 10.1016/j.clinbiochem.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Bell SC, De BK, Amaral MD. New pharmacological approaches for cystic fibrosis: promises, progress, pitfalls. Pharmacol Ther 145C: 19–34, 2015. doi: 10.1016/j.pharmthera.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Bertoncini E, Colomb-Lippa D. Pulmonology: CFTR modulators for cystic fibrosis. JAAPA 26: 59–60, 2013. doi: 10.1097/01720610-201302000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Derichs N. Targeting a genetic defect: cystic fibrosis transmembrane conductance regulator modulators in cystic fibrosis. Eur Res Rev 22: 58–65, 2013. doi: 10.1183/09059180.00008412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu H, Burton B, Huang CJ, Worley J, Cao D, Johnson JP Jr, Urrutia A, Joubran J, Seepersaud S, Sussky K, Hoffman BJ, Van Goor F. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros 11: 237–245, 2012. doi: 10.1016/j.jcf.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Kotha K, Clancy JP. Ivacaftor treatment of cystic fibrosis patients with the G551D mutation: a review of the evidence. Ther Adv Respir Dis 7: 288–296, 2013. doi: 10.1177/1753465813502115. [DOI] [PubMed] [Google Scholar]
- 8.Kapoor H, Koolwal A, Singh A. Ivacaftor: a novel mutation modulating drug. J Clin Diagn Res 8: SE01–SE05, 2014. doi: 10.7860/JCDR/2014/6486.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molloy K, McElvaney NG. Ivacaftor: from bench to bedside … and back again. Am J Respir Crit Care Med 190: 128–129, 2014. doi: 10.1164/rccm.201406-1122ED. [DOI] [PubMed] [Google Scholar]
- 10.Wainwright CE. Ivacaftor for patients with cystic fibrosis. Expert Rev Respir Med 8: 533–538, 2014. doi: 10.1586/17476348.2014.951333. [DOI] [PubMed] [Google Scholar]
- 11.Whiting P, Al M, Burgers L, Westwood M, Ryder S, Hoogendoorn M, Armstrong N, Allen A, Severens H, Kleijnen J. Ivacaftor for the treatment of patients with cystic fibrosis and the G551D mutation: a systematic review and cost-effectiveness analysis. Health Technol Assess 18: 1–106, 2014. doi: 10.3310/hta18180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle MP, Bell SC, Konstan MW, McColley SA, Rowe SM, Rietschel E, Huang X, Waltz D, Patel NR, Rodman D; VX09-809-102 study group. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir Med 2: 527–538, 2014. doi: 10.1016/S2213-2600(14)70132-8. [DOI] [PubMed] [Google Scholar]
- 13.Jones AM, Barry PJ. Lumacaftor/ivacaftor for patients homozygous for Phe508del-CFTR: should we curb our enthusiasm? Thorax 70: 615–616, 2015. doi: 10.1136/thoraxjnl-2015-207369. [DOI] [PubMed] [Google Scholar]
- 14.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, Davies JC, De Boeck K, Flume PA, Konstan MW, McColley SA, McCoy K, McKone EF, Munck A, Ratjen F, Rowe SM, Waltz D, Boyle MP; TRAFFIC Study Group; TRANSPORT Study Group. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 373: 220–231, 2015. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, Ramsey BW, Rowe SM, Sass LA, Tullis E, McKee CM, Moskowitz SM, Robertson S, Savage J, Simard C, Van Goor F, Waltz D, Xuan F, Young T, Taylor-Cousar JL. VX16-445-001 Study Group. VX-445-Tezacaftor-Ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med 379: 1612–1620, 2018. doi: 10.1056/NEJMoa1807120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donaldson SH, Pilewski JM, Griese M, Cooke J, Viswanathan L, Tullis E, Davies JC, Lekstrom-Himes JA, Wang LT. VX11-661-101 Study Group. Tezacaftor/Ivacaftor in subjects with cystic fibrosis and F508del/F508del-CFTR or F508del/G551D-CFTR. Am J Respir Crit Care Med 197: 214–224, 2018. doi: 10.1164/rccm.201704-0717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, Wang LT, Ingenito EP, McKee C, Lu Y, Lekstrom-Himes J, Elborn JS. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med 377: 2013–2023, 2017. doi: 10.1056/NEJMoa1709846. [DOI] [PubMed] [Google Scholar]
