Abstract
Dysregulated B-cell activation plays pivotal roles in systemic lupus erythematosus (SLE), which makes B-cell depletion a potential strategy for SLE treatment. The clinical success of anti-CD19 CAR-T cells in treating B-cell malignancies has attracted the attention of researchers. In this study, we aimed to investigate the feasibility of applying anti-CD19 CAR-T cell therapy to SLE treatment in a mouse disease model. We constructed murine anti-CD19 CARs with either CD28 or 4-1BB as the intracellular costimulatory motif and evaluated the therapeutic function of the corresponding CAR-T cells by infusing them into MRL-lpr mice. Furthermore, anti-CD19 CAR-T cells were transferred to MRL-lpr mice before the onset of disease to determine their role in SLE prevention. According to our observations, compared with antibody treatment, the adoptive transfer of our anti-CD19 CAR-T cells showed a more sustained B-cell-depletion effect in MRL-lpr mice. The transfer of syngeneic anti-CD19 CAR-T cells not only prevented disease pathogenesis before the onset of disease symptoms but also displayed therapeutic benefits at a later stage after disease progression. We also tried to optimize the treatment strategy and found that compared with CAR-T cells with the CD28 costimulatory motif, CAR-T cells with the 4-1BB costimulatory motif showed better therapeutic efficiency without cell enrichment. Taken together, these results show that anti-CD19 CAR-T cell therapy was effective in the prevention and treatment of a murine model of SLE, indicating its potential for clinical use in patients.
Keywords: Systemic lupus erythematosus, Autoimmune disease, Treatment, T cells, B cells
Subject terms: Immunotherapy, Autoimmunity
Introduction
Systemic lupus erythematosus (SLE) is a complex and heterogeneous autoimmune disease characterized by a relapsing course of flares, alternating periods of remission and highly varied clinical manifestations apparent in multiple organs.1 The pathogenesis of SLE relies on the complex interplay between genetic, endogenous, and environmental factors.2 Hyperactivation of autoreactive B cells is the key process in SLE pathogenesis and induces plasma cells to produce large amounts of autoantibodies that subsequently circulate and form immune complexes with encountered self-antigens and complement. As these immune complexes may then be deposited in small vessels or distal sites, they can eventually cause organ destruction or dysfunction.
Current treatments for SLE mainly depend on the use of nonsteroidal anti-inflammatory drugs, antimalarial drugs, glucocorticoids, and immunosuppression for severe symptoms with organ dysfunction.3 As B cells play a central role in the pathogenesis of SLE, many B-cell-directed immunotherapies have been recently developed. However, these therapies have shown only modest success in the subgroup of SLE patients with serologically active disease. The anti-BAFF (B-cell-activating factor) agent belimumab, which was the first targeted biological treatment for SLE, has obtained approval from the Food and Drug Administration and the European Medicines Agency but can only partially deplete naive B cells. In contrast, two other BAFF-blocking agents, tabalumab and blisibimod, showed negative results in clinical trials for SLE treatment.4,5 Rituximab, an antibody against CD20, can deplete B cells more efficiently, but response rates in SLE patients vary widely between studies. Disease relapse after treatment with rituximab remains a problem.6–9
CD19 expression is maintained at a high level throughout all stages of B-cell differentiation. Thus, it is considered a good target to achieve more efficient and long-lasting therapeutic responses in SLE patients.10,11 The transfer of autologous T cells expressing anti-CD19 chimeric antigen receptors has achieved complete and sustained B-cell depletion in the treatment of B-cell lymphoblastic leukemia patients and was more effective than antibody therapies that target CD19.
Until recently, the feasibility of CAR-T cell transfer as a therapy for autoimmune disease had been tested in only very limited circumstances. Jyothi et al. used receptor-modified T cells expressing a heterodimeric chimeric receptor containing autoantigenic peptide-MHCII and CD3ζ to kill autoreactive T cells and therefore prevent or treat experimental autoimmune encephalomyelitis.12 Similarly, Ellebrecht et al. designed an autoantigen-based chimeric receptor that can direct T cells to eliminate autoreactive B cells in autoimmune pemphigus vulgaris.13 Furthermore, Zhang et al. delayed or prevented disease in a NOD mouse model of type 1 diabetes by generating NOD MHCII-specific CAR-T cells.14 In addition, CAR-Treg cells have shown therapeutic efficacy in treating several different autoimmune disorders.15–17 We therefore asked whether anti-CD19 CAR-T cells can be used to deplete pathogenic B cells after SLE disease progression and aimed to understand their potential utility as a therapy for this autoimmune disease. Recently, Kansal et al. showed the effectiveness of anti-CD19 CAR-T cells in B-cell depletion in murine lupus models, as well as the prevention of disease progression when they were transferred before the onset of the disease; however, their therapeutic efficacy for an ongoing disease is still unknown.18
In our experiment, we infused syngeneic anti-mouse CD19 CAR-T cells into MRL-lpr mice, a spontaneous murine SLE disease model with severe lupus nephritis. Both before and after the onset of the disease, the transfer of anti-CD19 CAR-T cells effectively depleted B cells in vivo with persistent capacity, prolonged the life span of the mice, improved the symptoms of lupus, and delayed the progress of pathogenesis. In addition, we tested different therapeutic strategies and compared the therapeutic effects of CAR-T cells bearing different second-generation CARs with CD28 or 4-1BB as the costimulatory motif. In summary, we have addressed a novel approach for SLE treatment and provided important information regarding potential improvements, including the use of different CAR designs, which may be vital for transferring this protocol to clinical studies in human patients.
