Early indicators suggest that highly effective messenger RNA (mRNA) vaccinations (BNT 162b2 [Pfizer-BioNTech] and mRNA-1273 [NIH-Moderna]) are beginning to curb the coronavirus disease 2019 (COVID-19) pandemic that has already taken the lives of 3 million individuals worldwide. However, questions about vaccine effectiveness remain for individuals with inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, who are frequently treated with immune suppression.1 An early study among transplant recipients indicated low humoral immune response after an initial vaccination, in contrast to the robust response observed in healthy individuals in phase III clinical trials.2 Similarly, a recent study in IBD patients suggested that treatment with infliximab is associated with an attenuated level of anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike after a single dose of mRNA vaccine.3
However, the more clinically relevant question relates to seroprotection after completing the approved 2-part vaccination series. In a follow-up study of 658 transplant recipients, antispike antibody was detected in 54% of participants at a median of 29 days after the second vaccine dose.4 Preliminary results suggest that IBD patients may fare better. In a single-center US study all patients (n = 26) who completed 2-dose vaccine schedules had detectible antibody.5 Additionally, in a multicenter UK study, 85% (17/20) of infliximab-treated patients and 86% (6/7) of vedolizumab-treated patients seroconverted after the second vaccine dose.3
Despite this early reassuring data, larger studies evaluating COVID-19 vaccine effectiveness in IBD patients are urgently needed to guide optimal approaches to the COVID-19 vaccination. We assessed serologic response after completion of the 2-part mRNA vaccination series in a geographically diverse US IBD population.
Methods
The Partnership to Report Effectiveness of Vaccination in populations Excluded from iNitial Trials of COVID (PREVENT-COVID) is a prospective, observational, cohort study of patients with IBD who have received any COVID-19 vaccine granted Emergency Use Authorization in the United States. Eligibility criteria were a diagnosis of IBD, receipt of 1 or more doses of any COVID-19 vaccine within the prior 90 days, age 16 years or older, US residence, access to the internet and ability to complete surveys in English, and willingness to remain in study for 18 months. Participants were recruited through education, social media, and other outreach efforts in collaboration with the Crohn’s & Colitis Foundation and by referral at selected clinical sites (Appendix 1) and will be followed through internet surveys for up to 18 months to ascertain outcomes of COVID-19 infection and safety events.
We performed quantitative measurement of antireceptor binding domain IgG antibodies specific to SARS-CoV-2 at approximately 8 weeks after completing the vaccination series using the LabCorp Cov2Quant IgG assay. Results of 1.0 μg/mL or greater suggest vaccination and/or prior infection with SARS-CoV-2. We also performed qualitative assessment of nucleocapsid in a subset of participants as an indicator of past infection.
This analysis included all participants who completed their 2-part vaccination series and underwent laboratory testing before May 14, 2021. Participants who reported prior COVID-19 infection and/or had positive nucleocapsid antibody indicating prior native infection were excluded. We performed descriptive statistics to characterize the study population and antibody response, including subgroup analyses stratified by age, vaccine type, and reported medication use at the time of initial immunization. We used box and whisker plots to display mean, median, and interquartile ranges of antibody values overall and across subgroups. The study protocol was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
Results
The study population included 317 participants (mean age, 50.9 years; 75% women). Antibody testing was obtained at a median of 64 days (interquartile range, 59–73) after the second vaccination. Additional demographic characteristics and medication use are shown in Table 1 .
Table 1.
