Abstract
The quantification of airway compliance (Caw) is essential to the study of airway alterations in disease models. However, the required measurements of airway pressure and volume are difficult to acquire in mice. We hypothesized that the inflation limb of full-range pressure-volume (PV) curves could be used to quantify Caw, as it contains a segment where only the airway tree is distended. The study objective was to assess the feasibility of the approach by analysis of full-range PV curves previously collected in three mouse models: an elastase model of emphysema, a genetic model spontaneously developing emphysema (leukotriene C4 synthase knockout; LTC4S-KO), and a bleomycin model of lung fibrosis. Attempts to validate results included Caw change relative to respiratory system compliance (ΔCaw/ΔC), the minute work of breathing (mWOB), and the elastance at 20.5 Hz (Ers_20.5) from prior respiratory mechanics measurements in the same subjects. Caw was estimated at 3% of total compliance in healthy mice or 2.3 ± 1 μL/cmH2O (n = 17). The technique detected changes in models of respiratory obstructive and restrictive diseases relative to control mice as well as differences in the two emphysema models studied. The changes in Caw were consistent with those seen in ΔCaw/ΔC, mWOB, or Ers_20.5, with some variations according to the model, as well as with results reported in the literature in humans and mice. Direct Caw measurements in subjects as small as mice could prove useful to further characterize other respiratory disease models associated with airway remodeling or to assess treatment effects.
Keywords: airway compliance, emphysema, fibrosis, LTC4 synthase, pressure-volume curves
INTRODUCTION
The pressure-volume (PV) curve is an invaluable tool in respiratory research. It is frequently used to characterize models of respiratory diseases as it provides information on elastic recoil and its relationship to lung volumes (1–4). PV curves also have greatly contributed to the determination and understanding of the role of surfactant in pulmonary function (5–8) as well as to the calculation of the work of breathing (9). Given the highly nonlinear and variable shape of the inflation limb of a PV curve, the analysis is commonly only done on the deflation limb to provide information about the intrinsic elastic properties of the respiratory system. The determination of the slope of the pressure-volume relationship within the linear range of the deflation curve gives rise to the term compliance, a parameter that is sometimes also referred to as static or quasi-static compliance and that describes the distensibility of the entire respiratory system. Included in the compliance of the respiratory system are contributions from the chest wall, the lung tissue, and the airways as, like the chest wall and the lung parenchyma, the airways are also distensible (10–12). Establishing the specific contribution of each component has been of interest especially for the comprehensive understanding of physiological or pathophysiological mechanisms. Although the measurement conditions (e.g., extra pressure transducer, open vs. closed chest) can help establish the chest wall involvement, numerous studies have tried to address the contribution of the mechanical properties of the airways to lung function through varied approaches (11, 13–23). The airways are the conduits to the alveoli and their distensibility will determine both their volume and diameter. Each of these can have a significant impact on pulmonary function (9, 11), as the volume directly affects the alveolar ventilation (fresh gas going to the alveoli), and the diameter directly impacts the ability of gas to flow in and out of alveoli (i.e., the airway resistance).
The measurement of airway compliance through the PV relationship has been reported in large animals (13, 14, 23). This was either done by extracting the airway volume from an imaging approach (13, 14) or by limiting the evaluation to the trachea (23). To the best of our knowledge, there are no reports currently available in the literature of direct PV-derived measurements of airway compliance in small animal subjects like mice where the compliance of the entire airway tree was determined. Having access to such a technique would be beneficial for the physiological assessment as well as for the characterization of the underlying pathophysiological mechanisms, given the high number of respiratory disease models being developed in this species.
Partial and full-range PV curves are frequently constructed in mice to assess the mechanical properties of the entire respiratory system (1, 3, 4, 24, 25). During a PV measurement, both the inflation and deflation limbs are typically recorded. In addition, when performed on degassed lungs to generate a full-range PV curve, the first PV inflation arm contains a segment where, before the collapsed parenchyma starts to open, only the airway tree is distended. We reasoned that the initial PV inflation segment could be used to quantify airway compliance in mice. The main objective of the study was to determine the feasibility of the approach. We thus established a procedure to determine airway compliance from the initial inflation portion of mouse full-range PV curves and evaluated changes in airway characteristics in three models of obstructive and restrictive respiratory diseases.
