Abstract
The PIK3C3/VPS34 subunit of the class III phosphatidylinositol 3-kinase (PtdIns3K) complex plays a role in both canonical and noncanonical autophagy, key processes that control immune-cell responsiveness to a variety of stimuli. Our previous studies found that PIK3C3 is a critical regulator that controls the development, homeostasis, and function of dendritic and T cells. In this study, we investigated the role of PIK3C3 in myeloid cell biology using myeloid cell-specific Pik3c3-deficient mice. We found that Pik3c3-deficient macrophages express increased surface levels of major histocompatibility complex (MHC) class I and class II molecules. In addition, myeloid cell-specific Pik3c3 ablation in mice caused a partial impairment in the homeostatic maintenance of macrophages expressing the apoptotic cell uptake receptor TIM-4. Pik3c3 deficiency caused phenotypic changes in myeloid cells that were dependent on the early machinery (initiation/nucleation) of the classical autophagy pathway. Consequently, myeloid cell-specific Pik3c3-deficient animals showed significantly reduced severity of experimental autoimmune encephalomyelitis (EAE), a primarily CD4+ T-cell-mediated mouse model of multiple sclerosis (MS). This disease protection was associated with reduced accumulation of myelin-specific CD4+ T cells in the central nervous system and decreased myeloid cell IL-1β production. Further, administration of SAR405, a selective PIK3C3 inhibitor, delayed disease progression. Collectively, our studies establish PIK3C3 as an important regulator of macrophage functions and myeloid cell-mediated regulation of EAE. Our findings also have important implications for the development of small-molecule inhibitors of PIK3C3 as therapeutic modulators of MS and other autoimmune diseases.
Keywords: PIK3C3, Autophagy, Myeloid cells, Experimental autoimmune encephalomyelitis, IL-1β
Subject terms: Autoimmunity, Macroautophagy
Introduction
Macroautophagy (called autophagy hereafter) is an evolutionarily highly conserved nutrient-sensing system that promotes the degradation of cytoplasmic proteins and damaged organelles by lysosomes.1,2 The resulting degradation products are then used in cellular remodeling and in regenerating molecular building blocks during conditions of stress. Over 30 autophagy-related gene (ATG) products that were initially identified in yeast but are largely conserved in higher eukaryotes are required for autophagy. Autophagy plays a critical role in directly regulating immune-cell responsiveness to a variety of stimuli, and defects in this process have been linked to many diseases, including inflammatory bowel disease, metabolic syndrome, cancer, and neurodegeneration.1,2 One prominent target of autophagy modulation in the innate branch of the immune system is the macrophage, which can phagocytose pathogens, debris, infected cells, and apoptotic cells, present antigens to T cells by displaying processed antigens in association with major histocompatibility complex (MHC) molecules, and produce a variety of cytokines, including IL‐1, IL‐6, and TNF-α.
The PIK3C3/VPS34 subunit of the class III phosphatidylinositol 3-kinase (PtdIns3K) complex is a key early player in autophagy3 and has also been implicated in other cellular processes, including endocytosis,4 intracellular vesicular trafficking,5 microtubule-associated protein 1A/1B-light-chain 3 (LC3)-associated phagocytosis (LAP),6 and LC3-associated endocytosis (LANDO).7 VPS34 (yeast homolog of mammalian PIK3C3, the only PtdIns3K in yeast) was first described in yeast and is evolutionarily conserved. In yeast, two distinct complexes generate phosphatidylinositol 3-phosphate to act in autophagosome formation (complex I involving VPS34, VPS15 [PIK3R4, equivalent to p150 in mammalian cells], VPS30 [ATG6, equivalent to mammalian BECN1/Beclin 1], and ATG14 [equivalent to mammalian ATG14L] or vacuolar protein-sorting complex II involving VPS34, VPS15, VPS30, and VPS38 [equivalent to mammalian UVRAG]).8 In mammalian cells, homologs of complex I (involving PIK3C3, p150, BECN1, and ATG14L) and complex II (involving PIK3C3, p150, BECN1, and UVRAG) play critical roles in regulating autophagy and endolysosomal and autophagolysosomal maturation.9 Studies with various conditional knockout mouse models have reported conflicting conclusions regarding the role of PIK3C3 in autophagy and other physiological processes, ranging from the absence of autophagy phenotypes in sensory neurons10 and T lymphocytes11 to impaired autophagy in T lymphocytes,12,13 fibroblasts,14,15 cardiomyocytes, hepatocytes,14 podocytes,16,17 lens cells,18 and dendritic cells (DCs).19 Consequently, impaired endosomal formation or endocytosis,10,17 rather than autophagy, was identified as the main cause of altered phenotypes observed in some studies. Our lab previously reported that PIK3C3 controls the homeostasis and function of T cells and antigen cross-presenting CD8α+ DCs in an autophagy-dependent manner.13,19 We also demonstrated that T-cell-specific PIK3C3 controls the pathogenicity of central nervous system (CNS) autoimmunity independently of LAP.20 However, the role of PIK3C3 in the inflammatory functions of myeloid cells during experimental autoimmune encephalomyelitis (EAE) remains unknown. In this study, we generated mice with a myeloid cell-specific deletion of Pik3c3 to study the role of PIK3C3 in macrophage homeostasis in the context of EAE, an animal model of human multiple sclerosis (MS).
Results
Characterization of autophagy and CD11b+ myeloid cells during EAE
Dysregulated autophagy has been reported in MS and EAE.21–23 To evaluate whether autophagy levels are altered during inflammatory demyelination, we analyzed the levels of the autophagy marker LC3-II and the autophagy substrate polyubiquitin SQSTM1/p62 in total cells extracted from brain, spleen, and peritoneal cavity (PerC) of mice immunized with myelin oligodendrocyte glycoprotein (MOG) peptide 35–55 (MOG35–55) to induce clinical signs of EAE. During induction of autophagy, LC3-I is conjugated to phosphatidylethanolamine to form LC3-II, which is subsequently recruited to autophagosomal membranes. We found that spleen LC3-II levels were increased and p62 levels were decreased in EAE (Fig. 1a). However, no difference in brain autophagy levels was observed between naive and diseased mice (Fig. 1a), which could be due to high levels of basal autophagy in the brain compared to other metabolically active tissues.24 Interestingly, western blot analysis showed that mice with EAE had similar PIK3C3 protein levels compared with control mice, suggesting that the activation of autophagy during EAE is independent of alterations in PIK3C3 protein expression (Fig. 1a). The causal association of autophagy induction with EAE is unclear as abnormal autophagy activity has also been implicated in many other autoimmune settings.25
Fig. 1. Characterization of autophagy and CD11b+ cells during EAE.
a EAE was induced and brain cells, splenocytes, and peritoneal cell lysates were prepared for immunoblotting. One representative image is shown; each column represents one mouse. Actin beta (ACTB) was used as a loading control. b Immune cells were isolated from spleen and brain of mice at the peak of EAE disease. The percentages of CD11b+ myeloid cells and CD4+ T cells in each tissue were measured by flow cytometry. c Quantification of immune cells analyzed in b. d Representative histogram comparing brain CD11b+ cells from EAE and control mice for surface CD86 and CD206 expression. e EAE mice exhibit significantly increased frequencies of Ly-6CintLy-6G+ cells in the spleen and CNS. f Schematic for monitoring autophagic flux using retroviral RFP-EGFP-LC3 construct. g Representative flow cytometry plots and quantification of autophagy flux under the indicated conditions by measuring the loss of EGFP in RFP+ populations. Data are means ± SEM and are representative of two independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Previous studies have shown that the frequency of mononuclear cells expressing CD11b, representing monocytes, neutrophils, macrophages, or microglia, is increased after immunization in a MOG35–55 peptide-independent manner and that these cells can suppress CD4+ T-cell proliferation in vitro.26 Consistent with these findings, we found that spleen and brain CD11b+ and CD4+ T cells were significantly increased during active EAE (Fig. 1b, c), confirming the historical dogma that EAE is a CD4+ T-cell-mediated demyelinating disease of the CNS where macrophages are the end-stage effector cell. The increased CD11b+ cells in the brain were polarized toward a pro-inflammatory M1 phenotype (Fig. 1d), and there was a significant expansion of Ly-6CintLy-6G+ granulocyte and Ly-6ChighLy6G– pro-inflammatory monocyte subpopulations at the early peak phase of disease (Fig. 1e).
To evaluate autophagy levels in CD11b+ myeloid cells and CD4+ T cells during CNS inflammation, we induced EAE in CAG-RFP-EGFP-LC3 reporter mice. In these reporter animals, RFP and EGFP fluorescence are maintained before autophagosome fusion with lysosomes, whereas EGFP is quenched and RFP signals persist in the low pH environment of autolysosomes (pH = 4–5) (Fig. 1f).27 Concurrent flow cytometric analyses showed that EAE results in a greater proportion of RFP+EGFP– cells in spleen CD11b+ cells and CD4+ T cells, suggesting an ongoing autophagic flux (Fig. 1g). We also measured LC3 and p62 levels in these cells isolated from the brain or spleen from diseased wild-type (WT) mice by flow cytometry. Levels of LC3 and p62 were decreased in spleen-derived CD4+ T cells from mice with EAE compared with unimmunized control mice (Supplementary information, Fig. S1). However, levels of LC3 and p62 increased in brain-derived CD11b+ myeloid cells from mice with EAE (Supplementary information, Fig. S1). Together, these results suggest distinct autophagy levels of immune cells between spleen and CNS in mice with EAE.
