Abstract
The non-dipping pattern is nighttime systolic blood pressure (SBP) fall of less than 10%. Several studies showed that the non-dipping pattern, increased mean platelet volume (MPV), and platelet distribution width (PDW) are associated with elevated cardiovascular risk. Hypertensives with the non-dipping pattern have higher MPV than the dippers but this relationship was never investigated among people with type 1 diabetes mellitus (T1DM). This study aimed to investigate the association between the central dipping pattern and platelet morphology in T1DM subjects. We measured the central and brachial blood pressure with a validated non-invasive brachial oscillometric device—Arteriograph 24—during twenty-four-hour analysis in T1DM subjects without diagnosed hypertension. The group was divided based on the central dipping pattern for the dippers and the non-dippers. From a total of 62 subjects (32 males) aged 30.1 (25.7–37) years with T1DM duration 15.0 (9.0–20) years, 36 were non-dippers. The non-dipper group had significantly higher MPV (MPV (10.8 [10.3–11.5] vs 10.4 [10.0–10.7] fl; p = 0.041) and PDW (13.2 [11.7–14.9] vs 12.3 [11.7–12.8] fl; p = 0.029) than dipper group. Multivariable logistic regression revealed that MPV (OR 3.74; 95% CI 1.48–9.45; p = 0.005) and PDW (OR 1.91; 95% CI 1.22–3.00; p = 0.005) were positively associated with central non-dipping pattern adjusting for age, sex, smoking status, daily insulin intake, and height. MPV and PDW are positively associated with the central non-dipping pattern among people with T1DM.
Subject terms: Cardiology, Diabetes
Introduction
Type 1 diabetes mellitus (T1DM) is a chronic metabolic illness, where insulin treatment is obligatory1. People with T1DM have a higher risk of developing cardiovascular disease than those from the general population2. Women with T1DM die 12.5 years earlier than women without diabetes. In the case of men, the difference is 11.6. years3. However, the pathophysiology of elevated cardiovascular risk in this population is still uncertain.
The circadian rhythm of blood pressure (BP) is useful to predict cardiovascular events4,5. The non-dipping pattern is defined as nighttime systolic blood pressure (SBP) fall of less than 10%6. It is usually described based on brachial systolic blood pressure but central compared with brachial SBP is more strongly associated with preclinical organ damage7. Non-dippers have more cardiovascular events and higher cardiovascular mortality than dippers, even if they are normotensives4.
Until now mean platelet volume (MPV) and platelet distribution width (PDW) could not be used for diagnosis or prognosis in any disease because of poor standardization and wide variability8. Mean platelet volume is dependent on numerous physiological and pathological factors9 However, many studies showed a positive relation between MPV, the presence of cardiovascular disease, and future prognosis. Based on metanalysis (40 studies, 12 285 individuals) subjects with coronary artery disease have higher MPV than healthy controls10. Higher values of MPV and PDW were significantly related to higher risks of cardiovascular disease11. MPV could predict long-term outcomes and cardiovascular events after the percutaneous coronary intervention12. The morphology and function of platelets in diabetes (including T1DM) are disturbed13,14. Rodriguez et al. showed that MPV was positively related to the presence of T2DM independently of age, sex, hypertension, BMI, and use of anti-platelet medicaments15. Children with T1DM have significantly higher MPV and PDW than healthy controls16.
Previous studies revealed the association between elevated MPV, PDW, and non-dipping pattern among people with hypertension17–22. However, a large study by Pusuroglu et al.23 did not confirm that thus this relationship remains controversial. There is no data according to people without diagnosed hypertension. This association was never investigated in the T1DM population with a non-dipping pattern based on central SBP.
Methods
Data collection
The study aimed to investigate the association between central dipping pattern and platelet morphology in subjects with T1DM. The data comes from the Poznan Atherosclerosis in Adult Patients with long-term Type 1 Diabetes Mellitus Study (PARADISE T1DM Study), which was conducted according to the decision of the Ethics Committee at Poznan University of Medical Sciences (approval No. 67/19). The study complies with the Declaration of Helsinki. In all cases, we obtained written informed consent before inclusion in the study.
The participants of the study were managed by the Department of Internal Medicine and Diabetology. We recruited them consecutively from February 2019 to March 2020. We included people aged between 18 and 45 years with T1DM confirmed in the past by positive antibodies, at least 5-year diabetes duration, and no less than 70% successful blood pressure measurements. The exclusion criteria were: cardiovascular disease, hypertension (or using antihypertensives), malignancy, chronic kidney disease (stages 2–5), sleep apnea, thrombocythemia, or thrombocytopenia. Each subject was asked to fill two forms, including diabetes duration, diabetic complications, drugs taken, coexisting diseases, and lifestyle.
Every participant underwent anamnesis and a standard physical examination. Specific investigations were performed to detect diabetic complications: retinopathy, neuropathy, and diabetic kidney disease. Besides, we repeated the brachial blood pressure measurement. The measurement was performed three times by an upper-arm manual sphygmomanometer on both arms after 10 min of rest in the sitting position, at the level of the participant’s heart. Basic anthropometric parameters (weight, height, waist, and hip circumferences) were measured. We calculated body mass index (BMI) and waist-to-hip ratio (WHR) using the following equations: BMI = weight [kg]/ squared height [m2]; WHR = waist circumference [cm]/ hip circumference [cm].
All blood samples were taken from participants in the morning (between 7 and 8 AM), after an 8–12-h overnight fast. They were drawn into standardized tubes with dipotassium ethylenedinitrotetraacetic acid (EDTA). The laboratory measured MPV, PDW, and all other lab parameters within 120 min after blood collection. Then the blood samples were stored at room temperature (18–25 °C) Complete blood count was carried out on analyzer Sysmex XN1000 (Sysmex Corporation, Japan). To eliminate pre-analytical and analytical errors, the technicians in the laboratory were not informed if the patient is the dipper or the non-dipper. Each blood sample was collected, handled, and processed in the same way. MPV reflects the average size of platelets. PDW was counted from a platelet histogram at the level of 20% of the distribution peak. The same blood counters were used to mark the morphology of red blood cells and white blood cells.
We also obtained the following laboratory results: lipid profile, thyroid-stimulating hormone, creatinine, transaminases. Low-density lipoprotein cholesterol (LDL-C) level was estimated by the Friedewald formula24. Glycated hemoglobin (HbA1c) was evaluated with a turbidimetric inhibition immunoassay (Cobas 6000, Roche Diagnostics). Furthermore, the urine albumin to urine creatinine ratio (ACR) was calculated. Glucose Disposal Rate (eGDR) formula was chosen to calculate insulin resistance25: eGDR [mg/kg/min] = 24.31 − (12.22 × WHR) – (3.29 × hypertension) – (0.57 × HbA1c) where: WHR—waist to hip ratio, hypertension—if present count as 1, if not count as 0, HbA1c—glycated hemoglobin [%].
Skin autofluorescence (which reflects advanced glycation end products accumulation and long-term diabetic control26) was measured using a non-invasive device (AGE-Reader, DiagnOptics BV, Groningen, The Netherlands). This method was validated by Meerwaldt et al.27.
24-h BP measurements were taken using Arteriograph 24 (TensioMed Ltd., Budapest, Hungary). We marked: aortic systolic blood pressure (SBP Ao), brachial systolic blood pressure (SBP Br), brachial diastolic blood pressure (DBP Br), and pulse. The operating principle of Arteriograph 24 is detecting and processing oscillations on the arm cuff by a special high-fidelity sensor during a complete occlusion of the brachial artery28. Arteriograph 24 was validated using invasive and non-invasive methods of AS assessment28,29.
An appropriate cuff was chosen based on arm circumference. We measured the distance from the jugular notch to the pubic symphysis in the supine position to estimate the length of the aorta (required to calculate Pulse wave velocity). Pulse wave velocity is the gold standard of assessment of arterial stiffness. More rigid arterial walls result in faster pulse wave velocity30.
We used TensioWin software to program Arteriograph 24. Measurements were taken every 30 min daily and every 1 h nightly for 24 h during the hospitalization in the clinic. Participants were instructed to start manual measurement in case of failed automatic measurement. After 24 h, the device was removed, and the results were transferred to our database.
Each participant was asked to fill another questionnaire concerning hour by hour activity during 24 h of measurement, including physical activity, number of cigarettes, number of (caffeine-containing) coffee cups, body position in the moment of measurement, glycemia (at least 5 measurements per day), and insulin dosages. We also marked the time of sleeping. Sleeping time was double-checked—using data from Arteriograph 24 and self-reported information from the questionnaire filled by the participant.