MATERIALS AND METHODS
Experimental Data
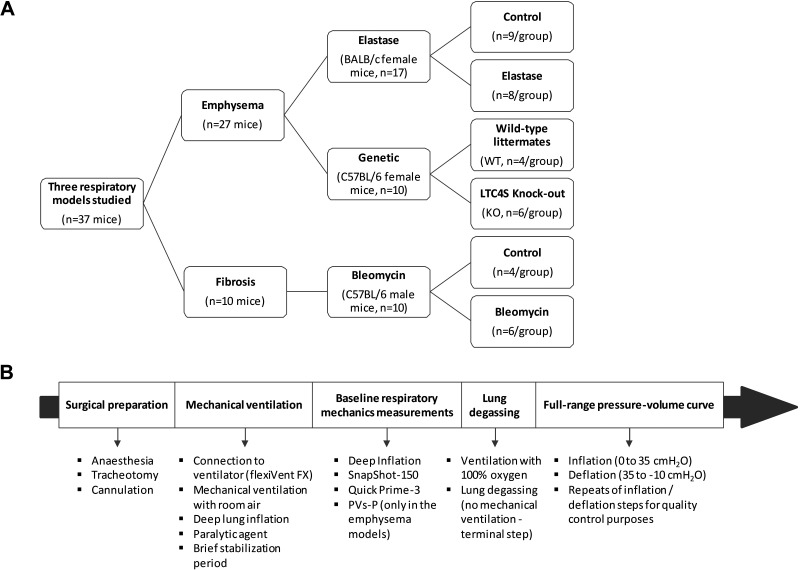
The study was performed by analysis of full-range PV curves and respiratory mechanics measurements previously collected in mice in two independent laboratories (3, 26, 27). Experimental data from a total of 37 mice (19.4–28.3 g, at the time of the study) were analyzed (Fig. 1). The data were derived from three different animal studies and included models of obstructive and restrictive respiratory diseases. Experimental data from two models of emphysema were included: 1) an elastase-induced model comprising of control (n = 9) and elastase-treated (n = 8) female BALB/c mice (3), and 2) a genetic model of spontaneous emphysema, resulting from a deficiency in leukotriene C4 synthase knockout (LTC4S-KO) in 10-wk-old female mice of a C57BL/6 background (n = 6), and their wild-type (WT) littermates (n = 4) (27). In addition, experimental data from a bleomycin-induced model of lung fibrosis in 7-mo-old male C57BL/6 mice (26) were also analyzed. All three protocols were approved by animal care committees at the respective institutions and included for standardization the same sequence of experimental procedures, as represented in Fig. 1. The same equipment (flexiVent FX, SCIREQ Inc., Montreal, QC, Canada) was also used across the three studies to record respiratory mechanics parameters, and construct full-range PV curves. The elastase-induced emphysema model represented a total of five control and five diseased animals. However, given that the study had demonstrated superimposable experimental PV deflation traces between the classic (manual) and the automated (flexiVent FX) methods (3), subjects having undergone the construction of the full-range PV curve using the classic (manual) method were also included in the analysis. This represented at total of eight additional mice, five control and three elastase-treated mice. Since the analysis was not blinded due to the obvious differences in the shape of the full-range PV curves between control subjects and animals with obstructive or restrictive respiratory diseases, one control subject associated with the classic method was excluded as a result of an unexplained low lung opening pressure on the first inflation limb of the PV curve. This can occasionally happen if the animal dies before complete lung degassing. Finally, in all three studies, accepted baseline measurements of respiratory mechanics with a coefficient of determination >0.90 (Quick Prime-3, PVs-P) or >0.95 (SnapShot-150) were used in the data analysis and values were reported for the exact same animals having been analyzed for airway compliance.
Figure 1.
Study design used for the determination of airway compliance in three mouse models of respiratory diseases. A: diagram representation of the study design including the number of animals (n) per experimental group. B: diagram summarizing of the experimental procedures used in each study included in the analysis.
Experimental Outcomes
The primary outcome of the study was airway compliance (Caw). It was determined from full-range PV curves collected in previously degassed lungs (Fig. 1) as follows. First, the lung opening pressure (Pop) was obtained from the initial section of the first PV inflation limb by the recognition of a characteristic elbow shape in the PV curve segment, associated with the sudden opening of the lung parenchyma. This was done by using a peak detection algorithm applied to the pressure signal to find the target pressures indicated within the full-range PV maneuver settings and identifying the specific segments of the curve. Pop was then determined from the first inflation limb (between 0 and 35 cmH2O) as a local maximum on that specific section of the full-range PV curve. The peak detection algorithm was incorporated in the flexiVent operating software (flexiWare version 8), which was used to determine Pop, except for the few animals in the elastase-induced emphysema study having undergone the construction of a full-range PV curve using the classic method where Pop was manually estimated in a similar manner. A common segment of analysis was then identified before Pop for the experimental group and its control based on the minimal Pop value observed. More specifically, the segment was determined between 3 cmH2O (positive end expiratory pressure, PEEP) and a pressure approximating 0.8 times the minimal Pop. Caw was then determined by linear regression, as the slope of this initial PV segment. Individual values of Caw were determined for each subject and then averaged per group. Caw could also be expressed as a percentage of the quasi-static compliance of the respiratory system (C), which was calculated as the slope of the deflation limb of the full-range PV curve at 5 ± 2 cmH2O.
Validation
In an attempt to validate the findings of Caw, the ratio of absolute change in Caw and total compliance of the respiratory system (ΔCaw/ΔC) was calculated. In addition, two parameters thought to potentially display changes under conditions of altered airway mechanical properties were calculated in the same mice from respiratory mechanics measurements collected before the lung degassing and construction of the full-range PV curve. First, the work done on the lung during inspiration, or inspiratory work of breathing (WOB), was determined from the area under the inflation curve of a tidal volume maneuver (SnapShot-150). Note that this differs from several previous reports where WOB was extracted from large amplitude maneuvers (24, 28), but it is consistent with what is typically calculated in human patients during regular quiet breathing (9, 29, 30). WOB was then normalized to the volume at maximal pressure and expressed per minute to yield a parameter referred to as the minute work of breathing (mWOB). The elastance at 20.5 Hz (Ers_20.5) was also calculated, as previously reported, from the imaginary part of respiratory input impedance (or reactance) (31). Ers_20.5 was used in the present study to confirm the results of Caw since, at a frequency of around 20 Hz, the contribution from the central airways is thought to dominate in the resistance part of impedance, with negligible influence in mice from the gas inertance or the chest wall (32, 33). The resistance at the same frequency (Rrs_20.5) was also extracted from the respiratory impedance spectra to complement the evaluation.
Data Analysis
For all parameters, individual values and group averages (means ± SD) were calculated. Differences between control and experimental groups were assessed for statistical significance by t test, using a P value < 0.05 (GraphPad Prism v6; GraphPad Software, San Diego, CA). Correlations between two parameters were assessed by calculating the Pearson’s r value, also using a P value < 0.05 for statistical significance and the same statistical software. The best-fit curve was obtained by linear regression and plotted with its 95% confidence intervals. The coefficient of determination assessing the quality of the fit (R2) was also reported as describing the fraction of the variation shared by the two variables. Unless specified otherwise, group sizes of 4–9 were used.
