Abstract
Background
Elevated serum lactate is associated with higher mortality in sepsis, whereas liver dysfunction is associated with higher serum lactate levels. We assessed the predictive ability of serum lactate in patients with liver cirrhosis and sepsis.
Methods
This retrospective study included 12 281 cases of suspected infection with initial serum blood lactate drawn during January 2007–December 2013.
Results
Using one-to-two propensity score matching analysis, 1053 and 2106 septic patients with and without underlying liver cirrhosis, respectively, were successfully matched. Lactate levels of survivors and nonsurvivors were 2.58 and 5.93 mmol/L, respectively, in patients without liver cirrhosis (WLC), 2.96 and 7.29 mmol/L, respectively, in patients with nondecompensated liver cirrhosis (NDLC), and 4.08 and 7.16 mmol/L, respectively, in patients with decompensated liver cirrhosis (DLC). In receiver operating characteristic curve analysis, the sensitivity and specificity for predicting mortality were 0.81 and 0.55, respectively, in the WLC group, 0.85 and 0.45, respectively, in the NDLC group, and 0.86 and 0.33, respectively, in the DLC group, using serum lactate levels >2.0 mmol/L.
Conclusions
The serum lactate level can be used to predict the severity of sepsis in patients with liver cirrhosis; however, its specificity would be lower at a cutoff of 2.0 mmol/L.
Keywords: lactic acid, liver cirrhosis, mortality, prognosis, sepsis
Introduction
Sepsis is a life-threatening organ dysfunction affecting more than 30 million people worldwide annually, with up to 5.3 million deaths [1]. The blood serum lactate level has been recognized as an independent prognostic marker of mortality in patients admitted to the emergency department (ED) with sepsis [2,3]. As a predictor of mortality for patients with sepsis, the blood serum lactate level has similar accuracy to the Sequential Organ Failure Assessment (SOFA) score and a superior discriminative power to the quick SOFA (qSOFA) score [4]. An elevated lactate level is considered to be due to oxidative stress and anaerobic glycolysis in tissues [5] or metabolic changes through epinephrine-related sarcolemmal Na+/K+-ATPase stimulation [6]. The Surviving Sepsis Campaign and the National Quality Forum suggested using lactate measurement as guidance for the treatment septic patients [7].
Lactate is mainly metabolized by the liver (around 60%) and kidney (around 30%) [6], and the rate of lactate clearance in the normal liver exceeds the rate of lactate production of other tissues [8]. Episodes of hepatic decompensation, such as hemorrhage or sepsis, might disturb the equilibrium of the metabolic acid-base state in cirrhotic patients [9]. Previous studies have implied that critically ill patients with cirrhosis have higher lactate levels, and early hepatic dysfunction has been related to elevated serum lactate in acute circulatory failure [10]. Patients with acute liver failure have a splanchnic release of lactate due to accelerated glycolysis in the splanchnic region [11]. Patients with chronic liver disease also have higher fasting lactate levels, owing to the alteration of excretory liver function and portal pressure. Moreover, higher circulating lactate levels are observed in Child class C patients compared to the other Child classes, suggesting that the lactate level increases with the degree of cirrhosis [12].
Although previous studies have demonstrated that liver disease is associated with lactate elevation, the impact of liver cirrhosis on the lactate level for predicting mortality in sepsis remains unknown. The objective of this study was to determine the influence of liver cirrhosis on serum lactate levels in predicting the severity of patients with sepsis.
Materials and methods
Study design
This was a single-center retrospective cohort study conducted between 1 January 2007 and 31 December 2013 in the Kaohsiung Chang Gung Memorial Hospital, a 2692-bed acute-care medical center, and the largest tertiary-level care hospital in southern Taiwan. The study protocol was approved by the Ethics Committee of Chang Gung Memorial Hospital (IRB No.: 103-0053B). Owing to the retrospective nature of the study, the requirement for written informed patient consent was waived.
