Abstract
In pulmonary arterial hypertension, plexiform lesions are associated with severe arterial obstruction and right ventricular failure. Exploring their structure and position is crucial for understanding the interplay between hemodynamics and vascular remodeling. The aim of this research was to use synchrotron-based phase-contrast micro-CT to study the three-dimensional structure of plexiform lesions. Archived paraffin-embedded tissue samples from 14 patients with pulmonary arterial hypertension (13 idiopathic, 1 with known BMPR2-mutation) were imaged. Clinical data showed high-median PVR (12.5 WU) and mPAP (68 mmHg). Vascular lesions with more than 1 lumen were defined as plexiform. Prior radiopaque dye injection in some samples facilitated 3-D rendering. Four distinct types of plexiform lesions were identified: 1) localized within or derived from monopodial branches (supernumerary arteries), often with a connection to the vasa vasorum; 2) localized between pulmonary arteries and larger airways as a tortuous transformation of intrapulmonary bronchopulmonary anastomoses; 3) as spherical structures at unexpected abrupt ends of distal pulmonary arteries; and 4) as occluded pulmonary arteries with recanalization. By appearance and localization, types 1–2 potentially relieve pressure via the bronchial circulation, as pulmonary arteries in these patients were almost invariably occluded distally. In addition, types 1–3 were often surrounded by dilated thin-walled vessels, often connected to pulmonary veins, peribronchial vessels, or the vasa vasorum. Collaterals, bypassing completely occluded pulmonary arteries, were also observed to originate within plexiform lesions. In conclusion, synchrotron-based imaging revealed significant plexiform lesion heterogeneity, resulting in a novel classification. The four types likely have different effects on hemodynamics and disease progression.
Keywords: imaging, lung, plexiform lesion, pulmonary arterial hypertension, synchrotron
INTRODUCTION
Pulmonary hypertension (PH) is a condition with high morbidity and mortality. Pulmonary arterial hypertension (PAH) is a subgroup of PH where the primary pathology resides predominantly in the precapillary arterioles (1, 2). This is also the group where treatment options have expanded significantly, but these are still far from curative and further advancement in the field is desperately needed (3).
Among the vascular changes seen in PAH, the plexiform lesion is considered the histopathological hallmark. In their 1958 paper, Heath and Edwards described plexiform lesions as one of four types of dilatation lesions, with the other three being vein-like branches of hypertrophied, usually occluded, muscular pulmonary arteries, angiomatoid lesions, and cavernous lesions (4). The plexiform lesion was further studied and defined by Wagenvoort: “In its classical form it consists of a plexus of small vessels within a focal dilatation of the arterial branch” (5, 6). However, the terms plexiform and “plexogenic” are used interchangeably and there is a lack of consensus in recent literature, indicating uncertainty in the PH field. Furthermore, the complexity increases as a lesion can present as either angiomatoid or plexiform depending on how it was sectioned. New techniques for studying these lesions are therefore needed.
Plexiform lesions are associated with a high disease burden (4, 7). However, it is crucial to determine whether plexiform lesions are markers of advanced disease or if they have roles in disease progression. PH-related vascular remodeling appears unaffected by current pharmacological treatment (8, 9), but reversibility of plexiform lesions has been demonstrated in both humans and rats in response to hemodynamic unloading (10, 11).
Interestingly, plexiform lesions have been linked to intrapulmonary bronchopulmonary anastomoses that bridge the pulmonary and bronchial circulations (12, 13). It has also been shown that bronchial artery hypertrophy/dilatation and bronchial angiogenesis are enhanced in bone morphogenetic protein receptor type II (BMPR2) mutation carriers with PAH, and that they are more likely to suffer from severe hemoptysis. A newly described type of lesion, named singular millimetric fibrovascular lesions (SiMFis), which connect the pulmonary arteries to bronchial vessels and pulmonary veins, have also been identified and shown to be more common in mutation carriers (14). However, the anatomy of pulmonary vascular disease remains difficult to characterize. Voelkel and Bogaard commented in their editorial that “more extensive stereological studies of the PAH lung” should be performed (15).
Previous attempts to visualize and study plexiform lesions in three dimensions (3-D) have yielded interesting data, but the techniques used have limitations and allow only small tissue volumes to be processed (12, 13, 16, 17). We recently developed protocols for synchrotron-based phase-contrast micro-CT to explore pulmonary vascular pathology (18). Here, we used this technique to advance the understanding of the three-dimensional microanatomy of plexiform lesions.
METHODS
Tissue Selection
Formalin-fixed paraffin-embedded pulmonary tissue, obtained during transplantation, cataloged as idiopathic PAH (IPAH) was retrieved from the biobank at Skåne University Hospital. One section per paraffin block was analyzed by light microscopy by an experienced pathologist. Tissue with vascular remodeling in accordance with PAH was selected (19). In total, samples from 13 patients were selected and imaged: two blocks each from nine patients and one block each from four.
Data from three patients were excluded following imaging and review of clinical parameters. Two patients had been labeled as having primary pulmonary hypertension in the pathology database, but the review of clinical data revealed that one had been diagnosed with pulmonary veno-occlusive disease (no identified plexiform lesions from imaging) and the other had been diagnosed with systemic sclerosis and secondary PAH. The third exclusion was a male diagnosed with IPAH at age 59 yr, where no plexiform lesions were identified in the imaged material.
In addition, four tissue blocks from a known BMPR2 mutation carrier, retrieved from the biobank at Children’s Hospital Colorado, were included. Previously published data suggest no significant differences in plexiform lesion density or lesion morphology between IPAH and BMPR2-mutation carriers (14, 17).
Healthy paraffin-embedded lung tissue, explanted at the time of hamartoma resections, from three patients were used as control tissue (Skåne University Hospital biobank).
Permission to access clinical data was granted by the Skåne County Council (KVB Dnr 235-20), and the study was approved by the Colorado Multiple Institutional Review Board and by the regional ethical review board in Lund, Sweden (Dnr 2017/597 and Dnr 2019-01769).
Tissue Preparation and Dye Injection
The tissue samples from 13 of the 14 patients were imaged without any pretreatment. The lung tissue from the BMPR2 mutation carrier had been injected with radiopaque dye during autopsy. The bronchial arteries (BA) were isolated after clamping of the aortic arch and distal thoracic aorta. The isolated aortic segment containing the BA branches was injected with blue dye. Unfortunately, the blue dye seems to not have penetrated very far into the distal lung tissue since the imaged blocks that were sectioned following imaging did not contain blue dye. Subsequently, the pulmonary arteries were injected with green dye. The dyes (CDI’s Tissue Marking Dye; Cancer Diagnostics, Durham, NC) were diluted with an equal volume of tap water to ensure low viscosity. Twenty milliliter syringes equipped with 22-gauge needles were used for the injections. After needle insertion, clamps were used to secure its position in the vessel before initiation of injections. The injections were performed with manually applied semigentle pressure and lasted ∼5 min. The lungs were subsequently inflated with formalin and embedded in paraffin.
Synchrotron-Based Phase Contrast Micro-CT and Image Processing
Image acquisition and processing was performed as previously published (18). In summary, one section from each sample was histologically stained to locate areas (volumes of interest) containing characteristic vascular pathology, as this is necessary for sample alignment. Each volume of interest was scanned with 80-ms single-projection exposure time and 1,801 tomographic projections (180° rotation), resulting in a total scan time of ∼2.4 min. Phase information retrieval was done using the single-image phase and intensity extraction algorithm by Paganin et al. (20). Subsequently, tomographic reconstruction was done using a Fourier-based regridding algorithm (21). Qualitative and quantitative visual analyses were then performed in ImageJ as described below (22). Video clips of non-dye injected tissue were also generated in ImageJ.
