ABSTRACT
Effects of isocaloric (sweetness differences but constant calories) preloads and isosweet (caloric differences but constant sweetness) preloads, as well as preloads that were neither isosweet nor isocaloric (sweetness and caloric differences) on subsequent ad libitum meal and total (preload + ad libitum) energy intakes were investigated. Thirty-five crossover studies were eligible for inclusion, representing 116 comparisons (41, isocaloric; 41, isosweet; and 34, neither isosweet nor isocaloric). References of existing reviews and literature from 4 databases were searched. The calculated raw mean differences in ad libitum and total energy intakes were pooled in meta-analyses using a random-effects model and the inverse of the variance as the weighting factor. Energy intakes at an ad libitum meal were significantly lower for low-/no-calorie sweetener (LNCS)–sweetened compared with unsweetened preloads in the isocaloric comparison (−55.5 kcal; 95% CI: −82.9, −28.0 kcal; P < 0.001); however, the difference in energy intake was not significant in additional sensitivity analyses (i.e., removal of comparisons where the matrix was a capsule and when xylitol was the LNCS). For the isosweet comparison, although the pooled energy intake at the ad libitum meal was significantly greater with the LNCS-sweetened preload compared with the caloric sweetener (CS)–sweetened preload (58.5 kcal; 95% CI: 35.4, 81.7 kcal; P < 0.001), the pattern was reversed when total energy intake was considered (−132.4 kcal; 95% CI: −163.2, −101.6 kcal; P < 0.001), explained by only partial compensation from the CS-sweetened preload. The results were similar when assessing ad libitum and total energy intakes when unsweetened compared with CS-sweetened preloads were consumed. Unsweetened or LNCS-sweetened preloads appear to have similar effects on intakes when compared with one another or with CS-sweetened preloads. These findings suggest that LNCS-sweetened foods and beverages are viable alternatives to CS-sweetened foods and beverages to manage short-term energy intake.
Keywords: caloric sweetener, low-calorie sweetener, noncaloric sweetener, food intake, energy intake, postprandial, preload, acute, short-term, ad libitum
Introduction
Consumption of low-/no-calorie sweeteners (LNCSs) from foods and beverages is prevalent in the US. Based on an evaluation of the 2009–2010 and 2011—2012 US NHANES, 25.1% of children and 41.4% of adults reported consuming LNCSs at least once during the 2-d survey (1). A similar prevalence also was observed in an evaluation of adults using the 2007–2008, 2009–2010, and 2011–2012 US NHANES, in which 47.8% of adults reported the intake of at least 1 LNCS-sweetened food or beverage or the addition of an LNCS to a food or beverage (2). LNCSs allow consumers to enjoy sweet-tasting foods without calories from caloric sweeteners (CSs), such as sucrose or glucose. As the caloric value of LNCSs is negligible, the usefulness of LNCSs as substitutes for CSs in managing calories and body weight has garnered much interest over the past decade and remains highly debated within the scientific community, despite the evidence.
A number of investigators have shown that those who consume LNCS-sweetened foods and beverages generally have higher-quality diets (3–8). Several evidence-based reviews that draw only from randomized clinical trials show either a beneficial effect or no detrimental effect on body weight when LNCS-sweetened foods and beverages are consumed compared with either unsweetened or CS-sweetened foods and beverages (9–13). Nevertheless, observational evidence has suggested otherwise (14–20). However, observational studies are known to have significant limitations, including the possibility of reverse causality (10, 21), as individuals who are watching their body weight (e.g., overweight or obese individuals) could be consuming LNCS-sweetened foods and beverages to help manage their body weights (as opposed to the LNCSs causing weight gain in these individuals). Consequently, greater confidence should be drawn from, and more scientific weight should be placed on, findings from evidence-based reviews that rely strictly on gold-standard clinical trials and/or intervention/experimental study designs.
As the body of evidence grows, so, too, do questions on the use of LNCSs. Several scientific and regulatory agencies have taken varied positions on LNCSs, due to differing views on the implications of sweetness on short- and long-term energy intakes (22–24). In a 2017 WHO-sponsored evidence-mapping exercise, 60 acute-feeding studies were identified wherein energy intakes and/or appetite ratings were assessed following the administration of different preloads (10). In these short-term studies, the subjects were provided with a “preload” (i.e., a test food vehicle that was unsweetened or sweetened either with an LNCS or CS) and were then requested to consume an ad libitum meal after a predefined time interval. Either total energy intake (i.e., from the preload and ad libitum meal) or energy intake from the ad libitum meal was subsequently measured. Lohner et al. (10) reported that in 39, 11, and 10 studies, there was no effect, a statistically significant decrease, or a statistically significant increase in energy intake and/or appetite, respectively, following the consumption of a preload sweetened with an LNCS versus a CS or placebo.
Interpretation of studies on the effects of sweetened (e.g., sucrose-sweetened beverage or LNCS-sweetened beverage) preloads and unsweetened preloads (e.g., water) on energy intakes and appetite can be challenging due to differences in study methodology. Insights on effects from sweetness, however, may be gleaned by comparing differences between isocaloric but not isosweet preloads (i.e., comparing preloads that are identical in macronutrient composition but that are either sweetened with an LNCS or unsweetened), isosweet but not isocaloric preloads, and preloads that are neither isocaloric nor isosweet observed in studies with relatively similar study designs. Together, these comparisons may provide further clarity on the effects of sweetness—in the absence or presence of calories—on subsequent energy intakes. While Rogers et al. (11) first suggested that sweetness does not affect acute energy intakes, a more stringent analysis using more rigorous study eligibility criteria is undertaken here, to include an investigation into effects on energy intakes from all permutations of sweetness and caloric preloads.
Methods
Literature search strategy
From existing reviews, a total of 115 short-term intervention studies were identified, 59 by Lohner et al. (10) and 56 by Rogers et al. (11). The full-length publications of these 115 studies were obtained and reviewed for eligibility for inclusion in our systematic evidence-based review and meta-analysis.
As literature searches for LNCS studies were conducted in 2015 and 2016 in the Lohner et al. (10) review and in 2015 in the Rogers et al. (11) review, we conducted an updated literature search on 26 May 2019 to identify additional relevant studies published in or subsequent to 2015 and another updated literature search on 13 April 2020 to identify relevant studies published in or subsequent to 2019. We also conducted a literature search for CS studies on 13 April 2020, with no restrictions on the publication year, as neither Lohner et al. (10) nor Rogers et al. (11) undertook this specific search. Four literature databases (BIOSIS Previews®, CAB ABSTRACTS, Embase®, and MEDLINE) were searched, using the electronic search tool ProQuest Dialog® (ProQuest LLC). Keywords used in each of the literature searches are provided in Supplemental Table 1.
Inclusion and exclusion criteria
For the 59 and 56 human intervention studies that were included in the scoping review by Lohner et al. (10) and the systematic review and meta-analysis by Rogers et al. (11), respectively, study eligibility was assessed by reviewing the full texts. The eligibility of the studies identified in the 3 literature searches we conducted was determined in a stepwise manner by first reviewing the titles, then the abstracts of titles determined to be potentially relevant, and then the full texts of abstracts determined to be potentially relevant. A study was included if it met all of the following inclusion criteria: 1) human intervention study; 2) effects of an unsweetened preload or a preload sweetened with either an LNCS or a CS compared with each other on subsequent, ad libitum food intakes were assessed; 3) direct assessment of ad libitum food intake was conducted (as opposed to the subjective measurement of hunger or appetite); 4) a single preload was administered, or if multiple preloads were administered, effects of the first preload could be isolated; 5) a crossover (within-subject) study design (i.e., within a study, each subject consumed each preload, thus serving as his/her own control); 6) independent effects of each preload on subsequent food intake could be isolated (i.e., results were not confounded by, for example, differences in the volumes/amounts of the preloads, time of consumption prior to the ad libitum meal, etc.); 7) energy intake at a single meal was assessed (i.e., a single meal—either breakfast, lunch, or dinner—had to be offered, ad libitum, either concurrently or shortly after the consumption of the preload); 8) statistical analyses were completed and documented, and sufficient data (either in table or figure format) were provided to permit the inclusion of the study results in a meta-analysis; 9) full-length article (e.g., not an abbreviated study report or a conference abstract); and 10) published in English.
Data extraction and study quality assessment
The following data were extracted from the included studies: study population (i.e., number of subjects and gender); study design (i.e., whether the crossover study was randomized and blinded, as well as the length of the washout period), preload physical form (i.e., whether solid food or liquid beverage), preload amount (grams or milliliters), time interval (minutes) between consumption of the preload and the ad libitum meal testing, sweetener used (none, LNCS or CS, and corresponding dose/concentration), and study findings [namely, whether there were any significant differences in energy intakes when isocaloric preloads (i.e., an LNCS-sweetened vs. unsweetened preload), isosweet preloads (i.e., an LNCS- vs. CS-sweetened preload), or preloads that were neither isosweet nor isocaloric (i.e., an unsweetened vs. CS-sweetened preload) were consumed]. Both ad libitum and total energy intakes (where appropriate) were examined. Total energy intake is defined as the sum of calories from both the preload and ad libitum meal; total energy intakes were examined only if the preloads being compared differed in energy content. Two reviewers equally shared the task of data entry, and a third reviewer verified data entries.
The 14-item “Quality Assessment of Controlled Intervention Studies” developed by the NIH's National, Heart, Lung, and Blood Institute (NHLBI) was used to assess study quality (25). Question 4 of the 14-item quality-appraisal tool, which relates to the blinding of study participants, was omitted due to the nature of this particular evidence-based review; blinding is not possible when comparing an LNCS- or CS-sweetened versus unsweetened preloads, nor is it always achievable despite the intention when comparing an LNCS- versus CS-sweetened preloads, as these may be distinguishable by participants.
Question 10 of the 14-item NHLBI quality-appraisal tool was related to the similarity of other interventions (i.e., similar background treatments) between groups. Given that the objective of our evidence-based review was to assess caloric intakes at an ad libitum meal following consumption of different preloads, there were potential confounders that would have to be controlled for on the day of testing and on the day before testing. Therefore, NHLBI question 10 was modified to revised question 10 and added a new question 11, and studies were appraised on whether potential confounders were controlled for on the day of testing and the day before testing, respectively. For example, in order to be awarded a point for revised question 10, a fasting period and administration of a standardized meal prior to consumption of the preload had to have been explicitly reported. If both criteria were not satisfied, the study was afforded a “no,” thereby scoring zero points for this question. To be awarded a point for the added new question 11, at least 2 of the following 3 confounders had to be controlled: 1) overnight fast and either 2) avoidance of alcohol and/or 3) physical activity restrictions.
According to the NHLBI's guidance on the application of the quality-assessment tool, each question should be rated as “yes,” “no,” or “other” (i.e., “not reported,” “could not determine,” or “not applicable”) for each study, and each study should then be rated for overall quality as either “good,” “fair,” or “poor.” Due to the inherent nature of the studies evaluated herein, the qualitative ratings of “good,” “fair,” or “poor” were too limited to allow for adequate differentiation among studies. To facilitate such differentiation, a quantitative score (either “1” for “yes” or “0” for “no,” “not reported,” or “could not determine”) was assigned to each checklist item, and the overall score for each study was then quantified based on the total number (or percentage) of criteria accounted for.
Statistical analysis
The preloads were categorized as either unsweetened or sweetened with an LNCS or a CS. Several meta-analyses were conducted to better understand how energy intakes at ad libitum meals were affected by the prior consumption of these preloads. For comparisons classified as isosweet but not isocaloric (i.e., LNCS- vs. CS-sweetened preload) and as neither isocaloric nor isosweet (i.e., unsweetened such as water vs. CS-sweetened preload), we separately pooled total energy intakes (defined as the intake of energy from the preload plus the ad libitum meal) as well as the intakes of energy at the ad libitum meal only. For comparisons classified as isocaloric but not isosweet (i.e., LNCS vs. an unsweetened preload), we pooled only the intakes of energy at the ad libitum meal as energy from preloads would be identical.
