ABSTRACT
To explore the role of coffee on health outcomes in the United States, where coffee consumption is common, we conducted a meta-analysis of prospective studies investigating the magnitude (any compared with no consumption) and the dose–response shape (cups per day) of the associations between caffeinated coffee consumption and incidence/mortality of cardiovascular disease (CVD), as well as incidence of type 2 diabetes (T2D), hepatocellular carcinoma (HCC), endometrial cancer, melanoma, and nonmelanoma skin cancer. We selected the desirable health outcomes that have been shown to be positively associated with coffee consumption. Studies were identified by searching PubMed/Embase databases up to September 2019. Inclusion criteria included prospective studies that investigated the relation of ≥3 categories of caffeinated coffee consumption and the outcomes of interest. Twenty-six studies (42 distinct cohorts), with 93,706 cases/deaths and 3,713,932 participants, met the inclusion criteria. In any coffee consumers, there was a significant inverse association with the risk of CVD (RR = 0.90; 95% CI: 0.84, 0.96), T2D (RR = 0.90; 95% CI: 0.85, 0.96), endometrial cancer (RR = 0.85; 95% CI: 0.78, 0.92), melanoma (RR = 0.89; 95% CI: 0.80, 0.99), and nonmelanoma skin cancer (RR = 0.92; 95% CI: 0.89, 0.95). Coffee consumption was also inversely associated with HCC (RR = 0.93; 95% CI: 0.80, 1.08), without reaching statistical significance. The dose–response relation was nonlinear uniquely for CVD (P-nonlinearity = 0.01). In particular, the largest risk reduction was observed for 3–4 cups/d (∼120 mL/cup) and no reduction thereafter. For other outcomes, the risk decreased linearly over the whole coffee consumption range. Current patterns of consumption in the United States would account for a fraction of avoided cases/deaths ranging from 6% to 12% according to the outcome considered. This study confirms the beneficial health effects of caffeinated coffee consumption in the US population on the health outcomes considered, and quantifies their possible magnitude.
Keywords: US population, caffeinated coffee consumption, health outcomes, dose–response shape, attributable fraction
Introduction
Coffee is a complex mixture of hundreds of different compounds (1). The most widely known is caffeine, a natural stimulant, that has been associated with increased blood pressure (2), decreased triglyceride and total cholesterol concentrations (3), and insulin resistance (4). Because of its caffeine content, coffee has been considered unhealthy in the past. However, coffee also contains phenolic acids, diterpenes, minerals, nicotinamide equivalent, and other compounds with potentially beneficial effects, such as insulin-sensitizing (5), antioxidative (6), and anti-inflammatory (7). Several reviews and meta-analyses have shown a protective role of coffee consumption on both incidence and/or mortality of several chronic diseases, including cardiovascular disease (CVD) (6), type 2 diabetes (T2D) (8), and different cancer types (9, 10). In these studies, meta-analytic estimates were obtained by pooling RRs for the highest compared with the lowest category. This approach, however, does not use information on intermediate categories, failing to provide a comprehensive description of the dose–response relation between coffee and health outcomes. Using a dose–response meta-analytic approach, some studies have estimated the shape of the relation for incidence or mortality of CVD (37, 38), incidence of T2D (39), incidence of selected cancers (40, 41), and overall and cause-specific mortality (42).
To explore the role of caffeinated coffee on health outcomes in the United States, where coffee consumption is common, we conducted a dose–response meta-analysis of US prospective studies investigating the magnitude and the shape of the associations between caffeinated coffee consumption and incidence or mortality of CVD, as well as incidences of T2D, hepatocellular carcinoma (HCC), endometrial cancer, melanoma, and nonmelanoma skin cancer. We selected the desirable health outcomes that have been shown to be positively associated with coffee consumption. For each outcome, we also estimated the fraction of cases/deaths avoided due to caffeinated coffee consumption.
Methods
Outcome selection and literature search
We considered the following health outcomes that have been shown to be positively associated with coffee consumption: CVD incidence/mortality and T2D incidence according to findings of previous reviews and meta-analyses (6, 8, 37–39, 42, 43); HCC incidence, endometrial cancer incidence, melanoma incidence, and nonmelanoma skin cancer incidence according to the latest report of the World Cancer Research Fund (44).
We performed a literature search up to September 2019 in the Medline/PubMed and Embase databases using the terms “prospective” and “cohort” for study design; “hot beverages,” “coffee,” and “caffeine” for coffee consumption; “chronic diseases,” “cardiovascular disease,” “coronary heart disease,” “stroke,” “myocardial infarction,” “ischemic heart disease,” “mortality,” and “heart failure” for CVD outcome; “diabetes” for T2D outcome; “liver,” “hepatocellular,” “biliary tract,” “gallbladder,” and “extrahepatic” for HCC outcome; “female,” “hormonal,” “endometrium,” and “endometrial” for endometrial outcome; “melanoma” and “skin” for melanoma outcome; “skin,” “basal cell,” and “squamous cell” for nonmelanoma skin outcome. In addition, outcomes involving cancer shared the terms “cancer,” “carcinoma,” and “neoplasm” (appendix). We followed the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines for conducting meta-analyses and reporting results (45). Two authors (MDM and FB) separately reviewed studies and discrepancies were discussed and solved. Studies were eligible for inclusion in the meta-analyses if they met the following criteria: 1) the study had a prospective design investigating the relation of caffeinated coffee consumption and outcomes of interest on humans in the United States; 2) the study reported RRs with 95% CIs for ≥3 categories of caffeinated coffee consumption; and 3) RRs had been adjusted at least for sex, age, and smoking.
Data extraction
From each study, we extracted data on selected outcomes, first author's surname, publication year, study name, period of enrollment, duration of follow-up (years), number of study participants, number of cases, sex and age of participants, adjustment factors, and RRs with their 95% CIs for each category of caffeinated coffee consumption (Table 1). When studies reported the adjusted RRs but not the corresponding 95% CIs (11), we calculated the CIs for crude RRs and related them to the adjusted ones.
TABLE 1.