Subjects with a nighttime SBP fall of less than 10% were included in the group of non-dippers. Entities with the nighttime SBP fall of at least 10% were involved in the group of dippers.
Data analysis
R-programming language (version 3.6.1.; Vienna, R Project) was used for statistical analysis. The data is presented either as number and percentage (categorical) or where appropriate as a median and interquartile range (numerical). We used the Chi-square and the Mann–Whitney U tests to compare the dippers and the non-dippers based on central and brachial SBP. p value was set as two-sided.
We performed a logistic regression analysis. The dependent variable was the presence of a central or brachial non-dipping pattern. All variables independent of BP were chosen for univariate regression analysis. Univariate regression results with p < 0.1 and other known non-dipping pattern risk factors were included in multivariable regression analysis31. PDW was included in another model because it was highly positively associated with MPV. We also excluded potassium because of the collinearity with the height.
Results
Study group
80 participants were recruited. However, we finally included 62 subjects in the final analysis. The scheme of the study is presented in Fig. 1. The participants were aged 30.1 (25.7–37.0) years, 32 (51.6%) of them were males. The median T1DM duration was 15.0 (9.0–20.0) years. 36 (58.1%) were non-dippers based on SBP Ao; 25 (40.3%) were non-dippers based on SBP Br.
Figure 1.
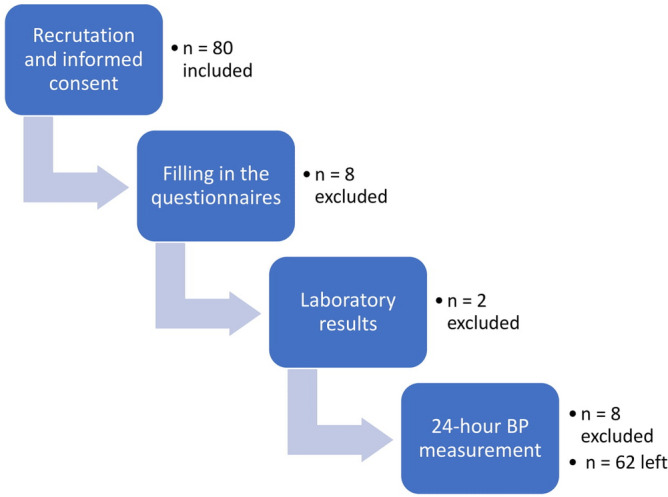
Flow diagram of the study and order of the procedures. BP blood pressure. All figures made by authors of the manuscript. GraphPad Prism 9 (https://www.graphpad.com/) was used to create forest plots.
Central non-dipping pattern
The groups (non-dippers vs dippers based on central SBP) did not differ significantly in general characteristics, diabetes complications, methods of treatment, and activity during the day of measurement. The non-dipper group had significantly higher MPV (10.8 [10.3–11.5] vs 10.4 [10.0–10.7] fl; p = 0.041) and PDW (13.2 [11.7–14.9] vs 12.3 [11.7–12.8] fl; p = 0.029) than the dipper group. The non-dippers consumed more alcohol than the dippers(1.5 [0.0–4.0] vs 0.8 [0.0–1.0] units/week; p = 0.015). The groups did not differ significantly in hemodynamic parameters like pulse wave velocity, aortic systolic blood pressure, brachial systolic and diastolic blood pressure. The differences in other morphology parameters like the number of platelets, red and white blood cells, hemoglobin, hematocrit, platelet volume/ platelet count ratio, as well as lipids, were also insignificant. The inverse associations between platelet count and MPV (Rs = − 0.17; p = 0.18) as well as PDW (Rs = − 0.15; p = 0.24) were not significant. Results are presented in Table 1. In univariate regression analysis (dependent variable—presence of SBP Ao non-dipping pattern) MPV, PDW and daily insulin intake were significant (p < 0.05). Sex, height, potassium, and alanine aminotransferase had a trend nearing significance (p < 0.1). Potassium concentration was positively associated with height (Rs = 0.34; p = 0.007), therefore potassium was excluded from the final model of multivariable logistic regression. PDW was included in another model because it was highly positively associated with MPV (Rs = 0.94; p < 0.001). Multivariable logistic regression revealed that MPV (OR 3.94; 95% CI 1.45–10.72; p = 0.007) and daily insulin intake (OR 253.23; 95% CI 2.99–21,420.55; p = 0.01) were positively associated with non-dipping pattern based on SBP Ao adjusting for age, sex, smoking status and height (Fig. 2). The model with PDW showed the positive relationship between PDW (OR 1.91; 95% CI 1.22–3.00; p = 0.005), daily insulin intake (OR 445.11; 95% CI 4.25–46,597.42; p = 0.01) and central non-dipping pattern adjusting for age, sex, smoking status and height (Fig. 3).
Table 1.
Comparison of groups with and without the central non-dipping pattern according to the Arteriograph 24 results.
| All participants n = 62 (100%) |
SBP Ao dippers n = 26 (41.9%) |
SBP Ao non–dippers n = 36 (58.1%) |
p value | |
|---|---|---|---|---|
| General characteristics | ||||
| Males n (%) | 32 (51.6%) | 10 (38.5%) | 22 (61.1%) | 0.08 |
| Age [years] | 30.1 (25.7–37.0) | 29.6 (22.3–39.4) | 32.8 (27.5–36.8) | 0.59 |
| Diabetes duration [years] | 15.0 (9.0–20.0) | 16.0 (9.0–22.0) | 15.0 (8.5–18.5) | 0.43 |
| BMI [kg/m2] | 24.8 (22.7–28.2) | 25.9 (23.2–28.7) | 24.3 (22.1–28.2) | 0.31 |
| WHR | 0.9 (0.8–0.9) | 0.9 (0.8–0.9) | 0.9 (0.8–0.9) | 0.92 |
| Family history for CVD n (%) | 17 (27.4%) | 5 (19.2%) | 12 (33.3%) | 0.22 |
| Systolic blood pressure [mmHg] | 123.0 (120.0–131.0) | 123.0 (116.0–131.0) | 123.0 (120.0–132.0) | 0.84 |
| Diastolic blood pressure [mmHg] | 80.0 (76.0–86.0) | 80.0 (76.0–85.0) | 81.0 (77.0–86.5) | 0.43 |
| Lifestyle | ||||
| Current smoker n (%) | 15 (24.2%) | 6 (23.1%) | 9 (25%) | 0.86 |
| Packyears | 0.0 (0.0–68.0) | 0.0 (0.0–40.0) | 2.3 (0.0–95.0) | 0.28 |
| Sport activity [hours/week] | 2.4 (0.0–6.0) | 2.0 (1.0–7.0) | 3.0 (0.0–6.0) | 0.86 |
| Alcohol intake [units/week] | 1.0 (0.0–2.0) | 0.8 (0.0–1.0) | 1.5 (0.0–4.0) | 0.015 |