Comprehensive Meta-Analysis Software (version 2.2.064, Biostat, Inc., Englewood, New Jersey, US) was utilized to conduct meta-analyses and generate forest plots. The pooled effect was the raw difference in means. The raw differences in means were calculated by subtracting the ad libitum energy intake following consumption of the unsweetened preload from that after consumption of the preload sweetened with the LNCS; by subtracting the ad libitum energy intake following consumption of the preload sweetened with a CS from that after consumption of the preload sweetened with an LNCS; and by subtracting the ad libitum energy intake following consumption of the preload sweetened with a CS from that after consumption of the unsweetened preload. For comparisons involving a preload sweetened with a CS, the differences in total energy intakes (preload + ad libitum meal) also were calculated and pooled. For all meta-analyses, a random-effects model, which takes into consideration the variability in response both within and between studies, was used, according to the methods described by DerSimonian and Laird (26). The inverse of the variance was used as the weighting factor. In calculating variance for the difference in energy intakes between interventions, a correlation coefficient of 0.5 was assumed, as recommended by the Agency for Healthcare Research and Quality (27). For interventions in which different demographic groups were enrolled (e.g., obese/lean, male/female, younger/older children), results within the study were first pooled. For interventions with multiple testing conditions (e.g., different volumes of preloads, different time intervals between preload consumption and ad libitum meal testing, different CS and/or LNCS), each comparison was considered an independent comparison; however, as all studies were crossover in design, the total number of participants was divided by the number of comparisons and subsequently used in the calculation of the variance so as to avoid double-counting subjects and a unit-of-analysis error (28). Publication bias was assessed using the trim-and-fill method developed by Duval and Tweedie (29); for all assessments of publication bias, the default was to look for missing studies on the opposite side of the pooled effect (e.g., if the pooled effect was a reduction in energy intake, missing studies were searched for to the right of the pooled effect). Heterogeneity was assessed by considering the I2 statistic, such that values of 0% to 40% were considered unimportant, 30% to 60% represented moderate heterogeneity, 50% to 90% represented substantial heterogeneity, and 75% to 100% represented considerable heterogeneity, according to the Cochrane Handbook for Systematic Reviews of Interventions (30).
Several sensitivity analyses were conducted for study population (adults, children), LNCS examined (aspartame, sucralose, stevia, all other LNCSs, including LNCS mixtures and LNCSs not further detailed), CS examined (sucrose, glucose, all other CSs, including CS mixtures and CSs not further detailed), preload physical form (liquid, solid/semi-solid, capsules), and preload volume (≤355 mL vs. >355 mL). A sensitivity analysis was not conducted for the time interval between preload consumption and ad libitum meal testing, as included studies varied in how “time interval” was defined (e.g., upon initiation of, after, or not adequately defined from preload consumption).
Results
Study identification
The literature searches resulted in the identification of 2937 titles, from which 177 abstracts and ultimately 73 full texts were retrieved (Figure 1). Combined with the 115 articles from the reviews by Lohner et al. (10) and Rogers et al. (11), a total of 188 potentially relevant full-length articles were reviewed for eligibility. Of the 188 articles, 35 were eligible for inclusion in the evidence-based review—16 from Lohner et al. (10), 9 from Rogers et al. (11), 8 from the CS literature search, and 2 from the LNCS literature searches. One of the primary reasons for study exclusion (42 of 188 full-length articles) was that the independent effects on subsequent food intakes of either an LNCS- or a CS-sweetened preload could not be disentangled, given that the preload in these excluded studies consisted of a mixture (e.g., the addition of an LNCS to a CS-sweetened preload to mask taste). A detailed breakdown of the results from each literature search is provided in Supplemental Table 2.
FIGURE 1.
Literature search process to identify studies in which effects of LNCS, CS, or unsweetened preloads on energy intakes were assessed. CS, caloric sweetener; LNCS, low-/no-calorie sweetener.
Overview of included studies
Key attributes and findings of the 35 studies that met the inclusion criteria are summarized in Table 1 (31–65). All studies followed a similar experimental design. Study participants were provided with an unsweetened preload or a preload sweetened with an LNCS or CS on separate test days. After a predefined time interval, participants were invited to consume an ad libitum test meal. Of the 35 studies, 31 were conducted in adults and 4 in children. The time interval between preload consumption and ad libitum meal testing ranged from 0 min (i.e., preload and ad libitum meal co-consumed) to 240 min, with the most common time interval being 1 h. The ad libitum meals were provided as lunch in all but 2 studies. For these 2 studies, the ad libitum meal was (or was assumed to be) breakfast (38, 57). The washout period between test days, which was reported in 28 of the 35 studies, ranged from a single day to 4 wk.
TABLE 1.
Key study attributes and results of the studies in which the effects of CS, LNCS, and UNS preloads on energy intakes were assessed1
| Energy intakes—study results3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PL | LNCS vs. CS | UNS vs. CS | ||||||||||
| Reference | Sample size and gender2 | Study design (WO) | Vehicle (amount) | Time (min) before ad libitum lunch | CS (wt:vol or wt:wt) | LNCS (wt:vol or wt:wt) | UNS | LNCS vs. UNS | Meal only | PL + meal | Meal only | PL + meal |
| Studies in adults | ||||||||||||
| Akhavan et al. (2011)—experiment 1 (31) | 14 M | R, C, DB, X (1 wk) | BEV (300 mL) | 60 bf lunch | — | SUCL (0.04%) | H2O | NSD | — | — | — | — |
| BEV + gelatin (300 mL) | SUCL (0.04%) | H2O | NSD | — | — | — | — | |||||
| Akhavan et al. (2011)—experiment 2 (31) | 15 M | R, C, DB, X (1 wk) | BEV (300 mL) | 60 bf lunch | SUC (25%) | SUCL (0.04%) | — | — | NSD | ↓ | — | — |
| GLU + FRU (25%) | SUCL (0.04%) | — | — | NSD | ↓ | — | — | |||||
| Almiron-Roig andDrewnowski (2003) (32) | 14 M | C, X (1 wk) | BEV (591 mL) | 135 bf lunch | GLU (4.33%) + FRU (4.77%) | — | H2O | — | — | — | NSD | ↓ |
| 18 F | GLU (4.33%) + FRU (4.77%) | — | H2O | — | — | — | NSD | ↓ | ||||
| Anton et al. (2010) (33) | 31 GD NR | C, SB, X (2 d) | Cream cheese (NR) | 20 bf lunch | SUC (NR) | Stevia (NR) | — | — | NSD | NR | — | — |
| ASP (NR) | — | — | NSD | NR | — | — | ||||||
| Björvell and Rössner(1982) (34) | 12 M + F | R, C, DB, X (1 wk) | BEV (30 mL) | 20 bf lunch | GLU (25%) | — | H2O | — | — | — | NSD | NR |
| Glycerol (25%) | — | H2O | — | — | — | ↑ | NR | |||||
| Black et al. (1991) (35) | 20 M | R, C, SB, X (NR) | BEV (280 mL) | 65 bf lunch | — | ASP (0.6%) | H2O | NSD | — | — | — | — |
| Black et al. (1993) (36) | 18 M | R, C, X (1 wk) | BEV (280 mL) | 65 bf lunch | — | ASP (0.12%) | H2O | NSD | — | — | — | — |
| BEV (560 mL) | 65 bf lunch | — | ASP (0.12%) | H2O | NSD | — | — | — | — | |||
| Canty and Chan (1991) (37) | NR, M + F | R, C, DB, X (1 to 4 d) | BEV (200 mL) | 60 bf lunch | SUC (10%) | ASP (0.06%) | H2O | NSD | NSD | NR | NSD | NR |
| SUC (10%) | SACC (0.03%) | H2O | NSD | NSD | NR | |||||||
| Cuomo et al. (2011) (38) | 10 M + F | R, SB, C, X (≥1 wk) | Carbonated BEV (300 mL) | 0 bf solid meal (assumed breakf) | — | ASP (0.04%) + ACE-K (0.04%) | H2O | NSD | — | — | — | — |
| — | ASP (0.04%) + ACE-K (0.04%) | H2O | NSD | — | — | — | — | |||||
| Noncarbonated BEV (300 mL) | 0 bf liquid meal (assumed breakf) | — | ASP (0.04%) + ACE-K (0.04%) | H2O | NSD | — | — | — | — | |||
| — | ASP (0.04%) + ACE-K (0.04%) | H2O | NSD | — | — | — | — | |||||
| DellaValle et al. (2005) (39) | 44 F | C, X (NR) | BEV (360 mL) | w lunch | Cola | Diet cola | H2O | NSD | NSD | ↓ | NSD | ↓ |
| Drewnowski et al. (1994) (40) | 24 M + F | C, X (1 wk) | Cream cheese (500 g) | 180 bf lunch | SUC (10%) | ASP (0.1%) | H2O | ↓ | ↑ | NR | ↑ | NR |
| Drewnowski et al. (1994) (41) | 24 F | C, X (1 wk) | White cheese (500 g) | 180 bf lunch | SUC (10%) | ASP (0.1%) | H2O | NR | NR | NR | NR | NR |
| Farhat et al. (2019) (42) | 30 M + F | R, C, SB, X (4–5 d) | BEV (300 mL) | 30 bf lunch | SUC (20%) | Stevia (0.33%) | H2O | NSD | NSD | NR | NSD | NR |
| Ford et al. (2011) (43) | 8 M + F | R, C, SB, X (≥3 d) | BEV (50 mL) | 120 bf lunch | — | SUCL (0.08%) | H2O | NSD | — | — | — | — |
| Gadah et al. (2016) (44) | 69 M + F | R, C, X (NR) | BEV (300 mL) | 20 bf lunch | SUC (14%) | SUCL (NR) | — | — | ↑ | NR | — | — |
| Kim (2006) (45) | 12 M | R, C, X (≥1 wk) | BEV (400 mL) | 180 bf test meal (assumed lunch) | GLU (18.75%) | SUCL (0.05%) | — | — | NSD | NR | — | — |
| Lavin et al. (2002) (46) | 20 M + F | C, X (≥3 d) | BEV (150 g) | 5 bf lunch | SUC (10%) | — | H2O | — | — | — | NSD | NR |
| Maersk et al. (2012) (47) | 24 GD NR | R, C, X (≥2 wk) | BEV (500 mL) | 240 bf lunch | SUC (11%) | ASP (NR) | H2O | NSD | NSD | ↓ | NSD | ↓ |
| Monsivais et al. (2007) (48) | 37 M + F | C, SB, X (1 wk) | BEV (475 mL) | 140 bf lunch | HFCS (12%) | ASP (NR) | — | — | NSD | ↓ | — | — |