Characteristics of prospective studies included in the meta-analysis of caffeinated coffee consumption and selected diseases. United States, 1987–2017
| Reference | Participants, n (sex) | Study name | Age range, y | Cases or deaths, n | Enrollment period | Follow-up, y | Adjustments |
|---|---|---|---|---|---|---|---|
| Cardiovascular disease incidence/mortality | |||||||
| Legrady et al., 1987 (11) | 1910 (men) | Chicago Western Electric Company Study | 40–56 | 220 | 1957–8 | 19 | Age, smoking, blood pressure, and serum cholesterol concentration |
| Klatsky et al., 1990 (12) | 11,990 (both) | Northern California Kaiser Permanente Medical Care Program | — | 1914 | 1978–85 | 8 | Age, sex, race, education, smoking, alcohol, and personal history of disease |
| Andersen et al., 2006 (13) | 27,312 (women) | Iowa Women's Health Study (IWHS) | 55–69 | 1411 | 1986 | 15 | Age, education, BMI, waist-to-hip ratio, physical activity, smoking, alcohol, energy intake, intakes of wholegrain, refined grain, red meat, fish, seafood, fruit and vegetables, use of multivitamin supplement, and use of hormone replacement therapy |
| Lopez-Garcia et al., 2008 (14) | 41,736 (men) | Health Professionals Follow-up Study (HPFS) | 40–75 | 2049 | 1986 | 18 | Age, BMI, physical activity, smoking, alcohol, energy intake, intakes of polyunsaturated, saturated and fish n–3 fats, intakes of trans fat and folate, glycemic load, use of multivitamin or vitamin E supplements, and family history of myocardial infarction |
| Lopez-Garcia et al., 2008 (14) | 86,214 (women) | Nurses’ Health Study (NHS) | 30–55 | 2368 | 1980 | 24 | Age, BMI, physical activity, smoking, alcohol, energy intake, intakes of polyunsaturated, saturated, and fish n–3 fats, intakes of trans fat and folate, glycemic load, use of multivitamin or vitamin E supplements, family history of myocardial infarction, menopausal status, and use of hormone replacement therapy |
| Freedman et al., 2012 (15) | 229,119 (men) | National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study | 50–71 | 9454 | 1995–6 | 14 | Age, race, education, marital status, BMI, physical activity, smoking, alcohol, energy intake, intakes of saturated fats, fruit, vegetables, red meat and white meat, use of vitamin supplement, and health status |
| Freedman et al., 2012 (15) | 173,141 (women) | National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study | 50–71 | 4667 | 1995–6 | 14 | Age, race, education, marital status, BMI, physical activity, smoking, alcohol, energy intake, intakes of saturated fats, fruit, vegetables, red meat and white meat, use of vitamin supplement, and health status |
| Loftfield et al., 2015 (16) | 90,317 (both) | Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial | 55–74 | 2220 | 1998–2001 | 11 | Age, sex, race, education, marital status, employment status, BMI, smoking, alcohol, energy intake, intakes of meat (red, white, and processed), fruit, vegetables, and saturated fats, use of any vitamin supplement, regular use of ibuprofen or aspirin, use of hormone replacement therapy, and personal history of diabetes |
| Tsujimoto et al., 2017 (17) | 8170 (men) | NHANES | 20–79 | 203 | 1999–2010 | 12 | Age, race, education, BMI, smoking, energy intake, intakes of carbohydrates, fats, and proteins, and personal histories of diabetes or cancer |
| Tsujimoto et al., 2017 (17) | 9424 (women) | NHANES | 20–79 | 100 | 1999–2010 | 12 | Age, race, education, BMI, smoking, energy intake, intakes of carbohydrates, fats, and proteins, and personal histories of diabetes or cancer |
| Type 2 diabetes incidence | |||||||
| Greenberg et al., 2005 (18) | 7006 (both) | NHANES-1 Epidemiologic Follow-up Study (NHEFS) | 32–60 | 170 | 1982–4 | 10 | Age, sex, race, education, income, BMI, physical activity, smoking, alcohol, and type of diet |
| Paynter et al., 2006 (19) | 5415 (men) | Atherosclerosis Risk in Communities (ARIC) Study | 45–64 | 718 | 1987–9 | 9 | Age, race, education, BMI, waist-to-hip ratio, physical activity, smoking, alcohol, energy intake, intake of fibers, serum magnesium concentration, personal history of hypertension, and family history of diabetes |
| Paynter et al., 2006 (19) | 6790 (women) | Atherosclerosis Risk in Communities (ARIC) Study | 45–64 | 719 | 1987–9 | 9 | Age, race, education, BMI, waist-to-hip ratio, physical activity, smoking, alcohol, energy intake, intake of fibers, serum magnesium concentration, personal history of hypertension, and family history of diabetes |
| Pereira et al., 2006 (20) | 28,212 (women) | Iowa Women's Health Study (IWHS) | 55–69 | 1418 | 1986 | 11 | Age, education, BMI, waist-to-hip ratio, physical activity, smoking, alcohol, energy intake, intakes of fats, magnesium, phytate, tea, and soda, Keys score, and personal history of hypertension |
| van Dam et al., 2006 (21) | 88,259 (women) | Nurses’ Health Study II (NHS-II) | 26–46 | 1263 | 1989 | 12 | Age, BMI, physical activity, smoking, alcohol, energy intake, polyunsaturated-to-saturated fat intake ratio, intakes of cereal fibers, processed meat, soft drinks and punch, glycemic index, personal histories of hypertension or hypercholesterolemia, family history of diabetes, and use of oral contraceptive or hormone replacement therapy |
| Boggs et al., 2010 (22) | 46,906 (women) | Black Women's Health Study (BWHS) | 30–69 | 3055 | 1995 | 12 | Age, questionnaire cycle, education, BMI, physical activity, smoking, energy intake, intakes of fibers, cereals, and soft drinks, glycemic index, personal history of hypertension or hypercholesterolemia, and family history of diabetes |
| Zhang et al., 2011 (23) | 1141 (both) | Strong Heart Study | 45–74 | 188 | 1989–92 | 10 | Age, sex, BMI, physical activity, smoking, alcohol, and family history of diabetes |
| Bhupathiraju et al., 2013 (24) | 39,059 (men) | Health Professionals Follow-up Study (HPFS) | 40–75 | 2865 | 1986 | 22 | Age, time interval, BMI, reported weight change, physical activity, smoking, alcohol, energy intake, intakes of soft drinks and punch, adherence to a low-calorie diet, Alternate Healthy Eating Index, personal histories of hypertension or hypercholesterolemia, and family history of diabetes |
| Bhupathiraju et al., 2013 (24) | 74,749 (women) | Nurses’ Health Study (NHS) | 30–55 | 7343 | 1984 | 24 | Age, time interval, BMI, reported weight change, physical activity, smoking, alcohol, energy intake, intakes of soft drinks and punch, adherence to a low-calorie diet, Alternate Healthy Eating Index, personal histories of hypertension |
| or hypercholesterolemia, family history of diabetes, and use of hormone replacement therapy | |||||||
| Doo et al., 2014 (25) | 36,127 (men) | Multiethnic Cohort Study (MEC) | 45–75 | 4541 | 1993–6 | 14 | Age, race, education, BMI, physical activity, smoking, alcohol, energy intake, intakes of fibers, processed meat and soft drinks, and personal history of hypertension |
| Doo et al., 2014 (25) | 39,042 (women) | Multiethnic Cohort Study (MEC) | 45–75 | 4051 | 1993–6 | 14 | Age, race, education, BMI, physical activity, smoking, alcohol, energy intake, intakes of fibers, processed meat, and soft drinks, and personal history of hypertension |
| Hepatocellular carcinoma incidence | |||||||
| Petrick et al., 2015 (26) | 1,212,893 (both) | Liver Cancer Pooling Project (LCPP)1 | — | 860 | — | — | Age, sex, race, study of origin, BMI, smoking, and alcohol |
| Setiawan et al., 2015 (27) | 162,022 (both) | Multiethnic Cohort Study (MEC) | 45–75 | 451 | 1993–6 | 18 | Age, sex, race, education, BMI, smoking, alcohol, and personal history of diabetes |
| Endometrial cancer incidence | |||||||
| Giri et al., 2011 (28) | 45,696 | Women's Health Initiative-Observational Study (WHI-OS) | 50–79 | 427 | 1993–8 | 12 | Age, race, BMI, smoking, and use of oral contraceptive or hormone replacement therapy |