Results
Construction of mouse anti-CD19 CAR-T cells and in vitro cytotoxic assay
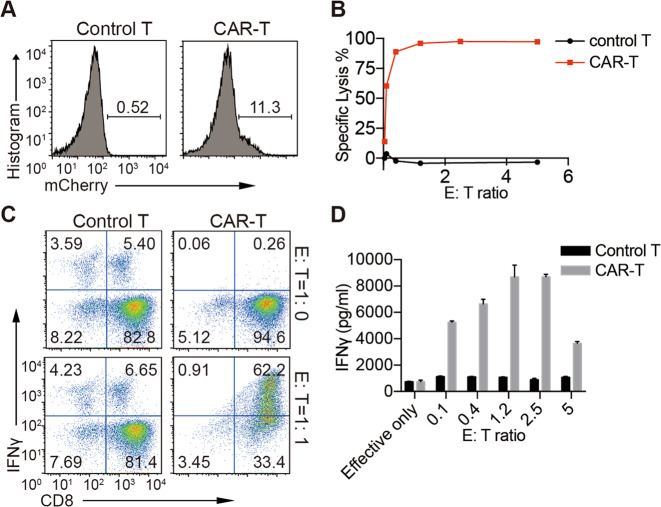
We constructed a murine anti-mouse-CD19 CAR-P2A-mCherry lentiviral expression vector (Supplementary Fig. 1A) consistent with the design of a second-generation 1D3 anti-mouse CD19 CAR.19 This CAR consisted of the extracellular 1D3 single-chain variable fragment, a portion of the murine CD28 transmembrane and cytoplasmic region, and the cytoplasmic region of murine CD3ζ with two inactivated ITAMs, which have been shown to decrease the exhaustion and apoptosis of T cells.19,20 After lentiviral infection, CAR expression, as indicated by mCherry expression, was detected in primary T cells from MRL-lpr mice and the 293FT cell line (Fig. 1a and Supplementary Fig. 1B). Live mCherry+ T cells were sorted by FACS and showed a purity of ~90% (Supplementary Fig. 1C). After the sorted cells were cultured for 3–5 days, their specific killing ability was tested (Fig. 1b–d). The CAR-T cells showed highly specific killing efficacy, and a killing efficacy close to 100% was reached at an E:T ratio of 1:1. FACS analysis and enzyme-linked immunosorbent assay (ELISA) revealed substantial IFNγ secretion by the CAR-T cells after their coculture with the target cells, and IFNγ production by CAR-T cells was specifically induced when CAR-T cells encountered their target cells.
Fig. 1.
CAR-T cell construction. a Flow cytometry showing mCherry-CAR expression on mouse primary T cells 2 days after transduction. b–d Effector cells, sorted CAR-T cells cultured for another 3–5 days; target cells, freshly sorted splenocytes labeled with CellTrace Violet. After coculture at the indicated ratios for 4 h, the percentage of lysed B cells (b) and levels of intracellular IFNγ expressed by effector cells (c) were analyzed by flow cytometry. d The supernatant IFNγ concentration after 24 h of coculture was analyzed by ELISA. The data are representative of at least two independent experiments with similar results
Mouse anti-CD19 CAR-T cells show efficient and sustained B-cell depletion in vivo
To test the therapeutic effect of CAR-T cells on SLE, sorted and functionally verified CAR-T cells were injected into MRL-lpr mice, a spontaneous SLE murine model.
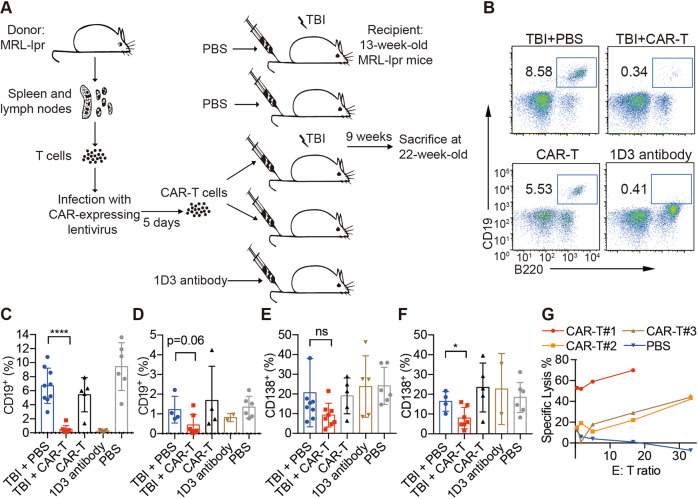
As indicated by both serum anti-dsDNA antibody and ANA levels, MRL-lpr mice had autoantibody levels similar to those of MRL-MpJ mice at the age of 8 weeks, and autoantibody levels were increased at the age of 13 weeks (Supplementary Fig. 4A, B). The therapeutic interventions were applied to 13-week-old MRL-lpr mice in which clear clinical symptoms had developed. The mice were randomly divided into five experimental groups (Fig. 2a), with the extra MRL-MpJ group used as the negative control group. We adapted the use of low-dose total body irradiation (TBI) as a preconditioning agent for CAR-T infusion, as several studies have suggested that TBI is required before CAR-T cell infusions for mice bearing leukemia.19,21 In this regard, a CAR-T cell-treated group without TBI was also included to check its necessity, and a non-TBI-treated PBS group was added to serve as the baseline. A 1D3 antibody treatment group was used as a positive control for short-term B-cell depletion.22 1D3 antibodies were injected twice a week for 2 weeks. The CAR-T groups received only one transfer.
Fig. 2.