| Shift work n (%) | 22 (35.5%) | 10.0 (38.5%) | 12.0 (33.3%) | 0.68 |
| Sleeping [hours/day] | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 0.90 |
| Physical work [hours/day] | 3.0 (0.0–8.0) | 2.0 (0.0–8.0) | 3.0 (0.0–8.0) | 0.51 |
| Complications | ||||
| At least one diabetic complication n (%) | 34 (54.8%) | 13 (50%) | 21 (58.3%) | 0.52 |
| Diabetic retinopathy n (%) | 12 (19.4%) | 4 (15.4%) | 8 (22.2%) | 0.50 |
| Diabetic nephropathy n (%) | 2 (3.2%) | 1 (3.8%) | 1 (2.8%) | 0.81 |
| Diabetic neuropathy n (%) | 28 (45.2%) | 13 (50%) | 15 (41.7%) | 0.52 |
| Treatment | ||||
| Insulin pump n (%) | 25 (40.3%) | 10 (38.5%) | 15 (41.7%) | 0.95 |
| Daily insulin intake [insulin units/day/kg] | 0.6 (0.5–0.7) | 0.6 (0.5–0.6) | 0.6 (0.5–0.8) | 0.09 |
| Metformin n (%) | 6 (9.7%) | 2 (7.7%) | 4 (11.1%) | 0.65 |
| Lipid profile | ||||
| Triglycerides [mmol/l] | 1.0 (0.7–1.3) | 1.0 (0.7–1.4) | 0.9 (0.6–1.3) | 0.61 |
| Total cholesterol [mmol/l] | 4.6 (4.2–5.1) | 4.6 (4.2–5.1) | 4.4 (4.2–5.2) | 0.95 |
| LDL–C [[mmol/l] | 2.4 (2.0–2.8) | 2.4 (2.0–2.8) | 2.4 (2.0–3.1) | 0.89 |
| HDL–C [mmol/l] | 1.7 (1.5–1.9) | 1.7 (1.4–1.8) | 1.8 (1.5–2.0) | 0.33 |
| Triglycerides / HDL–C ratio | 1.4 (0.8–2.1) | 1.3 (0.8–2.1) | 1.4 (0.9–2.1) | 0.53 |
| Laboratory results | ||||
| Red blood cells [T/l] | 4.8 (4.6–5.2) | 4.8 (4.6–5.0) | 4.8 (4.6–5.3) | 0.33 |
| Hemoglobin [g/l] | 14.3 (13.3–15.2) | 14.3 (12.8–15.1) | 14.2 (13.5–15.2) | 0.52 |
| Hematocrit [%] | 42.2 (39.2–44.1) | 42.0 (38.5–44.1) | 42.7 (39.5–44.8) | 0.41 |
| Mean red blood cells volume [fl] | 85.8 (82.9–88.2) | 85.8 (83.3–87.3) | 85.5 (82.9–87.9) | 0.96 |
| White Blood Cells [G/l] | 6.3 (5.5–7.3) | 6.0 (5.4–7.2) | 6.4 (5.5–7.3) | 0.49 |
| Platelets [G/l] | 262.0 (245.0–308.0) | 269.0 (245.0–315.0) | 260.5 (246.0–305.0) | 0.68 |
| Mean platelet volume [fl] | 10.6 (10.1–11.2) | 10.4 (10.0–10.7) | 10.8 (10.3–11.5) | 0.041 |
| Platelet distribution width [%] | 12.5 (11.7–14.2) | 12.3 (11.7–12.8) | 13.2 (11.7–14.9) | 0.029 |
| HbA1c [%] | 8.2 (7.0–9.1) | 7.9 (7.2–8.8) | 8.5 (7.0–9.7) | 0.24 |
| ALT [UI/l] | 16.0 (12.0–20.0) | 15.0 (11.0–17.0) | 16.5 (12.5–24.5) | 0.24 |
| AST [UI/l] | 17.0 (15.0–21.0) | 17.0 (15.0–21.0) | 17.0 (15.0–20.5) | 0.70 |
| Creatinine [µmol/L] | 73.4 (67.2–85.7) | 73.8 (66.3–84.9) | 72.9 (68.1–86.6) | 0.73 |
| ACR [mg/g] | 3.6 (3.0–7.0) | 4.0 (3.0–9.0) | 3.5 (3.0–6.0) | 0.38 |
| Estimated glucose disposal rate [mg/kg/min] | 9.2 (8.0–10.3) | 9.1 (8.1–10.4) | 9.3 (7.8–10.2) | 0.59 |
| Skin autofluorescence [IU] | 2.1 (1.8–2.4) | 2.0 (1.8–2.2) | 2.1 (1.9–2.5) | 0.35 |
| MPV/PLT ratio | 0.041 (0.034–0.044) | 0.039 (0.031–0.043) | 0.041 (0.036–0.045) | 0.24 |
| Activity during the day of measurement | ||||
| Time of physical activity [min] | 20.0 (0.0–40.0) | 20.0 (0.0–50.0) | 20.0 (0.0–40.0) | 0.93 |
| Number of cigarettes [n] | 0.0 (0.0–2.0) | 0.0 (0.0–0.0) | 0.0 (0.0–4.0) | 0.52 |
| Number of coffee cups [n] | 2.0 (1.0–3.0) | 2.0 (1.0–2.0) | 2.0 (1.0–3.0) | 0.48 |
| Mean glycemia [mmol/l] | 7.5 (6.7–8.8) | 7.7 (6.7–8.9) | 7.2 (6.8–8.0) | 0.50 |
| SD of glycemia [mmol/l] | 2.5 (2.0–3.0 | 2.6 (2.0–3.5) | 2.5 (2.0–2.9) | 0.25 |
| Insulin/day (u) | 38.1 (30.5–46.0) | 36.0 (30.5–44.5) | 39.0 (31.5–48.0) | 0.27 |
| Carbohydrates/day (g) | 15.3 (11.8–18.5) | 14.0 (12.0–17.5) | 16.0 (11.5–19.0) | 0.56 |
| Hemodynamic parameters | ||||
| Aortic systolic blood pressure [mmHg] | 118.9 (113.9–125.5) | 119.1 (113.9–125.5) | 118.7 (113.9–126.0) | 0.75 |
| Brachial systolic blood pressure [mmHg] | 130.3 (123.9–138.2) | 129.2 (121.5–138.2) | 130.6 (124.4–138.2) | 0.64 |
| Brachial diastolic blood pressure [mmHg] | 75.5 (71.1–79.4) | 75.2 (68.4–77.6) | 75.6 (73.0–79.5) | 0.19 |
| Pulse wave velocity [ms/s] | 7.7 (7.1–8.5) | 7.8 (7.1–8.4) | 7.7 (7.1–8.5) | 0.88 |
Data presented as median (IQR)/n(%). Bold values denote statistical significance at the p < 0.05 level.
ACR albumin to creatinine ratio, ALT alanine transaminase, Ao aortic, AST aspartate transaminase, BMI body mass index, HbA1c glycated hemoglobin, HDL-C high-density lipoprotein, LDL-C low-density lipoprotein, SBP systolic blood pressure, WHR waist-to-hip ratio.
Figure 2.
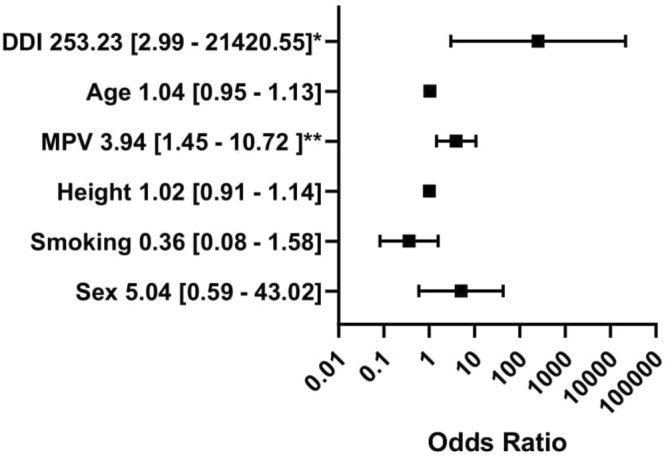
Multivariable logistic regression analysis with mean platelet volume. The dependent variable: the presence of central non-dipping pattern (coded one). The outcomes presented as a dependent variable (odds ratio, 95% confidence interval). *Significant at the 0.05 level; **significant at the 0.01 level. MPV mean platelet volume, DDI daily insulin intake. All figures made by authors of the manuscript. GraphPad Prism 9 (https://www.graphpad.com/) was used to create forest plots.
Figure 3.
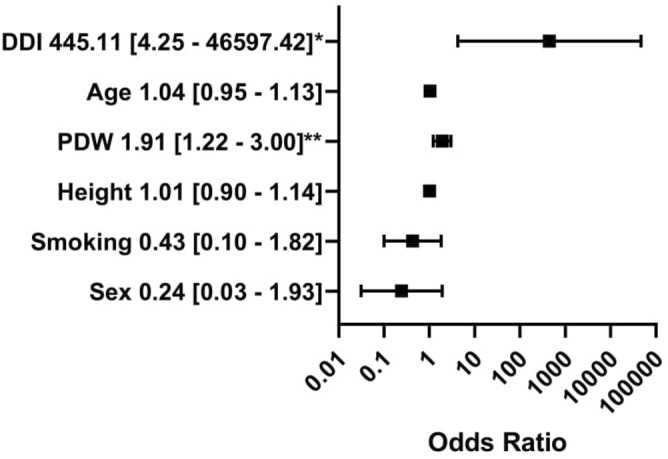
Multivariable logistic regression analysis with platelet distribution width. The dependent variable: the presence of central non-dipping pattern (coded one). The outcomes presented as a dependent variable (odds ratio, 95% confidence interval). *Significant at the 0.05 level. **Significant at the 0.01 level. PDW platelet distribution width, DDI daily insulin intake. All figures made by authors of the manuscript. GraphPad Prism 9 (https://www.graphpad.com/) was used to create forest plots.