RESULTS
Airway Compliance in Healthy Mice
The analysis included a total of 17 healthy control mice from two commonly used strains (Fig. 1). On average, airway compliance (Caw) was estimated at 2.3 ± 1 μL/cmH2O (n = 17, ranging from 0.6 to 4.2 μL/cmH2O) in healthy mice and represented ∼3% of the quasi-static compliance of the respiratory system. Strain-specific variability was however observable in all related parameters, and differences between the BALB/c and C57BL/6 reached statistical significance for Pop, Caw, C as well as the Caw/C ratio (Table 1, P < 0.05).
Table 1.
Airway compliance and related parameters in healthy control mice
| Parameter |
||||
|---|---|---|---|---|
| Strain | Pop, cmH2O | Caw, μL/cmH2O | C, mL/cmH2O | Caw/C, % |
| BALB/c | ||||
| Mean ± SD | 24 ± 2 | 1.7 ± 0.9 | 0.094 ± 0.009 | 2 ± 1 |
| Range | 21–28 | 0.6–3.7 | 0.08–0.11 | 1–4 |
| n | 9 | 9 | 9 | 9 |
| C57BL/6 | ||||
| Mean ± SD | 27 ± 1 | 3 ± 0.6 | 0.067 ± 0.012 | 5 ± 2 |
| Range | 25–29 | 2.2–4.2 | 0.05–0.083 | 3–8 |
| n | 8 | 8 | 8 | 8 |
| Strain comparison | ||||
| P value | 0.0021 | 0.0041 | 0.0001 | 0.0006 |
Strain comparisons were done by t test, with P < 0.05 as significant. C, total quasi-static compliance of the respiratory system; Caw, airway compliance; Caw/C, airway compliance expressed as a percentage of the total quasi-static compliance of the respiratory system; Pop, lung opening pressure.
PV Curves and Lung Opening Pressure in Models of Respiratory Diseases
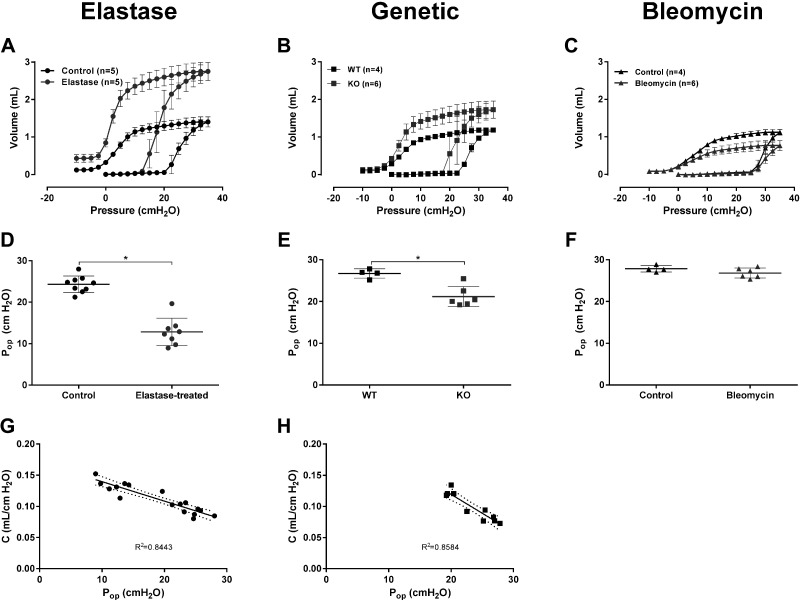
In BALB/c mice, the administration of elastase was previously reported to generate a severe model of emphysema relative to other mouse strains (2, 3). This is observable by a shift in the shape of the full-range PV curve (Fig. 2), a significant increase in the static compliance of the respiratory system, as well as a near doubling of total lung capacity (3). Also, in the elastase-treated mice, the lung opening pressure was determined to be approximately half of that of the control mice, with a mean Pop of 12.84 ± 3.31 versus 24.33 ± 2 cmH2O, respectively, resulting in a statistically significant difference between the two experimental groups (Fig. 2, P < 0.0001). In the elastase-treated mice, Pop ranged from as low as 8.97 cmH2O and there was a strong statistically significant negative correlation between Pop and C (Pearson’s r = −0.9189, P < 0.0001) and a large portion of the variability in Pop could be tied to the variation in C (R2 = 0.8443).
Figure 2.
Full-range pressure-volume curves and lung opening pressures in three models of respiratory diseases. A–C: average (±SD) full-range pressure-volume (PV) curves. A: control (n = 5) and elastase-treated mice (n = 5). B: wild-type (WT, n = 4) and leukotriene C4 synthase knockout (KO, n = 6) mice. C: control (n = 4) and bleomycin-treated mice (n = 6). In the elastase-induced model of emphysema (A), only the full-range PV curves constructed using the automated method (flexiVent FX) were included (3). D–F: lung opening pressure (Pop) in the three mouse models studied and their respective controls. Individual results (n = 4–9/group) and group means (±SD) are presented for each group. G and H: correlation between the lung opening pressure (Pop) and the compliance of the respiratory system in the elastase (G) and the genetic (H) models of emphysema. The compliance of the respiratory system (C) was calculated as the slope of the PV deflation limb between 3 and 7 cmH2O. The dashed lines represent the 95% confidence interval of the best-fit line (solid line) and R2 is the coefficient of determination assessing the quality of the fit. *P < 0.05, t test.
This analysis also included a genetic model that spontaneously develops an emphysematous phenotype as a result of the deletion of LTC4S (27). The deletion of LTC4S led to an upward shift of the full-range PV curves and an increase in the slope of the deflation limb, translating into an emphysematous phenotype (Fig. 2). As for the elastase-treated mice, Pop was significantly reduced in the LTC4S-KO group compared with the WT one (Fig. 2). However, the extent of the change in Pop was of smaller magnitude in the LTC4S-KO mice, with the minimal Pop value being at 19.24 cmH2O. A strong and statistically significant negative correlation between Pop and C was also found in this model (Pearson’s r = −0.9265, P < 0.0001) as well as a high fraction of shared variance (Fig. 2; R2 = 0.8584).