Single-cell transcriptomics reveals autophagy signatures in CNS inflammation
Given our observations that autophagy levels are altered during EAE, we performed a more global analysis of autophagy signatures of the CNS immune-cell compartment using single-cell transcriptomic data retrieved from Jordão et al.,28 in which cells from naive and MOG35–55-immunized mice were isolated and their mRNA sequenced. We represented these data with dimensionality reduction t-distributed stochastic neighbor embedding plots. Cells from both naive mice (homeostasis phase) and EAE-diseased mice at different stages (preclinical, onset, and acute phases) were partitioned into 19 clusters (Fig. 2a). CNS-associated macrophages (CAM), monocyte-derived cells, DCs, and microglia were defined and validated by their expression of genes representing key cellular features (Supplementary information, Fig. S2). Cells from EAE-diseased mice showed distinct clustering with increased numbers of each of these identified immune-cell types (Fig. 2b), confirming the flow cytometry data (Fig. 1b, c).
Fig. 2. Single-cell autophagy gene transcriptome analysis of CNS inflammation.
a Cells from both naive mice (homeostasis phase) and EAE-diseased mice at different stages (preclinical, onset, and acute phases) were partitioned into 19 clusters. Each dot represents an individual cell. Dashed lines indicate different hematopoietic populations. CAMs CNS-associated macrophages, DCs dendritic cells. Different colors represent different cell clusters. b t-SNE representation of analyzed brain immune cells from homeostasis and different stages of EAE. Different colors represent different EAE phases. c t-SNE plot depicting the expression levels of autophagy-related genes involved in different stages of the autophagy process (initiation: Ulk1, Ulk2, and Rb1cc1; nucleation: Pik3c3 and Becn1; elongation: Atg3, Atg5, and Atg7; fusion: Gabarap and Map1lc3bp). The original data for this figure were retrieved from ref. 28 and reanalyzed as described in “Materials and methods”.
Although expression levels were low, monocyte-derived myeloid cells but no other immune cells from mice with acute EAE generally expressed increased levels of genes involved in different stages of the autophagy process (initiation: Ulk1, Ulk2, and Rb1cc1; elongation: Atg3, Atg5, and Atg7; fusion: Gabarap and Map1lc3bp, Fig. 2c and Supplementary information, Table S1 and Fig. S3). Interestingly, monocyte-derived cells from mice with or without EAE expressed similar levels of rubcn that is involved in LAP (Supplementary information, Table S1 and Fig. S3). Pik3c3 expression levels were maintained across the different disease stages, suggesting that alterations in autophagy levels during EAE are independent of changes in Pik3c3 expression (Fig. 2c and Supplementary information, Table S1 and Fig. S3).
Pik3c3 deletion in myeloid cells modulates autophagy and metabolic parameters
To create a model in which autophagy is specifically ablated in myeloid cells, we bred mice in which exon 4 of the Pik3c3 gene is flanked by loxP sites (Pik3c3f/f) to mice expressing the Cre recombinase transgene at the lysozyme 2 (Lyz2) locus to yield mice in which Pik3c3 is conditionally ablated from circulating myeloid cells (pik3c3f/f;Lyz2-Cre mice). The efficacy of Lyz2-Cre has been reported to be approximately 90–100% in alveolar and peritoneal macrophages, 40% of spleen red pulp or spleen marginal zone macrophages, and 40–90% in microglia.29–31 The genotype and efficacy of Cre-mediated gene deletion of PIK3C3 from peritoneal macrophages were confirmed by western blotting (Fig. 3a) and immunofluorescence staining (Fig. 3b). These mice displayed no developmental abnormalities, exhibited normal reproductive ability, and were grossly indistinguishable from littermate controls. They also had indistinguishable spleen size, weight, and cellularity, compared to control littermates (Supplementary information, Fig. S4a). Hematoxylin–eosin staining revealed no gross abnormalities but accumulated autophagic vacuoles in the spleen, liver, and brain (Supplementary information, Fig. S4b). Increased infiltration of inflammatory cells in the lung tissue was observed (Fig. 3c), as has been reported in mice with myeloid cell-specific deletion of Atg5, Atg7, or Becn1.32–35 We used a cytometric bead array kit to measure the production of an array of cytokines in the serum of pik3c3f/f;Lyz2-Cre mice. All of the surveyed cytokines (TNF, IFN-γ, MCP-1, IL-6, IL-10, and IL-12 p70) were elevated in 6-week-old pik3c3f/f;Lyz2-Cre mice as compared with control mice (Fig. 3d).
Fig. 3. Effects of myeloid-specific Pik3c3 ablation on autophagy and immune parameters.
a Western blot analysis of freshly isolated peritoneal macrophage protein lysates confirmed a significant reduction of PIK3C3 in peritoneal macrophages of pik3c3f/f;Lyz2-Cre conditional knockout mice. Actin beta (ACTB) was used as a loading control. b Immunofluorescence microscopy confirms efficient ablation of PIK3C3 in spleen sections from pik3c3f/f;Lyz2-Cre mice. Scale bar: 50, 20 μm (magnified). c Lung tissues from pik3c3f/f;Lyz2-Cre mice were analyzed by H&E staining. Arrows indicate lung pathology. Scale bar: 100 μm. d Quantification of serum IL-12 p70, TNF, IFN-γ, MCP-1, IL-10, and IL-6 levels measured by cytometric bead array. The results from three independent experiments (6-week-old mice) were pooled and plotted. e Peritoneal cells were prepared and stained with Mitoview Green (100 nM) or TMRE (200 nM) with anti-CD11b and anti-F4/80 mAbs according to the manufacturer’s protocols. f Peritoneal macrophages isolated from Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice were cultured with 200-nM rapamycin for 18 h. Cell lysates were separated by SDS-PAGE and blotted with the indicated antibodies. g Macrophages from Pik3c3f/fCAG-RFP-EGFP-LC3 mice or pik3c3f/f;Lyz2-Cre+CAG-RFP-EGFP-LC3 mice were left untreated (NS) or cultured with 200-nM rapamycin. RFP and EGFP signals were assessed by confocal microscopy at 18 h. DAPI stains were performed to visualize nuclei. Representative images are shown, scale bar: 50 μm. h Quantification data from g for two independent experiments that were pooled and plotted. *P < 0.05, **P < 0.01, ***P < 0.001.
Because PIK3C3 plays a critical role in autophagosome formation during autophagy,3 we analyzed autophagy-related functions in Pik3c3-deficient macrophages. We first stained cells with Mitoview green, which localizes to mitochondria. We found that Pik3c3-deficient macrophages display increased mitochondrial mass compared with WT macrophages (Fig. 3e), suggesting defective autophagy.36 We also observed an increase in the mitochondrial potential- and volume-dependent dye tetramethylrhodamine ethyl ester perchlorate staining (TMRE) in Pik3c3-deficient macrophages when compared with Pik3c3-sufficient macrophages, consistent with an expansion of the functional mitochondrial content in these cells (Fig. 3e). In addition, western blot analyses of Pik3c3-deficient macrophages showed higher levels of LAMP1 during the steady state or in response to autophagic stimuli (Fig. 3f), indicating insufficient autophagosome formation and impaired lysosomal degradation. In agreement with defective autophagosome formation in Pik3c3-deficient macrophages, we observed a substantial increase in the levels of the autophagy substrate p62 and the autophagosome marker LC3-II, both of which were reduced following treatment with the autophagy inducer rapamycin (Fig. 3f). To further examine if Pik3c3 ablation in macrophages impairs autophagy, we bred pik3c3f/f;Lyz2-Cre with CAG-RFP-EGFP-LC3 reporter mice. Confocal microscopy of peritoneal macrophages treated with rapamycin showed increased RFP fluorescence in EGFP puncta in the presence of Pik3c3, whereas no change in RFP fluorescence was observed in the absence of Pik3c3, further indicating impaired autophagosome–lysosome fusion (Fig. 3g, h). Therefore, we conclude that Pik3c3-deficient macrophages exhibit defective autophagy.
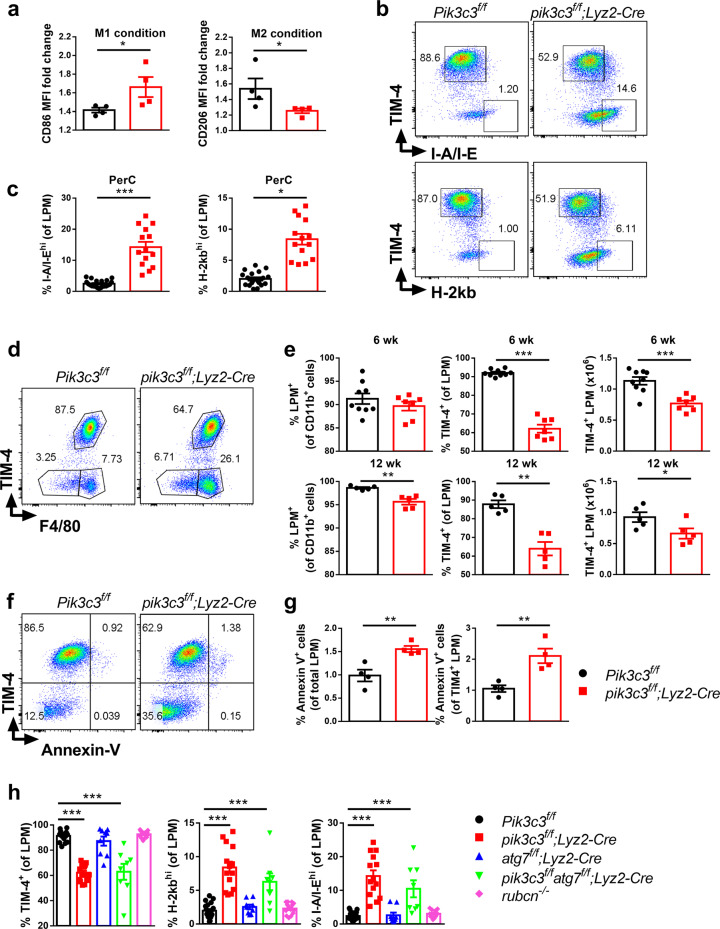
Autophagy is now a well-recognized pathway for metabolism and differentiation of immune cells including macrophages.37 To explore the effects of Pik3c3 deficiency on macrophage metabolism, we analyzed the rate of extracellular acidification as a measure of glycolysis in Pik3c3-deficient peritoneal macrophages. Pik3c3-deficient macrophages tended to be more “glycolytic” (i.e., had numerically higher basal glycolysis and maximal glycolytic capacity) as compared with WT macrophages (Supplementary information, Fig. S4c). Metabolic changes are strongly associated with macrophage polarization, and inhibition of glycolysis or oxidative phosphorylation can impair M1 or M2 macrophage activation, respectively.38,39 We hypothesized that the altered metabolism in Pik3c3-deficient macrophages could also skew macrophage polarization. To test this, we stimulated Pik3c3-deficient and control macrophages with IFN-γ (M1 culture conditions) or IL-4 (M2 culture conditions). IFN-γ or IL-4 treatment dramatically increased signature surface markers of M1 (co-stimulatory molecule CD86) or M2 (mannose receptor CD206) macrophage phenotypes, respectively. However, Pik3c3-deficient macrophages displayed increased CD86 and reduced CD206 expression as compared with macrophages from littermate controls (Fig. 4a). These data suggest that Pik3c3 deletion in macrophages promotes M1 and inhibits M2 macrophage polarization at homeostasis. In addition to these alterations, we found increased surface levels of both MHC class I and class II molecules in Pik3c3-deficient macrophages (Fig. 4b, c), a phenotype also observed for Pik3c3-deficient DCs,19 suggesting activation during homeostasis.