Study setting and population
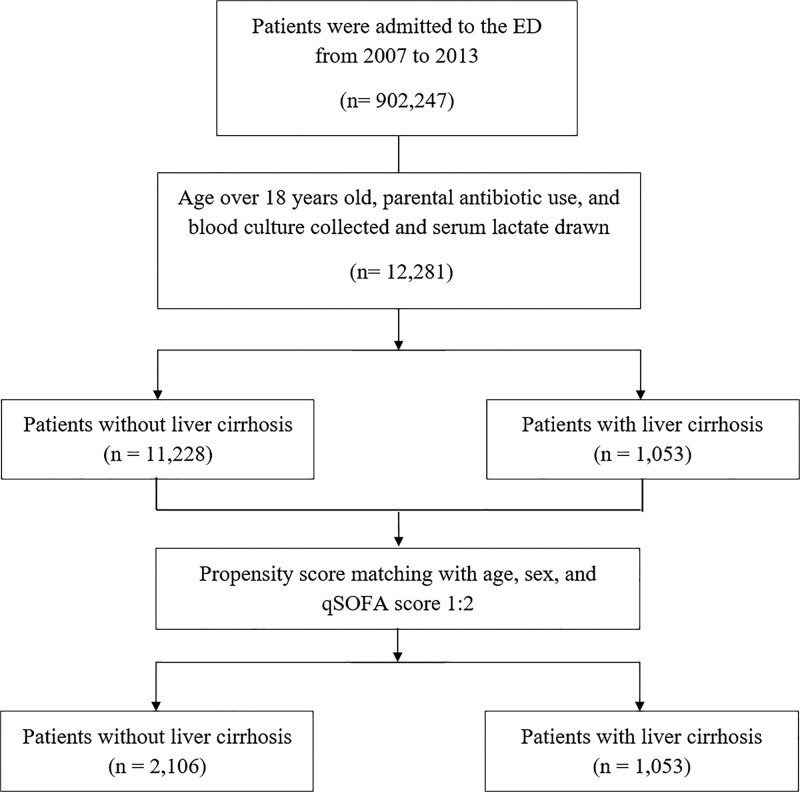
During the study period, we enrolled all adult patients (≥18 years old) who visited the ED with systemic inflammatory response syndrome (SIRS), received parenteral antibiotics, and had their blood culture and serum lactate levels tested. All patients with liver cirrhosis were enrolled and propensity score matched with age and sex, and the qSOFA score was performed with one-to-two ratios for control group selection. The study flowchart is shown in Fig. 1. We reviewed electronic medical records, including chart and nursing documentation, and documented the following data: demographic characteristics, vital signs at triage, major preexisting comorbidities, qSOFA scores, laboratory test results, major infection source, mechanical ventilation requirement, and 28-day in-hospital mortality.
Fig. 1.
Study flowchart.
The major pre-existing comorbidities and infection sites were determined according to the International Statistical Classification of Diseases and Related Health Problems Ninth Revision (ICD–9) coding. The pre-existing comorbidities included liver cirrhosis (571.2, 571.5, and 571.6), diabetes mellitus (250.00–250.99), hypertension (401.0–405.9), malignancy (140.00–199.99), cerebrovascular disease (430.00–438.99), congestive heart failure (428.0–428.9), and chronic renal insufficiency (582.00–589.99). The major infection sources included respiratory tract (481.0–486.9), urinary tract (590.00–590.99 and 601.0–601.9), intra-abdominal (562.11, 567.0–567.9, 5761, 574.00–574.19, 574.30–574.49, and 574.60–574.89), and soft tissue (680.0–686.9 and 728.86) infections.
Serum lactate levels were drawn within 6 h of the ED visit if sepsis was suspected by the ED physician. Serum lactate (mmol/L) levels were examined by serum-based immunoassay (Unicel DxC 880i Synchron, Beckman Coulter Inc., Brea, California, USA).
Definitions
Sepsis was diagnosed when an ED physician suspected infection with two or more SIRS criteria as indicated by the patient’s vital signs at triage as well as the initial laboratory results according to their electronic medical records. SIRS criteria were defined as body temperature >38°C or <36°C, heart rate >90 beats/min, respiratory rate >20 breaths/min, and abnormal white blood cell count (>1200/mm3, <4000/mm3, or bandemia ≥10%) [13]. To calculate qSOFA scores, one point was awarded for each of the following: systolic blood pressure of 100 mmHg or less, respiratory rate of at least 22 breaths/min, and Glasgow Coma Scale score of up to 13, giving a score of 0–3 [14]. Septic shock was defined as sepsis-induced hypotension despite adequate fluid resuscitation, in the absence of other causes of hypotension, and required inotropic agent usage [15]. Blood culture with potential contaminating pathogens, such as coagulase-negative Staphylococci, Micrococcus spp., Corynebacterium spp., and Propionibacterium acnes were considered as contaminations [16,17]. Decompensated liver cirrhosis (DLC) was defined by the development of overt clinical signs including ascites, bleeding, encephalopathy, and jaundice [18,19].