Demographics, Treatment Characteristics, and Humoral Immune Response to COVID-19 Immunization Among Patients With IBD Enrolled in the PREVENT-COVID Study
| Overall Population (N = 317) | Anti-TNF Monotherapy (n = 108) | Anti-TNF Combination Therapy (n = 24) | 6MP/AZA/MTX Alone (n = 20) | 5ASA, Sulfasalazine, Budesonide, or No Medication (n = 65) | Vedolizumab Monotherapy (n = 46) | Ustekinumab Monotherapy (n = 39) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median time from second vaccine dose to antibody test, days (interquartile range) | 64.0 | (59.0–72.5) | 65.0 | (61.0–72.0) | 67.5 | (61.5–75.5) | 69.0 | (63.0–75.5) | 64.0 | (59.0–72.0) | 61.0 | (55.0–70.0) | 63.0 | (56.0–71.0) |
| Mean age, y (SD) | 50.9 | (16.7) | 48.0 | (16.5) | 43.9 | (16.0) | 56.5 | (18.9) | 57.2 | (15.4) | 53.3 | (16.7) | 48.0 | (16.1) |
| Female | 238 | (75) | 79 | (73) | 19 | (79) | 15 | (75) | 52 | (80) | 33 | (72) | 30 | (77) |
| Type of vaccine at first dose | ||||||||||||||
| Pfizer | 173 | (55) | 57 | (53) | 11 | (46) | 14 | (70) | 33 | (51) | 26 | (57) | 22 | (56) |
| Moderna | 144 | (45) | 51 | (47) | 13 | (54) | 6 | (30) | 32 | (49) | 20 | (43) | 17 | (44) |
| Race | ||||||||||||||
| White | 301 | (95) | 102 | (94) | 23 | (96) | 19 | (95) | 63 | (97) | 43 | (93) | 37 | (95) |
| Black/African American | 1 | (0) | 1 | (1) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) |
| Asian | 5 | (2) | 3 | (3) | 0 | (0) | 0 | (0) | 1 | (2) | 1 | (2) | 0 | (0) |
| Native Hawaiian/Pacific | 1 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 1 | (3) |
| More than 1 race | 4 | (1) | 1 | (1) | 1 | (4) | 0 | (0) | 0 | (0) | 1 | (2) | 1 | (3) |
| Other | 5 | (2) | 1 | (1) | 0 | (0) | 1 | (5) | 1 | (2) | 1 | (2) | 0 | (0) |
| Hispanic | ||||||||||||||
| Yes | 9 | (3) | 1 | (1) | 1 | (4) | 1 | (5) | 1 | (2) | 1 | (2) | 3 | (8) |
| No | 308 | (97) | 107 | (99) | 23 | (96) | 19 | (95) | 64 | (98) | 45 | (98) | 36 | (92) |
| Region | ||||||||||||||
| Northeast | 80 | (25) | 32 | (30) | 6 | (25) | 4 | (20) | 16 | (25) | 12 | (26) | 8 | (21) |
| South | 108 | (34) | 39 | (36) | 6 | (25) | 8 | (40) | 19 | (29) | 13 | (28) | 18 | (46) |
| Midwest | 76 | (24) | 22 | (20) | 7 | (29) | 4 | (20) | 16 | (25) | 16 | (35) | 8 | (21) |
| West | 53 | (17) | 15 | (14) | 5 | (21) | 4 | (20) | 14 | (22) | 5 | (11) | 5 | (13) |
| Mean antispike antibody level (SD) | 28.6 | (47.5) | 15.1 | (18.4) | 11.5 | (9.4) | 24.0 | (25.2) | 44.2 | (79.0) | 45.2 | (51.0) | 34.6 | (47.2) |
| Median antispike antibody level (interquartile range) | 17.0 | (7.8–30.0 | 10.0 | (4.6–18.0 | 8.5 | (5.6–18.0 | 15.5 | (7.0–30.0 | 24.0 | (14.0–42.0 | 30.0 | (20.0–40.0 | 22.0 | (10.0–35.0 |
| Proportion with detectible antispike antibody | 300 | (95) | 101 | (94) | 21 | (88) | 19 | (95) | 61 | (94) | 46 | (100) | 38 | (97) |
Values are n (%) unless otherwise defined. 5ASA, 5-aminosalicylic acid; 6MP, 6-mercaptopurine; AZA, azathioprine; MTX, methotrexate; TNF, tumor necrosis factor.