Segmentation and volumetric rendering of the vasculature and airways were performed in Amira (Thermo Fisher Scientific).
The injected radiopaque dye enabled easy segmentation of the filled vessels by using simple thresholding methods. Manual segmentation was required for 3-D rendering of airways and non-dye injected vessels. For manual segmentation, the airways and vessels of interest were traced within the tomographic volume and the lumen was manually marked on every 5th to 20th slice, depending on the uniformity of the lumen and course of the structure. The complete 3-D course was then rendered by interpolating the intermediate slices.
To unite computing and aid researchers at synchrotron facilities, the raw data set used for Fig. 5B, Fig. 8C, and Supplemental Video S3 has been made publicly available at doi.psi.ch/detail/10.16907/d699e1f7-e822-4396-8c64-34ed405f07b7.
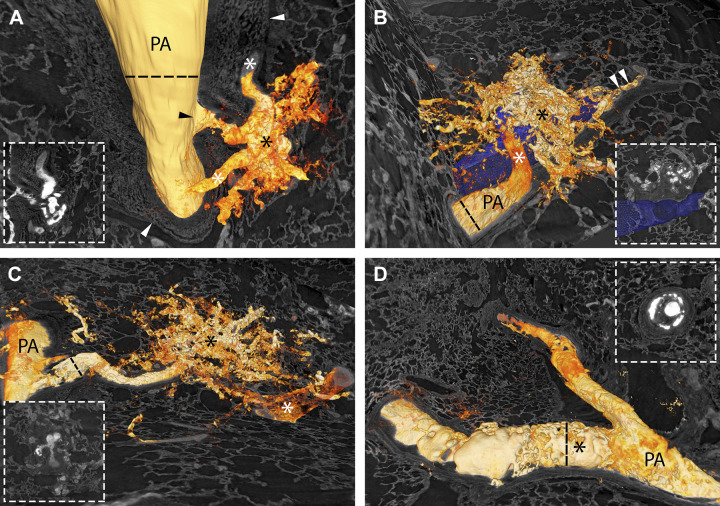
Figure 5.
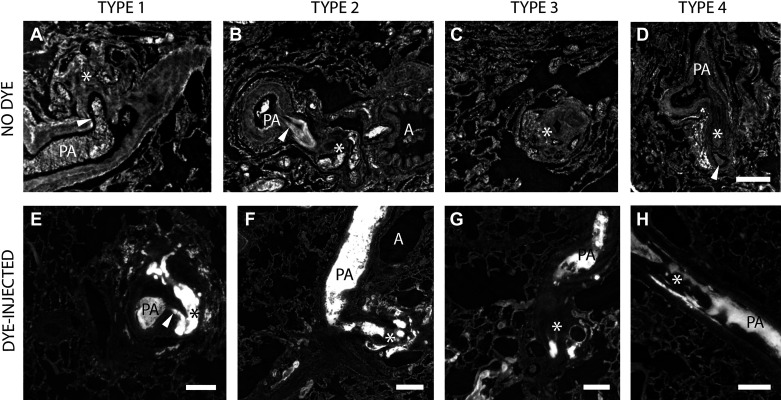
A–D: the four types of plexiform lesions from four different patients with IPAH. PA indicates pulmonary artery, and the asterisks mark the lesions. The arrowheads in A and B indicate the monopodial branch leaving the parent artery. In D, the arrowhead indicates the open lumen distal to the obstruction. Supplemental Videos S2–S5 scan through the four different lesions in A–D. The scale bar in D (200 µm) is valid for A–D. E–H: all four types of plexiform lesions from the dye-injected lung from the BMPR2-mutation carrier. PA marks open lumen proximal to the plexiform lesion in all four images and the asterisks mark the lesions. The arrowhead in E indicates the monopodial branch leaving the parent artery. Scale bars in E–H = 200 µm. IPAH, idiopathic pulmonary arterial hypertension.
Figure 8.
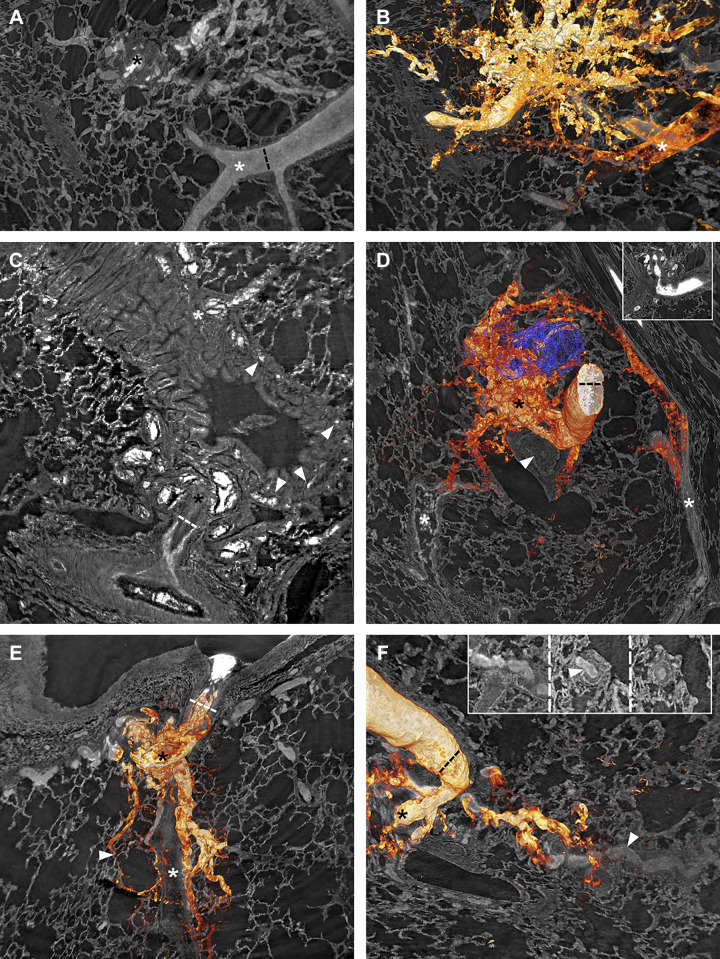
Vascular connections from plexiform lesions. Black asterisks mark plexiform lesions. A and B: a type 3 lesion, with and without 3-D rendering of the vasculature. In B, a pulmonary vein (white asterisk) can be seen to drain the lesion, possibly through connections larger than regular capillaries. C: a type 2 lesion that connects with the peribronchial microvessels (white arrowheads) which then drains to bronchial veins (white asterisk). D: a 3-D rendered type 2 lesion next to an airway reconstructed in blue. The white arrowhead marks a distal complete occlusion of the PA, and the white asterisks mark veins that drain the plexiform lesion. E: dilated thin-walled vessels originating from a type 1 lesion that follow the occluded monopodial branch (white asterisk) distally while separate vessels supply a nearby alveolar duct (white arrowhead). F: thin-walled collateral vessels from the same type 2 lesion as in D can be seen to reenter (white arrowhead) the lumen distal to complete occlusion. The insets, viewed from left (occluded) to right (patent), show the collateral entering (white arrowhead) the PA. As images A–F are 2-D images of 3-D projections, regular scale bars are inapplicable. Dashed lines indicate a measurement at a given point. In A and B, the lumen diameter of the nearby vein is 120 µm; the lumen diameter of the parent vessel is given for D and F = 173 µm; the parent vessel diameter is given for C = 144 µm and E = 228 µm. 2-D, two-dimensional; 3-D; three-dimensional.