| Gunter et al., 2012 (29) | 226,732 | National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study | 50–71 | 1486 | 1995–6 | 11 | Age, BMI, physical activity, smoking, personal history of diabetes, use of oral contraceptive or hormone replacement therapy, age at menarche, age at first child's birth, parity, and age at menopause |
| Je et al., 2011 (30) | 67,470 | Nurses’ Health Study (NHS) | 34–59 | 672 | 1980 | 26 | Age, BMI, smoking, alcohol, energy intake, use of oral contraceptive or hormone replacement therapy, age at menarche, age at last birth, parity, and age at menopause |
| Uccella et al., 2013 (31) | 23,356 | Iowa Women's Health Study (IWHS) | 55–69 | 542 | 1986 | 20 | Age, BMI, waist-to-hip ratio, smoking, alcohol, personal histories of hypertension or diabetes, use of hormone replacement therapy, age at menarche, and age at menopause |
| Melanoma incidence | |||||||
| Loftfield et al., 2015 (32) | 447,357 (both) | National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study | 50–71 | 2904 | 1995–6 | 11 | Age, sex, education, BMI, physical activity, smoking, alcohol, family history of cancer, and July erythemal exposure |
| Wu et al., 2015 (33) | 39,424 (men) | Health Professionals Follow-up Study (HPFS) | 40–75 | 771 | 1986 | 22 | Age, BMI, physical activity, smoking, alcohol, energy intake, personal history of nonskin cancer, family history of melanoma, natural hair color, number of moles on legs and arms, sunburn reaction as a child or adolescent, number of blistering sunburns, time spent in direct sunlight since high school, and cumulative ultraviolet flux since baseline |
| Wu et al., 2015 (33) | 163,886 (women) | Nurses’ Health Study (NHS) and Nurses’ Health II Study (NHS-II) | 30–55 and 25–42 | 1483 | 1980 and 1991 | 28 and 18 | Age, BMI, physical activity, smoking, alcohol, energy intake, personal history of nonskin cancer, family history of melanoma, use of hormone replacement therapy, menopausal status, natural hair color, number of moles on legs and arms, sunburn reaction as a child or adolescent, number of blistering sunburns, time spent in direct sunlight since high school, and cumulative ultraviolet flux since baseline |
| Wu et al., 2015 (34) | 66,484 (women) | Women's Health Initiative-Observational Study (WHI-OS) | 50–79 | 398 | 1993–8 | 12 | Age, education, income, region of residence, height, waist-to-hip ratio, smoking, alcohol, personal history of nonmelanoma skin cancer, skin reaction to sun, summer sunlight exposure at age 30, use of sunscreen, and use of aspirin |
| Nonmelanoma skin cancer incidence | |||||||
| Abel et al., 2007 (35) | 93,676 (women) | Women's Health Initiative-Observational Study (WHI-OS) | 50–79 | 7775 | 1993–8 | 12 | Age, education, income, region of residence, BMI, smoking, alcohol, intake of β-carotene, and use of hormone replacement therapy |
| Song et al., 2012 (36) | 39,976 (men) | Health Professionals Follow-up Study (HPFS) | 40–75 | 9727 | 1986 | 22 | Age, BMI, physical activity, smoking, personal history of nonskin cancer, natural hair color, number of moles, sunburn reaction as a child, number of blistering sunburns, ultraviolet index at birth, sunlight exposure at age 15 and age 30 |
| Song et al., 2012 (36) | 72,921 (women) | Nurses’ Health Study (NHS) | 30–55 | 15,273 | 1984 | 24 | Age, BMI, physical activity, smoking, personal history of nonskin cancer, natural hair color, number of moles, sunburn reaction as a child, number of blistering sunburns, ultraviolet index at birth, sunlight exposure at age 15 and age 30 |
The Liver Cancer Pooling Project (LCPP) included National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study, Agricultural Health Study (AHS), United States Radiologic Technologists Study (USRTS), Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Women's Health Study (WHS), Cancer Prevention Study-II (CPS-II) Nutrition Cohort, Iowa Women's Health Study (IWHS), Black Women's Health Study (BWHS), and Women's Health Initiative (WHI).
We used the method of Hamling et al. (46) to account for within-study covariance between risk estimates relating to the same reference category or to combine results for studies (12, 15, 16, 31, 36) that reported estimates by category of disease (e.g., coronary heart disease and stroke, type I and type II endometrial cancer, basal cell and squamous cell skin cancer).
We used median or mean values to represent each category of coffee consumption, when reported; alternatively we used the midpoint of the category. When the highest coffee consumption category was open-ended, we assumed it had the same width of the adjacent one.
Statistical analysis
We used a random-effects meta-analysis to estimate the pooled RRs of any caffeinated coffee consumption compared with no consumption. We also performed a 2-stage random-effects dose–response meta-analysis to evaluate the shape of the relation (cups per day), according to the method proposed by Greenland and Longnecker (47) and Orsini et al. (48), which has been applied in a similar context by Crippa et al. (42). Briefly, the 2-stage approach consisted of: 1) fitting a restricted cubic spline model with 3 knots at fixed percentiles (25%, 50%, and 75%) of the coffee distribution for each study (the restricted spline model was fit with a generalized least-squares regression model taking into account the correlation within each set of published RRs) (42, 48); and 2) combining the study-specific estimates obtained in the previous step using the maximum likelihood method in a multivariate random-effects meta-analysis (48, 49). In the spline model, an extra binary term (consumption/no consumption) was added to take into account spike at zero (i.e., the proportion of individuals having zero exposure) for coffee consumption (50).
Statistical heterogeneity among studies was quantified using the I2 statistic (51). In particular, I2 values <25% indicated low heterogeneity, whereas values >75% indicated high heterogeneity. Publication bias was assessed by the Egger regression test (52).
Under the assumption that the results of meta-analyses reflect causal and unbiased associations, we estimated, for each outcome, the fraction and the number of cases/deaths that would be attributable to lack of coffee consumption. These can be interpreted as the fraction and number of cases/deaths that were avoided based on actual consumption patterns. In particular, 2 different counterfactual prevalences of caffeinated coffee consumption were considered: 1) maximum prevalence reduction scenario assuming no coffee consumption (53); and 2) shifted prevalence consumption scenario indicating that all coffee drinkers would reduce their consumption by 1 cup/d (53, 54). The prevalence of US coffee consumption was obtained by combining data from multiple nonconsecutive 24-h dietary recalls and an FFQ of 2003–2004 and 2005–2006 NHANES surveys (55).
Coffee prevalence estimates were computed by using SAS (version 9.4; SAS Institute) according to the method described by Loftfield et al. (56). Meta-analyses were conducted with the dosresmeta (57) and metafor (58) packages in R (59).
Results
Study description
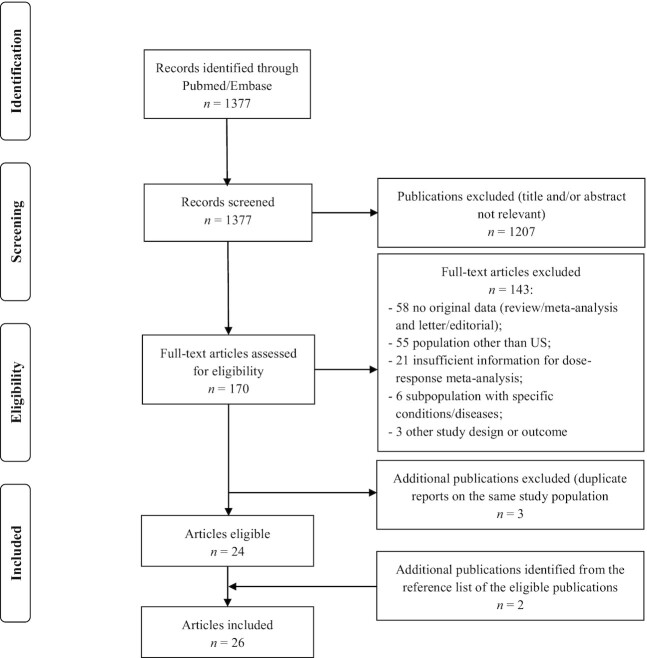
The literature search identified 1377 articles; of these 1207 were excluded after review of title or abstract, leaving 170 articles assessed for eligibility (Figure 1). Twenty-seven articles were selected after the exclusion of 143 articles for ≥1 of the following reasons: not reporting original results (58 articles); not conducting in the United States (55 articles); not reporting sufficient information for a dose–response meta-analysis (21 articles); analyzing subpopulations with specific conditions/diseases (6 articles); or not prospective design or different outcomes (3 articles). We excluded 3 additional articles that were duplicate reports on the same population. In addition, we included 2 articles identified from the reference list of the eligible pool.
