Abstract
Denisova Cave, a Pleistocene site in the Altai Mountains of Russian Siberia, has yielded significant fossil and lithic evidence for the Pleistocene in Northern Asia. Abundant animal and human bones have been discovered at the site, however, these tend to be highly fragmented, necessitating new approaches to identifying important hominin and faunal fossils. Here we report the results for 8253 bone fragments using ZooMS. Through the integration of this new ZooMS-based data with the previously published macroscopically-identified fauna we aim to create a holistic picture of the zooarchaeological record of the site. We identify trends associated with climate variability throughout the Middle and Upper Pleistocene as well as patterns explaining the process of bone fragmentation. Where morphological analysis of bones from the site have identified a high proportion of carnivore bones (30.2%), we find that these account for only 7.6% of the ZooMS assemblage, with large mammals between 3 and 5 more abundant overall. Our analysis suggests a cyclical pattern in fragmentation of bones which sees initial fragmentation by hominins using percussive tools and secondary carnivore action, such as gnawing and digestion, likely furthering the initial human-induced fragmentation.
Subject terms: Archaeology, Palaeontology
Introduction
Studying highly fragmented archaeological and paleontological bone assemblages is a particularly challenging task. Robust enough that they can survive a range of depositional environments, bones are still susceptible to numerous taphonomic processes1–4. Research into large assemblages of fragmented bones has been instrumental in exploring depositional histories and accumulation5–8 and the impact of freezing and thawing9, weathering10,11, trampling and gnawing12, and the role of humans modifying and processing bones, either for subsistence reasons (e.g. bone marrow exploitation and fuel use)13–16 or for the production of osseous tools17,18. Fragmentation adversely affects taxonomic and anatomical attribution and it is estimated that only a third of bones found in Pleistocene archaeological contexts can be identified using traditional methodologies, i.e. through visual inspection19,20. Without a reliable means of determining taxonomically highly fragmented bones these are often recorded as “unidentified” fauna excluding their integration into detailed zooarchaeological studies and the possibility of identifying new faunal groups and uncommon taxa.
Recently the potential of highly fragmented Pleistocene-age bone assemblages has been explored through the use of peptide mass fingerprinting, specifically using ZooMS (Zooarchaeology by Mass Spectrometry)21. ZooMS provides an efficient means of screening and taxonomically identifying bones through the targeted analysis of type I collagen (COL1). The method has been applied to large Pleistocene assemblages22–24, including the Middle and Late Pleistocene site, Denisova Cave, in the Russian Altai25,26. ZooMS analysis of fragmented bones at Denisova Cave has so far resulted in the identification of nine new hominin remains25–27, including two Neanderthals (Denisova 15 and Denisova 17), three Denisovans (Denisova 19, Denisova 20, and Denisova 21), and the offspring of a Neanderthal mother and a Denisovan father (Denisova 11)25,28. In the course of identifying these individuals a large assemblage of fauna has been identified which has not been discussed thus far.
Here we report the results of 8253 fragmented bones from Denisova Cave that were analysed using ZooMS. These bones were initially studied with the aim of identifying hominin remains and, in light of recent publications detailing the chronology of the site26,29, zooarchaeological findings29–34, and palaeoenvironmental reconstructions35,36 we aim to identify what contribution, if any, these fragmented bones may play in our understanding of Denisova Cave’s faunal record by reincorporating them into research at the site.
The addition of ZooMS-based determinations into the more traditional zooarchaeological analysis is inherently complicated, particularly when analysing morphologically non-diagnostic bones. A first limitation stems from the broad taxonomic groupings created through ZooMS analysis which do not match the often more detailed macroscopic identifications. For large mammals, ZooMS is typically only able to make genus level identifications, with some exceptions in which species can be determined37. Second, because NISP (Number of Individual/Identified Specimens) is the only reliable measurement through which these highly fragmentary bones can be counted, this inevitably leads to an overestimation of the presence/absence of fauna19,20. With these limitations in mind, and using different approaches we detail below, we use ZooMS-identified bones to create a holistic view of the fauna assemblage from Denisova Cave and determine broad trends and variation through time.
Materials
Denisova Cave, situated in the Altai Mountains of Russian Siberia, is unique in its hominin record as the only site where both Neanderthal and Denisovan fossils have been identified (Supplementary Fig. 1)38–41. The cave, situated in the ledge of a southwest-facing rock wall and overlooking the Anui River, has the longest stratigraphic sequence for northern Eurasia, with contexts dating from the Middle Pleistocene. This extensive sequence has made Denisova Cave a type-site for northern Eurasia, allowing for palynological42 and palaeoecological43,44 reconstructions of the region and several zooarchaeological studies29–34,36. Despite particularly favorable conditions that ensure a high degree of biomolecular preservation at the site, less than 5% of bones excavated can be identified macroscopically29–34,36, making Denisova Cave cave a good candidate for the application of ZooMS. We analysed 8,253 non-diagnostic bone fragments which were excavated from each of the three interconnecting chambers of Denisova Cave. The majority of these bones come from the East Chamber (n = 6288), followed by the Main (n = 1143) and South (n = 822) chambers (Supplementary Information; Supplementary Fig. 1). These were sampled with the permission of and in collaboration with the Institute of Archeology and Ethnography of the Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia.
Bones were specifically chosen as they could not be identified on the basis of morphology and, where possible, fragments > 2 cm in length were preferentially selected, although smaller bones were also part of our analysed assemblage. Assemblages of bones of this size were also assumed to contain morphologically indistinct hominin remains since all previously identified hominins at the site were no bigger than ~ 3 cm in length. Samples were drilled in preparation for analysis and from each bone approximately 20 mg was removed using a diamond covered disc. Care was taken to clean equipment between drilling samples to minimise cross-contamination. Sampling was undertaken at two laboratories, at the School of Archaeology, University of Oxford, U.K. and at the Department of Archaeology, Max Planck Institute for the Science of Human History (MPI-SHH), Jena, Germany.
Results
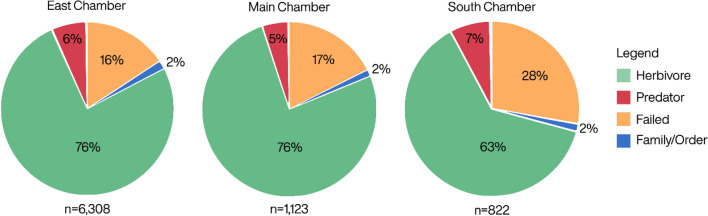
Overall, of the 8253 analysed bones, 74% were assigned to a specific ZooMS taxon whereas an additional 5% could only be identified to family or order level due to low quality spectra (Supplementary Information). The South Chamber had a high rate of samples that failed to produce enough collagen for taxonomic identification (28%), in comparison with lower rates of failure for the East (16%) and Main (17%) chambers (Fig. 1).