Brachial non-dipping pattern
The comparison of non-dippers and dippers based on brachial SBP revealed the difference in PDW—which was higher in the non-dipper group—(13.9 [12.1–15.1] vs 12.3 [11.7–13.4] fl; p = 0.020) but not in MPV (10.7 [10.3–11.4] vs 10.4 [10.0–10.9] p = 0.11). In the SBP Br non-dipper group were significantly more males than in the dipper group (17 [68.0%] vs 15 [40.5%] p = 0.034) (Table 2). The groups did not differ significantly in aortic and brachial blood pressure and pulse wave velocity. The differences in other morphology parameters like the number of platelets, red and white blood cells, hemoglobin, hematocrit, platelet volume/ platelet count ratio, as well as lipids, were also insignificant. In univariate regression analysis (dependent variable—presence of SBP Br non-dipping pattern) PDW, sex, potassium, and height were significant (p < 0.05). Multivariable logistic regression revealed that only MPV (OR 2.50; 95% CI 1.09–5.71; p = 0.008) and PDW in another model (OR 1.69; 95% CI 1.15–2.49; p = 0.008) were positively associated with non-dipping pattern based on SBP Br adjusting for age, sex, smoking status, and height (Figs. 4 and 5).
Table 2.
Comparison of groups with and without the brachial non-dipping pattern according to the Arteriograph 24 results.
| All participants n = 62 (100%) |
SBP Br dippers n = 37 (57.7%) |
SBP Br non–dippers n = 25 (40.3%) |
p value | |
|---|---|---|---|---|
| General characteristics | ||||
| Males n (%) | 32 (51.6%) | 15 (40.5%) | 17 (68.0%) | 0.034 |
| Age [years] | 30.1 (25.7–37.0) | 30.0 (26.3–37.3) | 30.3 (24.0–36.4 | 0.38 |
| Diabetes duration [years] | 15.0 (9.0–20.0) | 16.0 (9.0–20.0) | 15.0 (8.0–18.0) | 0.38 |
| BMI [kg/m2] | 24.8 (22.7–28.2) | 25.8 (23.2–28.4) | 23.8 (21.5–28.0) | 0.28 |
| WHR | 0.9 (0.8–0.9) | 0.8 (0.8–0.9) | 0.9 (0.8–0.9) | 0.71 |
| Family history for CVD n (%) | 17 (27.4%) | 10 (27.0%) | 7 (28.0%) | 0.93 |
| Systolic blood pressure [mmHg] | 123.0 (120.0–131.0) | 121.0 (115.0–130.0) | 123.0 (120.0–136.0) | 0.22 |
| Diastolic blood pressure [mmHg] | 80.0 (76.0–86.0) | 82.0 (76.0–85.0) | 80.0 (77.0–87.0) | 0.48 |
| Lifestyle | ||||
| Current smoker n (%) | 15 (24.2%) | 10 (27.0%) | 5 (20.0%) | 0.53 |
| Packyears | 0.0 (0.0–68.0) | 0.0 (0.0–80.0) | 0.0 (0.0–60.0) | 0.93 |
| Sport activity [hours/week] | 2.4 (0.0–6.0) | 2.0 (0.0–6.0) | 3.3 (0.0–6.0) | 0.76 |
| Alcohol intake [units/week] | 1.0 (0.0–2.0) | 1.0 (0.0–1.0) | 2.0 (0.0–3.0) | 0.050 |
| Shift work n (%) | 22 (35.5%) | 13 (35.1%) | 9 (36.0%) | 0.94 |
| Sleeping [hours/day] | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 0.80 |
| Physical work [hours/day] | 3.0 (0.0–8.0) | 3.3 (0.0–8.0) | 3.0 (0.0–8.0) | 0.87 |
| Complications | ||||
| At least one diabetic complication n (%) | 34 (54.8%) | 21 (56.8%) | 13 (52.0%) | 0.71 |
| Diabetic retinopathy n (%) | 12 (19.4%) | 5 (13.5%) | 7 (28.0%) | 0.16 |
| Diabetic nephropathy n (%) | 2 (3.2%) | 1 (2.7%) | 1 (4.0%) | 0.78 |
| Diabetic neuropathy n (%) | 28 (45.2%) | 19 (51.4%) | 9 (36.0%) | 0.23 |
| Treatment | ||||
| Insulin pump n (%) | 25 (40.3%) | 14 (37.8%) | 11 (44.0%) | 0.75 |
| Daily insulin intake [insulin units/day/kg] | 0.6 (0.5–0.7) | 0.6 (0.5–0.6) | 0.6 (0.5–0.7) | 0.72 |
| Metformin n (%) | 6 (9.7%) | 3 (8.1%) | 3 (12%) | 0.61 |
| Lipid profile | ||||
| Triglycerides [mmol/l] | 1.0 (0.7–1.3) | 0.9 (0.6–1.3 | 1.0 (0.8–1.4) | 0.39 |
| Total cholesterol [mmol/l] | 4.6 (4.2–5.1) | 4.6 (4.2–5.1) | 4.6 (4.2–5.0) | 0.70 |
| LDL–C [[mmol/l] | 2.4 (2.0–2.8) | 2.5 (2.2–2.9) | 2.2 (1.9–2.8) | 0.25 |
| HDL–C [mmol/l] | 1.7 (1.5–1.9) | 1.7 (1.5–1.9) | 1.8 (1.4–1.9) | 0.76 |
| Triglycerides / HDL–C ratio | 1.4 (0.8–2.1) | 1.3 (0.8–2.1) | 1.4 (0.9–2.1) | 0.65 |
| Laboratory results | ||||
| Red Blood Cells [T/l] | 4.8 (4.6–5.2) | 4.8 (4.6–5.0) | 4.8 (4.6–5.5) | 0.30 |
| Hemoglobin [g/l] | 14.3 (13.3–15.2) | 14.2 (13.2–15.1) | 14.3 (13.5–15.6) | 0.32 |
| Hematocrit [%] | 42.2 (39.2–44.1) | 42.1 (38.7–44.0) | 42.5 (39.8–45.5) | 0.26 |
| Mean red blood cells volume [fl] | 85.8 (82.9–88.2) | 85.8 (83.3–87.3) | 85.8 (82.9–87.5) | 1.00 |
| White blood cells [G/l] | 6.3 (5.5–7.3) | 6.3 (5.5–7.4) | 6.3 (5.5–6.9) | 0.95 |
| Platelets [G/l] | 262.0 (245.0–308.0) | 263.0 (246.0–314.0) | 259.0 (230.0–302.0) | 0.45 |
| Mean platelet volume [fl] | 10.6 (10.1–11.2) | 10.4 (10.0–10.9) | 10.7 (10.3–11.4) | 0.11 |
| Platelet distribution width [%] | 12.5 (11.7–14.2) | 12.3 (11.7–13.4) | 13.9 (12.1–15.1) | 0.020 |
| HbA1c [%] | 8.2 (7.0–9.1) | 7.9 (7.2–8.9) | 8.5 (7.0–9.9) | 0.24 |
| ALT [UI/l] | 16.0 (12.0–20.0) | 15.0 (13.0–19.0) | 16.0 (11.0–24.0) | 0.67 |
| AST [UI/l] | 17.0 (15.0–21.0) | 17.0 (15.0–20.0) | 16.0 (15.0–21.0) | 0.75 |
| Creatinine [µmol/L] | 73.4 (67.2–85.7) | 71.6 (67.2–80.4) | 75.1 (70.7–86.6) | 0.25 |
| ACR [mg/g] | 3.6 (3.0–7.0) | 3.2 (3.0–7.0) | 5.0 (3.0–7.0) | 0.93 |
| Estimated glucose disposal rate [mg/kg/min] | 9.2 (8.0–10.3) | 9.4 (8.1–10.3) | 8.8 (8.0–10.1) | 0.38 |
| Skin Autofluorescence [IU] | 2.1 (1.8–2.4) | 2.1 (1.9–2.4) | 2.0 (1.7–2.3) | 0.50 |
| MPV/PLT ratio | 0.041 (0.034–0.044) | 0.039 (0.033–0.043) | 0.041 (0.036–0.045) | 0.24 |
| Activity during the day of measurement | ||||
| Time of physical activity [min] | 20.0 (0.0–40.0) | 30.0 (10.0–40.0) | 10.0 (0.0–30.0) | 0.09 |
| Number of cigarettes [n] | 0.0 (0.0–2.0) | 0.0 (0.0–3.0) | 0.0 (0.0–1.0) | 0.89 |
| Number of coffee cups [n] | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.50 |
| Mean glycemia [mmol/l] | 7.5 (6.7–8.8) | 7.4 (6.8–8.0) | 7.6 (6.5–9.0) | 0.82 |
| SD of glycemia [mmol/l] | 2.5 (2.0–3.0) | 2.5 (1.9–2.9) | 2.5 (2.2–3.1) | 0.26 |
| Insulin/day (u) | 38.1 (30.5–46.0) | 37.9 (30.5–44.9) | 39.0 (31.7–46.0) | 0.79 |
| Carbohydrates/day (g) | 15.3 (11.8–18.5) | 15.0 (12.8–17.8) | 16.8 (11.0–20.0) | 0.82 |
| Hemodynamic parameters | ||||
| Aortic systolic blood pressure [mmHg] | 118.7 (113.9–126.0) | 118.9 (113.9–125.5) | 119.0 (113.9–125.4) | 0.97 |
| Brachial systolic blood pressure [mmHg] | 130.6 (124.4–138.2) | 130.6 (123.9–135.8) | 129.4 (124.0–138.3) | 0.79 |
| Brachial diastolic blood pressure [mmHg] | 75.6 (73.0–79.5) | 75.7 (69.5–78.4) | 75.5 (73.3–79.6) | 0.47 |
| Pulse wave velocity [ms/s] | 7.7 (7.1–8.5) | 7.7 (7.1–8.3) | 7.8 (6.8–8.7) | 0.95 |
Data presented as median (IQR) / n(%). Bold values denote statistical significance at the p < 0.05 level.