Identification of Plexiform Lesions
Based on previous literature, any vascular structure with a clear connection to a muscular pulmonary artery or arteriole, with at least two channels for the blood to pass, was identified as plexiform. The primary identification and subgrouping of plexiform lesions, including measurements, was solely based on the IPAH tissue. Each IPAH data set (volume of interest) was opened as a virtual stack of TIFF files in ImageJ (22) and visually dissected by three of the coauthors independently, and the position of each identified plexiform lesion was compared and recorded. Based on visual presentation and spatial location, different subtypes of lesions were identified. The lesions were subsequently divided into four groups by two authors. Another two authors reviewed and confirmed the classification. The dye-injected tissue was used to visualize and study the characteristics of the four lesion types.
Measurement of Vessel Diameter
The diameters, excluding adventitia, of the vessels that gave rise to plexiform lesions were measured just proximal to the lesions for the first three groups. For the fourth group (type 4) the diameter was measured at the site of the lesion. When the vessel was asymmetrical, the mean of two perpendicular measurements was used. Measurements were performed in ImageJ (22).
Histology
Serial sectioning, followed by histological and immunohistochemical staining, was performed after image acquisition. Hematoxylin-eosin, Elastica van Gieson, and Alcian blue/periodic acid-Schiff stainings were performed according to standard protocols (used also for clinical samples). Lymphatic vessels were stained using mouse antipodoplanin monoclonal (clone D2-40) antibody (Biocare Medical LLC, Pacheco, CA) diluted 1:25. Sections were pretreated with Ventana Cell Conditioning 1 (CC1; EDTA pH 8) with amplification. All stainings were performed by the Department of Pathology, Skåne University Hospital, Lund, Sweden.
Statistical Analysis
Statistical analysis was carried out using IBM SPSS 25. Spearman’s rank correlation was used to evaluate the relationship between lesion density (lesions/mm3) and clinical parameters.
RESULTS
Virtual Histology by Synchrotron Tomography
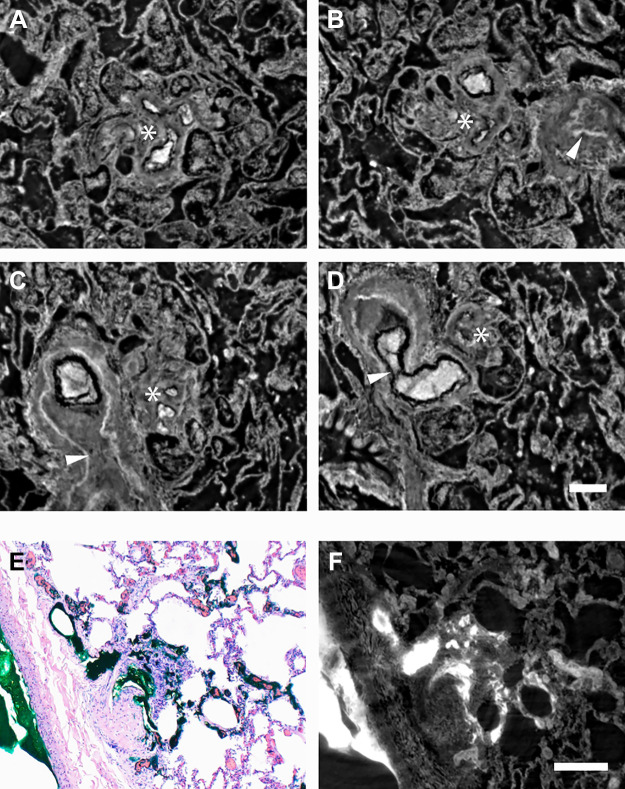
Synchrotron-based phase-contrast micro-CT enables virtual histology on a micrometer scale (effective pixel size 1.63 × 1.63 µm2) as shown in Fig. 1. As the tissue is imaged as a volume, it can be virtually dissected (Supplemental Video S1; all supplemental videos are available at https://doi.org/10.6084/m9.figshare.12951251.v1) as well as viewed from any angle of choice. The value of this technique when studying plexiform lesions is demonstrated in Fig. 2, A–D, which illustrates how the appearance of a lesion can differ depending on how it was sectioned. Radiopaque dye can be used to better visualize plexiform lesions, by both histology and tomography, as shown in Fig. 2, E and F.
Figure 1.
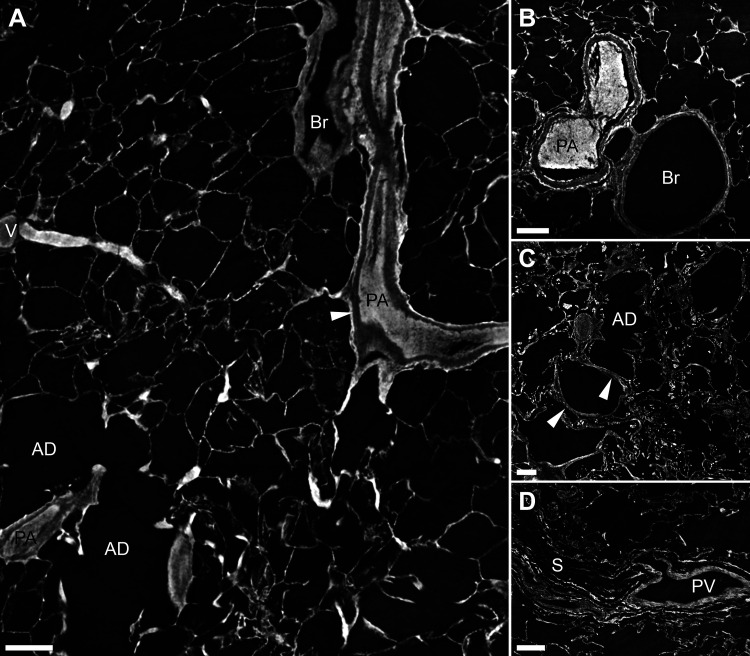
Normal lung imaged with synchrotron-based phase-contrast micro-CT. A: a bronchovascular bundle with a pulmonary artery (PA) and a bronchiole (Br) is seen to the right. The resolution allows for individual layers of the vascular wall to be discerned (white arrowhead). To the left, a vein (V) that collects oxygenated blood is shown, and below the vein two alveolar ducts (AD). Supplemental Video S1 scans through the 3-D volume from which the 2-D image in A has been captured. B: a cross section of a bifurcating muscular pulmonary artery (PA) and a bronchiole (Br). C: a longitudinal virtual section of an alveolar duct (AD) is shown. The white arrowheads mark small vessels within the wall of the alveolar duct. D: a pulmonary vein (PV), positioned within a septa (S) as expected. Scale bars in A–D = 200 µm. 2-D, two-dimensional; 3-D; three-dimensional.
Figure 2.
A–D: four virtual imaging planes of one plexiform lesion to illustrate the limitation of studying these lesions in 2-D. *The center of the plexiform lesion in all four images. A: no larger parent vessel is visualized. B: next to the plexiform lesion a completely occluded artery is seen (white arrowhead). C: the pulmonary artery next to the lesion is shown to be patent more proximally and then completely occluded (both dichotomous branches are occluded). The white arrowhead marks the internal elastic lamina and the area of complete occlusion. D: a 90° branch (white arrowhead) which exits the pulmonary artery proximal to the obstruction and appears to communicate with the plexiform lesion. The scale bar in D (200 µm) is valid for A–D. E and F: the radiopaque dye by histology and synchrotron tomography respectively. In E, hematoxylin-eosin staining of the BMPR2 lung injected with green dye in the pulmonary arteries is shown. In F, the same imaging plane has been localized in a tomography volume to illustrate the high attenuation of the dye, seen in white. The scale bar in F (200 µm) is valid for E and F.