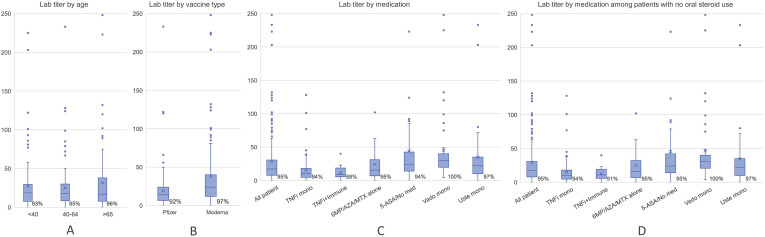
Overall, 300 of 317 participants (95% confidence interval [CI], 92–97) had detectable antibodies. The distribution of antibody response across medication classes and other patient characteristics are shown in Table 1. Participants receiving systemic corticosteroids appeared to have somewhat diminished antibody response, although formal hypothesis testing was not done in this exploratory analysis. Of 13 patients taking corticosteroids, the proportion with detectible antibodies was 85% (95% CI, 58–96) versus 95% (95% CI, 92–97) among nonsteroid users (mean antibody level, 22 μg/mL vs 29 μg/mL) among nonusers (Supplementary Table 1). Antibody response was generally similar across age group, vaccine type, and use of other classes of IBD medications (Supplementary Figure 1). Of the 10 participants with positive nucleocapsid antibody indicative of prior infection who were excluded from the above analyses, all had detectable antispike antibodies (mean, 70 μg/mL; median, 32 μg/mL).
Supplementary Figure 1.
Antispike antibody levels among IBD patients enrolled in the PREVENT-COVID study. Box and whisker plots illustrating mean (X), median, interquartile range, overall range of antispike antibody levels (μg/mL), and the proportion of participants with detectible antibody stratified by (A) age group, (B) type of vaccination, (C) IBD medication use (all participants), and (D) medication use among patients not taking corticosteroids.
Discussion
In this study of the humoral response to 2 doses of mRNA SARS-CoV-2 vaccine in a geographically diverse cohort of over 300 patients with IBD, most had detectable antibody responses after the second dose. Overall, these results reinforce the findings of 2 small reports (<30 participants each) indicating positive humoral immune response with complete vaccination.3 , 4 Taken together, these emerging data provide reassurance that most medications for IBD do not markedly reduce the response to COVID-19 immunization and support recent consensus recommendations to vaccinate all patients with IBD regardless of immune-modifying therapies.6 Our finding of somewhat attenuated humoral immune response in patients receiving corticosteroids requires further prospective evaluation and may ultimately warrant special consideration regarding timing of vaccination efforts, utility of antibody testing after vaccination, and/or the possible need for booster vaccination beyond the standard 2-dose series.
Study limitations include a convenience sample that lacks racial and ethnic diversity and under-represents men and the reliance of self-report in our direct-to-patient cohort. We did not conduct formal hypothesis testing in this exploratory analysis. Additionally, no threshold has been established for protective immunity in the quantitative antibody testing.
Although many questions remain and ongoing research efforts will help to further optimize immunization strategies for patients with IBD, these findings provide reassurance that most patients mount detectable humoral immune response to mRNA vaccinations and support current recommendations to vaccinate patients regardless of immunosuppressive treatment.
Acknowledgments
Partnership to Report Effectiveness of Vaccination in populations Excluded from iNitial Trials of COVID (PREVENT COVID) Study Group: K. Chun, M. Fernando, M. Zikry, LabCorp; X. Dai, University of North Carolina; R. Watkins, Division of Pediatric Gastroenterology & Nutrition, University of Maryland School of Medicine; J. Adler, Susan B. Meister Child Health Evaluation and Research Center and Department of Pediatrics, University of Michigan; M. C. Dubinsky, Department of Pediatrics, Susan and Leonard Feinstein IBD Center, Icahn School of Medicine, Mount Sinai, New York; A. Kastl, Division of Gastroenterology, Children’s Hospital of Philadelphia, Perelman School of Medicine, Philadelphia, PA; A. Bousvaros, Children’s Hospital Boston; J. A. Strople, Ann & Robert H. Lurie Children’s Hospital of Chicago; R. K. Cross, University of Maryland School of Medicine; P. D. R. Higgins, University of Michigan; R. Ungaro, Susan and Leonard Feinstein IBD Center, Icahn School of Medicine, Mount Sinai, New York; M. Bewtra, University of Pennsylvania; E. Bellaguarda, Division of Gastroenterology and Hepatology, Northwestern University, Chicago, IL; F. A. Farraye, Mayo Clinic Florida.