Efficacy of CAR-T cells in an SLE mouse model. a Working scheme of CAR-T cell manufacture and adoptive transfer in a mouse experiment. The percentage of blood CD19+ cells at 1 week (b, c) or 9 weeks (d) after transfer was analyzed by flow cytometry; the percentage of blood CD138+ cells at 1 week (e) or 9 weeks (f) after transfer was analyzed by flow cytometry. Live CD45+ cells were gated and plotted (b–f). g Effector cells, lymphocytes isolated from TBI + CAR-T cell-treated mice 9 weeks after transfer; target cells, fresh splenocytes labeled with CellTrace Violet; the percentage of lysed B cells was analyzed by flow cytometry after 24 h of coculture. The data are representative of at least two independent experiments with similar results. The data were analyzed by Student’s t test, and significance is indicated by *P < 0.05, ****P < 0.0001, and ns no significant differences
The CD19+ B-cell depletion efficiency was then examined at two different time points after CAR-T cell treatment. One week after transfer, we found that anti-CD19 CAR-T adoptive transfer with TBI preconditioning (TBI + CAR-T) successfully eradicated almost all circulating CD19+ B cells in the blood, achieving results equivalent to those of antibody-mediated depletion. Interestingly, a substantial number of B cells remained among CAR-T cells in the treated group without TBI (Fig. 2b, c). Furthermore, the B-cell-depletion effect of CAR-T treatment clearly lasted longer than that of antibody treatment, since the number of B cells in the TBI + CAR-T group remained low at 9 weeks after transfer but was substantially increased in the antibody-treated group (Fig. 2d).
In addition, we observed a slight decrease in CD19− CD138+ plasma cells in the blood of MRL-lpr mice after 1 week of CAR-T cell treatment (with TBI) compared with that of mice that received TBI + PBS (Fig. 2e). The reduction in CD138+ cells was more pronounced after 9 weeks (Supplementary Fig. 3A and Fig. 2f), which may have resulted from the sustained low proportion of B cells. However, mice that received 1D3 antibody treatment did not demonstrate changes in the CD138+ cell percentage (Fig. 2f).
The lytic activity of lymphocytes in mice in the TBI + CAR-T group was detected at the age of 22 weeks, although this activity was subject to individual variation (Fig. 2g and Supplementary Fig. 2), indicating the long-lasting killing capacity of CAR-T cells after transfer.
Therapeutic effects of anti-CD19 CAR-T cells in a mouse SLE model
Generally, MRL-lpr mice died at an average age of 20 weeks, which is what we also observed in the nontreated control group (TBI + PBS group). However, all mice that received TBI + CAR-T cells survived the 22nd week (Fig. 3a). Mice that received CAR-T cells without TBI also showed improved survival rates. In contrast, mice that received 1D3 antibodies showed a survival rate similar to that of mice in the control group.
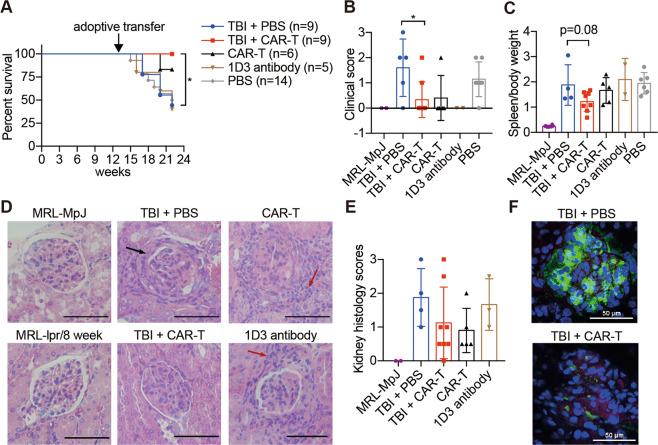
Fig. 3.
CAR-T cell treatment shows therapeutic benefits in mouse SLE. Mice received different treatments at 13 weeks of age and were sacrificed at 22 weeks of age for subsequent examinations. a The survival rate of MRL-lpr mice that received different treatments; the difference in survival between the TBI + PBS group and TBI + CAR-T group was compared by logrank (Mantel–Cox test) calculation. b The mouse skin lesions were scored, and the scores were plotted. c The ratios of spleen weight to total body weight were determined. d Representative hematoxylin and eosin (H&E)-stained sections of glomerular areas of the kidneys. Black arrow, immune complex formation; red arrow, infiltration of numerous lymphocytes. Original magnification, ×400. Bars represent 50 µm. e Statistical histology scores of H&E-stained kidney sections. f C3 and IgG deposition in the glomerulus was detected by immunofluorescence and is presented in a merged manner; frozen sections of the kidneys were stained with anti-mouse-C3-APC, anti-mouse-IgG-FITC, and DAPI. Original magnification, ×400. Bars represent 50 µm. The data are representative of at least two independent experiments with similar results. b, c The data were analyzed by Student’s t test, and significance is indicated by *P < 0.05
To evaluate the therapeutic effects compared with the nontreated survivors, we chose 22 weeks, during which we still found living mice in the control group, as the endpoint analysis time. Until 21 weeks of age, 80% of survived mice in the TBI + PBS group showed different degrees of skin ulceration, but only 22% of mice in the TBI + CAR-T group showed mild skin lesions (Fig. 3b). To evaluate lymphoproliferation and splenomegaly, spleen weight was examined at 22 weeks of age. A slightly reduced spleen weight was observed in mice in the TBI + CAR-T group (Fig. 3c).