Clinical Data
Clinical data for the included patients with IPAH are listed in Table 1. All ten included patients fulfilled the clinical criteria for pulmonary arterial hypertension. Three had comorbidities or histories of specific interest to the IPAH diagnosis. Patient 8 underwent surgery for a ventricular septal defect at age 4 yr (no residual shunt) and was later diagnosed with IPAH and patient 2 had a patent atrial septal defect (8 mm) which was not considered to have any hemodynamical impact. Patient 9 was suspected to have a familial form of PAH but had not been genetically tested.
Table 1.
Clinical characteristics of patients with IPAH
| IPAH | |
|---|---|
| (n = 10) | |
| Sex, M/F | 1/9 |
| Age at diagnosis, yr | 27 (22–44)a |
| Disease duration, yr | 2.5 (1–7.3)a |
| LTX | |
| Age at LTX, yr | 32 (24–45)a |
| BMI at LTX, kg/m2 | 22.8 (20.4–24.4)a |
| WHO functional class at LTX | |
| II, n (%) | 0 (0%) |
| III, n (%) | 4 (40%) |
| IV, n (%) | 6 (60%) |
| 6MWT before LTX, m | 308 (205–385)a |
| Medication | |
| ERA + PDE5i, n (%) | 1 (10%) |
| ERA + PGI2, n (%) | 4 (40%) |
| ERA + PDE5i + PGI2, n (%) | 5 (50%) |
| Hemodynamics before LTX | |
| , % | 93 (89–96)a |
| Hb, g/L | 140 (131–156)a |
| mAP, mmHg | 83 (75–93)a |
| mPAP, mmHg | 68 (58–82)a |
| PVR, WU | 12.5 (11.5–25.4)a |
| Suprasystemic mPAP at rest, n (%) | 3 (30%) |
| SV, mL | 45 (34.3–50.5)a |
Median with interquartile range (IQR).
BMI, body mass index; ERA, endothelin receptor antagonist; Hb, hemoglobin; LTX, lung transplantation; mAP, mean arterial pressure; mPAP, mean pulmonary arterial pressure; PDE5i, phosphodiesterase type 5 inhibitor; PGI2, prostaglandin I2/prostacyclin; PVR, pulmonary vascular resistance; , arterial oxygen saturation; SV, stroke volume; WHO, World Health Organization; WU, wood units; 6MWT, nonencouraged 6-min walk test.
The included patients were predominantly female with a median age of 27 yr at diagnosis and a median disease duration of 2.5 yr when transplanted. All had high mPAP and PVR as expected, and were either WHO functional class III or IV; 50% had triple therapy at the time of transplantation (Table 1).
Identification of Plexiform Lesions
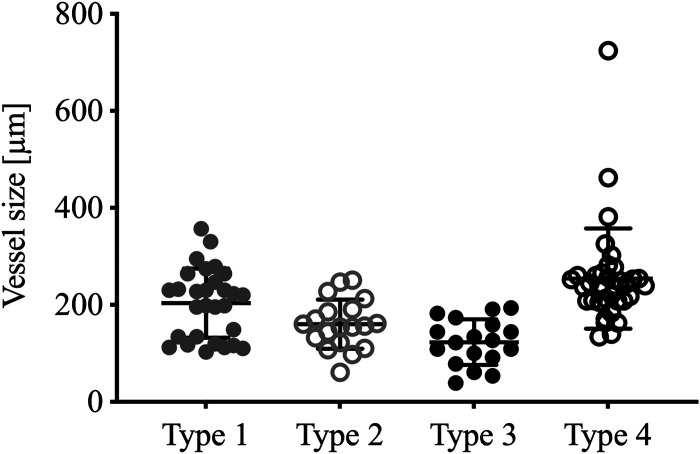
A total of 108 plexiform lesions were identified in the IPAH blocks, whereas none were observed in the control tissue. The lesions identified demonstrated great heterogeneity, even though they all fit within the above-stated definition of a plexiform/plexogenic lesion. There were however striking similarities between subgroups of lesions that became apparent during analysis. Based on this cohort of patients, we could categorize all identified lesions into one of four distinct subgroups (types) of plexiform lesions, presented schematically in Fig. 3 and described in detail in the following section. Table 2 shows the distribution of the different lesion types between the individual patients, as well as the density of lesions in the imaged volumes (lesions/mm3). Five of the eleven patients with IPAH studied had all four types of lesions and all four types were found in the known BMPR2-mutation carrier. Figure 4 shows the size of the artery or arteriole just proximal to the lesion (for type 1–3) or at the site of the lesion (for type 4).
Figure 3.
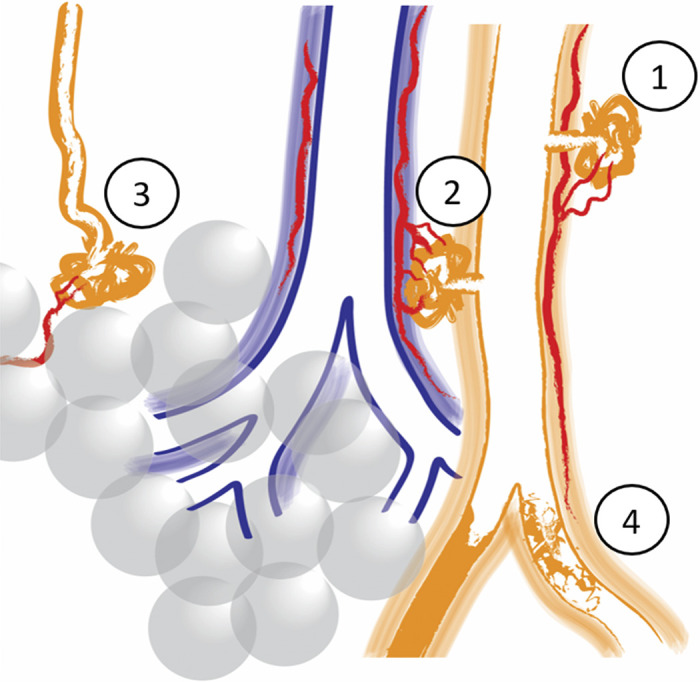
Schematic illustration of the four different plexiform lesion types. Type 1: in monopodial branches, often with a connection to the vasa vasorum (in red). Type 2: between pulmonary arteries (orange) and airways (blue) as a tortuous transformation of intrapulmonary bronchopulmonary anastomoses (IBA), connecting the pulmonary artery with peribronchiolar vessels (red). Type 3: at unexpected abrupt ends of distal pulmonary arteries/arterioles. Often, small dilated vessels are seen leaving type 3 lesions (red). Type 4: completely occluded pulmonary arteries with recanalization, or incomplete blockage.
Table 2.