Experimental data collected in a bleomycin-induced model of lung fibrosis were also analyzed (Fig. 2). As illustrated by the more convex shape of the full-range PV curves, the group of analyzed bleomycin-treated mice displayed the characteristic stiffening associated with the development of lung fibrosis. In this restrictive respiratory disease model, no change in Pop was observed relative to the control mice, with the minimal Pop value in the bleomycin-treated mice being at 25.34 cmH2O (Fig. 2).
Airway Compliance in Models of Obstructive and Restrictive Respiratory Diseases
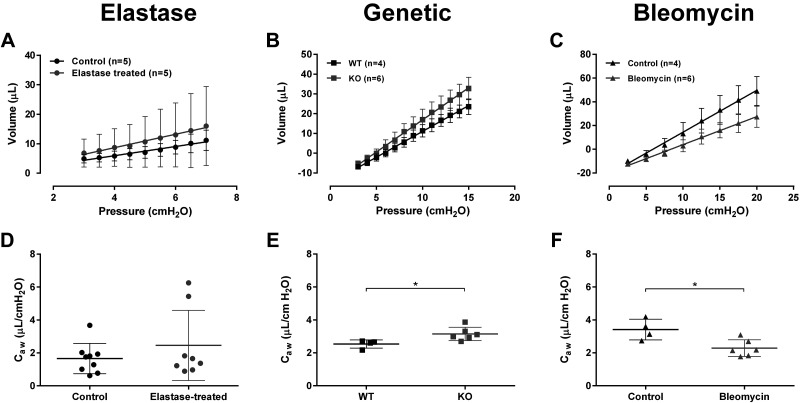
In the elastase-model, a segment of the initial PV inflation limb was isolated between 3 and 7 cmH2O for the analysis of Caw (Fig. 3). As observable from the shape of the Caw analysis PV segments, as well as from the comparison of the slopes, there was no statistical difference in Caw between the two study groups (Fig. 3). In the LTC4S-KO mice, consistent with the change in Pop, a longer portion of the initial PV inflation curve was isolated, between 3 and 15 cmH2O, for the determination of Caw (Fig. 3). The slope of the PV inflation analysis segment was steeper in the LTC4S-KO animals and translated into a statistically significant increase in Caw relative to the control mice (Fig. 3, P = 0.0275). In the restrictive respiratory disease model of lung fibrosis, the PV inflation segment for the determination of Caw was set from 3 to 20 cmH2O (Fig. 3). The PV inflation segment for the bleomycin-treated group was shifted down compared with that of the group of control mice. The difference in slope between the two experimental groups reached statistical significance, resulting in a significantly reduced Caw in the bleomycin-treated mice (Fig. 3, P = 0.0137).
Figure 3.
Determination of airway compliance. A–C: average (±SD) pressure-volume (PV) inflation curve segments for the determination of airway compliance. A: control (n = 5) and elastase-treated mice (n = 5). B: wild-type (WT, n = 4) and leukotriene C4 synthase knockout (KO, n = 6) mice. C: control (n = 4) and bleomycin-treated (n = 6) mice. In the elastase-induced model of emphysema (A), only the curves from the animals subjected to the automated method (flexiVent FX) were included (3). D–F: airway compliance (Caw) calculated by linear regression as the slope of the PV inflation segment. Individual results (n = 4–9/group) and group means (±SD) are presented for each group. *P < 0.05, t test.
Validation
To validate the methodology, the magnitude of Caw change was calculated and expressed as a percentage of that seen for the total respiratory system quasi-static compliance (ΔCaw/ΔC) in all three models studied (Table 2). In the elastase-model, where no significant change in airway compliance was seen, the ΔCaw/ΔC ratio was at 2.1%. Relative to that value, the ΔCaw/ΔC ratio moved in opposite directions in the genetic model of spontaneous emphysema and the bleomycin model of lung fibrosis, as did the values of Caw (Table 2).
Table 2.
Changes in compliance in models of respiratory diseases
| Phenotype | Model | ΔCaw, μL/cmH2O | ΔC, μL/cmH2O | ΔCaw/ΔC, % |
|---|---|---|---|---|
| Emphysema | Elastase | 0.80 | 38.24 | 2.1 |
| Genetic | 0.61 | 35.95 | 1.7 | |
| Fibrosis | Bleomycin | 1.13 | 20.41 | 5.5 |
ΔC, absolute change in magnitude for total quasi-static compliance of the respiratory system relative to control condition; ΔCaw, absolute change in magnitude for airway compliance relative to control condition; ΔCaw/ΔC, magnitude of Caw change expressed as a percentage of that seen for the total respiratory system quasi-static compliance.