- 18.Rowe SM, Daines C, Ringshausen FC, Kerem E, Wilson J, Tullis E, Nair N, Simard C, Han L, Ingenito EP, McKee C, Lekstrom-Himes J, Davies JC. Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med 377: 2024–2035, 2017. doi: 10.1056/NEJMoa1709847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Middleton PG, Mall MA, Drevinek P, Lands LC, McKone EF, Polineni D, Ramsey BW, Taylor-Cousar JL, Tullis E, Vermeulen F, Marigowda G, McKee CM, Moskowitz SM, Nair N, Savage J, Simard C, Tian S, Waltz D, Xuan F, Rowe SM, Jain R; VX17-445-102 Study Group. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 381: 1809–1819, 2019. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, Mall MA, Welter JJ, Ramsey BW, McKee CM, Marigowda G, Moskowitz SM, Waltz D, Sosnay PR, Simard C, Ahluwalia N, Xuan F, Zhang Y, Taylor-Cousar JL, McCoy KS; VX17-445-103 Trial Group. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 394: 1940–1948, 2019. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19: 939–945, 2013. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 22.Dekkers R, Vijftigschild LA, Vonk AM, Kruisselbrink E, de Winter-de Groot KM, Janssens HM, van der Ent CK, Beekman JM. A bioassay using intestinal organoids to measure CFTR modulators in human plasma. J Cyst Fibros 14: 178–181, 2015. doi: 10.1016/j.jcf.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 23.de Winter-de Groot KM, Janssens HM, van Uum RT, Dekkers JF, Berkers G, Vonk A, Kruisselbrink E, Oppelaar H, Vries R, Clevers H, Houwen RHJ, Escher JC, Elias SG, de Jonge HR, de Rijke YB, Tiddens H, van der Ent CK, Beekman JM. Stratifying infants with cystic fibrosis for disease severity using intestinal organoid swelling as a biomarker of CFTR function. Eur Respir J 52: 1702529, 2018. doi: 10.1183/13993003.02529-2017. [DOI] [PubMed] [Google Scholar]
- 24.Brewington JJ, Filbrandt ET, LaRosa FJ 3rd, Moncivaiz JD, Ostmann AJ, Strecker LM, Clancy JP. Brushed nasal epithelial cells are a surrogate for bronchial epithelial CFTR studies. JCI Insight 3: e99385, 2018. doi: 10.1172/jci.insight.99385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramalho AS, Furstova E, Vonk AM, Ferrante M, Verfaillie C, Dupont L, Boon M, Proesmans M, Beekman JM, Sarouk I, Cordero CV, Vermeulen F, De Boeck K; Belgian Organoid Project. Correction of CFTR function in intestinal organoids to guide treatment of cystic fibrosis. Eur Respir J 57: 1902426, 2021. doi: 10.1183/13993003.02426-2019. [DOI] [PubMed] [Google Scholar]
- 26.McCravy MS, Quinney NL, Cholon DM, Boyles SE, Jensen TJ, Aleksandrov AA, Donaldson SH, Noone PG, Gentzsch M. Personalised medicine for non-classic cystic fibrosis resulting from rare CFTR mutations. Eur Respir J 56: 2000062, 2020. doi: 10.1183/13993003.00062-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birket SE, Chu KK, Houser GH, Liu L, Fernandez CM, Solomon GM, Lin V, Shastry S, Mazur M, Sloane PA, Hanes J, Grizzle WE, Sorscher EJ, Tearney GJ, Rowe SM. Combination therapy with cystic fibrosis transmembrane conductance regulator modulators augment the airway functional microanatomy. Am J Physiol Lung Cell Mol Physiol 310: L928–L939, 2016. doi: 10.1152/ajplung.00395.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birket SE, Chu KK, Liu L, Houser GH, Diephuis BJ, Wilsterman EJ, Dierksen G, Mazur M, Shastry S, Li Y, Watson JD, Smith AT, Schuster BS, Hanes J, Grizzle WE, Sorscher EJ, Tearney GJ, Rowe SM. A functional anatomic defect of the cystic fibrosis airway. Am J Respir Crit Care Med 190: 421–432, 2014. doi: 10.1164/rccm.201404-0670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu KK, Mojahed D, Fernandez CM, Li Y, Liu L, Wilsterman EJ, Diephuis B, Birket SE, Bowers H, Martin Solomon G, Schuster BS, Hanes J, Rowe SM, Tearney GJ. Particle-tracking microrheology using micro-optical coherence tomography. Biophys J 111: 1053–1063, 2016. doi: 10.1016/j.bpj.