ABSTRACT
Ellagic acid (EA) is a dietary polyphenol present in various fruits, vegetables, herbs, and nuts. It exists either independently or as part of complex structures, such as ellagitannins, which release EA and several other metabolites including urolithins following absorption. During the past few decades, EA has drawn considerable attention because of its vast range of biological activities as well as its numerous molecular targets. Several studies have reported that the oxidative stress–lowering potential of EA accounts for its broad-spectrum pharmacological attributes. At the biochemical level, several mechanisms have also been associated with its therapeutic action, including its efficacy in normalizing lipid metabolism and lipidemic profile, regulating proinflammatory mediators, such as IL-6, IL-1β, and TNF-α, upregulating nuclear factor erythroid 2-related factor 2 and inhibiting NF-κB action. EA exerts appreciable neuroprotective activity by its free radical–scavenging action, iron chelation, initiation of several cell signaling pathways, and alleviation of mitochondrial dysfunction. Numerous in vivo studies have also explored the neuroprotective attribute of EA against various neurotoxins in animal models. Despite the increasing number of publications with experimental evidence, a critical analysis of available literature to understand the full neuroprotective potential of EA has not been performed. The present review provides up-to-date, comprehensive, and critical information regarding the natural sources of EA, its bioavailability, metabolism, neuroprotective activities, and underlying mechanisms of action in order to encourage further studies to define the clinical usefulness of EA for the management of neurological disorders.
Keywords: ellagic acid, natural sources, neuroprotection, bioavailability, metabolism, nanoformulations, molecular targets
Introduction
Neurodegenerative disorders have become a critical health concern throughout the world and are primarily identified by continuous destruction of neural cells and tissues as well as the associated dysfunction of the nervous system with aging (1). These disorders have diverse etiologies and mostly affect the peripheral nerves, spinal cord, and brain. The distinguishing symptoms of these disorders are memory loss, depression, motor dysfunction, cognitive impairment, and anxiety. Multiple sclerosis, prion diseases, Parkinson disease (PD), Alzheimer disease (AD), and Huntington disease are well-established neuronal disorders (2, 3). Some of the leading causes of neuronal degeneration are associated with toxin insults, heredity, metabolism, or attack by infectious pathogens (4). Previous studies documented that dysfunction of mitochondria, oxidative stress, neuroinflammation, and protein misfolding are major factors that account for neurodegenerative disorders (5). Transcription factors, such as cAMP-response element binding protein, nuclear factor erythroid 2-related factor-2 (Nrf2), NF-κB, Wnt/β-catenin, Janus kinase/signal transducer and activator of transcription, mitogen-activated protein kinases (MAPKs), and Toll-like receptor-4, play vital roles in pathophysiological alterations of neuronal cells (6, 7). Cholinesterase inhibitors, dopaminergic treatments, brain stimulation, and antipsychotic drugs are frequently used in treating neurodegenerative problems (8, 9). Additionally, riluzole, CERE120 (an experimental drug that consists of an adeno-associated virus), nonsteroidal anti-inflammatory drugs, and caffeine A2A receptor antagonists have also been suggested to minimize the risk of neurodegenerative complications (10). However, these drugs also produce several adverse effects with prolonged use. Hence there is a need to discover and prepare multitarget, safe, and more potent drugs, especially of a natural origin, for the management of neurodegenerative disorders (11, 12).
Plant-based natural products are the source for complex chemical molecules having multiple target sites in the human body. These molecules have been studied thoroughly for their diverse medicinal attributes (13). In the past decade, biological actions, nutritional features, and noteworthy health and therapeutic benefits of natural products and their phytoconstituents have been extensively evaluated (14, 15). Apart from their efficacy to prevent impairment caused by oxidative stress and inflammation, natural products have also been shown to alter multiple signal transduction pathways via direct actions on enzymes, such as kinases, receptors, and regulatory proteins (16, 17). Moreover, several herbal drugs and natural agents, such as Ginkgo biloba, Bacopa monnieri (brahmi), Celastrus paniculatus, curcumin, galantamine, coffee, and lavender essential oil, are preclinically as well as clinically tested for a neuroprotective role and have promising efficacy (18, 19). Ellagitannins (ETs) are polyphenolic compounds that are plentiful in seeds, fruits, and nuts. ETs are categorized as hydrolyzable tannins and are complex derivatives of ellagic acid (EA). EA is a polyphenolic compound that is present in a wide range of nuts, vegetables, and fruits, including pomegranates, walnuts, black raspberries, raspberries, almonds, and strawberries (20, 21). It possesses broad-spectrum physiological activities as confirmed by in vitro (22, 23), in vivo (24, 25), and clinical studies (26), including antioxidant, anti-inflammatory, antibacterial, anticarcinogenic, antiplasmodial, antiviral, hepatoprotective, antifibrotic, immunomodulatory, and neuroprotective activities (27, 28). Berries have been extensively used for neuroprotective studies because they contain several kinds of polyphenolic compounds that can work together and have a synergistic effect in the central nervous system (CNS) (29).
The majority of the presently established pharmacological attributes of EA depend on its antioxidant activity, which is primarily governed by its basic structure containing 4 hydroxyl groups that are responsible for scavenging both superoxide and hydroxyl anion radicals (30). EA has shown anti-inflammatory effects by its modulatory action on the cyclooxygenase (COX) enzyme and production of TNF-α, IL-1β, and IL-6 in a mouse model (31). Furthermore, it can improve AD-mediated dementia by suppressing oxidative and inflammatory cell damage and improving antioxidant content. EA inhibits cognitive abnormality following traumatic brain injury (TBI) in rats through its anti-inflammatory and antioxidant properties (32). EA restored redox homeostasis in several animal models of dementia by reducing inflammation and oxidative damage as well as augmenting antioxidant concentrations in the brain (14, 33). Because EA has gained remarkable attention for its multipurpose therapeutic properties, a systematic review of its therapeutic features, especially as a neuroprotective agent, is warranted. Recent studies have revealed the pharmacological role of EA in countering numerous cellular abnormalities (26, 34, 35). de Oliveira (36) has provided an overview of the limited number of neurological studies. In the present review, an effort has been made to provide up-to-date, comprehensive, and critical information on natural sources of EA, its dietary intake, metabolism, and therapeutic roles in various neurological disorders, and its underlying mechanisms of action.
Literature Search Strategy and Study Selection
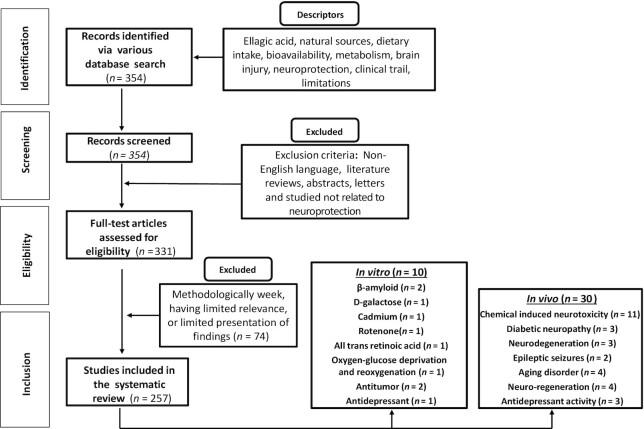
Various mainstream scholarly databases, including PubMed, ScienceDirect, EBSCOhost, and Scopus, were used for search and collection of literature. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) criteria suggested for writing systematic reviews (37) was followed. Articles published in peer-reviewed journals between 1922 and 2020 (up to April) in the English language were incorporated. Exclusion criteria for the elimination of articles involved conference abstracts, reports in a non-English language, letters to editors, and unpublished data. Major keywords, such as ellagic acid, bioavailability, neurodegenerative disorders, brain injury, prevention, pharmacological effect, metabolism, neuroprotection, and in vivo, in vitro, and clinical studies were used during the literature survey. A scheme for literature search and study selection is presented as Figure 1.
FIGURE 1.
PRISMA flowchart showing the procedure of literature searching and selection with numbers of articles at each stage. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Chemistry of EA
In 1831, Braconnot first discovered a new form of ET and termed it EA (2,3,7,8-tetrahydroxy[1]-benzopyranol[5,4,3-cde]benzopyran-5,10-dione). It is an extremely thermostable molecule (melting point 350°C) and has a molar mass of 302.197. It is also moderately soluble in ether, alcohol, and water but completely soluble in caustic potash (38). Structurally, the presence of 4 rings signifies the lipophilic domain. Representing the hydrophilic domain are 2 lactones and 4 phenolic groups, which act as electron acceptors and form hydrogen-bond sides, respectively (39). EA is a polyphenolic compound present in various fruits, nuts, and seeds, including raspberries, strawberries, baheda, grapes, pomegranate, walnuts, blackcurrants, camu-camu, longan seeds, mango, guava, almonds, and green tea (Table 1) (40–42).
TABLE 1.
List of plants reported for the presence of ellagic acid and their medicinal properties
| Family name | Plant name | Plant part used | Model used | Medicinal properties | Reference |
|---|---|---|---|---|---|
| Apocynaceae | Decalepis hamiltonii | Roots | In vivo | Anticancer | (52) |
| Macrosiphonia longiflora | Xylopodium | Clinical | Anti-inflammatory | (53) | |
| Juglandaceae | Carya illinoinensis | Kernels and shells | In vivo | Toxicological effect and antioxidant | (54) |
| Juglans regia | Kernels | — | Not determined | (55) | |
| Malvaceae | Thespesia lampas | Roots | In vitro and in vivo | Antioxidant and hepatoprotective | (56) |
| In vitro and in vivo | |||||
| Sterculia striata | Nut | In vitro | Antioxidant | (57) | |
| Sapindaceae | Dimocarpus longan | Seeds | In vitro | Antioxidant and antimicrobial | (58) |
| Nephelium lappaceum | Husk | In vitro | Antioxidant | (59) | |
| Rosaceae | Geum rivale | Aerial | — | Not determined | (60) |
| Rubus parvifolius | Whole plant | In vivo | Hepatoprotective and antioxidant | (61) | |
| Sanguisorba officinalis | In vitro | Antiadipogenic | (62) | ||
| Phyllanthaceae | Emblica officinalis | Fruits | In vitro, in vivo, and clinical | Antioxidant, antihepatotoxic, anti-inflammatory, and antidiabetic | (63) |
| Phyllanthus acuminatus | Leaves | In vitro | Antioxidant and cytotoxic | (64) | |
| Myrtaceae | Myrciaria dubia | Fruit | In vitro | Antioxidant | (65) |
| Psidium friedrichsthalianum | Antioxidant and metabolomic | (66) | |||
| Syzygium calophyllifolium | Antioxidant and antibacterial | (67) | |||
| Syzygium cumini | Antidiabetic and antioxidant | (68) | |||
| Myrciaria floribunda | Antioxidant | (69) | |||
| Eugenia uniflora | Leaves | In vitro and in vivo | Anti-inflammatory, antioxidant, and antibacterial | (70) | |
| Myrtus communis | — | Not determined | (71) | ||
| Campomanesia adamantium | Leaves and root | In vitro | Apoptotic death of leukemic cells | (72) | |
| Eucalyptus globulus | Bark, stem, leaves, and fruit | In vitro | Antioxidant, bioherbicide | (73) | |
| Acca sellowiana | Fruits, pulp, and peel | In vitro | Antimicrobial | (74) | |
| Euphorbiaceae | Chrozophora senegalensis | Leaves and stem | In vitro and in vivo | Cytotoxicity and antimalarial | (75) |
| Acalypha hispida | In vitro and in vivo | Anti-inflammatory and antioxidant | (76) | ||
| Gymnanthes lucida | Leaves | In vitro | Antimicrobial and cytotoxic | (77) | |
| Euphorbia pekinensis | Root | In vitro and in vivo | Antidiabetic | (78) | |
| Euphorbia supina | Herb | In vitro | Antioxidant | (79) | |
| Sebastiania chamaelea | Whole plant | In vitro and in vivo | Cytotoxicity and antimalarial | (75) | |
| Lythraceae | Trapa taiwanensis | Fruit | In vitro and in vivo | Antioxidant and hepatoprotective | (80) |
| Woodfordia fruticosa | Flower | In vivo | Antiulcer | (81) | |
| Lafoensia pacari | Leaves | In vitro and in vivo | Cytotoxicity and wound-healing | (82) | |
| Lagerstroemia speciosa | Leaves and stem | In vitro | Antiviral | (83) | |
| Combretaceae | Terminalia chebula | Fruit | In vitro | Antioxidant, antibacterial, and neuroprotective | (84) |
| Terminalia bellirica | In vitro and in vivo | Antioxidant, hepatoprotective, and antidiabetic | (85) | ||
| Cistaceae | Cistus laurifolius | Leaves | In vitro and in vivo | Antioxidant, prostaglandin inhibitory, and antimicrobial | (86) |
| Lecythidaceae | Barringtonia racemosa | Leaves and stems | In vitro | Antioxidant | (87) |
| Bixaceae | Cochlospermum angolensis | Bark | In vitro | Antioxidant and antidepressant | (88) |
| Fabaceae | Delonix elata | Stem and bark | In vitro and in vivo | Antioxidant and hepatoprotective | (89) |
| Moraceae | Ficus glomerata | Fruit and leaf | In vitro and in vivo | Antioxidant and gastroprotective | (90) |
| Gentianaceae | Gentiana scabra | Rhizome | In vitro and in vivo | Antioxidant and hepatoprotective | (91) |
| Geraniaceae | Geranium carolinianum | Aerial | In vitro | Anti-hepatitis B virus | (92) |
| Irvingiaceae | Irvingia gabonensis | Seed | — | Not determined | (93) |
| Anacardiaceae | Mangifera indica | Flower and fruit | In vitro | Antioxidant and antiplatelet | (94) |
| Moringaceae | Moringa oleifera | Leaves | In vitro and clinical | Antioxidant, antimicrobial, and photoprotective preparations | (95, 96) |
| Polygonaceae | Polygonum chinense | Whole plant | In vitro | Antiviral | (97) |
| Vitaceae | Vitis rotundifolia | Fruit | In vitro | Antioxidant | (98, 99) |
| Tamaricaceae | Tamarix aphylla | Leaves and stem | — | Not determined | (46) |
| Punicaceae | Punica granatum | Husk, fruit, and seeds | In vitro and in vivo | Antioxidant, anti-inflammatory, and vasculoprotective | (100, 101) |
EA Content in Food Products and Dietary Intake
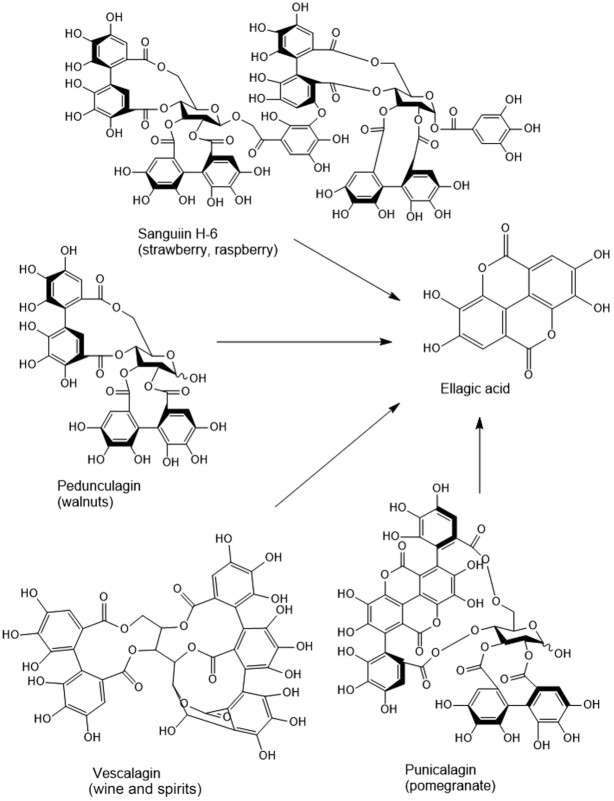
Epidemiological evidence has revealed that EA shows therapeutic action against certain chronic diseases (43). Table 2 summarizes results of quantitative analyses of the EA content in various fruits, nuts, and beverages. Wada and Ou (44) suggested that in all berries except boysenberries the concentration of free EA was 40–50% of the total EA. The concentration of total EA ranged from 47 to 90 mg/g in red raspberries and black raspberries, respectively. The consumption of EA can exceed the above estimations if EA-rich foods other than berries (e.g., pomegranate juice and walnuts) are also present in the regular diet or if EA is taken as a supplement (45). In honey, EA has been suggested to be a floral marker for honey production from the heather plant (46). Free EA and its glycoside derivatives, including arabinosides, glucosides, rhamnosides, and the corresponding conjugates of acetyl esters, have also been found in honey-related food supplements (47). Various other studies reported that 100 g of raspberries or a glass of pomegranate juice can provide ≤300 mg EA, 4 walnuts provide 400 mg, and a strawberry provides 70 mg EA (48). Additionally, it was found that punicalagin is found predominantly in pomegranate, sanguiin H-6 in strawberry and raspberry, vescalagin in oak-aged wines and spirits, and pedunculagin in walnuts. Upon hydrolysis, these compounds produce EA (Figure 2); however, several other metabolites can also be produced that are discrete from individual ETs (i.e., gallagic and tergallagic acid-O-glucoside) (49, 50).
TABLE 2.
Ellagic acid concentration in fruits, nuts, and beverages
| Food item/beverage | Ellagic acid concentration | References |
|---|---|---|
| Fresh fruit/nuts, mg/g dry weight | ||
| Arctic bramble | 0.7–3.2 | (102) |
| Blackberry | 0.015–0.022 | (20) |
| 11 | (103) | |
| Cloudberry | 0.56–3.60 | (104, 105) |
| Grapes | 0.36–0.91 | (102) |
| Pomegranate | 0.35–0.75 | (106) |
| Raspberry | 0.51–3.30 | (104, 105) |
| Strawberry | 0.25–0.85 | (105, 107) |
| Chestnut | 0.0016–0.025 | (108) |
| Pecan | 21–86 | (109) |
| Walnut | 16 | (110) |
| Fruit jam, mg/g | ||
| Raspberry jam | 0.76 | (111) |
| Strawberry jam | 0.24 | (111) |
| Fruit juice/wines/spirits, mg/100 mL | ||
| Muscadine grape juice | 0.08–0.84 | (112) |
| Pomegranate juice | 570 | (113) |
| Muscadine grape wine | 0.2–6.5 | (112) |
| Oak-aged red wine | 0.94–5 | (25, 114) |
| Cognac | 3.1–5.5 | (20) |
| Whisky (sour mash) | 2.38 | (103) |
| Whisky | 0.1–0.2 | (115) |
FIGURE 2.
Sanguiin H-6 is typical of strawberry and raspberry, pedunculagin of walnuts, punicalagin of pomegranate, and vescalagin of oak wine. All of them release ellagic acid upon hydrolysis in the gastrointestinal tract.
A survey performed in different geographical regions throughout the world suggested that citizens of Western Europe have the highest predictable daily dietary consumption of EA in both sexes (7.6 mg/d in men and 7.9 mg/d in women), followed by Americans and Australians (6.7 mg/d in women, 7.0 mg/d in men) (45, 51). In these regions strawberries accounted for >60% of the daily intake of EA. However, the expected consumption of EA was low (<1 mg/d) in African, Asian, and South American regions, probably due to inadequate availability of berries (51). Researchers reported that strawberries provided 0.2–0.3 mg of EA daily in France because strawberries are one of the major sources of EA (20). Conclusively, a literature survey confirmed that no harm from EA and ET intake has been observed in humans when these compounds are taken as part of the diet or as nutritional supplements.
