Abstract
A developing finding from the novel coronavirus 2019 (COVID-19) pandemic is the burden of neuropsychiatric symptoms seen in COVID-19 survivors. While studies have shown clinically significant rates of depression, anxiety, insomnia, and trauma-related symptoms such as post-traumatic stress disorder (PTSD) after COVID-19, little is known about how these symptoms evolve over time. Here, we report findings from a cohort study of 52 participants recruited from the greater New York City area following acute COVID-19 infection. Participants completed the Patient Health Questionnaire-9 (PHQ-9) for depressive symptoms, the Generalized Anxiety Disorder-7 (GAD-7) for anxiety-related symptoms, the Insomnia Severity Scale (ISS) for sleep-related symptoms, and the PTSD Checklist-Civilian version (PCL-C) for trauma-related symptoms both at baseline and at long-term (24–60 weeks post-infection) follow-up. We found a high degree of correlation between psychiatric symptom scales within participants. More participants met established cutoffs for clinically significant insomnia and post-traumatic stress at follow-up compared to baseline. Symptom scales for depression, insomnia, and PTSD were increased at long-term follow-up, with only increased PCL-C scores surviving correction for multiple comparisons (Z = 2.92, W = 434, p = 0.004). Our results present evidence from a small cohort that neuropsychiatric symptoms, particularly those related to PTSD, may worsen over time in COVID-19 survivors. Future studies should continue to investigate these questions in broader populations, while additionally exploring the potential biological and sociological mechanisms that may contribute to neuropsychiatric pathology after COVID-19 infection.
Keywords: COVID-19, Post-traumatic stress, Depression, Anxiety, Insomnia, Long-term
1. Introduction
The novel coronavirus 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected over 190 million persons worldwide and caused over 4 million deaths as of this writing (Dong et al., 2020). While the pandemic continues to occasion unprecedented stress on global healthcare systems, numerous outstanding questions remain regarding the long-term consequences of SARS-CoV-2 infection. Principal among these questions is the long-term impact on mental and physical health in survivors of COVID-19 (Del Rio et al., 2020; Nalbandian et al., 2021).
An emerging finding from the COVID-19 pandemic is the burden of neuropsychiatric symptoms in survivors (Huang et al., 2021; Taquet et al., 2021a). An analysis of electronic health records showed increased odds of receiving a diagnosis of depression, anxiety, and insomnia in COVID-19 survivors compared to other respiratory infections (Taquet et al., 2021a). Short-term follow-up studies of COVID-19 survivors show that symptoms of anxiety, depression, fatigue, and insomnia are prominent in the month following discharge (Mazza et al., 2020; Townsend et al., 2020). Patients recovering from COVID-19 demonstrated elevated Patient Health Questionnaire-9 (PHQ-9) scores, used to measure symptoms of depression, at 2-to-3-month follow-up after discharge (Raman et al., 2021). Additionally, 23 % of participants reported symptoms of anxiety and/or depression and 26 % reported sleep difficulties in a large quality-of-life survey at 6-month follow-up (Huang et al., 2021). These findings from COVID-19 are similar to the long-term neuropsychiatric symptoms observed after the original SARS outbreak (Lam et al., 2009). Interestingly, the majority of these studies have found little to no correlation with initial illness severity and inflammatory markers during acute illness on lasting psychiatric symptoms.
An outstanding question in this emerging research domain is whether post-COVID neuropsychiatric symptoms evolve over time within individual survivors. In order to address this key point, we recruited participants who had recovered from confirmed COVID-19 infection and followed them for at least 24 weeks with measures in multiple neuropsychiatric domains. We hypothesized that COVID-19 survivors would demonstrate a high burden of psychiatric symptoms, and that these symptoms might worsen over time given ongoing psychosocial stressors secondary to the pandemic. Here, we report longitudinal findings from an observational cohort study of recovered COVID-19 participants with baseline and follow-up measures of depression, anxiety, insomnia, and post-traumatic stress.
2. Methods
2.1. Cohort recruitment and methodology
Participants recovered from COVID-19 infection were recruited by community advertisement and referral from other ongoing COVID-19 studies from April 15th, 2020 to February 23rd, 2021 from the greater New York City area. Inclusion criteria were age >7 years and confirmation of previous COVID-19 infection. Enrolled participants were offered an online survey with medical and psychiatric questionnaires, as well as in-person visits for laboratory monitoring and physical examinations. In order to establish bona fide COVID-19 infection status, serum antibody testing was performed and participants who were antibody-negative without proof of acute COVID-19 infection (e.g., positive PCR test during acute infection) were excluded from our analysis.