Mice that received TBI + CAR-T cells showed mild glomerulonephritis, as indicated by the histological scores of hematoxylin and eosin (H&E)-stained kidney sections (Fig. 3d, e). Increased lymphocyte infiltration and crescent formation were observed in the kidneys of mice in the TBI + PBS group; however, no overall statistically significant difference in the histology scores was found due to the high variation between individuals. Less immune complex deposition was seen in the glomeruli of mice in the TBI + CAR-T group, but weak signals for IgG and C3 could still be detected (Fig. 3f).
We also checked the serum anti-dsDNA and antinuclear antibody concentrations, urine protein to creatinine ratio, and blood urea nitrogen to creatinine ratio. Surprisingly, no differences were found between the groups (Supplementary Fig. 3B–F). These results correspond with the variation in kidney histology score.
According to these data, although CAR-T cell treatment could effectively deplete B cells and prolong the life span of SLE-bearing mice, its therapeutic effect was not reflected in the results of biochemical tests. Our cohort observation found that the proportion of CD19+ B cells in the circulation was positively correlated with survival time in mice (Supplementary Fig. 3G), which explains why the biochemical test results of survivors in the TBI + PBS group and mice in the TBI + CAR-T group were similar. This finding also supports the feasibility of targeting CD19+ B cells as an SLE treatment and implies the requirements of further optimization to achieve better efficiency of CAR-T cell-mediated eradication of B cells in vivo.
Prevention of SLE by anti-CD19 CAR-T cell treatment
To test whether CAR-T cell transfer plays a role in SLE prevention, for which B cells could be removed at an earlier stage, we transferred CAR-T cells (with TBI preconditioning) to 8-week-old MRL-lpr mice before the onset of disease. A group treated with TBI and PBS was included as a control. Five weeks later, disease pathogenesis was evaluated. At the age of 13 weeks, only 11% of CAR-T cell-treated mice showed skin lesions, compared with 44% of PBS-treated mice (Supplementary Fig. 4C). In addition, mice in the PBS-treated group showed significantly increased proteinuria at 13 weeks, while the urine protein levels of the CAR-T cell-treated group remained the same as those of the control group (Supplementary Fig. 4D).
Until 22 weeks of age, the endpoint of our observation, mice that received CAR-T cells showed complete survival (Supplementary Fig. 4E). Furthermore, the symptoms of MRL-lpr mice from the CAR-T cell-treated group appeared to be ameliorated, unlike those in the PBS-treated mice. The circulating autoantibody levels at the age of 22 weeks indicated that the autoimmune response in the CAR-T cell-treated group was less strong than that in the control group (Supplementary Fig. 4F, G). Among the surviving mice, all individuals in the PBS control group showed varying degrees of skin lesions, yet only 11% of the mice that received CAR-T cell transfer had skin ulceration (Supplementary Fig. 4H). Milder proteinuria and a healthier glomerular structure were also observed in the treated mice (Supplementary Fig. 4I, J).
Taken together, these results show that anti-CD19 CAR-T cell treatment has the ability to prevent SLE effects in MRL-lpr mice. The onset of SLE pathogenesis was delayed, and the symptoms of the disease were ameliorated.
SLE treatment with anti-CD19 CAR-T cells bearing a 4-1BB costimulatory motif
CD28 and 4-1BB have been the most widely used costimulatory domains in CAR studies. However, the exact characteristics of second-generation CAR-T cells containing CD28 and 4-1BB have not yet been fully studied.
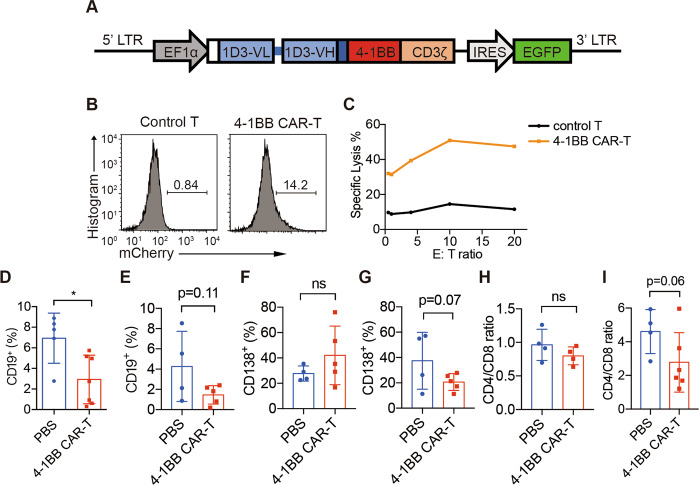
To compare the therapeutic effects of CD28 or 4-1BB as the costimulatory domain in our anti-CD19 CAR-T cells, we generated another CAR with a 4-1BB costimulatory motif (Fig. 4a). The 4-1BB CAR was expressed in MRL-lpr T cells, and 4-1BB CAR-T cells showed specific killing effects in vitro (Fig. 4b, c).
Fig. 4.