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu KK, Unglert C, Ford TN, Cui D, Carruth RW, Singh K, Liu L, Birket SE, Solomon GM, Rowe SM, Tearney GJ. In vivo imaging of airway cilia and mucus clearance with micro-optical coherence tomography. Biomed Opt Express 7: 2494–2505, 2016. doi: 10.1364/BOE.7.002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L, Chu KK, Houser GH, Diephuis BJ, Li Y, Wilsterman EJ, Shastry S, Dierksen G, Birket SE, Mazur M, Byan-Parker S, Grizzle WE, Sorscher EJ, Rowe SM, Tearney GJ. Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography. PloS One 8: e54473, 2013. doi: 10.1371/journal.pone.0054473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuggle KL, Birket SE, Cui X, Hong J, Warren J, Reid L, Chambers A, Ji D, Gamber K, Chu KK, Tearney G, Tang LP, Fortenberry JA, Du M, Cadillac JM, Bedwell DM, Rowe SM, Sorscher EJ, Fanucchi MV. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PloS One 9: e91253, 2014. doi: 10.1371/journal.pone.0091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Anderson JD, Deng L, Mackay S, Bailey J, Kersh L, Rowe SM, Guimbellot JS. Human nasal epithelial organoids for therapeutic development in cystic fibrosis. Genes (Basel) 11: 603, 2020. doi: 10.3390/genes11060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guimbellot JS, Leach JM, Chaudhry IG, Quinney NL, Boyles SE, Chua M, Aban I, Jaspers I, Gentzsch M. Nasospheroids permit measurements of CFTR-dependent fluid transport. JCI Insight 2: e95734, 2017. doi: 10.1172/jci.insight.95734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen PS, Braunstein TH, Jorgensen A, Larsen PL, Holstein-Rathlou NH, Frederiksen O. Stimulation of aquaporin-5 and transepithelial water permeability in human airway epithelium by hyperosmotic stress. Pflugers Arch 453: 777–785, 2007. doi: 10.1007/s00424-006-0157-3. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen PS, Frederiksen O, Holstein-Rathlou NH, Larsen PL, Qvortrup K. Ion transport in epithelial spheroids derived from human airway cells. Am J Physiol 276: L75–L80, 1999. doi: 10.1152/ajplung.1999.276.1.L75. [DOI] [PubMed] [Google Scholar]
- 37.Brewington JJ, Filbrandt ET, LaRosa FJ, 3rd, Ostmann AJ, Strecker LM, Szczesniak RD, Clancy JP. Detection of CFTR function and modulation in primary human nasal cell spheroids. J Cyst Fibros 17: 26–33, 2018. doi: 10.1016/j.jcf.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson JD, Liu Z, Odom LV, Guimbellot JS. HNE organoids closely recapitulate short-circuit current and clinical responses from patients. In: The 34th Annual North American Cystic Fibrosis Conference. Hoboken, NJ: Pediatric Pulmonology, 2020: p. S1–S385. [Google Scholar]
- 39.Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, Haddad BR, Rhim JS, Dritschilo A, Riegel A, McBride A, Schlegel R. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol 180: 599–607, 2012. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avramescu RG, Kai Y, Xu H, Bidaud-Meynard A, Schnur A, Frenkiel S, Matouk E, Veit G, Lukacs GL. Mutation-specific downregulation of CFTR2 variants by gating potentiators. Hum Mol Genet 26: 4873–4885, 2017. doi: 10.1093/hmg/ddx367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neuberger T, Burton B, Clark H, Van Goor F. Use of primary cultures of human bronchial epithelial cells isolated from cystic fibrosis patients for the pre-clinical testing of CFTR modulators. Methods Mol Biol 741: 39–54, 2011. doi: 10.1007/978-1-61779-117-8_4. [DOI] [PubMed] [Google Scholar]