Participants were instructed to fill out follow-up surveys at visits from 24 to 60 weeks following initial enrollment. A total of 52 participants filled out both the baseline and at least one follow-up survey consisting of the PHQ-9 for depressive symptoms, the Generalized Anxiety Disorder-7 (GAD-7) for anxiety-related symptoms, the Insomnia Severity Scale (ISS) for sleep-related and insomnia symptoms, and the PTSD Checklist-Civilian version (PCL-C) for trauma-related symptoms. All investigations were approved by local Institutional Review Board (IRB) at Columbia University Irving Medical Center.
2.2. Statistical analysis
PHQ-9, GAD-7, ISS, and PCL-C scores were examined with regard to established cutoff scores. A cutoff of 10 for PHQ-9 and GAD-7 is commonly used both clinically and in research studies, while we used a cutoff of 10 for the ISS based on a previous analysis (Morin et al., 2011). A cutoff of 35 was selected for the PCL-C to maximize sensitivity in a population with an identifiable stressor (McDonald and Calhoun, 2010; Walker et al., 2002). Scores were averaged for participants who filled out surveys at multiple follow-up visits. We analyzed the effects of age, sex, self-defined racial and Hispanic identity, smoking status, weight, hospital admission, and time from symptom onset to study enrollment on PHQ-9, GAD-7, ISS, and PCL-C scores at baseline and follow-up using multivariate generalized linear modeling. PHQ-9, GAD-7, ISS, and PCL-C scores were analyzed for within-subject correlation with Pearson's test. Baseline and follow-up values were compared with Wilcoxon signed rank test or t-test depending on data distribution. Initial significance was set at p < 0.05, and Bonferroni correction was utilized for multiple comparisons. Exact p-values are reported up to p < 0.001.
3. Results
3.1. Cohort demographics and multivariate analysis
Basic demographic for our cohort (n = 52) and mean PHQ-9, GAD-7, ISS, or PCL-C scores at baseline and follow-up are reported in Table 1. Multivariate analysis was performed to determine the effects of age, sex, self-defined racial and Hispanic identity, smoking status, weight, hospital admission, and time from symptom onset to study enrollment on PHQ-9, GAD-7, ISS, and PCL-C scores at baseline and at follow-up. Regression equations meeting initial significance values (α = 0.05) were notable for GAD-7 scores at baseline (F(8,41) = 2.62, R2 = 0.34, p = 0.02) and follow-up (F(8,41) = 2.67, R2 = 0.34, p = 0.02) and for PCL-C scores at baseline (F(8,40) = 4.16, R2 = 0.45, p = 0.001) and follow-up (F(8,40) = 4.24, R2 = 0.46, p < 0.001), but not for PHQ-9 scores or ISS scores. Regression equations for PCL-C scores were significant after Bonferroni correction for multiple comparisons, with subject weight (β = 0.07, p = 0.004) most likely to predict PCL-C scores at baseline and weight (β = 0.07, p = 0.003), self-identified racial identity (β = −2.76, p = 0.005) and self-identified Hispanic identity (β = 7.92, p = 0.009) most likely to predict PCL-C scores at long-term follow-up.
Table 1.
Cohort demographic data. ∗n = 51, ∗∗n = 50. Abbreviations: PHQ-9 - Patient Health Questionnaire-9; GAD-7 - Generalized Anxiety Disorder-7; ISS - Insomnia Severity Scale; PCL-C - PTSD Checklist-Civilian version.