Figure 1.
Comparison of overall ZooMS results by chamber of Denisova Cave showing samples which could be identified to their most specific ZooMS taxon (shown here as herbivores and predators) in comparison with samples which could only be identified to family/order or those which failed analysis.
In total we identified 18 ZooMS taxa amongst the bones we analysed (Table 1). A ZooMS taxon is defined here as the most specific identification ZooMS is able to provide, generally to a genus or family level. For instance, several Canids have been identified within the morphological assemblage at Denisova Cave, including Canis lupus (wolf), Vulpes corsak (corsak fox), Cuon alpinus (dhole), and Alopex lagopus (arctic fox), all of which could only be classified as ‘Canidae’ through ZooMS analysis as their peptide mass fingerprints are identical. Vulpes vulpes (red fox) is the only Canid that can be differentiated using ZooMS, as a result of a small difference in their COL1ɑ2 484‒498 peptide45 (Supplementary Table 1). The assemblage is dominated by large vertebrates, with small vertebrates and birds accounting for less than 1% of the identified material examined. This is likely in part a result of our selection of bones which were preferentially 2 cm in length or more.
Table 1.
The results of ZooMS analysis of 8,253 fragmented bones from Denisova Cave.
| ZooMS taxon | East chamber | Main chamber | South chamber | Total |
|---|---|---|---|---|
| Bison/Yak | 1629 | 337 | 221 | 2187 |
| Canidae | 140 | 12 | 8 | 160 |
| Capra | 209 | 75 | 30 | 314 |
| Cervidae | 18 | 18 | ||
| Cervidae/Gazella/Saiga | 1189 | 35 | 32 | 1256 |
| Crocuta/Panthera | 99 | 21 | 19 | 139 |
| Elephantidae | 181 | 40 | 35 | 256 |
| Equidae | 669 | 123 | 58 | 850 |
| Felidae | 7 | 1 | 8 | |
| Hominin | 6 | 1 | 7 | |
| Leporidae | 2 | 2 | ||
| Muridae | 2 | 2 | ||
| Mustelidae | 2 | 2 | ||
| Ovis | 147 | 108 | 40 | 295 |
| Ovis/Capra | 302 | 67 | 54 | 423 |
| Rangifer | 34 | 3 | 1 | 38 |
| Rhinocerotidae | 401 | 98 | 48 | 547 |
| Ursidae | 123 | 20 | 33 | 176 |
| Vulpes vulpes | 19 | 19 | ||
| Bird | 9 | 1 | 10 | |
| Capra/Rangifer | 12 | 2 | 3 | 17 |
| Cervidae/Gazella/Saiga/Equidae | 15 | 1 | 16 | |
| Crocuta/Panthera/Mustelidae | 11 | 5 | 4 | 20 |
| Equidae/Rhinocerotidae | 1 | 1 | ||
| Felidae/Crocuta/Panthera | 1 | 1 | ||
| Felidae/Crocuta/Panthera/Mustelidae | 2 | 2 | ||
| Felidae/Ursidae | 18 | 4 | 1 | 23 |
| Ovis/Capra/Cervidae/Gazella/Saiga | 25 | 25 | ||
| Ovis/Capra/Rangifer | 4 | 2 | 6 | |
| Ovis/Cervidae/Gazella/Saiga | 25 | 3 | 28 | |
| Unknown | 19 | 3 | 3 | 25 |
| Failed | 968 | 183 | 229 | 1380 |
| Total | 6288 | 1143 | 822 | 8253 |
For the species most likely included in the “ZooMS-taxon” see Table S1.
Comparing datasets
In order to compare the ZooMS identified fauna with previously-published zooarchaeological datasets from Denisova Cave we have compiled those datasets, referred to as the ‘morphological assemblage’, from the literature and converted them into ZooMS-style groupings (following Supplementary Table 1) which can then be directly compared with the new ZooMS results we present here (Fig. 2). In our discussion of percentages, we have excluded samples which failed ZooMS analysis or those which could not be assigned to the most specific ZooMS taxon possible, with the exception of Ovis/Capra and Crocuta/Panthera (see Supplementary Information) in order to make them more compatible with zooarchaeological results. We have also excluded small vertebrates as they were not identified in sufficient numbers for direct comparison with the morphological assemblage. While some results are not included in our comparisons with the zooarchaeological datasets, all results are reported in the Supplementary Information (Supplementary Table 2, 3, 4; External Database 1).
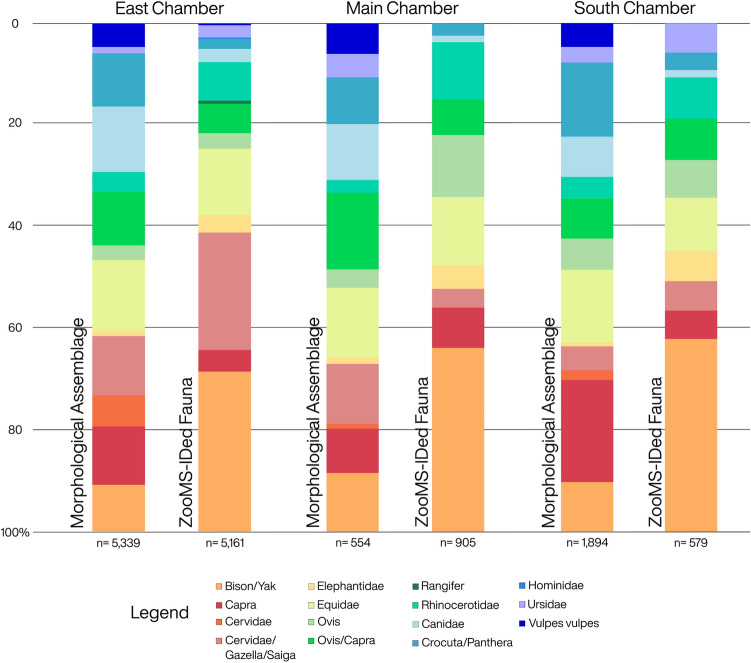
Figure 2.
Comparison of the overall abundance of fauna between the morphological assemblage and the ZooMS-IDed fauna for the three chambers of Denisova Cave. Only the largest faunal groups have been included. Small mammals from both the morphological assemblage and ZooMS-IDed component, samples which failed ZooMS analysis, and “Unknown” spectra are not included.