- 42.Guhr Lee TN, Cholon DM, Quinney NL, Gentzsch M, Esther CR Jr.. Accumulation and persistence of ivacaftor in airway epithelia with prolonged treatment. J Cyst Fibros 19: 746–751, 2020. doi: 10.1016/j.jcf.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cholon DM, Quinney NL, Fulcher ML, Esther CR Jr, Das J, Dokholyan NV, Randell SH, Boucher RC, Gentzsch M. Potentiator ivacaftor abrogates pharmacological correction of DeltaF508 CFTR in cystic fibrosis. Sci Transl Med 6: 246ra96, 2014. doi: 10.1126/scitranslmed.3008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matthes E, Goepp J, Carlile GW, Luo Y, Dejgaard K, Billet A, Robert R, Thomas DY, Hanrahan JW. Low free drug concentration prevents inhibition of F508del CFTR functional expression by the potentiator VX-770 (ivacaftor). Br J Pharmacol 173: 459–470, 2016. doi: 10.1111/bph.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chin S, Hung M, Won A, Wu YS, Ahmadi S, Yang D, Elmallah S, Toutah K, Hamilton CM, Young RN, Viirre RD, Yip CM, Bear CE. Lipophilicity of the cystic fibrosis drug, ivacaftor (VX-770), and its destabilizing effect on the major CF-causing mutation: F508del. Mol Pharmacol 94: 917–925, 2018. doi: 10.1124/mol.118.112177. [DOI] [PubMed] [Google Scholar]
- 46.Veit G, Avramescu RG, Perdomo D, Phuan PW, Bagdany M, Apaja PM, Borot F, Szollosi D, Wu YS, Finkbeiner WE, Hegedus T, Verkman AS, Lukacs GL. Some gating potentiators, including VX-770, diminish DeltaF508-CFTR functional expression. Sci Transl Med 6: 246ra297, 2014. doi: 10.1126/scitranslmed.3008889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guimbellot JS, Ryan KJ, Anderson JD, Liu Z, Kersh L, Esther CR, Rowe SM, Acosta EP. Variable cellular ivacaftor concentrations in people with cystic fibrosis on modulator therapy. J Cyst Fibros 19: 742–745, 2020. doi: 10.1016/j.jcf.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rowe SM, Pyle LC, Jurkevante A, Varga K, Collawn J, Sloane PA, Woodworth B, Mazur M, Fulton J, Fan L, Li Y, Fortenberry J, Sorscher EJ, Clancy JP. DeltaF508 CFTR processing correction and activity in polarized airway and non-airway cell monolayers. Pulm Pharmacol Ther 23: 268–278, 2010. doi: 10.1016/j.pupt.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Booij TH, Price LS, Danen EHJ. 3D Cell-based assays for drug screens: challenges in imaging, image analysis, and high-content analysis. SLAS Discov 24: 615–627, 2019. doi: 10.1177/2472555219830087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan CJ. Use of proper statistical techniques for research studies with small samples. Am J Physiol Lung Cell Mol Physiol 313: L873–L877, 2017. doi: 10.1152/ajplung.00238.2017. [DOI] [PubMed] [Google Scholar]
- 51.Debley JS, Barrow KA, Rich LM, Singh P, McKone EF, Nichols DP. Correlation between Ivacaftor-induced CFTR activation in airway epithelial cells and improved lung function: a proof-of-concept study. Ann Am Thorac Soc 17: 1024–1027, 2020. doi: 10.1513/AnnalsATS.202001-082RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pranke IM, Hatton A, Simonin J, Jais JP, Le Pimpec-Barthes F, Carsin A, Bonnette P, Fayon M, Stremler-Le Bel N, Grenet D, Thumerel M, Mazenq J, Urbach V, Mesbahi M, Girodon-Boulandet E, Hinzpeter A, Edelman A, Sermet-Gaudelus I. Correction of CFTR function in nasal epithelial cells from cystic fibrosis patients predicts improvement of respiratory function by CFTR modulators. Sci Rep 7: 7375, 2017. doi: 10.1038/s41598-017-07504-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teramoto S, Suzuki M, Matsui H, Ishii T, Matsuse T, Ouchi Y. Influence of age on diurnal variability in measurements of spirometric indices and respiratory pressures. J Asthma 36: 487–492, 1999. doi: 10.3109/02770909909054554. [DOI] [PubMed] [Google Scholar]