Statistical analysis
Continuous variables are expressed as mean ± SD and were analyzed using a t-test. Categorical variables are shown as actual numbers or percentages and were compared using Pearson’s chi-squared test or Fisher’s exact test. For the imbalance between patients with and without liver cirrhosis, propensity scores were estimated with liver cirrhosis as the dependent variable. Covariates, including age, sex, and qSOFA score were entered into the multivariable models, and matching was performed using the two-to-one matching protocol. Standardized differences of more than 0.10 were considered as evidence of imbalance. Based on the mean standardized differences and variance ratios of the two groups, a total of 1053 pairs of patients were successfully matched. Area under the receiver operating characteristic curve (AUROC) analysis was performed for serum lactate levels to predict the accuracy for 28-day in-hospital mortality. A regression model was used to calculate the crude odds ratio of serum lactate >2 to have sepsis-related mortality in each subgroup of patients. Age, sex, and qSOFA score were further adjusted in the regression model to calculate the adjusted odds ratio. All statistical tests were two-tailed with P <0.05 considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 24 (IBM Corp., Armonk, New York, USA).
Results
Baseline characteristics
During the 7-year study period, 12 281 sepsis patients with initial serum blood lactate drawn were included (Fig. 1). In total, there were 1053 and 11 228 patients with and without underlying liver cirrhosis, respectively. As a result of one-to-two propensity score matching, 1053 and 2106 patients with and without underlying liver cirrhosis, respectively, were successfully matched. There was no significant difference in age, sex, and qSOFA score between the two groups.
Table 1 shows that the patients with liver cirrhosis had significantly higher incidences of solid tumor, chronic kidney disease, intra-abdomen infection, and bacteremia than those without, whereas patients without liver cirrhosis had higher incidences of hypertension, cerebral vascular disease, heart failure, respiratory tract infection, and urinary tract infection. Furthermore, the liver cirrhosis group had significantly different physiological changes such as lower heart rate, systolic blood pressure, and diastolic blood pressure.
Table 1.
Demographic data of infected patients with and without liver cirrhosis
| Patients without liver cirrhosis n = 2106 | Patients with liver cirrhosis n = 1053 | P value | |
|---|---|---|---|
| Sex (male, %) | 67.4% | 69.3% | 0.29 |
| Age (mean ± SD) (years) | 60.6 ± 16.8 | 61.1 ± 14.6 | 0.41 |
| Vital sign at triage (mean ± SD) | |||
| Systolic blood pressure | 129 ± 36 | 125 ± 35 | 0.003* |
| Diastolic blood pressure | 78 ± 34 | 73 ± 29 | <0.001* |
| Pulse rate | 109 ± 319 | 103 ± 22 | <0.001* |
| Body temperature | 37.6 ± 1.7 | 37.5 ± 1.3 | 0.07 |
| Respiratory rate | 20 ± 4 | 20 ± 3 | 0.06 |
| Underlying disease (%) | |||
| Diabetes mellitus | 31.2% | 30.8% | 0.84 |
| Hypertension | 27.3% | 21.3% | <0.001* |
| Malignancy | 22.6% | 42.1% | <0.001* |
| Cerebrovascular disease | 13.1% | 4.7% | <0.001* |
| Congestive heart failure | 7.2% | 3.3% | <0.001* |
| Chronic kidney disease | 19.6% | 26.0% | <0.001* |
| qSOFA score | |||
| 0 | 48.4% | 47.3% | 0.40 |
| 1 | 33.9% | 36.7% | |
| 2 | 14.9% | 13.8% | |
| 3 | 2.8% | 2.3% | |
| Laboratory data (mean ± SD) | |||
| White blood cell count | 13.2 ± 10.8 | 11.1 ± 8.8 | <0.001* |
| Neutrophil (%) | 77.9 ± 15.8 | 78.8 ± 12.5 | 0.109 |
| Lymphocyte (%) | 12.4 ± 10.9 | 11.5 ± 9.0 | 0.029* |
| Band (%) | 1.7 ± 4.2 | 2.1 ± 4.6 | 0.019* |
| C-reactive protein | 125.5 ± 109.4 | 79.6 ± 81.7 | <0.001* |
| Lactate | 2.99 ± 3.17 | 4.65 ± 5.18 | <0.001* |
| Major infection focus | |||
| Respiratory tract infection | 41.5% | 25.4% | <0.001* |
| Urinary tract infection | 21.7% | 17.8% | 0.01* |
| Intra-abdomen infection | 10.1% | 22.4% | <0.001* |
| Soft tissue infection | 9.5% | 7.7% | 0.10 |
| Bacteremia | 14.0% | 24.5% | <0.001* |
| Septic shock within 72 h | 14.7% | 19.8% | <0.001* |
| Intubation within 72 h | 20.4% | 22.2% | 0.23 |
| 28-day in-hospital mortality | 12.2% | 23.8% | <0.001* |
*P < 0.05.
NS, not significant.