Fig. 4. Myeloid-specific Pik3c3 deficiency modulates macrophage phenotypes.
a Peritoneal macrophages were purified from Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice and differentiated into M1-polarized or M2-polarized macrophages by the addition of mouse-recombinant IFN-γ or IL-4, respectively, for 48 h. Summary of CD86 and CD206 mean fluorescence intensity (MFI) change after differentiation is shown. The results from two independent experiments were pooled. b, c Expression of MHC class I and class II molecules on Pik3c3-deficient macrophages. The results from three to six independent experiments were pooled and plotted. d Representative flow cytometric plots of TIM-4 and F4/80 surface expression on peritoneal macrophages. e Quantification of peritoneal myeloid cells in Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice analyzed by flow cytometry. Results from three independent experiments were pooled. f Peritoneal macrophages were prepared from Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice and stained with anti-TIM-4, -F4/80, and -CD11b antibodies and annexin V. g Quantification of the results from f, using data from two independent experiments that were pooled and plotted. h Expression of TIM-4 and MHC class I and class II molecules on peritoneal macrophages derived from Pik3c3f/f, pik3c3f/f;Lyz2-Cre, atg7f/f;Lyz2-Cre, pik3c3f/fatg7f/f;Lyz2-Cre, and rubcn–/– mice. Results from at least four independent experiments were pooled and plotted as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Myeloid-specific Pik3c3 deficiency alters macrophage phenotypes
Spleen immune-cell phenotyping revealed similar frequencies of CD3+, CD4+ and CD8+ T cells, iNKT cells, NK cells, B cells, and CD11b+ myeloid cells between Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice (Supplementary information, Fig. S4a). The cellularity and frequency of CD11b+, CD3+, CD8+, NK, and B cells were also similar in PerC of the two groups of mice (Supplementary information, Fig. S5a, b). The frequency but not the cellularity of CD4+ T cells was increased in PerC of 6-week-old mice (Supplementary information, Fig. S5a) and returned to normal by 12 weeks of age (Supplementary information, Fig. S5b). No difference was observed in the frequencies of small peritoneal macrophages (SPM, CD11bhiF4/80lo) and large peritoneal macrophages (LPM, CD11bhiF4/80hi) in 6-week-old mice (Fig. 4d, e), although a modest but significant decrease in LPM frequency was observed in 12-week-old mice (Fig. 4e). TIM-4, a phosphatidylserine receptor, mediates phagocytosis of apoptotic cells by resident peritoneal macrophages and is essential for the maintenance of the homeostatic state of peritoneal macrophages.40 We observed a significant reduction of TIM-4+ macrophages in PerC from 6-week-old pik3c3f/f;Lyz2-Cre mice (Fig. 4d, e), a phenotype that was retained in 12-week-old mice (Fig. 4e). This reduced prevalence of TIM-4+ macrophages was not observed in spleen (Supplementary information, Fig. S5c). A similar reduction in the prevalence of cells expressing another phosphatidylserine receptor, CD14, was observed between Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice, which was accompanied by a compensatory increase in cells expressing the phosphatidylserine-binding scavenger receptor CD36 (Supplementary information, Fig. S5d). To understand the mechanism of TIM-4+ macrophage loss in PerC, we first tested whether these cells were dying due to enhanced apoptosis. Staining of PerC macrophages with annexin V showed that Pik3c3-deficient TIM-4+ cells consistently contained a higher percentage of apoptotic cells, even though the rate of apoptosis was low in both groups of animals (Fig. 4f, g). No difference in the constitutive levels of TIM-4+ cell proliferation was observed between pik3c3f/f;Lyz2-Cre and Pik3c3f/f mice (Supplementary information, Fig. S5e), suggesting that the reduced prevalence of PerC TIM-4+ cells in the absence of Pik3c3 is likely due to increased apoptosis.
Because PIK3C3 has versatile cellular functions, we explored another essential autophagy factor, ATG7, which also contributes to both autophagy and LAP.41,42 However, in contrast with PIK3C3’s role in autophagy nucleation, ATG7 is essential for the elongation of autophagosomes and assists in the formation of LC3-II, which is subsequently recruited to autophagosomal membranes. Thus, we next asked whether molecular players downstream of autophagy nucleation exhibit alterations in the prevalence of PerC TIM-4+ cells and macrophage activation similar to those seen in pik3c3f/f;Lyz2-Cre mice. We generated atg7f/f;Lyz2-Cre single conditional knockout and pik3c3f/fatg7f/f;Lyz2-Cre double conditional knockout mice and analyzed TIM-4, MHC class I, and MHC class II expression on peritoneal macrophages. In atg7f/f;Lyz2-Cre mice, we observed variation in macrophage TIM-4 expression but, on average, atg7f/f;Lyz2-Cre mice had higher levels of TIM-4+ macrophages compared with pik3c3f/f;Lyz2-Cre mice (Fig. 4h). TIM-4+ macrophage levels were lower in pik3c3f/fatg7f/f;Lyz2-Cre mice compared with control mice. Interestingly, MHC class Ihigh and MHC class IIhigh macrophages were absent in atg7f/f;Lyz2-Cre mice but were present in both pik3c3f/f;Lyz2-Cre mice and pik3c3f/fatg7f/f;Lyz2-Cre mice (Fig. 4h). One allele of Pik3c3 or Atg7 was sufficient to maintain macrophage homeostasis, as macrophages from Pik3c3f/+;Lyz2-Cre and Atg7f/+;Lyz2-Cre mice displayed similar levels of TIM-4, MHC class I, and MHC class II expression compared with WT mice (data not shown). Global deficiency of rubcn, a gene whose product (RUBICON) is required for LAP and LANDO but not autophagy,43 did not affect peritoneal macrophage TIM-4, MHC class I, or MHC class II expression (Fig. 4h). Previous studies also reported the presence of MHC class IIhigh macrophages in becn1f/f;Lyz2-Cre mice, rb1cc1f/f;Lyz2-Cre mice, and to a lesser extent atg14f/f;Lyz2-Cre mice, but not in atg5f/f;Lyz2-Cre mice, atg7f/f;Lyz2-Cre mice, atg16l1f/f;Lyz2-Cre mice, and global rubcn–/– mice.35 Becn1-deficient macrophages also expressed lower levels of Timd4 (encoding TIM-4).35 Together, these data indicate that reduced TIM-4 expression and elevated MHC class I and MHC class II expression in the absence of Pik3c3 are mediated by a mechanism dependent on the early machinery (initiation/nucleation) of the classical autophagy pathway.
Pik3c3 deficiency in myeloid cells reduces the severity of EAE
Consistent with our observation that macrophage autophagy is modulated during EAE, myeloid cell-specific deletion of Atg5 or Atg7 reduces the severity of actively induced EAE44 and myeloid-specific deletion of Atg7 also delays the onset of passively induced EAE.34 To evaluate the inflammatory potential of Pik3c3-deficient myeloid cells in EAE, we first induced active EAE in pik3c3f/f;Lyz2-Cre mice with Pik3c3f/f mice as controls. We found that Pik3c3 deletion does not alter the kinetics of EAE onset and both groups of mice showed 100% disease incidence. However, disease severity was lower in pik3c3f/f;Lyz2-Cre mice (Fig. 5a). We next tested whether the reduction in the severity of EAE resulted from impaired induction of peripheral antigen-specific CD4+ effector T-cell responses. To test this possibility, we isolated splenocytes from Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice following EAE induction (day 10) and incubated them with MOG35–55 peptide (20 μg/ml) to measure antigen-specific cytokine production. Interestingly, no significant differences were observed for the frequency of IL-17+CD4+ and IFN-γ+CD4+ T cells (Fig. 5b, c). To evaluate the possibility that Pik3c3 deficiency in myeloid cells changes T-cell pathogenicity that reduces EAE severity, we immunized WT mice with MOG35–55 peptide and transferred primed myelin-specific CD4+ T cells to induce EAE in recipient Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice to model the effector phase of EAE. pik3c3f/f;Lyz2-Cre mice exhibited attenuated disease severity and incidence compared with their littermate controls upon adoptive transfer of encephalitogenic T cells (Fig. 5d), suggesting that the reduced severity of actively induced EAE in pik3c3f/f;Lyz2-Cre mice is due to the lack of PIK3C3 in CNS-resident myeloid cells.