We use the term “predator”, rather than “carnivore”, to group the activity of hominins, Canidae, Crocuta/Panthera, Ursidae and V. vulpes, some of which have varied diets. The term “herbivores” includes Bison/Yak, Capra, Cervidae/Gazella/Saiga, Elephantidae, Equidae, Ovis, and Rhinocerotidae.
East chamber
ZooMS analysis was carried out on 6,288 bone fragments from most archaeological layers of the East Chamber (Table 1; Supplementary Table 2). Predators account for 20% of the morphological assemblage of the East Chamber, in comparison with only 7.7% of the ZooMS-IDed assemblage. The overall proportion of predator remains does not vary significantly throughout the layers of the East Chamber in the morphological assemblage. Cave hyaena (Crocuta crocuta spelaea) are the dominant predators between layers 9–13, alongside smaller numbers of Panthera, Canidae, and Felidae29,31. Their behaviours are particularly visible in the archaeological material from layers 9–11 where a large number of bones have clearly passed through the digestive tracts of hyaenas or wolves, leaving acid corrosion marks on bones and dissolving the enamel on teeth29,31. In contrast, in the ZooMS-IDed component, Crocuta/Panthera are present in relatively low numbers, accounting for approximately 1.9% of all identified bones (Fig. 2). Canidae on the other hand are consistently the dominant predator group in the ZooMS-IDed component for each of the studied layers of the East Chamber. Within the morphological assemblage, Canidae only exceed Crocuta/Panthera in layers 15–1729,31 a period during which forest species like Capreolus pygargus (Siberian roe deer) and Cervus elaphus (red deer) are the major herbivore groups present in the assemblage (see below). In the ZooMS-IDed fauna, Canidae account for 2.7% of all the bones identified within the East Chamber.
The two most significant herbivore taxa in the ZooMS-IDed component for the East Chamber are Cervidae/Gazella/Saiga and Bison/Yak. The Cervidae/Gazella/Saiga ZooMS-taxon includes C. pygargus, C. elaphus, Megaloceros giganteus (Irish elk), Alces alces (elk), Saiga tatarica borealis (saiga antelope), and Procapra gutturosa (Mongolian gazelle). The only cervid species present at Denisova Cave which can be separated from this group is the Siberian roe deer on the basis of their COL1ɑ2 757–789 (ɑ2 757)45 peptide marker. The ɑ2 757 peptide for Siberian roe deer is present at m/z 3043.4/3059.4 but at m/z 3017.5/3033.5 for Cervidae/Gazella/Saiga. Since this differentiation is based on a single marker it is possible that many of the generically identified Cervidae/Gazella/Saiga are actually Siberian roe deer which were missing their ɑ2 757 marker in poorer preserved specimens. The ɑ2 757 marker is easily lost as a result of collagen degradation, a common problem for instance in the separation of Ovis and Capra which is done on the basis of the same peptide46.
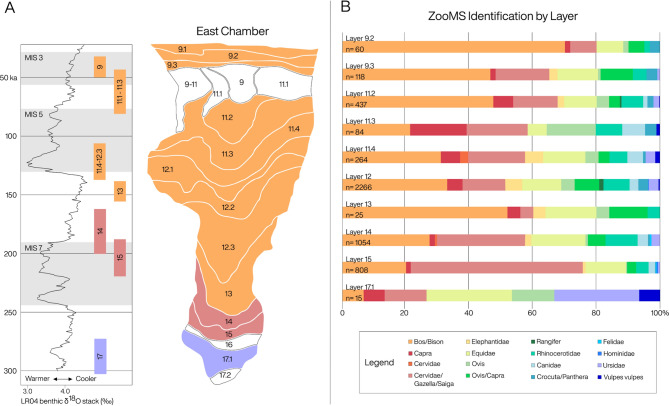
Cervidae/Gazella/Saiga bones account for 54% of the ZooMS-IDed component for layer 15. This number declines significantly in the overlying archaeological layers. In comparison, Bison/Yak, a taxonomic group that contains the remains of steppe bison (Bison priscus) and the less common Baikal yak (Poёphagus mutus), continue to increase by percentage throughout the stratigraphic layers. Bison/Yak eventually account for 70% of the ZooMS IDed fauna in layer 9.226 (Fig. 3; Supplementary Table 2).
Figure 3.
Relative abundance of fauna identified using ZooMS per layer of the East Chamber of Denisova Cave. This information can also be found in Supplementary Table 2. (A) The stratigraphy and corresponding age ranges are highlighted with the most abundant fauna for each layer. The marine-oxygen isotope curve was compiled from benthic δ18O records64; marine-oxygen isotope stages 3, 5, and 7 are highlighted in grey29. (B) Each bar depicts the major taxa identified. Only the largest groups of fauna have been included, excluding small mammals, “Unknown” identifications, and failed samples.
Main chamber
We carried out ZooMS analysis for 1,143 bones from layers 9–12 of the Main Chamber (Table 1; Supplementary Table 3). Predators dominate the morphological assemblage, accounting for more than 30% of all identifiable bone for layers 9–12. In comparison, the dominant predators, Canidae, Crocuta/Panthera, and Ursidae, make up only 5.5% of the ZooMS-IDed component. Our Crocuta/Panthera taxon likely includes C. spelaea, although Panthera spelaea are also present in the earliest Pleistocene layers of the Main Chamber. Crocuta/Panthera dominate the morphological assemblage for layers 9–11, accounting for 9.4% of identifiable bones (Fig. 2)29. In the ZooMS-IDed component, Crocuta/Panthera account for 1.68% of the fragmented bones for layers 9–11 making them the most commonly identified predators for these contexts. The morphological assemblage identifies Canidae as the major predator group in layer 1229,34, however none were identified in the ZooMS-IDed component for this context.
The major herbivore groups do not differ significantly between the morphological and ZooMS identified fauna. Equidae, Ovis/Capra, and Cervidae/Gazella/Saiga are present in relatively large numbers in each dataset. Bison/Yak remains increase significantly within the ZooMS-IDed fauna, accounting for 35% of the assemblage, more than four times the amount identified within the morphological bones (8.35%).
South chamber
We analysed 822 bones from the South Chamber using ZooMS (Table 1; Supplementary Table 4). Crocuta/Panthera, Canidae, and Ursidae are the dominant predator groups in the ZooMS fauna in this Chamber. They account, however, for only 10.5% of this assemblage, three times less than predators in the morphological assemblage (Fig. 2). Ursidae (Ursus (Spelaearctos) savini) account for 9.7% of the ZooMS-IDed fauna for layer 11. Previous zooarchaeological analysis suggested the cave may have been used for hibernation by the small cave bear (U. spelaearctos)32 which could explain the unusually high number of bear bones. Crocuta/Panthera were the dominant predator group for the ZooMS-IDed fauna in layer 12, accounting for 2.7% of the assemblage which is largely in line with dominant predator groups identified in the morphological assemblage. Bison/Yak (36.6%) and Equidae (10.3%) were the most common herbivores present within the ZooMS-IDed component.