ACR albumin to creatinine ratio, ALT alanine transaminase, AST aspartate transaminase, BMI body mass index, Br brachial, HbA1c glycated hemoglobin, HDL-C high-density lipoprotein, LDL-C low-density lipoprotein, SBP systolic blood pressure, WHR waist-to-hip ratio.
Figure 4.
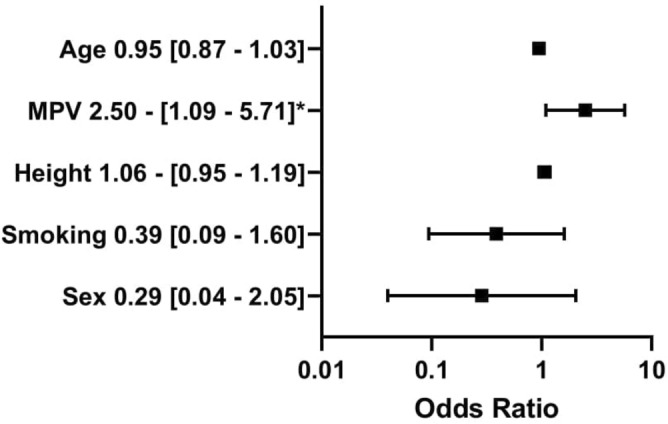
Multivariable logistic regression analysis with mean platelet volume. The dependent variable: the presence of brachial non-dipping pattern (coded one). The outcomes presented as a dependent variable (odds ratio, 95% confidence interval). *. significant at the 0.05 level; **significant at the 0.01 level; MPV mean platelet volume, DDI daily insulin intake. All figures made by authors of the manuscript. GraphPad Prism 9 (https://www.graphpad.com/) was used to create forest plots.
Figure 5.
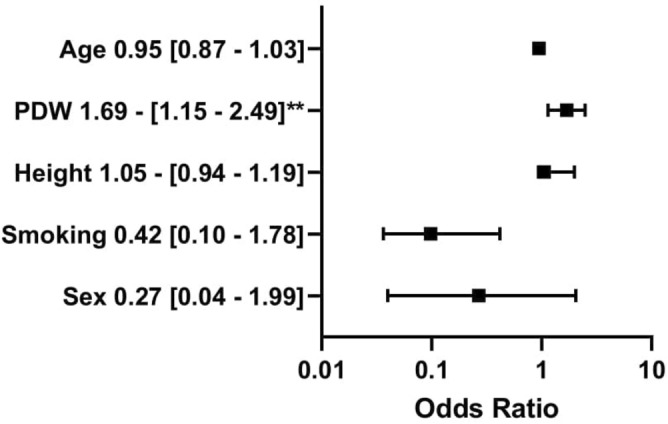
Multivariable logistic regression analysis with platelet distribution width. The dependent variable: the presence of brachial non-dipping pattern (coded one). The outcomes presented as a dependent variable (odds ratio, 95% confidence interval). *Significant at the 0.05 level; **significant at the 0.01 level; PDW platelet distribution width, DDI daily insulin intake. All figures made by authors of the manuscript. GraphPad Prism 9 (https://www.graphpad.com/) was used to create forest plots.
Hypertension in 24-h measurement
All of our participants had no diagnosed hypertension but 61.3% of them had elevated BP values in 24-h measurement. Therefore, we performed an additional analysis for participants without hypertension in 24-h measurement. SBP Ao non dippers had significantly higher MPV (11.0 [10.5–11.6] vs 10.5 [10.0–10.7] fl; p = 0.026) and PDW (14.0 [12.5–15.1] vs 12.0 [11.7–12.6] fl; p = 0.035) (Table 3).
Table 3.
Participants without hypertension in 24-h BP measurement. Comparison of the dippers and the non-dippers.
| All participants n = 24 (100%) |
SBP Ao dippers n = 13 (54.2%) |
SBP Ao non–dippers n = 11 (45.8%) |
p value | |
|---|---|---|---|---|
| Laboratory results | ||||
| Mean Platelet Volume [fl] | 10.7 (10.4–11.3) | 10.5 (10.0–10.7) | 11.0 (10.5–11.6) | 0.026 |
| Platelet distribution Width [%] | 12.5 (11.9–14.4) | 12.0 (11.7–12.6) | 14.0 (12.5–15.1) | 0.035 |
Data presented as median (IQR)/n (%). Bold values denote statistical significance at the p < 0.05 level.
Discussion
To our knowledge, this is the first study investigating the relationship between platelet morphology and the central non-dipping pattern in the T1DM population. We found that MPV and PDW were significantly higher in the central non-dippers compared to the dippers among people with T1DM. MPV and PDW were positively associated with the presence of the central non-dipping pattern adjusting for age, sex, smoking status, and height.
There are different methods to assess platelet reactivity, but many of them are complicated and high-priced32. The gold standard of platelet reactivity measurement is turbidimetric platelet aggregation, but there are many other techniques like flow cytometry, shear-stress mediated systems, imaging-based techniques33,34. Some studies suggest that the alternative could be MPV, as an indirect method of platelet activity measurement. Higher MPV correlates to a higher number of dense bodies per platelet, LDH activity, speed of ADP-induced aggregation, serotonin uptake, and release35. However, the relationship between MPV, PDW, and platelet aggregation was not proved in healthy volunteers36. De Luca et al. demonstrated that MPV value does not accompany platelet reactivity in the population of 1016 diabetic patients37. This study had several limitations. The diagnosis of diabetes was based only on medical history and fasting plasma glucose. There were no exclusion criteria and no differentiation for the type of diabetes. Furthermore, the results showed the positive association between MPV and U46619-stimulated P-selectin increase with the trend nearing significance (r = 0.24, p = 0.11). Therefore, we need more studies to verify the association between platelet activation and MPV, especially in the T1DM population. The limitation of MPV is that many other factors could influence its value: genetic variants, race, ethnicity, age, sex, and lifestyle9. PDW and MPV are not simply surrogates of more specific platelet reactivity tests. They reflect different aspects of platelet biology, not only the reactivity of platelets but also their age, generation, and turnover rates33.
Hypertension may partially derive from a hypercoagulable state, one component of which may be increased platelet activity. Hypertensives have higher MPV and a higher quantity of P-selectin and beta-thromboglobulin than normotensives. These proteins take part in platelet activation38. Gang et al. in a prospective study with a 9-years follow-up proved that elevated MPV allows predicting the development of hypertension independently of other risk factors like age, gender, and SBP39. Prehypertension is also associated with higher MPV40.
A Chinese prospective study in a large group of 31,751 people revealed that higher values of MPV were associated with a higher risk of stroke, cardiovascular disease, and coronary heart disease after 6 years of follow-up. Higher PDW was related to a higher risk of cardiovascular disease and coronary heart disease11. Higher MPV is also associated with a higher risk of restenosis after percutaneous transluminal coronary angioplasty12.
Both the non-dipping pattern and increased MPV are associated with elevated cardiovascular risk5,41. However, the pathophysiology of the relationship between the non-dipping pattern and MPV is unknown. The non-dipping pattern is related to higher nighttime SBP and the same higher mean diurnal SBP. Increased BP may lead to endothelium damage which results in elevated shear stress, an imbalance between free radicals and antioxidants, platelet activation, and aggregation42. Another possible pathomechanism is the impairment of the autonomic nervous system—low parasympathetic and high sympathetic activity. Catecholamines may stimulate alpha-2 adrenoreceptors which induce platelet aggregation43. This theory also suggests that adrenaline causes the release of the large activated platelets sequestered in the spleen44.