With regard to laboratory results of the liver cirrhosis group, the white blood cell count and C-reactive protein level were significantly lower, while the band neutrophil and serum lactate level were significantly higher. Patients in the liver cirrhosis group had a significantly higher septic shock rate within 72 h (19.8% vs 14.7%; P < 0.001) and a higher 28-day mortality rate (23.8% vs 12.2%; P < 0.001) than those without liver cirrhosis.
Subgroup analysis of serum lactate level in patients with different disease severities
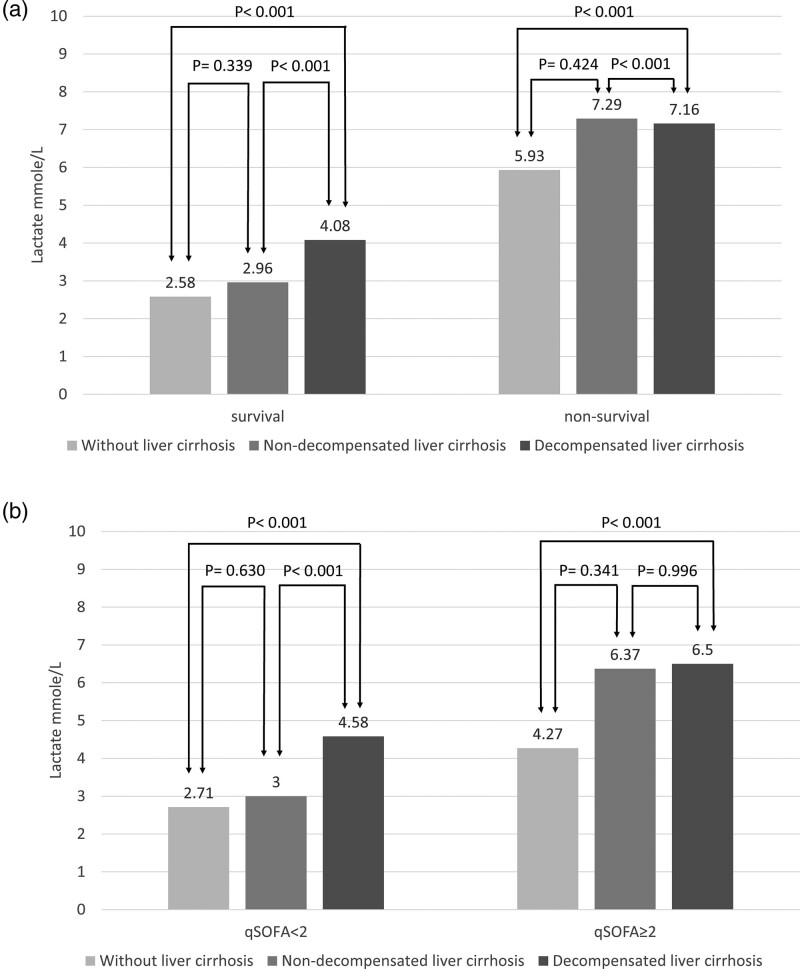
Table 2 and Figure 2 shows the subgroup analysis of the initial serum lactate level between different disease severities in patients with different stages of liver cirrhosis and those without liver cirrhosis. After categorizing the subgroup patients by hospital mortality, the serum lactate level between survivors and nonsurvivors was 2.58 vs 5.93 (P < 0.001) in patients without liver cirrhosis (WLC), 2.96 vs 7.29 (P = 0.023) in patients with nondecompensated liver cirrhosis (NDLC), and 4.08 vs 7.16 (P < 0.001) in patients with DLC. Using the qSOFA score to define disease severity, we found that the serum lactate level between the qSOFA <2 and qSOFA ≥2 groups was 2.71 vs 4.27 (P < 0.001) in patients WLC, 3.00 vs 6.37 (P < 0.003) in patients with NDLC, and 4.58 vs 6.50 (P < 0.001) in patients with DLC.
Table 2.
Subgroup analysis of the initial serum lactate level in patients with or without liver cirrhosis
| Patients without liver cirrhosis (n = 2106) | Nondecompensated liver cirrhosis patients (n = 173) | Decompensated liver cirrhosis patients (n = 879) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Survival (n = 1808) | Nonsurvival (n = 298) | P value | Survival (n = 156) | Nonsurvival (n = 17) | P value | Survival (n = 612) | Non-survival (n = 268) | P value | |
| Lactate (mmol/L) | 2.58 ± 2.43 | 5.93 ± 5.51 | <0.001 | 2.96 ± 3.13 | 7.29 ± 5.93 | 0.023 | 4.08 ± 4.68 | 7.16 ± 6.48 | <0.001 |
| qSOFA <2 (n = 1734) | qSOFA >2 (n = 372) | P value | qSOFA <2 (n = 158) | qSOFA >2 (n = 15) | P value | qSOFA <2 (n = 726) | qSOFA >2 (n = 154) | P value | |
| Lactate (mmol/L) | 2.71 ± 2.69 | 4.27 ± 4.64 | <0.001 | 3.00 ± 3.24 | 6.37 ± 5.36 | <0.03 | 4.58 ± 5.08 | 6.50 ± 6.48 | <0.001 |
Data present as mean ± SD.