| n | 52 |
| Age (median [IQR]) | 46.0 years (32.0 – 51.5) |
| Gender (Percent Female) | 32/52 (62.5%) |
| Race | |
| White | 25/52 (48.1%) |
| Black | 10/52 (19.2%) |
| Asian | 8/52 (15.4%) |
| Other | 6/52 (11.5%) |
| 2 or more races | 3/52 (5.8%) |
|
Hispanic |
11/52 (21.2%) |
| Smoking status | |
| Never smoker | 39/52 (75.0 %) |
| Former smoker | 11/52 (21.2 %) |
|
Current smoker |
2/52 (3.8%) |
|
Weight∗ (median [IQR]) |
160 lbs (138 – 210) |
| Disease severity | |
| Percent admitted to hospital | 7/52 (13.5%) |
|
Percent admitted to ICU |
2/52 (3.8%) |
|
Time from symptom onset to survey (median [IQR]) |
5 weeks (4 – 9) |
| Prior psychiatric symptoms | |
| Depression | 0 |
| Anxiety | 1/52 (1.9%) |
| Insomnia | 1/52 (1.9%) |
|
Psychosis |
0 |
| PHQ-9 (mean ± SEM) | |
| Baseline | 3.5 ± 0.6 |
|
Follow-up |
4.2 ± 0.6 |
| GAD-7∗ (mean ± SEM) | |
| Baseline | 2.9 ± 0.5 |
|
Follow-up |
3.0 ± 0.4 |
| ISS (mean ± SEM) | |
| Baseline | 6.8 ± 1.0 |
|
Follow-up |
7.7 ± 1.0 |
| PCL-C∗∗ (mean ± SEM) | |
| Baseline | 23.6 ± 1.2 |
| Follow-up | 25.6 ± 1.2 |
3.2. Correlation of PHQ-9, GAD-7, ISS, and PCL-C scores within participants
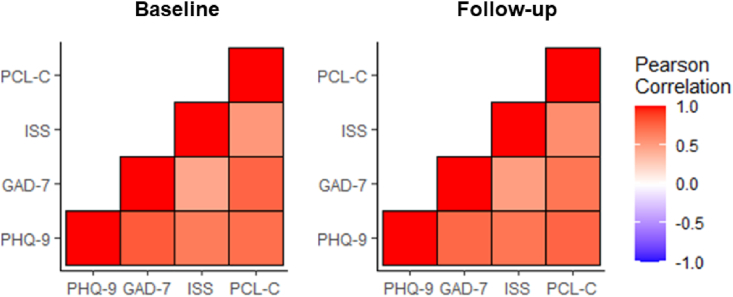
We next examined the relationship and independence of the psychometric tests used in our analysis. Pearson correlation confirmed that PHQ-9, GAD-7, ISS, and PCL-C scores were correlated both at baseline and at long-term follow-up, with all tested correlations meeting criteria for significance after correction for multiple comparisons (Fig. 1).
Fig. 1.
Correlation analysis of PHQ-9, GAD-7, ISS, and PCL-C scores. Pearson correlation matrix for PHQ-9, GAD-7, ISS, and PCL-C scores within individual participants generated in R (R-project,org; Vienna, Austria). Pearson correlation (r) values are represented in cool-warm colors (see legend) and all p < 0.001 (significant after Bonferroni correction for multiple comparisons)
Abbreviations: PHQ-9 - Patient Health Questionnaire-9; GAD-7 – Generalized Anxiety Disorder-7; ISS - Insomnia Severity Scale; PCL-C - PTSD Checklist-Civilian version. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. PHQ-9, GAD-7, ISS, and PCL-C scores at long-term follow-up compared to baseline
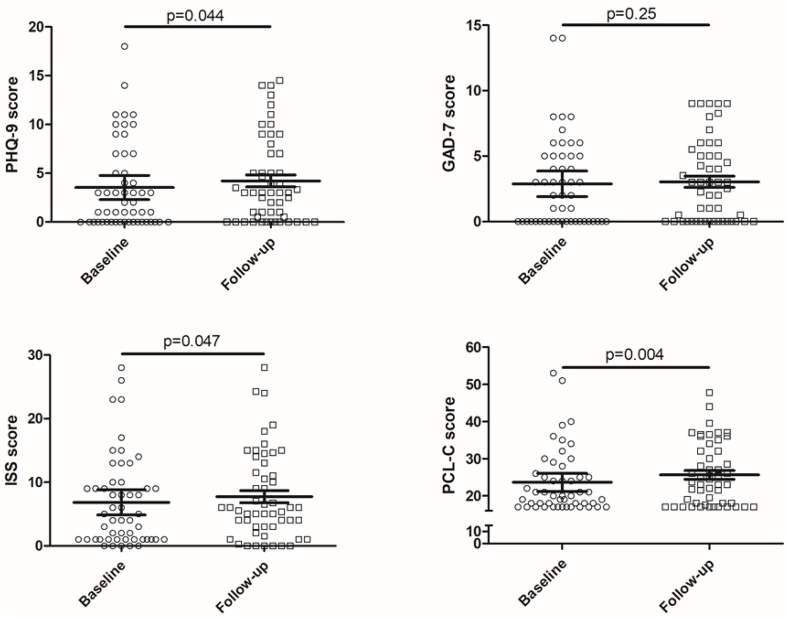
Overall, more participants met defined cut-off values for insomnia by ISS score and trauma-related symptoms by PCL-C score at follow-up compared to baseline (Table 2). PHQ-9 (Z = 2.02, W = 257, n = 52, p = 0.044), ISS (t(51) = -2.04, n = 52, p = 0.047), and PCL-C scores (Z = 2.92, W = 434, n = 50, p = 0.004) met initial criteria for a significant increase at long-term follow-up compared to baseline values (Fig. 2). However, only PCL-C scores survived correction for multiple comparisons. GAD-7 scores were not significantly altered at follow-up compared to baseline.