It is known that the presence of diabetes is associated with disturbed platelet biology. MPV was positively associated with the presence of T2DM in both sexes, especially in males15. Elevated MPV was related to insulin treatment, lowered MPV was related to sulphonylureas and metformin. In another sample, the researchers observed no relationship between platelet aggregation traits (ADP, collagen, epinephrine) and the risk of diabetes development after 18.1 years of follow-up. King et al. also found no significant association between T2DM and platelet reactivity tests45. It may suggest that MPV is a better predictor of T2DM than platelet reactivity tests. Shimodaira et al. proved that MPV was positively related to fasting plasma glucose among both individuals with prediabetes and healthy people with normal glucose levels46 In children with T1DM MPV was related to aortic intima-media thickness which is an early indicator of subclinical atherosclerosis47. Abdel-Moneim et al. compared three groups of children: with established T1DM (duration: 0.66 ± 0.4 years), with newly diagnosed T1DM (duration: 0.66 ± 0.4 years), and healthy controls. MPV and PDW were significantly higher in children with T1DM than those in the control group48. Platelet number was lower in children with established T1DM than in children with the newly diagnosed disease and controls. MPV was related to HbA1C among people with T1DM as opposed to T2DM subjects13. Children with HbA1C above 7.5% had significantly higher MPV and PDW than children with HbA1C below 7.5%. MPV was positively correlated with T1DM duration14.
The relationship between platelet morphology and the dipping pattern among T1DM subjects is uninvestigated. Our study is the first one and revealed the need for further research. Nevertheless, there are some works focused on the dipping pattern in this population. Theilade et al. investigated for the first time the central dipping among subjects with T1DM49. They proved that nocturnal central and brachial SBP fall decreased with diabetes duration and albuminuria. There was an association between HbA1C and the non-dipping pattern50. However, Mamta et al. observed no relationship between the brachial non-dipping pattern and glucose variability in subjects with T1DM51. Another study showed that nephropathy (but not other diabetic complications) was positively associated with the brachial non-dipping pattern52. Spallone et al. suggested the role of autonomic neuropathy in the development of nephropathy and the non-dipping pattern in the T1DM subjects, but further studies did not confirm that relationship52,53. In our study, we did not observe such associations. The groups did not differ in long-term diabetic control (HbA1C, skin autofluorescence), presence of diabetic complications, and insulin resistance (eGDR). However, the central non-dipping pattern was positively related to daily insulin intake. The whole study group was relatively young and had a low level of albumin to creatine ratio. It suggests that the non-dipping pattern may develop independently of glycemic control.
The prevalence of the non-dipping pattern among people with T1DM is uninvestigated because few studies have been published on this topic. It was noted by Jaiswal et al.51. In their study, the prevalence of the non-dipping pattern in 41 T1DM subjects was only 10%. A previous study by Stella et al. showed that 27.8% of 61 T1DM entities were the non-dippers52. The prevalence was 40.3% for the brachial non-dipping pattern and 58.1% for the central non-dipping pattern in our study.
In the literature, several works describe the association between MPV and the non-dipping pattern, but all of them are based only on brachial BP. MPV is thought to be higher in the non-dippers than the dippers among people with hypertension treated at least 6 months17. Inanc et al. demonstrated that the non-dippers have higher MPV than the dippers and normotensives18. Other studies confirmed the positive relationship between MPV and the non-dipping patterns among hypertensives19,20,54. A similar finding was obtained among hypertensive children, people with uncontrolled hypertension, and prehypertensive non-smokers21,55 Meric et al. observed that the mean platelet volume/ platelet count (MPV/PC) ratio was significantly higher in the non-dippers than the dippers and it could be used to predict non-dipping pattern with 95 sensitivity and 95 specificity22. In our study MPV was higher in central the non-dippers than the dippers. There were no significant differences in MPV based on the brachial dipping pattern. However, MPV was associated with a brachial non-dipping pattern in the multivariable regression model. We found no association between dipping pattern and MPV/PC ratio.
Previously described studies were performed in relatively small populations. On the contrary, Pusuroglu et al. found no difference in MPV between the hypertensive dippers and the non-dippers in the group of 840 participants23. However, MPV was significantly higher in hypertensives compared to normotensives. The non-dipping pattern was diagnosed only in hypertensives, so there was no information on how many subjects without hypertension were the non-dippers. We included only subjects with T1DM and without diagnosed hypertension. In our study group, there was no difference in MPV between the dippers and the non-dippers based on peripheral BP, but MPV was significantly higher in the central BP non-dippers than the dippers. Further studies are needed to confirm if MPV is more strongly associated with central than brachial dipping.
Cicek et al. found that hypertensives with the non-dipping pattern have higher pulse wave velocity than those with the dipping pattern56. Increased pulse wave velocity means higher arterial stiffness which is a strong cardiovascular risk factor. However, our results showed no relationship between the non-dipping pattern and pulse wave velocity. It could be a result of a smaller number of participants in our study or a different population (with type 1 diabetes and without hypertension). The relationship between the non-dipping pattern and pulse wave velocity is poorly documented yet and it needs further investigation.
In our study, we excluded people with alcoholism. However, the non-dippers consumed significantly more standard units of alcohol per week than the dippers. Even small amounts of alcohol may be associated with the non-dipping pattern but because of the cross-sectional character of our study, we cannot find any cause and effect relationships.
We showed for the first time the relationship between the non-dipping pattern and MPV among people with T1DM. Moreover, this is the first study investigating not only the brachial but central non-dipping pattern and its association with platelet morphology. Several studies proved that the brachial non-dippers have higher MPV than the dippers. We also showed that PDW (which was not investigated earlier) is higher in people without appropriate nocturnal fall in BP.
Those results bring practical implications because people with the non-dipping pattern have elevated cardiovascular risk. Thanks to 24-h BP measurement, PDW, and MPV, it is possible to identify them. We know that in the non-dippers impaired platelet biology should be suspected. These people should be watchfully observed. Non-pharmacological treatment could be useful because 20-weeks lifestyle modification (decreased sodium intake, intensified physical activity, reduction of alcohol consumption, and DASH diet) may reduce MPV in prehypertensive subjects57. Aspirin and other antiplatelet drugs are not recommended in primary prevention among T1DM subjects with low or moderate cardiovascular risk58. According to the ASCEND study, aspirin brings more side effects than benefits, in primary cardiovascular prevention among people with diabetes59. However, this data is based mostly on subjects with type 2 diabetes mellitus and the topic remains controversial. People with diabetes with the non-dipping pattern and increased MPV may have benefits from using antiplatelet drugs. Further studies are needed to establish proper treatment for T1DM subjects with a non-dipping pattern.
Our study has some limitations. We did not measure cardiac autonomic neuropathy, which could influence on dipping pattern. However, studies did not confirm the relationship between non-dipping pattern and autonomic neuropathy52. We did not perform more complicated, direct methods of platelet activity assessment. Another limitation of our study is the lack of standardization of MPV and PDW assessment. MPV measurements are cheap and widely available but susceptible to many factors. Firstly, the contact of the platelets with the standard anticoagulant—ethylenediaminetetraacetic acid (EDTA) may cause an increase in diameter60,61 Secondly, the MPV measurements are highly variable across analyzers61,62. Therefore, the results should be treated with caution cause MPV measurements may be not consistent across different studies. Our results were obtained using the same method and the same analyzer. The groups did not differ significantly in age and gender. The technicians in the laboratory were unconscious if the participant was the dipper or the non-dipper. The time between blood sampling and analysis was similar (within 120 min) but it was not accurately measured63. Each participant had only 1 day of 24-h BP measurement. Our results are limited because of the relatively small sample size, modest p values, and no account of multiple test correction in analysis.
Conclusions
Type 1 diabetes subjects with the central non-dipping pattern have higher values of MPV and PDW than the dippers. MPV and PDW are positively associated with the central non-dipping pattern adjusting for age, sex, smoking status, and height.
Supplementary Information
Author contributions
M.K. wrote the main manuscript text. He was also responsible for the conceptualization, methodology, formal analysis, investigation, and methodology of the study. D.N. was responsible for project administration, conceptualization, investigation, and resources. M.K. was responsible for formal analysis, supervision, investigation, and resources. D.K. was responsible for conceptualization, investigation, and resources. P.L., D.K., M.K., and J.F. were responsible for the investigation and resources. D.N., A.U., and D.Z.-Z. supervised the project. All authors reviewed the manuscript.
Funding
This work was supported by the Poznan University of Medical Sciences [Grant Numbers: 3551].