1D3-4-1BB CAR-T cells partially deplete B cells. a Diagram of the DNA encoding 1D3-4-1BB CAR. It encodes the antibody light chains (blue boxes), including their native signal peptides (white box), joined by a flexible linker (blue bar) to the VH and fused to the transmembrane region of CD8a (navy blue), signaling domains of 4-1BB (red box) and cytosolic domain of CD3ζ (orange box). The construct also contains IRES-driven EGFP to allow the detection of transduced cells. b Flow cytometry of GFP+ expression on mouse primary T cells after lentiviral transduction. Cells were FVD− TCRβ+ gated. c Unsorted 4-1BB CAR-T cells (effector) and CellTrace Violet-labeled splenocytes (target) were cocultured for 4 h and analyzed by flow cytometry. Blood was collected from the femoral arteries of MRL-lpr mice at 1 week (d, f) or 9 weeks (e, g) after transfer, and CD19+ and CD138+ percentages among FVD− CD45+ cells were analyzed by flow cytometry. CD4/CD8 ratios in the blood were also analyzed by flow cytometry at 1 week (h) or 9 weeks (i) after transfer. The data are representative of at least two independent experiments with similar results. The data were analyzed by Student’s t test, and significance is indicated by *P < 0.05
A similar experimental strategy was used for 4-1BB-bearing CAR-T cells as previously described (Fig. 2a). MRL-lpr mice at 13 weeks of age received 4-1BB CAR-T cells or PBS after low-dose TBI. To simplify the procedure, unsorted 4-1BB CAR-T cells with 12–30% CAR-GFP+ cells were used for infusion in this experiment. One week after CAR-T cell treatment, the percentage of circulating B cells decreased, but the percentage of plasma cells was maintained (Fig. 4d, f). Nine weeks later, the percentage of both CD19+ B cells and CD138+ plasma cells in the blood of mice that received 4-1BB CAR-T cells was decreased (Fig. 4e, g). Although the 4-1BB CAR-T cells were less enriched in this experiment, their long-term in vivo efficiency in depleting CD19+ B cells was similar to that of enriched CD28 CAR-T cells.
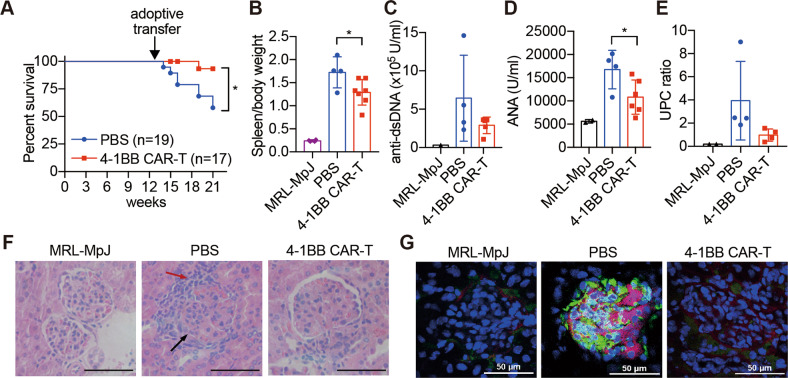
Until 22 weeks of age, mice infused with 4-1BB CAR-T cells showed an increased life span compared with the mice injected with PBS (Fig. 5a) and the regression of splenomegaly (Fig. 5b). The levels of autoantibodies were measured, and both anti-dsDNA antibodies and antinuclear antibodies in the serum were found to be decreased after 4-1BB CAR-T cell transfer (Fig. 5c, d). In addition, attenuated proteinuria levels indicated a therapeutic effect on nephritis (Fig. 5e). The results of kidney histology also showed less lymphocyte infiltration and less crescent deposition after the adoptive transfer of 4-1BB CAR-T cells (Fig. 5f). Granular immune complexes were reduced or absent in 4-1BB CAR-T cell-treated mice (Fig. 5g). Generally, MRL-lpr mice showed an increased CD4/CD8 ratio compared with that of SLE-free control mice, and we found that the transfer of 4-1BB CAR-T cells gradually reduced the CD4/CD8 ratio during disease development (Fig. 4h, i).
Fig. 5.
Therapeutic effects of 4-1BB CAR-T cell treatment. Mice that received low-dose TBI as preconditioning and adoptive transfer with 4-1BB CAR-T cells or PBS at 13 weeks of age were sacrificed at 22 weeks of age for subsequent examinations. a The survival rate of the mice; the difference in survival between the two groups was compared by logrank (Mantel–Cox test) calculation. b Total body weight and spleen weight. Sera were isolated from MRL-lpr mice; anti-dsDNA antibody (c) and ANA (d) levels were detected by ELISA. e Urine protein to creatinine ratios were analyzed by a chemistry analyzer. f Representative H&E-stained sections of glomerular areas of the kidneys. Black arrow, immune complex formation; red arrow, infiltration of numerous lymphocytes. Original magnification, ×400. Bars represent 50 µm. g C3 and IgG deposition in the glomerulus was detected by immunofluorescence and is presented in a merged manner; frozen sections of the kidney were stained with anti-mouse-C3-APC, anti-mouse-IgG-FITC, and DAPI. Original magnification, ×400. Bars represent 50 µm. The data are representative of at least two independent experiments with similar results. b, d The data were analyzed by Student’s t test, and significance is indicated by *P < 0.05
To examine whether 4-1BB CAR-T cell therapy has a more prolonged treatment effect on mouse diseases, we extended the observation period of the mice to 30 weeks (Supplementary Table 1). Although some of the mice treated with CAR-T cells died, their overall survival was better than that of the control group.
In addition, the CD19+ percentage after 4-1BB CAR-T cell treatment remained lower than that of the control mice until the mice were 30 weeks of age. These observations suggested the persistent efficacy of the treatment. However, again, the improvements in biochemical parameters were not as obvious. The autoantibody level and proteinuria after 4-1BB CAR-T cell treatment were similar to those in the PBS group.
In summary, through the use of both CD28 CAR-T and 4-1BB CAR-T cells, our study confirms the efficacy of anti-CD19 CAR-T cell transfer in the treatment of SLE in a mouse model. CAR-T cell treatment could effectively control the number of B cells, increase the survival of mice, and improve most clinical indications. This study can serve as a proof-of-principle study and suggests CAR-T cell transfer as a new option for clinical autoimmune disease treatment.