Fig. 5. Pik3c3 deficiency in myeloid cells attenuates the severity of active and passive EAE.
a EAE induced by active immunization with MOG35–55 peptide. AUC area under the curve. b Splenocytes from Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice induced for EAE (day 10) were cultured with MOG35–55 peptide (20 μg/ml). After 18 h of culture, cells were harvested and intracellular staining was performed (IL-17A and IFN-γ), and cytokine-producing CD4++ T cells were analyzed by flow cytometry. c Quantification of antigen-specific cytokine production. d Passive EAE induced in Pik3c3f/f and pik3c3f/f;Lyz2-Cre recipient mice by adoptive transfer of MOG35–55-specific T cells isolated from WT donor mice. e Fourteen days after adoptive transfer EAE induction, pik3c3f/f;Lyz2-Cre mice contained significantly fewer CD45+ cells in the brain. Furthermore, quantification of immune-cell subsets showed significantly lower frequencies of CD4+ and CD4+CD44+ T cells in pik3c3f/f;Lyz2-Cre mice at the peak of disease in the brain. The results from three independent experiments were pooled. f Lumbar spinal cord was assayed for inflammation (H&E stain) and demyelination (Luxol fast blue [LFB] stain). Pathology was scored on a 0–3 scale, n = 4 (Pik3c3f/f mice) and 5 (pik3c3f/f;Lyz2-Cre mice). Representative images are shown, scale bar: 50 μm. Arrows indicate foci of inflammation (H&E) or demyelination (LFB). The data shown are the average ± SEM. *P < 0.05; **P < 0.01.
Lyz2-Cre-mediated gene deletion is not specific to macrophages and can cause gene deletion in other cell lineages, including neutrophils, DCs, and neurons.45,46 To better attribute EAE resistance to Pik3c3 deficiency in macrophages, we transferred bone marrow-derived macrophages (BMDMs) from WT mice into Pik3c3f/f or pik3c3f/f;Lyz2-Cre mice 4 h prior to EAE induction and found similar disease severity between Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice (Supplementary information, Fig. S6). These results further reinforce the importance of macrophages in EAE development, and are consistent with a key role of macrophage PIK3C3 to the EAE phenotype of pik3c3f/f;Lyz2-Cre mice.
To determine the extent of CNS inflammation, we examined lumbar spinal cord histology and demyelination, as well as frequencies of CNS-infiltrating leukocytes in pik3c3f/f;Lyz2-Cre mice compared with Pik3c3f/f littermates. To this end, mice were sacrificed at day 15 following adoptive transfer of myelin-specific CD4+ T cells. pik3c3f/f;Lyz2-Cre mice showed substantially reduced total and CD44hi CNS-infiltrating CD4+ T cells (Fig. 5e) and exhibited significantly reduced inflammation and demyelination (Fig. 5f). Because of the importance of the imbalance between activation and polarization of M1 and M2 cells in the CNS for EAE progression,47 we next examined M1 and M2 markers on CD11b+ cells from the brain of diseased Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice. We found that the increased numbers of CD11b+ cells in the brain of pik3c3f/f;Lyz2-Cre mice are less polarized toward an M1 phenotype compared with similar cells from Pik3c3f/f mice (data not shown).
Impaired efferocytosis by Pik3c3-deficient macrophages
Reduced EAE in pik3c3f/f;Lyz2-Cre mice could be due to reduced reactivation of myelin-specific CD4+ T cells by CNS-resident myeloid cells that sampled myelin antigens from oligodendrocytes. To investigate whether the absence of Pik3c3 in macrophages impairs their capacity to stimulate myelin antigen-specific T cells, we sorted macrophages from LPS-treated and untreated Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice. Isolated peritoneal macrophages were pulsed with MOG35–55 peptide or a longer fragment of MOG (MOG1–125) that requires processing for its presentation on MHC class II, and these cells were cocultured with naive CD4+ T cells from transgenic mice carrying a T-cell receptor specific for the MOG35–55 peptide (2D2 cells). We found similar proliferation levels of 2D2 cells in response to coculture with MOG35–55- or MOG1–125-pulsed Pik3c3-sufficient and -deficient macrophages, suggesting that the EAE phenotype of pik3c3f/f;Lyz2-Cre is unlikely due to defects in the antigen-presenting capacity of macrophages (Supplementary information, Fig. S7).
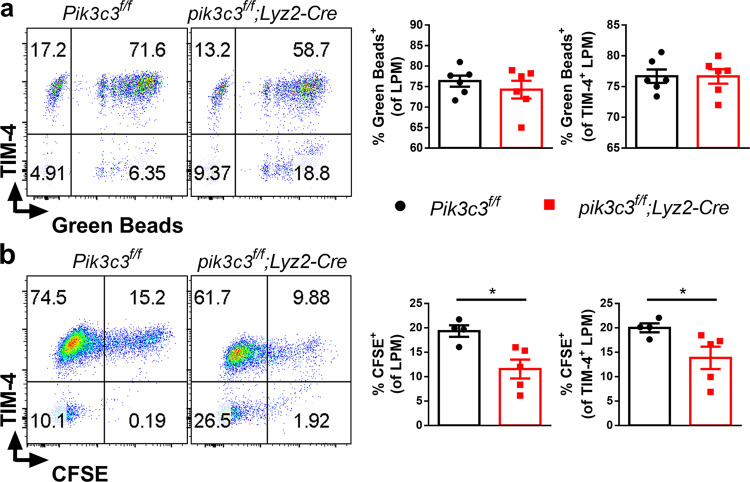
EAE development is driven by extracellular myelin antigens not expressed by professional antigen-presenting cells (APCs) and therefore requires endocytosis, followed by myelin antigen processing and presentation.48 In addition to autophagy, PIK3C3 has been implicated in endocytosis in mammals.4 Myelin uptake by CNS myeloid cells (especially infiltrated DCs and macrophages, and to a lesser extent microglia) is required for EAE development, and frequencies of these CNS myeloid cells are significantly correlated with EAE severity.49 Although DCs are important for the priming of autoreactive lymphocytes, these cells are not strictly required and other APCs can substitute for them in the induction of EAE.50,51 Here, we determined whether Pik3c3-deficient macrophages are impaired in endocytosis. First, we assessed general phagocytosis by culturing macrophages with fluorescent latex beads, and quantifying ingested beads by flow cytometry. We found that Pik3c3-deficient macrophages are similarly efficient in phagocytosing fluorescent latex beads compared with control macrophages (Fig. 6a), indicating that the general capacity of Pik3c3-deficient macrophages to phagocytose extracellular material is not compromised. Phagocytosis of extracellular materials requires triggering through a variety of phagocytic receptors such as those that can recognize “eat-me” signals on apoptotic cells.52 As oligodendrocyte apoptosis caused by demyelination and nerve damage is a significant hallmark of EAE development,53 we hypothesized that reduced EAE severity in pik3c3f/f;Lyz2-Cre mice is associated with impaired uptake of damaged oligodendrocytes by Pik3c3-deficient macrophages. This is supported by studies from several labs, including our own, showing that PIK3C3 complex subunit deletion impairs the capacity of phagocytic cells to clear apoptotic cells.19,54,55 Thus, we next determined the capacity of Pik3c3-deficient macrophages to take up apoptotic cells in vivo. We injected CFSE-labeled B6.SJL (CD45.1+) apoptotic thymocytes into groups of pik3c3f/f;Lyz2-Cre and Pik3c3f/f mice and found that, similar to Pik3c3-deficient DCs, Pik3c3-deficient macrophages are defective in taking up apoptotic cells (Fig. 6b). In addition, uptake of apoptotic cells by Pik3c3-deficient TIM-4+ macrophages was decreased compared with control TIM-4+ macrophages (Fig. 6b).
Fig. 6. Pik3c3-deficient macrophages display defective efferocytosis.
a Fluorescent latex beads were incubated with peritoneal cells (2 × 106/ml cells, 5-μg/mL beads, about 2.6 × 108 beads/mL final concentration) for 2 h at 37 °C. Percent of fluorescent cells was determined by flow cytometry. b CFSE-labeled B6.SJL (CD45.1+) apoptotic thymocytes (1 × 106) were injected into groups of mice. After 2 h, peritoneal cavity cells were harvested, and CFSE+CD45.1–CD45.2+ macrophage subsets were determined. A summary of the experiment in a is shown. Results from two independent experiments were pooled and plotted as mean ± SEM. *P < 0.05.