P < 0.05 indicated a significant difference.
qSOFA, quick sepsis-related organ failure assessment.
Fig. 2.
Subgroup analysis by survival and qSOFA score. (a) The serum lactate level was higher in patients with liver cirrhosis than WLC group, and correlated with severity of liver cirrhosis in the survival group. In the non-survival group, the serum lactate level was higher in liver cirrhosis than WLC group, but the NDLC group had higher lactate levels than the DLC group. (b) The serum lactate level increased with liver cirrhosis severity in both the qSOFA <2 and qSOFA ≥2 groups. DLC, decompensated liver cirrhosis; NDLC, nondecompensated liver cirrhosis; qSOFA, quick sequential organ failure assessment; WLC, without liver cirrhosis.
Table 3 shows that the adjusted odds ratios of the initial serum lactate level >2 mmol/L in 28-day in-hospital mortality of infected patients were 4.99, 4.74, and 3.33 in the WLC, NDLC, and DLC groups, respectively. Furthermore, the severity of liver cirrhosis showed a gradually decreased influence of the serum lactate level on sepsis-related mortality.
Table 3.
Odds ratio of initial serum lactate level in 28-day in-hospital mortality
| Patients without liver cirrhosis (n = 2106) | Nondecompensated liver cirrhosis patients (n = 173) | Decompensated liver cirrhosis patients (n = 880) | ||||
|---|---|---|---|---|---|---|
| Crude odds ratio (95% CI) | Adjusted odds ratio (95% CI) | Crude odds ratio (95% CI) | Adjusted odds ratio (95% CI) | Crude odds ratio (95% CI) | Adjusted odds ratio (95% CI) | |
| Lactate >2 mmol/L | 5.18* (3.75–7.15) | 4.99* (3.61–6.90) | 4.85* (1.04–22.59) | 4.74 (1.00–22.53) | 3.11* (2.07–4.68) | 3.33* (2.20–5.04) |
*P < 0.05.
CI, confidence interval; qSOFA, quick sepsis-related organ failure assessment.
Adjusted age, sex, and qSOFA score.
Receiver operating characteristic curve analysis for lactate in predicting 28-day mortality
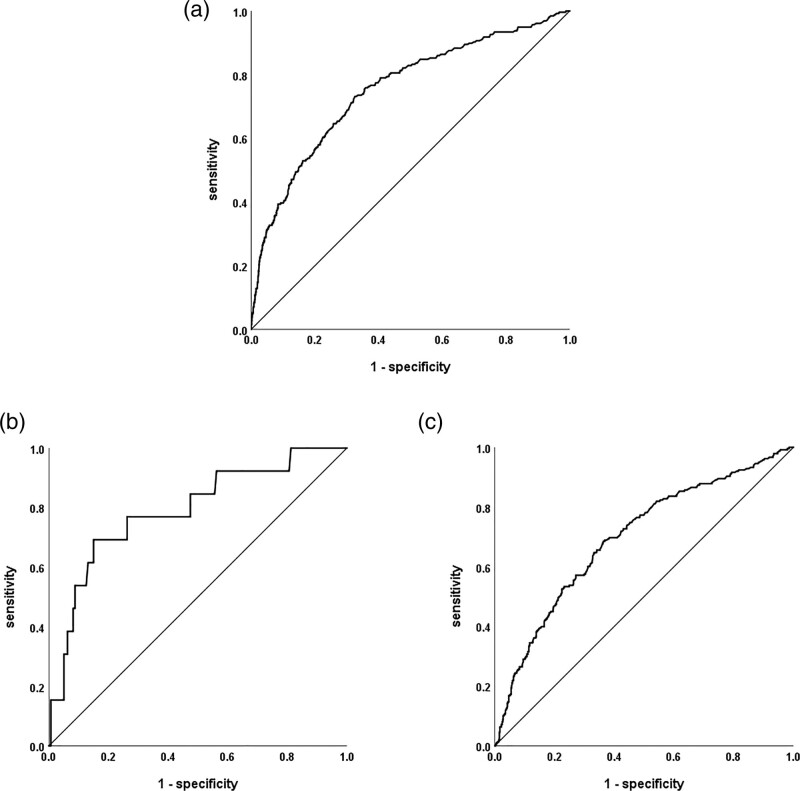
In ROC curve analysis (Table 4 and Figure 3), the AUROC of serum lactate level in predicting the 28-day mortality was 0.752 [95% confidence interval (CI), 0.719–0.786] in the WLC group, 0.790 in the NDLC group (95% CI, 0.655–0.925), and 0.696 (95% CI, 0.656–0.735) in the DLC group. Using a serum lactate level cutoff point of 2 mmol/L, the sensitivity and specificity for predicting mortality were 0.81 and 0.55 in the WLC group, 0.85 and 0.45 in the NDLC group, and 0.86 and 0.33 in the DLC group, respectively; the sensitivity and specificity became gradually less specific as the liver condition worsened. Using serum lactate levels higher than 4.0 mmol/L as the cutoff point, the sensitivity and specificity for predicting mortality were 0.49 and 0.86 in the WLC group, 0.69 and 0.84 in the NDLC group, and 0.57 and 0.73 in the DLC group, respectively.