FIGURE 1.
Flowchart of studies selection on caffeinated coffee consumption and selected outcomes.
Thus, the meta-analysis included 26 independent prospective studies corresponding to 42 distinct cohorts as reported in Table 1. In particular, 7 studies (11, 12, 15, 16, 13, 14, 17) (10 cohorts) comprised 24,606 CVD cases/deaths and 679,333 participants, 8 studies (18–25) (11 cohorts) 26,331 T2D cases and 372,706 participants, 2 studies (26, 27) (10 cohorts) 1311 HCC cases and 1,374,915 participants, 4 studies (31, 28–30) (4 cohorts) 3127 endometrial cancer cases and 363,254 participants, 3 studies (32–34) (4 cohorts) 5556 melanoma cases and 717,151 participants, and 2 studies (36, 35) (3 cohorts) 32,775 nonmelanoma skin cancer cases and 206,573 participants.
Any consumption meta-analysis
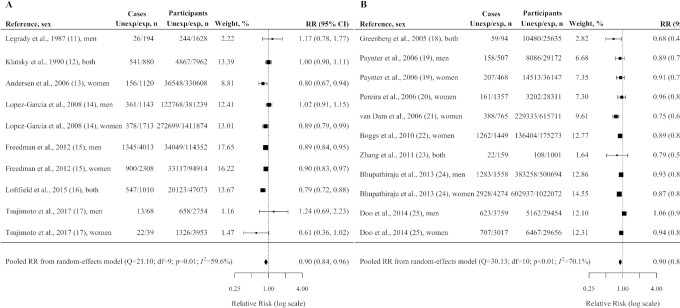
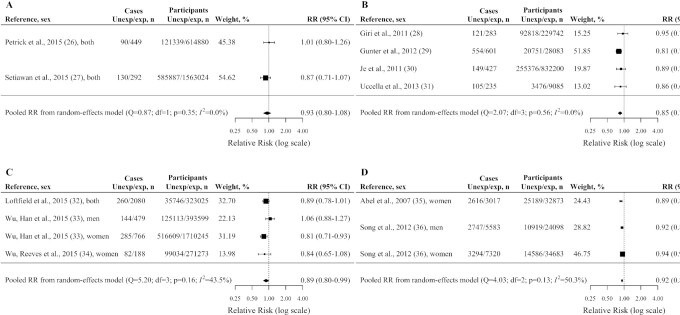
Figure 2 reports pooled estimates for CVD incidence/mortality and TD2 incidence, Figure 3 reports pooled estimates for selected cancer incidence. Compared with no consumption, (caffeinated) coffee drinkers showed a significant reduced risk of CVD incidence/mortality with a pooled RR of 0.90 (95% CI: 0.84, 0.96; Figure 2A), T2D incidence with a pooled RR of 0.90 (95% CI: 0.85, 0.96; Figure 2B), endometrial cancer incidence with a pooled RR of 0.85 (95% CI: 0.78, 0.92; Figure 3A), melanoma incidence with a pooled RR of 0.89 (95% CI: 0.80, 0.99; Figure 3B), and nonmelanoma skin cancer incidence with a pooled RR of 0.92 (95% CI: 0.89, 0.95; Figure 3C). Coffee drinkers showed a nonsignificant reduced risk of HCC incidence (pooled RR = 0.93; 95% CI: 0.80, 1.08; Figure 3D).
FIGURE 2.
Pooled adjusted RRs and corresponding 95% CIs (from random-effects meta-analysis) of cardiovascular disease incidence/mortality (A) and type 2 diabetes incidence (B) according to caffeinated coffee consumption (any vs. no consumption). United States, 1987–2017. exp, represented people with any consumption; Unexp, represented people with no consumption.
FIGURE 3.
Pooled adjusted RRs and corresponding 95% CIs (from random-effects meta-analysis) of hepatocellular carcinoma incidence (A), endometrial cancer incidence (B), melanoma incidence (C), and nonmelanoma skin cancer incidence (D) according to caffeinated coffee consumption (any vs. no consumption). United States, 1987–2017. exp, represented people with any consumption; Unexp, represented people with no consumption. Note: results of the Liver Cancer Pooling Project (LCPP) depicted in (A) included the National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study, Agricultural Health Study (AHS), United States Radiologic Technologists Study (USRTS), Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Women's Health Study (WHS), Cancer Prevention Study-II (CPS-II) Nutrition Cohort, Iowa Women's Health Study (IWHS), Black Women's Health Study (BWHS), and Women's Health Initiative (WHI).
Between-study heterogeneity was moderate for TD2 incidence (I2 = 70.1%; P < 0.01), for CVD incidence/mortality (I2 = 59.6%; P = 0.01), for nonmelanoma skin cancer incidence (I2 = 50.3%; P = 0.13), and for melanoma incidence (I2 = 43.5%; P = 0.16); there was no evidence of between-study heterogeneity for HCC incidence (I2 = 0.0%; P = 0.35) and for endometrial cancer incidence (I2 = 0.0%; P = 0.56). The Egger regression test provided no significant evidence of publication bias, with P values ranging from 0.07 to 0.81.
Dose–response meta-analysis
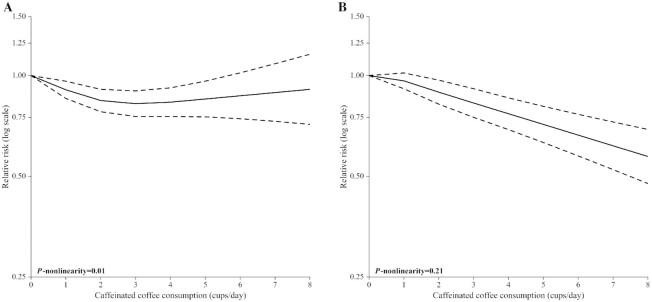
A nonlinear association emerged between caffeinated coffee consumption and CVD incidence/mortality (P-nonlinearity = 0.01; Figure 4A). Compared with no coffee consumption, the pooled RRs for CVD incidence/mortality were 0.91 (95% CI: 0.85, 0.96) for 1 cup/d (a standard American cup of coffee corresponded to ∼120 mL), 0.84 (95% CI: 0.78, 0.91) for 2 cups/d, 0.82 (95% CI: 0.75, 0.90) for 3 cups/d, 0.83 (95% CI: 0.75, 0.92) for 4 cups/d, 0.87 (95% CI: 0.74, 1.02) for 6 cups/d, and 0.91 (95% CI: 0.71, 1.16) for 8 cups/d (Table 2). There was between-study heterogeneity (I2 = 65.6%; P < 0.01).
FIGURE 4.
Pooled dose–response association (from 2-stage random-effects dose–response meta-analysis) between caffeinated coffee consumption (cups per day) and cardiovascular disease incidence/mortality (A) and type 2 diabetes incidence (B). United States, 1987–2017. Coffee consumption was modeled with restricted cubic spline. In the spline model, a binary term (consumption/no consumption) was added to take into account spike at zero for coffee.
TABLE 2.