Table 2.
Distribution of PHQ-9, GAD-7, ISS, and PCL-C scores at baseline and at long-term follow-up.
| Baseline | Follow-up | |
|---|---|---|
| Patient Health Questionnaire-9 (PHQ-9) | ||
| Minimal (0–4) | 37/52 (71.2 %) | 34/52 (65.4 %) |
| Mild (5–9) | 7/52 (13.5 %) | 10/52 (19.2 %) |
| Moderate (10–14) | 7/52 (13.5 %) | 8/52 (15.4 %) |
| Severe (≥15) | 1/52 (1.9 %) | 0 |
| Threshold (≥10) | 8/52 (15.4 %) | 8/52 (15.4 %) |
| Generalized Anxiety Disorder-7 (GAD-7) | ||
| Minimal (0–4) | 36/51 (70.6 %) | 36/51 (70.6 %) |
| Mild (5–9) | 13/51 (25.5 %) | 15/51 (29.4 %) |
| Moderate (10–14) | 2/51 (3.9 %) | 0 |
| Severe (≥15) | 0 | 0 |
| Threshold (≥10) | 2/51 (3.9 %) | 0 |
| Insomnia Severity Scale (ISS) | ||
| Minimal (0–7) | 30/52 (57.7 %) | 33/52 (63.5 %) |
| Mild (8–14) | 15/52 (28.8 %) | 10/52 (19.2 %) |
| Moderate (15–21) | 3/52 (5.8 %) | 6/52 (11.5 %) |
| Severe (≥22) | 4/52 (7.7 %) | 3/52 (5.8 %) |
| Threshold (≥10) | 13/52 (25.0 %) | 17/52 (32.7 %) |
| PTSD Checklist-Civilian (PCL-C) | ||
| Minimal (<30) | 40/50 (80.0 %) | 35/50 (70.0 %) |
| Mild (30–39) | 7/50 (14.0 %) | 9/50 (18.0 %) |
| Moderate (40–49) | 1/50 (2.0 %) | 1/50 (2.0 %) |
| Severe (≥50) | 2/50 (4.0 %) | 0 |
| Threshold (≥35) | 6/50 (12.0 %) | 11/50 (22.0 %) |
Fig. 2.
Comparison of PHQ-9, GAD-7, ISS, and PCL-C scores at baseline and at long-term follow-up. Scores were compared within-subject with Wilcoxon signed rank test (for PHQ-9, GAD-7, and PCL-C) or t-test (for ISS) depending of data distribution, with exact p values shown in figure.
Abbreviations: PHQ-9 - Patient Health Questionnaire-9; GAD-7 - Generalized Anxiety Disorder-7; ISS - Insomnia Severity Scale; PCL-C - PTSD Checklist-Civilian version.
4. Discussion
Here, we report longitudinal symptoms across multiple neuropsychiatric domains including depression, anxiety, insomnia, and trauma-related symptoms in a cohort of participants recovering from COVID-19. We observed a high degree of correlation between symptom domains within individual participants in our study (Fig. 1). Previous studies have shown substantial comorbidity between depression, anxiety, insomnia, and PTSD specifically in trauma-exposed populations (Muller et al., 2014), and a latent class analysis of trauma-exposed soldiers demonstrated specific subclasses of individuals with extensive symptom burden and comorbidity across psychiatric domains (Contractor et al., 2015).