Absorption, Bioavailability, Metabolism, and Excretion of EA
EA is found in a variety of ET-rich foods and also formed during food processing. Previous reports indicated that free EA is relatively less water soluble and precipitates in juices and liquors, hence its bioavailability is low based on animal and human studies (116, 117). After initial absorption in the stomach, ETs quickly undergo methyl conjugation by catechol O-methyltransferase. The breakdown of ETs starts in the stomach and the breakdown products can be detected in peripheral blood (118). In the gastrointestinal tract and at other sites, specifically in the liver, microbial metabolites of EA and ETs are again metabolized by phase I (hydroxylation) and phase II (sulfation, methylation, and glucuronidation) enzymes to yield more water-soluble metabolites that can be stored in tissues or eliminated via urine (119). EA can be transformed by the gut microbiota to urolithin-D, urolithin-C, urolithin-A, and urolithin-B in the intestine; these then move into the blood circulation via intestinal epithelial cells as their lipophilicity increases (120) (Figure 3). On the basis of microbial metabolism, individuals are stratified into 3 urolithin metabotypes: metabotype A—individuals synthesizing only urolithin-A; metabotype B—yielding isourolithin-A and/or urolithin-B apart from urolithin-A; and metabotype 0—unable to synthesize any urolithin (121).
FIGURE 3.
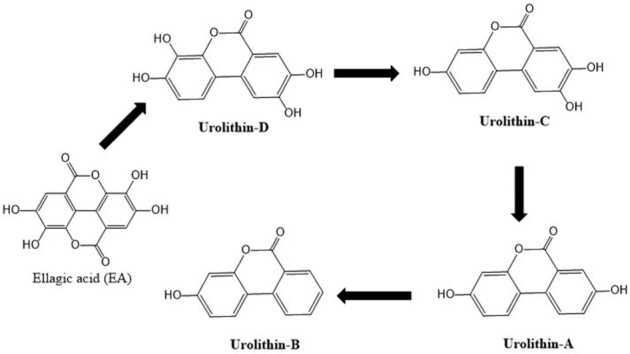
Ellagic acid metabolism by gut microbiota in humans and animals produces several metabolites (urolithin-A, urolithin-B, urolithin-C, and urolithin-D). These urolithins are further largely absorbed by the intestinal cells and glucuronidated.
The examination of Iberian pig samples (plasma and urine) suggested that urolithin-A glucuronide and sulfate were the major metabolites, along with urolithin-C whereas urolithin-B glucuronides and sulfates were minor metabolites. Additionally, the dimethyl ether glucuronide of EA was also a noteworthy metabolite. An enterohepatic recirculation was clearly observed in the Iberian pig. Analysis of gall bladder and bile suggested that tetrahydroxy-urolithin was absorbed in the first segment of the gut and was identified in hepatocytes, where it was conjugated and eliminated with the bile to the small intestine (122). In Iberian pigs urolithin metabolites were stored only in the gall bladder and the urinary bladder, where they attained high concentrations; however, they were not stored in any of the tested tissues, such as kidney, muscle, heart, adipose tissue, and liver (122).
Urolithin-A is stored in colon, prostate, and intestinal tissues, whereas urolithin-A glucuronide is chiefly found in kidney and liver tissues of the mouse (123). Metabolites derived from EA, primarily urolithin-A and urolithin-B, are eliminated by urine. The urinary elimination of EA and EA-O-glucuronide is <1% of intake in the case of humans (124). Additionally, urolithin-A is the chief EA metabolite identified in the feces of humans and pigs (125). Recently, Selma et al. (126) suggested that the monocultured bacteria Gordonibacter urolithinfaciens and G. pamelaeae account for the breakdown of EA to produce luteic acid, urolithin-M-5, urolithin-M-6, and urolithin-C.
In a pharmacokinetic study, EA was observed at a maximum concentration in plasma (Cmax) 1 h postingestion of 180 mL pomegranate juice (containing 318 mg ETs and 25 mg EA) in a human volunteer. The Cmax was 32 ng/mL (0.1 μM), and the EA was excreted within 4 h from the circulatory system (127). In another study conducted in Spain, 6 healthy volunteers were provided with 1 L of pomegranate juice (4.4 g punicalagins and no free EA). Despite the much higher dose, no EA was observed in plasma in the 4-h period following the juice intake (113).
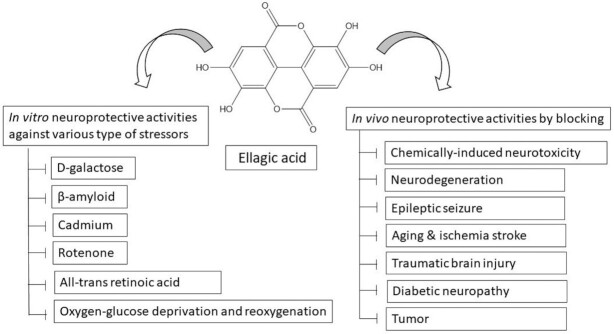
Preclinical Evidence for Neuroprotective Roles of EA
EA and EA-enriched products, including Ellagic Active tablets (Source Naturals, Santa Cruz, CA, USA), Ellagic Acid Capsules (Biotech Nutritions, Novi, MI, USA), and PomActiv (H&B Wellness, Chandigarh, India), are widely consumed as nutritional supplements owing to their health-promoting activities (128). Previous studies reported that EA substantially prevented the action of acetylcholinesterase (AChE) and hence upregulated cognitive potential through acetylcholine (88, 104). EA modulated the brain monoaminergic and GABAergic transmission pathways that have healing actions on memory and learning in experimental models of depression. Moreover, the stimulation of noradrenergic and serotonergic transmission in the brain is correlated with the antidepressant property of EA (129). The therapeutic efficacy of EA on various neurological complications or disorders and its in vitro and in vivo importance are discussed below.
Neurodegenerative Disorders Caused by Biological Processes
β-Amyloid-induced AD
The neuropathological hallmarks of AD are the accumulation of extracellular “senile plaques,” which are derived from β-amyloid (Aβ) fibrils, and intracellular “neurofibrillary tangles,” which contain hyperphosphorylated tau (τ) protein. Selective inhibition of Aβ oligomer offers a prime target for AD treatment. Rojanathammanee et al. (105) reported that EA (10 μM/L) alone or with punicalagin (10 μM/L) effectively prevented the Aβ-mediated TNF-α secretion and nuclear factor of activated T cells in cultured primary murine cortical microglia (Table 3). Feng et al. (130) demonstrated in vitro the effects of EA (100 and 300 μM) on Aβ42 aggregation, formation of β-sheets, Aβ oligomer concentrations, and cytotoxicity of Aβ42 in 5H-SY5Y neuroblastoma cells. The results revealed that both methylene blue and EA strengthen a noncovalent interaction within β-sheet structures and promote fibril assembly kinetics (130). Additionally, the fluorescence emission of thioflavin T (ThT) is shifted when ThT binds to β-sheet structures and is extensively used to measure Aβ fibril concentration. EA (0.1–0.4 mM) substantially prevented the ThT fluorescence of Aβ fibrils, showing suppressed binding of ThT to the fibrils by EA action. Moreover, EA prevented neurotoxicity by stimulating Aβ42 aggregation into fibrils with remarkable oligomer deficiency by conjugating with Aβ, aromatic stacking, or hydrophobic forces to stimulate fibrilization (107).
TABLE 3.
In vitro neuroprotective role of ellagic acid against various types of stressors1
| Stressor | Experimental model | EA concentration | Observations | References |
|---|---|---|---|---|
| Aβ | Primary murine cortical microglia | 10 μM/L | Inhibited microglial activation via attenuation of TNF-α, and NFAT activity | (105) |
| SH-SY5Y cells | 2 mg/mL | Prevented Aβ neurotoxicity by promoting Aβ aggregation into fibrils with significant oligomer loss | (130) | |
| 0.1–0.4 mM | Suppressed proinflammatory and disease aggravation markers | (107) | ||
| D-gal | SH-SY5Y cells | 0.01–10μM | Increased cell proliferation and GSH concentration, while decreasing concentrations of ROS, MDA, TNF-α, β-GAL, and AGEs | (131) |
| ATRA and TPA | SH-SY5Y cells | 30–100 μM | EA induced cell detachment, decreased cell viability, and induced apoptosis | (132) |
| 50 μM | EA decreased cell detachment, loss of viability, and activation of apoptosis | (133) | ||
| Cadmium | Rat primary astrocytes | 30 μM | Decreased ROS production and astrocyte cell death | (134) |
| Rotenone | PC12 pheochromocytoma | 10 μM | Decreased ROS and RNS production, PARP1, HSP70, and α-synuclein aggregation | (135) |
| OGD/R | Primary culture of rat cortical neurons | 10 and 30μg/mL | Decreased the number of apoptotic/necrotic cells, and remedied the decrease in the ratio of Bcl-2/Bax expression | (136) |
| Tumor | Human glioblastoma and rat glioma cell line | 5.5 mg or 10 mg | Chitosan-EA composite films induced the accumulation of the tumor suppressor protein p53 and increased caspase-3 activation, which preceded induction of apoptosis | (95) |
| 5.5 mg or 10 mg | EA induced apoptosis in cancer cells as well as suppressing angiogenesis in dose-dependent manner | (94) | ||
| Antidepressant | AChE, BuChE, and MAO-A | EA exhibited appreciable MAO-A inhibition activity compared with cholinesterase inhibitors | (88) |
1Aβ, β-amyloid; AChE, acetylcholinesterase; AGE, advanced glycation end-product; ATRA, all-trans retinoic acid; BuChE, butyrylcholinesterase; D-gal, d-galactose; EA, ellagic acid; GSH, reduced glutathione; HSP70, heat shock protein 70; MAO-A, monoamine oxidase A; MDA, malondialdehyde; NFAT, nuclear factor of activated T-cells; OGD/R, oxygen-glucose deprivation and reoxygenation; PARP, poly(ADP-ribose) polymerase; RNS, reactive nitrogen species; ROS, reactive oxygen species; TPA, 12-O-tetradecanoylphorbol-13-acetate; β-GAL, β-galactose.
EA and aging
Aging is a physiological process and is related to broad-spectrum chronic disorders, like atherosclerosis, cancer, retinopathy, and PD. Oxidative impairment triggered by reactive oxygen species (ROS) plays a critical role in aging (137). Excessive ROS have a damaging impact on humans, such as increasing membrane lipid peroxidation (LPO), damaging DNA, and initiating apoptosis, ultimately leading to cellular damage (138). Chen et al. (139) demonstrated the antioxidative, anti-inflammatory, and antiapoptotic effects of EA on d-galactose (d-gal)-induced aging in rats. The brain contains the most complex tissue of all organs of the body and requires large amounts of oxygen and energy to maintain its normal activities; however, only small amounts of antioxidative enzymes are present within it (140, 141). Results of this study revealed that subcutaneous injection of d-gal caused a significant decrease in antioxidant enzyme activities of catalase (CAT), GPx, superoxide dismutase (SOD), and total antioxidant capacity (TAC), and an increased malondialdehyde (MDA) concentration. Interestingly, the antioxidant enzyme activities were effectively increased by EA supplementation (50 mg/kg), which also significantly reduced the production of MDA in the brain (139). Among the cytokines, TNF-α, IL-6, and IL-1β are essential in the development and progression of oxidative stress because they are associated with ROS. The concentrations of these inflammatory cytokines were significantly lower in rats receiving EA. These results indicate that EA also has a strong anti-inflammatory efficacy in d-gal-induced aging in rats. Bcl-2 plays a key role during inhibition of apoptosis; it is a known factor in cell aging, and its overexpression can effectively prevent the apoptosis induced by hydrogen peroxide, free radicals, and microbial contamination (114). Meanwhile, the main function of Bax is to accelerate apoptosis and, together with Bcl-2, regulate cell apoptosis. EA intervention significantly downregulates the expression of Bcl-2 and Bax proteins and upregulates the expression of caspase-3 (139).
d-gal is a reducing sugar present in many foods, such as honey, beets, cheese, yoghurt, butter, milk, kiwi fruit, soy sauce, plums, dry figs, cherries, and celery. In classical galactosemia, the normal concentration of d-gal in the human blood is >10 mg/dL. For a healthy adult the maximal recommended daily dose is 50 g d-gal, and most of it can be eliminated from the body within ∼8 h after ingestion (142). Excessive administration of d-gal could contribute to generation of ROS through oxidative metabolism of d-gal as well as through glycation end-products (143). During the process of aging, a higher concentration of d-gal accumulates in the body and associates with the amines of proteins and peptides. This can produce advanced glycation end-products (AGEs), which bind to the receptor of advanced glycation end-products (RAGE). It has been reported that AGEs cause inflammation and oxidative stress through RAGE (144). Rahimi et al. (131) evaluated the effect of EA (0.01–10 μM) on aging by analyzing inflammation (TNF-α), cell proliferation, MDA, intracellular ROS, β-galactosidase (β-gal), reduced glutathione (GSH), and AGE concentrations in neuroblastoma cells (SH-SY5Y). During d-gal metabolism, higher amounts of d-gal can produce AGEs and ROS, which cause neurotoxicity by promoting neurogenesis suppression, neuronal apoptosis, and inflammatory response (145, 146). d-gal remarkably enhanced AGE concentrations via nonenzymatic glycation, oxidative stress activation, and alteration of inflammatory cytokine concentrations. The SH-SY5Y cells co-cultured with EA (0.01–10 μM) improved the AGE concentration in d-gal-induced aging (131). β-gal contributes to the breakdown of β-galactose–containing compounds like sphingolipids and lactose. A separate group of researchers presented β-gal as a critical marker for analyzing the process of aging in both in vitro and in vivo models (147, 148). Further, d-gal–induced SH-SY5Y cells showed a remarkable decline in cell proliferation, GSH concentration, and SOD activity, along with increased ROS and MDA concentrations (144). Following d-gal–induced aging, EA increased the concentrations of GSH and promoted cell proliferation, as well as reducing the concentrations of AGEs, ROS, β-gal, MDA, and TNF-α. Results also suggested that EA might act by modulating signaling pathways through the involvement of both heme oxygenase 1 (HO-1) and peroxisome proliferator-activated receptor-γ activation (131).
Fjaeraa and Nanberg (132) reported that EA (30–100 μM) lowered the viability of SH-SY5Y human neuroblastoma cells. It arrested cell cycle progression in all-trans retinoic acid (ATRA)–treated SH-SY5Y cells in a concentration-dependent manner. Additionally, EA increased the number of detached cells although co-treatment of EA with ATRA did not promote such parameters. EA activity in reducing numbers of adherent cells and growing cell detachment was much higher compared with ATRA. EA together with ATRA led to a more effective diminution in SH-SY5Y cell viability when compared with ATRA or EA individually. EA accelerated apoptosis in SH-SY5Y cells, as confirmed by a terminal deoxynucleotidyl transferase nick end labeling assay. Surprisingly, EA did not affect the number of ATRA-differentiated SH-SY5Y cells. EA produced only an insignificant reduction in viability of ATRA-differentiated cells. Therefore, the impact of EA on neuroblastoma cells is probably reliant on their differentiation state (132). Alfredsson et al. (133) investigated whether phorbol ester 12-O-tetradecanoylphorbol-13-acetate alters the sensitivity to EA in neuronal differentiation in SH-SY5Y neuroblastoma cells. Their findings revealed that differentiation diminished the sensitivity to EA (50 μM) with respect to cell detachment, loss of viability, and initiation of apoptosis. Differentiation-associated upregulation of integrin expression and Bcl-2 protein are probable protective mechanisms. The curative action was phenotype-specific and most prominent in ATRA-differentiated cultures (133).
S-Nitrosylation of protein disulfide isomerase and PD
The housekeeping chaperone protein disulfide isomerase (PDI) is usually involved in protein maturation and hence controls traffic flow in the cell (149). Post-mortems have shown that PDI (housekeeping chaperone) undergoes S-nitrosylation (SNO) of its catalytic cysteines in reaction to nitrosative stress in the brains of patients with PD (150). Kabiraj et al. (135) explored whether EA would prevent SNO of PDI in PC12 cells (a murine catecholaminergic cell line). EA (10 μM) mitigated peroxynitrite (ONOO−) and protected PC12 cells from rotenone-triggered cell death. EA also lowered the rotenone-induced ROS and reactive nitrogen species generation in these cells. The researchers suggested that EA inhibited rotenone-mediated apoptosis and endoplasmic reticulum stress by downregulating heat shock protein 70 and poly(ADP-ribose) polymerase-1 cleavage. EA was also capable of decreasing the concentrations of synphilin-1 and S-nitrosylation of protein disulfide isomerase (SNO-PDI; an index of nitrosative stress) and aggregation of α-synuclein. Accordingly, EA action suppressed the production of α-synuclein–synphilin-1 Lewy body–like inclusions in that experimental model, suggesting a capability to defend dopaminergic cells from hallmarks of PD by inhibiting SNO-PDI production and aggregation of protein and its accumulation within neuronal cells. It would also be beneficial to explore the function of EA in differentiated PC12 cells because of molecular discrepancies that can increase due to cellular differentiation, as recommended by other investigators (151, 152). Moreover, previously it was stated that EA derivatives (3,3′-di-O-methylellagic acid and 3,3′-di-O-methyl ellagic acid-4-O-β-d-xylopyranoside) were capable of promoting neuronal differentiation in epidermal growth factor–responsive neural stem cells (153). Nevertheless, further information is required to reveal the mechanisms by which EA promotes protection in these neuron-like cells. Assessment of neurotrophin metabolism (like expression and secretion) and signaling pathways involved in neuroprotection can be studied further.
Ischemic stroke reperfusion/hypoperfusion
Ischemic stroke is the most common cause of severe morbidity and the second leading cause of death in developed countries (154). Nearly one-third of patients affected by an acute ischemic stroke develop early neurological deterioration or neuropathy, which is related to enhanced mortality and long-term functional disability (155). Although a degree of spontaneous recovery of lost functions takes place in some stroke patients, the majority never regain full functional independence and ultimately suffer from a reduced quality of life (156). A group of researchers evaluated the neuroprotective mechanism of EA in ischemic stroke. In an oxygen-glucose deprivation and reoxygenation model in cultured cortical neurons, it was found that EA (10 and 30 μg/mL) appreciably increased survival rates, reduced the number of apoptotic/necrotic cells, and increased the ratio of Bcl-2/Bax expression (136).
EA treatment in a photothrombotic cerebral ischemia model significantly reduced infarct volumes, and increased the number of Bcl-2–positive neurons and the ratio of Bcl-2/Bax expression in the semidarkness zone near the brain ischemic focus, and remedied neurological deficit scores in rats (157). Nejad et al. (158) reported that ischemia-reperfusion reduced blood pressure (BP) and augmented heart rate in rats whereas EA (100 mg/kg) pretreatment enhanced BP and decreased heart rate in the ischemic rats. Furthermore, the electrocardiogram indicated that ischemia shortened the QRS and PR intervals. Conversely, EA-treated rats showed improved QRS voltage and enhanced PR interval compared with the ischemic group. In another study, Maryam and Khadijeh (159) demonstrated the neuroprotective effect of EA (50 mg/kg) on brain oxidative stress indices after permanent bilateral common carotid artery occlusion or ischemia/hypoperfusion in rats. Cerebral ischemia caused ROS overproduction and ultimately activated the pathways leading to cellular death in vulnerable areas of the brain (160). The outcome of this study indicated that EA appreciably reduced the MDA and thiol concentrations in hippocampus (HPC) tissue in the ischemia group, which was correlated with its antioxidant efficacy.
Antitumor effect of EA
Glioblastoma is the most common type of malignant tumor of the nervous system reported in humans. Kim et al. (161) studied the chitosan/β-glycerophosphate (Ch/β-GP) thermosensitive gel to transport EA as an approach to manage brain cancer. Previously, chitosan had been used to sustain drug release to the target sites (162). The EA-containing Ch/β-GP gel (5.5 mg or 10 mg EA) remarkably diminished the viability of human glioblastoma (U87) and rat glioma cell lines (C6) in a time- and concentration-dependent manner. The mechanisms of these effects were not explored by the researchers but they suggested that they could include both extrinsic and intrinsic pathways of apoptosis. In a separate in vitro study, Kim et al. (163) demonstrated that EA/Ch biomaterial suppressed glioma growth by upregulation of p53 (C6 cells) and activation of caspase-3 in both U87 and C6 cell lines. EA/Ch also diminished angiogenesis in an experimental model using human umbilical vein endothelial cells. Furthermore, Fourier transform infrared spectra suggested that protonation of phosphate groups in the Ch gel–forming solution occurred with the rise in temperature that triggers gelation. Thus, the Ch/EA combined film is a very curative technique to suppress tumor growth by promoting apoptotic cell death.