Pooled adjusted RRs and corresponding 95% CIs1 of selected outcomes according to caffeinated coffee consumption (cups per day). United States, 1987–2017
| Caffeinated coffee consumption, cups/d | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Outcome | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) |
| CVD incidence/mortality (10 cohorts) | 0.91 (0.85, 0.96) | 0.84 (0.78, 0.91) | 0.82 (0.75, 0.90) | 0.83 (0.75, 0.92) | 0.85 (0.75, 0.96) | 0.87 (0.74, 1.02) | 0.89 (0.73, 1.08) | 0.91 (0.71, 1.16) |
| T2D incidence (11 cohorts) | 0.96 (0.91, 1.02) | 0.89 (0.82, 0.97) | 0.83 (0.75, 0.91) | 0.77 (0.69, 0.86) | 0.71 (0.63, 0.81) | 0.66 (0.58, 0.77) | 0.62 (0.52, 0.73) | 0.57 (0.48, 0.69) |
| HCC incidence (10 cohorts2) | 1.02 (0.72, 1.45) | 0.82 (0.52, 1.29) | 0.71 (0.52, 0.96) | 0.61 (0.47, 0.80) | 0.53 (0.35, 0.78) | 0.45 (0.25, 0.81) | 0.39 (0.18, 0.86) | 0.34 (0.12, 0.93) |
| Endometrial cancer incidence (4 cohorts) | 0.94 (0.84, 1.06) | 0.91 (0.81, 1.02) | 0.82 (0.73, 0.92) | 0.74 (0.64, 0.85) | 0.67 (0.55, 0.81) | 0.60 (0.47, 0.77) | 0.55 (0.40, 0.74) | 0.49 (0.34, 0.71) |
| Melanoma incidence (4 cohorts) | 0.91 (0.81, 1.03) | 0.89 (0.77, 1.03) | 0.86 (0.75, 0.98) | 0.83 (0.72, 0.95) | 0.79 (0.67, 0.94) | 0.76 (0.62, 0.95) | 0.73 (0.56, 0.96) | 0.71 (0.51, 0.98) |
| Nonmelanoma skin cancer incidence (3 cohorts) | 0.96 (0.93, 0.98) | 0.92 (0.89, 0.94) | 0.88 (0.86, 0.91) | 0.85 (0.82, 0.88) | 0.82 (0.78, 0.86) | 0.78 (0.73, 0.84) | 0.75 (0.69, 0.83) | 0.73 (0.65, 0.82) |
From 2-stage random-effects dose–response meta-analysis. Coffee consumption was modeled with restricted cubic spline. In the spline model, a binary term (consumption/no consumption) was added to take into account spike at zero for coffee. CVD, cardiovascular disease; HCC, hepatocellular carcinoma; T2D, type 2 diabetes.
The Liver Cancer Pooling Project (LCPP) included the National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study, Agricultural Health Study (AHS), United States Radiologic Technologists Study (USRTS), Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Women's Health Study (WHS), Cancer Prevention Study-II (CPS-II) Nutrition Cohort, Iowa Women's Health Study (IWHS), Black Women's Health Study (BWHS), and Women's Health Initiative (WHI).
For other outcomes considered, we found no evidence of nonlinear relations (Figure 4B, Figure 5). In particular, the pooled RRs for T2D incidence were 0.96 (95% CI: 0.91, 1.02) for 1 cup/d, 0.89 (95% CI: 0.82, 0.97) for 2 cups/d, 0.83 (95% CI: 0.75, 0.91) for 3 cups/d, 0.77 (95% CI: 0.69, 0.86) for 4 cups/d, 0.66 (95% CI: 0.58, 0.77) for 6 cups/d, and 0.57 (95% CI: 0.48, 0.69) for 8 cups/d. There was between-study heterogeneity (I2 = 71.3%; P < 0.01).
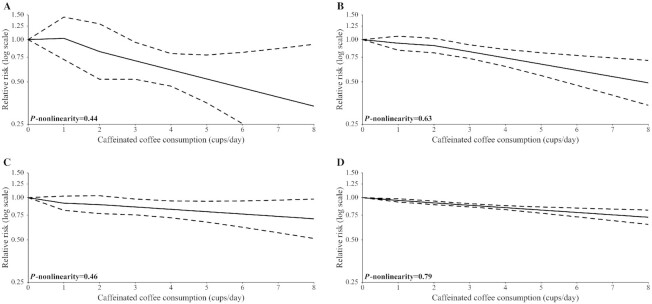
FIGURE 5.
Pooled dose–response association (from 2-stage random-effects dose–response meta-analysis) between caffeinated coffee consumption (cups per day) and hepatocellular carcinoma incidence (A), endometrial cancer incidence (B), melanoma incidence (C), and nonmelanoma skin cancer incidence (D). United States, 1987–2017. Coffee consumption was modeled with restricted cubic spline. In the spline model, a binary term (consumption/no consumption) was added to take into account spike at zero for coffee. Note: results of the Liver Cancer Pooling Project (LCPP) depicted in (A) included the National Institutes of Health-American Association of Retired Persons (NIH-AARP) Diet and Health Study, Agricultural Health Study (AHS), United States Radiologic Technologists Study (USRTS), Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Women's Health Study (WHS), Cancer Prevention Study-II (CPS-II) Nutrition Cohort, Iowa Women's Health Study (IWHS), Black Women's Health Study (BWHS), and Women's Health Initiative (WHI).
The pooled RRs for HCC incidence were 1.02 (95% CI: 0.72, 1.45) for 1 cup/d, 0.82 (95% CI: 0.52, 1.29) for 2 cups/d, 0.71 (95% CI: 0.52, 0.96) for 3 cups/d, 0.61 (95% CI: 0.47, 0.80) for 4 cups/d, 0.45 (95% CI: 0.25, 0.81) for 6 cups/d, and 0.34 (95% CI: 0.12, 0.93) for 8 cups/d. Between-study heterogeneity was moderate (I2 = 48.4%; P = 0.09).
The pooled RRs for endometrial cancer incidence were 0.94 (95% CI: 0.84, 1.06) for 1 cup/d, 0.91 (95% CI: 0.81, 1.02) for 2 cups/d, 0.82 (95% CI: 0.73, 0.92) for 3 cups/d, 0.74 (95% CI: 0.64, 0.85) for 4 cups/d, 0.60 (95% CI: 0.47, 0.77) for 6 cups/d, and 0.49 (95% CI: 0.34, 0.71) for 8 cups/d. There was no evidence of between-study heterogeneity (I2 = 0.0%; P = 0.72).
The pooled RRs for melanoma incidence were 0.91 (95% CI: 0.81, 1.03) for 1 cup/d, 0.89 (95% CI: 0.77, 1.03) for 2 cups/d, 0.86 (95% CI: 0.75, 0.98) for 3 cups/d, 0.83 (95% CI: 0.72, 0.95) for 4 cups/d, 0.76 (95% CI: 0.62, 0.95) for 6 cups/d, and 0.71 (95% CI: 0.51, 0.98) for 8 cups/d. There was no evidence of between-study heterogeneity (I2 = 0.0%; P = 0.65).
The pooled RRs for nonmelanoma skin cancer incidence were 0.96 (95% CI: 0.93, 0.98) for 1 cup/d, 0.92 (95% CI: 0.89, 0.94) for 2 cups/d, 0.88 (95% CI: 0.86, 0.91) for 3 cups/d, 0.85 (95% CI: 0.82, 0.88) for 4 cups/d, 0.78 (95% CI: 0.73, 0.84) for 6 cups/d, and 0.73 (95% CI: 0.65, 0.82) for 8 cups/d. There was low between-study heterogeneity (I2 = 4.5%; P = 0.61).