Zooarchaeological analysis of bones from the South Chamber32,33 suggests intense predator presence for layers 9–12, in particular Crocuta/Panthera. Their bones are disproportionately represented in the morphologically identifiable bones for South Chamber (n = 255), second only to Capra sp. remains, which are identified as the likely prey for cave hyena and snow leopard32,33. Fragments of cave hyena coprolites were identified throughout layers 9–12 of the South Chamber and many of the bones of prey taxa show traces of acid corrosion, indicating that they have passed through the digestive tracts of predators32,33. It is noteworthy that only 3.7% of bones recovered from this part of the cave are larger than 5 cm33. We may hypothesize that this large degree of fragmentation is due to predation. In turn, such a high concentration of carnivore processing could explain the low level of protein preservation in this section of the cave; 28% of the fragmented remains analysed using ZooMS failed to produce enough collagen for identification as opposed to 17% and 16% for the East and Main Chambers, respectively (Fig. 1; Table 1; Supplementary Table 4).
Discussion
Patterns of faunal variability
ZooMS has previously been applied to larger microfauna assemblages and older individual faunal samples47,48, yet our present work is the largest application of ZooMS to an assemblage of fragmented bones with an archaeological association. The oldest archaeological layers we study here (layers 15 and 14) date to between 217 and 163 ka29. For layer 15, from which we analysed nearly 1000 bones, the success rate was 86% (Supplementary Table 2). This is particularly encouraging and paves the way for the future analysis of bone assemblages of similar biomolecular preservation and antiquity.
Significant differences in success rates were observed across layers and the three non-connecting chambers. Bones from the East Chamber were the most successful, however an average of 35% of samples from the youngest Pleistocene contexts (layers 9.2 and 9.3) failed to produce enough collagen for ZooMS analysis. This high failure rate is likely due to the heavy phosphatisation in the soil36,49 which has previously impeded OSL, radiocarbon dating, and genetic analyses for these layers26,29. At present, it seems identifying fossils suitable for biomolecular analysis from Upper Pleistocene contexts at Denisova Cave will continue to be challenging. This is unfortunate given the persistent questions regarding which hominin populations were present in the Altai Mountains during this period26.
At Denisova Cave a high degree of bone fragmentation is observed overall. Of the 177,000 bones excavated at the East Chamber more than 95% were less than 2–5 cm in length and could not be identified macroscopically29,31. Using ZooMS however, on average 74% of the fragmented bones we analysed could be assigned to a specific ZooMS taxon and an additional 5% of samples that produced low quality spectra could be identified to family or order levels. This contrast in identification success (< 5% identified morphologically versus ~ 80% based on ZooMS) highlights once again the merits of large-scale application of ZooMS on highly fragmented archaeological assemblages.
Bones analysed in this study from the Main Chamber were selected from the Upper Palaeolithic layers 9 and 11 (Supplementary Fig. 1), whereas bones from the South Chamber came from both Upper Palaeolithic layer 11 and Middle Palaeolithic layers 12 and 21. For the East Chamber, bones were selected from all major stratigraphic units, from the early Middle Palaeolithic through to the Upper Palaeolithic layers (15–9), and the archaeologically sterile layer 17. The majority of bones from the ZooMS-IDed component from all three chambers were the remains of prey, suggesting that the formation of the assemblage is not entirely relegated to equifinality in that some processes of the accumulation can be unravelled3,4. In the East Chamber, the earliest evidence for hominins processing carcasses and modifying bone is present in layers 15 and 14 of the East Chamber which coincides with the arrival of the first Denisovans to the site27,35,50. From this period lithic technologies are continuously present throughout the contexts of this chamber and the layers studied for the Main and South chambers35,51. Alongside hominins, previous zooarchaeological studies for Denisova Cave have highlighted the major role carnivores likely played in the formation of the site’s fragmented bone assemblage30,33. Carnivore remains are found in all contexts for the site but their behaviours are particularly visible in Upper Palaeolithic contexts with extensive gnawing marks and digestion of bones likely by hyenas, panthers, leopards, and wolves31,33,35. The overlap and relationship between hominins and predators at Denisova Cave has been investigated through multiple studies. While periods of intensive hominin presence are evident, particularly during the Early Middle and Middle Palaeolithic contexts where anthropogenic marks are frequently found on bones29,31, evidence for other predators at the site is continuously documented throughout the Palaeolithic30–33.
Recent analysis of the microstratigraphy of Denisova Cave has furthered this, showing that due to slow sedimentation accumulation rates it is currently not possible to identify when predators like bears, wolves, and hyaenas were occupying the cave as opposed to hominin groups like Denisovans and Neanderthals36. Rather, it seems that many groups were alternating as the site’s main occupants throughout the Pleistocene32,36. Further analyses of the bone assemblage, such as detailed taphonomic studies, bone refitting exercises and extensive dating of specific locations to assess the contemporaneity of various species at the site, will shed further light on this. Early evidence however suggests that at times hominins and other predators may have even benefited from each other’s presence. The close stratigraphic relationship between carnivores and hominins at Denisova Cave and our inability to specifically discern when these groups were dominant at the site may indicate the role scavenging played at Denisova Cave, particularly in regards to cave hyaenas and wolves taking advantage of the intensive hunting practices of Denisovans and Neanderthals36. Additionally, cut marked carnivore bones, particularly of red and polar foxes52, suggests hominins might have been targeting them for their fur. This practice is common in other Pleistocene sites in Russia, such as in the Kostenki region53,54.
The most notable difference between the morphological assemblage and the ZooMS-IDed component of Denisova Cave is the abundance of predators in comparison with herbivores. In the morphological assemblage of all three chambers, on average, predator groups account for 30.2% of all identified bones29–34, whereas in the ZooMS-IDed component predators account for only 7.6%. This discrepancy helps to inform us of the main factor influencing fragmentation rates at the site. Both the ZooMS ID-ed component and the morphological assemblage suggest that carnivores were the driving force behind the high rate of herbivore bone fragmentation. Given that the hominin bones recovered so far are similarly fragmented to herbivore bones (none exceed a few cm in length), we hypothesize that although humans were the main agent responsible for the accumulation and initial dismembering, butchering, and fragmentation of (most) herbivores in the cave, a secondary agent, that is carnivores such as hyenas, caused further processing and reduction in size of both animal and human bone deposited there. This hypothesis is largely in line with archaeological evidence for the site, where bones and teeth are frequently found with gnawing and digestion marks32,33.