Antidepressant activity of EA
Depression is the most common condition that develops after TBI and usually occurs due to an alteration in monoaminergic functions (164). Several drugs recommended for the management of depression are inhibitors of monoamine oxidase A (MAO-A); these exert therapeutic action by enhancing synaptic monoamine concentrations (165) and also by controlling hydrogen peroxide overproduction (an end-product of the deamination reaction catalyzed by MAO-A) (166). In fact, most prescribed antidepressant drugs directly affect serotonin turnover in the brain (167), inhibit serotonin reuptake, and interact with serotonin (5-hydroxytryptamine) 5-HT1 and 5-HT2 receptors (168). The in vitro antidepressant efficacy of EA was evaluated by AChE, butyrylcholinesterase (BuChE), and MAO-A inhibition assays. Observations revealed that EA showed appreciable MAO-A inhibitory activity (EC50 = 1.6 μg/mL). However, even at the highest concentration tested (250 mg/mL), EA exhibited 16.0% and 23.8% of BuChE and AChE inhibition, respectively (88). Girish et al. (129) evaluated the acute and chronic antidepressant activity of EA using a forced swimming test (FST) and tail suspension test (TST) in mice. During this study, EA showed a dose-dependent (20, 50, and 100 mg/kg) reduction in the duration of immobility time, which was comparable to that observed with the marketed antidepressant drug fluoxetine, a selective serotonin reuptake inhibitor. Pretreatment with p-chlorophenylalanine (PCPA) prevented the anti-immobility effect induced by EA in the FST, suggesting the involvement of the serotonergic system in the antidepressant-like effect of EA (129). Furthermore, repeated administration of antidepressants increased the number and responsiveness of α-1 receptors in the rat brain, thus increasing the release of noradrenaline at certain synapses (169, 170). The decrease in immobility time elicited by EA was reversed by pretreatment of mice with prazosin (an α-1 adrenoceptor blocker) and yohimbine (an α-2 adrenoceptor blocker), indicating the involvement of these receptors in the antidepressant-like action of EA in the FST (129). The exact mechanism by which EA modulates the monoaminergic system is unclear. It might inhibit the MAO enzymes and cause an increase in the amount of monoamines stored and released from the nerve terminals, thus increasing monoaminergic activity (171).
Bedel et al. (172) demonstrated that sertraline (an antidepressant drug) and EA did not alter the impulsive locomotor behavior of mice. EA exhibited a significant reduction in immobility time in the TST. EA (2.5 mg/kg) appreciably reduced immobility time compared with other test concentrations (1 and 5 mg/kg) in the FST. EA treatment enhanced the hippocampal brain-derived neurotrophic factor (BDNF) concentration. Moreover, the antidepressant-like activity of EA appears to be facilitated by an enhanced concentration of BDNF in mouse HPC (172). In a separate study, the antianxiety-like property of acute and chronic administration of EA was evaluated by an elevated plus-maze (EPM) test in mice. This assay provided an excellent tool for analyzing benzodiazepine/γ-aminobutyric acid (GABA) and glutamate-related compounds (173). The anxiolytic-like effect of EA (at 25 mg/kg) was observed in the elevated plus-maze test, suggesting the absence of tolerance to this effect after chronic administration. However, lower (6 and 12.5 mg/kg) and higher concentrations (50 and 100 mg/kg) of EA did not exhibit a significant anxiolytic effect. It is possible that EA could be acting at additional sites that could mask or block its anxiolytic-like actions at high doses (173). The anxiolytic property noticed with EA treatment (25 mg/kg) was antagonized by pretreatment with flumazenil (a benzodiazepine site antagonist) and picrotoxin (a noncompetitive GABAA receptor antagonist) but not with pindolol (a β-adrenoceptor blocker/5-HT1A/1B receptor antagonist) or PCPA (a serotonin synthesis inhibitor). Pretreatment with picrotoxin completely blocked the actions of EA, but not those of diazepam. It was suggested that EA could interact with a GABAA receptor to mediate its anxiolytic-like actions (173).
EA and epileptic seizures
Epileptic seizures are associated with high energy formation and consumption. These changes are related to raised oxidative stress due to overproduction of free radicals, predominantly from the mitochondria (174). Picrotoxin is a GABAA-receptor antagonist that produces seizures by inhibiting the chloride ion channels associated with GABAA receptors. Pentylenetetrazol (PTZ) triggered seizures by inhibiting GABAA receptors and preventing chloride ion influx, promoting activation of excitatory neurons and the development of seizures (175). Administration of EA (20 and 40 mg/kg) appreciably reversed both the PTZ- and picrotoxin-triggered convulsions and the reduction of GABA concentrations in the brain. Therefore, EA displayed noteworthy antiepileptic activity, probably by enhancing brain GABA concentrations in mice (176). El-Missiry et al. (177) demonstrated that EA coated in calcium-alginate nanoparticles (Ca2+-EA-ALG NPs) displayed more curative anticonvulsant and neuroprotective potential than unencapsulated EA (50 mg/kg) against PTZ-mediated brain impairment and convulsions in mice. In PTZ-abused mice, Ca2+-EA-ALG NPs significantly ameliorated oxidative stress, as demonstrated by decreased 4-hydroxynonenal concentrations and augmented GSH content, with a rise in the activities of glutathione reductase and glutathione peroxidase (GPx) in the brain. Homocysteine is an endogenous glutamate receptor agonist that provokes the intraneuronal fibrillar conformation of Aβ, thus triggering neurodegeneration (178). Ca2+-EA-ALG NPs normalized the upsurge in homocysteine and Aβ in plasma and brains and protected GABA activity, probably by augmentation of GABAergic neurotransmission. A combination of oxidative stress and mitochondrial membrane destabilization leads to the discharge of cytochrome c (cyt c) and activation of apoptotic proteins. These activities are evaluated through the upregulation of cyt c, Bax, caspase-3, and caspase-9 with suppressed antiapoptotic protein Bcl-2 expression in PTZ-harmed mice brain. EA might act as an inhibitor of caspase, protecting DNA from damage and the release of apoptosis-inducing factors because of its antioxidant capacity. Additionally, histopathological analysis suggested that Ca2+-EA-ALG NPs decreased PTZ-induced morphological alterations and degeneration in hippocampal tissue (177).
EA and diabetic neuropathy
Diabetic encephalopathy and neuropathy are serious health concerns in humans associated with diabetes mellitus (179). These complications occur because of the metabolic repercussions of abnormal glucose regulation, particularly sorbitol accumulation, enhanced glycosylated protein concentrations, and oxidative/antioxidative imbalance within the nerve tissues and ultimately in the brain. Additionally, diabetes-associated oxidative stress can lead to apoptosis in nervous tissues (180). Uzar et al. (181) explored the therapeutic attributes of EA in sciatic nerve tissues and in the brain of diabetic rats by analyzing the TAC, CAT, and paraoxonase (PON-1) activities, oxidative stress index, total oxidant status (TOS) and NO and MDA concentrations. NO overproduction leads to the formation of peroxynitrite with superoxide anions that result in oxidative injury to the brain and sciatic nerve tissues. PON-1 is related to HDL and is found to decrease the susceptibility of LDL to LPO. EA (50 mg/kg) administration in diabetic rats exerts a notable decrease in TOS, NO, and MDA formation in sciatic tissues and brain. The decline in NO concentrations caused by EA could elucidate its role in the prevention of inducible nitric oxide synthase (iNOS) expression (157). Moreover, it has been noted that EA exhibited protection against oxidized LDL-mediated atherogenic signaling by prevention of ROS production (182).
Kumar and Bansal (183) demonstrated the role of phosphoinositide 3-kinase (PI3K) and endothelial nitric oxide synthase (eNOS) actions in the prevention of streptozotocin (STZ)-mediated memory dysfunction in rats by EA. They performed EPM and Morris water maze tests and quantified the oxidative stress markers in the brain (GSH, SOD, CAT, and lipid peroxides), nitrite, AChE, lactate dehydrogenase (LDH), TNF-α, and PI3K-eNOS. Previous studies reported that STZ-activated insulin resistance and decline in cerebral blood circulation are congruous with loss of eNOS and PI3K signaling (184, 185). Furthermore, excessive lipid peroxidation and decreased concentrations of antioxidant enzymes (CAT, SOD, and GSH) in brain altered the neuronal coordination and cognitive proficiency (186, 187). Moreover, TNF-α production by astrocytes and reactive microglia in response to immunogenic brain injury (e.g., STZ, TBI, or Aβ) is a distinctive hallmark of the AD-affected brain (188). STZ, a derivative of nitrosourea, is metabolized to NO, which stimulates the activity of iNOS (183) and reduces the expression and action of eNOS by PI3K dysfunction (189). Administration of EA (35 mg/kg) attenuated the thiobarbituric acid-reactive substances and prevented the reduction of TNF-α, CAT, GSH, and SOD activity in diabetic rats. EA suppressed the STZ-mediated abnormal upsurge of nitrite content and downregulated eNOS concentration in rat brain. Oxidative stress compromises the neuronal integrity that is quantified by measurement of LDH activity within the brain. EA treatment attenuated the elevated LDH activity and concomitant neurodegeneration in diabetic rats (183).
Jha et al. (107) explored the therapeutic effect of EA in STZ-associated sporadic Alzheimer disease (SAD) in the context of altered biochemical and behavioral features in rats. They analyzed the effects of STZ upon the whole HPC, that is, the cornus ammonis-1 (CA-1), CA-2, and CA-3, dentate gyrus (DG), and entorhinal cortex. STZ-induced SAD displayed a pathological resemblance to human SAD, particularly with regard to oxidative damage, cholinergic abnormality, neuroinflammation, neural death, and cognitive impairments. The role of EA in the inhibition of proinflammatory and disease augmentation markers was assessed by quantifying glial fibrillary acidic protein (GFAP), whereas enhancement of synaptic connectivity was investigated via quantification of synaptophysin. Findings suggested that EA (50 mg/kg) treatment suppressed STZ-triggered SAD and its related biochemical irregularities in rats. EA reduced oxidative stress status, proinflammatory markers (i.e., GFAP and C-reactive protein), AChE concentration, and Aβ plaque concentration. Additionally, a higher concentration of synaptophysin showed recovered synaptic connectivity, and integral neural construction demonstrated the neuroprotective role of EA. Moreover, behavioral analysis using maze paradigms showed declined locomotor behavior, unbalanced spontaneous changes, reduced memory score, and elevated memory errors in SAD rats. EA medication normalized these SAD-triggered irregular behavioral features in rats (107). These findings provide a clear picture that EA-rich supplements might act as a tonic for the brain or have an enormous therapeutic potential for neurodegenerative disorders and memory deficits.
TBI
TBI causes sickness and mortality throughout the world. It occurs in 2 stages: primarily, through the destruction of the brain tissue and blood vessels, which stimulates complex physiological processes including cellular and molecular events; and secondarily, through the processes that occur hours to days after TBI, which lead to further alteration of neurons and axons (190, 191). A variety of studies reported that TBI causes permanent physical, cognitive, social, and functional impairments along with chronic pain disorders (192). Mild TBI is the most easily identified form, which is responsible for ∼80% of total cases. Falls and deliberate self-harm are the main causes of all TBI-associated deaths (33%) and hospitalizations (52%) (193). Farbood et al. (194) evaluated the therapeutic potential of EA for cognitive impairments, long-term potentiation (LTP) deficits in the HPC, and brain inflammation triggered by diffuse TBI in rats. They evaluated the concentrations of IL-1β and IL-6, and blood–brain barrier (BBB) permeability in the rat. TBI causes notable deficits in passive avoidance memory, which has been associated with a remarkable decline in hippocampal LTP, as well as reduced BBB permeability with an upsurge in concentrations of IL-1β and IL-6 in brain tissue. Previous studies suggested that IL-6 and IL-1β are involved in the cellular and molecular mechanisms of learning and memory (194, 195). Further, IL-6 caused a noteworthy decline in expression of LTP, which affects hippocampal synaptic plasticity (196). IL-1β acts as a neuromodulator in the HPC (197), and the maintenance of LTP in the HPC relies on it (198, 199). A separate group of researchers found that 3 events altering the magnitude of LTP can be influenced by IL-1β: the function of N-methyl-d-aspartate receptors, glutamate release modulation, and calcium channel influx (200). Administration of EA (100 mg/kg) before TBI induction preserved the hippocampal LTP due to a marked reduction in IL-1β and IL-6 content of the brain. Moreover, EA pretreatment decreased concentrations of IL-1β and IL-6 toward normal concentrations in the brain (194).
Mashhadizadeh et al. (201) demonstrated the therapeutic attributes of EA for HPC electrophysiology deficits, memory, and higher concentration of TNF-α in TBI. They analyzed BBB permeability, passive avoidance task data, hippocampal LTP, and TNF-α concentration in brain tissues. Various studies described that TNF-α has both homeostatic as well as pathophysiological functions in the CNS (202, 203). The homeostatic action of TNF-α controls vital physiological activities, including learning and memory (204, 205), synaptic plasticity (206, 207), and astrocyte-triggered synaptic strengthening (208). During the pathological condition, TNF-α is produced in higher amounts by microglia and astrocytes, which take part in the neuroinflammatory response related to a wide range of neurological disorders (209, 210). Further, the growth of the HPC is regulated by TNF-α (211), possibly via activation of tumor necrosis factor receptor 2, which does not lead to caspase-3 activation, but is known to transduce the trophic effect of TNF-α (212). It has been observed that at pathophysiological concentrations TNF-α prevented LTP initiation in the CA1 and DG areas of the rat HPC (213, 214). EA treatment (100 mg/kg) appreciably restored TBI-mediated behavioral aberrations (impaired passive avoidance memory), and restored the field excitatory postsynaptic potential slope and population spike amplitude in TBI rats. Moreover, EA re-established the BBB activity along with the restoration of TNF-α, which could explain the effects of EA on astrocyte enhancement due to its anti-inflammatory actions (202).
Chemical Agent–Induced Neurodegenerative Disorders
Cadmium-induced oxidative stress
Cadmium ions (Cd2+) are extensively used in a large number of industrial sectors and are well-known carcinogens that lead to the development of various types of tumors in humans (215). Cd2+-mediated injury of astrocytes apparently resulted from GSH depletion (216). Astrocytes are the most abundant of glial cells that perform several functions in the CNS. They maintain ionic and neurotransmitter homeostasis, regulate synaptic transmission, provide energy substrates to neurons, and trigger neurogenesis (176). Yang et al. (134) reported that treatment with EA (30 μM) appreciably suppressed ROS formation and astrocytic cell death due to Cd2+ exposure in rats.
Doxorubicin-induced neurotoxicity
Previous studies reported that prolonged administration of doxorubicin (DOX) causes neurotoxicity and leads to the development of neuropsychiatric disorders, such as depression and impaired cognition function (217–219). DOX-triggered free radical formation in brain tissue enhanced LPO and challenged the antioxidant defense system, thus confirming the pro-oxidative effect of DOX (219, 220). Furthermore, DOX-mediated overproduction of superoxide anions could stimulate circulating concentrations of TNF-α, which can directly cross the BBB. Increased production of superoxide anions can also stimulate glial cells to trigger the formation of other proinflammatory cytokines, including NF-κB, IL-1, IL-6, and iNOS, which exacerbate the oxidative stress and neural apoptosis (221, 222). Rizk et al. (223) demonstrated that co-administration of EA (10 mg/kg) along with DOX suppressed brain MDA, reduced TNF-α and caspase-3 concentrations, and improved the GSH concentration in rats (Table 4). Additionally, EA prevented PGE2 action and suppressed the production of COX-2, and thus reduced TNF-α concentration. Further, EA elevated brain monoamines, which supports its antidepressant-like activity, which is facilitated by an interaction of EA with the noradrenergic and serotonergic systems (223). Other studies revealed that the radical-scavenging mechanism of EA might be associated with the direct scavenging action on both hydroxyl anions and superoxide anions, as well as indirect action through the induction of antioxidant enzymes in rats (224, 225).
TABLE 4.