Avoided fraction estimates
In the United States, 25.3% (corresponding to 52.2 million individuals) did not consume coffee, 40.6% (83.3 million) consumed 1 cup/d, 17.3% (35.7 million) 2 cups/d, 8.6% (17.8 million) 3 cups/d, 3.6% (7.4 million) 4 cups/d, 1.8% (3.7 million) 5 cups/d, and 3% (6 million) consumed ≥6 cups/d (Table 3).
TABLE 3.
Prevalence of caffeinated coffee consumption in the US population. NHANES, 2003–2004 and 2005–2006
| Caffeinated coffee consumption,1 cups/d | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No consumption | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||||||||
| US population | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
| Overall | 52.2 | 25.3 | 83.8 | 40.6 | 35.7 | 17.3 | 17.8 | 8.6 | 7.4 | 3.6 | 3.7 | 1.8 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 |
| Men | 22.2 | 23.6 | 36.3 | 38.5 | 17.2 | 18.2 | 8.6 | 9.1 | 4.5 | 4.7 | 2.3 | 2.4 | 1.1 | 1.2 | 1.1 | 1.2 | 1.1 | 1.2 |
| Women | 30.0 | 26.7 | 47.5 | 42.3 | 18.5 | 16.5 | 9.2 | 8.2 | 3.0 | 2.7 | 1.5 | 1.3 | 0.9 | 0.8 | 0.9 | 0.8 | 0.9 | 0.8 |
Absolute numbers, n expressed in million.
The fractions of cases/deaths avoided because of caffeinated coffee consumption (maximum prevalence reduction scenario) were 8.1% for CVD and T2D, 5.5% for HCC, 12.4% for endometrial cancer, 9.0% for melanoma, and 6.4% for nonmelanoma skin cancer. These figures correspond to 67,520 avoidable CVD cases/deaths, 121,500 T2D cases, 2355 HCC cases, 8137 endometrial cases, 9032 melanoma cases, and 345,600 nonmelanoma cases (Table 4).
TABLE 4.
Attributable fraction (AF) and number of cases or deaths1 avoided of selected outcomes according to 2 different counterfactual prevalences of caffeinated coffee consumption. United States, 1987–2017 and NHANES 2003–2004 and 2005–2006
| Maximum prevalence reduction scenario | Shifted prevalence consumption scenario | |||
|---|---|---|---|---|
| Outcome | AF, % | Cases or deaths avoided, n | AF, % | Cases or deaths avoided, n |
| CVD incidence/mortality | 8.1 | 67,520 | 4.5 | 41,145 |
| T2D incidence | 8.1 | 121,500 | 3.8 | 57,000 |
| HCC incidence | 5.5 | 2355 | 4.8 | 2055 |
| Endometrial cancer incidence | 12.4 | 8137 | 3.6 | 2362 |
| Melanoma incidence | 9.0 | 9032 | 4.0 | 4014 |
| Nonmelanoma skin cancer incidence | 6.4 | 345,600 | 2.7 | 145,800 |
According to disease incidence/mortality statistics of the American Heart Association, Centers for Disease Control and Prevention, and American Cancer Society. CVD, cardiovascular disease; HCC, hepatocellular carcinoma; T2D, type 2 diabetes.
The fractions of cases/deaths avoided under the shifted prevalence consumption scenario were 4.5% (∼41,145 avoidable cases/deaths) for CVD, 3.8% (57,000 cases) for T2D, 4.8% (2055 cases) for HCC, 3.6% (2362 cases) for endometrial cancer, 4.0% (4014 cases) for melanoma, and 2.7% (145,800 cases) for nonmelanoma cancer.
Discussion
This meta-analysis of 26 US prospective studies, including 42 distinct cohorts, indicates a significant inverse association between caffeinated coffee consumption and CVD incidence/mortality, T2D incidence, endometrial cancer incidence, melanoma incidence, and nonmelanoma skin cancer incidence. Similarly, coffee consumption was inversely associated with HCC incidence, without reaching the level of statistical significance. The dose–response relation was nonlinear uniquely for CVD incidence/mortality. In particular, the largest risk reduction for CVD incidence/mortality was observed for 3–4 cups/d and no risk reduction was observed thereafter. Conversely, the risk for other outcomes decreased linearly over the whole coffee consumption range. Assuming these associations are causal and the risk estimates unbiased, the actual patterns of coffee consumption would account for a fraction of avoided cases/deaths ranging from 6% to 12% according to the outcome considered.
Comparable nonlinear associations between coffee consumption and CVD incidence/mortality in the US population were reported in previous dose–response meta-analyses (37, 38, 42) where no further risk reduction was observed for a consumption of ≥4 cups/d. Likewise for other outcomes, no evidence of nonlinear relation emerged in previous reviews and meta-analyses (39–41, 60), not restricted to the US population.
Coffee is widespread in the United States, with three-quarters of the population being regular consumers. In the 2018/2019 fiscal year, US coffee consumption amounted to nearly 26.5 million 60-kg bags (61). Habitual coffee consumers seem to develop a partial tolerance to the effects of caffeine (62). Recent reviews reported no association between caffeine levels and adverse effect in healthy adults (63, 64). Furthermore, coffee contains other bioactive compounds, which acting synergically could be responsible for some beneficial health effects. Various studies found an inverse association between coffee consumption and the concentrations of systemic immune and inflammatory markers (65, 66). Phenolic compounds (i.e., chlorogenic, caffeic, ferulic, and cumaric acids) and diterpenes (i.e., cafestol and kahweol) have an important role in preventing, delaying, and protecting against oxidative stress, which can damage cells, proteins, and DNA. A recent umbrella review of the evidence across meta-analyses of observational and interventional studies on the association between coffee consumption and any outcome concluded that “coffee consumption seems generally safe within usual levels of intake” (6). In our study, we estimated an amount of >550,000 avoided cases/deaths under the maximum prevalence reduction scenario, and >250,000 under the shifted prevalence consumption scenario considering all outcomes jointly.
Our study has several strengths. First, the dose–response meta-analytic approach provides a complete description of the relation between coffee consumption and outcomes considered. Second, including studies with prospective design only should have minimized potential selection and recall biases. Another strength is the large number of cases/deaths, which should guarantee stable estimates. Lastly, we did not find evidence of publication bias.
A limitation consists of the low number of studies included in the meta-analyses for detecting publication bias. In particular, the statistical power of the Egger test is limited when the number of studies is <10 (67). Only the meta-analysis of CVD and T2D outcomes included >10 cohorts. A second limitation is the potential misclassification of the exposure due to self-reporting coffee consumption. We cannot exclude the presence of residual confounding from observational study design. However, we included only studies adjusted for sex, age, and smoking—the most important potential confounders for coffee consumption; in addition, most of the studies were further adjusted for major risk factors of the corresponding outcomes (e.g., physical activity and diet for CVD; BMI for T2D; alcohol use for HCC; oral contraceptive or replacement therapy for endometrial cancer; sunlight exposure for melanoma and nonmelanoma skin cancer) limiting this type of bias. Moreover, studies included were not adjusted for the use of additives (e.g., milk, cream, sugar) which might contribute to the variability of the results. Lastly, for the purpose of obtaining a more reliable estimation of coffee consumption in United States, we used surveys of NHANES 2003–2004 and 2005–2006, which were the only surveys that allowed combining information of multiple nonconsecutive 24-h dietary recalls and an FFQ. Over the years, the prevalence of coffee consumption might have changed. However, Loftfield et al. (56) showed no significant difference (P = 0.09) of adjusted means of coffee intakes over the NHANES survey cycle (i.e., from 2003–2004 to 2011–2012).
In conclusion, this study confirms the beneficial health effects of caffeinated coffee consumption in the US population on CVD, T2D, HCC, endometrial cancer, melanoma, and nonmelanoma skin cancer, and quantifies their possible magnitude.