CRediT Authorship Contributions
Michael D Kappelman, MD, MPH (Conceptualization: Lead; Data curation: Lead; Funding acquisition: Lead; Investigation: Lead; Methodology: Lead; Supervision: Lead; Writing – original draft: Lead; Writing – review & editing: Lead). Kimberly Weaver, MD (Data curation: Equal; Investigation: Equal; Supervision: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting). Margie Boccieri, MA (Data curation: Supporting; Project administration: Equal; Writing – review & editing: Supporting). Ann Firestine, MS (Data curation: Equal; Project administration: Equal; Writing – review & editing: Supporting). Xian Zhang, PhD (Formal analysis: Lead; Methodology: Equal; Visualization: Lead; Writing – review & editing: Supporting). Millie Long, MD, MPH (Conceptualization: Lead; Data curation: Lead; Funding acquisition: Lead; Investigation: Lead; Methodology: Lead; Writing – review & editing: Equal).
Footnotes
Conflicts of interest These authors disclose the following: Michael D. Kappelman has consulted for Abbvie, Janssen, Pfizer, and Takeda; is a shareholder in Johnson & Johnson; and has received research support from Pfizer, Takeda, Janssen, Abbvie, Lilly, Genentech, Boehringer Ingelheim, Bristol Myers Squibb, Celtrion, and Arenapharm. Millie Long has received research/grants from Pfizer Inc and has consulted for AbbVie Inc, Bristol-Myers Squibb Company, Calibr, Eli Lilly and Company, Genentech, Inc, Gilead Sciences, Inc, Janssen Pharmaceuticals, Inc, Pfizer Inc, Roche, Takeda Pharmaceuticals U.S.A., Inc, TARGET PharmaSolutions, Inc, and Theravance Biopharma. These authors from the PREVENT-COVID Study Group disclose the following: JA has consulted for Janssen and has received research support from The Gary and Rachel Glick Charitable Fund, Shaevsky Family Research Fund for Crohn's Disease, the Crohn’s & Colitis Foundation, and The Leona M. and Harry B. Helmsley Charitable Trust. MCD has received consultant fees from Abbvie, Arena, Bristol Myers Squibb, Celgene, Gilead, Janssen, Pfizer, Prometheus Labs, and Takeda; grant support from Abbvie and Prometheus Labs; and license fees from Takeda. AB has been a subinvestigator on trials for Prometheus, Janssen, Abbvie, Takeda, Buhlmann, Arena, and Eli Lilly in the past 36 months and has consulted for Arena, Tekeda, Best Doctors, and Eli Lilly. JAS has consulted for Janssen and has received research support from The Gary and Rachel Glick Charitable Fund, Shaevsky Family Research Fund for Crohn's Disease, the Crohn’s & Colitis Foundation, and The Leona M. and Harry B. Helmsley Charitable Trust. RKC has participated in advisory boards and has consulted for Abbvie, Bristol Myers Squibb, Janssen, LabCorp, Pfizer, Samsung Bioepis, and Takeda. PDRH has consulted for Abbvie, Pfizer, and Takeda and has received grant support from the National Institutes of Health, CCF, Abbvie, Pfizer, Takeda, Genentech, Eli Lilly, Arena, and the Rainin Foundation. RCU has served as an advisory board member or consultant for AbbVie, Bristol Myers Squibb, Eli Lilly, Janssen, Pfizer, and Takeda and has received research support from AbbVie, Boehringer Ingelheim, and Pfizer. RCU is funded by a National Institutes of Health Career Development Award (K23KD111995-01A1). MB discloses research funding from Janssen, GlaxoSmithKline, and Takeda; has served as a consultant for Janssen, AbbVie, BMS, and Pfizer; and has received honorarium for participation in a CME program sponsored by AbbVie. EB has consulted for Abbvie, Pfizer, and Bristol Myers Squibb. FAF is a consultant for Arena, BMS, Braintree Labs, Gilead, GI Reviewers, Innovation Pharmaceuticals, Iterative Scopes, Janssen, Pfizer, and Sebela and sits on a Data Safety Monitoring Board for Lilly and Theravance. The remaining authors disclose no conflicts.
Funding This research was funded by the Helmsley Charitable Trust.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org and at https://doi.org/10.1053/j.gastro.2021.06.016.