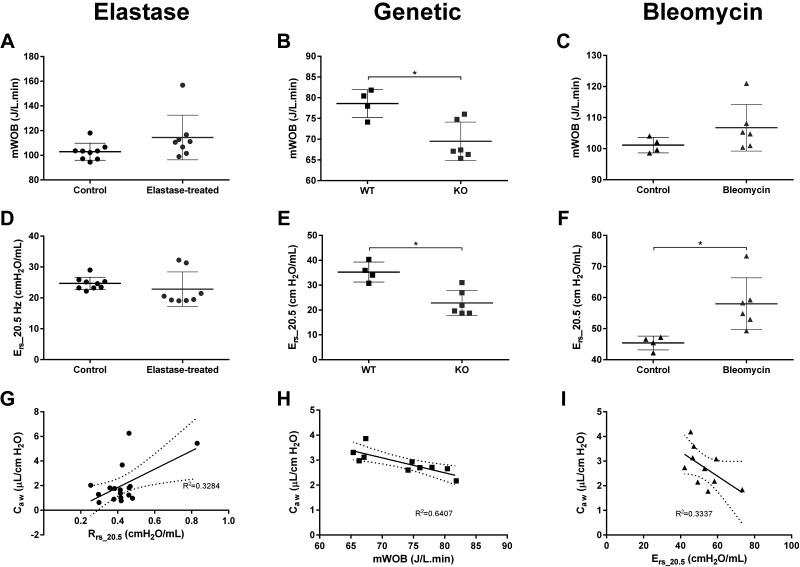
In addition, consistent with Caw findings, mWOB and Ers_20.5 remained unchanged in the elastase-induced model of emphysema (Fig. 4). Similarly, LTC4S-KO mice displayed a statistically significant decrease in mWOB and Ers_20.5 relative to the WT animals (Fig. 4), in agreement with the Caw changes observed in that model (Fig. 3E). A statistically significant correlation was found between Caw and either parameters, with the strongest correlation being with mWOB (Pearson’s r = −0.8004, P = 0.0054). However, only a moderate fraction of variance was shared between the two parameters (R2 = 0.6407), indicating that other variables mattered in this relationship (Fig. 4). Like for the two other experimental models studied, mWOB and Ers_20.5 were also calculated in the model of lung fibrosis (Fig. 4). Compared with the control animals, both parameters were numerically increased in the bleomycin-treated mice, in line with Caw results (Fig. 3F). However, under the present conditions, the increase only reached the level of statistical significance with Ers_20.5 (P = 0.0197), which showed a moderate level of correlation with Caw (Pearson’s r = −0.5777, P = 0.0803) and explained only a third of its variation (R2 = 0.3337, Fig. 4). In all three models studied, the resistance at the same frequency (Rrs_20.5) was also extracted to complement the analysis. Although Rrs_20.5 remained unchanged in the genetic model of emphysema and in the bleomycin model of lung fibrosis (data not shown), a statistically significant correlation was found with Caw in the elastase-induced model of emphysema (Pearson’s r = 0.5731, P = 0.0162). However, that correlation appears to be mostly driven by a single point slightly outside the group, as evidenced by the low coefficient of determination for the linear fit of the curve (R2 = 0.3284) and the wide 95% confidence band around the best-fit curve (Fig. 4).
Figure 4.
Inspiratory work of breathing and respiratory elastance at 20.5 Hz. A–C: normalized inspiratory work of breathing per units of time (mWOB). A: control (n = 9) and elastase-treated (n = 8) mice. B: wild-type (WT, n = 4) and leukotriene C4 synthase knockout (KO, n = 6) mice. C: control (n = 4) and bleomycin-treated (n = 6) mice. D–F: respiratory elastance at 20.5 Hz (Ers_20.5) in the three mouse models studied and their respective controls. Individual results (n = 4–9/group) and group means (±SD) are presented for each group. G–I: correlation between airway compliance (Caw) and mWOB in the genetic model of emphysema (H) or Ers_20.5 in the bleomycin model of lung fibrosis (I). Resistance at the same frequency (Rrs_20.5) was also extracted to complement the evaluation. A statistical correlation (Pearson’s r = 0.5731, P = 0.0162) was found between Rrs_20.5 and Caw in the elastase-induced model of emphysema (G). However, the low coefficient of determination (R2 = 0.3284) for the best-fit curve (solid line) and the wide 95% confidence interval (dash lines) suggest that the correlation is driven by a single point slightly outside of the group. *P < 0.05, t test.
DISCUSSION
Airways are distensible entities that expand as air flows into the lungs during inhalation. Because they are compliant, factors such as tissue composition, muscle tone, parenchymal tethering, intrathoracic pressure, resting lung volume or inflammation can all modify airway function. Many investigators have tried to characterize the elastic properties of the airways, as being able to study the changes in airway compliance could provide insight into underlying physiological or pathophysiological mechanisms. Indeed, altered airway compliance could lead to expiratory airflow limitation, with compliant airways being more susceptible to collapse, or to an augmented inspiratory work of breathing, as a result of an increase in airway stiffness or heterogeneous opening.
Direct measurements of airway compliance require recordings of pressure and volume changes pertaining only to the airways. The acquisition of such data is technically challenging in mice, given their small body size. Sera et al. (21, 22) previously reported a technique using synchrotron computed tomography (CT)-imaging to capture deformations of airway diameter, and thus airway volume, during an inflation from functional residual capacity (FRC) to total lung capacity (TLC). The method allowed the determination of a specific Caw (sCaw), or a normalized airway compliance to airway volume at FRC, which was used to assess changes in a mouse model of asthma (21). Although it would be possible in theory to extract a value for airway compliance from that measurement, provided a known airway volume at FRC, a more widely accessible method could be very useful for the evaluation of Caw in mice given the increasing number of respiratory disease models generated in that species. The present work aimed at assessing Caw in mice from full-range PV curves. In these curves, the inflation starts immediately following a lung degassing procedure. The initial segment of the first inflation limb, before the opening of the lungs, involves only the inflation of the airway tree, thus providing a PV curve that could be used for the determination of Caw. Using this approach, we analyzed inflation segments from full-range PV curves and determined Caw in three mouse models of obstructive and restrictive respiratory diseases. The results demonstrated that the technique has the sensitivity to detect changes and is, to our knowledge, the first report of Caw through direct PV measurements in laboratory animals as small as a mouse.