Discussion
In this study, we tested the possible use of anti-CD19 CAR-T cells to treat SLE in a mouse model. Our approach achieved sustained B-cell depletion in vivo and reduced disease symptoms, indicating the long-term efficacy of this therapeutic approach.
The efficacy of B-cell depletion and disease amelioration due to CAR-T cell transfer and the use of anti-CD19 antibodies were compared. Although anti-CD19 antibody 1D3 treatment of 13-week-old MRL-lpr mice achieved levels of B-cell depletion similar to those of anti-CD19 CAR-T cell transfer, it failed to improve the disease symptoms of SLE (Fig. 3a–e), indicating the advantage of CAR-T cell transfer over antibody usage for SLE treatment. Indeed, in a study of B lymphoma, CAR-T cells showed therapeutic efficacy superior to that of antibody therapy. The effect of CAR-T cells is generally believed to be more durable than that of antibody therapy, and our findings are consistent with this notion.
Plasma cells produce autoantibodies. Surprisingly, we observed only a slight reduction in CD19− CD138+ plasma cells as a consequence of CAR-T cell transfer, yet symptoms were nonetheless alleviated. One reason may be that anti-CD19 CAR-T cells do not directly target plasma cells but instead interrupt the accumulation of CD19− plasma cells; thus, it may take longer to observe changes in plasma cells. However, this then suggests that preventing the continued production of plasma cells is sufficient to alleviate the symptoms of the disease, which brings some hope for the successful targeting of CD19+ B cells in autoimmune diseases. If true, more significant effects could be achieved through the simultaneous targeting of both B cells and plasma cells, though this remains to be tested.23
Although TBI + CD28 CAR-T cell treatment led to a significant survival advantage, the improvements in clinical symptoms compared with those in survived mice in the PBS group were not as obvious. Since most of the mice in the control group did not survive to the endpoint of our analysis and because the survived mice exhibited a lower B-cell percentage in the first place (Supplementary Fig. 3F), the survived mice might have been the mice with relatively mild symptoms, reducing these differences. On the other hand, it suggests the necessity to optimize the treatment strategy to further improve its therapeutic efficacy.
Compared with an experimental strategy with CD28 CAR-T cells, the transfer of CAR-T cells bearing 4-1BB improved amelioration of lupus nephritis. The choice of costimulatory motif seems to be the main reason for the improved therapeutic effects. Several studies have discussed the efficacy and persistence of CD28 CAR and 4-1BB CAR in human T cells.24–28 CD28 CAR showed stronger signals than 4-1BB CAR, but 4-1BB CAR showed more persistent effects and lower exhaustion levels.24,25,27 Due to the persistent autoactivation of B cells in SLE, the long-term efficiency of CAR-T cells is essential in severe, refractory cases, which explains the increased therapeutic effects of 4-1BB CAR-T cells. However, experiments with more comparable conditions and a subtle CAR design need to be performed to further understand the underlying mechanism.
During the course of this work, Kansal et al. recently showed the effectiveness of anti-CD19 CAR-T cells for B-cell depletion in a murine lupus model, as well as the prevention of disease progression upon their transfer at an early period of the disease.18 Nevertheless, this study is not yet sufficient to support the use or effectiveness of SLE treatment for two reasons. First, clinical considerations of CAR-T cell therapies depend on clear disease symptoms; thus, disease prevention is not practical. Second, treating disease progression is much more difficult since the deletion of preexisting pathogenic plasma cells could not be achieved. Our study, however, shows that the transfer of syngeneic anti-CD19 CAR-T cells not only can prevent disease pathogenesis before the onset of disease symptoms but also has therapeutic benefits in disease treatment at a later stage after disease progression. Moreover, we have provided more information on potential improvements via different CAR designs, which may be vital for transferring this protocol to clinical studies in human patients.
As explained in the results, low-dose TBI is necessary as a preconditioning for CAR-T cell treatment. Not only can TBI benefit the homeostasis and expansion of transferred cells, but also high-dose or repeated TBI may reduce the number of circulating lymphocytes.29 In our case, we found no difference in the percentage of B cells, survival rate, or disease progression between the TBI + PBS group and the non-TBI + PBS group with the dose we were using (Figs. 2c–f and 3a–c).
Several problems remain to be solved before this treatment can be translated into clinical study. First, although it is acceptable for leukemia patients to receive lymphodepleting chemotherapy as preconditioning before CAR-T administration, the necessity of TBI in SLE treatment introduces a potential obstacle for its clinical use. Second, the disease indications of the application of CAR-T cell treatment for individuals with SLE need to be further discussed and determined. Finally, more tests on CARs of different designs, as well as different targeting strategies, need to be performed to identify whether better therapeutic effects for SLE can be achieved.
Materials and methods
Mice
MRL-lpr, MRL/MpJ, and C57BL/6 mice were purchased from the Shanghai Laboratory Animal Center, CAS, and bred at the Zhejiang University Laboratory Animal Center under specific pathogen-free conditions. The experimental protocols were approved by the Review Committee of the Zhejiang University School of Medicine and followed.
Construction of 4-1BB CARs
Total RNA was extracted from 1D3 hybridoma cells (ATCC), which secrete a rat monoclonal antibody that specifically reacts with mouse CD19 and cDNAs encoding the heavy and light chains amplified by SMARTer RACE 5′/3′ (Clontech).
The 4-1BB CAR components were as follows: the signal peptide of mCD8a, the variable region of the light chain and heavy chain of 1D3 antibody, the transmembrane region of mCD8a, and the cytoplasmic region of m4-1BB and mCD3ζ. The 4-1BB CAR sequence was ligated into a lentiviral backbone.