| Ranawana and Henry(2010) (49) | 23 M | R, C, X (≥2 d) | BEV (∼325 mL4) | 60 bf lunch | SUC (3%) | ASP + ACE-K (NR) | — | — | ↑ | NSD | — | — |
| 24 F | R, C, X (≥2 d) | BEV (∼325 mL4) | 60 bf lunch | SUC (3%) | ASP + ACE-K (NR) | — | — | NSD | ↓ | — | — | |
| Rodin (1990) (50) | 24 M + F | R, C, X (1 wk) | BEV (500 mL) | 38 bf lunch | FRU (10%) | ASP (0.05%) | H2O | NSD | ↑ | NSD | ↑ | NSD |
| GLU (10%) | ASP (0.05%) | NSD | ↓ | NSD | ↓ | |||||||
| Rogers and Blundell(1989) (51) | 24 M + F | C, SB, X (1 wk) | Yogurt (235 g) | 65 bf lunch | GLU (21%) | SACC (0.07%) | Plain | ↑ | NR | NSD | NR | NSD |
| Rogers et al. (1988) (52) | 12 M + F | R, C, SB, X (NR) | BEV (200 mL) | 65 bf lunch | GLU (25%) | SACC (0.07%) | H2O | NSD | NSD | NR | ↑ | NR |
| ASP (0.08%) | H2O | NSD | NSD | NR | ||||||||
| ACE-K (0.12%) | H2O | NSD | ↑ | — | ||||||||
| Rogers et al. (1990)—experiment 1 (53) | 12 M + F | C, DB, X (1 wk) | BEV (200 mL) + CAP | 60 bf lunch | — | ASP (0.12%) in BEV | H2O | NSD | — | — | — | — |
| CAP | 60 bf lunch | — | ASP (0.12%) | CAP | ↓ | — | — | — | — | |||
| Rogers et al. (1990)—experiment 2 (53) | 15 M + F | C, DB, X (1 wk) | BEV (200 mL) | 60 bf lunch | — | ASP (0.12%) | H2O | NSD | — | — | — | — |
| CAP | 60 bf lunch | — | ASP (0.12%) | CAP | ↓ | — | — | — | — | |||
| CAP | 60 bf lunch | — | ASP (0.24%) | CAP | ↓ | — | — | — | — | |||
| Rogers et al. (1991) (54) | 16 M + F | C, DB, X (1 wk) | CAP | 60 bf lunch | — | ASP (400 mg) | CAP | ↓ | — | — | — | — |
| Rolls et al. (1990) (55) | 14 M | R, C, X (≥3 d) | BEV (8 oz) | w lunch | SUC (10%) | ASP (0.05%) | H2O | NSD | NSD | ↓ | NSD | ↓ |
| 30 bf lunch | SUC (10%) | ASP (0.05%) | H2O | NSD | NSD | NSD | NSD | NSD | ||||
| 60 bf lunch | SUC (10%) | ASP (0.05%) | H2O | NSD | NSD | NSD | NSD | NSD | ||||
| BEV (16 oz) | w lunch | SUC (10%) | ASP (0.05%) | H2O | NSD | NSD | ↓ | NSD | ↓ | |||
| 30 bf lunch | SUC (10%) | ASP (0.05%) | H2O | NSD | NSD | NSD | NSD | NSD | ||||
| 60 bf lunch | SUC (10%) | ASP (0.05%) | H2O | NSD | NSD | NSD | NSD | NSD | ||||
| Shafer et al. (1987)—experiment 1 (56) | 9 M + F | R, DB, C, X (NR) | BEV (NR) | 60 bf lunch | GLU (25 g) | Xylitol (25g) | H2O | ↓ | NSD | — | NSD | — |
| FRU (25 g) | Xylitol (25g) | H2O | NSD | — | NSD | — | ||||||
| SUC (25 g) | Xylitol (25g) | H2O | NSD | — | NSD | — | ||||||
| Shafer et al. (1987)—experiment 2 (56) | 5 F | NR | BEV (NR) | 60 bf lunch | — | ASP (250 mg/100 mL) | H2O | NSD | — | — | — | — |
| — | Xylitol (5g) | H2O | NSD | — | — | — | — | |||||
| — | Xylitol (15g) | H2O | NSD | — | — | — | — | |||||
| — | Xylitol (25g) | H2O | ↓ | — | — | — | — | |||||
| Soenen and Westerterp-Platenga (2007) (57) | 40 M + F | R, C, SB, X (4 d) | BEV (800 mL) | 50 bf breakfast | SUC (11.3%) | ASP + ACE-K + cyclamate (NR) | — | — | ↑ | ↓ | — | — |
| HFCS (11.3%) | — | ↑ | ↓ | — | — | |||||||
| Stamataki et al. (2020) (58) | 20 M + F | R, C, DB, X (5 d) | BEV (330 mL) | 30 bf lunch | GLU (12.1%) | Stevia (240 ppm) | H2O | ↓ | NSD | ↓ | ↑ | NSD |
| SUC (12.1%) | Stevia (240 ppm) | H2O | ↓ | NSD | ↓ | ↑ | NSD | |||||
| Tey et al. (2017) (59) | 30 M | R, C, DB, X (≥5 d) | BEV (500 mL) | 60 bf lunch | SUC (13%) | ASP (0.09%) | — | — | ↑ | NSD | — | — |
| SUC (13%) | MFE (0.13%) | — | — | ↑ | NSD | — | — | |||||
| SUC (13%) | Stevia (0.07%) | — | — | ↑ | NSD | — | — | |||||
| Vozzo et al. (2002)—experiment 1 (60) | 10 M + F (Nondiabetics) | R, C, SB, X (≥5 d) | BEV (300 mL) | 180 bf lunch | GLU (25%) | — | H2O | — | — | — | ↑ | NR |
| FRU (25%) | — | H2O | — | — | — | ↑ | NR | |||||
| Vozzo et al. (2002)—experiment 2 (60) | 9 M + F (Diabetics) | R, C, SB, X (≥5 d) | BEV (300 mL) | 180 bf lunch | GLU (25%) | — | H2O | — | — | — | NSD | NR |
| FRU (25%) | — | H2O | — | — | — | NSD | NR | |||||
| Woodend and Anderson(2001) (61) | 15 M | R, C, X (NR) | BEV (360 mL) | 60 bf lunch | SUC (21%) | SUCL (0.08%) | H2O | NSD | ↑ | NR | ↑ | NR |
| Studies in children | ||||||||||||
| Anderson et al. (1989) (62) | 20 M + F | R, C, DB, X (1 d) | BEV (300 mL) | 90 bf lunch | SUC (18%) | ASP (0.1%) | — | — | NSD | NR | — | — |
| Bennett et al. (2018) (63) | 28 F | R, C, X (≥1 wk) | BEV (350 mL) | 60 bf lunch | GLU (5.14%) + FRU (4.57%) | — | H2O | — | — | — | NSD | NSD |
| GLU (4.57%) + FRU (6.29%) | — | H2O | — | — | — | ↑ | NSD | |||||
| Hetherington et al. (2000)—experiment 1 (64) | 25 M + F | C, DB, X (2 to 4 wk) | Gelatin dessert (100 g) | 120 bf lunch | SUC (17%) | ASP (0.025%) | — | — | NSD | NR | — | — |
| Hetherington et al. (2000)—experiment 2 (64) | 31 M + F | C, DB, X (2 to 4 wk) | Gelatin dessert (150 or 225 g) | 120 bf lunch | SUC (17%) | ASP (0.025%) | — | — | ↑ | NR | — | — |
| Poirier et al. (2019) (65) | 32 M | R, C, X (NR) | BEV (350 mL) | 60 bf lunch | GLU (5.14%) + FRU (4.57%) | — | H2O | — | — | — | NSD | ↓ |
| GLU (4.57%) + FRU (6.29%) | — | H2O | — | — | — | ↑ | NSD | |||||
1ACE-K, acesulfame potassium; ASP, aspartame; BEV, beverage; bf, before; breakf, breakfast; C, controlled; CAP, capsules; CS, caloric sweetener; DB, double-blind; FRU, fructose; GD, gender distribution; GLU, glucose; HFCS, high-fructose corn syrup; MFE, monk fruit extract; LNCS, low-/no-calorie sweetener; NR, not reported; NSD, no significant differences; PL, preload; R, randomized; SACC, saccharin; SB, single-blind; SUC, sucrose; SUCL, sucralose; UNS, unsweetened; w, with; WO, washout; X, crossover; —, not applicable; ↑, statistically significant increase; ↓, statistically significant decrease.
2The number of participants who completed the study is indicated. When the number of participant “completers” was not indicated, the number of participants randomized is provided.
3For the results on LNCS vs. CS and LNCS vs. UNS comparisons, ↑ indicates that there was a statistically significant increase in energy intake in the LNCS group, relative to the CS or UNS groups, and ↓ indicates that there was a statistically significant decrease in energy intake in the LNCS group, relative to the CS or UNS groups. For the results on UNS vs. CS comparisons, ↑ indicates that there was a statistically significant increase in energy intake in the UNS group, relative to the CS group, and ↓ indicates that there was a statistically significant decrease in energy intake in the UNS group, relative to the CS group.
4The volumes of the CS and LNCS fruit drinks were 349 mL and 325 mL, respectively.
In 5 studies (31, 53, 56, 60, 64), the effects of an LNCS-sweetened preload on subsequent energy intakes were assessed in 2 different cohorts of study participants. Thus, in total, there were 40 independent experiments across the 35 publications, where an “experiment” is defined as a trial in a single cohort of study participants, irrespective of the number of preloads tested in that cohort. In 18 of 40 experiments, the influence of variations in study parameters was assessed, including preload vehicle [experiments 1 and 2 of (53), experiment 1 of (31), 38], preload volume (36, 55), CS used in preload [experiment 2 of (31), experiment 1 of (32), 34, 50, 56, 57, 60], LNCS used in preload [experiment 2 of (33), 37, 52, 56, 59], time interval between preload consumption and ad libitum meal testing (55), and type of ad libitum meal (38).
Across the 40 experiments, there were 116 comparisons, including 41 isocaloric comparisons, 41 isosweet comparisons, and 34 comparisons that were neither isosweet nor isocaloric (i.e., unsweetened vs. CS-sweetened preload). The preload was a beverage (ranging in volume from 30 to 800 mL) in all of the experiments except for 9, which included as the preload vehicle either cream cheese (33, 40, 41), capsules [experiments 1 and 2 of (53), 54], gelatin dessert [experiments 1 and 2 of (64)], or yogurt (51).
Among the 41 isocaloric comparisons, the unsweetened preload was water in all except the following 7 comparisons: plain yogurt (51), capsules [(52), experiments 1 and 2 of (53), 54], or cream cheese (40, 41). Relevant unsweetened preloads were compared with either diet cola (1 comparison) or to preloads sweetened with the following LNCSs: aspartame (23 comparisons), sucralose (3 comparisons), xylitol (4 comparisons), mixture of aspartame and acesulfame-potassium (4 comparisons), saccharin (3 comparisons), stevia (2 comparisons), or acesulfame-potassium (1 comparison).
Among the 41 isosweet comparisons, the preloads compared were sweetened with aspartame versus sucrose (15 comparisons), stevia versus sucrose (4 comparisons), sucralose versus sucrose (3 comparisons), LNCS mixtures versus sucrose (2 comparisons), aspartame versus glucose (2 comparisons), and saccharin versus glucose (2 comparisons). The remaining 13 comparisons were of preloads sweetened with aspartame versus fructose or high-fructose corn syrup (HFCS); sucralose versus glucose or a mixture of glucose and fructose; mixture of LNCS versus HFCS; xylitol versus fructose, glucose, or sucrose; stevia versus glucose; saccharin versus sucrose; monk fruit extract versus sucrose; acesulfame-potassium versus glucose; and diet cola versus regular cola. The most common LNCS and CS used to sweeten preloads were aspartame (19 comparisons) and sucrose (27 comparisons), respectively. Sugar content for CS-sweetened preloads ranged between 10% and 25% sucrose (wt:vol or wt:wt).
Among the 34 unsweetened versus CS-sweetened preload comparisons, the preload vehicle was either a beverage (31 comparisons), cream cheese (2 comparisons), or yogurt (1 comparison). The CS used to sweeten the preload was either sucrose (15 comparisons), glucose (8 comparisons), mixture of glucose and fructose (5 comparisons), fructose (4 comparisons), glycerol (1 comparison), or HFCS (1 comparison).