In the present study, Caw was determined to represent ∼3% of the quasi-static compliance of the respiratory system in healthy mice, which is in the same order of magnitude as previously estimated by Mead (11) in humans. As in the technique described by Sera et al. (21, 22), Caw was determined during inflation whereas lung compliance is typically calculated during deflation. This is not a major concern since, in the segment analyzed for Caw, only the airway tree is inflated. Consistent with the airway volume range recorded in the present study, Kim et al. (34) recently reported the volume of larger airways (≥200 μm) in mice to be in the order of 40 μL, with a variation between end-of-expiration and peak-of-inspiration of ∼20 μL. One potential issue, which we are unable to assess, is the possibility of recruitment of collapsed small airways. Indeed, changes in airway volume were reported to be more significant in smaller airways and this effect could be exacerbated with disease (21, 22). The higher subject-to-subject variability seen in the elastase model of emphysema, relative to the other experimental groups analyzed in this study, could possibly be the result of this effect. An uneven opening of the very small airways stemming from heterogeneity in the parenchymal tethering could be a factor in this model. Indeed, an increased disease severity was previously reported (2, 3) and the change in the shape of the full-range PV curve (Fig. 2A) and in Pop (Fig. 2D) were more pronounced in that group. Interestingly, the results showed that variations in Pop correlated with that in the compliance of the respiratory system in the two models of emphysema evaluated (Fig. 2, G and H). Although the mechanism for this correlation is unclear, based on this observation, Pop could potentially provide a parameterized approach to evaluate or compare mouse models of emphysema across studies and laboratories based on disease severity. The Pop parameter, accessible from full-range PV curves and occasionally monitored, has to our knowledge rarely, if ever, been extracted from these curves. Since it captures a functional aspect of the respiratory system upon the opening of an atelectatic lung, it reflects the “stickiness” of the very peripheral airways and lining fluid and, thus, may be affected by alterations in the level of surfactant.
Since in this study, the minimal Pop value was used to determine the length of the Caw analysis segment, this may have had a negative impact on the precision of the measurement in the elastase model by limiting the PV inflation section. Other factors that could have affected Caw measurements could include the supine position of the subject, which could impact airway compliance, or the fact that the measurements were performed following lung degassing, which is a terminal procedure. However, relative to the studies by Sera et al. (21, 22), where a postmortem approach was also used, the present measurements were done at a much earlier time point (few minutes vs. 1 h) and hence may be closer to physiological conditions. An advantage of the present technique is that it does not require sophisticated imaging equipment. It, therefore, has the potential to make the measurement of Caw in mouse models of respiratory diseases more accessible as well as to extend its use to other species (e.g., rats) where full-range PV loops can also be performed (3).
The evolution of the change in Caw relative to that of the total respiratory system compliance (ΔCaw/ΔC, Table 2) was evaluated in the disease models studied to validate the methodology. In agreement with the direct measurements of Caw, a consistent relationship in ΔCaw/ΔC was also observed, with the latter parameter moving in opposite directions in the genetic model of spontaneous emphysema and the bleomycin model of lung fibrosis. Further validation of the measurement of airway compliance from the initial inflation limb of the PV curve could come from an estimation of a specific airway compliance, as previously reported by Sera et al. (21, 22). Normalizing the average Caw obtained in the present study in healthy adult mice (2.3 μL/cmH2O) using the airway volume reported by Kim et al. (34) for larger airways (40 μL) provided an estimate of sCaw at 0.06 cmH2O−1, which falls within the range of values reported for that parameter in mice (21). In addition, we calculated the amount of work involved during inspiration (mWOB) to extend the validation of the proposed method of Caw determination, using prior respiratory mechanics measurements done in the same subjects. Indeed, airway compliance is affected by variations in bronchomotor tone, airway stability, or heterogeneity in ventilation distribution, which can influence the degree of mechanical work required to inflate the lungs. We also reasoned that the elastance at 20.5 Hz (Ers_20.5) could be another parameter to consider, as it could provide insight into the stiffness of the larger conducting airways, given that the airway contribution is thought to dominate in the resistance part of the input impedance at higher frequencies (35, 36). The changes in mWOB and Ers_20.5 were generally consistent with the results obtained for Caw in the experimental models studied, perhaps more so in the emphysematous models where mWOB showed a stronger correlation with Caw in the LTC4S KO mouse model. Taken together, these results suggest that the methodology herein described provides a realistic assessment of airway mechanics. However, one must keep in mind that the respiratory mechanics measurements from which mWOB or Ers_20.5 were extracted came from mice that were anesthetized, paralyzed, and in the supine position. Although these conditions were considered advantageous in trying to tease out the intrinsic contribution of airway compliance to the respiratory function, the respiratory muscles were relaxed and the distortion of the chest wall was minimal such that the amount of work involved during inspiration may not reflect that obtained under conditions of spontaneous breathing. Also, the work of breathing was not calculated using a large amplitude maneuver, as previously reported in mice (24, 28). Instead, it was derived from a novel method of calculating it from a dynamic maneuver of tidal volume amplitude, as done clinically in patients (9, 29, 30).
In the present study, Caw changes were evaluated in two models of emphysema: an elastase-induced model as well as a genetically modified mouse model (LTC4S-KO) spontaneously developing of an emphysematous phenotype. Although both models displayed a similar respiratory phenotype, the results indicated that the airway involvement was not the same. In the elastase-induced model of emphysema, no change in Caw was observed, and that despite important increases in the compliance of the respiratory system as well as in various lung volumes (3). That was not the case in the genetically modified LTC4S-KO mouse model where an increase in Caw was detected. Similarly, a significant airway contribution was also noted in the bleomycin model of lung fibrosis, where a decrease in Caw was found. These results indicate that the method of directly assessing Caw in mice via the inflation limb of a full-range PV curve can detect disease-related changes and that different underlying mechanisms can be observed for a given respiratory phenotype. More research would be needed to further evaluate these differences.