Faunal patterns and palaeoclimate
Recent work on building chronologies for the site using radiocarbon and optical dating26,29 has enabled the three chambers of Denisova Cave to be securely dated and cross-correlated. As a result, stratigraphic contexts from separate sections of the site can now be attributed to specific MIS stages and compared to one another. We identify several trends in the ZooMS-IDed component with reference to depositional age and the environmental conditions prevailing at the time. We note, for instance, that despite the wide variety of taxa the Cervidae/Gazella/Saiga ZooMS identification encompasses, they remain in relatively low abundance throughout the majority of the Pleistocene, except for the earliest layers which correspond to MIS 9–7 (337–191 ka). The majority of bones from this phase were excavated in layer 15 of the East Chamber which is attributed to MIS 7 and the Penultimate Interglacial, and coincides with the arrival of the first Denisovans to the site27,35,50. Layers 15 and 14 of the East Chamber have the highest abundance of stone tools of any other phase at Denisova Cave and a large proportion of humanly modified bone27,35. The morphological assemblage from these layers is dominated by the remains of Siberian roe deer (19%) and red deer (10%) that live in forest and grasslands29,31. Cervids were clearly favoured by these early Denisovans, an observation which remains in stark contrast to the faunal record of all later phases documented at the site.
Bison/Yak are the most abundant fauna for almost all subsequent (post-MIS 7) layers (Fig. 3; Supplementary Tables 1, 2, and 3). Steppe bison and Baikal yak are known to have favoured more open, steppe and forest-steppe environments31,55. Despite the expansion of forests during interglacial periods however, their remains continue to dominate the ZooMS-IDed component at 39% (MIS 3) and 31% (MIS 5) (Fig. 3; Supplementary Tables 1, 2, and 3). This suggests that rather than the environment being a driving force behind the abundance of fragmented Bos and Bison bones, predators, likely hominins, were preferentially targeting them, mirroring a trend identified in other Pleistocene assemblages analysed with ZooMS22.
With regards to predators in the ZooMS-IDed component, Canidae are the dominant group for the majority of the Pleistocene layers we studied. From MIS 9–4 their bones account for approximately 5% of the identified remains in our study. They are found alongside smaller numbers of other predators, including Felidae and V. vulpes. Crocuta/Panthera remains are first identified in the ZooMS-IDed component in layer 15 and they have been macroscopically identified in the morphological assemblage in layers covering the entire Pleistocene. The majority of these bones are probably cave hyaena, however, smaller numbers of snow leopard and Eurasian cave lion are also present throughout the stratigraphy29–34. Crocuta/Panthera become more common than Canidae during MIS 5–3, alongside archaeological evidence of intensive carnivore presence during the same period, particularly in the South Chamber (Fig. 3; Supplementary Tables 1, 2, and 3). Bear remains are identified throughout the Pleistocene layers of Denisova Cave both in the ZooMS-IDed component and the morphological assemblage. The extinct cave bears, U. savini, were mostly vegetarian, and used the cave for hibernation32,33.
Animal size and impact on ZooMS-based fauna patterns
Body mass and size class, assigned based on previously published data56, probably play a disproportionate role in the number of taxa identified using ZooMS. For instance, Elephantidae, Bison/Yak, and Rhinocerotidae remains, all within body class size 6, are 3–5 times more abundant in the ZooMS-IDed component than the morphological dataset (Fig. 2). Bison/Yak remains are the most sensitive to this shift, accounting for only 6.3% of the morphological assemblage29–34 as opposed to 31% of the ZooMS-IDed component. A normalisation of the dataset based on body mass is not easy since it requires a high degree of confidence on other information, such as age and size of the targeted individuals, and carcass processing practices (e.g. transfer of specific body parts to the cave), which are unknown and/or fluctuate through time in the case of Denisova Cave.
In the case of mammoths, which were likely present in the Altai region in very small numbers and are not considered to have been a major contributor to hominin diet57, their bones are three times more abundant in the ZooMS identified megafauna (3.84% versus 1.08%). The only exception to this is the ratio of horse bones, for which no significant change in the percentage of their remains exists between the zooarchaeological dataset29–34 and the ZooMS-IDed component. With these factors in mind, any tentative predictive model for the abundance of large herbivores within a fragmented bone assemblage should consider both body class size and the likelihood that some taxa targeted by hominins were processed both in situ and at the cave site.
Conclusions
The application of ZooMS at Denisova Cave highlights the potential of the method to elucidate early hunting practices and adaptation to new environments. The new data expands our understanding of the site’s faunal record and taphonomy, and the high success rate is illustrative of the potential of the method to other sites and regions with comparable biomolecular preservation and to material of the same or even greater antiquity, extending further back into the Middle Pleistocene.
Aside from its effectiveness in screening large numbers of bones for the identification of specific taxa, e.g. hominin fossils, ZooMS is complementary to traditional zooarchaeological practices as it allows a large part of the morphologically non-identifiable component to be diagnosed taxonomically. This is particularly true at Denisova Cave where traditional zooarchaeological analyses identified less than 5% of the excavated fauna versus ~ 80% identification success using ZooMS in this study.
The new data is also useful for elucidating the taphonomic history of the bones recovered from a site. In our case, this is shown by the differences in prey-predator ratios in the ZooMS-IDed versus the morphologically-identified assemblages. These differences reveal that the main influence in the fragmentation of herbivores/prey bones at Denisova was two-fold and operated at different levels. Hominin processing of carcasses was the main factor for the introduction, deposition and initial fragmentation of many of the herbivore and some of the carnivore bones at the site. This was followed by secondary and more extreme fragmentation, of both animal and human bones, as a result of predator scavenging and gnawing.
Methods
Analysis was carried out at the ZooMS facility of the Department of Archaeology at the Max Planck Institute for the Science of Human History (MPI-SHH), Jena, Germany and the Manchester Institute of Biotechnology at the University of Manchester, UK. Samples analysed at the MPI-SHH followed published protocols using ammonium bicarbonate as the means of collagen extraction after which failed samples were re-attempted following acid soluble protocols23,58–61. Samples analysed at the University of Manchester followed published acid soluble protocols60,61. These protocols have all been optimised for the analysis of COL1 for taxonomic identification using ZooMS despite differences in initial protein extraction. The resulting spectra were screened for diagnostic markers using flexAnalysis 3.4 (Bruker Daltonics) and mMass software62. The spectra were compared against a reference library of known peptide markers21,23,63 which was informed further by previous zooarchaeological analysis carried out at Denisova Cave29–34.