In vivo neuroprotective effects of ellagic acid in various animal models1
| Neurotoxin | Animal model | Duration of study | Dose of EA (mg/kg) | Route of administration | Biomarkers | Observations | References |
|---|---|---|---|---|---|---|---|
| DOX | Male Sprague Dawley rats | 14 d | 10 | Oral | Brain MDA, TNF-α, iNOS, caspase-3, COX, cholinesterase GSH, monoamines | ↓MDA, ↓TNF-α, ↓iNOS, ↓caspase-3, ↓COX | (223) |
| ↓cholinesterase | |||||||
| ↑GSH, ↑monoamines | |||||||
| SA | Male Wistar rats | 21 d | 10 and 30 | Oral | MDA, NO, PCO, TNF-α, and IL-1β production, TAC, GSH, and GPx activity | ↓MDA, ↓NO, ↓TNF-α, ↓IL-1β, ↓PCO↑TAC, ↑GSH, ↑GPx | (228) |
| Arsenic-induced neuroinflammation | Wistar rats | 11 d | 20 and 40 | Oral | Total ROS, DNA fragmentation, BAX, IL-1β, TNF-α, IFN-γ, and mitochondrial membrane potential | ↓Total ROS, ↓TNF-α, ↓IFN-γ, ↓DNA fragmentation, ↓BAX, ↓Bcl-2, ↓IL-1β | (230) |
| ↑Mitochondrial membrane potential | |||||||
| ACR | Male Wistar rats | 30 d | 30 | Oral | MDA, NO, IL-1β and TNF-α concentrations, SOD, GPx, CAT activity | ↓MDA, ↓NO, ↓TNF-α, ↓IL-1β ↑Glutathione, ↑SOD, ↑GPx, ↑CAT | (234) |
| Cup | C57BL/6J mice | 4 wk | 40 and 80 | Oral | Oligodendrocyte apoptosis, IL-11, IL-17, SDF-1a or Cxcl12, | ↓Apoptosis, ↓macrophage activity, ↓IL-17 | (239) |
| ↑Mature oligodendrocyte population, ↑IL-11 | |||||||
| TCDD | Sprague Dawley female rats | 13 wk | 1 | Oral | Superoxide anion, LPO, and DNA single-strand breaks | ↓Superoxide anion, ↓LPO, ↓DNA single-strand breaks | (242) |
| Male Wistar rats | 10 d | 50 | Antioxidant enzyme activities and glutathione concentrations | ↑SOD, ↑CAT, ↑GSH, and ↑GPx | (243) | ||
| CCl4-induced brain injury | Male Wistar rats | 8 wk | 10 | Intraperitoneal | TNF-α, NF-κB, Nrf2, caspase-3, VEGF and Bcl-2 protein expression along with MDA, CAT, and GSH concentrations | ↓VEGF, ↓NF-κB, ↓TNF-α, ↓Bcl-2, ↓MDA↑Caspase-3, ↑Nrf2, ↑CAT, ↑GSH | (244) |
| Scopolamine and diazepam | Male Wistar rats and mice | 10 d | 10, 30, and 100 | Oral | Elevated plus maze and passive avoidance | ↓Amnesia and restored memory dysfunction | (245) |
| 6-OHDA | Wistar rats | 10 d | 50 | Oral | Stride length and cylinder tests and analysis of TNF-α and IL-1β concentrations | ↓Contralateral rotation, ↓TNF-α, ↓IL-1β | (246) |
| ↑Stride-length | |||||||
| Male Wistar rats | 14 d | 50 | MDA, SOD, GPx, stride-length, bar decent latency and frequency bands' power of pallidal EEG | ↓MDA, ↓stride-length, ↓bar decent latency and frequency bands' power of pallidal EEG | (247) | ||
| ↑SOD, ↑GPx | |||||||
| 10 d | Tail-flick and hot-plate tests and Morris water maze test | ↓Oxidative stress | (248) | ||||
| 1 wk | 50 | Rotational test, elevated narrow beam test, oxidative stress and MAO-B, S100, Nrf2, DNA damage, and HO-1 assessment | ↓MDA, ↓ROS, ↓DNA fragmentation↑MAO-B, ↑Nrf2, ↑HO-1 | (249) | |||
| PTZ | Swiss male albino mice | 14 d | 20 and 40 | Oral | Onset of convulsions, brain GABA concentration | ↑Onset of convulsions, ↑brain GABA concentration | (250) |
| Swiss male albino mice | 33 d | 50 | Homocysteine, Aβ1–42, GABA, glutamate, 4HNE, GSH, GR, GPx, TNF-α, IL-6, and cyt c concentration assessment | ↑GABA, ↑GSH, ↑GR, and ↑GPx↓Glutamate, ↓homocysteine, ↓ 4HNE, ↓cyt c, ↓p53, ↓Bax, ↓Bcl-2, ↓caspase-3, ↓caspase-9, and ↓DNA damage | (177) | ||
| D-gal-induced aging | Male Sprague Dawley rats | 8 wk | 50 | Oral | Antioxidative, anti-inflammatory, and anti-apoptotic potential | ↑SOD, ↑CAT, ↑GPx, and ↑TAC | (139) |
| ↓MDA, ↓TNF-α, ↓IL-6, and ↓IL-1β | |||||||
| Diabetic neuropathy | Female Wistar rats | 28 d | 50 | Oral | Analysis of CAT and PON-1 activities, TAS, TOS, OSI, MDA, and NO | ↓MDA, ↓TOS, ↓OSI, and ↓NO | (181) |
| ↑CAT, ↑PON-1, and ↑TAS | |||||||
| Wistar rats | 4 wk | 35 | Assessment of brain oxidative stress markers, nitrite, LDH, TNF-α, AChE, and eNOS | ↓Brain oxidative stress, ↓nitrite, ↓TNF-α, ↓AChE, and ↓LDH | (183) | ||
| Sporadic Alzheimer disease | Wistar rats | 5 wk | 50 | Oral | Evaluation of oxidative stress, AchE pool, Aβ plaque formation and inflammatory response, elevated synaptic plasticity, and mitochondrial energetics | ↓Oxidative stress profile, ↓proinflammatory markers ↑Synaptophysin | (107) |
| Ischemic stroke/reperfusion/hypoperfusion | Male Sprague Dawley rats | 2 d | 10 and 30 | Oral | Photothrombotic nerve injury and determination of the neurological function score | ↓Volume of cerebrum infarction, ↓neurological deficit scores | (136) |
| ↑Neuronal viability, ↑cell nuclear viability | |||||||
| Male Wistar rats | 10 d | 100 | Blood pressure, heart rate, MDA and EEG determination | ↓MDA concentration and restored the heart rate and blood pressure | (158) | ||
| 14 d | 50 | Measurement of MDA and thiol (-SH) group | ↓MDA, ↓thiol (-SH) | (159) | |||
| TBI | Male Wistar rats | 7 d | 100 | Oral | Analysis of passive avoidance memory and hippocampal LTP, brain content of IL-1β and IL-6, and BBB permeability | ↓Memory and hippocampal LTP impairments, ↓IL-1β, ↓IL-6, and ↓BBB permeability | (194) |
| 4 d | Intraperitoneal | PAT, hippocampal LTP, BBB permeability, and TNF-α content | ↓Neurologic severity score, ↓BBB permeability, ↓cognitive and hippocampal LTP abnormalities, ↓TNF-α | (201) | |||
| Antidepressant activity | Female albino mice | 14 d | 25, 50, and 100 | Oral | Forced swimming test and tail suspension test | Antidepressant-like effect of EA is mediated by the serotonergic and noradrenergic systems | (129) |
| Mice | 1, 2.5, and 5 | EA (2.5 mg/kg) reduced immobility time with increased hippocampal BDNF concentration | (172) | ||||
| Male albino mice | 25, 50, and 100 | Elevated plus-maze test and involvement of the GABAergic and serotonergic systems in antianxiety activity | ↑Percentage of time spent and entry into the open arms | (173) |
1Aβ, β-amyloid; AChE, acetylcholinesterase; ACR, acrylamide; BBB, blood–brain barrier; BDNF, brain-derived neurotrophic factor; CAT, catalase; COX, cyclooxygenase; Cup, cuprizone; Cxcl12, C-X-C motif chemokine 12; cyt c, cytochrome C; D-gal, d-galactose; DOX, doxorubicin; EA, ellagic acid; EEG, electroencephalography; eNOS, endothelial nitric oxide synthase; GABA, γ-aminobutyric acid; GPx, glutathione peroxidase; GR, glutathione reductase; GSH, reduced glutathione; HNE, hydroxynonenal; HO-1, heme oxygenase-1; iNOS, nitric oxide synthase; LDH, lactate dehydrogenase; LPO; lipid peroxidation; LTP, long-term potentiation; MAO-B, monoamine oxidase-B; MDA, malondialdehyde; Nrf2, nuclear factor erythroid 2-related factor-2; OSI, oxidative stress index; PAT, passive avoidance task; PCO, protein carbonylation; PON-1, paraoxonase; PTZ, pentylenetetrazol; ROS, reactive oxygen species; SA, sodium arsenite; SDF-1a, stromal cell-derived factor 1a; SOD, superoxide dismutase; TAC, total antioxidant capacity; TAS, total antioxidant status; TBI, traumatic brain injury; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TOS, total oxidant status; VEGF, vascular endothelial growth factor; 6-OHDA, 6-hydroxydopamine.
Sodium arsenite/arsenic–induced neurotoxicity
Arsenic is an environmental neurotoxin that is ubiquitous due to its natural existence and anthropogenic sources. Slow nerve conduction velocity and sensory and motor dysfunctions have also been documented in populations exposed to arsenic (195, 196). It can cross the BBB and accumulate in the brain tissue, which further leads to the development of neurobehavioral abnormalities (226, 227). Furthermore, sodium arsenite (SA) induces substantial neurotoxicity as confirmed by increased oxidative stress parameters, namely protein carbonylation (PCO), MDA, and nitric oxide concentrations along with a decline in GPx, GSH, and TAC concentrations in the brain tissue. Goudarzi et al. (228) observed that exposure of rat brain to SA caused neurotoxicity and adversely altered GSH content, GPx activity, and TAC, MDA, TNF-α, PCO, nitric oxide, and IL-1β concentrations. EA treatment improved the biochemical profile and gave rise to both morphological and functional modifications of the rat brain. Another study suggested that EA induced γ-glutamylcysteine synthetase activity and regulated intracellular GSH concentration in mouse brain tissue (229).
Firdaus et al. (230) determined the therapeutic efficacy of EA in arsenic-mediated neurotoxicity associated with mitochondrial dysfunction and inflammation in the HPC of rats. Their observations indicated that EA (20 and 40 mg/kg) restored the alteration in mitochondrial membrane potential in arsenic-treated rats in a dose-dependent manner, which could be attributed to its antioxidant activity, probably by a direct action on the mitochondrial membrane redox status. Further, there was enhanced ROS control of the expression of proinflammatory cytokines by stimulation of MAPK and protein kinase C, as confirmed by enhanced mRNA abundances of proinflammatory markers (IL-1β, IFN-γ, and TNF-α) and protein synthesis of IFN-γ and TNF-α in arsenic-insulted rats. Co-administration of EA upregulated Bcl-2 mRNA expression, decreased DNA fragmentation, downregulated caspase-3 activity, and decreased mRNA expression of IL-1β, IFN-γ, TNF-α, and Bax in arsenic-exposed rats in a dose-dependent manner (230).
Acrylamide-induced neurotoxicity
Tobacco smoke is a leading cause of acrylamide (ACR) exposure, which can lead to central and peripheral neurotoxicity (231). ACR decreased hindlimb grip strength, reduced locomotor activity, and enhanced heel splay in rats (232). Moreover, it exerted a negative impact on the growth and development of hippocampal neurons in juvenile rats (233). Goudarzi et al. (234) studied the curative efficacy of EA against ACR-mediated neurotoxicity in rats and suggested that treatment with EA (30 mg/kg) improved the locomotor discrepancies and muscle weakness within a limited range via anti-inflammatory activity (234). EA also restored the concentrations of cellular antioxidants in brain tissue and demonstrated that ACR toxicity targeted the antioxidant enzymes, whereas EA re-established the redox equilibrium by augmenting the antioxidant property (234). ACR triggered oxidative stress and upregulated the inflammatory responses by excessive production of inflammatory factors, including IL-1β and TNF-α (235, 236). EA suppressed the upsurge of TNF-α and IL-1β in rat brains exposed to ACR and prevented neurotoxicity (234).
Cuprizone-induced neurotoxicity
Cuprizone (bis-cyclohexanone-oxalyldihydrazone; Cup) is a copper-based chelating agent and is commonly used to study the factors involved in myelin loss and oligodendrocyte (OLG) death (237). Cup alters the normal metabolism of OLG and causes primary OLG dystrophy rather than autoimmunity (238). Sanadgol et al. (239) studied the effect of EA in Cup-induced specific apoptosis in OLGs along with key neuroimmune mediators like IL-11, IL-17, and CXCL12 during harmful demyelination in mice. The CXCL12 chemokine performs a vital role in the maturation of both the right and left hemispheres of the adult brain. CXCL12 binds to its receptor (Cxcr4) on the surface of the OLG precursor cell and triggers its maturation and differentiation processes (240). Cup upregulates the expression of CXCL12 and is frequently accompanied by reactive microgliosis and monocyte infiltration into the damaged site. Overexpression of IL-11 can restrict the Cup-mediated demyelination process by reducing microgliosis and OLG cell death, and promoting spontaneous repair (241). EA administration (40 and 80 mg/kg) triggered the expression of IL-11, controlled OLG loss, and avoided the consequent demyelination. Although EA did not noticeably alter the CXCL12 concentration in the corpus callosum region, it did notably decrease monocyte infiltration and microgliosis. Also, EA reduced IL-17 expression and lymphocyte transmigration across the BBB, at mRNA and protein levels. During EA treatment, remarkable reduction of IL-17 concentrations was observed with the maintenance of BBB integrity and inhibition of microglial movement (241).
Tetrachlorodibenzo-p-dioxin–induced neurotoxicity
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a notorious environmental pollutant that accumulates in the brain (250). TCDD binds with the aryl hydrocarbon receptor and forms a complex that subsequently translocates into the nucleus and alters regulation of numerous genes, which can lead to various biochemical changes (251, 252). A study on brain region specificity of TCDD-triggered oxidative stress showed marked formation of ROS and LPO in the HPC and cerebral cortex but not in the brainstem or cerebellum of rats (253). Hassoun et al. (242) studied the neuroprotective potential of EA against TCDD-mediated oxidative stress in the cerebral cortex, HPC, cerebellum, and brainstem regions of rat brain by analyzing superoxide anion, LPO, and DNA single-strand breaks (SSBs). EA (1 mg/kg) reduced the concentration of superoxide anions, LPO, and SSBs. The binding of EA to DNA, which prevents the interaction of the TCDD-receptor complex with the nucleus, might be a possible reason for its neuroprotective activity. A further study performed by Hassoun et al. (243) revealed that co-treatment of EA (50 mg/kg) with TCDD for 3 mo did not show marked alteration in CAT and SOD activities in cerebral cortex, HPC, cerebellum, or brainstem regions of the rat brain, whereas an appreciable rise in GSH and GPx concentrations was observed in all 4 regions (254, 255).
Carbon tetrachloride–induced brain injury
Neuronal and hepatic tissues rapidly take up and metabolize carbon tetrachloride (CCl4) into highly toxic ROS-like trichloromethyl (•CCl3) and trichloromethyl peroxy (•OOCCl3) radicals (256, 257). Brain tissues are presumed to be more prone to LPO caused by ROS, which leads to accumulation of PUFAs in the neuronal membranes (258, 259). Aslan et al. (244) validated the neuroprotective role of EA (10 mg/kg) in CCl4-induced brain injury in rats using apoptotic, inflammatory, and antioxidant markers, such as TNF-α, NF-κB, COX-2, Nrf2, caspase-3, vascular endothelial growth factor, Bcl-2, MDA, CAT, and GSH. Amplified TNF-α expression is associated with numerous pathological conditions including AD, cancer, and inflammatory responses (260). Co-administration of EA restored the concentration of various biomarkers, suggesting neuroprotective attributes (244).
Scopolamine- and diazepam-induced cognitive impairments
Cognitive impairment is a condition when an individual experiences difficulty in learning new things, memorizing, focusing, or decision-making that affects their everyday life. It can range from mild to severe (261). Scopolamine is commonly used to treat motion sickness and postoperative nausea and vomiting, whereas diazepam is used to treat anxiety, alcohol withdrawal, and seizures. Prolonged treatment with these drugs can lead to memory loss. Mansouri et al. (245) studied the efficacy of EA as a memory booster in rats and mice to counter scopolamine- and diazepam-induced cognitive impairments. They observed that EA (10, 30, and 100 mg/kg) pretreatment produced a noteworthy drop in the latency times in a retention trial of the EPM and passive avoidance tests. Moreover, in mice, long-term intake of EA reinstated diazepam-mediated damage of memory retention in an EPM task. In normal mice and rats, EA did not cause any alteration in the acquisition/retention of memory. Furthermore, the investigators observed that the radical scavenging activity is one of the characteristic features of EA which is accountable for its cognitive impairment activity. This suggests that EA can suppress the memory impairment caused by scopolamine and or diazepam, but it is not a true memory booster (245).
6-Hydroxydopamine-induced PD
PD is described as an age-related neurodegenerative disorder that develops due to progressive death of striatum-projecting dopaminergic neurons in the substantia nigra pars compacta (SNc). This results in various types of movement insufficiency, such as slowed motion, resting tremor, impaired gait functioning, muscle stiffness, and postural instability (262). Although the etiology of PD is not fully described, it is assumed that some factors that trigger oxidative imbalance, such as genetic susceptibility, free radical production, mitochondrial dysfunction, brain aging, and environmental toxins, play a role (263). Most notably, the oxidation of dopamine produces ROS, and this uncontrolled formation of ROS leads to oxidative stress and neuronal death. Pathogenesis of PD has also been linked to dietary habits, where deficiency of antioxidant components, such as vitamins (A, C, E, and niacin) and selenium, were shown to increase the risk of PD (264). 6-Hydroxydopamine (6-OHDA) exerts a neurotoxic effect primarily because of its oxidation by monoamine oxidase or molecular oxygen in the brain. This, in turn, produces intracellular hydrogen peroxide, which can be converted into more reactive hydroxyl radicals. Additionally, this hydrogen peroxide reduces GSH and SOD activity and elevates MDA concentrations, and the production of superoxide free radicals causes neuronal damage (265). Another study demonstrated that injection of 6-OHDA into the medial forebrain bundle (MFB) enhanced contralateral apomorphine-induced rotation and could exert a neurotoxic effect by impairing the nigrostriatal pathway, leading to enhanced contralateral rotations (266).
Farbood et al. (246) studied the neuroprotective potential of EA by analyzing motor activity via stride-length and cylinder tests as well as estimating IL-1β and TNF-α concentrations in both striatum and HPC tissues in 6-OHDA–induced MFB-lesioned rat brains. Their findings indicated that EA (50 mg/kg) significantly lowered the IL-1β and TNF-α concentrations in the striatum and HPC tissue and restored the increased contralateral rotation. Moreover, EA improved the cerebral antioxidant defense system that reduces oxidative stress and hence exerts a neuroprotective action against 6-OHDA–mediated neural oxidative damage. Further examination by Sarkaki et al. (247) revealed that EA (50 mg/kg) administration appreciably restored the concentration of MDA and elevated GPx and SOD activities in both striatum and HPC tissues in a 6-OHDA–induced PD rat model. In MFB-lesioned rats, the electroencephalographic (EEG) γ and β powers were remarkably reduced. However, EA treatment significantly increased EEG γ and β frequency band powers (247). These observations could provide an experimental base for the use of EA in the treatment and prevention of free radical–associated neural impairment in PD.
Dolatshahi et al. (248) examined the activity of EA (50 mg/kg) in the treatment of hyperalgesia and cognitive deficiency in 6-OHDA–insulted rats. Analgesia was evaluated by hot-plate and tail-flick tests; the passive avoidance task was analyzed by a shuttle box apparatus to record the initial and step-through latency. A Morris water maze test evaluated the spatial cognition ability, which determined the path length, escape latency time, swimming speed, and time spent in the target quadrant (267). Outcomes revealed that EA improved hyperalgesia in such a way that the pain sensation threshold was increased in both tail-flick and hot-plate tests. Additionally, administration of EA to lesioned rats diminished the 6-OHDA–mediated injury of the dopaminergic system, which can be proposed as a healing effect (248). These findings strongly support that EA has therapeutic potential in the management of cognitive and nociceptive deficiencies in PD.
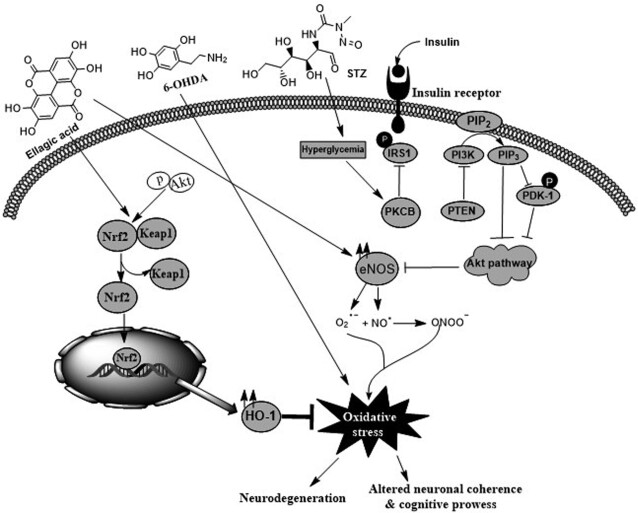
In another study of a 6-OHDA–induced rat model of PD, Baluchnejadmojarad et al. (249) demonstrated that EA (50 mg/kg) diminished apomorphine-mediated rotational bias and reduced the latency to initiate and the total time in the narrow beam task. This advantageous effect was partially abrogated following intracerebroventricular microinjection of estrogen receptor-β (ERβ) antagonist. ERβ receptors are commonly found in hippocampal and cortical regions in rodents and their activation could employ a neuroprotective action (268). EA suppressed striatal MDA, ROS, and DNA damage, and enhanced the concentrations of MAO-B, Nrf2, and HO-1. In the nigrostriatal system, damage of tyrosine hydroxylase-positive neurons in the SNc following 6-OHDA reduced synthesis of dopamine (269). EA prevented tyrosine hydroxylase–positive neuron loss within the SNc. Furthermore, a markedly higher striatal concentration of MAO-B in 6-OHDA–lesioned rats was noticed; MAO-B is responsible for metabolizing dopamine in the brain. It was found that EA pretreatment significantly reduced MAO-B. Additionally, EA has a therapeutic action against 6-OHDA–induced oxidative stress via activation and mobilization of the Nrf2 pathway and enhanced HO-1 expression that could exert neuroprotection against oxidative injury in the neural cells (Figure 4) (249).
FIGURE 4.
Neuroprotective effect of EA against 6-OHDA–induced neuropathy and diabetic encephalopathy. 6-OHDA, by the process of auto-oxidation, enhances oxidative stress in brain cells and consequently decreases ATP formation, which in turn leads to free radical production. EA inhibits the overproduction of free radicals and reduces the levels of MDA and hydroxyl radicals as well as DNA damage with an increase in GSH, SOD, and tyrosine hydroxylase concentrations in the brain. EA also upregulates the Nrf2 signaling pathway. In diabetic complications, EA reduces oxidative stress and oxidation of lipid membranes, but increases the cellular antioxidant response (enhances SOD, CAT, and GSH activities). EA also alters signaling pathways associated with diabetes, such as Akt pathways. Akt, protein kinase B; CAT, catalase; EA, ellagic acid, eNOS, endothelial nitric oxide synthase; GSH, reduced glutathione; HO-1, heme oxygenase-1; IRS1, insulin receptor substrate 1; Keap1, Kelch-like ECH-associated protein 1; MDA, malondialdehyde; Nrf2, nuclear factor erythroid 2-related factor 2; PDK1, 3-phosphoinositide-dependent kinase 1; PI3K, phosphatidylinositol-3 kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PTEN, phosphatase and tensin homolog; SOD, superoxide dismutase; STZ, streptozotocin; 6-OHDA, 6-hydroxydopamine.
