Barrière et al. reported less effective immune responses after COVID-19 vaccination in cancer patients versus patients without cancers.1 Cancer patients are at high risk of death from COVID-19,2 but also develop less effective antiviral immune responses after COVID-19 or vaccination.1 , 3 , 4 In this report we analyze the clinical efficacy of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in cancer patients receiving active cancer treatment in 1503 cancer patients receiving one or two doses of COVID-19 vaccine in the Centre Léon Bérard.
From 4 January to 6 April 2021, 1503 cancer patients without previously documented COVID-19 infection [female N = 735 (48.9%)], median age: 64.8 years, (range 16.7-95.4 years), under active cancer treatment received at least one dose of SARS-CoV-2 vaccine. <10% of patients refused vaccination. 1127 (74.9%), 317 (21.1%) and 59 (4%) received BNT162b2, messenger RNA (mRNA)-1273 and Chadox1 vaccines respectively as first doses, depending on availability. 1203 (80%) patients had a solid tumor and 300 (20%) had hematological malignancy, including 72 patients with chronic lymphocytic leukemia. 1081 (71.9%) had metastatic disease. 1003 (66.7%), 60 (3.9%), 245 (16.3%) and 189 (12.5%) had received cytotoxic chemotherapy, anti-CD20, radiotherapy, or surgery, respectively, in the previous 3 months.
1091 (72.6%) patients received two injections of COVID-19 vaccine at a median interval of 26 days (range 13-80 days), and 412 (27.4%) received only one injection (median follow-up after the day of vaccination for this group was 43 days, range 1-130 days).
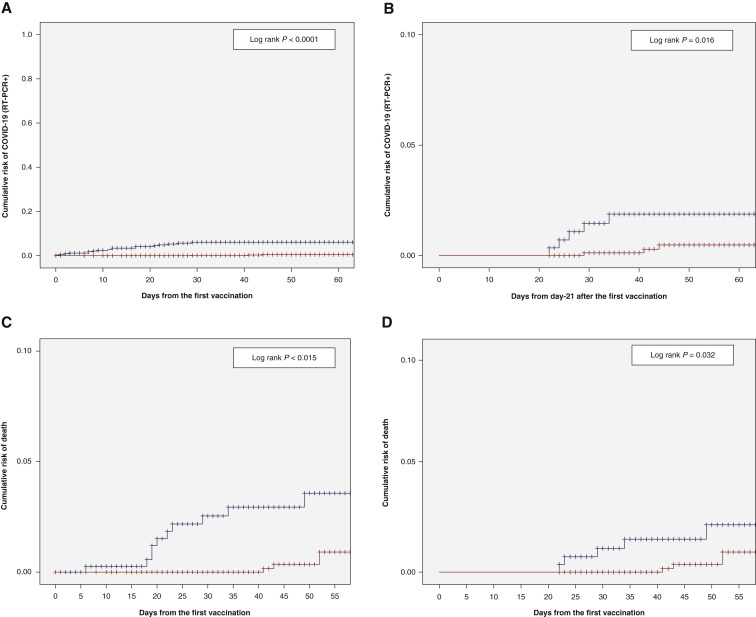
With a median follow-up of 44 (range 1-130) days for the whole group of 1503 patients, 24 of the 1503 (1.5%) patients developed COVID-19 symptoms with documented SARS-CoV-2 on RT-PCR: 4/1091 (0.4%) in patients who received two doses of vaccine versus 20/412 (5%) for those who received a single dose (P < 0.0001). With a landmark analysis at 21 days after first dose, these numbers were 4/1001 (0.4%) versus 5/283 (1.7%) for patients who received two versus one dose of vaccine (P = 0.016). Figure 1 A and 1B show the cumulative risk of documented COVID-19 with positive RT-PCR for SARS-CoV-2. The same differences were observed when mRNA vaccines were selected (not shown). Diagnosis of RT-PCR documented SARS-CoV-2 was not correlated with age, comorbidities (e.g. diabetes, renal failure, obesity), solid or hematological malignancies (not shown).
Figure 1.
Documented SARS-CoV-2 infection and death after one dose versus two doses of COVID-19 vaccines in cancer patients.
(A) Risk of SARS-CoV-2 RT-PCR+ from the first vaccine injection (1 dose in blue versus 2 doses in red). (B) Risk of SARS-CoV-2 RT-PCR+ from day-21 after the first vaccine injection (1 dose in blue versus 2 doses in red). (C) Survival from the first vaccine dose (1 dose in blue versus 2 doses in red). (D) Survival from day 21 after the first vaccine dose (1 dose in blue versus 2 doses in red).
COVID-19, coronavirus disease; SARS-Co-V2, severe acute respiratory syndrome coronavirus 2.
Three of the 24 (12.5%) RT-PCR+ patients died of COVID-19; 2 of 5 (40%) versus 1 of 19 (5%) patients with hematological and solid tumors, respectively (P = 0.036), representing an overall mortality rate of 0.7% and 0.08% in these two groups. The overall survival within 2 months from the date of the first vaccination was inferior for patients vaccinated with one dose versus patients vaccinated twice (Figure 1C, log rank P = 0.015) in the overall population, as well as with a landmark analysis at 21 days (Figure 1D, P = 0.032).
A total of 96 of the 1503 (6%) were tested for antispike antibody (Ab) after vaccination at a median time of 55 days after the first vaccine; 61/96 (63%) had detectable antispike Ab. Among these, four of the eight (50%) patients who later presented a documented SARS-CoV-2 RT-PCR had a detectable antispike Ab. Among the 96 tested patients, 4 of the 5 (80%) patients who died had undetectable antispike Ab after vaccination [versus 31/91 (34%) of the remaining patients, P = 0.038]. Two of the 5 who died had a RT-PCR documented SARS-CoV-2 infection.
In this analysis, COVID-19 vaccination was found to be effective in cancer patients. Documented COVID-19 was, however, more frequent in patients who received only one dose of vaccine. Overall death rate in the 2 months following the first vaccination was significantly higher in patients receiving only one dose and in patients with hematological cancers.
Consistent with Barrière et al. and another recent report,5 two doses of COVID-19 vaccines at 21- to 28-day intervals, according to the methods of the published randomized clinical trials, must be recommended in cancer patients receiving active treatment.
Acknowledgments
Funding
This work was supported by NetSARC (INCA & DGOS) and RREPS (INCA & DGOS); RESOS (INCA & DGOS); LYRICAN [grant number INCA-DGOS-INSERM 12563]; Association DAM's (no grant name); Eurosarc [grant number FP7-278742]; la Fondation ARC (grant UNICANCER); Infosarcome; InterSARC (INCA); LabEx DEvweCAN [grant number ANR-10-LABX 0061]; PIA Institut Convergence Francois Rabelais PLAsCAN [grant number PLASCAN, 17-CONV-0002]; La Ligue de L'Ain contre le Cancer (grant CANOPEE); La Ligue contre le Cancer (grant UNICANCER) and EURACAN [grant number EC 739521].
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Barrière J., Chamorey E., Adjtoutah Z., et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;32(8):1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assaad S., Avrillon V., Fournier M.-L., et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur J Cancer. 2020;135:251–259. doi: 10.1016/j.ejca.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solodky M.L., Galvez C., Russias B., et al. Lower detection rates of SARS-COV2 antibodies in cancer patients versus health care workers after symptomatic COVID-19. Ann Oncol. 2020;31(8):1087–1088. doi: 10.1016/j.annonc.2020.04.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roeker L.E., Knorr D.A., Thompson M.C., et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021 doi: 10.1038/s41375-021-01270-w. 1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]