- 54.Ferec C, Cutting GR. Assessing the disease-liability of mutations in CFTR. Cold Spring Harb Perspect Med 2: a009480, 2012. doi: 10.1101/cshperspect.a009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Green DM, McDougal KE, Blackman SM, Sosnay PR, Henderson LB, Naughton KM, Collaco JM, Cutting GR. Mutations that permit residual CFTR function delay acquisition of multiple respiratory pathogens in CF patients. Respir Res 11: 140, 2010. doi: 10.1186/1465-9921-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dekkers JF, Berkers G, Kruisselbrink E, Vonk A, de Jonge HR, Janssens HM, Bronsveld I, van de Graaf EA, Nieuwenhuis EE, Houwen RH, Vleggaar FP, Escher JC, de Rijke YB, Majoor CJ, Heijerman HG, de Winter-de Groot KM, Clevers H, van der Ent CK, Beekman JM. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med 8: 344ra384, 2016. doi: 10.1126/scitranslmed.aad8278. [DOI] [PubMed] [Google Scholar]
- 57.de Winter-de Groot KM, Berkers G, Marck-van der Wilt REP, van der Meer R, Vonk A, Dekkers JF, Geerdink M, Michel S, Kruisselbrink E, Vries R, Clevers H, Vleggaar FP, Elias SG, Heijerman HGM, van der Ent CK, Beekman JM. Forskolin-induced swelling of intestinal organoids correlates with disease severity in adults with cystic fibrosis and homozygous F508del mutations. J Cyst Fibros 19: 614–619, 2019. doi: 10.1016/j.jcf.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 58.Berkers G, van Mourik P, Vonk AM, Kruisselbrink E, Dekkers JF, de Winter-de Groot KM, Arets HGM, Marck-van der Wilt REP, Dijkema JS, Vanderschuren MM, Houwen RHJ, Heijerman HGM, van de Graaf EA, Elias SG, Majoor CJ, Koppelman GH, Roukema J, Bakker M, Janssens HM, van der Meer R, Vries RGJ, Clevers HC, de Jonge HR, Beekman JM, van der Ent CK. Rectal organoids enable personalized treatment of cystic fibrosis. Cell Rep 26: 1701–1708.e3, 2019. doi: 10.1016/j.celrep.2019.01.068. [DOI] [PubMed] [Google Scholar]
- 59.Noordhoek J, Gulmans V, van der Ent K, Beekman JM. Intestinal organoids and personalized medicine in cystic fibrosis: a successful patient-oriented research collaboration. Curr Opin Pulm Med 22: 610–616, 2016. doi: 10.1097/MCP.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 60.de Poel E, Lefferts JW, Beekman JM. Intestinal organoids for cystic fibrosis research. J Cyst Fibros 19: S60–S64, 2020. doi: 10.1016/j.jcf.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 61.Berkers G, van der Meer R, van Mourik P, Vonk AM, Kruisselbrink E, Suen SW, Heijerman HG, Majoor CJ, Koppelman GH, Roukema J, Janssens HM, de Rijke YB, Kemper EM, Beekman JM, van der Ent CK, de Jonge HR. Clinical effects of the three CFTR potentiator treatments curcumin, genistein and ivacaftor in patients with the CFTR-S1251N gating mutation. J Cyst Fibros 19: 955–961, 2020. doi: 10.1016/j.jcf.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 62.Berkers G, de Winter-de Groot KM, Dekkers JF, Vonk AM, Kruisselbrink E, Hagemeijer MC, Heida-Michel S, Geerdink M, Arets HG, van der Wilt RE. Intestinal organoid swelling and relation with disease severity and response to therapy. Pediatr Pulmonol 51: 159, 2016. [Google Scholar]
- 63.Fidler M, Sullivan J, Boj S, Vries R, Munck A, Higgins M, Moretto-Zita M, Negulescu P, Van Goor F, De Boeck K. WS18. 5 Evaluation of the contributions of splicing and gating defects to dysfunction of G970R-CFTR. J Cyst Fibros 16: S31, 2017. doi: 10.1016/S1569-1993(17)30259-X. [DOI] [Google Scholar]
- 64.Pranke I, Hatton A, Masson A, Flament T, Le Bourgeois M, Chedevergne F, Bailly C, Urbach V, Hinzpeter A, Edelman A, Sermet-Gaudelus I. Might brushed nasal cells be a surrogate for cftr modulator clinical response? Am J Respir Crit Care Med 199: 123–126, 2019. doi: 10.1164/rccm.201808-1436LE. [DOI] [PubMed] [Google Scholar]
- 65.McGarry ME, Illek B, Ly NP, Zlock L, Olshansky S, Moreno C, Finkbeiner WE, Nielson DW. In vivo and in vitro ivacaftor response in cystic fibrosis patients with residual CFTR function: N-of-1 studies. Pediatr Pulmonol 52: 472–479, 2017. doi: 10.1002/ppul.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]