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Michal Kulecki and Dariusz Naskret.
These authors jointly supervised this work: Aleksandra Uruska and Dorota Zozulinska-Ziolkiewicz.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-94414-y.
References
- 1.Association AD. Standards of medical care in diabetes—2020 abridged for primary care providers. Clin. Diabetes. 2020;38:10–38. doi: 10.2337/cd20-as01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schofield J, Ho J, Soran H. Cardiovascular risk in type 1 diabetes mellitus. Diabetes Ther. 2019;10:773–789. doi: 10.1007/s13300-019-0612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huo L, Harding JL, Peeters A, Shaw JE, Magliano DJ. Life expectancy of type 1 diabetic patients during 1997–2010: a national Australian registry-based cohort study. Diabetologia. 2016;59:1177–1185. doi: 10.1007/s00125-015-3857-4. [DOI] [PubMed] [Google Scholar]
- 4.Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J. Hypertens. 2002;20:2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Ohkubo T, Imai Y, Tsuji I, et al. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am. J. Hypertens. 1997;10:1201–1207. doi: 10.1016/S0895-7061(97)00274-4. [DOI] [PubMed] [Google Scholar]
- 6.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. The task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 7.Kollias A, Lagou S, Zeniodi ME, Boubouchairopoulou N, Stergiou GS. Association of central versus brachial blood pressure with target-organ damage: systematic review and meta-analysis. Hypertension. 2016;67:183–190. doi: 10.1161/HYPERTENSIONAHA.115.06066. [DOI] [PubMed] [Google Scholar]
- 8.Noris P, Melazzini F, Balduini CL. New roles for mean platelet volume measurement in the clinical practice? Platelets. 2016;27:607–612. doi: 10.1080/09537104.2016.1224828. [DOI] [PubMed] [Google Scholar]
- 9.Korniluk, A., Koper-Lenkiewicz, O. M., Kamińska, J., Kemona, H., Dymicka-Piekarska, V. Mean platelet volume (MPV): New perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediat. Inflamm. e9213074 (2019) [DOI] [PMC free article] [PubMed]
- 10.Sansanayudh N, Anothaisintawee T, Muntham D, McEvoy M, Attia J, Thakkinstian A. Mean platelet volume and coronary artery disease: a systematic review and meta-analysis. Int. J. Cardiol. 2014;175:433–440. doi: 10.1016/j.ijcard.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 11.He S, Lei W, Li J, et al. Relation of platelet parameters with incident cardiovascular disease (The Dongfeng-Tongji Cohort Study) Am. J. Cardiol. 2019;123:239–248. doi: 10.1016/j.amjcard.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Yang A, Pizzulli L, Lüderitz B. Mean platelet volume as marker of restenosis after percutaneous transluminal coronary angioplasty in patients with stable and unstable angina pectoris. Thromb. Res. 2006;117:371–377. doi: 10.1016/j.thromres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Zaccardi F, Rocca B, Rizzi A, Ciminello A, Teofili L, Ghirlanda G, De Stefano V, Pitocco D. Platelet indices and glucose control in type 1 and type 2 diabetes mellitus: a case-control study. Nutr. Metab. Cardiovasc. Dis. 2017;27:902–909. doi: 10.1016/j.numecd.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Venkatesh V, Kumar R, Varma DK, Bhatia P, Yadav J, Dayal D. Changes in platelet morphology indices in relation to duration of disease and glycemic control in children with type 1 diabetes mellitus. J. Diabetes Complicat. 2018;32:833–838. doi: 10.1016/j.jdiacomp.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez BAT, Johnson AD. Platelet measurements and type 2 diabetes: investigations in two population-based cohorts. Front. Cardiovasc. Med. 2020;7:118. doi: 10.3389/fcvm.2020.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malachowska B, Tomasik B, Szadkowska A, Baranowska-Jazwiecka A, Wegner O, Mlynarski W, Fendler W. Altered Platelets’ morphological parameters in children with type 1 diabetes—a case-control study. BMC Endocr. Disord. 2015 doi: 10.1186/s12902-015-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ordu S, Ozhan H, Caglar O, Alemdar R, Basar C, Yazici M, Erden I. Mean platelet volume in patients with dipper and non-dipper hypertension. Blood Press. 2010;19:26–30. doi: 10.3109/08037050903416402. [DOI] [PubMed] [Google Scholar]
- 18.Inanc T, Kaya MG, Yarlioglues M, et al. The mean platelet volume in patients with non-dipper hypertension compared to dippers and normotensives. Blood Press. 2010;19:81–85. doi: 10.3109/08037050903516284. [DOI] [PubMed] [Google Scholar]
- 19.Kaya MG, Yarlioglues M, Gunebakmaz O, Gunturk E, Inanc T, Dogan A, Kalay N, Topsakal R. Platelet activation and inflammatory response in patients with non-dipper hypertension. Atherosclerosis. 2010;209:278–282. doi: 10.1016/j.atherosclerosis.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Surgit O, Erturk M, Akgul O, Pusuroglu H, Korkmaz AF, Isiksacan N, Gul M, Uzun F, Ozal E, Eksik A. Assessment of mean platelet volume and soluble CD40 ligand levels in patients with non-dipper hypertension, dippers and normotensives. Clin. Exp. Hypertens. 2015;37:70–74. doi: 10.3109/10641963.2014.897725. [DOI] [PubMed] [Google Scholar]
- 21.Erdogan D, Icli A, Aksoy F, Akcay S, Ozaydin M, Ersoy I, Varol E, Dogan A. Relationships of different blood pressure categories to indices of inflammation and platelet activity in sustained hypertensive patients with uncontrolled office blood pressure. Chronobiol. Int. 2013;30:973–980. doi: 10.3109/07420528.2013.790045. [DOI] [PubMed] [Google Scholar]
- 22.Meric M, Yuksel S, Coksevim M, Gulel O. The effect of mean platelet volume/platelet count ratio on dipper and non-dipper blood pressure status. Medicina (Kaunas) 2019;55(11):742. doi: 10.3390/medicina55110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pusuroglu H, Cakmak HA, Erturk M, Akgul O, Akkaya E, Tosu AR, Celik O, Gul M, Yildirim A. Assessment of the relation between mean platelet volume, non-dipping blood pressure pattern, and left ventricular mass index in sustained hypertension. Med. Sci. Monit. 2014;20:2020–2026. doi: 10.12659/MSM.891040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Cordova CMM, Schneider CR, Juttel ID, de Cordova MM. Comparison of LDL-cholesterol direct measurement with the estimate using the Friedewald formula in a sample of 10,664 patients. Arq. Bras. Cardiol. 2004;83:476–481. doi: 10.1590/S0066-782X2004001800006. [DOI] [PubMed] [Google Scholar]
- 25.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49:626–632. doi: 10.2337/diabetes.49.4.626. [DOI] [PubMed] [Google Scholar]
- 26.Sugisawa E, Miura J, Iwamoto Y, Uchigata Y. Skin autofluorescence reflects integration of past long-term glycemic control in patients with type 1 diabetes. Diabetes Care. 2013;36:2339–2345. doi: 10.2337/dc12-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meerwaldt R, Graaff R, Oomen PHN, Links TP, Jager JJ, Alderson NL, Thorpe SR, Baynes JW, Gans ROB, Smit AJ. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47:1324–1330. doi: 10.1007/s00125-004-1451-2. [DOI] [PubMed] [Google Scholar]
- 28.Baulmann J, Schillings U, Rickert S, Uen S, Düsing R, Illyes M, Cziraki A, Nickering G, Mengden T. A new oscillometric method for assessment of arterial stiffness: comparison with tonometric and piezo-electronic methods. J. Hypertens. 2008;26:523–528. doi: 10.1097/HJH.0b013e3282f314f7. [DOI] [PubMed] [Google Scholar]
- 29.Horváth IG, Németh A, Lenkey Z, Alessandri N, Tufano F, Kis P, Gaszner B, Cziráki A. Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J. Hypertens. 2010;28:2068–2075. doi: 10.1097/HJH.0b013e32833c8a1a. [DOI] [PubMed] [Google Scholar]
- 30.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 31.de la Sierra A, Redon J, Banegas JR, et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466–472. doi: 10.1161/HYPERTENSIONAHA.108.124008. [DOI] [PubMed] [Google Scholar]