Methodological robustness of studies
Study quality appraisals resulting from the application of the NHLBI tool are presented in Table 2 (31–65). The percentage of accounted items (from the 14-item modified checklist) across the 40 experiments ranged from 29% to 93%, with 28 of the 40 experiments accounting for ≥50% of the 14 items in the checklist. As all the studies were crossover in design and the preload and ad libitum meal were consumed at the research facility, all studies received points for similar background demographics (question 6), high protocol adherence (question 9), and valid outcome measures (question 12). Method of randomization (question 2), allocation concealment (question 3), blinding of researchers (question 5), and sample-size calculation (question 13), however, were mostly not reported, with these variables accounted for in 3%, 3%, 28%, and 33% of experiments, respectively. In many studies, only 1 value was provided for the number of participants; therefore, it was not clear whether this represented the number enrolled or completed. Nonetheless, in the 60% of experiments that did report on numbers of subjects enrolled and completed, all received points for the acceptability of the participant attrition rate, which was <20% in all these experiments, and for providing reasons for subject attrition (questions 7 and 8, respectively). Thus, given the nature of these studies, which include at least 2 test days, each separated by a given washout period, it seems that subject attrition is generally not a problem. Only in 53% and 45% of experiments were details provided on controlling for potential confounders on and before test days, respectively (questions 10 and 11, respectively).
TABLE 2.
Quality appraisals of studies in which the effects of CS, LNCS, and UNS preloads on energy intakes were assessed1
| Reference | Q1 | Q2 | Q3 | Q5 | Q6 | Q7 | Q8 | Q9 | Q102 | Q113 | Q12 | Q13 | Q14 | Q15 | Total, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akhavan et al. (2011)—experiment 1 (31) | Yes | NR | NR | NR | Yes | NR | NR | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 (64) |
| Akhavan et al. (2011)—experiment 2 (31) | Yes | NR | NR | NR | Yes | NR | NR | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 (64) |
| Almiron-Roig and Drewnowski (2003) (32) | No | NR | NR | NR | Yes | Yes | Yes | Yes | NA | Yes | Yes | Yes | Yes | Yes | 9 (69) |
| Anderson et al. (1989) (62) | Yes | NR | NR | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | 11 (79) |
| Anton et al. (2010) (33) | No | NR | NR | NR | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | Yes | 9 (64) |
| Bennett et al. (2018) (63) | Yes | NR | NR | NR | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | 10 (71) |
| Björvell and Rössner (1982) (34) | Yes | NR | NR | Yes | Yes | Yes | Yes | Yes | NR | NR | Yes | NR | Yes | Yes | 9 (64) |
| Black et al. (1991) (35) | Yes | NR | NR | NR | Yes | NR | NR | Yes | Yes | NR | Yes | Yes | Yes | NR | 7 (50) |
| Black et al. (1993) (36) | Yes | NR | NR | NR | Yes | NR | NR | Yes | Yes | NR | Yes | NR | Yes | NR | 6 (43) |
| Canty and Chan (1991) (37) | Yes | NR | NR | Yes | Yes | NR | NR | Yes | Yes | NR | Yes | Yes | Yes | NR | 8 (57) |
| Cuomo et al. (2011) (38) | Yes | NR | NR | Yes | Yes | NR | NR | Yes | NA | NR | Yes | Yes | Yes | NR | 7 (54) |
| DellaValle et al. (2005) (39) | No | NR | NR | NR | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NR | Yes | No | 8 (57) |
| Drewnowski et al. (1994) (40) | No | NR | NR | NR | Yes | NR | NR | Yes | NA | NR | Yes | NR | Yes | NR | 4 (31) |
| Drewnowski et al. (1994) (41) | No | NR | NR | NR | Yes | Yes | Yes | Yes | NA | NR | Yes | NR | Yes | Yes | 7 (54) |
| Farhat et al. (2019) (42) | Yes | NR | NR | No | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | Yes | 10 (71) |
| Ford et al. (2011) (43) | Yes | NR | NR | No | Yes | Yes | Yes | Yes | NR | Yes | Yes | NR | Yes | Yes | 9 (64) |
| Gadah et al. (2016) (44) | Yes | NR | NR | NR | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 (79) |
| Hetherington et al. (2000)—exp 1 (64) | No | NR | NR | Yes | Yes | NR | NR | Yes | NR | Yes | Yes | NR | Yes | NR | 6 (43) |
| Hetherington et al. (2000)—exp 2 (64) | No | NR | NR | NR | Yes | Yes | Yes | Yes | NR | Yes | Yes | NR | Yes | Yes | 8 (57) |
| Kim (2006) (45) | Yes | NR | NR | NR | Yes | Yes | Yes | Yes | NR | Yes | Yes | NR | Yes | Yes | 9 (64) |
| Lavin et al. (2002) (46) | No | NR | NR | NR | Yes | NR | NR | Yes | NR | NR | Yes | NR | Yes | NR | 4 (29) |
| Maersk et al. (2012) (47) | Yes | NR | NR | NR | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | Yes | Yes | 10 (71) |
| Monsivais et al. (2007) (48) | No | NR | NR | NR | Yes | Yes | Yes | Yes | NR | NR | Yes | Yes | Yes | Yes | 8 (57) |
| Poirier et al. (2019) (65) | Yes | NR | NR | NR | Yes | Yes | Yes | Yes | NR | Yes | Yes | NR | Yes | Yes | 9 (64) |
| Ranawana and Henry (2010) (49) | Yes | NR | NR | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NR | Yes | No | 9 (64) |
| Rodin (1990) (50) | Yes | NR | NR | NR | Yes | Yes | Yes | Yes | NR | NR | Yes | NR | Yes | No | 7 (50) |
| Rogers and Blundell (1989) (51) | No | NR | NR | Yes | Yes | Yes | Yes | Yes | Yes | NR | Yes | NR | Yes | No | 8 (57) |
| Rogers et al. (1988) (52) | No | NR | NR | No | Yes | NR | NR | Yes | Yes | NR | Yes | NR | Yes | NR | 5 (36) |
| Rogers et al. (1990)—exp 1 (53) | No | NR | NR | NR | Yes | NR | NR | Yes | Yes | NR | Yes | NR | Yes | NR | 5 (36) |
| Rogers et al. (1990)—exp 2 (53) | No | NR | NR | Yes | Yes | NR | NR | Yes | Yes | NR | Yes | NR | Yes | NR | 6 (43) |
| Rogers et al. (1991) (54) | No | NR | NR | Yes | Yes | NR | NR | Yes | Yes | NR | Yes | NR | Yes | NR | 6 (43) |
| Rolls et al. (1990) (55) | No | NR | NR | NR | Yes | NR | NR | Yes | NR | NR | Yes | NR | Yes | NR | 4 (29) |
| Shafer et al. (1987)—exp 1 (56) | Yes | NR | NR | Yes | Yes | NR | NR | Yes | NR | NR | Yes | NR | Yes | NR | 6 (43) |
| Shafer et al. (1987)—exp 2 (56) | No | NR | NR | NR | Yes | NR | NR | Yes | NR | NR | Yes | NR | Yes | NR | 4 (29) |
| Soenen and Westerterp-Platenga (2007) (57) | Yes | NR | NR | NR | Yes | Yes | Yes | Yes | NA | Yes | Yes | No | Yes | Yes | 9 (69) |
| Stamataki et al. (2020) (58) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | Yes | 13 (93) |
| Tey et al. (2017) (59) | Yes | NR | NR | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | Yes | No | 10 (71) |
| Vozzo et al. (2002)—exp 1 (60) | Yes | NR | NR | No | Yes | Yes | Yes | Yes | NR | Yes | Yes | NR | Yes | Yes | 9 (64) |
| Vozzo et al. (2002)—exp 2 (60) | Yes | NR | NR | No | Yes | Yes | Yes | Yes | NR | Yes | Yes | NR | Yes | Yes | 9 (64) |
| Woodend and Anderson (2001) (61) | No | NR | NR | NR | Yes | Yes | Yes | Yes | NA | NR | Yes | NR | Yes | No | 6 (46) |
| Total, n (%) | 23 (58) | 1 (3) | 1 (3) | 11 (28) | 40 (100) | 24 (60) | 24 (60) | 40 (100) | 18 (53) | 18 (45) | 40 (100) | 13 (33) | 40 (100) | 20 (50) |
1Questions were as follows: Q1. Was the study described as randomized, a randomized trial, a randomized clinical trial, or an RCT? Q2. Was the method of randomization adequate (i.e., use of randomly generated assignment)? Q3. Was the treatment allocation concealed (so that assignments could not be predicted)? Q4. Were study participants and providers blinded to treatment group assignment? [Omitted because of the nature of the studies and the challenges in blinding (participants can distinguish sweetened from unsweetened; participants likely can also distinguish CS and LNCS)]. Q5. Were the people assessing the outcomes blinded to the participants’ group assignments? Q6. Were the groups similar at baseline on important characteristics that could affect outcomes (e.g., demographics, risk factors, comorbid conditions)? Q7. Was the overall drop-out rate from the study at endpoint 20% or lower of the number allocated to treatment? Q8. Was the differential drop-out rate (between treatment groups) at endpoint 15 percentage points or lower? Q9. Was there high adherence to the intervention protocols for each treatment group? Q10. Were variables on the day of testing controlled (i.e., were participants requested to fast between breakfast and consumption of the preload and/or consume a standardized breakfast)? Q11. Were variables on the day before testing controlled (i.e., was an overnight fast required and were alcohol and/or physical activity restrictions similar in the groups)? Q12. Were outcomes assessed using valid and reliable measures, implemented consistently across all study participants? Q13. Did the authors report that the sample size was sufficiently large to be able to detect a difference in the main outcome between groups with at least 80% power? Q14. Were outcomes reported or subgroups analyzed prespecified (i.e., identified before analyses were conducted)? Q15. Were all randomized participants analyzed in the group to which they were originally assigned (i.e., did they use an intention-to-treat analysis)? CS, caloric sweetener; exp, experiment; LNCS, low-/no-calorie sweetener; NA, not applicable; NR, not reported; Q, question; UNS, unsweetened.
2Q10 was analyzed based on 2 criteria; a “yes” was required for each of the following: 1) participants were requested to fast and 2) participants consumed a standardized breakfast. If the publication did not account for both criteria, the publication received an “NR” for Q10. In the case where the breakfast was the preload or the ad libitum meal, question 10 is NA as the fasting period between the breakfast and preload could not be measured. In this circumstance, overnight fasting was mandatory the day prior to testing and was required in order to score question 11 as a “yes.”
3Q11 was analyzed based on 3 criteria; a “yes” was awarded if: i) participants were requested to fast overnight, AND EITHER 2) physical activity was standardized the night before each testing session OR 3) alcohol consumption was standardized the night before each testing session; otherwise, the publication received an “NR” for Q11.