Number and density of plexiform lesions by type and patient
| Type 1 | Type 2 | Type 3 | Type 4 | Total | |
|---|---|---|---|---|---|
| Plexiform lesions | 31 (29%) | 21 (19.5%) | 21 (19.5%) | 35 (32%) | 108 (100%) |
| Patient 1 | 3 [0.016] | 2 [0.011] | 2 [0.011] | 4 [0.021] | 11 [0.059] |
| Patient 2 | 2 [0.011] | 3 [0.017] | 1 [0.006] | 3 [0.017] | 9 [0.050] |
| Patient 3 | 0 [0] | 0 [0] | 0 [0] | 2 [0.014] | 2 [0.014] |
| Patient 4 | 8 [0.055] | 5 [0.035] | 1 [0.007] | 7 [0.048] | 21 [0.145] |
| Patient 5 | 4 [0.018] | 2 [0.009] | 6 [0.027] | 7 [0.031] | 19 [0.085] |
| Patient 6 | 3 [0.016] | 2 [0.010] | 1 [0.005] | 5 [0.026] | 11 [0.057] |
| Patient 7 | 8 [0.045] | 3 [0.017] | 10 [0.056] | 0 [0] | 21 [0.118] |
| Patient 8* | 2 [0.028] | 0 [0] | 0 [0] | 0 [0] | 2 [0.028] |
| Patient 9* | 1 [0.009] | 2 [0.018] | 0 [0] | 5 [0.045] | 8 [0.072] |
| Patient 10* | 0 [0] | 2 [0.017] | 0 [0] | 2 [0.017] | 4 [0.034] |
One tissue block imaged. Number of lesions found per patient by type; lesion density (lesions/mm3) in brackets.
Figure 4.
Vessel size by lesion type. Distribution of vessel diameter measured proximal to each identified plexiform lesion, in micrometers. Type 4 measured at the site of the lesion. Lines represent mean and standard deviation (GraphPad Prism 8).
Four Distinct Types of Plexiform Lesions
The top panel in Fig. 5 shows two-dimensional (2-D) images of all four types of lesions from four different patients with IPAH and Supplemental Videos S2–S5 scan the same lesions. The bottom panel illustrates all four lesion types from the BMPR2-mutation carrier, where the tissue had been injected with dye. In Fig. 6, the dye has been used for 3-D reconstructions of all four types of lesions. The same lesions are also shown in Supplemental Videos S6–S9.
Figure 6.
Segmentation and 3-D rendering of dye-injected vessels (the injected dye is white in the tomograms and orange in the 3-D renderings) to illustrate the four types of plexiform lesions. Insets show 2-D cross sections of the same lesions. Black asterisk, plexiform lesion; PA, pulmonary artery. A: type 1 lesion. The black arrowhead marks the monopodial branch connecting the PA to the lesion, and white asterisks connections to the vasa vasorum. White arrowheads mark the adventitia. B: type 2 lesion. The white asterisk marks the intrapulmonary bronchopulmonary anastomoses (IBA), proximal to the occlusion of the PA. The lesion connects with the peribronchiolar vascular network surrounding the airway (3-D reconstructed in blue). White arrowheads mark collateral flow to the poorly perfused area distal to the obstruction. C: type 3 lesion. The white asterisk shows a pulmonary vein that connects with the lesion. D: type 4 lesion within the PA. The cross section shown in the inset is captured approximately where the black asterisk is positioned, to illustrate how it would look in a 2 D histological slide. Supplemental Videos S6–S9 show the same lesions as in A–D. As images A through D are 2-D images of 3-D projections, regular scale bars are inapplicable. For reference, the lumen diameter of parent vessels are indicated by dashed lines: A = 359 µm; B = 228 µm; C = 137 µm; D = 251 µm. 2-D, two-dimensional; 3-D; three-dimensional.
Type 1 lesions (Fig. 5, A and E and Fig. 6A) were localized within, or derived from, monopodial branches of an average size of 202 µm. Connections between type 1 lesions and the vasa vasorum of the larger parent pulmonary artery were frequently, but not invariably, observed and indicates connections with the systemic circulation as previously suggested (Fig. 6A, white asterisks) (12, 13). A near-complete occlusion of the vessel lumen was often observed within the lesion, possibly indicating a thrombotic event as previously described (4). Type 1 lesions constituted 29% of the lesions identified and were evenly distributed in the cohort.
Type 2 lesions (Fig. 5, B and F and Fig. 6B) were observed to originate from 90° branches (average size 160 µm) of pulmonary arteries that were directed toward airways (terminal bronchiole or larger). Connections to peribronchial (systemic) vessels were invariably observed. Therefore, this type is interpreted as tortuous transformations of intrapulmonary bronchopulmonary anastomoses. The lesions were evenly distributed in the cohort and constituted 19.5% of all identified lesions.
Type 3 lesions (Fig. 5, C and G and Fig. 6C) appeared at abrupt ends of distal pulmonary arteries/arteriole (average size 123 µm) next to alveolar ducts, as spherical structures that terminated the vessel. A normal arterial branching pattern distal to the lesion was rarely observed (sometimes completely occluded branches were seen distal to the lesion). The lesions were often connected to, and surrounded by, dilated thin-walled vessels. Type 3 lesions were unevenly distributed in the cohort. Almost half of the lesions found were identified in one patient (Table 2, patient 7).
Any occluded pulmonary artery with at least two lumens was defined as a type 4 lesion. (Fig. 5, D and H and Fig. 6D). These lesions appear plexiform when the vessel is viewed in cross section at the site where recanalization of an occlusion, or incomplete blockage, has created two or more new lumens. These lumens differ from the original lumen by their significantly smaller diameter and their tortuous route, seldomly infringing the elastic lamina. These lesions often appeared at, or distal to, bifurcations of arteries with a mean diameter of 253 µm. These lesions constituted 32% of the plexiform lesions identified and were present in all but two of the patients examined (Table 2).
Distal Vascular Remodeling and Vascular Connections from Plexiform Lesions
When the parent pulmonary artery could be followed distally from the sites of type 1 and 2 lesions, complete or near-complete lumen occlusions were always seen. Figure 7 shows a manual 3-D reconstruction of a type 1 lesion (non-dye injected IPAH tissue). The complete obstruction distal to the monopodial branch entering the lesion (Fig. 7G), the near-complete occlusion within the lesion (white and black asterisks in Fig. 7, tomography and histology, respectively), and the associated dilated thin-walled vessels (reconstructed in blue) are representative of many of the type 1 and 2 lesions observed. For type 1, these thin-walled vessels were often observed to connect to the vasa vasorum (arrowhead in Fig. 7I) and infrequently to vessels surrounding alveolar ducts (arrowhead in Fig. 8E). Thin-walled vessels seen around type 2 and 3 lesions often had venous connections, either to pulmonary venules (as shown for a type 3 lesion in Fig. 8, A and B) or to bronchial venules via peribronchial vascular plexuses (as shown for type 2 lesions in Fig. 8, C and D), likely bypassing areas of alveolar gas exchange.
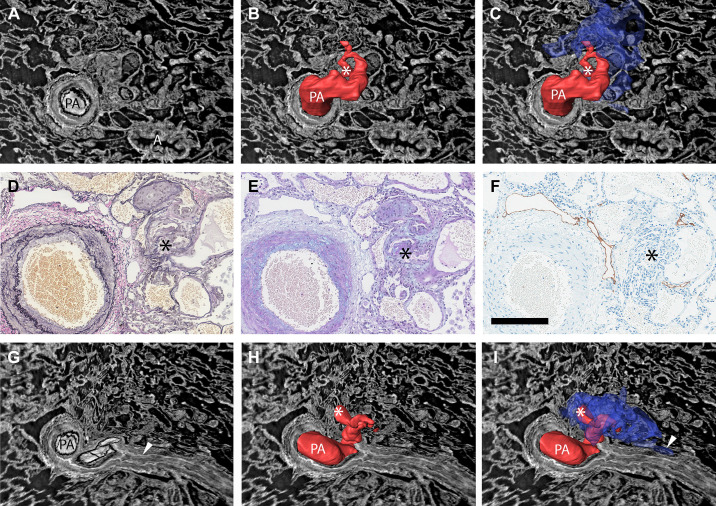
Figure 7.