Formulation Strategies To Increase EA Bioavailability
Numerous agents developed as dietary supplements suffer from poor solubility in aqueous media (270, 271), which can considerably limit their bioavailability. Even though EA exhibits appreciable therapeutic attributes, it has solubility problems and has been categorized under the biopharmaceutical classification system as a class IV substance with low solubility (<10 mg/mL in phosphate buffer, pH 7.4) and low permeability (0.13 × 10−6) (272). EA also has poor stability at a physiological pH of 7.4 (273, 274). To resolve these limitations, several approaches for oral consumption, comprising micro- and nanoparticles, self-emulsifying systems, solid dispersions, inclusion complexes, and polymorphs, have been evaluated (275). El-Missiry et al. (177) observed that Ca2+-EA-ALG NPs were superior to free EA in ameliorating increased IL-6 and TNF-α in mouse brain. The chitosan gels containing EA (1% w/v) significantly reduced viability of U87 and C6 cells (196). The chitosan/EA composite films were enzymatically degradable and exhibited a sustained slow release of EA. These materials could inhibit cancer cell growth in a concentration-dependent manner by inducing apoptosis of cancer cells as well as suppressing angiogenesis (198). Other researchers examined the bioactivity and bioavailability of poly(ε-caprolactone) (PCL)–formulated EA nanoparticles (EA-NPs) and free EA in New Zealand rabbits. Encapsulation in PCL NPs protected the entrapped EA from proteolytic breakdown and allowed a selective intracellular targeting by a “nanocitose” uptake mechanism that could have bypassed the discharging carriers (protein transporters). Free EA (50 mg/kg) was measured only for ≤12 h with a peak concentration (Cmax) of ∼0.159 μg/mL at 0.65 h after the oral administration. However, a similar concentration of EA-NPs extended the plasma concentration for ≥48 h. The observed increase in cellular uptake of EA entrapped in EA-NPs indicated that this nanoformulation is protecting the drug, therefore camouflaging the NPs from the efflux transporters (276).
Furthermore, mixtures of EA and polyvinylpyrrolidone (PVP), hydrophobic carboxymethyl cellulose acetate butyrate (CMCAB), or hydrophilic hydroxypropylmethyl cellulose acetate succinate (HPMCAS) at different weight ratios (1:9 and 1:3), prepared in acetone:ethanol (1:4 v/v) solution, were spray-dried with yields of 50–60%. Amorphous solid dispersions (ASDs) from very hydrophobic cellulose acetate adipate propionate (CAAdP) were instead prepared by the co-precipitation method (277). EA was amorphous ≤25% w/w in solid dispersions with PVP and HPMCAS, and ≤10% with CMCAB and CAAdP. Accordingly, EA-PVP and EA-HPMCAS attained the highest solution concentrations of 1500 and 280 μg/mL, respectively. In a stability study, pure EA exhibited a degradation degree by lactone ring opening of 20%, whereas lower values—16% for CMCAB, 14% for CAAdP, 6% for PVP, and just 5% for HPMCAS—were reported in the ASD samples. The dissolution rate was significantly higher with PVP and HPMCAS ASDs, whereas from CAAdP or CMCAB ASDs it was very slow and did not achieve adequate EA concentration at pH 6.8. As predicted, under acidic conditions EA release from PVP was quite fast, whereas from cellulose esters it was minimal. However, this advantage was nullified by crystallization of a large amount of the EA released from PVP (277). Because the 1:9 ASD with HPMCAS effectively stabilized EA against crystallization and degradation, the researchers concluded that this could be considered the most promising formulation. Several drug delivery systems have been developed to increase the bioavailability of EA, such as polymer-based nanoparticles, human BSA-EA complexes encapsulated into thermosensitive liposomes, complexes with cyclodextrins, encapsulation within niosomes and nanosized metalla cages, molecular dispersion within dendrimers, and EA-encapsulations (278).
Limitations, Future Prospects, and Conclusion
The available literature on EA has revealed that it is a natural dietary agent present in a broad spectrum of natural products and has potential to prevent or treat numerous diseases including neurological disorders. Undoubtedly, EA has positive impacts on antioxidant and anti-inflammatory mediators, reduces lipid peroxidation, and scavenges free radicals. Based on various in vitro and in vivo results, EA acts as a strong neuroprotective agent against different types of stressors, chemically induced neurotoxicity, neurodegeneration, epileptic seizures, aging, ischemic strokes, TBI, neural tumors, and diabetic neuropathy (Figure 5). EA also possesses therapeutic potential against AD and PD associated with neural injury, and has been suggested to repair motor damage and recover electrophysiological functions in rats. These activities can all be characterized by decrease of the neuroinflammatory biomarkers, such as TNF-α and IL-1β, as well as protection of the brain from free radical–induced damage. These findings suggest that EA has the capability for memory reinstatement in the management of dementia and other cognitive impairments. The low absorption from the gut could limit EA bioavailability and thereby affect its clinical efficacy. To overcome these challenges, several delivery systems and preparations have been developed to enhance its bioavailability. These approaches include EA-phospholipid complexes, polymer-based nanoparticles, nanomedicine (thermosensitive liposomes), and nano-sized metalla cages. Additionally, the comparatively limited clinical studies, with diversified protocols and relatively few subjects, indicate a key challenge for establishing the actual efficacy of EA as a promising medicinal agent. To overcome these issues, more pharmacokinetic studies, specifically in humans, are needed before the full therapeutic potential of EA is realized. To date, information regarding the pharmacological properties of EA in the context of neuroprotection is very limited; hence it is essential to explore the mechanisms of action of EA and to perform additional preclinical experiments.
FIGURE 5.
An overview of various neuroprotective attributes of ellagic acid.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—AKP and AG: conceptualized the idea; AG and RK: performed the literature search; AG: wrote the first draft of the manuscript; AG and AKS: prepared the figures; SJ: performed language editing; AB and AKP: critically reviewed and revised the manuscript; AB: supervised the project; and all authors: read and approved the final manuscript.
Notes
AG, RK, and AKS acknowledge the University Grants Commission (UGC) and Council of Scientific and Industrial Research for their research fellowships. AG, RK, AKS, and AKP also acknowledge the UGC-Special Assistance Programme and Department of Science and Technology Fund for Improvement of Science and Technology facilities of the Biochemistry Department, University of Allahabad, Prayagraj, India.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: Aβ, β-amyloid; AChE, acetylcholinesterase; ACR, acrylamide; AD, Alzheimer disease; AGE, advanced glycation end-product; ASD, amorphous solid dispersion; ATRA, all-trans retinoic acid; BBB, blood–brain barrier; BDNF, brain-derived neurotrophic factor; BP, blood pressure; BuChE, butyrylcholinesterase; Cmax, maximum concentration in plasma; CA, cornus ammonis; CAAdP, cellulose acetate adipate propionate; Ca2+-EA-ALG NP, ellagic acid encapsulated in calcium-alginate nanoparticles; CAT, catalase; Ch/β-GP, chitosan/β-glycerophosphate; CMCAB, carboxymethyl cellulose acetate butyrate; CNS, central nervous system; COX, cyclooxygenase; Cup, cuprizone; cyt c, cytochrome c; DG, dentate gyrus; d-gal, d-galactose; DOX, doxorubicin; EA, ellagic acid; EA-NP, ellagic acid nanoparticle; EEG, electroencephalographic; eNOS, endothelial nitric oxide synthase; EPM, elevated plus-maze; ERβ, estrogen receptor β; ET, ellagitannin; FST, forced swimming test; GABA, γ-aminobutyric acid type; GFAP, glial fibrillary acidic protein; GPx, glutathione peroxidase; GSH, reduced glutathione; HPMCAS, hydroxypropylmethyl cellulose acetate succinate; HPC, hippocampus; HO-1, heme oxygenase-1; iNOS, nitric oxide synthase; LDH, lactate dehydrogenase; LPO, lipid peroxidation; LTP, long-term potentiation; MAO, monoamine oxidase; MAPK, mitogen-activated protein kinase; MDA, malondialdehyde; MFB, medial forebrain bundle; Nrf2, nuclear factor erythroid 2-related factor-2; OLG, oligodendrocyte; PCL, poly(ε-caprolactone); PCO, protein carbonylation; PCPA, p-chlorophenylalanine; PD, Parkinson disease; PDI, protein disulfide isomerase; PI3K, phosphoinositide 3-kinase; PON-1, paraoxonase; PTZ, pentylenetetrazol; PVP, polyvinylpyrrolidone; RAGE, receptor of advanced glycation end-products; ROS, reactive oxygen species; SA, sodium arsenite; SAD, sporadic Alzheimer disease; SNc, substantia nigra pars compacta; SNO, S-nitrosylation; SNO-PDI, S-nitrosylation of protein disulfide isomerase; SOD, superoxide dismutase; SSB single-strand break; STZ, streptozotocin; TAC, total antioxidant capacity; TBI, traumatic brain injury; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; ThT, thioflavin T; TOS, total oxidant status; TST, tail suspension test; β-gal, β-galactosidase; 5-HT, 5-hydroxytryptamine; 6-OHDA, 6-hydroxydopamine.
Contributor Information
Ashutosh Gupta, Department of Biochemistry, University of Allahabad, Prayagraj, Uttar Pradesh, India.
Amit Kumar Singh, Department of Biochemistry, University of Allahabad, Prayagraj, Uttar Pradesh, India.
Ramesh Kumar, Department of Biochemistry, University of Allahabad, Prayagraj, Uttar Pradesh, India.
Sarah Jamieson, Lake Erie College of Osteopathic Medicine, Bradenton, FL, USA.
Abhay Kumar Pandey, Department of Biochemistry, University of Allahabad, Prayagraj, Uttar Pradesh, India.
Anupam Bishayee, Lake Erie College of Osteopathic Medicine, Bradenton, FL, USA.
References
- 1. Tejada S, Setzer WN, Daglia M, Nabavi SF, Sureda A, Braidy N, Gortzi O, Nabavi SM. Neuroprotective effects of ellagitannins: a brief review. Curr Drug Targets. 2017;18:1518–28. [DOI] [PubMed] [Google Scholar]
- 2. Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson's disease. Parkinsonism Relat Disord. 2012;18:S210–2. [DOI] [PubMed] [Google Scholar]
- 3. Chen W-W, Zhang X, Huang W-J. Role of neuroinflammation in neurodegenerative diseases (review). Mol Med Rep. 2016;13:3391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Losada-Barreiro S, Bravo-Diaz C. Free radicals and polyphenols: the redox chemistry of neurodegenerative diseases. Eur J Med Chem. 2017;133:379–402. [DOI] [PubMed] [Google Scholar]
- 5. Hsieh H-L, Yang C-M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res Int. 2013;2013:484613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ansari N, Khodagholi F. Natural products as promising drug candidates for the treatment of Alzheimer's disease: molecular mechanism aspect. Curr Neuropharmacol. 2013;11:414–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sowndhararajan K, Kim S. Neuroprotective and cognitive enhancement potentials of Angelica gigas nakai root: a review. Sci Pharm. 2017;85:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mizuno Y. Recent research progress in and future perspective on treatment of Parkinson's disease. Integr Med Int. 2014;1:67–79. [Google Scholar]
- 9. Okun MS. Deep-brain stimulation—entering the era of human neural-network modulation. N Engl J Med. 2014;371:1369–73. [DOI] [PubMed] [Google Scholar]
- 10. Chen X, Pan W. The treatment strategies for neurodegenerative diseases by integrative medicine. Integr Med Int. 2015;1:223–5. [Google Scholar]
- 11. Fang J, Li Y, Liu R, Pang X, Li C, Yang R, He Y, Lian W, Liu A-L, Du G-H. Discovery of multitarget-directed ligands against Alzheimer's disease through systematic prediction of chemical-protein interactions. J Chem Inf Model. 2015;55:149–64. [DOI] [PubMed] [Google Scholar]
- 12. Fang J, Pang X, Yan R, Lian W, Li C, Wang Q, Liu A-L, Du G-H. Discovery of neuroprotective compounds by machine learning approaches. RSC Advances. 2016;6:9857–71. [Google Scholar]
- 13. Gupta A, Singh AK, Kumar R, Ganguly R, Rana HK, Pandey PK, Sethi G, Bishayee A, Pandey AK. Corilagin in cancer: a critical evaluation of anticancer activities and molecular mechanisms. Molecules. 2019;24:3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gupta A, Kumar R, Bhattacharyya P, Bishayee A, Pandey AK. Terminalia bellirica (Gaertn.) roxb. (Bahera) in health and disease: a systematic and comprehensive review. Phytomedicine. 2020;77:153278. [DOI] [PubMed] [Google Scholar]
- 15. Silva RFM, Pogacnik L. Polyphenols from food and natural products: neuroprotection and safety. Antioxidants. 2020;9(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Darvesh AS, Carroll RT, Bishayee A, Novotny NA, Geldenhuys WJ, Van der Schyf CJ. Curcumin and neurodegenerative diseases: a perspective. Expert Opin Investig Drugs. 2012;21:1123–40. [DOI] [PubMed] [Google Scholar]
- 17. Di Paolo M, Papi L, Gori F, Turillazzi E. Natural products in neurodegenerative diseases: a great promise but an ethical challenge. Int J Mol Sci. 2019;20:5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaur R, Mehan S, Khanna D, Kalra S. Polyphenol ellagic acid-targeting to brain: a hidden treasure. Int J Neurol Res. 2015;1:141–52. [Google Scholar]
- 19. Velander P, Wu L, Henderson F, Zhang S, Bevan DR, Xu B. Natural product-based amyloid inhibitors. Biochem Pharmacol. 2017;139:40–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clifford MN, Scalbert A. Ellagitannins—nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1118–25. [Google Scholar]
- 21. Galano A, Francisco Marquez M, Perez-Gonzalez A. Ellagic acid: an unusually versatile protector against oxidative stress. Chem Res Toxicol. 2014;27:904–18. [DOI] [PubMed] [Google Scholar]
- 22. González-Sarrías A, Miguel V, Merino G, Lucas R, Morales JC, Tomás-Barberán F, Alvarez AI, Epsin JC. The gut microbiota ellagic acid-derived metabolite urolithin A and its sulfate conjugate are substrates for the drug efflux transporter breast cancer resistance protein (ABCG2/BCRP). J Agric Food Chem. 2013;61:4352–9. [DOI] [PubMed] [Google Scholar]
- 23. Ceci C, Lacal PM, Tentori L, De Martino MG, Miano R, Graziani G. Experimental evidence of the antitumor, antimetastatic and antiangiogenic activity of ellagic acid. Nutrients. 2018;10:1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta A, Pandey AK. Aceclofenac-induced hepatotoxicity: an ameliorative effect of Terminalia bellirica fruit and ellagic acid. World J Hepatol. 2020;12:949–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Evtyugin DD, Magina S, Evtuguin DV. Recent advances in the production and applications of ellagic acid and its derivatives. A review. Molecules. 2020;25:2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baradaran Rahimi V, Ghadiri M, Ramezani M, Askari VR. Antiinflammatory and anti-cancer activities of pomegranate and its constituent, ellagic acid: evidence from cellular, animal, and clinical studies. Phytother Res. 2020;34:685–720. [DOI] [PubMed] [Google Scholar]
- 27. Rogerio AP, Fontanari C, Borducchi E, Keller AC, Russo M, Soares EG, Albuquerque DA, Faccioli LH. Anti-inflammatory effects of Lafoensia pacari and ellagic acid in a murine model of asthma. Eur J Pharmacol. 2008;580:262–70. [DOI] [PubMed] [Google Scholar]
- 28. Goodwin EC, Atwood WJ, DiMaio D. High-throughput cell-based screen for chemicals that inhibit infection by simian virus 40 and human polyomaviruses. J Virol. 2009;83:5630–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelly E, Vyas P, Weber JT. Biochemical properties and neuroprotective effects of compounds in various species of berries. Molecules. 2017;23:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pari L, Sivasankari R. Effect of ellagic acid on cyclosporine A-induced oxidative damage in the liver of rats. Fundam Clin Pharmacol. 2008;22:395–401. [DOI] [PubMed] [Google Scholar]
- 31. Marin M, Giner RM, Rios J-L, Recio MC. Intestinal anti-inflammatory activity of ellagic acid in acute and chronic dextran sulfate sodium model of mice colitis. J Ethnopharmacol. 2013;150:925–34. [DOI] [PubMed] [Google Scholar]
- 32. Ojeda-Sana AM, van Baren CM, Elechosa MA, Juarez MA, Moreno S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control. 2013;31:189–95. [Google Scholar]
- 33. Yu M-H, Choi J-H, Chae I-G, Im H-G, Yang S-A, More K, Lee-I-S Lee J. Suppression of LPS-induced inflammatory activities by Rosmarinus officinalis L. Food Chem. 2013;136:1047–54. [DOI] [PubMed] [Google Scholar]
- 34. Alfei S, Turrini F, Catena S, Zunin P, Grilli M, Pittaluga AM, Boggia R. Ellagic acid a multi-target bioactive compound for drug discovery in CNS? A narrative review. Eur J Med Chem. 2019;183:111724. [DOI] [PubMed] [Google Scholar]
- 35. Derosa G, Maffioli P, Sahebkar A. Ellagic acid and its role in chronic diseases. Adv Exp Med Biol. 2016;928:473–9. [DOI] [PubMed] [Google Scholar]
- 36. de Oliveira MR. The effects of ellagic acid upon brain cells: a mechanistic view and future directions. Neurochem Res. 2016;41:121–8. [DOI] [PubMed] [Google Scholar]
- 37. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Malini P, Kanchana G, Rajadurai M. Antidiabetic efficacy of ellagic acid in streptozotocin-induced diabetes mellitus in albino Wistar rats. Asian J Pharm Clin Res. 2011;4:5. [Google Scholar]
- 39. Sepulveda L, Ascacio A, Rodriguez-Herrera R, Aguilera-Carbo A, Aguilar CN. Ellagic acid: biological properties and biotechnological development for production processes. Afr J Biotechnol. 2011;10:4518–23. [Google Scholar]
- 40. Shakeri A, Zirak MR, Sahebkar A. Ellagic acid: a logical lead for drug development?. Curr Pharm Des. 2018;24:106–22. [DOI] [PubMed] [Google Scholar]
- 41. Plundrich N, Grace MH, Raskin I, Ann Lila M. Bioactive polyphenols from muscadine grape and blackcurrant stably concentrated onto protein-rich matrices for topical applications. Int J Cosmet Sci. 2013;35:394–401. [DOI] [PubMed] [Google Scholar]