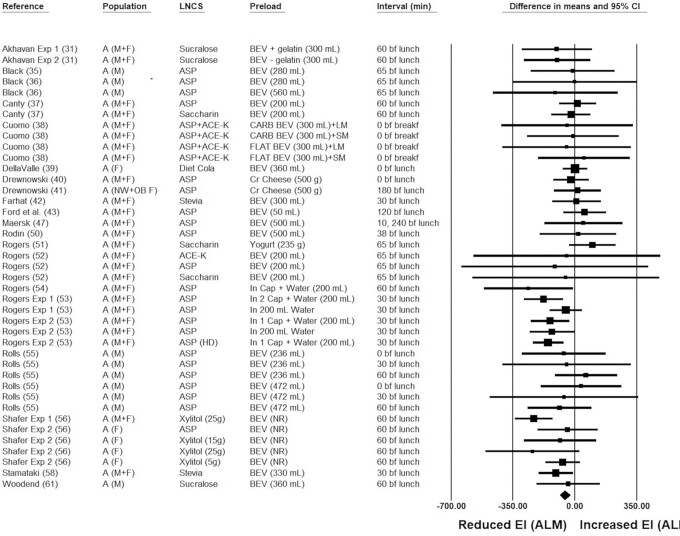
Ad libitum energy intakes following consumption of isocaloric preloads
The pooled raw mean difference in ad libitum energy when an LNCS-sweetened compared with an unsweetened preload was consumed was −55.5 kcal (95% CI: −82.9, −28.0 kcal; P < 0.001), indicating significantly lower ad libitum energy intakes following LNCS-sweetened versus unsweetened preload consumption (Figure 2). Neither heterogeneity (I2 = 21.0; P = 0.121) nor publication bias was detected. In 4 of the 41 isocaloric comparisons, LNCSs (i.e., aspartame) were provided in capsule form, thereby bypassing sweetness detection in the oral cavity. Unlike the other LNCS preload vehicles, such as foods (cream cheese, yogurt) or beverages, in which comparisons would have been considered isocaloric but not isosweet to the unsweetened preloads, aspartame capsules would qualify as both isocaloric and isosweet relative to unsweetened placebo capsule preloads. With these 4 comparisons removed from the meta-analysis, ad libitum energy intake following consumption of the LNCS-sweetened versus unsweetened preloads remained statistically significant, although the magnitude was lower (−35.9 kcal; 95% CI: −60.7, −11.1 kcal; P = 0.005). In 37 of the 41 comparisons, the LNCS used was noncaloric; in the 4 remaining comparisons, 5 to 25 g of xylitol were used as the LNCS, which equates to an energy contribution of 12 to 60 kcal. In none of the other preloads did the LNCS contribute calories; thus, as a sensitivity analysis, the 4 comparisons in which xylitol was used as the LNCS were removed. The pooled effect remained statistically significant (−44.3 kcal; 95% CI: −69.4, −19.2 kcal; P = 0.001). After removing comparisons in which the preload was aspartame capsules (n = 4 comparisons) or xylitol-sweetened beverages (n = 4 comparisons), the pooled effect of the remaining isocaloric comparisons was no longer statistically significant (pooled mean difference = −18.3 kcal; 95% CI: −45.1, 8.5 kcal; P = 0.181; see Supplemental Table 3). Given the different physiological responses to specific LNCSs, and given that the pooled effect is no longer statistically significant when aspartame capsule and xylitol comparisons are removed, effects of LNCS-sweetened preloads on acute energy intake are at least equivalent to unsweetened preloads and do not increase caloric intakes relative to unsweetened preloads. Ad libitum energy intakes were significantly or nearly significantly lower following consumption of an LNCS-sweetened versus unsweetened preload in all sensitivity analyses, except for when the preload LNCS was sucralose (−75.9 kcal; P = 0.168), the preload was cream cheese or yogurt (i.e., semi-solid preloads; 22.2 kcal; P = 0.531), and when, for beverage preloads, the volume was ≥355 mL (−12.8 kcal; P = 0.607). Of note, however, these sensitivity analyses were associated with the fewest number of comparisons, ranging from 3 to 8 (see Supplemental Table 3), whereas all the other sensitivity analyses in which pooled effects were significant/nearly significant and in favor of a reduced ad libitum energy intake following consumption of an LNCS-sweetened versus unsweetened preload were associated with 15 to 41 comparisons. Separately, the pooled ad libitum energy intake for aspartame capsules versus placebo capsules was statistically significant (−158.5 kcal; P < 0.001).
FIGURE 2.
Forest plot of the ad libitum energy intakes following consumption of LNCS-sweetened versus unsweetened preloads. Each square symbol is proportional to the weight of the comparison. The diamond represents the pooled effect. Energy intakes at the ALM were significantly lower (by ∼ −55.5 kcal; 95% CI: −82.9, −28.0 kcal; P < 0.001) following the consumption of an LNCS-sweetened versus unsweetened preload. A, adults; ACE-K, acesulfame potassium; ALM, ad libitum meal; ASP, aspartame; BEV, beverage; bf, before; breakf, breakfast; Cap, capsule(s); CARB, carbonated; Cr, cream; CS, caloric sweetener; EI, energy intake; Exp, experiment; F, females; HD, high-dose; LM, liquid meal; LNCS, low-/no-calorie sweetener; M, males; NW, normal-weight; OB, obese; SM, solid meal; UNS, unsweetened.
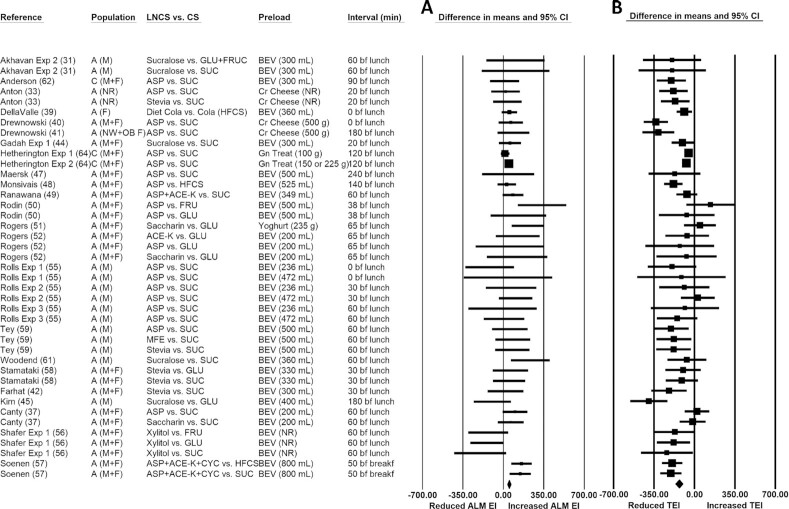
Ad libitum energy intakes following consumption of isosweet preloads
The pooled raw mean difference in ad libitum energy intake following LNCS- versus CS-sweetened preload consumption was 58.5 kcal (95% CI: 35.4, 81.7 kcal; P < 0.001), indicating significantly greater ad libitum energy intakes following LNCS- versus CS-sweetened preload consumption (Figure 3A). Statistically significant heterogeneity was identified (I2 = 32.8; P = 0.024), categorized as unimportant to moderate. Publication bias was detected, and using the trim-and-fill method of Duval and Tweedie (29), 8 studies were found to be missing to the left of the pooled effect. Imputing these 8 studies yielded a lower (but statistically significant) pooled raw mean difference of 35.1 kcal (95% CI: 9.6, 60.3 kcal). In most sensitivity analyses (see Supplemental Table 4), all the pooled raw mean differences in ad libitum energy intakes were greater following LNCS- versus CS-sweetened preload consumption and statistically significant, except when the LNCS was sucralose (73.2 kcal; P = 0.191) and the CS was either glucose (50.0 kcal; P = 0.364) or another CS (e.g., fructose) or CS mixtures (84.7 kcal; P = 0.100).
FIGURE 3.
Forest plot of the ad libitum meal (A) and total (preload + ALM) (B) energy intakes with consumption of LNCS- versus CS-sweetened preloads. Each square symbol is proportional to the weight of the comparison. The diamond represents the pooled effect. EIs at the ALM were significantly greater (by ∼58.5 kcal; 95% CI: 35.4, 81.7 kcal; P < 0.001) following the consumption of LNCS- versus CS-sweetened preloads (forest plot A). However, total energy intake (from the preload + ALM) was significantly lower (by ∼ −132.4 kcal; 95% CI: −163.2, −101.6 kcal; P < 0.001) (forest plot B). A, adults; ALM, ad libitum meal; ACE-K, acesulfame potassium; ASP, aspartame; BEV, beverage; bf, before; breakf, breakfast; C, children; Cr, cream; CS, caloric sweetener; CYC, cyclamate; EI, energy intake; Exp, experiment; F, females; FRU, fructose; GLU, glucose; Gn, gelatin; HFCS, high-fructose corn syrup; LNCS, low-/no-calorie sweetener; M, males; MFE, monk fruit extract; NR, not reported; NW, normal-weight; OB, obese; SUC, sucrose; TEI, total energy intake.
Total energy intakes with consumption of isosweet preloads
The pooled raw mean difference in total (preload + ad libitum meal) energy intake with LNCS- compared with CS-sweetened preload consumption was −132.4 kcal (95% CI: −163.2, −101.6 kcal; P < 0.001), indicating significantly lower total energy intakes with LNCS- versus CS-sweetened preload consumption (Figure 3B). Statistically significant heterogeneity was identified (I2 = 62.8; P < 0.001), categorized as moderate to substantial. Publication bias also was detected; using the trim-and-fill method of Duval and Tweedie (29), nine studies were found to be missing to the right of the pooled effect, and with these studies imputed, the pooled raw mean difference of −95.8 kcal remained statistically significant (95% CI, −127.4 to −64.3 kcal). In all sensitivity analyses, total energy intakes were significantly lower following LNCS- versus CS-sweetened preload consumption (Supplemental Table 5). The pooled raw mean difference in total energy intake in adults was 2-fold greater (−138.3 kcal vs. −75.1 kcal) than in children, though comparisons involving children were limited (n = 3 for children versus n = 38 for adults).
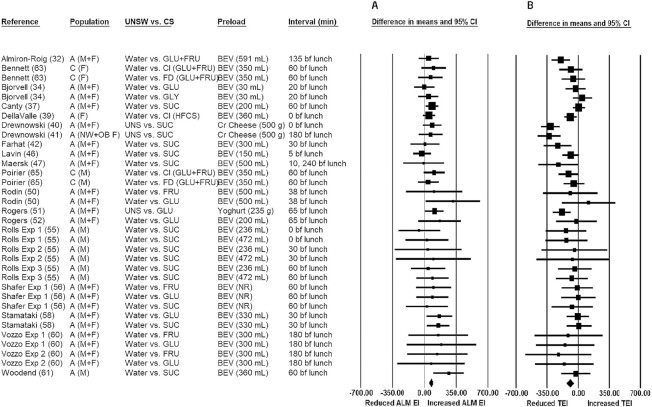
Ad libitum energy intakes following consumption of preloads that were neither isosweet nor isocaloric
The pooled raw mean difference in ad libitum energy intake with unsweetened compared with CS-sweetened preload consumption was 73.0 kcal (95% CI: 49.5, 96.5 kcal; P < 0.001), indicating significantly greater ad libitum energy intakes following unsweetened versus CS-sweetened preload consumption (Figure 4A). Although there was no significant heterogeneity (I2 = 0.0; P = 0.873), publication bias was detected. Using the trim-and-fill method of Duval and Tweedie (29), 7 studies were found to be missing to the left of the pooled effect. Imputing these 7 studies yielded a lower (but statistically significant) pooled raw mean difference of 58.3 kcal (95% CI: 35.4, 81.2 kcal). In all sensitivity analyses (see Supplemental Table 6), the pooled raw mean differences in ad libitum energy intakes were significantly greater with unsweetened compared with CS-sweetened preload consumption. There were notable observations in the sensitivity analyses (see Supplemental Table 6)—namely, the pooled raw mean difference was greater in magnitude when the CS was glucose (114.4 kcal, P < 0.001, compared with 71.9 kcal, P < 0.001, for sucrose or 67.8 kcal, P < 0.001, for other CS and CS mixtures).
FIGURE 4.
Forest plot of the ad libitum meal (A) and total (preload + ALM) (B) energy intakes with consumption of unsweetened versus CS-sweetened preloads. Each square symbol is proportional to the weight of the comparison. The diamond represents the pooled effect. EIs at the ad libitum meal were significantly greater (by ∼73.0 kcal; 95% CI: 49.5, 96.5 kcal; P < 0.001) following the consumption of UNS versus CS-sweetened preloads (forest plot A). However, total energy intake (from the preload + ALM) was significantly lower (by ∼ −94.3 kcal; 95% CI: −132.1, −56.4 kcal; P < 0.001) (forest plot B). A, adults; ALM, ad libitum meal; BEV, beverage; bf, before; C, children; Cl = cola; Cr, cream; CS, caloric sweetener; EI, energy intake; Exp, experiment; F, females; FD, fruit drink; FRU, fructose; HFCS, high-fructose corn syrup; M, males; NW, normal-weight; OB, obese; TEI, total energy intake; UNS/UNSW, unsweetened.