Contributor Information
PREVENT-COVID Study Group:
K. Chun, M. Fernando, M. Zikry, X. Dai, R. Watkins, J. Adler, M.C. Dubinsky, A. Kastl, A. Bousvaros, J.A. Strople, R.K. Cross, P.D.R. Higgins, R. Ungaro, M. Bewtra, E. Bellaguarda, and F.A. Farraye
Supplementary Methods
LabCorp’s Cov2Quant IgG assay uses electrochemiluminescence immunoassay technology for the quantitative measurement of IgG antibodies to SARS-CoV-2. The coronavirus spike glycoprotein is a viral fusion protein on the outer envelope of the virion that plays a critical role in viral infection by recognizing host cell receptors and mediating the fusion of viral and host cell membranes. Specifically, the receptor binding domain (RBD) of the spike protein is the moiety that interacts directly with the ACE2 receptor on a host cell to enable viral entry. Because the RBD is poorly conserved among other coronaviruses, antibodies to the RBD are SARS-specific antibodies in humans. Additionally, the spike protein is the target of mRNA vaccination. Internal validation indicated an assay sensitivity of 99% (95% CI, 97–100). Although positive results do not necessarily indicate protective immunity, prior studies have observed strong correlations between levels of RBD-binding antibodies and SARS-COV-2 neutralizing antibodies in patient sera.
On a subset of participants, we also performed qualitative detection of high affinity antibodies to SARS-CoV-2 nucleocapsid protein using the LabCorp assay. This test indicates recent or prior infection but does not detect antibodies induced by currently available SARS-CoV-2 vaccines. Although this assay in principle can detect high affinity antibodies of all isotypes (ie, IgG, IgA, IgM), it preferentially detects IgG antibodies because these are more likely to evolve to become high affinity.
For analyses of spike protein antibody levels, we computed the mean, median, and proportion of participants with detectible antibody levels in the overall population. For calculation of mean and medians, those with undetectable antibody levels were considered as a 0 value. We also conducted stratified analyses by age group (<40 years, 40–64 years, and ≥65 years), vaccine type (Pfizer vs Moderna), use of systemic corticosteroids at time of first dose, and the following mutually exclusive categories of medication use: (1) anti-tumor necrosis factor without concomitant use of immunomodulator (6-mercaptopurine, azathioprine, and methotrexate); (2) anti-tumor necrosis factor with concomitant use of immunomodulator, vedolizumab, and ustekinumab; (3) immunomodulator without biologic; (4) vedolizumab; (5) ustekinumab; and (6) no medications or 5-aminosalicylate/sulfasalazine only. Patients taking tofacitinib (n = 3) and tacrolimus (n = 2) were excluded from medication subgroup analyses. Stratified analyses by medication class were conducted overall and in the subgroup of participants not taking corticosteroids.
Appendix 1
Clinical Sites Referring Participants to PREVENT-COVID
University of North Carolina
Maryland
Michigan
Mount Sinai
University of Pennsylvania
Children’s Hospital Philadelphia
Children’s Hospital Boston
Brigham and Women’s
Northwestern
Ann & Robert Lurie Children’s Hospital of Chicago
Mayo Clinic Jacksonville
Supplementary Table 1.
Humoral Immune Response to COVID-19 Immunization, Stratified by Corticosteroid Use, Among Patients With IBD Enrolled in the PREVENT-COVID Study
| Corticosteroids (n = 13) | No corticosteroids (n = 304) | |
|---|---|---|
| Positive antispike antibody, % (95% CI) | 84.6 (57.8–95.7) | 95.1 (92.0–97.0) |
| Mean antispike antibody level (SD) | 21.6 (24.8) | 28.9 (48.3) |
| Median antispike antibody level (interquartile range) | 14.0 (3.7–26.0) | 17.5 (7.8–30.5) |
References
- 1.Farraye F.A., et al. Am J Gastroenterol. 2017;112:241–258. doi: 10.1038/ajg.2016.537. [DOI] [PubMed] [Google Scholar]
- 2.Boyarsky B.J., et al. JAMA. 2021;325:1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy N.A., et al. Gut. 2021 doi: 10.1136/gutjnl-2021-324789. [DOI] [Google Scholar]
- 4.Boyarsky B.J., et al. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong S.Y., et al. Gastroenterology. 2021;161:715–718. doi: 10.1053/j.gastro.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel C.A., et al. Gut. 2021;70:635–640. doi: 10.1136/gutjnl-2020-324000. [DOI] [PMC free article] [PubMed] [Google Scholar]