EAE attenuation due to myeloid-specific Pik3c3 deficiency is associated with reduced IL-1β production
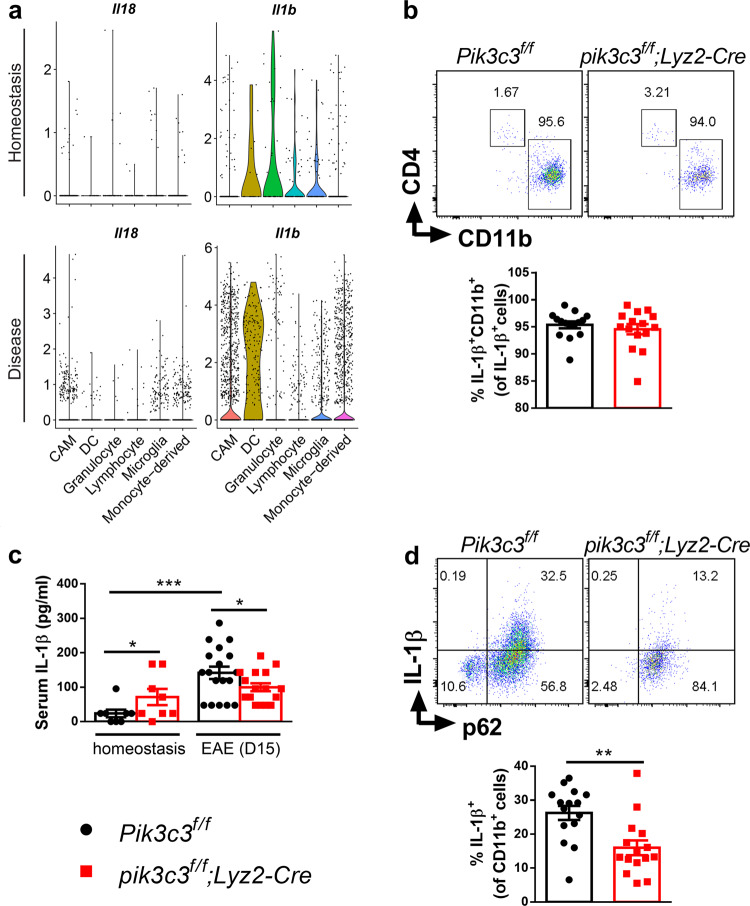
Finally, we investigated the possible mechanisms underlying the partial resistance to EAE in pik3c3f/f;Lyz2-Cre mice. It has been documented that production of IL-1β by myeloid cells is required for the development of EAE.56,57 Thus, we focused on inflammatory mediators derived from macrophages and noticed a clear increase of IL-1β and IL-18 protein expression in spleen and peritoneal cell lysates from mice with EAE (Fig. 1a). scRNA analysis also revealed elevated Il1b and Il18 expression by macrophages from mice with EAE (Fig. 7a), in accordance with previous studies demonstrating that inflammasomes are activated during EAE.58 In addition, more than 90% of IL-1β was produced by CD11b+ myeloid cells (Fig. 7a, b). Consistent with published results that basal autophagy restricts IL-1β secretion,59,60 we found that serum IL-1β levels are higher in pik3c3f/f;Lyz2-Cre mice during homeostasis (Fig. 7c). We further compared IL-1β levels in CNS cells from diseased Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice and found that the majority of IL-1β is produced by p62+CD11b+ cells (Fig. 7d). IL-1β production in diseased pik3c3f/f;Lyz2-Cre mice was significantly lower than in diseased Pik3c3f/f mice (Fig. 7c, d). Surprisingly though, pik3c3-deficient macrophages produced higher levels of IL-1β in response to LPS stimulation compared with pik3c3-sufficient macrophages (Supplementary information, Fig. S8). Collectively, these results suggest that reduced IL-1β levels in pik3c3f/f;Lyz2-Cre mice during EAE are a consequence rather than a cause of ameliorated EAE development in pik3c3f/f;Lyz2-Cre mice.
Fig. 7. Pik3c3 deficiency in myeloid cells inhibits EAE-induced IL-1β production in the CNS.
a Violin plots of Il1b and Il18 gene expression distribution of different cell clusters at homeostasis or nonhomeostasis states (preclinical, onset, and acute EAE). The original data for this figure were retrieved from ref. 28 and reanalyzed as described in “Materials and methods”. b At day 15 post EAE induction, CNS IL-1β+ cells were analyzed for expression of CD11b and CD4. Results from at least three independent experiments were pooled and plotted as mean ± SEM. c Quantification of serum IL-1β levels measured by ELISA. d IL-1β production from CNS cells. Cells were taken from the CNS and IL-1β-producing and p62-expressing CD11b+ cells were assessed. Results from at least three independent experiments were pooled and plotted as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
PIK3C3 inhibitor SAR405 delays EAE onset
Our data provide evidence that PIK3C3 is an important regulator of myeloid cell function and a potential therapeutic target for treating EAE. We therefore explored the capacity of the selective PIK3C3 inhibitor SAR405 to modulate EAE. We induced EAE in WT mice and treated the animals daily with SAR405 from days 3 to 13 after immunization. We found a delayed disease onset and reduced incidence and cumulative disease score in SAR405-treated mice (Fig. 8).
Fig. 8. SAR405 treatment delays the onset of EAE.
EAE was induced by active immunization with MOG35–55 peptide and SAR405 (10 mg/kg daily) was administered orally starting 3 days after immunization for 11 consecutive days. a Daily clinical score. b Cumulative disease score. AUC area under the curve. c Disease onset. d Peak disease score. Combined results from two independent experiments. The data shown are the average ±\ SEM. *P < 0.05.
Discussion
We showed that PIK3C3 is essential for macrophage homeostasis and TIM-4 expression, which plays an important anti-inflammatory role by promoting engulfment of phosphatidylserine-expressing apoptotic cells.61 Consistent with our published findings with Pik3c3-deficient DCs,19 we observed defective uptake of apoptotic cells by Pik3c3-deficient macrophages. Although our findings suggest defective TIM-4 expression as a likely cause for the impaired efferocytosis by Pik3c3-deficient macrophages, additional phagocytic receptors for apoptotic cells may be involved. Importantly, uptake of myelin antigens derived from apoptotic oligodendrocytes by myeloid cells is a prerequisite step in the development of EAE.49 The loss of TIM-4+ macrophages can be at least partly explained by defective autophagy because this phenotype was also observed in becn1f/f;Lyz2-Cre mice.35
We found increased surface levels of MHC class I and MHC class II molecules in Pik3c3-deficient macrophages, a phenotype also observed in Pik3c3-deficient DCs.19 The elevated MHC class II expression is likely dependent on the early machinery of the canonical autophagy pathway, as this phenotype was also observed in mice with myeloid cell-specific deletion of Becn1, Rb1cc1, and to a lesser extent Atg14, but not Atg5, Atg7, and Atg16l.35 PIK3C3 has been implicated in both canonical and noncanonical autophagy.6 The results from our lab and others35 with mice deficient in RUBICON, which in association with the BECN1–PIK3C3–UVRAG-containing PtdIns3K complexes mediates LAP and LANDO but not autophagy,43 suggest that alterations in the phenotype of Pik3c3-deficient macrophages are not caused by defective LAP or LANDO. Because Pik3c3 and other autophagy genes have pleiotropic functions,41,62 defects in other cellular processes that involve PIK3C3 may also contribute to the enhanced MHC class I and MHC class II surface molecule expression.
Autophagy has a central role in the development and function of many immune-cell subsets that contribute to the pathophysiology of MS and EAE.63 Patients with active relapsing–remitting MS or secondary progressive MS showed significantly elevated T-cell-derived ATG5 expression compared to nondisease controls.21 A recent study also found higher serum and cerebrospinal fluid ATG5 and Parkin levels in MS patients compared to patients with other inflammatory or noninflammatory neurological diseases.22 The increase of autophagy in circulating cells, especially T cells, may protract their survival, contributing to the pathogenesis of MS. Expression of ATG16L2, an isoform of ATG16L that inhibits ATG12–ATG5–ATG16L1 complex formation, is reduced in the serum of MS patients and this reduction is likely to perturb the homeostasis of T cells in MS.64 Another study that extensively analyzed expression levels of several genes involved in the autophagy pathway using the Fluidigm BioMark™HDSystem 96.96 Dynamic Array showed that 29 of 78 ATGs analyzed were significantly altered in MS patients, with 14 genes downregulated (including ATG16L2, ATG9A, FAS, GAA, HGS, and RAB24) and 15 genes upregulated (including ULK1, PIK3R1, BLC2, FOXO1, HTT, and RGS19),65 suggesting the involvement of autophagy, or more specifically, ATGs in MS pathogenesis. However, these changes in autophagy are not specific to MS as abnormal autophagy activity has also been implicated in many other autoimmune diseases.25 In EAE, Atg5 transcript and protein levels in peripheral blood positively correlate with disease severity in MOG35–55 peptide-induced EAE.21 Spinal cord p62 levels were reduced in EAE.23 Consistently, we observed a significant increase in the expression of autophagy-associated genes in CNS-infiltrating monocyte-derived cells, especially during the acute phase of EAE (Supplementary information, Table S1), but not in CNS-infiltrating lymphocytes. Together, these results suggest distinct autophagy levels in different immune cells during EAE development.
We found that Pik3c3 deficiency in myeloid cells reduces the severity of EAE. In addition to macrophages, Lyz2-Cre might drive Pik3c3 deletion in a subset of neutrophils and DCs, and some neurons.45,46 Consistent with this possibility, the ameliorated EAE in mice with a myeloid cell-specific deficiency of Atg7 may be partly explained by defective neutrophil function.44 Our experiments with adoptive transfer of WT BMDMs into Pik3c3f/f and pik3c3f/f;Lyz2-Cre recipient mice provide evidence that macrophages make a significant contribution to reduced EAE development in pik3c3f/f;Lyz2-Cre mice.
The ameliorated EAE phenotype of pik3c3f/f;Lyz2-Cre mice was associated with reduced accumulation of total CD4+ T cells, and CD44+CD4+ T cells in the brain, which could be due to reduced reactivation by CNS myeloid cells that have sampled myelin protein from oligodendrocytes. However, peripheral MOG35–55 antigen-specific activation of T cells was unchanged. Intact peripheral T-cell activation may reflect unaltered functions of peripheral APCs during EAE in these animals. These findings are in agreement with a study reporting that myeloid cell-specific deletion of Atg7 reduces the severity of actively induced EAE without altering the generation of antigen-specific T-cell responses in the periphery.44
Mechanistically, multiple lines of evidence have shown that IL-1β production by myeloid cells is crucial for EAE development.56,57 First, IL-1β expression by CCR2hi monocytes is necessary for their transmigration across CNS blood vessels in vivo. Second, IL-1β enables CNS endothelial cells to release GM-CSF, thus converting infiltrated monocytes into APCs, which further participate in the activation of peripheral CD4+ T cells. Furthermore, production of IL-1β by APCs in the presence of myelin-reactive CD4+ T cells is critical to the release of highly neurotoxic factors. In agreement with previous studies,56,57 we found enhanced IL-1β levels in mice with EAE, and deletion of Pik3c3 impaired the capacity of myeloid cells to produce IL-1β in response to EAE induction. Nevertheless, precise mechanisms remain unclear. Although basal autophagy restricts IL-1β secretion59,60 and macrophage (or hematopoietic cell)-specific deletion of Atg5, Atg16l1, or Atg7 enhancing constitutive IL-1β secretion in mice,52,66,67 autophagy-dependent IL-1β restriction may be unmasked during certain stress conditions such as inflammasome activation triggered by lysosomal membrane damage68 or the bacterial toxin nigericin,69 or CNS inflammation in the present study. It is also possible that the reduced IL-1β levels in pik3c3f/f;Lyz2-Cre mice during EAE are a consequence rather than a cause of ameliorated EAE development in pik3c3f/f;Lyz2-Cre mice.