Interestingly, symptoms of PTSD appeared to worsen at long-term (24–60 week) follow-up in COVID-19 survivors in our cohort (Fig. 2). Our results are similar to a recent study showing increased PCL-5 scores in COVID-19 survivors at 6 month follow-up compared to earlier time points, and this same study showed increased volume and functional activity of limbic structures including the bilateral amygdalae and hippocampi in survivors compared to controls (Tu et al., 2021). An additional report found that greater than 30 % of subjects who had recovered from COVID-19 met criteria for PTSD (Janiri et al., 2021). Another group reported a lower prevalence of PTSD (~10 %), but interestingly found that obesity increased risk for PTSD diagnosis (Tarsitani et al., 2021) similar to our findings that weight was a predictive factor for PCL-C scores by multivariate analysis. A recent large-scale analysis of electronic health records showed an increased risk of worsening PHQ-9 scores over time in COVID-19 survivors (Perlis et al., 2021). Our cohort demonstrated some potential worsening of PHQ-9 and ISS scores, although this did not meet criteria for significance after comparison for multiple comparisons.
Future studies should explore whether biological effects such as ongoing immune activation or sequelae from possible neuroinflammatory insults may underlie any potential worsening of neuropsychiatric outcomes over time. For example, direct central nervous system (CNS) action of COVID-19 (Meinhardt et al., 2021; Rhea et al., 2021) may result in long-term sequelae such as microglial activation. Indeed, widespread microglial activation has been noted in postmortem brain tissue after COVID-19 infection, although this did not correlate with the presence of viral RNA (Thakur et al., 2021). Severe COVID-19 infection leads to a diverse production of autoantibodies, with the CNS being the most common target tissue affected (Wang et al., 2021). Alternatively, peripheral effects of COVID-19 on vasculature and other organ systems may perturb central feedback loops and result in somatic symptoms that contribute to neuropsychiatric symptomatology, similar to reports of increased cardiovascular risk in subjects with high perceived stress (Tawakol et al., 2017). A recent transcriptomic study found evidence of widespread gene expression changes in the brain by single nucleus RNA-sequencing (snRNA-seq) but no evidence of direct CNS neuroinvasion, which may indicate that peripheral inflammation is sufficient to grossly impact brain function during and after COVID-19 infection (Yang et al., 2021). Further research is needed to more fully understand the effects of COVID-19 on the brain.
Another possibility is that sociocultural factors including social isolation and lockdowns may contribute to the observed worsening of select psychiatric symptoms. A large population study consisting mostly of participants without COVID-19 infection showed that PHQ-9 and GAD-7 scores decreased over time during the pandemic (Fancourt et al., 2021). Additionally, a population-based study from early 2020 in China showed a diminished psychological impact of COVID-19 stress as measured by the Impact of Event Scale (IEG) at 4 weeks compared to a baseline measure, along with unchanged scores on the Depression, Anxiety, and Stress Scale (DASS) (Wang et al., 2020). The impact of sociocultural stress from COVID-19 may also impact survivors differently from unaffected members of the general population. A latent class analysis of population-level data from the UK showed that those with worsening mental health outcomes were more likely to have had COVID-19 and to identify as non-white (Pierce et al., 2021), which is interesting given our findings that self-identified racial and Hispanic identity are predictive of PCL-C scores in this cohort.
Our study was limited by a number of important factors including self-selection bias, lack of a control group, and lack of information on socioeconomic factors such as income level that may contribute to mental health outcomes. While our cohort was notable for a low burden of self-reported psychiatric symptoms prior to COVID-19 infection (Table 1), we lacked detailed clinical information on pre-COVID psychiatric diagnoses which have been associated with post-COVID sequelae (Taquet et al., 2021b). While our limited sample size allowed us to collect more nuanced longitudinal information on psychiatric symptoms in our participants, our findings necessitate replication in larger samples in order to more fully understand the clinical consequences of potentially worsening mental health outcomes in patients recovering from COVID-19. Long-term anxiety, depression, insomnia, PTSD, and other neuropsychiatric symptoms following COVID-19 warrant further detailed investigation.
Declaration of competing interest
The authors of this submitted manuscript have no financial conflicts of interest.