Demonstration of how manual 3-D reconstructions of non-dye injected tissue and comparisons of imaging and histology and/or immunohistochemistry can make pulmonary vascular lesions easier to interpret. A: the type 1 plexiform lesion in Fig. 2, A–D is shown in cross section, in a view that shows both the patent parent pulmonary artery (PA) and a nearby airway (A). B: the PA above the imaging plane and a monopodial branch that enters the plexiform lesion have been manually reconstructed in red. At the site marked by the white asterisk, the passage for the blood to pass becomes very narrow (stenosis) and distal from this point the vessels within and around the plexiform lesion were thin-walled and dilated. C: the dilated thin-walled vessels have been reconstructed in blue. Black asterisk marks the center of the plexiform lesion in D–F. D and E: histology of sections that match the imaging plane seen in A–C. D: Elastin van Gieson staining with elastic fibers in black. E: Alcian blue/Periodic acid-Schiff staining, with acidic mucins in bright blue. F: Immunohistochemistry to localize lymphatic vessels (podoplanin in brown). G–I: Showing the same lesion but in a different imaging plane. From this angle, it is clear that the pulmonary artery is occluded distal from the plexiform lesion (white arrowhead in G). Asterisks in H and I mark the narrow passage within the plexiform lesion and the arrowhead in I marks a site where the dilated thin-walled vessels in blue appear to communicate with the vasa vasorum. As images A–C and G–I show 3-D projections, regular scale bars are inapplicable. The size of the vessel shown in images A–I can be gauged by the scale bar in F (200 µm). 2-D, two-dimensional; 3-D; three-dimensional.
Some of the thin-walled vessels from plexiform lesions were also observed to follow occluded pulmonary artery branches distally (Fig. 8, E and F) and could sometimes be demonstrated to reopen the vessel-like collaterals in the systemic circulation (Fig. 8F). A few sections were stained for podoplanin to identify lymphatic vessels. This showed that synchrotron-based imaging can be combined with immunohistochemistry and confirmed that lymphatic vessels are localized around but not within plexiform lesions (Fig. 7F) as previously demonstrated by Jonigk et al. (17).
Correlation with Clinical Data
Spearman’s rank correlation was used to investigate a possible relationship between lesion types and clinical parameters. As shown in Table 3, a correlation coefficient of 0.64 indicated a possible correlation between PVR and the amount of type 1 lesions. The correlation did however not reach statistical significance, possibly because of the small and homogenous cohort.
Table 3.
Spearman’s rank correlation between lesion types and clinical parameters
| Type 1 | Type 2 | Type 3 | Type 4 | Total | |
|---|---|---|---|---|---|
| mPAP | 0.24 | 0.15 | −0.57 | 0.42 | 0.12 |
| PVR | 0.64 | 0.22 | 0.51 | 0.33 | 0.48 |
| 6MWT | 0.32 | −0.46 | 0.26 | −0.44 | −0.13 |
| Age at diagnosis | 0.18 | −0.29 | 0.19 | 0.13 | 0.13 |
| Disease duration | −0.38 | 0.04 | −0.22 | −0.59 | −0.31 |
Correlation coefficients for lesion density (lesion/mm3) correlated to clinical parameters through Spearman’s rank correlation. mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; 6MWT, nonencouraged 6-min walk test.
DISCUSSION
This study investigated the value of synchrotron-based phase-contrast micro-CT for three-dimensional imaging of plexiform lesions. As Voelkel and Bogaard stated, plexiform lesions are not “important mostly as an entertainment to a handful of pathologists” (15), but are an integral part of the vascular remodeling of PAH that needs to be understood in the process of finding a cure. This new technique provided a unique opportunity to explore the microanatomy of hypertensive lungs and resulted in a novel classification of lesions of PAH.
A key challenge was selecting which lesions to analyze in the 3-D volumes, as all previous definitions of plexiform lesions, including the one used for this study, were based on 2-D histology. Dilatation lesions previously defined as angiomatoid, cavernous, or plexiform (4), as well as recanalized obstructive lesions, would invariably be included as they all have multiple lumens and are connected to a muscular pulmonary artery/arteriole. However, as it has been difficult to clearly identify and separate these lesions from each other previously (19, 23), this broad inclusion was not considered negative.
As mentioned in introduction, plexiform lesions have previously been linked to intrapulmonary bronchopulmonary anastomoses (12, 13). This relationship is debated however and a recent publication argues against this connection based on data from an experimental rat model of PAH (24). With this project, we have proved that certain types of plexiform lesions connect with the systemic circulation and that these connections are common. Here, lesions with systemic connections (type 1 and 2) were divided into two separate groups as they connect to different segments of the bronchial circulation. Whether there is a functional (hemodynamic) difference between these types remains to be investigated. We hypothesize that the bronchopulmonary shunts within these lesions could play a role in pressure offloading when the local pulmonary pressure exceeds that of bronchial precapillary vessels. This right-to-left shunting can potentially cause desaturation, especially during exercise, which has been linked to poor prognosis in PAH (25). However, the poor prognosis of these patients is most likely due to the advanced state of the peripheral occlusive disease and the intrapulmonary shunting potentially delays right ventricular failure. Unfortunately, because of the limited cohort used, this hypothesis could not be evaluated in this study.
It is also not possible at this stage to say how efficient a fully developed type 1 or 2 lesion is as a shunt compared to an innate pressure relief valve within a bronchopulmonary anastomosis (which has just opened because of an increase in pulmonary artery pressure). Does the evolution of lesions create additional connections? An increase of both flow and pressure within a shunt likely leads to vascular growth and arterial buckling/tortuosity similar to what is seen in collaterals in the systemic circulation (26), which would fit well with the angiogenic markers found to be upregulated within plexiform lesions (17, 27). Increased shear stress may however also cause endothelial injury and the presence of thrombi within plexiform lesions [as described by Heath and Edwards and Wagenvoort and Wagenvoort (4, 6), but interestingly found absent by Jonigk et al. (17)] would likely obstruct flow and affect the lesions structure and function.
Large type 2 lesions without significant obstruction to flow may be the equivalent of SiMFis, as they have been described to occur next to airways in both IPAH and BMPR2-mutation carriers with a prominent bronchial circulation (14). This possible sameness needs to be confirmed by future studies.
Type 3 lesions appear to be a heterogeneous group and calls for further investigation. Visually, the globular shape of the lesion, with surrounding thin-walled vessels, indicates that some could be equivalent to what Heath and Edwards (4) described as angiomatoid and cavernous lesions. The venous and airway-coupled vascular connectivity associated with type 3 lesions were similar to those seen for type 2 lesions but more peripheral. However, because of the anatomical characteristics mentioned previously and, importantly, as a systemic connection could not be verified this type was classified separately. Patients 5 and 7 appeared as outliers in terms of type 3 lesions compared with the rest of the cohort, but no obvious explanation for this was found. They had no similar comorbidities and did not differ significantly from the rest of the cohort in terms of clinical data or hemodynamics. However, it is important to note that these patients do not necessarily represent outliers, as no such conclusions can be drawn from this limited study population.