Samples were analysed following established protocols. The ammonium bicarbonate buffer protocol23,58,59 involved sample rinsing in ammonium bicarbonate overnight and incubation for 1 h at 65 ℃. The supernatant was treated with 0.4 µg trypsin (Thermo Scientific Pierce ™ Trypsin Protease) and allowed to digest at 37 ℃ for 18 h. The incubated samples were concentrated and desalted using C18 ZipTips (Thermo Scientific Pierce™ C18 Tips) and eluted in a final solution of 50 µl of 50% acetonitrile and 0.1% TFA. 0.5 µl of the resulting solution was mixed with 0.5 µl of α cyano-4-hydroxycinnamic acid solution (10 mg/mL in 50% acetonitrile [ACN] and 0.1% trifluoroacetic acid [TFA]) and allowed to crystallise. The samples were analysed using a Bruker Autoflex Speed LRF MALDI ToF/ToF mass spectrometer.
Samples analysed using acid soluble protocols25,60 were demineralised in 0.6 M hydrochloric acid (HCl) for 18 h. The supernatant was removed into 30 kDa molecular weight cut-off (MWCO) ultrafilters and centrifuged at 3700 rpm for 1 h. The filtrate was then twice washed through with 500 μL of 50 mM ammonium bicarbonate (AmBic) and further centrifuged at 3700 rpm for half an hour after each treatment. The final residue was resuspended with additional AmBic (200 μL), half of which was removed to create a backup sample set before digestion. The remaining 100 μL was then treated with 0.2 μg trypsin (sequencing grade; Promega UK) and incubated at 37 °C for 18 h. The resulting solution was then diluted to 1:10 using TFA and mixed with a matrix solution of 1 μl of α-cyano-4-hydroxycinnamic acid solution (10 mg/mL in 50% ACN/0.1% TFA), allowed to crystallise and analysed using a Bruker Ultraflex II MALDI ToF/ToF mass spectrometer. For samples identified as ovicaprids, the tryptic peptide solution was further purified and fractionated using C18 Ziptips into 10% ACN and 50% ACN fractions (both in 0.1% TFA) and further analysed using MALDI as described above.
Supplementary Information
Acknowledgements
We would like to thank the European Research Council, the Max Planck Society, the Oxford Radiocarbon Accelerator Unit (ORAU), the University of Manchester and Manchester Institute of Biotechnology, and the Institute of Archeology and Ethnography, Russian Academy of Sciences Siberian Branch for their ongoing support. We would like to thank our volunteers (Miriam Jenkins, Esther Gillespie, Lauren Bell, Marine Caldarola, Raija Heikkila, Laura Doody, Saltanat Amirova, Geoff Church, Lucy Koster, Rachael Holmes, Luke Ghent, Phoebe Ewles-Bergeron, Nicholas Siemens, Marion Sandilands, and Julianna Zavodski) who helped sample the material as well as Sandra Heberstreit, Jana Zech, and Kristine Richter for discussions and help in the laboratory.
Author contributions
S.B., N.W., A.O., D.C., B.J.S., V.L.H., M.B. performed the laboratory work; S.B., N.W., A.O., B.J.S., V.L.H., M.P.C. analyzed the data; S.B.. created the figures; M.K., M.S., A.D. provided the samples and site-specific expertise; K.D. and T.H. designed the study; S.B. and K.D. wrote the manuscript with the assistance and input of all authors.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work has received funding from the ERC under the European Union’s Horizon 2020 Research and Innovation Programme, grant agreement no. 715069 (FINDER) to KD and under the European Union’s Seventh Framework Programme (FP7/2007–2013), grant agreement no. 324139 (PalaeoChron) to TH. This project was supported further through the Royal Society and research fellowship funding to MB (UF120473) and the archaeological field studies were funded by the Russian Foundation for Basic Research (no. 18-09-40100 and 20-29-01011). Many thanks also go to the University of Manchester for the Dean’s Award Scholarship funding to VLH.
Data availability
All ZooMS spectra for identified samples are available on Mendeley Data and are located in two databases. External Database 1: http://dx.doi.org/10.17632/5bwmbhs3fs.1. External Database 2: http://dx.doi.org/10.17632/bgm2k6gt3j.1.
Competing interests
The authors declare no competing interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Samantha Brown, Email: brown@shh.mpg.de.
Katerina Douka, Email: douka@shh.mpg.de.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-94731-2.
References
- 1.Brain C. K. The Hunters Or the Hunted?: An Introduction to African Cave Taphonomy. Chicago: University of Chicago Press; 1983. [Google Scholar]
- 2.Villa P, Mahieu E. Breakage patterns of human long bones. J. Hum. Evol. 1991;21:27–48. doi: 10.1016/0047-2484(91)90034-S. [DOI] [Google Scholar]
- 3.Rogers AR. On equifinality in faunal analysis. Am. Antiq. 2000;65:709–723. doi: 10.2307/2694423. [DOI] [Google Scholar]
- 4.Lyman R. L. Vertebrate Taphonomy. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- 5.Madgwick R, Mulville J. Reconstructing depositional histories through bone taphonomy: Extending the potential of faunal data. J. Archaeol. Sci. 2015;53:255–263. doi: 10.1016/j.jas.2014.10.015. [DOI] [Google Scholar]
- 6.Bonnichsen R. Pleistocene Bone Technology in the Beringian Refugium. Ottawa: University of Ottawa Press; 1979. [Google Scholar]
- 7.Bartram Jr E. L ., Marean C. W. Explaining the ‘Klasies Pattern’: Kua ethnoarchaeology, the die Kelders middle stone age archaeofauna, long bone fragmentation and carnivore ravaging. J. Archaeol. Sci. 1999;26:9–29. doi: 10.1006/jasc.1998.0291. [DOI] [Google Scholar]
- 8.Stiner M. C. Honor Among Thieves a Zooarchaeological Study of Neandertal Ecology. Princeton University Press; 1994. [Google Scholar]
- 9.Pokines JT, et al. The effects of experimental freeze-thaw cycles to bone as a component of subaerial weathering. J. Archaeol. Sci. Rep. 2016;6:594–602. [Google Scholar]
- 10.Behrensmeyer AK. Taphonomic and ecologic information from bone weathering. Paleobiology. 1978;4:150–162. doi: 10.1017/S0094837300005820. [DOI] [Google Scholar]
- 11.Madgwick R, Mulville J. Investigating variation in the prevalence of weathering in faunal assemblages in the UK: A multivariate statistical approach: Investigating variation in weathering in UK faunal assemblages. Int. J. Osteoarchaeol. 2012;22:509–522. doi: 10.1002/oa.1274. [DOI] [Google Scholar]
- 12.Marean CW. Measuring the post-depositional destruction of bone in archaeological assemblages. J. Archaeol. Sci. 1991;18:677–694. doi: 10.1016/0305-4403(91)90029-O. [DOI] [Google Scholar]
- 13.Outram AK. A new approach to identifying bone marrow and grease exploitation: Why the ‘indeterminate’ fragments should not be ignored. J. Archaeol. Sci. 2001;28:401–410. doi: 10.1006/jasc.2000.0619. [DOI] [Google Scholar]
- 14.Morin E, Soulier M-C. New criteria for the archaeological identification of bone grease processing. Am. Antiq. 2017;82:96–122. doi: 10.1017/aaq.2016.16. [DOI] [Google Scholar]
- 15.Théry-Parisot, I., Costamagno, S., Brugal, J.-P., Fosse, P. & Guilbert, R. The use of bone as fuel during the Palaeolithic, experimental study of bone combustible properties. Archaeol. Milk Fats 50–59 (2005).