We conclude from our data that PIK3C3 controls macrophage phenotype and function during EAE in a manner that depends on the early machinery of autophagy and not LAP. Autophagy in macrophages might also be relevant to the perpetuation of neuroinflammation in patients with MS. Our findings have implications for the development of small-molecule inhibitors of PIK3C3 to treat MS and other autoimmune diseases.
Materials and methods
Mice
Pik3c3f/f 13,14 and rubcn–/–43 mice have been described. Atg7f/f mice were obtained from Dr Masaaki Komatsu at The Tokyo Metropolitan Institute of Medical Science. Lyz2-Cre mice (JAX stock #004781), CAG-RFP-EGFP-LC3 transgenic mice (JAX stock #027139), B6.SJL-Ptprca Pepcb/BoyJ (JAX stock #002014), and C57BL/6-Tg(Tcra2D2,Tcrb2D2)1Kuch/J (designated 2D2 TCRMOG, Stock No: 006912) were obtained from The Jackson Laboratory (Bar Harbor, ME). Macrophage-specific deletion of Pik3c3 was achieved by crossing the Pik3c3f/f mice with Lyz2-Cre transgenic mice. Six- to twelve-week-old animals of both sexes were used in this study. All breeders and experimental mice were on a sterile diet and housed in specific pathogen-free conditions in compliance with guidelines from the Institutional Animal Care and Use Committee at Vanderbilt University.
Induction and evaluation of active EAE
Six- to eight-week-old mice were used for EAE induction by s.c. injection of 200 μl of 2-mg/ml MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) peptide (Biomatik) emulsified in Freund’s complete adjuvant (2-mg/ml Mycobacterium tuberculosis extract H37Ra in incomplete Freund’s adjuvant [BD bioscience, 263910]). At immunization and 48 h later, all mice received 400 ng of pertussis toxin (Calbiochem, 516560) by i.p. injection. Mice were monitored for clinical signs of EAE, scored as follows: 0, no clinical signs; 0.5, partially limp tail; 1, paralyzed tail; 1.5, paralyzed tail and hind-leg inhibition; 2, loss in coordinated movement, hind-limb paresis; 2.5, one hind-limb paralyzed; 3, both hind limbs paralyzed; 3.5, hind limbs paralyzed, weakness in forelimbs; 4, forelimbs paralyzed; 5, moribund. For SAR405 (MedChemExpress, HY-12481) treatment, mice were randomly divided into two groups and treated before the onset of the disease, starting from day 3 after immunization. Mice received daily oral gavage of 10-mg/kg SAR405 (dissolved in 10% DMSO + 90% corn oil, final working concentration 2.5 mg/ml) for 11 consecutive days. Control groups received vehicle (10% DMSO + 90% corn oil) only.
Generation and adoptive transfer of BMDMs
Bone marrow cells from femur cavities were flushed and incubated in RPMI 1640 supplemented with 10% FBS, 10-μM HEPES (Gibco, 15630), 2-mM glutamine (Corning Inc., 25-005-Cl), 1% streptomycin/penicillin G (Corning Inc., 30-001-Cl), 50-mM β-mercaptoethanol (Sigma-Aldrich Corporation, M-7522), and 20-ng/ml recombinant mouse CSF (R&D Systems Inc., 416-ML). Fresh medium was added to the culture on day 3. Cells were harvested on day 7 using cold PBS and the cell number was adjusted to 2 × 106 cells in 200 μl of PBS. Two-hundred microliter BMDMs were injected i.p. into naive Pik3c3f/f or pik3c3f/f;Lyz2-Cre recipient mice 4 h before active EAE induction.
Induction of passive EAE
For induction of adoptively transferred EAE, Pik3c3f/f mice were used as donors. Active EAE was induced in these animals as described above. On day 10, spleens were harvested, and single-cell suspensions were prepared. The cells were stimulated with 50-μg/ml MOG35–55 at 1 × 107 cells/ml in 10 ml of RPMI 1640 complete medium in a 25-cm2 flask. After 72 h, cells were harvested and resuspended in PBS (1 × 108 cells/ml) for adoptive transfer. Recipient mice (6–8 weeks of age) were irradiated sublethally at 400 rads to generate a lymphopenic environment 6 h prior to injection of MOG35–55-specific T cells. Cells were injected i.p. (250 µl/mouse). The clinical scores were graded similarly to active EAE.
Preparation of cells from spleen, brain, and PerC
Cells from spleens were obtained by mashing the organ through a 70-μm cell strainer (Fisher Scientific International, Inc., 22-363-548) into RPMI 1640 medium (Fisher Scientific International, Inc., MT10040CM) supplemented with 5% fetal bovine serum (Sigma-Aldrich Corporation, F2442). For brain cells, whole brains were excised and incubated for 1 h at 37oC in RPMI medium containing 0.1-mg/ml DNase I (Sigma-Aldrich Corporation, 4536282001) and 1-mg/ml Collagenase type I (Worthington Biochemical Corporation, LS004196). After incubation, tissues were mechanically disrupted and cells were passed through a 70-μm strainer. Peritoneal cells were harvested by lavage with 5 ml of cold RPMI 1640 medium supplemented with 5% FBS (Sigma-Aldrich Corporation, F2442). RBC was lysed with ACK lysing buffer (Lonza bioscience, 10-548E).
Phagocytosis assay
Phagocytosis of apoptotic cells was assayed as previously described.70 Briefly, B6.SJL (CD45.1+) thymocytes (107 cells/ml) were cultured with 0.1-μM dexamethasone overnight to induce apoptosis. Apoptotic cells were subsequently labeled with the cell-permeable fluorescent dye 5′-carboxyfluorescein succinyl ester (CFSE, Sigma-Aldrich Corporation, 21888) and then injected i.p. (106 cells/mouse). After 2 h, PerC cells were harvested, and CFSE+CD45.1–CD45.2+ macrophage subsets were determined by flow cytometry. For in vitro assays, phagocytic activity was measured using fluorescent latex beads (1-μm diameter, green-labeled, Sigma-Aldrich Corporation, L4655). The beads were preincubated at a final concentration of 55 μg/mL for 30 mins at 37 °C in a 1:1 (v/v) PBS/FBS solution. They were then incubated with cells (2 × 106/ml peritoneal cells, 5 μg/mL beads, about 2.6 × 108 beads/mL final concentration) for 2 h at 37 °C. The percent of fluorescent cells was determined by flow cytometry.
In vitro differentiation of macrophages
MACS (Miltenyi Biotec, 130-110-434)-sorted peritoneal macrophages were seeded in 24-well plates at a density of 250,000 cells/well and differentiated into M1-polarized or M2-polarized macrophages by the addition of mouse-recombinant IFN-γ (10 ng/ml, PeproTech, 315-05) or IL-4 (20 ng/ml, PeproTech, 214-14) for 48 h, respectively.
T-cell proliferation assay
Peritoneal macrophages from LPS-challenged (1 mg/kg for 48 h) or unchallenged Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice were isolated using a PerC macrophage isolation kit (Miltenyi Biotec, 130-110-434) according to the manufacturer’s protocols. Naive MOG35–55-specific CD4+ T cells were isolated from 2D2 TCR-transgenic mouse splenocytes using a naive CD4+ T-cell isolation kit (Miltenyi Biotec, 130-104-453) according to the manufacturer’s protocols. T cells were labeled with CFSE according to the manufacturer’s instructions (Thermo Fisher Scientific, C34554). Macrophages were pulsed with 20 μg/ml MOG35–55 or MOG1–125 (AnaSpec Inc., AS-55158-100) for 4 h and cultured with T cells for 72 h at a ratio of 1:5 with media. T-cell proliferation was assessed by flow cytometry at the end of culture.
Immunoblotting analyses
Macrophages and T or B cells from the PerC were purified by magnetic sorting and washed three times with PBS before the preparation of cellular proteins for western blotting. Cells from brain, spleen, and PerC were lysed with ice-cold lysis buffer containing 1% protease inhibitor cocktail (Sigma-Aldrich Corporation, P8340). Protein concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories Ltd, 500-0116). Protein was separated by 10–15% SDS-PAGE and transferred to nitrocellulose membranes. Blot membranes were subsequently blocked with 5% milk, incubated with primary and secondary antibodies, and then visualized using ECLTM western blotting detection reagents (Amersham Bioscience) and exposure to the film. Antibodies against PIK3C3 (D9A5), LC3B (2775), p62 (5114), and actin beta (13E5) were purchased from Cell Signaling Technology. Antibodies against IL-1β (16806-1-AP) and IL-18 (5C6F8) were from Proteintech, and the antibody against LAMP1 (1D4B) was from BioLegend.
Histology and IHC staining
For lung, spleen, liver, and brain histology analysis, tissues from 6-week-old mice were fixed in 10% formalin overnight. For lumbar spinal cords, mice were sacrificed at day 15 post active EAE induction, and lumbar spinal cords were dissected and fixed in 10% formalin overnight. Fixed tissues were flattened, dehydrated, and embedded. Serial sections of 5-µm thickness were used for H&E staining for assessment of inflammation and Luxol fast blue (LFB) for assessment of demyelination. Stained tissues were coverslipped and scanned using a Leica SCN400 Slide Scanner. Slides were analyzed and scored in a blinded fashion for inflammation: 0, normal; 1, a few inflammatory cells; 2, moderate cellular infiltrate in parenchyma; 3, severe cellular infiltrate in parenchyma. Myelin breakdown was assessed as pale staining with LFB and scored as follows: 0, no demyelination; 1, mild demyelination; 2, moderate demyelination; 3, severe demyelination. For frozen sections, mouse spleen samples were fixed in 4% paraformaldehyde for 3.5 h, rinsed in PBS for 30 min, and equilibrated in PBS containing 30% sucrose overnight at 4 °C before being embedded in OCT (Sakura Finetek, 4583) for cryosectioning. Serial sections of 5-μm thickness were used for immunofluorescence staining with anti-LC3B, -p62, -LAMP1, and -CD11b antibodies, as specified in the figures. Immunofluorescence-stained slides were coverslipped using a mounting media containing DAPI (Vector Laboratories, H-1800) to identify cell nuclei. All images were acquired using an LSM 880 confocal microscope and viewed using ZEN microscopy software from Zeiss.