In summary, a PV-based method for the direct determination of Caw in mice was established from an existing measurement technique (3, 25). Its ability to detect change was evaluated in models of obstructive and restrictive respiratory diseases where significant changes were observed. The findings could contribute to an enhanced understanding of the underlying pathophysiological mechanisms in these models. As such, the method, which is more convenient than previously described approaches, is likely to be of value to further characterize disease models and assess treatment efficacy. Its utility could be expanded to evaluate other disease-related changes in mice (e.g., associated with airway remodeling), as well as in other species.
DATA AVAILABILITY
The data that support this study are within the paper and available upon request.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute (NHBLI) Grants R01-HL141490 and R01-HL140623 as well as Canadian Institutes of Health Research (CIHR) Grant MOP-93747.
DISCLOSURES
A. Robichaud and L. Fereydoonzad are employed by SCIREQ Inc., a commercial entity with interests in a subject area related to the content of this article. SCIREQ Inc. is an emka TECHNOLOGIES company. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
A.R. and W.M. conceived and designed research; A.R., L.F., S.L.C., J.M.L., and Y.I. performed experiments; A.R., L.F., J.G.M., and W.M. analyzed data; A.R., L.F., J.G.M., and W.M. interpreted results of experiments; A.R. prepared figures; A.R. drafted manuscript; A.R., L.F., S.L.C., J.G.M., and W.M. edited and revised manuscript; A.R., L.F., S.L.C., J.M.L., Y.I., M.R.H., J.G.M., and W.M. approved final version of manuscript.
REFERENCES
- 1.Dimori M, Heard-Lipsmeyer ME, Byrum SD, Mackintosh SG, Kurten RC, Carroll JL, Morello R. Respiratory defects in the Crtap KO mouse model of osteogenesis imperfecta. Am J Physiol Lung Cell Mol Physiol 318: L592–L605, 2020. doi: 10.1152/ajplung.00313.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Limjunyawong N, Craig JM, Lagassé HA, Scott AL, Mitzner W. Experimental progressive emphysema in BALB/cJ mice as a model for chronic alveolar destruction in humans. Am J Physiol Lung Cell Mol Physiol 309: L662–L676, 2015. doi: 10.1152/ajplung.00214.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robichaud A, Fereydoonzad L, Limjunyawong N, Rabold R, Allard B, Benedetti A, Martin JG, Mitzner W. Automated full-range pressure-volume curves in mice and rats. J Appl Physiol (1985) 123: 746–756, 2017. doi: 10.1152/japplphysiol.00856.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soutiere SE, Mitzner W. On defining total lung capacity in the mouse. J Appl Physiol (1985) 96: 1658–1664, 2004. doi: 10.1152/japplphysiol.01098.2003. [DOI] [PubMed] [Google Scholar]
- 5.Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child 97: 517–523, 1959. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- 6.Clements JA, Hustead RF, Johnson RP, Gribetz I. Pulmonary surface tension and alveolar stability. J Appl Physiol 16: 444–450, 1961. doi: 10.1152/jappl.1961.16.3.444. [DOI] [PubMed] [Google Scholar]
- 7.Johnson JW, Permutt S, Sipple JH, Salem ES. Effect of intra-alveolar fluid on pulmonary surface tension properties. J Appl Physiol 19: 769–777, 1964. doi: 10.1152/jappl.1964.19.4.769. [DOI] [PubMed] [Google Scholar]
- 8.Mitzner W, Johnson JW, Scott R, London WT, Palmer AE. Effect of betamethasone on pressure-volume relationship of fetal rhesus monkey lung. J Appl Physiol Respir Environ Exerc Physiol 47: 377–382, 1979. doi: 10.1152/jappl.1979.47.2.377. [DOI] [PubMed] [Google Scholar]
- 9.Otis AB, Fenn WO, Rahn H. Mechanics of breathing in man. J Appl Physiol 2: 592–607, 1950. doi: 10.1152/jappl.1950.2.11.592. [DOI] [PubMed] [Google Scholar]
- 10.Bates JHT, Rossi A, Milic-Emili J. Analysis of the behavior of the respiratory system with constant inspiratory flow. J Appl Physiol 58: 1840–1848, 1985. doi: 10.1152/jappl.1985.58.6.1840. [DOI] [PubMed] [Google Scholar]
- 11.Mead J. Contribution of compliance of airways to frequency-dependent behavior of lungs. J Appl Physiol 26: 670–673, 1969. doi: 10.1152/jappl.1969.26.5.670. [DOI] [PubMed] [Google Scholar]
- 12.West JB. Respiratory Physiology: The Essentials. Philadelphia: Lippincott Williams & Wilkins, 2012. [Google Scholar]
- 13.Brown RH, Mitzner W. Effect of lung inflation and airway muscle tone on airway diameter in vivo. J Appl Physiol 80: 1581–1588, 1996. doi: 10.1152/jappl.1996.80.5.1581. [DOI] [PubMed] [Google Scholar]
- 14.Brown RH, Mitzner W, Bulut Y, Wagner EM. Effect of lung inflation in vivo on airways with smooth muscle tone or edema. J Appl Physiol (1985) 82: 491–499, 1997. doi: 10.1152/jappl.1997.82.2.491. [DOI] [PubMed] [Google Scholar]
- 15.Hughes JM, Hoppin FG Jr, Mead J. Effect of lung inflation on bronchial length and diameter in excised lungs. J Appl Physiol 32: 25–35, 1972. doi: 10.1152/jappl.1972.32.1.25. [DOI] [PubMed] [Google Scholar]
- 16.Kelly VJ, Brown NJ, King GG, Thompson BR. A method to determine in vivo, specific airway compliance, in humans. Med Biol Eng Comput 48: 489–496, 2010. doi: 10.1007/s11517-010-0576-3. [DOI] [PubMed] [Google Scholar]