Total energy intakes with consumption of preloads that were neither isosweet nor isocaloric
The pooled raw mean difference in total energy intake with unsweetened compared with CS-sweetened preload consumption was −94.3 kcal (95% CI: −132.1, −56.4 kcal; P < 0.001), indicating that total energy intakes (i.e., from the preload + ad libitum meal) were significantly lower with unsweetened versus CS-sweetened preload consumption (Figure 4B). Although no publication bias was identified, there was significant heterogeneity (I2 = 52.1; P < 0.001), categorized as moderate to substantial. In all sensitivity analyses (see Supplemental Table 7), total energy intakes were significantly lower with unsweetened versus CS-sweetened preload consumption, except when the CS was glucose (−51.0 kcal; P = 0.120).
Total energy intakes with either LNCS-sweetened or unsweetened preloads compared with CS-sweetened preloads
In each of Figures 3 and 4, the forest plots show total energy intakes (ad libitum meal + preload) to be significantly lower with consumption of either an LNCS-sweetened or an unsweetened preload, in comparison to a CS-sweetened preload. Using unweighted raw mean data, Figure 5 illustrates the difference in total energy intake with consumption of either an LNCS- versus a CS-sweetened preload (−130.5 kcal) or an unsweetened preload versus a CS-sweetened preload (−93.5 kcal). Although the ad libitum energy intake for either an LNCS-sweetened or unsweetened preload is greater than that of the CS-sweetened preload, the reverse is true when total energy is considered and the calories of the preload are accounted for (Figure 5).
FIGURE 5.
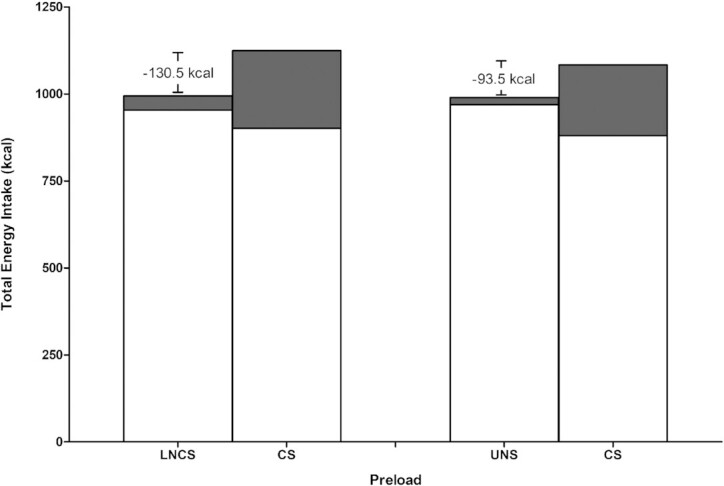
Graphical representation of the energy intakes with consumption of LNCS- versus CS-sweetened preloads and UNS versus CS-sweetened preloads. The white portion of bar represents the energy intake from the ad libitum meal and the gray portion of the bar represents the energy content of the preload. The numerical energy difference noted is of differences in total energy intake (from the preload + ad libitum meal) between the 2 preload conditions. CS, caloric sweetener; LNCS, low-/no-calorie sweetener; UNS, unsweetened.
Discussion
The role of sweeteners in the current obesity epidemic is intensely debated. While much of the well-controlled sweetener studies occur over longer periods of time—from weeks to months to years—and focus on anthropometric and physiological measures such as body weight and glycemic control, respectively (9, 11, 66–68), the analyses presented herein build on limited evidence-based reviews that attempt to better understand the effect of sweetness on immediate, acute (within a few hours) energy intakes. The findings from this systematic review and meta-analysis provide necessary insights into the effects of (un)sweetened preloads on subsequent acute energy intakes. These findings lend support to the hypothesis that unsweetened or LNCS-sweetened alternatives to CS-sweetened foods and beverages may benefit body weight and glycemic control by limiting caloric intakes.
Unsweetened and LNCS-sweetened preloads each resulted in a decrease in total energy intake when compared with a CS-sweetened preload, which suggests that effects observed are due to the lower (or no) calorie alternatives, not sweetness per se. Additionally, although ad libitum energy intake was significantly reduced following consumption of LNCS-sweetened preloads versus unsweetened preloads (isocaloric comparison), the difference between preloads was small and the statistical significance disappears when certain LNCS-sweetened preloads are removed from the pooled effect—namely, the comparisons in which the aspartame preload was administered in capsule form and when the preload was xylitol. Ad libitum energy intakes following the consumption of aspartame versus placebo capsules were significantly reduced, indicating an effect on energy intake that is independent of sweetness perception in the oral cavity. It has been hypothesized that aspartame may affect appetite through phenylalanine, generated upon aspartame digestion. Phenylalanine has been associated with the production of peptide hormones (e.g., cholecystokinin) known to influence appetite and increase satiation (53). These findings should be interpreted with caution, however, as there were only 4 comparisons in which aspartame was provided in capsule form, and all 4 were generated by the same research group [experiments 1 and 2 of (53), 54]. Xylitol is a low-calorie sugar alcohol that is associated with delayed gastric emptying and gastrointestinal intolerance (56, 69). Gastrointestinal intolerance may, itself, affect the amount of food consumed. After removing comparisons in which the preload was aspartame capsules or xylitol-sweetened beverages, the pooled effect of the remaining isocaloric comparisons, though still a reduction, was no longer statistically significant. Given the different physiological responses to specific LNCSs, and given that the pooled effect is no longer statistically significant when aspartame capsule and xylitol comparisons are removed, effects of LNCS-sweetened preloads on acute energy intake are at least equivalent to unsweetened preloads and do not increase caloric intakes relative to unsweetened preloads.
Based on the evidence base that was reviewed, which is mostly generalizable to normal-weight individuals (given that the majority of the studies were conducted in normal-weight subjects), differences in total energy intakes (preload + ad libitum meal) and ad libitum energy intakes were similar between isosweet/non-isocaloric comparisons (LNCS- vs. CS-sweetened preloads) and non-isosweet/non-isocaloric comparisons (unsweetened vs. CS-sweetened preloads), further reinforcing the notion that sweetness per se—compared with the absence of sweetness—does not contribute to a differential effect on appetite and acute energy intake. Rather, differences in energy intakes observed for the relevant comparisons noted are primarily driven by caloric differences in the preloads. The greater ad libitum energy intakes observed for LNCS-sweetened and unsweetened preloads (compared with CS-sweetened preloads) are easily explained by the fact that the LNCS-sweetened and unsweetened preloads had less calories than the CS-sweetened preload, which afforded some satiation during the ad libitum energy intake. The magnitude of the difference in total energy intake in each case suggests only partial (not full) compensation from CS-sweetened preloads. As noted previously, if these differences in energy intakes observed for the LNCS-sweetened or unsweetened preloads (compared with the CS-sweetened preloads) persist in chronic feeding trials, then substitution of CSs with LNCSs or unsweetened preloads should, theoretically, reduce the risk of weight gain. The latter hypothesis is, indeed, supported by numerous well-designed clinical trials conducted in adults (47, 67, 70–83) and children (84–88). There are, however, well-designed clinical trials in which no significant changes in body weight (i.e., neither a detriment nor a benefit) were observed with the use of LNCSs (89–91).
Of all of the studies that were evaluated, subjects who were overweight or obese were exclusively assessed in only 3 of the experiments (47, 60); therefore, sensitivity analyses based on body-weight status could not be performed. In the 5 experiments where subgroup analyses based on body-weight status were performed (33, 41, 50), the preload-by-weight status interaction was not statistically significant.
The characteristics of the ad libitum meal, which is a potential confounder to the evaluation, could not be evaluated. There were differences across studies in how the ad libitum meals were presented to the subjects; for instance, the subjects were presented with a single food (e.g., pizza) as the ad libitum meal in some studies while the subjects were presented with multiple foods to choose from in others. In addition, there could have been differences in the energy densities of the ad libitum meal across studies. Due to gaps in the data provided across studies and due to complexities in evaluating the different meal types, sensitivity analyses on the ad libitum meal type could not be performed.
Limitations of this systematic review and meta-analysis hinge on limitations of included primary research studies. Examples include the following: authors’ failure to report sample-size power calculations or disclose the washout period and inadequate standardization of pertinent factors that could independently affect energy intakes (e.g., the evening before the test day: standardization of dinner and consumption time, standardization of fasting period, restrictions on alcohol consumption and physical activity; the day of testing: standardization of breakfast and consumption time). Finally, most studies had adult participants who were provided with beverage preloads and for whom ad libitum meal energy intakes were measured at lunch; the latter factors are possibly both strengths and limitations—strengths as there was consistency in demographics, preloads, and measurement times enabling cross-study comparisons and limitations because it is not clear whether findings are generalizable across varying parameters, for example, would similar findings be observed in children and/or for meals other than lunch.
Strengths of this meta-analysis comprise the inclusion of only studies with a crossover design (which eliminates interindividual variability), the inclusion of only studies in which ad libitum meal energy intakes were reported (so that uncertainties associated with subjective measures of hunger could be eliminated), separate consideration of ad libitum meal and total energy intakes (which facilitates interpretation of findings), and separate analyses for isocaloric, isosweet, and neither isocaloric nor isosweet comparisons (which disentangles sweetness from calories and enables affect attribution to either sweetness and/or calories). Moreover, the acute-feeding trials increased confidence in the meta-analyses’ output in view of the well-controlled environment of study conduct such as preload volume, preload composition, and time interval between preload consumption and measurement of the ad libitum meal energy intake. Only those studies in which intakes at a single ad libitum meal were measured were considered within scope, which some may argue is a limitation because of a potential lack of generalizability across the day; however, this approach limited confounding and heterogenous study methodologies that would otherwise be introduced by multiple eating occasions. Finally, studies with preloads consisting of a mixture of LNCSs and CSs (i.e., to attempt to taste-match and mask preloads) were excluded, given the inability to isolate the independent effects of the LNCSs and CSs on subsequent energy intakes.
Factors influencing appetite, satiation, and energy intake are complex; moreover, distinguishing between hedonic and hunger triggers is challenging. Despite these challenges, the evidence base continues to grow and show that consumption of LNCS-sweetened or unsweetened beverages as an alternative to CS-sweetened beverages may contribute to reduced energy intakes, acutely. In longer-term studies, the substitution of CS-sweetened beverages with LNCS-sweetened or unsweetened beverages does not have any detrimental effect on body weight. Instead, these viable substitutions appear to be a tool (when used in conjunction with other tools) that facilitates weight management, both in adults (47, 70–83) and children (84–88).
In conclusion, in this systematic review and meta-analysis, the effects of LNCS-sweetened preloads, unsweetened preloads, and CS-sweetened preloads on subsequent food intake were examined. Specifically, we investigated whether there were differences in subsequent food intake between preloads when calories were controlled but not sweetness (i.e., isocaloric comparison), when sweetness was controlled but not calories (i.e., isosweet comparison), and when neither sweetness nor calories were controlled (i.e., unsweetened vs. CS-sweetened preloads). Although the consumption of LNCS-sweetened preloads resulted in lower ad libitum energy intakes when compared with unsweetened preloads, differences in appetite modulation may be explained by mechanisms other than sweetness perception, such as generating satiating peptide hormones upon aspartame digestion or delayed gastric emptying and/or gastrointestinal intolerance associated with sugar alcohols, like xylitol. Importantly, LNCS-sweetened preloads did not contribute any more to energy intake than the unsweetened preload counterpart. Similarly, effects on energy intake observed for both the LNCS- versus CS-sweetened preloads and the unsweetened versus CS-sweetened preloads were comparable in that the consumption of CS-sweetened preloads resulted in lower energy intake during the ad libitum meal in both cases but greater total energy intakes due to incomplete compensation from calories in the CS-sweetened preload. The totality of the evidence suggests, which is mostly generalizable to normal-weight individuals, that LNCS-sweetened foods and beverages (just as unsweetened foods and beverages) are viable alternatives to CS-sweetened foods and beverages to help manage caloric intake acutely. Additional studies are needed to better understand the mechanism by which substitution of CS-sweetened foods with unsweetened or LNCS-sweetened foods leads to decreased total energy intake.