ACKNOWLEDGEMENTS
We thank Dr A Crippa for his valuable comments.
The authors’ responsibilities were as follows—PB: conceptualized the research idea; MDM and FB: reviewed the literature; MDM: conducted the statistical analysis and drafted the first version of the manuscript; FB: contributed to interpretation of the results; FB, CLV, and EN: drafted the final version of the manuscript; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: CVD, cardiovascular disease; HCC, hepatocellular carcinoma; T2D, type 2 diabetes.
Contributor Information
Matteo Di Maso, Department of Clinical Sciences and Community Health, Branch of Medical Statistics, Biometry and Epidemiology “G.A. Maccacaro,” Università degli Studi di Milano, Milan, Italy.
Paolo Boffetta, Tish Cancer Institute, Ichan School of Medicine at Mount Sinai, New York, NY, USA; Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.
Eva Negri, Department of Biomedical and Clinical Sciences, Università degli Studi di Milano, Sacco Hospital, Milan, Italy.
Carlo La Vecchia, Department of Clinical Sciences and Community Health, Branch of Medical Statistics, Biometry and Epidemiology “G.A. Maccacaro,” Università degli Studi di Milano, Milan, Italy.
Francesca Bravi, Department of Clinical Sciences and Community Health, Branch of Medical Statistics, Biometry and Epidemiology “G.A. Maccacaro,” Università degli Studi di Milano, Milan, Italy.
References
- 1. Jeszka-Skowron M, Krawczyk M, Zgola-Grzeskowiak A. Determination of antioxidant activity, rutin, quercetin, phenolic acids and trace elements in tea infusions: influence of citric acid addition on extraction of metals. J Food Compos Anal. 2015;40:70–7. [Google Scholar]
- 2. Noordzij M, Uiterwaal CS, Arends LR, Kok FJ, Grobbee DE, Geleijnse JM. Blood pressure response to chronic intake of coffee and caffeine: a meta-analysis of randomized controlled trials. J Hypertens. 2005;23:921–8. [DOI] [PubMed] [Google Scholar]
- 3. Jee SH, He J, Appel LJ, Whelton PK, Suh I, Klag MJ. Coffee consumption and serum lipids: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol. 2001;153:353–62. [DOI] [PubMed] [Google Scholar]
- 4. Rebello SA, van Dam RM. Coffee consumption and cardiovascular health: getting to the heart of the matter. Curr Cardiol Rep. 2013;15:403. [DOI] [PubMed] [Google Scholar]
- 5. van Dam RM, Feskens EJ. Coffee consumption and risk of type 2 diabetes mellitus. Lancet. 2002;360:1477–8. [DOI] [PubMed] [Google Scholar]
- 6. Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ. 2017;359:j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kempf K, Herder C, Erlund I, Kolb H, Martin S, Carstensen M, Koenig W, Sundvall J, Bidel S, Kuha Set al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr. 2010;91:950–7. [DOI] [PubMed] [Google Scholar]
- 8. Carlstrom M, Larsson SC. Coffee consumption and reduced risk of developing type 2 diabetes: a systematic review with meta-analysis. Nutr Rev. 2018;76:395–417. [DOI] [PubMed] [Google Scholar]
- 9. Bravi F, Tavani A, Bosetti C, Boffetta P, La Vecchia C. Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta-analysis of prospective studies. Eur J Cancer Prev. 2017;26:368–77. [DOI] [PubMed] [Google Scholar]
- 10. Alicandro G, Tavani A, La Vecchia C. Coffee and cancer risk: a summary overview. Eur J Cancer Prev. 2017;26:424–32. [DOI] [PubMed] [Google Scholar]
- 11. LeGrady D, Dyer AR, Shekelle RB, Stamler J, Liu K, Paul O, Lepper M, Shryock AM. Coffee consumption and mortality in the Chicago Western Electric Company Study. Am J Epidemiol. 1987;126:803–12. [DOI] [PubMed] [Google Scholar]
- 12. Klatsky AL, Friedman GD, Armstrong MA. Coffee use prior to myocardial-infarction restudied—heavier intake may increase the risk. Am J Epidemiol. 1990;132:479–88. [DOI] [PubMed] [Google Scholar]
- 13. Andersen LF, Jacobs DR, Carlsen MH, Blomhoff R. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women's Health Study. Am J Clin Nutr. 2006;83:1039–46. [DOI] [PubMed] [Google Scholar]
- 14. Lopez-Garcia E, van Dam RM, Li TY, Rodriguez-Artalejo F, Hu FB. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. N Engl J Med. 2012;366:1891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loftfield E, Freedman ND, Graubard BI, Guertin KA, Black A, Huang WY, Shebl FM, Mayne ST, Sinha R. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am J Epidemiol. 2015;182:1010–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsujimoto T, Kajio H, Sugiyama T. Association between caffeine intake and all-cause and cause-specific mortality: a population-based prospective cohort study. Mayo Clin Proc. 2017;92:1190–202. [DOI] [PubMed] [Google Scholar]
- 18. Greenberg JA, Axen KV, Schnoll R, Boozer CN. Coffee, tea and diabetes: the role of weight loss and caffeine. Int J Obes. 2005;29:1121–9. [DOI] [PubMed] [Google Scholar]
- 19. Paynter NP, Yeh HC, Voutilainen S, Schmidt MI, Heiss G, Folsom AR, Brancati FL, Kao WH. Coffee and sweetened beverage consumption and the risk of type 2 diabetes mellitus: the atherosclerosis risk in communities study. Am J Epidemiol. 2006;164:1075–84. [DOI] [PubMed] [Google Scholar]
- 20. Pereira MA, Parker ED, Folsom AR. Coffee consumption and risk of type 2 diabetes mellitus: an 11-year prospective study of 28 812 postmenopausal women. Arch Intern Med. 2006;166:1311–6. [DOI] [PubMed] [Google Scholar]
- 21. van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, caffeine, and risk of type 2 diabetes: a prospective cohort study in younger and middle-aged U.S. women. Diabetes Care. 2006;29:398–403. [DOI] [PubMed] [Google Scholar]
- 22. Boggs DA, Rosenberg L, Ruiz-Narvaez EA, Palmer JR. Coffee, tea, and alcohol intake in relation to risk of type 2 diabetes in African American women. Am J Clin Nutr. 2010;92:960–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Lee ET, Cowan LD, Fabsitz RR, Howard BV. Coffee consumption and the incidence of type 2 diabetes in men and women with normal glucose tolerance: the Strong Heart Study. Nutr Metab Cardiovasc Dis. 2011;21:418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhupathiraju SN, Pan A, Malik VS, Manson JE, Willett WC, van Dam RM, Hu FB. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97:155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doo T, Morimoto Y, Steinbrecher A, Kolonel LN, Maskarinec G. Coffee intake and risk of type 2 diabetes: the Multiethnic Cohort. Public Health Nutr. 2014;17:1328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petrick JL, Freedman ND, Graubard BI, Sahasrabuddhe VV, Lai GY, Alavanja MC, Beane-Freeman LE, Boggs DA, Buring JE, Chan ATet al. Coffee consumption and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma by sex: the Liver Cancer Pooling Project. Cancer Epidemiol Biomarkers Prev. 2015;24:1398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Setiawan VW, Wilkens LR, Lu SC, Hernandez BY, Le Marchand L, Henderson BE. Association of coffee intake with reduced incidence of liver cancer and death from chronic liver disease in the US multiethnic cohort. Gastroenterology. 2015;148:118–25.; quiz e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giri A, Sturgeon SR, Luisi N, Bertone-Johnson E, Balasubramanian R, Reeves KW. Caffeinated coffee, decaffeinated coffee and endometrial cancer risk: a prospective cohort study among US postmenopausal women. Nutrients. 