- 42. Turk G, Sonmez M, Ceribasi AO, Yuce A, Atessahin A. Attenuation of cyclosporine A-induced testicular and spermatozoal damages associated with oxidative stress by ellagic acid. Int Immunopharmacol. 2010;10:177–82. [DOI] [PubMed] [Google Scholar]
- 43. Seeram NP. Berry fruits for cancer prevention: current status and future prospects. J Agric Food Chem. 2008;56(3):630–5. [DOI] [PubMed] [Google Scholar]
- 44. Wada L, Ou B. Antioxidant activity and phenolic content of Oregon caneberries. J Agric Food Chem. 2002;50:3495–500. [DOI] [PubMed] [Google Scholar]
- 45. Tomas-Barberan FA, Espin JC, Garcia-Conesa MT. Bioavailability and metabolism of ellagic acid and ellagitannins. In: Quideau S, editor. Chemistry and biology of ellagitannins. Singapore: World Scientific; 2009.. p. 273–97. [Google Scholar]
- 46. Mahfoudhi A, Prencipe FP, Mighri Z, Pellati F. Metabolite profiling of polyphenols in the Tunisian plant Tamarix aphylla (L.) Karst. J Pharm Biomed Anal. 2014;99:97–105. [DOI] [PubMed] [Google Scholar]
- 47. Zafrilla P, Ferreres F, Tomas-Barberan FA. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J Agric Food Chem. 2001;49:3651–5. [DOI] [PubMed] [Google Scholar]
- 48. Lorenzo JM, Munekata PE, Putnik P, Kovacevic DB, Muchenje V, Barba FJ. Sources, chemistry, and biological potential of ellagitannins and ellagic acid derivatives. Studies in Natural Products Chemistry. 2019;60:189–221. [Google Scholar]
- 49. Vattem DA, Shetty K. Biological functionality of ellagic acid: a review. J Food Biochem. 2005;29(3):234–66. [Google Scholar]
- 50. Kim ND, Mehta R, Yu W, Neeman I, Livney T, Amichay A, Poirier D, Nicholls P, Kirby A, Jiang Wet al. . Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res Treat. 2002;71(3):203–17. [DOI] [PubMed] [Google Scholar]
- 51. Murphy MM, Barraj LM, Spungen JH, Herman DR, Randolph RK. Global assessment of select phytonutrient intakes by level of fruit and vegetable consumption. Br J Nutr. 2014;112:1004–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Srivastava A, Jagan Mohan Rao L, Shivanandappa T. Isolation of ellagic acid from the aqueous extract of the roots of Decalepis hamiltonii: antioxidant activity and cytoprotective effect. Food Chem. 2007;103:224–33. [Google Scholar]
- 53. da Silva AO, Alves AD, De Almeida DAT, Balogun SO, de Oliveira RG, Aguiar AA, Soares IM, Marson-Ascencio PG, Ascencio SD, de Oliveira Matins DT. Evaluation of anti-inflammatory and mechanism of action of extract of Macrosiphonia longiflora (Desf.) Mull. Arg J Ethnopharmacol. 2014;154:319–29. [DOI] [PubMed] [Google Scholar]
- 54. Jia X, Luo H, Xu M, Zhai M, Guo Z, Qiao Y, Wang L. Dynamic changes in phenolics and antioxidant capacity during pecan (Carya illinoinensis) kernel ripening and its phenolics Profiles. Molecules. 2018;23:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vu DC, Vo PH, Coggeshall MV, Lin C-H. Identification and characterization of phenolic compounds in black walnut kernels. J Agric Food Chem. 2018;66:4503–11. [DOI] [PubMed] [Google Scholar]
- 56. Ambrose SS, Solairaj P, Subramoniam A. Hepatoprotective activity of active fractions of Thespesia lampas Dalz and Gibs (Malvaceae). J Pharmacol Pharmacother. 2012;3:326–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. de Britto Policarpi P, Turcatto L, Demoliner F, Ferrari RA, Bascunan VLAF, Ramos JC, Jachmanian I, Vtali L, Micke GA, Block JM. Nutritional potential, chemical profile and antioxidant activity of Chicha (Sterculia striata) nuts and its by-products. Food Res Int. 2018;106:736–44. [DOI] [PubMed] [Google Scholar]
- 58. Tseng H-C, Wu W-T, Huang H-S, Wu M-C. Antimicrobial activities of various fractions of longan (Dimocarpus longan Lour. Fen Ke) seed extract. Int J Food Sci Nutr. 2014;65:589–93. [DOI] [PubMed] [Google Scholar]
- 59. Hernandez C, Ascacio-Valdes J, De la Garza H, Wong-Paz J, Aguilar CN, Martinez-Avila GC, Castro-Lopez C, Aguilera-Carbo A. Polyphenolic content, in vitro antioxidant activity and chemical composition of extract from Nephelium lappaceum L. (Mexican rambutan) husk. Asian Pac J Trop Med. 2017;10:1201–5. [DOI] [PubMed] [Google Scholar]
- 60. Owczarek A, Gudej J. Investigation into biologically active constituents of Geum rivale L. Acta Pol Pharm. 2013;70:111–4. [PubMed] [Google Scholar]
- 61. Gao J, Sun CR, Yang JH, Shi JM, Du YG, Zhang Y-Y, Li J-H, Wan H-T. Evaluation of the hepatoprotective and antioxidant activities of Rubus parvifolius L. J Zhejiang Univ Sci B. 2011;12:135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Im SH, Wang Z, Lim SS, Lee O-H, Kang I-J. Bioactivity-guided isolation and identification of anti-adipogenic compounds from Sanguisorba officinalis. Pharm Biol. 2017;55:2057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kapoor MP, Suzuki K, Derek T, Ozeki M, Okubo T. Clinical evaluation of Emblica officinalis Gatertn (Amla) in healthy human subjects: health benefits and safety results from a randomized, double-blind, crossover placebo-controlled study. Contemp Clin Trials Commun. 2020;17:100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Navarro M, Moreira I, Arnaez E, Quesada S, Azofeifa G, Vargas F, Alvarado D, Chen P. Flavonoids and ellagitannins characterization, antioxidant and cytotoxic activities of Phyllanthus acuminatus Vahl. Plants. 2017;6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fracassetti D, Costa C, Moulay L, Tomás-Barberán FA. Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia). Food Chem. 2013;139:578–88. [DOI] [PubMed] [Google Scholar]
- 66. Cuadrado-Silva CT, Pozo-Bayon MA, Osorio C. Targeted metabolomic analysis of polyphenols with antioxidant activity in sour guava (Psidium friedrichsthalianum Nied.) fruit. Molecules. 2016;22:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sathyanarayanan S, Chandran R, Thankarajan S, Abrahamse H, Thangaraj P. Phytochemical composition, antioxidant and anti-bacterial activity of Syzygium calophyllifolium Walp. fruit. J Food Sci Technol. 2018;55:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gajera HP, Gevariya SN, Hirpara DG, Patel SV, Golakiya BA. Antidiabetic and antioxidant functionality associated with phenolic constituents from fruit parts of indigenous black jamun (Syzygium cumini L.) landraces. J Food Sci Technol. 2017;54:3180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. de Oliveira LM, Porte A, de Oliveira Godoy RL, da Costa Souza M, Pacheco S, de Araujo Santiago MCP, Gouvea ACMS, Nascimento LSM, Borguini RG. Chemical characterization of Myrciaria floribunda (H. West ex Willd) fruit. Food Chem. 2018;248:247–52. [DOI] [PubMed] [Google Scholar]
- 70. Falcao TR, de Araujo AA, Soares LAL, de Moraes Ramos RT, Bezerra ICF, Ferreira MRA, Neto MA, Melo MCN, Arajuo Jr RF, Guerra ACVet al. . Crude extract and fractions from Eugenia uniflora Linn leaves showed anti-inflammatory, antioxidant, and antibacterial activities. BMC Complement Altern Med. 2018;18:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Diaz-de-Cerio E, Arraez-Roman D, Segura-Carretero A, Ferranti P, Nicoletti R, Perrotta GM, Gomez-Caravaca AM. Establishment of pressurized-liquid extraction by response surface methodology approach coupled to HPLC-DAD-TOF-MS for the determination of phenolic compounds of myrtle leaves. Anal Bioanal Chem. 2018;410:3547–57. [DOI] [PubMed] [Google Scholar]
- 72. Campos JF, Espindola PP de T, Torquato HFV, Vital WD, Justo GZ, Silva DB, Corollo CA, Souza K, Paredes-Gamero EJ, Santos ED. Leaf and root extracts from Campomanesia adamantium (Myrtaceae) promote apoptotic death of leukemic cells via activation of intracellular calcium and caspase-3. Front Pharmacol. 2017;8:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Puig CG, Reigosa MJ, Valentao P, Andrade PB, Pedrol N. Unravelling the bioherbicide potential of Eucalyptus globulus Labill: biochemistry and effects of its aqueous extract. PLoS One. 2018;13:e0192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Phan ADT, Chaliha M, Sultanbawa Y, Netzel ME. Nutritional characteristics and antimicrobial activity of Australian grown feijoa (Acca sellowiana). Foods. 2019;8:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Garcia-Alvarez M-C, Moussa I, Njomnang Soh P, Nongonierma R, Abdoulaye A, Nicolau-Travers M-L, Fabre A, Wdzieczak-Bakala J, Ahond A, Poupat Cet al. . Both plants Sebastiania chamaelea from Niger and Chrozophora senegalensis from Senegal used in African traditional medicine in malaria treatment share a same active principle. J Ethnopharmacol. 2013;149:676–84. [DOI] [PubMed] [Google Scholar]
- 76. Siraj MA, Shilpi JA, Hossain MG, Uddin SJ, Islam MK, Jahan IA, Hossain H. Anti-inflammatory and antioxidant activity of Acalypha hispida leaf and analysis of its major bioactive polyphenols by HPLC. Adv Pharm Bull. 2016;6:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ochoa-Pacheco A, Escalona Arranz JC, Beaven M, Peres-Roses R, Gamez YM, Camacho-Pozo MI, Maury GL, de Macedo MB, Cos P, Tavares JFet al. . Bioassay-guided in vitro study of the antimicrobial and cytotoxic properties of the leaves from Excoecaria lucida Sw. Pharmacogn Res. 2017;9:396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee I-S, Jung S-H, Kim JS. Polyphenols from Euphorbia pekinensis inhibit AGEs formation in vitro and vessel dilation in larval zebrafish in vivo. Planta Med. 2018;84:176–81. [DOI] [PubMed] [Google Scholar]
- 79. Nugroho A, Rhim T-J, Choi M-Y, Choi JS, Kim Y-C, Kim M-S, Park H-J. Simultaneous analysis and peroxynitrite-scavenging activity of galloylated flavonoid glycosides and ellagic acid in Euphorbia supina. Arch Pharm Res. 2014;37:890–8. [DOI] [PubMed] [Google Scholar]
- 80. Wang S-H, Kao M-Y, Wu S-C, Lo D-Y, Wu J-Y, Chang J-C, Chiou RY-Y. Oral administration of Trapa taiwanensis nakai fruit skin extracts conferring hepatoprotection from CCl4-caused injury. J Agric Food Chem. 2011;59:3686–92. [DOI] [PubMed] [Google Scholar]
- 81. Syed YH, Khan M. Chromatographic profiling of ellagic acid in Woodfordia fruticosa flowers and their gastroprotective potential in ethanol-induced ulcers in rats. Pharmacognosy Res. 2016;8:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pereira LOM, Vilegas W, Tangerina MMP, Arunachalam K, Balogun SO, Orlandi-Mattos PE, Colodel EM, Martins DTO. Lafoensia pacari A. St.-Hil.: Wound healing activity and mechanism of action of standardized hydroethanolic leaves extract. J Ethnopharmacol. 2018;219:337–50. [DOI] [PubMed] [Google Scholar]
- 83. Park SW, Kwon MJ, Yoo JY, Choi H-J, Ahn Y-J. Antiviral activity and possible mode of action of ellagic acid identified in Lagerstroemia speciosa leaves toward human rhinoviruses. BMC Complement Altern Med. 2014;14:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shen Y-C, Juan C-W, Lin C-S, Chen C-C, Chang C-L. Neuroprotective effect of Terminalia chebula extracts and ellagic acid in pc12 cells. Afr J Tradit Complement Altern Med. 2017;14:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gupta A, Kumar R, Pandey AK. Antioxidant and antidiabetic activities of Terminalia bellirica fruit in alloxan induced diabetic rats. South Afr J Bot. 2020;130:308–15. [Google Scholar]
- 86. Mahmoudi H, Aouadhi C, Kaddour R, Gruber M, Zargouni H, Zaouali W, Hamida NB, Nasri MB, Ouerghi Z, Hosni K. Comparison of antioxidant and antimicrobial activities of two cultivated Cistus species from Tunisia. Biosci J. 2016;32:226–37. [Google Scholar]
- 87. Kong KW, Mat-Junit S, Ismail A, Aminudin N, Abdul-Aziz A. Polyphenols in Barringtonia racemosa and their protection against oxidation of LDL, serum and haemoglobin. Food Chem. 2014;146:85–93. [DOI] [PubMed] [Google Scholar]
- 88. Ferreres F, Grosso C, Gil-Izquierdo A, Valentão P, Andrade PB. Ellagic acid and derivatives from Cochlospermum angolensis Welw. Extracts: HPLC-DAD-ESI/MS(n) profiling, quantification and in vitro anti-depressant, anti-cholinesterase and anti-oxidant activities. Phytochem Anal. 2013;24:534–40. [DOI] [PubMed] [Google Scholar]
- 89. Krishnappa P, Venkatarangaiah K, Venkatesh Rajanna SKS, Gupta RKP. Antioxidant and prophylactic effects of Delonix elata L., stem bark extracts, and flavonoid isolated quercetin against carbon tetrachloride-induced hepatotoxicity in rats. Biomed Res Int. 2014;2014:507851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sumi SA, Siraj MA, Hossain A, Mia MS, Afrin S, Rahman MM. Investigation of the key pharmacological activities of Ficus racemosa and analysis of its major bioactive polyphenols by HPLC-DAD. Evid Based Complement Alternat Med. 2016;2016:3874516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ko H-J, Chen J-H, Ng LT. Hepatoprotection of Gentiana scabra extract and polyphenols in liver of carbon tetrachloride-intoxicated mice. J Environ Pathol Toxicol Oncol. 2011;30:179–87. [DOI] [PubMed] [Google Scholar]
- 92. Wu Q-Y, Zhou Y, Jin X, Guan Y, Xu M, Liu L-F. Chromatographic fingerprint and the simultaneous determination of five bioactive components of Geranium carolinianum L. water extract by high performance liquid chromatography. Int J Mol Sci. 2011;12:8740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sun J, Chen P. Ultra high-performance liquid chromatography with high-resolution mass spectrometry analysis of African mango (Irvingia gabonensis) seeds, extract, and related dietary supplements. J Agric Food Chem. 2012;60:8703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Alanon ME, Palomo I, Rodriguez L, Fuentes E, Arraez-Roman D, Segura-Carretero A. Antiplatelet activity of natural bioactive extracts from mango (Mangifera Indica L.) and its by-products. Antioxidants. 2019;8:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Abbas RK, Al-Mushhin AA, Elsharbasy FS, Ashiry KO. Reduce the risk of oxidation and pathogenic bacteria activity by Moringa oleifera different leaf extract grown in Sudan. J Microb Biochem Technol. 2020;12:427. [Google Scholar]
- 96. Baldisserotto A, Buso P, Radice M, Dissette V, Lampronti I, Gambari R, Manfredini S, Vertuani S. Moringa oleifera leaf extracts as multifunctional ingredients for “natural and organic” sunscreens and photoprotective preparations. Molecules. 2018;23:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tran TT, Kim M, Jang Y, Lee HW, Nguyen HT, Nguyen TN, Park HW, Dang QL, Kim J-C. Characterization and mechanisms of anti-influenza virus metabolites isolated from the Vietnamese medicinal plant Polygonum chinense. BMC Complement Altern Med. 2017;17:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Talcott ST, Lee J-H. Ellagic acid and flavonoid antioxidant content of muscadine wine and juice. J Agric Food Chem. 2002;50:3186–92. [DOI] [PubMed] [Google Scholar]
- 99. Lee J-H, Talcott ST. Fruit maturity and juice extraction influences ellagic acid derivatives and other antioxidant polyphenolics in muscadine grapes. J Agric Food Chem. 2004;52:361–6. [DOI] [PubMed] [Google Scholar]
- 100. Utomo B, Daningtia NR, Yuliani GA, Yuniarti WM. Effects of a standardized 40% ellagic acid pomegranate (Punica granatum L.) extract on seminiferous tubule histopathology, diameter, and epithelium thickness in albino Wistar rats after heat exposure. Vet World. 2019;12:1261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang D, Ozen C, Abu-Reidah IM, Chigurupati S, Patra JK, Horbanczuk JO, Jozwik A, Tzvetkov NT, Uhrin P, Atanasov AG. Vasculoprotective effects of pomegranate (Punica granatum L.). Front Pharmacol. 2018;9:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Torronen R. Sources and health effects of dietary ellagitannins. In: Quideau S, editor. Chemistry and biology of ellagitannins. Singapore: World Scientific; 2009.. p. 298–319. [Google Scholar]
- 103. Bakkalbasi E, Mentes O, Artik N. Food ellagitannins—occurrence, effects of processing and storage. Crit Rev Food Sci Nutr. 2009;49:283–98. [DOI] [PubMed] [Google Scholar]
- 104. Nag G, De B. Acetylcholinesterase inhibitory activity of Terminalia chebula, Terminalia bellerica and Emblica officinalis and some phenolic compounds. Int J Pharm Pharm Sci. 2011;3:4. [Google Scholar]
- 105. Rojanathammanee L, Puig KL, Combs CK. Pomegranate polyphenols and extract inhibit nuclear factor of activated T-cell activity and microglial activation in vitro and in a transgenic mouse model of Alzheimer disease. J Nutr. 2013;143:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–9. [DOI] [PubMed] [Google Scholar]
- 107. Jha AB, Panchal SS, Shah A. Ellagic acid: insights into its neuroprotective and cognitive enhancement effects in sporadic Alzheimer's disease. Pharmacol Biochem Behav. 2018;175:33–46. [DOI] [PubMed] [Google Scholar]
- 108. Goncalves B, Borges O, Costa HS, Bennett R, Santos M, Silva AP. Metabolite composition of chestnut (Castanea sativa Mill.) upon cooking: proximate analysis, fibre, organic acids and phenolics. Food Chem. 2010;122:154–60. [Google Scholar]
- 109. Malik NS, Perez JL, Lombardini L, Cornacchia R, Cisneros-Zevallos L, Braford J. Phenolic compounds and fatty acid composition of organic and conventional grown pecan kernels. J Sci Food Agric. 2009;89:2207–13. [Google Scholar]
- 110. Anderson KJ, Teuber SS, Gobeille A, Cremin P, Waterhouse AL, Steinberg FM. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J Nutr. 2001;131:2837–42. [DOI] [PubMed] [Google Scholar]
- 111. Koponen JM, Happonen AM, Mattila PH, Törrönen AR. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J Agric Food Chem. 2007;55:1612–9. [DOI] [PubMed] [Google Scholar]
- 112. Lee J-H, Talcott ST. Ellagic acid and ellagitannins effect on sedimentation in muscadine juice and wine. J Agric Food Chem. 2002;50:3971–6. [DOI] [PubMed] [Google Scholar]
- 113. Cerda B, Soto C, Albaladejo MD, Martínez P, Sanchez-Gascon F, Tomas-Barberan F, Epsin JC. Pomegranate juice supplementation in chronic obstructive pulmonary disease: a 5-week randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2006;60:245–53. [DOI] [PubMed] [Google Scholar]
- 114. Shaikh NH, Deshmukh VM, Walvekar MV. Alteration in testicular morphology and sperm count due to glycowithanolides treatment during aging. Asian J Pharm Clin Res. 2015;8:72–7. [Google Scholar]