Supplementary Material
Acknowledgments
We thank Judith E Hill for her assistance in assembling the reference list and numbering the references and Emily Booth for her assistance formatting the manuscript. All authors have read and approved the final manuscript.
Notes
The American Beverage Association provided funding for the work presented herein.
Author disclosures: MJ is a paid employee of the American Beverage Association. Intertek Health Sciences, Inc.(HYL, TP, DN, CV, SH, KMV), works for the American Beverage Association as paid scientific and regulatory consultants.
Supplemental Tables 1–7 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CS, caloric sweetener; HFCS, high-fructose corn syrup; LNCS, low-/no-calorie sweetener; NHLBI, National Heart, Lung, and Blood Institute.
Contributor Information
Han Youl Lee, Intertek Health Sciences, Inc., Mississauga, Ontario Canada.
Maia Jack, American Beverage Association, Science and Regulatory Affairs, Washington, DC, USA.
Theresa Poon, Intertek Health Sciences, Inc., Mississauga, Ontario Canada.
Daniel Noori, Intertek Health Sciences, Inc., Mississauga, Ontario Canada.
Carolina Venditti, Intertek Health Sciences, Inc., Mississauga, Ontario Canada.
Samer Hamamji, Intertek Health Sciences, Inc., Mississauga, Ontario Canada.
Kathy Musa-Veloso, Intertek Health Sciences, Inc., Mississauga, Ontario Canada.
References
- 1. Sylvetsky AC, Jin Y, Clark EJ, Welsh JA, Rother KI, Talegawkar SA. Consumption of low-calorie sweeteners among children and adults in the United States. J Acad Nutr Diet. 2017;117:441–8., e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malek AM, Hunt KJ, DellaValle DM, Greenberg D, St Peter JV, Marriott BP. Reported consumption of low-calorie sweetener in foods, beverages, and food and beverage additions by US adults: NHANES 2007–2012. Curr Dev Nutr. 2018;2:nzy054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ratliff JC, Riedt CS, Fulgoni VL III. Consumption of low-calorie sweetened beverages and water is associated with lower intake of carbohydrates and sugars and not associated with glycemic response in U.S. non-diabetic adolescents: results from the 2001–2014 National Health and Nutrition Examination Surveys. Nutrition X. 2019;1:100003. [DOI] [PubMed] [Google Scholar]
- 4. Catenacci VA, Pan Z, Thomas JG, Ogden LG, Roberts SA, Wyatt HR, Wing RR, Hill JO. Low/no calorie sweetened beverage consumption in the National Weight Control Registry. Obesity. 2014;22:2244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drewnowski A, Rehm CD. Consumption of low-calorie sweeteners among U.S. adults is associated with higher Healthy Eating Index (HEI 2005) scores and more physical activity. Nutrients. 2014;6:4389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rehm CD, Peñalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999–2012. JAMA. 2016;315:2542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leahy M, Ratliff JC, Riedt CS, Fulgoni VL III. Consumption of low-calorie sweetened beverages compared to water is associated with reduced intake of carbohydrates and sugar, with no adverse relationships to glycemic responses: results from the 2001–2012 National Health and Nutrition Examination Surveys. Nutrients. 2017;9:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barraj LM, Bi X, Murphy MM, Scrafford CG, Tran NL. Comparisons of nutrient intakes and diet quality among water-based beverage consumers. Nutrients. 2019;11:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toews I, Lohner S, Küllenberg de Gaudry D, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ. 2019;364:k4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lohner S, Toews I, Meerpohl JJ. Health outcomes of non-nutritive sweeteners: analysis of the research landscape. Nutr J. 2017;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rogers PJ, Hogenkamp PS, de Graaf C, Higgs S, Lluch A, Ness AR, Penfold C, Perry R, Putz P, Yeomans MRet al. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes. 2016;40:381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr. 2014;100:765–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Azad MB, Abou-Setta AM, Chauhan C, Rabbani R, Lys J, Copstein L, Mann A, Jeyaraman MM, Reid AE, Fiande Met al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189:E929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benton D. Can artificial sweeteners help control body weight and prevent obesity?. Nutr Res Rev. 2005;18:63–76. [DOI] [PubMed] [Google Scholar]
- 15. Renwick AG. Intense sweeteners, food intake, and the weight of a body of evidence. Physiol Behav. 1994;55:139–43. [DOI] [PubMed] [Google Scholar]
- 16. Renwick AG, Molinary SV. Sweet-taste receptors, low-energy sweeteners, glucose absorption and insulin release. Br J Nutr. 2010;104:1415–20. [DOI] [PubMed] [Google Scholar]
- 17. Rolls BJ. Effects of intense sweeteners on hunger, food intake, and body weight: a review. Am J Clin Nutr. 1991;53:872–8. [DOI] [PubMed] [Google Scholar]
- 18. Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. 2009;89:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown RJ, de Banate MA, Rother KI. Artificial sweeteners: a systematic review of metabolic effects in youth. Int J Pediatr Obes. 2010;5:305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pepino MY, Bourne C. Non-nutritive sweeteners, energy balance, and glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2011;14:391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mela DJ, McLaughlin J, Rogers PJ. Perspective: standards for research and reporting on low-energy (“artificial”) sweeteners. Adv Nutr. 2020;11:484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Health Canada . Canada's Dietary Guidelines for Health Professionals and Policy Makers. [Internet]. Cat.: H164-231/2019E-PDF. Ottawa (Canada): Health Canada; 2019; [cited 2020 Jun 20]. Available from: https://food-guide.canada.ca/static/assets/pdf/CDG-EN-2018.pdf. [Google Scholar]
- 23. Pan American Health Organization . Nutrient profile model. [Internet]. Washington (DC): Pan American Health Organization (PAHO), World Health Organization (WHO); 2016; [cited 2020 Jun 20]. Available from: https://www.paho.org/hq/index.php?option=com_content&view=article&id = 11662:paho-nutrient-profile-model&Itemid=41739&lang=en. [Google Scholar]
- 24. Dietary Guidelines Advisory Committee . 2015-2020 Dietary guidelines for Americans. 8th ed. [Internet]. Washington (DC): Dietary Guidelines Advisory Committee (DGAC), Departments of Health and Human Services (DHHS) and Agriculture (USDA); 2015; [cited 2020 Jun 20]. Available from: https://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 25. National Heart, Lung and Blood Institute . Study quality assessment tools. [Internet]. National Heart, Lung and Blood Institute (NHLBI), National Institutes of Health (NIH); 2020; [cited 2020 Jun 20]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. [Google Scholar]
- 26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 27. Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ. Empirical assessment of within-arm correlation imputation in trials of continuous outcomes. Methods Research Report. [Internet]. Prepared by the Tufts Evidence-based Practice Center under contract no. 290-2007-10055-I; AHRQ Publication No. 12(13)-EHC141-EF. Rockville (MD): Agency for Healthcare Research and Quality (AHRQ); 2012; [cited 2020 Jun 20]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK115797/. [PubMed] [Google Scholar]
- 28. Higgins JPT, Thomas JU, Chandler J, Cumpston M, Li T, Page M, Welch Veditors. Chapter 6: Choosing effect measures and computing estimates of effect. 6.2.9. Multiple intervention groups; Chapter 23: Including variants on randomized trials. 23.3.4. How to include multiple groups from one study. In: Cochrane handbook for systematic reviews of interventions. 2nd ed. Version 6 [Internet]. Chichester (UK): John Wiley & Sons; 2019; [cited 2020 Jun 20]. Available from: https://training.cochrane.org/handbook/current. [Google Scholar]
- 29. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 30. Deeks JJ, Higgins JPT, Altma DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas JU, Chandler J, Cumpston M, Li T, Page M, Welch Veditors. Cochrane handbook for systematic reviews of interventions. 2nd ed. Version 6 [Internet]. Chichester (UK): John Wiley & Sons; 2019; [cited 2020 Jun 20]. Available from: https://training.cochrane.org/handbook/current. [Google Scholar]
- 31. Akhavan T, Luhovyy BL, Anderson GH. Effect of drinking compared with eating sugars or whey protein on short-term appetite and food intake. Int J Obes. 2011;35:562–9. [DOI] [PubMed] [Google Scholar]
- 32. Almiron-Roig E, Drewnowski A. Hunger, thirst, and energy intakes following consumption of caloric beverages. Physiol Behav. 2003;79:767–73. [DOI] [PubMed] [Google Scholar]
- 33. Anton SD, Martin CK, Han H, Coulon S, Cefalu WT, Geiselman P, Williamson DA. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite. 2010;55:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Björvell H, Rössner S. Effects of oral glycerol on food intake in man. Am J Clin Nutr. 1982;36:262–5. [DOI] [PubMed] [Google Scholar]
- 35. Black RM, Tanaka P, Leiter LA, Anderson GH. Soft drinks with aspartame: effect on subjective hunger, food selection, and food intake of young adult males. Physiol Behav. 1991;49:803–10. [DOI] [PubMed] [Google Scholar]
- 36. Black RM, Leiter LA, Anderson GH. Consuming aspartame with and without taste: differential effects on appetite and food intake of young adult males. Physiol Behav. 1993;53:459–66. [DOI] [PubMed] [Google Scholar]
- 37. Canty DJ, Chan MM. Effects of consumption of caloric vs noncaloric sweet drinks on indices of hunger and food consumption in normal adults. Am J Clin Nutr. 1991;53:1159–64. [DOI] [PubMed] [Google Scholar]
- 38. Cuomo R, Savarese MF, Sarnelli G, Nicolai E, Aragri A, Cirillo C, Vozzella L, Zito FP, Verlezza V, Efficie Eet al. The role of a pre-load beverage on gastric volume and food intake: comparison between non-caloric carbonated and non-carbonated beverage. Nutr J. 2011;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DellaValle DM, Roe LS, Rolls BJ. Does the consumption of caloric and non-caloric beverages with a meal affect energy intake?. Appetite. 2005;44:187–93. [DOI] [PubMed] [Google Scholar]
- 40. Drewnowski A, Massien C, Louis-Sylvestre J, Fricker J, Chapelot D, Apfelbaum M. Comparing the effects of aspartame and sucrose on motivational ratings, taste preferences, and energy intakes in humans. Am J Clin Nutr. 1994;59:338–45. [DOI] [PubMed] [Google Scholar]
- 41. Drewnowski A, Massien C, Louis-Sylvestre J, Fricker J, Chapelot D, Apfelbaum M. The effects of aspartame versus sucrose on motivational ratings, taste preferences, and energy intakes in obese and lean women. Int J Obes Relat Metab Disord. 1994;18:570–8. [PubMed] [Google Scholar]