Cytokine analysis
Serum levels of IL-6, IL-10, MCP-1, IFN-γ, TNF, and IL-12 p70 were measured with the Cytometric Bead Array Mouse Inflammation Kit (BD Biosciences, 552364) according to the manufacturer’s instructions. Samples were acquired on a Canto II flow cytometer. Data were analyzed using FACP array version 3.0 (BD Biosciences) software. IL-1β in serum was measured with an ELISA kit (Fisher Scientific International, Inc., 88-7013).
Seahorse assay
Experiments were performed in the Vanderbilt High-throughput Screening Core Facility. Metabolic analysis was carried out using an Extracellular Flux Analyzer XFe96 (Seahorse Bioscience). Peritoneal macrophages were purified by negative selection using magnetic microbeads as described by the manufacturer (Miltenyi Biotec, 130-110-434). Sorted macrophages (1 × 105 cells/well) were transferred to an analysis plate (Seahorse Bioscience, 101085-004) and the plate was centrifuged to accumulate cells at the bottom. The plate was incubated in a CO2-free incubator at 37 °C for 1 h and later transferred to the Seahorse machine for metabolic analyses. Glycolysis was measured via extracellular acidification rate (ECAR) (mpH/min) with the use of real-time injections. Cells were resuspended in XF assay medium supplemented with 1 mM glutamine (pH = 7.4, Sigma-Aldrich Corporation, G7513-100ML) with the use of injections of glucose (10 mM, Fisher Scientific International, Inc., D16-500), oligomycin (1 µM, Sigma-Aldrich Corporation, 75351-5MG), and 2-deoxy-D-glucose (2-DG, 100 mM, Sigma-Aldrich Corporation, D8375-5G). ECAR values were calculated using the program provided by the manufacturer and the data file was exported as a GraphPad Prism file.
Analysis of single-cell RNA-seq data
Previously published mouse single-cell transcriptomics data in C57BL/6 samples from several CNS compartments (including leptomeninges, perivascular space, parenchyma, and choroid plexus) during different disease stages, including naive (homeostasis), preclinical, onset, and peak (acute) disease28 were reanalyzed for autophagy signatures. The original single-cell RNA sequencing profiles (GSE118948) were downloaded from Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/). The analyses were completed with Seurat (v3.1.5) packages in R with steps compiled from the original literature.28 For quality control, different sets of genes were measured for samples in different regions. For all sequential analyses, only the common genes measured in all regions were considered for additional analysis. Cells with either a total number of transcripts <1500 or expressing >2% of gene Kcnq1ot1 were abandoned for further analysis. In addition, genes correlating to Kcnq1ot1 above the specific Pearson’s correlation coefficient (>0.65) were removed as well. Cells with mitochondrial content >15% were removed. Data were normalized using a scaling factor of 10,000. Functions FindIntegrationAnchors and IntegrateData in Seurat R package were used for batch-effect correction with default parameters, and principal component analysis was performed using the top 3000 most variable genes and clustering was performed using the top 15 PCAs and resolution of 0.9. The markers provided in Table S2 of the original manuscript28 were used for cluster cell-type identification. The identified labeled clusters include 1873 CAM cells, 688 microglia, 2100 monocyte-derived cells, 213 DCs, 540 granulocytes, and 420 lymphocytes. Function FindMarkers in Seurat R package (v3)71 was used for differential expression analysis by comparing samples between disease and nondisease status for each cell type.
Flow cytometry
In all experiments, dead cells were excluded from the analysis by electronic gating, and propidium iodide (PI, Sigma-Aldrich Corporation, P4170-1G) staining. Fluorescently labeled Abs against mouse CD3, CD4, CD8α, CD11b, CD11c, CD19, CD44, CD45, CD45.1, CD45.2, CD62L, CD86, CD206, IgM, F4/80, TIM-4, NK1.1, MHC class II (I–A/I–E), MHC class I (H-2kb), Ki67, IL-1β, IL-17A, IFN-γ, p62, LC3, and appropriate isotype controls were obtained from BD Biosciences, eBioscience, or Proteintech. PBS57-loaded mouse CD1d tetramer was obtained from the National Institutes of Health Tetramer Facility (Emory University, Atlanta, GA). Annexin V staining set was obtained from BD Biosciences (561431). For measurement of mitochondrial content or mitochondrial membrane potential in peritoneal macrophages by flow cytometry, PerC cells were prepared and stained with Mitoview Green (100 nM, Biotium, 70054-T) or TMRE (200 nM, Biotium, 115532-52-0) with anti-CD11b and anti-F4/80 mAbs according to the manufacturer’s protocols. For measuring MOG35–55-specific IFN-γ and IL-17A production during EAE, splenocytes from Pik3c3f/f and pik3c3f/f;Lyz2-Cre mice induced for EAE (day 10) were cultured with MOG35–55 peptide (20 μg/ml) for 18 h in the presence of GolgiStop (BD Biosciences, 554724). Flow cytometric analyses were performed using a BD FACSCanto II or BD LSRFortessa™ flow cytometer depending on the number of targeted markers. The acquired data were analyzed using FlowJo software (Version 10.0.7, Treestar, Palo Alto, CA).
Statistical analysis
Statistical analysis was performed with GraphPad Prism 6.0 (GraphPad Software). Throughout the paper, the distribution of data points is expressed as average ± SEM. Normally distributed data were analyzed by Student’s t test, and nonparametric comparisons were performed by the Mann–Whitney U test. EAE clinical scores were analyzed using ANOVA and Kaplan–Meier curves by log-rank test. p < 0.05 was considered significant.
Supplementary information
Acknowledgements
Work in the authors’ lab was supported by grants from the NIH (AI139046 to L.V.K. and 1ZIAES10328601 to J.M.) and the National Multiple Sclerosis Society (60006625 to L.V.K.). Core Services were performed through the Vanderbilt Digestive Disease Research Center (NIH grant P30DK058404), the Vanderbilt Ingram Cancer Center (NIH grant P30CA68485), and the Vanderbilt Diabetes Research and Training Center (NIH grant P60DK020593). J.L.P. was supported by predoctoral NIH training grants (T32HL069765 and T32AR059039).
Author contributions
G.Y. and L.V.K designed research; G.Y., W.S., J.X., J.L.P., F.C., and L.W. performed research; G.Y. analyzed data; J.M. and J.Z. contributed new reagents/analytic tools; G.Y. and L.V.K. wrote the paper.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Supplementary information
The online version of this article (10.1038/s41423-020-00589-1) contains supplementary material.
References
- 1.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev. Cell Dev. Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 2.Ktistakis NT, Tooze SA. Digesting the expanding mechanisms of autophagy. Trends Cell Biol. 2016;26:624–635. doi: 10.1016/j.tcb.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Axe EL, et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaber N, Zong WX. Class III PI3K Vps34: essential roles in autophagy, endocytosis, and heart and liver function. Ann. N. Y. Acad. Sci. 2013;1280:48–51. doi: 10.1111/nyas.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J. 2008;410:1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- 6.Boyle KB, Randow F. Rubicon swaps autophagy for LAP. Nat. Cell Biol. 2015;17:843–845. doi: 10.1038/ncb3197. [DOI] [PubMed] [Google Scholar]
- 7.Heckmann BL, et al. LC3-associated endocytosis facilitates beta-amyloid clearance and mitigates neurodegeneration in murine alzheimer’s disease. Cell. 2019;178:536–551.e14. doi: 10.1016/j.cell.2019.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J. Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol. Biol. Cell. 2008;19:5360–5372. doi: 10.1091/mbc.e08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X, et al. Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc. Natl Acad. Sci. U.S.A. 2010;107:9424–9429. doi: 10.1073/pnas.0914725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLeod IX, Zhou X, Li QJ, Wang F, He YW. The class III kinase Vps34 promotes T lymphocyte survival through regulating IL-7Ralpha surface expression. J. Immunol. 2011;187:5051–5061. doi: 10.4049/jimmunol.1100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willinger T, Flavell RA. Canonical autophagy dependent on the class III phosphoinositide-3 kinase Vps34 is required for naive T-cell homeostasis. Proc. Natl Acad. Sci. U.S.A. 2012;109:8670–8675. doi: 10.1073/pnas.1205305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parekh VV, et al. Impaired autophagy, defective T cell homeostasis, and a wasting syndrome in mice with a T cell-specific deletion of Vps34. J. Immunol. 2013;190:5086–5101. doi: 10.4049/jimmunol.1202071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaber N, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc. Natl Acad. Sci. U.S.A. 2012;109:2003–2008. doi: 10.1073/pnas.1112848109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devereaux K, et al. Regulation of mammalian autophagy by class II and III PI 3-kinases through PI3P synthesis. PloS ONE. 2013;8:e76405. doi: 10.1371/journal.pone.0076405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bechtel W, et al. The class III phosphatidylinositol 3-kinase PIK3C3/VPS34 regulates endocytosis and autophagosome-autolysosome formation in podocytes. Autophagy. 2013;9:1097–1099. doi: 10.4161/auto.24634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bechtel W, et al. Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J. Am. Soc. Nephrology. 2013;24:727–743. doi: 10.1681/ASN.2012070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morishita H, et al. Deletion of autophagy-related 5 (Atg5) and Pik3c3 genes in the lens causes cataract independent of programmed organelle degradation. J. Biol. Chem. 2013;288:11436–11447. doi: 10.1074/jbc.M112.437103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parekh VV, et al. Autophagy-related protein Vps34 controls the homeostasis and function of antigen cross-presenting CD8alpha(+) dendritic cells. Proc. Natl Acad. Sci. U.S.A. 2017;114:E6371–E6380. doi: 10.1073/pnas.1706504114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang, G. et al. Autophagy-related protein PIK3C3/VPS34 controls T cell metabolism and function. Autophagy 1–12, 10.1080/15548627.2020.1752979 (2020).