Further investigation of type 3 lesions will require a larger cohort. To determine the vascular connections (systemic or pulmonary), stitching of adjacent volumes of dye-injected tissue imaged at a higher magnification would be needed.
A study by Pietra et al. (19) exemplified the difficulty of classifying plexiform lesions where analysis by three pathologists revealed discrepancies concerning the interpretation of recanalized thrombi as plexiform lesions. It is our understanding that the lesions discussed would correspond to those classified as type 4 in this study. Some argue that these occlusive lesions within the pulmonary artery should not be called plexiform. Type 4 lesions have however often been called plexiform in previous publications and there will likely be no practical negative consequences from including them in the classification, as long as the community understands the definitions and hemodynamic effects. Intimal thickening, type 4 lesions, and complete occlusions will inevitably increase the pulmonary vascular resistance, likely generating type 1 and 2 lesions upstream.
Venous connections from plexiform lesions have been described previously (12, 14). Here, we could confirm that dilated venules appear to drain plexiform lesions. To discern whether the connections are preexisting capillary beds which have dilated to accommodate more flow or if new vascular connections are formed, studies in animal models would be needed. Another type of connection is collateral vessels that originate within or in connection with plexiform lesions and follow the course of occluded arteries. Sometimes the reentry site of the “collaterals” could be visualized within the imaged volumes as shown in Fig. 8F. Poststenotic dilatations, both distal from obstructions within plexiform lesions and distal from collateral vessel reentry sites, were observed more frequently in some patients. The mechanisms and consequences of this phenomenon warrant further investigation.
It is debated whether PAH is predominantly a proliferative or degenerative disease (28). Heath and Edwards (4) described how intimal reactions evolve over time from a cellular to a fibrotic state, and a recent study showed that pulmonary vascular remodeling in a shunt model is reversible until cells become senescent (29). This indicates a dynamic, interdependent, presence of both proliferative and degenerative processes. An important focus for future studies is peripheral vessel loss (30, 31). Are the vessels still there, occluded but with an intact vascular wall structure, or do they go through a similar process as the ductus arteriosus and transform into a ligament-like fibrotic structure (32)? The specific locations of important processes such as metabolic changes, pro-proliferative signaling, senescence, inflammation, extracellular matrix accumulation, and enzymatic cleavage within the diseased vascular tree could be mapped with the help of synchrotron imaging (29, 33–37). This would likely give crucial clues for drug development. Here, synchrotron imaging combined with histology and immunohistochemistry (Fig. 7) showed that lymphatics are located around but not within plexiform lesions, confirming data by Jonigk et al. (17) and demonstrating the value of using the technique together with other standard methods.
Based on the morphological findings discussed above, it is probable that the process that causes peripheral vascular occlusions, including type 4 lesions, should be the main target for curative pharmacological intervention. However, to facilitate shunting through type 1 and 2 lesions, as well as modifying collateral formation, could be therapeutic options to rescue a failing right ventricle.
The main limitations of this study were the relatively low number of samples. Future studies are needed to corroborate this classification as well as to detail the cellular and molecular characteristics of each type. Larger and less homogenous cohorts could perhaps correlate the lesion types to patient-specific hemodynamics and other clinical parameters.
In conclusion, four distinct types of plexiform lesions were identified. The morphological findings suggest that the separate types have differing effects on hemodynamics and disease progression. We believe that novel imaging techniques like synchrotron-based phase-contrast micro-CT will be key for understanding the microanatomy of pulmonary vascular disease, directing PAH research toward novel and more efficient therapies.
SUPPLEMENTAL DATA
Supplemental Videos S1–S9: https://doi.org/10.6084/m9.figshare.12951251.
GRANTS
This work was supported by the Swedish Heart-Lung Foundation, the Swedish Society of Medicine, Sten K. Johnson Foundation, Fanny Ekdahl’s Foundation for Pediatric Research, the Crafoord Foundation, the Knut and Alice Wallenberg Foundation and the Skane County Council. The work was also supported by the National Institutes of Health Grant R01HL06702 and the Jayden DeLucia Foundation for Pulmonary Hypertension.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.W., C.N., and K.T.-L. conceived and designed research; C.W., C.N., N.P., O.v.d.H., G.L., I.J., R.M., M.B., and K.T.-L. performed experiments; C.W., C.N., N.P., O.v.d.H., G.L. C.G., and K.T.-L. analyzed data; C.W., C.N., O.v.d.H., V.D.J.P., H.B., C.G., and K.T.-L. interpreted results of experiments; C.W., C.N., N.P., O.v.d.H., P.-K.T., and K.T.-L. prepared figures; C.W. and K.T.-L. drafted manuscript; C.W., C.N., N.P., O.v.d.H., G.L., I.J., P.-K.T., R.M., V.D.J.P, H.B., M.B., C.G., and K.T.-L. edited and revised manuscript; C.W., C.N., N.P., O.v.d.H., G.L., I.J., P.-K.T., R.M., V.D.J.P, H.B., M.B., C.G., and K.T.-L. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the Paul Scherrer Institute, Villigen, Switzerland, for the provision of synchrotron radiation beam time at the TOMCAT beamline X02DA of the Swiss Light Source. We also acknowledge Alexander Tran for help with illustrations.
REFERENCES
- 1.Tuder RM. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res 367: 643–649, 2017. doi: 10.1007/s00441-016-2539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuder RM, Lee SD, Cool CC. Histopathology of pulmonary hypertension. Chest 114: 1S–6S, 1998. [Erratum in Chest 114: 1499, 1998]. doi: 10.1378/chest.114.1_supplement.1s-a. [DOI] [PubMed] [Google Scholar]
- 3.Barst RJ, Chung L, Zamanian RT, Turner M, McGoon MD. Functional class improvement and 3-year survival outcomes in patients with pulmonary arterial hypertension in the REVEAL Registry. Chest 144: 160–168, 2013. doi: 10.1378/chest.12-2417. [DOI] [PubMed] [Google Scholar]
- 4.Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 18: 533–547, 1958. doi: 10.1161/01.cir.18.4.533. [DOI] [PubMed] [Google Scholar]
- 5.Wagenvoort CA. Plexogenic arteriopathy. Thorax 49 Suppl: S39–S45, 1994. doi: 10.1136/thx.49.suppl.s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagenvoort CA, Wagenvoort N. Primary pulmonary hypertension a pathologic study of the lung vessels in 156 clinically diagnosed cases. Circulation 42: 1163–1184, 1970. doi: 10.1161/01.CIR.42.6.1163. [DOI] [Google Scholar]
- 7.Wagenvoort CA. The pathology of primary pulmonary hypertension. J Pathol 101: Pi, 1970. doi: 10.1002/path.1711010408. [DOI] [PubMed] [Google Scholar]