Primary CAR-T cell generation
293FT cells were cotransfected with the CAR-expressing lentiviral core vectors psPAX2 and pMD2.G at a ratio of 4:3:1 using the calcium phosphate transfection method. Virus-containing supernatants were collected 48–72 h after transfection. Supernatants were filtered through a 0.45 µm PES membrane before use.
For primary CAR-T cell generation, mouse T cells were isolated from the lymph nodes of MRL-lpr mice by negative selection (Stem Cell Technologies) and activated with plate-bound anti-CD3 (0.5 μg/mL) and anti-CD28 (1 μg/mL) for 36 h in lymphocyte culture medium (IMDM containing 10% [vol/vol] FBS, supplemented with penicillin and streptomycin, HEPES, sodium pyruvate 2-mercaptoethanol, and 50 U/mL IL-2). Cells were transferred to 24-well plates and spun-infected for 2 h at 1500 × g and 32 °C with 2 mL of viral supernatant in the presence of polybrene (8 μg/mL) and 10 mM HEPES. After spin infection, cells were resuspended at 1 × 106/mL in infection medium (50% lymphocyte culture medium, 50% viral supernatant with 8 μg/mL polybrene). Control T cells were processed with the same methods, but mock-transfected 293FT cell culture medium was used instead of viral supernatant. Twelve to sixteen hours later, the supernatant was discarded, and the cells were resuspended in fresh lymphocyte culture medium. TCRβ+ mCherry+ cells were sorted with a BD Aria II 2 days later. The cells were passaged at 1 × 106/mL every 2 days for 5 days before adoptive transfer.
Specific killing of CAR-T cells
After being sorted and cultured for 4 days, CAR-T cells or control T cells were cocultured with CellTrace Violet-labeled splenocytes in U-bottom 96-well plates for specific CAR-T killing tests. A total of 5 × 104 CAR-T cells or control T cells were added to each well as effector cells, and 1 × 106, 5 × 105, 1 × 105, 4 × 104, or 2 × 104 splenocytes were added to achieve E:T ratios of 0.1, 0.5, 1, 2.5, or 5, respectively. After 4 h of coculture, the cells were collected for CD19+ cell lysis and intracellular IFNγ expression analysis by FACS. After 24 h of coculture, the supernatant was collected for IFNγ secretion.
Flow cytometry analysis
The following monoclonal antibodies and reagents were used for surface staining: Fixable Viability Dye, CellTrace Violet, anti-CD19, anti-CD138, anti-B220, anti-TCRβ, anti-CD4, anti-CD8, anti-CD44, anti-CD62L, and anti-IFNγ. All antibodies were from eBioscience or BioLegend. Samples were run on an LSR Fortessa (BD) or NovoCyte (ACEA), and data were analyzed using FlowJo vX.0.7 software.
Adoptive transfer into MRL-lpr mice
For the indicated groups, 1.5 Gy TBI was applied on day 0. CAR-T cells were resuspended in PBS at 6.7 × 106/mL. The 1D3 antibody (BioXcell) was diluted to 833 μg/mL. The mice were injected with 150 μL of cells or antibody in PBS via the tail vein on day 1. For the antibody group, injections were repeated on days 4, 7, and 10.
Measurement of autoantibodies and cytokine levels
Standard methods were used for ELISA. The concentrations of serum anti-double-stranded DNA antibodies and antinuclear antibodies were measured by ELISA (Alpha Diagnostic International).
Analysis of renal histology and immune complex deposition
Mouse kidneys were harvested, fixed in 10% buffered formalin, and embedded in paraffin. Sections (4 μm) were stained with H&E. Pathologic changes in the kidneys were semiquantitatively scored on a scale of 0–3 (0 = no changes; 3 = severe cell proliferation/infiltration and crescent formation) as described previously.30
APC-conjugated anti-mouse complement C3 (Cedarlane) and FITC-conjugated anti-mouse total IgG (eBioscience) were used to examine immune complex deposition in the kidneys.
Skin lesion scoring
Macroscopic lupus erythematosus-like skin lesions and the spontaneous development of skin lesions were evaluated in MRL-lpr mice. Skin lesions were scored on a scale from 0 to 3 (0, none; 1, mild; 2, moderate (<2 cm); 3, severe (≥2 cm)) to assess changes in the nose, ears, and interscapular region.
Statistical analysis
Data are reported as the mean ± SD. Student’s unpaired t test was used to compare variables between two groups. All P values were 2-tailed. P values less than 0.05 indicated significance. GraphPad Prism 7 was used for statistical analyses.
Supplementary information
Acknowledgements
The authors thank Dr. Richard Sloan (University of Edinburgh) for manuscript editing, Yingying Huang from the core facilities (ZJU School of Medicine) for technical assistance in FACS analysis, and Huihui Su from the ZJU Affiliated Animal Hospital for technical assistance in the analysis of mouse samples. This work was supported by grants from the National Natural Science Foundation of China (31770954, 31530019 to L.L. and 31900628 to Q.X.) and the Fundamental Research Funds for the Central Universities (2018XZZX001-12 to L.L.).
Author contributions
X.J., C.P., C.L., L.X., and L.L. designed the research; X.J., Q.X., K.Z., and Y.J. performed the research; X.J. and L.L. analyzed the data; and X.J., Y.H., L.X., and L.L. wrote the paper.
Competing interests
The authors declare no competing interests.
Contributor Information
Yongmei Han, Email: 3408235@zju.edu.cn.
Linrong Lu, Email: lu_linrong@zju.edu.cn.
Supplementary information
The online version of this article (10.1038/s41423-020-0472-1) contains supplementary material.