- 115. Glabasnia A, Hofmann T. Sensory-directed identification of taste-active ellagitannins in American (Quercus alba L.) and European oak wood (Quercus robur L.) and quantitative analysis in bourbon whiskey and oak-matured red wines. J Agric Food Chem. 2006;54:3380–90. [DOI] [PubMed] [Google Scholar]
- 116. Romo-Vaquero M, Garcia-Villalba R, Gonzalez-Sarrias A, Beltrán D, Tomas-Barberan FA, Espin JC, Selma MV. Interindividual variability in the human metabolism of ellagic acid: contribution of Gordonibacter to urolithin production. J Funct Foods. 2015;17:785–91. [Google Scholar]
- 117. Saha P, Yeoh BS, Singh R, Chandrasekar B, Vemula PK, Haribabu B, Matam VK, Jala VR. Gut microbiota conversion of dietary ellagic acid into bioactive phytoceutical urolithin a inhibits heme peroxidases. PLoS One. 2016;11:e0156811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bialonska D, Ramnani P, Kasimsetty SG, Muntha KR, Gibson GR, Ferreira D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int J Food Microbiol. 2010;140:175–82. [DOI] [PubMed] [Google Scholar]
- 119. Singh AK, Cabral C, Kumar R, Ganguly R, Rana HK, Gupta A, Lauro MR, Carbone C, Reis F, Pandey AK. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients. 2019;11(9):2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nunez-Sanchez MA, Garcia-Villalba R, Monedero-Saiz T, Garcia-Talavera NV, Gomez-Sanchez MB, Sanchez-Alvarez C, Gracia-Albert AM, Rodriguez-Gil FJ, Ruiz-Marin M, Pastor-Quirante FAet al. . Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol Nutr Food Res. 2014;58:1199–211. [DOI] [PubMed] [Google Scholar]
- 121. Kujawska M, Jodynis-Liebert J. Potential of the ellagic acid-derived gut microbiota metabolite-urolithin A in gastrointestinal protection. World J Gastroenterol. 2020;26:3170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Espin JC, Gonzalez-Barrio R, Cerda B, Lopez-Bote C, Rey AI, Tomas-Barberan FA. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J Agric Food Chem. 2007;55:10476–85. [DOI] [PubMed] [Google Scholar]
- 123. Landete JM. Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res Int. 2011;44:1150–60. [Google Scholar]
- 124. Seeram NP, Aronson WJ, Zhang Y, Henning SM, Moro A, Lee R-P, Sartippour M, Harris DM, Rettig M, Suchard MAet al. . Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem. 2007;55:7732–7. [DOI] [PubMed] [Google Scholar]
- 125. Gonzalez-Barrio R, Edwards CA, Crozier A. Colonic catabolism of ellagitannins, ellagic acid, and raspberry anthocyanins: in vivo and in vitro studies. Drug Metab Dispos. 2011;39:1680–8. [DOI] [PubMed] [Google Scholar]
- 126. Selma MV, Beltrán D, Garcia-Villalba R, Espin JC, Tomas-Barberan FA. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014;5:1779–84. [DOI] [PubMed] [Google Scholar]
- 127. Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin Chim Acta. 2004;348:63–8. [DOI] [PubMed] [Google Scholar]
- 128. Lipinska L, Klewicka E, Sojka M. The structure, occurrence and biological activity of ellagitannins: a general review. Acta Sci Pol Technol Aliment. 2014;13:289–99. [DOI] [PubMed] [Google Scholar]
- 129. Girish C, Raj V, Arya J, Balakrishnan S. Evidence for the involvement of the monoaminergic system, but not the opioid system in the antidepressant-like activity of ellagic acid in mice. Eur J Pharmacol. 2012;682:118–25. [DOI] [PubMed] [Google Scholar]
- 130. Feng Y, Yang S, Du X, Zhang X, Sun X, Zhao M, Sun G-Y, Liu R-T. Ellagic acid promotes Abeta42 fibrillization and inhibits Abeta42-induced neurotoxicity. Biochem Biophys Res Commun. 2009;390:1250–4. [DOI] [PubMed] [Google Scholar]
- 131. Rahimi VB, Askari VR, Mousavi SH. Ellagic acid reveals promising anti-aging effects against d-galactose-induced aging on human neuroblastoma cell line, SH-SY5Y: a mechanistic study. Biomed Pharmacother. 2018;108:1712–24. [DOI] [PubMed] [Google Scholar]
- 132. Fjaeraa C, Nanberg E. Effect of ellagic acid on proliferation, cell adhesion and apoptosis in SH-SY5Y human neuroblastoma cells. Biomed Pharmacother. 2009;63:254–61. [DOI] [PubMed] [Google Scholar]
- 133. Alfredsson CF, Rendel F, Liang Q-L, Sundstrom BE, Nanberg E. Altered sensitivity to ellagic acid in neuroblastoma cells undergoing differentiation with 12-O-tetradecanoylphorbol-13-acetate and all-trans retinoic acid. Biomed Pharmacother. 2015;76:39–45. [DOI] [PubMed] [Google Scholar]
- 134. Yang C-S, Tzou B-C, Liu Y-P, Tsai M-J, Shyue S-K, Tzeng S-F. Inhibition of cadmium-induced oxidative injury in rat primary astrocytes by the addition of antioxidants and the reduction of intracellular calcium. J Cell Biochem. 2008;103:825–34. [DOI] [PubMed] [Google Scholar]
- 135. Kabiraj P, Marin JE, Varela-Ramirez A, Zubia E, Narayan M. Ellagic acid mitigates SNO-PDI induced aggregation of Parkinsonian biomarkers. ACS Chem Neurosci. 2014;5:1209–20. [DOI] [PubMed] [Google Scholar]
- 136. Liu Q-S, Deng R, Li S, Li X, Li K, Kebaituli G, Li X, Liu R. Ellagic acid protects against neuron damage in ischemic stroke through regulating the ratio of Bcl-2/Bax expression. Appl Physiol Nutr Metab. 2017;42(8):855–60. [DOI] [PubMed] [Google Scholar]
- 137. Zhen Y-Z, Lin Y-J, Li K-J, Zhang G-L, Zhao Y-F, Wang M-M, Wei J-B, Wei J, Hu G. Effects of rhein lysinate on D-galactose-induced aging mice. Exp Ther Med. 2016;11:303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Aydın AF, Çoban J, Dogan-Ekici I, Betul-Kalaz E, Dogru-Abbasoglu S, Uysal M. Carnosine and taurine treatments diminished brain oxidative stress and apoptosis in D-galactose aging model. Metab Brain Dis. 2016;31:337–45. [DOI] [PubMed] [Google Scholar]
- 139. Chen P, Chen F, Zhou B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci Rep. 2018;8:1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wu N, Zu Y, Fu Y, Kong Y, Zhao J, Li X, Li J, Wink M, Efferth T. Antioxidant activities and xanthine oxidase inhibitory effects of extracts and main polyphenolic compounds obtained from Geranium sibiricum L. J Agric Food Chem. 2010;58:4737–43. [DOI] [PubMed] [Google Scholar]
- 141. Wang T, Di G, Yang L, Dun Y, Sun Z, Wan J, Peng B, Liu C, Xiong G, Zhang Cet al. . Saponins from Panax japonicus attenuate D-galactose-induced cognitive impairment through its anti-oxidative and anti-apoptotic effects in rats. J Pharm Pharmacol. 2015;67:1284–96. [DOI] [PubMed] [Google Scholar]
- 142. Shwe T, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp Gerontol. 2018;101:13–36. [DOI] [PubMed] [Google Scholar]
- 143. Parameshwaran K, Irwin MH, Steliou K, Pinkert CA. D-Galactose effectiveness in modeling aging and therapeutic antioxidant treatment in mice. Rejuvenation Res. 2010;13:729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Liu Y-Y, Nagpure BV, Wong PT-H, Bian J-S. Hydrogen sulfide protects SH-SY5Y neuronal cells against d-galactose induced cell injury by suppression of advanced glycation end products formation and oxidative stress. Neurochem Int. 2013;62:603–9. [DOI] [PubMed] [Google Scholar]
- 145. Aguer C, Gambarotta D, Mailloux RJ, Moffat C, Dent R, McPherson R, Harper ME. Galactose enhances oxidative metabolism and reveals mitochondrial dysfunction in human primary muscle cells. PloS One. 2011;6:e28536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Sadigh-Eteghad S, Majdi A, McCann SK, Mahmoudi J, Vafaee MS, Macleod MR. Correction: D-galactose-induced brain ageing model: a systematic review and meta-analysis on cognitive outcomes and oxidative stress indices. PloS One. 2017;12:e0190328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Biran A, Zada L, Abou Karam P, Vadai E, Roitman L, Ovadya Y, Porat Z, Krizhanovsky V. Quantitative identification of senescent cells in aging and disease. Aging Cell. 2017;16:661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Griveau A, Wiel C, Le Calve B, Ziegler DV, Djebali S, Warnier M, Martin N, Marvel J, Vindrieux D, Bergo Met al. . Targeting the phospholipase A2 receptor ameliorates premature aging phenotypes. Aging Cell. 2018;17:e12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Narayan M. Disulfide bonds: protein folding and subcellular protein trafficking. FEBS J. 2012;279:2272–82. [DOI] [PubMed] [Google Scholar]
- 150. Nakamura T, Lipton SA. S-nitrosylation of critical protein thiols mediates protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Antioxid Redox Signal. 2011;14:1479–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hayakawa N, Shiozaki M, Shibata M, Koike M, Uchiyama Y, Matsuura N, Gotow T. Resveratrol affects undifferentiated and differentiated PC12 cells differently, particularly with respect to possible differences in mitochondrial and autophagic functions. Eur J Cell Biol. 2013;92:30–43. [DOI] [PubMed] [Google Scholar]
- 152. Pera M, Camps P, Munoz-Torrero D, Perez B, Badia A, Clos Guillen MV. Undifferentiated and differentiated PC12 cells protected by huprines against injury induced by hydrogen peroxide. PloS One. 2013;8:e74344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tabopda TK, Ngoupayo J, Liu JW, Mitaine-Offer A-C, Ngadjui BT, Lacaille-Dubois M-A, Luu B. Induction of neuronal differentiation in neurosphere stem cells by ellagic acid derivatives. Nat Prod Commun. 2009;4:517–20. [PubMed] [Google Scholar]
- 154. Pirzad Jahromi G, Shabanzadeh Pirsaraei A, Sadr SS, Kaka G, Jafari M, Seidi S, Charish J. Multipotent bone marrow stromal cell therapy promotes endogenous cell proliferation following ischemic stroke. Clin Exp Pharmacol Physiol. 2015;42:1158–67. [DOI] [PubMed] [Google Scholar]
- 155. Khandelwal P, Yavagal DR, Sacco RL. Acute ischemic stroke intervention. J Am Coll Cardiol. 2016;67:2631–44. [DOI] [PubMed] [Google Scholar]
- 156. Lee S, Kim W, Park J, Jang HH, Lee SM, Woo JS, Kim HS, Lee KH, Kwon Y-J, Lee Uet al. . Effects of electroacupuncture on endothelial function and circulating endothelial progenitor cells in patients with cerebral infarction. Clin Exp Pharmacol Physiol. 2015;42:822–7. [DOI] [PubMed] [Google Scholar]
- 157. Umesalma S, Sudhandiran G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-kappaB, iNOS, COX-2, TNF-alpha, and IL-6 in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin Pharmacol Toxicol. 2010;107:650–5. [DOI] [PubMed] [Google Scholar]
- 158. Nejad KH, Dianat M, Sarkaki A, Naseri MKG, Badavi M, Farbood Y. Ellagic acid improves electrocardiogram waves and blood pressure against global cerebral ischemia rat experimental models. Electron Physician. 2015;7:1153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Maryam R, Khadijeh GD. Effect of ellagic acid on oxidative stress due to brain ischemia/hypoperfusion in male rat. J Herbal Drugs. 2014;5:1–6. [Google Scholar]
- 160. Puyal J, Ginet V, Clarke PGH. Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: a challenge for neuroprotection. Prog Neurobiol. 2013;105:24–48. [DOI] [PubMed] [Google Scholar]
- 161. Kim S, Nishimoto SK, Bumgardner JD, Haggard WO, Gaber MW, Yang Y. A chitosan/beta-glycerophosphate thermo-sensitive gel for the delivery of ellagic acid for the treatment of brain cancer. Biomaterials. 2010;31:4157–66. [DOI] [PubMed] [Google Scholar]
- 162. Le Tien C, Lacroix M, Ispas-Szabo P, Mateescu M-A. N-acylated chitosan: hydrophobic matrices for controlled drug release. J Controlled Release. 2003;93:1–13. [DOI] [PubMed] [Google Scholar]
- 163. Kim S, Gaber MW, Zawaski JA, Zhang F, Richardson M, Zhang XA, Yang Y. The inhibition of glioma growth in vitro and in vivo by a chitosan/ellagic acid composite biomaterial. Biomaterials. 2009;30:4743–51. [DOI] [PubMed] [Google Scholar]
- 164. Yi L-T, Li C-F, Zhan X, Cui C-C, Xiao F, Zhou L-P, Xie Y. Involvement of monoaminergic system in the antidepressant-like effect of the flavonoid naringenin in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1223–8. [DOI] [PubMed] [Google Scholar]
- 165. Yamada M, Yasuhara H. Clinical pharmacology of MAO inhibitors: safety and future. Neurotoxicology. 2004;25:215–21. [DOI] [PubMed] [Google Scholar]
- 166. Dreiseitel A, Korte G, Schreier P, Oehme A, Locher S, Domani M, Hajak G, Sand PG. Berry anthocyanins and their aglycons inhibit monoamine oxidases A and B. Pharmacol Res. 2009;59:306–11. [DOI] [PubMed] [Google Scholar]
- 167. Kreiss DS, Lucki I. Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-hydroxytryptamine measured in vivo. J Pharmacol Exp Ther. 1995;274:866–76. [PubMed] [Google Scholar]
- 168. Bruning CA, Souza ACG, Gai BM, Zeni G, Nogueira CW. Antidepressant-like effect of m-trifluoromethyl-diphenyl diselenide in the mouse forced swimming test involves opioid and serotonergic systems. Eur J Pharmacol. 2011;658:145–9. [DOI] [PubMed] [Google Scholar]
- 169. Maj J, Rogoz Z, Dlaboga D, Dziedzicka-Wasylewska M. Pharmacological effects of milnacipran, a new antidepressant, given repeatedly on the alpha1-adrenergic and serotonergic 5-HT2A systems. J Neural Transm (Vienna). 2000;107:1345–59. [DOI] [PubMed] [Google Scholar]
- 170. Yi L-T, Xu H-L, Feng J, Zhan X, Zhou L-P, Cui C-C. Involvement of monoaminergic systems in the antidepressant-like effect of nobiletin. Physiol Behav. 2011;102:1–6. [DOI] [PubMed] [Google Scholar]
- 171. Xu Y, Ku B-S, Yao H-Y, Lin Y-H, Ma X, Zhang Y-H, Li X-J. The effects of curcumin on depressive-like behaviors in mice. Eur J Pharmacol. 2005;518:40–6. [DOI] [PubMed] [Google Scholar]
- 172. Bedel HA, Manas CK, Ozbey G, Usta C. The antidepressant-like activity of ellagic acid and its effect on hippocampal brain derived neurotrophic factor levels in mouse depression models. Nat Prod Res. 2018;32:2932–5. [DOI] [PubMed] [Google Scholar]
- 173. Girish C, Raj V, Arya J, Balakrishnan S. Involvement of the GABAergic system in the anxiolytic-like effect of the flavonoid ellagic acid in mice. Eur J Pharmacol. 2013;710:49–58. [DOI] [PubMed] [Google Scholar]
- 174. Mares J, Stopka P, Nohejlova K, Rokyta R. Oxidative stress induced by epileptic seizure and its attenuation by melatonin. Physiol Res. 2013;62:S67–74. [DOI] [PubMed] [Google Scholar]
- 175. Cavalcante TMB, De Melo J de MA, Lopes LB, Bessa MC, Santos JG, Vasconcelos LC, Neto AEV, Borges LTN, Fonteles MMF, Filho AJMCet al. . Ivabradine possesses anticonvulsant and neuroprotective action in mice. Biomed Pharmacother. 2019;109:2499–512. [DOI] [PubMed] [Google Scholar]
- 176. Ransom B, Behar T, Nedergaard M. New roles for astrocytes (stars at last). Trends Neurosci. 2003;26:520–2. [DOI] [PubMed] [Google Scholar]
- 177. El-Missiry MA, Othman AI, Amer MA, Sedki M, Ali SM, El-Sherbiny IM. Nanoformulated ellagic acid ameliorates pentylenetetrazol-induced experimental epileptic seizures by modulating oxidative stress, inflammatory cytokines and apoptosis in the brains of male mice. Metab Brain Dis. 2020;35:385–99. [DOI] [PubMed] [Google Scholar]
- 178. Baldelli E, Leo G, Andreoli N, Fuxe K, Biagini G, Agnati LF. Homocysteine potentiates seizures and cell loss induced by pilocarpine treatment. Neuromolecular Med. 2010;12:248–59. [DOI] [PubMed] [Google Scholar]
- 179. Manschot SM, Biessels GJ, Rutten GEHM, Kessels RPC, Kessels RCP, Gispen WH, Kappalle LJ. Peripheral and central neurologic complications in type 2 diabetes mellitus: no association in individual patients. J Neurol Sci. 2008;264:157–62. [DOI] [PubMed] [Google Scholar]
- 180. Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C, Mwangi S, Jones DP, Sitaraman SV, Srinivasan S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131–8.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Uzar E, Alp H, Cevik MU, Fırat U, Evliyaoglu O, Tufek A, Altun Y. Ellagic acid attenuates oxidative stress on brain and sciatic nerve and improves histopathology of brain in streptozotocin-induced diabetic rats. Neurol Sci. 2012;33:567–74. [DOI] [PubMed] [Google Scholar]
- 182. Ou H-C, Lee W-J, Lee S-D, Huang C-Y, Chiu T-H, Tsai K-L, Hsu W-C, Sheu WH-H. Ellagic acid protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol Appl Pharmacol. 2010;248:134–43. [DOI] [PubMed] [Google Scholar]
- 183. Kumar M, Bansal N. Ellagic acid prevents dementia through modulation of PI3-kinase-endothelial nitric oxide synthase signalling in streptozotocin-treated rats. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:987–1001. [DOI] [PubMed] [Google Scholar]
- 184. Agrawal R, Tyagi E, Shukla R, Nath C. Insulin receptor signaling in rat hippocampus: a study in STZ (ICV) induced memory deficit model. Eur Neuropsychopharmacol. 2011;21:261–73. [DOI] [PubMed] [Google Scholar]
- 185. Rajasekar N, Nath C, Hanif K, Shukla R. Intranasal insulin improves cerebral blood flow, Nrf-2 expression and BDNF in STZ (ICV)-induced memory impaired rats. Life Sci. 2017;173:1–10. [DOI] [PubMed] [Google Scholar]
- 186. Sharma UK, Kumar R, Gupta A, Ganguly R, Singh AK, Ojha AK, Pandey AK. Ameliorating efficacy of eugenol against metanil yellow induced toxicity in albino Wistar rats. Food Chem Toxicol. 2019;126:34–40. [DOI] [PubMed] [Google Scholar]
- 187. Sharma UK, Kumar R, Gupta A, Ganguly R, Pandey AK. Renoprotective effect of cinnamaldehyde in food color induced toxicity. 3Biotech. 2018;8:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Calsolaro V, Edison P. Neuroinflammation in Alzheimer's disease: current evidence and future directions. Alzheimers Dement. 2016;12:719–32. [DOI] [PubMed] [Google Scholar]
- 189. Bedse G, Di Domenico F, Serviddio G, Cassano T. Aberrant insulin signaling in Alzheimer's disease: current knowledge. Front Neurosci. 2015;9:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Al Nimer F, Lindblom R, Strom M, Guerreiro-Cacais AO, Parsa R, Aeinehband S, Mathiesen T, Lidman O, Piehl F. Strain influences on inflammatory pathway activation, cell infiltration and complement cascade after traumatic brain injury in the rat. Brain Behav Immun. 2013;27:109–22. [DOI] [PubMed] [Google Scholar]
- 191. Albert-Weissenberger C, Siren A-L. Experimental traumatic brain injury. Exp Transl Stroke Med. 2010;2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA. 2008;300:711–9. [DOI] [PubMed] [Google Scholar]
- 193. Centers for Disease Control and Prevention. TBI: get the facts [Internet]. CDC Injury Center; 2019; [cited 2020 May 10]. Available from: https://www.cdc.gov/traumaticbraininjury/get_the_facts.html