- 16.Bovy KM, Etnier MA, Butler VL, Campbell SK, Shaw JD. Using bone fragmentation records to investigate coastal human ecodynamics: A case study from Čḯxwicən (Washington State, USA) J. Archaeol. Sci. Rep. 2019;23:1168–1186. doi: 10.1038/s41598-018-37308-w. [DOI] [Google Scholar]
- 17.Gummesson S, Molin F, Sjöström A. The spatial organization of bone crafting during the middle and late Mesolithic at Ringsjöholm and Strandvägen in Sweden. J. Field Archaeol. 2019;44:165–179. doi: 10.1080/00934690.2019.1580093. [DOI] [Google Scholar]
- 18.Smith AB, Poggenpoel C. The technology of bone tool fabrication in the south-western Cape, South Africa. World Archaeol. 1988;20:103–115. doi: 10.1080/00438243.1988.9980059. [DOI] [Google Scholar]
- 19.Morin E, Ready E, Boileau A, Beauval C, Coumont M-P. Problems of identification and quantification in archaeozoological analysis, Part I: Insights from a blind test. J. Archaeol. Method Theory. 2017;24:886–937. doi: 10.1007/s10816-016-9300-4. [DOI] [Google Scholar]
- 20.Morin E, Ready E, Boileau A, Beauval C, Coumont M-P. Problems of identification and quantification in archaeozoological analysis, Part II: Presentation of an alternative counting method. J. Archaeol. Method Theory. 2017;24:938–973. doi: 10.1007/s10816-016-9301-3. [DOI] [Google Scholar]
- 21.Buckley M, Collins M, Thomas-Oates J, Wilson JC. Species identification by analysis of bone collagen using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2009;23:3843–3854. doi: 10.1002/rcm.4316. [DOI] [PubMed] [Google Scholar]
- 22.Sinet-Mathiot V, et al. Combining ZooMS and zooarchaeology to study Late Pleistocene hominin behaviour at Fumane (Italy) Sci. Rep. 2019;9:12350. doi: 10.1038/s41598-019-48706-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welker F, et al. Palaeoproteomic evidence identifies archaic hominins associated with the Châtelperronian at the Grotte du Renne. Proc. Natl. Acad. Sci. USA. 2016;113:11162–11167. doi: 10.1073/pnas.1605834113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prüfer K, et al. A high-coverage Neandertal genome from Vindija Cave in Croatia. Science. 2017;358:655–658. doi: 10.1126/science.aao1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown S, et al. Identification of a new hominin bone from Denisova Cave, Siberia using collagen fingerprinting and mitochondrial DNA analysis. Sci. Rep. 2016;6:23559. doi: 10.1038/srep23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douka K, et al. Age estimates for hominin fossils and the onset of the Upper Palaeolithic at Denisova Cave. Nature. 2019;565:640–644. doi: 10.1038/s41586-018-0870-z. [DOI] [PubMed] [Google Scholar]
- 27.Brown, S. et al. Earliest evidence for Denisovans identified using peptide mass fingerprinting and mitochondrial DNA analysis. Nat. Eco. Evol.(submitted).
- 28.Slon V, et al. The genome of the offspring of a Neanderthal mother and a Denisovan father. Nature. 2018;561:113–116. doi: 10.1038/s41586-018-0455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs Z, et al. Timing of archaic hominin occupation of Denisova Cave in southern Siberia. Nature. 2019;565:594–599. doi: 10.1038/s41586-018-0843-2. [DOI] [PubMed] [Google Scholar]
- 30.Vasiliev S.K., Shunkov M.V., Kozlikin M.B. Megafaunal remains from the eastern chamber of Denisova Cave and problems of reconstructing the Pleistocene environments in the Northwestern Altai. in Problems of Archaeology, Ethnography, Anthropology of Siberia and Neighboring Territories Vol. XXIII (2017).
- 31.Vasiliev SK, Shunkov MV, Kozlikin MB. Preliminary results for the balance of megafauna from pleistocene layers of the east gallery, Denisova Cave. Probl. Archaeol. Ethnogr. Anthropol. Siberia Adjacent Territories. 2013;19:32–38. [Google Scholar]
- 32.Vasiliev S. K, Shunkov M. V. Large Pleistocene mammals in the southern gallery of Denisova Cave. Probl. Archaeol. Ethnogr. Anthropol. Siberia Neighboring Territories. 2009;25:63–69. [Google Scholar]
- 33.Vasiliev, S. K., Kozlikin, M. B. & Shunkov, M. V. Megafaunal remains from the upper portion of Pleistocene deposits in south chamber of Denisova Cave. Probl. Archaeol. Ethnogr. Anthropol. Siberia Neighboring Territories569 (2018).