- 42. Farhat G, Berset V, Moore L. Effects of stevia extract on postprandial glucose response, satiety and energy intake: a three-arm crossover trial. Nutrients. 2019;11:3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, Bloom SR. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr. 2011;65:508–13. [DOI] [PubMed] [Google Scholar]
- 44. Gadah NS, Brunstrom JM, Rogers PJ. Cross-over studies underestimate energy compensation: the example of sucrose-versus sucralose-containing drinks. Appetite. 2016;107:398–405. [DOI] [PubMed] [Google Scholar]
- 45. Kim S-Y. Effect of glucose-sweetened drinks on blood glucose, energy, and water intake at a meal 3h later in healthy males. Nutr Sci. 2006;9:280–7. [Google Scholar]
- 46. Lavin JH, French SJ, Ruxton CH, Read NW. An investigation of the role of oro-sensory stimulation in sugar satiety?. Int J Obes. 2002;26:384–8. [DOI] [PubMed] [Google Scholar]
- 47. Maersk M, Belza A, Holst JJ, Fenger-Grøn M, Pedersen SB, Astrup A, Richelsen B. Satiety scores and satiety hormone response after sucrose-sweetened soft drink compared with isocaloric semi-skimmed milk and with non-caloric soft drink: a controlled trial. Eur J Clin Nutr. 2012;66:523–9. [DOI] [PubMed] [Google Scholar]
- 48. Monsivais P, Perrigue MM, Drewnowski A. Sugars and satiety: does the type of sweetener make a difference?. Am J Clin Nutr. 2007;86:116–23. [DOI] [PubMed] [Google Scholar]
- 49. Ranawana DV, Henry CJ. Are caloric beverages compensated for in the short-term by young adults? An investigation with particular focus on gender differences. Appetite. 2010;55:137–46. [DOI] [PubMed] [Google Scholar]
- 50. Rodin J. Comparative effects of fructose, aspartame, glucose, and water preloads on calorie and macronutrient intake. Am J Clin Nutr. 1990;51:428–35. [DOI] [PubMed] [Google Scholar]
- 51. Rogers PJ, Blundell JE. Separating the actions of sweetness and calories: effects of saccharin and carbohydrates on hunger and food intake in human subjects. Physiol Behav. 1989;45:1093–99. [DOI] [PubMed] [Google Scholar]
- 52. Rogers PJ, Carlyle JA, Hill AJ, Blundell JE. Uncoupling sweet taste and calories: comparison of the effects of glucose and three intense sweeteners on hunger and food intake. Physiol Behav. 1988;43:547–52. [DOI] [PubMed] [Google Scholar]
- 53. Rogers PJ, Pleming HC, Blundell JE. Aspartame ingested without tasting inhibits hunger and food intake. Physiol Behav. 1990;47:1239–43. [DOI] [PubMed] [Google Scholar]
- 54. Rogers PJ, Keedwell P, Blundell JE. Further analysis of the short-term inhibition of food intake in humans by the dipeptide L-aspartyl-L-phenylalanine methyl ester (aspartame). Physiol Behav. 1991;49:739–43. [DOI] [PubMed] [Google Scholar]
- 55. Rolls BJ, Kim S, Fedoroff IC. Effects of drinks sweetened with sucrose or aspartame on hunger, thirst and food intake in men. Physiol Behav. 1990;48:19–26. [DOI] [PubMed] [Google Scholar]
- 56. Shafer RB, Levine AS, Marlette JM, Morley JE. Effects of xylitol on gastric emptying and food intake. Am J Clin Nutr. 1987;45:744–7. [DOI] [PubMed] [Google Scholar]
- 57. Soenen S, Westerterp-Plantenga MS. No differences in satiety or energy intake after high-fructose corn syrup, sucrose, or milk preloads. Am J Clin Nutr. 2007;86:1586–94. [DOI] [PubMed] [Google Scholar]
- 58. Stamataki NS, Scott C, Elliott R, McKie S, Bosscher D, McLaughlin JT. Stevia beverage consumption prior to lunch reduces appetite and total energy intake without affecting glycemia or attentional bias to food cues: a double-blind randomized controlled trial in healthy adults. J Nutr. 2020;150:1126–34. [DOI] [PubMed] [Google Scholar]
- 59. Tey SL, Salleh NB, Henry J, Forde CG. Effects of aspartame-, monk fruit-, stevia- and sucrose-sweetened beverages on postprandial glucose, insulin and energy intake. Int J Obes. 2017;41:450–7. [DOI] [PubMed] [Google Scholar]
- 60. Vozzo R, Baker B, Wittert GA, Wishart JM, Morris H, Horowitz M, Chapman I. Glycemic, hormone, and appetite responses to monosaccharide ingestion in patients with type 2 diabetes. Metabolism. 2002;51:949–57. [DOI] [PubMed] [Google Scholar]
- 61. Woodend DM, Anderson GH. Effect of sucrose and safflower oil preloads on short term appetite and food intake of young men. Appetite. 2001;37:185–95. [DOI] [PubMed] [Google Scholar]
- 62. Anderson GH, Saravis S, Schacher R, Zlotkin S, Leiter LA. Aspartame: effect on lunch-time food intake, appetite and hedonic response in children. Appetite. 1989;13:93–103. [DOI] [PubMed] [Google Scholar]
- 63. Bennett LJ, Totosy de Zepetnek JO, Brett NR, Poirier K, Guo Q, Rousseau D, Bellissimo N. Effect of commercially available sugar-sweetened beverages on subjective appetite and short-term food intake in girls. Nutrients. 2018;10:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hetherington MM, Wood C, Lyburn SC. Response to energy dilution in the short term: evidence of nutritional wisdom in young children?. Nutr Neurosci. 2000;3:321–9. [DOI] [PubMed] [Google Scholar]
- 65. Poirier KL, Totosy de Zepetnek JO, Bennett LJ, Brett NR, Boateng T, Schwartz A, Luhovyy BL, Bellissimo N. Effect of commercially available sugar-sweetened beverages on subjective appetite and short-term food intake in boys. Nutrients. 2019;11:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. 2012;346:e7492. [DOI] [PubMed] [Google Scholar]
- 67. Peters JC, Beck J, Cardel M, Wyatt HR, Foster GD, Pan Z, Wojtanowski AC, Vander Veur SS, Herring SJ, Brill Cet al. The effects of water and non-nutritive sweetened beverages on weight loss and weight maintenance: a randomized clinical trial. Obesity. 2016;24:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nichol AD, Holle MJ, An R. Glycemic impact of non-nutritive sweeteners: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2018;72:796–804. [DOI] [PubMed] [Google Scholar]
- 69. Culbert SJ, Wang YM, Fritsche HA, Carr D Jr, Lantin E, Eys JV. Oral xylitol in American adults. Nutr Res. 1986; 6(8):913–22. [Google Scholar]
- 70. Blackburn GL, Kanders BS, Lavin PT, Keller SD, Whatley J. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. Am J Clin Nutr. 1997;65:409–18. [DOI] [PubMed] [Google Scholar]
- 71. Kanders BS, Lavin PT, Kowalchuk MB, Greenberg I, Blackburn GL. An evaluation of the effect of aspartame on weight loss. Appetite. 1988;11(Suppl 1):73–84. [PubMed] [Google Scholar]
- 72. Raben A, Vasilaras TH, Møller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721–9. [DOI] [PubMed] [Google Scholar]
- 73. Reyna NY, Cano C, Berm£dez VJ, Medina MT, Souki AJ, Ambard M, Nuñez M, Ferrer MA, Inglett GE. Sweeteners and beta-glucans improve metabolic and anthropometrics variables in well controlled type 2 diabetic patients. Am J Ther. 2003;10:438–43. [DOI] [PubMed] [Google Scholar]
- 74. Kim EJ, Kim MY, Kim J-S, Cho K-D, Han C-K, Lee B-H. Effects of fructooligosaccharides (FOS) intake on body weight, lipid profiles, and calcium status among Korean college students. FASEB J. 2011;25(1 Suppl):Abstract 771.4. [Google Scholar]
- 75. Raben A, Moller BK, Flint A, Vasilaris TH, Christina Moller A, Juul Holst J, Astrup A. Increased postprandial glycaemia, insulinemia, and lipidemia after 10 weeks' sucrose-rich diet compared to an artificially sweetened diet: a randomised controlled trial. Food Nutr Res. 2011;55:5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tate DF, Turner-McGrievy G, Lyons E, Stevens J, Erickson K, Polzien K, Diamond M, Wang X, Popkin B. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr. 2012;95:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Peters JC, Wyatt HR, Foster GD, Pan Z, Wojtanowski AC, Vander Veur SS, Herring SJ, Brill C, Hill JO. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity. 2014;22:1415–21. [DOI] [PubMed] [Google Scholar]
- 78. Sørensen LB, Vasilaras TH, Astrup A, Raben A. Sucrose compared with artificial sweeteners: a clinical intervention study of effects on energy intake, appetite, and energy expenditure after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2014;100:36–45. [DOI] [PubMed] [Google Scholar]
- 79. Madjd A, Taylor MA, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR. Effects on weight loss in adults of replacing diet beverages with water during a hypoenergetic diet: a randomized, 24-wk clinical trial. Am J Clin Nutr. 2015;102:1305–12. [DOI] [PubMed] [Google Scholar]
- 80. Kassi EN, Landis G, Pavlaki A, Lambrou G, Mantzou E, Androulakis I, Giannakou A, Papanikolaou E, Chrousos GP. Long-term effects of Stevia rebaudiana on glucose and lipid profile, adipocytokines, markers of inflammation and oxidation status in patients with metabolic syndrome. Endocr Rev. 2016;37(Suppl 1):Abstract SUN-577. [Google Scholar]
- 81. Vázquez-Durán M, Orea-Tejeda A, Castillo-Martínez L, Cano-García Á, Téllez-Olvera L, Keirns-Davis C. A randomized control trial for reduction of caloric and non-caloric sweetened beverages in young adults: effects in weight, body composition and blood pressure. Nutr Hosp. 2016;33:1372–8. [DOI] [PubMed] [Google Scholar]
- 82. Madjd A, Taylor MA, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR. Effects of replacing diet beverages with water on weight loss and weight maintenance: 18-month follow-up, randomized clinical trial. Int J Obes. 2018;42:835–40. [DOI] [PubMed] [Google Scholar]
- 83. Higgins KA, Mattes RD. A randomized controlled trial contrasting the effects of 4 low-calorie sweeteners and sucrose on body weight in adults with overweight or obesity. Am J Clin Nutr. 2019;109:1288–301. [DOI] [PubMed] [Google Scholar]
- 84. de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367:1397–406. [DOI] [PubMed] [Google Scholar]
- 85. Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics. 2006;117:673–80. [DOI] [PubMed] [Google Scholar]
- 86. Rodearmel SJ, Wyatt HR, Stroebele N, Smith SM, Ogden LG, Hill JO. Small changes in dietary sugar and physical activity as an approach to preventing excessive weight gain: the America on the Move Family Study. Pediatrics. 2007;120:e869–79. [DOI] [PubMed] [Google Scholar]
- 87. Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, Ludwig DS. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med. 2012;367:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Katan MB, de Ruyter JC, Kuijper LD, Chow CC, Hall KD, Olthof MR. Impact of masked replacement of sugar-sweetened with sugar-free beverages on body weight increases with initial BMI: secondary analysis of data from an 18 month double-blind trial in children. PLoS One. 2016;11:e0159771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Markey O, Le Jeune J, Lovegrove J. Energy compensation following consumption of sugar-reduced products: a randomized controlled trial. Eur J Nutr. 2016;55(6): 2137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gostner A, Schäffer V, Theis S, Menzel T, Lührs H, Melcher R, Schauber J, Kudlich T, Dusel G, Dorbath Det al. Effects of isomalt consumption on gastrointestinal and metabolic parameters in healthy volunteers. Br J Nutr. 2005;94(4):575–81. [DOI] [PubMed] [Google Scholar]
- 91. Njike V, Faridi Z, Shuval K, Dutta S, Kay C, West S, Kris-Etherton P, Katz D. Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. Int J Cardiol. 2011;149(1):83–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.