Table 4.
Receiver operating characteristic curve analysis of the initial serum lactate level in predicting 28-day in-hospital mortality
| Group | AUC | 95% CI | Cutoff point | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|---|---|
| Patients without liver cirrhosis | 0.752* | 0.719–0.786 | 2.00 | 0.81 | 0.55 | 0.36 |
| 4.00 | 0.49 | 0.86 | 0.35 | |||
| Nondecompensated liver cirrhosis | 0.790* | 0.655–0.925 | 2.00 | 0.85 | 0.45 | 0.30 |
| 4.00 | 0.69 | 0.84 | 0.53 | |||
| Decompensated liver cirrhosis | 0.696* | 0.656–0.735 | 2.00 | 0.86 | 0.33 | 0.19 |
| 4.00 | 0.57 | 0.73 | 0.30 |
*P < 0.05.
AUC, area under the curve; CI, confidence interval.
Fig. 3.
Receiver operating characteristic curve analysis of serum lactate levels for predicting 28-day mortality. (a) without liver cirrhosis, (b) nondecompensated liver cirrhosis, and (c) decompensated liver cirrhosis.
Discussion
Our study demonstrated that the serum lactate levels in patients with sepsis were significantly higher in liver cirrhotic patients than noncirrhotic patients, in both survivors and nonsurvivors. In addition, the serum lactate level increased with increasing liver cirrhosis severity in the survival group. In a subgroup analysis by qSOFA score, the serum lactate level increased with increasing liver cirrhosis severity in both the qSOFA <2 and qSOFA ≥2 groups. In ROC curve analysis, serum lactate could be used to predict sepsis-related mortality in all groups of patients, with the AUC around 0.7; however, with a cutoff point of 2 mmol/L, the liver cirrhotic group demonstrated higher sensitivity but lower specificity compared to those without liver cirrhosis, and the trend appeared more significant with increasing liver cirrhosis severity.
Previous studies demonstrated that hepatic dysfunction is associated with elevated lactate levels, independent from parameters correlated with acute circulatory failure [10]. Despite tissue hypoperfusion, hepatic dysfunction was considered to contribute to blood lactate elevation in patients with shock. Drolz’s study demonstrated that, in a cohort of 816 critically ill patients admitted to the ICU with cirrhosis, lactate was a useful and independent predictor of outcome [20]. Sterling et al. [21] conducted a multicenter randomized clinical trial of 187 patients undergoing early sepsis resuscitation, and revealed that liver dysfunction was significantly associated with higher baseline lactate levels. Although impaired lactate clearance and normalization was observed in advanced liver disease, the result did not translate into a difference in mortality [21]. However, categorized by past medical history, there were only 12 patients with liver cirrhosis in Sterling’s study. Categorized by the liver component of the sequential organ failure assessment score, only 32 patients with moderate-to-severe liver dysfunction were included. In contrast, the patient number is relatively larger in our study, in that there were 173 patients with NDLC and 879 patients with DLC. Similarly, we also found that the serum lactate level in patients with sepsis was significantly higher in liver cirrhotic patients. For predicting mortality, higher sensitivity and lower specificity were observed in patients with liver cirrhosis, and the trend appeared to correlate with increasing liver cirrhosis severity.