- 34.Agadjanian AK, Serdyuk NV. The history of mammalian communities and paleogeography of the Altai Mountains in the Paleolithic. Paleontol. J. 2005;39:645–821. [Google Scholar]
- 35.Shunkov MV, Kozlikin MB, Derevianko AP. Dynamics of the Altai Paleolithic industries in the archaeological record of Denisova Cave. Quat. Int. 2020 doi: 10.1016/j.quaint.2020.02.017. [DOI] [Google Scholar]
- 36.Morley MW, et al. Hominin and animal activities in the microstratigraphic record from Denisova Cave (Altai Mountains, Russia) Sci. Rep. 2019;9:13785. doi: 10.1038/s41598-019-49930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckley M, et al. Species identification of archaeological marine mammals using collagen fingerprinting. J. Archaeol. Sci. 2014;41:631–641. doi: 10.1016/j.jas.2013.08.021. [DOI] [Google Scholar]
- 38.Prüfer K, et al. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature. 2014;505:43–49. doi: 10.1038/nature12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krause J, et al. A complete mtDNA genome of an early modern human from Kostenki, Russia. Curr. Biol. 2010;20:231–236. doi: 10.1016/j.cub.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 40.Reich D, et al. Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature. 2010;468:1053–1060. doi: 10.1038/nature09710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mednikova MB. A proximal pedal phalanx of a Paleolithic hominin from denisova cave, Altai. Archaeol. Ethnol. Anthropol. Eurasia. 2011;39:129–138. doi: 10.1016/j.aeae.2011.06.017. [DOI] [Google Scholar]
- 42.Bolikhovskaya NS, Shunkov MV. Pleistocene environments of northwestern Altai: Vegetation and climate1. Archaeol. Ethnol. Anthropol. Eurasia. 2014;42:2–17. doi: 10.1016/j.aeae.2015.01.001. [DOI] [Google Scholar]
- 43.Baryshnikov, G. Large mammals and Neanderthal paleoecology in the Altai Mountains (Central Asia, Russia). Préhist. Eur. (1999).
- 44.Derevianko A. P, et al. Paleoenvironment and Paleolithic Human Occupation of Gorny Altai: Subsistence and Adaptation in the Vicinity of Denisova Cave. SB RAS Press; 2003. [Google Scholar]
- 45.Brown, S., Douka, K., Collins, M. & Richter, K. K. On the standardization of ZooMS nomenclature. J. Proteom. 104041 (2021). [DOI] [PubMed]
- 46.Buckley M, et al. Distinguishing between archaeological sheep and goat bones using a single collagen peptide. J. Archaeol. Sci. 2010;37:13–20. doi: 10.1016/j.jas.2009.08.020. [DOI] [Google Scholar]
- 47.Buckley M, Harvey VL, Chamberlain AT. Species identification and decay assessment of Late Pleistocene fragmentary vertebrate remains from Pin Hole Cave (Creswell Crags, UK) using collagen fingerprinting. Boreas. 2017;46:402–411. doi: 10.1111/bor.12225. [DOI] [Google Scholar]
- 48.Rybczynski N, et al. Mid-Pliocene warm-period deposits in the High Arctic yield insight into camel evolution. Nat. Commun. 2013;4:1550. doi: 10.1038/ncomms2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shunkov, M. V., Kulik, N. A., Kozlikin, M. B. & Sokol, E. V. The phosphates of Pleistocene–Holocene sediments of the Eastern Gallery of Denisova Cave. Dokl. Earth Sci. (2018).
- 50.Slon V, et al. Neandertal and Denisovan DNA from Pleistocene sediments. Science. 2017;356:605–608. doi: 10.1126/science.aam9695. [DOI] [PubMed] [Google Scholar]
- 51.Derevianko, A. P. & Shunkov, M. V. Formation of the Upper Paleolithic traditions in the Altai. Archaeol. Ethnol. Anthropol. Eurasia 12–40 (2004).
- 52.Vasiliev SA. Faunal exploitation, subsistence practices and Pleistocene extinctions in Paleolithic Siberia. Deinsea. 2003;9:513–556. [Google Scholar]
- 53.Vereshchagin NK, Kuzmina IE. Remains of mammals from the Palaeolithic sites on the Don and upper Desna. Trudy Zool. Inst. AN SSSR. 1977;72:77–110. [Google Scholar]
- 54.Borgia V. Hunting high and low: Gravettian hunting weapons from Southern Italy to the Russian Plain. Open Archaeol. 2017;3:376–391. doi: 10.1515/opar-2017-0024. [DOI] [Google Scholar]
- 55.Vasiliev S. K. Late Pleistocene Bison (Bison p. priscus Bojanis, 1827) from the southeastern part of Western Siberia. Archaeol., Ethnol. Anthropol. Eurasia. 2008;34:34–56. doi: 10.1016/j.aeae.2008.07.004. [DOI] [Google Scholar]
- 56.Smith FA, et al. Body mass of late Quaternary mammals. Ecology. 2003;84:3402. doi: 10.1890/02-9002. [DOI] [Google Scholar]
- 57.Agadjanian AK, Shunkov MV. Late Pleistocene mammals of the Northwestern Altai: Report 2. Charysh Basin. Paleontol. J. 2018;52:1461–1472. doi: 10.1134/S0031030118120055. [DOI] [Google Scholar]
- 58.van Doorn NL, Hollund H, Collins MJ. A novel and non-destructive approach for ZooMS analysis: Ammonium bicarbonate buffer extraction. Archaeol. Anthropol. Sci. 2011;3:281. doi: 10.1007/s12520-011-0067-y. [DOI] [Google Scholar]
- 59.Brown, S. et al.Zooarchaeology by Mass Spectrometry (ZooMS) for Bone Material—AmBiC Protocol v1 (protocols.io.bffdjji6) (2020).
- 60.van der Sluis LG, et al. Combining histology, stable isotope analysis and ZooMS collagen fingerprinting to investigate the taphonomic history and dietary behaviour of extinct giant tortoises from the Mare aux Songes deposit on Mauritius. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014;416:80–91. doi: 10.1016/j.palaeo.2014.06.003. [DOI] [Google Scholar]
- 61.Brown, S. et al.Zooarchaeology by Mass Spectrometry (ZooMS) for Bone Material—Acid Soluble Protocol. (protocols.io.bf5bjq2n). (2020)
- 62.Strohalm M, Hassman M, Kosata B, Kodícek M. mMass data miner: An open source alternative for mass spectrometric data analysis. Rapid Commun. Mass Spectrom. 2008;22:905–908. doi: 10.1002/rcm.3444. [DOI] [PubMed] [Google Scholar]
- 63.Buckley M, Kansa SW. Collagen fingerprinting of archaeological bone and teeth remains from Domuztepe, South Eastern Turkey. Archaeol. Anthropol. Sci. 2011;3:271–280. doi: 10.1007/s12520-011-0066-z. [DOI] [Google Scholar]
- 64.Lisiecki, L. E. & Raymo, M. E. A Pliocene–Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography20 (2005).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All ZooMS spectra for identified samples are available on Mendeley Data and are located in two databases. External Database 1: http://dx.doi.org/10.17632/5bwmbhs3fs.1. External Database 2: http://dx.doi.org/10.17632/bgm2k6gt3j.1.