- 32.Michelson AD. Methods for the measurement of platelet function. Am J Cardiol. 2009;103:20A–26A. doi: 10.1016/j.amjcard.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Puurunen M, Johnson AD. Mean platelet volume—a controversial marker of platelets that requires further unpacking. Thromb. Res. 2017;153:118–119. doi: 10.1016/j.thromres.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Cattaneo M. Light transmission aggregometry and ATP release for the diagnostic assessment of platelet function. Semin. Thromb. Hemost. 2009;35:158–167. doi: 10.1055/s-0029-1220324. [DOI] [PubMed] [Google Scholar]
- 35.Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br. J. Haematol. 1982;50:509–519. doi: 10.1111/j.1365-2141.1982.tb01947.x. [DOI] [PubMed] [Google Scholar]
- 36.Beyan C, Kaptan K, Ifran A. Platelet count, mean platelet volume, platelet distribution width, and plateletcrit do not correlate with optical platelet aggregation responses in healthy volunteers. J. Thromb. Thrombolysis. 2006;22:161–164. doi: 10.1007/s11239-006-9014-7. [DOI] [PubMed] [Google Scholar]
- 37.De Luca G, Verdoia M, Cassetti E, et al. Mean platelet volume is not associated with platelet reactivity and the extent of coronary artery disease in diabetic patients. Blood Coagul. Fibrinolysis. 2013;24:619–624. doi: 10.1097/MBC.0b013e328360c75a. [DOI] [PubMed] [Google Scholar]
- 38.Nadar SK, Blann AD, Kamath S, Beevers DG, Lip GYH. Platelet indexes in relation to target organ damage in high-risk hypertensive patients: a substudy of the Anglo-Scandinavian cardiac outcomes trial (ASCOT) J Am Coll Cardiol. 2004;44:415–422. doi: 10.1016/j.jacc.2004.03.067. [DOI] [PubMed] [Google Scholar]
- 39.Gang L, Yanyan Z, Zhongwei Z, Juan D. Association between mean platelet volume and hypertension incidence. Hypertens Res. 2017;40:779–784. doi: 10.1038/hr.2017.30. [DOI] [PubMed] [Google Scholar]
- 40.Yuksel C, Celik T, Demirkol S, Celik M, Bugan B, Iyisoy A, Yaman H. Increased platelet activation in young patients with prehypertension. Clin. Exp. Hypertens. 2011;33:381–387. doi: 10.3109/10641963.2010.549263. [DOI] [PubMed] [Google Scholar]
- 41.Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, Mohler ER, Reilly MP, Berger JS. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J. Thromb. Haemost. 2010;8:148–156. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blann AD, Nadar S, Lip GYH. Pharmacological modulation of platelet function in hypertension. Hypertension. 2003;42:1–7. doi: 10.1161/01.HYP.0000077901.84467.E1. [DOI] [PubMed] [Google Scholar]
- 43.Siess W, Lapetina EG. Platelet aggregation induced by alpha 2-adrenoceptor and protein kinase C activation. A novel synergism. Biochem. J. 1989;263:377–385. doi: 10.1042/bj2630377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL. Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am. J. Hypertens. 2002;15:111–118. doi: 10.1016/S0895-7061(01)02251-8. [DOI] [PubMed] [Google Scholar]
- 45.Kring C, Rasmussen LM, Lindholt JS, Diederichsen ACP, Vinholt PJ. Platelet aggregation is not altered among men with diabetes mellitus. Acta Diabetol. 2020;57:389–399. doi: 10.1007/s00592-019-01438-y. [DOI] [PubMed] [Google Scholar]
- 46.Shimodaira M, Niwa T, Nakajima K, Kobayashi M, Hanyu N, Nakayama T. Correlation between mean platelet volume and fasting plasma glucose levels in prediabetic and normoglycemic individuals. Cardiovasc. Diabetol. 2013;12:14. doi: 10.1186/1475-2840-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ersoy M, Selcuk Duru HN, Elevli M, Ersoy O, Civilibal M. Aortic Intima-media thickness and mean platelet volume in children with type 1 diabetes mellitus. Iran J. Pediatr. 2015;25(2):e368. doi: 10.5812/ijp.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdel-Moneim A, Zanaty MI, El-Sayed A, Khalil RG, Rahman HA. Relation between oxidative stress and hematologic abnormalities in children with type 1 diabetes. Can. J. Diabetes. 2020;44:222–228. doi: 10.1016/j.jcjd.2019.07.153. [DOI] [PubMed] [Google Scholar]
- 49.Theilade S, Lajer M, Joergensen C, Persson F, Andresdottir G, Reinhard H, Nielsen S, Lacy P, Williams B, Rossing P. 67 first ever 24-hour central blood pressure in patients with type 1 diabetes. J Hypertens. 2012;30:21. doi: 10.1097/01.hjh.0000419892.35120.97. [DOI] [Google Scholar]
- 50.Vílchez-López FJ, Carral-Sanlaureano F, Coserria-Sánchez C, Nieto A, Jiménez S, Aguilar-Diosdado M. Alterations in arterial pressure in patients with type 1 diabetes are associated with long-term poor metabolic control and a more atherogenic lipid profile. J. Endocrinol. Invest. 2011;34:e24–29. doi: 10.1007/BF03347057. [DOI] [PubMed] [Google Scholar]
- 51.Mamta J, Lynn A, Kara M-S, Rodica P-B. Is there an association between non-dipping blood pressure and measures of glucose variability in type 1 diabetes? J. Diabetes Complicat. 2018;32:947–950. doi: 10.1016/j.jdiacomp.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stella P, Tabak AG, Zgibor JC, Orchard TJ. Late diabetes complications and non-dipping phenomenon in patients with Type 1 diabetes. Diabetes Res. Clin. Pract. 2006;71:14–20. doi: 10.1016/j.diabres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Spallone V, Gambardella S, Maiello MR, Barini A, Frontoni S, Menzinger G. Relationship between autonomic neuropathy, 24-h blood pressure profile, and nephropathy in normotensive IDDM patients. Diabetes Care. 1994;17:578–584. doi: 10.2337/diacare.17.6.578. [DOI] [PubMed] [Google Scholar]
- 54.Yildiz G, Hür E, Özçiçek A, Candan F, Kayatas M. The mean platelet volume and atherogenic index of plasma in nondipper normotensive individuals compared to dippers. Clin. Exp. Hypertens. 2013;35:35–39. doi: 10.3109/10641963.2012.689043. [DOI] [PubMed] [Google Scholar]
- 55.Taban Sadeghi M, Soroureddin Z, Nouri-Vaskeh M, Nazarpoori P, Aghayari Sheikh Neshin S. Association of the mean platelet volume and red cell distribution width with dipper and non-dipper blood pressure in prehypertensive non-smokers. BMC. Res. Notes. 2019;12:824. doi: 10.1186/s13104-019-4868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cicek Y, Durakoglugil ME, Kocaman SA, Cetin M, Erdogan T, Dogan S, Ugurlu Y, Canga A. Non-dipping pattern in untreated hypertensive patients is related to increased pulse wave velocity independent of raised nocturnal blood pressure. Blood Press. 2013;22:34–38. doi: 10.3109/08037051.2012.701409. [DOI] [PubMed] [Google Scholar]
- 57.Yazici M, Kaya A, Kaya Y, Albayrak S, Cinemre H, Ozhan H. Lifestyle modification decreases the mean platelet volume in prehypertensive patients. Platelets. 2009;20:58–63. doi: 10.1080/09537100802613449. [DOI] [PubMed] [Google Scholar]
- 58.Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASDThe Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD) Eur. Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 59.ASCEND Study Collaborative Group Effects of aspirin for primary prevention in persons with diabetes mellitus. New Engl. J. Med. 2018;379:1529–1539. doi: 10.1056/NEJMoa1804988. [DOI] [PubMed] [Google Scholar]
- 60.Jackson SR, Carter JM. Platelet volume: laboratory measurement and clinical application. Blood Rev. 1993;7:104–113. doi: 10.1016/S0268-960X(05)80020-7. [DOI] [PubMed] [Google Scholar]
- 61.Lancé MD, Sloep M, Henskens YMC, Marcus MAE. Mean platelet volume as a diagnostic marker for cardiovascular disease: drawbacks of preanalytical conditions and measuring techniques. Clin. Appl. Thromb. Hemost. 2012;18:561–568. doi: 10.1177/1076029612458147. [DOI] [PubMed] [Google Scholar]
- 62.Jelenik T, Séquaris G, Kaul K, et al. Tissue-specific differences in the development of insulin resistance in a mouse model for type 1 diabetes. Diabetes. 2014;63:3856–3867. doi: 10.2337/db13-1794. [DOI] [PubMed] [Google Scholar]
- 63.Harrison P, Goodall AH. Studies on mean platelet volume (MPV)—new editorial policy. Platelets. 2016;27(7):605–606. doi: 10.1080/09537104.2016.1225467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.