References
- 1.Rahman A, Isenberg DA. Systemic lupus erythematosus. N. Engl. J. Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 2.Arbuckle MR, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 3.Xiong W, Lahita RG. Pragmatic approaches to therapy for systemic lupus erythematosus. Nat. Rev. Rheumatol. 2014;10:97–107. doi: 10.1038/nrrheum.2013.157. [DOI] [PubMed] [Google Scholar]
- 4.Isenberg D, et al. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial) Ann. Rheum. Dis. 2015;74:2006–2015. doi: 10.1136/annrheumdis-2013-205067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furie RA, et al. A phase 2, randomised, placebo-controlled clinical trial of blisibimod, an inhibitor of B cell activating factor, in patients with moderate-to-severe systemic lupus erythematosus, the PEARL-SC study. Ann. Rheum. Dis. 2015;74:1667–1675. doi: 10.1136/annrheumdis-2013-205144. [DOI] [PubMed] [Google Scholar]
- 6.Vital EM, et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum. 2011;63:3038–3047. doi: 10.1002/art.30466. [DOI] [PubMed] [Google Scholar]
- 7.Jonsdottir T, Sundelin B, Welin Henriksson E, van Vollenhoven RF, Gunnarsson I. Rituximab-treated membranous lupus nephritis: clinical outcome and effects on electron dense deposits. Ann. Rheum. Dis. 2011;70:1172–1173. doi: 10.1136/ard.2010.129288. [DOI] [PubMed] [Google Scholar]
- 8.Arce-Salinas CA, Rodriguez-Garcia F, Gomez-Vargas JI. Long-term efficacy of anti-CD20 antibodies in refractory lupus nephritis. Rheumatol. Int. 2012;32:1245–1249. doi: 10.1007/s00296-010-1755-0. [DOI] [PubMed] [Google Scholar]
- 9.Gregersen JW, Jayne DR. B-cell depletion in the treatment of lupus nephritis. Nat. Rev. Nephrol. 2012;8:505–514. doi: 10.1038/nrneph.2012.141. [DOI] [PubMed] [Google Scholar]
- 10.Odendahl M, et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J. Immunol. 2000;165:5970–5979. doi: 10.4049/jimmunol.165.10.5970. [DOI] [PubMed] [Google Scholar]
- 11.Mei HE, Schmidt S, Dorner T. Rationale of anti-CD19 immunotherapy: an option to target autoreactive plasma cells in autoimmunity. Arthritis Res. Ther. 2012;14(Suppl 5):S1. doi: 10.1186/ar3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jyothi MD, Flavell RA, Geiger TL. Targeting autoantigen-specific T cells and suppression of autoimmune encephalomyelitis with receptor-modified T lymphocytes. Nat. Biotechnol. 2002;20:1215–1220. doi: 10.1038/nbt758. [DOI] [PubMed] [Google Scholar]
- 13.Ellebrecht CT, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, et al. Chimeric antigen receptor (CAR) T cells targeting a pathogenic MHC class II:peptide complex modulate the progression of autoimmune diabetes. J. Autoimmun. 2019;96:50–58. doi: 10.1016/j.jaut.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fransson M, et al. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J. Neuroinflammation. 2012;9:112. doi: 10.1186/1742-2094-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blat D, Zigmond E, Alteber Z, Waks T, Eshhar Z. Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol. Ther. 2014;22:1018–1028. doi: 10.1038/mt.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald KG, et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Investig. 2016;126:1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kansal R, et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med. 2019;11:eaav1648. doi: 10.1126/scitranslmed.aav1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kochenderfer JN, Yu Z, Frasheri D, Restifo NP, Rosenberg SA. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116:3875–3886. doi: 10.1182/blood-2010-01-265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feucht J, et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 2019;25:82–88. doi: 10.1038/s41591-018-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheadle EJ, et al. Natural expression of the CD19 antigen impacts the long-term engraftment but not antitumor activity of CD19-specific engineered T cells. J. Immunol. 2010;184:1885–1896. doi: 10.4049/jimmunol.0901440. [DOI] [PubMed] [Google Scholar]
- 22.Abraham PM, Quan SH, Dukala D, Soliven B. CD19 as a therapeutic target in a spontaneous autoimmune polyneuropathy. Clin. Exp. Immunol. 2014;175:181–191. doi: 10.1111/cei.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmann K, Clauder AK, Manz RA. Targeting B cells and plasma cells in autoimmune diseases. Front. Immunol. 2018;9:835. doi: 10.3389/fimmu.2018.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Z, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, et al. CD33-specific chimeric antigen receptor T cells with different co-stimulators showed potent anti-leukemia efficacy and different phenotype. Hum. Gene Ther. 2018;29:626–639. doi: 10.1089/hum.2017.241. [DOI] [PubMed] [Google Scholar]
- 26.Priceman SJ, et al. Co-stimulatory signaling determines tumor antigen sensitivity and persistence of CAR T cells targeting PSCA+ metastatic prostate cancer. Oncoimmunology. 2018;7:e1380764. doi: 10.1080/2162402X.2017.1380764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du H, et al. Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell. 2019;35:221–37 e8. doi: 10.1016/j.ccell.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamieh M, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568:112–116. doi: 10.1038/s41586-019-1054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Bekkum DW. Effectiveness and risks of total body irradiation for conditioning in the treatment of autoimmune disease with autologous bone marrow transplantation. Rheumatology. 1999;38:757–761. doi: 10.1093/rheumatology/38.8.757. [DOI] [PubMed] [Google Scholar]
- 30.Zhao J, et al. P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum. 2013;65:3176–3185. doi: 10.1002/art.38174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.