Nowadays, plasma lactate levels are considered to be predictive for prognosis in patients with sepsis [22] pulmonary embolism [23], trauma [24], and in those undergoing cardiac surgery [25]. However, many underlying diseases and medications were found to be associated with an elevated lactate level, including asthma [26], liver dysfunction, kidney disease [27], diabetes on metformin treatment [28], and alcoholism. In septic patients using metformin, the serum lactate level remains useful for predicting mortality, with higher lactate levels (5.9 mmol/L) required to obtain more precise results [28]. For patients with cirrhosis, many factors may be useful as mortality predictors. In cirrhotic elderly patients admitted to the ICU, the lactate level and Acute Physiology and Chronic Health Evaluation II score were found to be the two best predictive factors for mortality compared to the Child–Pugh, Sequential Organ Failure Assessment, and Model for End-stage Liver Disease scores [29]. Similarly, our study implied that although the serum lactate level in patients with sepsis was significantly higher in liver cirrhotic patients, the lactate level remains a useful predictor of mortality. Thus, physicians should be aware of the impact of underlying disease and medication use on lactate levels, and avoid misinterpretations and inappropriate diagnostic or therapeutic activities.
In the nonsurvival group, the serum lactate level was higher in the NDLC group compared to the DLC group. One possible reason for this result could be that the sample size was relatively small. In the subgroup analysis for nonsurvival patients, there were only 17 patients in the NDLC group, but 268 patients in the DLC group. The small number of patients in the NDLC group might contribute to a dominant effect of the lactate mean value. In our study, more than 70% of nonsurvival patients in the NDLC group had a lower lactate level than the mean value of the DLC group. Moreover, in-hospital mortality was also affected by diverse factors including nosocomial infections, catheterization during hospitalization, and multiple comorbidities [30,31]. Therefore, the serum lactate level may have different predictive abilities in early and late in-hospital mortality. In contrast, when categorizing by qSOFA <2 or qSOFA ≥2, the serum lactate level increased with increasing liver cirrhosis severity; this could be explained by the fact that the timing of the measurement of serum lactate was closer to that of the qSOFA.
This study has several limitations. First, this is a retrospective study, and septic patients whom did not drown serum lactate were excluded. Patients who had lactate drawn were considered to have higher sepsis severity than those who did not. In other words, the patients included in our study tended to have a higher disease severity. Second, we only had initial lactate data instead of serial lactate measurements. Although lactate clearance and normalization during the early resuscitation of sepsis was unavailable, all patients with sepsis received aggressive treatment according to sepsis bundle in our hospital. The majority of patients with sepsis had reduced lactate levels. Finally, the study was conducted in a single city in Asia, and the results may not be generalizable to other ethnicities. Further studies should be conducted in other regions, with increased patient numbers, and with serial lactate measurement.
Conclusion
The serum lactate level in patients with sepsis was significantly higher in liver cirrhotic patients in both survivors and nonsurvivors. The serum lactate levels in the liver cirrhotic group had higher sensitivity but lower specificity for predicting mortality compared to patients without liver cirrhosis, and the trend appeared more significant with increasing liver cirrhosis severity.
Acknowledgements
We appreciated the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistics work.
C.Y.C. and C.M.S. conceived the study. C.T.K., K.H.W., and F.C.C. managed the data, including quality control. H.H.C., F.J.C., and J.B.H. provided statistical advice on the study design and analyzed the data. C.Y.C. and C.T.K. chaired the data oversight committee. C.Y.C. drafted the manuscript, and all of the authors contributed substantially to its revision. C.M.S. assumed responsibility for the paper as a whole. All authors read and approved the final manuscript.
The Institutional Review Committee on Human Research of Chang Gung Memorial Hospital approved the study. The Institutional Review Board waived the need for informed consent because the study has a retrospective design. The reference number is 103-0053B.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. ; International Forum of Acute Care Trialists. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016; 193:259–272. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care. 2014; 18:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryoo SM, Lee J, Lee YS, Lee JH, Lim KS, Huh JW, et al. Lactate level versus lactate clearance for predicting mortality in patients with septic shock defined by sepsis-3. Crit Care Med. 2018; 46:e489–e495. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Meng Z, Li Y, Zhao J, Wu S, Gou S, Wu H. Prognostic accuracy of the serum lactate level, the SOFA score and the qSOFA score for mortality among adults with sepsis. Scand J Trauma Resusc Emerg Med. 2019; 27:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013; 88:1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy B. Lactate and shock state: the metabolic view. Curr Opin Crit Care. 2006; 12:315–321. [DOI] [PubMed] [Google Scholar]