- 8.Rich S, Pogoriler J, Husain AN, Toth PT, Gomberg-Maitland M, Archer SL. Long-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertension. Chest 138: 1234–1239, 2010. doi: 10.1378/chest.09-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 261–272, 2012. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe K, Shinoda M, Tanaka M, Kuwabara Y, Yoshida K, Hirooka Y, McMurtry IF, Oka M, Sunagawa K. Haemodynamic unloading reverses occlusive vascular lesions in severe pulmonary hypertension. Cardiovasc Res 111: 16–25, 2016. doi: 10.1093/cvr/cvw070. [DOI] [PubMed] [Google Scholar]
- 11.Levy NT, Liapis H, Eisenberg PR, Botney MD, Trulock EP. Pathologic regression of primary pulmonary hypertension in left native lung following right single-lung transplantation. J Heart Lung Transplant 20: 381–384, 2001. doi: 10.1016/s1053-2498(00)00153-4. [DOI] [PubMed] [Google Scholar]
- 12.Galambos C, Sims-Lucas S, Abman SH, Cool CD. Intrapulmonary bronchopulmonary anastomoses and plexiform lesions in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 193: 574–576, 2016. doi: 10.1164/rccm.201507-1508LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaginuma G, Mohri H, Takahashi T. Distribution of arterial lesions and collateral pathways in the pulmonary hypertension of congenital heart disease: a computer aided reconstruction study. Thorax 45: 586–590, 1990. doi: 10.1136/thx.45.8.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghigna MR, Guignabert C, Montani D, Girerd B, Jais X, Savale L, Herve P, Thomas de Montpreville V, Mercier O, Sitbon O, Soubrier F, Fadel E, Simonneau G, Humbert M, Dorfmuller P. BMPR2 mutation status influences bronchial vascular changes in pulmonary arterial hypertension. Eur Respir J 48: 1668–1681, 2016. doi: 10.1183/13993003.00464-2016. [DOI] [PubMed] [Google Scholar]
- 15.Voelkel NF, Bogaard HJ. Adding complexity to plexogenic arteriopathy. Eur Respir J 48: 1553–1555, 2016. doi: 10.1183/13993003.01867-2016. [DOI] [PubMed] [Google Scholar]
- 16.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol 155: 411–419, 1999. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonigk D, Golpon H, Bockmeyer CL, Maegel L, Hoeper MM, Gottlieb J, Nickel N, Hussein K, Maus U, Lehmann U, Janciauskiene S, Welte T, Haverich A, Rische J, Kreipe H, Laenger F. Plexiform lesions in pulmonary arterial hypertension composition, architecture, and microenvironment. Am J Pathol 179: 167–179, 2011. doi: 10.1016/j.ajpath.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norvik C, Westoo CK, Peruzzi N, Lovric G, van der Have O, Mokso R, Jeremiasen I, Brunnstrom H, Galambos C, Bech M, Tran-Lundmark K. Synchrotron-based phase-contrast micro-CT as a tool for understanding pulmonary vascular pathobiology and the 3-D microanatomy of alveolar capillary dysplasia. Am J Physiol Lung Cell Mol Physiol 318: L65–L75, 2020. doi: 10.1152/ajplung.00103.2019. [DOI] [PubMed] [Google Scholar]
- 19.Pietra GG, Edwards WD, Kay JM, Rich S, Kernis J, Schloo B, Ayres SM, Bergofsky EH, Brundage BH, Detre KM. and Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation 80: 1198–1206, 1989. doi: 10.1161/01.cir.80.5.1198. [DOI] [PubMed] [Google Scholar]
- 20.Paganin D, Mayo SC, Gureyev TE, Miller PR, Wilkins SW. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J Microsc 206: 33–40, 2002. doi: 10.1046/j.1365-2818.2002.01010.x. [DOI] [PubMed] [Google Scholar]
- 21.Marone F, Stampanoni M. Regridding reconstruction algorithm for real-time tomographic imaging. J Synchrotron Radiat 19: 1029–1037, 2012. doi: 10.1107/S0909049512032864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi ES, Kim H, Ahn H, Strother J, Morris T, Masliah E, Hansen LA, Park K, Friedman PJ. Distribution of obstructive intimal lesions and their cellular phenotypes in chronic pulmonary hypertension. A morphometric and immunohistochemical study. Am J Respir Crit Care Med 162: 1577–1586, 2000. doi: 10.1164/ajrccm.162.4.9912131. [DOI] [PubMed] [Google Scholar]
- 24.Oshima K, Crockett ES, Joshi SR, McLendon JM, Matsumoto Y, McMurtry IF, Abe K, Oka M. Aneurysm-type plexiform lesions form in supernumerary arteries in pulmonary arterial hypertension: potential therapeutic implications. Am J Physiol Lung Cell Mol Physiol 317: L805–L815, 2019. doi: 10.1152/ajplung.00121.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paciocco G, Martinez FJ, Bossone E, Pielsticker E, Gillespie B, Rubenfire M. Oxygen desaturation on the six-minute walk test and mortality in untreated primary pulmonary hypertension. Eur Respir J 17: 647–652, 2001. doi: 10.1183/09031936.01.17406470. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, Han HC. Mechanical buckling of arterioles in collateral development. J Theor Biol 316: 42–48, 2013. doi: 10.1016/j.jtbi.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 195: 367–374, 2001. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhary KR, Taha M, Cadete VJ, Godoy RS, Stewart DJ. Proliferative versus degenerative paradigms in pulmonary arterial hypertension: have we put the cart before the horse? Circ Res 120: 1237–1239, 2017. doi: 10.1161/CIRCRESAHA.116.310097. [DOI] [PubMed] [Google Scholar]
- 29.van der Feen DE, Berger RMF, Bartelds B. Converging paths of pulmonary arterial hypertension and cellular senescence. Am J Respir Cell Mol Biol 61: 11–20, 2019. doi: 10.1165/rcmb.2018-0329TR. [DOI] [PubMed] [Google Scholar]
- 30.Campbell AI, Zhao Y, Sandhu R, Stewart DJ. Cell-based gene transfer of vascular endothelial growth factor attenuates monocrotaline-induced pulmonary hypertension. Circulation 104: 2242–2248, 2001. doi: 10.1161/hc4201.097838. [DOI] [PubMed] [Google Scholar]
- 31.Rabinovitch M, Haworth SG, Castaneda AR, Nadas AS, Reid LM. Lung biopsy in congenital heart disease: a morphometric approach to pulmonary vascular disease. Circulation 58: 1107–1122, 1978. doi: 10.1161/01.cir.58.6.1107. [DOI] [PubMed] [Google Scholar]
- 32.Rabinovitch M. Cell-extracellular matrix interactions in the ductus arteriosus and perinatal pulmonary circulation. Semin Perinatol 20: 531–541, 1996. doi: 10.1016/s0146-0005(96)80067-x. [DOI] [PubMed] [Google Scholar]
- 33.Archer SL. Pyruvate kinase and Warburg metabolism in pulmonary arterial hypertension: uncoupled glycolysis and the cancer-like phenotype of pulmonary arterial hypertension. Circulation 136: 2486–2490, 2017. doi: 10.1161/CIRCULATIONAHA.117.031655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim YM, Haghighat L, Spiekerkoetter E, Sawada H, Alvira CM, Wang L, Acharya S, Rodriguez-Colon G, Orton A, Zhao M, Rabinovitch M. Neutrophil elastase is produced by pulmonary artery smooth muscle cells and is linked to neointimal lesions. Am J Pathol 179: 1560–1572, 2011. doi: 10.1016/j.ajpath.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 115: 165–175, 2014. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tannenberg P, Tran-Lundmark K. The extracellular matrix in early and advanced pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 315: H1684–H1686, 2018. doi: 10.1152/ajpheart.00620.2018. [DOI] [PubMed] [Google Scholar]
- 37.Weiss A, Neubauer MC, Yerabolu D, Kojonazarov B, Schlueter BC, Neubert L, Jonigk D, Baal N, Ruppert C, Dorfmuller P, Pullamsetti SS, Weissmann N, Ghofrani HA, Grimminger F, Seeger W, Schermuly RT. Targeting cyclin-dependent kinases for the treatment of pulmonary arterial hypertension. Nat Commun 10: 2204, 2019. doi: 10.1038/s41467-019-10135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]