- 21.Alirezaei M, et al. Elevated ATG5 expression in autoimmune demyelination and multiple sclerosis. Autophagy. 2009;5:152–158. doi: 10.4161/auto.5.2.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patergnani S, et al. Autophagy and mitophagy elements are increased in body fluids of multiple sclerosis-affected individuals. J. Neurol., Neurosurg., Psychiatry. 2018;89:439–441. doi: 10.1136/jnnp-2017-316234. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharya A, Parillon X, Zeng S, Han S, Eissa NT. Deficiency of autophagy in dendritic cells protects against experimental autoimmune encephalomyelitis. J. Biol. Chem. 2014;289:26525–26532. doi: 10.1074/jbc.M114.575860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckley KM, et al. Rapamycin up-regulation of autophagy reduces infarct size and improves outcomes in both permanent MCAL, and embolic MCAO, murine models of stroke. Exp. Transl. Stroke Med. 2014;6:8. doi: 10.1186/2040-7378-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonam SR, Wang F, Muller S. Autophagy: a new concept in autoimmunity regulation and a novel therapeutic option. J. Autoimmun. 2018;94:16–32. doi: 10.1016/j.jaut.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Zhu B, et al. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J. Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Wang ZV, Hill JA, Lin F. New autophagy reporter mice reveal dynamics of proximal tubular autophagy. J. Am. Soc. Nephrol. 2014;25:305–315. doi: 10.1681/ASN.2013040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordao, M. J. C. et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science363, 10.1126/science.aat7554 (2019). [DOI] [PubMed]
- 29.Abram CL, Roberge GL, Hu Y, Lowell CA. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J. Immunol. Methods. 2014;408:89–100. doi: 10.1016/j.jim.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferro A, et al. Inhibition of NF-kappaB signaling in IKKbetaF/F;LysM Cre mice causes motor deficits but does not alter pathogenesis of Spinocerebellar ataxia type 1. PloS ONE. 2018;13:e0200013. doi: 10.1371/journal.pone.0200013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulido-Salgado M, et al. Myeloid C/EBPbeta deficiency reshapes microglial gene expression and is protective in experimental autoimmune encephalomyelitis. J. Neuroinflammation. 2017;14:54. doi: 10.1186/s12974-017-0834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdel Fattah E, Bhattacharya A, Herron A, Safdar Z, Eissa NT. Critical role for IL-18 in spontaneous lung inflammation caused by autophagy deficiency. J. Immunol. 2015;194:5407–5416. doi: 10.4049/jimmunol.1402277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanayama M, He YW, Shinohara ML. The lung is protected from spontaneous inflammation by autophagy in myeloid cells. J. Immunol. 2015;194:5465–5471. doi: 10.4049/jimmunol.1403249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanayama M, Danzaki K, He YW, Shinohara ML. Lung inflammation stalls Th17-cell migration en route to the central nervous system during the development of experimental autoimmune encephalomyelitis. Int. Immunol. 2016;28:463–469. doi: 10.1093/intimm/dxw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang YT, et al. Select autophagy genes maintain quiescence of tissue-resident macrophages and increase susceptibility to Listeria monocytogenes. Nat. Microbiol. 2020;5:272–281. doi: 10.1038/s41564-019-0633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pua HH, Guo J, Komatsu M, He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J. Immunol. 2009;182:4046–4055. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- 37.Riffelmacher T, Richter FC, Simon AK. Autophagy dictates metabolism and differentiation of inflammatory immune cells. Autophagy. 2018;14:199–206. doi: 10.1080/15548627.2017.1362525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vats D, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tannahill GM, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong K, et al. Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proc. Natl Acad. Sci. U.S.A. 2010;107:8712–8717. doi: 10.1073/pnas.0910929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176:11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang G, Driver JP, Van Kaer L. The role of autophagy in iNKT cell development. Front. Immunol. 2018;9:2653. doi: 10.3389/fimmu.2018.02653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez J, et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat. Cell Biol. 2015;17:893–906. doi: 10.1038/ncb3192. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Bhattacharya A, et al. Autophagy is required for neutrophil-mediated inflammation. Cell Rep. 2015;12:1731–1739. doi: 10.1016/j.celrep.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Shi J, Hua L, Harmer D, Li P, Ren G. Cre driver mice targeting macrophages. Methods Mol. Biol. 2018;1784:263–275. doi: 10.1007/978-1-4939-7837-3_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orthgiess J, et al. Neurons exhibit Lyz2 promoter activity in vivo: Implications for using LysM-Cre mice in myeloid cell research. Eur. J. Immunol. 2016;46:1529–1532. doi: 10.1002/eji.201546108. [DOI] [PubMed] [Google Scholar]
- 47.Jiang Z, Jiang JX, Zhang GX. Macrophages: a double-edged sword in experimental autoimmune encephalomyelitis. Immunol. Lett. 2014;160:17–22. doi: 10.1016/j.imlet.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slavin AJ, et al. Requirement for endocytic antigen processing and influence of invariant chain and H-2M deficiencies in CNS autoimmunity. J. Clin. Invest. 2001;108:1133–1139. doi: 10.1172/JCI13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sosa RA, Murphey C, Ji N, Cardona AE, Forsthuber TG. The kinetics of myelin antigen uptake by myeloid cells in the central nervous system during experimental autoimmune encephalomyelitis. J. Immunol. 2013;191:5848–5857. doi: 10.4049/jimmunol.1300771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yogev N, et al. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor(+) regulatory T cells. Immunity. 2012;37:264–275. doi: 10.1016/j.immuni.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 51.Isaksson M, Lundgren BA, Ahlgren KM, Kampe O, Lobell A. Conditional DC depletion does not affect priming of encephalitogenic Th cells in EAE. Eur. J. Immunol. 2012;42:2555–2563. doi: 10.1002/eji.201142239. [DOI] [PubMed] [Google Scholar]
- 52.Martinez J, et al. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl Acad. Sci. U.S.A. 2011;108:17396–17401. doi: 10.1073/pnas.1113421108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hovelmeyer N, et al. Apoptosis of oligodendrocytes via Fas and TNF-R1 is a key event in the induction of experimental autoimmune encephalomyelitis. J. Immunol. 2005;175:5875–5884. doi: 10.4049/jimmunol.175.9.5875. [DOI] [PubMed] [Google Scholar]
- 54.Wu, M. Y. et al. PI3KC3 complex subunit NRBF2 is required for apoptotic cell clearance to restrict intestinal inflammation. Autophagy1–16,10.1080/15548627.2020.1741332 (2020). [DOI] [PMC free article] [PubMed]
- 55.Konishi A, Arakawa S, Yue Z, Shimizu S. Involvement of Beclin 1 in engulfment of apoptotic cells. J. Biol. Chem. 2012;287:13919–13929. doi: 10.1074/jbc.M112.348375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levesque SA, et al. Myeloid cell transmigration across the CNS vasculature triggers IL-1beta-driven neuroinflammation during autoimmune encephalomyelitis in mice. J. Exp. Med. 2016;213:929–949. doi: 10.1084/jem.20151437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pare A, et al. IL-1beta enables CNS access to CCR2(hi) monocytes and the generation of pathogenic cells through GM-CSF released by CNS endothelial cells. Proc. Natl Acad. Sci. U.S.A. 2018;115:E1194–E1203. doi: 10.1073/pnas.1714948115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olcum M, Tastan B, Kiser C, Genc S, Genc K. Microglial NLRP3 inflammasome activation in multiple sclerosis. Adv. Protein Chem. Struct. Biol. 2020;119:247–308. doi: 10.1016/bs.apcsb.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi N, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galluzzi L, Green DR. Autophagy-independent functions of the autophagy machinery. Cell. 2019;177:1682–1699. doi: 10.1016/j.cell.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Kaer, L., Postoak, J. L., Wang, C., Yang, G. & Wu, L. Innate, innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE. Cell. Mol. Immunol.10.1038/s41423-019-0221-5 (2019). [DOI] [PMC free article] [PubMed]
- 64.Yin L, et al. Autophagy-related gene16L2, a potential serum biomarker of multiple sclerosis evaluated by bead-based proteomic technology. Neurosci. Lett. 2014;562:34–38. doi: 10.1016/j.neulet.2013.12.070. [DOI] [PubMed] [Google Scholar]
- 65.Igci M, et al. Gene expression profiles of autophagy-related genes in multiple sclerosis. Gene. 2016;588:38–46. doi: 10.1016/j.gene.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 66.Kimmey JM, et al. Unique role for ATG5 in neutrophil-mediated immunopathology during M. tuberculosis infection. Nature. 2015;528:565–569. doi: 10.1038/nature16451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saitoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 68.Kimura T, et al. Dedicated SNAREs and specialized TRIM cargo receptors mediate secretory autophagy. EMBO J. 2017;36:42–60. doi: 10.15252/embj.201695081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dupont N, et al. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1beta. EMBO J. 2011;30:4701–4711. doi: 10.1038/emboj.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cain DW, et al. Identification of a tissue-specific, C/EBPbeta-dependent pathway of differentiation for murine peritoneal macrophages. J. Immunol. 2013;191:4665–4675. doi: 10.4049/jimmunol.1300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.