- 7.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the Third International consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016; 315:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dishart MK, Schlichtig R, Tonnessen TI, Rozenfeld RA, Simplaceanu E, Williams D, Gayowski TJ. Mitochondrial redox state as a potential detector of liver dysoxia in vivo. J Appl Physiol (1985). 1998; 84:791–797. [DOI] [PubMed] [Google Scholar]
- 9.Funk GC, Doberer D, Kneidinger N, Lindner G, Holzinger U, Schneeweiss B. Acid-base disturbances in critically ill patients with cirrhosis. Liver Int. 2007; 27:901–909. [DOI] [PubMed] [Google Scholar]
- 10.De Jonghe B, Cheval C, Misset B, Timsit JF, Garrouste M, Montuclard L, Carlet J. Relationship between blood lactate and early hepatic dysfunction in acute circulatory failure. J Crit Care. 1999; 14:7–11. [DOI] [PubMed] [Google Scholar]
- 11.Clemmesen JO, Høy CE, Kondrup J, Ott P. Splanchnic metabolism of fuel substrates in acute liver failure. J Hepatol. 2000; 33:941–948. [DOI] [PubMed] [Google Scholar]
- 12.Jeppesen JB, Mortensen C, Bendtsen F, Møller S. Lactate metabolism in chronic liver disease. Scand J Clin Lab Invest. 2013; 73:293–299. [DOI] [PubMed] [Google Scholar]
- 13.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992; 101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 14.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. ; Sepsis Definitions Task Force. Developing a new definition and assessing new clinical criteria for septic shock: for the Third International consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016; 315:775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. ; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003; 31:1250–1256. [DOI] [PubMed] [Google Scholar]
- 16.Laupland KB, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev. 2014; 27:647–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006; 19:788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Amico G. The clinical course of cirrhosis. Population based studies and the need of personalized medicine. J Hepatol. 2014; 60:241–242. [DOI] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018; 69:406–460. [DOI] [PubMed] [Google Scholar]
- 20.Drolz A, Horvatits T, Rutter K, Landahl F, Roedl K, Meersseman P, et al. Lactate improves prediction of short-term mortality in critically ill patients with cirrhosis: a multinational study. Hepatology. 2019; 69:258–269. [DOI] [PubMed] [Google Scholar]
- 21.Sterling SA, Puskarich MA, Jones AE. The effect of liver disease on lactate normalization in severe sepsis and septic shock: a cohort study. Clin Exp Emerg Med. 2015; 2:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro NI, Trzeciak S, Hollander JE, Birkhahn R, Otero R, Osborn TM, et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med. 2009; 37:96–104. [DOI] [PubMed] [Google Scholar]
- 23.Vanni S, Viviani G, Baioni M, Pepe G, Nazerian P, Socci F, et al. Prognostic value of plasma lactate levels among patients with acute pulmonary embolism: the thrombo-embolism lactate outcome study. Ann Emerg Med. 2013; 61:330–338. [DOI] [PubMed] [Google Scholar]
- 24.Régnier MA, Raux M, Le Manach Y, Asencio Y, Gaillard J, Devilliers C, et al. Prognostic significance of blood lactate and lactate clearance in trauma patients. Anesthesiology. 2012; 117:1276–1288. [DOI] [PubMed] [Google Scholar]
- 25.Noval-Padillo JA, Serra-Gomez C, Gomez-Sosa L, Hinojosa-Perez R, Huici-Moreno MJ, Adsuar A, et al. Changes of lactate levels during cardiopulmonary bypass in patients undergoing cardiac transplantation: possible early marker of morbidity and mortality. Transplant Proc. 2011; 43:2249–2250. [DOI] [PubMed] [Google Scholar]
- 26.Lewis LM, Ferguson I, House SL, Aubuchon K, Schneider J, Johnson K, Matsuda K. Albuterol administration is commonly associated with increases in serum lactate in patients with asthma treated for acute exacerbation of asthma. Chest. 2014; 145:53–59. [DOI] [PubMed] [Google Scholar]
- 27.Bellomo R. Bench-to-bedside review: lactate and the kidney. Crit Care. 2002; 6:322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen F-C, Kung C-T, Cheng H-H, Tsai T-C, Hsiao S-Y, Wu C-H, Su C-M. Metformin affects serum lactate levels in predicting mortality of patients with sepsis and bacteremia. J Clin Med. 2019; 8:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tas A, Akbal E, Beyazit Y, Kocak E. Serum lactate level predict mortality in elderly patients with cirrhosis. Wien Klin Wochenschr. 2012; 124:520–525. [DOI] [PubMed] [Google Scholar]
- 30.Sheng WH, Wang JT, Lin MS, Chang SC. Risk factors affecting in-hospital mortality in patients with nosocomial infections. J Formos Med Assoc. 2007; 106:110–118. [DOI] [PubMed] [Google Scholar]
- 31.Abe T, Ogura H, Shiraishi A, Kushimoto S, Saitoh D, Fujishima S, et al. ; JAAM FORECAST Group. Characteristics, management, and in-hospital mortality among patients with severe sepsis in intensive care units in Japan: the FORECAST study. Crit Care. 2018; 22:322. [DOI] [PMC free article] [PubMed] [Google Scholar]