- 17.Kelly VJ, Brown NJ, Sands SA, Borg BM, King GG, Thompson BR. Effect of airway smooth muscle tone on airway distensibility measured by the forced oscillation technique in adults with asthma. J Appl Physiol 112: 1494–1503, 2012. doi: 10.1152/japplphysiol.01259.2011. [DOI] [PubMed] [Google Scholar]
- 18.Novali M, Shalaby KH, Robichaud A, Benedetti A, Fereydoonzad L, McGovern TK, Schuessler TF, Martin JG. Mechanical consequences of allergic induced remodeling on mice airway resistance and compressibility. Respir Physiol Neurobiol 218: 11–20, 2015. doi: 10.1016/j.resp.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Okazawa M, Paré PD, Lambert RK. Compliance of peripheral airways deduced from morphometry. J Appl Physiol (1985) 89: 2373–2381, 2000. doi: 10.1152/jappl.2000.89.6.2373. [DOI] [PubMed] [Google Scholar]
- 20.Penn RB, Wolfson MR, Shaffer TH. Effect of tracheal smooth muscle tone on collapsibility of immature airways. J Appl Physiol (1985) 65: 863–869, 1988. doi: 10.1152/jappl.1988.65.2.863. [DOI] [PubMed] [Google Scholar]
- 21.Sera T, Uesugi K, Himeno R, Yagi N. Small airway changes in healthy and ovalbumin-treated mice during quasi-static lung inflation. Respir Physiol Neurobiol 156: 304–311, 2007. doi: 10.1016/j.resp.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Sera T, Uesugi K, Yagi N. Localized morphometric deformations of small airways and alveoli in intact mouse lungs under quasi-static inflation. Respir Physiol Neurobiol 147: 51–63, 2005. doi: 10.1016/j.resp.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Shaffer TH, Bhutani VK, Wolfson MR, Penn RB, Tran NN. In vivo mechanical properties of the developing airway. Pediatr Res 25: 143–146, 1989. doi: 10.1203/00006450-198902000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Gremlich S, Roth-Kleiner M, Equey L, Fytianos K, Schittny JC, Cremona TP. Tenascin-C inactivation impacts lung structure and function beyond lung development. Sci Rep 10: 1–3, 2020. doi: 10.1038/s41598-020-61919-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limjunyawong N, Fallica J, Horton MR, Mitzner W. Measurement of the pressure-volume curve in mouse lungs. J Vis Exp 95: e52376, 2015. doi: 10.3791/52376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins SL, Chan-Li Y, Oh M, Vigeland CL, Limjunyawong N, Mitzner W, Powell JD, Horton MR. Vaccinia vaccine-based immunotherapy arrests and reverses established pulmonary fibrosis. JCI insight 1: e83116, 2016. doi: 10.1172/jci.insight.83116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishii Y, McGovern T, Fujii U, Nakada E, Farahnak S, Khazaei N, Martin J. Leukotriene C4 synthase deficiency causes spontaneous emphysematous changes in mice. Eur Respir J 54: PA5421, 2019. doi: 10.1183/13993003.congress-2019.PA5412. [DOI] [Google Scholar]
- 28.Phillips JE, Peng R, Burns L, Harris P, Garrido R, Tyagi G, Fine JS, Stevenson CS. Bleomycin induced lung fibrosis increases work of breathing in the mouse. Pulm Pharmacol Ther 25: 281–285, 2012. doi: 10.1016/j.pupt.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Milic-Emili J, Rocca E, D’Angelo E. Work of breathing. In: Basics of Respiratory Mechanics and Artificial Ventilation, edited by Milic-Emili J, Lucangelo U, Pesenti A, Zin WA.. Milano: Springer, 1999, p. 165–175. [Google Scholar]
- 30.Plato A. Respiratory physiology for intensivists. In: Critical Heart Disease in Infants and Children, edited by Ungerleider RM, Meliones JN, Nelson McMillan K, Cooper DS, Jacobs JP. Philadelphia PA: Elsevier, 2019, p. 134–149.e132. [Google Scholar]
- 31.Barnas GM, Heglund NC, Yager DE, Yoshino KA, Loring SH, Mead JE. Impedance of the chest wall during sustained respiratory muscle contraction. J Appl Physiol (1985) 66: 360–369, 1989. doi: 10.1152/jappl.1989.66.1.360. [DOI] [PubMed] [Google Scholar]
- 32.Südy R, Fodor GH, Dos Santos Rocha A, Schranc A, Tolnai J, Habre W, Peták F. Different contributions from lungs and chest wall to respiratory mechanics in mice, rats, and rabbits. J Appl Physiol (1985) 127: 198–204, 2019. doi: 10.1152/japplphysiol.00048.2019. [DOI] [PubMed] [Google Scholar]
- 33.Tomioka S, Bates JHT, Irvin CG. Airway and tissue mechanics in a murine model of asthma: alveolar capsule vs. forced oscillations. J Appl Physiol 93: 263–270, 2002. doi: 10.1152/japplphysiol.01129.2001. [DOI] [PubMed] [Google Scholar]
- 34.Kim EH, Preissner M, Carnibella RP, Samarage CR, Bennett E, Diniz MA, Fouras A, Zosky GR, Jones HD. Novel analysis of 4DCT imaging quantifies progressive increases in anatomic dead space during mechanical ventilation in mice. J Appl Physiol (1985) 123: 578–584, 2017. doi: 10.1152/japplphysiol.00903.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bates JHT. Lung Mechanics. An Inverse Modeling Approach. New York: Cambridge University Press, 2009. [Google Scholar]
- 36.Peters U, Kaminsky DA, Bhatawadekar S, Lundblad L, Maksym GN. Oscillometry for lung function testing. In: Lung Function Testing in the 21st Century, edited by C. Ionescu. London, UK: Academic Press, 2018, p. 25–47. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support this study are within the paper and available upon request.