2011;3:937–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gunter MJ, Schaub JA, Xue X, Freedman ND, Gaudet MM, Rohan TE, Hollenbeck AR, Sinha R. A prospective investigation of coffee drinking and endometrial cancer incidence. Int J Cancer. 2012;131:E530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Je Y, Hankinson SE, Tworoger SS, De Vivo I, Giovannucci E. A prospective cohort study of coffee consumption and risk of endometrial cancer over a 26-year follow-up. Cancer Epidemiol Biomarkers Prev. 2011;20:2487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uccella S, Mariani A, Wang AH, Vierkant RA, Cliby WA, Robien K, Anderson KE, Cerhan JR. Intake of coffee, caffeine and other methylxanthines and risk of type I vs type II endometrial cancer. Br J Cancer. 2013;109:1908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loftfield E, Freedman ND, Graubard BI, Hollenbeck AR, Shebl FM, Mayne ST, Sinha R. Coffee drinking and cutaneous melanoma risk in the NIH-AARP diet and health study. J Natl Cancer Inst. 2015;107(2):dju421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu S, Han J, Song F, Cho E, Gao X, Hunter DJ, Qureshi AA. Caffeine intake, coffee consumption, and risk of cutaneous malignant melanoma. Epidemiology. 2015;26:898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu H, Reeves KW, Qian J, Sturgeon SR. Coffee, tea, and melanoma risk among postmenopausal women. Eur J Cancer Prev. 2015;24:347–52. [DOI] [PubMed] [Google Scholar]
- 35. Abel EL, Hendrix SO, McNeeley SG, Johnson KC, Rosenberg CA, Mossavar-Rahmani Y, Vitolins M, Kruger M. Daily coffee consumption and prevalence of nonmelanoma skin cancer in Caucasian women. Eur J Cancer Prev. 2007;16:446–52. [DOI] [PubMed] [Google Scholar]
- 36. Song F, Qureshi AA, Han J. Increased caffeine intake is associated with reduced risk of basal cell carcinoma of the skin. Cancer Res. 2012;72:3282–9. [DOI] [PubMed] [Google Scholar]
- 37. Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129:643–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grosso G, Micek A, Godos J, Sciacca S, Pajak A, Martinez-Gonzalez MA, Giovannucci EL, Galvano F. Coffee consumption and risk of all-cause, cardiovascular, and cancer mortality in smokers and non-smokers: a dose-response meta-analysis. Eur J Epidemiol. 2016;31:1191–205. [DOI] [PubMed] [Google Scholar]
- 39. Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care. 2014;37:569–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lafranconi A, Micek A, Galvano F, Rossetti S, Del Pup L, Berretta M, Facchini G. Coffee decreases the risk of endometrial cancer: a dose-response meta-analysis of prospective cohort studies. Nutrients. 2017;9(11):1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Godos J, Micek A, Marranzano M, Salomone F, Rio DD, Ray S. Coffee consumption and risk of biliary tract cancers and liver cancer: a dose-response meta-analysis of prospective cohort studies. Nutrients. 2017;9(9):950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crippa A, Discacciati A, Larsson SC, Wolk A, Orsini N. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol. 2014;180:763–75. [DOI] [PubMed] [Google Scholar]
- 43. Grosso G, Godos J, Galvano F, Giovannucci EL. Coffee, caffeine, and health outcomes: an umbrella review. Annu Rev Nutr. 2017;37:131–56. [DOI] [PubMed] [Google Scholar]
- 44. World Cancer Research Fund International.. Diet, nutrition, physical activity and cancer: a global perspective—the third expert report. [Internet]. London (UK): World Cancer Research Fund International;2018. [Accessed 2020 Feb]. Available from: https://www.wcrf.org/dietandcancer. [Google Scholar]
- 45. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 46. Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Statist Med. 2008;27:954–70. [DOI] [PubMed] [Google Scholar]
- 47. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- 48. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata Journal. 2006;6:40–57. [Google Scholar]
- 50. Lorenz E, Jenkner C, Sauerbrei W, Becher H. Modeling variables with a spike at zero: examples and practical recommendations. Am J Epidemiol. 2017;185:650–60. [DOI] [PubMed] [Google Scholar]
- 51. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statist Med. 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 52. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ezzati M, Hoorn SV, Rodgers A, Lopez AD, Mathers CD, Murray CJ, Comparative Risk Assessment Collaborating Group. Estimates of global and regional potential health gains from reducing multiple major risk factors. Lancet. 2003;362:271–80. [DOI] [PubMed] [Google Scholar]
- 54. Bosetti C, Tzonou A, Lagiou P, Negri E, Trichopoulos D, Hsieh CC. Fraction of prostate cancer incidence attributed to diet in Athens, Greece. Eur J Cancer Prev. 2000;9:119–23. [DOI] [PubMed] [Google Scholar]
- 55. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Continuous NHANES. [Internet]. [Accessed 2020 Feb]. Available from: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. [Google Scholar]
- 56. Loftfield E, Freedman ND, Dodd KW, Vogtmann E, Xiao Q, Sinha R, Graubard BI. Coffee drinking is widespread in the United States, but usual intake varies by key demographic and lifestyle factors. J Nutr. 2016;146:1762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Crippa A, Orsini N. Multivariate dose-response meta-analysis: the dosresmeta R package. J Stat Softw. [Internet]2016;72. doi:10.18637/jss.v072.c01. [Google Scholar]
- 58. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 59. R Core Team.. R: a language and environment for statistical computing. [Internet]. Vienna (Austria): R Foundation for Statistical Computing; 2018; [cited 2019 Mar 19]. Available from: https://www.R-project.org [Google Scholar]
- 60. Micek A, Godos J, Lafranconi A, Marranzano M, Pajak A. Caffeinated and decaffeinated coffee consumption and melanoma risk: a dose-response meta-analysis of prospective cohort studies. Int J Food Sci Nutr. 2018;69:417–26. [DOI] [PubMed] [Google Scholar]
- 61. Statista. Coffee consumption worldwide from 2012/13 to 2018/19. [Internet]. [Accessed 2020 Feb]. Available from: https://www.statista.com/statistics/292595/global-coffee-consumption. [Google Scholar]
- 62. van Dam RM. Coffee consumption and coronary heart disease: paradoxical effects on biological risk factors versus disease incidence. Clin Chem. 2008;54:1418–20. [DOI] [PubMed] [Google Scholar]
- 63. Doepker C, Franke K, Myers E, Goldberger JJ, Lieberman HR, O'Brien C, Peck J, Tenenbein M, Weaver C, Wikoff D. Key findings and implications of a recent systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Nutrients. 2018;10(10):1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wikoff D, Welsh BT, Henderson R, Brorby GP, Britt J, Myers E, Goldberger J, Lieberman HR, O'Brien C, Peck Jet al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxicol. 2017;109:585–648. [DOI] [PubMed] [Google Scholar]
- 65. Lopez-Garcia E, van Dam RM, Qi L, Hu FB. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr. 2006;84:888–93. [DOI] [PubMed] [Google Scholar]
- 66. Loftfield E, Shiels MS, Graubard BI, Katki HA, Chaturvedi AK, Trabert B, Pinto LA, Kemp TJ, Shebl FM, Mayne STet al. Associations of coffee drinking with systemic immune and inflammatory markers. Cancer Epidemiol Biomarkers Prev. 2015;24:1052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–29. [DOI] [PubMed] [Google Scholar]