- 194. Farbood Y, Sarkaki A, Dianat M, Khodadadi A, Haddad MK, Mashhadizadeh S. Ellagic acid prevents cognitive and hippocampal long-term potentiation deficits and brain inflammation in rat with traumatic brain injury. Life Sci. 2015;124:120–7. [DOI] [PubMed] [Google Scholar]
- 195. Arisi GM. Nervous and immune systems signals and connections: cytokines in hippocampus physiology and pathology. Epilepsy Behav. 2014;38:43–7. [DOI] [PubMed] [Google Scholar]
- 196. Tancredi V, D'Antuono M, Cafe C, Giovedi S, Bue MC, D'Arcangelo G, Onofri F, Benfenati F. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J Neurochem. 2000;75:634–43. [DOI] [PubMed] [Google Scholar]
- 197. Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci U S A. 1998;95:7778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ross FM, Allan SM, Rothwell NJ, Verkhratsky A. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J Neuroimmunol. 2003;144:61–7. [DOI] [PubMed] [Google Scholar]
- 199. Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. J Biol Chem. 2000;275:26252–8. [DOI] [PubMed] [Google Scholar]
- 200. O'Connor JJ, Coogan AN. Actions of the pro-inflammatory cytokine IL-1 beta on central synaptic transmission. Exp Physiol. 1999;84:601–14. [PubMed] [Google Scholar]
- 201. Mashhadizadeh S, Farbood Y, Dianat M, Khodadadi A, Sarkaki A. Therapeutic effects of ellagic acid on memory, hippocampus electrophysiology deficits, and elevated TNF-α level in brain due to experimental traumatic brain injury. Iran J Basic Med Sci. 2017;20:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol. 2012;7:42–59. [DOI] [PubMed] [Google Scholar]
- 203. Santello M, Volterra A. TNFα in synaptic function: switching gears. Trends Neurosci. 2012;35:638–47. [DOI] [PubMed] [Google Scholar]
- 204. Baune BT, Wiede F, Braun A, Golledge J, Arolt V, Koerner H. Cognitive dysfunction in mice deficient for TNF- and its receptors. Am J Med Genet Part B Neuropsychiatr Genet. 2008;147B:1056–64. [DOI] [PubMed] [Google Scholar]
- 205. Beste C, Baune BT, Falkenstein M, Konrad C. Variations in the TNF-α gene (TNF-α -308G→A) affect attention and action selection mechanisms in a dissociated fashion. J Neurophysiol. 2010;104:2523–31. [DOI] [PubMed] [Google Scholar]
- 206. Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–5. [DOI] [PubMed] [Google Scholar]
- 207. Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Santello M, Bezzi P, Volterra A. TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron. 2011;69:988–1001. [DOI] [PubMed] [Google Scholar]
- 209. McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-α to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011;10:391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Golan H, Levav T, Mendelsohn A, Huleihel M. Involvement of tumor necrosis factor alpha in hippocampal development and function. Cereb Cortex. 2004;14:97–105. [DOI] [PubMed] [Google Scholar]
- 212. Yang L, Lindholm K, Konishi Y, Li R, Shen Y. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J Neurosci. 2002;22:3025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Tancredi V, D'Arcangelo G, Grassi F, Tarroni P, Palmieri G, Santoni A, Eusebi F. Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neurosci Lett. 1992;146:176–8. [DOI] [PubMed] [Google Scholar]
- 214. Butler MP, O'Connor JJ, Moynagh PN. Dissection of tumor-necrosis factor-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early- but not late-phase LTP. Neuroscience. 2004;124:319–26. [DOI] [PubMed] [Google Scholar]
- 215. Waalkes MP. Cadmium carcinogenesis. Mutat Res. 2003;533:107–20. [DOI] [PubMed] [Google Scholar]
- 216. Im J-Y, Paik S-G, Han P-L. Cadmium-induced astroglial death proceeds via glutathione depletion. J Neurosci Res. 2006;83:301–8. [DOI] [PubMed] [Google Scholar]
- 217. Merzoug S, Toumi ML, Boukhris N, Baudin B, Tahraoui A. Adriamycin-related anxiety-like behavior, brain oxidative stress and myelotoxicity in male Wistar rats. Pharmacol Biochem Behav. 2011;99:639–47. [DOI] [PubMed] [Google Scholar]
- 218. Christie L-A, Acharya MM, Parihar VK, Nguyen A, Martirosian V, Limoli CL. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18:1954–65. [DOI] [PubMed] [Google Scholar]
- 219. Merzoug S, Toumi ML, Tahraoui A. Quercetin mitigates adriamycin-induced anxiety- and depression-like behaviors, immune dysfunction, and brain oxidative stress in rats. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:921–33. [DOI] [PubMed] [Google Scholar]
- 220. Joshi G, Aluise CD, Cole MP, Sultana R, Pierce WM, Vore M, Clair DK, Butterfield DA. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: implications for oxidative stress-mediated chemobrain. Neuroscience. 2010;166:796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Mohamed RH, Karam RA, Amer MG. Epicatechin attenuates doxorubicin-induced brain toxicity: critical role of TNF-α, iNOS and NF-κB. Brain Res Bull. 2011;86:22–8. [DOI] [PubMed] [Google Scholar]
- 222. Keeney JTR, Miriyala S, Noel T, Moscow JA, St Clair DK, Butterfield DA. Superoxide induces protein oxidation in plasma and TNF-α elevation in macrophage culture: insights into mechanisms of neurotoxicity following doxorubicin chemotherapy. Cancer Lett. 2015;367:157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Rizk HA, Masoud MA, Maher OW. Prophylactic effects of ellagic acid and rosmarinic acid on doxorubicin-induced neurotoxicity in rats. J Biochem Mol Toxicol. 2017;31. doi:10.1002/jbt.21977. [DOI] [PubMed] [Google Scholar]
- 224. Iino T, Nakahara K, Miki W, Kiso Y, Ogawa Y, Kato S, Takeuchi K. Less damaging effect of whisky in rat stomachs in comparison with pure ethanol. Digestion. 2001;64:214–21. [DOI] [PubMed] [Google Scholar]
- 225. Palani S, Santhakumari D, Balachandar S, Ambika S. Protective role of ellagic acid in modulating iron induced nephrotoxicity in rats [Internet]. 2015; [cited 2020 May 10]. Available from: https://www.semanticscholar.org/paper/Protective-role-of-Ellagic-acid-in-modulating-Iron-Palani-Santhakumari/efd58808cf03d3b6c0839011f1483889f4dcae91 [Google Scholar]
- 226. Meyer JN, Leung MCK, Rooney JP, Sendoel A, Hengartner MO, Kisby GE, Bess AS. Mitochondria as a target of environmental toxicants. Toxicol Sci. 2013;134:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Prakash C, Soni M, Kumar V. Mitochondrial oxidative stress and dysfunction in arsenic neurotoxicity: a review. J Appl Toxicol. 2016;36:179–88. [DOI] [PubMed] [Google Scholar]
- 228. Goudarzi M, Amiri S, Nesari A, Hosseinzadeh A, Mansouri E, Mehrzadi S. The possible neuroprotective effect of ellagic acid on sodium arsenate-induced neurotoxicity in rats. Life Sci. 2018;198:38–45. [DOI] [PubMed] [Google Scholar]
- 229. Carlsen H, Myhrstad MCW, Thoresen M, Moskaug JO, Blomhoff R. Berry intake increases the activity of the γ-glutamylcysteine synthetase promoter in transgenic reporter mice. J Nutr. 2003;133:2137–40. [DOI] [PubMed] [Google Scholar]
- 230. Firdaus F, Zafeer MF, Anis E, Ahmad M, Afzal M. Ellagic acid attenuates arsenic induced neuro-inflammation and mitochondrial dysfunction associated apoptosis. Toxicol Rep. 2018;5:411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Riboldi BP, Vinhas AM, Moreira JD. Risks of dietary acrylamide exposure: a systematic review. Food Chem. 2014;157:310–22. [DOI] [PubMed] [Google Scholar]
- 232. He Y, Tan D, Bai B, Wu Z, Ji S. Epigallocatechin-3-gallate attenuates acrylamide-induced apoptosis and astrogliosis in rat cerebral cortex. Toxicol Mech Methods. 2017;27:298–306. [DOI] [PubMed] [Google Scholar]
- 233. Lai S, Gu Z, Zhao M, Li X, Ma Y, Luo L, Liu J. Toxic effect of acrylamide on the development of hippocampal neurons of weaning rats. Neural Regen Res. 2017;12:1648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Goudarzi M, Mombeini MA, Fatemi I, Aminzadeh A, Kalantari H, Nesari A, Najafzadehvarzi H, Mehrzadi S. Neuroprotective effects of ellagic acid against acrylamide-induced neurotoxicity in rats. Neurol Res. 2019;41:419–28. [DOI] [PubMed] [Google Scholar]
- 235. Pan X, Wu X, Yan D, Peng C, Rao C, Yan H. Acrylamide-induced oxidative stress and inflammatory response are alleviated by N-acetylcysteine in PC12 cells: involvement of the crosstalk between Nrf2 and NF-κB pathways regulated by MAPKs. Toxicol Lett. 2018;288:55–64. [DOI] [PubMed] [Google Scholar]
- 236. Zhao M, Lewis Wang FS, Hu X, Chen F, Chan HM. Acrylamide-induced neurotoxicity in primary astrocytes and microglia: roles of the Nrf2-ARE and NF-κB pathways. Food Chem Toxicol. 2017;106:25–35. [DOI] [PubMed] [Google Scholar]
- 237. Abakumova TO, Kuzkina AA, Zharova ME, Zharova MV, Pozdeeva DA, Gubskii IL, Shepeleva II, Antonova OM, Nokolova NV, Kekelidze ZIet al. . Cuprizone model as a tool for preclinical studies of the efficacy of multiple sclerosis diagnosis and therapy. Bull Exp Biol Med. 2015;159:111–5. [DOI] [PubMed] [Google Scholar]
- 238. Praet J, Guglielmetti C, Berneman Z, Van der Linden A, Ponsaerts P. Cellular and molecular neuropathology of the cuprizone mouse model: clinical relevance for multiple sclerosis. Neurosci Biobehav Rev. 2014;47:485–505. [DOI] [PubMed] [Google Scholar]
- 239. Sanadgol N, Golab F, Tashakkor Z, Taki N, Moradi Kouchi S, Mostafaie A, Mehdizadeh M, Abdollahi M, Tagizadeh G, Sharifzadeh M. Neuroprotective effects of ellagic acid on cuprizone-induced acute demyelination through limitation of microgliosis, adjustment of CXCL12/IL-17/IL-11 axis and restriction of mature oligodendrocytes apoptosis. Pharm Biol. 2017;55:1679–87. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 240. Zilkha-Falb R, Kaushansky N, Kawakami N, Ben-Nun A. Post-CNS-inflammation expression of CXCL12 promotes the endogenous myelin/neuronal repair capacity following spontaneous recovery from multiple sclerosis-like disease. J Neuroinflammation. 2016;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Zhang Y, Taveggia C, Melendez-Vasquez C, Einheber S, Raine CS, Salzer JL, Brosnan CF, John GR. Interleukin-11 potentiates oligodendrocyte survival and maturation, and myelin formation. J Neurosci. 2006;26:12174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Hassoun EA, Vodhanel J, Abushaban A. The modulatory effects of ellagic acid and vitamin E succinate on TCDD-induced oxidative stress in different brain regions of rats after subchronic exposure. J Biochem Mol Toxicol. 2004;18:196–203. [DOI] [PubMed] [Google Scholar]
- 243. Hassoun EA, Vodhanel J, Holden B, Abushaban A. The effects of ellagic acid and vitamin E succinate on antioxidant enzymes activities and glutathione levels in different brain regions of rats after subchronic exposure to TCDD. J Toxicol Environ Health A. 2006;69:381–93. [DOI] [PubMed] [Google Scholar]
- 244. Aslan A, Gok O, Beyaz S, Arslan E, Erman O, Agca CA. The preventive effect of ellagic acid on brain damage in rats via regulating of Nrf-2, NF-kB and apoptotic pathway. J Food Biochem. 2020;44(6):e13217. [DOI] [PubMed] [Google Scholar]
- 245. Mansouri MT, Farbood Y, Naghizadeh B, Shabani S, Mirshekar MA, Sarkaki A. Beneficial effects of ellagic acid against animal models of scopolamine- and diazepam-induced cognitive impairments. Pharm Biol. 2016;54:1947–53. [DOI] [PubMed] [Google Scholar]
- 246. Farbood Y, Sarkaki A, Dolatshahi M, Taqhi Mansouri SM, Khodadadi A. Ellagic acid protects the brain against 6-hydroxydopamine induced neuroinflammation in a rat model of Parkinson's disease. Basic Clin Neurosci. 2015;6:83–9. [PMC free article] [PubMed] [Google Scholar]
- 247. Sarkaki A, Farbood Y, Dolatshahi M, Mansouri SMT, Khodadadi A. Neuroprotective effects of ellagic acid in a rat model of Parkinson's disease. Acta Med Iran. 2016;54:494–502. [PubMed] [Google Scholar]
- 248. Dolatshahi M, Farbood Y, Sarkaki A, Mansouri SMT, Khodadadi A. Ellagic acid improves hyperalgesia and cognitive deficiency in 6-hydroxidopamine induced rat model of Parkinson's disease. Iran J Basic Med Sci. 2015;18:38–46. [PMC free article] [PubMed] [Google Scholar]
- 249. Baluchnejadmojarad T, Rabiee N, Zabihnejad S, Roghani M. Ellagic acid exerts protective effect in intrastriatal 6-hydroxydopamine rat model of Parkinson's disease: possible involvement of ERβ/Nrf2/HO-1 signaling. Brain Res. 2017;1662:23–30. [DOI] [PubMed] [Google Scholar]
- 250. Kakeyama M, Sone H, Miyabara Y, Tohyama C. Perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin alters activity-dependent expression of BDNF mRNA in the neocortex and male rat sexual behavior in adulthood. Neurotoxicology. 2003;24:207–17. [DOI] [PubMed] [Google Scholar]
- 251. Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–54. [DOI] [PubMed] [Google Scholar]
- 252. Safe S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs). Crit Rev Toxicol. 1990;21:51–88. [DOI] [PubMed] [Google Scholar]
- 253. Hassoun EA, Al-Ghafri M, Abushaban A. The role of antioxidant enzymes in TCDD-induced oxidative stress in various brain regions of rats after subchronic exposure. Free Radic Biol Med. 2003;35:1028–36. [DOI] [PubMed] [Google Scholar]
- 254. Di Mascio P, Murphy ME, Sies H. Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am J Clin Nutr. 1991;53:194S–200S. [PubMed] [Google Scholar]
- 255. Davies KJ. Oxidative stress: the paradox of aerobic life. Biochem Soc Symp. 1995;61:1–31. [DOI] [PubMed] [Google Scholar]
- 256. Sanzgiri UY, Srivatsan V, Muralidhara S, Dallas CE, Bruckner JV. Uptake, distribution, and elimination of carbon tetrachloride in rat tissues following inhalation and ingestion exposures. Toxicol Appl Pharmacol. 1997;143:120–9. [DOI] [PubMed] [Google Scholar]
- 257. Weber LWD, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–36. [DOI] [PubMed] [Google Scholar]
- 258. Del Maestro R, McDonald W. Distribution of superoxide dismutase, glutathione peroxidase and catalase in developing rat brain. Mech Ageing Dev. 1987;41:29–38. [DOI] [PubMed] [Google Scholar]
- 259. Adams JD, Klaidman LK, Odunze IN, Shen HC, Miller CA. Alzheimer's and Parkinson's disease. Brain levels of glutathione, glutathione disulfide, and vitamin E. Mol Chem Neuropathol. 1991;14:213–26. [DOI] [PubMed] [Google Scholar]
- 260. Bari SMI, Reis LG, Nestorova GG. Calorimetric sandwich-type immunosensor for quantification of TNF-α. Biosens Bioelectron. 2019;126:82–7. [DOI] [PubMed] [Google Scholar]
- 261. Bowie CR, Milanovic M, Tran T. Pathophysiology of cognitive impairment in depression. In: Quevedo J, Carvalho AF, Zarate CA, editors. Neurobiology of depression. Cambridge (MA): Academic Press; 2019.. p. 27–30. [Google Scholar]
- 262. Philippens IHCHM, Hart BA't, Torres G. The MPTP marmoset model of parkinsonism: a multi-purpose non-human primate model for neurodegenerative diseases. Drug Discov Today. 2010;15:985–90. [DOI] [PubMed] [Google Scholar]
- 263. Moreira ELG, Rial D, Aguiar AS, Figueiredo CP, Siqueira JM, DalBo S, Horst H, de Oliveira J, Mancini G, Santos TSet al. . Proanthocyanidin-rich fraction from Croton celtidifolius Baill confers neuroprotection in the intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine rat model of Parkinson's disease. J Neural Transm (Vienna). 2010;117:1337–51. [DOI] [PubMed] [Google Scholar]
- 264. Chaturvedi RK, Shukla S, Seth K, Chauhan S, Sinha C, Shukla Y, Agrawal AK. Neuroprotective and neurorescue effect of black tea extract in 6-hydroxydopamine-lesioned rat model of Parkinson's disease. Neurobiol Dis. 2006;22:421–34. [DOI] [PubMed] [Google Scholar]
- 265. Hritcu L, Ciobica A, Artenie V. Effects of right-unilateral 6-hydroxydopamine infusion-induced memory impairment and oxidative stress: relevance for Parkinson's disease. Cent Eur J Biol. 2008;3:250–7. [Google Scholar]
- 266. Kelly E, Jenner P, Marsden CD. Behavioural effects mediated by unilateral nigral dopamine receptor stimulation in the rat. Exp Brain Res. 1984;55:243–52. [DOI] [PubMed] [Google Scholar]
- 267. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. [DOI] [PubMed] [Google Scholar]
- 268. Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. [DOI] [PubMed] [Google Scholar]
- 269. Macchi B, Di Paola R, Marino-Merlo F, Felice MR, Cuzzocrea S, Mastino A. Inflammatory and cell death pathways in brain and peripheral blood in Parkinson's disease. CNS Neurol Disord Drug Targets. 2015;14:313–24. [DOI] [PubMed] [Google Scholar]
- 270. Wong SM, Kellaway IW, Murdan S. Enhancement of the dissolution rate and oral absorption of a poorly water soluble drug by formation of surfactant-containing microparticles. Int J Pharm. 2006;317:61–8. [DOI] [PubMed] [Google Scholar]
- 271. Naseem A, Olliff CJ, Martini LG, Lloyd AW. Effects of plasma irradiation on the wettability and dissolution of compacts of griseofulvin. Int J Pharm. 2004;269:443–50. [DOI] [PubMed] [Google Scholar]
- 272. Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20. [DOI] [PubMed] [Google Scholar]
- 273. Bala I, Bhardwaj V, Hariharan S, Kharade SV, Roy N, Ravi Kumar MNV. Sustained release nanoparticulate formulation containing antioxidant-ellagic acid as potential prophylaxis system for oral administration. J Drug Target. 2006;14:27–34. [DOI] [PubMed] [Google Scholar]
- 274. Bala I, Bhardwaj V, Hariharan S, Kumar MNVR. Analytical methods for assay of ellagic acid and its solubility studies. J Pharm Biomed. 2006;40:206–10. [DOI] [PubMed] [Google Scholar]
- 275. Zuccari G, Baldassari S, Ailuno G, Turrini F, Alfei S, Caviglioli G. Formulation strategies to improve oral bioavailability of ellagic acid. Appl Sci. 2020;10:3353. [Google Scholar]
- 276. Mady FM, Shaker MA. Enhanced anticancer activity and oral bioavailability of ellagic acid through encapsulation in biodegradable polymeric nanoparticles. Int J Nanomedicine. 2017;12:7405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Li B, Harich K, Wegiel L, Taylor LS, Edgar KJ. Stability and solubility enhancement of ellagic acid in cellulose ester solid dispersions. Carbohydr Polym. 2013;92:1443–50. [DOI] [PubMed] [Google Scholar]
- 278. Ceci C, Graziani G, Faraoni I, Cacciotti I. Strategies to improve ellagic acid bioavailability: from natural or semisynthetic derivatives to nanotechnological approaches based on innovative carriers. Nanotechnology. 2020;31:382001. [DOI] [PubMed] [Google Scholar]