Acknowledgements
EJK is supported by grant R25MH086466 from National Institute of Mental Health (NIH-NIMH). LJP was supported by the National Institute of Allergy and Infectious Diseases of the NIH under Award T32AI114398. ASN is supported by T32 NS007153-36 and P30 AG066462-01 awards. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors would like to thank all research participants for their participation in this ongoing cohort.
References
- Contractor A.A., Elhai J.D., Fine T.H., Tamburrino M.B., Cohen G., Shirley E., Chan P.K., Liberzon I., Galea S., Calabrese J.R. Latent profile analyses of posttraumatic stress disorder, depression and generalized anxiety disorder symptoms in trauma-exposed soldiers. J. Psychiatr. Res. 2015;68:19–26. doi: 10.1016/j.jpsychires.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Del Rio C., Collins L.F., Malani P. Long-term health consequences of COVID-19. J. Am. Med. Assoc. 2020;324:1723–1724. doi: 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancourt D., Steptoe A., Bu F. Trajectories of anxiety and depressive symptoms during enforced isolation due to COVID-19 in England: a longitudinal observational study. Lancet Psychiatry. 2021;8:141–149. doi: 10.1016/S2215-0366(20)30482-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiri D., Carfi A., Kotzalidis G.D., Bernabei R., Landi F., Sani G., Gemelli Against COVID-19 Post-Acute Care Study Group Posttraumatic stress disorder in patients after severe COVID-19 infection. JAMA Psychiatry. 2021;78:567–569. doi: 10.1001/jamapsychiatry.2021.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M.H., Wing Y.K., Yu M.W., Leung C.M., Ma R.C., Kong A.P., So W.Y., Fong S.Y., Lam S.P. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch. Intern. Med. 2009;169:2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., Melloni E.M.T., Furlan R., Ciceri F., Rovere-Querini P., COVID-19 BioB Outpatient Clinic Study group. Benedetti F. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S.D., Calhoun P.S. The diagnostic accuracy of the PTSD checklist: a critical review. Clin. Psychol. Rev. 2010;30:976–987. doi: 10.1016/j.cpr.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., Laue M., Schneider J., Brunink S., Greuel S., Lehmann M., Hassan O., Aschman T., Schumann E., Chua R.L., Conrad C., Eils R., Stenzel W., Windgassen M., Rossler L., Goebel H.H., Gelderblom H.R., Martin H., Nitsche A., Schulz-Schaeffer W.J., Hakroush S., Winkler M.S., Tampe B., Scheibe F., Kortvelyessy P., Reinhold D., Siegmund B., Kuhl A.A., Elezkurtaj S., Horst D., Oesterhelweg L., Tsokos M., Ingold-Heppner B., Stadelmann C., Drosten C., Corman V.M., Radbruch H., Heppner F.L. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- Morin C.M., Belleville G., Belanger L., Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M., Vandeleur C., Rodgers S., Rossler W., Castelao E., Preisig M., Ajdacic-Gross V. Factors associated with comorbidity patterns in full and partial PTSD: findings from the PsyCoLaus study. Compr. Psychiatr. 2014;55:837–848. doi: 10.1016/j.comppsych.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis R., Santillana M., Ognyanova K., Green G., Druckman J., Lazer D., Baum M. Comparison of post-COVID depression and major depressive disorder. 2021. medRxiv [preprint] [DOI] [PMC free article] [PubMed]
- Pierce M., McManus S., Hope H., Hotopf M., Ford T., Hatch S.L., John A., Kontopantelis E., Webb R.T., Wessely S., Abel K.M. Mental health responses to the COVID-19 pandemic: a latent class trajectory analysis using longitudinal UK data. Lancet Psychiatry. 2021;8:610–619. doi: 10.1016/S2215-0366(21)00151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman B., Cassar M.P., Tunnicliffe E.M., Filippini N., Griffanti L., Alfaro-Almagro F., Okell T., Sheerin F., Xie C., Mahmod M., Mozes F.E., Lewandowski A.J., Ohuma E.O., Holdsworth D., Lamlum H., Woodman M.J., Krasopoulos C., Mills R., McConnell F.A.K., Wang C., Arthofer C., Lange F.J., Andersson J., Jenkinson M., Antoniades C., Channon K.M., Shanmuganathan M., Ferreira V.M., Piechnik S.K., Klenerman P., Brightling C., Talbot N.P., Petousi N., Rahman N.M., Ho L.P., Saunders K., Geddes J.R., Harrison P.J., Pattinson K., Rowland M.J., Angus B.J., Gleeson F., Pavlides M., Koychev I., Miller K.L., Mackay C., Jezzard P., Smith S.M., Neubauer S. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea E.M., Logsdon A.F., Hansen K.M., Williams L.M., Reed M.J., Baumann K.K., Holden S.J., Raber J., Banks W.A., Erickson M.A. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat. Neurosci. 2021;24:368–378. doi: 10.1038/s41593-020-00771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Geddes J., Husain M., Luciano S., Harrison P. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarsitani L., Vassalini P., Koukopoulos A., Borrazzo C., Alessi F., Di Nicolantonio C., Serra R., Alessandri F., Ceccarelli G., Mastroianni C.M., d'Ettorre G. Post-traumatic stress disorder among COVID-19 survivors at 3-month follow-up after hospital discharge. J. Gen. Intern. Med. 2021;36:1702–1707. doi: 10.1007/s11606-021-06731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawakol A., Ishai A., Takx R.A., Figueroa A.L., Ali A., Kaiser Y., Truong Q.A., Solomon C.J., Calcagno C., Mani V., Tang C.Y., Mulder W.J., Murrough J.W., Hoffmann U., Nahrendorf M., Shin L.M., Fayad Z.A., Pitman R.K. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389:834–845. doi: 10.1016/S0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur K.T., Miller E.H., Glendinning M.D., Al-Dalahmah O., Banu M.A., Boehme A.K., Boubour A.L., Bruce S.S., Chong A.M., Claassen J., Faust P.L., Hargus G., Hickman R.A., Jambawalikar S., Khandji A.G., Kim C.Y., Klein R.S., Lignelli-Dipple A., Lin C.C., Liu Y., Miller M.L., Moonis G., Nordvig A.S., Overdevest J.B., Prust M.L., Przedborski S., Roth W.H., Soung A., Tanji K., Teich A.F., Agalliu D., Uhlemann A.C., Goldman J.E., Canoll P. 2021. COVID-19 Neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L., Dyer A.H., Jones K., Dunne J., Mooney A., Gaffney F., O'Connor L., Leavy D., O'Brien K., Dowds J., Sugrue J.A., Hopkins D., Martin-Loeches I., Ni Cheallaigh C., Nadarajan P., McLaughlin A.M., Bourke N.M., Bergin C., O'Farrelly C., Bannan C., Conlon N. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PloS One. 2020;15 doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y., Zhang Y., Li Y., Zhao Q., Bi Y., Lu X., Kong Y., Wang L., Lu Z., Hu L. Post-traumatic stress symptoms in COVID-19 survivors: a self-report and brain imaging follow-up study. Mol. Psychiatr. 2021 doi: 10.1038/s41380-021-01223-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E.A., Newman E., Dobie D.J., Ciechanowski P., Katon W. Validation of the PTSD checklist in an HMO sample of women. Gen. Hosp. Psychiatr. 2002;24:375–380. doi: 10.1016/s0163-8343(02)00203-7. [DOI] [PubMed] [Google Scholar]
- Wang C., Pan R., Wan X., Tan Y., Xu L., McIntyre R.S., Choo F.N., Tran B., Ho R., Sharma V.K., Ho C. A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav. Immun. 2020;87:40–48. doi: 10.1016/j.bbi.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., Liu F., Zhou T., Israelow B., Wong P., Coppi A., Lucas C., Silva J., Oh J.E., Song E., Perotti E.S., Zheng N.S., Fischer S., Campbell M., Fournier J.B., Wyllie A.L., Vogels C.B.F., Ott I.M., Kalinich C.C., Petrone M.E., Watkins A.E., Yale I.T., Dela Cruz C., Farhadian S.F., Schulz W.L., Ma S., Grubaugh N.D., Ko A.I., Iwasaki A., Ring A.M. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- Yang A.C., Kern F., Losada P.M., Agam M.R., Maat C.A., Schmartz G.P., Fehlmann T., Stein J.A., Schaum N., Lee D.P., Calcuttawala K., Vest R.T., Berdnik D., Lu N., Hahn O., Gate D., McNerney M.W., Channappa D., Cobos I., Ludwig N., Schulz-Schaeffer W.J., Keller A., Wyss-Coray T. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595:565–571. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]